Abstract

Polymeric materials produced from fossil fuels have been intimately linked to the development of industrial activities in the 20th century and, consequently, to the transformation of our way of living. While this has brought many benefits, the fabrication and disposal of these materials is bringing enormous sustainable challenges. Thus, materials that are produced in a more sustainable fashion and whose degradation products are harmless to the environment are urgently needed. Natural biopolymers—which can compete with and sometimes surpass the performance of synthetic polymers—provide a great source of inspiration. They are made of natural chemicals, under benign environmental conditions, and their degradation products are harmless. Before these materials can be synthetically replicated, it is essential to elucidate their chemical design and biofabrication. For protein-based materials, this means obtaining the complete sequences of the proteinaceous building blocks, a task that historically took decades of research. Thus, we start this review with a historical perspective on early efforts to obtain the primary sequences of load-bearing proteins, followed by the latest developments in sequencing and proteomic technologies that have greatly accelerated sequencing of extracellular proteins. Next, four main classes of protein materials are presented, namely fibrous materials, bioelastomers exhibiting high reversible deformability, hard bulk materials, and biological adhesives. In each class, we focus on the design at the primary and secondary structure levels and discuss their interplays with the mechanical response. We finally discuss earlier and the latest research to artificially produce protein-based materials using biotechnology and synthetic biology, including current developments by start-up companies to scale-up the production of proteinaceous materials in an economically viable manner.
1. Introduction
Polymer materials represent a central aspect of everyday life and advanced technologies, from consumer goods to textiles, from high-performance composites to packaging, and from adhesives to electronic components, to name just a few examples. There is literally no modern human activity that does not depend on classical and more advanced polymers, and their production nowadays exceeds that of steel. Yet we have a fundamental ambivalence with respect to polymers, because they are also perceived as emblematic of a non-sustainable way of life. Indeed, despite our enormous reliance on plastics for every aspect of our modern life,1 they generate much disdain, being perceived as materials that are “cheap” and polluting.2 This perception arises from two fundamental reasons. First, polymers are in their large majority synthesized from fossil fuels, i.e. petro-chemical derived chemicals. And second, many are non-biodegradable and exhibit poor recyclability. Their degradation products can be toxic, which raises serious concerns about their end-of-life disposal,2 not to mention their accumulation in large quantities in the Oceans that badly affect marine ecosystems3,4 or in landfills.5,6 Because of these issues, there is an urgent need to develop new polymeric materials that can be more sustainable, i.e. their fabrication does not fully rely on petro-chemical derived chemicals, they use less energetic resources, and they can degrade into non-harmful chemicals in the environment.
In our quest toward alternative and more sustainable polymer solutions, Nature perhaps represents the best source of inspiration, exhibiting key advantages over fossil-fuel derived polymers. Biological materials are produced in an eco-friendly fashion using natural chemicals. Indeed, the complex machinery of living cells that produces extracellular tissues operates in an aqueous environment and at room temperature and pressure. The degradable products of these materials are harmless, such as amino acids in the case of protein-based biopolymers. Thus, the living world abounds in biopolymers with remarkable mechanical and physical characteristics that can compete or sometimes exceed the best synthetic polymers.7−9 For example, silk has been known for decades to exhibit outstanding mechanical properties10,11 that surpass those of many synthetic fibers,12,13 and there has been an outburst of activity in producing artificial versions of silk using biotechnology processes in the past decade, with a wide range of potential applications explored.14,15 Furthermore, biological polymers are produced via bottom-up “biofabrication” processes that seamlessly exploit the multi-scale self-assembly and supramolecular chemistry afforded by the versatility of the 20 amino acids of proteins and their post-translational modifications (PTMs).16 The end products are typically multi-functional, and when reproduced artificially with de novo gene design, innovative properties can in principle be achieved such as bioactivity and programmed degradability. Altogether, these synergistic characteristics are still unmatched in synthetic polymers.
This review is organized in five sections. In Section 2, we present methods of protein sequencing in the field of protein materials, a critical initial step that has traditionally been a major bottleneck in the field. Earlier methods are first described, in particular those still in use today that yield information that cannot be readily obtained with “omics” methods, before moving to latest developments that are relevant to materials discoveries. In Section 3, essential molecular designs of protein-based materials are described, with an emphasis on sequence/structure property relationships and common biochemical and structural features. The materials presented in this section are organized based on their structural functionality and illustrate that protein-based materials utilize the whole spectrum of secondary structures at the disposal of polypeptides. Some load-bearing proteins are mostly intrinsically disordered,17 such as the bioelastomeric elastin (Section 3.2.1), or in their storage glands prior to secretion, e.g. mussel foot proteins (MFPs, Section 3.3.1) or the slime of the velvet worm (Section 3.1.3). Others are dominated by β-sheets that are preferentially aligned along the macroscopic fiber such as silk fibroins (Section 3.1.1) or perpendicular to the fiber in the case of amyloid-like fibrils; for example barnacle cement proteins (Section 3.3.2) or curli filaments (Section 3.3.4). Yet other proteins are mostly made of α-helical coiled-coil regions that are reminiscent of intermediate filaments (IFs), including marine snail egg capsule proteins (ECPs, Section 3.2.4) and hagfish filaments (Section 3.1.2), or contain collagenous triple-helical domains, such as the core of mussel adhesive filaments (Section 3.2.3). And finally, some extracellular structures are built from proteins with both ordered and disordered regions as illustrated by the squid sucker ring teeth proteins called suckerins (Section 3.4.4).
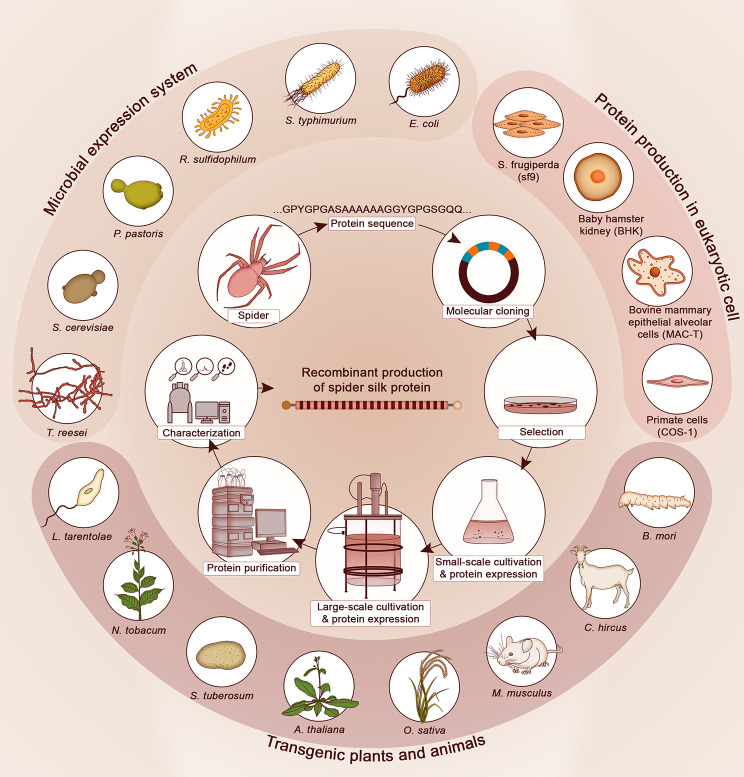
In Section 4, we survey the bioengineering fabrication of protein-based materials presented in Section 3. The different types of living hosts used for biofabrication are compared, including their key respective advantages/disadvantages and current limitations. Methods of purification are also described here as this has been, and remains in many cases, a bottleneck toward scaling-up of protein-based materials. Recent efforts by spin-off companies to fabricate protein materials are described in this section to highlight potential routes for future commercialization. In the final Section 5, we provide a perspective on future developments and major hurdles that will need to be overcome in order to achieve protein materials produced though biotechnology a viable alternative to synthetic polymers.
2. Sequencing of Extracellular Proteins: Historical Perspective
Nature abounds with extracellular tissues with structural (load-bearing) functionalities that are predominantly made of proteins and which are now increasingly exploited to artificially design and produce protein-based materials.18,19 But before such materials can be replicated via synthetic biology fabrication processes, it is critical to carefully identify their building blocks at the molecular level. Traditionally, such investigations have been very time-consuming because they have often been considered tedious and technically challenging. In the case of biological materials, there are two main reasons for these hurdles. First, these extracellular tissues are usually highly cross-linked in order to sustain the aggressive mechanical, chemical, and biological stresses (pathogens, bacterial degradation) to which they are subjected in their natural environment. The covalent bonds between the proteins’ side chains result in highly insoluble polymeric networks, thwarting isolation of intact proteins and complicating subsequent sequencing efforts. Typically, only short peptide fragments could be isolated and in low yields. Historically, such studies would thus take many years to identify only partial sequences of the proteins of interest. Second, while the genomic era and associated technological advances have helped overcome some of these challenges and have accelerated discoveries, there is still a sparse number of genomes available for many organisms used as model systems in the field. As a result, obtaining the complete biochemical characterization of proteinaceous building blocks of biological materials (full-length sequences, PTMs, and cross-links that stabilize the final tissue) remained until recently a lengthy endeavor carried out by a handful of research groups.
Rapid developments in next-generation sequencing (NGS) and proteomic technologies as well as their associated bioinformatic softwares in the past decade have considerably alleviated these limitations, providing a range of powerful tools that accelerate discoveries, especially when used synergistically. While these advance approaches have undoubtedly taken the center stage of protein discoveries, traditional biochemical and molecular biology methods are still needed to identify important molecular features that regulate the final function of protein-based materials, such as PTMs or cross-linking chemistry. Once this information is available, together with in-depth understanding of sequence-property relationships, it can be translated into artificial fabrication of protein-based materials via DNA recombinant technology. Four extracellular fibrous and elastomeric protein materials that exemplify the extensive timeline that was required to fully identify their end-to-end sequences are highlighted in this section namely: (i) resilin, (ii) elastin, (iii) silk fibroins, and (iv) mussel adhesive proteins, as summarized in the timeline shown in Figure 1.
Figure 1.
Timeline discoveries of several load-bearing proteins that have gathered broad research interest as biological model systems in biomimetic materials engineering.
2.1. Discovery and Sequencing of Resilin (1951–2001)
Resilin, the protein that constitutes the hinge connecting the wings of insects to their thorax, is historically interesting because it was the first structure protein to be investigated in what can be broadly referred as “biomimetic” or “bioinspired” materials. Thus, in 1951, Krogh and Weis-Fogh20 published a study on the physiology of desert locus flight that highlighted the importance of resilin, an insoluble, rubber-like substance in the wing-hinge ligament whose amino acid composition was analyzed 10 years later.21 The same year, Weis-Fogh demonstrated—for the first time for a structural protein—that resilin was a rubber-like material.22,23 A few years later, Andersen identified dityrosine and trityrosine24,25 as the cross-links that stabilize the rubbery resilin network. It was only about 30 years later, in 1992, that sequences of peptide fragments of resilin were obtained by Lombardi and Kaplan.26 They isolated and trypsin-digested resilin from the cockroach Periplaneta americana (P. americana) and subjected the trypsin digests to Edman sequencing, obtaining a dozen peptide sequences 5 to 15 amino acid long. In 2001, Ardell and Andersen27 also sequenced resilin peptide fragments from the desert locust Shistocera gregaria (S. gregaria) using Edman sequencing. By then, however, they benefited from a major scientific breakthrough since the complete genome of Drosophilia melanogaster (D. melanogaster commonly known as the fruit fly) had recently been published.28 Searching the genome with their tryptic peptides, as well as those previously obtained by Lombardi and Kaplan, they identified two candidate genes encoding for pro-resilin, the precursor protein of resilin prior to cross-linking.27 Finally in 2005, Elvin and co-workers29 expressed an exon of one of these genes encoding multiple copies of a putative elastic repeat motif. By inducing dityrosine cross-linking using Ruthenium-mediated photo-cross-linking, they were able to artificially recapitulate the elastomeric properties of native resilin.
2.2. Discovery and Sequencing of (Tropo)elastin (1949–1988)
Elastin is the main structural protein providing elasticity to various tissues of vertebrates. Given its importance for human health, studies on elastin were initiated as early as the late 1940s/early 1950s, starting by its partial amino acid composition by Neumann30 and Partridge and Davis.31 One year later, Partridge and co-workers32 were able to isolate soluble fragments of elastin and demonstrated that the solution turned into a viscous layer made of liquid droplets upon raising the temperature above 25 °C. This was probably the first evidence of the coacervation process of a biomacromolecule—or liquid liquid phase separation (LLPS)—which has since been recognized as a hallmark of tropoelastin33 and synthetic elastin-like polypeptides (ELPs).34 Early biochemical investigations were hampered by the inherent insolubility of mature elastin attributed to the high degree of chemical cross-links between polypeptide chains. As a result, it took more that 10 years to identify these unusual cross-links as the heterocyclic desmosine, isodemosine, and lysinorleucine.35,36
Further advances in elastin biochemistry were achieved in 1969 when its soluble precursor, tropoelastin, was isolated by Sandberg et al.37 from Cu-deficient porcine aorta. Electrophoresis and quantitative amino acid analysis estimated the molecular weight (MW) of tropoelastin to be around 60–70 kDa, and the authors were also able to obtain the first 5 amino acids of the N-terminus by Edman sequencing. By digesting soluble tropoelastin with trypsin and subjecting the tryptic fragments to Edman sequencing, the same group identified Ala-rich peptides that were involved in cross-linking formation,38 followed by the identification39 of tetra-, penta-, and hexapeptide repeats [GGVP], [PGVGV], and [PGVGVA] in the tropoelastin sequence. These repeating sequences were exploited decades later to design ELPs.40 Using similar strategies of enzymatic digestion and Edman sequencing of tryptic peptides, Gerber and Anwar41,42 identified the specific Lys-rich region involved in cross-linking.
Advances in molecular biology methods in the 1980s finally allowed researchers to obtain the complete primary sequence of tropoelastin. Thus, in 1987, Indik et al.43 isolated human mRNA from embryonic aorta and used it as a template to synthesize cDNA, from which three overlapping cDNA clones were identified and combined into a single cDNA sequence that included a Met initiation site and stop codon. The encoded primary sequence had a MW of 72 kDa and included all previously identified short peptides including the hydrophobic cross-linking domains, the pentapeptide repeats, as well as several sequences that were previously not identified. The study also established that elastin mRNA exhibited alternative splicing. With the full-length elastic cDNA clone available, a few years later the same team successfully produced the first human tropoelastin in a recombinant bacterial system,44 paving the way for a wide range of regenerative medicine and tissue engineering applications that have since been demonstrated.45 In parallel, Urry and co-workers34 synthesized ELPs made of the pentapeptide repeat [VPGVG]n and demonstrated their temperature-induced coacervation. Upon increasing the temperature above 25 °C, soluble ELPs phase-separated in two phases: a dense and viscous phase and a diluted phase, with a transition temperature that depended on the MW of the polypeptides, thus establishing the critical role of the pentapeptide repeats in triggering coacervation. Furthermore, Urry et al. also established the ability to broadly modulate the transition temperature of ELPs from 5 °C up to 70 °C by varying the guest residue composition.46 This lower-critical solution temperature (LCST) behavior has since been exploited in biomedical and drug delivery applications, as reviewed elsewhere.40 Despite the long-held view that tropoelastin has mostly a random-coil structure, it is also noteworthy that small-angle X-ray and neutron scattering studies (SAXS and SANS, respectively) later established that tropoelastin seems to display a well-defined shape in solution consisting of an asymmetric coil with a protruding foot-like structure,47−49 as presented in more details in Section 3.2.1.
2.3. Discovery and Sequencing of Spider Silk Fibroins (1960–2007)
Owing to its remarkable physical properties,50−53 spider silk has long attracted the interest of scientists from diverse fields (biologists, chemists, material scientists, etc.). A central goal of these efforts has been to establish how the mechanical properties are linked to the molecular design and the secondary structure of silk fiber proteins (called spidroins). It was more than a century ago that Fisher demonstrated spider silk fibers to be made of proteins.54 The first X-ray diffraction measurements of silks were obtained in 1955 from silkworm (Bombyx mori, B. mori) fibers by Marsh, Correy and Pauling,55 who suggested the β-pleated sheet crystal structure. Then, five years later Warwicker56 reported the first X-ray diffraction measurements of spider silk fibers and confirmed the presence of β-sheets oriented along the fiber axis. Since then, numerous structural studies of silks have been conducted using techniques such as wide angle X-ray spectroscopy (WAXS),57−59 infrared spectroscopy,60,61 solid-state Nuclear Magnetic Resonance (ssNMR) spectroscopy,62 or Raman spectroscopy.63 This large body of work has been extensively reviewed elsewhere.64,65
Identifying the primary structure of spider silks has been an arduous path largely related to their extremely large MWs, to the presence of highly repetitive peptide sequences, and to the fact that there exist many different types of silks within a single species.11 In 1970, Andersen66 reported the amino acid composition from several silk glands in the European garden spider Araneus diadematus (A. diadematus). For a few years, research into the molecular structure of spider silk fibroins remained rather quiet, until the late 1980s/early 1990s when Work and Young67 and Lombardi and Kaplan68 reported the amino acid composition of silk glands from the golden silk spider Nephila Clavipes (N. clavipes). Then, a breakthrough arose in 1990 when Xu and Lewis69 isolated penta- and hexapeptides from N. clavipes dragline silk using a partial hydrolysis treatment and obtained their sequences with the automated Edman method, from which they designed DNA probes. Concurrently, they built a cDNA library of the major spider ampullate glands (MaSP) that concentrates the precursor of dragline silk and screened it with their DNA probes. They managed to deduce for the first time partial amino acid sequences of a spider silk fibroin (spidroin-1), notably recognizing the presence of highly repetitive peptide domains such as stretches of poly-Ala and Gly-Gly-Xaa peptides (where Xaa is typically Tyr, Ala, or Leu). They also realized the highly biased codon design of fibroin-encoding genes that avoids the use of cytosine and guanine in the third base position. Using the same method, Hinman and Lewis70 later isolated a second partial cDNA clone for another silk protein (spidroin-2) containing multiple pentapeptide repeats rich in Gly, Tyr, and Pro (e.g., GYGPG or GPGGY motifs) that explained the Pro content measured by amino acid analysis (AAA). Building up on this work, a few years later Guerette et al.71 constructed cDNA libraries from the major ampullate glands of A. diadematus that secrete and store specific silks, namely the tough dragline silk, the extensible viscid silk, and the cocoon silk. Screening these cDNA libraries with probes designed from spidroins-1 and -2, they identified a gene family of at least 4 fibroin genes, each of which encoding a distinct silk protein characterized by various proportions of amorphous (Gly-rich) and crystalline (poly-Ala and poly-Gly-Ala) domains. This study established that silk expression is gland-specific and linked the mechanical properties of the different silks to the peptide domains that make up their modular primary structure. Using similar approaches, Hayashi and Lewis72 later obtained the partial sequence of the flagelliform gland silk protein that constitutes the core fiber of the catching spiral. The complete gene sequences for any spider silk remained unknown until 2007, when the full-length gene encoding the dragline silk of the black widow (Latrodectus hesperus) was obtained by Ayoub et al.73 through construction of a genomic library. This study notably revealed N- and C-terminal domains that turned out to be instrumental to explain natural silk biofabrication.
The molecular cloning approaches highlighted above identified the main modular peptide repeats governing the wide diversity of mechanical properties of silks, which subsequently became important tools in the biotechnology of artificial silk proteins. However, the molecular strategy controlling the ability of spiders to stockpile precursor proteins at high concentration prior to rapid spinning and solidification remained unknown for another decade. Many efforts to recombinantly express silk proteins resulted in irreversible protein aggregation,74,75 partly because they were designed from shorter and incomplete cDNAs made of the central repeat regions of silks. Two breakthrough studies published in 2010 provided critical answers to this question. Thus, Hagn et al.76 solved the three-dimensional (3D) structure of the non-repetitive C-terminal (CT) domain—which is highly conserved across species—by solution NMR. Three key findings were identified in this study. First, the CT exhibited a thermoresponsive LCST, transitioning from the soluble to the aggregated state as the temperature increased. Second, NaCl (present in the ampullate glands) increased protein solubility and regulated the LCST. And third, shear forces were shown to trigger aggregation of oligomeric silk assemblies through partial unfolding of the CT domain, which exposed the hydrophobic repeat region of the silk proteins that formed well-defined β-sheet-rich aggregates. Concurrently, Askarieh et al.77 solved the 3D structure of the conserved N-terminus (NT) domain of spidroin-1 from the web spider (Euprosthenos australis, E. australis) by X-ray crystallography. They found that aggregation of silk constructs containing the NT domain was strongly pH-dependent. The protein transited from a soluble state to an aggregation state as the pH dropped below 7, a behavior fully consistent with the intrinsic pH gradient along the spider’s silk extrusion duct that is maintained by carbonic anhydrase.78 Based on these findings, the role of charged residues in controlling pH-triggered aggregation of silk fibroins was precisely identified both experimentally78−81 and through molecular dynamics simulations.82 A unified model explaining the rapid spinning and solidification of spider silk as a function of pH and ionic gradients was proposed.83 Exploiting these mechanisms, Andersson et al.84 designed a recombinant spider silk construct containing NT and CT domains from two different species exhibiting an unprecedented solubility >500 mg/mL in aqueous solutions. Remarkably, continuous fibers as long as 1,000 m could be spun from 1 mL of this solution (see also Section 4.1). This study exemplifies the need to fully understand the molecular mechanisms of assembly in order to replicate natural material fabrication.
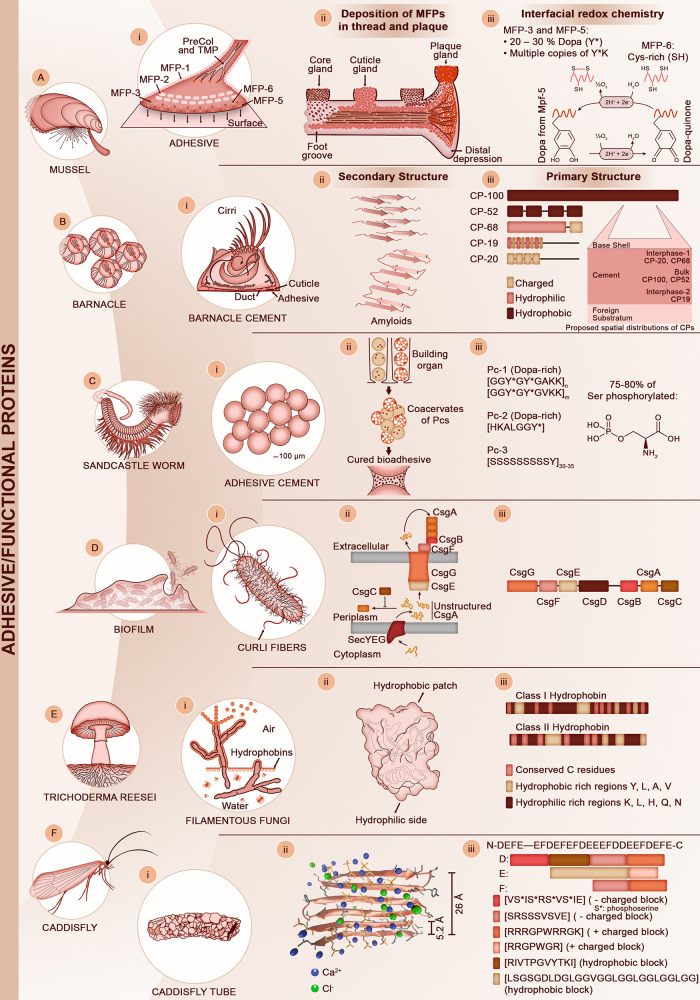
2.4. Discovery and Sequencing of Mussel Adhesive Proteins (1952–2006)
A final illustrative example of a load-bearing biological material that has attracted a lot of attention in the biomimetic community in the past two decades is mussel adhesive fibers (byssal threads). These fibers exhibit multi-functional properties, including underwater adhesion,85 the presence of a protective yet extensible coating86,87 or elastomeric gradients along the threads88,89 to name some of the unique biomechanical features identified in mussel byssus. The byssus is a multi-protein complex containing at least four distinct families of MFPs, namely: (i) adhesive proteins located at the distal extremity and in contact with solid substrates; (ii) collagen-like proteins (CLPs) with silk-like and elastin-like domains that constitute the core of the fibers; (iii) matrix proteins that connect the adhesive to the core; and (iv) cuticle proteins that coat the fibers. Comprehensive reviews on mussel adhesive fibers and their bioinspired applications can be found elsewhere.90−92 As above, we focus here on the meticulous efforts undertaken to obtain a comprehensive picture of mussel thread biochemistry, which were primarily conducted by Waite and co-workers. Identification of β-sheet and collagen-like structural features in byssal threads was obtained as early as the 1950s by X-ray diffraction93,94 and later confirmed by histochemical95 and amino acid96 analyses. Subsequent research focused on morphological and ultrastructural studies of the byssal threads, the mussel foot from which the threads are secreted, and the secretory glands.97,98 In 1980, Waite and Tanzer first reported, using AAA, the unusual presence of the post-translated 3,4-dihydroxy-l-phenylalanine (Dopa) amino acid in the mussel threads,99 and a year later, the same authors identified Dopa (11%), as well as 4-hydroxyproline (Hyp) (13%), in the secreting phenolic glands of the foot,100 which sparked further research over a span of three decades to identify and sequence MFPs. The first sequence data were obtained in 1983 by isolating MFP-1 from the phenolic gland and generating peptide fragments by enzymatic digestion, which were subsequently sequenced by automated Edman analysis.101,102 These studies revealed MFP-1 to consist of multiple repeats of the decapeptide [A-K-P/Hyp-S-Y/Dopa-Hyp-Hyp-T-Dopa-K]. Although MFP-1 was the first mussel adhesive protein for which primary sequences were obtained, it was only in 2005 that its location in the coating of byssal threads was corroborated.103
In 1992, MFP-2 (the most abundant component in the adhesive plaque) was the next protein to be isolated from the mussel foot.104 Automated Edman sequencing of tryptic-digested peptides revealed that it was enriched in Cys, had a lower Dopa content than MFP-1 (5 mol % vs 15 mol % for MFP-1), and contained three distinct types of peptide repeats including Gly- and Tyr-rich penta-repeats as well as a Hyp- and Dopa-rich deca-repeats. The complete full-length sequence of MFP-2 was obtained three years later by Inoue et al.,105 who isolated a cDNA clone encoding MFP-2. This study revealed MFP-2 to be a 45 kDa protein consisting of 11 tandem repeats of an epidermal growth factor (EGF) stabilized by disulfide bonds. Another protein from the adhesive plaque, MFP-3, was next to be sequenced.106 MFP-3 is characterized by a high polymorphism, with high variability in Dopa content between polymorphs (from 5 mol % to 20 mol % and MWs ranging from 5 to 7.5 kDa). Owing to its low MW, the entire sequence of a MFP-3 polymorph could be achieved by thoroughly mapping enzymatically digested peptides obtained from purified MFP-3, whose sequences were deduced from automated Edman sequencing, tandem mass spectroscopy (MS/MS), and Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) mass spectroscopy. In contrast to MFP-1 and -2, MFP-3 is not made of repetitive motifs. The complete sequences of the other main plaque proteins (MFP-5 and MFP-6) were obtained in 2001107 and 2006,108 respectively, by combining molecular approaches and peptide mapping. In short, a cDNA library of the mussel foot was prepared and was probed with peptides isolated directly from the adhesive plaque or from the mussel feet. In addition to the high level of Dopa modification (up to 30 mol %), MFP-5 is also phosphorylated. In contrast, MFP-6 has a low content of Dopa but a relatively high amount of Cys and was later demonstrated to protect Dopa residues in MFP-5 from being oxidized into the less adhesive Dopa-quinone form.109
Finally, the family of MFPs constituting the core of the byssal threads are the prepolymerized collagens (PreCols), which were isolated and sequenced from 1995 to 1998.88,110−112 Partial sequences of PreCol-D (located in the distal region of the thread) and PreCol-P (located in the proximal region) were initially identified by automated Edman sequencing of peptide fragments obtained from purified PreCols.110 A cDNA library from a mussel foot was later prepared and screened by polymerase chain reaction (PCR) using primers designed from the partial sequences of PreCols.88,111 These studies revealed that the PreCols are 230 to 240 kDa block copolymer type proteins consisting of a central collagen-like domain flanked by either rigid silk-like domains (PreCol-D) or more flexible elastin-like domains (PreCol-P), thus explaining the early collagenous X-ray diffraction patterns obtained by Rudall.94 These PreCols are distributed in a graded fashion along the thread88 such that the proximal region is mechanically stiffer whereas the distal region of the threads is more compliant (see Section 3.2.3). A third PreCol (PreCol-NG) was subsequently identified and proposed to be evenly distributed along byssal threads.112
Biotechnological efforts to produce recombinant versions of MFPs started in the early 1990s in expression systems such as Escherichia coli (E. coli) and yeast,113,114 but the yields were extremely low. The first successful recombinant expression of a MFP in E. coli was reported in 2004 by Hwang and co-workers,115 who were also able to modify Tyr residues into Dopa by treating MFP-5 with mushroom tyrosinase.
2.5. High-Throughput RNA-Sequencing and Proteomics (2013–Present)
Unsurprisingly, obtaining the complete primary structures of extracellular, highly insoluble proteins has been intimately linked to technological advances. As highlighted above, early efforts in the 1950s started by obtaining the amino acid composition of structural proteins. The development of stepwise N-terminal degradation by Pehr Edman116 and the subsequent automatization of the method117 were quickly adopted by the community, leading to the discovery of partial peptide sequences in elastin,37,41 resilin,26 silks,69 and MFPs.101,102 However, obtaining the full primary structure of these proteins remained an elusive target. This changed in the late 1980s/early 1990s with advances in molecular biology, exemplified by tools such as cDNA libraries, the increasing availability of a broad range of molecular cloning methods, and the rapid development of PCR, which collectively greatly enhanced our ability to obtain the primary structures of structural proteins. Progress in proteomic mass spectroscopy techniques, such as electrospray ionization mass spectroscopy (ESI-MS),118 MS/MS,119 in-gel digestion,120 and MALDI-TOF,121−123 also considerably improved identification of peptide fragments from the precursors or processed tissues.
At the beginning of the millennium, the genomic revolution made an immediate impact. In the case of resilin, for example, probing the genome of the fruit fly with known partial sequences was sufficient to identify the resilin gene.27 Genomic libraries were also critical to obtain the full sequences of silk-encoding genes. Because of their large size, extreme modularity, and codon-bias, sequencing these genes proved highly challenging to achieve even with the full battery of molecular cloning techniques. For example, despite decades of research, it was only in 2007 that the full-length sequence of a spider silk protein was published.73 Nevertheless, significant challenges remained. First, owing to the inherent structural functionality of biological materials and their exposure to aggressive external environments, extracting proteins or even shorter peptide fragments is exceedingly difficult. Harsh solvents or chemicals are usually employed, such as hexafluoroisopropanol (HFIP) for silk fibers or strong acids for sclerotized tissues, often leading to moderate extraction yields. Second, the complete genomes of many model organisms that serve as a source of inspiration in biomimetic research have not been sequenced.
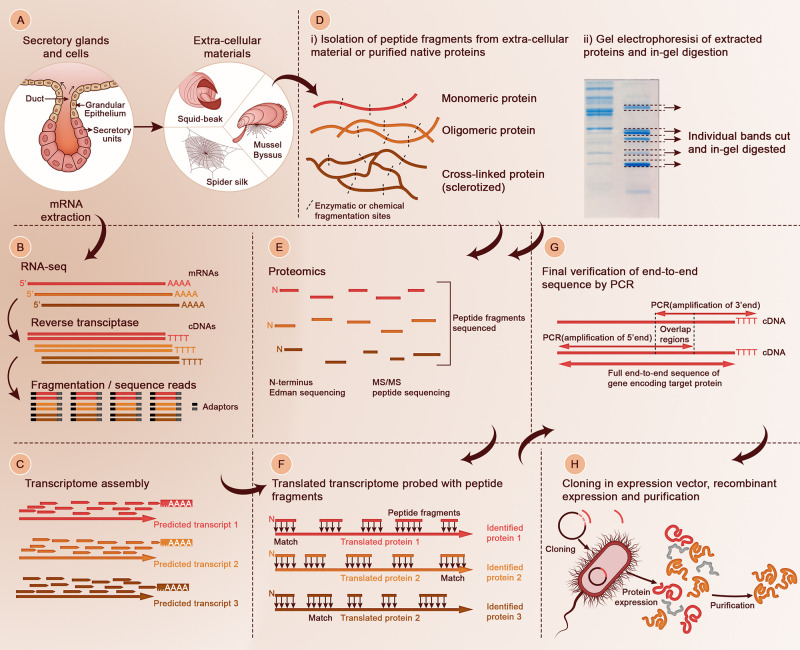
In the early 2010s, next-generation sequencing (NGS) methods were another technological leap that greatly expediated genomic and transcriptomic studies.124 Among NGS technologies, RNA-sequencing (RNA-seq) has had a major impact in accelerating molecular discoveries for biomimetic research. A detailed description of RNA-seq technologies is beyond the scope of this review and can be found elsewhere,125−127 and we only provide a quick overview. Briefly, mRNAs are extracted from specific tissues or from single cells128,129 in the latest developments (Figure 2A). A cDNA library is then constructed by reverse transcriptase (RT), followed by fragmentation into small reads 30–400 base-pairs long (depending on the specific sequencing equipment), to which universal adaptors are added to both ends (Figure 2B). Alternatively, fragmentation can first be carried out on the isolated mRNAs before RT. Each short cDNA read is then sequenced on high-throughput sequencers. In the final stage, reads are aligned to a reference genome or assembled de novo using bioinformatic tools.130 This latter feature is particularly attractive in basic biomimetic research as many model organisms have yet their genomes to be sequenced.
Figure 2.
Overall approach to obtain complete sequences of load-bearing structural proteins. (A) Extraction of mRNAs from the cells and glands where proteins are stockpiled prior to secretion in the extracellular milieu. (B) Construction of cDNA library from mRNAs extracted in the secretory cells and glands. (C) Transcriptome assembly. (D) Isolation of proteins or peptide fragments from the biological material of interest (i) and gel electrophoresis of isolated compounds (ii). In-gel digestion of isolated proteins or protein fragments is carried out for subsequent proteomic studies. (E) High-throughput proteomic of digested peptides from (D). In the past decade, tandem mass spectroscopy (MS/MS) has been the most-used tool, but more traditional tools such as N-terminus sequencing can still provide highly valuable information. (F) Peptide fragments sequenced by proteomic methods are probed against the translated transcriptome using bioinformatic tools. (G) The full sequences of the candidates genes of interest coding for identified proteins in (F) are verified by RACE PCR. (H) The newly identified proteins can be cloned in suitable vectors and recombinantly expressed, for example in E. coli (shown), yeast, or other types of host.
In 2013, we first demonstrated how RNA-seq, in combination with high-throughput proteomic tools, drastically fast-tracked the discovery of proteins constituting the building blocks of biological materials or regulating biofabrication processes such as biomineralization.131 The approach consists in assembling a transcriptome of the secreting glands (or epithelial tissues) containing the cells that secrete the precursors proteins using the Trinity suite developed by Grabherr et al.,132 which allows researchers to assemble a transcriptome from RNA-seq data without a reference genome (Figure 2C). In parallel, proteins from the mature structure or from the secreting glands are extracted and purified (Figure 2D). In many cases, owing to the densely cross-linked nature of extracellular tissues, intact proteins cannot be isolated. However, peptide fragments isolated by harsh chemical degradation can be sufficient, as long as their sequences can be identified by MS/MS (Figure 2E). Alternatively, extracted proteins from the mature tissues can be separated by gel electrophoresis. Separated protein bands are then cut from the gel, in-gel digested with different enzymes,120 and subjected to MS/MS. Traditionally, MS/MS relied on available databases to match MS/MS spectra to known peptides, but here again this approach is not suitable for proteins obtained from organisms with unknown genomes. Thanks to de novo peptide sequencing bioinformatic tools such as the PEAKS software133 or other propriety softwares developed in conjunction with MS equipments, peptide sequencing from MS/MS spectra has been greatly facilitated. Finally, the translated transcriptome can be uploaded as a reference database in these softwares and probed with the sequenced peptide fragments to identify the target proteins (Figure 2F). For highly expressed proteins from extracellular tissues, the full-length transcripts are usually assembled and a small fraction of peptide coverage is sufficient to unambiguously identify the full-length protein. However, structural proteins often have highly repetitive sequences, and in such cases the predicted transcripts are usually fragmented, precluding identification of the complete protein sequence. In such cases, the entire full-length protein is obtained by Rapid Amplification of cDNA Ends (RACE)-PCR, using designing primers based on the partial transcripts and using the cDNAs as a template (Figure 2G). Once the entire end-to-end sequence of the target protein has been obtained, its encoding gene can be cloned in expression vectors, expressed, and purified for downstream applications (Figure 2H), as discussed in Section 4 of this review.
This high-throughput RNA-seq/proteomic integrative platform has enabled the complete identification of dozens of unknown proteins from biological materials in just a few years, an endeavor to be compared with the decades of research it took to sequence other structural proteins of biomimetic interest (Figure 1). For example, Guerette et al.131 sequenced most MFPs from the Asian green mussel (Perna viridis, P. viridis) as well as the native tyrosinase enzymes that convert Tyr to Dopa in MFPs. Other newly identified load-bearing proteins of interest included coiled-coil proteins from the egg case membrane of marine snails that exhibit unique elastomeric properties. In a follow-up study,134 we revealed that the hard sucker ring teeth (SRT) that line up the tentacles and arms of squids135 are entirely made of a family of structural proteins, dubbed “suckerins”, and fully sequenced the 21 suckerins comprising SRT of the jumbo squid (Dosidicus gigas, D. gigas), as well as 17 suckerins from SRT of other species of squids. More details on the primary structure design of suckerins are presented in Section 3.4.4. Using the same approach, Tan et al.136 were able to elucidate the complete sequences of the proteins that constitute the squid beak,137,138 a very hard, yet unmineralized structure whose densely cross-linked nature138 had until then precluded any sequence identification and is discussed in Section 3.4.3. In this specific case, the chemical hydroxylamine was used to liberate short peptide fragments from the beak as other harsh chemicals or enzymes failed to release peptide fragments suitable for MS/MS analysis. As another example of the versatility of the approach, Amini et al.139 obtained the complete sequence of the main protein that regulates mineralization of the mantis shrimp dactyl club, an impact-resistant hunting appendage140−144 that has generated much interest in the bioinspired materials community in recent years.145,146
The integrative RNA-seq/proteomic approach has been adopted by other researchers to identify and characterize extracellular structural proteins of interest and has been particularly successful in studies of biological adhesives,147,148 For example, Hennebert and co-workers149 sequenced a sea star footprint protein that is secreted by sea star tube feet to mediate adhesion to solid surfaces, while Wunderer et al.150 sequenced the adhesive proteins involved in the temporary adhesion of the flatworm. Likewise, DeMartini et al.151 identified new MFPs from the Californian blue mussel by conducting transcriptomic analyses of the different glandular tissues that express and secrete MFPs. Altogether, the advent of high-throughput omics methods has undoubtedly opened unprecedented opportunities in the discovery of structural proteins, which once reproduced artificially may have the potential to replace polymers derived from fossil fuels in various applications, as discussed in Section 4.
3. Molecular Design of Natural Protein-Based Materials
3.1. Fibrous Proteins
3.1.1. Silk Fibroins
Silk-producing arthropods have evolved to produce fibers whose designs and functions are as diverse as their material needs, with a broad range of mechanical responses, from highly extensible and elastomeric-like to stiff and strong.152 The variety of uses for silk among spiders is much larger than that among other insects such as silkworms which mainly use it to make cocoons.13 Orb-weaving spiders, in particular, produce up to seven different types of silk, as well as a glue substance for various ecological functions, each of which is expressed and secreted in specific glands (Figures 3A-i to 3A-vii).153,154 Mechanically strong silks including the dragline and auxiliary silks are produced in the major and minor ampullate glands, respectively, whereas viscid and aciniform silks produced in the flagelliform and aciniform glands are weaker but show substantially greater extensibility. In addition, female spiders secrete very special silk from their cylindriform glands as a protective eggs sac.83 Also, spiders have evolved to produce a rather unexplored set of adhesive silks, namely pyriform adhesive silk and aggregate silk glue, which can outperform man-made cyanoacrylate-based glues in certain applications.155,156
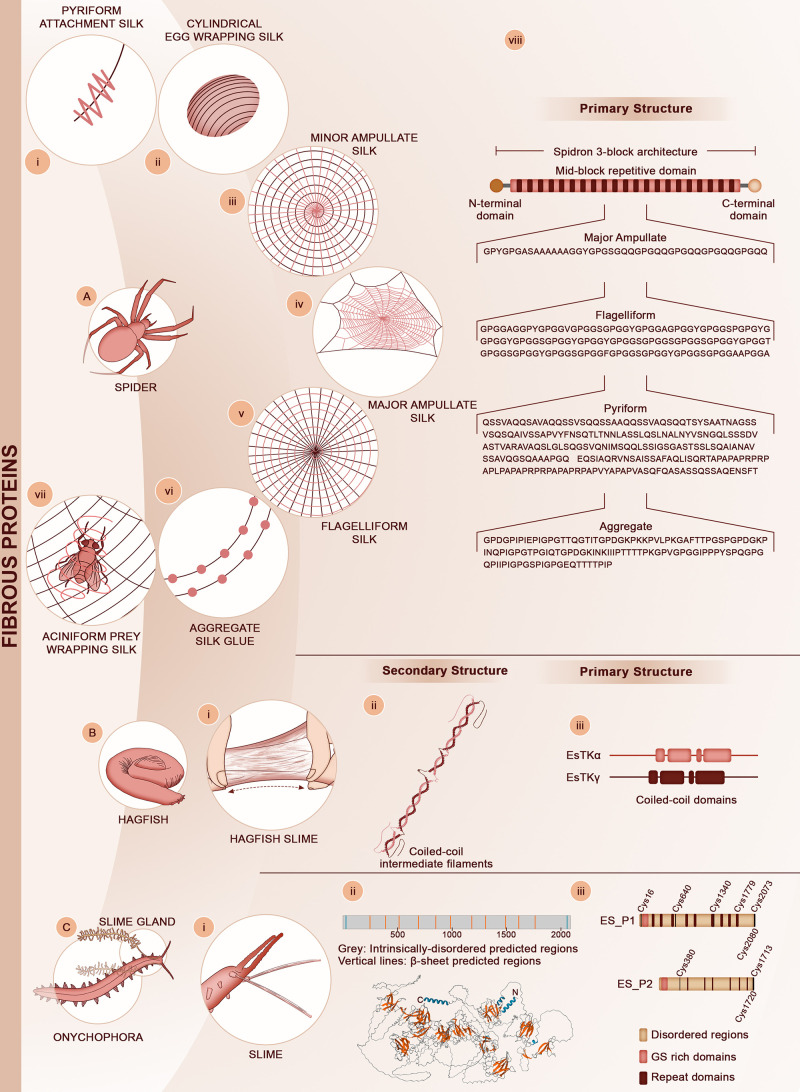
Figure 3.
Fibrous protein materials. (A) Orb-weaving spiders can produce up to seven different silk types, namely (i) pyriform attachment silk, (ii) cylindrical egg wrapping silk, (iii) minor ampullate silk, (iv) major ampullate silk, (v) flagelliform silk, (vi) aggregate silk glue, and (vii) aciniform prey wrapping silk. Schematic representation of the 3-block spidroin architecture consisting of the non-repetitive N- and C-terminal domains and the long central repetitive block, which is shared among all silk types. (viii) Small portions of the amino acid sequences for the repeating units of four major silks described in the text are presented: Araneus ventricosus Dragline spidroin-1 (UniProtKB - A0A090BQB1); Latrodectus hesperus Pyriform spidroin-1 (UniProtKB - C7T5D2); Nephila clavipes Flagelliform spidroin-1 (UniProtKB - Q9NHW4); Latrodectus hesperus Aggregate glue spidroin-1 (UniProtKB - A0A140DL44). These sequences are from refs (157−160), respectively. (B) Hagfish (here the pacific hagfish (Eptatretus stoutii is represented) secrete a two-component viscous slime when threatened by predators. (i) The tough slime is made of mucins and fibrous proteins. (ii) The proteins are heterodimeric coiled-coil intermediate filaments. (iii) Primary architecture of the two coiled-coil proteins EsTKα and EsTKβ. The solid rectangles represent the coiled-coil regions. Adapted with permission from ref (161). Copyright 2017 Royal Society of Chemistry. (C) Velvet worms (onychophora) swiftly eject (i) proteinaceous fibers to capture their prey. Adapted with permission from ref (162). Copyright 2018 American Chemical Society. (ii) The fibers consist of a multi-protein complex, and the dominant proteins in the complex are large MW proteins (180–250 kDa) that are mostly disordered in solution, with a few short β-sheet domains predicted in silico. (iii) The primary structure of the main slime proteins can be divided into three main domains, i.e. long disordered domains, low sequence complexity domains enriched in Gly and Ser at the N-terminus, and interspersed repeat domains 20–30 amino acid long. Adapted from ref (163). Creative Commons CC BY.
The mechanical properties such as adhesion, strength, toughness, and elasticity are highly tunable and uniquely altered by spiders depending on the environmental conditions and ecological needs.164 For example, dragline silk is stiff and has a high ultimate tensile strength in dry conditions but supercontracts and becomes elastomeric in the hydrated state.165−168 Such a diverse range of function and material performance is based on the interplay of (1) highly optimized molecular architecture; (2) intermixing of various building blocks; (3) hierarchical structure–property interactions at multiple length scales; and (4) unique physiochemical processing steps.83 The main types of silks and the link with their molecular architecture are briefly discussed below.
3.1.1.1. Dragline Silk
Spidroins, the constituents of all spider silks, are a family of structural proteins characterized by a 3-block protein architecture with an overall MW of 250–350 kDa (Figure 3A-viii).169 It includes an exceptionally long and highly repetitive central section (midblock) flanked by two relatively small and highly conserved globular N- and C-terminal domains.83 This molecular architecture is shared among all the spidroins and is critical to enhance solubility and prevent premature crystallization/aggregation.77,170−172 While the midblock provides the basis for the diversity of function and material properties of the different silk types, the terminal domains remain highly conserved and play central roles in the end-to-end polymerization of the spidroins.83
The core region of the load-bearing spidroins is rich in hydrophobic poly-Ala and hydrophilic Gly residues (Figure 3A-viii).169,173−176 The sequence arrangement highly resembles the architecture of a diblock copolymer in which 8–10 consecutively arranged Ala stretches are segregated from the Gly-rich blocks. The poly-Ala repeat motifs form about one-quarter of the sequences. In contrast, poly-Gly motifs are the dominant feature in the sequence. During spinning, Ala-rich sequences undergo conformational transitions into the relatively short anisotropic β-crystallites that are needed to provide strength and stiffness. Another key feature of the Gly-rich block is the high Pro content. Pro and Gly are known to be secondary structure breakers. Therefore, the presence of both contributes to the formation of largely expanded isotropic amorphous structures that provide extensibility and resilience to the fibers.169,173−176
3.1.1.2. Flagelliform Silk
Flagelliform silk, also commonly known as viscid silk, is the most elastic and extensible silk fiber.177 It forms the capturing spiral treads (Figure 3A-v). As the name suggests, the impact of the prey on the web is mainly dissipated through flagelliform silk. The midblock region of the viscid spidroin completely lacks the presence of any Ala residues (Figure 3A-viii).178 Instead, the core sequence is almost entirely formed from poly-Gly repeating motifs and Pro. In a most simplistic view, flagelliform is dragline silk with no crystalline domain and a 100% amorphous domain. This results in a substantially high elastic and stretchable response as high as 1000% but also in supercontraction when hydrated to 50% of its original length.178
3.1.1.3. Adhesive Silk
While pyriform and aggregate silk glues remain the least studied silk types, they can be considered as one of the most unique biological glues.179−181 The pyriform adhesive is made from fast-curing sticky fibrils (Figure 3A-i). It is brushed into elaborated patterns that fasten dragline silk threads tightly to the substrates using a minimal amount of materials. It can form strong adhesion even to surfaces with low surface energy, such as Teflon. The most striking difference between pyriform spidroin and other spidroin family members is the lack of the commonly available repeating motif rich in Gly or Pro (Figure 3A-viii). In addition, the pyriform repetitive mid-blocks contain sequence motifs with distinctive stretches of polar and charged amino acids including Glu, Arg, and Gln.158 The abundance of such charged residues enables desirable properties such as high water solubility of the spidroin, strong interaction with the substrate, and self-assembly to fibers. Furthermore, the pyriform exhibits much shorter poly-Ala stretches with only three consecutive Ala residues.
It is important to note that pyriform adhesive is uniquely optimized to be used on dried surfaces and lacks performance on semi or fully hydrated surfaces.158 In contrast, the aggregate glue is a wet adhesive with the function to retain prey upon impact with the web (Figure 3A-vi).182 It is completely different from all the other spider silks. Instead of forming solid filaments, it tends to form specific suspended viscoelastic amorphous microdroplets on the axial capture spiral threads.183 Its arrangement highly resembles beads on a string. The aggregate glue exhibits strong hygroscopic features with an interior glycoprotein core and an exterior aqueous shell. The mid-block exhibits low complexity and a highly repetitive nature similar to other spidroins. While its sequence is less biased toward Gly, Pro, and Gln, Ala residues are almost absent from the mid-block (Figure 3A-viii).156 What makes the mid-block distinctly different from that of any other spidroin is that it has a substantially higher content of Ser and Thr. These are found to be prone to O-glycosylation and directly reflect the hygroscopic properties, as well as the stickiness of the aggregate glue.
3.1.2. Hagfish Filaments
Hagfish (Figure 3B) are ancient benthic fish that have evolved a peculiar defense mechanism against predators. When threatened by predators (for instance sharks), specialized cells located on their epidermis evict a viscous slime that rapidly swells upon contact with seawater and can clog the gills of the attacker.184 The slime (shown in Figure 3B-i) is mechanically robust as well as slippery. In an infamous example featured in the popular press,185 containers of hagfish spilled onto a highway in Oregon, leading to chain-reaction crashes of multiple vehicles. The culprit was the hagfish slime that was rapidly released by hundreds of scared hagfishes that fell on the road, rending it slippery. The slime is composed of two main components secreted by two specific sets of gland thread cells,186 namely the mucus and proteinaceous fibers. The mucus is secreted by gland mucus cells (GMCs) and composed mostly of proteins (77% dry weight), carbohydrates (12%), lipid (5%), and sulfate (6%). Whereas the sequence of the mucus proteins has remained unknown to date, some authors have suggested on the basis of these data that the mucus is made up of glycoproteins. However, what is clear is that mucin vesicles from the mucus swell very fast upon encountering seawater, with swelling occurring as fast as 100 ms in some species.
The second component of the mucus, secreted by the gland threads cells (GTCs),187 are proteinaceous fibers188−190 made of keratin-like fibers first sequenced by Koch and co-workers.191 Slime proteins’ molecular architectures are reminiscent of intermediate filaments (IFs) from the cell’s cytoskeleton:191,192 they form long α-helical coiled-coil rods intervened by flexible linkers that self-assemble into nanofilaments193 as illustrated in Figures 3B-ii and 3B-iii (keratins also belong to IFs, which is why hagfish threads are referred as “keratin-like”). In the Pacific hagfish Eptatretus stouti (E. stouti), the filaments are heterodimers comprised of two proteins called E. stoutii thread keratins α (EsTKα) and γ (EsTKγ).
In the native state, synchrotron WAXS has revealed that hagfish threads exhibit an α-helical coiled-coil to β-sheet transition (α → β transition) when the whole slime is strained in the dry state.193 The resulting β-sheet-reinforced fibers exhibit remarkable mechanical properties that rival silk and approach Kevlar, with an elastic modulus as high as 8 GPa, an ultimate tensile strength (UTS) of ca. 700 MPa, and an energy to failure approaching 200 MJ m–3.194 These elevated mechanical properties are a direct consequence of the presence of β-sheet nanocrystals that are also the source of the remarkable mechanical performance of spider silks.195
3.1.3. Velvet Worm Slime
Velvet worms are ancient terrestrial carnivores that are classified within their own phylum, the Onychophora (which means “claw bearers” in Greek).196 They are divided in two families, the Peripatidae found in tropical and subtropical regions and the Peripatopsidae distributed in more temperate climates. Both families of velvet worms employ a peculiar hunting strategy: they swiftly evict a stream of sticky proteinaceous fibers (the slime) from their oral papillae, which undergo a rapid liquid-to-solid transition, thus hardening in a few seconds to capture and immobilize their prey (Figure 3C). The slime is initially stored as a highly concentrated protein solution in elongated slime glands (Figure 3C-i),197 and solidifies first into a gel and eventually into stiff fibers exhibiting a stiffness on par with Nylon. Early studies197 proposed the slime to be made of collagenous proteins based on the high content of Pro amino acid. This hypothesis was unambiguously disproved by Haritos and co-workers,198 who obtained partial sequences of the main slime proteins by constructing a cDNA library of the slime gland and probing it with tandem MS/MS of the slime. This study resulted in the identification of fragments of Pro-rich (20 mol %) proteins (with MW > 200 Da based on SDS-PAGE) with low sequence complexity predicted to be largely unstructured. The classical sequence pattern of collagens (GX1X2)n where X2 is usually Pro was not observed in these sequences. However, the full-length sequence was not achieved. That study also established that the mature slime contains additional mid-size MW (∼55–65 kDa by SDS-PAGE) as well as low MW (<20 kDa) proteins.
In a series of studies, Baer and co-workers provided first insights into the biofabrication process of slime fibers. This team observed that drawn slime fibers are made of a mostly proteinaceous core covered by a sheet of nanoglobular protein/lipids complexes.199 Upon prolonged exposure to water they also showed that the slime could be fully redissolved, forming a colloidal suspension made of near-monodispersed nanoglobules about 50 nm in diameter as detected by AFM and DLS studies, and new fibers could be drawn from the dissolved colloidal solution. Lipids were proposed to stabilize slime proteins into soluble complexes, thereby preventing aggregation. During fiber drawing, the protein/lipid nanoglobules were suggested to segregate on the outside coating whereas the core of the fiber is mostly protein-based. This biofabrication process thus provides remarkable “green processing” lessons, because strong fibers are drawn from an aqueous solution under ambient conditions with minimum postprocessing. In a follow-up study,162 the same team established that some of the proteins are phosphorylated and proposed that the zwitterionic nature of the slime proteins (with alternate positively charged and negatively charged patches) may facilitate fiber formation by electrostatic interactions, with phosphorylated residues mediating assembly by electrostatic complexation with divalent ions such as Ca2+ and/or Mg2+. Using WAXS, Fourier transform infrared spectroscopy (FTIR), and Raman spectroscopy, Baer et al.200 also detected the presence of β-sheet crystallites in the crude slime, protein droplets, and dried fibers. On this basis, they suggested that slime proteins may be stored as β-sheet-containing droplets that transition first into an intermediate viscoelastic gel and then into the final solid fibers by water loss.
Very recently, using the integrative transcriptomic/proteomic approach highlighted in Figure 2, our team obtained the complete sequences of the major slime proteins from a species (which is yet to be fully identified) from the Peripatidae family.163 This study revealed that proteins detected in the high-MW region by electrophoresis are in fact multi-protein complexes linked by intermolecular disulfide bonding, which are dominated by two high-MW proteins called Eoperipatus slime proteins 1 and 2 (ES_P1 and ES_P2) and linked to mid-MW Eoperipatus slime proteins 5 and 6 (ES_P5 and ES_P6). The primary structures of ES_P1 and ES_P2 unveiled four noteworthy features that may be critical for slime fabrication, as highlighted in Figure 3C-ii and iii. First, using bioinformatic tools for intrinsically disordered proteins (IDPs), they were predicted to be largely disordered as previously envisioned (Figure 3C-ii).198 However, it is noteworthy that predictions with AlphaFold201 supported the ability of both proteins to locally acquire short β-sheet structures. Second, both proteins contain repeat motifs (30 and 20 amino acids long for ES_P1 and ES_P2, respectively) made of basic dipeptides (RR, RK, and KK), short Pro-rich motifs (PP, IP, IIP, PI, and IP), as well as single acidic residues that are however found only in the repeats of ES_P1 (Figure 3C-iii). Third, the N-termini contain low complexity (LC) domains dominated by Gly and Ser amino acids (GS-rich domains, Figure 3C-iii). And fourth, Cys residues are mostly segregated at the termini of the primary structures. Despite their low abundance, Cys residues are likely to play a key role in slime assembly, by providing sites for disulfide bonding that maintain the multi-protein complexes together. Interestingly, the LC domains of the N-termini share homology with the fused in sarcoma (FUS) RNA-binding protein, a ribonucleoprotein that is well-known to exhibit LLPS.202,203 A recombinant construct of this domain for ES_P1 was established to exhibit LLPS, indicating that the N-termini of ES_P1 and ES_P2 may also promote LLPS, enabling the slime proteins to be concentrated in the gland while preventing premature aggregation, which would impede efficient slime ejection.
Slime proteins are also characterized by a high occurrence of PTMs, including phosphorylation, glycosylation, as well as Pro hydroxylation. These modifications must undoubtedly play an important function in slime biofabrication and properties, but their roles to date remain speculative. Baer et al.162 have suggested that phosphorylation of slime proteins could help mediate nanoglobules’ charge–charge attractive interactions with positively charged domains as well as forming ionic bridges with divalent cations (Ca2+ and Mg2+). Solid state NMR of the slime suggested that glycosylation occurs on Ser residues primarily located in flexible regions of the slime, and an adhesive function—which would help prey capture—has been proposed, similar to the case of pyriform silk. With regard to Hyp, it is known to increase the thermal stability of collagens and other structural proteins, but here again, it is yet unclear why ES_P1 and ES_P2 contain a high abundance of Hyp. Overall, while the full-length sequence of the large slime proteins is a critical first step to understand the biofabrication of the slime, much remains to be done to understand the liquid-to-solid transition as well as how the sequences are correlated to the mechanical properties of the slime. Open questions include the role of PTMs, namely phosphorylation, glycosylation, Pro hydroxylation, and disulfide bonds. The role of lower MW slime proteins is also unknown. As we have highlighted for spider silk and mussel adhesives, understanding the primary structure/property relationships of these proteinaceous fibers has been ongoing for decades and researchers are still discovering important biochemical and structural features associated with biofabrication.204−206 The slime of the velvet worm is as complex as these extracellular fibers and will require similar research efforts to achieve the same level of understanding.
3.1.4. Comparative Overview
The fibrous proteins described in this section display rather diverse primary sequences but also structural conformations (Figure 3). This can be comprehended considering that they originated from organisms that are evolutionarily distinct and that operate in significantly different environments. Thus, their sequences are evolutionarily optimized for very specific ecological functions. Nevertheless, despite this broad diversity, they share several similarities at the molecular scale as well as in terms of biofabrication conditions. The key function of these fibrous proteins is mainly to capture prey or as a line of defense against predators. Thus, the survival of the organism relies on the activation of the materials in a matter of seconds. Here, activation is referred to as the transition of the preassembled building block from a liquid or a weak gel phase to a solid phase that can be rapidly deployed by the animal. This hierarchical process originates at the molecular scale and primarily involves conformational transitions from random coils or α-helices to β-sheets during the physicochemical processing steps that harden the material in a matter of few seconds. Broadly speaking, this process can be divided into five key steps, starting from gland secretion and ending with the cured material:
-
(i)
The first insight is that expression of the core proteins takes place in very dedicated secretion glands. Expression is seemingly followed by PTMs such as phosphorylation, hydroxylation, and glycosylation. It has become distinctively evident with advances in non-invasive molecular characterization methods that almost all of these glands co-express smaller proteins, glycoproteins, or polysaccharides and secrete lipids or waxes with important functions during storage or processing and to optimize the material functionalities. PTMs as well as co-expression of other polymers seem to be a common molecular strategy enhancing solubility and preventing premature crystallization/aggregation that would clog the glands. The other key function of PTMs is related to fine-tuning the material properties by altering the hydration level of the fibers after curing. Furthermore, spider silk and velvet worm adopt a similar strategy of producing large MW complexes from the core proteins, which is carried out by covalently linking the extremities of the proteins via disulfide bridges using Cys residues localized at the terminal regions. The resulting higher aspect ratio complexes significantly enhance the mechanical performance of the final materials compared to the monomeric building blocks.
-
(ii)
The second commonality is that the colloidal solutions in the glands are stored at high concentrations (25–50% w/v) by actively pumping water molecules out and increasing the overall biopolymeric mass during storage. This results in the preorganization of the components in the slime or the spinning dope into intermediate assemblies that are crucially important for fiber formation. The nature of this assembly state is not completely understood. Two theories have been put forward associated with their micellar or liquid crystal nature; however, neither can fully explain the complexity of the assemblies. A more comprehensive mechanism has recently been formulated based on coacervation, which is built upon multiple pieces of evidence that under controlled conditions, most naturally occurring structural proteins can undergo LLPS, resulting in the formation of condensed liquid droplets. Thus, there is growing awareness that LLPS of structural proteins represents a ubiquitous transient mechanism underlying structural assemblies toward the fabrication of natural structural materials.
-
(iii)
The glands of these organisms are very sophisticated machineries In addition to being able to pump water out, they have the capability to simultaneously change the solution conditions, such as altering the pH and tuning the cationic/anionic balance to modulate the solubility of the core proteins. As described earlier, divalent Ca and Mg ions can form salt bridges between acidic amino acids and cross-link the velvet worm slime core proteins. A similar chemistry may be expected during the formation of IFs in the slime-producing glands of the hagfish. The major difference is that, in addition to the co-secreted ions, the mechanical properties of the filaments may be quickly adjusted upon encountering seawater due to the abundance of divalent ions that can be taken up from the environment. The process can be facilitated as the filaments may initially adopt a more expanded conformation at the higher pH (8–8.2) of seawater. Furthermore, an added advantage for the velvet worm is that the addition of ions is an energetically favorable strategy to increase the slime viscosity without increasing the polymer content, which may enable researchers to optimize the distance of projection during slime ejection. Spiders, on the other hand, use monovalent ions such as potassium. This has an inverse effect on the viscosity since the spinning dope requires passing through a very long and thin spinning duct, and the biomechanics of the gland is not capable of generating backpressure, which is why the silk spinning is based on a pulling mechanism and not a pushing one.
-
(iv)
Primarily weak hydrogen bonding networks and hydrophobic interactions appear to be the main stabilizing inter- and intramolecular interactions, both in the preassembled state of the core proteins and in the final material. This distinctive characteristic is important not only to create sacrificial bonds for energy dissipation during mechanical deformation but also for self-healing, hydration, and adhesion of the fibers. Charged side chains seem to impart important characteristics to the core proteins by forming ion pairs, but also other less specific electrostatic interactions. This comes with the added advantage that by altering the pH, phosphorylation and dephosphorylation can be modulated, which can significantly contribute to conformational switches.
-
(v)
Finally, mechanical forces (extensional flow, elongation, or shear) all trigger molecular alignment and crystallization of the proteins. For instance, under such conditions spidroins form a classical block copolymer structure, with nanoconfined β-sheets enabling stiffness and rigidity and the amorphous domains providing extensibility and resilience. Moreover, the anisotropic orientation of the β-sheets allows to fine-tune the load-bearing characteristics during uniaxial tension. The slime of the worm seems to adopt a similar transition during self-assembly, with crystallization also directly linked to drying of the fibers. Likewise in the hagfish slime fibers, simultaneous pulling and drying can be used to artificially trigger the formation of β-sheets dominated fibers with an exceptional combination of stiffness, strength, and toughness, although this draw-processing is not directly used by the animal.
3.2. Elastomeric Proteins
The ability of some tissues to sustain large reversible deformation is critical for their biological function. These elastomeric properties are provided by proteins that are usually in a random-coil conformation and cross-linked into 3D networks via side-chain-mediated covalent cross-linking, although as presented below there are some exceptions of proteins that adopt well-defined secondary structures and yet exhibit large reversible elastic deformation. The molecular design of such proteins is presented and discussed in this section. The thermodynamic and polymer physics framework used to describe bioelastomeric properties is based on entropic elasticity, and the detailed formalism can be found elsewhere.11,207,208 Briefly, the retracting force on an elastomeric network f arises from two contributions, namely the internal energy component fU and the entropic component fS, expressed as
 |
1 |
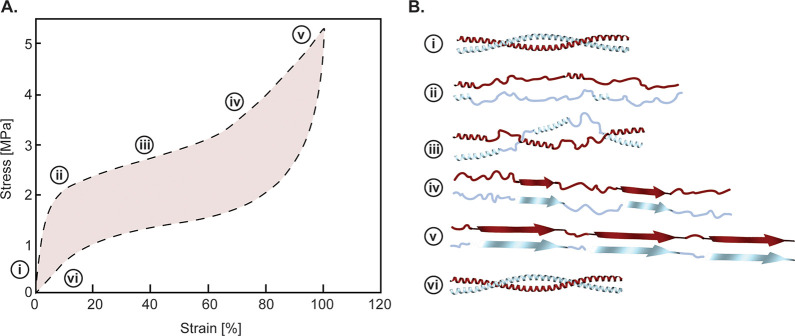
where (∂U/∂L) is the change in internal energy U by unit extension L and (∂S/∂L) is the change in entropy S per unit extension L, which using the Maxwell relationship is equal to the negative change in force f with temperature T, −(∂f/∂T). At high strains, the entropic component fS is larger than the internal energy component fU, hence the term “entropic elasticity”. In elastomeric materials and at a given strain, the force increases with temperature and the thermoelastic coefficient (∂f/∂T) can be experimental obtained from a series of stress–strain curves at different temperatures.11,209 Because the thermoelastic coefficient is positive, a classical result of entropic elasticity is that at a fixed strain, the force on an elastomeric fiber increases with temperature, a characteristic that is useful to differentiate non-entropic bioelastomers described in Section 3.2.4.
3.2.1. Elastin
Elastin is the principal protein responsible for the elasticity of vertebrate structures, notably skin, cardiovascular tissues, cartilage, etc. (Figure 4A-i). As discussed in Section 2.2, the soluble precursor of elastin tropoelastin, a 72 kDa protein which after extracellular secretion is stabilized by hydrophobic cross-links, forms a 3D random network that protects the material against chemical and enzymatic degradation. The primary structure of tropoelastin consists of alternative hydrophobic and cross-linking blocks with low complexity sequence,210 as illustrated in Figures 4A-iii. The hydrophobic blocks are typically made of short (4 to 6 residues long) GVP-rich peptides, such as [GGVP], [PGVGV], or [PGVGVA]. On the other hand, the cross-linking domains contain Lys residues that are flanked either by stretches of poly-Ala on both sides (KA domains) or by the hydrophobic motif [PGVGGL] (KP) domain (Figure 4A-iii).
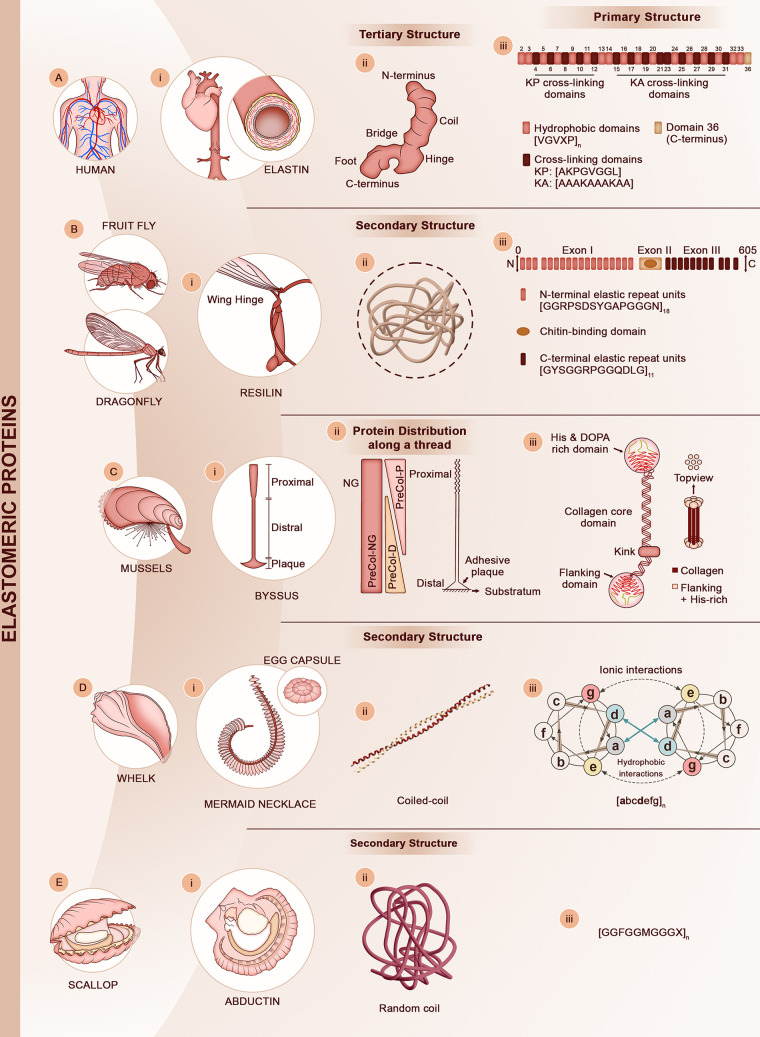
Figure 4.
Bioelastomeric protein materials. (A) Human elastin provides elasticity to vascular organs, lungs, and skin (i). (ii) Despite its high molecular mobility, the tropoelastin molecule (the precursor of elastin) has a defined tertiary structure consisting of an elongated asymmetric coil connected to a foot-like protrusion by a hinge domain. Adapted from ref (47). Copyright 2011 National Academy of Sciences. (iii) The primary structure of tropoelastin is made of alternative blocks of hydrophobic and cross-linking (KA and KP) domains. Representative peptide motifs for each domain are shown. (B) In flying insects, such as the fruit fly and the dragonfly, the hinge of wings is made of the resilin protein (i). (ii) The secondary structure of resilin is largely disordered. (iii) The primary structure of resilin has three main regions. The N- and C-terminus providing elasticity are highly repetitive (consensus motifs shown) while the central region has a Rebers & Riddiford (R&R) chitin binding domain. Adapted with permission from ref (218). Copyright 2012 Springer-Nature. (C) Mussel byssal threads are (i) mechanically graded fibers that transition from a stiff proximal region to an elastomeric distal region. (ii) The core of a thread is made of three main proteins (PreCol-P, PreCol-NG, and PreCol-D) with different spatial distributions along the thread as schematically illustrated. Adapted with permission from ref (89). Copyright 2004 American Chemical Society. (iii) PreCols consist of a central collagenous core flanked by either stiff, silk-like domains (PreCol-D) or flexible, elastin-like domains (PreCol-P) that are packed into regular lattices. The termini of the PreCols contain His- and Dopa-rich domains that enable cross-linking via either metal ion coordination bonds or Di-Dopa covalent bonds (see Figure 5 for details on the cross-linking chemistry). Adapted with permission from ref (219). Copyright 2021 American Chemical Society (D) Marine snails (here a whelk) lay their embryos within (i) elastomeric egg capsules. During deposition, a female will deposit a single string of egg capsules attached to a central filament (mermaid necklace). (ii) Egg capsules are made of coiled-coil intermediate filaments bundling together to form the macroscopic capsules. (iii) Coiled-coil domains are made of heptad repeats [abcdefg] where a and d are hydrophobic residues. (E) In scallop and other bivalves, the adductor muscle is made of the abductin protein (i), which is the only documented bioelastomer to work under compression loading. (ii) Abductin has been found to have random coil structure. (iii) The primary structure consists of multiple repeats of a Gly-rich motif that has a central Meth residue.
The hydrophobic blocks, exemplified by the pentapeptide motif [VGVXP], drive the coacervation process34,211,212 during which tropoelastin concentrates at the cell surface following secretion. At low temperatures, tropoelastin is in the monomeric state, with a network of clathrate water molecules surrounding the hydrophobic blocks and preventing aggregation.33 Above a critical temperature, the H-bonded network of water molecules is disrupted, exposing hydrophobic blocks and triggering aggregation in the form of microdroplets (coacervates). Earlier studies of tropoelastin structural fluctuations during coacervation relied on low-resolution spectroscopy methods and solid-state NMR studies.213−216 More recently, solution NMR combined with magic angle spinning (MAS) solid-state NMR studies of ELPs comprising of alternative hydrophobic and cross-linking domains have refined our molecular understanding of elastin coacervation.217 In the solution (non-coacervated state), Nuclear Overhauser Effect Spectroscopy (NOESY) experiments determined that β-turns of [VPGV] and [GVGV] domains form short-range intramolecular interactions mediated by the proton backbone. After coacervation, the hydrophobic domains retain disordered and transient interactions, but these intermolecular interactions are stronger and attributed to multiple non-specific hydrophobic contacts between Gly, Val, and Pro residues. Furthermore, the cross-linking domains are also disordered. After cross-linking, on the other hand, whereas hydrophobic domains are still disordered, cross-linking domains adopt a helical conformation.
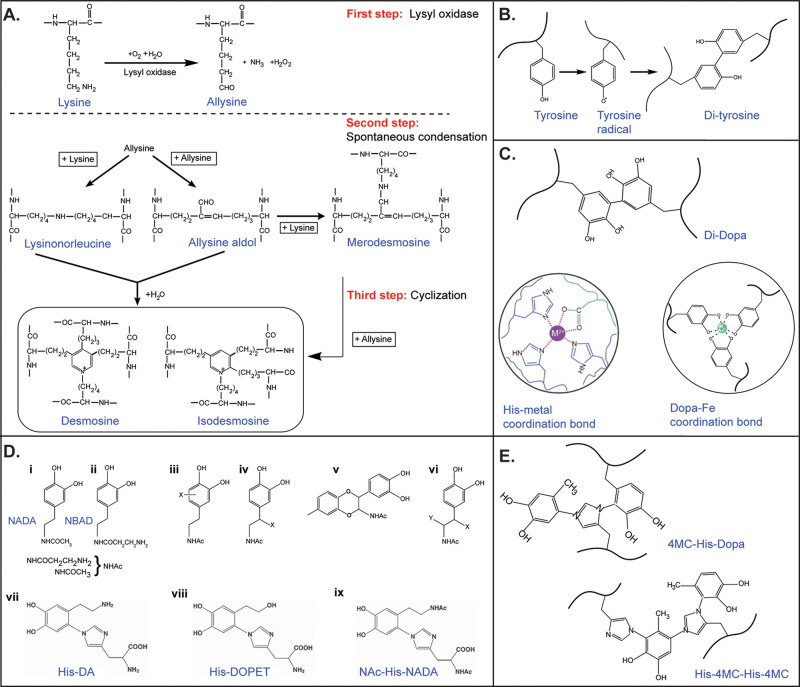
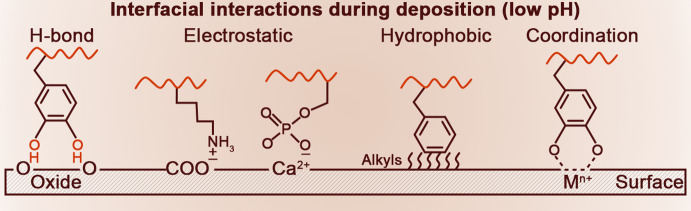
Lys chemistry is central to the cross-linking and stabilization of tropoelastin into a 3D elastomeric network, which occurs in 3 steps displayed in Figure 5A.35,36 In the first step, Lys side-chains are converted by a lysine oxidase (LOX) enzyme to the semi-aldehyde allysine (ALys).220 In the second spontaneous step, either a Schiff base reaction occurs between one Lys and one ALys side-chain to form a lysinonorleucine cross-link or two ALys react through an aldol condensation to give rise to the allysine aldol cross-link. In the third step, the bifunctional cross-links undergo a cyclization condensation reaction to form the final desmosine or isodesmosine cross-links, which can thus be viewed as a four-Lys ring structure. The sequence patterns of cross-link domains are thought to promote the four-Lys ring in the following ways.208 In the KA domain, the tendency of Ala to form α-helical segments leads to the exposure of Lys side-chains to one side of the helix, whereas in the KP domain, the Pro residues may favor a poly-Pro helical segment that may also expose Ala side-chains on one side of the segment. In both cases, the structural conformations result in two pairs of Lys emerging on one side of two helical segments, thus enabling the formation of the tetra-cross-links from the four Lys/ALys side-chains.
Figure 5.
Cross-linking chemistry in various protein-based biological materials. (A) In elastin, cross-linking is initiated on Lys residues that are converted into allysine (ALys) by the lysyl oxidase enzyme (top panel). Spontaneous condensation between Lys and ALys leads to the intermediate cross-links lysinonorleucine, ALys aldol, and merodesmosine (middle panel). In the final step, cyclization condensation results in the cyclic cross-links desmosine and isodesmosine (bottom panel). Adapted with permission from ref (221). Copyright 1998 John Wiley & Sons. (B) Cross-links in resilin are simpler and consist of dityrosine, triggered by enzymatic oxidation of Tyr residues with peroxidase that leads to oxidative phenolic coupling. (C) Cross-links in the PreCol proteins of mussel threads are Di-Dopa and His-Metal coordination bonds. The Dopa-Fe3+ coordination bonds are particularly prominent in the protective coating of the byssus made of MFP-1. (D) In insect cuticles, cross-linking starts by oxidation of Tyr amino acids into Dopa, which is subsequently decarboxylated to dopamine and then acylated to either N-acetyldopamine (NADA) (i) or N-β-alanyldopamine (NBAD) (ii) resulting in acyldopamine derivatives. These compounds are enzymatically oxidized into o-quinones, which are then isomerized to quinone methides that can subsequently react with nucleophiles (X) on the aromatic ring (iii) or with the side-chain (iv) to produce catecholic-based cross-links. Alternatively, cross-links can be formed by reaction of the quinone methide intermediates with another available NADA (v) or other nucleophilic reactants (Y and X, vi). (i-vi) Adapted with permission from ref (222). Copyright 2010 Elsevier. Catecholamine-His adducts with covalent attachment between the imidazole and aromatic rings have also been identified as cross-links in insect cuticles, including His-dopamine (His-DA, vii), His-dihydroxylphenyl ethanol (His-DOPET, viii), and N-acetyl-His-NADA (NAc-His-NADA, ix). (vii-ix) Adapted with permission from ref (223) Copyright 1999 Elsevier. (E) Identified cross-links in squid beak proteins include covalent adducts between His side-chains with either the low MW compound 4-methyl catechol (4MC) or Dopa side-chains. Adapted with permission from ref (138). Copyright 2010 American Society for Biochemistry and Molecular Biology.
In earlier studies, tropoelastin was considered to adopt a fully disordered structure in solution. However, a series of studies by the Weiss and Buehler groups, combining scattering studies with MD and ab initio simulations, have refined this early assumption and found that tropoelastin is not completely disordered. SAXS and SANS investigations47 established that the tropoelastin monomer takes the shape of an elongated asymmetric coil connected by a hinge to a foot-like protrusion, as illustrated in Figure 4A-ii. The central coil region contributes to the remarkable elasticity of tropoelastin, whereas the foot region comprises the cell-interacting C-terminal region. In addition, the authors also proposed the cross-link domains to be located between the hinge and a “spur” region, leading them to propose a head-to-tail assembly of tropoelastin molecules compatible with both elastic fiber assembly and tetra-cross-link chemistry discussed above. Yeo et al.224 then verified that the hinge region imparted flexibility to tropoelastin, which was attributed to a helix predicted by MD simulations. Furthermore, this flexible helix was found to be important to facilitate molecular assembly, as the addition of a spliced-out exon to wild-type tropoelastin led to the prediction of a more rigid β-sheet motif, which altered the elastic fiber assembly and resulted in aberrant hydrogels during macroscopic processing. Using replica exchange MD (REMD) simulations, the same team validated the experimentally obtained the shape of tropoelastin,49 corroborating the roles of the 3 main domains of tropoelastin, namely the extended coil in the N-terminus contributing to elasticity, the C-terminus protruded foot interacting with cells, and the hinge region driving assembly. REMD simulations also predicted that localized folded regions, such as helices in the hinge and foot, confer the overall shape to the molecule, although a large fraction of tropoelastin remained in the disordered conformation as also predicted by large-scale MD simulation of the ELP [GVPGV]7.225 The structure obtained by REMD was also converted into a coarse grain model to better identify nucleation events associated with tropoelastin coacervation.48 These computational efforts corroborated that the hydrophobic domains drive coacervation, which is nucleated through head-to-head, head-to-tail, and tail-to-tail intermolecular contacts. Furthermore, they also established that the sequence length of the hydrophobic domains is directly correlated with coacervation tendency.
3.2.2. Resilin
As pointed out in Section 2.1, resilin is the first elastomeric protein that was investigated through a rigorous biomechanical framework in the pioneer work of Weis-Fogh.22,23 Located in the hinge of insect wings as shown in Figure 4B-i (locusts, dragonflies, mosquitos, fruit flies, etc.), resilin plays a central role in insect flies by storing elastic energy provided by thoracic muscles and transmitting it to the wings for efficient flapping. While the original studies on resilin were conducted on the wing-hinge ligaments from locusts and dragonflies, the full-length sequence of resilin was first reported from the fruit fly D. melanogaster.27 The molecular architecture comprises three main domains (Figure 4B-iii), namely elastic N- and C-terminal domains (translated by exons 1 and 3 of the resilin gene)226,227 flanking a central chitin-binding domain (translated by exon 2 of the resilin gene). The chitin-binding domain was identified by the presence of a Rebers & Riddiford (RR) consensus motif found in insect cuticle proteins (see Section 3.4.2) and is shown to display a high affinity to chitin.228 Both elastic domains consist of highly repetitive yet distinct peptide motifs. The N-terminus contains 18 repeats of the 15-amino-acid long consensus motif [GGRPSDSYGAPGGGN], whereas the C-terminus contains 11 repeats of the 13-amino-acid long consensus motif [GYSGGRPGGQDLG]. Resilin genes have also been identified in other insect species with some variations among the consensus repeats of the elastic domains.226,229 Notably, in the African malaria mosquito (Anopheles gambiae, A. gambiae), Lyons and co-workers230 identified the 11-amino-acid long consensus repeat [AQTPSSQYGAP] that has a lower Gly content than in the consensus motif from the resilin of D. melanogaster. Despite this significant difference consensus sequence, recombinant constructs made of multiple copies of this shorter repeat exhibit comparable elastomeric characteristics after cross-linking as those from D. melanogaster.231
Using shorter resilin-like polypeptides (RLPs) from the N-terminus elastic domain,29,232 Dutta and co-workers233 established that RLPs exhibit an unusual dual-phase temperature behavior: this RLP is soluble between 6 to 70 °C but insoluble below 6 and above 70 °C. Thus, RLPs are characterized by both an upper critical solution temperature (UCST) and a LCST phase behavior, forming spherical coacervate-like aggregates above its LCST with a size in the 100–150 nm range. In the soluble regime, combined circular dichroism (CD) spectroscopy and SAXS studies on a RLP designed from the elastic domain of A. cambiae (chosen over D. melanogaster due to its shorter consensus sequence and higher expression yield) established that the protein is intrinsically disordered,234 a result also determined with full-length resilin.228 The IDP nature of the elastic resilin motifs are in line with their low complexity sequences containing a high amount of Pro and Gly residues, since structural proteins enriched with these two amino acids are known to promote conformational disorder at the expense of structural order.235 The stimuli-responsivity behavior of RLPs to temperature, pH, or ionic strength is regulated by sequence design, amino acid composition within the repeats (e.g., hydrophobic vs polar residues, the type of aromatic side-chains, etc.), and polypeptide length. For a detailed discussion of these interrelationships, the reader is referred to the recent review by Balu et al.227
Resilin cross-linking is central to the elastomeric properties and chemical stability of the mature material and is mediated by Tyr residues forming di- and trityrosine (Figure 5B), as established in the early studies by Andersen.24,25 In native resilin, these cross-links are catalyzed by peroxidase enzymes and readily identified owing to their blue color emission under UV irradiation.29,236 In artificial resilin, cross-linking can be mediated both enzymatically (peroxidase, laccase, or tyrosinase) or with a photoinitiator such as ruthenium salt29,237 or riboflavin.238
Conducting FTIR and SAXS measurements as a function of temperature on both full-length recombinant resilin as well as exons 1 and 3, Qin et al.218 proposed a molecular mechanism to explain the elasticity of resilin. They suggested that full-length resilin assembles into irregular and hydrated micellar structures driven by the repeat hydrophobic and hydrophilic blocks of resilin. According to their model, exon 1 has longer hydrophilic segments that are in random-coil conformation and are softer, and it connects the more hydrophobic segments of exon 3, which are more tightly packed and stiffer. Upon mechanical loading, exon 1 domains first uncoil before exon 3 domains undergo a structural and reversible transformation into more ordered β-turn structures. However, this model relies mostly on structural transitions induced by temperature as energy input and remains to be fully validated with stress-induced spectroscopic characterization.
3.2.3. Mussel Thread Proteins with Collagen-, Silk-, and Elastin-like Domains
To attach themselves to substrates, mussels use the byssus as a holdfast with a bundle of threads attached at one end to a surface and at the other end to a stem of the mussel. Each mussel thread is a shock absorber with a strong and stiff distal end and an elastomeric proximal end (Figure 4C-i). The main components of a mussel thread are collagenous proteins known as the PreCols (see Section 2.4) with triple helical collagen cores, which make up 96% of the distal thread and 66% of the proximal thread.85 The three variants are present in various relative contents along the length of the thread. PreCol-D is highly abundant at the distal end of the thread, and its relative concentration decreases gradually toward the proximal end, whereas the distribution of PreCol-P starts at approximately the middle of the thread and dominates the end of the proximal region. PreCol-NG appears uniformly in the thread (Figure 4C-ii.). All three PreCols form triple helices, which assemble into 6 + 1 bundles with hexagonal packing. The bundles further arrange into an axially staggered semi-crystalline structure with quasi-hexagonal lateral spacing (Figure 4C-iii).239,240 Each of the collagen precursor variants mentioned above contains several distinct functional domains that share sequence homology with known structural proteins.
All PreCol variants have an ABCBA pentablock copolymer-like primary structure (Figure 4C-iii) with a central triple helical collagen core (approximately 150 nm in length) that is abundant in [Gly-X-Y] repeats, interrupted by single deletion of Gly. These deletions are believed to cause bends in the fibrillar collagens.88,241 Pro and Hyp residues are frequent in the X and Y positions, which stabilize the triple helix. However, there is a large number of Gly residues in the X position of the triplet, together with other destabilizing amino acid residues (Ala, Leu, and Asp) in the X and Y positions. The three PreCol variants have different flanking domains at both ends of the collagen core followed by His-rich domains at the N- and C-termini. PreCol-D contains flanking domains resembling spider dragline silk motifs with poly-Ala repeats and [GGX] repeats.111 PreCol-P is flanked by elastin-like domains with a pentapeptide [GXGPG] repeat, in which X could be a Gly or a hydrophobic residue.111 PreCol-NG has flanking domains that are reminiscent of the Gly-rich protein of plant cell walls with [XGly]n repeats, in which X can be Ala, Leu, or Asn. Beyond the flanking domains are the His-rich domains, which also contain two to four Dopa residues112 that are proposed to form both Dopa-metal complexes and covalent cross-links via Di-Dopa bonds (Figure 5C).242 PreCol bundles are stored in mussel collagen glands in secretory vesicles with fluid smectic liquid crystal (LC) phase. Upon secretion, the pH change from mild acidic pH to seawater pH will trigger the hierarchical assembly of the PreCol bundles.219
Mussel threads are well-engineered elastomeric fibers with gradient mechanical properties and an excellent capacity of adsorbing and dissipating energies. They have both high toughness and high energy dissipation, as a thread can withhold and recover as high as 200% strain and dissipates up to 70% of the energy absorbed.243,244 Mussels achieve this remarkable feat by engineering byssal threads with gradient mechanical properties: the distal thread is stronger and stiffer, with high hysteresis (70%), whereas the proximal portion is softer and weaker, with a lower hysteresis (40%). The silk fibroin-like flanking domains of PreCol-D can form stacked β-sheets, and they possess significantly different mechanical properties from the elastin-like elastin domains.85 The gradient distribution of PreCol-D and -P is expected to play a critical role in the gradient mechanical properties of the mussel thread.
Mussel threads exhibit time-dependent self-healing properties that do not involve the cellular repair machinery. In situ SAXS measurements during tensile mechanical testing of the thread have suggested that the PreCol bundles assemble into a semi-crystalline structure that can withhold large reversible deformation via the unfolding and refolding of the folded protein domains surrounded by a sacrificial network of His-metal coordination complexes (Figure 5C).239,240,245,246 Indeed, His residues at the N- and C-termini form metal coordination complexes with transition metal ions, such as Zn2+ and Cu2+, that can be mechanically broken and reformed, so that the extensible collagen domains in the thread are mechanically protected via the sacrificial and recoverable His-metal coordination complexes. The structural recovery is rather rapid, while the recovery of the mechanical properties is much slower. The mechanical and self-healing properties of the thread are impaired upon treating the thread with the chelating agent EDTA or under low-pH conditions.245,247,248
Mussel threads and PreCols provide very useful insights into the design of load-bearing materials. By employing a gradient distribution of PreCols with different mechanical properties built-in intrinsically, the byssal threads display gradient mechanical properties. Such graded structures can smoothen the stress distribution, reduce stress concentrations, and eliminate stress singularities, collectively improving the bonding strength between interfaces and the fracture toughness.85 The ABCBA pentablock copolymer-like design of PreCols highlights the idea of modular design of multi-domain functional proteins, which can be adopted by synthetic biology. The concept of sacrificial reversible cross-linking has been explored in many synthetic self-healing polymers. Finally, the ability of PreCols to form smectic LC phase that can be easily processed into hierarchically structured fibers is meaningful for the design of various load-bearing polymeric and supramolecular materials. Recombinant mussel byssal PreCols and/or PreCol derived load-bearing proteins can be useful for different applications.249
3.2.4. Coiled-Coil Proteins
All proteinaceous materials described in this section thus far can be described within the framework of classical entropic-driven elasticity,11,207,208 wherein retraction forces are mostly governed by conformational entropy as described in Section 3.2. Noteworthy exceptions are the egg capsules of certain oviparous animals, such as sea snails, which exhibit long-range reversible elasticity but with a fundamentally different mechanism of deformation at the molecular level. Notably, whelks (illustrated in Figure 4D) deposit their embryos within protective egg capsules, which they lay out as a long string (up to one meter long) of multiple capsules (Figure 4D-i) on the Ocean floor of the intertidal zone. Embryos develop within these capsules for months and must thus be protected against a variety of physical, chemical, and biological threats, including predatory attacks, high-velocity breakers, or hydration/dehydration stresses during tidal cycles. Rapoport and Shadwick250,251 first reported the unusual tensile response of egg capsules from various species of the large whelks Busycon canaliculatum (B. canaliculatum), Busycon carica, (B. carica), and Kelletia kelletii (K. kelletii). The stress–strain curves of these capsules in the hydrated state can be separated into 6 distinct regimes, illustrated in Figure 6A: (i) an initial linear elastic regime with an elastic modulus on the order of 50 to 100 MPa (depending on the species), which is much higher than those of other bioelastomers, whose moduli are typically in the range 0.5–1 MPa; (ii) a pseudo-yield regime that initiates at tensile stresses and strains above 1.5–2 MPa and 4–5%, respectively; (iii) a large extension with modest increase in stress (“plateau” region); (iv) a strain-hardening regime above ca. 70% strain, during which the stress rapidly increases with strain until the (v) peak stress; (vi) upon unloading, a return to the initial length occurring at smaller stress values than during loading, resulting in significant stress–strain hysteresis. That is, the loading and unloading paths do not coincide (Figure 6A), such that a large fraction of the elastic energy stored during loading is internally dissipated during unloading. In other words, the capsular material has shock-absorbing capability and this has important biological functionality: if the stored elastic energy of the strained capsule was fully released upon unloading (as in elastin and resilin), it would constitute a death trap for the growing embryos since they would be on the receiving end of the restored elastic energy.
Figure 6.
Uniaxial tensile response of coiled-coil elastomeric proteins. (A) The whelk egg capsules (shown in Figure 4D) can display large reversible deformation, but unlike most elastomers, the elasticity is not entropically dominated. (B) During straining, coiled-coils gradually unravel and eventually transition into β-sheets. Upon unloading the process is reversible and coiled-coils reform. The whole process dissipates significant elastic energy as highlighted by the large hysteresis of the stress–strain curve in (A). Adapted with permission from ref (252). Copyright 2009 Nature Publishing Group.
At the microstructural level, the capsules comprise stacked layers ca. 10–20 μm thick, which are themselves made of aligned fibers 200–500 nm in diameter that are rotated by 90° from one layer to the next, resulting in a plywood-like structure. The deformation mechanism linked to the egg capsules stress–strain curves was identified by in situ laboratory and synchrotron WAXS investigations252 and later confirmed by Raman spectroscopy studies.253,254 In the unstrained state, the capsular proteins within the fibers adopt an α-helical coiled-coil conformation (Figure 6B-i), a result confirming earlier X-ray studies by Gathercole,255 with an orthotropic diffraction pattern due to the plywood arrangement of the layered structure. As the material is strained, the α-helical coiled-coils unravel and then gradually transition toward extended β-sheets oriented in the loading direction (Figures 6B-ii to 6B-v), with maximum strains of up to 150% achievable. However, upon unloading, the coiled-coils reform (Figure 6B-vi) such that the long-range elasticity can be considered a reversible phase transition from α-helical coiled-coils to extended β-sheets. In contrast to the classical rubber elasticity, this mechanism is mostly driven by changes in internal energy associated with the phase transition, which can be described by a Clausius–Clapeyron type of relationship:252,256
| 2 |
where σt is defined as the critical transition stress for the α–β transition, εt is the total transformation strain, V is the volume of material, and ΔH is the enthalpy of the α–β phase transition. Conducting stress–strain curves at different temperatures and plotting (σt/T) vs (1/T) yielded a linear relationship (where the slope is directly proportional to ΔH), thus validating the theory proposed by Flory decades earlier257 that some elastic proteins can achieve high extensibility through an internal energy-dominated mechanism. This mechanism occurs in marine snail egg capsules because in contrast to most bioelastomeric materials, the building blocks of these capsules exhibit a well-ordered secondary structure in the form of long coiled-coil fibrils.
The coiled-coil propensity was substantiated when the complete sequences of ECPs were obtained for the tropical marine snail spiral melongena (Pugilina cochlidium, P. cochlidium)131 and for B. canalicatum.258 The egg capsules are built from two or three ECPs made a central rod domain containing two or three helical domains, each of which comprise multiple consecutive segments of the coiled-coil heptad repeat [abcdefg], where a and d are hydrophobic residues that stabilize the helical core through hydrophobic interactions (Figure 4D-ii and 4D-iii). The helical segments are interrupted by linker regions, whereas the N- and C-termini constitute the head and tail domains. With this tripartite molecular architecture (head-central rod-tail), ECPs can be classified as IFs,259,260 a family of proteins whose well-known members include the cytoskeleton desmin and vimentin as well as the extracellular keratins.261,262
Verification that ECPs can be self-assembled into coiled-coil nanofilaments was established by Fu et al.,263 who recombinantly expressed distinct ECPs, solubilized the proteins from the inclusion body in 6 M urea, and refolded them into coiled-coil filaments, with the coiled-coil signature corroborated by CD and FTIR spectroscopy analyses. This study also established that the coiled-coil filaments are most likely comprised of heterodimeric coils (as opposed to homodimeric coils), which was concluded based on a stronger coiled-coil signature for heterodimers as well as their enhanced thermal stability by CD spectroscopy measurements. In contrast to IFs, ECPs did not assemble into higher order structures, very likely because the highly conserved motif R/KLLEGE, located at the end of the central coiled-coiled domains in IFs and that mediates lateral assembly, is absent in ECPs. On the other hand, Loke et al.264 identified and sequenced a smaller, 11.5 kDa “bundling” protein in the nidamental gland of P. cochlidium in ECPs that mediate oligomerization and in vitro gelation of native ECPs. Controlled bundling via accessory proteins is commonly observed in self-assembled biological polymers and gels265,266 and plays an important role to regulate the mechanical response of the extracellular matrix.
There are similarities in the mechanical response and deformation mechanisms of egg capsule proteins and fibrous α-keratins, such as wool keratins.267−269 In both cases, the tensile response is characterized by an initial linear elastic region until a pseudo-yielding stress value, followed by a stress plateau and then a strain-hardening region.256 Upon unloading, both biopolymers exhibit significant hysteresis and, thus, shock-absorbing capacity. The most prominent resemblance is the α-helical coiled-coil to β-sheet transition that has been identified in keratins using micromechanical testing in conjunction with X-ray261,270,271 and Raman spectroscopy.272 As expected for biopolymeric materials, the water content strongly affects the mechanical response, lowering the pseudo-yielding stress and the stress at which phase transition occurs. But there are also substantial differences: in keratins strains are reversible only until 30–40% and the process is not instantaneous. Keratins filaments are stabilized by extensive disulfide cross-linking, resulting in higher tensile stresses but at the same time restricting the total achievable reversible strain. For this reason, although the mechanically induced α–β transition is common in both systems, keratins are not classified as bioelastomeric. However, the reversible α-to-β transition was recently exploited in natural wool keratin.273 In this work, reversibility was achieved by rehydrating fibers initially strained in the dry state, thus achieving a hydration-driven shape-memory effect. This study highlighted the importance of extracting intact individual coiled-coil units from the native material without denaturing them and offers interesting perspectives for the development of shape-memory biopolymeric materials.
3.2.5. Abductin
Abductin is a rubberlike protein found in bivalve mollusks such as scallops (Figure 4E). It is the major protein located in the triangular rubberlike pad, called the adductor muscle, in the inner hinge ligament of the shell (Figure 4E-i). The adductor functions as an elastic pivot that controls opening and closure of the shell.274,275 Scallops can use this opening-closing action at a frequency of about 4 Hz to expel water from the shell interior, which provides scallops the ability of swimming by jet propulsion to escape predators.208 Abductin is the only natural rubberlike protein that shows compressible elasticity. The compression modulus of scallop abductin is around 3.5–4.5 MPa.208 In the adductor muscle of scallops, abductin needs to store a large amount of elastic energy to open the shell and also needs to have a high resilience to recover the muscle energy for the next opening cycle. The functional resilience of the adductor hinge ligament from scallop Placopecten magellanicus was measured by Bowie et al.276 as well as Denny and Miller,277 and the results show temperature-dependent resilience with an average resilience of 0.8–0.9.
The primary structure of abductin was first obtained by Cao et al.,278 who deduced it from the cDNA sequence of Argopecten irradians (A. irradians), the bay scallop. It contains an Ala-rich N terminus with 2 conserved Cys as well as 2 Tyr residues. The first 19 amino acids in the N-terminus likely represent the signal peptide for the secretion of the protein and are not included in the secreted protein in the extracellular matrix. The second domain of abductin mainly contains a repeating motif, [GGFGGMGGGX], which has di- and tri-Gly blocks separated by a single amino acid, and a methionine (Met) located at the center of the conserved motif (Figures 4E-ii and 4E-iii). As a consequence, abductin has an unusually high Met content, a feature not found in other known elastomeric proteins such as elastin and resilin. Compared to other elastomeric proteins, abductin shows a very different amino acid composition, containing 58 mol % Gly and only 1 mol % Pro.275 The content of polar and charged residues, at 13 mol %, is higher than elastin (7 mol %) but much lower than resilin (30–35 mol %). This amino acid composition suggests that the hydration level of abductin is likely between those of elastin and resilin.208
Owing to its high Gly content, abductin is likely to maintain an overall random coil structure. Unlike other rubber-like proteins, abductin contains a very low Pro content and is thus unlikely to contain β-turns. The elasticity of abductin arises from a network of random protein chains and can also be described by classical rubber elasticity. Since abductin in the extracellular matrix is only 116 amino acids long, multiple cross-links are required to provide it with these elastomeric properties. It has been proposed that the 3 Lys’s and 14 Meth’s may form cross-links via sulfilimine cross-links.279 Given the fluorescence of abductin, it may also have dityrosine cross-links similar to those of resilin through the two terminal Tyr groups, but this has not been validated yet.
Compared to other elastomeric proteins, relatively few attempts have been made to produce functional recombinant abductin. Bochicchio et al. produced two polypeptides with the repeating motif of abductin using solid state peptide synthesis.280 The peptides exhibited temperature-dependent assembly, transiting from a coil-structure to a type II β-turn at higher temperature, and they assembled into fibrillar supramolecular structures. The fibers could be further assembled into a biphasic gel matrix with a honeycomb-like structure. Su et al. designed a recombinant polypeptide that contained abductin-like sequences derived from A. irradians.281 The polypeptide showed a dual-phase transition behavior with both UCST and LCST properties, similar to those of RLPs. The abductin-based proteins/peptides showed good cytocompatibility and may be used in tissue engineering and drug delivery applications.
3.2.6. Comparative Overview
In entropically driven bioelastomers, the proteins are mostly disordered, although as mentioned in the case of tropoelastin, localized regions can be structured and the overall shape of the molecule is not random. These proteins share the commonality of a high (Gly + Pro) content, between 45–60 mol %,282 with Gly favoring chain mobility and Pro acting as β-sheet breaker, thus preventing the formation of unfavorable amyloid-like structures.235 Indeed, random-coil domains are more readily extendable than rigid β-sheet amyloids upon application of an external mechanical load, in turn resulting in higher macroscopic extensibility. Resilin and elastin, on the other hand, have very different contents of hydrophobic and polar residues. Elastin, with 60% of non-polar residues and 7% polar and charged residues, is one of the most hydrophobic proteins. In contrast, resilins contain between 25–30% hydrophobic residues depending on the species and up to 35% of charged + polar residues. Consequently, the resilin network is much more hydrated than elastin, resulting in significant differences in their viscoelastic properties.282 The Gly content of abductin is the highest among all elastomeric proteins at around 60 mol %. Long stretches of poly-Gly are known to form stable β-sheet structures; however, in abductin’s primary sequence, di- and tri-Gly peptides are interspersed with other amino acids, as seen in the consensus repeat shown in Figure 4E-iii, notably Meth. Since abductin is also known to be in a random-coil configuration, it is likely that the non-polar Meth residues prevent the formation of a stable secondary structure, instead promoting disorder, whereas Gly maintains a high chain mobility. In mussel threads, the proximal region of the thread is elastomeric, which is attributed to the predominance of PreCol-P in this region (Figure 4C-iii). PreCol-P also contains an overall high Gly content, but in this case the Gly residues in different blocks of the protein have very different mobilities. In the central collagen-like domain, Gly’s are buried within the rigid triple helix where their mobility is severely restricted. However, the central block is flanked by elastin-like domains (GXGPG repeat), which are likely in random-coil configuration with Gly having a much higher mobility, resulting in the flexible attributes of PreCol-P compared to PreCol-D and PreCol-NG.
The cross-link chemistry that mechanically and chemically stabilizes mature elastomeric materials is highly diverse. The resilin network occurs through dityrosine cross-linking (Figure 5B), whereas in elastin a more complex cross-linking chemistry operates, involing the initial oxidation of Lys residues into ALys followed by a series of condensation and cyclization reactions (Figure 5A) that eventually results in the desmosine and isodesmosine heterocyclic cross-links. The cross-links in abductin are still unknown; albeit, there is speculation of the existence of dityrosine between the Tyr residues located at the extremities of the primary sequences. It has also been speculated that Lys and Meth may form sulfilimine cross-links given the presence of both amino acids in abductin. In mussel threads, His-metal and Dopa-metal coordination bonds have been identified (Figure 5C) and contribute to the self-healing properties, and Di-Dopa has also been isolated.
The molecular design of a bioelastomeric structure made of coiled-coil proteins is entirely different, relying on the self-assembly of IF-like structures containing heptad repeat domains interspersed by linker regions. In these materials, the elastomeric response is governed by the reversible α–β transition. The range of fully reversible deformations likely depends on the fraction of coiled-coil regions in the primary structure, the assembly of these domains, as well as on the cross-link density,256 but generally speaking the initial elastic modulus is higher than that for elastomers made of disordered proteins. The sequence design within the heptad repeats may also play a role in regulating the elastomeric properties, but systematic investigations of these variables have yet to be carried out. Likewise, coiled–coiled filament cross-links that stabilize the whole network are still unknown at this time.
3.3. Adhesive Proteins
3.3.1. Mussel Adhesive (Foot) Proteins
Marine mussels use byssus-mediated adhesion to anchor on various wet surfaces (Figure 7A-i). A byssus typically contains tens to more than a hundred threads with diameters of 100–250 μms and lengths of 2–6 cm.91 Each thread contains three parts: an extensible proximal end, a stiff distal portion, and a spatulate adhesive plaque that attaches to the substrate. A mussel uses an organ called the mussel foot to make the threads one at a time in a period of 30 s to 8 min by depositing a series of byssal proteins. The secretion of the proteins is spatially and temporally regulated, and the whole process is reminiscent of the injection molding process widely applied in polymer processing.206,283,284 The byssal proteins from different species of mussels show both similarities and differences in their sequences between species, with the California mussel, Mytilus californianus, and the Asian Green mussel, P. viridis, being the most studied systems.91,285,286 Many of the byssal proteins from both species, especially the adhesive MFPs, are rich in Dopa.100,108,151,287
Figure 7.
Bioadhesive proteins. (A) At the distal end of mussel threads, the adhesive plaque is constructed from different plaque proteins. (i) In Mytilus genus schematically represented here, MFP-3 and MFP-5 are the adhesive primers that are initially secreted and are in direct contact with the substrate. MFP-6 is a Cys-rich protein located in the adhesive layer. Adapted with permission from ref (91). Copyright 2017 The Company of Biologists Ltd. The plaque is linked to the core of the thread (made of PreCols, see Figure 4C) through the linker proteins MFP-2 and MFP-4. (ii) The byssus of mussels is assembled in a process similar to polymer injection molding. MFPs are stored in different secretory glands and secreted into the foot groove in a spatiotemporal controlled manner. Adapted from ref (283). Creative Commons CC BY. (iii) MFP-3 and -5 are rich in Dopa and contain multiple copies of the dipeptide Dopa-Lys (Y*K). Cys residues of MFP-6 are oxidized as disulfide bonds, ensuring that Dopa residues from MFP-5 remain reduced and not oxidized into Dopa-quinone. (B) The barnacle cement (i) is a permanent adhesive made of cement proteins (CPs) that ensures robust bonding of the barnacle shell to solid substrates. (ii) The cement is made of amyloid-like cross-β nanofibrils. (iii) In acorn barnacles (M. rosa), at least five CPs have been identified. A spatial distribution of CPs across the cement has been proposed,292 which has however been questioned in recent years. Adapted with permission from ref (292). Copyright 2013 Taylor & Francis. (C) The sandcastle worms construct a tubular shell (i) with sand grains, comminuted shells, and a secreted polyelectrolytic bioadhesive. (ii) Oppositely charged proteins are stored in homogeneous and heterogeneous granules. Co-secretion of the preassembled adhesive packets leads to the homogeneous curing of the cement. Adapted with permission from ref (293). Copyright 2013 American Chemical Society. (iii) Polycationic protein Pc-1 and polyanionic protein Pc-2 are rich in Dopa. Pc-3 contains an extremely high (∼70%) phosphoserine (pSer) content. (D) Biofilm formation and interfacial adhesion of the enterobacteria is mediated through (i) curli fibers on the cell surface. (ii) The self-assembly of curli’s is carried with a complex outer membrane machinery. (iii) It is composed of multiple Csg subunits each with very distinct functionality crucially important for the controlled growth of the curli fibers and for host cell adhesion, invasion, and colonization. Adapted with permission from ref (294). Copyright 2018 Elsevier. (E). Many fungi such as (i) Trichoderma reesei secret a group of surface-active amphiphilic structural proteins known as (ii) hydrophobins at the air–water interface. Their primary function is to act as water repellent coatings but also mediating communication of the hyphal network with their surrounding environment during growth and development. Their interfacial assemblies and the ability to make strong adhesion to various surfaces are also linked to infections related to pathogenic fungi. (iii) There are two major classes of hydrophobins, Class I (UniProtKB - P52754)(295) and Class II (UniProtKB - A0A023WG46),296 each with very distinct sequence, molecular structure, hydropathy plots, and solubility, resulting in different biophysical characteristics. (F) Caddisfly larvae construct a protective casing tube (i) made of inorganic sediments. (ii) The inorganic particles are glued together with silk-like fibroins that form antiparallel β-hairpins stabilized by Ca2+ ions. (iii) Silk fibroins are very large MW proteins comprised of large modular blocks (D, E, and F), which are themselves made of smaller positively and negatively charged submotifs as well as hydrophobic motifs. In the negatively charged motif, Ser residues are often phosphorylated (pSer). (ii) and (iii) Adapted with permission from ref (297). Copyright 2013 American Chemical Society.
Byssal proteins are synthesized and stored in three major glands, the phenol, collagen, and accessory glands that contain protein components for the plaque, the thread core, and the cuticle of a thread (Table 1). Upon thread deposition, the mussel foot secrets the protein in a manner similar to a microfluidic delivery device.206,283 The adhesive primers MFP-3 and MFP-5 are the first batch of proteins deposited followed by MFP-6, a cysteine-rich protein which forms the adhesive layer of the plaque, followed by the matrix proteins, MFP-2 and MFP-4 as well as the cuticular protein MFP-1 (Figure 7A-i and 7A-ii).109,288,289 PreCols including PreCol-D, -P, and -NG where D, P, and NG represent distal, proximal, and nongradient, respectively, are then secreted together with thread matrix proteins TMP and proximal TMP.88,110−112,290,291
Table 1. Comparison of the Known MFPs of Mytilus Byssus with Regard to Localization in the Foot Glands and Byssus, Mass, PI, Modification and Adhesion Energy (Ead) to Mica.
| Protein | Localization | Mass (kDa) | PI | Modifications (mol %) | Ead(mJ m–2) | Ref |
|---|---|---|---|---|---|---|
| MFP-1 | Cuticle | ∼110 | 10.5 | Dopa (15) Hyp; DiHyp | 1 | (288,300,301) |
| MFP-2 | Plaque core | 45 | 9.5 | Dopa (5) | 1 | (288,299,302) |
| MFP-3f | Plaque interface | 6 | 8–10 | Dopa (20) HyArg | 6 | (109,287,299) |
| MFP-3s | Plaque interface | 6 | 7–8 | Dopa (10) | 3 | (303,304) |
| MFP-4 | Plaque core | 90 | 8.4 | Dopa (2) | 0 | (298) |
| MFP-5 | Plaque interface | 11 | 9.8 | Dopa (30); pSer | 15 | (108,305) |
| MFP-6 | Plaque interface | 12 | 9.3 | Dopa (5) | 0.5 | (109) |
| MFP-7 | Plaque core | 3.8–4.2 | 10.1–10.4 | Dopa (0.2) | n.d. | (151) |
| PreCol-D | Thread core (distal) | 240 (trimer) | 9.5 | Hyp; Dopa (0.1) | n.d. | (110) |
| PreCol-P | Thread core (proximal) | 240 (trimer) | 11.4 | Hyp; Dopa (0.1) | n.d. | (88) |
| PreCol-NG | Thread core | 240 (trimer) | 8.8 | Hyp; Dopa (0.1) | n.d. | (112) |
| PTMP-1 | Thread core (proximal) | 45 | 5.9 | Glycosylation | n.d. | (291) |
| TMP-1 | Thread core | 56.5 | 9.5 | Dopa | n.d. | (290,291) |
As mentioned, all the MFPs contain various contents of Dopa in the sequences, with the adhesive primers MFP-3 and -5 having the highest Dopa contents (Figure 7A-iii). In the mussel glands, Dopa is co- or post-translationally modified from Tyr; albeit, neither the modification process nor the enzyme(s) involved in this process are fully understood.91,131 Apart from Dopa, most of mussel foot proteins share some other comment features: most MFPs have a high Gly content, and the isoelectric points (IEPs) of all the MFPs are above 8, indicating these proteins are positively charged in the seawater environment. So far, no polyanionic protein has been successfully identified in the mussel adhesive plaques.151 Apart from their polycationic nature, most of the MFPs are also disordered or at least contain intrinsically disordered regions. The adhesive primers MFP-3 and MFP-5 mostly show random coil structure in pH 3 solution, and so does MFP-4.298,299 Unlike the adhesive proteins, the collagenous proteins PreCol-D, -P, and -NG are highly structured with triple helical cores.110−112 The structures and properties of PreCols are discussed separately in Section 3.2.3.
The byssal proteins are secreted as protein-rich fluids that form via LLPS or coacervation. This liquid condensation can occur for both highly structured (PreCols) and disordered (MFPs) byssal proteins.206,283,285,306 The separated liquid phase allows for the direct delivery of the adhesives underwater. Other than phase separation, coacervates possess some physiochemical properties that are critical to underwater adhesives. Their ultralow interfacial tension allows coacervates to spread on all kinds of surfaces. The shear thinning property facilitates the delivery of the coacervate in the injection molding thread forming process.307 The fluid state of coacervates also facilitates the diffusion of cargos and enzymatic (such as catechol oxidase) triggered solidification.293
Since Waite and co-workers first reported the adhesive properties of mussel byssal proteins almost 4 decades ago, mussel adhesion has gradually become a model system and inspiration source for the development of next-generation wet adhesives. There are many review articles on the topic of mussel-inspired adhesives, and many of them contain detailed discussions on the properties of major MFPs. We refer the readers to some excellent review articles for the details of MFPs.91,92,308 In the following sections, we will focus on two important aspects related to the role of Dopa in mussel adhesion, namely Dopa-mediated molecular interactions and the redox chemistry of Dopa. Undoubtedly, Dopa plays essential roles in mussel adhesion; however, we would like to emphasize that the belief that Dopa is the only important aspect of mussel adhesion is inaccurate and unfortunate. Mussels tightly regulate the fabrication, secretion, delivery, as well as assembly and curing process, and use a spectrum of adhesive and cohesive interactions to achieve a firm and durable adhesive.109,206,283
3.3.1.1. Adhesive and Cohesive Properties of Dopa
Dopa is abundant in MFP-1, MFP-3, and MFP-5 and plays essential roles in the biological functions of the proteins. In the mussel plaque, Dopa’s roles are many-faceted, providing both adhesive and cohesive strength as well as surface protection to the plaque. Since Dopa-mediated molecular interactions are critical to the properties and functions of MFPs and mussel-inspired synthetic adhesives, we highlight the important interactions in the mussel adhesive proteins. Most of these interactions were identified by the team of Waite and Israelachvili using the surface forces apparatus (SFA) technique (refs (109, 300, 302, 303, 305, and 308−310)).
The first critical function of Dopa in the adhesive MFPs is to evict the surface hydration layer on surfaces. Synthetic MFP-3 peptides with Dopa modification can dehydrate titanium oxide (TiO2) and hydroxyapatite (HAP) surfaces, while the same peptides with Tyr residues cannot remove the hydration water on both surfaces.306 With the two hydroxyl groups in the aromatic ring, Dopa can form bidentate hydrogen bonding with hydrophilic substrates such as mica and mineral surfaces (Figure 8).309 Dopa can also form coordination bonds with metal oxides, which shows a pH-dependent binding.301,311 At neutral pH, the coordination bond between Dopa and TiO2 can have a bond energy of 100 kJ/mol,312 which is nearly half the strength of a covalent bond. Apart from the bidentate interactions through the two hydroxyl groups of the catechol, the aromatic ring of Dopa can also interact with hydrophobic surfaces via the hydrophobic and π–π interactions and with positively charged surfaces through π-cation interactions.310,313,314 Recently, it has been found that marine tunicates also secrete Dopa or gallol-containing adhesive proteins to achieve adhesion on a variety of underwater substrates.315,316
Figure 8.
Side-chain molecular interactions of MFPs with solid substrates during adhesive plaque deposition under the mussel foot. Dopa can mediate H-bonds and coordination bonds with oxide surfaces (although the latter is usually not occurring at the low pH under the mussel foot) as well as hydrophobic interactions with hydrophobic surfaces. Additionally Lys and pSer can electrostatically interact with negatively charged and positively charged surfaces, respectively. Adapted with permission from ref (91). Copyright 2017 The Company of Biologists Ltd.
The coordination chemistry of Dopa also plays a critical role in the cohesive strength of mussel plaque. Dopa can form pH-dependent mono, bis, and tris complexes with Fe3+. The catechol-iron coordination complexes greatly contribute to the mechanical properties of the MFP-1 coating layer in the cuticle of the thread.87,317 Recently, it was found that V3+ or VO2+ can also form charge transfer complexes with Dopa.284,318,319 These charge transfer complexes have been utilized in many synthetic Dopa-containing polymers to provide bulk strength as well as self-healing properties to the materials.90 Dopa-mediated interactions, such as bidentate hydrogen binding and π-cation interactions, can also contribute to the cohesive strength of the mussel plaque.313,320
In seawater, a surface is likely to be patchy and have heterogeneous chemistry.321 The many-faceted nature of Dopa-mediated molecular interactions provides mussels opportunistic adhesion mechanisms on various types of surfaces with different chemical compositions. However, Dopa is not the only amino acid residue that contributes to the adhesion of MFPs. It has been shown that many synergies exist between Dopa and other amino acid residues in various adhesives proteins. Charged Lys can have cooperative effects with neighboring Dopa residues on both surface dehydration and adhesion of MFPs and synthetic polymers.322,323 Phosphorylated serine can form a salt bridge with Ca2+ via electrostatic interactions, which may explain why mussels have included this post-translational modification in MFP-5.324 Additionally, various hydrophobic residues, including Phe, Tyr, and Trp, contribute to the adhesion of MFPs on hydrophobic surfaces, as illustrated in Figure 8.310
Under neutral to basic pH, such as in seawater, Dopa undergoes a two-electron oxidation, forming Dopaquinone with the existence of oxygen or other oxidants.109 The product Dopaquinone loses the ability of forming bidentate binding with various surfaces and therefore is less adhesive. To solve the oxidation problem, mussels tightly control the redox environment of Dopa by secreting the Dopa-rich proteins MFP-3 and MFP-5 in an acidic coacervate form together with thiol-rich proteins such as MFP-6 and MFP-16 to -19 (Figure 7A-iii).109,325 The Cys thiols can sacrificially reduce the Dopaquinone to Dopa. Inside the plaque and in the thread, Dopaquinone is also considered to be essential to the protein cross-linking of the MFPs and PreCols. Quinone groups can form Michael additions with different amino acid residues such as Dopa, Cys, Lys, and His to form di-, S-cysteinyl-, lysyl-, and histidyl-Dopa adducts that facilitate cross-linking between MFPs.109,138
3.3.2. Barnacle Cement Proteins
Barnacles are some of the most successful hard macro-fouling organisms in marine environments. They are small crustaceans that construct a protective biomineralized shell, which in their adult phase is permanently attached to immersed substrates via a multi-protein adhesive cement292,326 as illustrated in Figure 7B-i. At different stages of their life cycle, barnacles secrete both temporary as well as permanent adhesives.327 In the early stage, barnacle larvae (called “cyprids”) explore substrata with a temporary adhesive, leaving footprints behind as they move around surfaces.328 Once the cyprids have identified a suitable substrate for attachment, they secrete a permanent adhesive329 and quickly initiate metamorphosis to the adult stage, whereby they grow a biomineralized shell and eventually settle permanently by means of the multi-protein adhesive cement. The permanent cyprid adhesive is a biphasic system consisting of a lipid phase and phosphorylated proteins, and it has been suggested that the lipidaceous phase may dehydrate the surface and protect the adhesive cement against bacterial degradation.329 At the moment, neither the lipid composition nor the primary sequence of the phosphoprotein is known. In addition, during molting and thus shell growth, barnacles secrete a complex fluid330 consisting of phenolic compounds, lipids, and proteins that is thought to clean and prime the surface prior to deposition of the next cuticle layer of the barnacle shell. Similar to the permanent cyprid adhesive cement, the detailed composition and sequences of the proteins present in this fluid are currently unknown.
In contrast, the biochemistry of barnacle cement proteins (CPs) that make up the permanent adhesive of the adult cement is better established. Sequence invitations of CPs were instigated for the acorn barnacle Megabalanus rosa (Mr) by Kamino and co-workers, who obtained the full-length sequences of five MrCPs of the permanent adhesive,331 called MrCP-19,332MrCP-20,333MrCP-52,334MrCP-68,335 and MrCP-100,336 where the number indicates the MW, and whose primary architectures are shown in Figure 7B-iii. On the basis of their amino acid compositions, Kamino proposed to classify these proteins as hydrophobic (MrCP-100 and MrCP-52), hydrophilic (MrCP-20) enriched with both acidic and basic residues (42 mol % of charged residues) as well as Cys (17 mol %), and two hydrophilic proteins (MrCP-19 and MrCP-68) with a bias composition toward six residues, namely Ser, Thr, Gly, Ala, Val, and Lys. Homologous CPs for other species have since been obtained by other groups as reviewed by Liang et al.327 Further, using additional biochemical insights and biophysical experiments, Kamino suggested a model of spatial arrangement of CPs across the multi-layer adhesive joint.292 Because the overall amino acid composition of the cement is close to those of MrCP-52 and MrCP-100, the latter were proposed to comprise the bulk of the adhesive cement. Conducting adsorption studies of purified MrCPs (from both native and recombinantly expressed sources) on various substrates, including calcite, gold, glass, and polystyrene,332,337MrCP-20 was proposed to be located at the barnacle base shell/cement interface due to its adsorption specificity toward calcite, whereas MrCP-19 was suggested to constitute the primary layer in direct contact with the foreign substrate. Recent experimental338,339 and computational340 studies have provided a more refined picture, indicating that MrCP-20 may not only be involved with adsorption to the calcitic base of the barnacle shell but also may regulate biomineralization of the latter, as discussed in a subsequent paragraph below.
Surface spectroscopy and nanoscale structural investigations of the adult barnacle cement by Rittschof, Wahl, and co-workers341−345 have provided key insights into the nanoscale interactions of CPs with solid substrates. These studies have identified that the barnacle cement consists of highly insoluble amyloid-like fibrils, a possibility that was first suggested by Kamino on the basis of barnacle cement amino acid composition bias.335 Experimental evidence that the barnacle cement contains a significant amount of amyloid structures has been obtained using Congo red and thioflavin-T staining assays specific for the characteristic cross-β structure of amyloids343,47 (Figure 7B-ii), by in situ attenuated total reflection FTIR of barnacle cement341,342,344,345 and by far UV CD spectroscopy.342 Atomic Force Microscopy (AFM) imaging has also confirmed the nanofibrillar nature of the barnacle cement,343,346 which provides an interesting parallel with amyloid-like structures associated with bioadhesion in other organisms, most notably in bacterial curli fibers347 and in subaerial algae.348 Short peptides from the hydrophobic MrCP-52, possibly located in the bulk of the cement, have also been shown to self-assemble into amyloids.349
More recent investigations by So et al.350 on Amphibalanus amphitrite have identified likely cross-linking and polymerization pathways of CPs that may help stabilize and cure the whole cement, pointing toward a more complex picture of cement stabilization and hardening than the early model of Kamino presented in Figure 7B-iii. This study suggested that cement polymerization is triggered by enzymatic oxidation of low MW catechols induced by a peroxinectin oxidase identified and sequenced from the cement gland, forming a semi-quinone adduct that can spontaneously react with amine side-chains of CPs. This mechanism shares similarities with insect cuticle sclerotization presented in Section 3.4.2. This study also identified the presence of aldehydes and ketones in solubilized proteins attributed to a 43 kDa silk-like protein, which is modified by peroxinectin and/or by a LOX-like enzyme that oxidizes Lys to ALys similar to elastin cross-linking. Thus, there are strong indications that cement curing involves enzymatically triggered interprotein cross-linking.
Among CPs of adult barnacles, MrCP-20 has received particular attention because it can be readily expressed in E. coli in the soluble state, allowing researchers to conduct in-depth biophysical and structural studies. The primary structure of MrCP-20 (Figure 9A) is distinct from other CPs. It contains an unusually high amount of charged residues (40 mol % of acidic and basic residues) and is enriched in Cys (17 mol %). Upon alignment of Cys residues, 6 conserved repeats have been suggested.333 Using recombinant MrCP-20, So et al.338 highlighted the dynamic interactions between MrCP-20 and calcite surfaces leading to the templating of protein nanofibrils, as well as the crystal-orientation specificity of MrCP-20/calcite interfaces. This study suggested a disordered-to order transition of MrCP-20 upon contact with the periodic feature of crystalline calcite. Because of the relatively small size of MrCP-20, Mohanram et al.351 were able to obtain its three-dimensional solution structure using multi-dimensional NMR, as shown in Figure 9B. This study revealed that MrCP-20 is organized into three β-sheet-containing folded domains interrupted by two flexible dynamic loops, imparting structural integrity while at the same time maintaining a certain level of conformational flexibility. Furthermore, a prominent feature of the tertiary structure of MrCP-20 is that 12 of the 32 Cys residues form disulfide bonds, thus enhancing packing of the monomer. These disulfide bonds are usually located directly adjacent to Pro residues, which could mitigate the destabilization effect of Pro on the tertiary structure. Interestingly, MrCP-20 is one of the few known examples of an extracellular adhesive protein that forms a folded or semi-folded protein in solution and whose structure has been experimentally obtained, the other one being PVFP-5, a mussel foot protein of the Asian green mussel.286,352
Figure 9.
Structural studies of the barnacle cement protein MrCP20. (A) Primary structure with the identified secondary structure regions identified by solution NMR. (B) Tertiary structure obtained by solution NMR (i) and calculated electrostatic surface potential from (ii). (i) reproduced with permission from ref (351). Copyright 2019 Royal Society. (ii) reproduced with permission from ref (340). Copyright 2020 American Chemical Society. (C) Molecular dynamics (MD) simulations of MrCP20 in the presence of CO32– and Ca2+ ions, predicting the formation of ion clusters around the surface of the protein. (D) MD simulations of MrCP20 on calcite surfaces. (C) and (D) reproduced with permission from ref (340). Copyright 2020 American Chemical Society. (E) Calcium carbonate (CaCO3) crystallization in the absence (i) or presence (ii) of MrCP20. In the presence of MrCP20, CaCO3 crystallizes in the metastable vaterite polymorphism. (F) In the presence of CaCO3, MrCP20 self-assembles into nanofibrils as seen by AFM (i) and TEM (ii) imaging. (E) and (F) reproduced with permission from ref (339). Copyright 2021 American Chemical Society.
Building upon this work, Kumar et al.340 conducted comprehensive MD computations of the interactions between MrCP-20 and inorganic ions involved in shell biomineralization, as well as with inorganic surfaces representative of marine substrates. This study predicted that MrCP-20 sequestrate both CO32– and Ca2+ ions via electrostatic interactions as well as with coordination and salt-mediated bonds, forming ion clusters around the protein surface that elicit a semi-ordered to a more ordered conformation of the protein (Figure 9C). MD simulations also revealed that conformational flexibility of the dynamic loops enables efficient adsorption with model calcite surfaces in a non-specific manner (Figure 9D), not only through electrostatic interactions but also through water-mediated interactions. In a follow-up experimental study339 combining NMR techniques, EM, and AFM observation, it was shown that MrCP-20 regulates calcium carbonate biomineralization through an unusual pathway. Indeed, the protein initially stabilizes the metastable vaterite polymorphism of calcium carbonate (Figure 9E), delaying the crystallization of the thermodynamically stable calcitic phase. In addition, this study demonstrated that calcium carbonate crystallization concurrently triggers MrCP-20 to form nanofibrils, in line with the findings that the cement surface is made of amyloid-like nanofibrils (Figure 9F). Therefore, MrCP-20 appears to be a multi-functional protein that concentrates inorganic ions around its surface, thus regulating calcification of the barnacle shell, while at the same time mediating interfacial adhesion on inorganic substrates.
Given the putative role of MrCP-19 to mediate interfacial adhesion,292 self-assembly and biophysical studies of this CP and homologous variants have also been undertaken. Using deep proteomic analysis of multiple CPs, So et al.350 identified a 19 kDa consensus repeat of alternating charged and non-charged stretches found in multiple CPs, which was found in 10 instances along these CPs. This pattern—which consists of a mostly non-charged block of 20–25 AA long followed by a shorter block (10–15 AA long) containing 30% of charged residues—exhibited a strong propensity to form cross-β amyloid fibrils. Within this pattern, the central sequences [GSVTAX], where X is a charged residue, were identified to play a critical role in templating and regulating the growth of larger microfibrils. Peptides lacking the charged domains instead formed irregular aggregates. Thus, this bipartite sequence pattern from CP-19 and homologous 19 kDa sequences from other CPs were suggested to form the molecular basis for the self-assembly of amyloid-like nanofibrils.
Overall, the sequence/structure/adhesive property relationships in barnacle adhesion are not as established as in mussel adhesion, but there has been significant progress in recent years. The functional role of amyloid-like nanofibrils to mediate adhesion is well-accepted, and several CPs have been shown experimentally to self-assemble in nanofibrils under specific conditions, including MrCP-52, MrCP-20, and MrCP-19. Furthermore, enzymatically triggered oxidative cross-linking appears to play an important role in stabilizing and curing the overall cement. These molecular principles may be exploited in synthetic bioadhesives in the near future.
3.3.3. Sandcastle Worm Cement Proteins
Sandcastle worms, Phragmatopoma californica (Fewkes), use protein-based glue to bond mineral particles together (Figure 7C-i).353−355 The glue is composed of distinct sets of condensed polycationic and polyanionic proteins and polysaccharides with particularly high charge densities.293 The polycations and polyanions lead to complex coacervation, a form of LLPS caused by the spontaneous association of oppositely charged polyelectrolytes that is mainly entropically driven by the release of small counterions.
Five main glue proteins, including polycationic Pc-1, -2, -4, and -5 and polyanionic Pc-3, have been identified.293,324,354 The polycationic glue proteins are rich in Lys, His, Gly, and Tyr, with the latter being often post-translationally modified to Dopa in Pc-1 and Pc-2. The polyanionic Pc-3 proteins (Pc-3A and Pc-3B) contain an unusually high content of phosphoserine (pSer) (Figure 7C-ii). Pc-3A contains a pSer enriched N-terminus and a Lys and Arg-rich C-terminal block, whereas Pc-3B is almost entirely composed of 30 to 35 blocks of 6 to 12 pSer separated by a single Tyr. The proteins are synthesized and stored by two major cell types that produce homogeneous and heterogeneous secretory granules, distinguished by their morphology and differential staining. Pc-2 and Pc-5 are located in the homogeneous granules together with a sulfated polysaccharide, while Pc-1, -3, and -4 are located in the heterogeneous granules.293,356 The polyanionic Pc-3A and B proteins are only located in the sub-granule of the heterogeneous granules, with a high concentration of Mg2+ (∼0.5 M) neutralizing the charge of the pSer residues.356,357
In the ocean, the worms build their tubes by applying aliquots of glue on the contact points of the sand grains.353 The adhesive contains two major coacervate components stored in micron-sized droplets. The homogeneous granules contain a uniformly distributed complex coacervate of polycationic proteins Pc-2/-5 and sulfated polysaccharide, whereas the heterogeneous granules contain a single-component coacervate formed by the complexation of pSer-rich Pc-3A/B and Mg2+, surrounded by a matrix of polycationic Pc-1 and -4. Upon glue secretion, the worms simultaneous secret the granules, which may be mechanically captured and mixed, creating a single coacervate phase (Figure 7C-iii).293,356 The functional groups in the proteins and polysaccharides, including primary amines, phosphates, aromatic compounds, and catechols, can all contribute to the adhesive properties of this coacervate. After secretion, seawater triggers a phase inversion due to the substantial changes in the pH and ionic environments between the regulated secretory system and seawater. The worms also co-secrete catechol oxidase into the granules, which provide rapid Dopa oxidation to Dopaquinone and subsequent quinonic cross-linking or curing, as discussed in the mussel adhesive section. Such an enzyme-facilitated cross-linking mechanism can promote curing of the underwater bioadhesive.293 Since its discovery, the sandcastle worm underwater adhesive has been an inspiration source for the design of medical wet adhesives.358−361 The enzymatically triggered curing mechanism can provide additional insights into the development of novel underwater adhesives that require both strong adhesion and rapid curing.
3.3.4. Curli Filaments
The curli filaments are a unique class of extracellular proteinaceous fimbriae functional amyloids (Figure 7D).362−366 Curlis are produced in enteric bacteria such as E. coli and Salmonella spp.362,363,366 They mediate the host cell interfacial adhesion, invasion, and colonization and play a key role in biofilm formation.367−371 Curli filaments are formed on the surface of the Enterobacteriaceae and exhibit a lateral dimension of about 4–7 nm but can expand to a few μm in length (Figure 7D-i).364,372 Such a high aspect ratio results in the formation of a highly entangled and dense interconnected three-dimensional fibrillar scaffold. This facilitates persistent infection by providing high tolerance against various environmental stresses, chemical treatments, host immune systems, and most antimicrobial compounds. Various characterization techniques have identified that proteins in the curli filaments adopt the cross-β-sheet conformation typical for all amyloid fibrils.364,372 Intermolecular β-sheets are highly stable structures and arranged along the filament axis with the β-strands aligned perpendicularly to the filament axis,373−376 enabling curli filaments to resist harsh chemical treatments that readily dissolve most proteins.
The formation of curli filaments involves a complex but highly optimized molecular machinery (Figure 7D-ii). Each curli filament consists of two subunits, CsgA and CsgB (Figure 7D-iii).377−379 Both subunits are extracellular secreted proteins with similar MWs of about 13 kDa.380 CsgA and CsgB both consist of Gln/Asn-rich repeat motifs, a typical biochemical characteristic of most amyloids. CsgA constitutes the major portion of the filaments, whereas CsgB represents only a minor contribution. In vivo polymerization of CsgA exhibits a nucleation-dependent assisted kinetic.377−382 CsgB acts as a precursor that initiates nucleation and growth of the CsgA into extended filaments at the surface of the bacterial cell. The initiation of curli formation occurs only when both proteins are co-expressed. Given that CsgA shows an intrinsically disordered confirmation, it appears that CsgB guides CsgA into a conformation that can link onto other copies of CsgA.377−382 Without CsgB as a nucleation protein, secreted CsgA in the extracellular milieu remains soluble without undergoing polymerization.380 This seems to be an evolutionary adaptation since the cells can control when and where to trigger the polymerization of curli. The added advantage of using an auxiliary nucleation protein is to ensure that polymerization of CsgA will be regulated outside the cell, in contrast with disease-related amyloid proteins associated with many pathological conditions in humans such as Alzheimer’s disease, Parkinson’s disease, and diabetes.383,384 Pathogenic amyloids are formed from aberrant structures that result from protein-folding mishaps. Such proteins uncontrollably and intercellularly self-coalesce into plaques that interfere with the healthy function of cells and ultimately result in cell death.373−376
The overall process of the curli’s self-assembly pathway is efficiently controlled by an outer membrane-bound oligomeric lipoprotein called CsgG,381 which ensures extracellular transportation and stabilization of both CsgA and CsgB to the cell surface.381 Other chaperone-like proteins are also found to be involved in curli’s’ biogenesis pathway.382,385 CsgE and CsgF are shown to interact with and assist CsgG at the outer membrane. Cooperatively, they ensure that the secreted nucleation protein CsgB is properly localized at the interface of the cell and they also provide CsgB with protease resistance to avoid premature fibrillation termination.382,385 However, to avoid excess periplasmic CsgA amyloidosis, cells use an inhibitory chaperone known as CsgC.386 CsgC transiently interacts with prefibrillar CsgA, which in return suppresses its progression and discontinues the polymerization. The overall process of the curli formation is tightly regulated by a master transcription factor regulator, i.e., CsgD.386 CsgD regulates the CsgBAC operon that promotes transcription of both CsgA and CsgB.387 CsgD also regulates the post-transcriptional regulator of cellulose synthesis adrA.388,389 Therefore, CsgD controls and modulates two major structural components of the biofilms, curli and cellulose.
3.3.5. Hydrophobins
Hydrophobins are a group of secreted surface-active amphiphilic structural proteins produced by most filamentous fungi with surfactant-like activity (Figure 7E).390,391 They form a hydrophobic coating at the interface of the hyphal or conidia wall (Figure 7E-i).392 However, they can also self-assemble at any hydrophilic–hydrophobic surfaces.393−395 The primary function of hydrophobins is to repel water but also to mediate the communication of the hyphal with their surrounding environment during growth and development.396 For example, they are found to be involved in the formation of aerial structures and the attachment of hyphae to hydrophobic surfaces.393,397 In pathogenic fungi, hydrophobins are involved in masking recognition by the immune system, therefore preventing immune response.398
There are two classes of hydrophobins, each with very different biophysical characteristics, i.e., Class I and Class II (Figure 7E-ii and 7E-iii). Such classification has been introduced to account for distinct differences in their sequence, molecular structure, hydropathy plots, and solubility that change the overall self-assembly of each hydrophobin class.399 Class I hydrophobins typically form highly insoluble amyloid-like rodlets at interfaces. In contrast, Class II hydrophobins form highly ordered 2D crystalline monolayers. Recently, intermediate hydrophobins have been discovered that share the structures and functions of both Class I and Class II.400−403 Hydrophobins generally have a relatively small MW of about 8–10 kDa and contain eight conserved Cys residues398,404 that stabilize their amphipathic structure through four disulfide bridges.295,405−411 These disulfide bonds are significantly important. They provide not only structural stability but also conformational plasticity to efficiently self-assembles and packs the proteins with the interacting interface. The role of the disulfide bonds differs between the two classes.295,405−411 Class I needs disulfide bonds to maintain solubility without affecting the overall self-assembling. In contrast, Class II is required not only for structural stability but also for function during interfacial assembly.
3.3.6. Caddisfly Silk
Caddisflies (Figure 7F) are aquatic insects that belong to the Triochptera order. In the larva stage, they secrete an underwater fibrous casing decorated with solid sediments for protection and food storage,412,413 as schematically illustrated in Figure 7F-i. Due to the homologies with B. mori silk (see below), the fibrous proteins are called caddisfly silk fibroins.
Constructing gland-specific cDNA libraries and probing them by RACE PCR, Yonemura et al.413,414 disclosed the first sequences of caddisfly fibroins (from the species Hydropsyche angustipennis). Later on, fibroin sequences from Stenopsyche marmorata (S. marmomrata) were obtained using a similar approach by Wang et al.,415 whereas Ashton et al.297 investigated fibroins from the case maker Hesperophylax consimilis (H. consimilis) species by a transcriptomic and MS/MS approach, the latter allowing researchers to identify PTMs. In all these species of caddisfly, fibroins share homology with B. mori silk, being composed of heavy (H) and light (L) chains larger than 300 kDa and of 25 kDa, respectively. The H-fibroins all share an overall primary structure consisting of non-repetitive C- and N-termini that flank a long central and repetitive domain made of alternating hydrophobic and hydrophilic blocks. However, there are also significant differences with B. mori silk, notably the conspicuous absence of poly(Ala) and poly(Gly-Ala) blocks that are a hallmark of the central repeat sequences of B. mori and spider silks. Caddisfly fibroins also contain a much higher content of basic residues, which may have important implications for fiber assembly and extracellular stabilization. Furthermore, structural and biochemical investigations (using EM, immunostaining, and tandem MS/MS) by Stewart and Wang412 revealed that Ser residues of caddisfly silk fibroins are densely phosphorylated, which is likely an adaptation to the underwater environment in which caddisfly larvae develop.
A representative molecular architecture of a caddisfly H-fibroin for H. consimilis is shown in Figure 7F-ii. The primary structure consists of a block copolymer-like architecture made of large blocks called “D”, “E”, and “F” (Figure 7F-iii), which are themselves constructed from 6 types of smaller sub-repeat motifs, as illustrated in Figure 7F-iii: two of these motifs are negatively charged and enriched in Ser, two are positively charged, and two are hydrophobic. Noteworthy features of these motifs are the following: one of the negatively charged motifs contains 25% of PSer; the positively charged motifs are very rich in Arg (ca. 50%), whereas the hydrophobic motifs are very distinct from each other. The first one is a relatively short, 11 residue motif with sequence [RIVTPGVYTKI], while the second, longer motif contains multiple copies of the tripeptide GGX, where X is often L or V.
In a series of studies, Stewart and co-workers297,416−418 have revealed the central molecular structure–property relationships of caddisfly silk. Thus, Ca2+ ions, that are present in nearly stoichiometric concentration with phosphate groups, were found to mechanically stabilize caddisfly silk fibers.416 During tensile stress–strain testing, native fibers exhibit maximum tensile strength on the order of 30–40 MPa with a marked mechanical hysteresis during cyclic testing. Once treated with EDTA (which chelates Ca2+ ions), the fibers lost all mechanical integrity and were no longer birefringent, and the amount of β-sheets decreased as measured by FTIR. Complementing these results with MD simulations, a model of antiparallel β-hairpins constructed from the motif [PSerX]4 located in the D sub-repeat of H-fibroin was proposed, whereby Ca2+ ions electrostatically bridge adjacent hairpins as illustrated in Figure 7F-ii. Additional WAXS and 31P NMR studies416,417 confirmed the stabilization role of Ca2+/PO43– interactions: upon Ca2+ chelation, PSer transitioned from a rigid environment assigned to β-sheets to a flexible one. In addition, WAXS spectra confirmed the fibers to be semi-crystalline with a D-spacing consistent with β-sheets. Interestingly, in B. mori and dragline spider silk, β-sheet crystallites are assigned to interdigitation of poly(Ala) and poly(Gly-Ala) domains, which are absent in caddisfly fibroins. Thus, the authors suggested that in caddisfly silk, the (Ca2+/[PSerX]4) motif has replaced the poly(Ala) and poly(Gly-Ala) domains to construct β-sheet crystallites. Further, these interactions have been shown to be reversible and Ca2+ can be substituted with other multivalent ions such as Mg2+ and Fe3+.417 In subsequent research,418 the mechanical hysteresis and viscoelastic behavior of caddisfly fibers were attributed to the presence of double Ca2+/stabilized networks. The first network is formed by reversible Ca2+/phosphorylated Ser bonds mentioned above, whereas the second network occurs by complexation of Ca2+ with carboxylate groups from the N-terminus of H-fibroin. Upon uniaxial loading, the initial linear elastic regime was followed by pseudo-yielding above a stress threshold, which was attributed to rupture of Ca2+-containing sacrificial bonds forming the double networks, leading to dissipation of strain energy. While immediate loading cycles did not follow the initial loading/unloading cycles, the mechanical response could be almost fully recovered after some time owing to the reformation of the Ca2+-mediated reversible bonds, a behavior that parallels His-metal coordination bonds of mussel byssal fibers.245,253 Overall, caddisfly silk fibroins may thus provide molecular design guidelines to fabricate self-repair fibrous materials with a high capacity for absorption of mechanical energy.
3.3.7. Comparative Overview
Biological adhesives have gathered increased fundamental research interest in the past decades. Mussels widely use post-translated Dopa in their adhesive proteins to enhance wet-resistant adhesion, but recent studies have indicated that non-modified aromatic residues may be as efficient313,323,419 for this function. Dopa is also found in the sandcastle worm cement proteins, where it may have a similar physicochemical role in addition to trigger cross-linking and curing the adhesive cement. Another abundant post-translational modification of sandcastle worm adhesive proteins is phosphorylation, which is used to regulate gelation kinetics and to form fluid-like complex coacervates that facilitate wetting and the formation of a concentrated cement. Phosphorylation is also highly used in caddisfly silks, albeit for a different purpose as it mediates electrostatic interactions with Ca2+ to form reversible networks that increase mechanical dissipation of the fibers. Phosphorylated proteins have also been identified in the permanent adhesive of barnacle cyprids, but their function remains unclear. In adult barnacles, the permanent adhesive is made of amyloid-like (cross-β) nanofibrils, and an increasing number of barnacle CPs have been documented to be able to self-assemble into amyloids, a design strategy that is used by other organisms for adhesive purposes. Notably, adhesive curli fibers as well as hydrophobins I are also made of elongated cross-β nanofibrils. Dopa is absent as a post-translated modification of CPs, but in recent studies the involvement of low-MW catechols in hardening and sclerotization has been established.
The biofabrication of bioadhesives also shares commonalities across the living world. Thus, coacervation has been identified in the sandcastle worm324,420 and mussel adhesive proteins.285,304,306 At least one barnacle CP can form microdroplets in the presence of inorganic ions339 before transitioning into nanofibrils, suggesting a similar processing pathway. Although the presence of a phase-separated intermediate has not yet been identified in caddisfly silk, the homology of caddisfly silk fibroins to other silks that are known to self-assemble via LLPS205 makes it possible that this mechanism also operates in this case.
Clearly, these studies have opened many opportunities in biomimetic engineering of bioadhesives, and a wide range of molecular designs exists that can be replicated in synthetic analogs.
3.4. Proteins Assembling into Bulk Materials
The extracellular proteins described in Sections 3.1 to 3.3 are mostly exposed to tensile loading in their natural environments (such as fibrous and elastomeric materials, except for abductin, which has a compressive loading functionality) or are deposited as thin adhesive layers. While these structures are the most abundant and studied proteinaceous materials, there also exist “bulk” biological materials containing a high volume fraction of proteins and that are subjected to compressive or shearing mechanical loading regimes, notably exoskeletons and mouthparts. In many living organisms, such functionality is usually obtained through biomineralization, such as vertebrates’ bones and teeth or seashells. These types of structures have been abundantly reviewed elsewhere421−424 and are not covered here. We instead concentrate on biomacromolecular structures in which proteins are the dominant or even the only phase, and we focus on the description of their primary structures that can potentially be reproduced artificially. For a detailed description of their biophysical and mechanical characteristics, the reader is referred to other recent reviews.422,425−427
3.4.1. Marine Worm Jaw Proteins
Polychaetae are carnivorous annelid worms that live in the intertidal benthic mud. Their proboscises contain hard jaws (Figure 10) that are used to grasp prey and inject venom during hunting. Studies initiated by Lichtenegger et al.428,429 showed that the jaws of two species of polychaete, the bloodworm Glycera dibranchiata (G. dibranchiata) and the scavenger clam worm Nereis virens (N. virens), are largely unmineralized and mostly organic-based yet exhibit a mechanical performance that can compete with hard polymers and even mineralized structures.425,430 The main components of the jaws are species-specific proteins that are reinforced with different transition metals, Cu for Glycera jaws and Zn for Nereis, which are present as ionic ligands and as a mineralized phase. The biomolecular composition of both materials is now presented, with emphasis on the molecular design of the protein components.
Figure 10.
Hard bulk materials predominantly made of proteins. (A) The polychaete bloodworm (Glycera dibranchiata) is equipped with two pairs of hard jaws (i) for grasping prey. (ii) The jaw is a composite material made of at least one multi-task protein (MTP), melanin, Cu2+ ions, as well as the Cu-based atacamite biomineral nanofibers (not shown in this cartoon). The melanin network is interspersed with MTP and Cu2+. (iii) MTP is highly enriched in Gly and His residues arranged in short modular tri- and tetrapeptide repeats. The chemical structure of melanin is also shown. (ii) and (iii) Adapted with permission from ref (431). Copyright 2022 Elsevier. (B) The Nereis genus of polychaete (i) has one pair of jaws. (ii) The jaws are made of cross-linked proteins and Zn2+ ions. In the mature state the proteins are mostly in the β-sheet conformation and form a cross-linked network with Zn2+ ions as coordination centers. Adapted with permission from ref (432). Copyright 2008 American Chemical Society. (iii) Nereis jaw proteins are highly enriched in Gly and His residues, the latter forming coordination bonds with Zn2+ ions. (C) The exoskeleton (cuticle) of insects, here represented by a beetle, is a multi-layer composite (i) structure. The procuticle is divided into different layers (exo-, meso-, and endocuticle) made of chitin and proteins in different ratios and levels of cross-linking depending on the layer. (ii) Cuticular proteins (CuPs) contain chitin-binding domains (CBDs) that fold (a CBD from D. gigas beak is illustrated, left) in the presence of chitin. A predicted CBD/chitin interface is also shown (right). Reproduced with permission from ref (433). Copyright 2021 Elsevier. (iii) A common primary sequence feature of cuticle proteins is the RR2 consensus. (D) Squids are equipped with two hard biotools for predatory purposes. (i) D. gigas squid beak is a biomolecular composite of proteins and chitin that are distributed in a graded fashion along the beak (ii). Adapted from ref (136). (iii) Two protein families are found in the squid beak, namely chitin-binding proteins (here illustrated with DgCBP-1, with CBDs shown as orange rectangles) and His-rich proteins (here illustrated with DgHBP-1). DgCBPs are most abundant in the proximal, soft region of the beak, while DgHBPs are more concentrated in the hard tip (rostrum) region, where they are heavily cross-linked. The C-termini of DgHBPs are highly repetitive, containing multiple copies of pentapeptide motifs enriched in Gly, His, Tyr, Phe, and Ala. (iv) The second hard tissue in squids is sucker ring teeth (SRT) that are entirely made of a protein family dubbed suckerins. (v) Suckerins self-assemble into hexagonally packed fibrils made of nanoconfined β-sheets. Adapted from ref (434). Creative Commons CC BY. (vi) Suckerins exhibit a block copolymer primary structure with alternative blocks of β-sheet forming domains enriched in Ala, His, and Thr and longer amorphous domains enriched in Gly and Tyr. Adapted with permission from ref (134). Copyright 2014 American Chemical Society.
3.4.1.1. Glycera Jaw Proteins
Glycera worms are equipped with two pairs of jaws (Figure 10A-i) consisting of an organic matrix made of proteins (ca. 45 wt %) and melanin (ca. 40 wt %), within which Cu is incorporated both in the Cu-containing mineral atacamite (Cu2Cl(OH)3) and as coordinated ions (Figure 10A-ii). Atacamite takes the form of elongated crystallite fibrils preferentially oriented parallel to the jaw surface, which were initially proposed to enhance the abrasion resistance.428 It was later recognized through nanoscale scratching and wear testing435 that atacamite fibers do not in fact improve the tribological performance of the jaws and are more likely to increase their bending resistance. Instead, the abrasion resistance is higher in the surface layer435 enriched with unmineralized Cu ions that are expected to form coordination complexes with His residues from jaw proteins. In support of the extreme stability of Cu complexes providing mechanical strengthening, EDTA treatment cannot remove Cu ions from the Glycera jaw.436 Another intriguing aspect of Glycera jaws is the significant contribution of melanin to the mechanical integrity of their jaws. Indeed, following protein hydrolysis the jaws retain their shape and microstructure whereas their hardness (H) and elastic modulus (E) are only retained by 50%.
The protein composition was found to be dominated by two amino acids, Gly and His, comprising ca. 50 mol % and 30 mol %, respectively,428,436,437 with the remaining amino acids being Ala, Asp, and Lys. The complete amino acid sequence of Glycera jaw proteins was only recently obtained by Wonderly et al.431 using the strategy described in Figure 2. The team built a transcriptome of the pulp tissue where the jaws are secreted and interrogated it with short peptides isolated from the mature jaws. As additional criteria, they searched for fully assembled transcripts with a high Gly and His bias, containing start and stop codons, as well as a signal peptide. With this approach, they identified the main Glycera jaw protein, dubbed “multi-tasking protein” (MTP), having a MW of 30 kDa. As expected from earlier amino acid composition measurements, MTP is highly enriched in Gly (49 mol %) and His (40 mol %), with the remaining being the charged amino acids Asp, Glu, and Lys as well as the hydrophobic Ala. Interestingly, long stretches of poly-Gly or poly-His, which may have been anticipated from the amino acid composition, are completely absent such that no more than two consecutive Gly or His residues are found, with the sequence consisting almost entirely of [GGH/GHG], [GGHH], or [HHG/GHH] repeats interspersed by charged residues and a few isolated Ala (Figure 10A-iii).
Despite this low sequence complexity, MTP features remarkable multi-functional properties. First, it can bind and recruit a high number of Cu2+ ions, which not only enables intermolecular bridging between adjacent chains but also triggers LLPS of the MTP-Cu2+ complexes (Figure 10A-iii). Second, the MTP-Cu complex catalyzes the oxidation of Dopa (independently secreted by melanosomes) in a manner reminiscent of catechol oxidase, a mechanism that may subsequently facilitate sclerotization and hardening of the jaw. Third, the MTP-Cu2+ droplets template polymerization of melanin at the droplets interface or within the droplets, resulting in a viscous solution from which solid films and fibers can be drawn. While the fabrication of these materials is still in the early proof-of-concept stage compared to artificial silk, the MTP-Cu2+ complex system provides new insights for bioinspired polymer fabrication using a relatively simple protein design.
3.4.1.2. Nereis Jaw Proteins
Nereid polychaete have a pair of fang-like jaws that are used as primary tools for feeding and defending (Figure 10B-i). These jaws are fiber-reinforced biomolecular composites containing proteins, Zn2+ ions, and halogens such as Cl, Br, and I429,438,439 that are arranged as bundles of fibers made of closely packed fibrils aligned parallel to the long axis of the jaw. Zn ions account for 2% of the mass of the jaw440 and have been found by X-ray adsorption spectroscopy (XAS) to be present in a single metal coordination environment. Each Zn2+ is coordinated by three His residues (Figure 10B-ii) and a chloride ion.429,441 This Zn2+-mediated three-coordinated cross-linking has been confirmed by DFT calculations.442 The content of Zn increases toward the tip and cutting-edges of the jaw and correlates with increasing hardness H and modulus E, indicating that the Zn2+-histidine metal chelation is essential to the impact-resistant properties of the jaw.89,443 The importance of metal chelation was further supported by careful chelation experiments, whereby Zn2+ ions were removed by EDTA treatment, resulting in ca. 80% decrease in E and H as measured by nanoindentation. These properties could be almost fully recovered by reintroducing not only Zn2+ into the jaw but also other divalent transition metals such as Cu2+ and Mn2+.443,444
Proteins are the most abundant component of N. virens jaws, accounting for ∼90% of the total mass.444 The distribution of His and Ala residues within the jaw is spatially graded with almost complementary trends, suggesting gradients of proteins within the jaws. The complete sequence of N.virens jaw proteins (NVJPs) has so far not been obtained because sequencing efforts of these proteins precluded the advent of RNA-seq and transcriptomics for biomaterials. Nevertheless, Broomell and Waite have obtained the nearly complete sequences of the main NVJPs.432 They constructed a cDNA library of the pulp that secretes the jaws, which was probed with short peptides purified from the jaw material that were sequenced using Edman sequencing and MS/MS. The main NVJPs, which are highly similar and likely isoforms, have predicted MWs of 32 to 40 kDa.
The partial sequence of NVJP-1a is shown in Figure 10B-iii. While NVJPs share some similarity with MTP, in particular high His (27 mol %) and Gly (36 mol %) contents that are often present as variations of Gly- and His-rich repeats such as [GGH] or [GH)]n (where n = 1 to 5), there are significant differences both in terms of amino acid composition and primary structure. NVJPs contain a broader diversity of amino acids, notably the acidic residues Asp (9.1 mol %) and Glu (6.4 mol %), as well as the aromatic residue Tyr (7.3 mol %), a feature shared with squid beak proteins discussed in Section 3.4.3. In the N-terminus, Tyr residues are very often flanked between two Gly residues (in motifs such as [GYGHG] or [GYGGH]) or by a Gly and an Asp residue (DYG motif). Unlike MTP, occurrences of poly-Gly longer than two residues long are found, as well as His-rich motifs free of Gly. Recombinant NVJP-1 was produced in the same study and displayed mainly random coil and extended turn structures at pH 5 as measured by CD and FTIR. However, increasing the pH to that of seawater resulted in a structural transition toward β-sheet-rich structures. Furthermore, incubation with Zn2+ triggered its self-assembly into amyloid-like fibrils that remained insoluble even after re-acidification of the medium.432 Recombinant NVJP-1 has been shown to be photo-cross-linkable to form hydrogels that partially dehydrate and contract upon introduction of Zn2+.442
3.4.2. Insect Cuticle Proteins
The exoskeletons of arthropods, also called cuticles, have been extensively investigated for several decades, starting with the earlier studies by Pryor.445−448 Their primary function is to act as barriers against the natural environment and to protect the organism against chemical, biological, and mechanical degradation. This central requirement has made biochemical and molecular characterizations of cuticles a daunting and slow task since it inherently renders the materials highly resistant against isolation of their biomolecular building blocks, although recent developments facilitate such work, as described in Section 3.4.3 below. Insect cuticles, exemplified in Figure 10C-i for a beetle, are essentially biomolecular composites consisting of units of the polysaccharide chitin (N-acetyl-glucosamine) bonded together by hydrogen bonds, forming nanofibrillar crystallites that are embedded within a protein matrix (Figure 10C-ii).448 Cuticular proteins (CuPs) are particularly challenging to study owing to their highly sclerotized nature; that is, the protein chains are densely covalently cross-linked222,449,450 through complex quinone-based oxidative chemistry involving catecholamines, in particular N-acetyldopamine (NADA) and N-β-alaninedopamine (NBAD) that are incorporated inside the cuticular matrix and subsequently oxidatively cross-linked, leading to cuticle stabilization and hardening (Figure 5D-i to 5D-vi). Using solid-state and 2D NMR, ESI MS/MS, and MALDI-TOF, it has been demonstrated that catecholamines can also form adducts with His residues of cuticular proteins through Michael addition on either nitrogen of the imidazole ring of His side-chains.223,451−455 The different types of His-catecholamine cross identified in insect cuticles are shown in Figures 5D-vii to 5D-ix.
With the rapid improvement and availability in sequencing and proteomic technologies in the past decade, thousands of CuPs coding genes have been identified in arthropods456−458 and added to sequence databases459 (http://aias.biol.uoa.gr/CutProtFam-Pred/home.php). Despite this abundance, many CuPs exhibit commonalities at the primary structure level, which were initially assigned to two protein families460 but later expanded to several more.461 We restrict our description to the most abundant families of CuPs, namely those containing the well-established RR consensus domains that play the key structural function of binding to chitin.462,463 The RR-1 domain was identified in 1988464 in several CuPs and consists of a 35–36 amino acid motif with the following consensus:
This RR-1 consensus was later revised as the extended or RR-2 consensus, which is found in a wide range of insects, crustaceans, as well as mollusks as highlighted in Figure 11:
Figure 11.
Sequence homology of CPs from different arthropods containing the RR consensus (green residues). Reproduced with permission from ref (433). Copyright 2021 Elsevier.
Given the central role of His in catecholamine-inducted cuticle sclerotization, a hallmark of many CuPs is their high His content. For example, in B. mori and the tobacco cutworm (Spodoptera litura) the majority of CuPs are His-rich, with a His content ranging from 6% up to a staggering 45%. These CuPs belong to the RR-2 family, but it is important to note that the RR-2 consensus itself is not enriched in His residues (see Figure 10C-iii and Figure 11), which are instead segregated in other regions of the primary structure, suggesting that chitin-binding and sclerotization occur in distinct domains of CuPs. Interestingly, the high His content is a recurrent feature of many other proteins identified in hard proteinaceous materials430 due to biochemical features of the imidazole side-chain that are discussed in Sections 3.4.3 to 3.4.5.
3.4.3. Squid Beak Proteins
Cephalopods (squids, octopus, and cuttlefish) are soft-body animals, with the exception of two hard biopolymeric structures that have garnered significant interest from a bioinspired material perspective in the past decade, namely their beaks (Figure 10D-i) and their SRT, found in squids and cuttlefish but not in octopuses (Figure 10D-iv). Extensive biomechanical and biochemical investigations by our team have revealed that the squid beak of D. gigas, despite its hunting and chewing functionality, is wholly organic and does not contain any mineral phase.7,137,138 In contrast to polychaete jaws, it is devoid of transition metals. On the other hand, and common to insect cuticles, the beak is a biocomposite made of densely cross-linked proteins and chitin nanofibrils, which are gradually distributed to form a biomechanically graded structure137 with a roughly 2-order of magnitude variation in mechanical properties from the soft proximal part to the hard distal tip (called the “rostrum”), illustrated in Figure 10D-i. Deeply embedded and attached within the buccal musculature465,466 are the soft proximal wings, which mostly consist of hydrated chitin and chitin-binding proteins. Moving along the increasingly hard rostrum, the relative protein content increases together with the cross-link density, whereas at the same time the bound water is removed from the chitin scaffold by a molecular dehydration process. The hardness H (0.5–0.87 GPa) and elastic modulus E (4–7 GPa) of the hard rostrum in hydrated conditions are on par with those of hard thermoset polymers, combined with a higher fracture resistance,7 which is noteworthy given the inverse correlation between hardness/modulus and fracture toughness, including for biological materials.467 The correlation between the gradients in mechanical properties and biomolecular components is not intuitive. Since chitin is as a load-bearing fibrous polysaccharide, one would expect the relative content of chitin to be higher near the hard rostrum region, but the opposite is seen in the beak, with the proteinaceous component clearly dominant in the rostrum (Figure 10D-ii). This can be explained by the chemical nature of the cross-links, which occur by covalent bonding between squid beak proteins. Thus, hardening has been proposed to occur via a dual mechanism of interprotein cross-linking that concomitantly induces chitin fibril dehydration (Figure 10D-ii):137 the mechanical properties increase not only because of the formation of a densely cross-linked network but also because during cross-linking, molecular-bound water is evicted from the network owing to the hydrophobic nature of the cross-links. Since dry chitin is stiffer and stronger than hydrated chitin, dehydration of the protein/chitin network increases the mechanical properties of the composite. Furthermore, dehydration of the cross-linked network is largely irreversible, explaining the moderate decay in mechanical properties of the rostrum under hydrated conditions.7,137
The major cross-links in the squid beak have been identified by MS/MS and solution NMR of acid-hydrolyzed samples.138 They consist of a family of His-catechol adducts, with the catechols arising both from low-MW phenolic compounds as well as from Dopa side-chains (Figure 5E). These cross-links, which remain insoluble after hard acid hydrolysis, not only provide mechanical stability but are also responsible for the dark pigmentation of the beak.137 They also precluded early attempts to sequence squid beak proteins since the dense cross-linked density thwarted protein extraction. This challenge was overcome as discussed in Section 2.5 using the integrative approach shown in Figure 2 and led to the discovery that proteins comprising the squid beak can be divided into two main families.136 The first family comprises four chitin-binding proteins (CBPs, Figure 10D-iii) based on the predicted presence of chitin-binding domains (CBDs), which was also corroborated by a lectin-binding assay specific to chitin oligomers.468 It is noteworthy that the RR2 consensus of arthropod cuticles was identified in a chitin-binding domain of CBP-3.433 In this study, chitin oligomers were shown by solution NMR to strongly interact with the CBD (which harbors the RR-2 consensus) and to trigger a conformational transition of this domain into an antiparallel β-sheet/α-helix fold containing a hollow groove, in which chitin fibrils can be stabilized (Figure 10D-ii). The CBP/chitin complexes have been proposed to form the beak scaffold within which the second family is incorporated during growth.
The second family comprises 3 proteins (and multiple isoforms) enriched in Gly (20–35 mol %), Ala (16–19 mol %), and His (10–16 mol %) amino acids and to a lesser extent in Tyr and Phe. They have been named His-rich beak proteins (HBPs) due to the key role of His in regulating pH-induced LLPS. They share a two-domain architecture (Figure 10D-iii), consisting of a non-repetitive N-terminal domain with stretches of Ala- and His-rich regions, and a highly repetitive C-terminus domain made of tandem pentapeptide repeats enriched in Gly, Ala, His, Tyr, and Phe. More specifically, two types of pentapeptides have been identified: [GHGXY] or [GHGX′Y], where X is a hydrophobic residue, usually Val and Leu, and X′ is generally Gly or Ala; and [GAGFA]. The modular regions of HBP-1 and -3 are constructed from both types of pentapeptides, whereas HBP-2 only contains the first type, making it more hydrophilic since it lacks the more hydrophobic motif [GAGFA]. Since the pentapeptide repeat motifs enriched in Gly- and hydrophobic-rich residues share some analogy with the tandem repeats of ELPs, it was postulated that HBPs may also exhibit self-coacervation. This was demonstrated both for full-length HBPs as well as for shorter consensus peptides (HBpeps) made of a limited number of pentapeptide motifs.136,469,470 There are however two key differences with the phase behavior of ELPs. First, HBPs and HBpeps feature an UCST; that is, above a critical temperature the coacervates disassemble and the proteins or peptides form a homogeneous solution.471 And second, their phase behavior is pH-dependent due to the high content of His, which is the only charged residue in wild-type pentapeptide repeats of HBPs. With a pKa of ca. 6.5, the repeats are positively charged in acidic conditions and thus repel each other to maintain solubility. Around neutral pH, the imidazole cycle becomes deprotonated, in turn enabling HBPs and HBpeps to self-associate through hydrophobic and π–π interactions arising from Tyr residues, leading to phase separation. In HBpeps, the pH at which phase separation occurs can be finely tuned by single mutations or by varying the number of tandem repeats, offering interesting possibilities for “smart” drug delivery applications.472 Since the phase behavior of HBPs and HBpeps is regulated by electrostatic interactions, it can also be modulated by varying the ionic strength of the buffer solution.473
A systematic study of HBpeps469 identified the motif GHGLY as critical to drive LLPS, with at least two motifs required to induce phase separation that have to be separated by a flexible linker region a few amino acids long. In the same study, solution NMR studies precisely identified the molecular mechanism of pH-induced coacervation: it is triggered by His-Tyr transient H-bonding under acidic pH, followed by π–π stacking between Tyr side-chains as the pH raises, emphasizing the critical role of both His and Tyr amino acids in the phase separation process. Owing to their relatively simple sequence complexity, HBP derived peptides have emerged as convenient model systems to study the effect of single amino acid mutations on the phase behavior. For example, our team found that His and Tyr residues located near the peptides’ termini drive phase separation, prompting us to suggest that these residues mediate the formation of a molecular topological network, which upon expansion forms the microdroplets. In addition, the incorporation of the more hydrophobic pentapeptide GAGFA470 or even single-amino-acid mutations474 (such as Phe → Ala) significantly alter the viscoelastic properties of the droplets, allowing researchers to tune the viscosity and storage modulus or trigger phase transitions of liquid droplets into hydrogels.
Based on the coacervation properties of HBPs, the current model of squid beak biofabrication136 is parallel to synthetic composites produced by infiltration of a fibrous preform with a polymer resin. In this analogy, shown in Figure 10D-ii, the scaffold (or preform) consists of the chitin/CBPs complexes and forms a flexible, hydrophilic, and transparent material. HBPs coacervate microdroplets can then wet and infiltrate this scaffold, which is enabled by the liquid-like nature of coacervates with low interfacial tension as well as their shear-thinning behavior postulated to facilitate flow through the porous scaffold. In the last step, sclerotization (curing by interprotein cross-linking) occurs, either spontaneously at the basic pH of seawater or assisted by catechol oxidase, resulting in the cross-links shown in Figure 5E. We emphasize that this simplified model of beak growth has not been experimentally validated, which is extremely challenging since it would require dissection of beaks from animals at different stages of development. Studies on smaller species such as the common squid (Loligo vulgaris) may be able to address this question in the future.
3.4.4. Suckerins
The second type of hard protein polymers evolved by squids and cuttlefish for their predatory strategies are SRT (shown in Figure 10D-iv) that are encased within the sucker cuptions lining up their arms and tentacles. In the large D. gigas, each arm and tentacle contains hundreds of SRT, such that a single animal is armed with thousands of razor-sharp SRT to latch and hold firmly onto prey. In contrast to the squid beak, SRT are entirely proteinaceous with a heavy bias toward Gly, Tyr, and His135 but do not contain polysaccharides. In addition, and unlike polychaete jaws, transition metals are also absent. A remarkable and unique feature for a biopolymer-based hard tissue is that SRT consist of a supramolecular network held together by a high density of weak interactions, without any intraprotein covalent bonding stabilizing the structure. This was demonstrated by the pliable nature of SRT in a hot water bath—making SRT a thermoplastic protein polymer—and by the fact that SRT are fully soluble in chaotropic solutions such as urea or formic acid, but without protein degradation.135 Despite the absence of interchain covalent linkage, SRT exhibit high mechanical properties with an isotropic elastic modulus on the order of 7–7.5 GPa and 2–2.5 GPa in the dry and hydrated states, respectively. Exploiting the combined RNA-seq/proteomics approach, Guerette et al.131,134 demonstrated that SRT are entirely assembled by a protein family called “suckerins”. In D. gigas, 21 suckerins have been identified, with the majority sharing a modular architecture of primary structure consisting of short [M1] modules that are typically 10–15 amino acids long (enriched in Ala, Thr, and His) and longer [M2] modules 20 to 30 amino acids long rich in Gly, Tyr, and Leu as highlighted in Figure 10D-vi. The sequence motif [AATAVSHTTHHA] is the most prominent in the M1 module while the M2 module contains multiple copies of the tripeptides [GGY], [GHY], and [GGL].131,475 Another key feature, which critically influences the nanostructure and mechanical properties of SRT, is the presence of Pro residues that usually separate the M1 and M2 modules. As a result, most suckerins can be represented with the following general sequence design: [Pro-(M1)-Pro-(M2)]n, where n = 3–13 depending on the individual suckerin.
Using WAXS and Raman spectroscopy,134,256 the central structural component of SRT has been found to be randomly oriented β-sheets of well-defined nanoscale size, about 2.5 nm wide and 3–3.5 nm long, embedded within an amorphous matrix, resulting in an overall structure that can be defined as a semi-crystalline biopolymer with thermoplastic characteristics.434,476 Critically, the well-defined size of β-sheets has been proposed to be regulated by Pro: indeed, the length of M1 modules flanked by a Pro residue on each side is highly conserved at 12–13 residues.134 Since Pro is a well-known β-sheet disruptor,235 it restricts the size of M1 modules to ca. 10–11 amino acids, which corresponds to 3.1 to 3.5 nm long in the extended conformation state. The β-sheet propensity of M1 modules was corroborated by investigating short peptides from this module, which were shown to self-assemble into amyloid-like fibrils.475,477 SAXS studies434 further indicated that the randomly oriented β-sheets of SRT are organized at the next hierarchical level into a hexagonally packed nanofibrillar lattice, as illustrated in Figure 10D-v. Using all atom discrete MD (DMD) simulations, Ding and co-workers478,479 later confirmed the self-assembly of the [M1-M2] block copolymer architecture of suckerins, in particular corroborating that [M1] modules formed cross-β oligomers whereas [M2] modules exhibited a stronger self-assembly propensity but remained largely unstructured. These computational studies led the authors to propose a refined self-assembly model of suckerins driven by microphase separation between [M1] and [M2] modules, with [M2] acting as molecular glue and confining the β-sheet-rich [M1] modules into amyloid-like nano-assemblies.
3.4.5. Comparative Overview
Structural proteins that are the dominant components of bulk biopolymeric materials share noteworthy commonalities. First, all these proteins without exception are enriched in His and Gly amino acids. The recurrent theme of His enrichment in many sclerotized proteins430 reflects the chemical versatility of the imidazole side-chain in mediating inter- or intraprotein interactions. With a pKa of 6.5, the imidazole moiety is the only amino acid that is titratable around physiological pH, such that in the acidic intracellular environment it is protonated, whereas at the neutral or basic pH of the seawater environment it is partially deprotonated. As His is often the dominant charged residue (as is the case in many of the examples highlighted here), this may allow proteins to enhance solubility prior to extracellular secretion through charge–charge repulsion. The higher solubility of His-rich proteins at acidic pH has indeed been demonstrated in suckerins and squid beak proteins,136,473,480 as well as in shorter peptides derived from these proteins.469,475,477,481 Once in the external environment, deprotonation can lead to Michael addition with polyphenols, resulting in interprotein cross-linking mediated by His-catechol covalent adducts, as identified in insect cuticles and the squid beak. Second, deprotonated imidazole can form coordination bonds with transition metals to stabilize the network, which has been identified in polychaete jaws429,432,435,444,482 as well as in the hard coating that protects mussel fibers.87,245,483 More recently, it has been shown in squids’ HBPs that the protonated form of imidazole enables transient H-bonding interactions with H-bond acceptors, in particular with the hydroxyl group of Tyr,469 which was identified to be a critical nucleation step in the pH-induced LLPS of His-rich proteins and peptides. While such interactions have not been explored in other systems, it is plausible they may also exist once carefully investigated. Lastly, in suckerins His residues are located in the conserved β-sheet-forming M1 module475 and provide amphiphilic characteristics to short M1 peptides, thereby enhancing their solubility477 and enabling supramolecular assembly into different fibrillar polymorphisms depending on solvent and concentration.481 His has also been shown by dynamic MD simulations to regulate the pH-dependent fibrillization of M1 peptides.478 From a biomimetic perspective, the pH-responsivity of His-rich proteins and peptides offers exciting opportunities for biomedical applications, including in bioadhesives,419 hydrogels,484,485 and drug delivery systems,471,472,486,487 as discussed in more details in Sections 4.6 and 4.7.
Gly is the other amino acid that is highly abundant in all structural proteins presented in this section, with a content from 20 up to 50 mol % depending on the protein. Gly provides a high level of conformational flexibility and may enable the proteins to maintain intrinsically disordered configurations under given pH and ionic strength conditions. During biofabrication, this feature may be critical to form concentrated phases by a LLPS mechanism triggered by various external stimuli, while preventing premature aggregation. This mechanism has been demonstrated in various recombinant proteins, including pH-induced LLPS in squids’ HBPs,136,470 Cu2+-induced LLPS in Glycera’s MTPs,431 and temperature-induced LLPS in a Gly-rich insect cuticle protein from the wing of the swallowtail butterfly.488 Therefore, it is tempting to suggest that LLPS/coacervation constitutes a widespread strategy by which living organisms construct protein-based hard materials.
It is also noteworthy that squid beak, insect cuticle, and polychaete jaw proteins all contain a significant fraction of aromatic Tyr and Phe, as well as a small content of post-translated Dopa. While Tyr is absent in Glycera MTP, this is largely compensated by the secretion of low-MW polyphenols that are used to create the melanin-based scaffold of the jaw.431 Aromatic residues and low-MW polyphenols are especially critical during sclerotization and hardening of the final hard tissue through quinone-based oxidative cross-linking. In addition, there is increasing evidence that aromatic residues have an important role to play in the biofabrication of hard protein-based materials through their involvement in LLPS of the precursor proteins, in particular by H-bonding and π–π interactions identified in the coacervate phase.469,474 In suckerins, Tyr is absent from the β-sheet forming module M1 and systematically located in the flexible M2 modules,131,484 perhaps also mediating a LLPS process, although this remains to be identified for this protein family. It should be highlighted that LLPS has been proposed to be involved not only in the fabrication of hard bulk tissues but also in bioelastomers (tropoelastin, Section 3.2.1), fibrous proteins (silk and slime of the velvet worm, Section 3.1), and adhesive proteins (MFPs and sandcastle cement, Section 3.3). This suggests that LLPS may be a universal mechanism coevolved by many living organisms to fabricate both hard and soft tissues. Similar to His, the chemical properties of Tyr can also be exploited in biomimetic materials fabrication. Notably, dityrosine cross-linking, induced either photochemically or enzymatically,227,238,484 is a popular gelation mechanism in protein and peptide hydrogels.
4. Bioengineering of Protein-Based Materials
4.1. Silk- and Silk-Based Materials
It can be argued that many petroleum-based synthetic fibers were inspired by silk.489 For example, nylon was invented to replicate the properties of natural silk but also to overcome its techno-economic challenges for mass production. Despite a century of research and innovation into engineering synthetic polymers, natural silk is still preferred due to its unique physiochemical characteristics that are challenging to replicate. Nevertheless, harvesting silk naturally remains economically unviable and it does not provide substantial quantities with consistent qualities, and there is always the risk of pathogen transmission.
One radical solution with disruptive potential is the use of advanced biotechnology platforms with cutting-edge synthetic biology tools. These promising opportunities have not yet been fully exploited. With advancements in synthetic biology, biological manufacture will inevitably become mainstream and replaces much of the current technologies in accordance with the more sustainable use of natural resources. Taken together, its impact is predicted to grow significantly scientifically, economically, and in terms of societal benefits. In the past few decades, microbial recombinant production of silk proteins has taken steps toward this green transition. It has enabled the manufacturing of biosynthetic silk fibers that mimic not only the molecular building blocks but also their key material properties. Interestingly, many of these recombinantly produced silk variants are becoming commercially available for different medical and industrial applications. In this section, we highlight some of the key publications in the field of silk mimicry. It is worth mentioning that most recombinant silk proteins are based on sequences from spider silk rather than silkworm silk. This is mainly driven by the diversity of spider species and silk types but most importantly by the greater mechanical performance of spider silks.
For a few decades, the size, repetitive nature, and overall complexity of the spidroins core sequences created significant challenges that hampered the progress toward bioengineering of silk and silk-like proteins (see Section 3.1.1).490,491 One of the most challenging issues related to the recombinant production of silk proteins is the discrepancy between the spider’s codon usage and the expression host organism. This is exacerbated in bacterial hosts as they inherently exhibit low tolerance to repetitive sequences and actively try to eliminate such repeats through homologous recombination.492 One solution to overcome this challenge was to optimize the dsDNA oligonucleotide sequences to achieve matching codon usage as the host.493 This approach enabled the production of several variants of naturally occurring major ampullate silk originating from A. diadematus commonly known as ADF3 and ADF4.75 A similar approach was also adopted later toward other synthetic spider silk genes.494−496 Today, codon optimization is done more effectively using advanced computational algorithms.497−499 The other major issue with the expression of repetitive spidroin core sequences is their extreme bias toward specific amino acids. Therefore, upregulation in the use of only a subset of tRNA during translation induces stress on the host by interfering with the normal flow of metabolic flux.492 Cells in spider silk glands evolutionarily adapted to maintain their metabolic balance by creating specific tRNAs pools for the most abundant amino acids used in each silk type.500 A similar approach has been shown to be effective for the recombinant expression of silk MaSp1 variants.501,502 This has resulted in the expression of MWs close to those of native spidroins, substantially higher yields, and ultimately better mechanical properties. Xia et al.503 for the first time demonstrated overexpression of glyVXY genes encoding tRNAgly for GGU and GGC triplets resulting in the expression of MaSp1 constructs with 96 repeating modules and a MW of about 285 kDa.
For a long time, the recombinant production of spidroins was centered mainly around the midblock load-bearing domains originating from N. clavipes or A. diadematus.504,505 It took nearly 10 years from the first cloning of spidroin genes by Hinman et al.70 without a terminal domain to make variants flanked with non-repetitive folded N- and C-terminal domains (see Section 3.1.1).493 This enabled investigating the effect of both terminal regions on storage and self-assembly76,493,506 and more importantly the unveiling of how the terminal domains modulate the mechanical properties of the engineered materials.81,84,495 These discoveries led to the production of variants with different lengths, different core repeats motifs, and the presence or absence of N- and/or C-terminal domains. The emerging conclusion from these studies indicated an increase in strength, stiffness, extensibility, and overall toughness of the artificial fibers in the presence of N- and C-terminal domains.
In addition to fibrous materials, recombinantly produced spidroins have been processed into miscellaneous structural materials including high-performance films, aerogels, hydrogels, nanocomposites, adhesives, microspheres, etc. as summarized in Figure 12.75,507−511 In this review, the terms “aerogels” and “foams” are used interchangeably to describe solids consisting of interconnected network open-celled mesoporous materials with at least more than half their volumes non-solid (empty air pockets).
Figure 12.
Diverse material applications from biotechnologically produced spider silk proteins. Various fabrication methodologies such as aerosol, electrospinning, immersion, microfluidics, lithography, and many more have been employed to fabricate materials with length scales ranging from nanometer- to millimeter-scale. This includes nanospheres, non-woven nanofibrils, composites, films, fibers, adhesives, hydrogels, and aerogels with potential medical and industrial applications.
The manufacturing process to fabricate such a versatile range of materials can be achieved from aqueous solutions by altering conditions such as pH, temperature, protein concentration, ionic strength, and composition.496,507,508 For instance, Leal-Egaña et al.512 and DeSimone et al.513 employed electrospinning to manufacture non-woven mesh from engineered ADF3. The authors demonstrated the advantage of using electrospun silk filaments with diameters ranging from 700 to 900 nm to support the immobilization of biological components in the spinning solution. Foams remain one of the most ideal classes of material as they exhibit a high strength-to-weight ratio. Many research groups have demonstrated the use of recombinantly produced spidroins to manufacture highly porous and interconnected foam-like structures, used for example as 3D cell culture scaffolds.514−517 Wang et al.516 fabricated porous foams from a recombinantly produced spider silk protein called pNSR-16 containing the RGD motif cell-binding domain.518 The authors made the foams by dissolving the proteins with formic acid followed by the addition of NaCl. Once the solvents were evaporated and salt granules dissolved, pore sizes ranging from 250 to 350 nm were obtained. In another study, Johansson et al.515 built a 3D network of silk foam by using recombinant spider silk 4RepCT engineered with the cell adhesion RGD-binding motif. The foams were fabricated through a very simple and scalable process, which involved gently introducing air bubbles into the 4RepCT solution. The process was continued until the stable foam was formed by the self-assembly of 4RepCT proteins at the air–water interface.519
The other promising applications of recombinant silk are hydrogels with 95–97% w/w water content and a high swelling ratio.520 Schacht et al.521 demonstrated the use of ADF4 hydrogel as cytocompatible bioinks in additive manufacturing of hierarchical tissue-like structures by incorporating living cells. The authors obtained the best-performing ink formulation by altering the concentrations of the silk proteins, cross-linking, and chemical functionalization. For more comprehensive coverage of protein- and silk-based bioinks, the reader is referred to the recent reviews by Kaplan and co-workers.522,523 It is also possible to produce nano- or microspheres from recombinant spidroins, for example drug delivery vehicles.524−526 This new class of materials was manufactured for the first time by Slotta et al.,527 who produced different variants of MaSp1, resulting in spheres with tunable β-sheet content and rigidity. The spheres were produced through rapid solvent evaporation. However, another method later also proposed by Slotta et al.506 was found to be more versatile. The authors demonstrated the use of a biologically relevant salt (potassium phosphate) for the formation of spheres from the engineered version of ADF4 proteins. More importantly, the size range (250 nm to 3 μm) and molecular conformation of the spheres could be effectively controlled in a more reproducible manner. The authors also demonstrated the use of microspheres for the encapsulation of water-soluble drugs.
4.2. Recombinant Mussel Adhesive Proteins
Due to the complicated purification procedures and high cost of extracting natural mussel foot proteins from the mussel plaques, various methods to recombinantly produce mussel foot proteins (rMFPs) have been developed. rMFPs are proven to be an alternative and effective way to study the properties and applications of mussel adhesive proteins. E. coli is the most commonly used prokaryotic expression host for rMFP expression.528,529 However, as E. coli lacks the genetic machinery to post-translationally modify the expressed protein, a post-expression enzymatic conversion using mushroom tyrosinase is typically needed to convert Tyr to Dopa in the expressed rMFPs.530
Most of the early works on rMFPs were conducted by Cha, Hwang and co-workers. Using E. coli, Hwang et al. expressed recombinant MFP-3 and MFP-5 by adding a constructed vector with a hexa-histidine (His6) tag at the N-terminus of the native MFP-3 or MFP-5 sequence.528,531 However, this approach suffered from low protein production yield (∼13.7%) as during the expression, the accumulation of the soluble rMFP-3 and rMFP-5 is toxic to E. coli. To improve the expression yield and purification efficiency, a recombinant hybrid fusion of MFP-1 and MFP-5 proteins was developed named as RFP-151 which added 6 repeats of decapeptide [AKPSYPPTYK] from MFP-1 to both the N- and C-termini of MFP-5 with a hexa-histidine affinity ligand.529,531 A similar version of the MFP-1/MFP-3 hybrid protein, RFP-131, was also developed.530 The hybrid proteins showed much higher expression yield (∼40%) as the formation of an insoluble inclusion body is non-toxic to the cells, thus ensuring cell growth.529 The two polycationic recombinant proteins can form complex coacervates with polyanionic hyaluronic acid (HA).532 The RFP-151/HA coacervate exhibited very low interfacial energy (<1 mJ/m2) and shear thinning properties.307 After Dopa modification, the two rMFP coacervates also showed improved shear adhesion strength of ∼3–4 MPa on aluminum surface compared to the modified recombinant proteins (∼2 MPa).532
Recombinant MFP-1 (rMFP-1) was successfully expressed using 12 tandem repeats of the MFP-1 consensus decapeptide [AKPSYPPTYK]12.317 Without Dopa conversion, the polycationic rMFP-1 formed coacervate with polycationic poly(2-(trimethylamino) ethyl methacrylate) (MADQUAT) via π-cation interactions533 and underwent self-coacervation with salt concentrations at or above the seawater level (>0.7 M NaCl) via intrinsic π-cation interactions from the Lys and Tyr residues.534 After Dopa modification, rMFP-1 could also form adhesive hydrogels via both Dopa oxidation-induced covalent cross-linking and metal coordination bonds.535
The biggest drawback of using wild-type E. coli for the expression of rMFPs is that Dopa cannot be directly incorporated into the recombinant proteins. A post-expression mushroom tyrosinase treatment needs to be performed to convert Tyr residues in the rMFPs to Dopa. To solve this problem, an E. coli system with endogenous tyrosyl-tRNA synthetase (TyrRS) has been developed to use TyrRS for Dopa incorporation without interfering with Tyr incorporation.536,537 Such an engineered E. coli system allows Dopa to be co-translationally introduced into the recombinant proteins in a Tyr-depleted medium.285,537 While these approaches are promising, the expression yields achieved are lower than those for proteins made of canonical amino acids and will need to be significantly increased in larger-scale expression efforts. Alternatively, it may be possible to co-express the enzyme with the substrate protein to achieve post-expression enzymatic conversion, as demonstrated for ELPs post-translationally modified with a lipid tail.538
Modular adhesive proteins with a fused MFP domain and other functional proteins or peptides have also been developed to realize multi-functionalities. The first attempt was to fuse rMFP-151 with an RGD peptide, the GRGDSP peptide.539 With the RGD cell recognition motif, RFP-151-RGD not only shares the advantages of rMFP-151, such as high adhesion and adsorption capacities, but also shows superior cell-adhesion and spreading abilities. Zhong et al.540,541 have developed an E. coli-based expression system that can realize modular design of multidomain functional nanofibers. By fusing the curli fiber protein GsgA (see Section 3.3.4), with MFP-3 or MFP-5, the CsgA-MFP-3/MFP-5 proteins could self-assemble into amyloid nanofibers with strong underwater adhesion.540 The same approach can be used to construct biofilms as living glues with self-healing properties.541 More functional domains, such as CBDs can be incorporated into multi-domain amyloid proteins with strong chitin-binding ability.542
4.3. Hagfish Filaments
A main interest in replicating hagfish filaments has been their ability to achieve a mechanical performance on par or even better than that of dragline silk,543 but with much smaller MWs building blocks, i.e. hagfish thread keratins described in Section 3.1.2. Since hagfish slime proteins can classified as IFs, protocols used for self-assembly have been largely inspired by those developed for IFs such as desmin, vimentin, or keratins,259,544 which consist in fully denaturing the expressed keratinous proteins into 8 M urea and then subjecting them to stepwise dialysis in decreasing concentrations of urea. In the final step where the denaturing agent has been completely dialyzed out, coiled-coil nanofilaments with homogeneous size distribution can be achieved. Using this approach, Fu et al.545 expressed both (EsTKα) and (EsTKγ) in E. coli and established the microenvironmental conditions (buffer type, pH, temperature) enabling the self-assembly of IF-like filaments from 1:1 mixtures of EsTKα and EsTKγ, as verified by the α-helical signature in CD spectra and the presence of nanofilaments by TEM imaging. Above a threshold concentration, the self-assembled EsTK filaments exhibited a rheological response (storage modulus vs concentration scaling law) very similar to that of desmin IFs,546 indicating that recombinant hagfish filaments can form an elastic network of entangled filaments that share strong similarities with IFs. Furthermore, when subjected to oscillatory shear strains in the rheometer, EsTK filaments underwent a conformational transition from α-helical- to β-sheet-rich structure as verified by CD spectroscopy before and after shear strain. Thus, this proof-of-concept study established the ability of EsTK filaments to mimic the α → β transition observed in native haggish threads.
In follow-up work,161 lyophilized self-assembled filaments were resolubilized in formic acid at the very high concentration of 100 mg/mL. By applying this concentrated dope solution onto an electrolyte buffer, fibers could be produced from the air/liquid interface and subsequently drawn up to 100% their initial length. The draw-processing increased the elastic moduli of the fibers from 0.5 to 4 GPa and the Ultimate Tensile Strength (UTS) from 25 to 150 MPa, although this increase in load-bearing ability came at the expense of the maximum strain to failure. Raman spectroscopy, synchrotron WAXS, and Congo red staining established the predominance of elongated β-sheet crystallites in the drawn fibers, thus demonstrating the critical important of β-sheet formation in strengthening the fibers. The mechanical properties could be further enhanced by chemical cross-linking using glutaraldehyde (which promotes Lys-Lys interprotein cross-linking), with elastic modulus reaching as high as 20 GPa and UTS ca. 250 MPa. These values are in the range of the stiffest artificial silk spiders reported,503 showing that artificial hagfish fibers can compete with synthetic spider silks while benefiting from easier expression in E. coli.
Following these studies, Oliveira et al. were able to scale-up the expression of both EsTKα and EsTKγ in E. coli using a 100 l bioreactor,547 and they reported the substantial amount of 8 to 10 g/L of recovered proteins. The hagfish thread proteins were also expressed in the inclusion body. However, stepwise dialysis protocols, while enabling the formation of homogeneous IFs that are suitable for fundamental biophysical studies, are not practical to produce large-scale amounts of self-assembled filaments. Thus, in contrast to Fu et al., the inclusion body (after washing steps) was solubilized in HFIP, resulting in highly concentrated dopes of hagfish protein solutions. Fibers were then produced by extruding the HFIP-solubilized dope into a coagulation bath made of isopropanol (IPA), followed by draw-processing the coagulated fibers into IPA or IPA/water solutions up to 300% the initial fiber length, which resulted in an increased β-sheet content. The mechanical properties achieved with this processing method were comparable to those reported from the stepwise self-assembly method, with elastic moduli of 5.5–6 GPa and UTS of 150–160 MPa.
Stepwise refolding or solubilization of the expressed hagfish proteins in a strong denaturing agent such as HFIP is not the biological strategy by which hagfish IFs are self-assembled and subsequently aggregated into macroscopic fibers. From that perspective, a comprehensive understanding of slime self-assembly fabrication in the native system across multiple scales, from the intracellular molecular level to the macroscopic scale, will probably be required before we can fully replicate native hagfish fiber production. Research works in this direction, such as carried out by Winegard et al.187 that looked at secretion of hagfish slime proteins from the GTCs, are important as they may reveal critical biofabrication steps that could be replicated in artificial production of hagfish fibers.
4.4. Reslilin-like Proteins (RLPs)
Since the discovery of the resilin gene in D. melanogaster, various attempts have been made to develop recombinant resilin-like proteins or polypeptides (RLPs). The first RLP developed, Rec1-resilin, was expressed using E. coli. Rec1-resilin contains the 17 repeats of the putative elastic repeat motif, [GGRPSDSYGAPGGGN], from the Drosophila CG 15920 gene.29 After photo-cross-linking, Rec1-resilin formed hydrogels that contained Di-Tyr blue fluorescence, similar to that of natural resilin. The cross-linked Rec1-resilin hydrogels also exhibited 97% resilience, which is higher than that of synthetic polybutadiene (80%). This resilin-mimetic protein can also form organized nanopatterns on different substrates, including mica, Si wafer, and graphite.548
E. coli. is the most commonly used host for the expression of RLPs with three different induction methods: isopropyl β-D-1-thiogalactopyranoside (IPTG) induction, autoinduction, and lactose induction.226,227 The expressed RLPs are typically soluble products and can be purified by heating and salting-out, nickel-nitrilotriacetate (Ni-NTA) affinity chromatography, and temperature-induced coacervation.549 Affinity tags are often introduced to simplify the purification process. The yield of the RLPs depends on the sequence and characteristics of individual proteins, and they range from 15–450 mg/mL.226 A combination of IPTG and lactose induction in a large-scale fermentation could increase the yield of Rec1-resilin by 20-fold (300 mg/L).550
Recombinant RLPs exhibit rubber-like mechanical properties. Recombinant exon 1 protein from the D. melanogaster CG 15920 gene showed resilience of 90% for uncross-linked and 93% for horseradish peroxidase (HRP)-mediated cross-linked exon 1 protein. The corresponding values were 63% and 82% for uncross-linked and cross-linked exon 3 protein, respectively.228,551 Recombinant full length resilin can bind the cuticle polysaccharide chitin via the chitin-binding RR-2 domain (exon 2). The protein can be further polymerized through oxidation of the tyrosine residues resulting in a high-performance protein–carbohydrate composite material.228 Tamburro et al. designed a series of polypeptides containing the repeating sequence [GGRPSDSYGAPGGGN] of exon 1 (RES9n, RES15n, RES24n, and RES60n), and a polypeptide with the repeating sequence [GYSGGRPGGQDLG] of exon 3 (RES39c). Structural characterization indicated that the peptides mainly contain β-turns and an extended coils structure, similar to the secondary structures of other elastomeric proteins.552 Two RLPs, An 16 and Dros 16, contain 16 copies of an 11-residue sequence of [AQTPSSQYGAP] from Anopheles gambiae and 16 copies of a 15-residue sequence of [GGRPSDSYGAPGGGN] from D. melanogaster, respectively, and exhibit material properties similar to those of natural resilin.231
4.4.1. Multi-functional RLP-Based Materials
In biological systems, resilin is rarely present by itself. It typically colocalize with other cuticular proteins as well as with chitin fibers in the functional components of the insect exoskeleton structures. Such composite structures result in a combination of properties from the individual components.553,554 Artificial modular RLPs combining the elastomeric repeating sequence of resilin with other functional motifs provide new functionalities to RLPs. Using modular cloning schemes, the number of repeats in RLPs can be precisely tuned and combined with additional motifs with different functionalities, including tuning the assembly and mechanical properties of the hydrogels, promoting cell adhesion and differentiation, growth factor delivery, and regulating material degradation.227,555
A suite of modular RLPs were first developed by Kiick and colleagues.556 Their RLPs contained 12 repeats of the putative resilin sequence derived from D melanogaster resilin repeat [GGRPSDSYGAPGGGN] with the Tyr replacing the Phy in order to introduce photo-cross-linking. The first modular RLP expressed, namely RLP12, also contained three functional domains including a cell-binding ligand [RGD], a matrix metalloproteinase (MMP), and a heparin binding domain (HBD) in the sequence. The authors further developed RLPs with individual function motifs including the cell-binding sequence (RLP12-RGD), the matrix metalloproteinase (RLP12-MMP), and the heparin binding domain (RLP12-HBD). Hydrogels can be prepared by using each or a mixture of the modular RLPs, with tunable mechanical properties, which allows for the independent control over the function of the hydrogels for regenerative medicine applications.555,557,558 By incorporating Cys thiols in the functional RLPs, the polypeptides can be cross-linked with vinyl sulfone terminated four-arm start PEG macromers to form RLP-PEG hybrid hydrogels. These hybrid RLP-PEG hydrogels display rubber-like properties, and they can be used as cell-instructive, structured tissue engineering scaffolds.559,560
Liu and co-workers have also developed a series of modular RLPs based on the consensus sequence derived from the African mosquito A. gambiae, with different functional domains, including a cell-binding sequence (RZ-RGD), a peptide derived from bone morphogenetic protein-2 (RZ-BMP), and a vascular endothelial growth factor (VEGF) mimicking peptide (RZ-QK).561−565 The modular RZ-RGD could form hydrogels using tris(hydroxymethyl)phosphine (THP) and/or transglutaminase enzyme (TGase)-mediated cross-linking with tunable mechanical properties. The shear modulus of the hydrogels ranged from 0.1 to 2.2 kPa.563,566 The RZ-RGD hydrogels increased the spreading, viability, and proliferation of human mesenchymal stem cells (hMSCs).561,564 Surfaces treated with the RZ-BMP peptide accelerated osteogenic differentiation in a sequence-specific manner, while surfaces treated with RZ-QK peptide promoted endothelial differentiation of hMSCs.564,565
The combination of the consensus sequence of resilin with repetitive sequences from other structural proteins, such as elastin, spider silk, and collagen, represents an effective method for obtaining recombinant polypeptides with tunable mechanical and self-assembly properties and biological functions.567 Artificial elastomeric proteins combining the small non-mechanical protein GB1(G) and the 15-residue putative resilin sequence derived from D. melanogaster (R), namely (GR)4 and GRG5RG4R, can form hydrogels with muscle-like mechanical properties, exhibiting high resilience (>90%) at low strain (<15%) but extensibility and toughness at high strain.568 The elastic modulus of the hydrogels was in the range of 50–70 kPa at 15% strain, which is close to that of myofibrils/myocytes. The tunable mechanical properties of the hydrogels make them suitable materials for tissue engineering. A resilin–elastin–collagen chimeric polypeptide (REC) has also been designed and could self-assemble into fibrils with a Young’s modulus between 0.1 and 3 MPa.569 Genetically engineered resilin-silk copolymer could self-assemble into micelle-like particles or nanofibrils in a temperature- and time-dependent manner. Resilin-silk copolymers can also form physically or photo-cross-linked hydrogels with different stiffnesses.570 Weitzhandler et al. designed a series diblock co-polypeptides of hydrophilic ELP domain and hydrophobic RLP block.571 The self-assembly of the ELP-RLPs was dictated by their chain length, degree of hydrophilicity, and hydrophilic weight fraction of the ELP block. A minimum threshold in the length of the hydrophobic RLP block was required for self-assembly of the diblock co-polypeptides. A spherical-to-cylindrical micelle transition occurred by increasing the length of the RLP block or increasing the hydrophobicity of the corona-forming ELP block. The diblock co-polypeptides could form stable micelles after core-cross-linking.572 Different anticancer ligands, such as a GRGDSPAS peptide targeting αvβ3 integrin or an engineered variant of a death receptor 5 agonist, have also been incorporated at the end of the ELP-RLP for cancer treatment.572
4.5. Elastin- and Elastin-like Polypeptides (ELPs)
As the name suggests, elastin is a naturally occurring biological elastomer that provides elastic recoil property to vertebrates tissue.573 The primary physiological function of elastin is to maintain structural stability after repetitive contraction and extension of the tissues over a lifetime. In contrast, abnormal mutations in elastin genes are associated with a handful of severe syndromes and wide obstructive vascular disease.221 Elastin is made from 3 to 4 nm filaments that grow up to 5–8 μm in diameter fibrils.574−576 The filaments are highly aligned parallel to the longitudinal axis of the elastin fibers. The primary building block of elastin is the monomeric protein precursor known as tropoelastin, discussed in details in Sections 2.2 and 3.2.1, and a negligible fraction of other non-elastic proteins such as fibrillins, fibulins, and some microfibril-associated glycoproteins.577,578 Elastin formation starts with the extracellular self-assembly of tropoelastin into the highly elongated bicontinuous scaffold of interconnected mesoglobules.217
Since the discovery of elastin, the extraction of homogeneous tropoelastin was found to be a highly inconsistent process. This often resulted in yields with low quality and high polydispersity. The primary limiting was the insolubility of elastin, owing to the high density of covalent cross-linking which often required harsh chemical or enzymatic digestion. However, the prevalence of the recombinant production of some of the key repeat motifs has provided an alternative approach toward studying fundamental structure–function relationships. Taking inspiration from these repeats has led to the biosynthesis of a new class of proteins commonly known as ELPs. In the past few decades, ELPs have contributed to many medical and technological innovations, enabling the formulation of robust materials with a unique set of physicochemical properties that are challenging to replicate synthetically.
The use of ELPs as a tag to purify recombinant proteins can be argued to be one of the most used applications of ELPs,579 and it has proven to be one of the easiest, cost-effective, and scalable purification strategies that bypass the need for advanced chromatography protocols.579 The method relies on the fact that ELPs exhibit a sharp and reversible phase separation above their LCST.580 Below the LCST, ELPs are well solvated and adopt a random-coil conformation. This makes it completely soluble under biologically relevant solution conditions. However, above the LCST, ELPs become partially insoluble and form coacervates that turn the solution opaque to the naked eye. The process is completely reversible: if the temperature reaches below the LCST, resolubilization takes place and the phenomenon still occurs even when the ELP is fused to other proteins. Therefore, target proteins tagged with ELPs can be purified from cell debris by inverse transition cycling. This involves cycling the cell lysate through the ELP’s insoluble–soluble phase. In each cycle, phase-separated insoluble ELP-fusions are collected and other soluble contaminants are discarded and washed away. The process is repeated multiple times until the desired purity is achieved. In addition, the LCST boundary can be fine-tuned to maximize the purification efficiency specifically in each purification case depending on the origin of the cell lysate and the quantity of the contaminants. For example, the LCST can be decreased with increasing ionic strength, ELP concentration, and ELP length.579−582 Also, the core residue of the selected ELP tag affects the transition temperature. While the hydrophobic residues lower the transition temperature, charged or hydrophilic amino acids have an inverse effect.582 Finally if needed, the ELP tags can be cleaved from the target proteins using rationally introduced proteolytic sites between the target protein and ELP tag.583
The use of ELPs has also been demonstrated in the fabrication of high-performance structural materials, in particular the use of ELPs and ELP-fusions for templating biomineralization. For instance, Elsharkawy et al.584 investigated the effect of modularity between the order–disorder ratio of an ELP toward programmed biomineralization of hierarchically ordered hydroxyapatite minerals. Their process was based on recombinantly produced ELPs that are aligned and organized into mechanically stable 2D-hydrogels. The authors demonstrated multi-scale spatiotemporal control over the growth and morphological orientation of the mineral, which provided new insights to overcome some of the challenges associated with regenerative mineralized tissues. In a more complex approach, we have also demonstrated, in a proof-of-concept study, how supramolecular self-assembly of ELP-fusion proteins can be used to manufacture 3D-shaped dental implant crowns.146 The work was inspired by the molecular and architectural design of the impact-resistant dactyl club of the stomatopod. This new material consisted of highly expanded helicoidal arranged cellulose nanocrystals (CNCs). The CNC network was infiltrated using ELP-fusion in their phase-separated microdroplets preassembly state and cross-linked to mediate the growth of apatite crystals. The resulting nanocomposite materials showed high stiffness, strength, and fracture toughness.
The use of ELPs in biomedical applications has a long history. In fact, in the past few decades, there has been a significant accumulation of literature on the topic (234,952 articles, 178,574 patent applications, 182 clinical trials, and 110 policy documents, source: https://www.dimensions.ai, accessed on 25 Aug 2022). The large body of literature on biomedical applications has been regularly reviewed before, including recently by Varanko et al.40 Here we only highlight a few examples of recent progress in the use of genetically engineered ELPs for medical applications. Urosev et al.585 demonstrated a hemostatic-ELP protein variant by introducing charge and polar residues at the N- and C-termini. The ELP could be used to selectively bind to blood clots and modulate their physicochemical properties, such as improved resistance to enzymatic degradation and mechanical stability. In addition, the pore size of the clots could be fine-tuned according to the phase separation profile of the ELP variant. This enabled researchers to create either smaller pore sizes or arbitrary pore shapes to prevent bleeding more effectively. This approach holds great potential as an alternative treatment of bleeding disorders and perioperative bleeding. Another use of engineered ELPs in medical applications is related to novel injectable tissue integrating depots with the capability to encapsulate and release drugs in a controlled fashion. Roberts et al.586 demonstrated how alternating the amino acid composition of the hydrophilic cross-linking domains and the hydrophobic disordered region could result in a diverse range of interconnected fibrillar networks, which could be integrated seamlessly into the tissue weeks after integration. This team created a library of recombinantly produced ELPs, each with unique polarity, charge distribution, hydrophobicity, and overall composition of ordered-disordered regions, leading to the identification of a distinct ensemble of LLPS behaviors with tunable physicochemical properties.
There has been a long interest in the development of tough and highly stretchable hydrogels from naturally occurring proteins with inherent elasticity. To this end, the use of pentapeptide repeats of ELPs followed by chemical or enzymatic cross-linking has been demonstrated, resulting in hydrogels with up to about 400% reversible strain.587,588 In another effort, Gonzalez et al.589 illustrated the engineering of multi-functional ELPs through rational incorporation of covalent and reversible cross-linking domains, such as metal coordination and hydrophobic interactions, resulting in hydrogels with staggering strength (>2.5 MPa), toughness (>1300 J m–2), strain (>500%), and self-healing achieved in the presence of the divalent cation Zn2+.
4.6. Suckerins
SRT, made of a family of suckerin proteins (see Section 3.4.4), exhibit many physicochemical and mechanical characteristics that make them particularly attractive from a sustainability perspective and for biomedical applications. In addition to their robust and isotropic mechanical properties, they consist of a semi-crystalline supramolecular network, leading to a thermoplastic response. This means that biopolymers made of suckerins can in principle be thermally melted and remolded into new objects. Further, they feature a similar molecular design as the repeat regions of silks but with a lower MW, thereby in principle facilitating full-length recombinant production. The first forays into recombinant expression of suckerins were conducted by Ding et al.,480 who expressed codon-optimized D. gigas suckerin-19 (shown in Figure 13A) cloned in E. coli BL 21 cells. Suckerin-19 was chosen because of its representative diblock copolymer architecture and medium MW of 39 kDa among all D. gigas suckerins (note that in early nomenclature, suckerin-19 was called “suckerin-39”, but it was later renamed suckerin-19 following the comprehensive identification of all D. gigas suckerins134). In these early attempts, both His-tagged suckerin-19 and suckerin-19 were explored and both proteins were concentrated in the inclusion body, providing a prepurification step that facilitated protein purification using microfluidization and resolubilization of the inclusion body into strong denaturing agents. In following research, only suckerin-19 was investigated, as the His tag did not provide any benefit during purification. A few noteworthy characteristics of recombinant rec-suckerin-19 were identified in this study, which laid the foundations for subsequent studies of suckerins. Following purification, rec-suckerin-19 was found to exhibit a high solubility limit of up to 70 mg/mL in mild acidic conditions (5% acetic acid) but a much lower solubility near neutral pH, with the formation of nanoscale aggregates observed. At low concentration (1 mg/mL) and acidic pH, CD and FTIR studies indicated that suckerins remained in random coil but oligomerized into β-sheet-rich nanoparticles at higher concentration and near neutral conditions. These results thus provided microenvironment conditions to be used in order to tailor solubility, β-sheet content, and controlled aggregation of suckerins.
Figure 13.
Applications of recombinant suckerins (rec-suckerins) and suckerin peptides. (A) Sucker ring teeth (SRT) and modular architecture of suckerin-12 and suckerin-19. (B) Rec-suckerin-19 can be processed into Di-Tyr cross-linked hydrogels and stiff materials (i) with a broad range of elastic modulus. Cell culture studies have shown that rec-suckerins are non-cytotoxic, with cells rapidly proliferating on rec-suckerins substrates. Reproduced with permission from ref (484). Copyright 2015 Wiley-VCH. (ii) Rec-suckerin-12 can also form hydrogels with tunable elastic modulus by incubation in various salts of the Hofmeister series. Reproduced with permission from ref (590). Copyright 2019 Wiley-VCH. (C) Rec-suckerin-19 self-assembled into drug-loaded nanoparticles (NPs) for nanomedicine applications (i). Reproduced with permission from ref (591). Copyright 2017 American Chemical Society. (ii) Rec-suckerin-12 can also be self-assembled into ca. 100 nm NPs with quasi-monodisperse size distribution when incubating in weakly kosmotropic salts. Reproduced with permission from ref (592). Copyright 2020 American Chemical Society. (D) In a reductionist approach, short peptides derived from suckerins can also be used as building blocks to construct nano- and biomaterials. (i) The short peptide A1H1 can be self-assembled into stiff mesoscale fibers (left, reproduced with permission from ref (477). Copyright 2017 American Chemical Society) made of amyloid-like cross-β nanofibrils as predicted by MD simulations (right, reproduced from ref (479). Creative Commons CC BY-NC 4.0. (ii) The short peptide GV8 can form stiff hydrogels in water (left, reproduced from ref (485). Creative Commons CC BY). Large macromolecular therapeutics, such as proteins, can be encapsulated in GV8 hydrogels and released in a controlled fashion, for example in wounds (right, reproduced with permission from ref (593). Copyright 2021 Elsevier).
Following these initial investigations, Ding et al.484 fabricated rec-suckerin-19 biomaterials exhibiting a broad range of elastic moduli E, including hydrogels (E = 40–500 Pa) and cross-linked films with moderate moduli (0.15–1.5 MPa) as well as in the few GPa range (1–3 GPa) as illustrated in Figure 13B-i. This large variation in elastic moduli was obtained by exploiting the high Tyr content of suckerin-19, which enabled researchers to induce dityrosine cross-linking catalyzed by Ru2+ salt as previously demonstrated with resilin. Notably, the elastic modulus could be tuned by manipulating the β-sheet content, which was itself governed by modulating the dityrosine cross-link density. The highest moduli correlated with the β-sheet content but, counterintuitively, not with the cross-link density. Indeed, above a certain dityrosine cross-link density, β-sheet formation was frustrated because the cross-links prevented spatial presentation of β-strands. Thus, there exists an optimum cross-link density for which maximum stiffness can be achieved. Overall, this work demonstrated that rec-suckerin-19 can be tailored to match soft tissues, ligaments, and muscle tissues. Under optimized cross-linking conditions, the modulus can even approach that of bone. Importantly, the study also established that suckerin-based materials exhibit promising biocompatibility, since primary cells, human dermal fibroblast (HDF), human mesenchymal stem cells (hMSCs), and human embryonic kidney (HEK293) cells all rapidly proliferated on suckerin-19 films and no cell death was observed for any of these cells.
Other biomedical applications of recombinant suckerins that have been demonstrated include drug and gene delivery carriers and wet-resistant adhesives. Recognizing the ability of rec-suckerins-19 to be assembled into β-sheet stabilized nanoparticles, Ping et al.591 prepared rec-suckerin-19 nanocarriers using a salting-out method, resulting in nanoparticles with a controlled particle size below 200 nm in optimized conditions of salt type, concentration, and fabrication temperature (Figure 13C-i). The nanoparticles were loaded with the hydrophobic anticancer drug doxorubicin (Dox) and could be delivered into HeLa cells by clathrin-mediated endocytosis. Once in the cytoplasm, Dox was released by the proton-sponge594 effect activated by the high His content of rec-suckerin-19, resulting in cell death. The Dox-loaded rec-suckerin-19 nanoparticles were also tested in vivo on mice with HeLa xenografts. Peritumoral injection of Dox-loaded formulation led to a strong decrease of tumor size and tumor necrosis, whereas control experiments with Dox-free suckerin-19 nanoparticles did not elicit adverse reaction to the mice, further pointing toward the biocompatibility of suckerins. In addition, this study also demonstrated that rec-suckerin-19 could condense plasmid DNA (encoding for luciferase) into nanoparticles, with a complexation mechanism largely driven by hydrophobic interactions between β-sheets and the major groove of the DNA helix, which is advantageous over electrostatic-mediated complexation since it enhanced the colloidal stability of the complexes. Luciferase expression was demonstrated in vitro, establishing that suckerins may also be used for gene delivery applications.
The adhesive properties of artificial suckerins were established by Deepankumar et al.,419 who expressed the shorter suckerin-12 and measured its surface adsorption and adhesion energy on wet mica surfaces. Using the SFA, normalized adhesion forces as high as 70 mN/m were obtained, which exceeded the SFA wet adhesion values of all mussel adhesive proteins, and it was found that these strong adhesive properties were mediated by the cross-β amyloid motif of suckerins. Indeed, upon disrupting the β-sheets with denaturing agents, the adhesive forces were completely suppressed. Given the well-known wet adhesion properties of mussel adhesive proteins,92 these results highlight the potential of suckerins as a bioadhesive material.
By varying the salt type, protein concentration, pH, and ionic strength conditions, the biophysical properties of suckerin hydrogels and nanoscale assemblies of suckerins can be controlled. For example, Buck et al.590 fabricated hydrogels from rec-suckerin-12 (Figure 13B-ii) and found that the contraction and elastic modulus of these hydrogels could be tuned by incubating them in various salts of the Hofmeister series. The governing parameter appeared to be deprotonation of His residues, which is either promoted in kosmotropic anions (citrate, phosphate, and acetate) even below the pKa of His (∼6) or prevented in chaotropic anions (sulfate and nitrate). Depending on the protonation state of His residues, the aromatic character of their imidazole side-chain becomes a regulating parameter in controlling the mechanical properties through π-stacking. Along the same line, Hershewe et al.592 showed that β-sheets can be reversibly unfolded and refolded even in acidic conditions (where suckerins are usually unfolded) by adding the less kosmotropic KCl salt. In this study, the authors also introduced the non-canonical amino acid para-L-azido-phenylalanine (pAzF) in rec-suckerin-12 using a site-specific biorthogonal strategy, subsequently enabling researchers to fluorescently label the protein using biorthogonal conjugation. The β-sheet content, aggregation, and particle size could all be modulated by adding different salts of the Hofmeister series into the protein solution. In the most kosmotropic salts (citrate and sulfate), suckerin formed aggregates with little control of the size and polydispersity of the particles regardless of pH, whereas in the lesser kosmotropic salts (chloride and acetate), the particle size in acidic conditions could be better controlled. Under optimized conditions, the authors achieved ca. 100 nm nanoparticles with a narrow size distribution illustrated in Figure 13C-ii. Altogether, these studies indicate that there is a large latitude to tune the mechanical, biophysical, and self-assembly characteristics of suckerin-based materials, which largely depends on the ability to control the (reversible) β-sheet formation of the [M1] domains of suckerins, a process in which the abundant amino acids His and Tyr play a central role.
It has also been demonstrated that biomaterials can be fabricated not only from full-length suckerin proteins but also with a reductionist approach using shorter peptides designed after the [M1] and [M2] modules of the suckerin family475 (see Section 3.4.4). Hiew et al.475 systematically screened shorter domains from these modules that preferentially interact. Among those, the most abundant repetitive motif, the Ala-rich and His-rich peptide [AATAVSHTTHHA] (A1H1), was identified as having a strong propensity to assemble into cross-β amyloid-like fibrous materials exhibiting robust mechanical properties,477 with the His-rich motif [HTHHA] helping to maintain high solubility under acidic conditions and the Ala-rich motif driving β-sheet assembly (Figure 13D-i). Depending on solvent type and removal and peptide concentration,481 this peptide can be self-assembled in multiple chiral polymorphisms. Another notable peptide identified in suckerins is the octapeptide [GLYGGYGV] (abbreviated GV8).485 As illustrated in Figure 13D-ii, this peptide can form stiff hydrogels (storage modulus between 30–40 kPa) in water without any cross-linking or chemical modifications. With solution and solid-state NMR studies, combined with WAXS, CD, and FTIR, GV8 was found to gel via an unusual 310 helix-to-β-sheet transition. In solution, GV8 initially adopts a 310 helical conformation stabilized by π–π stacking of Tyr residues, which upon gelation rearranges into antiparallel β-sheets. In follow-up work, it was demonstrated that GV8 could encapsulate larger protein therapeutics in a simple one-pot formulation.593 By simply varying the peptide concentration, a tunable release profile of therapeutics from the hydrogel was shown. It was also found with in vivo studies on mice models that therapeutic-loaded GV8 hydrogels could find promising applications for chronic wound healings.
All the work described in this section has been carried out on suckerins from D. gigas. In other efforts, suckerin constructs from the common squid Loligo vulgaris have also been recombinantly produced in E. coli(595) and subsequently used to achieve self-healing materials with tunable mechanical properties.596
4.7. Cuticle and Squid Beak Proteins
Whereas there has been abundant research trying to mimic insect cuticles using chitin or chitosan as the initial scaffold,597−599 most likely based on the belief that chitin is the main load-bearing phase in cuticles,460 very little attention has been given in producing artificial CuPs for biomaterial applications and there is undoubtedly untapped potential in this direction. In one case, a Gly-rich cuticle protein from the wings of the swallowtail butterfly was demonstrated to form reversible, temperature-dependent coacervates reminiscent of tropoelastin.488 In other more recent examples, a protein from the cuticle of red flour beetle containing the RR motif was shown to form complex coacervates with chitosan.600 Similarly, a Gly- and His-rich CuP from the head capsule of the Asian corn borer also exhibited coacervation behavior with chitosan despite lacking a clearly identified CBD.601
As far as we know, mimicking the potential biofabrication process of the squid beak using artificial proteins and aqueous-based chemistry has remained unexplored, although there have been attempts to mimic the squid beak graded structure with synthetic polymers.602 If successfully implemented, it may provide opportunities for fabrication of more sustainable composites. On the other hand, biomimetic HBpeps coacervates have recently emerged as promising biocompatible drug carriers with stimuli-responsivity for controlled release. The main idea behind these microcarriers is to exploit the pH-induced or temperature-induced self-coacervation, during which therapeutics initially solubilized in an aqueous buffer are recruited within the peptide coacervates with high efficiency. The therapeutic-loaded coacervates are then disassembled by an external stimulus or by an endogenic compound, resulting in demixing of the droplets and in release of the therapeutics. For example, Lim et al.486 co-recruited insulin and the enzyme glucose oxidase (GOx) within HBpeps coacervate microdroplets. Upon incubation with glucose, the latter reacted with GOx to form gluconic acid, thereby acidifying the microdroplets, which quickly disassembled and released insulin. HBpep microdroplets in this case can be viewed as simplified β-islets, with glucose-induced release of insulin. In another proof-of-concept study,471 the small anticancer molecule doxorubicin (Dox) was recruited together with iron oxide magnetic nanoparticles within HBpep coacervates. Applying an alternating magnetic field, localized heating could be induced within the droplets via the magnetic hyperthermia effect, which triggered microdroplet disassembly and thus release of entrapped Dox. Here, the coacervates not only released Dox on demand, but they were also found to readily cross the cell membrane to quickly reach the cytosol. Exploiting this useful cellular uptake feature, Sun et al.472 recently modified a HBpep with a self-immolative side-chain to tweak the phase behavior and successfully released intracellularly a broad range of macromolecular therapeutics, including anticancer stapled peptides, large MW proteins, and mRNAs.
4.8. Scaling-up Efforts
Historically, the main goal behind the use of recombinantly produced spider silk proteins has been toward the commercial production of synthetic fibers with physicochemical properties that closely resemble those of their naturally occurring counterparts. However, after years of research and development, many large corporations such as BASF and DuPont have pulled out their investment and dropped research in this field. Such decisions were mainly driven by economic considerations on the basis that production could not be achieved at a low enough cost for industrially relevant quantities. The size, repetitive nature, and overall complexity of the spidroins’ core sequences create significant challenges that hampered progress toward commercialization.490,491 Nonetheless, there is an emerging number of start-ups that are working on sustainable solutions for large-scale recombinant production of spider silk proteins and also on alternative applications other than reinforcing fibers. Although the efforts are still ongoing, recombinantly produced spider silk proteins can be expected to be produced in a cost-effective manner that makes them attractive for certain applications. Table 2 illustrates eight out of twenty-one promising start-ups worldwide that use their own version of spider silk proteins in applications ranging from textile, medical, composite, and cosmetics.
Table 2. Selection of Start-up Companies Currently Producing Recombinant Silk Proteins.
| Organization | Location | Founded | Fundinga | Technology | Use |
|---|---|---|---|---|---|
| AMSilk | Germany | 2006 | $42.2M | E. coli | Cosmetic, medical and textile |
| Araknitek | USA | 2012 | $4M | E. coli, Transgenic goat and alfalfa | Medical & textile |
| Bolt Threads | USA | 2009 | $214M | P. pastoris | Textile |
| Entogenetics | USA | 2007 | $540K | Transgenic Silkworm | Textile |
| Kraig Laboratories | USA | 2006 | $4M | Transgenic Silkworm | Textile & medical |
| Modern Meadow | USA | 2011 | $184M | E. coli | Textile |
| Spiber | Japan | 2007 | $424M | E. coli | Textile |
| Seevix | Israel | 2014 | E. coli | Medical & textile |
Total funding is estimated based on publicly available information. Undisclosed funding is not included. Only equity funding rounds before the Initial public offering (IPO) were considered. Source: Tracxn.
As an example, one of the well-known companies in the field is AMSilk, which produces its version of spider silk proteins in E. coli. The firm has generated 20 versions of spider silk sequences all originating from A. diadematus, but only four sequences are currently being sold in applications. According to AMSilk, it has successfully demonstrated half a ton of pilot-scale fermentation and purification feasibility tests. The proteins have been mainly used as an additive for personal care to formulate moisturizing hydrogels, shampoos, and lotions where the surface of hair or skin could be altered to feel smoother, shinier, and healthier. It appears that the use of spider silk in cosmetic applications provides the largest portion of revenue for the company. However, AMSilk has shown its intention to pivot toward wound-healing sprays and coatings for silicone breast implants. Other applications include microspheres as drug delivery vehicles and vaccine stabilizer scaffolds that eliminate the use of refrigeration and facilitate cold-chain management. Moreover, the company has joined forces with some of the biggest companies in the fields of fashion, wearables, sports equipment, and automobiles. In 2016 at the Biofabricate Conference in New York, Adidas unveiled the world’s first performance shoe made using Biosteel fiber made by AMSilk. The shoes were 15% lighter in weight, 100% biodegradable, and stronger than conventional synthetic fibers that are used today. Today, AMSilk, in partnership with Airbus and Omega, is constructing new prototypes demonstrating the capability of using Biosteel in highly technical applications.
While E. coli remains the most popular host, spidroin-encoding genes have been expressed in many other platforms with varying yields (Figure 14). Table 3 demonstrates the exploitation of a full spectrum of expression hosts for the heterologous production of spidroins. This includes protein production in microbes, eukaryotic cells, transgenic animals, and plants. Ease of molecular cloning, short generation time, low cost, and undemanding industrial scale-up have been the main driving forces behind the use of E. coli in both academic research and industry.603−605 That being said, the early issues with the use of E. coli were low yields, low stability, and the possibility of using only short spidroin genes.491,494 Most of these concerns relate to translational errors during expression resulting from depletion of the alanyl- and glycyl-tRNA pool in E. coli. tRNA depletion results in premature termination of the transcription that promotes low yields and low-quality truncated spidroins.500 This is mainly attributed to the highly repetitive nature of the spidroins’ midblock, which mostly contains poly-Gly and Ala residues.494 In light of these significant concerns, a variety of synthetic biology approaches including bioinformatics, codon optimization, cloning strategies, directed evolution, and metabolic engineering have been used to optimize the production of the spidroins in E. coli. Such efforts have resulted in the production of native-size spidroins with mechanical properties resembling those of the natural dragline silk of N. clavipes, in which the sequences were originated from refs (503 and 606).
Figure 14.
Schematic overview of the steps involved in the recombinant production of spider silk proteins in various expression hosts. The central region of the figure illustrates the key steps involved in the recombinant production of any structural proteins. For example, the gene encoding the desired spidroin from a spider is fused to the promoter-based expression vector and subsequently transformed into an expression host such as E. coli. The process involves the selection of positively transformed cells, and small- and large-scale cultivations. In the next step, the target proteins are purified. This is mainly done using fast protein liquid chromatography (FPLC) techniques. The structure and function of the purified proteins are then validated using techniques such as gel electrophoresis, MS/MS, solid or solution NMR, CD, FTIR, DLS, WAXS-SAXS, and many other activity assays. The outer region of the figure demonstrates the expression hosts used for the recombinant production of spider silk proteins which are categorized into three major groups, namely microbial protein expression systems, expression in eukaryotic cell systems, and transgenic plants and animals.
Table 3. Expression Hosts Used for the Recombinant Production of Spider Silk Proteins.
| Expression host | Spidroin homologue | Spider species | MW (kDa) | Yield | Refs |
|---|---|---|---|---|---|
| Microbial Protein Production through Industrial Biotechnology Platforms | |||||
| E. coli | ADF-3 | A. diadematus | 56 | 0.1 g/L | (493) |
| ADF-4 | A. diadematus | 34 | 0.3 g/L | ||
| MaSp1 | N. clavipes | 100–285 | 2.7 g/L | (503) | |
| MaSp1-chimeric | E. australis | 35 | 0.1 g/L | (84) | |
| MaSp1 | N. clavipes | 285–556 | 1.2 g/L | (606) | |
| S. typhimurium | ADF-1, ADF-2 & ADF-3 | A. diadematus | 25–56 | 0.01 g/L | (607) |
| R. sulfidophilum | MaSp1 | N. clavipes | 0.4–0.5g/L | (608) | |
| P. pastoris | MaSp1 | N. clavipes | 65 | 0.6 g/L | (609) |
| S. cerevisiae | MaSp1 | N. clavipes | 94 | 0.4 g/L | (610) |
| T. reesei | ADF-3 & ADF-4 | A. diadematus | 30–56 | 2–4g/L | (611) |
| Protein Production in Eukaryotic Cells | |||||
| S. frugiperda (sf9) | ADF-4 | A. diadematus | 60 | 0.05 g/L | (170) |
| Baby hamster kidney (BHK) | ADF-3 | A. diadematus | 60 | 0.05 g/L | (612) |
| Bovine mammary epithelial alveolar cells (MAC-T) | MaSp1, MaSp2 & ADF-3 | N. clavipes/A. diadematus | 60–140 | (612) | |
| Primate cells (COS-1) | MaSp1 | E. australis | 22–25 | (613) | |
| Transgenic Plant and Animal | |||||
| L. tarentolae | MaSp1 & MaSp2 | N. clavipes | 73–81 | (614) | |
| N. tobacum | FLAG | N. clavipes | 72–460 | 0.036–0.19 g/L | (615,616) |
| S. tuberosum | MaSp1-chimeric | N. clavipes | 12–100 | 0.5% of total proteins | (617) |
| A. thaliana | MaSp1 | N. clavipes | 64 | 18% of total proteins | (618) |
| O. sativa | AvMaSp | A. ventricosus | 22 | (618) | |
| M. musculus | MaSp1 & MaSp2 | N. clavipes | 40–55 | 0.011 g/L | (619) |
| C. hircus | MaSp1 & MaSp2 | N. clavipes | 65 | 0.01 g/L | (620) |
| B. mori (simultaneous expression and spinning of the recombinant proteins into fibers by the silkworms) | MaSp1-chimeric | N. clavipes | ∼120–300 | (621) | |
| MaSp1-chimeric | N. clavipes | 83 | (622) | ||
| MaSp2 & FLAG-chimeric | N. clavipes | 78–106 | 5% of the spun silk fiber | (623) | |
| MaSp1-chimeric | N. clavipes | 67 | 30% of the spun silk fiber | (624) | |
| MaSp1-chimeric | N. clavipes | 70 | 0.006 g/larva | (625) | |
S. typhimurium is another Gram-negative bacterium whose type III secretion system is capable of expressing and extracellularly secreting spidroins in the culture medium. This comes with the added advantage of forgoing easier downstream purification steps, unlike E. coli.607 Production of spider silk proteins has been also tested using an unconventional photosynthetic bacterium Rhodovulum sulfidophilum (R. sulfidophilum). What makes the protein production in the R. sulfidophilum platform unique is that it utilizes sunlight and abundantly available raw materials such as CO2, and N2 as carbon and nitrogen sources, respectively. This enables an economical and more sustainable microbial factory for the production of any biochemicals or biopolymers. In this case, R. sulfidophilum was engineered to produce MaSp1 under photoheterotrophic and photoautotrophic growth conditions with yields of about 0.4 and 0.5, respectively. Other unicellular microorganisms aside from bacteria have also be proven to be viable choices for heterologous production of spidroins. P. pastoris, S. cerevisiae, and T. reesei are attractive, industrially relevant strains optimized for the fermentation process.610,611,621 While historically these strains have been mainly exploited as a production host for industrial enzymes required for the production of second-generation biofuels,626−628 they have more recently been deployed toward the production of structural proteins and material components.629−632 What makes these hosts exceptional is their ability to achieve extracellular overexpression and secretion of up to 50–100 g/L of target proteins. This is mainly achieved thanks to the prolonged fermentation process that enables greater cell density and higher accumulation of target proteins. Unlike conventional hosts, extracellular secretion of the target proteins remains free of native contaminating proteins, making downstream processes such as purification easier and more cost-effective. In addition, recent advances have been made toward altering the biosynthesis of these strains with multiple protease deletions to avoid undesirable proteolytic activities, therefore maintaining the quality of the target proteins during large-scale batch or continuous fermentation.611,633−635 Furthermore, having the more sophisticated and reliable molecular machinery of eukaryotic cell enables the translation of larger recombinant genes without premature termination during expression that maintained their biological activity. Transient expression of spider silk fragments has been also tested both in mammalian and insect cells with varying results (Table 3).170,612−614 The major concerns in single-cell mammalian/insect cell cultures are the substantially greater operating costs of scaling-up and the potential contamination by animal-borne pathogens. In light of such concerns, the recombinant production of spider silk proteins in transgenic plants is also being investigated (Table 3).614−618 Transgenic plants provide an alternative eukaryotic expression system in terms of genetic stability for longer sequences, yield, and relatively easier scale-up in terms of production and purification.636 Expression in the plants enables protein production at different parts of the plants such as tuber, seed, and leave.636 This comes with the advantage that the length and complexity of the heterologous genes can be matched to a specific tissue and organelle type to improve the overall solubility and stability.637 Similar approaches have been also tested in transgenic mice and goats (Table 3).619,620
One of the most exciting areas of research in the field involves the incorporation of the spidroin genes into B. mori as the expression host. The technology is based on hijacking the evolutionary optimized silkworm’s spinning organ to simultaneously co-express spidroins along with fibroins and then to self-assemble the composite liquid proteins directly into high-quality solid fibers (Table 3).621−625 The resulting fibers surpass the mechanical properties of silkworm silk, achieving properties approaching those of dragline silk.621−625 This opens up an entirely new way to produce spider silk fibers in commercial quantities. The significant advantage is that the heterologously produced spidroins are spun into fibers under biomimetic spinning conditions that more closely resemble the actual silk spinning in spiders.84,495,638 In contrast, the spinning methods employed in all the other aforementioned expression platforms are not as efficient (Table 3), and of course this strategy bypasses the need for purification steps altogether. It is worth mentioning that various gene-editing strategies have been employed to create transgenic B. mori, including the early piggyBac approach and later the TALEN-ZFN system, which is currently the state-of-the-art gene-editing technology, i.e. CRISPR/Cas9.621−625 It is evident that advances in genome-editing technology have had a substantial impact on the mechanical properties of fibers made by transgenic B. mori.,621−625 and this is mainly attributed to the ability to precisely incorporate the native size chimeric version of spidroin genes. While the materials from the earlier transgenic B. mori exhibited inferior mechanical properties, more recent versions demonstrated stiffness and maximum strength greater than those of their native counterpart.621−625 Currently, Kraig Laboratories and Entogenetics are the two major companies actively pursuing this goal but a more widespread adaptation of such technology toward commercialization can be expected soon.
5. Outlook and Conclusions
Polymeric materials are intricately linked to all aspects of modern societies and technologies, contributing to countless essential goods of our daily life. But this dependence has come with undeniable caveats. Synthetic polymers are based on fossil fuels and their poor degradability and general lack of recyclability are causing major harm to our ecosystems. Thus, there is a pressing demand to develop more sustainable solutions that will mitigate these issues. Nature has countless examples of biopolymers synthesized using the complex machinery of living cells, and we have much to learn from such materials. Before one can attempt to replicate natural protein-based materials, a first important step is to identify the complete sequences of the protein building blocks. As discussed in the first section of this review, it took decades of work to fully sequence the early model systems of natural protein-based materials. Thanks to technological breakthroughs in NGS and proteomics, it is now possible to sequence structural proteins in a much shorter time scale. Multiple examples of natural protein-based materials exist in the living world, and while non-exhaustive, the model biological systems presented in this review are representative of the major classes of load-bearing materials found in Nature, including stiff or extensible (bioelastomers) fibers, adhesives, and bulk materials. More often than not, biological materials are multi-functional, such as mussel fibers: a single byssal thread is both stiff and extensible, and it contains multiple adhesive proteins for efficient underwater attachment as well as a hard proteinaceous coating. The molecular designs unveiled in the past decades (see Figure 2 for the recent discovery pathway) and described in this review offer countless opportunities to design from the bottom up to a whole new range of biopolymers. By combining building blocks from multiple model systems in a modular fashion, the range of achievable mechanical and physicochemical functional properties can be broadly expanded well beyond those observed in the native system, in principle enabling the fabrication of truly multi-functional materials that may find usage in a wide range of applications, as exemplified in Figures 12 and 13.
Biotechnology and genetic engineering are indispensable tools in our quest to harness the molecular designs of natural biological materials and replicate them synthetically using an increasing range of expression hosts (Figure 14), enabling the production of biopolymers with single-molecule precision and monodispersity. In the past decade, some of the most noticeable improvements in the field have been the cost and speed of gene synthesis. Currently, the cost of codon optimization and gene synthesis is as low as eight cents per base pair, and it is expected to decrease by another factor of two in the next five years, that is nearly 20-fold cheaper than a decade ago. Instead of making modifications to an existing gene, different variants of the same sequence can be easily and cheaply synthesized. Furthermore, laboratories with relatively low budgets can place an order for thousands of natural or mutated designs and screen for potentially viable candidates. It is now possible to quickly go beyond optimization of the original sequence and to create completely newly designed sequences de novo, which once expressed could potentially outperform naturally occurring structural proteins. The other important advancement in the field has been the integration of automation and one-step precision cloning techniques. Conventionally, it could take up to a month for a skillful molecular biologist to successfully carry out the cloning of a single gene using multi-step cloning techniques. This was further prone to complication depending on the complexity of the gene, sequence length, transformation efficiency, stability of cloning-expression vectors, and cloning-expression host. Today a single operator with a general understanding of molecular biology using a laboratory standard robotics arm can perform parallel cloning of hundreds of variants in a single week. Combined with accessibility to a substantially large selection of expression hosts, the chance of successful expression of any orthogonal genes has never been greater. To overcome some of the remaining challenges of expressing difficult proteins, metabolic engineering of the host using the synthetic biology toolkit is a favored option to improve the yield and quality of the desired proteins, which has been made possible thanks to the availability of the entire genomes of almost all the expression hosts and their sub-variants. Furthermore, state-of-the-art gene editing techniques such as CRISPR/Cas9 have been streamlined, as well as larger-scale and programmable chromosome fusion which can target thousands of genes at once. These exciting enabling technologies could result in the creation of completely new synthetic hosts that could be programmed and specifically designed to express the target proteins.
There are several challenges ahead before artificial materials replicating the model systems described in this review can become a reality in daily life applications. The spatiotemporal pathways by which biology precisely regulates molecular assembly from the cellular level to the final, multi-scale material structure are still largely unknown with a few exceptions such as silk fibers and mussel threads, for which breakthroughs in our understanding of their biofabrication have been achieved in recent years (see Rising and Harrington in this thematic issue1000). The role of many PTMs also remains elusive in many model systems. Toward replicating the green chemistry principles by which biologicals process extracellular materials, it will be crucial in the coming years to elucidate how structural precursor proteins are secreted in the extracellular milieu and transported to their final destination; how they specifically interact with other building blocks (other proteins, polysaccharides, metal ions, and minerals) of the mature structure; and how they are eventually chemically stabilized to build robust materials. Multiple length scales will need to be carefully investigated, including at the intracellular level (different vesicles secrete specific proteins), the glandular tissues where the precursor proteins are concentrated and subjected to various microenvironments (pH, ionic strength, etc.), conditions and/or mechanical stresses, and the tissue level during deposition.
With regard to artificial production of protein-based materials, current microbial fermentation processes mainly involve the conversion of readily available carbon sources such as plant-based simple sugars and starch into chemicals, fuels, and biopolymers. One alternative solution is to exploit biological conversion of low-cost CO2 (atmospheric, industrial waste emissions, and synthetic gas from gasification of non-food agricultural wastes biomass) to high-added-value chemicals and energy. The latest advances in synthetic biology are forecast to offer new tools to improve the properties and artificial production of these materials. For example, it may be possible to alter the metabolism of the microorganisms and increase yields significantly or to engineer strains that mostly rely on CO2 as a carbon source during fermentation.639 The incorporation of non-canonical amino acids to enhance the properties of the materials is another promising avenue. Thus, we anticipate that future research will likely require engineering and utilization of entirely new and novel microbial strains to fabricate complex biopolymers. In order to deliver the next generation of biopolymers in a cost-effective, efficient, and sustainable manufacturing manner, bioprocessing methods will also require ingenuity to interlink high-throughput screening platforms with automation for process optimization using advanced mini-reactors. These will then need to be seamlessly integrated into large-scale fermentation methods such as continuous flow reactors. We are also entering a new era of machine learning and advanced computational modeling. This new in silico toolbox may provide a wide source of data-driven inquiries and may find exciting applications in protein polymer design and manufacturing, for example to enhance metabolic engineering to optimize yields and the final product design.
Acknowledgments
AM acknowledges financial support from the Singapore Ministry of Education (MOE) through an Academic Research (AcRF) Tier 3 grant (Grant No. MOE 2019-T3-1-012) and an AcRF Tier 2 grant (Grant No. MOE 2018-T2-1-043) and from the strategic initiative on biomimetic and sustainable materials (IBSM) at Nanyang Technological University (NTU). JY thanks the Singapore National Research Fellowship (NRF-NRFF11-2019-0004) and the Singapore MOE Tier 2 Grant (MOE-T2EP30220-0006). PM would like to acknowledge financial support from the Academy of Finland project (Grant No. 348628), the Academy of Finland Center of Excellence Program (2022-2029) in Life-Inspired Hybrid Materials (LIBER) project number 346106, and the Jenny and Antti Wihuri Foundation (Centre for Young Synbio Scientists) as well as internal funding from the VTT Technical Research Centre of Finland.
Glossary
Abbreviations
- AAA
amino acid analysis
- ADF
Araneus diadematus ampullate silk
- A. diadematus
Araneus diadematus (garden spider)
- A. gambiae
Anopheles gambiae (african mosquito)
- A. irradians
Argopecten irradians (bay scallop)
- AFM
atomic force microscopy
- ALys
semi-aldehyde allysine
- B. canaliculatum
Busycon canaliculatum (whelk)
- B. mori
Bombyx mori (silkworm)
- BMP
bone morphogenetic protein
- CBD
chitin-binding domain
- CBP
chitin-binding protein
- CD
circular dichroism
- cDNA
complementary DNA
- CLPs
collagen-like proteins
- CNC
cellulose nanocrystal
- CPs
cement proteins
- CuPs
cuticular proteins
- CT domain
C-terminal domain
- D. gigas
Dosidicus gigas (jumbo squid)
- D. melanogaster
Drosophilia melanogaster (fruit fly)
- Dopa
3,4-dihydroxy-L-phenylalanine
- Dox
doxorubicin
- DLS
dynamic light scattering
- E
elastic modulus
- E. australis
Euprosthenos australis (web spider)
- E. coli
Escherichia coli
- ECP
egg capsule protein
- EGF
epidermal growth factor
- ELP
elastin-like polypeptide
- EM
electron microscopy
- EP_SP
Eoperipatus slime protein (velvet worm slime protein)
- ESI-MS
electrospray ionization mass spectroscopy
- E. stouti
Eptatretus stouii (pacific hagfish)
- EsTK
Eptatretus stoutii thread keratins
- FUS
fused in sarcoma RNA-binding protein
- FTIR
Fourier-transform infrared spectroscopy
- G. dibranchiata
Glycera dibranchiata (bloodworm)
- GOx
glucose oxidase
- GMCs
gland mucous cells
- GTC
gland threads cell
- H
hardness
- HA
hyaluronic acid
- HBP
His-rich beak protein
- H. consimilis
Hesperophylax consimilis (case maker caddisfly)
- HFIP
hexafluoroisopropanol
- HMSC
human mesenchymal stem cell
- Hyp
4-hydroxyproline
- IDP
intrinsically disordered protein
- IEP
isoelectric point
- IF
intermediate filament
- IPA
isopropanol
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- LC phase
liquid crystal phase
- LC domain
low complexity domain
- LCST
lower-critical solution temperature
- LLPS
liquid–liquid phase separation
- LOX
lysine oxidase
- MALDI-TOF
matrix-assisted laser desorption/ionization time-of-flight
- MAS
magic angle spinning
- MFP
mussel foot protein
- MMP
matrix metalloproteinase
- Mr
Megabalanus rosa
- mRNA
messenger RNA
- MS
mass spectroscopy
- MS/MS
tandem mass spectroscopy
- MTP
multi-tasking protein of Glycera jaw
- MW
molecular weight
- NGS
next-generation sequencing
- NMR
nuclear magnetic resonance spectroscopy
- NOESY
nuclear Overhauser effect spectroscopy
- N. virens
Nereis virens
- NVJP
Nereis virens jaw protein
- NT domain
N-terminus domain
- (P. americana
Periplaneta americana (cockroach)
- Pc
Phragmatopoma cement protein
- P.cochlidum
Pugilina chochlidum (spiral melongena)
- PCR
polymerase chain reaction
- PreCol
prepolymerized collagens
- pSer
phosphoserine
- PTMs
post-translational modifications
- P. viridis
Perna viridis (Asian green mussel)
- RACE-PCR
rapid amplification of cDNA ends-PCR
- REMD
replica exchange molecular dynamics
- RGD
Arg-Gly-Asp
- RLP
resilin-like polypeptide
- rMFP
recombinant mussel foot protein
- RNA-seq
RNA-sequencing
- RR consensus
Rebers & Riddiford consensus
- RT
reverse transcriptase
- R. sulfidophilum
Rhodovulum sulfidophilum
- SAXS
small-angle X-ray scattering
- SANS
small-angle neutron scattering
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SFA
surface forces apparatus
- S. gregaria
Shistocera gregaria (desert locus)
- SRT
sucker ring teeth
- tRNA
Ttransfer RNA
- TyrRS
tyrosyl-tRNA synthetase
- UCST
upper-critical solution temperature
- UTS
ultimate tensile strength
- VEGF
vascular endothelial growth factor
- WAXS
wide-angle X-ray scattering
- XAS
X-ray adsorption spectroscopy
Biographies
Ali Miserez is a Professor of Biomimetic and Bioinspired Materials at Nanyang Technological University (NTU) in Singapore. He obtained his Ph.D. in 2003 from the Ecole Polytechnique Fédérale de Lausanne (EPFL, Switzerland) in Materials Science and Engineering. In 2004, he received a Swiss National Science Foundation postdoc fellowship and moved to the University of California, Santa Barbara, where he expanded his research interest toward biomimetic engineering and biochemistry of extracellular tissues. In 2009, he was hired at NTU as an Assistant Professor, and in 2011 he was awarded the Singapore National Research Foundation (NRF) Fellowship. At NTU, he is the founding Director of the “Center for Sustainable Materials”. Dr. Miserez’s research aims at revealing the molecular, physicochemical, and structural principles from unique biological materials and at translating their molecular designs into novel biomimetic materials, including for biomedical applications. Pioneering the integrative usage of RNA-sequencing and proteomics for biological materials, his team has discovered and sequenced hundreds of extracellular structural proteins from a wide range of organisms. He has recently developed a new type of intracellular drug delivery carriers based on phase-separating peptides designed after his discoveries of extracellular liquid–liquid phase separation. He has delivered numerous invited lectures at international conferences, including at various Gordon Research Conferences in the fields of bioinspired materials, biointerfaces, and biomineralization.
Jing Yu is a Nanyang Assistant Professor in the School of Materials Science and Engineering (MSE) at Nanyang Technological University (NTU), Singapore. He obtained his Ph.D. in 2012 in Chemical Engineering from the University of California, Santa Barbara. Dr. Yu later conducted a postdoc at the California Institute of Technology, where he worked on engineered T cell immunotherapy. In 2014, Dr. Yu expanded his research interests toward polyelectrolytes brushes during his postdoc at the University of Chicago. In 2017, he became an Assistant Professor at NTU. The goal of Jing’s research is to characterize the dynamic properties of interfaces with hierarchical structures and to gain molecular-level control of soft interfaces to enable the design of integrated, multi-functional interfaces. He is a Singapore National Research Foundation (NRF) Fellow, Class of 2019, which allows him to explore interdisciplinary research in Singapore.
Pezhman Mohammadi is a Senior research scientist at VTT Technical Research Centre of Finland in the Enzyme & Material Biotechnology team, and the Sustainable Products & Materials Research area. He is also an Academy of Finland Research Fellow and Principal Investigator in the Natural Sciences and Engineering Council. He received his Ph.D. (2019) from the Department of Bioproducts and Biosystems at Aalto University in biomolecular material engineering using biotechnology solutions. During his 3 year postdoctoral fellowship at The Centre for Young Synbio Scientists he expanded his research interest toward molecular biomimetic and bioinspired materials, with a cross-multidisciplinary approach combining molecular biology, advanced protein engineering, soft-matter physics, nanocomposite, polymer chemistry, nanomechanics, and structure/property relationships across length scales. His current research at VTT Technical Research Centre of Finland is focused on merging synthetic biology, material engineering, advanced computational modelling, and machine learning.
Author Contributions
CRediT: Ali Miserez conceptualization, funding acquisition, investigation, project administration, writing-original draft, writing-review & editing; Jing Yu conceptualization, funding acquisition, investigation, writing-original draft, writing-review & editing; Pezhman Mohammadi conceptualization, funding acquisition, investigation, writing-original draft, writing-review & editing.
The authors declare no competing financial interest.
References
- Andrady A. L.; Neal M. A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. B 2009, 364, 1977–1984. 10.1098/rstb.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp J. C.; Ritchie R. J.; Allan J. E. M. Biobased Chemicals: The Convergence of Green Chemistry with Industrial Biotechnology. Trends Biotechnol. 2013, 31, 219–222. 10.1016/j.tibtech.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Cózar A.; Echevarría F.; González-Gordillo J. I.; Irigoien X.; Úbeda B.; Hernández-León S.; Palma Á. T.; Navarro S.; García-de-Lomas J.; Ruiz A.; et al. Plastic Debris in the Open Ocean. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 10239–10244. 10.1073/pnas.1314705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen M.; Lebreton L. C. M.; Carson H. S.; Thiel M.; Moore C. J.; Borerro J. C.; Galgani F.; Ryan P. G.; Reisser J. Plastic Pollution in the World’s Oceans: More Than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS One 2014, 9, 111913. 10.1371/journal.pone.0111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. D.; Reinert K. H.; Hagan J. V.; Lord W. V. Polymers as Solid Waste in Municipal Landfills. J. Air Waste Manag. Assoc. 1995, 45, 247–251. 10.1080/10473289.1995.10467364. [DOI] [PubMed] [Google Scholar]
- Barnes D. K. A.; Galgani F.; Thompson R. C.; Barlaz M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Philos. Trans. R. Soc. B 2009, 364, 1985–1998. 10.1098/rstb.2008.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserez A.; Li Y.; Waite J. H.; Zok F. Jumbo Squid Beaks: Inspiration for the Design of Robust Organic Composites. Acta. Biomater. 2007, 3, 139–149. 10.1016/j.actbio.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Hu X.; Cebe P.; Weiss A. S.; Omenetto F. G.; Kaplan D. L.. Protein-Based Composite Materials. Mater. Today 2012, 15, 208. 10.1016/S1369-7021(12)70091-3. [DOI] [Google Scholar]
- Ling S.; Kaplan D. L.; Buehler M. J. Nanofibrils in Nature and Materials Engineering. Nat. Rev. Mater. 2018, 3, 18016. 10.1038/natrevmats.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright S. A.; Biggs W. D.; Currey J. D.; Gosline J. W.. Mechanical Design in Organisms; Princeton University Press, 1976. [Google Scholar]
- Gosline J. M.; Denny M. W.; DeMont M. E. Spider Silk as Rubber. Nature 1984, 309, 551–552. 10.1038/309551a0. [DOI] [Google Scholar]
- Kummerlen J.; van Beek J. D.; Vollrath F.; Meier B. H. Local Structure in Spider Dragline Silk Investigated by Two-Dimensional Spin-Diffusion Nuclear Magnetic Resonance. Macromolecules 1996, 29, 2920–2928. 10.1021/ma951098i. [DOI] [Google Scholar]
- Shao Z.; Vollrath F. Surprising Strength of Silkworm Silk. Nature 2002, 418, 741–741. 10.1038/418741a. [DOI] [PubMed] [Google Scholar]
- Hardy J. G.; Römer L. M.; Scheibel T. R. Polymeric Materials Based on Silk Proteins. Polymer 2008, 49, 4309–4327. 10.1016/j.polymer.2008.08.006. [DOI] [Google Scholar]
- Tao H.; Kaplan D. L.; Omenetto F. G. Silk Materials - a Road to Sustainable High Technology. Adv. Mater. 2012, 24, 2824–2837. 10.1002/adma.201104477. [DOI] [PubMed] [Google Scholar]
- Mason T. O.; Shimanovich U. Fibrous Protein Self-Assembly in Biomimetic Materials. Adv. Mater. 2018, 30, 1706462. 10.1002/adma.201706462. [DOI] [PubMed] [Google Scholar]
- van der Lee R.; Buljan M.; Lang B.; Weatheritt R. J.; Daughdrill G. W.; Dunker A. K.; Fuxreiter M.; Gough J.; Gsponer J.; Jones D. T.; et al. Classification of Intrinsically Disordered Regions and Proteins. Chem. Rev. 2014, 114, 6589–6631. 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. J.; Holmberg A. L.; Olsen B. D. Artificially Engineered Protein Polymers. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 549–575. 10.1146/annurev-chembioeng-060816-101620. [DOI] [PubMed] [Google Scholar]
- Tang T.-C.; An B.; Huang Y.; Vasikaran S.; Wang Y.; Jiang X.; Lu T. K.; Zhong C. Materials Design by Synthetic Biology. Nat. Rev. Mater. 2021, 6, 332–350. 10.1038/s41578-020-00265-w. [DOI] [Google Scholar]
- Weis-Fogh T.; Krogh A. The Respiratory Exchange of the Desert Locust (Schistocerca Gregaria) before, During, and after Flight. J. Exp. Biol. 1951, 28, 344–357. 10.1242/jeb.28.3.344. [DOI] [Google Scholar]
- Bailey K.; Weis-Fogh T. Amino Acid Composition of a New Rubber-Like Protein, Resilin. Biochim. Biophys. Acta 1961, 48, 452–459. 10.1016/0006-3002(61)90043-9. [DOI] [PubMed] [Google Scholar]
- Weis-Fogh T. Thermodynamic Properties of Resilin, a Rubber-Like Protein. J. Mol. Biol. 1961, 3, 520–531. 10.1016/S0022-2836(61)80018-1. [DOI] [Google Scholar]
- Weis-Fogh T. Molecular Interpretation of the Elasticity of Resilin, a Rubber-Like Protein. J. Mol. Biol. 1961, 3, 648–667. 10.1016/S0022-2836(61)80028-4. [DOI] [Google Scholar]
- Andersen S. O. Characterization of a New Type of Cross-Linkage in Resilin, a Rubber-Like Protein. Biochim. Biophys. Acta 1963, 69, 249–262. 10.1016/0006-3002(63)91258-7. [DOI] [PubMed] [Google Scholar]
- Andersen S. O. The Crosslinks in Resilin Identified as Dityrosine and Trityrosine. Biochim. Biophys. Acta 1964, 93, 213–215. 10.1016/0304-4165(64)90289-2. [DOI] [PubMed] [Google Scholar]
- Lombardi E. C.; Kaplan D. L. Preliminary Characterization of Resilin Isolated from the Cockroach, Periplaneta Americana. Mater. Res. Soc. Symp. Proc. 1992, 292, 3–7. 10.1557/PROC-292-3. [DOI] [Google Scholar]
- Ardell D. H.; Andersen S. O. Tentative Identification of a Resilin Gene in Drosophila Melanogaster. Insect Biochem. Mol. Biol. 2001, 31, 965–970. 10.1016/S0965-1748(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Adams M. D.; Celniker S. E.; Holt R. A.; Evans C. A.; Gocayne J. D.; Amanatides P. G.; Scherer S. E.; Li P. W.; Hoskins R. A.; Galle R. F.; et al. The Genome Sequence of Drosophila Melanogaster. Science 2000, 287, 2185–2195. 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Elvin C. M.; Carr A. G.; Huson M. G.; Maxwell J. M.; Pearson R. D.; Vuocolo T.; Liyou N. E.; Wong D. C.; Merritt D. J.; Dixon N. E. Synthesis and Properties of Crosslinked Recombinant Pro-Resilin. Nature 2005, 437, 999–1002. 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- Neuman R. E. The Amino Acid Composition of Gelatine, Collagens and Elastins from Difference Sources. Arch. Biochem 1949, 24, 289–298. [PubMed] [Google Scholar]
- Partridge S. M.; Davis H. F. Preferential Release of Aspartic Acid During the Hydrolysis of Proteins. Nature 1950, 165, 62–63. 10.1038/165062a0. [DOI] [PubMed] [Google Scholar]
- Adair G. S.; Davis H. F.; Partridge S. M. A Soluble Protein Derived from Elastin. Nature 1951, 167, 605–605. 10.1038/167605a0. [DOI] [PubMed] [Google Scholar]
- Yeo G. C.; Keeley F. W.; Weiss A. S. Coacervation of Tropoelastin. Adv. Colloid Interface Sci. 2011, 167, 94–103. 10.1016/j.cis.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Urry D. W.; Trapane T. L.; Prasad K. U. Phase-Structure Transitions of the Elastin Polypentapeptide-Water System within the Framework of Composition-Temperature Studies. Biopolymers 1985, 24, 2345–2356. 10.1002/bip.360241212. [DOI] [PubMed] [Google Scholar]
- Partridge S. M.; Elsden D. F.; Thomas J. Constitution of the Cross-Linkages in Elastin. Nature 1963, 197, 1297–1298. 10.1038/1971297a0. [DOI] [PubMed] [Google Scholar]
- Franzblau C.; Sinex F. M.; Faris B. Isolation of an Unknown Component from Hydrolysates of Elastin. Nature 1965, 205, 802–803. 10.1038/205802a0. [DOI] [Google Scholar]
- Sandberg L. B.; Weissman N.; Smith D. W. Purification and Partial Characterization of a Soluble Elastin-Like Protein from Copper-Deficient Porcine Aorta. Biochemistry 1969, 8, 2940–2945. 10.1021/bi00835a037. [DOI] [PubMed] [Google Scholar]
- Sandberg L. B.; Weissman N.; Gray W. R. Structural Features of Tropoelastin Related to the Sites of Cross-Links in Aortic Elastin. Biochemistry 1971, 10, 52–56. 10.1021/bi00777a008. [DOI] [PubMed] [Google Scholar]
- Foster J. A.; Bruenger E.; Gray W. R.; Sandberg L. B. Isolation and Amino Acid Sequences of Tropoelastin Peptides. J. Biol. Chem. 1973, 248, 2876–2879. 10.1016/S0021-9258(19)44088-X. [DOI] [PubMed] [Google Scholar]
- Varanko A. K.; Su J. C.; Chilkoti A. Elastin-Like Polypeptides for Biomedical Applications. Annu. Rev. Biomed. Eng. 2020, 22, 343–369. 10.1146/annurev-bioeng-092419-061127. [DOI] [PubMed] [Google Scholar]
- Gerber G. E.; Anwar R. A. Structural Studies on Cross-Linked Regions of Elastin. J. Biol. Chem. 1974, 249, 5200–5207. 10.1016/S0021-9258(19)42348-X. [DOI] [PubMed] [Google Scholar]
- Gerber G. E.; Anwar R. A. Comparative Studies of the Cross-Linked Regions of Elastin from Bovine Ligamentum Nuchae and Bovine, Porcine and Human Aorta. Biochem. J. 1975, 149, 685–695. 10.1042/bj1490685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indik Z.; Yeh H.; Ornstein-Goldstein N.; Sheppard P.; Anderson N.; Rosenbloom J. C.; Peltonen L.; Rosenbloom J. Alternative Splicing of Human Elastin Mrna Indicated by Sequence Analysis of Cloned Genomic and Complementary DNA. Proc. Natl. Acad. Sci. U.S.A. 1987, 84, 5680–5684. 10.1073/pnas.84.16.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indik Z.; Abrams W. R.; Kucich U.; Gibson C. W.; Mecham R. P.; Rosenbloom J. Production of Recombinant Human Tropoelastin: Characterization and Demonstration of Immunologic and Chemotactic Activity. Arch. Biochem. Biophys. 1990, 280, 80–86. 10.1016/0003-9861(90)90521-Y. [DOI] [PubMed] [Google Scholar]
- Yeo G. C.; Aghaei-Ghareh-Bolagh B.; Brackenreg E. P.; Hiob M. A.; Lee P.; Weiss A. S. Fabricated Elastin. Adv. Healthc. Mater. 2015, 4, 2530–2556. 10.1002/adhm.201400781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W.; Gowda D. C.; Parker T. M.; Luan C.-H.; Reid M. C.; Harris C. M.; Pattanaik A.; Harris R. D. Hydrophobicity Scale for Proteins Based on Inverse Temperature Transitions. Biopolymers 1992, 32, 1243–1250. 10.1002/bip.360320913. [DOI] [PubMed] [Google Scholar]
- Baldock C.; Oberhauser A. F.; Ma L.; Lammie D.; Siegler V.; Mithieux S. M.; Tu Y.; Chow J. Y. H.; Suleman F.; Malfois M.; et al. Shape of Tropoelastin, the Highly Extensible Protein That Controls Human Tissue Elasticity. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 4322–4327. 10.1073/pnas.1014280108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarakanova A.; Ozsvar J.; Weiss A. S.; Buehler M. J. Coarse-Grained Model of Tropoelastin Self-Assembly into Nascent Fibrils. Mater. Today Bio. 2019, 3, 100016. 10.1016/j.mtbio.2019.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarakanova A.; Yeo G. C.; Baldock C.; Weiss A. S.; Buehler M. J. Molecular Model of Human Tropoelastin and Implications of Associated Mutations. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 7338–7343. 10.1073/pnas.1801205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. V. Spider Silk: The Unraveling of a Mystery. Acc. Chem. Res. 1992, 25, 392–398. 10.1021/ar00021a002. [DOI] [Google Scholar]
- Gosline J. M.; Guerette P. A.; Ortlepp C. S.; Savage K. N. The Mechanical Design of Spider Silks: From Fibroins Sequence to Mechanical Function. J. Exp. Biol. 1999, 202, 3295–3303. 10.1242/jeb.202.23.3295. [DOI] [PubMed] [Google Scholar]
- Omenetto F. G.; Kaplan D. New Opportunities for an Ancient Material. Science 2010, 329, 528–531. 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keten S.; Xu Z.; Ihle B.; Buehler M. J. Nanoconfinement Controls Stiffness, Strength and Mechanical Toughness of Beta-Sheet Crystals in Silk. Nat. Mater. 2010, 9, 359–367. 10.1038/nmat2704. [DOI] [PubMed] [Google Scholar]
- Fischer E. Über Spinnenseide. Z. Physiol. Chem. 1907, 53, 126–139. 10.1515/bchm2.1907.53.1-2.126. [DOI] [Google Scholar]
- Marsh R. E.; Corey R. B.; Pauling L. An Investigation of the Structure of Silk Fibroin. Biochim. Biophys. Acta 1955, 16, 1–34. 10.1016/0006-3002(55)90178-5. [DOI] [PubMed] [Google Scholar]
- Warwicker J. O. Comparative Studies of Fibroins: I. The Crystal Structures of Various Fibroins. J. Mol. Biol. 1960, 2, 350–351. 10.1016/S0022-2836(60)80046-0. [DOI] [PubMed] [Google Scholar]
- Lucas F.; Rudall K. M. Extracellular Fibrous Proteins: The Silks. Compr. Biochem. 1968, 26B, 475–558. [Google Scholar]
- Grubb D. T.; Jelinski L. W. Fiber Morphology of Spider Silk: The Effects of Tensile Deformation. Macromolecules 1997, 30, 2860–2867. 10.1021/ma961293c. [DOI] [Google Scholar]
- Martel A.; Burghammer M.; Davies R. J.; Di Cola E.; Vendrely C.; Riekel C. Silk Fiber Assembly Studied by Synchrotron Radiation Saxs/WAXS and Raman Spectroscopy. J. Am. Chem. Soc. 2008, 130, 17070–17074. 10.1021/ja806654t. [DOI] [PubMed] [Google Scholar]
- Boulet-Audet M.; Lefèvre T.; Buffeteau T.; Pézolet M. Attenuated Total Reflection Infrared Spectroscopy: An Efficient Technique to Quantitatively Determine the Orientation and Conformation of Proteins in Single Silk Fibers. Appl. Spectrosc. 2008, 62, 956–962. 10.1366/000370208785793380. [DOI] [PubMed] [Google Scholar]
- Boulet-Audet M.; Vollrath F.; Holland C. Identification and Classification of Silks Using Infrared Spectroscopy. J. Exp. Biol. 2015, 218, 3138–3149. 10.1242/jeb.128306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A. H.; Michal C. A.; Jelinski L. W. Molecular Orientation and Two-Component Nature of the Crystalline Fraction of Spider Dragline Silk. Science 1996, 271, 84–87. 10.1126/science.271.5245.84. [DOI] [PubMed] [Google Scholar]
- Lefèvre T.; Paquet-Mercier F.; Rioux-Dubé J.-F.; Pézolet M. Structure of Silk by Raman Spectromicroscopy: From the Spinning Glands to the Fibers. Biopolymers 2012, 97, 322–336. 10.1002/bip.21712. [DOI] [PubMed] [Google Scholar]
- Kajava A. V.; Squire J. M.; Parry D. A. D. Β-Structures in Fibrous Proteins. Adv. Protein Chem. 2006, 73, 1–15. 10.1016/S0065-3233(06)73001-7. [DOI] [PubMed] [Google Scholar]
- Squire J. M.; Parry D. A. D.. Fibrous Protein Structures: Hierarchy, History and Heroes. In Fibrous Proteins: Structures and Mechanisms; Parry D. A. D., Squire J. M., Eds.; Springer International Publishing, 2017; pp 1–33. [DOI] [PubMed] [Google Scholar]
- Andersen S. O. Amino Acid Composition of Spider Silks. Comp. Biochem. Physiol. 1970, 35, 705–711. 10.1016/0010-406X(70)90988-6. [DOI] [Google Scholar]
- Work R. W.; Young C. T. The Amino Acid Compositions of Major and Minor Ampullate Silks of Certain Orb-Web-Building Spiders (Araneae, Araneidae). J. of Arachnol. 1987, 15, 65–80. [Google Scholar]
- Lombardi S. J.; Kaplan D. L. The Amino Acid Composition of Major Ampullate Gland Silk (Dragline) of Nephila Clavipes (Araneae, Tetragnathidae). J. Arachnol. 1990, 18, 297–306. [Google Scholar]
- Xu M.; Lewis R. V. Structure of a Protein Superfiber: Spider Dragline Silk. Proc. Natl. Acad. Sci. U.S.A. 1990, 87, 7120–7124. 10.1073/pnas.87.18.7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman M. B.; Lewis R. V. Isolation of a Clone Encoding a Second Dragline Silk Fibroin. Nephila Clavipes Dragline Silk Is a Two-Protein Fiber. J. Biol. Chem. 1992, 267, 19320–19324. 10.1016/S0021-9258(18)41777-2. [DOI] [PubMed] [Google Scholar]
- Guerette P. A.; Ginzinger D.; Weber B.; Gosline J. M. Silk Properties Determined by Gland-Specific Expression of a Spider Fibroin Gene Family. Science 1996, 272, 112–115. 10.1126/science.272.5258.112. [DOI] [PubMed] [Google Scholar]
- Hayashi C. Y.; Lewis R. V. Evidence from Flagelliform Silk Cdna for the Structural Basis of Elasticity and Modular Nature of Spider Silks. J. Mol. Biol. 1998, 275, 773–784. 10.1006/jmbi.1997.1478. [DOI] [PubMed] [Google Scholar]
- Ayoub N. A.; Garb J. E.; Tinghitella R. M.; Collin M. A.; Hayashi C. Y. Blueprint for a High-Performance Biomaterial: Full-Length Spider Dragline Silk Genes. PLoS One 2007, 2, e514 10.1371/journal.pone.0000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman M. B.; Jones J. A.; Lewis R. V. Synthetic Spider Silk: A Modular Fiber. Trends Biotechnol. 2000, 18, 374–379. 10.1016/S0167-7799(00)01481-5. [DOI] [PubMed] [Google Scholar]
- Huemmerich D.; Scheibel T.; Vollrath F.; Cohen S.; Gat U.; Ittah S. Novel Assembly Properties of Recombinant Spider Dragline Silk Proteins. Curr. Biol. 2004, 14, 2070–2074. 10.1016/j.cub.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Hagn F.; Eisoldt L.; Hardy J. G.; Vendrely C.; Coles M.; Scheibel T.; Kessler H. A Conserved Spider Silk Domain Acts as a Molecular Switch That Controls Fibre Assembly. Nature 2010, 465, 239–242. 10.1038/nature08936. [DOI] [PubMed] [Google Scholar]
- Askarieh G.; Hedhammar M.; Nordling K.; Saenz A.; Casals C.; Rising A.; Johansson J.; Knight S. D. Self-Assembly of Spider Silk Proteins Is Controlled by a Ph-Sensitive Relay. Nature 2010, 465, 236–238. 10.1038/nature08962. [DOI] [PubMed] [Google Scholar]
- Andersson M.; Chen G.; Otikovs M.; Landreh M.; Nordling K.; Kronqvist N.; Westermark P.; Jörnvall H.; Knight S.; Ridderstråle Y.; et al. Carbonic Anhydrase Generates CO2 and H+ That Drive Spider Silk Formation Via Opposite Effects on the Terminal Domains. PLOS Biol. 2014, 12, e1001921 10.1371/journal.pbio.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze S.; Zwettler F. U.; Johnson C. M.; Neuweiler H. The N-Terminal Domains of Spider Silk Proteins Assemble Ultrafast and Protected from Charge Screening. Nat. Commun. 2013, 4, 2815. 10.1038/ncomms3815. [DOI] [PubMed] [Google Scholar]
- Ries J.; Schwarze S.; Johnson C. M.; Neuweiler H. Microsecond Folding and Domain Motions of a Spider Silk Protein Structural Switch. J. Am. Chem. Soc. 2014, 136, 17136–17144. 10.1021/ja508760a. [DOI] [PubMed] [Google Scholar]
- Kronqvist N.; Otikovs M.; Chmyrov V.; Chen G.; Andersson M.; Nordling K.; Landreh M.; Sarr M.; Jörnvall H.; Wennmalm S. Sequential Ph-Driven Dimerization and Stabilization of the N-Terminal Domain Enables Rapid Spider Silk Formation. Nat. Commun. 2014, 5, 3254. 10.1038/ncomms4254. [DOI] [PubMed] [Google Scholar]
- Wallace J. A.; Shen J. K. Unraveling a Trap-and-Trigger Mechanism in the Ph-Sensitive Self-Assembly of Spider Silk Proteins. J. Phys. Chem. Lett. 2012, 3, 658–662. 10.1021/jz2016846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rising A.; Johansson J. Toward Spinning Artificial Spider Silk. Nat. Chem. Biol. 2015, 11, 309–315. 10.1038/nchembio.1789. [DOI] [PubMed] [Google Scholar]
- Andersson M.; Jia Q.; Abella A.; Lee X.-Y.; Landreh M.; Purhonen P.; Hebert H.; Tenje M.; Robinson C. V.; Meng Q.; et al. Biomimetic Spinning of Artificial Spider Silk from a Chimeric Minispidroin. Nat. Chem. Biol. 2017, 13, 262–264. 10.1038/nchembio.2269. [DOI] [PubMed] [Google Scholar]
- Waite J. H.; Vaccaro E.; Sun C.; Lucas J. M. Elastomeric Gradients: A Hedge against Stress Concentration in Marine Holdfasts?. Philos. Trans. R. Soc. B 2002, 357, 143–153. 10.1098/rstb.2001.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten-Andersen N.; Fantner G. E.; Hohlbauch S.; Waite J. H.; Zok F. W. Protective Coatings on Extensible Biofibres. Nat. Mater. 2007, 6, 669–672. 10.1038/nmat1956. [DOI] [PubMed] [Google Scholar]
- Harrington M. J.; Masic A.; Holten-Andersen N.; Waite J. H.; Fratzl P. Iron-Clad Fibers: A Metal-Based Biological Strategy for Hard Flexible Coatings. Science 2010, 328, 216–220. 10.1126/science.1181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne K. J.; Qin X. X.; Waite H. Extensible Collagen in Mussel Byssus: A Natural Block Copolymer. Science 1997, 277, 1830–1832. 10.1126/science.277.5333.1830. [DOI] [PubMed] [Google Scholar]
- Waite H.; Lichtenegger H. C.; Stucky G. D.; Hansma P. Exploring Molecular and Mechanical Gradients in Structural Bioscaffolds. Biochemistry 2004, 43, 7653–7662. 10.1021/bi049380h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten-Andersen N.; Harrington M. J.; Birkedal H.; Lee B. P.; Messersmith P. B.; Lee K. Y. C.; Waite J. H. Ph-Induced Metal-Ligand Cross-Links Inspired by Mussel Yield Self-Healing Polymer Networks with near-Covalent Elastic Moduli. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 2651–2655. 10.1073/pnas.1015862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite J. H. Mussel Adhesion - Essential Footwork. J. Exp. Biol. 2017, 220, 517–530. 10.1242/jeb.134056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q.; Chen J.; Wang J.; Zeng H.; Yu J. Recent Progress in Synthesis and Application of Mussel-Inspired Adhesives. Nanoscale 2020, 12, 1307–1324. 10.1039/C9NR09780E. [DOI] [PubMed] [Google Scholar]
- Mercer H. Observations on the Molecular Structure of Byssus Fibres. Mar. Freshw. Res. 1952, 3, 199–204. 10.1071/MF9520199. [DOI] [Google Scholar]
- Rudall K. M.The Distribution of Collagen and Chitin. Symp. Soc. Exp. Biol. 1955, 9. [Google Scholar]
- Pujol J. P. Formation of the Byssus in the Common Mussel (Mytilus Edulis L.). Nature 1967, 214, 204–205. 10.1038/214204a0. [DOI] [Google Scholar]
- Pikkarainen J.; Rantanen J.; Vastamäki M.; Lampliaho K.; Kari A.; Kulonen E. On Collagens of Invertebrates with Special Reference to Mytilus Edulis. Eur. J. Biochem. 1968, 4, 555–560. 10.1111/j.1432-1033.1968.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Tamarin A.; Lewis P.; Askey J. The Structure and Formation of the Byssus Attachment Plaque in Mytilus. J. Morphol. 1976, 149, 199–221. 10.1002/jmor.1051490205. [DOI] [PubMed] [Google Scholar]
- Zuccarello L. V. The Collagen Gland of Mytilus Galloprovincialis: An Ultrastructural and Cytochemical Study on Secretory Granules. J. Ultrastruct. Res. 1980, 73, 135–147. 10.1016/S0022-5320(80)90119-7. [DOI] [PubMed] [Google Scholar]
- Waite J. H.; Tanzer M. L. The Bioadhesive of Mytilus Byssus: A Protein Containing L-Dopa. Biochem. Biophys. Res. Commun. 1980, 96, 1554–1561. 10.1016/0006-291X(80)91351-0. [DOI] [PubMed] [Google Scholar]
- Waite J. H.; Tanzer M. Polyphenolic Substance of Mytilus Edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline. Science 1981, 212, 1038–1040. 10.1126/science.212.4498.1038. [DOI] [PubMed] [Google Scholar]
- Waite J. H. Evidence for a Repeating 3,4-Dihydroxyphenylalanine- and Hydroxyproline-Containing Decapeptide in the Adhesive Protein of the Mussel, Mytilus Edulis L. J. Biol. Chem. 1983, 258, 2911–2915. 10.1016/S0021-9258(18)32805-9. [DOI] [PubMed] [Google Scholar]
- Waite J. H.; Housley T. J.; Tanzer M. L. Peptide Repeats in a Mussel Glue Protein - Theme and Variations. Biochemistry 1985, 24, 5010–5014. 10.1021/bi00340a008. [DOI] [PubMed] [Google Scholar]
- Sun C.; Waite J. H. Mapping Chemical Gradients within and Along a Fibrous Structural Tissue, Mussel Byssal Threads. J. Biol. Chem. 2005, 280, 39332–39336. 10.1074/jbc.M508674200. [DOI] [PubMed] [Google Scholar]
- Rzepecki L. M.; Hansen K. M.; Waite J. H. Characterization of a Cystine-Rich Polyphenolic Protein Family from the Blue Mussel Mytilus Edulis L. Biol. Bull. 1992, 183, 123–137. 10.2307/1542413. [DOI] [PubMed] [Google Scholar]
- Inoue K.; Takeuchi Y.; Miki D.; Odo S. Mussel Adhesive Plaque Protein Gene Is a Novel Member of Epidermal Growth Factor-Like Gene Family. J. Biol. Chem. 1995, 270, 6698–6701. 10.1074/jbc.270.12.6698. [DOI] [PubMed] [Google Scholar]
- Papov V. V.; Diamond T. V.; Biemann K.; Waite J. H. Hydroxyarginine-Containing Polyphenolic Proteins in the Adhesive Plaques of the Marine Mussel Mytilus Edulis. J. Biol. Chem. 1995, 270, 20183–20192. 10.1074/jbc.270.34.20183. [DOI] [PubMed] [Google Scholar]
- Waite J. H.; Qin X.-X. Polyphosphoprotein from the Adhesive Pads of Mytilus Edulis. Biochemistry 2001, 40, 2887–2893. 10.1021/bi002718x. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Waite J. H. Linking Adhesive and Structural Proteins in the Attachment Plaque of Mytilus Californianus. J. Biol. Chem. 2006, 281, 26150–26158. 10.1074/jbc.M604357200. [DOI] [PubMed] [Google Scholar]
- Yu J.; Wei W.; Danner E.; Ashley R. K.; Israelachvili J. N.; Waite J. H. Mussel Protein Adhesion Depends on Interprotein Thiol-Mediated Redox Modulation. Nat. Chem. Biol. 2011, 7, 588–590. 10.1038/nchembio.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X.; Waite J. H. Exotic Collagen Gradients in the Byssus of the Mussel Mytilus Edulis. J. Exp. Biol. 1995, 198, 633–644. 10.1242/jeb.198.3.633. [DOI] [PubMed] [Google Scholar]
- Qin X.-X.; Coyne K. J.; Waite J. H. Tough Tendons: Mussel Byssus Has Collagen with Silk-Like Domains. J. Biol. Chem. 1997, 272, 32623–32627. 10.1074/jbc.272.51.32623. [DOI] [PubMed] [Google Scholar]
- Qin X.-X.; Waite J. H. A Potential Mediator of Collagenous Block Copolymer Gradients in Mussel Byssal Threads. Proc. Natl. Acad. Sci. U.S.A. 1998, 95, 10517–10522. 10.1073/pnas.95.18.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filpula D. R.; Lee S.-M.; Link R. P.; Strausberg S. L.; Strausberg R. L. Structural and Functional Repetition in a Marine Mussel Adhesive Protein. Biotechnol. Prog. 1990, 6, 171–177. 10.1021/bp00003a001. [DOI] [PubMed] [Google Scholar]
- Salerno A. J.; Goldberg I. Cloning, Expression, and Characterization of a Synthetic Analog to the Bioadhesive Precursor Protein of the Sea Mussel Mytilus Edulis. Appl. Microbiol. Biotechnol. 1993, 39, 221–226. 10.1007/BF00228610. [DOI] [PubMed] [Google Scholar]
- Hwang D. S.; Yoo H. Y.; Jun J. H.; Moon W. K.; Cha H. J. Expression of Functional Recombinant Mussel Adhesive Protein Mgfp-5 in Escherichia Coli. Appl. Environ. Microbiol. 2004, 70, 3352–3359. 10.1128/AEM.70.6.3352-3359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman P. Method for Determination of the Amino Acid Sequence in Peptides. Acta Chemical Scandinavia 1950, 4, 283–293. 10.3891/acta.chem.scand.04-0283. [DOI] [Google Scholar]
- Edman P.; Begg G. A Protein Sequenator. Eur. J. Biochem. 1967, 1, 80–91. 10.1111/j.1432-1033.1967.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Wilm M.; Mann M. Analytical Properties of the Nanoelectrospray Ion Source. Anal. Chem. 1996, 68, 1–8. 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- Mann M. The Rise of Mass Spectrometry and the Fall of Edman Degradation. Clin. Chem. 2016, 62, 293–294. 10.1373/clinchem.2014.237271. [DOI] [PubMed] [Google Scholar]
- Shevchenko A.; Tomas H.; Havli J.; Olsen J. V.; Mann M. In-Gel Digestion for Mass Spectrometric Characterization of Proteins and Proteomes. Nat. Protoc. 2006, 1, 2856–2860. 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- Karas M.; Bachmann D.; Hillenkamp F. Influence of the Wavelength in High-Irradiance Ultraviolet Laser Desorption Mass Spectrometry of Organic Molecules. Anal. Chem. 1985, 57, 2935–2939. 10.1021/ac00291a042. [DOI] [Google Scholar]
- Tanaka K.; Waki H.; Ido Y.; Akita S.; Yoshida Y.; Yoshida T.; Matsuo T. Protein and Polymer Analyses up to M/Z 100 000 by Laser Ionization Time-of-Flight Mass Spectrometry. Rapid Commun. Mass. Spectrom. 1988, 2, 151–153. 10.1002/rcm.1290020802. [DOI] [Google Scholar]
- Karas M.; Bahr U. Laser Desorption Ionization Mass Spectrometry of Large Biomolecules. Trends Anal. Chem. 1990, 9, 321–325. 10.1016/0165-9936(90)85065-F. [DOI] [Google Scholar]
- Metzker M. L. Sequencing Technologies — the Next Generation. Nat. Rev. Genet. 2010, 11, 31–46. 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Gerstein M.; Snyder M. Rna-Seq: A Revolutionary Tool for Transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsolak F.; Milos P. M. Rna Sequencing: Advances, Challenges and Opportunities. Nat. Rev. Genet. 2011, 12, 87–98. 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R.; Grzelak M.; Hadfield J. Rna Sequencing: The Teenage Years. Nat. Rev. Genet. 2019, 20, 631–656. 10.1038/s41576-019-0150-2. [DOI] [PubMed] [Google Scholar]
- Saliba A.-E.; Westermann A. J.; Gorski S. A.; Vogel J. Single-Cell Rna-Seq: Advances and Future Challenges. Nucleic Acids Res. 2014, 42, 8845–8860. 10.1093/nar/gku555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A.; Engel J.; Teichmann S. A.; Lönnberg T. A Practical Guide to Single-Cell Rna-Sequencing for Biomedical Research and Clinical Applications. Genome Med. 2017, 9, 75. 10.1186/s13073-017-0467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber M.; Grabherr M. G.; Guttman M.; Trapnell C. Computational Methods for Transcriptome Annotation and Quantification Using Rna-Seq. Nat. Methods 2011, 8, 469–477. 10.1038/nmeth.1613. [DOI] [PubMed] [Google Scholar]
- Guerette P. A.; Hoon S.; Seow Y.; Raida M.; Masic A.; Wong F. T.; Ho V. H.; Kong K. W.; Demirel M. C.; Pena-Francesch A.; et al. Accelerating the Design of Biomimetic Materials by Integrating Rna-Seq with Proteomics and Materials Science. Nat. Biotechnol. 2013, 31, 908–915. 10.1038/nbt.2671. [DOI] [PubMed] [Google Scholar]
- Grabherr M. G.; Haas B. J.; Yassour M.; Levin J. Z.; Thompson D. A.; Amit I.; Adiconis X.; Fan L.; Raychowdhury R.; Zeng Q.; et al. Full-Length Transcriptome Assembly from Rna-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B.; Zhang K.; Hendrie C.; Liang C.; Li M.; Doherty-Kirby A.; Lajoie G. Peaks: Powerful Software for Peptide De Novo Sequencing by Ms/Ms. Rapid Commun. Mass. Spectrom. 2003, 17, 2337–2342. 10.1002/rcm.1196. [DOI] [PubMed] [Google Scholar]
- Guerette P. A.; Hoon S.; Ding D.; Amini S.; Masic A.; Venkatesh B.; Ravi V.; Weaver J. C.; Miserez A. Nanoconfined Β-Sheets Mechanically Reinforce the Supra-Biomolecular Network of Robust Squid Sucker Ring Teeth. ACS Nano 2014, 8, 7170–7179. 10.1021/nn502149u. [DOI] [PubMed] [Google Scholar]
- Miserez A.; Weaver J. C.; Pedersen P. B.; Schneeberk T.; Hanlon R. T.; Kisailus D.; Birkedal H. Microstructural and Biochemical Characterization of the Nano-Porous Sucker Rings from Dosidicus Gigas. Adv. Mater. 2009, 21, 401–406. 10.1002/adma.200801197. [DOI] [Google Scholar]
- Tan Y. P.; Hoon S.; Guerette P. A.; Wei W.; Hao C.; Ghadban A.; Miserez A.; Waite J. H. Infiltration of a Chitin Scaffold by Protein Coacervates Defines the Squid Beak Mechanical Gradient. Nat. Chem. Biol. 2015, 11, 488–495. 10.1038/nchembio.1833. [DOI] [PubMed] [Google Scholar]
- Miserez A.; Schneberk T.; Sun C.; Zok F. W.; Waite J. H. The Transition from Stiff to Compliant Materials in Squid Beak. Science 2008, 319, 1816–1819. 10.1126/science.1154117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserez A.; Rubin D.; Waite J. H. Cross-Linking Chemistry of Squid Beak. J. Biol. Chem. 2010, 285, 38115–38124. 10.1074/jbc.M110.161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini S.; Tadayon M.; Loke J. J.; Kumar A.; Kanagavel D.; Le Ferrand H.; Duchamp M.; Raida M.; Sobota R. M.; Chen L.; et al. A Diecast Mineralization Process Forms the Tough Mantis Shrimp Dactyl Club. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 8685. 10.1073/pnas.1816835116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J. C.; Milliron G. W.; Miserez A.; Evans-Lutterodt K.; Herrera S.; Gallana I.; Mershon W. J.; Swanson B.; Zavattieri P.; DiMasi E.; et al. The Stomatopod Dactyl Club: A Formidable Damage-Tolerant Biological Hammer. Science 2012, 336, 1275–1280. 10.1126/science.1218764. [DOI] [PubMed] [Google Scholar]
- Amini S.; Masic A.; Bertinetti L.; Teguh J. S.; Herrin J. S.; Zhu X.; Su H.; Miserez A. Textured Fluorapatite Bonded to Calcium Sulphate Strengthen Stomatopod Raptorial Appendages. Nat. Commun. 2014, 5, 3187. 10.1038/ncomms4187. [DOI] [PubMed] [Google Scholar]
- Grunenfelder L. K.; Suksangpanya N.; Salinas C.; Milliron G.; Yaraghi N.; Herrera S.; Evans-Lutterodt K.; Nutt S. R.; Zavattieri P.; Kisailus D. Bio-Inspired Impact-Resistant Composites. Acta. Biomater. 2014, 10, 3997–4008. 10.1016/j.actbio.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Amini S.; Tadayon M.; Idapalapati S.; Miserez A. The Role of Quasi-Plasticity on the Extreme Contact Damage Tolerance of the Stomatopod Dactyl Club. Nat. Mater. 2015, 14, 943–950. 10.1038/nmat4309. [DOI] [PubMed] [Google Scholar]
- Yaraghi N. A.; Guarín-Zapata N.; Grunenfelder L. K.; Hintsala E.; Bhowmick S.; Hiller J. M.; Betts M.; Principe E. L.; Jung J.-Y.; Sheppard L.; et al. A Sinusoidally Architected Helicoidal Biocomposite. Adv. Mater. 2016, 28, 6835–6844. 10.1002/adma.201600786. [DOI] [PubMed] [Google Scholar]
- Behera R. P.; Le Ferrand H. Impact-Resistant Materials Inspired by the Mantis Shrimp’s Dactyl Club. Matter 2021, 4, 2831–2849. 10.1016/j.matt.2021.07.012. [DOI] [Google Scholar]
- Mohammadi P.; Gandier J.-A.; Nonappa; Wagermaier W.; Miserez A.; Penttilä M. Bioinspired Functionally Graded Composite Assembled Using Cellulose Nanocrystals and Genetically Engineered Proteins with Controlled Biomineralization. Adv. Mater. 2021, 33, 2102658. 10.1002/adma.202102658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennebert E.; Maldonado B.; Ladurner P.; Flammang P.; Santos R. Experimental Strategies for the Identification and Characterization of Adhesive Proteins in Animals: A Review. Interface Focus 2015, 5, 20140064. 10.1098/rsfs.2014.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey P. A.; Power A. M.; Santos R.; Bertemes P.; Ladurner P.; Palmowski P.; Clarke J.; Flammang P.; Lengerer B.; Hennebert E.; et al. Omics-Based Molecular Analyses of Adhesion by Aquatic Invertebrates. Biol. Rev. 2021, 96, 1051. 10.1111/brv.12691. [DOI] [PubMed] [Google Scholar]
- Hennebert E.; Leroy B.; Wattiez R.; Ladurner P. An Integrated Transcriptomic and Proteomic Analysis of Sea Star Epidermal Secretions Identifies Proteins Involved in Defense and Adhesion. J. Proteomics 2015, 128, 83–91. 10.1016/j.jprot.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Wunderer J.; Lengerer B.; Pjeta R.; Bertemes P.; Kremser L.; Lindner H.; Ederth T.; Hess M. W.; Stock D.; Salvenmoser W.; et al. A Mechanism for Temporary Bioadhesion. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 4297–4306. 10.1073/pnas.1814230116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartini D. G.; Errico J. M.; Sjoestroem S.; Fenster A.; Waite J. H. A Cohort of New Adhesive Proteins Identified from Transcriptomic Analysis of Mussel Foot Glands. J. R. Soc. Interface 2017, 14, 20170151. 10.1098/rsif.2017.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig C. L. Evolution of Arthropod Silks. Annu. Rev. Entomol. 1997, 42, 231–267. 10.1146/annurev.ento.42.1.231. [DOI] [PubMed] [Google Scholar]
- Hu X.; Vasanthavada K.; Kohler K.; McNary S.; Moore A. M.; Vierra C. A. Molecular Mechanisms of Spider Silk. Cell. Mol. Life Sci. 2006, 63, 1986–1999. 10.1007/s00018-006-6090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. H.; Edmonds D. T.; Vollrath F. Structural Engineering of an Orb-Spider’s Web. Nature 1995, 373, 146–148. 10.1038/373146a0. [DOI] [Google Scholar]
- Sahni V.; Harris J.; Blackledge T. A.; Dhinojwala A. Cobweb-Weaving Spiders Produce Different Attachment Discs for Locomotion and Prey Capture. Nat. Commun. 2012, 3, 1106–1106. 10.1038/ncomms2099. [DOI] [PubMed] [Google Scholar]
- Stellwagen S. D.; Renberg R. L. Toward Spider Glue: Long Read Scaffolding for Extreme Length and Repetitious Silk Family Genes Agsp1 and Agsp2 with Insights into Functional Adaptation. G3: Genes Genomes Genet. 2019, 9, 1909–1919. 10.1534/g3.119.400065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana Y.; Sezutsu H.; Nakajima K.-i.; Tamada Y.; Kojima K. High-Toughness Silk Produced by a Transgenic Silkworm Expressing Spider (Araneus Ventricosus) Dragline Silk Protein. PLoS One 2014, 9, e105325–e105325. 10.1371/journal.pone.0105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasingame E.; Tuton-Blasingame T.; Larkin L.; Falick A. M.; Zhao L.; Fong J.; Vaidyanathan V.; Visperas A.; Geurts P.; Hu X. Pyriform Spidroin 1, a Novel Member of the Silk Gene Family That Anchors Dragline Silk Fibers in Attachment Discs of the Black Widow Spider, Latrodectus Hesperus. J. Biol. Chem. 2009, 284, 29097–29108. 10.1074/jbc.M109.021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi C. Y.; Lewis R. V. Molecular Architecture and Evolution of a Modular Spider Silk Protein Gene. Science 2000, 287, 1477–1479. 10.1126/science.287.5457.1477. [DOI] [PubMed] [Google Scholar]
- Collin M. A.; Clarke T. H.; Ayoub N. A.; Hayashi C. Y. Evidence from Multiple Species That Spider Silk Glue Component Asg2 Is a Spidroin. Sci. Rep. 2016, 6, 21589. 10.1038/srep21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J.; Guerette P. A.; Pavesi A.; Horbelt N.; Lim C. T.; Harrington M. J.; Miserez A. Artificial Hagfish Protein Fibers with Ultra-High and Tunable Stiffness. Nanoscale 2017, 9, 12908–12915. 10.1039/C7NR02527K. [DOI] [PubMed] [Google Scholar]
- Baer A.; Hänsch S.; Mayer G.; Harrington M. J.; Schmidt S. Reversible Supramolecular Assembly of Velvet Worm Adhesive Fibers Via Electrostatic Interactions of Charged Phosphoproteins. Biomacromolecules 2018, 19, 4034–4043. 10.1021/acs.biomac.8b01017. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Sharma B.; Soon W. L.; Shi X.; Zhao T.; Lim Y. T.; Sobota R. M.; Hoon S.; Pilloni G.; Usadi A.; et al. Complete Sequences of the Velvet Worm Slime Proteins Reveal That Slime Formation Is Enabled by Disulfide Bonds and Intrinsically Disordered Regions. Adv. Sci. 2022, 9, 2201444. 10.1002/advs.202201444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarger J. L.; Cherry B. R.; Van Der Vaart A. Uncovering the Structure-Function Relationship in Spider Silk. Nat. Rev. Mater. 2018, 3, 18008. 10.1038/natrevmats.2018.8. [DOI] [Google Scholar]
- Astbury W. T.; Woods H. J. X-Ray Studies of the Structure of Hair, Wool, and Related Fibres. II.- the Molecular Structure and Elastic Properties of Hair Keratin. Philos. Trans. R. Soc. A 1933, 232, 333–394. 10.1098/rsta.1934.0010. [DOI] [Google Scholar]
- Agnarsson I.; Boutry C.; Wong S.-C.; Baji A.; Dhinojwala A.; Sensenig A. T.; Blackledge T. A. Supercontraction Forces in Spider Dragline Silk Depend on Hydration Rate. Zoology 2009, 112, 325–331. 10.1016/j.zool.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Cohen N.; Levin M.; Eisenbach C. D. On the Origin of Supercontraction in Spider Silk. Biomacromolecules 2021, 22, 993–1000. 10.1021/acs.biomac.0c01747. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Shao Z.; Vollrath F. Relationships between Supercontraction and Mechanical Properties of Spider Silk. Nat. Mater. 2005, 4, 901–905. 10.1038/nmat1534. [DOI] [PubMed] [Google Scholar]
- Lewis R. V. Spider Silk: Ancient Ideas for New Biomaterials. Chem. Rev. 2006, 106, 3762–3774. 10.1021/cr010194g. [DOI] [PubMed] [Google Scholar]
- Ittah S.; Cohen S.; Garty S.; Cohn D.; Gat U. An Essential Role for the C-Terminal Domain of a Dragline Spider Silk Protein in Directing Fiber Formation. Biomacromolecules 2006, 7, 1790–1795. 10.1021/bm060120k. [DOI] [PubMed] [Google Scholar]
- Eisoldt L.; Thamm C.; Scheibel T. The Role of Terminal Domains During Storage and Assembly of Spider Silk Proteins. Biopolymers 2012, 97, 355–361. 10.1002/bip.22006. [DOI] [PubMed] [Google Scholar]
- Sponner A.; Vater W.; Rommerskirch W.; Vollrath F.; Unger E.; Grosse F.; Weisshart K. The Conserved C-Termini Contribute to the Properties of Spider Silk Fibroins. Biochem. Biophys. Res. Commun. 2005, 338, 897–902. 10.1016/j.bbrc.2005.10.048. [DOI] [PubMed] [Google Scholar]
- Vollrath F.; Knight D. P. Liquid Crystalline Spinning of Spider Silk. Nature 2001, 410, 541–548. 10.1038/35069000. [DOI] [PubMed] [Google Scholar]
- Ulrich S.; Glisovic A.; Salditt T.; Zippelius A. Diffraction from the Beta-Sheet Crystallites in Spider Silk. Eur. Phys. J. E. Soft Matter 2008, 27, 229–242. 10.1140/epje/i2008-10374-7. [DOI] [PubMed] [Google Scholar]
- Kubik S. High-Performance Fibers from Spider Silk. Angew. Chem., Int. Ed. Engl. 2002, 41, 2721–2723. . [DOI] [PubMed] [Google Scholar]
- Yamaguchi E.; Yamauchi K.; Gullion T.; Asakura T. Structural Analysis of the Gly-Rich Region in Spider Dragline Silk Using Stable-Isotope Labeled Sequential Model Peptides and Solid-State Nmr. Chem. Commun. 2009, 4176–4178. 10.1039/b906145b. [DOI] [PubMed] [Google Scholar]
- Adrianos S. L.; Teule F.; Hinman M. B.; Jones J. A.; Weber W. S.; Yarger J. L.; Lewis R. V. Nephila Clavipes Flagelliform Silk-Like Ggx Motifs Contribute to Extensibility and Spacer Motifs Contribute to Strength in Synthetic Spider Silk Fibers. Biomacromolecules 2013, 14, 1751–1760. 10.1021/bm400125w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker N.; Oroudjev E.; Mutz S.; Cleveland J. P.; Hansma P. K.; Hayashi C. Y.; Makarov D. E.; Hansma H. G. Molecular Nanosprings in Spider Capture-Silk Threads. Nat. Mater. 2003, 2, 278–283. 10.1038/nmat858. [DOI] [PubMed] [Google Scholar]
- Chaw R. C.; Saski C. A.; Hayashi C. Y. Complete Gene Sequence of Spider Attachment Silk Protein (Pysp1) Reveals Novel Linker Regions and Extreme Repeat Homogenization. Insect Biochem. Mol. Biol. 2017, 81, 80–90. 10.1016/j.ibmb.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Wolff J. O.; Grawe I.; Wirth M.; Karstedt A.; Gorb S. N. Spider’s Super-Glue: Thread Anchors Are Composite Adhesives with Synergistic Hierarchical Organization. Soft Matter 2015, 11, 2394–2403. 10.1039/C4SM02130D. [DOI] [PubMed] [Google Scholar]
- Geurts P.; Zhao L.; Hsia Y.; Gnesa E.; Tang S.; Jeffery F.; Mattina C. L.; Franz A.; Larkin L.; Vierra C. Synthetic Spider Silk Fibers Spun from Pyriform Spidroin 2, a Glue Silk Protein Discovered in Orb-Weaving Spider Attachment Discs. Biomacromolecules 2010, 11, 3495–3503. 10.1021/bm101002w. [DOI] [PubMed] [Google Scholar]
- Opell B. D.; Burba C. M.; Deva P. D.; Kin M. H. Y.; Rivas M. X.; Elmore H. M.; Hendricks M. L. Linking Properties of an Orb-Weaving Spider’s Capture Thread Glycoprotein Adhesive and Flagelliform Fiber Components to Prey Retention Time. Ecol. Evol. 2019, 9, 9841–9854. 10.1002/ece3.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla S.; Amarpuri G.; Dhopatkar N.; Blackledge T. A.; Dhinojwala A. Hygroscopic Compounds in Spider Aggregate Glue Remove Interfacial Water to Maintain Adhesion in Humid Conditions. Nat. Commun. 2018, 9, 1890. 10.1038/s41467-018-04263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.; Fudge D. S.; Levy N.; Gosline J. M. Hagfish Slime Ecomechanics: Testing the Gill-Clogging Hypothesis. J. Exp. Biol. 2006, 209, 702. 10.1242/jeb.02067. [DOI] [PubMed] [Google Scholar]
- Mele C.Chain-Reaction Crash with Minor Injuries, except for the Slime Eels. The New York Times, July 14th, 2017. [Google Scholar]
- Fudge D. S.; Schorno S.; Ferraro S. Physiology, Biomechanics, and Biomimetics of Hagfish Slime. Annu. Rev. Anal. Biochem. 2015, 84, 947–967. 10.1146/annurev-biochem-060614-034048. [DOI] [PubMed] [Google Scholar]
- Winegard T.; Herr J.; Mena C.; Lee B.; Dinov I.; Bird D.; Bernards M.; Hobel S.; Van Valkenburgh B.; Toga A.; et al. Coiling and Maturation of a High-Performance Fibre in Hagfish Slime Gland Thread Cells. Nat. Commun. 2014, 5, 3534. 10.1038/ncomms4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing S. W.; Spitzer R. H.; Salo W. L.; Downing J. S.; Saidel L. J.; Koch E. A. Threads in the Hagfish Slime Gland Thread Cells: Organization, Biochemical Features, and Length. Science 1981, 212, 326–328. 10.1126/science.212.4492.326. [DOI] [PubMed] [Google Scholar]
- Downing S. W.; Spitzer R. H.; Koch E. A.; Salo W. L. The Hagfish Slime Gland Thread Cell. I. A Unique Cellular System for the Study of Intermediate Filaments and Intermediate Filament-Microtubule Interactions. J. Cell. Biol. 1984, 98, 653–669. 10.1083/jcb.98.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R. H.; Downing S. W.; Koch E. A.; Salo W. L.; Saidel L. J. Hagfish Slime Gland Thread Cells. Ii. Isolation and Characterization of Intermediate Filament Components Associated with the Thread. J. Cell. Biol. 1984, 98, 670–677. 10.1083/jcb.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch E. A.; Spitzer R. H.; Pithawalla R. B.; Parry D. A. An Unusual Intermediate Filament Subunit from the Cytoskeletal Biopolymer Released Extracellularly into Seawater by the Primitive Hagfish (Eptatretus Stouti). J. Cell Sci. 1994, 107, 3133–3144. 10.1242/jcs.107.11.3133. [DOI] [PubMed] [Google Scholar]
- Koch E. A.; Spitzer R. H.; Pithawalla R. B.; Castillos F. A.; Parry D. A. Hagfish Biopolymer: A Type I/Type Ii Homologue of Epidermal Keratin Intermediate Filaments. Int. J. Biol. Macromol. 1995, 17, 283–292. 10.1016/0141-8130(95)98156-S. [DOI] [PubMed] [Google Scholar]
- Fudge D. S.; Gardner K. H.; Forsyth V. T.; Riekel C.; Gosline J. M. The Mechanical Properties of Hydrated Intermediate Filaments: Insights from Hagfish Slime Threads. Biophys. J. 2003, 85, 2015–2027. 10.1016/S0006-3495(03)74629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosline J. M.; Fudge D. S.; Guerette P.. Alpha-Helical Protein Based Materials and Methods for Making Same. US Patent 7,049,405 B2, 2006.
- Xu G.; Gong L.; Yang Z.; Liu X. Y. What Makes Spider Silk Fibers So Strong? From Molecular-Crystallite Network to Hierarchical Network Structures. Soft Matter 2014, 10, 2116–2123. 10.1039/C3SM52845F. [DOI] [PubMed] [Google Scholar]
- Blaxter M.; Sunnucks P. Velvet Worms. Curr. Biol. 2011, 21, R238–R240. 10.1016/j.cub.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Benkendorff K.; Beardmore K.; Gooley A. A.; Packer N. H.; Tait N. N. Characterisation of the Slime Gland Secretion from the Peripatus, Euperipatoides Kanangrensis (Onychophora: Peripatopsidae). Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 1999, 124, 457–465. 10.1016/S0305-0491(99)00145-5. [DOI] [Google Scholar]
- Haritos V. S.; Niranjane A.; Weisman S.; Trueman H. E.; Sriskantha A.; Sutherland T. D. Harnessing Disorder: Onychophorans Use Highly Unstructured Proteins, Not Silks, for Prey Capture. Proc. R. Soc. B. Biol. Sci. 2010, 277, 3255–3263. 10.1098/rspb.2010.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer A.; Schmidt S.; Haensch S.; Eder M.; Mayer G.; Harrington M. J. Mechanoresponsive Lipid-Protein Nanoglobules Facilitate Reversible Fibre Formation in Velvet Worm Slime. Nat. Commun. 2017, 8, 974. 10.1038/s41467-017-01142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer A.; Horbelt N.; Nijemeisland M.; Garcia S. J.; Fratzl P.; Schmidt S.; Mayer G.; Harrington M. J. Shear-Induced Β-Crystallite Unfolding in Condensed Phase Nanodroplets Promotes Fiber Formation in a Biological Adhesive. ACS Nano 2019, 13, 4992–5001. 10.1021/acsnano.9b00857. [DOI] [PubMed] [Google Scholar]
- Jumper J.; Evans R.; Pritzel A.; Green T.; Figurnov M.; Ronneberger O.; Tunyasuvunakool K.; Bates R.; Žídek A.; Potapenko A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D. T.; Kato M.; Lin Y.; Thurber K. R.; Hung I.; McKnight S. L.; Tycko R. Structure of Fus Protein Fibrils and Its Relevance to Self-Assembly and Phase Separation of Low-Complexity Domains. Cell 2017, 171, 615–627. 10.1016/j.cell.2017.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M.; Han T. W.; Xie S.; Shi K.; Du X.; Wu L. C.; Mirzaei H.; Goldsmith E. J.; Longgood J.; Pei J.; et al. Cell-Free Formation of Rna Granules: Low Complexity Sequence Domains Form Dynamic Fibers within Hydrogels. Cell 2012, 149, 753–767. 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valois E.; Mirshafian R.; Waite J. H. Phase-Dependent Redox Insulation in Mussel Adhesion. Sci. Adv. 2020, 6, eaaz6486 10.1126/sciadv.aaz6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malay A. D.; Suzuki T.; Katashima T.; Kono N.; Arakawa K.; Numata K. Spider Silk Self-Assembly Via Modular Liquid-Liquid Phase Separation and Nanofibrillation. Sci. Adv. 2020, 6, eabb6030 10.1126/sciadv.abb6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priemel T.; Palia G.; Förste F.; Jehle F.; Sviben S.; Mantouvalou I.; Zaslansky P.; Bertinetti L.; Harrington M. J. Microfluidic-Like Fabrication of Metal Ion-Cured Bioadhesives by Mussels. Science 2021, 374, 206–211. 10.1126/science.abi9702. [DOI] [PubMed] [Google Scholar]
- Treloar L. R. G.The Physics of Rubber Elasticity; Oxford University Press, 2005. [Google Scholar]
- Gosline J. M.Rubberlike Proteins. Mechanical Design of Structural Materials in Animals; Princeton University Press, 2018; pp 290–313. [Google Scholar]
- Shadwick R. E.; Gosline J. M. Physical and Chemical Properties of Rubber-Like Elastic Fibres from the Octopus Aorta. J. Exp. Biol. 1985, 114, 239–257. 10.1242/jeb.114.1.239. [DOI] [Google Scholar]
- Ozsvar J.; Yang C.; Cain S. A.; Baldock C.; Tarakanova A.; Weiss A. S.. Tropoelastin and Elastin Assembly. Front. Bioeng. Biotechnol. 2021, 9, 10.3389/fbioe.2021.643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W. Entropic Elastic Processes in Protein Mechanisms. I. Elastic Structure Due to an Inverse Temperature Transition and Elasticity Due to Internal Chain Dynamics. J. Protein Chem. 1988, 7, 1–34. 10.1007/BF01025411. [DOI] [PubMed] [Google Scholar]
- Miao M.; Bellingham C. M.; Stahl R. J.; Sitarz E. E.; Lane C. J.; Keeley F. W. Sequence and Structure Determinants for the Self-Aggregation of Recombinant Polypeptides Modeled after Human Elastin. J. Biol. Chem. 2003, 278, 48553–48562. 10.1074/jbc.M308465200. [DOI] [PubMed] [Google Scholar]
- Yao X. L.; Hong M. Structure Distribution in an Elastin-Mimetic Peptide (Vpgvg)3 Investigated by Solid-State Nmr. J. Am. Chem. Soc. 2004, 126, 4199–4210. 10.1021/ja036686n. [DOI] [PubMed] [Google Scholar]
- Tamburro A. M.; Bochicchio B.; Pepe A. Dissection of Human Tropoelastin: Exon-by-Exon Chemical Synthesis and Related Conformational Studies. Biochemistry 2003, 42, 13347–13362. 10.1021/bi034837t. [DOI] [PubMed] [Google Scholar]
- Ohgo K.; Niemczura W. P.; Kumashiro K. K. Probing the Natural-Abundance 13c Populations of Insoluble Elastin Using 13c-1h Heteronuclear Correlation (Hetcor) NMR Spectroscopy. Macromolecules 2009, 42, 7024–7030. 10.1021/ma900816k. [DOI] [Google Scholar]
- Ohgo K.; Niemczura W. P.; Seacat B. C.; Wise S. G.; Weiss A. S.; Kumashiro K. K. Resolving Nitrogen-15 and Proton Chemical Shifts for Mobile Segments of Elastin with Two-Dimensional NMR Spectroscopy. J. Biol. Chem. 2012, 287, 18201–18209. 10.1074/jbc.M111.285163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichheld S. E.; Muiznieks L. D.; Keeley F. W.; Sharpe S. Direct Observation of Structure and Dynamics During Phase Separation of an Elastomeric Protein. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, E4408-E4415 10.1073/pnas.1701877114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G.; Hu X.; Cebe P.; Kaplan D. L. Mechanism of Resilin Elasticity. Nat. Commun. 2012, 3, 1003. 10.1038/ncomms2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle F.; Priemel T.; Strauss M.; Fratzl P.; Bertinetti L.; Harrington M. J. Collagen Pentablock Copolymers Form Smectic Liquid Crystals as Precursors for Mussel Byssus Fabrication. ACS Nano 2021, 15, 6829–6838. 10.1021/acsnano.0c10457. [DOI] [PubMed] [Google Scholar]
- Schmelzer C. E. H.; Heinz A.; Troilo H.; Lockhart-Cairns M. P.; Jowitt T. A.; Marchand M. F.; Bidault L.; Bignon M.; Hedtke T.; Barret A.; et al. Lysyl Oxidase-Like 2 (Loxl2)-Mediated Cross-Linking of Tropoelastin. FASEB J. 2019, 33, 5468–5481. 10.1096/fj.201801860RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrhovski B.; Weiss A. S. Biochemistry of Tropoelastin. Eur. J. Biochem. 1998, 258, 1–18. 10.1046/j.1432-1327.1998.2580001.x. [DOI] [PubMed] [Google Scholar]
- Andersen S. A. Insect Cuticular Sclerotization: A Review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. 10.1016/j.ibmb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Kerwin J. L.; Turecek F.; Xu R.; Kramer K. J.; Hopkins T. L.; Gatlin C. L.; Yates J. R. Mass Spectrometric Analysis of Catechol-Histidine Adducts from Insect Cuticle. Anal. Biochem. 1999, 268, 229–237. 10.1006/abio.1998.3069. [DOI] [PubMed] [Google Scholar]
- Yeo G. C.; Tarakanova A.; Baldock C.; Wise S. G.; Buehler M. J.; Weiss A. S. Subtle Balance of Tropoelastin Molecular Shape and Flexibility Regulates Dynamics and Hierarchical Assembly. Sci. Adv. 2016, 2, e1501145 10.1126/sciadv.1501145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher S.; Pomès R. The Liquid Structure of Elastin. eLife 2017, 6, e26526 10.7554/eLife.26526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R. S.; Kim Y.; Liu J. C. Resilin: Protein-Based Elastomeric Biomaterials. Acta. Biomater. 2014, 10, 1601–1611. 10.1016/j.actbio.2013.06.038. [DOI] [PubMed] [Google Scholar]
- Balu R.; Dutta N. K.; Dutta A. K.; Choudhury N. R. Resilin-Mimetics as a Smart Biomaterial Platform for Biomedical Applications. Nat. Commun. 2021, 12, 149. 10.1038/s41467-020-20375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G.; Lapidot S.; Numata K.; Hu X.; Meirovitch S.; Dekel M.; Podoler I.; Shoseyov O.; Kaplan D. L. Expression, Cross-Linking, and Characterization of Recombinant Chitin Binding Resilin. Biomacromolecules 2009, 10, 3227–3234. 10.1021/bm900735g. [DOI] [PubMed] [Google Scholar]
- Lyons R. E.; Wong D. C. C.; Kim M.; Lekieffre N.; Huson M. G.; Vuocolo T.; Merritt D. J.; Nairn K. M.; Dudek D. M.; Colgrave M. L.; et al. Molecular and Functional Characterisation of Resilin across Three Insect Orders. Insect Biochem. Mol. Biol. 2011, 41, 881–890. 10.1016/j.ibmb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Lyons R. E.; Lesieur E.; Kim M.; Wong D. C. C.; Huson M. G.; Nairn K. M.; Brownlee A. G.; Pearson R. D.; Elvin C. M. Design and Facile Production of Recombinant Resilin-Like Polypeptides: Gene Construction and a Rapid Protein Purification Method. Protein Eng. Des. Sel. 2007, 20, 25–32. 10.1093/protein/gzl050. [DOI] [PubMed] [Google Scholar]
- Lyons R. E.; Nairn K. M.; Huson M. G.; Kim M.; Dumsday G.; Elvin C. M. Comparisons of Recombinant Resilin-Like Proteins: Repetitive Domains Are Sufficient to Confer Resilin-Like Properties. Biomacromolecules 2009, 10, 3009–3014. 10.1021/bm900601h. [DOI] [PubMed] [Google Scholar]
- Truong M. Y.; Dutta N. K.; Choudhury N. R.; Kim M.; Elvin C. M.; Nairn K. M.; Hill A. J. The Effect of Hydration on Molecular Chain Mobility and the Viscoelastic Behavior of Resilin-Mimetic Protein-Based Hydrogels. Biomaterials 2011, 32, 8462–8473. 10.1016/j.biomaterials.2011.07.064. [DOI] [PubMed] [Google Scholar]
- Dutta N. K.; Truong M. Y.; Mayavan S.; Roy Choudhury N.; Elvin C. M.; Kim M.; Knott R.; Nairn K. M.; Hill A. J. A Genetically Engineered Protein Responsive to Multiple Stimuli. Angew. Chem., Int. Ed. Engl. 2011, 50, 4428–4431. 10.1002/anie.201007920. [DOI] [PubMed] [Google Scholar]
- Balu R.; Dutta N. K.; Choudhury N. R.; Elvin C. M.; Lyons R. E.; Knott R.; Hill A. J. An16-Resilin: An Advanced Multi-Stimuli-Sesponsive Resilin-Mimetic Protein Polymer. Acta. Biomater. 2014, 10, 4768–4777. 10.1016/j.actbio.2014.07.030. [DOI] [PubMed] [Google Scholar]
- Rauscher S.; Baud S.; Miao M.; Keeley F. W.; Pomès R. Proline and Glycine Control Protein Self-Organization into Elastomeric or Amyloid Fibrils. Structure 2006, 14, 1667–1676. 10.1016/j.str.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Malencik D. A.; Anderson S. R. Dityrosine Formation in Calmodulin: Cross-Linking and Polymerization Catalyzed by Arthromyces Peroxidase. Biochemistry 1996, 35, 4375–4386. 10.1021/bi9526037. [DOI] [PubMed] [Google Scholar]
- Fancy D. A.; Kodadek T. Chemistry for the Analysis of Protein-Protein Interactions: Rapid and Efficient Cross-Linking Triggered by Long Wavelength Light. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 6020–6024. 10.1073/pnas.96.11.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partlow B. P.; Applegate M. B.; Omenetto F. G.; Kaplan D. L. Dityrosine Cross-Linking in Designing Biomaterials. ACS Biomater. Sci. Eng. 2016, 2, 2108–2121. 10.1021/acsbiomaterials.6b00454. [DOI] [PubMed] [Google Scholar]
- Krauss S.; Metzger T. H.; Fratzl P.; Harrington M. J. Self-Repair of a Biological Fiber Guided by an Ordered Elastic Framework. Biomacromolecules 2013, 14, 1520–1528. 10.1021/bm4001712. [DOI] [PubMed] [Google Scholar]
- Renner-Rao M.; Clark M.; Harrington M. J. Fiber Formation from Liquid Crystalline Collagen Vesicles Isolated from Mussels. Langmuir 2019, 35, 15992–16001. 10.1021/acs.langmuir.9b01932. [DOI] [PubMed] [Google Scholar]
- Waite J. H.; Qin X. X.; Coyne K. J. The Peculiar Collagens of Mussel Byssus. Matrix Biol. 1998, 17, 93–106. 10.1016/S0945-053X(98)90023-3. [DOI] [PubMed] [Google Scholar]
- Waite J. H. Adhesion À La Moule. Integr. Comp. Biol. 2002, 42, 1172–1180. 10.1093/icb/42.6.1172. [DOI] [PubMed] [Google Scholar]
- Bell E.; Gosline J. Mechanical Design of Mussel Byssus: Material Yield Enhances Attachment Strength. J. Exp. Biol. 1996, 199, 1005–1017. 10.1242/jeb.199.4.1005. [DOI] [PubMed] [Google Scholar]
- Smeathers J. E.; Vincent J. F. V. Mechanical Properties of Mussel Byssus Threads. J. Molluscan Stud. 1979, 45, 219–230. 10.1093/oxfordjournals.mollus.a065497. [DOI] [Google Scholar]
- Harrington M. J.; Gupta H. S.; Fratzl P.; Waite J. H. Collagen Insulated from Tensile Damage by Domains That Unfold Reversibly: In Situ X-Ray Investigation of Mechanical Yield and Damage Repair in the Mussel Byssus. J. Struct. Biol. 2009, 167, 47–54. 10.1016/j.jsb.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenau A.; Papadopoulos P.; Kremer F.; Scheibel T. Mussel Collagen Molecules with Silk-Like Domains as Load-Bearing Elements in Distal Byssal Threads. J. Struct. Biol. 2011, 175, 339–347. 10.1016/j.jsb.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Harrington M. J.; Waite J. H. Ph-Dependent Locking of Giant Mesogens in Fibers Drawn from Mussel Byssal Collagens. Biomacromolecules 2008, 9, 1480–1486. 10.1021/bm8000827. [DOI] [PubMed] [Google Scholar]
- Vaccaro E.; Waite J. H. Yield and Post-Yield Behavior of Mussel Byssal Thread: A Self-Healing Biomolecular Material. Biomacromolecules 2001, 2, 906–911. 10.1021/bm0100514. [DOI] [PubMed] [Google Scholar]
- Hagenau A.; Scheibel T. Towards the Recombinant Production of Mussel Byssal Collagens. J. Adhes. 2010, 86, 10–24. 10.1080/00218460903417701. [DOI] [Google Scholar]
- Rapoport H. S.; Shadwick R. E. Mechanical Characterization of an Unusual Elastic Biomaterial from the Egg Capsules of Marine Snails (Busycon Spp). Biomacromolecules 2002, 3, 42–50. 10.1021/bm0155470. [DOI] [PubMed] [Google Scholar]
- Rapoport H. S.; Shadwick R. E. Reversibly Labile, Sclerotization-Induced Elastic Properties in a Keratin Analog from Marine Snails: Whelk Egg Capsule Biopolymer (Wecb). J. Exp. Biol. 2007, 210, 12–26. 10.1242/jeb.02613. [DOI] [PubMed] [Google Scholar]
- Miserez A.; Wasko S.; Carpenter C.; Waite J. H. Non-Entropic and Reversible Long-Range Deformation of an Encapsulating Bioelastomer. Nat. Mater. 2009, 8, 910–916. 10.1038/nmat2547. [DOI] [PubMed] [Google Scholar]
- Harrington M. J.; Wasko S.; Masic A.; Fisher F. D.; Gupta H. S.; Fratzl P. Pseudoelastic Behaviour of a Natural Material Is Achieved Via Reversible Changes in Protein Backbone Conformation. J. R. Soc. Interface 2012, 9, 2911–2922. 10.1098/rsif.2012.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerette P. A. Z.; Tay G. Z.; Hoon S.; Loke J. J.; Hermawan A. F.; Schmitt C. N. Z.; Harrington M. J.; Masic A.; Karunaratne A.; Gupta H. S.; et al. Integrative and Comparative Analysis of Coiled-Coil Based Marine Snail Egg Cases - a Model for Biomimetic Elastomers. Biomater. Sci. 2014, 2, 710–722. 10.1039/c3bm60264h. [DOI] [PubMed] [Google Scholar]
- Gathercole L. J.Studies on the Protein of the Egg Capsule of Whelks; University of Leeds, Leeds, U.K., 1969. [Google Scholar]
- Miserez A.; Guerette P. A. Phase Transition-Induced Elasticity of Α-Helical Bioelastomeric Fibres and Networks. Chem. Soc. Rev. 2013, 42, 1973–1995. 10.1039/C2CS35294J. [DOI] [PubMed] [Google Scholar]
- Flory P. J. Theory of Elastic Mechanisms in Fibrous Proteins. J. Am. Chem. Soc. 1956, 78, 5222–5235. 10.1021/ja01601a025. [DOI] [Google Scholar]
- Wasko S.; Tay G.; Nowak C.; Schwaighofer A.; Waite J. H.; Miserez A. Structural Proteins from Whelk Egg Capsule with Long Range Elasticity Associated with a Solid-State Phase Transition. Biomacromolecules 2014, 15, 30–42. 10.1021/bm401598z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H.; Bär H.; Kreplak L.; Strelkov S. V.; Aebi U. Intermediate Filaments: From Cell Architecture to Nanomechanics. Nature Rev. Mol. Cell Biol. 2007, 8, 562–573. 10.1038/nrm2197. [DOI] [PubMed] [Google Scholar]
- Herrmann H.; Aebi U.. Intermediate Filaments: Structure and Assembly. Cold Spring Harb. Perspect. Biol. 2016, 8, a018242. 10.1101/cshperspect.a018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreplak L.; Doucet J.; Dumas P.; Briki F. New Aspects of the a-Helix to B-Sheet Transition in Stretched Hard a-Keratin Fibers. Biophys. J. 2004, 87, 640–647. 10.1529/biophysj.103.036749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. H.; Kim M. S.; Chung B. M.; Leahy D. J.; Coulombe P. A. Structural Basis for Heteromeric Assembly and Perinuclear Organization of Keratin Filaments. Nat. Struct. Mol. Biol. 2012, 19, 707–715. 10.1038/nsmb.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T.; Guerette P. A.; Tan R. Y. T.; Zhao H.; Schefer L.; Mezzenga R.; Miserez A. Biomimetic Self-Assembly of Recombinant Marine Snail Egg Capsule Proteins into Structural Coiled-Coil Units. J. Mater. Chem. B 2015, 3, 2671–2684. 10.1039/C4TB01434K. [DOI] [PubMed] [Google Scholar]
- Loke J. J.; Kumar A.; Hoon S.; Verma C.; Miserez A. Hierarchical Assembly of Tough Bioelastomeric Egg Capsules Is Mediated by a Bundling Protein. Biomacromolecules 2017, 18, 931–942. 10.1021/acs.biomac.6b01810. [DOI] [PubMed] [Google Scholar]
- Storm C.; Pastore J. J.; MacKintosh F. C.; Lubensky T. C.; Janmey P. A. Nonlinear Elasticity in Biological Gels. Nature 2005, 435, 191–194. 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- Muiznieks L. D.; Keeley F. W. Molecular Assembly and Mechanical Properties of the Extracellular Matrix: A Fibrous Protein Perspective. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 866–875. 10.1016/j.bbadis.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Feughelman M.Mechanical Properties and Structure of Alpha-Keratin Fibres: Wool, Human Hair, and Related Fibres; University of South Wales Press, 1997. [Google Scholar]
- Feughelman M. Natural Protein Fibers. J. Appl. Polym. Sci. 2002, 83, 489–507. 10.1002/app.2255. [DOI] [Google Scholar]
- Hearle J. W. S. A Critical Review of the Structural Mechanics of Wool and Hair Fibres. Int. J. Biol. Macromol. 2000, 27, 123–138. 10.1016/S0141-8130(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Kreplak L.; Doucet J.; Briki F. Unraveling Double Stranded Alpha-Helical Coiled Coils: An X-Ray Diffraction Study on Hard Alpha-Keratin Fibers. Biopolymers 2001, 58, 526–533. . [DOI] [PubMed] [Google Scholar]
- Kreplak L.; Franbourg A.; Briki F.; Leroy F.; Dallé D.; Doucet J. A New Deformation Model of Hard a-Keratin at the Nanometer Scale: Implications for Hard a-Keratin Intermediate Filament Mechanical Properties. Biophys. J. 2002, 82, 2265–2274. 10.1016/S0006-3495(02)75572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin R.; Colomban P. Nanomechanics of Single Keratin Fibres: A Raman Study of the a-Helix to B-Sheet Transition and the Effect of Water. J. Raman Spectrosc. 2007, 38, 504–514. 10.1002/jrs.1672. [DOI] [Google Scholar]
- Cera L.; Gonzalez G. M.; Liu Q.; Choi S.; Chantre C. O.; Lee J.; Gabardi R.; Choi M. C.; Shin K.; Parker K. K. A Bioinspired and Hierarchically Structured Shape-Memory Material. Nat. Mater. 2021, 20, 242–249. 10.1038/s41563-020-0789-2. [DOI] [PubMed] [Google Scholar]
- Kahler G. A.; Fisher F. M. Jr.; Sass R. L. The Chemical Composition and Mechanical Properties of the Hinge Ligament in Bivalve Molluscs. Biol. Bull. 1976, 151, 161–181. 10.2307/1540712. [DOI] [PubMed] [Google Scholar]
- Kelly R. E.; Rice R. V. Abductin: A Rubber-Like Protein from the Internal Triangular Hinge Ligament of Pecten. Science 1967, 155, 208–210. 10.1126/science.155.3759.208. [DOI] [PubMed] [Google Scholar]
- Bowie M. A.; Layes J. D.; Demont M. E. Damping in the Hinge of the Scallop Placopecten Magellanicus. J. Exp. Biol. 1993, 175, 311–315. 10.1242/jeb.175.1.311. [DOI] [Google Scholar]
- Denny M.; Miller L. Jet Propulsion in the Cold: Mechanics of Swimming in the Antarctic Scallop Adamussium Colbecki. J. Exp. Biol. 2006, 209, 4503–4514. 10.1242/jeb.02538. [DOI] [PubMed] [Google Scholar]
- Cao Q.; Wang Y.; Bayley H. Sequence of Abduction, the Molluscan Rubber Protein. Curr. Biol. 1997, 7, R677–R678. 10.1016/S0960-9822(06)00353-8. [DOI] [PubMed] [Google Scholar]
- Vanacore R.; Ham A.-J. L.; Voehler M.; Sanders C. R.; Conrads T. P.; Veenstra T. D.; Sharpless K. B.; Dawson P. E.; Hudson B. G. A Sulfilimine Bond Identified in Collagen Iv. Science 2009, 325, 1230–1234. 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochicchio B.; Jimenez-Oronoz F.; Pepe A.; Blanco M.; Sandberg L. B.; Tamburro A. M. Synthesis of and Structural Studies on Repeating Sequences of Abductin. Macromol. Biosci. 2005, 5, 502–511. 10.1002/mabi.200500007. [DOI] [PubMed] [Google Scholar]
- Su R. S.; Renner J. N.; Liu J. C. Synthesis and Characterization of Recombinant Abductin-Based Proteins. Biomacromolecules 2013, 14, 4301–4308. 10.1021/bm401162g. [DOI] [PubMed] [Google Scholar]
- Gosline J. M.Mechanical Design of Structural Materials in Animals; Princeton University Press, 2018. [Google Scholar]
- Priemel T.; Degtyar E.; Dean M. N.; Harrington M. J. Rapid Self-Assembly of Complex Biomolecular Architectures During Mussel Byssus Biofabrication. Nat. Commun. 2017, 8, 14539. 10.1038/ncomms14539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priemel T.; Palia R.; Babych M.; Thibodeaux C. J.; Bourgault S.; Harrington M. J. Compartmentalized Processing of Catechols During Mussel Byssus Fabrication Determines the Destiny of Dopa. Proc. Natl. Acad. Sci. U.S.A 2020, 117, 7613–7621. 10.1073/pnas.1919712117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepankumar K.; Guo Q.; Mohanram H.; Lim J.; Mu Y.; Pervushin K.; Yu J.; Miserez A. Liquid-Liquid Phase Separation of the Green Mussel Adhesive Protein Pvfp-5 Is Regulated by the Post-Translated Dopa Amino Acid. Adv. Mater. 2022, 34, 2103828. 10.1002/adma.202103828. [DOI] [PubMed] [Google Scholar]
- Petrone L.; Kumar A.; Sutanto C. N.; Patil N. J.; Kannan S.; Palaniappan A.; Amini S.; Zappone B.; Verma C.; Miserez A. Mussel Adhesion Is Dictated by Time-Regulated Secretion and Molecular Conformation of Mussel Adhesive Proteins. Nat. Commun. 2015, 6, 8737. 10.1038/ncomms9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.; Robertson N. B.; Jewhurst S. A.; Waite J. H. Probing the Adhesive Footprints of Mytilus Californianus Byssus. J. Biol. Chem. 2006, 281, 11090–11096. 10.1074/jbc.M510792200. [DOI] [PubMed] [Google Scholar]
- Miki D.; Takeuchi Y.; Inoue K.; Odo S. Expression Sites of Two Byssal Protein Genes of Mytilus Galloprovincialis. Biol. Bull. 1996, 190, 213–217. 10.2307/1542541. [DOI] [PubMed] [Google Scholar]
- Inoue K.; Takeuchi Y.; Miki D.; Odo S.; Harayama S.; Waite J. H. Cloning, Sequencing and Sites of Expression of Genes for the Hydroxyarginine-Containing Adhesive-Plaque Protein of the Mussel Mytilus Galloprovincialis. Eur. J. Biochem. 1996, 239, 172–176. 10.1111/j.1432-1033.1996.0172u.x. [DOI] [PubMed] [Google Scholar]
- Suhre M. H.; Gertz M.; Steegborn C.; Scheibel T. Structural and Functional Features of a Collagen-Binding Matrix Protein from the Mussel Byssus. Nat. Commun. 2014, 5, 3392. 10.1038/ncomms4392. [DOI] [PubMed] [Google Scholar]
- Sagert J.; Waite J. H. Hyperunstable Matrix Proteins in the Byssus of Mytilus Galloprovincialis. J. Exp. Biol. 2009, 212, 2224–2236. 10.1242/jeb.029686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamino K. Mini-Review: Barnacle Adhesives and Adhesion. Biofouling 2013, 29, 735–749. 10.1080/08927014.2013.800863. [DOI] [PubMed] [Google Scholar]
- Wang C. S.; Stewart R. J. Multipart Copolyelectrolyte Adhesive of the Sandcastle Worm, Phragmatopoma Californica (Fewkes): Catechol Oxidase Catalyzed Curing through Peptidyl-Dopa. Biomacromolecules 2013, 14, 1607–1617. 10.1021/bm400251k. [DOI] [PubMed] [Google Scholar]
- Deshmukh M.; Evans M. L.; Chapman M. R. Amyloid by Design: Intrinsic Regulation of Microbial Amyloid Assembly. J. Mol. Biol. 2018, 430, 3631–3641. 10.1016/j.jmb.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakanpää J.; Szilvay G. R.; Kaljunen H.; Maksimainen M.; Linder M.; Rouvinen J. Two Crystal Structures of Trichoderma Reesei Hydrophobin Hfbi - the Structure of a Protein Amphiphile with and without Detergent Interaction. Protein Sci. 2006, 15, 2129–2140. 10.1110/ps.062326706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettini P. P.; Frascella A.; Kolařík M.; Comparini C.; Pepori A. L.; Santini A.; Scala F.; Scala A. Widespread Horizontal Transfer of the Cerato-Ulmin Gene between Ophiostoma Novo-Ulmi and Geosmithia Species. Fungal Biol. 2014, 118, 663–674. 10.1016/j.funbio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Ashton N. N.; Roe D. R.; Weiss R. B.; Cheatham T. E.; Stewart R. J. Self-Tensioning Aquatic Caddisfly Silk: Ca2+ Dependent Structure, Strength, and Load Cycle Hysteresis. Biomacromolecules 2013, 14, 3668–3681. 10.1021/bm401036z. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Waite J. H. Proteins in Load-Bearing Junctions: The Histidine-Rich Metal-Binding Protein of Mussel Byssus. Biochemistry 2006, 45, 14223–14231. 10.1021/bi061677n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D. S.; Waite J. H. Three Intrinsically Unstructured Mussel Adhesive Proteins, Mfp-1, Mfp-2, and Mfp-3: Analysis by Circular Dichroism. Protein Sci. 2012, 21, 1689–1695. 10.1002/pro.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q.; Gourdon D.; Sun C.; Holten-Andersen N.; Anderson T. H.; Waite J. H.; Israelachvili J. N. Adhesion Mechanisms of the Mussel Foot Proteins Mfp-1 and Mfp-3. Proc. Natl. Acad. Sci. U.S.A 2007, 104, 3782–3786. 10.1073/pnas.0607852104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D. S.; Harrington M. J.; Lu Q.; Masic A.; Zeng H.; Waite J. H. Mussel Foot Protein-1 (Mcfp-1) Interaction with Titania Surfaces. J. Mater. Chem. 2012, 22, 15530–15533. 10.1039/c2jm32439c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D. S.; Zeng H.; Masic A.; Harrington M. J.; Israelachvili J. N.; Waite J. H. Protein- and Metal-Dependent Interactions of a Prominent Protein in Mussel Adhesive Plaques. J. Biol. Chem. 2010, 285, 25850–25858. 10.1074/jbc.M110.133157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W.; Yu J.; Broomell C.; Israelachvili J. N.; Waite J. H. Hydrophobic Enhancement of Dopa-Mediated Adhesion in a Mussel Foot Protein. J. Am. Chem. Soc. 2013, 135, 377–383. 10.1021/ja309590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W.; Tan Y.; Martinez Rodriguez N. R.; Yu J.; Israelachvili J. N.; Waite J. H. A Mussel-Derived One Component Adhesive Coacervate. Acta. Biomater. 2014, 10, 1663–1670. 10.1016/j.actbio.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner E. W.; Kan Y.; Hammer M. U.; Israelachvili J. N.; Waite J. H. Adhesion of Mussel Foot Protein Mefp-5 to Mica: An Underwater Superglue. Biochemistry 2012, 51, 6511–6518. 10.1021/bi3002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W.; Petrone L.; Cai H.; Israelachvili J. N.; Miserez A.; Waite J. H. An Underwater Surface Drying Peptide Inspired by a Mussel Adhesive Protein. Adv. Funct. Mater. 2016, 26, 3496–3507. 10.1002/adfm.201600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D. S.; Zeng H.; Srivastava A.; Krogstad D. V.; Tirrell M.; Israelachvili J. N.; Waite J. H. Viscosity and Interfacial Properties in a Mussel-Inspired Adhesive Coacervate. Soft Matter 2010, 6, 3232–3236. 10.1039/c002632h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. P.; Messersmith P. B.; Israelachvili J. N.; Waite J. H. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.; Wei W.; Danner E.; Israelachvili J. N.; Waite J. H. Effects of Interfacial Redox in Mussel Adhesive Protein Films on Mica. Adv. Mater. 2011, 23, 2362–2366. 10.1002/adma.201003580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.; Kan Y.; Rapp M.; Danner E.; Wei W.; Das S.; Miller D. R.; Chen Y.; Waite J. H.; Israelachvili J. N. Adaptive Hydrophobic and Hydrophilic Interactions of Mussel Foot Proteins with Organic Thin Films. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 15680–15685. 10.1073/pnas.1315015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.; Wei W.; Menyo M. S.; Masic A.; Waite J. H.; Israelachvili J. N. Adhesion of Mussel Foot Protein-3 to Tio2 Surfaces: The Effect of Ph. Biomacromolecules 2013, 14, 1072–1077. 10.1021/bm301908y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.; Scherer N. F.; Messersmith P. B. Single-Molecule Mechanics of Mussel Adhesion. Proc. Natl. Acad. Sci. U.S.A 2006, 103, 12999–13003. 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbie M. A.; Wei W.; Schrader A. M.; Cristiani T. R.; Dobbs H. A.; Idso M.; Chmelka B. F.; Waite J. H.; Israelachvili J. N. Tuning Underwater Adhesion with Cation-Π Interactions. Nat. Chem. 2017, 9, 473–479. 10.1038/nchem.2720. [DOI] [PubMed] [Google Scholar]
- Park S.; Kim S.; Jho Y.; Hwang D. S. Cation-Pi Interactions and Their Contribution to Mussel Underwater Adhesion Studied Using a Surface Forces Apparatus: A Mini-Review. Langmuir 2019, 35, 16002–16012. 10.1021/acs.langmuir.9b01976. [DOI] [PubMed] [Google Scholar]
- Li S.; Huang X.; Chen Y.; Li X.; Zhan A. Identification and Characterization of Proteins Involved in Stolon Adhesion in the Highly Invasive Fouling Ascidian Ciona Robusta. Biochem. Biophys. Res. Commun. 2019, 510, 91–96. 10.1016/j.bbrc.2019.01.053. [DOI] [PubMed] [Google Scholar]
- Zeng F.; Wunderer J.; Salvenmoser W.; Ederth T.; Rothbächer U. Identifying Adhesive Components in a Model Tunicate. Philos. Trans. R. Soc. B 2019, 374, 20190197. 10.1098/rstb.2019.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H.; Hwang D. S.; Israelachvili J. N.; Waite J. H. Strong Reversible Fe3+-Mediated Bridging between Dopa-Containing Protein Films in Water. Proc. Natl. Acad. Sci. U.S.A 2010, 107, 12850–12853. 10.1073/pnas.1007416107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle F.; Macias-Sanchez E.; Sviben S.; Fratzl P.; Bertinetti L.; Harrington M. J. Hierarchically-Structured Metalloprotein Composite Coatings Biofabricated from Co-Existing Condensed Liquid Phases. Nat. Commun. 2020, 11, 862. 10.1038/s41467-020-14709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesko M.; Xiang L.; Bohle S.; Hwang D. S.; Zeng H.; Harrington M. J. Catechol-Vanadium Binding Enhances Cross-Linking and Mechanics of a Mussel Byssus Coating Protein. Chem. Mater. 2021, 33, 6530–6540. 10.1021/acs.chemmater.1c02063. [DOI] [Google Scholar]
- Ahn B. K.; Lee D. W.; Israelachvili J. N.; Waite J. H. Surface-Initiated Self-Healing of Polymers in Aqueous Media. Nat. Mater. 2014, 13, 867–872. 10.1038/nmat4037. [DOI] [PubMed] [Google Scholar]
- Callow J. A.; Callow M. E. Biofilms. Prog. Mol. Subcell. Biol. 2006, 42, 141–169. 10.1007/3-540-30016-3_6. [DOI] [PubMed] [Google Scholar]
- Maier G. P.; Rapp M. V.; Waite J. H.; Israelachvili J. N.; Butler A. Adaptive Synergy between Catechol and Lysine Promotes Wet Adhesion by Surface Salt Displacement. Science 2015, 349, 628–632. 10.1126/science.aab0556. [DOI] [PubMed] [Google Scholar]
- Ou X.; Xue B.; Lao Y.; Wutthinitikornkit Y.; Tian R.; Zou A.; Yang L.; Wang W.; Cao Y.; Li J. Structure and Sequence Features of Mussel Adhesive Protein Lead to Its Salt-Tolerant Adhesion Ability. Sci. Adv. 2020, 6, eabb7620 10.1126/sciadv.abb7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.; Sun C.; Stewart R. J.; Waite J. H. Cement Proteins of the Tube-Building Polychaete Phragmatopoma Californica. J. Biol. Chem. 2005, 280, 42938–42944. 10.1074/jbc.M508457200. [DOI] [PubMed] [Google Scholar]
- Valois E.; Hoffman C.; Demartini D. G.; Waite J. H. The Thiol-Rich Interlayer in the Shell/Core Architecture of Mussel Byssal Threads. Langmuir 2019, 35, 15985–15991. 10.1021/acs.langmuir.9b01844. [DOI] [PubMed] [Google Scholar]
- Khandeparker L.; Anil A. C. Underwater Adhesion: The Barnacle Way. Int. J. Adhes. Adhes. 2007, 27, 165–172. 10.1016/j.ijadhadh.2006.03.004. [DOI] [Google Scholar]
- Liang C.; Strickland J.; Ye Z.; Wu W.; Hu B.; Rittschof D.. Biochemistry of Barnacle Adhesion: An Updated Review. Front. Mar. Sci. 2019, 6, 10.3389/fmars.2019.00565. [DOI] [Google Scholar]
- Aldred N.; Clare A. S. The Adhesive Strategies of Cyprids and Development of Barnacle-Resistant Marine Coatings. Biofouling 2008, 24, 351–363. 10.1080/08927010802256117. [DOI] [PubMed] [Google Scholar]
- Gohad N. V.; Aldred N.; Hartshorn C. M.; Jong Lee Y.; Cicerone M. T.; Orihuela B.; Clare A. S.; Rittschof D.; Mount A. S. Synergistic Roles for Lipids and Proteins in the Permanent Adhesive of Barnacle Larvae. Nat. Commun. 2014, 5, 441. 10.1038/ncomms5414. [DOI] [PubMed] [Google Scholar]
- Fears K. P.; Orihuela B.; Rittschof D.; Wahl K. J. Acorn Barnacles Secrete Phase-Separating Fluid to Clear Surfaces Ahead of Cement Deposition. Adv. Sci. 2018, 5, 1700762. 10.1002/advs.201700762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamino K.Diversified Material Designs in Biological Underwater Adhesives. In Structural Interfaces and Attachments in Biology; Thomopoulos S., Birman V., Genin G. M., Eds.; Springer: New York, 2013; pp 175–199. [Google Scholar]
- Urushida Y.; Nakano M.; Matsuda S.; Inoue N.; Kanai S.; Kitamura N.; Nishino T.; Kamino K. Identification and Functional Characterization of a Novel Barnacle Cement Protein. FEBS J. 2007, 274, 4336–4346. 10.1111/j.1742-4658.2007.05965.x. [DOI] [PubMed] [Google Scholar]
- Kamino K. Novel Barnacle Underwater Adhesive Protein Is a Charged Amino Acid-Rich Protein Constituted by a Cys-Rich Repetitive Sequence. Biochem. J. 2001, 356 (2), 503–507. 10.1042/bj3560503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamino K.; Nakano M.; Kanai S. Significance of the Conformation of Building Blocks in Curing of Barnacle Underwater Adhesive. FEBS J. 2012, 279, 1750–1760. 10.1111/j.1742-4658.2012.08552.x. [DOI] [PubMed] [Google Scholar]
- Kamino K.; Odo S.; Maruyama T. Cement Proteins of the Acorn Barnacle, Megabalanus Rosa. Biol. Bull. 1996, 190, 403–409. 10.2307/1543033. [DOI] [PubMed] [Google Scholar]
- Kamino K.; Inoue K.; Maruyama T.; Takamatsu N.; Harayama S.; Shizuri Y. Barnacle Cement Proteins. Importance of Disulfide Bonds in Their Insolubility. J. Biol. Chem. 2000, 275, 27360–27365. 10.1016/S0021-9258(19)61519-X. [DOI] [PubMed] [Google Scholar]
- Mori Y.; Urushida Y.; Nakano M.; Uchiyama S.; Kamino K. Calcite-Specific Coupling Protein in Barnacle Underwater Cement. FEBS J. 2007, 274, 6436–6644. 10.1111/j.1742-4658.2007.06161.x. [DOI] [PubMed] [Google Scholar]
- So C. R.; Liu J.; Fears K. P.; Leary D. H.; Golden J. P.; Wahl K. J. Self-Assembly of Protein Nanofibrils Orchestrates Calcite Step Movement through Selective Nonchiral Interactions. ACS Nano 2015, 9, 5782–5791. 10.1021/acsnano.5b01870. [DOI] [PubMed] [Google Scholar]
- Mohanram H.; Georges T.; Pervushin K.; Azaïs T.; Miserez A. Self-Assembly of a Barnacle Cement Protein (Mrcp20) into Adhesive Nanofibrils with Concomitant Regulation of CaCO3 Polymorphism. Chem. Mater. 2021, 33, 9715–9724. 10.1021/acs.chemmater.1c03477. [DOI] [Google Scholar]
- Kumar A.; Mohanram H.; Li J.; Le Ferrand H.; Verma C. S.; Miserez A. Disorder-Order Interplay of a Barnacle Cement Protein Triggered by Interactions with Calcium and Carbonate Ions: A Molecular Dynamics Study. Chem. Mater. 2020, 32, 8845–8859. 10.1021/acs.chemmater.0c02319. [DOI] [Google Scholar]
- Barlow D. E.; Dickinson G. H.; Orihuela B.; Rittschof D.; Wahl K. J. In Situ ATR-FTIR Characterization of Primary Cement Interfaces of the Barnacle Balanus Amphitrite. Biofouling 2009, 25, 359–366. 10.1080/08927010902812009. [DOI] [PubMed] [Google Scholar]
- Barlow D. E.; Dickinson G. H.; Orihuela B.; Kulp J. L. III; Rittschof D.; Wahl K. J. Characterization of the Adhesive Plaque of the Barnacle Balanus Amphitrite: Amyloid-Like Nanofibrils Are a Major Component. Langmuir 2010, 26, 6549–6556. 10.1021/la9041309. [DOI] [PubMed] [Google Scholar]
- Sullan R. M. A.; Gunari N.; Tanur A. E.; Chan Y.; Dickinson G. H.; Orihuela B.; Rittschof D.; Walker G. C. Nanoscale Structures and Mechanics of Barnacle Cement. Biofouling 2009, 25, 263–275. 10.1080/08927010802688095. [DOI] [PubMed] [Google Scholar]
- Barlow D. E.; Wahl K. J. Optical Spectroscopy of Marine Bioadhesive Interfaces. Annual Review of Analytical Chemistry 2012, 5, 229–251. 10.1146/annurev-anchem-061010-113844. [DOI] [PubMed] [Google Scholar]
- Golden J. P.; Burden D. K.; Fears K. P.; Barlow D. E.; So C. R.; Burns J.; Miltenberg B.; Orihuela B.; Rittshof D.; Spillmann C. M.; et al. Imaging Active Surface Processes in Barnacle Adhesive Interfaces. Langmuir 2016, 32, 541–550. 10.1021/acs.langmuir.5b03286. [DOI] [PubMed] [Google Scholar]
- Dickinson G.; Vega I. E.; Wahl K. J.; Orihuela B.; Beyley V.; Rodriguez E. N.; Everett R. K.; Bonaventura J.; Rittschof D. Barnacle Cement: A Polymerization Model Based on Evolutionary Concepts. J. Exp. Biol. 2009, 212, 3499–3510. 10.1242/jeb.029884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart M. M.; Chapman M. R. Curli Biogenesis and Function. Annu. Rev. Microbiol. 2006, 60, 131–147. 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostaert A. S.; Giordani C.; Crockett R.; Karsten U.; Schumann R.; Jarvis S. P. Characterisation of Amyloid Nanostructures in the Natural Adhesive of Unicellular Subaerial Algae. J. Adhes. 2009, 85, 465–448. 10.1080/00218460902996366. [DOI] [Google Scholar]
- Nakano M.; Kamino K. Amyloid-Like Conformation and Interaction for the Self-Assembly in Barnacle Underwater Cement. Biochemistry 2015, 54, 826–835. 10.1021/bi500965f. [DOI] [PubMed] [Google Scholar]
- So C. R.; Scancella J. M.; Fears K. P.; Essock-Burns T.; Haynes S. E.; Leary D. H.; Diana Z.; Wang C.; North S.; Oh C. S.; et al. Oxidase Activity of the Barnacle Adhesive Interface Involves Peroxide-Dependent Catechol Oxidase and Lysyl Oxidase Enzymes. ACS Appl. Mater. Interfaces 2017, 9, 11493–11505. 10.1021/acsami.7b01185. [DOI] [PubMed] [Google Scholar]
- Mohanram H.; Kumar A.; Verma C.; Pervushin K.; Miserez A. Three-Dimensional Structure of Megabalanus Rosa Cement Protein 20 Revealed by Multi-Dimensional NMR and MD Simulations. Philos. Trans. R. Soc. B 2019, 374, 20190198. 10.1098/rstb.2019.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santonocito R.; Venturella F.; Dal Piaz F.; Morando M. A.; Provenzano A.; Rao E.; Costa M. A.; Bulone D.; San Biagio P. L.; Giacomazza D.; et al. Recombinant Mussel Protein Pvfp-5β: A Potential Tissue Bioadhesive. J. Biol. Chem. 2019, 294, 12826–12835. 10.1074/jbc.RA119.009531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M. J.; Steren R. E.; Hlady V.; Stewart R. J. Multiscale Structure of the Underwater Adhesive of Phragmatopoma Californica: A Nanostructured Latex with a Steep Microporosity Gradient. Langmuir 2007, 23, 5045–5049. 10.1021/la063765e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite J. H.; Jensen R. A.; Morse D. E. Cement Precursor Proteins of the Reef-Building Polychaete Phragmatopoma Californica (Fewkes). Biochemistry 1992, 31, 5733–5738. 10.1021/bi00140a007. [DOI] [PubMed] [Google Scholar]
- Stewart R. J.; Wang C. S.; Song I. T.; Jones J. P. The Role of Coacervation and Phase Transitions in the Sandcastle Worm Adhesive System. Adv. Colloid Interface Sci. 2017, 239, 88–96. 10.1016/j.cis.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. S.; Stewart R. J. Localization of the Bioadhesive Precursors of the Sandcastle Worm, Phragmatopoma Californica (Fewkes). J. Exp. Biol. 2012, 215, 351–361. 10.1242/jeb.065011. [DOI] [PubMed] [Google Scholar]
- Stewart R. J.; Weaver J. C.; Morse D. E.; Waite J. H. The Tube Cement of Phragmatopoma Californica: A Solid Foam. J. Exp. Biol. 2004, 207, 4727–4734. 10.1242/jeb.01330. [DOI] [PubMed] [Google Scholar]
- Jones J. P.; Sima M.; O’Hara R. G.; Stewart R. J. Water-Borne Endovascular Embolics Inspired by the Undersea Adhesive of Marine Sandcastle Worms. Adv. Healthc. Mater. 2016, 5, 795–801. 10.1002/adhm.201500825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S.; Weerasekare G. M.; Stewart R. J. Multiphase Adhesive Coacervates Inspired by the Sandcastle Worm. ACS Appl. Mater. Interfaces 2011, 3, 941–944. 10.1021/am200082v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H.; Stewart R. J. Biomimetic Underwater Adhesives with Environmentally Triggered Setting Mechanisms. Adv. Mater. 2010, 22, 729–733. 10.1002/adma.200902380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.; Xu C.; Sebastin M.; Lee A.; Holwell N.; Xu C.; Miranda Nieves D.; Mu L.; Langer R. S.; Lin C.; et al. Bioinspired Nanoparticulate Medical Glues for Minimally Invasive Tissue Repair. Adv. Healthc. Mater. 2015, 4, 2587–2596. 10.1002/adhm.201500419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialegna A.; Lasa I.; Valle J. Amyloid Structures as Biofilm Matrix Scaffolds. J. Bacteriol. 2016, 198, 2579–2588. 10.1128/JB.00122-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovich S. A.; Gazit E.; Laor Bar-Yosef D. Two Decades of Studying Functional Amyloids in Microorganisms. Trends Microbiol. 2021, 29, 251–265. 10.1016/j.tim.2020.09.005. [DOI] [PubMed] [Google Scholar]
- Iadanza M. G.; Jackson M. P.; Hewitt E. W.; Ranson N. A.; Radford S. E. A New Era for Understanding Amyloid Structures and Disease. Nature Rev. Mol. Cell Biol. 2018, 19, 755–773. 10.1038/s41580-018-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada A.; Levin A.; Toprakcioglu Z.; Shen Y.; Lutz-Bueno V.; Baumann K. N.; Mohammadi P.; Linder M. B.; Mezzenga R.; Knowles T. P. J.. Modulating the Mechanical Performance of Macroscale Fibers through Shear-Induced Alignment and Assembly of Protein Nanofibrils. Small 2020, 16 ( (9), ), e1904190. 10.1002/smll.201904190 [DOI] [PubMed] [Google Scholar]
- Fowler D. M.; Koulov A. V.; Balch W. E.; Kelly J. W. Functional Amyloid-from Bacteria to Humans. Trends Biochem. Sci. 2007, 32, 217–224. 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Flemming H. C.; Wingender J.; Szewzyk U.; Steinberg P.; Rice S. A.; Kjelleberg S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- Dragoš A.; Kovács Á. T. The Peculiar Functions of the Bacterial Extracellular Matrix. Trends Microbiol. 2017, 25, 257–266. 10.1016/j.tim.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Koo H.; Allan R. N.; Howlin R. P.; Stoodley P.; Hall-Stoodley L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karygianni L.; Ren Z.; Koo H.; Thurnheer T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. 10.1016/j.tim.2020.03.016. [DOI] [PubMed] [Google Scholar]
- Koo H.; Yamada K. M. Dynamic Cell-Matrix Interactions Modulate Microbial Biofilm and Tissue 3d Microenvironments. Curr. Opin. Cell Biol. 2016, 42, 102–112. 10.1016/j.ceb.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles T. P. J.; Buehler M. J. Nanomechanics of Functional and Pathological Amyloid Materials. Nat. Nanotechnol. 2011, 6, 469–479. 10.1038/nnano.2011.102. [DOI] [PubMed] [Google Scholar]
- Gremer L.; Schenk C.; Reinartz E.; Ravelli R. B. G.; Tusche M.; Lopez-iglesias C.; Hoyer W.; Heise H.; Willbold D. Fibril Structure of Amyloid- β(1–42 by Cryo-Electron Microscopy. Science 2017, 358 (6359), 116–119. 10.1126/science.aao2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A.; Schmidt M.; Rennegarbe M.; Haupt C.; Liberta F.; Stecher S.; Puscalau-Girtu I.; Biedermann A.; Fändrich M.. AA Amyloid Fibrils from Diseased Tissue Are Structurally Differeqnt from in Vitro Formed Saa Fibrils. Nat. Commun. 2021, 12. 10.1038/s41467-021-21129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radamaker L.; Lin Y. H.; Annamalai K.; Huhn S.; Hegenbart U.; Schönland S. O.; Fritz G.; Schmidt M.; Fändrich M. Cryo-Em Structure of a Light Chain-Derived Amyloid Fibril from a Patient with Systemic Al Amyloidosis. Nat. Comms. 2019, 10, 1103. 10.1038/s41467-019-09032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmer M.; Close W.; Funk L.; Rasmussen J.; Bsoul A.; Schierhorn A.; Schmidt M.; Sigurdson C. J.; Jucker M.; Fändrich M. Cryo-EM Structure and Polymorphism of Aβ Amyloid Fibrils Purified from Alzheimer’s Brain Tissue. Nat. Comms. 2019, 10, 4760. 10.1038/s41467-019-12683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer N. D.; Schmidt J. C.; Chapman M. R. The Curli Nucleator Protein, Csgb, Contains an Amyloidogenic Domain That Directs Csga Polymerization. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 12494–12499. 10.1073/pnas.0703310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Q.; Crick S. L.; Pinkner J. S.; Ford B.; Hultgren S. J.; Frieden C. The E. Coli Csgb Nucleator of Curli Assembles to Β-Sheet Oligomers That Alter the Csga Fibrillization Mechanism. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 6502–6507. 10.1073/pnas.1204161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBenedictis E. P.; Liu J.; Keten S. Adhesion Mechanisms of Curli Subunit Csga to Abiotic Surfaces. Sci. Adv. 2016, 2, 1–11. 10.1126/sciadv.1600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. D.; Zhou Y.; Salgado P. S.; Patwardhan A.; McGuffie M.; Pape T.; Grabe G.; Ashman E.; Constable S. C.; Simpson P. J.; et al. Atomic Resolution Insights into Curli Fiber Biogenesis. Structure 2011, 19, 1307–1316. 10.1016/j.str.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loferer H.; Hammar M.; Normark S. Availability of the Fibre Subunit Csga and the Nucleator Protein Csgb During Assembly of Fibronectin-Binding Curli Is Limited by the Intracellular Concentration of the Novel Lipoprotein Csgg. Mol. Microbiol. 1997, 26, 11–23. 10.1046/j.1365-2958.1997.5231883.x. [DOI] [PubMed] [Google Scholar]
- Nenninger A. A.; Robinson L. S.; Hultgren S. J. Localized and Efficient Curli Nucleation Requires the Chaperone-Like Amyloid Assembly Protein Csgf. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 900–905. 10.1073/pnas.0812143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillmore J. D.; Hawkins P. N. Pathophysiology and Treatment of Systemic Amyloidosis. Nat. Rev. Nephrol. 2013, 9, 574–586. 10.1038/nrneph.2013.171. [DOI] [PubMed] [Google Scholar]
- Greenwald J.; Riek R. Biology of Amyloid: Structure, Function, and Regulation. Structure 2010, 18, 1244–1260. 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Chapman M. R.; Robinson L. S.; Pinkner J. S.; Roth R.; Heuser J.; Hammar M.; Normark S.; Hultgren S. J. Role of Escherichia Coli Curli Operons in Directing Amyloid Fiber Formation. Science 2002, 295, 851–855. 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gerven N.; Klein R. D.; Hultgren S. J.; Remaut H. Bacterial Amyloid Formation: Structural Insights into Curli Biogensis. Trends Microbiol. 2015, 23, 693–706. 10.1016/j.tim.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakikhany K.; Harrington C. R.; Nimtz M.; Hinton J. C. D.; Römling U. Unphosphorylated Csgd Controls Biofilm Formation in Salmonella Enterica Serovar Typhimurium. Mol. Microbiol. 2010, 77, 771–786. 10.1111/j.1365-2958.2010.07247.x. [DOI] [PubMed] [Google Scholar]
- Simm R.; Morr M.; Kader A.; Nimtz M.; Römling U. Ggdef and Eal Domains Inversely Regulate Cyclic Di-Gmp Levels and Transition from Sessibility to Motility. Mol. Microbiol. 2004, 53, 1123–1134. 10.1111/j.1365-2958.2004.04206.x. [DOI] [PubMed] [Google Scholar]
- Römling U.; Rohde M.; Olsén A.; Normark S.; Reinköster J. Agfd the Checkpoint of Multicellular and Aggregative Behaviour in Salmonella Typhimurium Regulates at Least Two Independent Pathways. Mol. Microbiol. 2000, 36, 10–23. 10.1046/j.1365-2958.2000.01822.x. [DOI] [PubMed] [Google Scholar]
- Wösten H. A. B.; De Vries O. M. H.; Wessels J. G. H. Interfacial Self-Assembly of a Fungal Hydrophobin into a Hydrophobic Rodlet Layer. Plant Cell 1993, 5, 1567–1574. 10.2307/3869739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B. W.; Sallada N. D. Hydrophobins: Multifunctional Biosurfactants for Interface Engineering. J. Biol. Eng. 2019, 13, 1–8. 10.1186/s13036-018-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X.; Kirui A.; Muszyński A.; Widanage M. C. D.; Chen A.; Azadi P.; Wang P.; Mentink-Vigier F.; Wang T. Molecular Architecture of Fungal Cell Walls Revealed by Solid-State NMR. Nat. Comms. 2018, 9, 1–12. 10.1038/s41467-018-05199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink M. F. B. G.; Claessen D.; Bouma B.; Dijkhuizen L.; Wösten H. A. B. Amyloids - a Functional Coat for Microorganisms. Nat. Rev. Microbiol. 2005, 3, 333–341. 10.1038/nrmicro1127. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Lienemann M.; Qiau M.; Linder M. B. Mechanisms of Protein Adhesion on Surface Films of Hydrophobin. Langmuir 2010, 26, 8491–8496. 10.1021/la101240e. [DOI] [PubMed] [Google Scholar]
- Whiteford J. R.; Spanu P. D. The Hydrophobin Hcf-1 of Cladosporium Fulvum Is Required for Efficient Water-Mediated Dispersal of Conidia. Fungal Genet. Biol. 2001, 32, 159–168. 10.1006/fgbi.2001.1263. [DOI] [PubMed] [Google Scholar]
- Wösten H. A. B.; van Wetter M.-A.; Lugones L. G.; van der Mei H. C.; Busscher H. J.; Wessels J. G. H. How a Fungus Escapes the Water to Grow into the Air. Curr. Biol. 1999, 9 (2), 85–88. 10.1016/S0960-9822(99)80019-0. [DOI] [PubMed] [Google Scholar]
- Wösten H. A. B. Hydrophobins: Multipurpose Proteins. Annu. Rev. Microbiol. 2001, 55, 625–646. 10.1146/annurev.micro.55.1.625. [DOI] [PubMed] [Google Scholar]
- Aimanianda V.; Bayry J.; Bozza S.; Kniemeyer O.; Perruccio K.; Elluru S. R.; Clavaud C.; Paris S.; Brakhage A. A.; Kaveri S. V.; et al. Surface Hydrophobin Prevents Immune Recognition of Airborne Fungal Spores. Nature 2009, 460, 1117–1121. 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- Wessels J. G. H. Hydrophobins: Proteins That Change the Nature of the Fungal Surface. Adv. Microb. Physiol. 1996, 38, 1–45. 10.1016/S0065-2911(08)60154-X. [DOI] [PubMed] [Google Scholar]
- Jensen B. G.; Andersen M. R.; Pedersen M. H.; Frisvad J. C.; Søndergaard I. Hydrophobins from Aspergillus Species Cannot Be Clearly Divided into Two Classes. BMC Res. Notes 2010, 3, 1–6. 10.1186/1756-0500-3-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandier J. A.; Langelaan D. N.; Won A.; O’Donnell K.; Grondin J. L.; Spencer H. L.; Wong P.; Tillier E.; Yip C.; Smith S. P.; et al. Characterization of a Basidiomycota Hydrophobin Reveals the Structural Basis for a High-Similarity Class I Subdivision. Sci. Rep. 2017, 7, 1–9. 10.1038/srep45863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl-Seiboth V.; Gruber S.; Sezerman U.; Schwecke T.; Albayrak A.; Neuhof T.; Von Döhren H.; Baker S. E.; Kubicek C. P. Novel Hydrophobins from Trichoderma Define a New Hydrophobin Subclass: Protein Properties, Evolution, Regulation and Processing. J. Mol. Evol. 2011, 72, 339–351. 10.1007/s00239-011-9438-3. [DOI] [PubMed] [Google Scholar]
- Littlejohn K. A.; Hooley P.; Cox P. W. Bioinformatics Predicts Diverse Aspergillus Hydrophobins with Novel Properties. Food Hydrocoll. 2012, 27, 503–516. 10.1016/j.foodhyd.2011.08.018. [DOI] [Google Scholar]
- Gandier J. A.; Master E. R. Pichia Pastoris Is a Suitable Host for the Heterologous Expression of Predicted Class I and Class II Hydrophobins for Discovery, Study, and Application in Biotechnology. Microorganisms 2018, 6, 3. 10.3390/microorganisms6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vocht M. L.; Reviakine I.; Wosten H. A. B.; Brisson A.; Wessels J. G. H.; Robillard G. T. Structural and Functional Role of the Disulfide Bridges in the Hydrophobin Sc3. J. Biol. Chem. 2000, 275, 28428–28432. 10.1074/jbc.M000691200. [DOI] [PubMed] [Google Scholar]
- Sallada N. D.; Dunn K. J.; Berger B. W. A Structural and Functional Role for Disulfide Bonds in a Class II Hydrophobin. Biochemistry 2018, 57, 645–653. 10.1021/acs.biochem.7b01166. [DOI] [PubMed] [Google Scholar]
- Hakanpää J.; Paananen A.; Askolin S.; Nakari-Setälä T.; Parkkinen T.; Penttilä M.; Linder M. B.; Rouvinen J. Atomic Resolution Structure of the HfbIIHydrophobin, a Self-Assembling Amphiphile. J. Biol. Chem. 2004, 279, 534–539. 10.1074/jbc.M309650200. [DOI] [PubMed] [Google Scholar]
- Hakanpää J.; Linder M.; Popov A.; Schmidt A.; Rouvinen J. Hydrophobin HfbIIin Detail: Ultrahigh-Resolution Structure at 0.75 Å. Acta Crystallogr. D 2006, 62, 356–367. 10.1107/S0907444906000862. [DOI] [PubMed] [Google Scholar]
- Kershaw M. J.; Thornton C. R.; Wakley G. E.; Talbot N. J. Four Conserved Intramolecular Disulphide Linkages Are Required for Secretion and Cell Wall Localization of a Hydrophobin During Fungal Morphogenesis. Mol. Microbiol. 2005, 56, 117–125. 10.1111/j.1365-2958.2005.04547.x. [DOI] [PubMed] [Google Scholar]
- Kwan A. H. Y.; Winefield R. D.; Sunde M.; Matthews J. M.; Haverkamp R. G.; Templeton M. D.; Mackay J. P. Structural Basis for Rodlet Assembly in Fungal Hydrophobins. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 3621–3626. 10.1073/pnas.0505704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q.; Kwan A. H.; Sunde M. Solution Structure and Interface-Driven Self-Assembly of Nc2, a New Member of the Class II Hydrophobin Proteins. Proteins 2014, 82, 990–1003. 10.1002/prot.24473. [DOI] [PubMed] [Google Scholar]
- Stewart R. J.; Wang C. S. Adaptation of Caddisfly Larval Silks to Aquatic Habitats by Phosphorylation of H-Fibroin Serines. Biomacromolecules 2010, 11, 969–974. 10.1021/bm901426d. [DOI] [PubMed] [Google Scholar]
- Yonemura N.; Sehnal F.; Mita K.; Tamura T. Protein Composition of Silk Filaments Spun under Water by Caddisfly Larvae. Biomacromolecules 2006, 7, 3370–3378. 10.1021/bm060663u. [DOI] [PubMed] [Google Scholar]
- Yonemura N.; Mita K.; Tamura T.; Sehnal F. Conservation of Silk Genes in Trichoptera and Lepidoptera. J. Mol. Evol. 2009, 68, 641–653. 10.1007/s00239-009-9234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Sanai K.; Wen H.; Zhao T.; Nakagaki M. Characterization of Unique Heavy Chain Fibroin Filaments Spun Underwater by the Caddisfly Stenopsyche Marmorata (Trichoptera; Stenopsychidae). Mol. Biol. Rep. 2010, 37, 2885–2892. 10.1007/s11033-009-9847-1. [DOI] [PubMed] [Google Scholar]
- Addison J. B.; Ashton N. N.; Weber W. S.; Stewart R. J.; Holland G. P.; Yarger J. L. Β-Sheet Nanocrystalline Domains Formed from Phosphorylated Serine-Rich Motifs in Caddisfly Larval Silk: A Solid State NMR and XRD Study. Biomacromolecules 2013, 14, 1140–1148. 10.1021/bm400019d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addison J. B.; Weber W. S.; Mou Q.; Ashton N. N.; Stewart R. J.; Holland G. P.; Yarger J. L. Reversible Assembly of Β-Sheet Nanocrystals within Caddisfly Silk. Biomacromolecules 2014, 15, 1269–1275. 10.1021/bm401822p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N. N.; Stewart R. J. Self-Recovering Caddisfly Silk: Energy Dissipating, Ca(2+)-Dependent, Double Dynamic Network Fibers. Soft Matter 2015, 11, 1667–1676. 10.1039/C4SM02435D. [DOI] [PubMed] [Google Scholar]
- Deepankumar K.; Lim C.; Polte I.; Zappone B.; Labate C.; De Santo M. P.; Mohanram H.; Palaniappan A.; Hwang D. S.; Miserez A. Supramolecular Β-Sheet Suckerin-Based Underwater Adhesives. Adv. Funct. Mater. 2020, 30, 1907534. 10.1002/adfm.201907534. [DOI] [Google Scholar]
- Stewart R. J.; Wang C. S.; Shao H. Complex Coacervates as a Foundation for Synthetic Underwater Adhesives. Adv. Colloid Interface Sci. 2011, 167, 85–93. 10.1016/j.cis.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers M.-A.; Chen P. Y.. Biological Materials Science: Biological Materials, Bioinspired Materials, and Biomaterials; Cambridge University Press, 2014. [Google Scholar]
- Wegst U. G. K.; Bai H.; Saiz E.; Tomsia A. P.; Ritchie R. O. Bioinspired Structural Materials. Nat. Mater. 2015, 14, 23–36. 10.1038/nmat4089. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Meyers M. A.; Zhang Z.; Ritchie R. O. Functional Gradients and Heterogeneities in Biological Materials: Design Principles, Functions, and Bioinspired Applications. Prog. Mater. Sci. 2017, 88, 467–498. 10.1016/j.pmatsci.2017.04.013. [DOI] [Google Scholar]
- Liu Z.; Zhang Z.; Ritchie R. O. On the Materials Science of Nature’s Arms Race. Adv. Mater. 2018, 30, 1705220. 10.1002/adma.201705220. [DOI] [PubMed] [Google Scholar]
- Amini S.; Miserez A. Wear and Abrasion Resistance Selection Maps of Biological Materials. Acta. Biomater. 2013, 9, 7895–7907. 10.1016/j.actbio.2013.04.042. [DOI] [PubMed] [Google Scholar]
- Naleway S. E.; Porter M. M.; McKittrick J.; Meyers M. A. Structural Design Elements in Biological Materials: Application to Bioinspiration. Adv. Mater. 2015, 27, 5455–5476. 10.1002/adma.201502403. [DOI] [PubMed] [Google Scholar]
- Harrington M. J.; Fratzl P. Natural Load-Bearing Protein Materials. Prog. Mater. Sci. 2021, 120, 100767. 10.1016/j.pmatsci.2020.100767. [DOI] [Google Scholar]
- Lichtenegger H. C.; Schöberl T.; Bartl M. H.; Waite H.; Stucky G. D. High Abrasion Resistance with Sparse Mineralization: Copper Biomineral in Worm Jaws. Science 2002, 298, 389–392. 10.1126/science.1075433. [DOI] [PubMed] [Google Scholar]
- Lichtenegger H. C.; Schöberl T.; Ruokolainen J. T.; Cross J. O.; Heald S. M.; Birkedal H.; Waite J. H.; Stucky G. D. Zinc and Mechanical Prowess in the Jaws of Nereis, a Marine Worm. Proc. Natl. Acad. Sci. U.S.A. 2003, 100 (16), 9144–9149. 10.1073/pnas.1632658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broomell C. C.; Khan R. K.; Moses D. N.; Miserez A.; Pontin M. G.; Stucky G. D.; Zok F. W.; Waite J. H. Mineral Minimization in Nature’s Alternative Teeth. Proc. R. Soc. Interface 2007, 4, 19–31. 10.1098/rsif.2006.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderly W. R.; Nguyen T. T. D.; Malollari K. G.; DeMartini D.; Delparastan P.; Valois E.; Messersmith P. B.; Helgeson M. E.; Waite J. H. A Multi-Tasking Polypeptide from Bloodworm Jaws: Catalyst, Template, and Copolymer in Film Formation. Matter 2022, 5, 1890–1908. 10.1016/j.matt.2022.04.001. [DOI] [Google Scholar]
- Broomell C. C.; Chase S. F.; Laue T.; Waite J. H. Cutting Edge Structural Protein from the Jaws of Nereis Virens. Biomacromolecules 2008, 9, 1669–1677. 10.1021/bm800200a. [DOI] [PubMed] [Google Scholar]
- Heymann D.; Mohanram H.; Kumar A.; Verma C. S.; Lescar J.; Miserez A. Structure of a Consensus Chitin-Binding Domain Revealed by Solution NMR. J. Struct. Biol. 2021, 213, 107725. 10.1016/j.jsb.2021.107725. [DOI] [PubMed] [Google Scholar]
- Latza V.; Guerette P. A.; Ding D.; Amini S.; Kumar A.; Schmidt I.; Keating S.; Oxman N.; Weaver J. C.; Fratzl F.; et al. Multi-Scale Thermal Stability of a Hard Thermoplastic Protein-Based Material. Nat. Comms. 2015, 6, 8313. 10.1038/ncomms9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontin M. G.; Moses D. N.; Waite J. H.; Zok F. W. A Nonmineralized Approach to Abrasion-Resistant Biomaterials. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 13559–13564. 10.1073/pnas.0702034104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses D. N.; Mattoni M.; Slack N. L.; Waite H.; Zok F. Role of Melanin in Mechanical Properties of Glycera Jaws. Acta. Biomater. 2006, 2, 521–530. 10.1016/j.actbio.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Moses D. N.; Harreld J. D.; Stucky G. D.; Waite J. H. Melanin and Glycera Jaws. Emerging Dark Side of a Robust Biocomposite Structure. J. Biol. Chem. 2006, 281, 34826–34832. 10.1074/jbc.M603429200. [DOI] [PubMed] [Google Scholar]
- Khan R. K.; Stoimenov P. K.; Mates T. E.; Waite J. H.; Stucky G. D. Exploring Gradients of Halogens and Zinc in the Surface and Subsurface of Nereis Jaw. Langmuir 2006, 22, 8465–8471. 10.1021/la061027k. [DOI] [PubMed] [Google Scholar]
- Birkedal H.; Khan R. K.; Slack N.; Broomell C.; Lichtenegger H. C.; Zok F.; Stucky G. D.; Waite J. H. Halogenated Veneers: Protein Cross-Linking and Halogenation in the Jaws of Nereis, a Marine Polychaete Worm. ChemBioChem. 2006, 7, 1392–1399. 10.1002/cbic.200600160. [DOI] [PubMed] [Google Scholar]
- Gibbs P. E.; Bryan G. W. Copper-the Major Metal Component of Glycerid Polychaete Jaws. J. Mar. Biolog. Assoc. U.K. 1980, 60, 205–2014. 10.1017/S0025315400024267. [DOI] [Google Scholar]
- Gupta M. K.; Becknell K. A.; Crosby M. G.; Bedford N. M.; Wright J.; Dennis P. B.; Naik R. R. Programmable Mechanical Properties from a Worm Jaw-Derived Biopolymer through Hierarchical Ion Exposure. ACS Appl. Mater. Interfaces 2018, 10, 31928–31937. 10.1021/acsami.8b10107. [DOI] [PubMed] [Google Scholar]
- Chou C.-C.; Martin-Martinez F. J.; Qin Z.; Dennis P. B.; Gupta M. K.; Naik R. R.; Buehler M. J. Ion Effect and Metal-Coordinated Cross-Linking for Multiscale Design of Nereis Jaw Inspired Mechanomutable Materials. ACS Nano 2017, 11, 1858–1868. 10.1021/acsnano.6b07878. [DOI] [PubMed] [Google Scholar]
- Broomell C. C.; Waite J. H.; Zok F. W. Role of Transition Metals in Sclerotization of Biological Tissue. Acta. Biomater. 2008, 4, 2045–2051. 10.1016/j.actbio.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Broomell C.; Khan R.; Mattoni M.; Waite J. H.; Stucky G. D.; Zok F. Critical Role of Zinc in Hardening of Nereis Jaws. J. Exp. Biol. 2006, 209 (16), 3219–3225. 10.1242/jeb.02373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor M. G. M.Sclerotisation. In Comparative Biochemistry; Florkin M., Mason H. S., Eds.; Academic Press, 1962; Vol. 4, pp 371–396. [Google Scholar]
- Pryor M. G. M. On the Hardening of the Cuticle of Insects. Proc. R. Soc. B. Biol. Sci. 1940, 128 (852), 393–407. 10.1098/rspb.1940.0018. [DOI] [Google Scholar]
- Hunt S.Polysaccharide-Protein Complexes in Invertebrates; Academic Press, 1970. [Google Scholar]
- Neville A. C.Biology of Fibrous Composites: Development Beyond the Cell Membrane; Cambridge University Press, 1993. [Google Scholar]
- Sugumaran M.; Semensi V.; Kalyanaraman B.; Bruce J. M.; Land E. J. Evidence for the Formation of a Quinone Methide During the Oxidation of the Insect Cuticular Sclerotizing Precursor 1,2-Dehydro-N-Acetyldopamine. J. Biol. Chem. 1992, 267, 10355–10361. 10.1016/S0021-9258(19)50026-6. [DOI] [PubMed] [Google Scholar]
- Andersen S. A.Sclerotization. In Physiology of the Insect Epidermis; Binnington K., Retnakaran A., Eds.; CSIRO Publications, 1991; pp 123–140. [Google Scholar]
- Schaefer J.; Kramer K. J.; Garbow J. R.; Jacob G. S.; Stejskal E. O.; Hopkins T. L.; Speirs R. D. Aromatic Cross-Links in Insect Cuticles: Detection by Solid-State 13C and 15N NMR. Science 1987, 235 (4793), 1200–1204. 10.1126/science.3823880. [DOI] [PubMed] [Google Scholar]
- Xu R.; Huang X.; Morgan T. D.; Prakash O.; Kramer K. J.; Hawley M. D. Characterization of Products from the Reactions of N-Acetyldopamine Quinone with N-Acetylhistidine. Arch. Biochem. Biophys. 1996, 329 (1), 56–64. 10.1006/abbi.1996.0191. [DOI] [PubMed] [Google Scholar]
- Xu R.; Huang X.; Hopkins T. L.; Kramer K. J. Catecholamine and Histidyl Protein Cross-Linked Structures in Sclerotized Insect Cuticle. Insect Biochem. Mol. Biol. 1997, 27, 101–108. 10.1016/S0965-1748(96)00083-5. [DOI] [Google Scholar]
- Kerwin J. L. Profiling Peptide Adducts of Oxidized N-Acetyldopamine by Electrospray Mass Spectrometry. Rapid Commun. Mass. Spectrom. 1997, 11, 557–566. . [DOI] [PubMed] [Google Scholar]
- Kramer E. J.; Kanost M. R.; Hopkins T. L.; Jiang H.; Zhu Y. C.; Xu R.; Kerwin J. L.; Turecek F. Oxidative Conjugation of Catechols with Proteins in Insect Skeletal Systems. Tetrahedron 2001, 57, 385–392. 10.1016/S0040-4020(00)00949-2. [DOI] [Google Scholar]
- Masson V.; Arafah K.; Voisin S.; Bulet P. Comparative Proteomics Studies of Insect Cuticle by Tandem Mass Spectrometry: Application of a Novel Proteomics Approach to the Pea Aphid Cuticular Proteins. Proteomics 2018, 18, 1700368. 10.1002/pmic.201700368. [DOI] [PubMed] [Google Scholar]
- Pan P.-L.; Ye Y.-X.; Lou Y.-H.; Lu J.-B.; Cheng C.; Shen Y.; Moussian B.; Zhang C.-X. A Comprehensive Omics Analysis and Functional Survey of Cuticular Proteins in the Brown Panthopper. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 5175–5180. 10.1073/pnas.1716951115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Li S.; Li W.; Peng L.; Chen Z.; Xiao Y.; Guo H.; Zhang J.; Cheng T.; Goldsmith M. R.; et al. Genome-Wide Annotation and Comparative Analysis of Cuticular Protein Genes in the Noctuid Pest Spodoptera Litura. Insect Biochem. Mol. Biol. 2019, 110, 90–97. 10.1016/j.ibmb.2019.04.012. [DOI] [PubMed] [Google Scholar]
- Magkrioti C. K.; Spyropoulos I. C.; Iconomidou V. A.; Willis J. H.; Hamodrakas S. J. Cuticledb: A Relational Database of Arthropod Cuticular Proteins. BMC Bioinform. 2004, 5, 138. 10.1186/1471-2105-5-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S. O.; Hojrup P.; Roepstorff P. Insect Cuticular Proteins. Insect Biochem. Mol. Biol. 1995, 25, 153–176. 10.1016/0965-1748(94)00052-J. [DOI] [PubMed] [Google Scholar]
- Willis J. H. Structural Cuticular Proteins from Arthropods: Annotation, Nomenclature, and Sequence Characteristics in the Genomics Era. Insect Biochem. Mol. Biol. 2010, 40, 189–204. 10.1016/j.ibmb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebers J. E.; Willis J. H. A Conserved Domain in Arthropod Cuticular Proteins Binds Chitin. Insect Biochem. Mol. Biol. 2001, 31, 1083–1093. 10.1016/S0965-1748(01)00056-X. [DOI] [PubMed] [Google Scholar]
- Hamodrakas S. J.; Willis J. H.; Iconomidou V. A. A Structural Model of the Chitin-Binding Domain of Cuticle Proteins. Insect Biochem. Mol. Biol. 2002, 32, 1577–1583. 10.1016/S0965-1748(02)00079-6. [DOI] [PubMed] [Google Scholar]
- Rebers J. E.; Riddiford L. M. Structure and Expression of a Manduca Sexta Larval Cuticle Gene Homologous to Drosophila Cuticle Genes. J. Mol. Biol. 1988, 203, 411–423. 10.1016/0022-2836(88)90009-5. [DOI] [PubMed] [Google Scholar]
- Dilly P. N.; Nixon M. The Cells That Secret the Beaks in Octopods and Squids (Mollusca, Cephalopoda). Cell Tissue Res. 1976, 167 (2), 229–241. 10.1007/BF00224330. [DOI] [PubMed] [Google Scholar]
- Uyeno T. A.; Kier W. M. Functional Morphology of the Cephalopod Buccal Mass: A Novel Joint Type. J. Morphol. 2005, 264, 211–222. 10.1002/jmor.10330. [DOI] [PubMed] [Google Scholar]
- Ritchie R. O. The Conflicts between Strength and Toughness. Nat. Mater. 2011, 10, 817–822. 10.1038/nmat3115. [DOI] [PubMed] [Google Scholar]
- Rubin D.; Miserez A.; Waite J. H.. Antecedents of Arthropod Sclerotization. In Advances in Insect Physiology; Casas J., Ed.; Elsevier, 2010; Vol. 38, pp 75–133. [Google Scholar]
- Gabryelczyk B.; Cai H.; Shi X.; Sun Y.; Swinkels P. J. M.; Salentinig S.; Pervushin K.; Miserez A. Hydrogen Bond Guidance and Aromatic Stacking Drive Liquid-Liquid Phase Separation of Intrinsically Disordered Histidine-Rich Peptides. Nat. Comms. 2019, 10 (1), 5465. 10.1038/s41467-019-13469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Gabryelczyk B.; Manimekalai M. S. S.; Grüber G.; Salentinig S.; Miserez A. Self-Coacervation of Modular Squid Beak Proteins - a Comparative Study. Soft Matter 2017, 13, 7740–7752. 10.1039/C7SM01352C. [DOI] [PubMed] [Google Scholar]
- Lim Z. W.; Varma V.; Ramanujan R. V.; Miserez A. Magnetically Responsive Peptide Coacervates for Dual Hyperthermia and Chemotherapy Treatments of Liver Cancer. Acta. Biomater. 2020, 110, 221–230. 10.1016/j.actbio.2020.04.024. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Lau S. Y.; Lim Z. W.; Chang S. C.; Ghadessy F.; Partridge A.; Miserez A. Phase-Separating Peptides for Direct Cytosolic Delivery and Redox-Activated Release of Macromolecular Therapeutics. Nat. Chem. 2022, 14, 274–283. 10.1038/s41557-021-00854-4. [DOI] [PubMed] [Google Scholar]
- Le Ferrand H.; Duchamp M.; Gabryelczyk B.; Cai H.; Miserez A. Time-Resolved Observations of Liquid-Liquid Phase Separation at the Nanoscale Using in Situ Liquid Transmission Electron Microscopy. J. Am. Chem. Soc. 2019, 141, 7202–7210. 10.1021/jacs.9b03083. [DOI] [PubMed] [Google Scholar]
- Lim J.; Kumar A.; Low K.; Verma C. S.; Mu Y.; Miserez A.; Pervushin K. Liquid-Liquid Phase Separation of Short Histidine- and Tyrosine-Rich Peptides: Sequence Specificity and Molecular Topology. J. Phys. Chem. B 2021, 125, 6776–6790. 10.1021/acs.jpcb.0c11476. [DOI] [PubMed] [Google Scholar]
- Hiew S. H.; Guerette P. A.; Zvarec O. J.; Phillips M.; Zhou F.; Su H.; Pervushin K.; Orner B. P.; Miserez A. Modular Peptides from the Thermoplastic Squid Sucker Ring Teeth Form Amyloid-Like Cross-Β Supramolecular Networks. Acta. Biomater. 2016, 46, 41–54. 10.1016/j.actbio.2016.09.040. [DOI] [PubMed] [Google Scholar]
- Rieu C.; Bertinetti L.; Schuetz R.; Salinas-Zavala C.; Weaver J. C.; Miserez A.; Masic A. The Role of Water on the Structure and Mechanical Properties of a Thermoplastic Natural Block Co-Polymer from Squid Sucker Ring Teeth. Bioinspir. Biomim 2016, 11 (5), 055003–055010. 10.1088/1748-3190/11/5/055003. [DOI] [PubMed] [Google Scholar]
- Hiew S. H.; Sánchez-Ferrer A.; Amini S.; Zhou F.; Adamcik J.; Guerette P.; Su H.; Mezzenga R.; Miserez A. Squid Suckerin Biomimetic Peptides Form Amyloid-Like Crystals with Robust Mechanical Properties. Biomacromolecules 2017, 18, 4240–4248. 10.1021/acs.biomac.7b01280. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Ding F. Thermo- and pH-Responsive Fibrillization of Squid Suckerin A1h1 Peptide. Nanoscale 2020, 12, 6307–6317. 10.1039/C9NR09271D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Wang Y.; Tong C.; Wei G.; Ding F.; Sun Y. Molecular Insights into the Self-Assembly of Block Copolymer Suckerin Polypeptides into Nanoconfined Β-Sheets. Small 2022, 18, 2202642. 10.1002/smll.202202642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D.; Guerette P. A.; Hoon S.; Kong K. W.; Cornvik T.; Nilsson M.; Miserez A. Biomimetic Production of Silk-Like Recombinant Squid Sucker Ring Teeth Proteins. Biomacromolecules 2014, 15 (9), 3278–3289. 10.1021/bm500670r. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ferrer A.; Adamcik J.; Handschin S.; Hiew S. H.; Miserez A.; Mezzenga R. Controlling Supramolecular Chiral Nanostructures by Self-Assembly of a Biomimetic Β-Sheet-Rich Amyloidogenic Peptide. ACS Nano 2018, 12, 9152–9161. 10.1021/acsnano.8b03582. [DOI] [PubMed] [Google Scholar]
- Moses D. N.; Pontin M. G.; Waite J. H.; Zok F. W. Effects of Hydration on Mechanical Properties of a Highly Sclerotized Tissue. Biophys. J. 2008, 94, 3266–3272. 10.1529/biophysj.107.120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyar E.; Harrington M. J.; Politi Y.; Fratzl P. The Mechanical Role of Metal Ions in Biogenic Protein-Based Materials. Angew. Chem., Int. Ed. Engl. 2014, 53, 12026–12044. 10.1002/anie.201404272. [DOI] [PubMed] [Google Scholar]
- Ding D.; Guerette P. A.; Fu J.; Zhang L.; Irvine S. A.; Miserez A. From Soft Self-Healing Gels to Stiff Films in Suckerin-Based Materials through Modulation of Cross-Link Density and Β-Sheet Content. Adv. Mater. 2015, 27 (26), 3953–3961. 10.1002/adma.201500280. [DOI] [PubMed] [Google Scholar]
- Hiew S. H.; Mohanram H.; Ning L.; Guo J.; Sánchez-Ferrer A.; Shi X.; Pervushin K.; Mu Y.; Mezzenga R.; Miserez A. A Short Peptide Hydrogel with High Stiffness Induced by 310-Helices to Β-Sheet Transition in Water. Adv. Sci. 2019, 6 (21), 1901173. 10.1002/advs.201901173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Z. W.; Ping Y.; Miserez A. Glucose-Responsive Peptide Coacervates with High Encapsulation Efficiency for Controlled Release of Insulin. Bioconjugate Chem. 2018, 29, 2176–2180. 10.1021/acs.bioconjchem.8b00369. [DOI] [PubMed] [Google Scholar]
- Ramos R.; Koh K.; Gabryelczyk B.; Chai L.; Kanagavel D.; Yan X.; Ganachaud F.; Miserez A.; Bernard J. Nanocapsules Produced by Nanoprecipitation of Designed Suckerin-Silk Fusion Proteins. ACS Macro. Lett. 2021, 10, 628–634. 10.1021/acsmacrolett.1c00171. [DOI] [PubMed] [Google Scholar]
- Yamanaka M.; Ishizaki Y.; Nakagawa T.; Taoka A.; Fukumori Y. Purification and Characterization of Coacervate-Forming Cuticular Proteins from Papilio Xuthus Pupae. Zool. Sci. 2013, 30 (7), 534–542. 10.2108/zsj.30.534. [DOI] [PubMed] [Google Scholar]
- Clarke A. J. Nylon: The Story of a Fashion Revolution (Review). Technol. Cult. 2002, 43, 446–448. 10.1353/tech.2002.0053. [DOI] [Google Scholar]
- Tokareva O.; Michalczechen-Lacerda V. A.; Rech E. L.; Kaplan D. L. Recombinant DNA Production of Spider Silk Proteins. Microb. Biotechnol. 2013, 6, 651–663. 10.1111/1751-7915.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrely C.; Scheibel T. Biotechnological Production of Spider-Silk Proteins Enables New Applications. Macromol. Biosci. 2007, 7, 401–409. 10.1002/mabi.200600255. [DOI] [PubMed] [Google Scholar]
- Arcidiacono S.; Mello C.; Kaplan D.; Cheley S.; Bayley H. Purification and Characterization of Recombinant Spider Silk Expressed in Escherichia Coli. Applied microbiology and biotechnology 1998, 49, 31–38. 10.1007/s002530051133. [DOI] [PubMed] [Google Scholar]
- Huemmerich D.; Helsen C. W.; Quedzuweit S.; Oschmann J.; Oschmann J.; Rudolph R.; Scheibel T. Primary Structure Elements of Spider Dragline Silks and Their Contribution to Protein Solubility. Biochemistry 2004, 43 (42), 13604–13612. 10.1021/bi048983q. [DOI] [PubMed] [Google Scholar]
- Heidebrecht A.; Scheibel T. Recombinant Production of Spider Silk Proteins. Adv. Appl. Microbiol. 2013, 82, 115–153. 10.1016/B978-0-12-407679-2.00004-1. [DOI] [PubMed] [Google Scholar]
- Heidebrecht A.; Eisoldt L.; Diehl J.; Schmidt A.; Geffers M.; Lang G.; Scheibel T. Biomimetic Fibers Made of Recombinant Spidroins with the Same Toughness as Natural Spider Silk. Adv. Mater. 2015, 27, 2189–2194. 10.1002/adma.201404234. [DOI] [PubMed] [Google Scholar]
- Aigner T. B.; DeSimone E.; Scheibel T. Biomedical Applications of Recombinant Silk-Based Materials. Adv. Mater. 2018, 30, 1704636–1704636. 10.1002/adma.201704636. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop T.; Claassens N. J.; van der Oost J. Improved Protein Production and Codon Optimization Analyses in Escherichia Coli by Bicistronic Design. Microb. Biotechnol. 2019, 12, 173–179. 10.1111/1751-7915.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H.; Liang Y.; Zhong X.; Pan Z.; Huang L.; Zhang H.; Xu Y.; Zhou W.; Liu Z. Codon Optimization with Deep Learning to Enhance Protein Expression. Sci. Rep. 2020, 10 (1), 17617. 10.1038/s41598-020-74091-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould N.; Hendy O.; Papamichail D. Computational Tools and Algorithms for Designing Customized Synthetic Genes. Front. Bioeng. Biotechnol. 2014, 2, 41–41. 10.3389/fbioe.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelas G. C.; Arroyo G.; Carrasco C.; Dompenciel R. Spider Silkglands Contain a Tissue-Specific Alanine Trna That Accumulates in Vitro in Response to the Stimulus for Silk Protein Synthesis. Dev. Biol. 1990, 140, 215–220. 10.1016/0012-1606(90)90069-U. [DOI] [PubMed] [Google Scholar]
- Cao H.; Parveen S.; Ding D.; Xu H.; Tan T.; Liu L. Metabolic Engineering for Recombinant Major Ampullate Spidroin 2 (Masp2) Synthesis in Escherichia Coli. Sci. Rep. 2017, 7, 11365. 10.1038/s41598-017-11845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.-X.; Qian Z.-G.; Zhong J.-J.; Xia X.-X. Hyper-Production of Large Proteins of Spider Dragline Silk Masp2 by Escherichia Coli Via Synthetic Biology Approach. Process Biochem. 2016, 51, 484–490. 10.1016/j.procbio.2016.01.006. [DOI] [Google Scholar]
- Xia X. X.; Qian Z. G.; Ki C. S.; Park Y. H.; Kaplan D. L.; Lee S. Y. Native-Sized Recombinant Spider Silk Protein Produced in Metabolically Engineered Escherichia Coli Results in a Strong Fiber. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 14059–14063. 10.1073/pnas.1003366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos-Pinto J. R. A.; Arcuri H. A.; Esteves F. G.; Palma M. S.; Lubec G. Spider Silk Proteome Provides Insight into the Structural Characterization of Nephila Clavipes Flagelliform Spidroin. Sci. Rep. 2018, 8, 14674. 10.1038/s41598-018-33068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humenik M.; Magdeburg M.; Scheibel T. Influence of Repeat Numbers on Self-Assembly Rates of Repetitive Recombinant Spider Silk Proteins. J. Struct. Biol. 2014, 186, 431–437. 10.1016/j.jsb.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Rammensee S.; Slotta U.; Scheibel T.; Bausch A. R. Assembly Mechanism of Recombinant Spider Silk Proteins. Proc. Natl. Acad. Sci. U.S.A 2008, 105, 6590–6595. 10.1073/pnas.0709246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblhofer E.; Heidebrecht A.; Scheibel T. To Spin or Not to Spin: Spider Silk Fibers and More. Applied microbiology and biotechnology 2015, 99, 9361–9380. 10.1007/s00253-015-6948-8. [DOI] [PubMed] [Google Scholar]
- Mohammadi P.; Jonkergouw C.; Beaune G.; Engelhardt P.; Kamada A.; Timonen J. V. I.; Knowles T. P. J.; Penttila M.; Linder M. B. Controllable Coacervation of Recombinantly Produced Spider Silk Protein Using Kosmotropic Salts. J. Colloid Interface Sci. 2020, 560, 149–160. 10.1016/j.jcis.2019.10.058. [DOI] [PubMed] [Google Scholar]
- Mohammadi P.; Aranko A. S.; Landowski C. P.; Ikkala O.; Jaudzems K.; Wagermaier W.; Linder M. B. Biomimetic Composites with Enhanced Toughening Using Silk-Inspired Triblock Proteins and Aligned Nanocellulose Reinforcements. Sci. Adv. 2019, 5 (9), 1–12. 10.1126/sciadv.aaw2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi P.; Beaune G.; Stokke B. T.; Timonen J. V. I.; Linder M. B. Self-Coacervation of a Silk-Like Protein and Its Use as an Adhesive for Cellulosic Materials. ACS Macro. Lett. 2018, 7, 1120–1125. 10.1021/acsmacrolett.8b00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi P.; Aranko A. S.; Lemetti L.; Cenev Z.; Zhou Q.; Virtanen S.; Landowski C. P.; Penttilä M.; Fischer W. J.; Wagermaier W.; et al. Phase Transitions as Intermediate Steps in the Formation of Molecularly Engineered Protein Fibers. Commun. Biol. 2018, 1, 86–86. 10.1038/s42003-018-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Egaña A.; Lang G.; Mauerer C.; Wickinghoff J.; Weber M.; Geimer S.; Scheibel T. Interactions of Fibroblasts with Different Morphologies Made of an Engineered Spider Silk Protein. Adv. Eng. Mater. 2012, 14, B67–B75. 10.1002/adem.201180072. [DOI] [Google Scholar]
- DeSimone E.; Aigner T. B.; Humenik M.; Lang G.; Scheibel T. Aqueous Electrospinning of Recombinant Spider Silk Proteins. Mater. Sci. Eng. C 2020, 106, 110145–110145. 10.1016/j.msec.2019.110145. [DOI] [PubMed] [Google Scholar]
- Widhe M.; Shalaly N. D.; Hedhammar M. A Fibronectin Mimetic Motif Improves Integrin Mediated Cell Biding to Recombinant Spider Silk Matrices. Biomaterials 2016, 74, 256–266. 10.1016/j.biomaterials.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Johansson U.; Widhe M.; Shalaly N. D.; Arregui I. L.; Nilebäck L.; Tasiopoulos C. P.; Åstrand C.; Berggren P.-O.; Gasser C.; Hedhammar M. Assembly of Functionalized Silk Together with Cells to Obtain Proliferative 3d Cultures Integrated in a Network of Ecm-Like Microfibers. Sci. Rep. 2019, 9 (1), 6291. 10.1038/s41598-019-42541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. X.; Xue Z. X.; Wei M. H.; Chen D. L.; Li M.. A Novel Scaffold from Recombinant Spider Silk Protein in Tissue Engineering. Advanced Materials Research; Trans Tech Publ, 2011; Vol. 152, pp 1734–1744. [Google Scholar]
- Schacht K.; Vogt J.; Scheibel T. Foams Made of Engineered Recombinant Spider Silk Proteins as 3d Scaffolds for Cell Growth. ACS Biomater. Sci. Eng. 2016, 2, 517–525. 10.1021/acsbiomaterials.5b00483. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E.; Pierschbacher M. D. New Perspectives in Cell Adhesion: Rgd and Integrins. Science 1987, 238, 491–497. 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Mohammadi P.; Zemke F.; Wagermaier W.; Linder M. B. Interfacial Crystallization and Supramolecular Self-Assembly of Spider Silk Inspired Protein at the Water-Air Interface. Materials 2021, 14, 4239–4239. 10.3390/ma14154239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungst T.; Smolan W.; Schacht K.; Scheibel T.; Groll J. r. Strategies and Molecular Design Criteria for 3d Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539. 10.1021/acs.chemrev.5b00303. [DOI] [PubMed] [Google Scholar]
- Schacht K.; Jüngst T.; Schweinlin M.; Ewald A.; Groll J.; Scheibel T. Biofabrication of Cell-Loaded 3d Spider Silk Constructs. Angew. Chem., Int. Ed. Engl. 2015, 54, 2816–2820. 10.1002/anie.201409846. [DOI] [PubMed] [Google Scholar]
- Mu X.; Agostinacchio F.; Xiang N.; Pei Y.; Khan Y.; Guo C.; Cebe P.; Motta A.; Kaplan D. L. Recent Advances in 3d Printing with Protein-Based Inks. Prog. Polym. Sci. 2021, 115, 101375. 10.1016/j.progpolymsci.2021.101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostinacchio F.; Mu X.; Dirè S.; Motta A.; Kaplan D. L. In Situ 3d Printing: Opportunities with Silk Inks. Trends Biotechnol. 2021, 39, 719–730. 10.1016/j.tibtech.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann B.; Hümmerich D.; Scheibel T.; Fehr M. Formulation of Poorly Water-Soluble Substances Using Self-Assembling Spider Silk Protein. Colloids Surf. A Physicochem. Eng. Asp. 2008, 331, 126–132. 10.1016/j.colsurfa.2008.04.005. [DOI] [Google Scholar]
- Spiess K.; Lammel A.; Scheibel T. Recombinant Spider Silk Proteins for Applications in Biomaterials. Macromol. Biosci. 2010, 10, 998–1007. 10.1002/mabi.201000071. [DOI] [PubMed] [Google Scholar]
- Lammel A.; Schwab M.; Hofer M.; Winter G.; Scheibel T. Recombinant Spider Silk Particles as Drug Delivery Vehicles. Biomaterials 2011, 32, 2233–2240. 10.1016/j.biomaterials.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Rabotyagova O. S.; Cebe P.; Kaplan D. L. Self-Assembly of Genetically Engineered Spider Silk Block Copolymers. Biomacromolecules 2009, 10, 229–236. 10.1021/bm800930x. [DOI] [PubMed] [Google Scholar]
- Hwang D. S.; Gim Y.; Cha H. J. Expression of Functional Recombinant Mussel Adhesive Protein Type 3a in Escherichia Coli. Biotechnol. Prog. 2005, 21, 965–970. 10.1021/bp050014e. [DOI] [PubMed] [Google Scholar]
- Kim D.; Hwang D. S.; Kang D. G.; Kim J. Y.; Cha H. J. Enhancement of Mussel Adhesive Protein Production in Escherichia Coli by Co-Expression of Bacterial Hemoglobin. Biotechnol. Prog. 2008, 24, 663–666. 10.1021/bp0703477. [DOI] [PubMed] [Google Scholar]
- Cha H. J.; Hwang D. S.; Lim S.; White J. D.; Matos-Perez C. A.; Wilker J. J. Bulk Adhesive Strength of Recombinant Hybrid Mussel Adhesive Protein. Biofouling 2009, 25, 99–107. 10.1080/08927010802563108. [DOI] [PubMed] [Google Scholar]
- Hwang D. S.; Gim Y.; Yoo H. J.; Cha H. J. Practical Recombinant Hybrid Mussel Bioadhesive Fp-151. Biomaterials 2007, 28, 3560–3568. 10.1016/j.biomaterials.2007.04.039. [DOI] [PubMed] [Google Scholar]
- Lim S.; Choi Y. S.; Kang D. G.; Song Y. H.; Cha H. J. The Adhesive Properties of Coacervated Recombinant Hybrid Mussel Adhesive Proteins. Biomaterials 2010, 31, 3715–3722. 10.1016/j.biomaterials.2010.01.063. [DOI] [PubMed] [Google Scholar]
- Kim S.; Huang J.; Lee Y.; Dutta S.; Yoo H. Y.; Jung Y. M.; Jho Y.; Zeng H.; Hwang D. S. Complexation and Coacervation of Like-Charged Polyelectrolytes Inspired by Mussels. Proc. Natl. Acad. Sci. U.S.A 2016, 113 (7), E847–853. 10.1073/pnas.1521521113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.; Yoo H. Y.; Huang J.; Lee Y.; Park S.; Park Y.; Jin S.; Jung Y. M.; Zeng H.; Hwang D. S.; et al. Salt Triggers the Simple Coacervation of an Underwater Adhesive When Cations Meet Aromatic Pi Electrons in Seawater. ACS Nano 2017, 11, 6764–6772. 10.1021/acsnano.7b01370. [DOI] [PubMed] [Google Scholar]
- Kim B. J.; Oh D. X.; Kim S.; Seo J. H.; Hwang D. S.; Masic A.; Han D. K.; Cha H. J. Mussel-Mimetic Protein-Based Adhesive Hydrogel. Biomacromolecules 2014, 15, 1579–1585. 10.1021/bm4017308. [DOI] [PubMed] [Google Scholar]
- Iraha F.; Oki K.; Kobayashi T.; Ohno S.; Yokogawa T.; Nishikawa K.; Yokoyama S.; Sakamoto K. Functional Replacement of the Endogenous Tyrosyl-Trna Synthetase-Trnatyr Pair by the Archaeal Tyrosine Pair in Escherichia Coli for Genetic Code Expansion. Nucleic Acids Res. 2010, 38, 3682–3691. 10.1093/nar/gkq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B.; Ayyadurai N.; Yun H.; Choi Y. S.; Hwang B. H.; Huang J.; Lu Q.; Zeng H.; Cha H. J. In Vivo Residue-Specific Dopa-Incorporated Engineered Mussel Bioglue with Enhanced Adhesion and Water Resistance. Angew. Chem., Int. Ed. Engl. 2014, 53, 13360–13364. 10.1002/anie.201406099. [DOI] [PubMed] [Google Scholar]
- Mozhdehi D.; Luginbuhl K. M.; Simon J. R.; Dzuricky M.; Berger R.; Varol H. S.; Huang F. C.; Buehne K. L.; Mayne N. R.; Weitzhandler I.; et al. Genetically Encoded Lipid-Polypeptide Hybrid Biomaterials That Exhibit Temperature-Triggered Hierarchical Self-Assembly. Nat. Chem. 2018, 10, 496–505. 10.1038/s41557-018-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D. S.; Sim S. B.; Cha H. J. Cell Adhesion Biomaterial Based on Mussel Adhesive Protein Fused with RGD Peptide. Biomaterials 2007, 28, 4039–4046. 10.1016/j.biomaterials.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Zhong C.; Gurry T.; Cheng A. A.; Downey J.; Deng Z.; Stultz C. M.; Lu T. K. Strong Underwater Adhesives Made by Self-Assembling Multi-Protein Nanofibres. Nat. Nanotechnol. 2014, 9, 858–866. 10.1038/nnano.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Huang J.; Zhang J.; Liu S.; Cui M.; An B.; Wang X.; Pu J.; Zhao T.; Fan C.; et al. Engineered Bacillus Subtilis Biofilms as Living Glues. Mater. Today 2019, 28, 40–48. 10.1016/j.mattod.2018.12.039. [DOI] [Google Scholar]
- Cui M.; Qi Q.; Gurry T.; Zhao T.; An B.; Pu J.; Gui X.; Cheng A. A.; Zhang S.; Xun D.; et al. Modular Genetic Design of Multi-Domain Functional Amyloids: Insights into Self-Assembly and Functional Properties. Chem. Sci. 2019, 10, 4004–4014. 10.1039/C9SC00208A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dance A. Will Hagfish Yield the Fibers of the Future?. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 7005–7006. 10.1073/pnas.1606279113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H.; Aebi U. Intermediate Filaments: Molecular Structure, Assembly Mechanism, and Integration into Functionally Distinct Intracellular Scaffolds. Annu. Rev. Anal. Biochem. 2004, 73, 749–789. 10.1146/annurev.biochem.73.011303.073823. [DOI] [PubMed] [Google Scholar]
- Fu J.; Guerette P. A.; Miserez A. Self-Assembly of Recombinant Hagfish Thread Keratins Amenable to a Strain-Induced Α-Helix to Β-Sheet Transition. Biomacromolecules 2015, 16, 2327–2339. 10.1021/acs.biomac.5b00552. [DOI] [PubMed] [Google Scholar]
- Schopferer M.; Bär H.; Hochstein B.; Sharma S.; Mücke N.; Herrmann H.; Willenbacher N. Desmin and Vimentin Intermediate Filament Networks: Their Viscoelastic Properties Investigated by Mechanical Rheometry. J. Mol. Biol. 2009, 388 (1), 133–143. 10.1016/j.jmb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Oliveira P. E.; Chen D.; Bell B. E.; Harris T. I.; Walker C.; Zhang H.; Grob B.; Lewis R. V.; Jones J. A. The Next Generation of Protein Super-Fibres: Robust Recombinant Production and Recovery of Hagfish Intermediate Filament Proteins with Fibre Spinning and Mechanical-Structural Characterizations. Microb. Biotechnol. 2021, 14, 1976–1989. 10.1111/1751-7915.13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta N. K.; Choudhury N. R.; Truong M. Y.; Kim M.; Elvin C. M.; Hill A. J. Physical Approaches for Fabrication of Organized Nanostructure of Resilin-Mimetic Elastic Protein Rec1-Resilin. Biomaterials 2009, 30, 4868–4876. 10.1016/j.biomaterials.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Lyons R. E.; Elvin C. M.; Taylor K.; Lekieffre N.; Ramshaw J. A. Purification of Recombinant Protein by Cold-Coacervation of Fusion Constructs Incorporating Resilin-Inspired Polypeptides. Biotechnol. Bioeng. 2012, 109, 2947–2954. 10.1002/bit.24565. [DOI] [PubMed] [Google Scholar]
- Kim M.; Elvin C.; Brownlee A.; Lyons R. High Yield Expression of Recombinant Pro-Resilin: Lactose-Induced Fermentation in E. Coli and Facile Purification. Protein Expr. Purif. 2007, 52, 230–236. 10.1016/j.pep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Qin G.; Rivkin A.; Lapidot S.; Hu X.; Preis I.; Arinus S. B.; Dgany O.; Shoseyov O.; Kaplan D. L. Recombinant Exon-Encoded Resilins for Elastomeric Biomaterials. Biomaterials 2011, 32, 9231–9243. 10.1016/j.biomaterials.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburro A. M.; Panariello S.; Santopietro V.; Bracalello A.; Bochicchio B.; Pepe A. Molecular and Supramolecular Structural Studies on Significant Repetitive Sequences of Resilin. ChemBioChem. 2010, 11, 83–93. 10.1002/cbic.200900460. [DOI] [PubMed] [Google Scholar]
- Appel E.; Heepe L.; Lin C. P.; Gorb S. N. Ultrastructure of Dragonfly Wing Veins: Composite Structure of Fibrous Material Supplemented by Resilin. J. Anat. 2015, 227, 561–582. 10.1111/joa.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels J.; Appel E.; Gorb S. N. Functional Diversity of Resilin in Arthropoda. Beilstein J. Nanotechnol. 2016, 7, 1241–1259. 10.3762/bjnano.7.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Tong Z.; Jia X.; Kiick K. L. Resilin-Like Polypeptide Hydrogels Engineered for Versatile Biological Functions. Soft Matter 2013, 9, 665–673. 10.1039/C2SM26812D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charati M. B.; Ifkovits J. L.; Burdick J. A.; Linhardt J. G.; Kiick K. L. Hydrophilic Elastomeric Biomaterials Based on Resilin-Like Polypeptides. Soft Matter 2009, 5, 3412–3416. 10.1039/b910980c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Mahara A.; Tong Z.; Levenson E. A.; McGann C. L.; Jia X.; Yamaoka T.; Kiick K. L. Recombinant Resilin-Based Bioelastomers for Regenerative Medicine Applications. Adv. Healthc. Mater. 2016, 5, 266–275. 10.1002/adhm.201500411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Teller S.; Clifton R. J.; Jia X.; Kiick K. L. Tunable Mechanical Stability and Deformation Response of a Resilin-Based Elastomer. Biomacromolecules 2011, 12, 2302–2310. 10.1021/bm200373p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann C. L.; Akins R. E.; Kiick K. L. Resilin-Peg Hybrid Hydrogels Yield Degradable Elastomeric Scaffolds with Heterogeneous Microstructure. Biomacromolecules 2016, 17, 128–140. 10.1021/acs.biomac.5b01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann C. L.; Levenson E. A.; Kiick K. L. Resilin-Based Hybrid Hydrogels for Cardiovascular Tissue Engineering. Macromolecules 2013, 214 (2), 203–213. 10.1002/macp.201200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner J. N.; Cherry K. M.; Su R. S.; Liu J. C. Characterization of Resilin-Based Materials for Tissue Engineering Applications. Biomacromolecules 2012, 13, 3678–3685. 10.1021/bm301129b. [DOI] [PubMed] [Google Scholar]
- Renner J. N.; Kim Y.; Cherry K. M.; Liu J. C. Modular Cloning and Protein Expression of Long, Repetitive Resilin-Based Proteins. Protein Expr. Purif. 2012, 82, 90–96. 10.1016/j.pep.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Gill E. E.; Liu J. C. Enzymatic Cross-Linking of Resilin-Based Proteins for Vascular Tissue Engineering Applications. Biomacromolecules 2016, 17, 2530–2539. 10.1021/acs.biomac.6b00500. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Liu J. C. Protein-Engineered Microenvironments Can Promote Endothelial Differentiation of Human Mesenchymal Stem Cells in the Absence of Exogenous Growth Factors. Biomater. Sci. 2016, 4, 1761–1772. 10.1039/C6BM00472E. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Renner J. N.; Liu J. C. Incorporating the Bmp-2 Peptide in Genetically-Engineered Biomaterials Accelerates Osteogenic Differentiation. Biomater. Sci. 2014, 2, 1110–1119. 10.1039/C3BM60333D. [DOI] [PubMed] [Google Scholar]
- Su R. S. C.; Gill E. E.; Kim Y.; Liu J. C. Characterization of Resilin-Like Proteins with Tunable Mechanical Properties. J. Mech. Behav. Biomed. Mater. 2019, 91, 68–75. 10.1016/j.jmbbm.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okesola B. O.; Lau H. K.; Derkus B.; Boccorh D. K.; Wu Y.; Wark A. W.; Kiick K. L.; Mata A. Covalent Co-Assembly between Resilin-Like Polypeptide and Peptide Amphiphile into Hydrogels with Controlled Nanostructure and Improved Mechanical Properties. Biomater. Sci. 2020, 8, 846–857. 10.1039/C9BM01796H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv S.; Dudek D. M.; Cao Y.; Balamurali M. M.; Gosline J.; Li H. Designed Biomaterials to Mimic the Mechanical Properties of Muscles. Nature 2010, 465, 69–73. 10.1038/nature09024. [DOI] [PubMed] [Google Scholar]
- Bracalello A.; Santopietro V.; Vassalli M.; Marletta G.; Del Gaudio R.; Bochicchio B.; Pepe A. Design and Production of a Chimeric Resilin-, Elastin-, and Collagen-Like Engineered Polypeptide. Biomacromolecules 2011, 12, 2957–2965. 10.1021/bm2005388. [DOI] [PubMed] [Google Scholar]
- Huang S. C.; Qian Z. G.; Dan A. H.; Hu X.; Zhou M. L.; Xia X. X. Rational Design and Hierarchical Assembly of a Genetically Engineered Resilin-Silk Copolymer Results in Stiff Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 1576–1585. 10.1021/acsbiomaterials.7b00353. [DOI] [PubMed] [Google Scholar]
- Weitzhandler I.; Dzuricky M.; Hoffmann I.; Garcia Quiroz F.; Gradzielski M.; Chilkoti A. Micellar Self-Assembly of Recombinant Resilin-/Elastin-Like Block Copolypeptides. Biomacromolecules 2017, 18, 2419–2426. 10.1021/acs.biomac.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P.; Dzuricky M.; Min J.; Jenkins I.; Chilkoti A. Concentration-Independent Multivalent Targeting of Cancer Cells by Genetically Encoded Core-Crosslinked Elastin/Resilin-Like Polypeptide Micelles. Biomacromolecules 2021, 22, 4347–4356. 10.1021/acs.biomac.1c00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muiznieks L. D.; Weiss A. S.; Keeley F. W. Structural Disorder and Dynamics of Elastin. Biochemistry and Cell Biology 2010, 88, 239–250. 10.1139/O09-161. [DOI] [PubMed] [Google Scholar]
- Gotte L.; Volpin D.; Horne R. W.; Mammi M. Electron Microscopy and Optical Diffraction of Elastin. Micron 1976, 7 (2), 95–102. 10.1016/0047-7206(76)90052-2. [DOI] [Google Scholar]
- Gotte L.; Giro M. G.; Volpin D.; Horne R. W. The Ultrastructural Organization of Elastin. J. Ultrastruct. Res. 1974, 46, 23–33. 10.1016/S0022-5320(74)80019-5. [DOI] [PubMed] [Google Scholar]
- Gotte L.; Mammi M.; Pezzin G. Scanning Electron Microscope Observatlons on Elastin. Connect. Tissue Res. 1972, 1, 61–67. 10.3109/03008207209152057. [DOI] [Google Scholar]
- Mithieux S. M.; Weiss A. S. Elastin. Adv. Protein Chem. 2005, 70, 437–461. 10.1016/S0065-3233(05)70013-9. [DOI] [PubMed] [Google Scholar]
- Visconti R. P.; Barth J. L.; Keeley F. W.; Little C. D. Codistribution Analysis of Elastin and Related Fibrillar Proteins in Early Vertebrate Development. Matrix Biol. 2003, 22, 109–121. 10.1016/S0945-053X(03)00014-3. [DOI] [PubMed] [Google Scholar]
- Scopes R. K.Protein Purification: Principles and Practice; Springer Science & Business Media, 1993. [Google Scholar]
- Meyer D. E.; Chilkoti A. Purification of Recombinant Proteins by Fusion with Thermally-Responsive Polypeptides. Nat. Biotechnol. 1999, 17, 1112–1115. 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- Cho Y.; Zhang Y.; Christensen T.; Sagle L. B.; Chilkoti A.; Cremer P. S. Effects of Hofmeister Anions on the Phase Transition Temperature of Elastin-Like Polypeptides. J. Phys. Chem. B 2008, 112, 13765–13771. 10.1021/jp8062977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. E.; Chilkoti A. Quantification of the Effects of Chain Length and Concentration on the Thermal Behavior of Elastin-Like Polypeptides. Biomacromolecules 2004, 5, 846–851. 10.1021/bm034215n. [DOI] [PubMed] [Google Scholar]
- Massodi I.; Raucher D. A Thermally Responsive Tat-Elastin-Like Polypeptide Fusion Protein Induces Membrane Leakage, Apoptosis, and Cell Death in Human Breast Cancer Cells. J. Drug Target. 2007, 15, 611–622. 10.1080/10611860701502780. [DOI] [PubMed] [Google Scholar]
- Elsharkawy S.; Al-Jawad M.; Pantano M. F.; Tejeda-Montes E.; Mehta K.; Jamal H.; Agarwal S.; Shuturminska K.; Rice A.; Tarakina N. V. Protein Disorder-Order Interplay to Guide the Growth of Hierarchical Mineralized Structures. Nat. Commun. 2018, 9, 2145. 10.1038/s41467-018-04319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urosev I.; Lopez Morales J.; Nash M. A. Phase Separation of Intrinsically Disordered Protein Polymers Mechanically Stiffens Fibrin Clots. Adv. Funct. Mater. 2020, 30, 2005245–2005245. 10.1002/adfm.202005245. [DOI] [Google Scholar]
- Roberts S.; Harmon T. S.; Schaal J. L.; Miao V.; Li K. J.; Hunt A.; Wen Y.; Oas T. G.; Collier J. H.; Pappu R. V. Injectable Tissue Integrating Networks from Recombinant Polypeptides with Tunable Order. Nat. Mater. 2018, 17 (12), 1164. 10.1038/s41563-018-0233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annabi N.; Mithieux S. M.; Zorlutuna P.; Camci-Unal G.; Weiss A. S.; Khademhosseini A. Engineered Cell-Laden Human Protein-Based Elastomer. Biomaterials 2013, 34, 5496–5505. 10.1016/j.biomaterials.2013.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-N.; Avery R. K.; Vallmajo-Martin Q.; Assmann A.; Vegh A.; Memic A.; Olsen B. D.; Annabi N.; Khademhosseini A. A Highly Elastic and Rapidly Crosslinkable Elastin-Like Polypeptide-Based Hydrogel for Biomedical Applications. Adv. Funct. Mater. 2015, 25, 4814–4826. 10.1002/adfm.201501489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. A.; Simon J. R.; Ghoorchian A.; Scholl Z.; Lin S.; Rubinstein M.; Marszalek P.; Chilkoti A.; López G. P.; Zhao X. Strong, Tough, Stretchable, and Self-Adhesive Hydrogels from Intrinsically Unstructured Proteins. Adv. Mater. 2017, 29, 1604743. 10.1002/adma.201604743. [DOI] [PubMed] [Google Scholar]
- Buck C. C.; Dennis P. B.; Gupta M. K.; Grant M. T.; Crosby M. G.; Slocik J. M.; Mirau P. A.; Becknell K. A.; Comfort K. K.; Naik R. R. Anion-Mediated Effects on the Size and Mechanical Properties of Enzymatically Crosslinked Suckerin Hydrogels. Macromol. Biosci. 2019, 19, 1800238. 10.1002/mabi.201800238. [DOI] [PubMed] [Google Scholar]
- Ping Y.; Ding D.; Ramos R. A. N. S.; Mohanram H.; Deepankumar K.; Gao J.; Tang G.; Miserez A. Supramolecular Β-Sheets Stabilized Protein Nanocarriers for Drug Delivery and Gene Transfection. ACS Nano 2017, 11, 4528–4541. 10.1021/acsnano.6b08393. [DOI] [PubMed] [Google Scholar]
- Hershewe J. M.; Wiseman W. D.; Kath J. E.; Buck C. C.; Gupta M. K.; Dennis P. B.; Naik R. R.; Jewett M. C. Characterizing and Controlling Nanoscale Self-Assembly of Suckerin-12. ACS Synth. Biol. 2020, 9, 3388–3399. 10.1021/acssynbio.0c00442. [DOI] [PubMed] [Google Scholar]
- Hiew S. H.; Wang J. K.; Koh K.; Yang H.; Bacha A.; Lin J.; Yip Y. S.; Vos M. I. G.; Chen L.; Sobota R. M.; et al. Bioinspired Short Peptide Hydrogel for Versatile Encapsulation and Controlled Release of Growth Factor Therapeutics. Acta. Biomater. 2021, 136, 111–123. 10.1016/j.actbio.2021.09.023. [DOI] [PubMed] [Google Scholar]
- Kichler A.; Mason A. J.; Bechinger B. Cationic Amphipathic Histidine-Rich Peptides for Gene Delivery. Biochim. Biophys. Acta. Biomembr. 2006, 1758, 301–307. 10.1016/j.bbamem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Jung H.; Pena-Francesch A.; Saadat A.; Sebastian A.; Kim D. H.; Hamilton R. F.; Albert I.; Allen B. D.; Demirel M. C. Molecular Tandem Repeat Strategy for Elucidating Mechanical Properties of High-Strength Proteins. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 6478–6483. 10.1073/pnas.1521645113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Francesch A.; Jung H.; Demirel M. C.; Sitti M. Biosynthetic Self-Healing Materials for Soft Machines. Nat. Mater. 2020, 19, 1230–1235. 10.1038/s41563-020-0736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K. M.; Kim E.; Wu L.-Q.; Liu Y.; Bentley W. E.; Payne G. F. Biomimetic Fabrication of Information-Rich Phenolic-Chitosan Films. Soft Matter 2011, 7, 9601–9616. 10.1039/c1sm05293d. [DOI] [Google Scholar]
- Zargar V.; Asghari M.; Dashti A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng. Rev. 2015, 2, 204–226. 10.1002/cben.201400025. [DOI] [Google Scholar]
- Shamshina J. L.; Berton P.; Rogers R. D. Advances in Functional Chitin Materials: A Review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. 10.1021/acssuschemeng.8b06372. [DOI] [Google Scholar]
- Vaclaw M. C.; Sprouse P. A.; Dittmer N. T.; Ghazvini S.; Middaugh C. R.; Kanost M. R.; Gehrke S. H.; Dhar P. Self-Assembled Coacervates of Chitosan and an Insect Cuticle Protein Containing a Rebers-Riddiford Motif. Biomacromolecules 2018, 19, 2391–2400. 10.1021/acs.biomac.7b01637. [DOI] [PubMed] [Google Scholar]
- Gong Q.; Chen L.; Wang J.; Yuan F.; Ma Z.; Chen G.; Huang Y.; Miao Y.; Liu T.; Zhang X.-X.; et al. Coassembly of a New Insect Cuticular Protein and Chitosan Via Liquid-Liquid Phase Separation. Biomacromolecules 2022, 23, 2562–2571. 10.1021/acs.biomac.2c00261. [DOI] [PubMed] [Google Scholar]
- Fox J. D.; Capadona J. R.; Marasco P. D.; Rowan S. J. Bioinspired Water-Enhanced Mechanical Gradient Nanocomposite Films That Mimic the Architecture and Properties of the Squid Beak. J. Am. Chem. Soc. 2013, 135, 5167–5174. 10.1021/ja4002713. [DOI] [PubMed] [Google Scholar]
- Venkatesan H.; Chen J.; Hu J. Fibers Made of Recombinant Spidroins—a Brief Review. AATCC J. Res. 2019, 6, 37–40. 10.14504/ajr.6.S1.8. [DOI] [Google Scholar]
- Chen R. Bacterial Expression Systems for Recombinant Protein Production: E. Coli and Beyond. Biotechnol. Adv. 2012, 30, 1102–1107. 10.1016/j.biotechadv.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Lewis R. V.; Hinman M.; Kothakota S.; Fournier M. J. Expression and Purification of a Spider Silk Protein: A New Strategy for Producing Repetitive Proteins. Protein Expr. Purif. 1996, 7, 400–406. 10.1006/prep.1996.0060. [DOI] [PubMed] [Google Scholar]
- Bowen C. H.; Dai B.; Sargent C. J.; Bai W.; Ladiwala P.; Feng H.; Huang W.; Kaplan D. L.; Galazka J. M.; Zhang F. Recombinant Spidroins Fully Replicate Primary Mechanical Properties of Natural Spider Silk. Biomacromolecules 2018, 19, 3853–3860. 10.1021/acs.biomac.8b00980. [DOI] [PubMed] [Google Scholar]
- Widmaier D. M.; Tullman-Ercek D.; Mirsky E. A.; Hill R.; Govindarajan S.; Minshull J.; Voigt C. A. Engineering the Salmonella Type III Secretion System to Export Spider Silk Monomers. Mol. Syst. Biol. 2009, 5, 309–309. 10.1038/msb.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong C. P.; Higuchi-Takeuchi M.; Malay A. D.; Oktaviani N. A.; Thagun C.; Numata K. A Marine Photosynthetic Microbial Cell Factory as a Platform for Spider Silk Production. Commun. Biol. 2020, 3, 357. 10.1038/s42003-020-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock S. R.; Bedzyk L. A. Production of Synthetic Spider Dragline Silk Protein in Pichia Pastoris. Appl. Microbiol. Biotechnol. 1997, 47, 33–39. 10.1007/s002530050884. [DOI] [PubMed] [Google Scholar]
- Sidoruk K. V.; Davydova L. I.; Kozlov D. G.; Gubaidullin D. G.; Glazunov A. V.; Bogush V. G.; Debabov V. G. Fermentation Optimization of a Saccharomyces Cerevisiae Strain Producing 1f9 Recombinant Spidroin. Appl. Biochem. Microbiol. 2015, 51, 766–773. 10.1134/S0003683815070066. [DOI] [Google Scholar]
- Linder M.; Penttilä M.; Szilvay G.; Ilmen M.; Kiema A.; Aro N.; Saloheimo M.; Westerholm-Parvinen A.; Vitikainen M.; Paananen A.; Joensuu J.. Production of fusion proteins in Trichoderma. WO2017115005A1, 2017.
- Lazaris A.; Arcidiacono S.; Huang Y.; Zhou J.-F.; Duguay F.; Chretien N.; Welsh E. A.; Soares J. W.; Karatzas C. N. Spider Silk Fibers Spun from Soluble Recombinant Silk Produced in Mammalian Cells. Science 2002, 295, 472–476. 10.1126/science.1065780. [DOI] [PubMed] [Google Scholar]
- Grip S.; Rising A.; Nimmervoll H.; Storckenfeldt E.; McQueen-Mason S. J.; Pouchkina-Stantcheva N.; Vollrath F.; Engström W.; Fernandez-Arias A. Transient Expression of a Major Ampullate Spidroin 1 Gene Fragment from Euprosthenops Sp. In Mammalian Cells. Cancer Genomics Proteomics 2006, 3 (2), 83–87. [PubMed] [Google Scholar]
- Lyda T. A.; Wagner E. L.; Bourg A. X.; Peng C.; Tomaraei G. N.; Dean D.; Kennedy M. S.; Marcotte W. R. A Leishmania Secretion System for the Expression of Major Ampullate Spidroin Mimics. PLoS One 2017, 12 (5), e0178201. 10.1371/journal.pone.0178201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann V.; Weichert N.; Menzel M.; Knoch D.; Paege N.; Scheller J.; Spohn U.; Conrad U.; Gils M. Native-Sized Spider Silk Proteins Synthesized in Planta Via Intein-Based Multimerization. Transgenic Res. 2013, 22, 369–377. 10.1007/s11248-012-9655-6. [DOI] [PubMed] [Google Scholar]
- Weichert N.; Hauptmann V.; Helmold C.; Conrad U. Seed-Specific Expression of Spider Silk Protein Multimers Causes Long-Term Stability. Front. Plant Sci. 2016, 7, 6. 10.3389/fpls.2016.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J.; Gührs K.-H.; Grosse F.; Conrad U. Production of Spider Silk Proteins in Tobacco and Potato. Nat. Biotechnol. 2001, 19, 573–577. 10.1038/89335. [DOI] [PubMed] [Google Scholar]
- Yang J.; Barr L. A.; Fahnestock S. R.; Liu Z.-B. High Yield Recombinant Silk-Like Protein Production in Transgenic Plants through Protein Targeting. Transgenic Res. 2005, 14, 313–324. 10.1007/s11248-005-0272-5. [DOI] [PubMed] [Google Scholar]
- Xu H. T.; Fan B. L.; Yu S. Y.; Huang Y. H.; Zhao Z. H.; Lian Z. X.; Dai Y. P.; Wang L. L.; Liu Z. L.; Fei J.; et al. Construct Synthetic Gene Encoding Artificial Spider Dragline Silk Protein and Its Expression in Milk of Transgenic Mice. Anim. Biotechnol. 2007, 18, 1–12. 10.1080/10495390601091024. [DOI] [PubMed] [Google Scholar]
- Copeland C. G.; Bell B. E.; Christensen C. D.; Lewis R. V. Development of a Process for the Spinning of Synthetic Spider Silk. ACS Biomater. Sci. Eng. 2015, 1, 577–584. 10.1021/acsbiomaterials.5b00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Xia L.; Day B. A.; Harris T. I.; Oliveira P.; Knittel C.; Licon A. L.; Gong C.; Dion G.; Lewis R. V. Crispr/Cas9 Initiated Transgenic Silkworms as a Natural Spinner of Spider Silk. Biomacromolecules 2019, 20 (6), 2252–2264. 10.1021/acs.biomac.9b00193. [DOI] [PubMed] [Google Scholar]
- Wen H.; Lan X.; Zhang Y.; Zhao T.; Wang Y.; Kajiura Z.; Nakagaki M. Transgenic Silkworms (Bombyx Mori) Produce Recombinant Spider Dragline Silk in Cocoons. Mol. Biol. Rep. 2010, 37, 1815–1821. 10.1007/s11033-009-9615-2. [DOI] [PubMed] [Google Scholar]
- Teulé F.; Miao Y.-G.; Sohn B.-H.; Kim Y.-S.; Hull J. J.; Fraser M. J.; Lewis R. V.; Jarvis D. L. Silkworms Transformed with Chimeric Silkworm/Spider Silk Genes Spin Composite Silk Fibers with Improved Mechanical Properties. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 923–928. 10.1073/pnas.1109420109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Dong Q.; Yu Y.; Niu B.; Ji D.; Li M.; Huang Y.; Chen X.; Tan A. Mass Spider Silk Production through Targeted Gene Replacement in Bombyx Mori. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 8757–8762. 10.1073/pnas.1806805115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Hu J.; Miao Y.; Zhao A.; Zhao T.; Wu D.; Liang L.; Miikura A.; Shiomi K.; Kajiura Z. Expression of EGFP-Spider Dragline Silk Fusion Protein in BmN Cells and Larvae of Silkworm Showed the Solubility Is Primary Limit for Dragline Proteins Yield. Mol. Biol. Rep. 2008, 35 (3), 329–335. 10.1007/s11033-007-9090-6. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Zhu Z.; Nielsen J.; Siewers V. Engineering Saccharomyces Cerevisiae Cells for Production of Fatty Acid-Derived Biofuels and Chemicals. Open Biol. 2019, 9, 190049–190049. 10.1098/rsob.190049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergün B. G.; Çalık P. Lignocellulose Degrading Extremozymes Produced by Pichia Pastoris: Current Status and Future Prospects. Bioprocess. Biosyst. Eng. 2016, 39 (1), 1–36. 10.1007/s00449-015-1476-6. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Zhang Z. Engineering Strategies for Enhanced Production of Protein and Bio-Products in Pichia Pastoris: A Review. Biotechnol. Adv. 2018, 36, 182–195. 10.1016/j.biotechadv.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Buijs N. A.; Siewers V.; Nielsen J. Advanced Biofuel Production by the Yeast Saccharomyces Cerevisiae. Curr. Opin. Chem. Biol. 2013, 17, 480–488. 10.1016/j.cbpa.2013.03.036. [DOI] [PubMed] [Google Scholar]
- Caspeta L.; Nielsen J. Toward Systems Metabolic Engineering of Aspergillus and Pichia Species for the Production of Chemicals and Biofuels. Biotechnology J. 2013, 8, 534–544. 10.1002/biot.201200345. [DOI] [PubMed] [Google Scholar]
- Schuster A.; Schmoll M. Biology and Biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. 10.1007/s00253-010-2632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusakov A. V. Alternatives to Trichoderma Reesei in Biofuel Production. Trends Biotechnol. 2011, 29, 419–425. 10.1016/j.tibtech.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Landowski C.; Huuskonen A.; Saarinen J.; Westerholm-Parvinen A.; Kanerva A.; Natunen J.; Hanninen A.-L.; Salovuori N.; Penttila M.; Saloheimo M.. Protease Deficient Filamentous Fungal Cells and Methods of Use Thereof. US10731168B2, 2019.
- Huuskonen A.; Saloheimo M.; Kanerva A.. Multiple Proteases Deficient Filamentous Fungal Cells and Methods of Use Thereof. EP3019602A2, 2016.
- Pakula T.; Saloheimo M.; Hakkinen M.; Westerholm-Parvinen A.; Penttila M.; Vitikainen M.. Method for Protein Production in Filamentous Fungi. US9512415B2, 2011.
- Moon K.-B.; Park J.-S.; Park Y.-I.; Song I.-J.; Lee H.-J.; Cho H. S.; Jeon J.-H.; Kim H.-S. Development of Systems for the Production of Plant-Derived Biopharmaceuticals. Plants 2020, 9, 30–30. 10.3390/plants9010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. Y.; Wi S. J. Potential of Plants to Produce Recombinant Protein Products. J. Plant Biol. 2016, 59, 559–568. 10.1007/s12374-016-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppel A.; Holland C. Progress and Trends in Artificial Silk Spinning: A Systematic Review. ACS Biomater. Sci. Eng. 2017, 3, 226–226. 10.1021/acsbiomaterials.6b00669. [DOI] [PubMed] [Google Scholar]
- Rising A.; Harrington M. J.. Biological Materials Processing: Time-Tested Tricks for Sustainable Fiber Fabrication. Chem. Rev. 2023, 10.1021/acs.chemrev.2c00465 [DOI] [PubMed] [Google Scholar]
- Liew F. E.; Nogle R.; Abdalla T.; Rasor B. J.; Canter C.; Jensen R. O.; Wang L.; Strutz J.; Chirania P.; De Tissera S.; et al. Carbon-Negative Production of Acetone and Isopropanol by Gas Fermentation at Industrial Pilot Scale. Nat. Biotechnol. 2022, 40, 335–344. 10.1038/s41587-021-01195-w. [DOI] [PubMed] [Google Scholar]