Abstract
In a series of studies designed to test the role of renal “work” in compensatory kidney growth we examined the relationship between absolute sodium reabsorption—which constitutes the bulk of renal energy expenditure, and growth of the remaining kidney at various intervals after contralateral nephrectomy.
The increase in weight of the remaining kidney preceded the rise in sodium reabsorption and these two processes took place at different rates between 24 hours and 21 days after uninephrectomy.
Absolute sodium reabsorption did not change during the first hours after contralateral nephrectomy, at a time when biochemical alterations are known to occur.
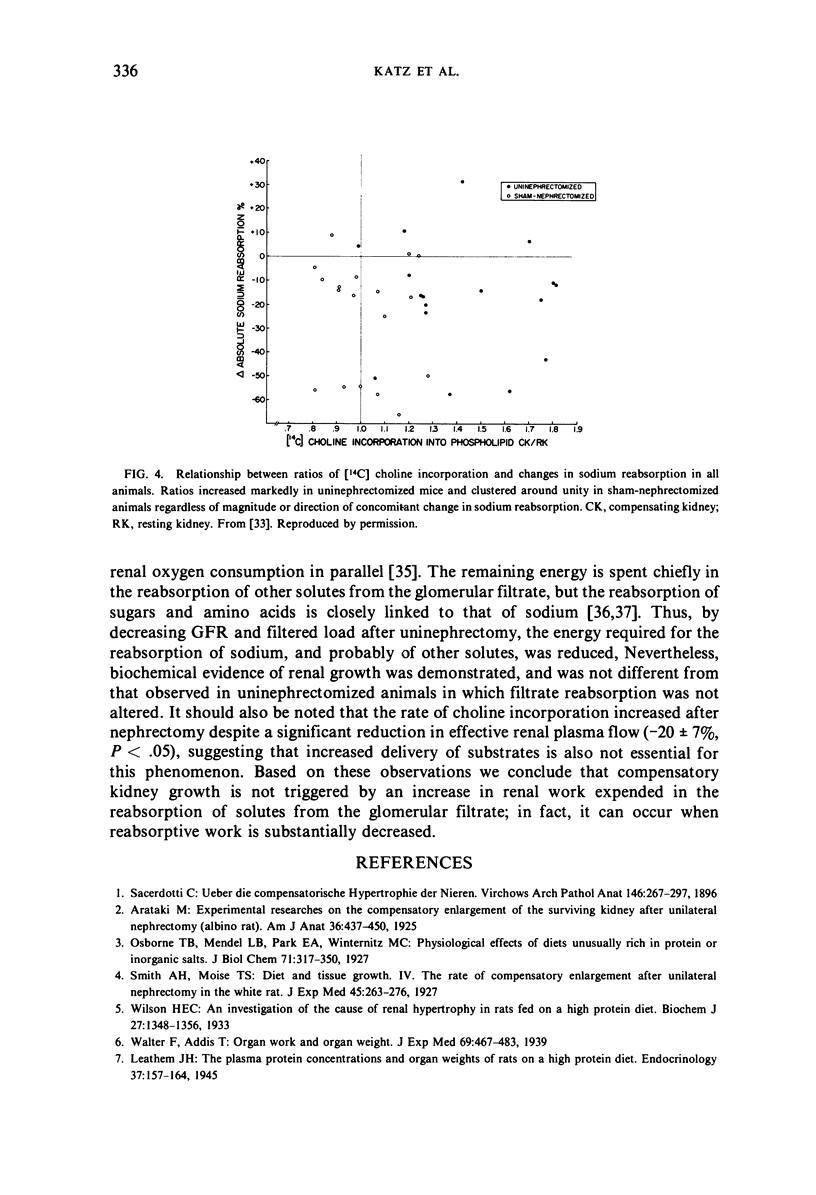
The rate of [14C] choline incorporation into renal phospholipid, an early biochemical indicator of compensatory kidney growth, increased significantly one hour after contralateral nephrectomy but remained unchanged after sham-nephrectomy, regardless of the magnitude or direction of the concomitant change in absolute sodium reabsorption (“kidney work”).
These results indicate that renal work expended in the reabsorption of glomerular filtrate is neither the initiating, nor the primary controlling factor, of the compensatory kidney growth that follows unilateral nephrectomy.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOCK M. A., WAKIM K. G., MANN F. C. Appraisal of certain factors influencing compensatory renal hypertrophy. Am J Physiol. 1953 Jan;172(1):60–66. doi: 10.1152/ajplegacy.1952.172.1.60. [DOI] [PubMed] [Google Scholar]
- Bugge-Asperheim B., Kiil F. Examination of growth-mediated changes in hemodynamics and tubular transport of sodium, glucose and hippurate after nephrectomy. Scand J Clin Lab Invest. 1968 Dec;22(4):255–265. doi: 10.3109/00365516809167062. [DOI] [PubMed] [Google Scholar]
- Coe F. L., Korty P. R. Protein synthesis during compensatory renal hypertrophy. Am J Physiol. 1967 Dec;213(6):1585–1589. doi: 10.1152/ajplegacy.1967.213.6.1585. [DOI] [PubMed] [Google Scholar]
- DE WARDENER H. E., MILLS I. H., CLAPHAM W. F., HAYTER C. J. Studies on the efferent mechanism of the sodium diuresis which follows the administration of intravenous saline in the dog. Clin Sci. 1961 Oct;21:249–258. [PubMed] [Google Scholar]
- Dicker S. E. Cyclic nucleotides in compensatory renal hypertrophy [proceedings]. J Physiol. 1976 Dec;263(1):192P–193P. [PubMed] [Google Scholar]
- Halliburton I. W., Thomson R. Y. The effect of diet and of unilateral nephrectomy on the composition of the kidney. Cancer Res. 1967 Sep;27(9):1632–1638. [PubMed] [Google Scholar]
- Johnson H. A., Amendola F. Mitochondrial proliferation in compensatory growth of the kidney. Am J Pathol. 1969 Jan;54(1):35–45. [PMC free article] [PubMed] [Google Scholar]
- Katz A. I., Epstein F. H. Relation of glomerular filtration rate and sodium reabsorption to kidney size in compensatory renal hypertrophy. Yale J Biol Med. 1967 Dec;40(3):222–230. [PMC free article] [PubMed] [Google Scholar]
- Katz A. I. Renal function immediately after contralateral nephrectomy: relation to the mechanism of compensatory kidney growth. Yale J Biol Med. 1970 Dec;43(3):164–172. [PMC free article] [PubMed] [Google Scholar]
- Katz A. I., Toback F. G., Lindheimer M. D. Independence of onset of compensatory kidney growth from changes in renal function. Am J Physiol. 1976 Apr;230(4):1067–1071. doi: 10.1152/ajplegacy.1976.230.4.1067. [DOI] [PubMed] [Google Scholar]
- Knox F. G., Fleming J. S., Rennie D. W. Effects of osmotic diuresis on sodium reabsorption and oxygen consumption of kidney. Am J Physiol. 1966 Apr;210(4):751–759. doi: 10.1152/ajplegacy.1966.210.4.751. [DOI] [PubMed] [Google Scholar]
- Mackay E. M., Mackay L. L., Addis T. THE DEGREE OF COMPENSATORY RENAL HYPERTROPHY FOLLOWING UNILATERAL NEPHRECTOMY : I. THE INFLUENCE OF AGE. J Exp Med. 1932 Jul 31;56(2):255–265. doi: 10.1084/jem.56.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malt R. A. Compensatory growth of the kidney. N Engl J Med. 1969 Jun 26;280(26):1446–1459. doi: 10.1056/NEJM196906262802606. [DOI] [PubMed] [Google Scholar]
- Malt R. A., Lemaitre D. A. Accretion and turnover of RNA in the renoprival kidney. Am J Physiol. 1968 May;214(5):1041–1047. doi: 10.1152/ajplegacy.1968.214.5.1041. [DOI] [PubMed] [Google Scholar]
- Phillips T. L., Leong G. F. Kidney cell proliferation after unilateral nephrectomy as related to age. Cancer Res. 1967 Feb;27(2):286–292. [PubMed] [Google Scholar]
- Potter D. E., Leumann E. P., Sakai T., Holliday M. A. Early responses of glomerular filtration rate to unilateral nephrectomy. Kidney Int. 1974 Feb;5(2):131–136. doi: 10.1038/ki.1974.17. [DOI] [PubMed] [Google Scholar]
- SHARE L. Effect of increased ureteral pressure on renal function. Am J Physiol. 1952 Jan;168(1):97–106. doi: 10.1152/ajplegacy.1951.168.1.97. [DOI] [PubMed] [Google Scholar]
- Schlondorff D., Weber H. Cyclic nucleotide metabolism in compensatory renal hypertrophy and neonatal kidney growth. Proc Natl Acad Sci U S A. 1976 Feb;73(2):524–528. doi: 10.1073/pnas.73.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. H., Moise T. S. DIET AND TISSUE GROWTH : IV. THE RATE OF COMPENSATORY RENAL ENLARGEMENT AFTER UNILATERAL NEPHRECTOMY IN THE WHITE RAT. J Exp Med. 1927 Jan 31;45(2):263–276. doi: 10.1084/jem.45.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toback F. G., Smith P. D., Lowenstein L. M. Phospholipid metabolism in the initiation of renal compensatory growth after acute reduction of renal mass. J Clin Invest. 1974 Jul;54(1):91–97. doi: 10.1172/JCI107754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Klöss S. Sodium dependence of the amino acid transport in the proximal convolution of the rat kidney. Pflugers Arch. 1974;351(1):49–60. doi: 10.1007/BF00603510. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Klöss S. Specificity and sodium dependence of the active sugar transport in the proximal convolution of the rat kidney. Pflugers Arch. 1974;351(1):35–48. doi: 10.1007/BF00603509. [DOI] [PubMed] [Google Scholar]
- WEISS A. G., KOEBELE F., OUDET P. Quelques remarques concernant l'exérèse des cancers bronchiques après radiothérapie. Strasb Med. 1952 Jun;3(6):487–488. [PubMed] [Google Scholar]
- Walter F., Addis T. ORGAN WORK AND ORGAN WEIGHT. J Exp Med. 1939 Feb 28;69(3):467–483. doi: 10.1084/jem.69.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman E. J., Renquist K., Stroup R., Kashgarian M., Hayslett J. P. Increased tubular reabsorption of sodium in compensatory renal growth. Am J Physiol. 1973 Mar;224(3):565–571. doi: 10.1152/ajplegacy.1973.224.3.565. [DOI] [PubMed] [Google Scholar]
- Wilson H. E. An investigation of the cause of renal hypertrophy in rats fed on a high protein diet. Biochem J. 1933;27(5):1348–1356. doi: 10.1042/bj0271348. [DOI] [PMC free article] [PubMed] [Google Scholar]


