Abstract
Background
Patellar (knee cap) dislocation occurs when the patella disengages completely from the trochlear (femoral) groove. It affects up to 42/100,000 people, and is most prevalent in those aged 20 to 30 years old. It is uncertain whether surgical or non‐surgical treatment is the best approach. This is important as recurrent dislocation occurs in up to 40% of people who experience a first time (primary) dislocation. This can reduce quality of life and as a result people have to modify their lifestyle. This review is needed to determine whether surgical or non‐surgical treatment should be offered to people after patellar dislocation.
Objectives
To assess the effects (benefits and harms) of surgical versus non‐surgical interventions for treating people with primary or recurrent patellar dislocation.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group's Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, AMED, CINAHL, Physiotherapy Evidence Database and trial registries in December 2021. We contacted corresponding authors to identify additional studies.
Selection criteria
We included randomised and quasi‐randomised controlled clinical trials evaluating surgical versus non‐surgical interventions for treating primary or recurrent lateral patellar dislocation in adults or children.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were recurrent patellar dislocation, and patient‐rated knee and physical function scores. Our secondary outcomes were health‐related quality of life, return to former activities, knee pain during activity or at rest, adverse events, patient‐reported satisfaction, patient‐reported knee instability symptoms and subsequent requirement for knee surgery. We used GRADE to assess the certainty of evidence for each outcome.
Main results
We included 10 studies (eight randomised controlled trials (RCTs) and two quasi‐RCTs) of 519 participants with patellar dislocation. The mean ages in the individual studies ranged from 13.0 to 27.2 years. Four studies included children, mainly adolescents, as well as adults; two only recruited children. Study follow‐up ranged from one to 14 years.
We are unsure of the evidence for all outcomes in this review because we judged the certainty of the evidence to be very low. We downgraded each outcome by three levels. Reasons included imprecision (when fewer than 100 events were reported or the confidence interval (CI) indicated appreciable benefits as well as harms), risk of bias (when studies were at high risk of performance, detection and attrition bias), and inconsistency (in the event that pooled analysis included high levels of statistical heterogeneity).
We are uncertain whether surgery lowers the risk of recurrent dislocation following primary patellar dislocation compared with non‐surgical management at two to nine year follow‐up. Based on an illustrative risk of recurrent dislocation in 348 people per 1000 in the non‐surgical group, we found that 157 fewer people per 1000 (95% CI 209 fewer to 87 fewer) had recurrent dislocation between two and nine years after surgery (8 studies, 438 participants).
We are uncertain whether surgery improves patient‐rated knee and function scores. Studies measured this outcome using different scales (the Tegner activity scale, Knee Injury and Osteoarthritis Outcome Score, Lysholm, Kujala Patellofemoral Disorders score and Hughston visual analogue scale). The most frequently reported score was the Kujala Patellofemoral Disorders score. This indicated people in the surgical group had a mean score of 5.73 points higher at two to nine year follow‐up (95% CI 2.91 lower to 14.37 higher; 7 studies, 401 participants). On this 100‐point scale, higher scores indicate better function, and a change score of 10 points is considered to be clinically meaningful; therefore, this CI includes a possible meaningful improvement.
We are uncertain whether surgery increases the risk of adverse events. Based on an assumed risk of overall incidence of complications during the first two years in 277 people out of 1000 in the non‐surgical group, 335 more people per 1000 (95% CI 75 fewer to 723 more) had an adverse event in the surgery group (2 studies, 144 participants).
Three studies (176 participants) assessed participant satisfaction at two to nine year follow‐up, reporting little difference between groups. Based on an assumed risk of 763 per 1000 non‐surgical participants reporting excellent or good outcomes, seven more participants per 1000 (95% CI 199 fewer to 237 more) reported excellent or good satisfaction.
Four studies (256 participants) assessed recurrent patellar subluxation at two to nine year follow‐up. Based on an assumed risk of patellar subluxation in 292 out of 1000 in the non‐surgical group, 73 fewer people per 1000 (95% CI 146 fewer to 35 more) had patellar subluxation as a result of surgery.
Slightly more people had subsequent surgery in the non‐surgical group. Pooled two to nine year follow‐up data from three trials (195 participants) indicated that, based on an assumed risk of subsequent surgery in 215 people per 1000 in the non‐surgical group, 118 fewer people per 1000 (95% CI 200 fewer to 372 more) had subsequent surgery after primary surgery.
Authors' conclusions
We are uncertain whether surgery improves outcome compared to non‐surgical management as the certainty of the evidence was very low. No sufficiently powered trial has examined people with recurrent patellar dislocation. Adequately powered, multicentre, randomised trials are needed. To inform the design and conduct of these trials, expert consensus should be achieved on the minimal description of both surgical and non‐surgical interventions, and the pathological variations that may be relevant to both choice of these interventions.
Keywords: Adolescent; Adult; Child; Humans; Young Adult; Fractures, Bone; Knee Joint; Patella; Patellar Dislocation; Patellar Dislocation/surgery; Quality of Life
Plain language summary
Surgery or non‐surgical treatments: which works better to treat people who have a dislocated knee cap?
Key messages
We did not find enough good‐quality evidence to show whether surgery or non‐surgical treatment works better to treat people who have a dislocated knee cap.
Good‐quality research is required that compare these treatments.
What is a dislocated knee cap?
The knee cap is a lens‐shaped bone at the front of the knee. A dislocation occurs when the knee cap completely moves out of the groove in the thigh‐bone at the knee. It typically occurs in young, physically active people when they twist their bent knee whilst their foot is fixed to the ground. The cause of a dislocation may be linked to an abnormal shape of the knee bones, weakness of the muscles around the hip or knees, or tightness of soft tissues on the outside of the knee.
After a knee cap dislocation, some people recover completely. But some people may have repeated dislocations, or a feeling of instability in their knee cap, or both. They may also have persistent pain or limited function.
How is a dislocated knee cap treated?
When the knee cap dislocates, the soft tissues around the knee are injured. People need to have treatment to help restore the knee back to full health. This may include treatments such as holding the knee in place (by wearing a kind of brace or bandage), exercises, manual therapy (such as physiotherapy) and taping the area around the knee. However, some doctors suggest that people may have a better outcome if surgery is performed. Surgery may be used to: repair or reconstruct the injured ligaments and muscles that hold the knee cap in the groove, reshape the groove, or change where the knee cap attaches to the shin‐bone to stop it from dislocating again.
What did we want to find out?
We wanted to find out whether surgery or non‐surgical treatment was better at preventing another knee cap dislocation and restoring knee function. We also looked at any unwanted effects of treatment, how satisfied people were with their treatment, symptoms of instability and the need for surgery after the initial treatment.
What did we do?
We searched the medical literature until December 2021 for studies that compared surgical with non‐surgical treatment for adults or children who had a patellar dislocation. We summarised and compared the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 10 relevant studies (519 adults and children). Studies randomly allocated people to receive surgery or a non‐surgical treatment. In nine studies, people were treated for a first‐time dislocation, one study treated people after repeated knee cap dislocations. People ranged from 13 to 27 years of age, with six studies including children. People in the studies were monitored from one to nine years after their injury.
Main results
We were very uncertain about whether surgery compared to non‐surgical treatment:
‐ reduced the number of repeat dislocations;
‐ affected how well the knee cap worked;
‐ increased or reduced the risk of side effects;
‐ made a difference to how satisfied people were with treatment;
‐ increased or reduced instability in the knee cap; or
‐ increased or reduced the need for additional surgery.
What are the limitations of the evidence?
These studies were small. Some had weaknesses in their design and conduct. The quality of the evidence is very low. We were very uncertain about these findings.
How up to date is this evidence?
This review updates our previous review. The evidence is up to date to December 2021.
Summary of findings
Summary of findings 1. Surgical compared with non‐surgical treatment for patellar dislocation.
| Surgical compared with non‐surgical treatment for patellar dislocation | ||||||
|
Population: people with lateral patellar dislocation Settings: hospital (surgical) and hospital/rehabilitation centres (non‐surgical). Countries where trials were conducted included Brazil, Denmark, Finland, Germany, Sweden and the UK. Intervention: surgical procedures, including: MPFL repair, MPFL reconstruction, medial retinacula repair, medial reefing, lateral release, tibial tuberosity transfer, modified Roux Goldwraithe procedure and osteochrondral fracture repair. Comparison: non‐surgical treatments, including bracing/orthoses and exercise‐based rehabilitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐surgical | Surgical | |||||
|
Number of participants sustaining recurrent patellar dislocationa Follow‐up: two to nine years |
348 per 1000b | 191 per 1000 (139 to 261) |
RR 0.55 (0.40 to 0.75) |
438 (8) | ⊕⊝⊝⊝c Very low | Surgery resulted in 157 fewer (95% CI 209 fewer to 87 fewer) people per 1000 having a recurrent dislocation during this time. |
|
Knee and physical functiona Measured using the Kujala patellofemoral disorders scorea Scale from: 0 to 100 (higher scores = better function) Follow‐up: two to nine years |
The mean Kujala patellofemoral disorders score in the non‐surgical group was 81.90points | The mean Kujala patellofemoral disorders score in the surgical groups was 5.73 points higher (2.91 point lower to 14.37 points higher) | MD 5.73 (‐2.91 to 14.37) | 401 (7) | ⊕⊝⊝⊝d Very low | The CI includes the putative MCID of 10 pointse in favour of surgery. Thus, this includes the possibility of a clinically important effect of surgery on outcome at two to nine years assessed using this score. |
|
Adverse effects of treatment Overall incidence of complications Follow‐up: less than two years |
277 per 1000b | 612 per 1000 (202 to 1000) |
RR 2.21 (0.73 to 6.66) |
144 (2) | ⊕⊝⊝⊝f Very low | Surgery resulted in 335 more (95% CI 75 fewer to 723 more) people per 1000 having an adverse event during this time. |
|
Patient satisfactiona Reported as 'good' or 'excellent'. Follow‐up: two to nine years |
763 per 1000b | 770 per 1000 (564 to 1000) |
RR 1.01 (0.74 to 1.38) |
176 (3) | ⊕⊝⊝⊝g Very low | Surgery resulted in 7 more (95% CI 199 fewer to 237 more) people per 1000 reporting a good or excellent outcome for satisfaction at this time. |
|
Patient‐reported knee instabilitya Incidence of patellar subluxation Follow‐up: two to nine years |
292 per 1000b | 219 per 1000 (146 to 327) |
RR 0.75 (0.50 to 1.12) |
256 (5) | ⊕⊝⊝⊝h Very low | Surgery resulted in 73 fewer (95% CI 146 fewer to 35 more) people per 1000 reporting patellar subluxation at this time. |
|
Subsequent requirement for surgery (reoperations) for complicationsa Incidence Follow‐up: two to nine years |
215 per 1000b | 97 per 1000 (15 to 587) |
RR 0.45 (0.07 to 2.73) |
195 (3) | ⊕⊝⊝⊝i Very low | Surgery resulted in 118 fewer (95% CI 200 fewer to 372 more) people per 1000 having subsequent surgery during this time. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCID: minimal clinically important difference; MD: mean difference; MPFL: medial patellofemoral ligament; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aTrials in this analysis recruited only people with primary (first time) dislocation. bDerived from the pooled estimate in the non‐surgical group. cEvidence downgraded by three levels: one level for imprecision as fewer than 100 events were reported, and two levels for very serious risk of bias (due to high risk of performance, detection and attrition bias). dEvidence downgraded by three levels: two levels for serious risk of bias (selection, performance and detection bias) and one level for inconsistency as this pooled analysis exhibited statistical heterogeneity (I² = 90%). eWhilst the MCID for the Kujala score has yet to be determined for the patellar dislocation population, a change exceeding 10 points is regarded as clinically meaningful for the anterior knee pain population (Bennell 2000; Crossley 2004). fEvidence downgraded one level due to imprecision with fewer than 100 events reported, one level for risk of bias (due to high risk of performance, detection and attrition bias) and one level for inconsistency though statistical heterogeneity where this pooled analysis exhibited substantial heterogeneity (I² = 87%). gEvidence downgraded three levels: two levels due to serious risk of bias (selection, performance and detection bias) and one level for serious imprecision. hEvidence downgraded three levels: one level due to imprecision with fewer than 100 events reported, and two levels due to risk of bias (due to high risk of performance, detection and attrition bias). iEvidence downgraded three levels: one level due to imprecision with fewer than 100 events reported, two levels due to risk of bias (due to high risk of selection, performance, detection and attrition bias).
Background
This is an update of our Cochrane Review last published in 2015 (Smith 2015).
Description of the condition
Patellar dislocation occurs when the patella (kneecap) disengages completely from the trochlear (femoral) groove, typically to the lateral side when the femur rotates internally on the tibia with the foot fixed on the ground. The patella may spontaneously slip back into its original position, or require manual reduction to push it back into place. The term 'patellar instability' is used to include both patellar dislocation and subluxation (partial dislocation), or a feeling that the patella is unstable. As a result, these people often do not 'trust' their knee not to be unstable. This leads to activity modification and restriction as people try to avoid dislocations or instability. This restriction can range from everyday tasks such as walking, housework or shopping and everyday activities to more physical tasks such as sports and exercise, particularly activities involving twisting and changing direction (Smith 2011a). Accordingly, patellar dislocation and instability can have a significant impact on a person's quality of life.
When the patella dislocates laterally, injury occurs to the soft tissues of the medial aspect of the knee joint, particularly to the medial patellofemoral ligament (Thompson 2019). This predisposes to subsequent episodes of patellar dislocation or subluxation, and eventually to degenerative change in the knee joint. As well as injury of the medial capsular structures, a range of anatomical factors may predispose to patellar instability. These include variations of limb alignment, such as excessive valgus knee (Huntington 2020; Smith 2011b), or of architecture/geometry of the patella and lower femur, particularly of the trochlear groove, such as trochlear dysplasia (Huntington 2020; Thompson 2019), excessive lateral positioning of the attachment of the patellar tendon onto the shinbone (tibial tuberosity) or connective tissue laxity, such as benign joint hypermobility syndrome (Beasley 2004).
The term 'primary patellar dislocation' refers to the first time a person experiences a patellar dislocation. Its incidence is highest in young and physically active people in the second and third decades of life (Merchant 2007). The annual incidence of primary patellar dislocation has been estimated at 43 per 100,000 in children under 15 years (Nietosvaara 1994), with the incidence across all age groups much lower (estimated at seven per 100,000 by Atkin 2000). Females are more likely to be affected than males (Mitchell 2015). Women are frequently more hypermobile than men (Scher 2010). Females also have a different muscle/body mass ratio (Strugnell 2014), meaning they are more susceptible to injuries such as anterior cruciate ligament rupture and patellar dislocation (Hsiao 2010). Recurrent patellar dislocation can occur in 15% to 60% of primary dislocation cases (Martinez‐Cano 2021; Woo 1998).
Description of the intervention
Following reduction of the patellar dislocation, people frequently undergo non‐surgical treatment consisting of physiotherapy and rehabilitation (Beasley 2004; Moiz 2018). This may include treatments such as immobilisation and bracing to limit knee movement, exercises, manual therapy, taping and electrotherapeutic modalities (Moiz 2018). Non‐surgical management is frequently exercise‐based, with the aim being to restore neuro‐musculoskeletal control of the patellofemoral joint at the hip, knee, ankle and foot through strengthening and muscle recruitment exercises and activities (Smith 2011b). If muscles and soft‐tissues are tight or restricted in length, most commonly the hamstrings, quadriceps, gastrocnemius or iliotibial band/tensor fascia lata, targeted stretching exercises are prescribed (Smith 2010; Smith 2011b). Non‐surgical management is most frequently delivered by a physiotherapist (Smith 2010; Smith 2011b).
Some surgeons advocate surgical intervention for primary, or more frequently, recurrent dislocation (Donell 2006a; Thompson 2019). Such orthopaedic surgical interventions are of three main types.
Proximal patellar realignment soft tissue procedures. These are designed to repair or tighten the capsular soft tissues and tendinous soft tissues on the medial side of the knee (repair or medial plication) or reconstruct the ligamentous structures, particularly the medial patellofemoral ligament (MPFL) to resist lateral displacement of the patella (Conlan 1993; Hautamaa 1998). If the lateral capsular soft tissues appear too tight, they may be incised (lateral release), but this is not recommended as an isolated procedure at present.
Distal patellar realignment procedures. This can include the tibial tubercle osteotomy (TTO) or Roux‐Goldthwaite procedures. In both instances, the surgeon alters where the patella attaches onto the tibia (Felli 2019). A TTO may be used where, most commonly, the patellar attachment is medialised (moved more centrally) and distalised (moved downwards) to correct abnormal patellar tracking in the distal femur (Cosgarea 2002; Dath 2006; Dejour 1994).
Osseous (bony) procedures. This includes a trochleoplasty where the surgeon constructs a groove in the femur for the patella to move within (Dejour 1994; Donell 2006b). This may also include femoral or tibial osteotomy for abnormal or excessive rotation of the tibia or femur.
These interventions may be performed separately or in combination. The choice of surgical intervention will be influenced by the specific anatomical abnormalities predisposing the individual to their instability problem (Thompson 2019). Physiotherapy rehabilitation is most often commenced following any of the above surgical interventions to rehabilitate people postoperatively (McGee 2017).
How the intervention might work
Non‐surgical ('conservative') treatments including physiotherapy aim to restore knee range of motion and improve patellar stability (Beasley 2004; Cosgarea 2002). It has been suggested that one principal cause of recurrent patellar dislocation is weakness of the vastus medialis, one of the four muscles forming the quadriceps (Dath 2006). By strengthening this muscle, it has been hypothesised that the patella will track more centrally in the trochlear groove, avoiding a more lateral position that may increase the likelihood of recurrent dislocation and instability symptoms (Donell 2006a). Similarly, strengthening muscle groups that control femoral internal rotation such as the glutaei muscle complex, has been suggested to reduce lateral patellar tracking through maintenance of femoral neutrality during activity (Donell 2006a; Smith 2010). Foot orthoses have also been recommended as a potential treatment adjunct, with the objective of controlling excessive tibial rotation, which may also influence patellar tracking through lateralisation of the patellar attachment on the tibia (Smith 2010). Finally, stretching shortened or tight soft tissues (such as of the hamstring, quadriceps, calf complex) through exercise or manual technique including mobilisation or massage, in addition to the lateral retinaculum/iliotibial band/tensor fascia lata, has also been proposed to reduce lateralisation of the patella within the patellofemoral joint (Smith 2010).
Surgical interventions, as described above, offer repair or reconstruction of soft tissues, or procedures to deepen the trochlear groove or to realign the patellar tendon, to stabilise the patella in a more medial position (Thompson 2019). The hypothesis is that by including an appropriate surgical procedure in addition to their postoperative rehabilitation programme, these interventions will be more effective than conservative treatment alone in reducing the recurrent instability that may substantially limit functional capabilities and quality of life.
Why it is important to do this review
Some authors have suggested that surgical interventions should be considered rather than physiotherapy alone (Boden 1997; Guhan 2009). Others have written that surgical interventions may be no better in preventing recurrent dislocation and functional restoration than non‐surgical approaches (Mears 2001; Nikku 1997). Determining the optimal management approach for this population is important for a number of reasons. Firstly, there is a high risk of recurrent patellar dislocation and instability symptoms if treatment is not effective. A second dislocation happens in around 40% of people within the first five years (Moiz 2018; Sanders 2018; Stefancin 2007). If a second (recurrent) dislocation occurs, ongoing restriction is highly likely and outcomes are poor (Liu 2018; Mäenpää 1997; Moiz 2018; Stefancin 2007). Secondly, there is a risk of cartilage lesions after repetitive subluxation and patellar dislocation (Salonen 2017). Repetitive injury of this nature can lead to early degenerative changes and osteoarthritis, resulting in long‐term pain and disability (Arendt 2016). Finally, patellar dislocation is more frequent in younger rather than older people (Huntington 2020; Merchant 2007). Ascertaining the most appropriate management strategy for this population is important to minimise the impact of this condition on their lifestyles and subsequent activities, and the impact of treatment for younger people could potentially have long‐lasting consequences.
The purpose of this systematic review is to inform clinical practice through the examination of the evidence from randomised trials comparing surgical to non‐surgical treatment approaches following patellar dislocation.
This is an update of our Cochrane Review last published in 2015, which identified five randomised studies and one quasi‐randomised study, including 344 people with primary (first‐time) patellar dislocation (Smith 2015). We found that, although there is some evidence to support surgical over non‐surgical management of primary patellar dislocation in the short term, the certainty of evidence was very low. We were very uncertain about the estimate of effect. We did not identify any trials that examined people with recurrent patellar dislocation. We concluded that adequately powered, multicentre, randomised controlled trials, conducted and reported to contemporary standards, were needed.
Objectives
To assess the effects (benefits and harms) of surgical versus non‐surgical interventions for treating people with primary or recurrent patellar dislocation.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised (use of a method of allocating participants to a treatment that is not strictly random, e.g. by date of birth, hospital record number, alternation) controlled clinical trials (RCTs) evaluating surgical versus non‐surgical interventions for treating patellar dislocation (either primary or recurrent) were eligible.
Types of participants
Eligible participants were people of any age (adults or children) with a reported history of patellar dislocation, either primary or recurrent, recorded either as a historical account from a participant, or observed by a healthcare professional. We excluded trials that recruited participants who presented with anterior knee pain or patellar subluxation rather than a clear, convincing history or evidence of a patellar dislocation.
Types of interventions
Non‐surgical intervention, or conservative management, is the control intervention in this review. Non‐surgical treatment strategies following patellar dislocation include: a period of immobilisation, bracing or splinting, manual therapy, exercise‐based treatments, education and advice, electrotherapeutic modalities and taping techniques.
Surgical treatment strategies include the following.
Proximal patellar realignment soft tissue procedures such as medial reefing, lateral release, MPFL repair or reconstruction.
Distal patellar realignment procedures such as the TTO or a Roux‐Goldthwaite operation.
Osseous (bony) procedures such as trochleoplasty or femoral or tibial osteotomy.
Types of outcome measures
We assessed the clinical and radiological outcome measures described below.
Primary outcomes
Recurrent patellar dislocation.
Validated patient‐rated knee and physical function scores for patellar dislocation outcomes (Paxton 2003), e.g. the Lysholm score (Lysholm 1982), the Tegner activity score (Tegner 1985), the Hughston visual analogue score (VAS) (Flandry 1991), the Norwich Patellar Instability score (Smith 2014), the Banff‐II Patellar Instability Instrument (Hiemstra 2013) and the Kujala Patellofemoral Disorders score (Kujala 1993).
We assessed these outcomes at three time points after treatment (short, medium and long term).
Secondary outcomes
Health‐related quality of life scores such as the EQ‐5D‐5L (Herdman 2011) and the Short Form‐12 (SF‐12) (Ware 1996).
Return to former activities: work and sports.
Knee pain during activity or at rest, as measured using a VAS or similar.
Adverse events (complications), e.g. deep or superficial infection, nerve palsy, allergies, rash or abrasion from taping or orthoses. These were assessed either as individual adverse events or as composite adverse event data.
Patient‐reported satisfaction such as measured with Likert scale, VAS or any other validated score.
Patient‐reported knee instability symptoms.
Subsequent requirement for knee surgery (reoperations) for complications such as infection, or mechanical instability.
These outcomes were assessed at each follow‐up time point presented within the included studies.
Search methods for identification of studies
Electronic searches
For this update, we revised all our search strategies in line with the current Cochrane Bone Joint and Muscle Trauma Group practices. We searched the following databases.
Cochrane Bone, Joint and Muscle Trauma Group's Specialised Register (15 December 2021)
Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Register of Studies (CRS‐Web) 15 December 2021, Issue 1)
MEDLINE Ovid (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 15 December 2021)
Embase Ovid (1980 to 15 December 2021)
Allied and Complementary Medicine (AMED) (1985 to 15 December 2021)
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1981 to 15 December, Week 1, 2021)
Physiotherapy Evidence Database (PEDro) (15 December 2021)
World Health Organization (WHO) International Clinical Trials Registry Platform (21 December 2021)
Clinicaltrials (21 December 2021)
There were no constraints based on language or publication status. The date of search was restricted from the date of the previous review (October 2014). Details of the previous search strategies are available in the previous review (Smith 2015).
In MEDLINE we combined a subject‐specific search with the Cochrane Highly Sensitive Search Strategy for identifying RCTs in MEDLINE (sensitivity‐maximising version) (Lefebvre 2019). Details of search strategies for all databases are shown in Appendix 1.
Searching other resources
We searched conference proceedings from the British Orthopaedic Association Annual Congress, the British Trauma Society meetings, the European Federation of National Associations of Orthopaedics and Traumatology (EFORT) and the British Association for Surgery of the Knee (BASK) via the supplements of the Bone and Joint Journal (December Week 1 2021). We also searched bibliographies of relevant articles and contacted trial investigators in this area.
Data collection and analysis
Selection of studies
Two review authors (TS and AG) independently selected the potentially eligible articles from citation titles and, if available, abstracts. Upon obtaining full articles, the same two authors independently performed the study selection. In cases of disagreement of paper inclusion/exclusion, a consensus was reached through discussion. Had that not been possible, we would have sought arbitration from a third author (CH).
Data extraction and management
Two review authors (TS and AG) independently extracted data from trial reports. We contacted corresponding authors when key information was missing. In cases of disagreement, we sought consensus through discussion or adjudication by a third author (CH). After the individual review authors had extracted the relevant data, these were collated to form a single, agreed and completed data extraction form with all the included trial's characteristics and results. The template data extraction form is presented in Appendix 2. This collected all key trial data and participant information from the included articles.
Assessment of risk of bias in included studies
Two review authors (TS and AG) independently assessed the risk of bias of the included trials using Cochrane's risk of bias tool (Higgins 2011). This consists of five domains: sequence generation (selection bias); allocation concealment (selection bias); blinding of participants, personnel (performance bias) and blinding of outcome assessors (detection bias); incomplete outcome data (attrition bias); selective reporting (reporting bias); as well as other risks of bias. We categorised risk of bias as low, unclear or high for each of the included trials. When no information was given by an included trial, we rated this domain as 'unclear' risk of bias. When differences between the ratings of the two assessors could not be resolved through discussion, we asked a third author (CH) to adjudicate.
Measures of treatment effect
We measured treatment effects using risk ratios (RR) for binary data and mean differences (MD) for continuous data. Should different scales or tools have been used to measure the same domain of a continuous outcome, we would have calculated standardised mean differences (SMDs). We used 95% confidence intervals (CI) throughout.
We categorised measurement of treatment effect time points as: short term (up to and including two years postrandomisation); medium term (over two years to less than 10 years postrandomisation); and long term (10 years or more postrandomisation). Where trials presented several follow‐up periods, we extracted and analysed data to inform short‐, medium‐ and long‐term results. Where authors reported multiple time points within the same time point category, we reported the later time point.
Unit of analysis issues
The unit of randomisation in the majority of trials included in this review was the individual participant. Exceptionally, as in the case of trials including people with bilateral patellar dislocations, data for trials may be presented for dislocations or knees rather than for an individual person. Where such unit of analysis issues arose, and appropriate corrections were not made, we presented the data for such trials only when the disparity between the units of analysis and randomisation was small.
Dealing with missing data
We contacted the corresponding study authors in respect of any missing key information from their publications. Where appropriate, we performed intention‐to‐treat analyses to include all people randomised to the intervention groups. We were alert to the potential mislabelling or misidentification of standard errors and standard deviations. Unless we could derive missing standard deviations from confidence interval data, we did not impute assumed values.
Assessment of heterogeneity
We appraised clinical diversity in terms of participants, interventions and outcomes for the included trials. We assessed statistical heterogeneity by visual inspection of the forest plot and by using the I² statistic and Chi² test. The Chi² test was interpreted as demonstrating substantial heterogeneity where P was 0.10 or less. I² was interpreted where 0% to 40% indicated potentially unimportant statistical heterogeneity, 30% to 60% represented moderate heterogeneity, 50% to 90% represented substantial heterogeneity, and 75% to 100 represented considerable statistical heterogeneity.
Assessment of reporting biases
We assessed outcome reporting bias by considering the data reported by trials against prospectively registered protocols. Where no prospectively registered protocol was available, we were unable to assess reporting biases. In the event that sufficient data were presented for a given outcome at a given time point (from at least 10 trials), we planned to assess publication bias using funnel plots.
Data synthesis
We considered clinical and methodological heterogeneity to determine whether it was appropriate to pool data in meta‐analysis. When judged appropriate, we pooled results from individual studies in meta‐analyses using fixed‐ or random‐effects models (depending on the results of heterogeneity tests), with 95% CI. We adopted a fixed‐effect model when there was no evidence of statistical heterogeneity (I² less than or equal to 30% and Chi² P > 0.01). We adopted a random‐effects model where there was no evidence of methodological diversity such as cohort, intervention or trial procedure, but statistical heterogeneity was evident that could not be readily explained (as denoted with an I² > 30% and Chi² P value equal to or less than 0.01). We were able to pool data in this review to determine short‐, medium‐ and long‐term outcomes.
Subgroup analysis and investigation of heterogeneity
We were unable to undertake all the planned formal subgroup analyses due to lack of studies. However, we were able to perform a formal subgroup analysis of males versus females. We made comparisons comparing results of participants under the age of 16 years following surgical and non‐surgical management.
Should data become available in a future update, we plan to carry out formal subgroup analyses to assess the difference in outcome between those who are hypermobile versus non‐hypermobile participants, in order to investigate whether this is an important prognostic variable in this patient group. We will also assess for a difference in outcome between different surgical treatments e.g. whether there is a difference in outcomes between repair versus reconstruction of MPFL. We also plan to undertaken formal subgroup analyses by participant age and primary versus recurrent patellar dislocation. We do not intend to analyse the effect of timing of surgery or conservative intervention in relation to the time since the participant's primary patellar dislocation.
Sensitivity analysis
We undertook sensitivity analyses on the primary outcomes to examine the impact of including trials at high risk of bias due to lack of allocation concealment by analysing studies at low risk of selection bias (for allocation concealment). We planned to undertake a sensitivity analysis of trials where the population was poorly defined. However, this was not a limitation within the included trials so was not undertaken.
Summary of findings and assessment of the certainty of the evidence
Two review authors (TS and AG) used the GRADE system to assess the certainty of the body of evidence associated with selected critical outcomes in the review. We did not construct summary of findings tables for all comparisons in this review. Instead, we summarised the evidence available for the two primary outcomes.
Recurrent patellar dislocation
Knee and physical function scores
We also summarised the evidence for four secondary outcome measures.
Incidence of complications (adverse effects of treatment)
Patient satisfaction
Patient‐reported knee instability (patellar subluxation)
Subsequent requirement for surgery
For these outcomes, we selected the follow‐up time point (short, medium or long term) for the outcome that provided the most substantial body of evidence. For knee and physical function scores, we reported the Kujala Patellofemoral Disorders score in the summary of findings table because this provided the most substantial body of evidence.
We used the GRADE approach to determine the certainty of evidence for each outcome (very low, low, moderate or high), as recommended by Cochrane (Schünemann 2021). The GRADE approach assesses the certainty of a body of evidence based on the extent to which we can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the certainty of a body of evidence considers within‐study risk of bias (study limitations), directness of the evidence (indirectness), heterogeneity of the data (inconsistency), precision of the effect estimates (imprecision), and risk of publication bias. The certainty of the evidence could be high, moderate, low or very low, being downgraded by one or two levels depending on the presence and extent of concerns in each of the five GRADE domains. We used footnotes to describe reasons for downgrading the certainty of the evidence for each outcome, and we used these judgements when drawing conclusions in the review. Of note, we assessed imprecision as occurring when fewer than 100 events were reported for a given analysis and/or where the confidence interval crossed both appreciable benefit and harm. We used GRADEpro GDT software to construct the summary of findings table.
Results
Description of studies
Results of the search
For this update we screened a total of 1712 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (15), CENTRAL (249), MEDLINE (410), Embase (783), AMED (23), CINAHL (105), PEDro (6), the WHO ICTRP (81), and ClinicalTrials.gov (40). Our searches of other resources, namely reference lists from potentially eligible and eligible papers, identified four additional studies that appeared to meet the inclusion criteria.
Once duplicates had been removed, we had a total of 1269 records. We excluded 1255 records based on titles and abstracts. We obtained the full text of the remaining 14 records and linked any references pertaining to the same study under a single study ID. Upon further analysis, we included four new studies (Askenberger 2018; Ji 2017; Rahman 2020; Regalado 2016). Including the previous six studies (Bitar 2012; Camanho 2009; Christiansen 2008; Nikku 1997; Petri 2013; Sillanpää 2009), 10 trials were eligible recruiting 519 participants. We excluded six new studies (Alvarez 2020; Kang 2017; Moström 2014; NCT02185001; Sillanpää 2011; Zheng 2019), and two were ongoing (Liebensteiner 2021; NCT02263807). No studies are awaiting classification. Studies excluded in the previous version of this review are reported in Smith 2015.
Further details of the process of screening and selecting trials for inclusion in the review are illustrated in Figure 1.
1.
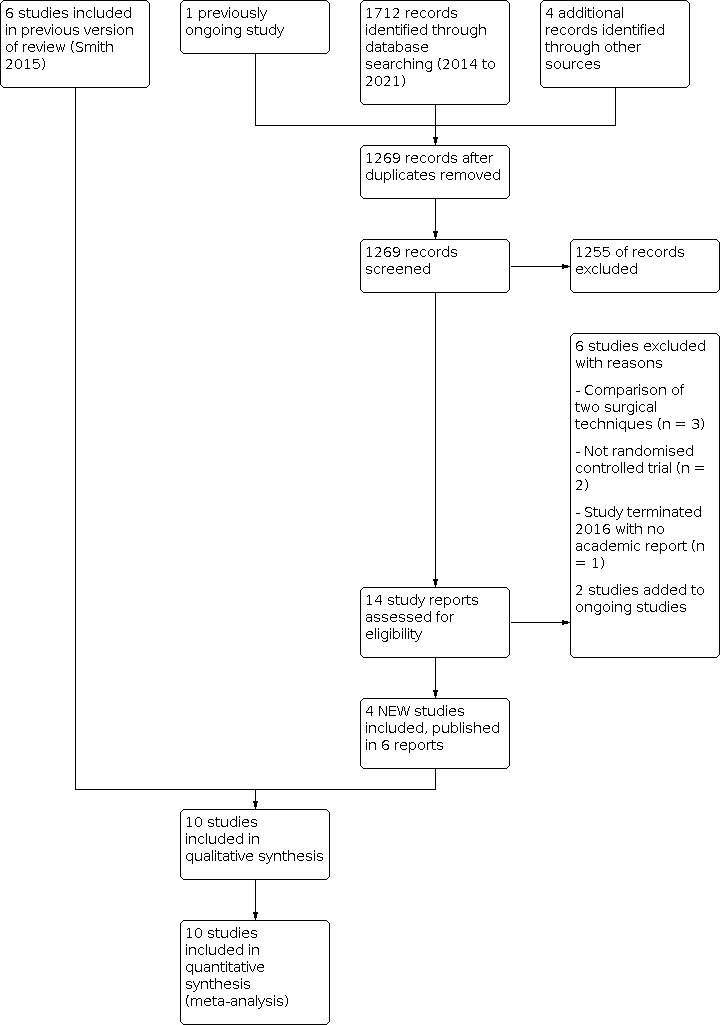
Study flow diagram
Included studies
We included 10 trials (recruiting 519 participants) published between 1997 and 2020. They were all written in English. Three trials were conducted in Finland (Nikku 1997; Regalado 2016; Sillanpää 2009), two in Brazil (Bitar 2012; Camanho 2009), one in Germany (Petri 2013), one in Denmark (Christiansen 2008), one in Sweden (Askenberger 2018), one in China (Ji 2017) and one in the UK (Rahman 2020).
Randomisation procedure
Eight trials, including 344 participants, reported that they were randomised trials (Askenberger 2018; Bitar 2012; Camanho 2009; Christiansen 2008; Petri 2013; Rahman 2020; Regalado 2016; Sillanpää 2009), and two were quasi‐randomised by odd or even birth year (Nikku 1997; Ji 2017).
Participant demographic characteristics
Of the 500 participants for whom demographic data are available, 263 people (146 females and 117 males) were allocated surgery and 237 people (118 females and 119 males) were allocated non‐surgical intervention. The mean age in the surgery groups ranged from 13.2 years (Askenberger 2018) to 27.2 years (Petri 2013). The mean age in the non‐surgical groups ranged from 13.0 years (Askenberger 2018) to 24.6 years (Camanho 2009). Rahman 2020 did not report gender or age characteristics by group.
In the individual trials, the mean age ranged from 13.1 years in Askenberger 2018 to 26.0 years in Rahman 2020; and the percentage of females from 7.5% in Sillanpää 2009, which included military recruits, to 65.6% in Nikku 1997. Four trials included children, who were mainly adolescents, as well as adults (Bitar 2012; Camanho 2009; Christiansen 2008; Nikku 1997). Askenberger 2018 and Regalado 2016 solely recruited children. The youngest participants were eight years old (Regalado 2016) and the oldest, who was an outlier, was 74 years old (Camanho 2009). Age was not reported in Ji 2017.
Nikku 1997 reported the outcomes of 127 knees in 125 participants, whilst Bitar 2012 reported the outcomes of 41 knees in 39 participants. In Bitar 2012, presenting trial data by patellar dislocation was unavoidable except for knee‐specific outcomes, such as the incidence of recurrent instability/dislocation. All remaining trials were analysed as one knee per person.
Four trials made reference to whether their participants presented with joint hypermobility (Askenberger 2018; Bitar 2012; Nikku 1997; Rahman 2020). Bitar 2012 reported that no patellar hypermobility was detected, and Nikku 1997 stated that one participant in each group presented with ligament laxity as assessed using the Beighton score (Carter 1964). Fourteen participants (38%) in the Askenberger 2018 non‐surgical group and 13 participants (35%) in their surgical group presented with Beighton scores of four and above to indicate joint hypermobility. Rahman 2020 reported that seven participants (38%) in their trial presented with joint hypermobility but did not present the breakdown of this by group allocation.
Patellar dislocation and eligibility criteria characteristics
Nine trials only recruited participants who had sustained primary patellar dislocation (Askenberger 2018; Bitar 2012; Camanho 2009; Christiansen 2008; Ji 2017; Nikku 1997; Petri 2013; Regalado 2016; Sillanpää 2009). One trial only recruited participants who had experienced recurrent patellar dislocations (Rahman 2020).
The diagnosis of patellar dislocation was made during initial clinical examination within the trial, on the basis of a variety of different combinations of signs and symptoms. These inclusion criteria included: patellar dislocation requiring reduction in two trials (Christiansen 2008; Camanho 2009), a history of acute knee trauma in seven trials (Askenberger 2018; Bitar 2012; Camanho 2009; Ji 2017; Nikku 1997; Petri 2013; Sillanpää 2009), and intra‐articular haematoma, tenderness on the medial epicondyle and positive lateral patellar apprehension test results in Christiansen 2008 and Ji 2017. Magnetic resonance imaging was used as an eligibility criterion in four trials (Askenberger 2018; Ji 2017; Rahman 2020; Sillanpää 2009). All participants in four trials underwent arthroscopy to aid diagnosis (Askenberger 2018; Christiansen 2008; Petri 2013; Regalado 2016). The diagnosis of recurrent patellar dislocation in Rahman 2020 was a self‐reported experience of two or more lateral patellar dislocations or one dislocation with a minimum of six‐month history of subjective patellar instability leading up to the time of recruitment.
The main exclusion criteria were the presence (and/or requirement for surgical fixation) of a large osteochondral fracture presented in eight trials (Askenberger 2018; Bitar 2012; Camanho 2009; Nikku 1997; Petri 2013; Rahman 2020; Regalado 2016; Sillanpää 2009), an inability to follow up the planned treatment regimens in three trials (Bitar 2012; Christiansen 2008; Rahman 2020), prior knee surgery in five trials (Bitar 2012; Christiansen 2008; Ji 2017; Nikku 1997; Regalado 2016) and a previously reported patellar dislocation or instability in nine trials (Askenberger 2018; Bitar 2012; Camanho 2009; Christiansen 2008; Ji 2017; Nikku 1997; Petri 2013; Regalado 2016; Sillanpää 2009). Other exclusion criteria were the co‐existence of a significant tibiofemoral ligament injury requiring (or not) surgical fixation (Askenberger 2018; Bitar 2012; Ji 2017; Rahman 2020; Regalado 2016), people with conditions associated with serious neuromuscular or congenital diseases (Bitar 2012; Ji 2017), a history of a non‐traumatic event such as walking or squatting with 'moderate' stress on the knee and in the absence of acute pain in the knee (Bitar 2012), open growth plates (Rahman 2020), open injury (Petri 2013) or women who were pregnant or lactating (Petri 2013). Ji 2017 excluded people with patellofemoral dysplasia (Dejour B‐D) and patellar alta (Insall‐Salvati > 1.2) or a tibial tuberosity‐trochlear groove (TT‐TG) distance greater than 20 mm. Rahman 2020 excluded people with severe trochlear dysplasia or rotational coronal or sagittal malalignment of the femur or tibia, which in the opinion of the treating surgeon required surgical correction. Rahman 2020 also specifically excluded people with medial patellar dislocation.
Non‐surgical management
Non‐surgical management in nine trials consisted of initial immobilisation in a cast, splint or locked orthosis, followed by active mobilisation with physiotherapy. Rahman 2020 was the only trial not to use a form of immobilisation in their non‐surgical cohort, proceeding directly onto active mobilisation postrandomisation; this may be because they were the only trial studying recurrent rather than first‐time dislocation. There was variation in the duration of immobilisation and in components of the physiotherapy programmes (see Characteristics of included studies). This is summarised in Table 2. Whilst participants in four trials underwent arthroscopy prior to randomisation (Askenberger 2018; Christiansen 2008; Petri 2013; Regalado 2016), this was a diagnostic arthroscopic procedure and not a therapeutic arthroscopy. Of note in Sillanpää 2009, all participants in the non‐operative group received knee aspiration to relieve pain and four underwent arthroscopic removal of an osteochondral fragment. Similarly, Ji 2017 reported that arthroscopy surgery was performed to remove any loose bodies, if required. However, they did not report how frequently this was required. All these trials were included given the non‐corrective nature of these procedures.
1. Summary of non‐surgical management.
| Study | Knee brace/cast | Knee strength/ROM exercises | Initial FWB permitted | Initial PWB/NWB | Education/advice | Home exercise programme | Electric stimulation | Analgesia | Manual therapy | Care stability exercises | Cryotherapy | Muscle stretching exercises | CBT | Foot orthoses |
| Askenberger 2018 | 4 weeks | X | X | ‐ | ‐ | X | ‐ | ‐ | ‐ | X | ‐ | ‐ | ‐ | ‐ |
| Bitar 2012 | 3 weeks | X | ‐ | X | ‐ | ‐ | X | X | ‐ | ‐ | X | ‐ | ‐ | ‐ |
| Camanho 2009 | 3 weeks | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | ‐ | ‐ |
| Christiansen 2008 | 2 weeks | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ji 2017 | 3 weeks | X | ‐ | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Nikku 1997 | 3 weeks | X | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Petri 2013 | 3 weeks | X | ‐ | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Rahman 2020 | ‐ | X | ‐ | ‐ | X | ‐ | X | X | X | ‐ | ‐ | ‐ | X | X |
| Regalado 2016 | 6 weeks | X | X | ‐ | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sillanpää 2009 | 6 weeks | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
CBT: cognitive behavioural therapy treatment; FWB: full weight bearing; NWB: non‐weight bearing; PWB: partial weight bearing; ROM: range of motion
Surgical management
A summary of the surgical management interventions is presented in Table 3. The predominant operative intervention was repair or reconstruction of the soft tissues of the medial aspect of the knee joint. Four trials reported that all participants in their surgical groups solely underwent MPFL repair (Askenberger 2018; Camanho 2009; Christiansen 2008; Ji 2017). This was an arthroscopic procedure in two trials (Askenberger 2018; Camanho 2009), and an open procedure in the other two trials (Christiansen 2008; Ji 2017). Nikku 1997 reported that all participants allocated to surgery in their trial received either a medial reefing with an MPFL augmentation using adductor magnus (six participants) or medial reefing with a lateral release (54 participants). Petri 2013 reported that their surgical intervention was repair of the medial soft tissues and a "MPFL‐plastic" procedure was not undertaken. Whilst they acknowledged that a lateral release was optional, they did not stipulate the frequency with which this procedure was undertaken. Sillanpää 2009 allocated 14 participants in the surgical group to receive a combined medial reefing procedure and MPFL suture repair, a Roux‐Goldwraithe (RG) procedure for four participants, and an arthroscopic repair was also required for an osteochondral fracture in six people. In Bitar 2012, the surgical procedure was an MPFL reconstruction using a medial slip of the patellar ligament, which was then sutured to the distal aspect of the vastus medialis muscle. Rahman 2020 did not report what specific surgical procedures were undertaken for their nine surgical participants. In Regalado 2016, surgical procedures were determined by clinical presentation against the Fulkerson classification (Fulkerson 1987). Through this, three participants with type I underwent lateral retinacula release (LLR); whilst 13 participants with a type II‐IV underwent a modified RG procedure (combination of proximal and distal realignment with LLR and medial imbrications).
2. Summary of surgical management.
| Study | MPFL repair | Medial reefing | Medial retinacula repair | MPFL reconstruction | Lateral release | modified Roux‐Goldwraite | Tibial tuberosity transfer | Osteochondral fracture repair |
| Askenberger 2018 | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Bitar 2012 | ‐ | ‐ | ‐ | X | ‐ | ‐ | ‐ | ‐ |
| Camanho 2009 | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Christiansen 2008 | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ji 2017 | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Nikku 1997 | ‐ | X | X | X | X | ‐ | ‐ | ‐ |
| Petri 2013 | X | X | X | ‐ | X | ‐ | X | ‐ |
| Rahman 2020a | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Regalado 2016 | ‐ | ‐ | ‐ | ‐ | X | X | ‐ | ‐ |
| Sillanpää 2009 | X | X | ‐ | ‐ | ‐ | X | ‐ | X |
MPFL: medial patellofemoral ligament
aRahman 2020 did not specifically state what surgical interventions were performed.
All participants allocated to the surgical management strategies received a period of postoperative rehabilitation. With the exception of four trials (Askenberger 2018; Camanho 2009; Ji 2017; Rahman 2020), the postoperative rehabilitation programme used in each study was identical to that used in the non‐operative group. Camanho 2009 immobilised participants in their surgical group in an inguinal‐malleolar splint for three weeks, permitted their surgical patients to wear a movable immobiliser for three weeks and to commence passive knee range of motion exercises during this early postoperative period. Askenberger 2018 immobilised participants in a soft cast splint for four weeks following surgery. The subsequent physiotherapy programmes were the same. Ji 2017 immobilised their participants postsurgery in a knee brace in full knee extension for the first two postoperative days and then permitted knee flexion from zero to 90 degrees until four weeks postoperatively. In Rahman 2020, surgical participants underwent a similar programme of rehabilitation to their non‐surgical group, except that there was less focus on goal‐setting in the postsurgical intervention, with progress more closely dictated by surgical milestones for tissue healing.
Follow‐up time points and outcome measures
The shortest follow‐up period was 12 months (Rahman 2020). The maximum follow‐up was three years in two studies (Askenberger 2018; Christiansen 2008; Petri 2013). The mean follow‐up was 44 months (range 24 to 61 months) in Bitar 2012, and 42 months (range 24 to 54) in Ji 2017. Follow‐up in Camanho 2009 was after two years and before five years, the mean follow‐ups in the surgical and non‐surgical groups being 40.4 and 36.3 months, respectively. Nikku 1997 presented data at mean follow‐up periods of 25 months (range 20 to 45 months), seven years (range 5.7 to 9.1 years) and, for a subgroup of children only, 14 years (range 11 to 15 years), across three publications. Regalado 2016 reported their follow‐up data on children at six years. The median follow‐up was seven years, range six to nine years, in Sillanpää 2009.
Primary outcomes for review
All included trials provided data for our primary outcome of recurrent dislocation. Eight trials reported data on validated patient‐rated knee and physical function or activity scores. Eight trials reported the Kujala Patellofemoral Disorders score (Askenberger 2018; Bitar 2012; Camanho 2009; Christiansen 2008; Ji 2017; Nikku 1997; Petri 2013; Sillanpää 2009). Three trials reported the Tegner activity score (Askenberger 2018; Nikku 1997; Sillanpää 2009). Validated patient‐completed outcome measures included the Knee Injury and Osteoarthritis Outcome Score (KOOS) (Christiansen 2008), the KOOS‐Child (Askenberger 2018), the Lysholm knee score (Nikku 1997), and the Hughston VAS knee score (Nikku 1997).
Secondary outcomes for review
Two trials reported other knee function and activities (Nikku 1997; Sillanpää 2009); return to former activities in one trial (Sillanpää 2009); knee pain using a visual analogue scale (VAS) in two trials (Nikku 1997; Sillanpää 2009); and adverse events relating to treatment in three trials (Nikku 1997; Rahman 2020; Regalado 2016). Participant satisfaction of outcome was reported in four trials (Ji 2017; Nikku 1997; Petri 2013; Regalado 2016). Askenberger 2018 assessed health‐related quality of life using the EQ‐5D‐Y (Wille 2010).
There was variation in the definitions used for 'instability'. Six trials reported the frequency of recurrent patellar subluxation (Bitar 2012; Camanho 2009; Ji 2017; Nikku 1997; Petri 2013; Sillanpää 2009). Three studies reported the number of participants in each group who underwent subsequent surgery (Nikku 1997; Regalado 2016; Sillanpää 2009).
Excluded studies
Studies excluded in the previous version of this review are reported in Smith 2015. In this review, we excluded six trials from the review (see Characteristics of excluded studies). We excluded three trials as they were not randomised or quasi‐randomised trials (Moström 2014; Sillanpää 2011; Zheng 2019), and two trials as some participants in the non‐surgical intervention received surgical interventions (Alvarez 2020; Kang 2017). We reclassified one trial, which was previously ongoing (NCT02185001), as excluded as it was terminated with no results presented.
Ongoing studies
Two trials are ongoing (Liebensteiner 2021; NCT02263807). Liebensteiner 2021 is an RCT being conducted in Austria and Germany, assessing outcomes of a tailored surgical approach to non‐surgical (bracing and physiotherapy) management for primary patellar dislocation. The surgical intervention is tailored to address the pathologic anatomy that predisposes participants to lateral patellar dislocation. Therefore, all participants randomised to this group will receive an MPFL reconstruction with or without trochleoplasty, tibial tuberosity transfer, derotational osteotomy, or varus osteotomy. The follow‐up period for the planned 160 participants is 24 months. NCT02263807 is an RCT taking place in Norway, comparing outcomes of surgical (MPFL reconstruction) with non‐surgical (physiotherapy) management for recurrent patellar dislocation. The follow‐up period for the planned 70 participants is 36 months. The trial commenced in 2010. At the last update, 75 participants had been enrolled.
Risk of bias in included studies
Our judgements of the risk of bias in the 10 included trials are summarised in the risk of bias graph (Figure 2) and the risk of bias summary (Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We judged two trials as being of low risk of selection bias (Petri 2013; Rahman 2020). This reflected the use of a computer‐generated randomisation sequence and sealed envelopes (Petri 2013), and computer‐generated randomisation through a telephone system (Rahman 2020). The quasi‐randomised trials of Nikku 1997 and Ji 2017, which allocated treatment according to year of birth, were assessed as being of high risk of selection bias relating to inadequate sequence generation and lack of allocation concealment. We assessed the other six trials as unclear risk of selection bias from sequence generation, which reflected inadequate information on randomisation methods (Askenberger 2018; Bitar 2012; Camanho 2009; Christiansen 2008; Regalado 2016; Sillanpää 2009). Bitar 2012 and Camanho 2009 probably used the same method involving drawing of a slip of paper specifying the treatment. Three trials referred to randomisation using a sealed envelope approach to minimise selection bias (Askenberger 2018; Christiansen 2008Sillanpaa 2009). However, no details were reported on adequate safeguards to ensure that allocation concealment were ensured throughout, hence we classified these as unclear risk of bias.
Blinding
No trials blinded their assessors to treatment allocation. Due to the design of these trials and the topic under investigation, it would have been very difficult, if not impossible, to blind treating clinicians to treatment allocation, or participants to their allocation intervention. We assessed all trials as being of high risk of performance and detection bias relating to lack of blinding.
Incomplete outcome data
We judged one trial as being of high risk of attrition bias as the numbers of participants lost to follow‐up differed between the groups where five non‐surgical and one surgical participant were lost to follow‐up (Regalado 2016). We considered eight trials as being of low risk of bias (Askenberger 2018; Bitar 2012; Camanho 2009; Christiansen 2008; Ji 2017; Nikku 1997; Petri 2013; Sillanpää 2009). Small losses to follow‐up were reported in five trials (Bitar 2012; Christiansen 2008; Nikku 1997; Petri 2013; Sillanpää 2009). There were no losses reported in Camanho 2009. Four trials reported reasons for their missing participants, which we considered adequate (Askenberger 2018; Ji 2017; Petri 2013; Sillanpää 2009). One trial was at unclear risk as the reason for attrition was not clear, and seven participants were missing from the analysis at the six‐month time point (Rahman 2020). Only Bitar 2012 confirmed that the data were analysed according to intention‐to‐treat principles.
Selective reporting
No protocols or prospective trial registration documents were available for eight trials. Two trials provided ISRCTN trial registration numbers (Askenberger 2018; Rahman 2020). Although all the planned outcomes defined in the methods section were reported in the results sections of the included trials, we judged seven trials not reporting adverse effects of surgery as having high risk of selective reporting bias (Askenberger 2018; Bitar 2012; Camanho 2009; Christiansen 2008; Ji 2017; Petri 2013; Sillanpää 2009). Two trials reported adverse events and therefore we judged them as being at low risk of selective reporting (Nikku 1997; Regalado 2016). Rahman 2020 presented adverse events but did not present the outcomes of their cohort by allocated group, and therefore were assessed as being of unclear risk of reporting bias.
Other potential sources of bias
We identified no other sources of bias in the included studies.
Effects of interventions
See: Table 1
Primary outcomes
Recurrent dislocation
All 10 trials reported the frequency of recurrent dislocation after surgery compared with non‐surgical interventions. Data for this outcome are presented for each follow‐up period; see Analysis 1.1; Figure 4.
1.1. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 1: Number of participants sustaining recurrent patellar dislocation
4.

Forest plot of comparison 1. Surgical versus non‐surgical management. Outcome: 1.1 Number of participants sustaining recurrent patellar dislocation
Pooled data from two trials showed little difference between the surgical and non‐surgical groups at short‐term follow‐up (Ji 2017; Rahman 2020) (2/39 versus 4/36; risk ratio (RR) 0.48 favouring surgery, 95% confidence intervals (CI) 0.10 to 2.38; 2 studies, 75 participants). This finding is consistent compared to participants with recurrent dislocation events alone prior to randomisation in Rahman 2020 (1/9 versus 1/10; RR 1.11, 95% CI 0.08 to 15.28; 19 participants). Pooled data from eight trials showed a smaller incidence of recurrent dislocation at medium‐term follow‐up in the surgical group (Askenberger 2018; Bitar 2012; Camanho 2009; Christiansen 2008; Nikku 1997; Petri 2013; Regalado 2016; Sillanpää 2009) (44/231 versus 72/207; RR 0.55 favouring surgery, 95% CI 0.40 to 0.75; 8 studies, 438 participants). This trend was consistent compared to an analysis involving children only in Askenberger 2018 and Regalado 2016 (13/52 versus 27/52; RR 0.48 favouring surgery, 95% CI 0.28 to 0.82; 2 studies, 110 participants; data not shown). There was little difference between surgical and non‐surgical groups at long‐term follow‐up (24/36 versus 20/28; RR 0.93 favouring surgery, 95 CI 0.67 to 1.30; 1 study, 64 participants). For these outcomes, using GRADE criteria, we downgraded the certainty of evidence three levels to very low‐certainty evidence; one level for imprecision as fewer than 100 events were reported, and two levels for very serious risk of bias (due to high risk of performance, detection and attrition bias).
Sensitivity analysis
Only two studies were at low risk of selection bias (for allocation concealment), one of which reported short‐term data (Rahman 2020), and one of which reported medium‐term data (Petri 2013). The findings from Rahman 2020 were consistent with the pooled result for short‐term data in Analysis 1.1 (RR 1.11, 95% CI 0.08 to 15.28; 1 study, 19 participants). However, the result in Petri 2013 indicated little or no difference in recurrent patellar dislocation in the medium term (RR 0.44, 95% CI 0.09 to 2.09; 1 study 20 participants), and this differed from our interpretation of the primary pooled analysis.
Validated patient‐rated knee and physical function scores for patellar dislocation outcomes
Three trials reported the Tegner activity score (0 to 10: higher values indicate improved outcome) (Askenberger 2018; Nikku 1997; Sillanpää 2009) (Analysis 1.2). Pooled data from these trials showed little difference between the groups at short‐term follow‐up (MD ‐0.56 favouring non‐surgery, 95% CI ‐1.08 to ‐0.04; 190 participants). This finding was the same for a subgroup involving children only in the Askenberger 2018 study (MD ‐0.50 favouring non‐surgery, 95% CI ‐1.33 to 0.33; 65 participants). This is consistent at medium‐term follow‐up (MD 0.00, 95% CI ‐1.15 to 1.15; 1 study, 40 participants); and, for children only, at long‐term follow‐up (MD ‐1.60 favouring non‐surgical treatment, 95% CI ‐2.44 to ‐0.76; 1 study, 64 participants). We downgraded the certainty of evidence three levels to very low‐certainty evidence; one level due to imprecision where the CIs include both appreciable benefit and appreciable harm, and two levels for very serious risk of bias (due to high risk of performance and detection bias and risk of selection bias in Nikku 1997).
1.2. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 2: Tegner activity score (0 to 10: best score)
The Knee Injury and Osteoarthritis Outcome Score (KOOS) (0 to 10 for total score: higher values indicate improved outcome) was assessed by Askenberger 2018 and Christiansen 2008 (Analysis 1.3). There was little difference between surgical and non‐surgical intervention groups at short‐term follow‐up in the KOOS subsections: symptoms (MD ‐3.35, 95% CI ‐11.09 to 4.39; 2 studies, 145 participants), pain (MD ‐1.01, 95% CI ‐10.18 to 8.15; 2 studies, 145 participants), activities of daily living (ADL) (MD ‐0.31, 95% CI ‐7.96 to 7.33; 2 studies, 145 participants), sports and recreation (MD ‐4.64, 95% CI ‐21.85 to 12.57; 2 studies, 145 participants) or quality of life (MD ‐4.14, 95% CI ‐18.69 to 10.41; 2 studies, 145 participants). The results from this analysis are presented in Analysis 1.3. However, for an analysis involving children only (Askenberger 2018), participants reported better outcomes in the non‐surgical group in symptoms (MD ‐7.20, 95% CI ‐14.21 to ‐0.19; 68 participants), sports and recreation (MD ‐14.00, 95% CI ‐24.06 to ‐3.94; 68 participants) and quality of life (MD ‐12.20, 95% CI ‐21.56 to ‐2.84; 68 participants). We downgraded the certainty of evidence three levels to very low‐certainty evidence; one level for serious risk of bias (performance and detection bias) and two levels for serious imprecision where the CIs include both appreciable benefit and appreciable harm.
1.3. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 3: KOOS (0 to 100: best outcome) at short term follow‐up
Nikku 1997 found no significant difference between the two groups in the Lysholm knee score (0 to 100: higher values indicate improved outcome) at short‐term follow‐up (MD ‐1.00, 95% CI ‐4.63 to 2.63; 125 participants; Analysis 1.4). We downgraded the certainty of evidence three levels to very low‐certainty evidence; one level due to imprecision where the CIs include both appreciable benefit and appreciable harm, and two levels for very serious risk of bias (due to high risk of performance and detection bias and risk of selection bias).
1.4. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 4: Lysholm score (0 to 100: best score) at short‐term follow‐up
Similarly Nikku 1997 reported the Hughston visual analogue scale (VAS) patellofemoral scores (28 to 100: higher values indicate improved outcome) (Analysis 1.5). At short‐term follow‐up there was no difference between the groups in these scores (MD ‐2.80, 95% CI ‐6.70 to 1.10; 125 participants; Analysis 1.5). At a medium‐term follow‐up in Nikku 1997, this remained the same (medians (interquartile range) surgical: 89 (74 to 95) versus non‐surgical: 94 (84 to 96); reported P value = 0.08). For an analysis of children only at long‐term follow‐up, reported scores favoured non‐surgical management (MD ‐7.00, 95% CI ‐13.95 to ‐0.05; 1 study, 64 participants; Analysis 1.5). We downgraded the certainty of evidence three levels to very low‐certainty evidence; two levels due to very serious risk of bias (selection, performance and detection bias) and one level due to imprecision with fewer than 100 events reported.
1.5. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 5: Hughston VAS patellofemoral score (28 to 100: best outcome)
The Kujala Patellofemoral Disorders score (0 to 100: higher values indicate improved outcome) was evaluated in eight trials (Askenberger 2018; Bitar 2012; Camanho 2009; Christiansen 2008; Ji 2017; Nikku 1997; Petri 2013; Sillanpää 2009). Data for this outcome are presented at short‐term, medium‐term and long‐term follow‐up periods (Analysis 1.6; Figure 5). At short‐term follow‐up, Ji 2017 found higher Kujala Patellofemoral Disorders scores in the surgical group (MD 13.38 favouring surgical treatment, 95% CI 10.96 to 15.80; 1 study, 56 participants). Pooled data from seven trials showed no difference between the surgical and non‐surgical groups at medium‐term follow‐up (MD 5.73 favouring surgical treatment, 95% CI ‐2.91 to 14.37; 7 studies, 401 participants; Analysis 1.6). When assessed as adult participants alone, there was no difference between the treatments at medium term follow‐up (MD 7.99 favouring surgical treatment, 95% CI ‐6.27 to 8.27; 5 studies, 318 participants). However, when the analysis was compared to a children‐only analysis, non‐surgical treatment offered favourable outcomes (MD ‐5.79 favouring non‐surgical treatment, 95% CI ‐9.38 to ‐2.19; 2 studies, 190 participants). Although based on data for people with anterior knee pain, this result does not reach a minimal clinically important difference (MCID) of 10 points (Bennell 2000; Crossley 2004). For an analysis including children only, data from one trial showed no difference between surgical and non‐surgical groups at long‐term follow‐up (MD ‐1.00 favouring non‐surgical treatment, 95% CI ‐8.60 to 6.60; 1 study, 64 participants; Analysis 1.6). For the medium‐term follow‐up of this outcome, we downgraded the certainty of evidence three levels to very low‐certainty evidence; two levels for very serious risk of bias (selection, performance and detection bias) and one level for inconsistency as this pooled analysis exhibited statistical heterogeneity (Chi² = 60.48, df = 6 (P < 0.0001), I² = 90%).
1.6. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 6: Kujala patellofemoral disorders score (0 to 100: best outcome)
5.

Forest plot of comparison: 1 Surgical versus non‐surgical management, outcome: 1.6 Kujala patellofemoral disorders score (0 to 100: best outcome)
Nikku 1997 conducted performance tests at short‐term (mean of two years) follow‐up consisting of timed 'figure‐of‐eight' running, one‐leg hop distance and maximum number of squat downs in one minute. There were insufficient data provided to calculate effect estimates. Study authors reported significantly better squat results (P = 0.03) and superior timed 'figure‐of‐eight' run performance (P = 0.004) in the non‐surgical group compared with the surgery group. They reported no significant difference in one‐leg hop quotient between the interventions (P = 0.8). Patient‐reported outcomes of activity level were evaluated in Sillanpää 2009. There were insufficient data provided to calculate effect estimates. They reported that there was no statistically significant difference between group differences in the subjective assessment of functional knee limitations for stairs, running and squatting (P > 0.05). We downgraded the certainty of evidence three levels to very low‐certainty evidence: one level for imprecision where the CIs included both appreciable benefit and appreciable harm, and two levels for very serious risk of bias (due to high risk of performance and detection bias and risk of selection bias).
Sensitivity analysis
One study had low risk of selection bias (Petri 2013). This reported no difference between the surgical and non‐surgical groups for Kujala Patellofemoral Disorders score at two year follow‐up (MD 6.20 favouring surgery, 95% CI ‐9.09 to 21.49; 20 participants).
Secondary outcomes
Health‐related quality of life (HRQoL)
Data for an analysis of children only in Askenberger 2018 showed little difference between surgical and non‐surgical groups at short‐term follow‐up (MD 1.70 favouring surgical treatment, 95% CI ‐6.60 to 10.00; 1 study, 67 participants; Analysis 1.7).
1.7. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 7: Health‐related quality of life
Return to former activities: work and sports
Sillanpää 2009 reported little between‐group difference in the frequency of participants who regained the same activity level as before their dislocation at the medium‐term follow‐up (13/17 versus 15/21; RR 1.07 favouring surgery, 95% CI 0.73 to 1.56; 1 study, 38 participants; Analysis 1.8).
1.8. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 8: Return to former activities: work and sports
Knee pain during activity or at rest
Two trials assessed knee pain using a VAS, one at short‐term (Nikku 1997), and the other at medium‐term follow‐up (Sillanpää 2009) (Analysis 1.9). Neither found a significant difference between treatment groups. The results for Nikku 1997 were: MD 0.20 favouring non‐surgical treatment, 95% CI ‐0.29 to 0.69; 125 participants. The results for Sillanpää 2009 were: MD 0.50 favouring non‐surgical treatment, 95% CI ‐0.28 to 1.28; 38 participants. We downgraded the certainty of evidence three levels to very low‐certainty evidence; two levels due to very serious risk of bias (selection, performance and detection bias) and one level for serious imprecision with the CIs including both appreciable benefit and appreciable harm.
1.9. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 9: Knee pain (VAS 0 to 10: worst outcome)
Adverse events of interventions
Three trials reported on adverse events of treatment (Nikku 1997; Rahman 2020; Regalado 2016). Overall complications were reported in all three trials at two time points: two studies at short‐term and one study at medium‐term follow‐up (Analysis 1.10). Pooled data show there was greater incidence of complications in the surgical group at short‐term follow‐up period (63/79 versus 18/65, RR 2.21 favouring non‐surgical treatment, 95% CI 0.73 to 6.66; 2 studies, 144 participants). An analysis of only those who were recruited after recurrent patellar dislocation and instability showed that there was little difference between the groups at short‐term follow‐up (7/9 versus 6/10, RR 1.30 favouring non‐surgical; 95% CI 0.70 to 2.40; 1 study, 19 participants). We downgraded the certainty of evidence three levels to very low‐certainty evidence: one level for imprecision with fewer than 100 events reported, two levels due to very serious risk of bias (due to high risk of performance, detection and attrition bias), and inconsistency though statistical heterogeneity where the pooled analysis for short‐term follow‐up exhibited some heterogeneity (Chi² = 7.60, df = 1 (P = 0.006), I² = 87%). For a children‐only analysis in Regalado 2016, there was a higher incidence of overall complications in the surgical group at medium‐term follow‐up (3/16 versus 0/20; RR 8.65 favouring non‐surgical, 95% CI 0.48 to 156.11; 1 study, 36 participants). We downgraded the certainty of evidence three levels to very low‐certainty evidence: two levels for imprecision with fewer than 100 events reported, and one level due to serious risk of bias (due to high risk of performance and detection bias).
1.10. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 10: Number of complications: overall complications
The results of specific complications are summarised in Table 4. Two trials reported the incidence of nerve injury (Nikku 1997; Regalado 2016) (Analysis 1.11). Pooled analysis showed a higher incidence of nerve injury following surgery (44/86 versus 3/75, RR 10.37 favouring non‐surgical treatment 95% CI 3.63 to 29.63; 2 studies, 161 participants; Analysis 1.11). For an analysis involving children only in Regalado 2016, this difference was less marked with fewer events (1/16 versus 0/20, RR 3.71 favouring non‐surgical, 95% CI 0.16 to 85.29; 1 study, 36 participants). We downgraded the certainty of evidence three levels to very low‐certainty evidence; two levels due to very serious risk of bias (selection, performance and detection bias) and one level for serious imprecision.
3. Summary of individual complications.
| Complication | Study | Surgical group | Non‐surgical group |
| Nerve injury | Nikku 1997 | 43/70 | 3/55 |
| Regalado 2016 | 1/16 | 0/20 | |
| Deep wound infection | Nikku 1997 | 2/70 | 0/55 |
| Regalado 2016 | 1/16 | 0/20 | |
| Cosmetically unsatisfactory scar | Nikku 1997 | 31/77 | 8/55 |
| Less than 90 degrees knee flexion at 6 weeks | Nikku 1997 | 18/70 | 5/55 |
1.11. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 11: Number of complications: nerve injury
Two trials reported the incidence of deep wound infection (Nikku 1997; Regalado 2016). Pooled analysis showed a higher incidence of deep wound infection following surgery (3/86 versus 0/75, RR 3.84 favouring non‐surgical treatment 95% CI 0.43 to 34.02; 2 studies, 161 participants; Analysis 1.12). For an analysis of children only (Regalado 2016), this difference was less marked, with fewer events (1/16 versus 0/20, RR 3.71 favouring non‐surgical, 95% CI 0.16 to 85.29; 1 study, 36 participants).
1.12. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 12: Number of complications: deep wound infections
Only Nikku 1997 reported the adverse effect of a cosmetically unsatisfactory scar at short‐term follow‐up. Study authors reported greater incidence of this complication in their surgical group (31/77 versus 8/55, RR 2.77 favouring non‐surgical treatment, 95% CI 1.38 to 5.55; 1 study, 132 participants; Analysis 1.13). Study authors also reported a greater incidence of knee flexion at less than 90 degrees at short term in the surgical group (18/70 versus 5/55, RR 2.83 favouring non‐surgical, 95% CI 1.12 to 7.14; 1 study, 125 participants; Analysis 1.14).
1.13. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 13: Number of complications: cosmetically unsatisfactory scar
1.14. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 14: Number of complications: less than 90 degrees knee flexion at six weeks
Patient‐reported satisfaction
Four trials assessed patient satisfaction (Ji 2017; Nikku 1997; Petri 2013; Regalado 2016). Data for this outcome were presented at short‐term, medium‐term and long‐term follow‐up (Analysis 1.15). Ji 2017 reported a higher incidence of satisfaction, reported as 'good or excellent' ratings of treatment outcome in the surgical group at short‐term follow‐up (24/30 versus 10/26, RR 2.08 favouring surgical treatment, 95% CI 1.24 to 3.49; 1 study, 56 participants; Analysis 1.15). Pooled data at medium‐term follow‐up showed little difference between the groups in 'good or excellent' ratings of treatment outcome by participants (68/96 versus 61/80; RR 1.01 favouring surgery, 95% CI 0.74 to 1.38; 3 studies, 176 participants; Analysis 1.15). This was the same when assessed in children only in Regalado 2016 (13/15 versus 11/15, RR 1.18 favouring surgical treatment, 95% CI 0.82 to 1.70; 1 study, 30 participants). This similar finding of little difference between the groups was also reported for children only in Nikku 1997 at long‐term follow‐up (21/32 versus 21/28, RR 0.88 favouring non‐surgical treatment, 95% CI 0.63 to 1.22; 1 study, 60 participants). We downgraded the certainty of evidence three levels to very low‐certainty evidence; two levels due to very serious risk of bias (selection, performance and detection bias) and one level for serious imprecision.
1.15. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 15: Patient satisfaction (reported good or excellent)
Patient‐reported knee instability symptoms
Patellar subluxation
Six trials recorded the numbers of participants reporting an episode or episodes of patellar subluxation during follow‐up (Bitar 2012; Camanho 2009; Ji 2017; Nikku 1997; Petri 2013; Sillanpää 2009). Data for this outcome were presented at short‐term and medium‐term follow‐ups. At short‐term follow‐up, Ji 2017 reported a lower incidence of patellar subluxation in the surgical group (2/30 versus 4/26, RR 0.43 favouring surgery, 95% CI 0.09 to 2.18; 1 study, 56 participants; Analysis 1.16). There was minimal difference between groups in the incidence of participants reporting patellar subluxation at medium‐term follow‐up (31/136 versus 35/120, RR 0.75 favouring surgery, 95% CI 0.50 to 1.12; 5 studies, 256 participants; Analysis 1.16). This pooled analysis exhibited some heterogeneity (Chi² = 5.60, df = 4 (P = 0.23), I² = 29%). We downgraded the certainty of evidence three levels to very low‐certainty evidence: one level for imprecision with fewer than 100 events reported and two levels for very serious risk of bias (due to high risk of performance, detection and attrition bias).
1.16. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 16: Number of participants sustaining recurrent patellar subluxation
Other knee instability symptoms
Four trials reported the number of participants in each group suffering episodes of instability (dislocation, subluxation or both) (Bitar 2012; Camanho 2009; Nikku 1997; Sillanpää 2009). The incidence of participants with instability was lower in the surgical group at medium‐term follow‐up (47/125 versus 64/112, RR 0.63 favouring surgery, 95% CI 0.49 to 0.82; 4 studies, 237 participants; Analysis 1.17). We downgraded three levels to very low‐certainty evidence: one level for imprecision with fewer than 100 events reported, two levels due to very serious risk of bias (high risk of selection, performance, detection and attrition bias) and inconsistency from statistical heterogeneity as both pooled analyses exhibited significant heterogeneity (Chi² = 16.07, df = 3 (P = 0.001), I² = 81%).
1.17. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 17: Number of participants sustaining any episode of instability
Subsequent requirement for surgery
Three trials reported the number of participants in each group who had undergone subsequent surgical intervention (Nikku 1997; Regalado 2016; Sillanpää 2009). Data for this outcome are presented at medium‐term and long‐term follow‐up periods. Pooled data at medium‐term follow‐up showed a marginally higher requirement for subsequent surgery in the non‐surgery group (20/102 versus 20/93, RR 0.45 favouring surgical group, 95% CI 0.07 to 2.73; 3 studies, 195 participants; Analysis 1.18). This was more pronounced when assessed in children only in Regalado 2016 (0/15 versus 4/15, RR 0.11 favouring surgical treatment, 95% CI 0.01 to 1.90; 1 study, 30 participants). However, at long‐term follow‐up for a children‐only analysis in Nikku 1997, those in the non‐surgery group reported a lower incidence of subsequent surgery (16/36 versus 11/28, RR 1.13 favouring non‐surgical, 95% CI 0.63 to 2.04; 1 study, 64 participants; Analysis 1.18). Several participants in Nikku 1997 had more than one operation. We downgraded three levels to very low‐certainty evidence: one level for imprecision with fewer than 100 events reported and two levels due to very serious risk of bias (due to high risk of selection, performance, detection and attrition bias).
1.18. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 18: Number of participants who underwent subsequent surgery
Discussion
Summary of main results
The findings of this review are based on 10 trials involving 519 participants with patellar dislocation. The mean ages in the individual studies ranged from 13.0 to 27.2 years, with four trials including children (patients under the age of 16 years), mainly adolescents, as well as adults, whilst two solely recruited children. Based on our assessment of the evidence using the GRADE approach, we rated the certainty of evidence for each reported outcome as very low (see Table 1). This means that we are very uncertain about whether surgical interventions improve outcomes compared with non‐surgical interventions.
The most frequently reported outcome was recurrent patellar dislocation (10 trials). Pooled medium‐term follow‐up data from eight trials (438 participants) indicated that, based on an assumed risk of recurrent dislocation in 348 people per 1000 in the non‐surgical group, 157 fewer people per 1000 (95% CI 209 fewer to 87 fewer) had recurrent dislocation as a result of surgery.
Very low‐certainty evidence was available on treatment effect for five validated patient‐rated knee and physical function scores for patellar dislocation: the Tegner activity scale, KOOS, Lysholm, Hughston VAS and Kujala Patellofemoral Disorders score. Of these, the most frequently reported outcome was the Kujala Patellofemoral Disorders score, with the most data reported at a medium‐term follow‐up. At this follow‐up time point there was no clear difference in outcome between the groups, although the putative MCID of 10 points (Bennell 2000; Crossley 2004) was included in the CI in favour of surgery (MD 5.73 points higher, 95% CI 2.91 points lower to 14.37 points higher; 7 studies, 401 participants). The CI included the MCID of 10 (estimated for people with anterior knee pain) and the point estimate was close to 10. Whilst this may point to the possibility of a clinically important effect, the conflicting results between the time point and the broad CIs suggest we cannot draw meaningful conclusions from these data.
Three trials reported adverse effects of treatment; all four major complications were attributed to the surgical treatment group. Pooled short‐term data from two trials (144 participants) indicated that, based on an assumed risk of adverse event in 277 out of 1000 in the non‐surgical group, 335 more people per 1000 (95% CI 75 fewer to 723 more) may have an adverse event during this time as a result of surgery.
There was little difference in patient satisfaction reported between the groups at medium‐term follow‐up. Pooled medium‐term follow‐up data from three trials (176 participants) indicated that, based on 763 out of 1000 participants reporting excellent or good outcomes in the non‐surgical group, seven more people per 1000 (95% CI 199 fewer to 237 more) may have excellent or good satisfaction with their outcome as a result of surgery.
We are uncertain whether surgery improves knee instability based on the data from four trials with medium‐term follow‐up data (256 participants). Pooled data indicated that, based on an assumed risk of patellar subluxation in 292 out of 1000 in the non‐surgical group, 73 fewer people per 1000 (95% CI 146 fewer to 35 more) may have patellar subluxation during this time as a result of surgery.
Slightly fewer people in the surgery group had subsequent surgery in the medium term after their randomised treatment. Pooled medium‐term follow‐up data from three trials (195 participants) indicated that, based on an assumed risk of subsequent surgery in 215 people per 1000 in the non‐surgical group, 118 fewer people per 1000 (95% CI 200 fewer to 372 more) had subsequent surgery after primary surgery.
Overall completeness and applicability of evidence
The objective of the review, to assess the benefits and harms of surgical compared with non‐surgical interventions for treating people following patellar dislocation, has been met in part. Our findings are largely relevant to the management of people who seek treatment following a first‐time or primary lateral patellar dislocation. Only one trial was included which assessed the outcomes of surgical or non‐surgical interventions following recurrent or secondary patellar dislocation (Rahman 2020). This was a feasibility study that recruited 19 adult participants and provided limited between‐group clinical outcome data. Only three trials measured and reported the frequency of adverse events (Nikku 1997; Rahman 2020; Regalado 2016). Furthermore, only three trials presented results for a children‐only subgroup (Askenberger 2018; Nikku 1997; Regalado 2016), with Nikku 1997 presenting this as a subgroup of their overall cohort. The findings of this review should therefore be interpreted with some caution for people under 16 years of age, and should not be used to justify the treatment of those people who are managed following recurrent lateral patellar dislocation. Furthermore, only Nikku 1997 reported long‐term outcomes, albeit for a subgroup of their patellar dislocation cohort. It therefore remains uncertain what the long‐term outcomes are for this population. Nikku 1997 noted that both treatment groups reported high recurrent dislocation rates but that functionally, children had good outcomes, and were able to perform all their activities of daily living (ADL), irrespective of recurrent patellar instability and dislocation events. Finally, no trials assessed whether the presence or absence of generalised joint or specific patellar hypermobility was an important variable on outcome.
The data were insufficient to perform prespecified subgroup analyses exploring whether the treatment effect differed importantly according to key participant characteristics. As the evidence‐base develops, it is hoped that such planned analyses may be undertaken.
The included trials used a number of different surgical and rehabilitative interventions. It was not possible to determine the relative efficacy of individual interventions such as MPFL repair versus reconstruction. This indirect comparison would be a valuable subgroup analysis as further data become available in a future update. In addition, there was a degree of clinical heterogeneity amongst participants. For instance, some individuals suffering patellar dislocation may have had predisposing factors (e.g. family history, particular anatomical morphology of the patellofemoral joint, soft tissue integrity or hypermobility). Whilst this offers benefit in being able to generalise the findings to the typical patellar dislocation community, it is not possible to infer whether results differ between people presenting with differing clinical presentations. Finally, some participants suffered complications resulting from their patellar dislocation, such as separation of osteochondral fragments into the knee joint. Although some trials reported these factors (Nikku 1997; Sillanpää 2009), the included trials were uneven in the description of anatomical pathology present in their participants, the diagnostic procedures used to investigate them or the rationale for choice of surgical technique.
As acknowledged in the Description of the condition, the aetiology of patellar dislocation is multifactorial. Consequently, there can be a degree of heterogeneity with respect to clinical presentation contributing to, or causing, the dislocation. As a result, the need for surgery may be slightly different between individuals. This may be regarded as a limitation. However, there was no evidence from the original papers of a significant level of clinical heterogeneity to negate appropriate meta‐analyses.
The included trials generally described their non‐surgical management poorly. This remains a recurrent limitation in the literature across the previous updates of this systematic review. Whilst most studies appropriately reported the method and duration of immobilisation, all included studies poorly described their rehabilitation regimens, such as type of exercises prescribed or their frequency, duration or intensity. This has been previously acknowledged and remains a widespread limitation within the patellar instability literature (Smith 2010). Consequently, it was not possible to assess clinical heterogeneity in the non‐surgical management of participants effectively. It should be noted that in four trials, all 'non‐surgical' group participants had diagnostic arthroscopy prior to randomisation (Askenberger 2018; Christiansen 2008; Petri 2013; Regalado 2016), and all participants received knee aspiration to relieve pain in Sillanpää 2009.
Quality of the evidence
All 10 trials had serious methodological weaknesses, in particular resulting from lack of blinding, that placed them all at high risk of performance and detection bias. Only Nikku 1997 included more than 100 participants; the other trials were small and insufficiently powered. The dominance of Nikku 1997 is evident in all the analyses, which is of particular note because it was quasi‐randomised and thus at high risk of selection bias. There may also have been a risk of publication and other reporting bias due to the small number of small trials included (Song 2010). Where reported, there were few losses to follow‐up; but differences in the follow‐up times between the treatment groups in two trials meant these were likely to be at high risk of attrition bias (Bitar 2012; Camanho 2009). Five trials had set follow‐up times (Askenberger 2018; Christiansen 2008; Petri 2013; Rahman 2020; Regalado 2016), whereas the period of follow‐up spanned three years in Nikku 1997 (medium‐term follow‐up) and Sillanpää 2009, and 24 to 54 months in Ji 2017. Another limitation is that the bulk of the evidence pertained to two to five years' follow‐up, without clear clinical or methodological justification for such.
There was clinical heterogeneity amongst the individual included studies, including in the surgical methods used. For the primary outcome of recurrent episodes of dislocation, it is notable that the included trials fell into two groups. In four trials, no recurrent dislocation occurred in the surgical group (Bitar 2012; Camanho 2009; Regalado 2016; Sillanpää 2009), whereas recurrent dislocation occurred in the surgical groups of the other trials. We cannot detect an obvious clinical reason for this difference, which may anyway reflect in part the small sample sizes of these trials.
We assessed the certainty of the evidence as very low for all outcomes. Two generalities applied. For all outcomes that included evidence from Nikku 1997, we downgraded the evidence two levels for serious limitations in trial design. For all outcomes with evidence from Nikku 1997 only, we further downgraded the evidence one level for serious imprecision. For all outcomes with evidence from a single trial that was not Nikku 1997, we downgraded the evidence one level for limitations in study design and two levels for serious imprecision. As the evidence was dominated by Nikku 1997 for all outcomes with pooled data, we downgraded the evidence for these two levels for serious limitations in study design. As there were often two or more reasons for downgrading of these outcomes, we have selected the main one in our account below. We downgraded the evidence for the outcome of recurrent dislocation one further level for imprecision. We downgraded the evidence for four outcomes (Kujala Patellofemoral Disorders score results, recurrent subluxation, any episode of instability and subsequent surgery) one further level for inconsistency. We downgraded the evidence for patient satisfaction one further level for indirectness. This grading means that we are very uncertain about the estimates of effect (Table 1).
We did not downgrade the certainty of evidence, using GRADE, for indirectness, since the evidence did not indicate that this occurred for the review outcomes. We also did not downgrade the certainty of evidence for publication bias. This was justified as no outcomes met the threshold of 10 studies with data for a specific time point to assess for the effect of publication bias using a funnel plot. As the evidence base develops, it is anticipated that both GRADE criteria will be explored in future updates of this review.
Potential biases in the review process
We consider that our search strategy was comprehensive and believe that we have identified all relevant published trials that met the inclusion criteria, although we cannot be completely sure. For example, we could not rule out a failure to identify trials published in non‐indexed journals or unpublished trials, although a lack of peer review would raise questions about the veracity of any such trials that were missed for this reason.
While we have consistently presented recurrent dislocation as our primary outcome, some have questioned whether it is correct to separate dislocations from subluxations and from episodes of instability rather than presenting these together as the primary measure of treatment success or failure. We consider that our approach continues to be correct and that our decision to separate patellar dislocation from subluxation and general perceived instability symptoms is justified through the distinction between the severity and impact of these injuries on individuals (Donell 2006a).
We have updated the 'Differences between protocol and review' section in this Cochrane Review. The changes reflect the limitations of conducting this review based on the limited available literature on this topic. As the evidence base develops in this field, it is anticipated that a number of the acknowledged differences from the protocol to review will be addressed.
Agreements and disagreements with other studies or reviews
Systematic reviews performed by the review authors of the outcomes of non‐operative rehabilitation interventions have been reported (Smith 2010; Moiz 2018). No relevant randomised trials were identified by these reviews. The previous version of this review reported outcomes of six studies (Smith 2015). We reported a statistically significant difference between surgical and non‐surgical interventions for the outcomes of frequency of recurrent dislocation, and Kujala Patellofemoral Disorders score. The updated systematic review allowed a comparison of outcome by age of participant and included the first study of participants with recurrent patellar dislocation and instability.
Reviews by Frosch 2011 and Sillanpää 2012 also reported limited differences in clinical outcomes between surgical and non‐surgical interventions, but suggested that decision‐making on treatment options should include an assessment of anatomical risk factors for recurrent dislocation. They suggested that people with a normal or minor dysplastic patellofemoral joint may be more suitable for non‐surgical treatment, whilst those with a higher grade of trochlear dysplasia or other significant morphological abnormalities may benefit from surgical treatment. Since the current evidence base has not provided sufficient information on morphological features, it is not possible to perform a subgroup analysis to test these hypotheses. Similar findings were reported by Baier 2011, Sillanpää 2012 and Tsai 2012.
Overall the findings from previous systematic reviews agree with those reported in this Cochrane Review. Our results were mirrored in Yang 2019, a systematic review which included both randomised and non‐randomised studies (16 studies, 918 participants). They reported a clearer signal in favour of surgical treatment on the Kujala Patellofemoral Disorders score (SMD 0.79, 95% CI 0.30 to 1.28; 10 studies, 565 participants) and low incidence of redislocation (odds ratio 0.44, 95% CI 0.30 to 0.63; 13 studies, 787 participants). However, these findings should be viewed with caution given the inclusion of non‐RCT data which had greater risk of selection and allocation bias. Whilst Tian 2020 only included studies which assessed non‐surgical intervention to repair of the MPFL (five studies, 300 participants), they reported higher Kujala Patellofemoral Disorders scores for participants randomised to the surgical group, compared with the non‐surgical group. They also reported no difference in redislocation rate or Tenger activity score, KOOS and subjective option, as reported in our review. The difference in outcomes by KOOS score found in our review were also reported in Zhang 2020, a systematic review which included six studies with 469 participants aged under 18 years. They reported poorer KOOS scores for participants who received surgical intervention but lower redislocation rate postsurgery within five years of treatment. The difference in outcomes between children and adults has become more clearly defined in this updated Cochrane Review compared with previous versions. This may be attributed to a difference in functional demand and physical activity requirements, expectations or the growing musculoskeletal system through adolescence. Further examination of the influence on age is therefore warranted in future updates, based on the signal of this outcome in this review and Zhang 2020.
Xing 2020 focused their review on acute primary patellar dislocations, including all studies identified in this review. They reported similar findings to our previous review (Smith 2015), highlighting caution in the interpretation given the certainty of the evidence. They also highlight the trend towards investigating MPFL reconstruction and that, if further evidence were to be presented on this, the effect of the comparison may change. Whilst we acknowledge this as a potential factor, there is also a clear indication that MPFL repair is a frequently investigated surgical option (Askenberger 2018; Camanho 2009; Sillanpää 2009), which may have important implications for a surgical versus non‐surgical comparison. This should also be considered in the future. Hussein 2018 highlighted that it was not possible to compare the outcomes of surgical versus non‐surgical management by MPFL repair or reconstruction in their review. Nonetheless, they reported the same broad findings from the analysis of their systematic review, which included a non‐pooled analysis of the same studies identified in this review.
Authors' conclusions
Implications for practice.
We are uncertain whether surgery improves outcomes compared to non‐surgical management, as the certainty of the evidence is very low. No high‐certainty, sufficiently powered randomised or quasi‐randomised controlled trials have assessed the outcomes of surgical compared with non‐surgical treatments in people who seek treatment following a secondary or recurrent patellar dislocation. Due to the very low certainty and incompleteness of the evidence, this finding must be viewed with caution until a stronger evidence base is established.
Implications for research.
The evidence from the currently published trials is very low certainty evidence, which means that we are very uncertain about the estimates and that further research is very likely to have an important impact on the estimates of effect.
Based on the incidence of recurrent dislocation in the studies included in this review, a case could be made for a multicentre RCT managed from a clinical research centre, enrolling in excess of 250 participants, conducted and reported to the standards of the CONSORT statement (CONSORT 2010). We suggest that before such a trial is conducted, expert consensus be achieved on the standards for future research in this area. This might include clearer definition of both surgical and non‐surgical interventions, and the outcomes that should be reported. These might include recurrent dislocation, and a measure of subluxation or instability episodes, validated functional and quality of life scores. Follow‐up should be assessed at set time points; we suggest that two, five and 10 years follow‐up would be suitable. As individuals with patellar instability may have multiple episodes, recording both the number of participants sustaining an event and the number of events in each group would be desirable, to allow calculation of both risk rate and rate ratio. Key anatomical or pathological factors particularly relevant to the natural history of patellar instability, and thus to the choice of intervention, should also be recorded. Such a consensus would inform the design and conduct of a large study of management of primary and recurrent patellar instability, and would also be useful in research evaluating the place of surgery in the management of recurrent dislocation.
Feedback
Presentational errors, 17 November 2011
Summary
We have used this new review for teaching purposes in our postgraduate programme and realized that Figure 3 is wrong and does not match with Analysis 1.1: 1. It does not contain all the graphical elements for sections 1.1.1 and 1.1.2. 2. The point estimates and diamonds shown are on the wrong side (i.e. favouring non‐surgical interventions). 3. In Analysis 1.3, the label of the x‐axis (exp/control) differs from the other forest plots.
We hope these errors can be corrected.
Reply
We thank Dr von Elm for contacting us and are glad with his use of our review. His observations are all correct. Regarding the mismatch between Analysis 1.1 and Figure 1, errors of reproduction appear to have occurred at some point in the processing of the review, including in the generation of the pdf files for publication. We have revised the scale of Analysis 1.1 and checked that Figure 1 accurately reflects this in RevMan before resubmission for publication. The Managing Editor of the Bone, Joint and Muscle Trauma Group has notified the RevMan support team and Wiley of this problem.
The inconsistent labelling of Analysis 1.3 has now been changed to read "surgical": "non‐surgical" for consistency.
Contributors
Comment from: Dr Erik von Elm Reply from: Professor William Gillespie and Dr Helen Handoll (Cochrane, Bone, Joint and Muscle Trauma Group), 22 November 2011
What's new
| Date | Event | Description |
|---|---|---|
| 23 January 2023 | New search has been performed | In this update, published in 2023, the following changes were made
|
| 23 January 2023 | New citation required but conclusions have not changed |
|
History
Protocol first published: Issue 4, 2009 Review first published: Issue 11, 2011
| Date | Event | Description |
|---|---|---|
| 24 December 2014 | New citation required but conclusions have not changed | Two new studies (Bitar 2012; Petri 2013) included. One study (Palmu 2008) included in the previous version was found to be a subgroup (children only) of another included study (Nikku 1997). Summary of findings table incorporated. |
| 18 October 2014 | New search has been performed | Search was updated to 18 October 2014 |
| 22 November 2011 | Feedback has been incorporated | Feedback incorporated and minor changes made. |
Acknowledgements
We thank Joanne Elliott and Sharon Lewis for their feedback on this review update. We thank Joanne Elliott for editorial support and Maria Clarke for her help with the database searches.
We thank Helen Handoll, Paul Jenkins and Jane Mackintosh for their constructive comments on the protocol and original review; and Lindsey Elstub for editorial support. We also acknowledge Joanne Elliott for her help in the development of the search strategies for the original review. We would particularly like to thank Professor William Gillespie, for his guidance on the first version of the review.
We would like to thank Alexandre Bitar, University of São Paulo, Brazil; Peter Balcarek, University Medical Center Göttingen, Germany; Marco Demange, University of São Paulo, Brazil; Risto Nikku, Helsinki University Central Hospital, Finland; Martin Lind, Aarhus University Hospital, Denmark; Maximilian Petri, Medizinische Hochschule, Hannover, Germany; Petri Sillanpaa, Central Military Hospital, Helsinki, Finland; and Fei Wang, Third Hospital of Hebei Medical University, Shijiazhuang, China for providing additional data used as part of the meta‐analysis and for screening the provisional list of included studies.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Editorial and peer‐reviewer contributions
Cochrane Bone, Joint and Muscle Trauma Group supported the authors in the development of this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Xavier Griffin, Co‐ordinating Editor, Cochrane Bone, Joint and Muscle Trauma Group
Editor (advised on methodology and review content, edited the article): Sharon Lewis, Deputy Co‐ordinating Editor, Cochrane Bone, Joint and Muscle Trauma Group
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Joanne Elliott, Managing Editor, Cochrane Bone, Joint and Muscle Trauma Group
Information Specialist (advised on search methods, ran searches, edited the article): Maria Clarke, Information Specialist, Cochrane Bone, Joint and Muscle Trauma Group
Copy Editor (copy‐editing and production): Andrea Takeda, Cochrane Central Production Service
Peer‐reviewer (provided comments and recommended an editorial decision): David Beard
Appendices
Appendix 1. Search strategies (October 2014 to December 2021)
The searches were run in two stages: the first search was run in January 2021 and a second top‐up search was run in December 2021.
CENTRAL (CRS‐Web)
Search 1
#1 MESH DESCRIPTOR Patellar Dislocation AND CENTRAL:TARGET (41) #2 MESH DESCRIPTOR Patella AND CENTRAL:TARGET (282) #3 MESH DESCRIPTOR Joint Dislocations AND CENTRAL:TARGET (304) #4 MESH DESCRIPTOR Joint Instability AND CENTRAL:TARGET (742) #5 #3 OR #4 (1023) #6 #5 AND #2 (32) #7 (patell* AND (dislocat* or sublux* or instability)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (370) #8 #1 OR #6 OR #7 (370) #9 01/10/2014_TO_07/01/2021:CRSCREATED AND CENTRAL:TARGET (1018149) #10 #8 AND #9 (223)
Search 2 (top‐up search)
#9 07/01/2021_TO_15/12/2021:CRSCREATED AND CENTRAL:TARGET (93772) #10 #8 AND #9 (26)
MEDLINE (Ovid interface)
Search 1
1 Patellar Dislocation/ (1187)
2 Patella/ and (Joint Dislocations/ or Joint Instability/) (1678)
3 (patell* and (dislocat* or sublux* or instability)).tw. (4319)
4 1 or 2 or 3 (5109)
5 randomized controlled trial.pt. (520386)
6 controlled clinical trial.pt. (94005)
7 randomi?ed.ab. (605787)
8 placebo.ab. (214452)
9 drug therapy.fs. (2266944)
10 randomly.ab. (349150)
11 trial.ab. (536539)
12 groups.ab. (2142728)
13 or/5‐12 (4905941)
14 exp animals/ not humans.sh. (4774680)
15 13 not 14 (4265275)
16 4 and 15 (674)
17 (201410* or 201411* or 201412* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021*).ed,dt. (8559363)
Search 2 (top‐up search)
17 2021*.ed,dt. (2266447) 18 16 and 17 (93)
Embase (Ovid interface)
Search 1
1 patella dislocation/ (2706) 2 Patella/ and (dislocation/ or joint instability/) (537) 3 (patell* and (dislocat* or sublux* or instability)).tw. (4945) 4 1 or 2 or 3 (5921) 5 Randomized controlled trial/ (635517) 6 Controlled clinical study/ (466429) 7 Random*.ti,ab. (1606274) 8 randomization/ (89502) 9 intermethod comparison/ (267583) 10 placebo.ti,ab. (312243) 11 (compare or compared or comparison).ti. (508464) 12 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (2223546) 13 (open adj label).ti,ab. (84227) 14 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (233222) 15 double blind procedure/ (177178) 16 parallel group*1.ti,ab. (26751) 17 (crossover or cross over).ti,ab. (106404) 18 ((assign* or match or matched or allocation) adj5 (alternate or group*1 or intervention*1 or patient*1 or subject*1 or participant*1)).ti,ab. (342923) 19 (assigned or allocated).ti,ab. (403819) 20 (controlled adj7 (study or design or trial)).ti,ab. (364263) 21 (volunteer or volunteers).ti,ab. (247434) 22 trial.ti. (312493) 23 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (6142557) 24 or/5‐22 (4829676) 25 24 not 23 (4180381) 26 4 and 25 (1124) 27 (2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021*).dc,yr. (11891732) 28 26 and 27 (647)
Search 2 (top‐up search)
27 2021*.dc,yr. (2284738) 28 26 and 27 (136)
CINAHL (NHS NICE Healthcare Databases)
Search 1
S1 MH patella dislocation (255) S2 MH dislocations (3,581) S3 MH patella (2,575) S4 S2 AND S3 (211) S5 S1 OR S4 (466) S6 TI ( patell* AND dislocat* ) OR AB ( patell* AND dislocat* ) (876) S7 TI ( patell* AND sublux* ) OR AB ( patell* AND sublux* ) (237) S8 TI ( patell* AND instability ) OR AB ( patell* AND instability ) (1,017) S9 S5 OR S6 OR S7 OR S8 (1,762) S10 (MH “Clinical Trials+”) (311,867) S11 PT Clinical Trial (108,600) S12 TI clinical trial* OR AB clinical trial* (118,751) S13 TI ( (single blind* or double blind*) ) OR AB ( (single blind* or double blind*) ) (49,753) S14 TI random* OR AB random* (363,221) S15 S10 OR S11 OR S12 OR S13 OR S14 (560,148) S16 S9 and S15 (146) S17 EM 20140101‐20210107 (2,850,614) S18 S16 AND S17 (81)
Search 2 (top‐up search)
S17 EM 20210107‐ (366,637) S18 S16 AND S17 (24)
AMED (Allied and Complementary Medicine)
Search 1
1 Patella/ (476) 2 Dislocations/ (552) 3 1 and 2 (22) 4 (dislocat* or sublux* or instability).ti,ab. (3755) 5 patell*.ti,ab. (1652) 6 4 and 5 (109) 7 3 or 6 (112) 8 (2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021*).up,yr. (69553) 9 7 and 8 (19)
Search 2 (top‐up search)
8 2021*.up,yr. (9661) 9 7 and 8 (4)
Physiotherapy Evidence Database (PEDro)
Patella AND dislocation (6)
WHO International Clinical Trials Registry Platform
Patella AND dislocation (81)
Cinicaltrials.gov
Patella AND dislocation | First posted from 01/01/2014 to 01/08/2021 (32) Patella AND dislocation | First posted from 01/08/2021 to 12/21/2021 (8)
Appendix 2. Data Extraction Template
Patellar Dislocation: Surgical versus Non‐Surgical Management ‐ Data Extraction Tool
Reviewer: Study ID:
Setting/recruitment frame
| Design | Country | Hospital | Recruitment dates | Follow‐up |
Participants
| N | N knees | Age (range, mean,SD) | Sex | Patellar dislocation defined? | Primary or recurrent dislocation | Duration symptoms (range, mean,SD) | |
| Group 1 | |||||||
| Group 2 |
| Inclusion criteria | Exclusion criteria |
| Intervention | Intervention details | Postsurgical rehabillitation | Notes |
| Outcomes | Tick if available | How ascertained? | Follow up duration |
| Recurrent patellar dislocation | |||
| Validated patient‐rated knee and physical function scores | |||
| Health related quality of life | |||
| Return to former activities: work and sports | |||
| Knee pain during activity or at rest | |||
| Adverse events (complications) | |||
| Patient‐reported satisfaction | |||
| Patient‐reported knee instability symptoms | |||
| Subsequent requirement for knee surgery |
Notes:
| Number analysed & data by time point | Group 1 | Group 2 |
| Recurrent patellar dislocation | ||
| Validated patient‐rated knee and physical function scores | ||
| Health related quality of life | ||
| Return to former activities: work and sports | ||
| Knee pain during activity or at rest | ||
| Adverse events (complications) | ||
| Patient‐reported satisfaction | ||
| Patient‐reported knee instability symptoms | ||
| Subsequent requirement for knee surgery |
Criteria for judging risk of bias in the risk of bias assessment tool – DELETE NON‐RATED BOXES
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | |
| Criteria for a judgement of ‘Low risk’ of bias. | The investigators describe a random component in the sequence generation process such as:
*Minimization may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for the judgement of ‘High risk’ of bias. | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorization of participants, for example:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment | |
| Criteria for a judgement of ‘Low risk’ of bias. | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| Criteria for the judgement of ‘High risk’ of bias. | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
|
BLINDING OF PARTICIPANTS AND PERSONNEL Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | Any one of the following:
|
|
BLINDING OF OUTCOME ASSESSMENT Detection bias due to knowledge of the allocated interventions by outcome assessors | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | Any one of the following:
|
|
INCOMPLETE OUTCOME DATA Attrition bias due to amount, nature or handling of incomplete outcome data | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | Any one of the following:
|
|
SELECTIVE REPORTING Reporting bias due to selective outcome reporting | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any of the following:
|
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. It is likely that the majority of studies will fall into this category. |
|
OTHER BIAS Bias due to problems not covered elsewhere in the table | |
| Criteria for a judgement of ‘Low risk’ of bias. | The study appears to be free of other sources of bias. |
| Criteria for the judgement of ‘High risk’ of bias. | There is at least one important risk of bias. For example, the study:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | There may be a risk of bias, but there is either:
|
Data and analyses
Comparison 1. Surgical versus non‐surgical management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Number of participants sustaining recurrent patellar dislocation | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Short‐term follow‐up | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.10, 2.38] |
| 1.1.2 Medium‐term follow‐up | 8 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.40, 0.75] |
| 1.1.3 Long‐term follow‐up | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.67, 1.30] |
| 1.2 Tegner activity score (0 to 10: best score) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Short‐term follow‐up | 2 | 190 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.08, ‐0.04] |
| 1.2.2 Medium‐term follow‐up | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.15, 1.15] |
| 1.2.3 Long‐term follow‐up | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐2.44, ‐0.76] |
| 1.3 KOOS (0 to 100: best outcome) at short term follow‐up | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 Symptoms | 2 | 145 | Mean Difference (IV, Random, 95% CI) | ‐3.35 [‐11.09, 4.39] |
| 1.3.2 Pain | 2 | 145 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐10.18, 8.15] |
| 1.3.3 Activities of daily living | 2 | 145 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐7.96, 7.33] |
| 1.3.4 Sports and recreation | 2 | 145 | Mean Difference (IV, Random, 95% CI) | ‐4.64 [‐21.85, 12.57] |
| 1.3.5 Quality of life | 2 | 145 | Mean Difference (IV, Random, 95% CI) | ‐4.14 [‐18.69, 10.41] |
| 1.4 Lysholm score (0 to 100: best score) at short‐term follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5 Hughston VAS patellofemoral score (28 to 100: best outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5.1 Short‐term follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5.2 Long‐term follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6 Kujala patellofemoral disorders score (0 to 100: best outcome) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.6.1 Short‐term follow‐up | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 13.38 [10.96, 15.80] |
| 1.6.2 Medium‐term follow‐up | 7 | 401 | Mean Difference (IV, Random, 95% CI) | 5.73 [‐2.91, 14.37] |
| 1.6.3 Long‐term follow‐up | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐8.60, 6.60] |
| 1.7 Health‐related quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.8 Return to former activities: work and sports | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.9 Knee pain (VAS 0 to 10: worst outcome) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.9.1 Two years (20 to 45 months) follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.9.2 Six to nine years follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10 Number of complications: overall complications | 3 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [0.91, 7.04] |
| 1.10.1 Short‐term follow‐up | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [0.73, 6.66] |
| 1.10.2 Medium‐term follow‐up | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 8.65 [0.48, 156.11] |
| 1.11 Number of complications: nerve injury | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.11.1 Short‐term follow‐up | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.37 [3.63, 29.63] |
| 1.12 Number of complications: deep wound infections | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.12.1 Short‐term follow‐up | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.84 [0.43, 34.02] |
| 1.13 Number of complications: cosmetically unsatisfactory scar | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.14 Number of complications: less than 90 degrees knee flexion at six weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.15 Patient satisfaction (reported good or excellent) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.15.1 Short‐term follow‐up | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [1.24, 3.49] |
| 1.15.2 Medium‐term follow‐up | 3 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.74, 1.38] |
| 1.15.3 Long‐term follow‐up | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.22] |
| 1.16 Number of participants sustaining recurrent patellar subluxation | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.16.1 Short‐term follow‐up | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.09, 2.18] |
| 1.16.2 Medium‐term follow‐up | 5 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.12] |
| 1.17 Number of participants sustaining any episode of instability | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.17.1 Medium‐term follow‐up | 4 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.49, 0.82] |
| 1.18 Number of participants who underwent subsequent surgery | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.18.1 Medium‐term follow‐up | 3 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.07, 2.73] |
| 1.18.2 Long‐term follow‐up | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.63, 2.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Askenberger 2018.
| Study characteristics | ||
| Methods | Single‐centre RCT Randomisation method: pre‐prepared envelopes (made by statistician so unknown to surgeon, patient or involved person). Block randomisation (blocks of 6), stratified by sex. Randomisation conducted directly after diagnostic arthroscopy whilst participant still under general anaesthetic. Follow‐up: mean follow‐up was two years (no data presented on range). |
|
| Participants | Trial performed in Sweden. Recruitment from December 2009 to April 2012. N = 74 participants (74 knees) Inclusion criteria: people aged nine to 14 years (skeletally immature); sustained an acute primary lateral patellar dislocation with hemarthrosis defined as effusion in knee joint within 12 hours after acute injury. Diagnosis of lateral patellar dislocation was based on history, clinical examination, radiographs and MRI. Imaging confirmation was made on 3 of 4 major MRI signs: (1) knee haemarthrosis; (2) an injury to the MPFL defined as an oedema at the injury site with or without visible disrupted fibres; (3) a bone bruise pattern in the medial patellar and/or; (4) lateral femoral condyle. When there was uncertainty over diagnosis based on history, clinical examination and radiographs, diagnosis was based solely on MRI where all four signs were required. Exclusion criteria: other lower limb disability; previous injury to affected knee; people with a first‐time lateral patellar dislocation who had osteochondral fragment > 1cm2 from the articulating lateral femoral condyle and/or patellar surface were managed with open surgery. |
|
| Interventions | Diagnostic arthroscopy was performed in all participants, irrespective of group allocation. This included joint irrigation, blood clot removal and removal of smaller osteochondral fragments. Surgery: (N = 37 participants/37 knees; mean age 13.19 ± 1.08; 18 females/19 males) Intervention: arthroscopic repair of MPFL (Twinfix Ti 3.5 Quick‐T Fixation System ‐ Smith & Nephew). A small skin incision was made at the medial side of the patellar for anchor insertion. Patellar was temporarily fixed in the trochlear groove with an A‐O 1.6‐mm pin, placed through the patellar into the trochlea to secure the position during the MPFL repair. The femoral attachment injury was repaired with the same type of anchors through a small extra‐articular skin incision at the medial femoral condyle. Fluoroscopy determined the femoral position of the MPFL at the Schöttle point and distal to the physis to avoid growth disturbance. Postoperatively, participants wore a soft‐cast splint for four weeks, were permitted to fully weight bear and were provided with physical therapy. This was focused on strength and functional training, including gluteal muscle training and core training for stability. Participants were provided with a home training programme. Physical therapy was provided by paediatric physical therapists who had specialist knowledge regarding paediatric patellofemoral rehabilitation. Non‐surgery: (N = 37 participants/37 knees; mean age 13.03 ± 1.14 years; 20 females/17 males) Intervention: participants were provided with a lateral stabilising soft knee brace which was advised to be worn for four weeks, day and night. Participants were permitted to fully weight‐bear immediately. Participants were referred to paediatric physical therapists who had specialist knowledge regarding paediatric patellofemoral rehabilitation. In this, they received a physical therapy programme that was focused on strength and functional training, including gluteal muscle training and core training for stability. Participants were provided with a home training programme. |
|
| Outcomes | Follow‐up: mean 24 months (no range values provided) Outcomes collected included: recurrent patellar dislocation; time to redislocation; physical examination (presence of positive apprehension test or patellar tilt, thigh circumference, knee range of motion), Beighton score; pain assessed using a VAS; knee function measured using the KOOS for children (KOOS‐Child), Kujala Patellofemoral Disorders Score; health‐related quality of life measured using the EQ‐5D‐Y; physical activity measured using the Tegnar activity score; physical performance measured using isokinetic dynamometer, to measure concentric thigh muscle torque; 1‐legged hop for distance, side hope and single‐limb 30‐second mini‐squat test, which was used to calculate the LSI. |
|
| Notes | Power calculation used, requiring 32 participants in each group. Intention‐to‐treat analysis principles were adopted. No strategy was established to analyse or impute missing data. No details provided on the frequency, duration, dosage or tailoring of the rehabilitation programme for either group. Number of surgeons was stated to be three. All participants underwent diagnostic arthroscopy. Whilst this is a surgical intervention, the non‐surgical group did not receive any surgical intervention in repairing or reconstructing the patellofemoral joint anatomy. Accordingly, this has been regarded as a non‐surgical intervention for the purposes of this review. Funding source: this study was supported by grants from H.R.H. King Oscar II’s and H.R.H. Queen Sophia’s Golden Wedding Foundation, the Research Committee of the Sophiahemmet Foundation, the Skandia Research Foundation, and the Swedish National Centre for Research in Sports Declarations of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Page 4: Quote: "Randomization was conducted directly after diagnostic arthroscopic surgery". No report of how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Page 4: Quote: "An envelope with the randomization details was previously prepared (from the statistician) unknown to the surgeon and all involved persons". Although blinding is mentioned, there is no mention of adequate safeguards. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the difference in knee brace used between the surgical and non‐surgical group, it was not possible to maintain participant or personnel blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Page 5, Figure 4: Four participants declined final physical test. Twelve participants waived physical test due to recent injury. The author excluded six participants from non‐surgical group, in final analysis, due to redislocation and cross‐over prior to two year follow‐up. However, this was not the trial primary outcome measure. |
| Selective reporting (reporting bias) | High risk | No protocol available but the planned outcomes defined in the Methods section (Page 4) were reported and reflect the trial registry (ISRCTN 39959729). Adverse events were not reported. |
| Other bias | Low risk | No other sources of bias. |
Bitar 2012.
| Study characteristics | ||
| Methods | Single‐centre RCT Randomisation method: drawing of paper slips Follow‐up: minimum was two years; mean follow‐up was 44 months (range 24 to 61 months) |
|
| Participants | Trial performed in Brazil. Recruitment from 2003 to 2006 N = 42 but presented in the text and table as 39 participants (41 knees) with three others "lost in the follow‐up period" Inclusion criteria: acute (up to three weeks postinjury) primary patellar dislocation with a history of laterally displaced patella and on physical examination: tenderness of the medial retinaculum, a positive apprehension test, effusion or haemarthrosis of the knee joint attributed to a patellar dislocation. Confirmation of diagnosis and assessment of injury to the MPFL made using MRI Exclusion criteria: participants excluded with previous history of knee surgery or serious knee lesion including patellar dislocation or symptoms of patellar instability; coexistence of tibiofemoral ligament injury requiring repair; large osteochondral fragments (diameter > 15 mm) requiring fixation; conditions associated with serious neuromuscular or congenital disease; participants younger than 12 years of age; a non‐traumatic patellar dislocation (e.g. dislocation during gait or squatting with moderate stress on the knee); inability/unwillingness to provide consent or comply with treatment protocol |
|
| Interventions |
Surgery (N = 21 participants/21 knees; mean age 24.0; 12 females/9 males) Intervention: open MPFL reconstruction performed by rotating a medial strip of the patellar ligament from the tibial tuberosity to the adductor tubercle of the femoral condyle, attached to this point with an absorbable interference screw. Suture attachment of the rotated graft with the distal end of the vastus medialis muscle also performed. No lateral release or other procedure undertaken. Postsurgical rehabilitation: all surgical participants were immobilised for three weeks in a knee immobiliser (knee position not stated). During this period, isometric quadriceps strengthening exercises, analgesics, cryotherapy and electronic stimulation was permitted. Immediate weight‐bearing permitted postoperatively, and passive knee range of motion exercises performed by a physiotherapist. At the third postoperative week, the knee immobiliser was dispelled and knee range of motion, proprioception and closed kinetic chain exercises commenced; these were progressed to open kinetic chain exercises over time. The overall objective was to progress surgical participants to return to previous sporting activities in approximately 10 to 12 weeks postoperation. Non‐surgery (N = 18 participants/20 knees; mean age 24.1; 9 females (11 knees)/9 males) Intervention: non‐weight‐bearing immobilised in an extension brace for three weeks, followed by a physiotherapy programme consisting of quadriceps strengthening and knee range of motion exercises. During the initial three weeks of immobilisation, participants were provided with analgesia, cryotherapy and electrical stimulation. Weight‐bearing was permitted after the three weeks of immobilisation. Initially proprioceptive and closed kinetic chain exercises were prescribed. These were progressed to open kinetic chain exercises, with the overall objective to progress the participants to their previous sporting activities within 16 to 24 weeks following commencement of non‐operative rehabilitation |
|
| Outcomes | Follow‐up: mean 44 months (range 24 to 61 months) Outcomes collected included: Kujala Patellofemoral Disorders Score; recurrent patellar dislocation; episodes of patellar subluxation; and participant satisfaction |
|
| Notes | Power calculation used, requiring 22 in each group. Intention‐to‐treat analysis principles were adopted. No strategy was established to analyse or impute missing data. Personal communication with Dr A Bitar (25 October 2013) who reviewed the search results. Funding source: not stated. Declarations of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "For randomisation...we conducted a draw for the 2 groups" (page 115). No report of how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No reference was made to concealment of allocation during randomisation (page 115). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not reported, but extremely unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not reported, and unlikely. Participants completing the Kujala Patellofemoral Disorders Score and reporting recurrent dislocation/subluxation were clearly unblinded. |
| Incomplete outcome data (attrition bias) | Low risk | Three lost to follow‐up postrandomisation and data not included. The follow‐up was 38 months for the surgical management group and 48 months for the non‐surgical management group, which is a likely source of bias. |
| Selective reporting (reporting bias) | High risk | No protocol available but the planned outcomes defined in the Methods section (page 115 to 116) were reported in the Results section (page 117 to 118). Adverse effects of surgery were not reported. |
| Other bias | Low risk | No other sources of bias. |
Camanho 2009.
| Study characteristics | ||
| Methods | Single‐centre RCT Randomisation method: blind drawing of slips of paper allocating group Follow‐up: "minimum follow‐up time of 25 months" (listed as part of inclusion criteria) to maximum 60 months; mean 40.4 months in surgical group and 36.3 months in non‐surgery group Location and person who randomised or assessed not stated |
|
| Participants | Trial performed in Brazil. The period in which the study was undertaken was not stated N = 33 participants Inclusion criteria: primary patellar dislocation with a convincing history of traumatic dislocation, requirement for reduction Exclusion criteria: osteochondral fracture, patellar fracture, previous knee surgery |
|
| Interventions |
Surgery (N = 17; mean age 24.6; 11 females/6 males) Intervention: arthroscopic MPFL repair. Postoperative rehabilitation: three weeks in a removable immobiliser and physiotherapy Non‐surgery (N = 16; mean age 26.8; 9 females/7 males) Intervention: immobilised in a cylinder cast for three weeks, followed by a physiotherapy programme consisting of strengthening exercises particularly of the vastus medialis obliquus. Hamstring and retinacular stretching begun after one month after dislocation |
|
| Outcomes | Follow‐up: aim between two and five years, mean 40.4 months in the surgery group and 36.3 months in the non‐surgical group. Outcomes collected included: recurrent patellar dislocation, positive Apprehension Test, recurrent instability symptoms, Smillie test results, and the Kujala Patellofemoral Disorders Score |
|
| Notes | Not concealed allocation; location and person who randomised not stated. No details provided on rehabilitation programme used. Sample size was not based on a power calculation. Number of surgeons not stated Personal communication with Dr A Bitar who reviewed the updated search results (25 October 2013) and provided standard deviation values for Kujala Patellofemoral Disorders score results (19 January 2010) Funding source: not stated. Declarations of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly divided into two groups by means of a drawing, by blindly selecting a slip of paper that assigned them to either the surgical treatment group or the conservative treatment group" (page 621) No report of how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote "Patients were randomly divided into two groups by means of a drawing, by blindly selecting a slip of paper that assigned them to either the surgical treatment group or the conservative treatment group" (page 621). Although blinding is mentioned, there is no mention of adequate safeguards. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not reported, but extremely unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not reported, and unlikely. Participants completing questionnaires for the Kujala Patellofemoral Disorders and Tegner scores were clearly unblinded. |
| Incomplete outcome data (attrition bias) | Low risk | The title indicates that it is a study on the management of acute patellar dislocation, and the text (page 621) states “All were operated on less than 1 month after the trauma causing the lesion had occurred.” However, in the inclusion criteria we find “a minimum follow‐up time of 25 months after the dislocation episode” (page 621), and in the exclusion criteria we find “follow‐up after the first dislocation shorter than 24 months” (page 621). This appears to mean that randomised participants from both groups were excluded from the analysis, but there is no report of losses. Follow‐up may have stretched from 25 to 60 months. Additionally, the follow‐up was 40.4 months for the surgical management group and 36.3 months for the non‐surgical management group; which may be a source of bias. |
| Selective reporting (reporting bias) | High risk | No protocol available but the planned outcomes defined in the methods section were reported. Adverse effects of surgery were not reported. |
| Other bias | Low risk | No other sources of bias. |
Christiansen 2008.
| Study characteristics | ||
| Methods | Single‐centre RCT Randomisation method: drawing of envelopes Follow‐up: two years |
|
| Participants | Trial performed in Denmark from April 1998 to September 2002 N = 80 participants (77 reported as three excluded as did not complete final follow‐up) Inclusion criteria: individuals with primary patellar dislocation, aged 13 to 30 years Exclusion criteria: history of patellofemoral instability or pain; unable to follow treatment regimen |
|
| Interventions | All participants underwent an arthroscopy. Surgery (N = 42; mean age 20.0; 18 females/24 males) Intervention: open repair of the MPFL performed on average 50 days after dislocation. Postoperative rehabilitation: no information provided Non‐surgery (N = 35; mean age 19.9; 17 females/18 males) Intervention: brace from zero to two weeks immobilised zero to 20 knee range of motion degrees |
|
| Outcomes | Follow‐up: 2 years (also 2 and 6 weeks, and 1 year) Outcomes collected included: incidence of redislocation at two years, Kujala Patellofemoral Disorders Score, and the KOOS |
|
| Notes | Power calculation used. Requiring 39 in each group. Intention‐to‐treat analysis principles were not adopted. Personal communication with Dr Martin Lind who reviewed the updated search results (22 October 2013). Funding source: not stated. Declarations of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization between surgery and conservative treatment was performed by random drawing of 100 envelopes" (page 883). No report of how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope system. Quote: "Randomization between surgery and conservative treatment was performed by random drawing of 100 envelopes" (page 883), but no report of whether these were securely sealed and allocated sequentially. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not mentioned in the study report. Treatment staff and participants unlikely to be blinded, as randomisation was conducted at arthroscopy. Aftercare clearly not identical in both groups (Quote: "Patients randomised to conservative treatment received no further treatment or brace usage" (page 882). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not reported, and unlikely. Participants completing questionnaires for the Kujala Patellofemoral Disorder score and KOOS scores were clearly unblinded. |
| Incomplete outcome data (attrition bias) | Low risk | Three lost to follow‐up postrandomisation (Figure 2). |
| Selective reporting (reporting bias) | High risk | No protocol available but the planned outcomes defined in the methods section were reported, although these did not include adverse effects of surgery. |
| Other bias | Low risk | No other sources of bias. |
Ji 2017.
| Study characteristics | ||
| Methods | Single‐centre RCT Randomisation method: quasi‐randomised. Used birth years as odd/even number allocation where 30 participants were born in odd‐number years (surgical group); 32 born in even‐number years (non‐surgical group). Follow‐up: minimum was 24 months; mean follow‐up was 42 months (range 24 to 54 months) |
|
| Participants | Trial performed in China. Recruitment from October 2008 to January 2011. N = 62 randomised, but 56 participants included in the study where five participants were excluded and one lost to follow‐up. Inclusion criteria: a time interval from the initial injury to the time of hospital admission of less than three weeks; a primary patellar dislocation with a positive apprehension test; a MPFL injury confirmed on MRI. Exclusion criteria: a previous knee injury or surgery; a coexistent tibiofemoral ligament injury of the involved knee; radiographic evidence of bone abnormalities such as patellofemoral dysplasia (Dejour B–D) and patella alta (Insall–Salvati > 1.2); a TT‐TG distance greater than 20 mm; patients whose contralateral knee could not move normally for reasons such as a fracture of the lower limb or sequelae from polymyositis (unhealthy other knee affects evaluation of walking/jumping). |
|
| Interventions | All participants underwent non‐surgical atraumatic patellar reduction to reduce risk of further osteochondral injury to the patellar or later femoral trochlea. If needed, for both groups, arthroscopic surgery was performed to remove loose bodies. Surgery (N = 30 participants/30 knees; mean age not recorded; 19 females/11 males) Intervention: open MPFL repair. 3 cm longitudinal skin incision between adductor tubercle and condylus medialis. Superficial fascia split and deeper dissection to MPFL performed. With the knee in extension, MPFL insertion site at the femur was identified distal to the medial condyle. The stump was debrided and anchored between the adductor tubercle and condylus medialis with a metal anchor and a baseball stitch. Wound irrigated and closed in layers. Postoperatively the knee was immobilised in a brace in full extension. Postoperatively patients were provided with immediate quadriceps strengthening exercises and commence mobilisation two days postoperatively (weight‐bearing with crutches). Knee flexion exercises were limited from full extension to 90 degrees knee flexion from Day 2 postoperatively to Week 4. Participants were permitted to fully weight‐bear from four weeks postoperatively onwards. Participants were permitted to return to normal work after two months, and resume sport within four months. Non‐surgery (N = 26 participants/26 knees; mean age not recorded; 17 females/9 males) Intervention: participants were provided a knee brace to be worn for at least three weeks. Early mobilisation with permitted with knee flexion range of motion limited from full extension to 60 degrees knee flexion; participants provided with crutches and instructed to partial weight‐bear. Exercise prescription made including straight leg raises, quadriceps isometric exercises, progressed as tolerated. In total, rehabilitation duration ranged from two to four months, until the participant no longer complained of pain and muscle strength was reported to have been restored. |
|
| Outcomes | Follow‐up: mean 42 months (range 24 to 54 months) Outcomes collected included: subjective knee function measured using the Kujala Patellofemoral Disorders Score; occurrence of redislocation and subluxation events; complications; radiographic outcomes including patellar tilt and the lateral shift ratio on plain x‐ray at 12‐months postoperatively; clinical stability using the Apprehension Test. |
|
| Notes | No power calculation; examiners not blinded risk of detection bias; no mention of blinding of anyone/surgeon/ patient; limited information on rehabilitation programme tailoring or modification or adherence. Funding source: the project was supported by a key project grant from the National Natural Science Foundation (approve number: 81371910). Declarations of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Page 379: Quote: participants were "randomly divided into two groups". No report of how sequence was generated. |
| Allocation concealment (selection bias) | High risk | Allocation by year of birth. No information provided on who allocated to groups and whether they were involved in screening and recruiting participants. Page 379: Quote: participants were "randomly divided into two groups according to their year of birth". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Page 380: No mention of blinding for patient/surgeon in the paper. Due to the nature of the intervention, this was likely to have not been blinded otherwise may have expected this to have been reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Page 380: Assessors were not blinded to group allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Page 279: One patient lost to follow‐up. This attrition is unlikely to have changed the outcome of the study. However, there is uncertainty about why five additional patients were randomised and not included in the study, as the reasons for the exclusions could have been determined prior to randomisation. |
| Selective reporting (reporting bias) | High risk | Outcomes not clearly defined before study. Study not registered with a trial registration and no protocol paper available. |
| Other bias | Low risk | No other sources of bias. |
Nikku 1997.
| Study characteristics | ||
| Methods | Multicentre RCT Quasi‐randomisation using year of birth Follow‐up: mean 25 months (range 20 to 45 months); mean 7 years (range 5.7 to 9.1 years), mean 14 years (range 11 to 15 years) for children only subgroup |
|
| Participants | Trial performed in Finland. Recruitment from January 1991 to December 1992 N = 125 participants (127 knees) Inclusion criteria: primary lateral patellar dislocation where injury was less than 14 days Exclusion criteria: previous major knee injury, previous knee surgery, ligament injuries needing repair, osteochondral fractures needing fixation |
|
| Interventions |
Surgery (N = 70; mean age 19.5, SD 9; 52 females/18 males) Intervention: medial reefing (18), repair of medial retinaculum (39) or augmentation of MPFL (6) or lateral release (54) Postoperative rehabilitation: thigh muscle exercises and full weight‐bearing. If patellar dislocatable on examination under anaesthesia, immobilised on splint/cast for three weeks. Mobilisation started with orthosis for three weeks and used during sporting activities for the first six months postdislocation Non‐surgery (N = 55; mean age 19.1, SD 7.5; 30 females/25 males) Intervention: identical rehabilitation programme to surgical group |
|
| Outcomes | Follow‐up (3 time periods): mean 25 months (range 20 to 45 months); mean 7 years (range 5.7 to 9.1 years); and, for a children‐only subgroup, mean 14 years (11 to 15 years) Outcomes collected included: patient satisfaction with outcome, Lysholm knee score, Hughston VAS knee score, Tegner activity score, recurrent dislocation rates, recurrent subluxation rates; subsequent surgical intervention, performance tests consisting of timed figure of eight running, one leg hop distance, maximum number of squat downs in one minute, and subsequent pain on VAS, thigh circumference knee range of motion, patellofemoral crepitus, apprehension test, prepatellar sensibility and scar sensibility |
|
| Notes | Two orthopaedic consultants and two registrars did 88% of operations. Assessment clinically performed by two surgeons. Intention‐to‐treat analysis principles were not adopted. Sample size was not based on a power calculation. Confirmation gained from Professor Simon Donell that Palmu 2008 (which was previously included as a separate study) reported the 14‐year follow‐up of a children‐only (including adolescents) subgroup of this trial (25 October 2014) (Donell 2014) Funding source: the study was supported by the Finnish Orthopedic Association, the Medical Society of Finland and the Finnish Office for Health Technology Assessment Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "randomization was based on the year of birth (even/odd)" (page 420) |
| Allocation concealment (selection bias) | High risk | Quote: "randomization was based on the year of birth (even/odd)" (page 420) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not mentioned in the study report. Treatment staff and participants unlikely to be blinded. To note though that: Quote: "After‐care was identical in both groups" (page 420) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor/data collection blinding is not mentioned in the study report. Quote: "Recurrences were asked about twice: by a mailed questionnaire and by the examiner at the final evaluation" (page 420). Quote: "The clinical examination was performed by two of the authors (YN, RN)" (page 420) Participants completing questionnaires for the Lysholm, Hughston VAS, Kujala Patellofemoral Disorders score and Tegner activity score were clearly unblinded. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "123/125 patients attended the performance test and clinical examination. 2 patients returned only the questionnaires" (page 420‐1) However, there was mention of exclusions: "4 had erroneous randomization and 1 was lost to follow‐up". |
| Selective reporting (reporting bias) | Low risk | No protocol available but the planned outcomes defined in the methods section were reported. Adverse effects of surgery were reported. The reporting of the children‐only subgroup at 14 years (Palmu 2008) did not appear to have been predetermined. |
| Other bias | Low risk | No other sources of bias. |
Petri 2013.
| Study characteristics | ||
| Methods | Multicentre RCT Randomisation method: sealed envelope system performed in the individual study centres Follow‐up: 24 months (questionnaire) |
|
| Participants | Trial performed in Germany N = 20 participants Inclusion criteria: isolated, unilateral first‐time traumatic patellar dislocation; aged between 15 and 40 years; provided informed consent to participate Exclusion criteria: recurrent dislocation; significant anatomical deformities (not specified); open injury; people who were pregnant or lactating; an osteochondral fracture which required fixation. |
|
| Interventions |
Surgery (N = 12; mean age 27.2; 4 females/8 males) Intervention: diagnostic arthroscopy performed, followed by open soft tissue repairs including mainly suture and optional tightening of ruptured medial structures. "MPFL‐plastics" were not performed. Lateral release was optional. Tibial tuberosity and bony correction was optional. Postoperative rehabilitation: a DonJoy range of motion brace was applied with 0 to 60 degrees extension‐flexion permitted from weeks zero to three, increased to zero to 90 degrees extension‐flexion permitted from weeks three to six. Participants were required to partial weight‐bear for initial three weeks up to 15 kg on crutches, followed by progressions to full weight‐bearing from week three onwards. No further information on rehabilitation provided Non‐surgery (N = 8; mean age 21.6; 3 females/5 males) Intervention: participants were provided with a DonJoy range of motion brace with zero to 60 degrees extension‐flexion permitted from weeks zero to three, increased to zero to 90 degrees extension‐flexion permitted from weeks three to six postrandomisation. Participants were required to partial weight‐bear for initial three weeks up to 15 kg on crutches, followed by progressions to full weight‐bearing from week three onwards. No further information on rehabilitation provided |
|
| Outcomes | Follow‐up: two years (also six and 12 months). Outcomes recorded included: Kujala Patellofemoral Disorders score; recurrent dislocation; episodes of patellar subluxation; and participant satisfaction |
|
| Notes | Sample size was not based on a power calculation. No statement on intention‐to‐treat analysis. No attempt was made to analyse missing data using imputation techniques. Personal communication with Dr P Balcarek (27 October 2013) and Dr M Petri who reviewed the updated search results (25 October 2013). Funding source: funding was received from Ormed‐DJO. Declarations of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "With use of a sealed envelope method utilising a software generated block randomisation patients were randomised in the individual centres" (page 210) |
| Allocation concealment (selection bias) | Low risk | Quote: "With use of a sealed envelope method utilising a software generated block randomisation patients were randomised in the individual centres" (page 210) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant blinding is not mentioned in the study report. Due to the nature of the interventions, treatment staff and participants unlikely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | All outcomes were self‐reported (thought questionnaires) by the participants. Blinding of participants not reported, but clearly unblinded. |
| Incomplete outcome data (attrition bias) | Low risk | Two participants lost to follow‐up in the surgical group; two participants in the non‐surgical group. Three participants had moved out of the area, whilst contact data for one participant were incomplete. |
| Selective reporting (reporting bias) | High risk | No protocol available but the planned outcomes defined in the methods section (page 210) were presented in the Results section (pages 211‐2). Adverse effects of surgery were not reported. |
| Other bias | Low risk | No other sources of bias. |
Rahman 2020.
| Study characteristics | ||
| Methods | Multicentre RCT (three UK NHS Trusts) Randomisation method: independent telephone randomisation service from a clinical trials unit. Block randomisation (blocks sizes of four or six) randomised 1:1, stratified by joint hypermobility (Beighton's score of four points or more), or the presence of patellar alta (Biedert ration of < 0.25 on sagittal MRI scan as determined by the treating clinician). Follow‐up: minimum of 12 months. |
|
| Participants | Trial performed in the UK. Recruitment from March 2017 to May 2018. N = 19 (19 knees) Inclusion criteria: people aged 16 and over with closed epiphyseal plates on MRI; experienced two or more lateral patellar dislocations or one dislocation with minimum six‐month history of subjective instability (self‐reported) leading up to the time of recruitment; provided written consent to participate. Exclusion criteria: another knee condition resulting in stability problems (e.g. ACL rupture, unstable meniscal tear which had not been treated); past knee surgery (except simple arthroscopy with or without lateral release or previous meniscal surgery); developmental abnormalities of lower limb requiring complex surgery either in the form of severe trochlea dysplasia requiring trochleoplasty or rotational, coronal or sagittal malalignment of the femur or tibia requiring surgical correction (i.e. osteotomy); previous entry into trial for other knee; osteochondral defects or chondral injury requiring surgery (including removal of a loose body); medial patellar dislocation; people unable to give written consent or expected to be unable to complete protocol. |
|
| Interventions |
Overall cohort characteristics: 11 females/8 males; mean age 26 years (standard deviation: 12) Surgery: (N = 9 participants/9 knees) Intervention: operation was determined by the surgeon's decision on the participant's clinical presentation. During the preparation for the study, a surgical consensus meeting was held to determine the proposed surgical interventions for the trial. No information was provided as to what the surgical interventions. Postsurgery, participants were referred for a standard package of physiotherapy. This consisted of physiotherapy, delivered by a qualified physiotherapist for a minimum of three months, with a maximum of six sessions. Session included advice regarding avoiding reconstruction stretching (medial tissue stretch), knee bracing, interventions for pain relief and reduce swelling and the provision of a home exercise programme. Treatments were exercise‐based including knee range of motion exercises, quadriceps strengthening; correction of biomechanical factors for proximal (hip) and distal (foot and ankle) factors which may be biomechanically contributing to instability and dislocation ‐ for example glutaei exercise programme. Progression of exercises was made respecting symptoms of pain, swelling and instability, as well as functional/physical capabilities. Intervention adjuncts which could be offered to participants included walking aids to reduce weight‐bearing, electrostimulation and exercise classes/group sessions. Non‐surgery: (N = 10 participants/10 knees) Intervention: personalised knee therapy (physiotherapy). This was a package of non‐surgical care designed as part of the study design from a consensus of physiotherapists, surgeons and researchers. Intervention was delivered by a qualified physiotherapist over a planned six sessions over a minimum of three months. If clinically required, this could be performed over more than six sessions if the treatment aims were not met after this time. The intervention included eduction and advice, goal‐setting, graduated activity/exercise approach to progression of treatment. The interventions were aimed to: reduce swelling; optimise knee range of motion; quadriceps strengthening; correction of biomechanical factors for proximal (hip) and distal (foot and ankle) factors which may be biomechanically contributing to instability and dislocation ‐ for example glutaei exercise programme or foot orthoses; and provision of home exercises (no more than three exercises at one given time). Progression of exercises was made respecting symptoms of pain, swelling and instability, as well as functional/physical capabilities. Treatment was exercise‐based but adjuncts which could be offered to participants included walking aids to reduce weight‐bearing, patellar stabilisation or orthoses, electrostimulation, exercise classes/group sessions, manual therapies and cognitive behavioural therapies. All participants in this group were provided with a treatment booklet which included physiotherapy instructions, an intervention diary and home exercise instructions. Full outline of the intervention is provided in Appendix 1 of the academic paper. |
|
| Outcomes | Follow‐up: mean 12 months. Intervals: 3, 6 and 12 months Outcomes collected included: Norwich Patellar Instability Score, Kujala Patellofemoral Disorder score, Banff Patellar Instability Instrument, EuroQol‐5D‐5L, self‐reported global assessment of change and satisfaction, resource use, adverse events. |
|
| Notes | Surgical fixation techniques differ based on surgeon preference ‐ no information provided in the paper regarding what interventions were actually performed and the number of surgeons who undertook these. The study was a feasibility study and was therefore not powered to answer a definitive question on clinical outcome. Both T Smith and A Metcalfe (authors of this Cochrane Review) were part of the Rahman 2020 trial team Funding source: Funding was provided by the West Midlands Clinical Research Network in a pump‐priming grant, the UHCW NHS Foundation Trust and the University of Warwick Clinical Trials Unit Declarations of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Page 4: "Randomisation by telephone system at Warwick Trials centre. Strictly sequentially 1:1 ratio." |
| Allocation concealment (selection bias) | Low risk | Quote: Page 4: "Randomisation by telephone system at Warwick Trials centre. Randomization list prepared by statistician who had no patient contact." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: Page 3: "Recruiters, clinicians and patients were all un‐blinded to the intervention received" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: Page 3: "Recruiters, clinicians and patients were all un‐blinded to the intervention received". No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Figure 1, Page 5: Four participants did not respond at three months. Seven participants did not response to follow up at six months. One participant did not respond to follow‐up at 12 months (following protocol change). Reasons for loss were not presented. Given the number of participants, this may have affected outcomes. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the clinical trial registration were reported in the paper. |
| Other bias | Low risk | No other sources of bias. |
Regalado 2016.
| Study characteristics | ||
| Methods | Single‐centre RCT Randomisation method: not documented. Follow‐up: minimum of 36 months clinical and functional assessment and functional assessment by questionnaire collected in a telephone interview at 72 months. |
|
| Participants | Trial performed in Finland. Study performed between 1998 and 2000. Recruitment period not specified. N = 36 participants consented and randomised. Inclusion criteria: adolescents; acute primary patellar dislocation (determined by ability to dislocate and lock the patellar laterally under anaesthesia); no previous knee surgery/substantial knee injury; no tibiofemoral ligamentous injury requiring surgery; no osteochondral fragment requiring fixation. Exclusion criteria: previous knee trauma; osteochondritic knee lesion. |
|
| Interventions |
Surgery (N = 16 participants/16 knees; mean age 13.5 range (8‐16); 11 females/5 males) Intervention: based on Fulkerson classification. Through this, participants (N=3) with type I: underwent LLR; whilst participants with a type II‐IV, underwent a modified Roux‐Goldwraithe (N=13) procedure (combination of proximal and distal realignment with LLR and medial imbrications). A midline incision with a subcutaneous lateral release was performed, a distal third of the lateral vastus lateralis tendon was released, and the incision was extended obliquely along the superolateral edge of the muscle. The patellar tendon was split longitudinally and the lateral held was detached from the tibial tubercle, brought medially underneath the intact medial half and sutured into the subperiostieal pocket beneath the pes anserinus insertion. The medial retinacula band was detached posteriorly, brought proximally up to the level of the vastus medialis muscle, pulled laterally through the tunnel across the distal quadriceps tendon and pulled back over to the medial side where it was anchored to the medial capsule. Postoperatively, participants wore a brace fixed between zero and 30 degrees knee flection for three weeks, and 90m degrees for the next three weeks. Participants received one to two visits physiotherapy per month for six months. During this, patients received exercises and advice on regaining full knee range motion, quadriceps strengthening with isometric and then isokinetic exercises once full and painful knee range of motion was achieved. Full weight‐bearing was permitted immediately postoperatively. Non‐surgery (N = 20 participants/20 knees; mean age 13.5 (range 8‐16); 11 females/9 males) Intervention: the rehabilitation programme was the same as the postsurgical rehabilitation programme. Participants wore a brace fixed between zero and 30 degrees knee flexion for three weeks, and 90 degrees for the next three weeks. Participants received one to two visits physiotherapy per month for six months. During this, patients received exercises and advice on regaining full knee range motion, quadriceps strengthening with isometric and then isokinetic exercises once full and painful knee range of motion was achieved. Full weight‐bearing was permitted immediately postrandomisation. |
|
| Outcomes | Follow‐up: 36 months clinical and functional assessment and functional assessment by questionnaire collected in a telephone interview at 72 months. Outcomes collected included: redislocation rate; reoperation rate; knee function assessed by self‐reported excellent/good/poor responses; postoperative complications; overall satisfaction with procedure. |
|
| Notes | No documentation of randomisation process or concealment. Unclear documentation of the power calculation to inform the study sample size. Study outcomes not clearly defined prior to the study taking place. No mention was made on who undertook the surgical or non‐surgical interventions. Analysis excludes participants with incomplete data with no approach to imputation or managing missing data. Non‐validated measure was used of functional outcomes. Funding source: the study was financially supported by EVO Grant from the Hospital District of Northern Savo, Finland. Declarations of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No report of how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No mention of adequate safeguards to selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not mentioned in the study report. Treatment staff and participants unlikely to be blinded. Aftercare is the same in both groups, but it is not clear if participants received equal physiotherapy. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcomes assessment not described. Participants completing questionnaires for the questionnaire‐scores were clearly unblinded. |
| Incomplete outcome data (attrition bias) | High risk | Six participants lost to follow up (five non‐surgical; one surgical). Final analysis completed from participants without missing data. Given the number of participants missing and difference between the groups, this may have affected outcome. |
| Selective reporting (reporting bias) | Low risk | No protocol available but the planned outcomes defined in the methods section were reported. |
| Other bias | Low risk | No other sources of bias. |
Sillanpää 2009.
| Study characteristics | ||
| Methods | Single‐centre RCT Randomisation method: sealed envelopes Follow‐up: median seven years (six to nine years) |
|
| Participants | Trial performed in Finland. Recruitment from 1998 to 2000 N = 40 participants (all military recruits) Inclusion criteria: individuals with a primary acute traumatic patellar dislocation Exclusion criteria: previous subluxation, pre‐existing ipsilateral or contralateral knee pathology, previous ligament injury or fracture of the involved knee, or large osteochondral lesion requiring open surgery |
|
| Interventions |
Surgery (N = 18; mean age 20.0; 1 female/17 males) Intervention: medial reefing and repair of MPFL (N = 14); Roux‐Goldthwaite procedure (N = 4) arthroscopic repair of osteochondral fracture (N = 6). Postoperative rehabilitation: no information provided Non‐surgery (N = 22; mean age 20.0; 2 females/20 males) Intervention: knee orthosis, guided isometric quadriceps exercises. First three weeks immobilised zero to 30 degrees knee flexion, three to six weeks immobilised form zero to 90 degrees and free‐range of motion from six weeks onwards. (All participants of this group received knee aspiration to relieve pain and four underwent arthroscopic removal of an osteochrondral fragment) |
|
| Outcomes | Follow‐up: median seven years (range six to nine years). Outcomes recorded included: recurrent dislocation rates, frequency of subluxation rates, Kujala Patellofemoral Disorders score, VAS pain, knee range of motion, Tegner score, quadriceps girth, MRI presence of patellar and femoral chondral lesions, participant‐reported outcomes of activity level, frequency of reoperation rate, severity of patellofemoral joint osteoarthritis, subjective assessment of pain and functional knee limitations for stairs, running, squatting, and pain, radiological findings for sulcus angle, lateral patellofemoral angle, lateral patellar displacement, Blackburne‐Peel ratio | |
| Notes | Operations performed by two orthopaedic surgeons Not clear whether the assessors were blinded. Sample size was based on power calculation. Personal communication with Dr P Sillanpaa who reviewed the updated search results (25 October 2013) and provided standard deviation values for Kujala Patellofemoral Disorders scores and Tegner scores (18 January 2010) Funding source: none received. Declarations of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...military recruits who had been admitted to a military hospital because of an acute primary traumatic patellar dislocation were randomized to treatment" (page 264). No report of how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Written informed consent was obtained from each patient. With use of a sealed‐envelope method, forty patients were randomly allocated to two treatment groups: (1) initial patellar stabilization surgery and (2) non‐operative treatment with a knee orthosis (as well as arthroscopic removal of an osteochondral fragment if necessary)" (page 264) No mention of adequate safeguards. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of personnel or participants was not described. Quote: "The post‐injury or postoperative rehabilitation protocols were identical for the two groups" (page 264). However, Quote: "Four patients in the nonoperatively treated group underwent arthroscopic removal of an osteochondral fragment, but no additional procedures were performed. Since primary traumatic patellar dislocations are frequently associated with osteochondral fractures, we believe that performing arthroscopy initially in some patients may be unavoidable, even in a randomized study. Ten patients (four treated nonoperatively and six treated with surgical stabilization) had removable fragments, and the osteochondral fractures were treated identically (i.e. with arthroscopic removal of the fragments) in the two treatment groups" (page 266). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcomes assessment not described. Participants completing questionnaires for the Kujala Patellofemoral Disorders score and Tegner activity score were clearly unblinded. |
| Incomplete outcome data (attrition bias) | Low risk | One participant lost from each group: one participant missing had moved to another country, and one could not be reached for follow‐up assessment. |
| Selective reporting (reporting bias) | High risk | No protocol available but the planned outcomes defined in the methods section were reported. Adverse effects of surgery were not reported. |
| Other bias | Low risk | No other sources of bias. |
ACL = anterior cruciate ligament; KOOS ‐ Knee Injury and Osteoarthritis Outcome Score; LLR = lateral retinacula release; LSI ‐ Limb Symmetric Index; MPFL = medial patellofemoral ligament; MRI = magnetic resonance imaging; N = number of participants; NHS = National Health Service; RCT = randomised controlled trial; TT‐TG = tibial tuberosity trochlear groove; VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez 2020 | No non‐surgical comparison group. |
| Kang 2017 | No non‐surgical comparison group. |
| Moström 2014 | Not a randomised controlled trial |
| NCT02185001 | Study terminated due to inadequate patient recruitment and coordinator left organisation ‐ 2016 |
| Sillanpää 2011 | No non‐surgical comparison group. |
| Zheng 2019 | Not a randomised controlled trial |
Characteristics of ongoing studies [ordered by study ID]
Liebensteiner 2021.
| Study name | Conservative versus tailored surgical treatment in patients with first time lateral patella dislocation: a randomized‐controlled trial |
| Methods | RCT Participants randomised to either non‐surgical treatment or to a tailored patella stabilizing treatment |
| Participants | Trial performed in Austria and Germany. N = 160 (planned) Inclusion criteria
Exclusion criteria
|
| Interventions | Surgical group: Surgical procedure selected based on addressing the respecting pathologic anatomy which predisposes lateral patellar dislocation. Surgical procedures will be applied in individual combinations, depending on clinical presentation, although all will receive MPFL reconstruction. Additional surgical procedures to MPFL reconstruction may include: trochleoplasty, tibial tuberosity transfer, derotational osteotomy, varus osteotomy. No details provided on postoperative rehabilitation programme. Non‐surgical group: Patients will use a motion‐restricting knee brace to limit patellar lateralisation and knee ROM (set to 0‐20‐40° for week 1 to 2, 0‐10‐60° for week 3 to 4, and 0‐0‐90° for week 5 to 8). Partial weight‐bearing recommended for initial two weeks. Patients will be prescribed outpatient physical therapy following a protocol of: Phase 1 (Week 1 + 2). Knee ROM limited to 0‐20‐40°, partial weight‐bearing maintained. Phase 2 (Week 3 + 4). Knee ROM limited to 0‐10‐60°, progression to full weight‐bearing, emphasis on quadriceps recruitment exercises (especially vastus medialis) Phase 3 (Week 5 to 8). Knee ROM limited to 0‐0‐90°, reacquiring activities of daily living, core stability, sensorimotor training (leg axes stabilization), strength training commenced Phase 4. Return to sports, dependent on the type and previous level of sports activity, gradual increase of training volume and intensity |
| Outcomes | Follow‐up period planned for 24 months postrandomisation in the first instance. Outcomes collected include: BPII 2.0 (German version); Kujala Patellofemoral Disorders score; SF‐12; Marx Activity Scale; reoperative; recurrent patellar dislocation; Apprehension Test; joint degeneration assessed by MRI, evaluated using the MOAKS scoring system; Patellar Instability Severity Score |
| Starting date | Not declared |
| Contact information | Dr Alexander Keiler: Department of Orthopaedics and Traumatology, Medical University of Innsbruck, Innsbruck, Austria |
| Notes | Awaiting listing on www.ClinicalTrials.gov No information on current status of trial Last update: 14 April 2022 |
NCT02263807.
| Study name | Knee function in patients with two or more episodes of patella dislocations (MPFL) |
| Methods | RCT Participants randomised to either surgical intervention (MPFL reconstruction) or non‐surgical (physiotherapy) intervention. |
| Participants | Trial performed in Norway. N = 70 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Surgical group: offered reconstruction of the MPFL followed by physiotherapy. No further information on rehabilitation was provided. Non‐surgical group: Diagnostic arthroscopy followed by physiotherapy. No further information on rehabilitation was provided. |
| Outcomes | Follow‐up period was 36 months postrandomisation. Outcomes collected include: reoperative; recurrent patellar subluxation; Kujala Patellofemoral Disorders score, KOOS, Lysholm score, Tegner activity score, VAS and Activity Scale. |
| Starting date | 1 May 2010 |
| Contact information | Professor Asbjørn Årøen MD, PhD: University Hospital, Akershus, Norway. |
| Notes | The study is currently active but not recruiting. At last update, 75 participants had fulfilled the inclusion criteria and were included in the study. Last update 11 March 2019. |
BPII = Banff Patellofemoral Instability‐Instrument; KOOS = Knee injury and Osteoarthritis Outcome Score; MOAKS = MRI Osteoarthritis Knee Score; MPFL = medial patellofemoral ligament; MRI = magnetic resonance imaging; RCT = randomised controlled trial; ROM = range of motion; SF‐12 = Short‐Form 12; TT‐TG = tibial tuberosity trochlear groove; VAS = visual analogue scale
Differences between protocol and review
For difference between protocol and the previous version of this review, please see Smith 2015.
We have separated health‐related quality of life as a separate outcome measure. This is now included as a secondary outcome measure rather than being included in the 'validated patient‐rated knee and physical function or activity score' outcome. We have also merged the previous 'other knee function and activity scores' secondary outcome as presented in the Smith 2015 review, into the 'validated patient‐rated knee and physical function' primary outcome measure.
We have removed range of knee motion as a separated secondary outcome as this is now reported in adverse events.
We planned to assess the difference in outcomes between time points through pooled subgroup analyses, e.g. short‐term data compared to medium‐term data for a given outcome. Due to the data available at specific time points, this was not conducted.
We planned to undertake formal subgroup analyses by participant age and primary versus recurrent patellar dislocation. However, due to the number of trials available, this was not possible.
We planned to perform worst‐ and best‐case analyses to assess missing data. Due to the data available in this review, this was not performed.
Contributions of authors
Toby Smith and Caroline Hing co‐ordinated and conceived the protocol, and, with assistance of Lesley Gillespie from the Cochrane Bone, Joint and Muscle Trauma Group, designed the search strategy. Professor Fujian Song (University of East Anglia) provided guidance on methodological and statistical analysis during the development of the protocol. Caroline Hing, Toby Smith and Professor Simon Donell (Norfolk and Norwich University Hospitals NHS Foundation Trust) provided a clinical perspective during the protocol development and review preparation. Caroline Hing, Toby Smith, Fujian Song and Simon Donell designed and wrote the protocol. Toby Smith and Andrew Gaukroger screened the search results and identified the studies, extracted the data and prepared the data extraction table for analysis. Toby Smith analysed the data. Toby Smith, Caroline Hing and Andrew Metcalfe provided a clinical perspective during the full review development and preparation. Toby Smith, Andrew Gaukroger, Caroline Hing and Andrew Metcalfe all revised and agreed the full review.
Toby Smith is the guarantor of the protocol and full review.
Sources of support
Internal sources
-
St George's University, London, UK, UK
Employing organisation for Hing
-
University of East Anglia, Norwich, UK
Employing organisation for Smith
-
Norfolk and Norwich University Foundation Hospital NHS Trust, Norwich, UK
Employing organisation for Smith
-
University of Warwick, UK
Employing organisation for Metcalfe
External sources
No sources of support provided
Declarations of interest
Toby O Smith: was an investigator of a trial included in the review (Rahman 2020). This trial was assessed independently by other review authors. He has received funding from the NIHR for randomised trials and clinical effectiveness research, including a current trial related to the review, on the management of recurrent patellar dislocation.
Andrew Gaukroger: none known.
Andrew Metcalfe: was an investigator of a trial included in the review (Rahman 2020). This trial was assessed independently by other review authors. He is research lead for the British Association for Surgery of the Knee and head of the patellofemoral working group, as well as research lead for the British Patellofemoral Society. None of these roles have any financial rewards associated with them. He has received funding from the NIHR for randomised trials and clinical effectiveness research, including a current trial related to the review, on the management of recurrent patellar dislocation. Some of these trials have received funding from Stryker for treatment, imaging and training costs but he has no personal financial relationship with Stryker and no relationship outside of these studies.
Caroline B Hing: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Askenberger 2018 {published data only}
- Askenberger M, Bengtsson Mostrom E, Ekstrom W, Arendt EA, Hellsten A, Mikkelsen C, et al. Operative repair of medial patellofemoral ligament injury versus knee brace in children with an acute first-time traumatic patellar dislocation: a randomized controlled trial. American Journal of Sports Medicine 2018;46(10):2328-40. [DOI: 10.1177/0363546518770616] [PMID: ] [DOI] [PubMed] [Google Scholar]
- ISRCTN39959729. Conservative versus arthroscopic refixation of the Medial Patellofemoral ligament (MPFL) after traumatic first time dislocation of the patella in children [Conservative versus Arthroscopic refixation of the Medial Patellofemoral ligament (MPFL) after traumatic first time dislocation of the patella in children: a prospective randomised study]. isrctn.com/ISRCTN39959729 (first received 25 February 2011). [ISRCTN NUMBER: ISRCTN39959729]
Bitar 2012 {published data only}
- Bitar A. Personal communication 25 October 2013.
- Bitar AC, Demange MK, D'Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with MPFL reconstruction using patellar tendon. American Journal of Sports Medicine 2012;40(1):114-22. [DOI] [PubMed] [Google Scholar]
Camanho 2009 {published data only}
- Bitar A. Personal communication 19 January 2010.
- Camanho GL, Viegas Ade C, Bitar AC, Demange MK, Hernandez AJ. Conservative versus surgical treatment for repair of the medial patellofemoral ligament in acute dislocations of the patella. Arthroscopy 2009;25(6):620-5. [DOI] [PubMed] [Google Scholar]
Christiansen 2008 {published data only}
- Christiansen SE, Jakobsen B, Lund B, Lind M. Isolated repair of the medial patellofemoral ligament in primary dislocation of the patella: a prospective randomized study. Arthroscopy 2008;24(8):881-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lind M. Personal communication 22 January 2010.
Ji 2017 {published data only}
- Ji G, Wang S, Wang X, Liu J, Niu J, Wang F. Surgical versus nonsurgical treatments of acute primary patellar dislocation with special emphasis on the MPFL injury patterns. Journal of Knee Surgery 2017;30(4):378-84. [DOI: 10.1055/s-0036-1592151] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wang F. Personal communication 5 May 2021.
Nikku 1997 {published data only}
- Donell S. Personal communication 25 October 2014.
- Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. Journal of Bone & Joint Surgery - American Volume 2009;91(Suppl 2 Pt 1):139-45. [DOI] [PubMed] [Google Scholar]
- Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome. A 7-year follow-up report and risk analysis of 127 randomised patients. Acta Orthopaedica 2005;76(5):699-704. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nikku R, Nietosvaara Y, Kallio PE, Aalto K, Michelsson JE. Operative versus closed treatment of primary dislocation of the patella. Similar 2-year results in 125 randomized patients. Acta Orthopaedica Scandinavica 1997;68(5):419-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomised clinical trial. Journal of Bone & Joint Surgery - American Volume 2008;90(3):463-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pieler-Bruha E. Acute patellar dislocation in children and adolescents: a randomized clinical trial: Commentary. Journal für Mineralstoffwechsel 2008;15(2):96-7. [Google Scholar]
Petri 2013 {published data only}
- Balcarek P. Personal communication 27 October 2013.
- Petri M, Liodakis E, Hofmeister M, Despang FJ, Maier M, Balcarek P, et al. Operative vs conservative treatment of traumatic patellar dislocation: results of a prospective randomized controlled clinical trial. Archives of Orthopaedics and Trauma Surgery 2013;133:209-13. [DOI] [PubMed] [Google Scholar]
- Petri M. Personal communication 25 October 2013.
Rahman 2020 {published data only}
- ISRCTN14950321. Patellar Instability: physiotherapy or Surgery? (PIPS feasibility trial). who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN14950321 (first received 22 December 2016). [ISRCTN NUMBER: 14950321]
- Rahman U, Gemperle-Mannion E, Qureshi A, Edwin C, Smith TO, Parsons H, et al. The feasibility of a randomised control trial to assess physiotherapy against surgery for recurrent patellar instability. Pilot and Feasibility Studies 2020;6:94. [DOI: 10.1186/s40814-020-00635-9] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Regalado 2016 {published data only}
- Regalado G, Lintula H, Kokki H, Kroger H, Vaatainen U, Eskelinen M. Six-year outcome after non-surgical versus surgical treatment of acute primary patellar dislocation in adolescents: a prospective randomized trial. Knee Surgery Sports Traumatology Arthroscopy 2016;24(1):6-11. [DOI: 10.1007/s00167-014-3271-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sillanpää 2009 {published data only}
- Sillanpää P. Personal communication 18 January 2010.
- Sillanpää PJ, Mattila VM, Mäenpää H, Kiuru M, Visuri T, Pihlajamäki H. Treatment with and without initial stabilizing surgery for primary traumatic patellar dislocation. A prospective randomized study. Journal of Bone & Joint Surgery - American Volume 2009;91(2):263-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alvarez 2020 {published data only}
- Alvarez O, Steensen RN, Rullkoetter PJ, Fitzpatrick CK. Computational approach to correcting joint instability in patients with recurrent patellar dislocation. Journal of Orthopaedic Research 2020;38(4):768-76. [DOI: 10.1002/jor.24526] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kang 2017 {unpublished data only}
- ChiCTR-IOR-17010528. Fixation versus unfixation of osteochondral fractures after patellar dislocations in adolescent patients: a randomized controlled trial. chictr.org.cn/showproj.aspx?proj=17416 (first received 26 January 2017).
Moström 2014 {published data only}
- Moström EB, Mikkelsen C, Weidenhielm L, Janarv PM. Long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients. Scientific World Journal 2014;2014:1-8. [DOI: 10.1155/2014/473281] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02185001 {published data only}
- NCT02185001. Repair of medial patellofemoral ligament compared to conservative treatment for first time patella dislocation. clinicaltrials.gov/show/NCT02185001 (first received 9 July 2014).
Sillanpää 2011 {unpublished data only}
- NCT00816647. A prospective randomized study of medial patellofemoral ligament (MPFL) reconstruction. clinicaltrials.gov/show/NCT00816647 (first received 1 January 2009).
Zheng 2019 {published data only}
- Zheng X, Hu Y, Xie P, Cui M, Ma X, Feng Yu-e, et al. Surgical medial patellofemoral ligament reconstruction versus non-surgical treatment of acute primary patellar dislocation: a prospective controlled trial. International Orthopaedics 2019;43(6):1495-1501. [DOI: 10.1007/s00264-018-4243-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Liebensteiner 2021 {published data only}
- Liebensteiner M, Keiler A, El Attal R, Balcarek P, Dirisamer F, Giesinger J, et al. Conservative versus tailored surgical treatment in patients with first time lateral patella dislocation: a randomized-controlled trial. Journal of Orthopaedic Surgery and Research 2021;16:378. [DOI: 10.1186/s13018-021-02513-3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02263807 {published data only}
- NCT02263807. Knee function in patients with two or more episodes of patella dislocations. clinicaltrials.gov/show/NCT02263807 (first received 13 October 2014).
Additional references
Arendt 2016
- Arendt EA, Berruto M, Filardo G, Ronga M, Zaffagnini S, Farr J, et al. Early osteoarthritis of the patellofemoral joint. Knee Surgery Sports Traumatology Arthroscopy 2016;24:1836-44. [DOI: 10.1007/s00167-016-4103-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Atkin 2000
- Atkin DM, Fithian DC, Marangi KS, Stone ML, Dobson BE, Mendelsohn C. Characteristics of patients with primary acute lateral patellar dislocation and their recovery within the first 6 months of injury. American Journal of Sports Medicine 2000;28(4):472-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baier 2011
- Baier C, Springorum HR, Beckmann J, Grifka J, Matussek J. Treatment of patellar instability in children and adolescents. Orthopade 2011;40(10):868-76. [DOI: 10.1007/s00132-011-1775-9] [DOI] [PubMed] [Google Scholar]
Beasley 2004
- Beasley LS, Vidal AF. Traumatic patellar dislocation in children and adolescents: treatment update and literature review. Current Opinion in Pediatrics 2004;16(1):29-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bennell 2000
- Bennell K, Bartam S, Crossley K, Green S. Outcome measures in patellofemoral pain syndrome: test retest reliability and inter-relationships. Physical Therapy in Sport 2000;1(2):32-41. [Google Scholar]
Boden 1997
- Boden BP, Pearsall AW, Garrett WE Jr, Feagin JA Jr. Patellofemoral instability: evaluation and management. Journal of the American Academy of Orthopaedic Surgeons 1997;5(1):47-57. [PMID: ] [DOI] [PubMed] [Google Scholar]
Carter 1964
- Carter C, Wilkinson J. Persistent joint laxity and congenital dislocation of the hip. Journal of Bone and Joint Journal - American Edition 1964;46:40-5. [PubMed] [Google Scholar]
Conlan 1993
- Conlan T, Garth WP Jr, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. Journal of Bone and Joint Surgery - American Volume. 1993;75(5):682-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology 2010;63(8):834-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cosgarea 2002
- Cosgarea AJ, Browne JA, Kim TK, McFarland EG. Evaluation and management of the unstable patella. Physician and Sportsmedicine 2002;30(10):33-40. [EMBASE: 2002360234] [DOI] [PubMed] [Google Scholar]
Crossley 2004
- Crossley KM, Bennell KL, Cowan SM, Green S. Analysis of outcome measures for persons with patellofemoral pain: which are reliable and valid? Archives of Physical Medicine and Rehabilitation 2004;85(5):815-22. [DOI] [PubMed] [Google Scholar]
Dath 2006
- Dath R, Chakravarthy J, Porter KM. Patella dislocations. Trauma 2006;8(1):5-11. [EMBASE: 2006239083] [Google Scholar]
Dejour 1994
- Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surgery, Sports Traumatology, Arthroscopy 1994;2(1):19-26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Donell 2006a
- Donell ST. Patellofemoral dysfunction-extensor mechanisms malalignment. Current Orthopaedics 2006;20(2):103-11. [Google Scholar]
Donell 2006b
- Donell ST, Joseph G, Hing CB, Marshall TJ. Modified Dejour trochleoplasty for severe dysplasia: operative technique and early clinical results. Knee 2006;13(4):266-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Donell 2014
- Donell S. Personal communication 25 October 2014.
Felli 2019
- Felli L, Capello AG, Lovisolo S, Chiarlone F, Alessio-Mazzola M. Goldthwait technique for patellar instability: surgery of the past or here to stay procedure? A systematic review of the literature. Musculoskeletal Surgery 2019;103:107-113. [DOI: 10.1007/s12306-018-0566-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Flandry 1991
- Flandry F, Hunt JP, Terry GC, Hughston JC. Analysis of subjective knee complaints using visual analog scales. American Journal of Sports Medicine 1991;19(2):112-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frosch 2011
- Frosch S, Balcarek P, Walde TA, Schüttrumpf JP, Wachowski MM, Ferleman KG, et al. The treatment of patellar dislocation: a systematic review [Die Therapie der Patellaluxation: eine systematische Literaturanalyse]. Zeitschrift fur Orthopadie und Unfallchirurgie 2011;149(6):630-45. [DOI: 10.1055/s-0030-1250691] [DOI] [PubMed] [Google Scholar]
Fulkerson 1987
- Fulkerson JP, Schutzer SF, Ramsby GR, Bernstein RA. Computerized tomography of the patellofemoral joint before and after lateral release or realignment. Arthroscopy 1987;3:19-24. [DOI: 10.1016/s0749-8063(87)80005-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed on 19 April 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guhan 2009
- Guhan B, Lee AS. Acute repair of medial patellofemoral ligament. Journal of Bone and Joint Surgery - British Volume 2009;91(Suppl 3):413-4. [Google Scholar]
Hautamaa 1998
- Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clinical Orthopaedics and Related Research 1998;349:174-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herdman 2011
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research 2011;20:1727-36. [DOI: 10.1007/s11136-011-9903-x] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hiemstra 2013
- Hiemstra LA, Kerslake S, Lafave MR, Heard SM, Buchko GM, Mohtadi NG. Initial validity and reliability of the Banff Patella Instability Instrument. American Journal of Sports Medicine 2013;41:1629-35. [DOI: 10.1177/0363546513487981] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hsiao 2010
- Hsiao M, Owens BD, Burks R, Sturdivant RX, Cameron KL. Incidence of acute traumatic patellar dislocation among active-duty United States military service members. American Journal of Sports Medicine 2010;38(10):1997-2004. [DOI] [PubMed] [Google Scholar]
Huntington 2020
- Huntington LS, Webster KE, Devitt BM, Scanlon JP, Feller JA. Factors associated with an increased risk of recurrence after a first-time patellar dislocation: a systematic review and meta-analysis. American Journal of Sports Medicine 2020;48:2552-62. [DOI: 10.1177/0363546519888467] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hussein 2018
- Hussein A, Sallam AA, Imam MA, Snow M. Surgical treatment of medial patellofemoral ligament injuries achieves better outcomes than conservative management in patients with primary patellar dislocation: a meta-analysis. Journal of ISAKOS 2018;3:98-104. [DOI: 10.1136/jisakos-2017-000152] [DOI] [Google Scholar]
Krämer 2014
- Krämer JA, Schmidt S, Jürgens KU, Lentschig M, Schmeling A, Vieth V. The use of magnetic resonance imaging to examine ossification of the proximal tibial epiphysis for forensic age estimation in living individuals. Forensic Science, Medicine and Pathology 2014;10:306-13. [DOI: 10.1007/s12024-014-9559-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kujala 1993
- Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy 1993;9(2):159-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook.
Liu 2018
- Liu JN, Steinhaus ME, Kalbian IL, Post WR, Green DW, Strickland SM, et al. Patellar instability management: a survey of the International Patellofemoral Study Group. American Journal of Sports Medicine 2018;46:3299-306. [DOI: 10.1177/0363546517732045] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lysholm 1982
- Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. American Journal of Sports Medicine 1982;10(3):150-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mäenpää 1997
- Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. American Journal of Sports Medicine 1997;25:213-7. [DOI: 10.1177/036354659702500213] [PMID: ] [DOI] [PubMed] [Google Scholar]
Martinez‐Cano 2021
- Martinez-Cano JP, Chica J, Martinez-Arboleda JJ, Rincón-Escobar E, Zamudio-Castilla L, Renjifo M, et al. Patellofemoral dislocation recurrence after a first episode: a case-control study. Orthopaedic Journal of Sports Medicine 2021;9(1):1-7. [DOI: 10.1177/2325967120981636] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGee 2017
- McGee TG, Cosgarea AJ, McLaughlin K, Tanaka M, Johnson K. Rehabilitation after medial patellofemoral ligament reconstruction. Sports Medicine and Arthroscopy Reviews 2017;25:105-13. [DOI: 10.1097/JSA.0000000000000147] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mears 2001
- Mears SC, Cosgarea AJ. Surgical treatment options in patellofemoral disorders. Current Opinion in Orthopaedics 2001;12(2):167-73. [EMBASE: 2001135456] [Google Scholar]
Merchant 2007
- Merchant ND, Bennett CH. Recent concepts in patellofemoral instability. Current Opinion in Orthopaedics 2007;18(2):153-60. [EMBASE: 2007090939] [Google Scholar]
Mitchell 2015
- Mitchell J, Magnussen RA, Collins CL, Currie DW, Best TM, Comstock RD, et al. Epidemiology of patellofemoral instability injuries among high school athletes in the United States. American Journal of Sports Medicine 2015;43:1676-82. [DOI: 10.1177/0363546515577786] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moiz 2018
- Moiz M, Smith N, Smith TO, Chawla A, Thompson P, Metcalfe A. Clinical outcomes after the nonoperative management of lateral patellar dislocations: a systematic review. Orthopaedic Journal of Sports Medicine 2018;6(6):1-17. [DOI: 10.1177/2325967118766275] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nietosvaara 1994
- Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. Journal of Pediatric Orthopaedics 1994;14(4):513-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palmu 2008
- Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. Journal of Bone and Joint Surgery 2008;90(3):463-70. [DOI: 10.2106/JBJS.G.00072] [PMID: ] [DOI] [PubMed] [Google Scholar]
Paxton 2003
- Paxton EW, Fithian DC, Stone ML, Silva P. The reliability and validity of knee-specific and general health instruments in assessing acute patellar dislocation outcomes. American Journal of Sports Medicine 2003;31(4):487-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salonen 2017
- Salonen EE, Magga T, Sillanpää PJ, Kiekara T, Mäenpää H, Mattila VM. Traumatic patellar dislocation and cartilage injury: a follow-up study of long-term cartilage deterioration. American Journal of Sports Medicine 2017;45:1376-82. [DOI: 10.1177/0363546516687549] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sanders 2018
- Sanders TL, Pareek A, Hewett TE, Stuart MJ, Dahm DL, Krych AJ. High rate of recurrent patellar dislocation in skeletally immature patients: a long-term population-based study. Knee Surgery Sports Traumatology Arthroscopy 2018;26:1037-43. [DOI: 10.1007/s00167-017-4505-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Scher 2010
- Scher DL, Owens BD, Sturdivant RX, Wolf JM. Incidence of joint hypermobility syndrome in a military population: impact of gender and race. Clinical Orthopaedics and Related Research 2010;458(7):1790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Sillanpää 2012
- Sillanpää PJ, Mäenpää HM. First-time patellar dislocation: surgery or conservative treatment? Sports Medicine and Arthroscopy Reviews 2012;20(3):128-35. [DOI: 10.1097/JSA.0b013e318256bbe5] [DOI] [PubMed] [Google Scholar]
Smith 2010
- Smith TO, Davies L, Chester R, Clark A, Donell ST. A systematic review of physiotherapy following lateral patellar dislocation. Physiotherapy 2010;96:269-81. [DOI: 10.1016/j.physio.2010.02.006] [DOI] [PubMed] [Google Scholar]
Smith 2011a
- Smith TO, Donell ST, Chester R, Clark A, Stephenson R. What activities do patients with patellar instability perceive makes their patella unstable? Knee 2011;18:333-9. [DOI: 10.1016/j.knee.2010.07.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 2011b
- Smith TO, Song F, Donell ST, Hing CB. Operative versus non-operative management of patellar dislocation. A meta-analysis. Knee Surgery, Sports Traumatology, Arthroscopy 2011;19(6):988-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 2014
- Smith TO, Donell ST, Clark A, Chester R, Cross J, Kader DF, et al. The development, validation and internal consistency of the Norwich Patellar Instability (NPI) score. Knee Surgery Sports Traumatology Arthroscopy 2014;22:324-35. [DOI: 10.1007/s00167-012-2359-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Song 2010
- Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al. Dissemination and publication of research findings: an updated review of related biases. Health Technology Assessment 2010;14(8):1-193. [DOI: 10.3310/hta14080] [DOI] [PubMed] [Google Scholar]
Stefancin 2007
- Stefancin JJ, Parker RD. First-time traumatic patellar dislocation: a systematic review. Clinical Orthopaedics and Related Research 2007;455:93-101. [DOI: 10.1097/BLO.0b013e31802eb40a] [PMID: ] [DOI] [PubMed] [Google Scholar]
Strugnell 2014
- Strugnell C, Dunstan DW, Magliano DJ, Zimmet PZ, Shaw JE, Daly RM. Influence of age and gender on fat mass, fat-free mass and skeletal muscle mass among Australian adults: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Journal of Nutrition, Health and Ageing 2014;18(5):540-6. [DOI] [PubMed] [Google Scholar]
Tegner 1985
- Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clinical Orthopaedics and Related Research 1985;198:43-9. [MEDLINE: ] [PubMed] [Google Scholar]
Thompson 2019
- Thompson P, Metcalfe AJ. Current concepts in the surgical management of patellar instability. Knee 2019;26:1171-81. [DOI: 10.1016/j.knee.2019.11.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tian 2020
- Tian G, Yang G, Zuo L, Li F, Wang F. Conservative versus repair of medial patellofemoral ligament for the treatment of patients with acute primary patellar dislocations: a systematic review and meta-analysis. Journal of Orthopaedic Surgery 2020;28(2):1-8. [DOI: 10.1177/2309499020932375] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tsai 2012
- Tsai CH, Hsu CJ, Hung CH, Hsu HC. Primary traumatic patellar dislocation. Journal of Orthopaedic Surgery and Research 2012;6(7):21. [DOI: 10.1186/1749-799X-7-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1996
- Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34(3):220-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wille 2010
- Wille N, Badia X, Bonsel G, Burstrom K, Cavrini G, Devlin N, et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Quality of Life Research 2010;19(6):875-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woo 1998
- Woo R, Busch MT. Management of patellar instability in children. Operative Techniques in Sports Medicine 1998;6(4):247-58. [Google Scholar]
Xing 2020
- Xing X, Shi H, Feng S. Does surgical treatment produce better outcomes than conservative treatment for acute primary patellar dislocations? A meta-analysis of 10 randomized controlled trials. Journal of Orthopaedic Surgery and Research 2020;15:118. [DOI: 10.1186/s13018-020-01634-5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2019
- Yang F, Guo W, Wang Q, Zhu Z, Guan C, Zhao S, et al. Surgical versus nonsurgical treatment of primary acute patellar dislocation: a systematic review and meta-analysis. Medicine 2019;98:e16338. [DOI: 10.1097/MD.0000000000016338] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2020
- Zhang K, Jiang H, Li J, Fu W. Comparison between surgical and nonsurgical treatment for primary patellar dislocations in adolescents: a systematic review and meta-analysis of comparative studies. Orthopaedic Journal of Sports Medicine 2020;8(9):1-10. [DOI: 10.1177/2325967120946446] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hing 2011
- Hing CB, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD008106. [DOI: 10.1002/14651858.CD008106.pub2] [DOI] [PubMed] [Google Scholar]
Smith 2015
- Smith TO, Donell S, Song F, Hing CB. Surgical versus non‐surgical interventions for treating patellar dislocation. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD008106. [DOI: 10.1002/14651858.CD008106.pub3] [DOI] [PubMed] [Google Scholar]


