Abstract
BACKGROUND
Valvular heart disease (VHD) is a common cause of cardiovascular morbidity and mortality worldwide; however, its epidemiological profile in Korea requires elucidation.
METHODS
In this nationwide retrospective cohort study from the Korean valve survey, which collected clinical and echocardiographic data on VHD from 45 medical centers, we identified 4,089 patients with VHD between September and October 2019.
RESULTS
The aortic valve was the most commonly affected valve (n = 1,956 [47.8%]), followed by the mitral valve (n = 1,598 [39.1%]) and tricuspid valve (n = 1,172 [28.6%]). There were 1,188 cases of aortic stenosis (AS) and 926 cases of aortic regurgitation. The most common etiology of AS was degenerative disease (78.9%). The proportion of AS increased with age and accounted for the largest proportion of VHD in patients aged 80–89 years. There were 1,384 cases of mitral regurgitation (MR) and 244 cases of mitral stenosis (MS). The most common etiologies for primary and secondary MR were degenerative disease (44.3%) and non-ischemic heart disease (63.0%), respectively, whereas rheumatic disease (74.6%) was the predominant cause of MS. There were 1,172 tricuspid regurgitation (TR) cases, of which 46.9% were isolated and 53.1% were associated with other valvular diseases, most commonly with MR. The most common type of TR was secondary (90.2%), while primary accounted for 6.1%.
CONCLUSIONS
This report demonstrates the current epidemiological status of VHD in Korea. The results of this study can be used as fundamental data for developing Korean guidelines for VHD.
Keywords: Valvular heart diseases, Epidemiology, Korea
INTRODUCTION
Valvular heart disease (VHD) is a common cause of cardiovascular morbidity and mortality worldwide.1) The majority of morbidity and mortality attributable to VHD is due to rheumatic VHD, especially in industrially underdeveloped countries, affecting an estimated 41 million people worldwide in 2019.1),2) On the other hand, VHD remains common in developed countries since the decreased prevalence of rheumatic VHD has been replaced by an increased prevalence of degenerative VHD.3) The most common types of degenerative VHD are aortic stenosis (AS) and mitral regurgitation (MR), with an estimated global prevalence of 9 million and 24 million, respectively, in 2019.1),2) Aging and population growth led to a 50% increase in the number of people affected by degenerative VHD, and the mortality rate due to degenerative VHD doubled between 1990 and 2017.4)
Over the past half-century, the burden of VHD has dramatically changed globally due to geographical differences and socioeconomic development. In addition, recent advances such as the use of transcatheter technology in VHD treatment have attracted the attention of clinicians seeking to change the treatment landscape. Therefore, VHD has become a major focus in cardiovascular medicine.5) At this point, understanding the current epidemiology of VHD is essential for the development of clinical practice guidelines and the establishment of health policies. Therefore, we developed the Korean valve survey (KVS) registry to evaluate the contemporary prevalence, etiology, and demographic profiles of VHD in Korea.
METHODS
Study design
This nationwide retrospective multicenter observational study using KVS data was designed by the Committee of Clinical Practice Guidelines of the Korean Society of Echocardiography (KSE) to investigate the contemporary national burden of VHD in Korea.
A total of 45 centers representing all regions of the country participated in the KVS. The recruitment period was 2 months (September to October, 2019). The list of participating sites and investigators is provided in Supplementary Table 1. No specific limit was imposed on the maximum number of patients enrolled in each center. The study protocol was reviewed and approved by the institutional review board of Seoul National Univerity Hospital (No H-2107-155-1237) and each participating center, which waived the requirement for written informed consent because of the retrospective nature of the study and the anonymized data analysis.
Study population
Individuals aged ≥ 18 years were screened for VHD. The main inclusion criterion of the KVS registry was moderate or severe native VHD, including MR, mitral stenosis (MS), AS, aortic regurgitation (AR), and tricuspid regurgitation (TR), which may draw more clinical attention than mild VHD (Figure 1). We did not collect data on tricuspid stenosis, pulmonary stenosis, or pulmonary regurgitation considering the relatively low prevalence of each. In cases of multiple VHD (a combination of at least moderate severity stenotic or regurgitant lesions occurring on ≥ 2 cardiac valves) and mixed VHD (a combination of stenotic and regurgitant lesions on the same valve), the presence of moderate or severe VHD as the dominant lesion(s) was a prerequisite for study inclusion eligibility. A more detailed definition of each VHD is described in the Supplementary Data 1.
Figure 1. Echocardiographic images of valvular heart disease. Degenerative calcific aortic stenosis is shown on a mid-esophageal aortic valve short-axis view on transesophageal echocardiography (A); rheumatic mitral stenosis is shown on the parasternal short-axis view aortic level on transthoracic echocardiography (B); aortic regurgitation detected on color Doppler in the parasternal long-axis view on transthoracic echocardiography (C); mitral valve posterior prolapse with significant mitral regurgitation on 2-dimensional (D) and color Doppler (E) in the mid-esophageal 2-chamber view on transesophageal echocardiography; and failure of tricuspid valve coaptation during systole (F) and significant tricuspid regurgitation on color Doppler (G) in the apical 4-chamber view on transthoracic echocardiography.
A single VHD was defined as moderate or severe VHD affecting a valve without concomitant VHD of the other valves, whereas multiple VHDs were defined as moderate or severe VHD with concomitant VHD of one or more valves. VHD etiologies were classified according to patient history and clinical and echocardiographic findings by an experienced attending echocardiologist at each center. VHD severity was estimated on echocardiography using an integrative approach according to international guidelines.6),7) The decision to perform further investigations, such as 2-dimensional (2D) speckle tracking echocardiography, dobutamine stress echocardiography, transesophageal echocardiography, cardiac computed tomography, coronary angiography, and catheterization, was left to the discretion of the attending physicians at each center.
Data collection
Data were collected using a password-protected web-accessible electronic case report form (eCRF) (Korea VHD; accessible at https://kmcecrf.kr/vhd/) that was created a priori by the Committee of Clinical Practice Guidelines of the KSE. Essential data included the clinical and echocardiographic variables. The collected data were coded and stored, and access to them was strictly controlled. The attending physicians completed the eCRF with assistance from the clinical research coordinators. Data in the eCRF were audited by 2 study investigators (Son JW and Park JB).
Statistical analysis
Continuous variables are expressed as mean and standard deviation, while categorical data are summarized as frequency and proportion. Student’s t-test and the χ2 test were used to compare continuous and categorical variables, respectively. Two-sided p-values < 0.05 were considered statistically significant. All analyses were performed using R Statistical Software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Clinical characteristics of study population
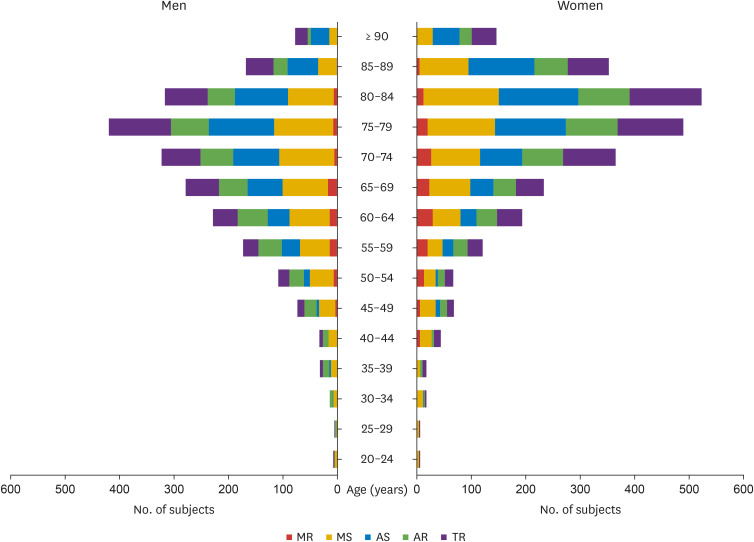
A total of 4,089 patients with VHD were enrolled at 45 centers in Korea, including 2,017 (49.3%) inpatients and 2,072 (50.7%) outpatients. The baseline clinical characteristics of patients are listed in Table 1. The mean age was 70.2 ± 13.3 years, and there were more women than men (53.2% vs. 46.8%; p < 0.001). The most common comorbidity was hypertension (n = 2,369 [57.9%]), followed by atrial fibrillation (n = 1,265 [30.9%]), dyslipidemia (n = 1,088 [26.6%]), and diabetes mellitus (n = 1,056 [25.8%]). A significant proportion of patients with VHD were 60–90 years of age; the most common age groups for men and women were 75–80 years and 80–84 years, respectively (Figure 2). The age group distribution according to VHD type is shown in Figure 3. The age distribution patterns were similar among the different VHD types. Specifically, more than 70% of patients with AS were older than 70 years. More than half of the patients with AR, MR, or TR were aged > 70 years, whereas more than half of those with MS were aged < 70 years.
Table 1. Demographic, clinical, and echocardiographic characteristics of patients with valvular heart disease in Korea.
| Variables | All cases (n = 4,089) | MS (n = 244) | MR (n = 1,384) | AS (n = 1,188) | AR (n = 926) | TR (n = 1,172) | |
|---|---|---|---|---|---|---|---|
| Demographic data | |||||||
| Age (years) | 72.0 ± 13.3 | 65.5 ± 10.9 | 69.8 ± 14.2 | 76.4 ± 10.5 | 70.4 ± 13.4 | 73.2 ± 12.8 | |
| Male | 1,914 (46.8) | 77 (31.6) | 667 (48.2) | 555 (46.7) | 441 (47.6) | 519 (44.3) | |
| Systolic blood pressure (mmHg) | 125.5 ± 19.7 | 118.1 ± 18.3 | 123.8 ± 19.6 | 127.8 ± 19.7 | 128.5 ± 19.0 | 122.0 ± 19.5 | |
| Diastolic blood pressure (mmHg) | 70.7 ± 12.9 | 70.7 ± 12.3 | 72.1 ± 13.1 | 69.4 ± 12.5 | 68.1 ± 12.7 | 71.5 ± 12.7 | |
| Body mass index (kg/m2) | 23.5 ± 3.8 | 23.6 ± 3.9 | 23.4 ± 3.8 | 23.9 ± 3.8 | 23.4 ± 3.6 | 23.2 ± 4.0 | |
| Body surface area (m2) | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | |
| Smoking | 252 (6.2) | 18 (7.4) | 99 (7.2) | 63 (5.3) | 74 (8.0) | 53 (4.5) | |
| Comorbidities | |||||||
| Hypertension | 2,369 (57.9) | 89 (36.5) | 758 (54.8) | 798 (67.2) | 541 (58.4) | 652 (55.6) | |
| Diabetes mellitus | 1,056 (25.8) | 47 (19.3) | 373 (27.0) | 402 (33.8) | 149 (16.1) | 282 (24.1) | |
| Dyslipidemia | 1,088 (26.6) | 50 (20.5) | 323 (23.5) | 410 (34.5) | 229 (24.7) | 273 (23.3) | |
| Atrial fibrillation | 1,265 (30.9) | 151 (61.9) | 472 (34.1) | 177 (14.9) | 180 (19.4) | 691 (59.0) | |
| Chronic dialysis | 249 (6.1) | 10 (4.1) | 113 (8.2) | 65 (5.5) | 39 (4.2) | 81 (6.9) | |
| Chronic pulmonary disease | 382 (9.3) | 15 (6.1) | 120 (8.7) | 103 (8.7) | 63 (6.8) | 147 (12.5) | |
| Previous myocardial infarction | 335 (8.2) | 8 (3.3) | 152 (10.0) | 104 (8.8) | 50 (5.4) | 89 (7.6) | |
| Echocardiographic parameters | |||||||
| LV end-diastolic dimension (mm) | 52.0 ± 8.7 | 48.6 ± 6.6 | 56.3 ± 8.8 | 48.9 ± 6.9 | 55.2 ± 8.6 | 50.0 ± 8.7 | |
| LV end-systolic dimension (mm) | 35.9 ± 10.1 | 32.3 ± 6.5 | 41.0 ± 11.2 | 32.0 ± 8.1 | 38.0 ± 9.1 | 35.0 ± 9.9 | |
| LV ejection fraction (%) | 55.1 ± 14.2 | 58.3 ± 9.8 | 49.1 ± 16.5 | 59.6 ± 11.7 | 55.9 ± 12.3 | 53.3 ± 14.4 | |
| LV ejection fraction ≤ 40% | 716 (17.5) | 16 (6.6) | 447 (32.3) | 111 (9.3) | 122 (13.2) | 233 (19.9) | |
| Interventricular septum thickness (mm) | 9.9 ± 2.0 | 9.0 ± 1.7 | 9.6 ± 2.0 | 10.9 ± 2.1 | 10.2 ± 1.9 | 9.4 ± 1.8 | |
| LV posterior wall thickness (mm) | 9.7 ± 1.8 | 9.0 ± 1.7 | 9.3 ± 1.8 | 10.5 ± 1.9 | 10.0 ± 1.7 | 9.2 ± 1.6 | |
Values are shown as number (%) or mean ± standard deviation.
AR: aortic regurgitation, AS: aortic stenosis, LV: left ventricular, MR: mitral regurgitation, MS: mitral stenosis, TR: tricuspid regurgitation.
Figure 2. Distribution of valvular heart disease in men and women by age.
AR: aortic regurgitation, AS: aortic stenosis, MR: mitral regurgitation, MS: mitral stenosis, TR: tricuspid regurgitation.
Figure 3. Proportion of age group by valvular heart disease type.
AR: aortic regurgitation, AS: aortic stenosis, LV: left ventricular, MR: mitral regurgitation, MS: mitral stenosis, TR: tricuspid regurgitation.
The aortic valve was the most commonly involved valve (n = 1,956 [47.8%]), followed by the mitral valve (n = 1,598 [39.1%]), and the tricuspid valve (n = 1,172 [28.7%]). When we separately analyzed 2,738 (66.9%) patients with single VHD, the aortic valve (n=1,370 [50.0%]) was most frequently affected, followed by the mitral valve (n = 818 [29.9%]) and the tricuspid valve (n = 550 [20.1%]). Multiple VHDs were present in 590 (14.4%) patients, including double-valve disease in 543 (13.3%) and triple-valve disease in 47 (1.1%). The most frequent combination of multiple VHDs was mitral and tricuspid valve disease (n = 297 [7.3%]), followed by mitral and aortic valve diseases (n = 186 [4.5%]). More specifically, the combination of MR and TR, AR and MR, and AS and MR occurred in 273 (6.7%), 103 (2.5%), and 54 (1.3%) cases, respectively.
Mitral valve disease
There were 244 patients with MS (mean age, 65.5 ± 10.9 years; 31.6% men) and 1,384 with MR (mean age, 69.8 ± 14.2 years; 48.2% men). Among 1,384 patients with MR, 795 had primary MR (mean age, 68.2 ± 15.2 years; 44.8% men), and the remaining 598 had secondary MR (mean age, 71.8 ± 12.5 years; 52.5% men) (Supplementary Table 2). Nine patients had a mixed MR etiology. Among the patients with MS, the proportion of women to men was more than 2:1 (68.4% vs. 31.6%). Atrial fibrillation accounted for more than half (n = 151 [61.9%]) of MS cases (Table 1).
Table 2 summarizes the echocardiographic parameters of VHD severity. Among patients with MS, the mean transmitral pressure gradient was 8.15 ± 3.59 mmHg. The mitral valve area was 1.17 ± 0.26 cm2 by 2D planimetry, while the mitral valve area by pressure half-time method was 1.23 ± 0.31 cm2 in rheumatic MS. In patients with MR, the effective regurgitant orifice area was 0.30 ± 0.19 cm2 and the regurgitant volume was 43.9 ± 22.1 mL. The etiologies of mitral valve disease are shown in Figure 4A-C.
Table 2. Echocardiographic parameters of mitral and aortic valve disease severity.
| Variables | MS (n = 244) | MR (n = 1,384) | AS (n = 1,188) | AR (n = 926) | TR (n = 1,172) | |
|---|---|---|---|---|---|---|
| Mitral valve | ||||||
| Trans valvular mean PG (mmHg) | 8.15 ± 3.59 | - | - | - | - | |
| MVA by 2D planimetry (cm2) | 1.17 ± 0.26 | - | - | - | - | |
| EROA (cm2) | - | 0.30 ± 0.19 | - | - | - | |
| Regurgitant volume (mL) | - | 43.94 ± 22.11 | - | - | - | |
| Aortic valve | ||||||
| Peak velocity (m/s) | - | - | 3.90 ± 0.89 | - | - | |
| Trans valvular mean PG (mmHg) | - | - | 37.30 ± 19.10 | - | - | |
| AVA by 2D planimetry (cm2) | - | - | 1.04 ± 0.23 | - | - | |
| AVA by continuity equation (cm2) | - | - | 0.96 ± 0.32 | - | - | |
| Aortic regurgitation PHT (msec) | - | - | - | 446.00 ± 143.01 | - | |
| Tricuspid regurgitation | ||||||
| Regurgitant jet area (mm2) | - | - | - | - | 15.00 ± 7.03 | |
| PISA radius (cm)* | - | - | - | - | 0.75 ± 0.40 | |
| RV basal diameter (mm) | - | - | - | - | 36.60 ± 8.20 | |
| RV mid diameter (mm) | - | - | - | - | 34.30 ± 10.70 | |
| RV fraction area (%) | - | - | - | - | 15.00 ± 7.03 | |
Values are presented as mean ± standard deviation.
AR: aortic regurgitation, AS: aortic stenosis, AVA: aortic valve area, EROA: effective regurgitant orifice area, MR: mitral regurgitation, MS: mitral stenosis, MVA: mitral valve area, PG: pressure gradient, PISA: proximal isovelocity surface area, PTH: pressure half-time, RV: right ventricle, TR: tricuspid regurgitation, 2D: 2-dimensional.
*The PISA radius was measured at Nyquist 30–40 cm/s.
Figure 4. Etiology of each valvular heart disease. Pie charts showing the distribution of the etiology of mitral stenosis (A), primary mitral regurgitation (B), secondary mitral regurgitation (C), aortic stenosis (D), aortic regurgitation (E), and tricuspid regurgitation (F) in Korea.
The most common etiology of MS was rheumatic valve disease (n = 181 [74.6%]), followed by degenerative valve disease (n = 50 [20.5%]). Degenerative valve disease (n = 352 [44.3%]) and mitral valve prolapse (n = 268 [33.7%]) were the most common causes of primary MR, while rheumatic valve disease accounted for only 11.6% (n = 92) of cases. More than half of secondary MR cases were related to non-ischemic heart disease (e.g., dilated cardiomyopathy) (n = 377 [63.0%]). The prevalence of ischemic heart disease was 29.3% in patients with secondary MR. In patients with secondary MR, left ventricular (LV) chamber was enlarged (mean LV end-diastolic dimension [LVEDD], 59.3 ± 9.2 mm; LV end-systolic dimension [LVESD], 47.0 ± 11.4 mm) and LV systolic function was reduced (LV ejection fraction [LVEF], 38.9 ± 15.1%). More than half of the patients with secondary MR (57.2%) had an LVEF ≤ 40% (Supplementary Table 2).
Aortic valve disease
There were 1,188 patients with AS (mean age, 76.4 ± 10.5 years; 46.7% men) and 926 with AR (mean age, 70.4 ± 13.4 years; 47.6% men). Hypertension was the most frequent comorbidity in both patients with AS (n = 798 [67.2%]) and AR (n = 541 [58.4%]). The LV chamber size (LVEDD, 48.9 ± 6.9 mm; LVESD, 32.0 ± 8.1 mm) and systolic function (LVEF, 59.6 ± 11.7%) were generally within the normal range in patients with AS, among whom only 9.3% had an LVEF ≤ 40%. In patients with AR, the LV chamber was enlarged (LVEDD, 55.2 ± 8.6 mm; LVESD, 38.0 ± 9.1 mm) with preserved LV systolic function (LVEF, 55.9 ± 12.3%) (Table 1).
Regarding AS severity, the transaortic peak velocity and transaortic mean pressure gradient were 3.90 ± 0.89 m/s and 37.3 ± 19.1 mmHg, respectively, and the aortic valve area was 1.04 ± 0.23 cm2 by 2D planimetry and 0.96 ± 0.32 cm2 by the continuity equation method (Table 2).
The etiologies of aortic valve disease are shown in Figure 4D and E. The most common cause of aortic valve disease was degenerative disease (78.9% in AS; 64.3% in AR), followed by congenital disease (e.g., bicuspid valve) in AS (9.1%) and rheumatic disease in AR (9.1%). The third most common cause of aortic valve disease was rheumatic disease (7.7%) in AS, and aortopathy (e.g., annuloaortic ectasia) (7.7%) in AR.
Tricuspid valve disease
Considering the extremely low prevalence of tricuspid stenosis, we collected only data on TR. There were 1,172 patients with TR (age, 73.2 ± 12.8 years; 44.3% men). Atrial fibrillation (50.9%) was the most frequent comorbidity associated with TR, followed by hypertension (55.6%), diabetes mellitus (24.1%), and dyslipidemia (23.3%). Compared to other VHD types, chronic pulmonary disease was relatively frequent in patients with TR (12.5%) (Table 1). Secondary TR was the most frequent etiology (90.2%), with primary TR accounting for 6.1% (Figure 4F). More than half of TR cases (n = 622 [53.1%]) were identified in patients with multiple VHD, in whom the most common concomitant VHD was MR (n = 342 [55.0%]), followed by AR (n = 215 [34.6%]), AS (n = 78 [12.5%]), and MS (n = 55 [8.8%]).
Investigations performed for patients with VHD
Table 3 summarizes the types and frequencies of the modalities used in addition to conventional echocardiography for assessing VHD. Specifically, 2D speckle tracking echocardiography was the most frequently performed advanced echocardiographic technique in all cases (36.3%) and across all different VHD types. Transesophageal echocardiography was most commonly used in patients with MS (20.5%), whereas cardiac computed tomography (23.4%) and coronary angiography (26.2%) were predominantly performed in patients with AS. Right heart catheterization for assessing cardiac hemodynamics was most frequently performed in patients with TR (6.1%), followed by those with AS (5.1%), and MR (3.6%).
Table 3. Additional modalities for assessing valvular heart disease.
| Variables | All cases (n = 4,089) | MS (n = 244) | MR (n = 1,384) | AS (n = 1,188) | AR (n = 926) | TR (n = 1,172) | |
|---|---|---|---|---|---|---|---|
| Advanced echocardiography | |||||||
| 2D speckle tracking echocardiography | 1,484 (36.3) | 80 (32.8) | 489 (35.3) | 462 (38.9) | 296 (32.0) | 435 (37.1) | |
| 3D transthoracic echocardiography | 538 (13.2) | 30 (12.3) | 218 (15.8) | 148 (12.5) | 154 (16.6) | 140 (11.9) | |
| Transesophageal echocardiography | 519 (12.7) | 50 (20.5) | 167 (12.1) | 211 (17.8) | 117 (12.6) | 109 (9.3) | |
| Stress echocardiography | 175 (4.3) | 11 (4.5) | 40 (2.9) | 71 (6.0) | 40 (4.3) | 39 (3.3) | |
| Other assessment modalities | |||||||
| Cardiac computed tomography | 627 (15.3) | 29 (11.9) | 151 (10.9) | 278 (23.4) | 140 (15.1) | 147 (12.5) | |
| Magnetic resonance imaging | 61 (1.5) | 3 (1.2) | 26 (1.9) | 14 (1.2) | 12 (1.3) | 20 (1.7) | |
| Coronary angiography | 784 (19.2) | 36 (14.8) | 299 (21.0) | 311 (26.2) | 150 (16.2) | 165 (14.1) | |
| Right heart catheterization | 180 (4.4) | 3 (1.2) | 50 (3.6) | 60 (5.1) | 18 (1.9) | 71 (6.1) | |
Values are shown as number (%).
AR: aortic regurgitation, AS: aortic stenosis, MR: mitral regurgitation, MS: mitral stenosis, TR: tricuspid regurgitation, 2D: 2-dimensional, 3D: 3-dimensional.
DISCUSSION
This is the first study to use data from the KVS registry, which aims to establish the epidemiological profiles of VHD in Korea. We observed significant differences in age and sex distributions, comorbidities, and etiologies among the different VHD types. The aortic valve was most commonly affected, of which AS was the most frequent VHD in elderly patients aged > 70 years, followed by MR. The majority of VHD cases in Korea are attributed to degenerative disease, whereas only a minor proportion is caused by rheumatic VHD. These findings suggest that the epidemiological profiles of VHD in Korea reflect an increased aging population and improved social demographics.
The aging population has increased the prevalence and incidence of degenerative VHD in almost all countries worldwide,1) and Korea is no exception. Korea is one of the world’s most rapidly aging countries, with a population aged 65 years and older accounting for 16.5% of the total population in 2021 (Statistics Korea; http://kostat.go.kr), which is beyond the definition of an aging society by the World Health Organization. As a result, the age-standardized cumulative prevalence of non-rheumatic VHD increased 1.81-fold in patients over 65 years of age in Korea over 6 years, from 2006 to 2011.8) This observation is consistent with that previously reported in other industrially developed countries, such as Europe and the United States.9),10) In all European regions, degenerative VHD was the leading cause of VHD, accounting for 3 of 4 cases, especially after 65 years of age.11) That is, the prevalence of VHD continues to increase with age, indicating the growing importance of aging-associated degenerative and pathological attributes of the valve tissue.
In the KVS registry, the aortic valve was the most affected site, among which more than 3-quarters of AS and more than half of AR cases had a degenerative etiology, while approximately 10% of cases were caused by rheumatic changes. This observation is also consistent with those of other continents. Degenerative AS is the most common VHD across 25 European countries.12) In the United States, AS is the second most common VHD, affecting approximately 5% of the 65-year-old population, with a prevalence that increases with age.13) Changes in life expectancy and socioeconomic conditions resulted in a dramatic increase in degenerative VHD cases as a consequence of an increased prevalence of atherosclerotic risk factors, such as hypertension, diabetes mellitus, dyslipidemia, smoking, and obesity.14) Although AR is the third most common degenerative VHD worldwide following AS and MR,9),15) it is not strongly associated with traditional atherosclerotic risk factors. Instead, AR is more often associated with heterogeneous causes such as endocarditis, congenital disease (e.g., bicuspid aortic valve), and aortopathy (e.g., annuloaortic ectasia). However, AR is also caused by degenerative pathogenesis such as aortic aneurysm, which is thought to reflect age-related changes in the vascular wall. Therefore, it is assumed that the trajectory of the incidence of AR is similar to that of other degenerative VHD types, with an overall increasing prevalence in the aging population.1)
The prevalence of primary MR increased significantly worldwide by 70% between 1990 and 2017.1) A population-based study of prospectively collected clinical and echocardiographic data reported that MR was the most common VHD in the United States.9) Intriguingly, degenerative MR has less regional variation than degenerative AS, probably as a result of its relative pathophysiological independence from atherosclerotic risk factors.4) In the current study, we separately analyzed primary and secondary MR because the diagnostic and treatment approaches are distinctly different from each other.16) Degenerative disease accounts for a significant proportion of the etiologies of primary MR, whereas rheumatic disease constituted approximately 10% of cases. Our study showed that 63.0% of secondary MR cases were associated with non-ischemic heart disease, whereas only 16.6% of cases were related to myocardial infarction. A previous population-based study showed that the prevalence of ischemic MR was 50% in the overall population; among these, MR was mild in 38% and moderate or severe in 12%.17) A recent study demonstrated that 43% of patients with secondary MR had ischemic cardiomyopathy.18) Given that the prevalence of ischemic heart disease continuously increases with age, secondary MR is expected to be increasingly common in the aging population.
Great progress has been made in understanding the pathophysiologic mechanisms, imaging characteristics, and clinical course of degenerative VHD over the past few decades, although no medical therapies have been developed to slow its progression. However, with the advancement of transcatheter technologies, more elderly or frail patients with degenerative VHD will benefit from nonsurgical intervention because physicians have faced difficulty deciding whether to offer surgical treatment to these patients due to their increased risk.19),20),21) Considering the significant medical economic burden associated with the state-of-the-art management of older patients with degenerative VHD, further studies investigating the current status and trends of degenerative VHD may be a fundamental cornerstone for preparing our health systems to respond adequately and in a timely manner to the anticipated increase in these diseases.
Rheumatic VHD is a sequela of untreated streptococcal pharyngitis caused by a group A streptococcal infection. It most commonly affects the mitral valves, followed by the aortic valve, and is usually present in young adults.22) Its major antecedents are the factors that influence its transmissions, such as poor sanitation, overcrowding, and poverty, thus mainly affecting industrially underdeveloped countries.23) In contrast, even in high-income countries, the complete control of rheumatic VHD remains elusive. Indeed, in Korea, there was no material change in the overall age-standardized cumulative prevalence of rheumatic VHD between 2006 and 2011 regardless of age or sex.8) In our study, although few individuals had MS, 3-quarters of MS cases were caused by rheumatic VHD. This might not stem from low-income economic conditions, as this is not the case in Korea. However, the high prevalence of rheumatic MS may be driven by a related reduction in premature mortality.
In the present study, more women than men overall had VHD. In particular, MS was twice as common in women than in men. Women have a higher prevalence of MS than men, with a ratio of 3:1; the prevalence of rheumatic VHD is also higher in women than in men.24) This can be explained by the differences in endogenous hormones, which are the key mediators of rheumatic disease progression.25) Furthermore, women providing childcare would have higher exposure to Streptococcus pyogenes. Our findings also demonstrated that the prevalence of MS in general and rheumatic MS, in particular, was higher in women than in men. Given the sex-related differences in the clinical presentation, etiologies, and prevalence of VHD,26),27),28) future research focusing on the influence of sex on its epidemiology, pathogenesis, progression, therapeutic indications, and outcomes is needed to better understand and manage VHD.
A great deal of attention has been given to TR, which is no longer called “a forgotten valvular disease” since it is reportedly associated with increased mortality.29) However, information remains limited on the current status and temporal trends in the epidemiology of TR. In our study, TR was frequent overall and mainly accompanied by other VHD types, particularly MR and AS. Moreover, more than half of the TR cases occurred in patients with a structurally normal tricuspid valve, the so-called functional TR. Functional TR is generally associated with left-sided VHD, myocardial disease, pulmonary hypertension, or atrial fibrillation.30) Therefore, it inevitably occurs more frequently in an aging population. In the past, no treatment strategies other than surgical repair were available for severe TR. However, encouraging preliminary experience with transcatheter tricuspid valve intervention may pave the way for TR management improvements. The ongoing CLASP II TR clinical trial (NCT04097145) is ongoing and aims to evaluate the safety and effectiveness of the transcatheter valve repair system for TR.
This study had several limitations. First, it was a cross-sectional study and could not assess the temporal patterns of prevalence and incidence. Second, despite our best efforts to determine the causes of VHD, it is difficult to attribute all VHD cases to a definitive etiology because surgical specimens were available for only a limited number of patients. In particular, it is difficult to distinguish between rheumatic and functional TR on echocardiography alone. Third, referral bias could have affected our findings because most participant centers were tertiary and/or referral hospitals. Finally, our data did not include detailed information on the presenting signs and symptoms of the patients with VHD. As a next step, a prospective registry study with a more comprehensive and extensive collection of clinical, biomarker, imaging, and outcome data on VHD is required.
In conclusion, this is the first study to evaluate the current epidemiological profile of VHD in Korea. Similar to other industrialized countries, degenerative VHD is the most common form in Korea. The aortic valve was the most commonly affected site and AS was the most common disease, followed by MR. Our results may be fundamental data for use by further studies of VHD patients and the development of Korean guidelines for VHD.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Son JW, Kim EK, Kim IC, Kim HY, Seo JS, Sun BJ, Shim CY, Yoon SJ, Lee S, Lee SH, Kang DH, Park JB.
- Data curation: Choi YJ, Son JW, Kim EK, Kim IC, Kim HY, Seo JS, Shim CY, Yoon SJ, Lee S, Lee SH, Park JB.
- Formal analysis: Choi YJ.
- Investigation: Son JW, Kim EK, Kim IC, Kim HY, Seo JS, Sun BJ, Shim CY, Lee S, Lee SH, Kang DH, Park JB.
- Methodology: Son JW, Kang DH.
- Project administration: Kang DH.
- Resources: Yoon SJ, Kang DH.
- Supervision: Kang DH, Park JB.
- Visualization: Choi YJ, Park JB.
- Writing - original draft: Choi YJ, Park JB.
- Writing - review & editing: Park JB.
SUPPLEMENTARY MATERIALS
Definition of valvule heart disease
List of participating centers and principal investigators
Demographic, clinical, and echocardiographic characteristics of patients with mitral regurgitation
References
- 1.Coffey S, Roberts-Thomson R, Brown A, et al. Global epidemiology of valvular heart disease. Nat Rev Cardiol. 2021;18:853–864. doi: 10.1038/s41569-021-00570-z. [DOI] [PubMed] [Google Scholar]
- 2.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010;85:483–500. doi: 10.4065/mcp.2009.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadgir S, Johnson CO, Aboyans V, et al. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990-2017. Circulation. 2020;141:1670–1680. doi: 10.1161/CIRCULATIONAHA.119.043391. [DOI] [PubMed] [Google Scholar]
- 5.Binder RK, Dweck M, Prendergast B. The year in cardiology: valvular heart disease. Eur Heart J. 2020;41:912–920. doi: 10.1093/eurheartj/ehz948. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner H, Hung J, Bermejo J, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. doi: 10.1016/j.echo.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Lancellotti P, Tribouilloy C, Hagendorff A, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14:611–644. doi: 10.1093/ehjci/jet105. [DOI] [PubMed] [Google Scholar]
- 8.Jang SY, Ju EY, Seo SR, et al. Changes in the etiology of valvular heart disease in the rapidly aging Korean population. Int J Cardiol. 2014;174:355–359. doi: 10.1016/j.ijcard.2014.04.112. [DOI] [PubMed] [Google Scholar]
- 9.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 10.Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8:162–172. doi: 10.1038/nrcardio.2010.202. [DOI] [PubMed] [Google Scholar]
- 11.Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol. 2014;30:962–970. doi: 10.1016/j.cjca.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 13.Ancona R, Pinto SC. Epidemiology of aortic valve stenosis (AS) and of aortic valve incompetence (AI): is the prevalence of AS/AI similar in different parts of the world? J Cardiol Pract. 2020;18:N° 10 [Google Scholar]
- 14.Yan AT, Koh M, Chan KK, et al. Association between cardiovascular risk factors and aortic stenosis: the CANHEART aortic stenosis study. J Am Coll Cardiol. 2017;69:1523–1532. doi: 10.1016/j.jacc.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Andell P, Li X, Martinsson A, et al. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart. 2017;103:1696–1703. doi: 10.1136/heartjnl-2016-310894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation. 2005;111:295–301. doi: 10.1161/01.CIR.0000151097.30779.04. [DOI] [PubMed] [Google Scholar]
- 18.Grayburn PA, Chandrashekhar YS. Functional mitral regurgitation: more questions than answers. JACC Cardiovasc Imaging. 2021;14:711–714. doi: 10.1016/j.jcmg.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Eggebrecht H, Mehta RH. Transcatheter aortic valve implantation (TAVI) in Germany: more than 100,000 procedures and now the standard of care for the elderly. EuroIntervention. 2019;14:e1549–e1552. doi: 10.4244/EIJ-D-18-01010. [DOI] [PubMed] [Google Scholar]
- 20.Mack MJ, Lindenfeld J, Abraham WT, et al. 3-Year outcomes of transcatheter mitral valve repair in patients with heart failure. J Am Coll Cardiol. 2021;77:1029–1040. doi: 10.1016/j.jacc.2020.12.047. [DOI] [PubMed] [Google Scholar]
- 21.Song C, Madhavan MV, Lindenfeld J, et al. Age-related outcomes after transcatheter mitral valve repair in patients with heart failure: analysis from COAPT. JACC Cardiovasc Interv. 2022;15:397–407. doi: 10.1016/j.jcin.2021.11.037. [DOI] [PubMed] [Google Scholar]
- 22.Zühlke L, Engel ME, Karthikeyan G, et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study) Eur Heart J. 2015;36:1115–22a. doi: 10.1093/eurheartj/ehu449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017;377:713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 24.Negi PC, Kandoria A, Asotra S, et al. Gender differences in the epidemiology of Rheumatic Fever/Rheumatic heart disease (RF/RHD) patient population of hill state of northern India; 9 years prospective hospital based, HP-RHD registry. Indian Heart J. 2020;72:552–556. doi: 10.1016/j.ihj.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubtsova K, Marrack P, Rubtsov AV. Sexual dimorphism in autoimmunity. J Clin Invest. 2015;125:2187–2193. doi: 10.1172/JCI78082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietz MF, Prihadi EA, van der Bijl P, et al. Sex-specific differences in etiology and prognosis in patients with significant tricuspid regurgitation. Am J Cardiol. 2021;147:109–115. doi: 10.1016/j.amjcard.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Summerhill VI, Moschetta D, Orekhov AN, Poggio P, Myasoedova VA. Sex-specific features of calcific aortic valve disease. Int J Mol Sci. 2020;21:5620. doi: 10.3390/ijms21165620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitsche C, Koschutnik M, Kammerlander A, Hengstenberg C, Mascherbauer J. Gender-specific differences in valvular heart disease. Wien Klin Wochenschr. 2020;132:61–68. doi: 10.1007/s00508-019-01603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lüscher TF. Valvular heart disease: tricuspid regurgitation is the new frontier. Eur Heart J. 2018;39:3555–3557. doi: 10.1093/eurheartj/ehy608. [DOI] [PubMed] [Google Scholar]
- 30.Prihadi EA, Delgado V, Leon MB, Enriquez-Sarano M, Topilsky Y, Bax JJ. Morphologic types of tricuspid regurgitation: characteristics and prognostic implications. JACC Cardiovasc Imaging. 2019;12:491–499. doi: 10.1016/j.jcmg.2018.09.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definition of valvule heart disease
List of participating centers and principal investigators
Demographic, clinical, and echocardiographic characteristics of patients with mitral regurgitation