Background:
In the era of the coronavirus disease 2019 (COVID-19) pandemic, surgeons and medical staff are often at a high risk of infection in the operating room, especially when the patient is spontaneously breathing. In this study, we examined the minimum requirements for personal protective equipment with double surgical masks to potentially reduce unnecessary waste of supplies.
Methods:
Two mannequins were each connected to a test lung machine simulating a surgeon and patient with spontaneous breathing. An aerosol generator containing severe acute respiratory syndrome coronavirus 2 virion particle substitutes was connected to the patient mannequin. The sampling points for the target molecules were set at different distances from the patient mannequin and sent for multiplex quantitative polymerase chain reaction analysis. Three clinical scenarios were designed, which differed in terms of the operating room pressure and whether a fabric curtain barrier was installed between the mannequins.
Results:
Analysis of the multiplex quantitative polymerase chain reaction results showed that the cycle threshold (Ct) value of the target molecule increased as the distance from the aerosol source increased. In the negative-pressure operating room, the Ct values were significantly increased at all sample points compared with the normal pressure room setting. The Ct value sampled at the surgeon mannequin wearing double face masks was significantly increased when a cloth curtain barrier was set up between the two mannequins.
Conclusion:
Double surgical masks provide elementary surgeon protection against COVID-19 in a negative pressure operating room, with a physical barrier in place between the surgeon and patient who is spontaneously breathing during local anesthesia or sedated surgery.
Takeaways
Question: What is the minimal requirement of personal protective equipment against COVID-19 in the operating room?
Findings: In this simulation study, two mannequins and lung machines were set up as spontaneous breathing surgeon and patient, with the surgeon wearing double surgical masks. The cycle threshold (Ct) value of aerosol sampling sites of SARS-CoV-2 virion particle substitutes generated by the patient mannequin had increased in a negative pressure operating room, with a physical barrier between the surgeon and patient.
Meaning: Double surgical masks can provide basic surgeon protection against COVID-19 in a negative-pressure operating room with a physical barrier between the surgeon and patient.
INTRODUCTION
The close proximity required for patient care places operating room staff at high risk of exposure and infection with severe acute respiratory syndrome coronavirus-2 (SARS-COV-2).1 The Omicron and Delta variants of coronavirus disease 2019 (COVID-19) have further increased the risk of transmission and the virulence of infection.2 Although most high-grade personal protective equipment (PPE) is effective at reducing transmission during brief exposure, variability in environmental conditions, such as ambient humidity, notably influences potential transmission, suggesting that more information on virus particle dispersal is needed under operating room conditions.3
Simulated tests using exposure chambers show that PPE was highly effective (>95%) against a uniform stream of particles smaller than 5 µm when the study subject was positioned at varying distances from the particle generator.4 However, this type of test does not reproduce the environmental conditions of operation or where there is a constant flow of suspended exhaled particulates of varying sizes at a defined distance from the source. Information on particulate load and the effectiveness of PPE over time would help healthcare providers choose the best possible protective strategies under these specific conditions.
A considerable number of elective procedures have been postponed and avoided during the COVID-19 outbreak to reserve medical resources for life-threatening conditions. However, elective and local anesthesia procedures are still necessary for patients in need, as well as for medical facilities to maintain essential healthcare function.
In this study, we examined viral vector transmission using simulated exposure to spontaneous respiration during surgery involving the thoracoabdominal regions while the patient is awake and spontaneously breathing. Our goal was to establish a reference point for transmission risk and the minimum requirement of PPE for other sites of care to use to assess the safety of environmental conditions for healthcare providers in the operating room.
MATERIAL AND METHODS
Target Molecule
An Interferon-γ (mIFN-γ) (NM_008337) mouse tagged open reading frame Clone expression plasmid (MR227155, OriGene Technologies) was selected as a substitute for the SARS-CoV-2 virion particle.5 The SARS-CoV-2 is a single-stranded RNA virus with genome length of approximately 29.9 kb.6,7 Considering the fragility and infectious risk of using the SARS-CoV-2 or its similar viruses, we chose the complementary deoxyribonucleic acid clone plasmid that has more stability compared with ribonucleic acid (RNA), and considering safety, availability issues, and to reduce the possibility of false positivity in future studies if the SARS-CoV-2 virion was used.
Two concentrations of the sample molecule were used in each scenario for validation: 275 µg/10 mL and 550 µg/10 mL.
Aerosol Generation
A large-volume nebulizer (Galamed, I-lan, Taiwan) was used to generate the aerosol particles containing the target molecule. The jet nebulizer was powered by medical O2 sources, with a flow rate of 5 L/min.
Mannequin Setup
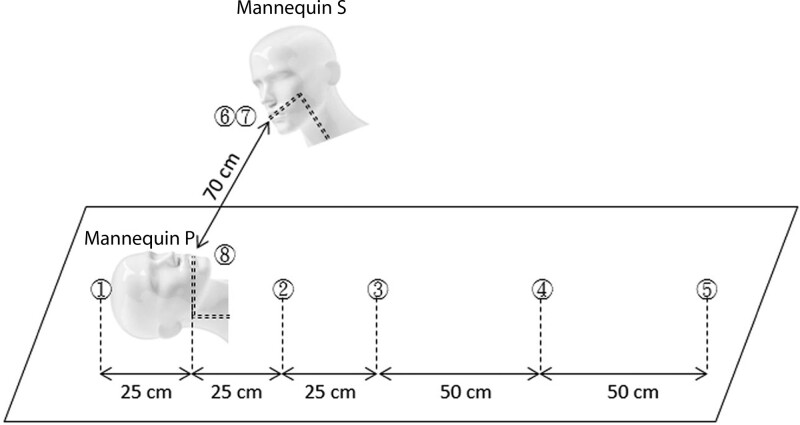
A mannequin head (mannequin P) (Male Styro Mannequin #0831, Hairess Corporation, Crown Point, Ind.) representing the surgical patient was placed on the surgical table. Another mannequin head (mannequin S) representing the surgeon was suspended 70 cm in the lateral caudal direction of mannequin P (Figs. 1, 2). The setup was based on our daily practice in the operating room with the surgeon operating while standing at the patient’s thoracoabdominal region (as shown in Fig. 2E). The distance between the surgeon and patient’s head was measured, while both of their heights were at an average of 171 cm.8
Fig. 1.
Design setup. Mannequin S represents the surgeon and mannequin P represents the patient, each is connected to an individual test lung. One of the two chambers of the test lung is connected to a driving ventilator. The nebulizer containing the target molecules is connected to mannequin P.
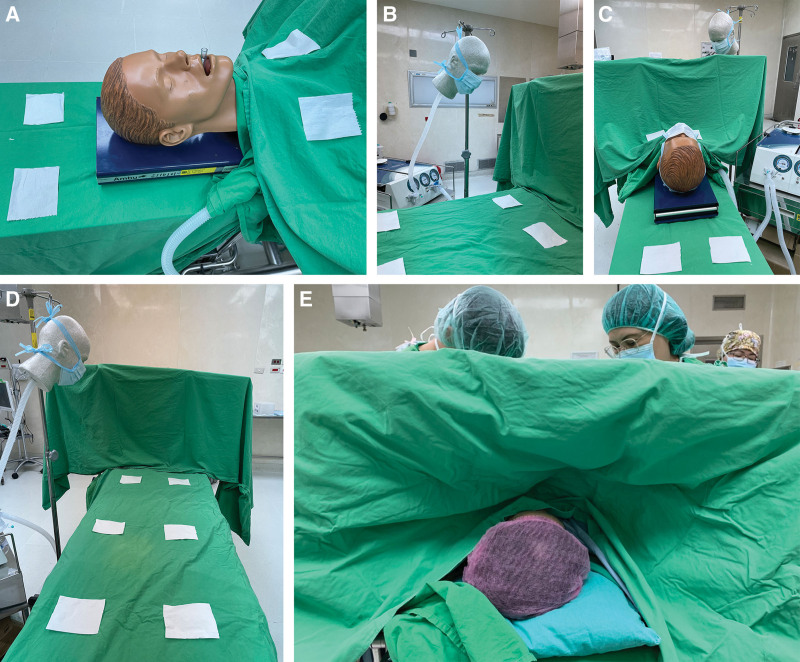
Fig. 2.
Design set up with actual images. A, Mannequin P as the patient. B, Mannequins S as the surgeon. C, Cloth curtain between the two mannequins with view from the patient’s cranial side. D, Cloth curtain between the two mannequins with view from the surgeon’s side. E, Operation scene in the real world.
The aforementioned large-volume nebulizer containing the target molecule was connected to the trachea of mannequin P and supplied with an oxygen flow rate of 5 L/min. The tracheas of the mannequins’ head were both connected to one side of a two-compartment test lung machine (Vent Aid TTL; Michigan Instruments, Grand Rapids, Mich.) using 7.0-mm endotracheal tubes (Mallinckrodt, Covidien, Mansfield, Mass.). Lung compliance was set at 50 mL/cm H2O and airway resistance at 8.2 cm H2O/(L * s). The second chamber of the test lung was connected to a driving ventilator (Siemens-Elema, Solna, Sweden), and the tidal volume and respiratory rate were set at 500 mL and 12 breaths/min. The inspiratory to expiratory ratio was set at 1:2. The two separate chambers of the test lung were physically connected by a rigid metal strap. When the ventilator delivered a tidal volume to the first chamber, the second chamber rose, drawing in a tidal volume through the trachea of both mannequin heads, thus simulating spontaneous breathing in the patient.
The outflow of aerosol was propelled by the respiration generated by the test lung machine and exited out of the mouth of the model patient via an endotracheal tube. The Murphy’s eye side hole segment was trimmed to allow homogenous unidirectional flow.
Scenario Design
We hypothesized that during times of insufficient supply of PPE, the minimal requirement would be to wear double surgical face masks (CSD brand, qualified for Taiwan national standard CNS14774, bacterial filtration efficiency, and BFE of 3 µm > 99%). Two measures were adopted to minimize exposure, including a lamellar flow ventilated negative-pressure operating room, and a physical barrier with fabric curtain to separate the space between aerosol outlets from the patient and the surgeon.
Therefore, we designed three separate scenarios to test these hypotheses (Table 1).
Table 1.
Scenario Design for Protective Equipment for COVID-19 in the Operating Room
| Scenario Design* | Ventilation System for Mannequin P | Laminar Airflow Negative Pressure in Operating Room | Cloth Curtain Barrier between Mannequins S and P |
|---|---|---|---|
| Scenario I | Self-ventilation | – | – |
| Scenario II | Self-ventilation | + | – |
| Scenario III | Self-ventilation | + | + |
| Control scenario | Closed ventilation | + | – |
Mannequin S wearing double surgical masks in all the scenarios.
P, patient; S, surgeon.
Scenario I: a self-ventilated patient wearing a surgical mask undergoing surgery on the thoracoabdominal region with the surgeon wearing double surgical masks in a regular operating room. The regular operating room is a positively pressured operating room, and the pressure difference between outside of the room is 8 Pa. The average air turnover rate is 35 per hour. The cleanroom class is 10,000 (less than 10,000 particles of size 0.5 um/ft3).
Scenario II: a self-ventilated patient wearing a surgical mask undergoing surgery on the thoracoabdominal region with the surgeon wearing double surgical masks in a laminar airflow negative-pressure operating room.
Scenario III: a self-ventilated patient wearing surgical masks undergoing surgery on the thoracoabdominal region with the surgeon wearing double surgical masks in a laminar airflow negative-pressure operating room, with a fabric curtain barrier placed at the patient’s neck level separating the space (Fig. 2C, D).
Control scenario: A patient ventilated in a closed system with the surgeon wearing double surgical masks in a negative-pressure operating room.
Sampling
Pieces of 10 cm × 10 cm cellulose filter pads (YFYCPG, Taipei, Taiwan) were used as passive air samplers placed at designated sampling points (Fig. 3). These included the outer surface of the surgeon’s mask, the innermost surface of the surgeon’s mask, the outer surface of the patient’s mask, and at the nebulizer outlet (sampled using a standard viral swab). Additional spaced sampling points were located at 0.25 m cephalad to the nebulizer outlet, and 0.25 m, 0.5 m, 1.0 m, and 1.5 m caudal of the nebulizer outlet.
Fig. 3.
Sampling sites. (1) 0.25-m cephalad to the aerosol outlet, (2) 0.25-m caudal to the aerosol outlet, (3) 0.5-m caudal to the aerosol outlet, (4) 1.0-m caudal to the aerosol outlet, (5) 1.5-m caudal to the aerosol outlet, (6) outer surface of the surgeon’s double mask, (7) inner surface of the surgeon’s double mask, and (8) inner surface of the aerosol outlet tubing.
The samplers were exposed to the aerosol in the operating room for 2 hours during the nebulization process, and samples were taken 40 minutes after the nebulization was stopped, allowing the aerosolized particles to settle. The loaded pads were placed inside viral transfer tubes (UTM, brand) as an extraction vessel, stored in 4 °C, and analyzed within 48 hours by multiplex quantitative polymerase chain reaction (qPCR).
Multiplex Quantitative-PCR
The reactions were performed using an ABI step-one-plus real-time PCR detection system (Applied Biosystems, Foster City, Calif.). Each experiment was performed in 20 μL of reaction volume comprising 10 μL of KAPA SYBR FAST qPCR Master Mix (2X) Kit (Sigma-Aldrich), 5 μL of the aforementioned target molecule, and 5 μL of each 2.5 μmol/L primer-probe mixture. The thermal cycling program consisted of an initial denaturation in one cycle of 10 minutes at 95 °C, followed by 40 cycles of 20 seconds at 95 °C, 20 seconds at 60 °C, and 20 seconds at 72 °C. Each sample was run in quadruplicate. Samples with equivocal amplifications underwent an additional run. The fold changes in target genes after multiplex qPCR were calculated as follows: cycle threshold (Ct) was chosen at the beginning of log phase amplification during PCR.
ΔCt = –(Cttarget1 – Cttarget2).
The relative copy ratio of sample mIFN-γ in comparison to control template was presented as
RCR = 2−ΔCt.
The control mIFN-γ plasmid DNA stock (up to 1.3 mg/mL) was diluted serially into 10−5, 10−7, 10−9 to 10−11 standards. These dilutions were amplified on the sample PCR plate, along with experimental samples, to produce a standard curve, and for copy number calculation. Since PCR amplifications tended to be irregular after extended cycles, the upper limit of Ct was determined to be 35. A Ct number of more than 30 was used as a cut off for positivity.
Each scenario was repeated three times, and two identical samples were taken from each sampling site each time. Data were presented as the mean of the six samples.
Statistical Analysis
Comparisons between the two groups were assessed using analysis of variance. P values less than 0.05 were considered statistically significant. All statistical analyses were performed using SPSS software (IBM, SPSS 24).
RESULTS
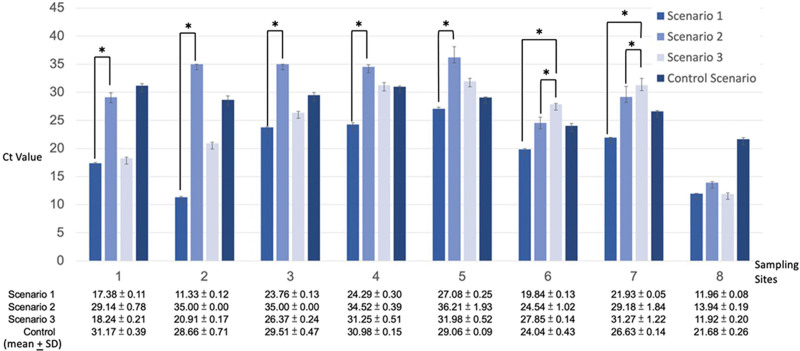
Analysis of the multiplex qPCR results revealed a consistent linear correlation indicating a decreased distribution of the target molecule with increased distance from the aerosol source. The results in scenarios I–III are shown in Figure 4.
Fig. 4.
Analysis of the multiplex qPCR results for all scenarios.
Comparison of scenarios I and II showed that in scenario II (negative-pressure operating room), the regular interval spaced samples (1 to 5) had low target molecule levels approaching the negative control level, while in scenario I (regular operating room), the same group of samples had much higher concentration compared with the negative control value.
In scenario I, there was a significant concentration of the target molecule on the inner face of the surgeon’s double surgical masks (7) (Ct value mean + SD = 21.93 + 0.05). The same site showed a significantly reduced concentration in scenario II (Ct value mean + SD = 29.18 + 1.84) and scenario III (Ct value mean + SD = 31.27 + 1.22).
In scenario III (cloth curtain barrier set up), a reduced concentration of target molecule was observed over the outer (6) and inner sides (7) of the surgeon’s double surgical masks (Ct value, outer side mean + SD = 27.85 + 0.14; inner side mean + SD = 31.27 + 1.22) compared with scenario II (Ct value, outer side mean + SD = 24.54 + 1.02; inner side mean + SD = 29.18 + 1.84) and the control scenario (Ct value, outer side mean + SD = 24.04 + 0.43; inner side mean + SD = 26.63 + 0.14).
DISCUSSION
To our understanding, this is the first simulation study investigating the transmission of SARS-CoV-2 virus by simulating both the patient and the surgeon with test lung machines in a scenario representing surgical procedures involving the thoracoabdominal areas in operating room settings. Operating rooms with laminar airflow ventilation and negative-pressure systems were recommended during the COVID-19 pandemic to prevent the risk of aerosol transmission.9–12 However, whether it is possible to reduce unnecessary use of PPE in these operation rooms has not been well studied.
Our study revealed that the Ct value in the laminar airflow negative-pressure operating room showed a decrease in target molecule distribution with increasing distance from the patient mannequin aerosol source compared to the normal operating room. This might indicate that the laminar airflow negative-pressure system could reduce the movement of most aerosols and droplets generated by the patient. Newsom et al13 found that the laminar airflow ventilation primarily reduced the distribution of droplets smaller than 1000 μm compared with conventional airflow systems using a cough generator model with a fluorescein staining method. These findings might imply that an operating room equipped with laminar airflow negative-pressure system could be an option to protect surgeons while performing surgical procedures when the patient is under spontaneous breathing effort.
Since it was recommended to avoid performing surgery on patients with a confirmed or undetermined diagnosis of COVID-19, elective procedures have been extensively postponed in recent years.10,14–16 This has raised interest in determining whether it is safe to perform elective procedures involving sections below the nuchal region away from the nasopharyngeal area, and what is the minimum requirement of PPE in these procedures.
Unlike previous studies which focused on the transmission of aerosols during intubation, extubation, and general anesthesia procedures,11,12,15–17 our study simulates both the patient and surgeon’s spontaneous breathing physiology using test lungs, mimicking procedures under local or spinal anesthesia while the patient is awake and breathing spontaneously.
When placing a fabric curtain barrier between the patient and surgeon mannequin, we found that the Ct value at the inner side of the surgeon mannequin’s double surgical mask increased, indicating that the transmission of aerosols had been blocked. This might also indicate that the minimum requirement of PPE could be as simple as wearing double surgical masks if there is an appropriate barrier preventing the transmission of aerosol generated by the patient’s spontaneous breathing while performing surgery in a laminar airflow negative pressure system operation room.
However, we also found that the curtain barrier did not increase the Ct value at sample points at sites away from the patient mannequin under the laminar airflow negative pressure room setting. Two hypotheses have been proposed to explain this finding. First, the curtain barrier might have caused the aerosols and droplets capable of traveling beyond the field to have different transmission paths due to altered aerodynamics. Second, the curtain drape we set up had a height difference between the central and lateral sides (Fig. 2C), and this might explain why the transmission was decreased over the surgeon mannequin set up at the right lateral side of the patient, but not the sample sites at the central distant parts of the patient mannequin.
Several previous studies have designed various types of surgical drapes to prevent the transmission of SARS-CoV-2 virus, mainly proposed in fields involving high risk aerosol generating procedures, such as anesthesiology, neurosurgery, ophthalmology, otorhinolaryngology, and dentistry.17–24 Our study found that similar effects could be achieved by a regular aseptic surgical fabric curtain to reduce the aerosol transmission and avoid unnecessary use of high level PPE. However, this was only limited in procedures involving areas away from the patient’s nuchal region.
In the early design phase of our study, we plan to include the N95 mask scenarios as comparison. However, due to the limited supply of plasmid we can acquire during the COVID-19 pandemic period in our country, we could only prepare enough plasmid for the most decisive scenarios, with variables of operating room pressure and a cloth barrier, instead of different types of masks between scenarios, which may lead to insufficient plasmid. Also, since we found the double surgical masks could already provide sufficient protection in certain scenarios, we assume that the simulation tests with the higher protection N95 masks would not impact our findings.
Engineered viral particles have been used in humans and laboratory animals to analyze the mechanism of particulate spread.25 Recent studies by Zhang et al26 showed that airborne exposure was the primary route of transmission. However, several questions remain regarding the role of smaller particles.23 This issue was recently clarified by Bourouiba,27 who showed that the distinction between droplets and aerosols might not be important in determining spread as respiratory exhalations have been shown to be a “multiphase turbulent gas cloud that entrains ambient air.”
The gas-entrapped viral particles contain variable suspension sizes that are propelled much farther than predicted in isolated droplets or aerosol spread. Entrapped viral particles have been shown to spread up to 7–8 m in a radial distance. Rapid evaporation in environments such as low-humidity operating rooms leaves residues or droplet nuclei that can remain suspended in the air for hours and spread using laminar airflow systems with viability influenced by environmental climate control.27 This recent evidence suggests that distances of 1 and 1–2 m recommended by the World Health Organization and Centers for Disease Control, respectively, likely underestimate the exposure of healthcare providers to respiratory transmission.
The complex relationship between environmental conditions, proximity of care providers, and whether patients breathe spontaneously or are ventilated suggest that further studies are needed to identify the risk of viral spread under defined healthcare conditions.
This study has several limitations, which warrant further research. First, the simulated operating condition only included procedures performed under local or spinal anesthesia in the thoracoabdominal region, while the patient was breathing spontaneously. Our results, albeit significant, could only be applied to this exact scenario. However, since during general anesthesia, the patient and the anesthesia machine form a closed ventilation system via the air-tight breathing circuit, we could assume that the same finding could indicate that procedures performed under closed circuit general anesthesia would be safer, if other conditions remained the same. Second, due to the increased tubing length from the nebulizer to the outlet in our setting, we could assume that larger particles would be deposited inside the tubing, and the ratio of smaller particles at the outlet would be increased compared with reference values. Finally, our study design did not take into account the infective potential of the particle, and it is important to note that the presence of particles does not necessarily translate directly into infectivity.
CONCLUSION
Our simulation study using aerosols containing mIFN-γ plasmid DNA demonstrated that during times of limited PPE supply, double surgical masks could provide basic protection against aerosol exposure for surgeons operating in the thoracoabdominal region while the patient is spontaneously breathing, provided that the patient wears a surgical mask, the operation takes place in a negative-pressure operation room, and there is a physical barrier, such as a fabric curtain, between the patient’s face and the surgical field.
ACKNOWLEDGMENTS
The authors acknowledge the support of the Y. L. Lin Hung Tai Education and Culture Charity Trust, the Y. L. Lin Hung Tai Education Foundation, and the Common Well Foundation. The authors thank professor Susan Mandell for reviewing and editing our manuscript.
Footnotes
Published online 18 January 2023.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Bor-Uei Shyr, Yi-Ting Yeh, and Wei-Nung Teng contributed equally as first author to this study. Chin-Su Liu and Hsu Ma contributed equally as corresponding author.
REFERENCES
- 1.Kibbe MR. Surgery and COVID-19. JAMA. 2020;324:1151–1152. [DOI] [PubMed] [Google Scholar]
- 2.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Ferro EG, Zhou G, et al. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. JAMA. 2020;324:703–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sickbert-Bennett EE, Samet JM, Clapp PW, et al. Filtration efficiency of hospital face mask alternatives available for use during the COVID-19 pandemic. JAMA Intern Med. 2020;180:1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ORIGENE. Ifng (NM_008337) Mouse Tagged ORF Clone 2022. Available at https://www.origene.com/catalog/cdna-clones/expression-plasmids/mr227155/ifng-nm_008337-mouse-tagged-orf-clone. 2022.
- 6.Bar-On YM, Flamholz A, Phillips R, et al. SARS-CoV-2 (COVID-19) by the numbers. Elife. 2020;9:e57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang MY, Zhao R, Gao LJ, et al. SARS-CoV-2: structure, biology, and structure-based therapeutics development. Front Cell Infect Microbiol. 2020;10:587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SC, Lin CW, Lee PF, et al. Anthropometric characteristics in Taiwanese adults: age and gender differences. Int J Environ Res Public Health. 2021;18:7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theodorou C, Simpson GS, Walsh CJ. Theatre ventilation. Ann R Coll Surg Engl. 2021;103:151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong J, Goh QY, Tan Z, et al. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anaesth. 2020;67:732–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welsh Surgical Research Initiative (WSRI) Collaborative. Recommended operating room practice during the COVID-19 pandemic:systematic review. BJS Open. 2020;4:748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh Surgical Research Initiative (WSRI) Collaborative. Surgery during the COVID-19 pandemic: operating room suggestions from an international Delphi process. Br J Surg. 2020;107:1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newsom RB, Amara A, Hicks A, et al. Comparison of droplet spread in standard and laminar flow operating theatres: SPRAY study group. J Hosp Infect. 2021;110:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrester JD, Nassar AK, Maggio PM, et al. Precautions for operating room team members during the COVID-19 pandemic. J Am Coll Surg. 2020;230:1098–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.COVIDSurg Collaborative Collaborators. Global guidance for surgical care during the COVID-19 pandemic. Br J Surg. 2020;107:1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Simone B, Chouillard E, Di Saverio S, et al. Emergency surgery during the COVID-19 pandemic: what you need to know for practice. Ann R Coll Surg Engl. 2020;102:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fidler RL, Niedek CR, Teng JJ, et al. Aerosol retention characteristics of barrier devices. Anesthesiology. 2021;134:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Amico RS, Khatri D, Kwan K, et al. Coronavirus neurosurgical/head and neck drape to prevent aerosolization of coronavirus disease 2019 (COVID-19): the Lenox Hill Hospital/Northwell Health Solution. World Neurosurg. 2020;142:314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown S, Patrao F, Verma S, et al. Barrier system for airway management of COVID-19 patients. Anesth Analg. 2020;131:e34–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David AP, Jiam NT, Reither JM, et al. Endoscopic skull base and transoral surgery during COVID-19 pandemic: minimizing droplet spread with negative-pressure otolaryngology viral isolation drape. Head Neck. 2020;42:1577–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matava CT, Yu J, Denning S. Clear plastic drapes may be effective at limiting aerosolization and droplet spray during extubation: implications for COVID-19. Can J Anaesth. 2020;67:902–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence RJ, O’Donoghue G, Kitterick P, et al. Recommended personal protective equipment for cochlear implant and other mastoid surgery during the COVID-19 era. Laryngoscope. 2020;130:2693–2699. [DOI] [PubMed] [Google Scholar]
- 23.Bianco F, Incollingo P, Grossi U, et al. Preventing transmission among operating room staff during COVID-19 pandemic: the role of the Aerosol Box and other personal protective equipment. Updates Surg. 2020;72:907–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chari DA, Workman AD, Chen JX, et al. Aerosol dispersion during mastoidectomy and custom mitigation strategies for otologic surgery in the COVID-19 era. Otolaryngol Head Neck Surg. 2021;164:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung NHL. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol. 2021;19:528–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R, Wang G, Guo S, et al. Formation of urban fine particulate matter. Chem Rev. 2015;115:3803–3855. [DOI] [PubMed] [Google Scholar]
- 27.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323:1837–1838. [DOI] [PubMed] [Google Scholar]