Significance
We showed that Pgr-Cre mediated deletion of the m6A writer Mettl3 results in complete infertility due to failure in implantation and decidualization. This study uncovers the physiological function of m6A modification in the female reproductive tract during pregnancy. We found that infertility phenotypes mainly result from decreased PGR protein expression following Mettl3 deletion. Mechanistically, our data showed that METTL3-mediated m6A modification in the 5′-UTR of Pgr mRNA is essential for its efficient translation. Besides uncovering a novel epigenetic mechanism required for normal progesterone signaling during implantation at the mRNA modification layer, our findings are of high clinical relevance, since aberrant progesterone signaling is often associated with uterine pathophysiology.
Keywords: METTL3, m6A, embryo implantation, progesterone receptor
Abstract
Embryo implantation, a crucial step in human reproduction, is tightly controlled by estrogen and progesterone (P4) via estrogen receptor alpha and progesterone receptor (PGR), respectively. Here, we report that N6-methyladenosine (m6A), the most abundant mRNA modification in eukaryotes, plays an essential role in embryo implantation through the maintenance of P4 signaling. Conditional deletion of methyltransferase-like 3 (Mettl3), encoding the m6A writer METTL3, in the female reproductive tract using a Cre mouse line with Pgr promoter (Pgr-Cre) resulted in complete implantation failure due to pre-implantation embryo loss and defective uterine receptivity. Moreover, the uterus of Mettl3 null mice failed to respond to artificial decidualization. We further found that Mettl3 deletion was accompanied by a marked decrease in PGR protein expression. Mechanistically, we found that Pgr mRNA is a direct target for METTL3-mediated m6A modification. A luciferase assay revealed that the m6A modification in the 5′ untranslated region (5′-UTR) of Pgr mRNA enhances PGR protein translation efficiency in a YTHDF1-dependent manner. Finally, we demonstrated that METTL3 is required for human endometrial stromal cell decidualization in vitro and that the METTL3-PGR axis is conserved between mice and humans. In summary, this study provides evidence that METTL3 is essential for normal P4 signaling during embryo implantation via m6A-mediated translation control of Pgr mRNA.
Embryo implantation is a crucial step in human reproduction. The rate of natural conception per menstrual cycle is only approximately 30%, mainly due to implantation failure (1). In assisted reproduction, despite the high success rate of in vitro fertilization, the implantation rate remains low (2). Moreover, many pregnancy complications are rooted in suboptimal implantation (3). Embryo implantation is tightly controlled by ovarian 17β-estradiol (E2) and progesterone (P4) acting via estrogen receptor alpha (ESR1) and progesterone receptor (PGR), respectively (4). In mice, the uterus enters a pre-receptive stage under the regulation of E2 on gestational day 1 (GD1, the day when the vaginal plug is seen) and GD2, which is marked by epithelial cell proliferation. On GD3 and GD4, under the regulation of P4, uterine epithelial cells stop proliferation and begin to differentiate, which is a hallmark of the receptive stage (5). Over the past decades, the components of the P4 signaling required for the implantation have been extensively studied using genetically modified mice (6). Nevertheless, a complete molecular atlas of P4 signaling is still lacking.
N6-methyladenosine (m6A) is the most prevalent form of mRNA modification in eukaryotes. Emerging evidence has shown that m6A modification is a powerful regulator of gene expression (7). The m6A modification is catalyzed by a methyltransferase complex consisting of the catalytic subunit methyltransferase-like 3 (METTL3), the target recognition subunit METTL14 and the regulatory subunit Wilms tumor 1-associated protein (8). The reverse reaction is mediated by two demethylases, fat mass and obesity-associated protein and alkB homolog 5 (9, 10). M6A modification is recognized by reader proteins, including YTH domain-containing proteins (YTHDC1/2 and YTHDF1/2/3), insulin-like growth factor 2 mRNA-binding proteins (IGF2BP1/2/3), and eukaryotic translation initiation factor 3 (EIF3) (11). Through binding with different reader proteins, the m6A modification regulates gene expression by affecting mRNA splicing, translocation, degradation, stabilization, and translation (12). Functional studies have shown that m6A is indispensable for a variety of biological processes, including spermatogenesis, oogenesis, and embryo development (13, 14). To date, however, whether the m6A modification is involved in embryo implantation has remained unclear.
Here, we specifically deleted Mettl3 in the female reproductive tract using the Pgr-Cre driver. Mice with conditional Mettl3 deletion were found to be completely infertile due to a failure in implantation and decidualization. We further found that the deletion of Mettl3 caused a marked decrease in PGR expression at the protein but not the mRNA level. Mechanistically, our data demonstrated that METTL3-mediated m6A modification in the 5′-UTR is essential for its efficient translation. In summary, our results provided evidence that METTL3 is crucial for normal P4 responsiveness during embryo implantation via the m6A-mediated translation control of Pgr mRNA.
Results
Pgr-Cre-Mediated Deletion of Mettl3 Resulted in Infertility due to Failure in Implantation and Decidualization.
As a first step, we determined the expression pattern of METTL3 in the mouse uterus during early pregnancy using immunohistochemistry. We found that METTL3 was expressed at low levels in epithelial cells and stromal cells on GD1 and its expression gradually increased from GD2 to GD4. With the onset of implantation on GD5, the expression of METTL3 was upregulated around the implanting blastocyst both in epithelial cells and stromal cells. On GD8, METTL3 was highly expressed in the entire decidual bed (SI Appendix, Fig. S1).
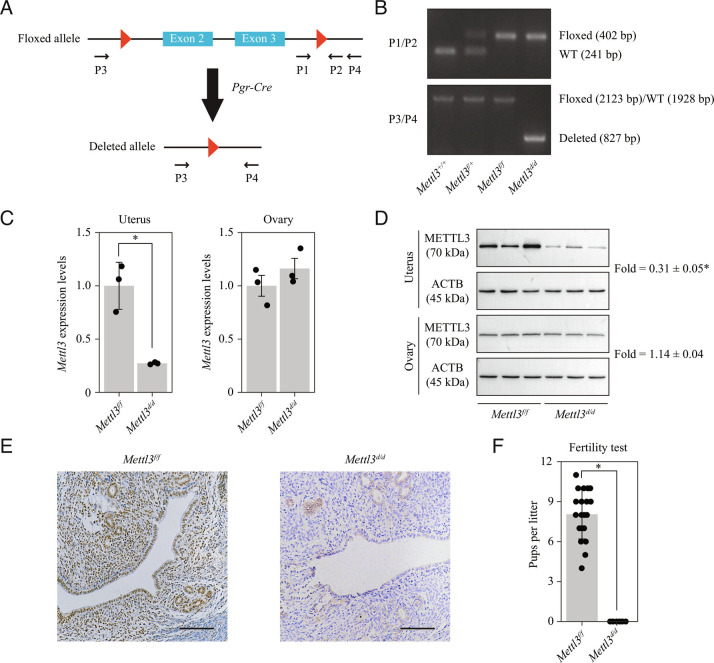
Given that Mettl3 null mice are embryonic lethal (14), transgenic mice with loxp sites flanking exons 2 and 3 of the Mettl3 gene (henceforth referred to as Mettl3f/f) were used in this study. Mice with conditional deletion of Mettl3 in the female reproductive tract (henceforth referred to as Mettl3d/d) were generated by mating Mettl3f/f mice with mice of the Pgr-Cre driver line (Fig. 1A). The deletion of Mettl3 in the uterus of Mettl3d/d mice was confirmed at the level of DNA (Fig. 1B), mRNA (Fig. 1C) and protein (Fig. 1 D and E). Notably, the expression of Mettl3 in the ovary was not affected (Fig. 1 C and D). According to RNA sequencing (RNA-seq) data, the deletion of exons 2 and 3 resulted in splicing events between exons 1 and 5 and, to a lesser extent, between exons 1 and 4. In either case, the open reading frame was shifted, leading to premature translation termination and the loss of METTL3 protein (SI Appendix, Fig. S2). In a 6-mo fertility test, Mettl3f/f females were found to be fertile (8.05 ± 1.85 pups/litter), whereas Mettl3d/d females were completely infertile (Fig. 1F).
Fig. 1.
Pgr-Cre-mediated deletion of Mettl3 leads to complete infertility. (A) Diagram showing the strategy used for conditional Mettl3 deletion. Mettl3 floxed (Mettl3f/f) mice have loxP sites flanking exons 2 and 3. Conditional Mettl3 deletion (Mettl3d/d) mice were generated by crossing Mettl3f/f mice with mice of the Pgr-Cre driver line. (B) Genotyping analysis showing the Mettl3 knockout efficiency at the DNA level in the uterus on gestational day 4 (GD4). (C) Quantitative RT-PCR analysis of Mettl3 mRNA levels in the uterus and the ovary of Mettl3f/f and Mettl3d/d mice on GD4. Data are presented as mean ± SD. *P < 0.05. (D) Western blot analysis of METTL3 protein levels in the uterus and the ovary on GD4. (E) Immunohistochemical staining of METTL3 protein in the uterus on GD4. (Scale bar, 100 μm.) (F) Analysis of litter sizes for 6 Mettl3f/f mice and 6 Mettl3d/d mice during a 6-mo fertility test. Data are presented as mean ± SD. *P < 0.05.
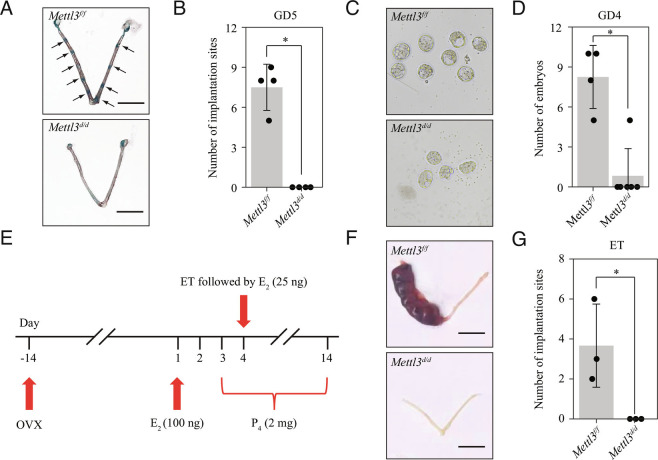
To determine the cause of infertility in Mettl3d/d females, we compared the pregnancy status of Mettl3d/d and Mettl3f/f female mice at different time points. We noticed that Mettl3d/d female mice exhibited complete implantation failure on GD5 (Fig. 2 A and B) and GD8 (SI Appendix, Fig. S3 A and B). On GD4, blastocysts were recovered from only 1 of 6 Mettl3d/d females and all these blastocysts were morphologically abnormal (Fig. 2 C and D). Histological examination of ovaries showed no differences between Mettl3d/d and Mettl3f/f mice (SI Appendix, Fig. S4A) while serum E2 and P4 levels were also comparable (SI Appendix, Fig. S4 B and C), indicating that ovary function was unaffected in Mettl3d/d mice. Embryos flushed from Mettl3d/d uteri on GD2 were normal (SI Appendix, Fig. S5A) and could be cultured to blastocysts in vitro (SI Appendix, Fig. S5B). Those embryos flushed from the oviduct of Mettl3d/d females on GD3 were mostly lost or deformed (SI Appendix, Fig. S6 A and B) suggested that pre-implantation embryo loss was likely caused by the oviduct environment. In wild-type mice, METTL3 was highly expressed in epithelial cells and weakly expressed in stromal cells in both the ampullary region and the isthmus region of the oviduct from GD1 to GD4 (SI Appendix, Fig. S7 A and B). Further analysis demonstrated that Mettl3 was efficiently deleted in the isthmus region of oviduct of Mettl3d/d mice on GD3, whereas no recombination was observed in the ampulla region of the oviduct (SI Appendix, Fig. S8 A and B). This was in line with previous studies reporting that the recombinatorial activity of Pgr-Cre could be detected in the isthmus region but not in the ampullary region of the oviduct (15, 16). Our results indicated that pre-implantation embryo loss resulted from Mettl3 deletion in the isthmus region of oviduct. Using an embryo transfer (ET) protocol (Fig. 2E), we found that the cultured blastocysts derived from Mettl3d/d donors were able to implant into Mettl3f/f recipient uteri, whereas the opposite was not observed (Fig. 2 F and G). These results indicated that embryo implantation failure in Mettl3d/d mice was due not only to pre-implantation embryo loss but also to defective uterine receptivity during the window of implantation.
Fig. 2.
Mettl3 deletion results in embryo implantation failure due to pre-implantation embryo loss and defective uterine receptivity. (A) Representative images of mouse uterus of Mettl3f/f mice and Mettl3d/d mice on gestational day (GD5). Implantation sites are marked by arrowheads. (Scale bar, 1 cm.) (B) Bar plot showing the no. of embryo implantation sites in Mettl3f/f mice and Mettl3d/d mice on GD5. Data are presented as mean ± SD. *P < 0.05. (C) Representative images of embryos collected from the uterus of Mettl3f/f mice and Mettl3d/d mice on GD4. (D) Bar plot showing the no. of embryos collected from the uterus of Mettl3f/f mice and Mettl3d/d mice on GD4. Data are presented as mean ± SD. *P < 0.05. (E) Experimental scheme for ET using ovariectomized (OVX) mice as recipients. (F) ET between Mettl3f/f and Mettl3d/d mice. Embryos were recovered from the oviduct of donors on GD2 and cultured to the blastocyst stage in vitro. Blastocysts from Mettl3d/d mice were transferred to the uterus of Mettl3f/f mice, while blastocysts from Mettl3f/f mice were transferred to the uterus of Mettl3d/d mice. For all ET experiments, 6 morphologically normal blastocysts were transferred to one uterine horn. (G) Bar plot showing the no. of embryo implantation sites in ET experiments. Data are presented as mean ± SD. *P < 0.05.
METTL3 has been reported to be highly expressed in decidual cells (17), which was also confirmed in our study (SI Appendix, Fig. S1), implying that METTL3 plays a role in decidualization. To address this possibility, we examined the impact of Mettl3 deletion on decidualization using an artificial decidualization model in which sesame seed oil was injected into the lumen of the uterine horn to trigger a decidual response in a manner similar to embryo implantation. We found that the decidual response was absence in Mettl3d/d mice based on the size and weight of uterine horns (SI Appendix, Fig. S9 A and B). Compromised decidualization was confirmed by the reduced expression levels of the decidualization markers, prolactin family 8 subfamily A member 2 (18), bone morphogenetic protein 2 (19), and Wnt family member 4 (20) (SI Appendix, Fig. S9C). Furthermore, alkaline phosphatase activity, a marker of stromal cell differentiation (2), was detected in the stroma of Mettl3f/f uteri, but not in that of Mettl3d/d uteri after artificial decidualization (SI Appendix, Fig. S9D). The decidualization process is accompanied by neovascularization (21). Here, we found that the expression of PECAM1, an endothelial cell-specific marker, was markedly diminished in Mettl3d/d uteri after artificial decidualization (SI Appendix, Fig. S9E). These results indicated that the deletion of Mettl3 in the mouse uterus leads to decidualization failure.
The Deletion of Mettl3 Led to Decreased Expression of PGR at the Protein Level.
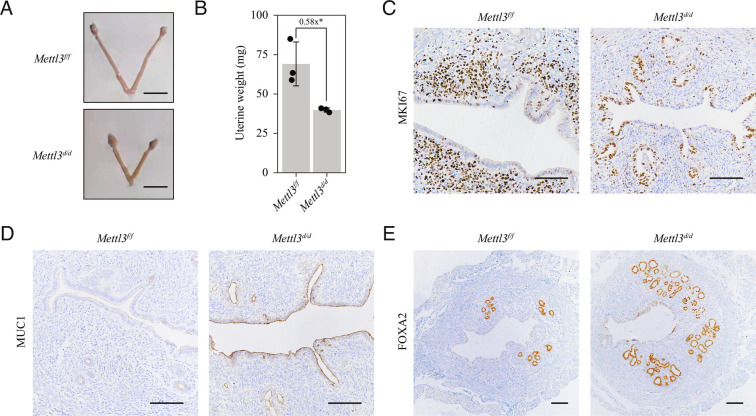
We next explored the reason for the defective uterine receptivity seen in Mettl3d/d mice. The uterine size and weight of Mettl3d/d mice were significantly lower than those of Mettl3f/f mice on GD4 (Fig. 3 A and B). Immunohistochemical staining for marker of proliferation Ki-67 (MKI67) showed epithelial cell growth arrest and stromal cell proliferation in Mettl3f/f mice, which is a hallmark of uterine receptivity (5, 22). However, in Mettl3d/d mice, proliferation persisted in a small population of luminal and glandular epithelial cells, whereas stromal cell proliferation was almost absent (Fig. 3C). Defective uterine receptivity in Mettl3d/d mice was further confirmed based on the high levels of mucin 1 (MUC1) expression detected in uterine epithelial cells (Fig. 3D). Previous studies showed that METTL3 is downregulated in endometriosis, adenomyosis and endometrial cancer (23–27). Here, we found that the no. of glands was increased in Mettl3d/d uteri compared to Mettl3f/f uteri (Fig. 3E), indicative of an anti-proliferation role of METTL3 in uterine epithelial cells.
Fig. 3.
Mettl3 deletion disrupts stromal proliferation and glandular development during the window of implantation. (A) Representative images of uteri collected from Mettl3f/f mice and Mettl3d/d mice on gestational day 4 (GD4). (B) The weight of uteri collected from Mettl3f/f mice and Mettl3d/d mice on GD4. Data are presented as mean ± SD. *P < 0.05. (C–E) Immunohistochemical staining for MKI67 (C), MUC1 (D) and FOXA2 (E) in uteri collected from Mettl3f/f mice and Mettl3d/d mice on GD4. (Scale bar, 100 μm.)
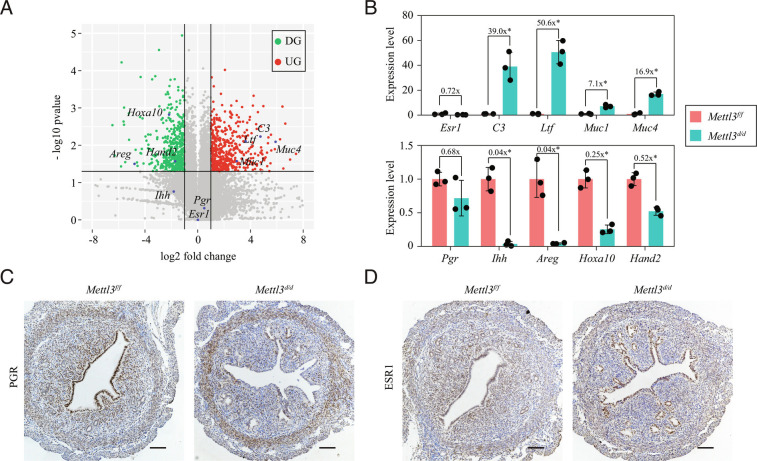
To identify the molecular mechanism underlying the defective uterine receptivity in Mettl3d/d mice, uterine tissues were collected from both groups of mice on GD4 and subjected to mRNA sequencing analysis. A total of 988 genes were found to be differentially expressed (fold change > 2 and P < 0.05), 489 of which were upregulated, and 499 genes downregulated in Mettl3d/d mice relative to that in Mettl3f/f mice (Fig. 4A and SI Appendix, Table S1). Analysis of the differentially expressed genes revealed that several P4 target genes, including amphiregulin (Areg), homeobox A10 (Hoxa10), and heart and neural crest derivatives expressed 2 (Hand2), were significantly downregulated in Mettl3d/d uteri compared with Mettl3f/f uteri. Another P4 target gene, Indian hedgehog (Ihh), was also downregulated (fold change = 0.28), although not significantly, likely due to large intra-group variation. Meanwhile, E2 target genes, such as complement C3 (C3), lactotransferrin (Ltf ), mucin 1 (Muc1) and mucin 4 (Muc4) were significantly up-regulated in Mettl3d/d uteri compared to Mettl3f/f uteri. Quantitative RT-PCR analysis using an independent set of uterine samples confirmed that P4 target genes Ihh, Areg, Hoxa10, and Hand2 were significantly downregulated, whereas E2 target genes C3, Ltf, Muc1, and Muc4 were significantly upregulated in Mettl3d/d uteri compared to Mettl3f/f uteri (Fig. 4B). Interestingly, both RNA-seq and quantitative RT-PCR results demonstrated that the expression of neither Esr1 nor Pgr was significantly changed at the mRNA level (Fig. 4 A and B). Immunohistochemical analysis further showed that although the ESR1 protein level was unchanged, PGR protein expression was decreased in the uteri of Mettl3d/d mice (Fig. 4 C and D). The possibility that decreased Pgr gene dosage was directly responsible for the reduced PGR protein in Mettl3d/d (Mettl3f/f; PgrCre/+) mice was excluded, as PGR protein levels were comparable between PgrCre/+ mice and Mettl3f/f mice (SI Appendix, Fig. S10). This was in line with previous studies (28, 29) and was likely due to protein dosage compensation (30). These results indicated that defective uterine receptivity in Mettl3d/d mice was due to altered E2 and P4 signaling resulting from decreased expression of PGR at the protein level.
Fig. 4.
PGR protein expression is decreased in the uterus of Mettl3-deleted mice during the window of implantation. (A) Volcano plot for differentially expressed genes between the Mettl3f/f uterus and the Mettl3d/d uterus on gestational day 4 (GD4) as determined by RNA-seq analysis. DG, down-regulated genes; UG, up-regulated genes. (B) Validation of E2 target genes and P4 target genes by quantitative RT-PCR. Data are presented as mean ± SD. *P < 0.05. (C and D) Immunohistochemistry staining of PGR (C) and ESR1 (D) in the uterus of Mettl3f/f mice and Mettl3d/d mice on GD4. (Scale bar,100 μm.)
P4 inhibits macrophage infiltration into the uterus, an effect that is compromised in Pgr knockout mice (31, 32). Gene ontology (GO) analysis of our RNA-seq data indicated that upregulated genes were significantly associated with inflammatory response (SI Appendix, Fig. S11A). Immunohistochemistry results showed that there were substantially more F4/80+ macrophages in Mettl3d/d uteri than in Mettl3f/f uteri on GD4 (SI Appendix, Fig. S11B). Notably, the no. of CD86+ inflammation-associated cells was specifically increased in regions proximal to the luminal epithelium, whereas that of CD206+ anti-inflammation-associated cells was specifically increased in regions distal to the luminal epithelium (SI Appendix, Fig. S11 C and D). It has been reported that PGR and forkhead box O1 (FOXO1) are reciprocally expressed in the mouse uterus during early pregnancy (33). Here, we observed that the expression of FOXO1 was upregulated in the Mettl3d/d uterus compared with that in the Mettl3f/f uterus on GD4 (SI Appendix, Fig. S12 A–C). Finally, by comparing our data with a published microarray dataset (34), we identified 18 commonly downregulated genes (including Areg) and 24 commonly upregulated genes (including C3 and Ltf) between the Mettl3d/d uterus and the Pgr knockout uterus (SI Appendix, Fig. S13 A and B). These data further validated the Pgr knockout-like phenotype in the Mettl3d/d uterus.
Next, we examined the relationship between Mettl3 deletion and PGR protein expression in the oviduct. In the ampulla region of the oviduct, where Mettl3 was intact, the PGR protein level was unchanged, whereas, in the isthmus region of oviduct, where Mettl3 was efficiently deleted, PGR protein expression was reduced (SI Appendix, Fig. S8 A and B). To mimic the effect of reduced PGR protein expression in the isthmus region of the oviduct, we subcutaneously injected RU486, a PGR antagonist, into pregnant wild-type mice on GD2 and GD3 [1 mg/(mouse∙day−1)]. All the embryos were lost in these RU486-treated mice on GD4 (n = 3), similar to that previously reported (35–37). RNA-seq analysis revealed that 605 genes were downregulated and 649 upregulated in the Mettl3d/d oviduct relative to the Mettl3f/f oviduct on GD3 (SI Appendix, Fig. S14A and Table S2). GO analysis showed that upregulated genes were significantly enriched in hydrolase activity and peptidase activity within the molecular function category (SI Appendix, Fig. S14B). Of the 76 genes with hydrolase activity or peptidase activity (SI Appendix, Fig. S14C), we validated 11 genes by using qRT-PCR, including cathepsins Ctsb/c/h/k/s/w and matrix metallopeptidases Mmp2/3/7/11/14 (SI Appendix, Fig. S14D). These results indicated that pre-implantation embryo loss in Mettl3d/d mice was likely caused by the increased expression of genes with hydrolase activity and peptidase activity in the isthmus region of oviduct resulting from decreased expression of PGR protein following Mettl3 deletion.
Pgr mRNA Is a Direct Target of METTL3-Mediated m6A Modification.
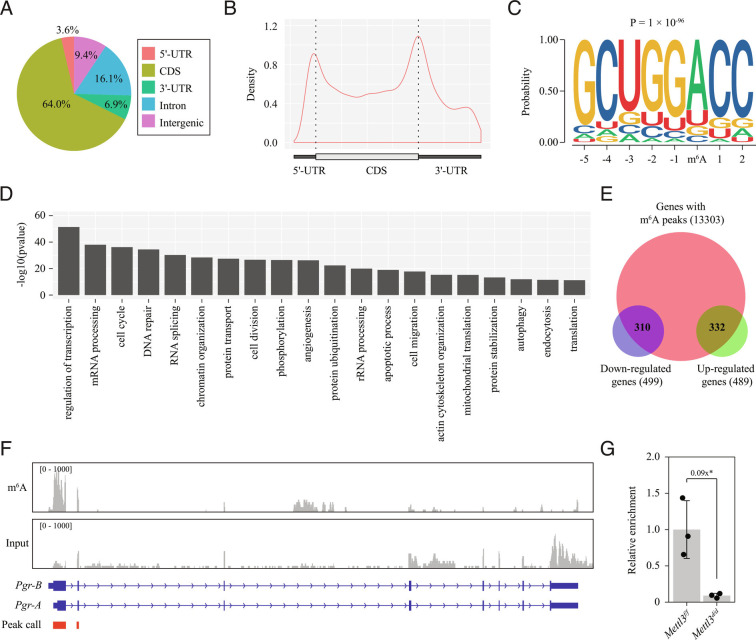
As METTL3 is a writer of m6A modification, we next investigated global m6A modification on mRNAs in the uteri of wild-type mouse (pooled uterine tissues from GD4 and GD8 with equal weight) using methylated RNA immunoprecipitation sequencing (MeRIP-seq). We identified 31,219 unique m6A peaks linked to 12,173 genes (q-value < 0.05; SI Appendix, Table S3). Most of these m6A peaks resided in the CDS (60.4%), followed by the intronic region (16.1%), the intergenic region (9.4%), the 3′-UTR (6.9%), and, finally, the 5′-UTR (3.6%) (Fig. 5A). Metagene analysis revealed a strong enrichment of m6A peaks around the start and stop codons based on peak density (Fig. 5B), which is consistent with a previous report (38). An unbiased motif search showed that the conserved m6A consensus motif RRACH (R = A/G, H = A/C/U) was significantly overrepresented in m6A peaks, indicating that our MeRIP-seq data were of high quality (Fig. 5C). GO analysis showed that regulation of transcription was the most enriched term among genes with m6A modification (Fig. 5D). By examining the RNA-seq data, we found that 310/499 (62.1%) downregulated genes and 332/489 (67.9%) upregulated genes were marked by m6A modification (Fig. 5E).
Fig. 5.
Pgr mRNA is a direct target of Mettl3-mediated m6A modification. (A–C) Global profiling of m6A modification in the wild-type uterus by methylated RNA immunoprecipitation sequencing (MeRIP-seq). (A) Pie chart presenting fractions of m6A peaks in different genomic segments. (B) The metagene distribution of m6A peaks in the gene body. (C) Sequence logo of the consensus motif. (D) Bar plot showing the top 20 enriched GO terms ranked by P-value. (E) Venn diagram showing the m6A peaks in differentially expressed genes on gestational day 4 (GD4). (F) Integrative genomics viewer displaying the coverage of m6A immunoprecipitation and input in Pgr mRNA based on MeRIP-seq data. Peak calling was performed by using the MACS3 software. Only peaks within exons are shown. (G) Quantitative MeRIP-PCR analysis of the Pgr-A 5′-UTR from the Mettl3f/f uterus and the Mettl3d/d uterus on GD4. Data are presented as means ± SD. *P < 0.05.
By MeRIP-seq data mining, we identified an m6A peak spanning the entire 5′-UTR and partial CDS (within exons 1 and 2) of the Pgr isoform A (Pgr-A) mRNA, which is the dominant Pgr mRNA isoform expressed in the mouse uterus (39, 40) (Fig. 5F). Additionally, we analyzed two previously reported MeRIP-seq datasets relating to the adult mouse brain (41, 42), and identified two m6A peaks, one covering the entire 5′-UTR of Pgr-A mRNA and the other the entire 5′-UTR of Pgr-B mRNA (SI Appendix, Fig. S15 A–D). This suggested that the m6A modification in the 5′-UTR of Pgr-A mRNA is conserved in both the uterus and the brain. Quantitative MeRIP-PCR analysis showed significantly decreased enrichment of the Pgr-A 5′-UTR in the uteri of Mettl3d/d mice relative to Mettl3f/f mice on GD4 (Fig. 5G), indicating that Pgr mRNA is a direct target of METTL3-mediated m6A modification in the mouse uterus.
The m6A modification has been reported to regulate many aspects of mRNA biology; however, its major function is to promote mRNA degradation in the cytoplasm (43). Indeed, integrative analysis of RNA-seq data and MeRIP-seq data revealed that m6A modification was slightly but significantly more prevalent among upregulated genes than downregulated genes following Mettl3 deletion (SI Appendix, Fig. S16 A and B). In this study, we observed that the protein level rather than the mRNA level of Pgr was changed when m6A was abolished by Mettl3 deletion, which was not consistent with the m6A-mediated mRNA degradation mechanism. Several studies have reported that translation is enhanced in genes with m6A modification in their 5′-UTR (44–46). Thus, we reasoned that m6A modification in the 5′-UTR of Pgr mRNA might be the key to explaining the decreased expression of PGR protein following Mettl3 deletion.
M6A Modification in the 5′-UTR of Pgr mRNA Enhanced PGR Protein Translation in a YTHDF1-Dependent Manner.
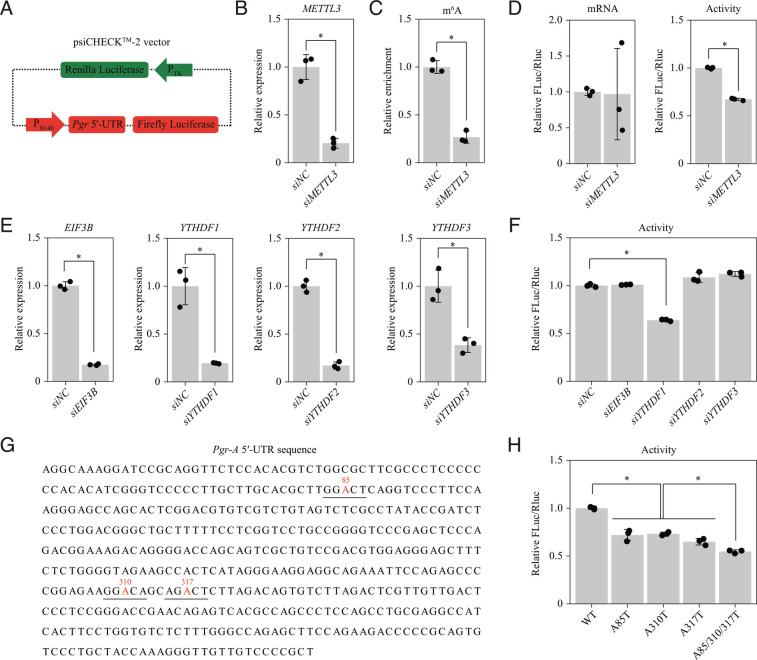
To dissect the role of the Pgr 5′-UTR in translation, we generated a luciferase reporter construct in which the complete Pgr-A 5′-UTR sequence was inserted into the 5′-UTR region of firefly luciferase driven by the SV40 promoter. The Renilla luciferase under the control of the TK promoter in the same plasmid served as a control to normalize transfection efficiency (Fig. 6A). This luciferase reporter plasmid was co-transfected with METTL3 small interfering (siRNA) or negative control siRNA into HEK293T cells. Following METTL3 knockdown (Fig. 6B), the m6A modification level in Pgr-A 5′-UTR within the luciferase reporter was significantly decreased (Fig. 6C). A luciferase assay revealed that m6A in Pgr-A 5′-UTR enhanced protein translation efficiency, but had no effect on mRNA stability (Fig. 6D). Studies showed that EIF3 (45), YTHDF1 (47, 48), YTHDF2 (46) and YTHDF3 (49, 50) can serve as m6A readers that enhance the translation efficiency of m6A-modified mRNAs. Using siRNA screening (Fig. 6E), we found that only YTHDF1 was involved in controlling the translation of luciferase reporter (Fig. 6F). These results indicated that m6A modification in the 5′-UTR of Pgr-A mRNA enhances PGR protein translation efficiency in a YTHDF1-dependent manner.
Fig. 6.
M6A modification in the 5′-UTR of Pgr mRNA enhances PGR protein translation efficiency in a YTHDF1-dependent manner. (A–D) Dissecting the role of the Pgr-A 5′-UTR in translation by dual-luciferase reporter assay. (A) Diagram of the dual-luciferase plasmid carrying the Pgr-A 5′-UTR sequence upstream of the firefly luciferase gene. The Renilla luciferase served as a control for normalization. (B) Quantitative RT-PCR for evaluating the knockdown effectiveness of siRNA targeting METTL3 in HEK293T cells. siNC, negative control. (C) Quantitative MeRIP-PCR analysis of Pgr-A 5′-UTR. (D) The effect of METTL3 knockdown on translation efficiency of the recombined dual-luciferase plasmid. (E and F) SiRNA screening to identify m6A reader proteins for the Pgr-A 5′-UTR. (E) Quantitative RT-PCR for evaluating the knockdown effectiveness of siRNAs targeting m6A reader proteins EIF3B and YTHDF1/2/3. (F) The effect of knockdown of m6A reader proteins on translation efficiency of the recombined dual-luciferase plasmid. (G and H) Point mutation analysis of m6A sites in Pgr-A 5′-UTR. (G) The location of m6A sites in the 5′-UTR of Pgr-A mRNA predicted by the SRAMP tool. Potential m6A sites are colored in red and the corresponding RRACH motifs are underlined. A-to-T mutations were introduced to abrogate each m6A site. (H) The effect of point mutations on translation efficiency of the recombined dual-luciferase plasmid. Data are presented as means ± SD. *P < 0.05.
We were particularly interested in the precise location of m6A-modified sites within the Pgr-A 5′-UTR. Three predicted sites (A85, A310, and A317) were retrieved via the SRAMP tool (51) (Fig. 6G). For each predicted site, we abolished m6A modification by generating A-to-T point mutation in the luciferase reporter plasmid containing wild-type Pgr-A 5′-UTR. A luciferase assay revealed that A85T, A310T, and A317T mutations significantly reduced translation efficiency compared with wild-type Pgr-A 5′-UTR. Moreover, the triple mutation (A85T/A310T/A317T) had a more potent effect than each mutation alone, indicating that multiple m6A modification sites might function synergistically (Fig. 6H).
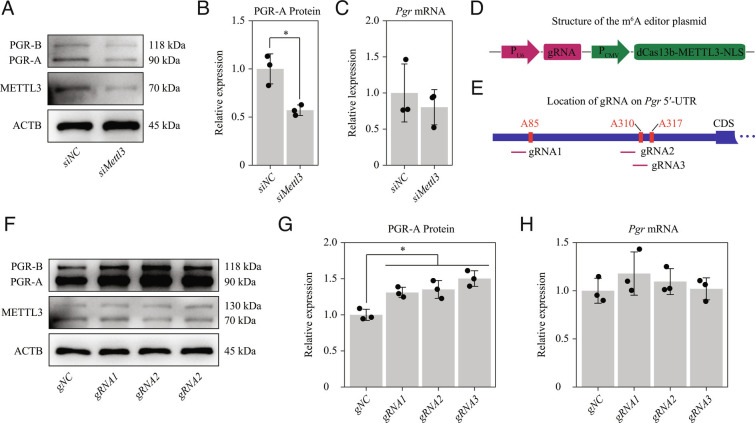
To further confirm the above findings from the luciferase reporter experiments, we examined the effect of m6A modification on the 5′-UTR of Pgr mRNA directly in vitro using primary endometrial stromal cells (ESCs) isolated from wild-type mice on GD4. The expression of PGR-A protein, but not that of total Pgr mRNA, was significantly inhibited when Mettl3 was knocked down by siRNA in primary mouse uterine stromal cells (Fig. 7 A–C), which was consistent with the in vivo gene deletion results. We then performed targeted m6A modification on the 5′-UTR of Pgr-A mRNA using the dCas13b-METTL3-NLS m6A editor (52) (Fig. 7D). Three guide RNAs (gRNAs) targeting distinct positions around the m6A sites were designed (Fig. 7E). We found that targeted m6A modification by all three sgRNAs could significantly increase PGR-A protein expression (Fig. 7 F–H). Notably, PGR-B protein expression showed a similar trend (Fig. 7A and Fig. 7F), implying that PGR-B might also be regulated by m6A modification. As PGR-B is dispensable for embryo implantation (39), the regulation of PGR-B by m6A was not further investigated in this study.
Fig. 7.
Targeted m6A modification in the Pgr 5′-UTR by dCas13b-METTL3-NLS increases the PGR protein expression in mouse uterine stromal cells in vitro. (A–C) Knockdown of Mettl3 in mouse uterine stromal cells. (A) Western blot analysis of METTL3 and PGR following transfection with negative control or Mettl3 siRNA. (B) The relative levels of PGR-A protein according to western blot band intensity. (C) Quantitative RT-PCR analysis of total Pgr mRNA levels. (D–H) Targeted m6A modification of Pgr-A 5′-UTR by dCas13b-METTL3-NLS in mouse uterine stromal cells. (D) Construction of a single plasmid containing the dCas13b-METTL3-NLS m6A editor and the gRNA. (E) Schematic representation of the positions of 3 gRNAs. (F) Western blot analysis of METTL3 expression in mouse uterine stromal cells transfected with negative control or gRNA1/2/3, respectively, for 48 h. (G) The relative expression levels of PGR-A protein according to western blot band intensity. (H) Quantitative RT-PCR analysis of total Pgr mRNA levels. Data are presented as means ± SD. *P < 0.05.
METTL3 Is Required for Human Endometrial Stromal Cell (HESC) Decidualization In Vitro.
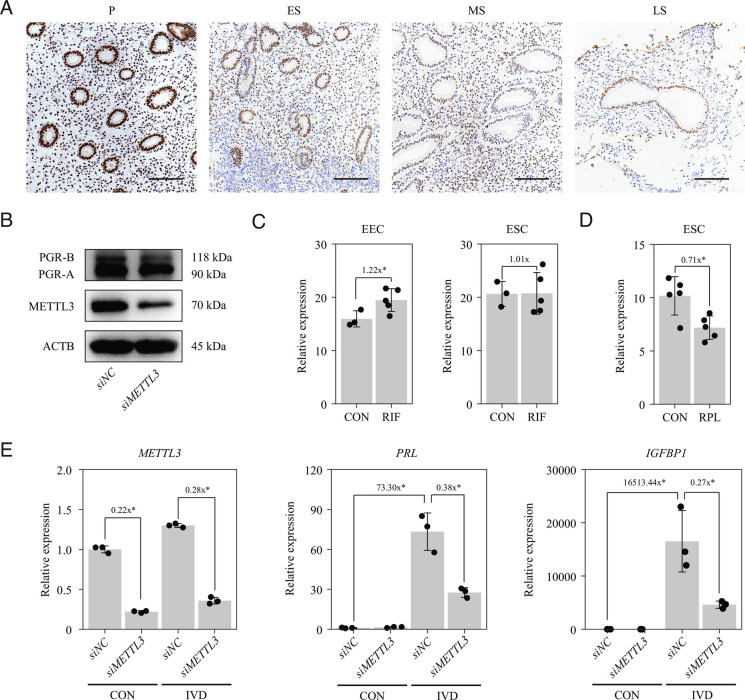
To investigate the role of METTL3 in human endometrium, we analyzed the expression of METTL3 in endometrial biopsy samples obtained from normal fertile women with a regular menstrual cycle. Endometrial samples were categorized as being in the proliferative, early-secretory, mid-secretory, or late-secretory phase. METTL3 protein expression was high in both epithelial cells and stromal cells in the proliferative phase, declined progressively during the secretory phase (Fig. 8A). The same expression trend was observed for PGR protein in a previous study (33). In primary HESCs, expression of PGR-A protein was inhibited when METTL3 was knocked down by siRNA (Fig. 8B), which was consistent with the findings from primary mouse endometrial cells (Fig. 7 A–C). These results indicated that the METTL3-PGR axis is likely conserved between mice and humans.
Fig. 8.
METTL3 is required for HESC decidualization in vitro. (A) Immunohistochemical analysis of endometrial METTL3 protein expression during the menstrual cycle. P, proliferative phase; ES, early secretory phase; MS, middle secretory phase; LS, late secretory phase. (Scale bar, 100 μm.) (B) Western blot analysis of METTL3 and PGR expression in primary HESCs following transfection with negative control or METTL3 siRNA for 48 h. (C) Expression of METTL3 in endometrial epithelial cells (EECs) and ESCs from control patients (CON) and patients with recurrent implantation failure (RIF). Data are presented as means ± SD. *P < 0.05. (D) Expression of METTL3 in ESCs from CON and patients with recurrent pregnancy loss (RPL). Data are presented as means ± SD. *P < 0.05. (E) Expression PRL and IGFBP1 in primary HESCs after METTL3 knockdown in the in vitro decidualization (IVD) model. Data are presented as means ± SD. *P < 0.05.
Single-cell RNA-seq has been used to analyze the endometrium of patients with recurrent implantation failure (RIF) in the mid-secretory phase (53) and the fetal–maternal interface of patients with recurrent pregnancy loss (RPL) during the first trimester (54). We generated pseudo-bulk RNA-seq data for epithelial cells and stromal cells by averaging single-cell RNA data, as described in our previous study (55). We found that METLL3 was unchanged in ESCs, but was significantly upregulated in endometrial epithelial cells (EECs) from patients with RIF compared with that from healthy controls (Fig. 8C). In contrast, METLL3 expression was significantly downregulated in ESCs from patients with RPL relative to that from healthy controls (Fig. 8D), suggesting that METTL3 is involved in decidualization in humans. Using the in vitro decidualization model with primary HESCs, we found that the expression of decidualization marker genes prolactin (PRL) and insulin-like growth factor binding protein 1 (IGFBP1) was significantly decreased with METTL3 knockdown after decidualization for 4 d (Fig. 8E). These results indicated that METTL3 is required for HESC decidualization in vitro.
Discussion
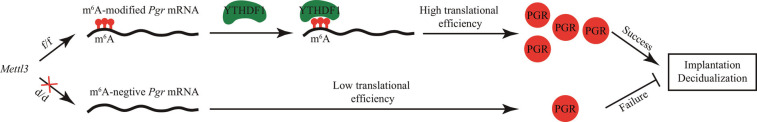
Studies have shown that mRNA m6A modification plays an important role in various biological processes (11). Here, we showed that the Pgr-Cre mediated deletion of the m6A writer Mettl3 in the female reproductive tract results in complete infertility. We further showed that the infertility phenotypes are mainly due to the decreased expression of PGR at the protein level. Mechanistically, we found that Pgr mRNA is a direct target for METTL3-mediated m6A modification and that m6A modification in the 5′-UTR of Pgr mRNA enhances PGR protein translation efficiency in a YTHDF1-dependent manner (Fig. 9). This study uncovers the physiological function of m6A modification in the female reproductive tract during pregnancy.
Fig. 9.
Working model. In Mettl3f/f mice, Pgr mRNA with m6A modification in the 5′-UTR is recognized by YTHDF1, which promotes PGR protein translation. However, in Mettl3d/d mice, m6A modification in Pgr mRNA is lost, and PGR protein cannot be efficiently translated; the low level of PGR protein eventually leads to the failure in implantation and decidualization.
The major phenotypes of Mett3d/d mice were pre-implantation embryo loss, defective uterine receptivity and compromised decidualization. Pre-implantation embryo loss was caused by the deletion of Mett3d/d in the isthmus region of the oviduct. We discovered that PGR protein was particularly decreased in the isthmus following Mettl3 deletion. It is well established that ESR1 in the oviduct is crucial for embryo survival and transport (16, 56, 57); however, the role of PGR in the oviduct is undefined (58). Indirect evidence that PGR might be required for pre-implantation embryo survival comes from liver receptor homolog 1 conditional knockout mice, which exhibit reduced P4 synthesis in the ovary. In these mice, although only a few embryos reached the blastocyst stage, most could form blastocysts if P4 was supplied (59, 60). In this study, we found that the pre-implantation embryo loss phenotype in Mettl3d/d mice could be fully recapitulated by the injection of RU486 into wild-type mice on GD2 and GD3. RNA-seq analysis revealed that several genes with hydrolase activity or peptidase activity were significantly upregulated in the oviduct of Mettl3d/d mice. Our results indicated that pre-implantation embryo loss in Mettl3d/d mice was likely caused by the increased expression of genes with hydrolase activity and peptidase activity in the isthmus region of oviduct resulting from decreased expression of PGR protein following Mettl3 deletion. Using mouse models of ET and artificial decidualization, we found that the uterine phenotypes in Mettl3d/d mice are defective uterine receptivity and compromised decidualization. Like in the isthmus region of the oviduct, the PGR protein level was markedly decreased in the uteri of Mettl3d/d mice during the window of implantation. Meanwhile, the P4 target genes, Ihh, Areg, Hoxa10, and Hand2, were significantly downregulated. Notably, although the level of ESR1 protein was unchanged, E2 target genes C3, Ltf, Muc1, and Muc4 were significantly upregulated. Given that P4 counteracts E2 in the uterus (61), the observed increase in E2 signaling could be explained by the decrease in PGR protein levels and, consequently, P4 signaling. In agreement with the reduced PGR protein levels, the uteri of Mettl3d/d mice exhibited persistent proliferation in a small population of epithelial cells on GD4, whereas stromal cell proliferation was almost absent. Reduced PGR protein expression and P4 signaling in the uterus of Mettl3d/d mice were further validated by the observed increase in macrophage infiltration and FOXO1 expression. Combined, our findings suggested that the major phenotypes of Mett3d/d mice result from a decrease in PGR protein levels. Given the essential role of PGR in the establishment and maintenance of pregnancy, our study places METTL3 and its associated m6A modification at the center of the master gene network that governs normal embryo implantation.
In this study, we also identified the mechanism underlying the reduction in PGR protein expression in Mettl3d/d mice. We found that METTL3-mediated m6A modification in the 5′-UTR mRNA is essential for efficient PGR protein translation. Removal of this modification by Mettl3 deletion results in decreased PGR protein expression, leading to a failure in implantation and decidualization. The biological function of m6A modification in mRNAs varies widely. Four distinct mechanisms have been proposed. First, m6A promotes mRNA decay through the reader protein YTHDF2, which is the best-established function for m6A (43); second, m6A regulates mRNA stability through the reader protein IGF2BP1/2/3 (11); third, m6A affects mRNA splicing and export by binding to the reader protein YTHDC1 in the nucleus (11); fourth, m6A, preferably in the 5′-UTR, enhances mRNA translation efficiency by binding to EIF3 (45), YTHDF1 (47, 48) or YTHDF3 (49, 50). Our results support the fourth mechanism, as we found that m6A modification in the 5′-UTR of Pgr mRNA enhanced translation efficiency in a YTHDF1-dependent manner. Studies have shown that the activity of PGR can be regulated at many layers, including DNA methylation (28), transcription factor (62, 63), microRNA (64), and protein modification (29, 65). By identifying m6A as a powerful regulator of Pgr translation, our study uncovered a epigenetic mechanism ensuring normal PGR activity during embryo implantation at the mRNA modification layer.
In addition, we found that METTL3 is required for HESC decidualization in vitro and that the METTL3-PGR axis is conserved between mice and humans. Aberrant P4 signaling is often associated with uterine pathophysiology in humans. It is well known that uterine P4 resistance resulting from decreased PGR protein expression is responsible for the development of endometriosis (66), adenomyosis (67), and endometrial cancer (68). In endometriosis, METTL3 expression and m6A levels are significantly lower in both eutopic and ectopic endometrium than in normal control endometrium (23, 24). In patients with adenomyosis, METTL3 expression and m6A levels are significantly decreased in the endometrium compared with that in healthy control (27). In endometrial cancer, m6A levels are significantly reduced due to either METTL14 mutation or decreased expression of METTL3 (25, 26). These observations suggested that the METTL3-PGR axis might contribute to the development of these diseases. Through mining public single-cell RNA-seq data, we found that METLL3 expression was significantly upregulated in EECs from patients with RIF relative to that from healthy controls, which is consistent with a previous study (69). Interestingly, it was reported that increased METTL3 expression and m6A levels lead to a reduction in HOXA10 expression in the mid-secretory endometrium of women with RIF (69). In our study, we found that the loss of m6A following Mettl3 deletion resulted in decreased expression of Hoxa10 in the mouse uterus. The reason for this discrepancy is unknown. In addition, through data mining, we found that METLL3 expression was significantly lower in ESCs from patients with RPL than in those from healthy controls. The role of the METTL3-PGR axis in uterine pathophysiology deserves further investigation.
In summary, we provide evidence that METTL3 is essential for normal P4 signaling during embryo implantation via m6A-mediated translation control of Pgr mRNA. Besides uncovering an epigenetic mechanism ensuring normal P4 signaling during embryo implantation at the mRNA modification layer, our findings are of high clinical relevance, as aberrant P4 signaling is often associated with uterine pathophysiology.
Materials and Methods
Mice.
Mettl3f/f mice (Cat. No. NM-CKO-190006) and PgrCre/+ mice (Cat. No. NM-KI-200117) were purchased from Shanghai Model Organisms Center, Inc. Mettl3d/d mice were generated by crossing Mettl3f/f mice with PgrCre/+ mice. The Mettl3f/f littermates were used as control. All mice were bred under the specific pathogen-free condition with free access to diet and water in a 12-h day and 12-h night cycle. All the animal procedures were approved by the Institutional Animal Care and Use Committee of South China Agricultural University (No. 2021B036, approved on 14/03/2021).
ET.
The two-cell embryos were flushed from the oviduct of donor mice on GD2 and cultured to the fully expanded blastocyst stage in KSOM medium (Millipore). Recipient mice were prepared as described previously (70). Briefly, adult female mice were ovariectomized and rested for 2 wk to clear endogenous ovarian hormones. Hormone supplementation started with subcutaneous injection of 100 ng E2 (Sigma) on day 1 and then 2 mg P4 (Sigma) on day 3. Six blastocysts were transferred into the uterine lumen of one horn on day 4, followed by injection of 25 ng E2 and 2 mg P4 to induce embryo implantation. P4 was supplemented for each day post-ET. Uterine samples were collected on day 14.
Artificial Decidualization.
Adult female mice were ovariectomized. After a 2-wk rest, mice were subcutaneously injected with 100 ng E2 (Sigma) on day 1 then 2 mg P4 (Sigma) on day 3. On day 4, after the injection of 2 mg P4 together with 25 ng E2, 10 μL of sesame oil (Sigma) was injected into the lumen of one uterine horn to induce decidualization. The contralateral horn serving as control. P4 was supplemented for each day. Uterine samples were collected on day 8.
Quantitative RT-PCR.
The TRIzol reagent (Invitrogen) was used to extract total RNA. Genomic DNA was eliminated by DNase I (Invitrogen) treatment. The PrimeScript reverse transcriptase reagent kit (TaKaRa) was used for cDNA synthesis. Quantitative PCR was performed on Applied Biosystems 7500 (Life Technologies) using the THUNDERBIRD SYBR qPCR Mix (Toyobo). Rpl7 gene served as reference for normalization. All primer sequences are listed in SI Appendix, Table S4.
Western Blotting.
Tissues were homogenized in lysis buffer. The concentration of protein was measured with the BCA reagent kit (Applygen). Protein samples were separated by electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% skim milk in Tris-buffered saline with Tween-20 for 1 h at room temperature and then incubated overnight at 4 °C with primary antibody. After three washes in 5% milk in TBS-T, membranes were incubated with horseradish peroxidase (HRP)-conjuncted secondary antibody for 1 h at room temperature. The signal was developed with the enhance chemiluminescence kit (Amersham Biosciences). Band intensities were analyzed with the ImageJ software. Uncropped and unprocessed scans of the blots are presented in SI Appendix, Fig. S17. Primary antibodies with detailed information are listed in SI Appendix, Table S5.
Immunohistochemistry.
Paraformaldehyde-fixed paraffin-embedded tissues were cut into 5-μm sections. Antigen retrieval was performed with citrate buffer pH 6.0. Sections were blocked with 10% horse serum in PBS and then incubated with primary antibody overnight at 4 °C. After washed by PBS for three times, sections were incubated with HRP-conjuncted secondary antibody for 1 h at room temperature. The signal was developed by using the diaminobenzidine kit (Zhongshan Golden Bridge Biotechnology Co.). Sections were counterstained with hematoxylin. Primary antibodies are listed in SI Appendix, Table S5.
Alkaline Phosphatase Activity Histochemistry.
To detect alkaline phosphatase activity, frozen tissue sections of 10 μm were used. The slides were fixed in cold PFA for 15 min and washed three times with phosphate-buffered saline (PBS). The BCIP/NBT kit (Zhongshan Golden Bridge Biotechnology Co.) was used for staining. Sections were counterstained with 1% methyl green.
RNA-seq.
TRIzol reagent (Invitrogen) was used to extract total RNA. The quality of total RNA was measured by using ND-1000 Nanodrop and Agilent 2200 TapeStation. RNA-seq libraries were generated with TruSeq RNA sample preparation kit (Illumina). High-throughput sequencing was conducted on the HiSeq 2500 system (Illumina). After quality control, clean reads were mapped to mouse genome (UCSC mm10) by using Hisat2 v2.2.1 (71). Mapped reads were assembled by using Cufflinks v2.2.1 (72). Differentially expressed genes were selected based on the criteria of fold change > 2 and P-value < 0.05.
GO Analysis.
GO analysis was performed by using the Database for Annotation, Visualization and Integrated Discovery (DAVID) online tools (73). Genes were classified according to the biological process category or the molecular function category. Redundant GO terms were removed manually. The cutoff for P-value was set at 0.05.
MeRIP-seq.
MeRIP-Seq was performed according to the published procedure (42). Briefly, fragmented mRNA was incubated with anti-m6A polyclonal antibody (Synaptic Systems, 202003) in immunoprecipitation buffer for 2 h at 4 °C and then immunoprecipitated by incubation with protein-A beads (Thermo Fisher) for another 2 h at 4 °C. Bound RNA was eluted from the beads with m6A (BERRY & ASSOCIATES) and extracted with the TRIzol reagent (Invitrogen). Fragmented mRNA without immunoprecipitation was used as input control. RNA-seq libraries were generated with the Next® Ultra™ II Directional RNA Library Prep Kit (New England Biolabs). Sequencing data were obtained from the HiSeq 2500 system (Illumina). After quality control, clean data were aligned to the reference genome (UCSC mm10) with Hisat2 software v2.2.1 (71). The read alignment on the genome was visualized by using the IGV tool v2.14.0 (74). MACS software v3.0.0a7 (75) was used for m6A peak calling with the significance cutoff q-value < 0.05. Peaks were annotated as located in 5′-UTR, CDS, 3′-UTR, intronic region and intergenic region. The metagene profile was drawn by the R package Guitar v2.12.0 (76). Motifs in m6A peaks were identified using HOMER v4.7 (77).
MeRIP-PCR.
Immunoprecipitated RNA and input RNA were prepared in the same way as described in MeRIP-Seq. RNAs were reversely transcribed with random hexamers. Quantitative PCR was performed using THUNDERBIRD SYBR qPCR Mix (Toyobo) on the Applied Biosystems 7500 (Life Technologies). The m6A enrichment in each sample was calculated by normalizing to the input. The primer sequences are listed in SI Appendix, Table S4.
Luciferase Reporter Assay.
The complete sequence of mouse Pgr-A 5′-UTR was synthesized and cloned into the psiCHECKTM-2 vector (Promega) by using the NheI site. Site-directed mutagenesis was used to generate three single-point mutants A85T, A310T and A317T, and a triple-point mutant A85T/A310T/A317T. Reconstructed plasmid and siRNA were co-transfected into HEK293T cells by using Lipofectamine 3000 (Invitrogen). Cell lysates were collected 48 h after transfection. Luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity. The siRNAs with detailed information are listed in SI Appendix, Table S6.
Targeted m6A Modification.
PspCas13b-compatible gRNAs targeting mouse Pgr-A 5′-UTR were designed as described previously (52). A scramble gRNA was used as negative control. For each gRNA, the U6-gRNA cassette based on the pU6-PspCas13b-gRNA-Actb1216 plasmid (#155368, Addgene) was synthesized and cloned into the pCMV-dCas13-M3nls plasmid (#155366, Addgene) using the NruI site. Targeted m6A modification experiment was performed in primary uterine stromal cells which were isolated as described previously (78, 79). Lipofectamine 3000 (Invitrogen) was used for plasmid transfection. Cells were harvested 48 h after transfection. The gRNA sequences are shown in SI Appendix, Table S7.
Human Endometrial Sample Collection.
A cohort of patients was recruited for endometrial biopsy to assess endometritis before ET in the First Affiliated Hospital of Sun Yat-sen University in China from July 2020 to October 2021. This study had been approved by the Ethical Committee of the First Affiliated Hospital of Sun Yat-sen University (No. 2018-266) and all participants signed an informed consent. Normal fertile participants who had no apparent endometrial pathology and had a confirmed clinical pregnancy after ET were selected for this study. The age of participants was between 25 and 38 y with a body mass index between 17.6 and 26.1. The menstrual cycle was 28 ± 7 d. Detailed information of patients is listed in SI Appendix, Table S8.
Isolation and Culturing of Primary HESCs.
Three normal fertile participants who had no apparent endometrial pathology and had a confirmed clinical pregnancy after ET were selected from the same cohort as described above. This study had been approved by the Ethical Committee of the First Affiliated Hospital of Sun Yat-sen University (No. 2018-266) and all participants signed an informed consent. Detailed information of patients is listed in SI Appendix, Table S9. The endometrial tissues were first cut into pieces as small as possible and subjected to type I collagenase (Gibco) digestion for 1 h. The EECs and ESCs were separated using membrane filters (100 µm cell filters and 40-µm cell filters, Corning). HESCs were cultured in DMEM/F12 (Gibco) containing 10% charcoal-stripped fetal bovine serum (cFBS, VivaCell). To induce decidualization, cells were treated with 0.5 mM 8-Br-cAMP (Sigma) and 1 μM medroxyprogesterone acetate in %2 cFBS for 4 d.
Statistical Analysis.
Statistical analysis was conducted with R v4.1.3 (https://www.r-project.org/). For data with two groups, Student's t test was employed. For data containing more than two groups, one-way ANOVA with Tukey's multiple comparison test was used. Data are presented as the mean ± SD of at least three independent samples. P < 0.05 was considered statistically significant.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S3 (XLSX)
Dataset S04 (XLSX)
Dataset S05 (XLSX)
Dataset S06 (XLSX)
Dataset S07 (XLSX)
Dataset S08 (XLSX)
Dataset S09 (XLSX)
Acknowledgments
This research was funded by National Natural Science Foundation of China (32070845 and 31771665), Guangdong Natural Science Funds for Distinguished Young Scholars (2021B1515020079), Innovation Team Project of Guangdong University (2019KCXTD001), Guangdong Special Support Program (2019BT02Y276) and National Key R&D Program of China (2018YFA0801404).
Author contributions
Y.-W.X., S.-H.Y., and J.-L.L. designed research; Z.-H.Z., G.-L.Z., R.-F.J., Y.-Q.H., Q.-Y.Z., and X.-R.L. performed research; Z.-H.Z., G.-L.Z., R.-F.J., Y.-Q.H., Q.-Y.Z., J.-P.H., X.-R.L., Z.-S.Y., L.Y., X.J., and L.-J.Q. contributed new reagents/analytic tools; Z.-H.Z., Q.-Y.Z., J.-P.H., Z.-S.Y., L.Y., X.J., L.-J.Q., C.-H.D., Y.-W.X., S.-H.Y., and J.-L.L. analyzed data; and Z.-H.Z., Y.-W.X., S.-H.Y., and J.-L.L. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Yan-Wen Xu, Email: xuyanwen@mail.sysu.edu.cn.
Shi-Hua Yang, Email: yangsh@scau.edu.cn.
Ji-Long Liu, Email: jilongliu@scau.edu.cn.
Data, Materials, and Software Availability
The sequencing data generated in this study are deposited in the Gene Expression Omnibus (GEO) under accession codes GSE211521 (80), GSE211614 (81) and GSE217174 (82).
Supporting Information
References
- 1.Norwitz E. R., Schust D. J., Fisher S. J., Implantation and the survival of early pregnancy. N. Engl. J. Med. 345, 1400–1408 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Wang H., Dey S. K., Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 7, 185–199 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Zhang S., et al. , Physiological and molecular determinants of embryo implantation. Mol. Aspects Med. 34, 939–980 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasquez Y. M., DeMayo F. J., Role of nuclear receptors in blastocyst implantation. Semin. Cell Dev. Biol. 24, 724–735 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha J., Sun X., Dey S. K., Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 18, 1754–1767 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu S. P., Li R., DeMayo F. J., Progesterone receptor regulation of uterine adaptation for pregnancy. Trends Endocrinol. Metab. 29, 481–491 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer K. D., Jaffrey S. R., The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 15, 313–326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oerum S., Meynier V., Catala M., Tisne C., A comprehensive review of m6A/m6Am RNA methyltransferase structures. Nucleic Acids Res. 49, 7239–7255 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia G., et al. , N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 7, 885–887 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng G., et al. , ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaccara S., Ries R. J., Jaffrey S. R., Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 20, 608–624 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Shi H., Wei J., He C., Where, when, and how: Context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell 74, 640–650 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang F., Wang X., Li Z., Ni K., Xiong C., Epigenetic regulation of mRNA N6-methyladenosine modifications in mammalian gametogenesis. Mol. Hum. Reprod. 27, gaab025 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Batista P. J., et al. , m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem. Cell 15, 707–719 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daikoku T., et al. , Conditional deletion of Tsc1 in the female reproductive tract impedes normal oviductal and uterine function by enhancing mTORC1 signaling in mice. Mol. Hum. Reprod. 19, 463–472 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrera G. G. B., et al. , Oviductal retention of embryos in female mice lacking estrogen receptor alpha in the isthmus and the uterus. Endocrinology 161, bqz033 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao S., et al. , Exploration of the potential roles of m6A regulators in the uterus in pregnancy and infertility. J. Reprod. Immunol. 146, 103341 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Soares M. J., Konno T., Alam S. M., The prolactin family: effectors of pregnancy-dependent adaptations. Trends Endocrinol. Metab. 18, 114–121 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Lee K. Y., et al. , Bmp2 is critical for the murine uterine decidual response. Mol. Cell Biol. 27, 5468–5478 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franco H. L., et al. , WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 25, 1176–1187 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das A., et al. , De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 106, 12542–12547 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukui Y., et al. , Uterine receptivity, embryo attachment, and embryo invasion: Multistep processes in embryo implantation. Reprod. Med. Biol. 18, 234–240 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L., et al. , Exploring diagnostic m6A regulators in endometriosis. Aging (Albany NY) 12, 25916–25938 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., et al. , Inhibition of METTL3/m6A/miR126 promotes the migration and invasion of endometrial stromal cells in endometriosisdagger. Biol. Reprod. 105, 1221–1233 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J., et al. , m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat. Cell Biol. 20, 1074–1083 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruan P., et al. , m(6)A mRNA methylation regulates the ERK/NF-kappaB/AKT signaling pathway through the PAPPA/IGFBP4 axis to promote proliferation and tumor formation in endometrial cancer. Cell Biol. Toxicol., 10.1007/s10565-022-09751-z (2022). [DOI] [PubMed] [Google Scholar]
- 27.Zhai J., et al. , m(6)A RNA methylation regulators contribute to eutopic endometrium and myometrium dysfunction in adenomyosis. Front. Genet. 11, 716 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su R. W., Strug M. R., Jeong J. W., Miele L., Fazleabas A. T., Aberrant activation of canonical Notch1 signaling in the mouse uterus decreases progesterone receptor by hypermethylation and leads to infertility. Proc. Natl. Acad. Sci. U.S.A. 113, 2300–2305 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xin Q., et al. , Polycomb subunit BMI1 determines uterine progesterone responsiveness essential for normal embryo implantation. J. Clin. Invest. 128, 175–189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schukken K. M., Sheltzer J. M., Extensive protein dosage compensation in aneuploid human cancers. Genome. Res. 32, 1254–1270 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aikawa S., et al. , Uterine deficiency of high-mobility group box-1 (HMGB1) protein causes implantation defects and adverse pregnancy outcomes. Cell Death. Differ. 27, 1489–1504 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tibbetts T. A., Conneely O. M., O’Malley B. W., Progesterone via its receptor antagonizes the pro-inflammatory activity of estrogen in the mouse uterus. Biol. Reprod. 60, 1158–1165 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Vasquez Y. M., et al. , FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLoS Genet. 14, e1007787 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong J. W., et al. , Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 146, 3490–3505 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Roblero L. S., Croxatto H. B., Effect of RU486 on development and implantation of rat embryos. Mol. Reprod. Dev. 29, 342–346 (1991). [DOI] [PubMed] [Google Scholar]
- 36.Batten B. E., Roh S. I., Kim M. H., The effects of the progesterone antagonist RU-486 on mouse preimplantation development in vitro and in vivo. Contraception 38, 365–371 (1988). [DOI] [PubMed] [Google Scholar]
- 37.Roblero L. S., Fernandez O., Croxatto H. B., The effect of RU486 on transport, development and implantation of mouse embryos. Contraception 36, 549–555 (1987). [DOI] [PubMed] [Google Scholar]
- 38.Mao Y., et al. , m(6)A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat. Commun. 10, 5332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conneely O. M., Mulac-Jericevic B., Lydon J. P., Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids 68, 771–778 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Shao R., et al. , Nuclear progesterone receptor A and B isoforms in mouse fallopian tube and uterus: Implications for expression, regulation, and cellular function. Am. J. Physiol. Endocrinol. Metab. 291, E59–72 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Schwartz S., et al. , Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep. 8, 284–296 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer K. D., et al. , Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 149, 1635–1646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murakami S., Jaffrey S. R., Hidden codes in mRNA: Control of gene expression by m(6)A. Mol. Cell 82, 2236–2251 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coots R. A., et al. , m(6)A facilitates eIF4F-independent mRNA translation. Mol. Cell 68, 504–514.e507 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer K. D., et al. , 5’ UTR m(6)A promotes cap-independent translation. Cell 163, 999–1010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J., et al. , Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z., et al. , N(6)-methyladenosine regulates glycolysis of cancer cells through PDK4. Nat. Commun. 11, 2578 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu K., et al. , N(6)-methyladenosine modification regulates imatinib resistance of gastrointestinal stromal tumor by enhancing the expression of multidrug transporter MRP1. Cancer Lett. 530, 85–99 (2022). [DOI] [PubMed] [Google Scholar]
- 49.Chang G., et al. , YTHDF3 induces the translation of m(6)A-enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell 38, 857–871.e857 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X., et al. , YTHDF3 modulates hematopoietic stem cells by recognizing RNA m6A modification on Ccnd1. Haematologica 107, 2381–2394 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y., Zeng P., Li Y. H., Zhang Z., Cui Q., SRAMP: Prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features. Nucleic Acids Res. 44, e91 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson C., Chen P. J., Miao Z., Liu D. R., Programmable m(6)A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat. Biotechnol. 38, 1431–1440 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai Z. Z., et al. , Single-cell transcriptome profiling of the human endometrium of patients with recurrent implantation failure. Theranostics 12, 6527–6547 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng Y., et al. , Characterization of placental and decidual cell development in early pregnancy loss by single-cell RNA sequencing. Cell Biosci. 12, 168 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y., Zhu Q. Y., Liu J. L., Deciphering mouse uterine receptivity for embryo implantation at single-cell resolution. Cell Prolif. 54, e13128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S., et al. , Estrogen receptor alpha is required for oviductal transport of embryos. FASEB J. 31, 1595–1607 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winuthayanon W., et al. , Oviductal estrogen receptor alpha signaling prevents protease-mediated embryo death. Elife 4, e10453 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lydon J. P., et al. , Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes. Dev. 9, 2266–2278 (1995). [DOI] [PubMed] [Google Scholar]
- 59.Zhang C., Murphy B. D., Progesterone is critical for the development of mouse embryos. Endocrine 46, 615–623 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Zhang C., et al. , Liver receptor homolog-1 is essential for pregnancy. Nat. Med. 19, 1061–1066 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Q., et al. , The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 331, 912–916 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rubel C. A., et al. , A Gata2-Dependent transcription network regulates uterine progesterone responsiveness and endometrial function. Cell Rep. 17, 1414–1425 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petz L. N., Nardulli A. M., Sp1 binding sites and an estrogen response element half-site are involved in regulation of the human progesterone receptor A promoter. Mol. Endocrinol. 14, 972–985 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Liu J. L., et al. , Combined analysis of microRNome and 3’-UTRome reveals a species-specific regulation of progesterone receptor expression in the endometrium of rhesus monkey. J. Biol. Chem. 287, 13899–13910 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang Y., et al. , P38alpha MAPK is a gatekeeper of uterine progesterone responsiveness at peri-implantation via Ube3c-mediated PGR degradation. Proc. Natl. Acad. Sci. U.S.A. 119, e2206000119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J. J., Kurita T., Bulun S. E., Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 34, 130–162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bulun S. E., Yildiz S., Adli M., Wei J. J., Adenomyosis pathogenesis: Insights from next-generation sequencing. Hum. Reprod. Update 27, 1086–1097 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel B., et al. , Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 21, 155–173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xue P., et al. , Increased METTL3-mediated m(6)A methylation inhibits embryo implantation by repressing HOXA10 expression in recurrent implantation failure. Reprod. Biol. Endocrinol. 19, 187 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kondoh E., et al. , Stress affects uterine receptivity through an ovarian-independent pathway. Hum. Reprod. 24, 945–953 (2009). [DOI] [PubMed] [Google Scholar]
- 71.Kim D., Paggi J. M., Park C., Bennett C., Salzberg S. L., Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trapnell C., et al. , Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang D. W., et al. , DAVID bioinformatics resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 35, W169–W175 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thorvaldsdottir H., Robinson J. T., Mesirov J. P., Integrative genomics viewer (IGV): High-performance genomics data visualization and exploration. Brief Bioinform. 14, 178–192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng J., Liu T., Qin B., Zhang Y., Liu X. S., Identifying ChIP-seq enrichment using MACS. Nat. Protoc 7, 1728–1740 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cui X., et al. , Guitar: An R/Bioconductor package for gene annotation guided transcriptomic analysis of RNA-related genomic features. Biomed Res. Int. 2016, 8367534 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heinz S., et al. , Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J. L., Wang T. S., Zhao M., Genome-wide association mapping for female infertility in inbred mice. G3 (Bethesda) 6, 2929–2935, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Q., et al. , Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J. Biol. Chem. 282, 31725–31732 (2007). [DOI] [PubMed] [Google Scholar]
- 80.He J. P., Liu J. L., Global m6A-modified mRNAs in the uterus of wild-type mouse. NCBI: GEO. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE211521. Deposited on 18 Aug 2022. [Google Scholar]
- 81.He J. P., Liu J. L., Gene expression changes in Mettl3-deleted mouse uterus on gestational day 4. NCBI: GEO. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE211614. Deposited on 19 Aug 2022. [Google Scholar]
- 82.He J. P., Liu J. L., Gene expression changes in Mettl3-deleted mouse oviduct on gestational day 3. NCBI: GEO. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE217174. Deposited on 3 Nov 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S3 (XLSX)
Dataset S04 (XLSX)
Dataset S05 (XLSX)
Dataset S06 (XLSX)
Dataset S07 (XLSX)
Dataset S08 (XLSX)
Dataset S09 (XLSX)
Data Availability Statement
The sequencing data generated in this study are deposited in the Gene Expression Omnibus (GEO) under accession codes GSE211521 (80), GSE211614 (81) and GSE217174 (82).