Abstract
Abnormal oxidative stress caused by human immunodeficiency virus (HIV) infection affects viral replication and causes non-acquired immune deficiency syndrome-related complications in infected individuals. The transcription factor NFE2-related factor 2 (NRF2), a key regulator of oxidative stress, responds to abnormal oxidative stress by regulating the expression of NRF2-dependent cytoprotective genes. The present study aimed to determine whether inhibition of oxidative stress could control HIV replication and improve cell survival. In this study, the NRF2 activator, methyl bardoxolone, was used to treat cells for HIV infection. The effects on HIV replication and apoptosis pathways were confirmed by NRF2 activation or knockdown. The results showed that NRF2 activation could block HIV replication in macrophages before the integration phase and inhibited the expression of apoptotic pathways in virus-exposed macrophages. The study presents an unconventional anti-viral strategy of activation antioxidant response for HIV infection blocking.
Keywords: HIV, Oxidative stress, Inflammation, Macrophage, Apoptosis
HIV; Oxidative stress; Inflammation; Macrophage; Apoptosis
1. Introduction
Although highly active antiretroviral therapy (HAART) therapy has improved the mortality rate of most patients with acquired immune deficiency syndrome (AIDS), the emergence of drug resistance and short- and long-term side effects induced by this therapy remains a challenge. Insight into cellular antiviral defense mechanisms may promote cross these limitations and support new prevention and treatment strategies.
Monocytes are important innate immune cells associated with immunopathogenesis in human immunodeficiency virus (HIV) infection. During their differentiation into macrophages, monocytes become extremely susceptible to HIV infection [1], carrying the virus into restricted compartments like the brain [2], and vagina [3] and constituting the viral reservoir. Importantly, macrophages increase T cell infection [4] and thereby exacerbate T cell depletion in infected individuals, thus increasing the patient's immunodeficiency. Therefore, preventing HIV replication in mononuclear macrophages is essential to prevent and control HIV infection, and alleviate complications.
During HIV infection, monocytes often exhibit enhanced sensitivity to inflammation and abnormal oxidative stress thereby enhancing HIV progression [5, 6, 7, 8]. Moreover, oxidative stress and inflammation are closely associated: for one thing inflammation due to infections leads to increased levels of reactive oxygen species (ROS) and induce oxidative stress [9, 10]; for another, inflammatory responses can also be induced by oxidative stress [11]. Thus, inflammation and oxidative stress act as major features in HIV monocyte-derived macrophage infection. A report suggested that inhibiting inflammation could curb HIV propagation [12]. And defense against oxidative stress is recognized to resist viral replication and disease progression [13, 14]. Therefore, understanding the oxidative stress and inflammation process after infection would benefit strategies to depress HIV replication and alleviate complications.
Transcription factor NFE2-related factor 2 (NRF2) is an important negative regulator of oxidative stress and inflammation. NRF2-activators dissociate NRF2 from its negative regulator, Keap1, allowing it to translocate into the nucleus, where it binds to antioxidant response elements (AREs) to express NRF2-dependent cytoprotective genes [15, 16]. Studies have confirmed that NRF2 activation is accompanied by increased antioxidants and decreased inflammatory cytokine levels, which is expected to reduce cellular oxidative damage and regulate cells’ antiviral defense.
Previous studies showed that the NRF2 pathway activator, sulforaphane (SFN), could effectively inhibit HIV infection in macrophages, but not in T cells [17]. In the present study, we used a new NRF2 pathway activator and nuclear factor kappa B (NF-κB) pathway suppressant, methyl bardoxolone (Bard), to treat HIV-infected macrophages, detecting the drug's effects on resistance to virus replication, and inflammatory cytokines and antioxidant levels, to verify that anti-inflammatory and antioxidant treatment could achieve viral suppression. Promoting HIV blocking and apoptosis suppressing in HIV-infected macrophages is expected to induce restraining HIV infection.
2. Materials and methods
2.1. Cell lines
The 293T, primary monocytes, and TZM-bl cells were cultured in DMEM and the THP-1 cell and primary T cells were cultured in RPMI (1640) medium (Sigma-Aldrich, St. Louis, MO, USA). The medium was supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin (Gibco®, Invitrogen, Waltham, MA, USA) at 37 °C and 5% CO2. THP-1 cells were differentiated into a macrophage-like state in media containing 100 ng/ml phorbol myristate acetate (PMA) for 2 days.
2.2. Primary cell isolation
Human Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of whole blood using Ficoll density gradient centrifugation. Monocytes were isolated by adhesion and differentiated into human monocyte-derived macrophages (hMDMs) for 7 days in RPMI1640 supplemented with hGM-CSF(1000U/ml) (PeproTech AF-300-03). Primary T cells were isolated using CD3-immunomagnetic beads (Miltenyi Biotech, Auburn, CA, USA) and maintained in RPMI1640 supplemented with 10% FBS, 10 U/ml interleukin-2 at 37 °C and 5% CO2. Cells were maintained for further analysis.
2.3. Virus preparation and infection
One day before transfection,2×106 HEK293T cells were inoculated in 10 cm plates for 16 h. The next day, 4 μg HIV-1 pNL4.3 env (−)GFP(+) were co-transfected with 1μg pCL-VSV-G by Lipofectamine 2000 Transfection Reagent. 48 h post-transfection, virus-containing supernatants were collected and monitored using a p24 enzyme-linked immunosorbent assay (ELISA) kit (R&D System, Minneapolis, MN USA) to estimate the virus concentration. THP-1 cells were infected via spinoculation at 2,000 rpm for 3 h using a series of virus concentrations and determined the optimal concentration of 40 ng/mL.
2.4. Reagents
Methyl bardoxolone (Bard) was purchased from MCE (Monmouth junction, NJ, USA; HY-13324) and dissolved in dimethyl sulfoxide (DMSO; 100 mM stock). PMA (P1585) and lipopolysaccharide (LPS) (L2630) were acquired from Sigma-Aldrich and dissolved in DMSO. Anti-heme oxygenase (HO-1) Rabbit antibody (ab68477), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mouse antibody (ab8245), Goat anti-rabbit antibody IgG H&L (HRP) (ab97051), and Rabbit anti-mouse antibody IgG H&L (HRP) (ab97046) were obtained from Abcam (Abcam Cambridge, UK). Rabbit anti-P65 antibody (8242), rabbit anti-phospho (p)–NF–κB p65 (Ser536) antibody (3033), rabbit anti-Phospho-IKKα/β (Ser176/180) antibody (2697), rabbit anti-IKKβ antibody (2678), rabbit anti-p-P38 antibody (4511), rabbit anti-extracellular kinase 1/2 (ERK1/2) antibody (4695), rabbit anti-p-ERK1/2 (p44/42) antibody (4376), rabbit anti-p-JUN N-Terminal Kinase (JNK) antibody (27747), rabbit anti-p-mitogen-activated protein kinase (MAPK) activated protein kinase 2 (MAPKAPK-2) antibody (3007), rabbit anti-p-p53 antibody (9286), mouse anti-p53 antibody (2524), mouse anti-BCL2 apoptosis regulator Bcl-2 antibody (15071), rabbit anti-BCL2 associated X, apoptosis regulator (Bax) antibody (5023), rabbit anti-cleaved Caspase 3 antibody (9664), rabbit anti-Caspase-3 antibody (14220), rabbit anti-cleaved Caspase 9 antibody (7237), and rabbit anti-cleaved poly (ADP-Ribose) polymerase (PARP) antibody (9664) and rabbit anti-PARP antibody (9532) were purchased from Cell Signaling Technology (CST, Danvers, MA, USA).
2.5. Quantitative real-time reverse transcription PCR (qRT-PCR)
Host cells' gene transcription levels and HIV DNA levels were analyzed by qRT-PCR. Real-time PCR for HIV Viral nucleic acids was performed as described previously [18]. Total RNA was extracted using Trizol and cDNA was synthesized using Takara reverse transcriptase (037A, Takara, Shiga, Japan). The total DNA of host cells was isolated using a Qiagen DNA Mini kit (QIAGEN, Hilden, Germany). DNA was analyzed using SYBR Green fluorescence (iQ SYBR Green Supermix, Bio-Rad, Hercules, CA, USA).
2.6. ELISA
The human inflammatory cytokines were detected using ELISA kits (Dakewe Biotech Co., Ltd., Shenzhen, China). Viral production was measured using P24 HIV-1 antigen quantification (R&D Systems Inc.). ELISA detection of supernatants was performed according to the manufacturer's instructions.
2.7. Western blotting
Protein lysates were prepared from a total of 6-well cells by using radioimmunoprecipitation assay (RIPA) buffer containing a 10% proa tease inhibitor cocktail (Sigma). Protein concentrations were quantified using the bicinchoninic acid (BCA) protein assay (Bio-Rad). The samples (20 μg) were added with 2 × sample buffer and heated to denature the proteins. The proteins were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked and incubated with primary antibodies (see section 2.4) overnight at 4 °C. For fluorescent detection of protein expression, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (see Section 2.4) for 1 h at room temperature and visualized using enhanced chemiluminescence reagents and the VersaDoc MP 5000 Imaging system (Bio-Rad). The protein band density values were normalized to GAPDH protein levels.
2.8. Assessment of oxidative stress
Catalase (CAT) activity in THP-1 cells was measured using a catalase assay kit (Cat. S0051). Superoxide dismutase 2 (SOD2) enzymatic activity was measured using Cu/Zn-SOD and Mn-SOD Assay Kits with WST-8 (Cat. S0103). Intracellular ROS were estimated by detecting oxidation of 2,7-dichlorofluorescein diacetate (DCFH-DA). The malondialdehyde (MDA) levels were evaluated using a Lipid Peroxidation MDA Assay Kit (Cat. S0131). The intracellular levels of glutathione (GSH) were measured using a GSH and glutathione disulfide (GSSG) Assay Kit (Cat. S0053). All the kits were performed according to the manufacturer's protocol (Beyotime Institute of Biotechnology, Shanghai, China).
2.9. Small interfering RNA (siRNA) transfection
An NRF2 siRNA and siRNA control (si-NC) were purchased from GenePharma (Shanghai, China) for temporary gene silencing. The targeted sequences of si-Nrf2 were as follows: GGAGAAGUGUUUGAGUUUATT. THP-1 cells were transfected siRNA using RNAiMAX Transfection Reagent (Cat. 13778075) (Invitrogen, San Diego, CA, USA) following the manufacturer's protocols. Cells were cultured for 48 h for subsequent experiments.
2.10. Flow cytometry
The percentages of type 1 macrophages (M1) (CD206−/CD86+), and type 2 macrophages (M2) (CD206+/CD86−) were measured using flow cytometry using a BD FACSCanto II instrument (Becton Dickinson, Franklin Lakes, NJ, USA). Single-cell pellets were stained with anti-CD86-PE, anti-CD206-FITC, and anti-CD11 b-APC. The data were analyzed using FlowJo v.10 software (FlowJo LLC, Ashland, OR, USA).
2.11. Statistical analysis
GraphPad Prism 8 (GraphPad Inc., La Jolla, CA, USA) and SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis and constructing of the plots. Data are presented as the mean ± SD. One-way ANOVA followed by Student–Newman–Keuls posthoc analysis was used to analyze the statistical differences. Paired samples were compared using a paired t-test. A P value <0.05 was considered statistically significant.
3. Results
3.1. Activation of NRF2 inhibits HIV replication in primary macrophages but not in T cells
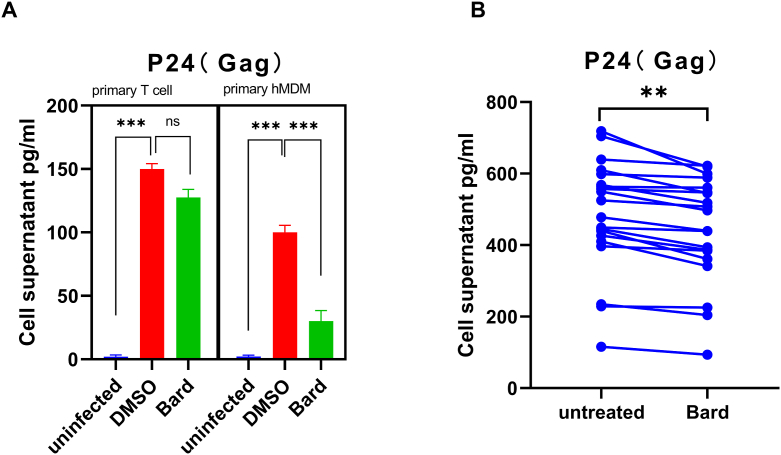
Previous reports demonstrated that NRF2 pathway activation affects macrophage virus infection. In the present study, to determine whether Bard has an antiviral effect on HIV by activating NRF2, normal adult primary monocytes and lymphocytes were isolated and infected with VSV-G pseudotyped HIV-1 GFP reporter virus strain in Figure 1A, then the level of P24 in the supernatant was measured for compared the level of HIV replication between Bard treated or non-treated cells. The result shows that the level of P24 from the supernatant of primary hMDMs was significantly suppressed by Bard treatment. However, the same result was not obtained in primary T lymphocytes. Meanwhile, we collected and activated monocytes from ART-treated well-controlled individuals, and treated them with Bard to compare the changes in viral replication before and after treatment. Similarly, Bard treatment inhibited virus replication in these cells to a certain extent (Figure 1B). Collectively, these results indicated that NRF2 activation reduces virus replication in hMDMs but not lymphocytes.
Figure 1.
NRF2 blocks HIV in primary macrophages but not in primary T cells. (A) Primary T cells and hMDMs were either mock-infected or infected with VSV-G pseudotyped HIV-1 GFP reporter virus for 24 h. (B) Primary hMDMs from HIV-infected patients were treated with media supplemented with vehicle only (DMSO) or with 0.1 μM Bard. After 24 h of treatment, the supernatant of the cells was harvested and the level of P24(Gag) was measured using ELISA. The bar graphs represent the data for replicate experiments (n = 3). The line represents the data from the same patient (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001). NRF2, NFE2-related factor 2; hMDMs, human monocyte-derived macrophages; DMSO, dimethyl sulfoxide; Bard, methyl bardoxolone; ELISA, enzyme-linked immunosorbent assay.
3.2. NRF2 inhibits virus replication by affecting early phase and transcription of the virus
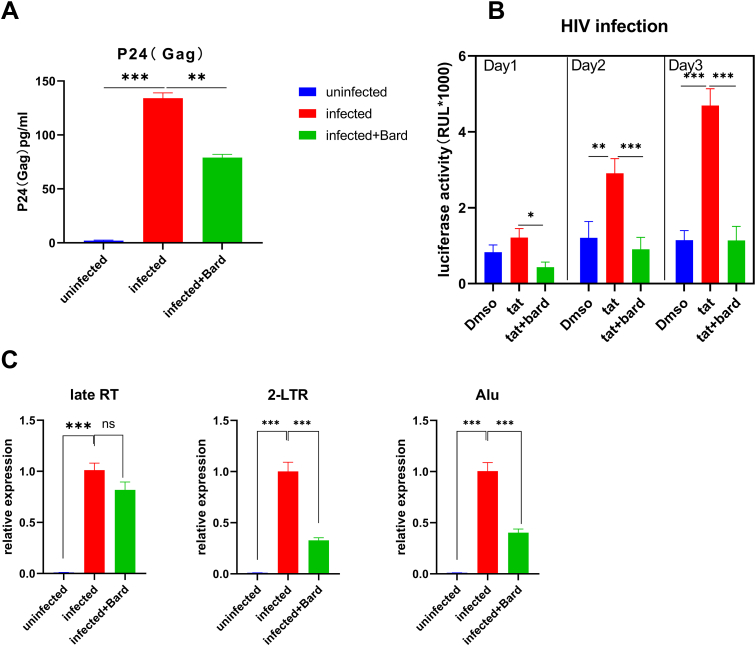
Next, we want to know the effect of NRF2 activation on the virus's life cycle. Firstly, we used the pNL4-3/VSV-G pseudovirus strain, a pseudovirus that alters its entry, to infect the PMA-stimulated Bard pretreatment mononuclear macrophage cell line THP-1 and detect the virus entry process and the release of virions. The level of P24 from the supernatant decreased under NRF2 activation after HIV infection, which showed that blocking HIV replication failed to affect the virus entry (Figure 2A). Next, to detect the blocking of HIV replication after entry, we used qPCR to measure the reverse transcription products of PMA-stimulated HIV-infected THP-1 cells with Bard pretreated. We found that the controls and cells with NRF2 activation showed similar levels of late reverse transcription products, indicating that activated NRF2 cannot hinder the replication steps from viral entry to viral reverse transcription (Figure 2C). By contrast, the result for 2-long terminal repeat (2-LTR) circle qPCR, an unproductive infection byproduct of viral preintegration complex transportation to the nucleus, was clearly decreased in NRF2 activation samples compared to untreated controls (Figure 2D). Besides, Alu-PCR of integrated proviral sequences showed a similar decrease compared with that in the vehicle-treated controls (Figure 2E,p < 0.05). Taken together, the result showed that NRF2 inhibits virus replication before the formation of the 2-LTR circles process. Also, its effect on HIV-1 LTR transcription activation was determinated in TZMbl cells, a HeLa-derived cell line that contains a stably integrated HIV-LTR-luciferase construct (Figure 2B). The result shows that Bard treatment significantly reduced Tat-induced transcriptional activation. Totally, these results proved that NRF2 activation affects the early phase and transcription of the life cycle of the virus.
Figure 2.
NRF2 blocks HIV after entry and before or at 2-LTR circle formation. (A) PMA-induced THP-1 cells were pretreated with media supplemented with vehicle only (DMSO) or with 0.1 μM Bard for 24 h, after cells, were infected with either mock-infected or VSV-G-pseudotyped HIV-1 encoding GFP virus. After 24 h, cells were harvested for P24(Gag) detection. The bar graphs represent the data for replicate experiments (n = 3). (B) TZM-bl cells were treated with media supplemented with 100 ng/ml Tat or with 0.1 μM Bard for 24 h, 48 h, or 72 h and measured for luciferase expression, the data are presented as the mean ± SD (n = 3). (C–E) PMA-induced THP-1 cells were infected with VSV-G pseudotyped HIV-1 GFP reporter virus for 24 h after pretreated with media supplemented with vehicle only (DMSO) or with 0.1 μM Bard for 24 h. Cells were then harvested and viral DNA products were detected using real-time PCR with primer sets specific for the stage of HIV reverse transcription. (C) Relative quantities of late reverse transcription products, (D) 2-LTR circles, and (E) integrated proviruses. The bar graph represents the data for replicate experiments (n = 3). NRF2, NFE2-related factor 2; PMA, phorbol myristate acetate; GFP, green fluorescent protein; DMSO, dimethyl sulfoxide; Bard, methyl bardoxolone; 2-LTR, 2-long terminal repeat.
3.3. The activation of NRF2 affects the level of redox substances
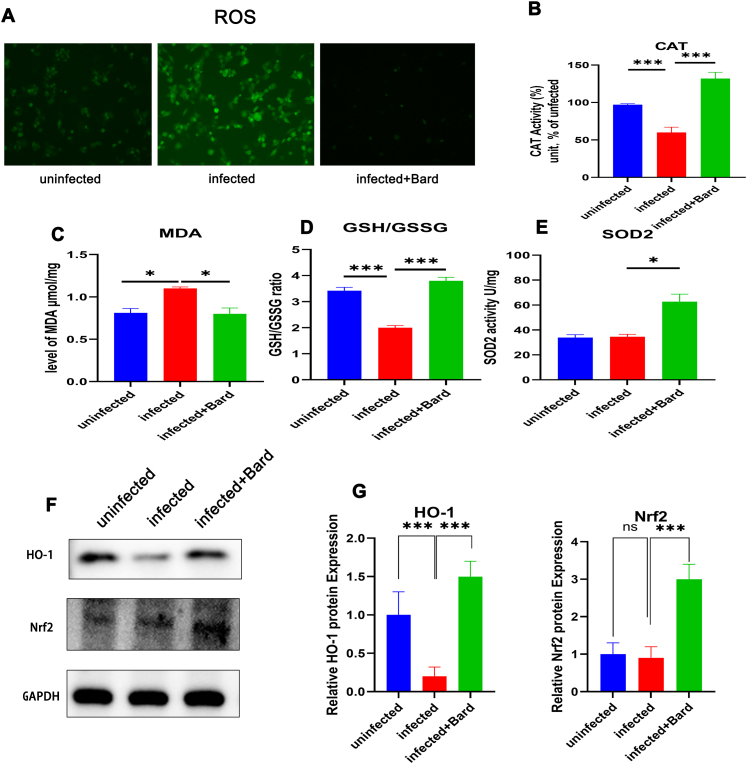
Generally, HIV infection induces oxidative stress and causes abnormal expression of redox substances and the release of reactive oxygen species. This is modulated by the activated NRF2 pathway. To test the influence of the NRF2 pathway on redox substances after HIV infection, we measured the levels of ROS, CAT, MDA, SOD2 activity, and the GSH/GSSG ratio in infected THP-1 cells. The results present that NRF2 activation could significantly decrease intracellular ROS (Figure 3A), the MDA content (Figure 3C) and increase the CAT activity (Figure 3B), GSH/GSSG ratio (Figure 3D), and SOD2 activity (Figure 3E) compared with HIV exposure alone. Similarly, the levels of HO-1 and NRF2 were also upregulated by NRF2 activation than controls infected with HIV alone (Figure 3F, G). These results indicated that activation of NRF2 alleviates the excessive oxidative stress caused by HIV infection, probably related to the reduction of virus replication.
Figure 3.
NRF2 activation promotes HIV induced the expression of oxides and antioxidants in macrophage cells.
THP-1 cells were either mock-infected or infected with VSV-G pseudotyped HIV-1 GFP reporter virus for 24 h and treated with media supplemented with vehicle only (DMSO) or with 0.1 μM Bard. After 24 h, the levels of ROS (A) CAT(B) MDA (C) GSH/GSSG (D) SOD2 (E) in the cells were measured using assay kit and HO-1 (F) was assessed using western blotting. The bar graphs represent quantification of the Western blot band density normalized to the control for replicate experiments (n = 3). Each experiment was repeated three times with similar results. (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001). NRF2, NFE2-related factor 2; DMSO, dimethyl sulfoxide; Bard, methyl bardoxolone; ROS, reactive oxygen species; CAT, catalase; MDA, malondialdehyde; GSH, glutathione; GSSG, glutathione disulfide; SOD, superoxide dismutase; HO-1, heme oxygenase 1.
3.4. NRF2 activation inhibits pro-inflammatory cytokine levels
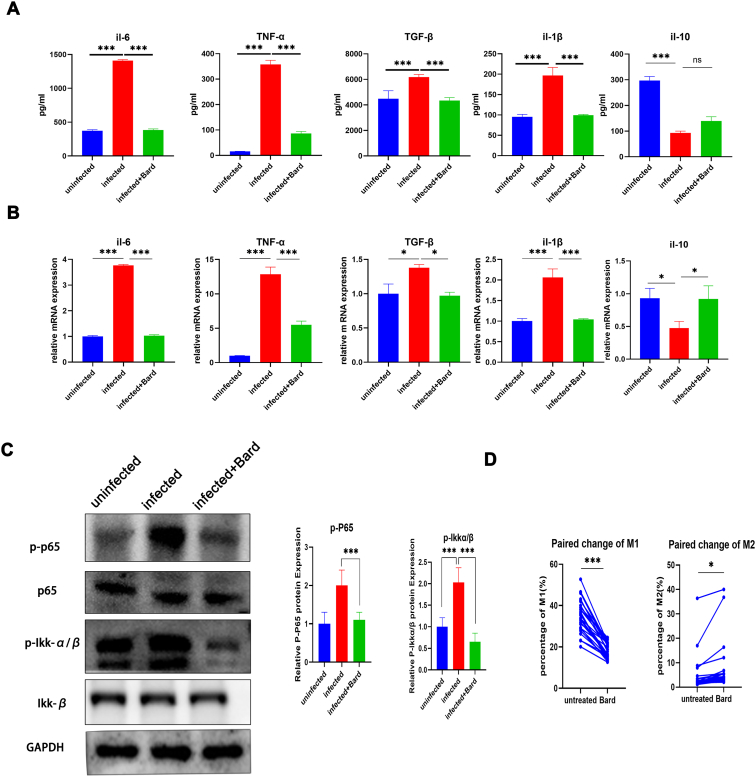
Normally, excessive oxidative stress is often accompanied by the abnormal activation of inflammatory factors and manifested as an increased secretion of pro-inflammatory cytokine and a decrease of anti-inflammatory cytokines as well as activation of the NF-κB pathway by macrophages. These are also identified as factors involved in regulatory virus replication. To verify the activation of NRF2 regulating the secretion of cytokines in macrophages, we detected the level of proinflammatory cytokines IL-1β, IL-6, TNF-α, TGF-β, anti-inflammatory cytokine IL-10 from macrophages supernatant and mRNA by ELISA and qPCR, and the level of Phospho-p65, Phospho-Ikkα/β by Western blot. From our research, the secretion of proinflammatory cytokines and the level of Phosphorylation p65, Ikkα/β of THP-1 cells following NRF2 activation and virus-infected was significantly lower than that of infected cells, especially for the two inflammatory factors, TNF-α and IL-6 (Figure 4A, C). qPCR detection of cytokines in the same cells showed consistent results with the supernatant (Figure 4B). However, we did not observe NRF2-induced upregulation of anti-inflammatory factors, probably because of cell dysfunction from the infection.
Figure 4.
NRF2 inhibits HIV induced pro-inflammatory effects in macrophage cells.
THP-1 cells were either mock-infected or infected with VSV-G pseudotyped HIV-1 GFP reporter virus for 24,h and then treated with media supplemented with vehicle only (DMSO) or with, 0.1 μM Bard. After 24 h of treatment, the supernatant and the cells were harvested and the level of cytokines was measured using ELISA (A) and cytokine mRNAs were measured using real-time PCR (B), the cellular proteins were harvested to meBiomedicineevel of phosphorylated P65 and Ikkα/β, the relative quantification of the Western blot band density was normalized to that of the control (C). The bar graphs represent the data for replicate experiments (n = 3). (D) Primary hMDMs cells from HIV-infected patients were treated with media supplemented with vehicle only (DMSO) or with 0.1 μM Bard. After 24 h of treatment, the cells were harvested for flow cytometry to measure the percentage of M1 type and M2 type macrophages; the lines represent the data from the same patient. The lines represent the data from the same patient (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001). NRF2, NFE2-related factor 2; hMDMs, human monocyte-derived macrophages; DMSO, dimethyl sulfoxide; Bard, methyl bardoxolone; ELISA, enzyme-linked immunosorbent assay.
Next, to further understand the effect of the increased secretion of pro-inflammatory cytokines in macrophages, flow cytometry was used to examine the phenotypic changes in virus-infected THP-1 cells and monocytes from patients with AIDS (Figure 4D). The M1 and M2 ratio changes in these cells before and after NRF2 activation were an anti-inflammatory trend. As the result, NRF2 activation alleviated the abnormal increase in the proportion of M1-type macrophages, whereas, and the decrease in the proportion of M2-type cells. These results suggested that NRF2 activation alleviates the abnormal inflammatory activation of macrophages caused by HIV infection, which also helps to suppress HIV infection of monocyte-macrophages.
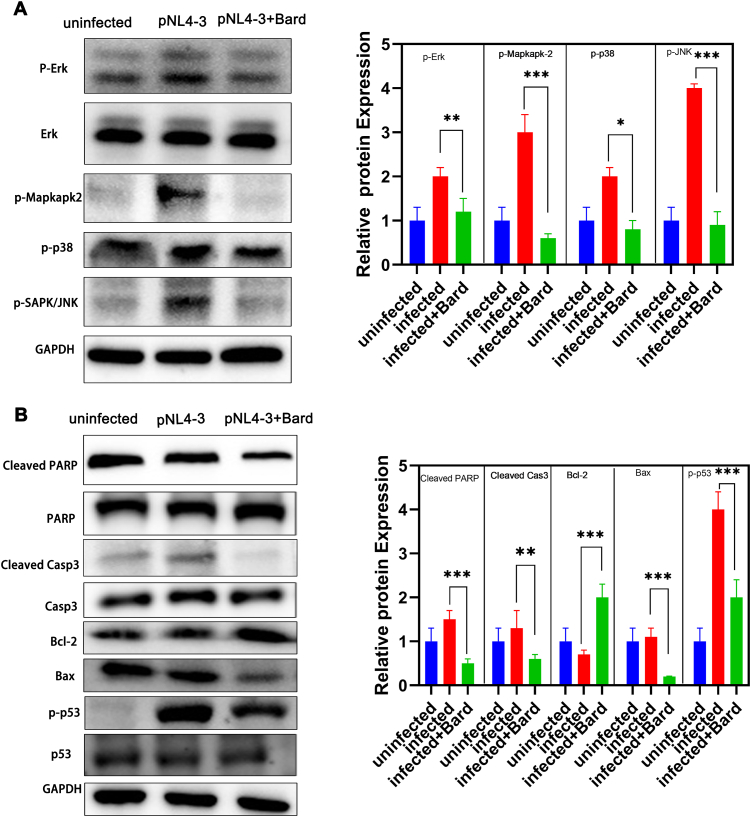
3.5. NRF2 activation inhibits the MAPK pathway and apoptosis pathway of THP-1 cells
Apoptosis is considered to be another key factor involved in the regulation of the NRF2 pathway after HIV replication. The important role of the MAPK pathway in regulating cell death prompts us to hypothesize that the MAPK cascade is involved in the caspase-independent apoptosis in Bard-treated and HIV-infected cells. For verification, we detected the levels of phosphorylated ERK, P38 MAPK, and JNK in the MAPK pathway using western blotting. After Bard treatment and infection with HIV, the levels of phosphorylated MAPK pathway-related proteins in THP-1 cells decreased significantly compared with those HIV-infected cells alone and were similar to the negative uninfected control levels (Figure 5A). As expected, the level of the anti-apoptotic protein Bcl2 was upregulated and the pro-apoptotic proteins p53, Bax, cleaved-caspase 3, and cleaved-PARP were downregulated in the NRF2 activated and infected samples compared with those infected cells alone, a nearly levels as those in the uninfected control samples (Figure 5B). These results indicated that NRF2 activation in macrophages inhibits the MAPK pathway and thus inhibits apoptosis, thereby affecting the function of hMDMs.
Figure 5.
NRF2 depress the MAPK pathway and apoptosis pathway in HIV infected macrophages cells.
THP-1 cells were either mock-infected or infected withp VSV-G pseudotyped HIV-1 GFP reporter virus for 24 h and then treated with media supplemented with vehicle only (DMSO) or with 5 μM Bard. After 24 h, the levels of apoptosis pathway proteins (A) and MAPK pathway proteins (B)were assessed using western blotting. The bar graphs represent the quantification of Western blot band density normalized to that of the control for replicate experiments (n = 3) Each experiment was repeated three times with similar results. (∗P < 0.05,∗∗P < 0.01, ∗∗∗P < 0.001). NRF2, NFE2-related factor 2; MAPK, mitogen activated protein kinase; DMSO, dimethyl sulfoxide; Bard, methyl bardoxolone.
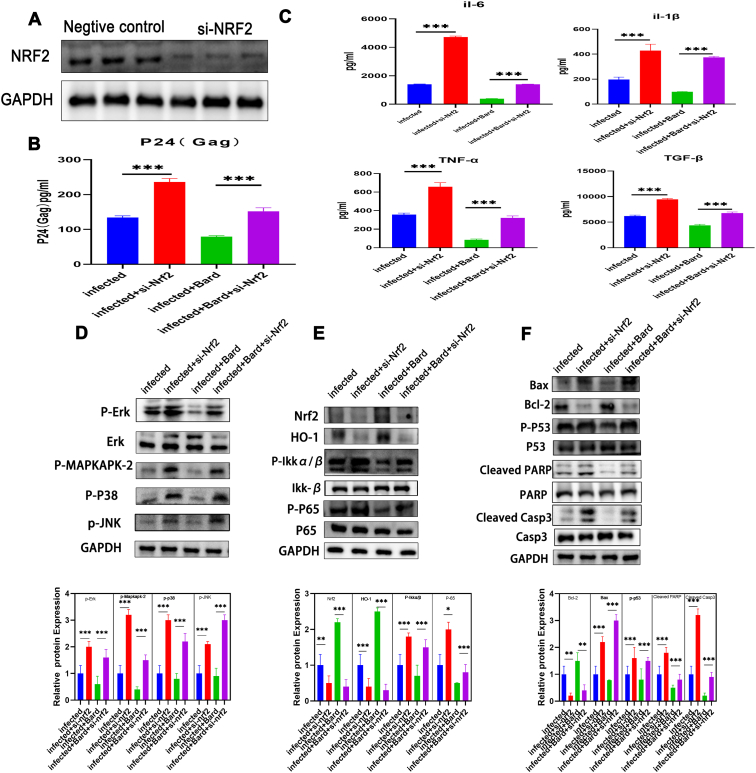
3.6. si-NRF2 restores anti-viral and anti-apoptotic effects
To further confirm the anti-inflammatory and anti-apoptotic effects of response to the NRF2 pathway, we performed a rescue experiment. THP-1 cells were transfected with si-NRF2 to knock down NRF2. Firstly, we observed that transfection of si-NRF2 significantly reduced the NRF2 protein level in cells compared with that transfected with si-NC (Figure 6A). At the same time, we detected the expression of viral protein pro-inflammatory factors of the supernatant. Compared with the si-NC transfected group, the levels of p24 and inflammatory cytokine in the cells after knocking down NRF2 were significantly increased, especially in these NRF2 activation cells (Figure 6B, C). This shows that NRF2 knockdown completely counteracted the inhibition of inflammatory cytokine and HIV virion secretion caused by NRF2 activation. In addition, western blotting experiments proved that the inhibition of the MAPK pathway, apoptosis pathway, and NF-κB pathway by NRF2 activation was also rescued by knockdown (Figure 6D-F). These results confirmed that it is NRF2 activation that improves resistance to viral replication and the inhibitory effect of related pathways after Bard treatment.
Figure 6.
si-NRF2 rescued the function of Bard in HIV infected macrophages cells.(A) THP-1 cells were transfected with si-NRF2 or si-NC, the expression of NRF2 were measured by Western blot for the efficiency of knowndown. THP-1 cells either mock-infected or infected with VSV-G pseudotyped HIV-1 GFP reporter virus were transfected with si-NRF2 or si-NC were treated for 48 h with media supplemented with either vehicle only (DMSO) or with 0.1 μM Bard. After 24 h the supernatant of the cell was harvested and measured by ELISA for the level of P24(Gag) (B) and cytokines (C). Proteins were extracted from the cells to determine the levels of MAPK pathway proteins (C) NF-κB, NRF2, and HO-1 (D) and apoptosis pathway proteins (E) by western blotting. The bar graphs represent quantification of the Western blot band density normalized to that of the control for replicate experiments (n = 3) Each experiment was repeated three times with similar results. (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001RF2, NFE2-related factor 2; Bard, methyl bardoxolone; DMSO, dimethyl sulfoxide; ELISA, enzyme-linked immunosorbent; MAPK, mitogen activated protein kinase; HO-1, heme oxygenase 1.
4. Discussion
Blocking viral replication and controlling aberrant inflammation and apoptosis in macrophages is considered crucial to controlling AIDS after HIV infection [19, 20]. In the present study, we explored the regulation and function of NRF2 activation in inhibiting viral replication in HIV-infected macrophage cells. We demonstrated that activation of NRF2, a transcription regulator of anti-oxidant genes, could block the secretion of pro-inflammatory cytokines after HIV infection, which would benefit the suppression of non-AIDS comorbidities in patients with HIV. Importantly, we found that NRF2 activation reduced macrophage apoptosis, which might improve macrophage immune ability. These results emphasized that elevated active NRF2 is critically important to HIV + individuals and ultimately affects integration and transcription. We focused on NRF2 activation in macrophage infection, intending to determine the possible benefits of blocking virus replication and other effects on HIV infection.
Normally, NRF2 is present in the cytoplasm and is degraded by Keap1-induced ubiquitination. Following abnormal oxidative stress, NRF2 responds quickly and migrates to the nucleus [21]. HIV infection reduces the antioxidant capacity, which also produces an increase in ROS and MDA levels, a reduction in GSH and SOD contents, and an increased GSSG/GSH ratio [5]. Our research was highly consistent with previous researches (Figure 2). Probably, this increased oxidation might be caused by HIV-1 inhibiting the NRF2/ARE axis, which is likely to be involved in the replication of HIV. Studies have reported a possible association between oxidative stress and HIV replication, and the expression of the NRF2 downstream regulatory protein HO-1 correlates negatively with the level of HIV replication in macrophages [22, 23]. Therefore, studying the effects of HIV infection on NRF2 and oxidative stress is essential to develop strategies to block viral replication.
Honestly, Noronha et al. observed that SFN blocked virus replication in macrophages by the upregulation of NRF2 expression [17]. Similarly, we showed a similar inhibition effect by Bard-induced activation of NRF2. We also demonstrated that NRF2 regulates HIV replication after entry before integration. However, we also found that NRF2 activation suppression the transcription of HIV. Therefore, our research demonstrated that NRF2 activation blocks the release of viral particles by blocking multiple viral phases. Besides, our result also proved that NRF2 activation alleviated the abnormal oxidative stress caused by cell infection. This might construct a poor cellular environment that can effectively prevent the virus from spreading. Concretely, the effect of this result was manifested in inhibiting the post-entry process of the virus and the function of Tat transcriptional activation. However, NRF2 directly regulates intracellular host proteins or viral proteins through anti-oxidative stress to block HIV replication remains to be determined.
Our result report that activation of NRF2 decreases HIV-1 LTR transactivation, similar to results conducted by previous researchers [24], This partly explains the result of the blocking effect on viral replication, indicating that viral transcription is impaired by NRF2 activation. Besides, our finding also found that NRF2 activation strongly inhibits infection at or before 2-LTR formation, which could account for the NRF2-mediated HIV block in another way. Likewise, this is a continuation of the previous results, confirming that the NRF2 pathway is involved in HIV post-entry block [17]. Whether these two methods are parallel or complementary, we cannot infer yet. Of course, this may also include other life cycle processes of diseases, but we still need further experimental verification.
HIV-1 infection affects the functions of macrophages significantly, altering cytokine expression [25] and activating the NF-κB and MAPK pathways [26]. Macrophage infection could have long-lasting adverse consequences for several tissue-specific diseases, including AIDS-related liver disease [27], cardiovascular diseases [28], and neurocognitive disorders [25]. Studies reported that NRF2 activation is often accompanied by NF-κB pathway inhibition and decreased levels of pro-inflammatory cytokines such as TNF-α and IL-6 [29-30]. In addition, inhibiting inflammation exerts a strong control of HIV replication [31, 32]. Therefore, the anti-inflammatory effects of NRF2 activation would further inhibit HIV replication.
In our research, NRF2 activation significantly reduced the proportion of M1 cells and decreased levels of pro-inflammatory factors in macrophages HIV-infected. This partly explains the blocking of HIV replication in macrophages by NRF2 activation. Understanding the role of NRF2 activation in inhibiting pro-inflammatory cytokines and HIV-1 pathogenesis could identify therapeutic strategies to treat HIV-1 infection.
Oxidative stress is associated with increased apoptosis in macrophages. HIV infection-induced apoptosis mediated by caspase 3 is characterized by the expression of apoptosis-related proteins caused by ROS generation in productive cells or bystander cells [22, 33, 34, 35]. HIV infection-induced apoptosis is closely related to the depletion of hMDMs after infection, which is also the key to strong immunodeficiency and tissue damage. Therefore, it would be significant to promote cell survival by blocking the signals that regulate and induce apoptosis, especially in non-infected cells. Typically, under oxidative stress, activation of the apoptosis pathway is closely related to the MAPK pathway: Activation of the MAPK/ERK pathway can trigger cell apoptosis [36]. Research showed that the activation of NRF2 also has a strong anti-apoptotic effect [37]. In other research, NRF2 inhibits the activation of P38, ERK, and JNK signaling pathways, which regulate the caspase-independent apoptosis of infected macrophages [38]. In our study, activation of NRF2 inhibited cell apoptosis and the MAPK pathway, including ERK, MAPK/ERK kinase, JNK, and p38. Especially, our results indicated that NRF2 activation promotes macrophage survival and relieves macrophage dysfunction caused by HIV infection; however, it is unknown whether the pro-survival effect is more conducive to productive cells or bystander cells, which requires further research.
Our study had limitations. Firstly, the clinical samples used lacked more comprehensive clinical testing data, and the number of participants was low. Secondly, the key proteins that affect viral replication after NRF2 activation need to be identified. Finally, the key mechanisms involving NRF2 remain to be further determined.
5. Conclusion
In conclusion, our results clarified that NRF2 activation blocks HIV replication and promotes survival in macrophages after infection, providing a potential therapeutic target for patients with AIDS.
Declarations
Author contribution statement
Dating han: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Wanpeng Yin: Analyzed and interpreted the data.
Haijing Fu; Xiaodi Zhang; Linfang Cheng; Fuming Liu; changzhong jin; Xuebin tian; Yiwen Xie: Contributed reagents, materials, analysis tools or data.
Nanping wu: Conceived and designed the experiments.
Funding statement
Nanping wu was supported by Key Technologies Research and Development Program [No.2018ZX10302-102].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
Supplementary content related to this article has been published online at https://doi.org/10.1016/j.heliyon.2022.e12575.
Acknowledgements
We thank Elixigen Corporation for reading our manuscript and providing native English professional support.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Wilson E.M., Singh A., Hullsiek K.H., Gibson D., Henry W.K., Lichtenstein K., Önen N.F., Kojic E., Patel P., Brooks J.T., et al. Monocyte-activation phenotypes are associated with biomarkers of inflammation and coagulation in chronic HIV infection. J. Infect. Dis. 2014;210:1396–1406. doi: 10.1093/infdis/jiu275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carvallo L., Lopez L., Fajardo J.E., Jaureguiberry-Bravo M., Fiser A., Berman J.W. HIV-Tat regulates macrophage gene expression in the context of neuroAIDS. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips D.M., Tan X., Perotti M.E., Zacharopoulos V.R. Mechanism of monocyte-macrophage-mediated transmission of HIV. AIDS Res. Hum. Retrovir. 1998;14(Suppl 1):S67–70. [PubMed] [Google Scholar]
- 4.Collins D.R., Lubow J., Lukic Z., Mashiba M., Collins K.L. Vpr promotes macrophage-dependent HIV-1 infection of CD4+ T lymphocytes. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivanov A.V., Valuev-Elliston V.T., Ivanova O.N., Kochetkov S.N., Starodubova E.S., Bartosch B., Isaguliants M.G. Oxidative stress during HIV infection: mechanisms and consequences. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/8910396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coaccioli S., Crapa G., Fantera M., Del Giorno R., Lavagna A., Standoli M.L., Frongillo R., Biondi R., Puxeddu A. Oxidant/antioxidant status in patients with chronic HIV infection. La Clinica terapeutica. 2010;161:55–58. [PubMed] [Google Scholar]
- 7.Nasi M., De Biasi S., Gibellini L., Bianchini E., Pecorini S., Bacca V., Guaraldi G., Mussini C., Pinti M., Cossarizza A. Ageing and inflammation in patients with HIV infection. Clin. Exp. Immunol. 2017;187:44–52. doi: 10.1111/cei.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh M.V., Kotla S., Le N.T., Ae Ko K., Heo K.S., Wang Y., Fujii Y., Thi Vu H., McBeath E., Thomas T.N., et al. Senescent phenotype induced by p90RSK-NRF2 signaling sensitizes monocytes and macrophages to oxidative stress in HIV-positive individuals. Circulation. 2019;139:1199–1216. doi: 10.1161/CIRCULATIONAHA.118.036232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korenaga M., Wang T., Li Y., Showalter L.A., Chan T., Sun J., Weinman S.A. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J. Biol. Chem. 2005;280:37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 10.Zuo L., Prather E.R., Stetskiv M., Garrison D.E., Meade J.R., Peace T.I., Zhou T. Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20184472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivanov A.V., Bartosch B., Smirnova O.A., Isaguliants M.G., Kochetkov S.N. HCV and oxidative stress in the liver. Viruses. 2013;5:439–469. doi: 10.3390/v5020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan X., Hu L., Luquette L.J., 3rd, Gao G., Liu Y., Qu H., Xi R., Lu Z.J., Park P.J., Elledge S.J. Systematic identification of synergistic drug pairs targeting HIV. Nat. Biotechnol. 2012;30:1125–1130. doi: 10.1038/nbt.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amjad S.V., Davoodi P., Goodarzi M.T., Abdolsamadi H., Poorolajal J., Parsa S., Paydari D., Ahmadi-Motamayel F. Salivary antioxidant and oxidative stress marker levels in HIV-positive individuals. Comb. Chem. High Throughput Screen. 2019;22:59–64. doi: 10.2174/1386207322666190306144629. [DOI] [PubMed] [Google Scholar]
- 14.Quaye O., Kuleape J.A., Bonney E.Y., Puplampu P., Tagoe E.A. Imbalance of antioxidant enzymes activities and trace elements levels in Ghanaian HIV-infected patients. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellezza I., Giambanco I., Minelli A., Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochimica et biophysica acta. Molecular cell research. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Chen W., Sun Z., Wang X.J., Jiang T., Huang Z., Fang D., Zhang D.D. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol. Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuya A.K., Sharifi H.J., Jellinger R.M., Cristofano P., Shi B., de Noronha C.M. Sulforaphane inhibits HIV infection of macrophages through Nrf2. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mbisa J.L., Delviks-Frankenberry K.A., Thomas J.A., Gorelick R.J., Pathak V.K. Real-time PCR analysis of HIV-1 replication post-entry events. Methods Mol. Biol. 2009;485:55–72. doi: 10.1007/978-1-59745-170-3_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruize Z., Kootstra N.A. The role of macrophages in HIV-1 persistence and pathogenesis. Front. Microbiol. 2019;10:2828. doi: 10.3389/fmicb.2019.02828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zicari S., Sessa L., Cotugno N., Ruggiero A., Morrocchi E., Concato C., Rocca S., Zangari P., Manno E.C., Palma P. Immune activation, inflammation, and non-AIDS Co-morbidities in HIV-infected patients under long-term ART. Viruses. 2019;11 doi: 10.3390/v11030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baird L., Yamamoto M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell Biol. 2020;40 doi: 10.1128/MCB.00099-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao P., Ande A., Sinha N., Kumar A., Kumar S. Effects of cigarette smoke condensate on oxidative stress, apoptotic cell death, and HIV replication in human monocytic cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill A.J., Kovacsics C.E., Cross S.A., Vance P.J., Kolson L.L., Jordan-Sciutto K.L., Gelman B.B., Kolson D.L. Heme oxygenase-1 deficiency accompanies neuropathogenesis of HIV-associated neurocognitive disorders. J. Clin. Invest. 2014;124:4459–4472. doi: 10.1172/JCI72279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H.S., Li H.Y., Zhou Y., Wu M.R., Zhou H.S. Nrf2 is involved in inhibiting Tat-induced HIV-1 long terminal repeat transactivation. Free Radic. Biol. Med. 2009;47:261–268. doi: 10.1016/j.freeradbiomed.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 25.Cobos-Jiménez V., Booiman T., Hamann J., Kootstra N.A. Macrophages and HIV-1. Curr. Opin. HIV AIDS. 2011;6:385–390. doi: 10.1097/COH.0b013e3283497203. [DOI] [PubMed] [Google Scholar]
- 26.Herbein G., Gras G., Khan K.A., Abbas W. Macrophage signaling in HIV-1 infection. Retrovirology. 2010;7:34. doi: 10.1186/1742-4690-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurice J.B., Garvey L., Tsochatzis E.A., Wiltshire M., Cooke G., Guppy N., McDonald J., Marchesi J., Nelson M., Kelleher P., et al. Monocyte-macrophage activation is associated with nonalcoholic fatty liver disease and liver fibrosis in HIV monoinfection independently of the gut microbiome and bacterial translocation. AIDS (London, England) 2019;33:805–814. doi: 10.1097/QAD.0000000000002133. [DOI] [PubMed] [Google Scholar]
- 28.Feinstein M.J., Lloyd-Jones D.M. Macrophage inflammation and cardiovascular disease in HIV: mechanistic insights and future directions. J. Infect. Dis. 2017;215:1343–1345. doi: 10.1093/infdis/jix085. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7 doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishida K., Kaji K., Sato S., Ogawa H., Takagi H., Takaya H., Kawaratani H., Moriya K., Namisaki T., Akahane T., et al. Sulforaphane ameliorates ethanol plus carbon tetrachloride-induced liver fibrosis in mice through the Nrf2-mediated antioxidant response and acetaldehyde metabolization with inhibition of the LPS/TLR4 signaling pathway. J. Nutr. Biochem. 2021;89 doi: 10.1016/j.jnutbio.2020.108573. [DOI] [PubMed] [Google Scholar]
- 31.Feria M.G., Taborda N.A., Hernandez J.C., Rugeles M.T. HIV replication is associated to inflammasomes activation, IL-1β, IL-18 and caspase-1 expression in GALT and peripheral blood. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poerio N., Caccamo N., La Manna M.P., Olimpieri T., Henrici De Angelis L., D’Andrea M.M., Dieli F., Fraziano M. Phosphatidylserine liposomes reduce inflammatory response, mycobacterial viability and HIV replication in coinfected human macrophages. J. Infect. Dis. 2021;225:1675–1679. doi: 10.1093/infdis/jiab602. [DOI] [PubMed] [Google Scholar]
- 33.Cao D., Khanal S., Wang L., Li Z., Zhao J., Nguyen L.N., Nguyen L.N.T., Dang X., Schank M., Thakuri B.K.C., et al. A matter of life or death: productively infected and bystander CD4 T cells in early HIV infection. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.626431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atluri V.S., Pilakka-Kanthikeel S., Garcia G., Jayant R.D., Sagar V., Samikkannu T., Yndart A., Nair M. Effect of cocaine on HIV infection and inflammasome gene expression profile in HIV infected macrophages. Sci. Rep. 2016;6 doi: 10.1038/srep27864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu D.M., Shi J., Liu S., Liu Y., Zheng D. HIV infection enhances TRAIL-induced cell death in macrophage by down-regulating decoy receptor expression and generation of reactive oxygen species. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cagnol S., Chambard J.C. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 37.Kohandel Z., Farkhondeh T., Aschner M., Samarghandian S. Nrf2 a molecular therapeutic target for Astaxanthin. Biomed. Pharmacotherapy Biomedecine Pharmacotherapie. 2021;137 doi: 10.1016/j.biopha.2021.111374. [DOI] [PubMed] [Google Scholar]
- 38.Bonay M., Roux A.L., Floquet J., Retory Y., Herrmann J.L., Lofaso F., Deramaudt T.B. Caspase-independent apoptosis in infected macrophages triggered by sulforaphane via Nrf2/p38 signaling pathways. Cell Death Disc. 2015;1 doi: 10.1038/cddiscovery.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.