Abstract
Objective.
The present study examined the association between Five Factor Model personality traits and lung function and dyspnea.
Methods.
Participants were middle aged and older adults aged 34 to 103 years old (N> 25,000) from the Midlife in the United States Study (MIDUS), the Health and Retirement Study (HRS), the English Longitudinal Study of Ageing (ELSA), the National Health and Aging Trends Survey (NHATS), and the Wisconsin Longitudinal Study graduate (WLSG) and sibling (WLSS) samples. Data on peak expiratory flow (PEF), dyspnea, personality traits, smoking, physical activity, body mass index (BMI), emotional/psychiatric problems, and demographic factors were obtained in each sample.
Results.
A meta-analysis indicated that higher neuroticism was related to lower PEF, higher risk of PEF less than 80% of predicted value, and higher risk of dyspnea. In contrast, higher extraversion and conscientiousness were associated with higher PEF, lower likelihood of PEF lower than 80% of the predicted value, and lower risk of dyspnea. Higher openness was related to higher PEF and lower risk of PEF less than 80%, whereas agreeableness was related to higher PEF and lower risk of dyspnea. Smoking, physical activity, BMI and emotional/psychiatric problems partially accounted for these associations. There was little evidence that lung disease moderated the association between personality and PEF and dyspnea.
Conclusions.
Across cohorts, this study found replicable evidence that personality is associated with lung function and associated symptomatology.
Keywords: Personality, lung function, dyspnea, peak expiratory flow
1. Introduction
Poor performance on measures of lung function, such as peak expiratory flow (PEF), are critical for the diagnosis of chronic pulmonary disease (COPD) [1], and are predictive of poor mental health [2], higher risk of limitations in activities of daily living[3], frailty [4], hospitalization [5], and mortality [6, 7]. Furthermore, lower PEF values have been related to cognitive impairment [8] and increased risk of incident dementia [9]. Given the impact of lung function on well-being, morbidity and mortality, there is a need for a better understanding of the factors associated with lung function. There is evidence that factors ranging from genetic to environmental contribute to lung function [10,11,12]. The present study extends existing knowledge by examining psychological factors and lung function. Specifically, it examines whether personality traits (relatively enduring patterns of thoughts, feelings and behaviors) are associated with measure of PEF.
The Five Factor Model of Personality [13] organizes personality traits along five broad dimensions: Neuroticism (the tendency to experience distress and negative emotions), extraversion (the tendency to be outgoing and active), openness (the tendency to be curious and imaginative), agreeableness (the tendency to be trusting and cooperative) and conscientiousness (the tendency to be disciplined and organized). Existing models and research suggest that personality contributes to health across adulthood [14]. Consistent with these conceptual models, pervasive associations have been found between personality and a range of health-related behaviors and outcomes, including cigarette smoking [15], self-rated health [16], biological markers [17,18], incident disease (e.g., Alzheimer’s disease) [19] , and mortality [20]. Furthermore, personality has been associated with objective measures of physical performance, including gait speed [21] and grip strength [22].
There are reasons to expect an association between personality and lung function. Indeed, personality is related to several clinical, behavioral, and biological factors that are implicated in lung function. For example, higher neuroticism, and lower extraversion, openness and conscientiousness have been linked with lower cardiorespiratory fitness [23], which may manifest into lower PEF. Furthermore, these traits as well as lower agreeableness have been associated with higher risk of frailty [24], which is indicated in part by lower PEF [25]. In addition, higher neuroticism and lower conscientiousness are related to smoking [15], physical inactivity [26], higher risk of obesity [27] and metabolic syndrome [28], that are known to increase the likelihood of worse lung function [29-32].
Consistent with these assumptions, one study conducted in the Health and Retirement Study (HRS) found that higher neuroticism was related to lower PEF and higher risk of PEF lower than 80% of predicted values (Terracciano et al., 2017). In contrast, higher extraversion, openness, agreeableness and conscientiousness were related to higher PEF and lower risk of PEF less than 80% of predicted value [33]. Furthermore, personality was related to associated symptomatology. Specifically, higher neuroticism was associated with higher shortness of breath (dyspnea), whereas higher extraversion, openness, agreeableness, and conscientiousness were related to lower risk of dyspnea [33]. Therefore, this study provides evidence of an association between personality and lung function and associated symptomatology. However, to our knowledge, no other study has examined this link, and the association between personality and lung function and dyspnea remains relatively unaddressed. As a result, the present study thought to extend this knowledge by using a multi-cohort design to test the replicability of the link between the FFM personality traits, lung function, and associated symptomatology.
Based upon six large samples of middle-aged and older adults, the present study examined the association between personality and lung function. Building upon existing research [33], it was hypothesized that higher neuroticism would be related to lower PEF, whereas higher extraversion, openness, agreeableness and conscientiousness would be associated with higher PEF. Consistent with previous research, the same hypotheses were tested to predict PEF below a frequently used clinical threshold, the predicted 80% PEF [34]. Complementary, these hypotheses were further tested with shortness of breath (e.g. dyspnea) as the outcome. Additional analyses examined whether the associations between personality and lung function and dyspnea were accounted for by BMI, smoking, physical inactivity and emotional/psychiatric problems. These variables could be considered confounding factors, but more properly potential mediators on the pathways from personality to lung function and symptoms. Finally, the study tested whether the associations were moderated by clinical status (lung disease) to evaluate whether the associations are present in the general population or limited to the subgroup with a known lung disease.
2. Method
2.1. Participants
Participants were drawn from six cohorts: the Midlife in the United States Study (MIDUS), the Health and Retirement Study (HRS), the English Longitudinal Study of Ageing (ELSA), the National Health and Aging Trends Survey (NHATS), and the Wisconsin Longitudinal Study graduate (WLSG) and sibling (WLSS) samples. The MIDUS Study was approved by the Education and Social/Behavioral Sciences and the Health Sciences Institutional Review Board at the University of Wisconsin-Madison. The HRS was approved by the University of Michigan IRB. ELSA was approved by the National Research and Ethics Committee of the UK National Health Service. The NHATS was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board. The WLS received approval from the Health Sciences IRB at University of Wisconsin–Madison. No IRB was required for the present study because it uses publicly available de-identified data. In each sample, written informed consent was obtained from participants. Participants were included if they had complete data on personality, PEF, demographic factors and height. Individuals with missing data were excluded. Additional analyses were conducted with individuals who provided also dyspnea data. Descriptive statistics for the six samples are presented in Table 1.
Table 1.
Descriptive Statistics for the Six Samples
| MIDUS | HRS | ELSA | WLSG | WLSS | NHATS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | M/% | SD | M/% | SD | M/% | SD | M/% | SD | M/% | SD | M/% | SD |
| Age (Years) | 55.28 | 11.79 | 67.61 | 10.30 | 65.85 | 8.05 | 71.20 | 0.91 | 69.21 | 6.67 | 79.11 | 7.32 |
| Sex (% women) | 55% | - | 59% | - | 54% | - | 54% | - | 54% | - | 58% | - |
| Race (% White) | 94% | - | 77% | - | 98% | - | 100% | - | 100% | - | 74% | - |
| Ethnicity (% Hispanic) | - | - | 15% | - | - | - | - | - | - | - | 4% | - |
| Education | 7.75 | 2.45 | 13.28 | 2.85 | 4.30 | 2.20 | 13.86 | 2.39 | 14.13 | 2.58 | 5.29 | 2.27 |
| Height | 1.69 | 0.09 | 1.66 | 0.10 | 1.66 | 0.09 | 1.67 | 0.09 | 1.68 | 0.09 | 1.67 | 0.11 |
| Neuroticism | 2.02 | 0.63 | 1.96 | 0.61 | 2.09 | 0.59 | 3.01 | 0.92 | 3.03 | 0.92 | 2.20 | 0.85 |
| Extraversion | 3.13 | 0.57 | 3.19 | 0.57 | 3.18 | 0.55 | 3.79 | 0.87 | 3.77 | 0.89 | 3.16 | 0.74 |
| Openness | 2.96 | 0.52 | 2.93 | 0.57 | 2.90 | 0.54 | 3.46 | 0.76 | 3.47 | 0.75 | 2.85 | 0.82 |
| Agreeableness | 3.43 | 0.51 | 3.51 | 0.50 | 3.51 | 0.47 | 4.80 | 0.71 | 4.79 | 0.71 | 3.59 | 0.52 |
| Conscientiousness | 3.40 | 0.45 | 3.37 | 0.48 | 3.32 | 0.48 | 4.75 | 0.71 | 4.72 | 0.71 | 3.25 | 0.71 |
| Peak expiratory flow(PEF)a | 421.61 | 129.41 | 380.44 | 135.17 | 6.71 | 2.17 | 403.03 | 131.57 | 414.61 | 134.36 | 332.46 | 137.83 |
| PEF lower than 80% | 47% | 48% | 44% | 38% | 37% | 58% | ||||||
| Dyspneab | 27% | 15% | 25% | 31% | 31% | 21% | ||||||
Note. HRS: N= 10,181; MIDUS: N= 997; ELSA: N= 5245; WLSG: N= 4734; WLSS:N= 2514; NHATS: N= 2448.
PEF was recorded in L/min in the MIDUS, HRS, NHATS and WLS, and in L/seconds in ELSA.
Due to missing data, HRS: N= 8998; MIDUS: N= 996; ELSA: N= 5050; WLSG: N= 4518; WLSS:N= 2403; NHATS: N= 2447
The MIDUS is a longitudinal study of non-institutionalized US adults. Data from the second wave (2004-2006, MIDUS II) were used in the present study. PEF and dyspnea were obtained among a subsample as a part of the biomarker project of the MIDUS II.The analyzed sample was composed of 997 individuals aged from 34 to 84 years old (55% women, Mean age= 55.28, SD= 11.79). Of this sample, 996 individuals also answered the dyspnea question.
The HRS is a nationally representative longitudinal study of Americans 50 years and older and their spouse. Half of the sample provided data on personality, demographic factors, height and PEF in 2014; Data were obtained from the other half in 2016. Both waves were combined, resulting in a total sample of 10,181 participants aged from 50 to 100 years old (59% women, mean age=67.61, SD=10.30). Within this sample, dyspnea data was available for 8,998 participants. We published previously on personality and PEF with different waves of data from HRS [33], but there is likely to be some overlap in the sample.
ELSA is a panel study of a representative cohort of men and women living in England aged 50 years and older. Data on personality and demographic factors were obtained at Wave 5 (2010-2011), and data on height and PEF were obtained in Wave 6 (2012-2013). The analyzed sample was composed of 5,245 individuals aged from 50 to 89 years (54% women, mean age = 65.85, SD = 8.05). Dyspnea data were obtained for 5,050 participants within this sample.
The WLS is a longitudinal study of men and women who graduated from Wisconsin high schools in 1957 (WLSG) and one of their selected siblings (WLSS). Data for WLS samples were obtained in the 2011 wave. In the WLSG, a total of 4,734 participants aged from 70 to 74 years old (54% women, mean age = 71.20, SD = 0.91) had complete data. Of this sample, 4,518 participants provided data on dyspnea. In the WLSS, complete data were obtained from a total of 2,514 participants aged from 40 to 92 years old (53% women, mean age = 69.21, SD = 6.67). Dyspnea data were available for 2,403 individuals of this sample.
The NHATS is a nationally representative survey of Medicare enrollees aged 65 years and older. One third of the sample provided data on personality, demographic factors, height and PEF in 2013, and data were obtained from a second third in 2014. Both waves were combined, resulting in a total sample of 2,448 participants aged from 67 to 103 years (58% women, mean age = 79.11, SD = 7.32). Within this sample, 2,447 participants had data on dyspnea.
2.2. Measures
2.2.1. Personality
Personality traits were assessed using the 26-item version of the Midlife Development Inventory (MIDI) [35] in the HRS, MIDUS, and ELSA. A 10-item version of the MIDI was used in the NHATS. Using a scale ranging from 1 (not at all) to 4 (a lot), participants were asked to rate how well adjectives representing the five traits described themselves. Example items are nervous (e.g., neuroticism), talkative (e.g., extraversion), imaginative (e.g., openness), warm (e.g., agreeableness), and organized (e.g., conscientiousness). A 29-item version of the Big Five Inventory (BFI) [36] was used in the WLSG and WLSS. Participants were asked to rate items assessing neuroticism (e.g., To what extent do you agree that you see yourself as someone who can be tense?), extraversion (e.g., To what extent do you agree that you see yourself as someone who is full of energy?), openness (e.g., To what extent do you agree that you see yourself as someone who has an active imagination?), agreeableness (e.g., To what extent do you agree that you see yourself as someone who is generally trusting?), and conscientiousness (e.g., To what extent do you agree that you see yourself as someone who does things efficiently?) on a scale ranging from 1 (disagree strongly) to 6 (agree strongly).
2.2.2. PEF
PEF was assessed using a peak flow meter in the MIDUS, HRS, NHATS, and WLS samples, and using a spirometer in ELSA. The best of three trials was used in the MIDUS, HRS, ELSA and WLS samples, whereas the best of two trials was used in NHATS. PEF was recorded in L/min in the MIDUS, HRS, NHATS and WLS, and in L/seconds in ELSA.
2.2.3. Dyspnea
In the MIDUS, participants were asked how much they felt short of breath during the past week including today, on a scale from 1 “not at all” to 5 “extremely”. Answers of a “little bit”, “moderately”, “quite a bit” and “extremely” were recoded as 1 and answers of not at all were recoded as 0. In the HRS, participants indicated whether they had persistent or troublesome shortness of breath while awake using a yes/no format. In ELSA, participants reported their symptoms of breathlessness and dyspnea using the Medical Research Council (MRC) dyspnea scale. Participants are usually classified as no dyspnea (coded as 0), grade 1 (coded as 1, “Short of breath when hurrying or walking up a slight hill”), grade 2 (coded as 2, “Walks slower than contemporaries on level ground because of breathlessness”), and grade 3 (coded as 3, “Has to stop for breath when walking at own pace on a level ground”). Grade 1, grade 2, and grade 3 were recoded as 1. Participants in the WLS samples, were asked whether they had shortness of breath in the last six months, using a yes/no format. In the NHATS, participants were asked to indicate whether they had any breathing problems, including shortness of breath or difficulty breathing in the last month (coded as 1 for yes, and 0 for no).
2.2.4. Covariates.
Demographic factors and height (in meters) were included as covariates. All six samples included self-reported age (in years) and sex (coded as 1 for female and 0 for male). Education was measured in years in HRS and WLS samples, using a scale from 1 (no grade school) to 12 (doctoral level degree) in the MIDUS, from 1 (No qualification) to 7 (NVQ4/NVQ5/Degree or equivalent) in ELSA, and from 1 (No schooling completed) to 9 (Master’s, professional or doctoral degree) in the NHATS. Race was assessed in HRS, and MIDUS, NHATS, and ELSA. In HRS and NHATS, race was coded as 1 for African American and 0 for other, and as 1 for white and 0 for other in the three remaining samples. Ethnicity (coded as 1 for Hispanic and 0 for not Hispanic) was also included as covariate in HRS and NHATS. Staff-assessed height was measured in MIDUS, HRS, ELSA, and WLS samples, and self-reported height was used in the NHATS. Additional analyses included BMI, smoking, physical activity, and emotional/psychiatric problems as covariates (see supplementary material for the measures).
2.3. Data Analysis
In each sample, regression analysis was used to regress PEF on the personality traits. Age, sex, education, and height were included as covariates in all samples, race was included as an additional covariate in the MIDUS, HRS, ELSA, and NHATS, and ethnicity was included as an additional covariate in the HRS and NHATS. Separate analyses were conducted for each personality trait. Logistic regression analyses tested whether personality was related to PEF less than 80% of the predicted value. Predicted PEF was calculated using the Nunn and Gregg formula [34]. Given that PEF less than 80% of the predicted value was derived from sex, age, and height, these variables were not included as covariates. Logistic regression was also used to test whether personality predicted risk of dyspnea, controlling for demographic factors and PEF. Personality traits were z-scored for the logistic regression analysis to faciliate interpretation. A random effect meta-analysis was conducted by combining the estimates from these analyses.
Supplementary analyses included BMI, physical activity, smoking and emotional/psychiatric problems as additional covariates. Sensitivity analyses were performed to test whether the associations between personality and PEF (both continuous and percent of predicted value) and dyspnea were dependent on a diagnosis of lung disease by testing an interaction between each trait and lung disease (see supplementary material for the measures of lung disease).
3. Results
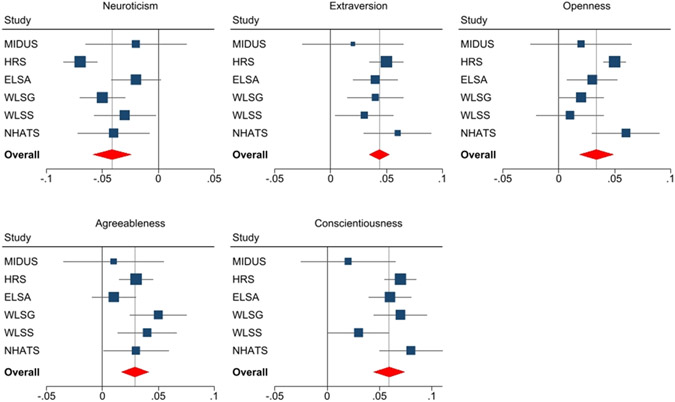
As expected, the meta-analysis indicated that higher neuroticism was associated with lower PEF, whereas higher extraversion, openness, agreeableness and conscientiousness were related to higher PEF (Table 2; Figure 1). The association between conscientiousness and PEF was observed in five out of six samples, whereas the association between neuroticism, extraversion, agreeableness and PEF was significant in four out of six samples. Openness was significantly related to PEF in three out of six samples. Additional analyses revealed that the overall pattern of associations between personality and PEF was almost unchanged when BMI, smoking, physical activity, and emotional/psychiatric problems were included as covariates (Supplementary Tables S1-S7).
Table 2.
Summary of Regression Analysis Predicting PEF from Personality Traits in the Six Samples
| MIDUS a β |
HRS b β |
ELSAa β |
WLSG c β |
WLSS c β |
NHATSb β |
Random Effect |
Heterogeneity I2 |
|
|---|---|---|---|---|---|---|---|---|
| Neuroticism | −0.02 (−0.06;0.03) |
−0.07*** (−0.08; −0.05) |
−0.02 (−0.04; 0.004) |
−0.05*** (−0.07;−0.03) |
−0.03* (−0.06;−0.005) |
−0.04* (−0.07;−0.006) |
−0.04*** (−0.062;−0.024) |
52.39 |
| Extraversion | 0.02 (−0.03;0.06) |
0.05*** (0.04; 0.07) |
0.04*** (0.02; 0.06) |
0.04*** (0.02;0.07) |
0.03 (−0.002;0.05) |
0.06*** (0.03;0.09) |
0.04*** (0.032;0.056) |
0 |
| Openness | 0.02 (−0.03;0.06) |
0.05*** (0.04; 0.06) |
0.03* (0.005; 0.05) |
0.02 (−0.00;0.04) |
0.01 (−0.02;0.04) |
0.06*** (0.03;0.09) |
0.03*** (0.019;0.050) |
30.06 |
| Agreeableness | 0.01 (−0.04;0.05) |
0.03*** (0.02; 0.05) |
0.01 (−0.009; 0.03) |
0.05*** (0.03;0.08) |
0.04* (0.007;0.06) |
0.03* (0.002;0.06) |
0.03*** (0.017;0.043) |
5.35 |
| Conscientiousness | 0.02 (−0.03;0.06) |
0 07*** (0.05; 0.08) |
0.06*** (0.04; 0.08) |
0.07*** (0.05;0.10) |
0.03* (0.002;0.06) |
0.08*** (0.05;0.11) |
0.06*** (0.051;0.075) |
1.28 |
Note. MIDUS: N= 997; HRS: N= 10,181; ELSA: N= 5245; WLSG: N= 4734; WLSS:N= 2514; NHATS: N= 2448
p < .05
p < .01
p <.001. β = Standardized regression coefficient
Adjusted for age, sex, education, race, and height
Adjusted for age, sex, education, race, ethnicity, and height
Adjusted for age, sex, education and height
Figure 1.
Forest plots of the associations between the five personality traits and PEF.
Consistent with the analysis of continuous PEF, the meta-analysis indicated that neuroticism was related to a higher risk of PEF lower than 80% of the predicted value, whereas extraversion, openness, and conscientiousness were associated with a lower risk of PEF less than 80% (Table 3). These associations were significant in four out of six samples. There was no significant relationship with agreeableness in the meta-analysis. A one SD higher score on neuroticism was associated with a 12% higher risk of PEF lower than 80% (the range of significant effects across samples was 13-20%). In contrast, a one SD higher score on extraversion, openness, or conscientiousness was respectively associated with a 7%, 10%, and 12% lower risk of PEF lower than 80% of the predicted value (the range of significant effects across samples was 9-10% for extraversion, 10-22% for openness, and 14-20% for conscientiousness). These associations mostly persisted when BMI, smoking, physical activity, and emotional/psychiatric problems were included as covariates (Supplementary Table S1, Tables S8-S13).
Table 3.
Summary of Logistic Regression Analysis Predicting PEF< 80% from Personality Traits
| MIDUS a Odds Ratio |
HRS b Odds Ratio |
ELSAa Odds Ratio |
WLSG c Odds Ratio |
WLSS c Odds Ratio |
NHATSb Odds Ratio |
Pooled Odds Ratio |
Heterogeneity I2 |
|
|---|---|---|---|---|---|---|---|---|
| Neuroticism | 1.05 (0.92-1.19) |
1.17*** (1.13-1.22) |
1.00 (0.94-1.06) |
1.20*** (1.13-1.28) |
1.13** (1.04-1.22) |
1.15** (1.06-1.25) |
1.12*** (1.05-1.17) |
81.97 |
| Extraversion | 1.01 (0.89-1.15) |
0.91*** (0.88-0.95) |
0.95 (0.90-1.01) |
0.92** (0.87-0.98) |
0.92* (0.84-1.00) |
0.92* (0.85-1.00) |
0.93*** (0.90-0.95) |
0 |
| Openness | 0.98 (0.86-1.12) | 0.87*** (0.83-0.91) | 0.92** (0.87-0.98) | 1.00 (0.93-1.06) | 0.88** (0.80-0.96) | 0.82*** (0.76-0.90) | 0.91*** (0.86-0.96) | 74.08 |
| Agreeableness | 1.17* (1.03-1.33) | 1.02 (0.98-1.07) | 1.02 (0.96-1.08) | 0.97 (0.92-1.03) | 1.02 (0.94-1.11) | 1.00 (0.92-1.09) | 1.02 (0.99-1.05) | 30.54 |
| Conscientiousness | 1.04 (0.92-1.18) | 0.88*** (0.85-0.92) | 0.87*** (0.82-0.91) | 0.85*** (0.80-0.90) | 0.95 (0.88-1.03) | 0.83*** (0.77-0.91) | 0 89*** (0.85-0.93) | 62.62 |
Note. MIDUS: N= 997; HRS: N= 10,181; ELSA: N= 5245; WLSG: N= 4734; WLSS:N= 2514; NHATS: N= 2448
p < .05
p < .01
p <.001
Adjusted for education and race
Adjusted for education, race, and ethnicity
Adjusted for education
The meta-analysis also indicated associations between higher neuroticism and a higher risk of dyspnea and between higher conscientiousness, agreeableness and extraversion and a lower likelihood of dyspnea, controlling for demographic factors and PEF (Table 4). The association with neuroticism and conscientiousness was statistically significant in all six samples, in five samples for extraversion, and in four samples for agreeableness. A one SD higher score on neuroticism was related to a 33% higher likelihood of dyspnea, whereas a one SD higher score on conscientiousness, extraversion or agreeableness was associated with a 39%, a 25%, and a 14% lower risk of shortness of breath, respectively. Openness was unrelated to shortness of breath in the meta-analysis. These associations persisted when BMI, physical activity, smoking, and emotional/psychiatric problems were included as additional covariates (Supplementary Tables S14-S19).
Table 4.
Summary of Logistic Regression Analysis Predicting Dyspnea from Personality Traits in the Six Samples
| MIDUS a Odds Ratio |
HRS b Odds Ratio |
ELSAa Odds Ratio |
WLSG c Odds Ratio |
WLSS c Odds Ratio |
NHATSb Odds Ratio |
Pooled Odds Ratio |
Heterogeneity I2 |
|
|---|---|---|---|---|---|---|---|---|
| Neuroticism | 1.39*** (1.19-1.62) |
1.41*** (1.33-1.49) |
1.30*** (1.21-1.39) |
1.29*** (1.20-1.38) |
1.38*** (1.26-1.52) |
1.25*** (1.13-1.38) |
1.33*** (1.28-1.39) |
33.48 |
| Extraversion | 0.76*** (0.66-0.88) |
0.74*** (0.70-0.79) |
0 70*** (0.66-0.75) |
0.84*** (0.79-0.90) |
0.78*** (0.71-0.86) |
1.04 (0.94-1.14) |
0.80*** (0.73-0.89) |
89.50 |
| Openness | 0.89 (0.77-1.03) |
0.87*** (0.82-0.92) |
0.85*** (0.79-0.91) |
1.06 (0.98-1.13) |
1.05 (0.95-1.15) |
1.01 (0.92-1.12) |
0.95 (0.87-1.04) |
84.99 |
| Agreeableness | 0.83* (0.71-0.96) |
0.89*** (0.84-0.94) |
1.00 (0.94-1.08) |
0.77*** (0.72-0.83) |
0.77*** (0.70-0.84) |
1.02 (0.93-1.13) |
0.88** (0.79-0.97) |
89.19 |
| Conscientiousness | 0.66*** (0.57-0.76) |
0.74*** (0.70-0.78) |
0.73*** (0.69-0.78) |
0.67*** (0.63-0.72) |
0.63*** (0.57-0.69) |
0.87** (0.79-0.96) |
0.72*** (0.66-0.78) |
83.38 |
Note. MIDUS: N= 996; HRS: N= 8998; ELSA: N= 5050; WLSG: N= 4518; WLSS:N= 2403; NHATS: N= 2447
p < .05
p < .01
p <.001
Adjusted for age, sex, education, race, and PEF
Adjusted for age, sex, education, race, ethnicity, and PEF
Adjusted for age, sex, education, and PEF
Sensitivity analyses tested whether lung disease moderated the association between personality and PEF. There was little replicable evidence for an interaction between personality traits and lung disease. There were three exceptions in the WLSG: higher extraversion (βInteraction= .02, p<.05), agreeableness (βInteraction= .02, p<.01) and conscientiousness (βInteraction= .02, p<.05) were more strongly related to higher PEF among individuals suffering from lung disease. In the MIDUS, higher extraversion (βInteraction= .06, p<.01) and higher openness were associated with higher PEF (βInteraction= .06, p<.05) among individuals with lung disease. There were no other significant interactions between personality traits and lung disease on PEF in the other samples. There was also no evidence that lung disease moderated the association between personality and the risk of PEF lower than 80% of predicted value or the likelihood of dyspnea. The only exception was observed in the WLSS: higher extraversion was more strongly related to lower risk of PEF lower than 80% among individuals without lung disease (Odds ratioInteraction= 1.34, p < .05).
4. Discussion
Based on six large samples of middle-aged and older adults, the present study examined the associations between personality and lung function and associated symptomatology. As expected, higher neuroticism was related to lower PEF, higher risk of PEF less than 80% of predicted value, and higher risk of dyspnea. In contrast, higher extraversion and conscientiousness were associated with higher PEF, lower likelihood of PEF lower than 80% of the predicted value, and lower risk of dyspnea. Higher openness was related to lower PEF and lower likelihood of PEF lower than 80%, and higher agreeableness was related to higher PEF and lower likelihood of dyspnea. While some of these associations were not statistically significant in all samples, the associations were robust when accounting for smoking, BMI, physical activity, and emotional/psychiatric problems. Furthermore, reported diagnosis of lung disease generally did not moderate the associations, suggesting that the observed associations are not limited to a subset of the population.
Neuroticism was consistently associated with worse lung function, which supports previous research on this association [33] and corroborates related evidence about cardiorespiratory fitness [23]. These associations with lung function should be interpreted in the broader context of the associations between higher neuroticism and a worse overall health profile [37], including a higher likelihood of health-risk behaviors, such as smoking [15] and lower physical activity [26], and health-related conditions such as obesity [27]. These health-related behaviors and conditions are in turn harmful for lung function and could be potential mechanisms that explain the association between personality and lung function. Consistent with these assumptions, smoking, physical activity, BMI, and emotional/psychiatric problems partially, but not completely, accounted for the link between neuroticism and PEF. Therefore, other factors may explain part of this association. For example, a stress-related pathway may also explain part of this association. Recent research found that higher neuroticism is related to higher risk of chronic conditions and functional limitations in part through its association with higher stress reactivity [38]. Given that stress is related to lung function [39], it may be one pathway between neuroticism and worse lung function. Furthermore, neuroticism has been related to lower muscular strength [22], which may manifest in worse respiratory muscle strength and ultimately lower lung function[40-42]. Complementary, neuroticism is related to higher chronic pain [43], which has been found to be related to lower lung function [5]. At the biological level, neuroticism is related to a higher risk of metabolic syndrome [28], which has been associated with worse lung function [29]. Finally, the link between neuroticism and lung function may be explained in part by shared genetics [44].
The present study also found consistent associations between neuroticism and dyspnea. This association was observed controlling for PEF and was only partially accounted by behavioral and health-related factors. The basic tendencies associated with neuroticism, including a propensity to experience distress and anxiety, may lead to subjective feelings of shortness of breath. For example, higher anxiety may exacerbate dyspnea, even without respiratory problems. The higher pain [43] and fatigue [45] experienced by individuals with high neuroticism may also lead to higher shortness of breath.
As expected, extraversion, openness, and conscientiousness were related to better lung function. This finding is consistent with previous research on these traits and higher PEF [33] and is broadly consistent with a report of their association with better cardiorespiratory fitness [23]. The behavioral, health-related and psychological profiles of extraverted, open, and conscientious individuals may explain in part their higher lung function. Indeed, extraversion, openness, and conscientiousness are related to higher levels of physical activity [26], which in turn may contribute to higher PEF. In addition, higher conscientiousness is associated with lower risk of smoking [15] and lower risk of obesity [27] which may benefit lung function. Furthermore, higher extraversion, openness, and conscientiousness have been related to lower stress reactivity [38,46, 47], which could be reflected in better lung function [39]. Of note, additional analyses indicated that the association between these traits and PEF was partially accounted by smoking, physical activity, BMI, and emotional/psychiatric problems. Other pathways may operate in these associations. For example, extraversion, openness, and conscientiousness have been associated with lower fatigability [48] which has been associated with better physical performance [49], and thus may lead to higher PEF. In addition, both extraversion and conscientiousness are associateed with lower likelihood of persistent pain [43], which contributes to better lung function [5]. At the biological level, higher conscientiousness and openness are associated with lower inflammation [17] which leads to better lung function [50]. Finally, extraversion, openness and conscientiousness are related to higher muscular strength [22], which may benefit PEF [40-42].
Higher extraversion and conscientiousness were also associated with lower dyspnea. Most importantly, these associations were observed while controlling for PEF, which suggests that even after accounting for differences in lung capacity, these personality traits are related to the dyspnea. The core characteristic of extraversion and conscientiousness may be directly reflected in dyspnea. Indeed, extraversion is characterized by a propensity to experience positive emotions and to be energetic, and conscientiousness is defined by a tendency to be industrious and organized, which are accompanied by lower fatigue [45], and may reduce the risk of experiencing shortness of breath. Furthermore, extraverted and conscientious individuals may be less likely to report shortness of breath because they experience less stress [46,47].
Agreeableness was related to lung function and dyspnea. However, this trait was not related to the risk of PEF less than 80% of predicted value. These associations support the link between agreeableness and better PEF and lower dyspnea [23]. Agreeable individuals present favorable behavioral, health-related, and psychological profiles which may contribute to better lung function and lower dyspnea. For example, higher agreeableness has been associated with more frequent physical activity [26], better self-rated health [16], lower fatigue and stress [45,47], which may lead to higher PEF and lower shortness of breath.
The present study has theoretical implications. The present study adds to existing lifespan models of personality and health [14] by identifying replicable associations between personality traits and lung function. In addition, this study adds to the existing literature on the predictors of lung function. Indeed, the identification of an association between personality and PEF complements the list of biological, behavioral, and environmental factors related to lung function [10-12] . Of note, the association between personality and PEF was independent of the diagnosis of lung disease, suggesting that personality traits are relevant to lung function across the whole population. In addition, this study provides robust evidence that shortness of breath not only reflects objective lung function and behavioral risk factors, but also an individuals’ characteristic ways of thinking, feeling, and behaving.
Perhaps the most significant strength of the current study is the inclusion of six large samples of middle-aged and older adults. Other major strengths include (a) the analyses of both a performance measure (PEF) and self-reported dyspnea; (b) the inclusion of all five major personality traits; (c) the inclusion of relevant covariates; (d) and an analytic approach consistent across samples, which reduces heterogeneity in the results due to analytic choices and facilitates meta-analytic syntheses of the evidence. There are also limitations. The observational design of the present study limits causal interpretations. Although personality may contribute to lung function, it is also likely that lung function may be related to personality change [51]. However, the majority of the samples include only one assessment of personality and/lung function, which limits the possibility to test for the reciprocal associations between between personality and lung function. Longitudinal research including repeated assessments of both personality and measures of lung function are needed to test for such model. Furthermore, the present study focused on PEF as a marker of lung function, and additional measures, such as forced expiratory volume (FEV) were unavailable in the majority of studies included. Therefore, future research is needed to examine whether the link observed between personality and PEF generalizes to other markers. The present study focused on middle-aged and older adults, and little is known about the extent to which the pattern of association generalizes to younger age groups. Finally, this study focused on broad personality domains. Additional research may examine the specific facets associated with lung function and dyspnea.
In sum, the present study found that personality traits have pervasive associations with lung function and associated symptomatology. Higher neuroticism was related to lower lung function and higher risk of dyspnea, whereas higher extraversion and conscientiousness were associated with better lung function and lower risk of dyspnea. From a practical perspective, personality assessment could help identify individuals at risk of poor lung function and improve the targeting and individualization of interventions. For example, individuals with high neuroticism, low extraversion, openness, and conscientiousness may be targeted by physical activity programs or cognitive behavioral therapy to maintain or improve lung function. In addition, personality traits are modifiable by interventions [52]. Therefore, such programs may be directed toward changing personality traits, such as high neuroticism and low conscientiousness, leading ultimately to better lung function.
Supplementary Material
Acknowledgments
The Health and Retirement Study (HRS) is sponsored by the National Institute on Aging (NIA-U01AG009740) and conducted by the University of Michigan. HRS data are publicly available at http://hrsonline.isr.umich.edu/. The Midlife in the United States (MIDUS) is sponsored by the MacArthur Foundation Research Network on Successful Midlife Development, the National Institute on Aging (P01-AG020166; U19-AG051426), and grants from the General Clinical Research Centers Program (M01-RR023942, M01-RR00865) and the National Center for Advancing Translational Sciences (UL1TR000427). MIDUS data are publicly available at https://midus.wisc.edu/. Funding for the English Longitudinal Study of Ageing is provided by the National Institute of Aging [grants 2RO1AG7644-01A1 and 2RO1AG017644] and a consortium of UK government departments coordinated by the Office for National Statistics. ELSA data are available from the UK Data Service (UKDS, https://www.ukdataservice.ac.uk/). The National Health and Aging Trends Study (NHATS) is sponsored by the National Institute on Aging (grant number NIA U01AG032947) through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health. NHATS data are available for public download at: http://www.nhats.org..The Wisconsin Longitudinal Study (WLS) has been supported principally by the National Institute on Aging (AG-9775, AG-21079, AG-033285, and AG-041868), with additional support from the Vilas Estate Trust, the National Science Foundation, the Spencer Foundation, and the Graduate School of the University of Wisconsin-Madison. A public use file of data is available at http://www.ssc.wisc.edu/wlsresearch/data/.
Financial Support:
The research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health (grant numbers R01AG068093, R01AG053297). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: None
References
- 1.Jackson H, Hubbard R. Detecting chronic obstructive pulmonary disease using peak flow rate: cross sectional survey. BMJ. 2003;327(7416):653–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodwin RD, Chuang S, Simuro N, Davies M, Pine DS. Association between lung function and mental health problems among adults in the United States: findings from the First National Health and Nutrition Examination Survey. Am J Epidemiol. 2007. 15;165(4):383–8. [DOI] [PubMed] [Google Scholar]
- 3.Fragoso CA, Gahbauer EA, Van Ness PH, Concato J, Gill TM. Peak expiratory flow as a predictor of subsequent disability and death in community-living older persons. J Am Geriatr Soc. 2008;56(6):1014–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trevisan C, Rizzuto D, Maggi S, Sergi G, Welmer AK, Vetrano DL. Cross-Sectional and Longitudinal Associations between Peak Expiratory Flow and Frailty in Older Adults. J Clin Med. 2019;8(11):1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts MH, Mapel DW. Limited lung function: impact of reduced peak expiratory flow on health status, health-care utilization, and expected survival in older adults. Am J Epidemiol. 2012;176(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith M, Zhou M, Wang L, Peto R, Yang G, Chen Z. Peak flow as a predictor of cause-specific mortality in China: results from a 15-year prospective study of ~170,000 men. Int J Epidemiol. 2013;42(3):803–815. [DOI] [PubMed] [Google Scholar]
- 7.Trevisan C, Rizzuto D, Sergi G, Maggi S, Welmer AK, Vetrano DL. Peak expiratory flow, walking speed and survival in older adults: An 18-year longitudinal population-based study. Exp Gerontol. 2020;135:110941. [DOI] [PubMed] [Google Scholar]
- 8.DeCarlo CA, MacDonald SW, Vergote D, Jhamandas J, Westaway D, Dixon RA. Vascular Health and Genetic Risk Affect Mild Cognitive Impairment Status and 4-Year Stability: Evidence From the Victoria Longitudinal Study. J Gerontol B Psychol Sci Soc Sci. 2016;71(6):1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russ TC, Kivimäki M, Batty GD. Respiratory Disease and Lower Pulmonary Function as Risk Factors for Dementia: A Systematic Review With Meta-analysis. Chest. 2020;157(6):1538–1558. [DOI] [PubMed] [Google Scholar]
- 10.Hallberg J, Iliadou A, Anderson M, et al. Genetic and environmental influence on lung function impairment in Swedish twins. Respir Res. 2010;11(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melbourne CA, Mesut Erzurumluoglu A, Shrine N, et al. Genome-wide gene-air pollution interaction analysis of lung function in 300,000 individuals. Environ Int. 2022;159:107041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarnoki DL, Tarnoki AD, Lazar Z, et al. Genetic and environmental factors on the relation of lung function and arterial stiffness. Respir Med. 2013;107(6):927–935. [DOI] [PubMed] [Google Scholar]
- 13.McCrae RR, John OP. An introduction to the five-factor model and its applications. J Pers 1992; 60(2) : 175–215. [DOI] [PubMed] [Google Scholar]
- 14.Friedman HS, Kern ML Personality, well-being, and health. Annu Rev Psychol. 2014;65:719–42. [DOI] [PubMed] [Google Scholar]
- 15.Hakulinen C, Hintsanen M, Munafò MR, Virtanen M, Kivimäki M, Batty GD, Jokela M. Personality and smoking: individual-participant meta-analysis of nine cohort studies. Addiction. 2015;110(11):1844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephan Y, Sutin AR, Luchetti M, Hognon L, Canada B, Terracciano A. Personality and self-rated health across eight cohort studies. Soc Sci Med. 2020;263:113245. [DOI] [PubMed] [Google Scholar]
- 17.Luchetti M, Barkley JM, Stephan Y, Terracciano A, Sutin AR. Five-factor model personality traits and inflammatory markers: new data and a meta-analysis. Psychoneuroendocrinology. 2014;50:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephan Y, Sutin AR, Luchetti M, Canada B, Terracciano A. Personality and HbA1c: Findings from six samples. Psychoneuroendocrinology. 2020;120:104782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aschwanden D, Strickhouser JE, Luchetti M, Stephan Y, Sutin AR, Terracciano A. Is personality associated with dementia risk? A meta-analytic investigation. Ageing Res Rev. 2021. ;67:101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham EK, Rutsohn JP, Turiano NA, et al. Personality Predicts Mortality Risk: An Integrative Data Analysis of 15 International Longitudinal Studies. J Res Pers. 2017;70:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephan Y, Sutin AR, Bovier-Lapierre G, Terracciano A. Personality and walking speed across adulthood: Prospective evidence from five samples. Soc Psychol Personal Sci. 2018; 9(7):773–780. [Google Scholar]
- 22.Stephan Y, Sutin AR, Canada B, Deshayes M, Kekäläinen T, Terracciano A. Five-factor model personality traits and grip strength: Meta-analysis of seven studies. J Psychosom Res. 2022. ;160:110961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terracciano A, Schrack JA, Sutin AR, Chan W, Simonsick EM, Ferrucci L. Personality, metabolic rate and aerobic capacity. PLoS One. 2013;8(1):e54746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephan Y, Sutin AR, Canada B, Terracciano A. Personality and Frailty: Evidence From Four Samples. J Res Pers. 2017. ;66:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magave JA, Bezerra SJS, Matos AP, Pinto ACPN, Pegorari MS, Ohara DG. Peak Expiratory Flow as an Index of Frailty Syndrome in Older Adults: A Cross-Sectional Study. J Nutr Health Aging. 2020;24(9):993–998. [DOI] [PubMed] [Google Scholar]
- 26.Sutin AR, Stephan Y, Luchetti M, Artese A, Oshio A, Terracciano A. The Five-Factor Model of personality and physical inactivity: A meta-analysis of 16 samples. J Res Pers. 2016; 63:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutin AR, Terracciano A. Personality traits and body mass index: Modifiers and mechanisms. Psychol Health. 2015;31:259–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutin AR, Costa PT Jr, Uda M, Ferrucci L, Schlessinger D, Terracciano A. Personality and metabolic syndrome. Age (Dordr). 2010;32(4):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baffi CW, Wood L, Winnica D, et al. Metabolic Syndrome and the Lung. Chest. 2016;149(6):1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benadjaoud MA, Menai M, van Hees VT, et al. The association between accelerometer-assessed physical activity and respiratory function in older adults differs between smokers and non-smokers. Sci Rep. 2019;9(1):10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Littleton SW, Tulaimat A. The effects of obesity on lung volumes and oxygenation. Respir Med. 2017;124:15–20 [DOI] [PubMed] [Google Scholar]
- 32.Oelsner EC, Balte PP, Bhatt SP, et al. Lung function decline in former smokers and low-intensity current smokers: a secondary data analysis of the NHLBI Pooled Cohorts Study. Lancet Respir Med. 2020;8(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terracciano A, Stephan Y, Luchetti M, Gonzalez-Rothi R, Sutin AR. Personality and Lung Function in Older Adults. J Gerontol B Psychol Sci Soc Sci. 2017. ;72(6):913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunn AJ, Gregg I. New regression equations for predicting peak expiratory flow in adults. BMJ. 1989. ;298(6680):1068–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimprich D, Allemand M, Lachman ME. Factorial structure and age-related psychometrics of the MIDUS personality adjective items across the life span. Psychol Assess. 2012. ;24(1):173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.John OP, Donahue EM, Kentle RL. The Big Five Inventory—Versions 4a and 54. Berkeley, CA: Institute of Personality and Social Research, University of California, 1991. [Google Scholar]
- 37.Strickhouser JE, Zell E, Krizan Z. Does personality predict health and well-being? A metasynthesis. Health Psychol. 2017;36(8):797–810. [DOI] [PubMed] [Google Scholar]
- 38.Leger KA, Turiano NA, Bowling W, Burris JL, Almeida DM. Personality Traits Predict Long-Term Physical Health via Affect Reactivity to Daily Stressors. Psychol Sci. 2021;32(5):755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plourde A, Lavoie KL, Raddatz C, Bacon SL. Effects of acute psychological stress induced in laboratory on physiological responses in asthma populations: A systematic review. Respir Med. 2017;127:21–32. [DOI] [PubMed] [Google Scholar]
- 40.Holmes SJ, Allen SC, Roberts HC. Relationship between lung function and grip strength in older hospitalized patients: a pilot study. Int J Chron Obstruct Pulmon Dis. 2017;12:1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanai M, Kanai O, Fujita K, Mio T, Ito M. Decreased handgrip strength can predict lung function impairment in male workers: a cross sectional study. BMC Pulm Med. 2020;20(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Son DH, Yoo JW, Cho MR, Lee YJ. Relationship Between Handgrip Strength and Pulmonary Function in Apparently Healthy Older Women. J Am Geriatr Soc. 2018;66(7):1367–1371. [DOI] [PubMed] [Google Scholar]
- 43.Sutin AR, Stephan Y, Luchetti M, Terracciano A. The prospective association between personality traits and persistent pain and opioid medication use. J Psychosom Res. 2019;123:109721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehto K, Pedersen NL, Almqvist C, Lu Y, Brew BK. Asthma and affective traits in adults: a genetically informative study. Eur Respir J. 2019;53(5):1802142. [DOI] [PubMed] [Google Scholar]
- 45.Stephan Y, Sutin AR, Luchetti M, Canada B, Terracciano A. Personality and fatigue: meta-analysis of seven prospective studies. Sci Rep. 2022;12(1):9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leger KA, Charles ST, Turiano NA, Almeida DM. Personality and stressor-related affect. J Pers Soc Psychol. 2016;111(6):917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo J, Zhang B, Cao M, Roberts BW. The Stressful Personality: A Meta-Analytical Review of the Relation Between Personality and Stress. Pers Soc Psychol Rev. 2022:10888683221104002. [DOI] [PubMed] [Google Scholar]
- 48.Chan T, Wanigatunga AA, Terracciano A, et al. Traits and treadmills: Association between personality and perceived fatigability in well-functioning community-dwelling older adults. Psychol Aging. 2021;36(6):710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simonsick EM, Glynn NW, Jerome GJ, Shardell M, Schrack JA, Ferrucci L. Fatigued, but Not Frail: Perceived Fatigability as a Marker of Impending Decline in Mobility-Intact Older Adults. J Am Geriatr Soc. 2016;64(6):1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hancox RJ, Gray AR, Sears MR, Poulton R. Systemic inflammation and lung function: A longitudinal analysis. Respir Med. 2016;111:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jokela M, Hakulinen C, Singh-Manoux A, Kivimäki M. Personality change associated with chronic diseases: pooled analysis of four prospective cohort studies. Psychol Med. 2014;44(12):2629–2640. [DOI] [PubMed] [Google Scholar]
- 52.Stieger M, Flückiger C, Rüegger D, Kowatsch T, Roberts BW, Allemand M. Changing personality traits with the help of a digital personality change intervention. Proc Natl Acad Sci U S A. 2021. ;118(8):e2017548118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.