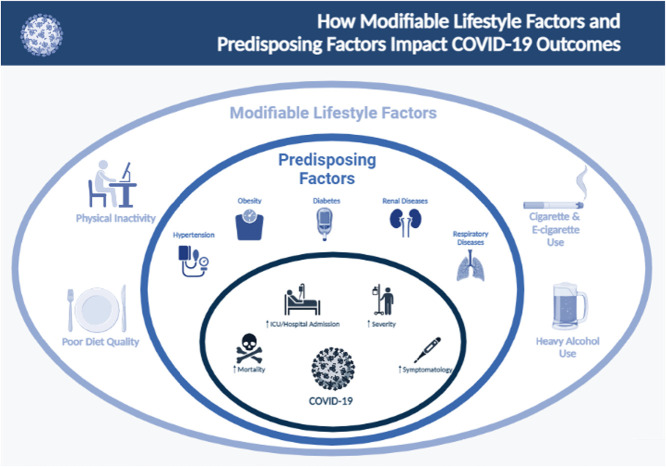
Abstract
The coronavirus disease 2019 (COVID-19) pandemic has affected >610 million people globally, exerting major social, economic, and health impacts. Despite the large number of global casualities and severe symptomatology associated with COVID-19, a large number of individuals remain at elevated risk of infection and severe outcomes related to poor lifestyle behaviors and/or associated comorbidities. Beyond the well-known social distance and masking policies, maintaining an active lifestyle, minimizing the consumption of tobacco products, and maintaining an adequate nutrition status are some of the key factors that, in an affordable and accessible way, have the potential to improve health and minimize COVID-19 impact. In addition, bringing awareness of the higher risks and poor prognosis of COVID-19 when other conditions are present seems to be essential to protect those individuals with the highest risks.
Keywords: COVID-19, Risk factors, Cardiovascular disease, Physical activity, Sedentary behavior
Graphical abstract
(Illustration created with BioRender)
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has affected >610 million people, causing over 6.5 million deaths globally.1 The infection, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, is characterized, in severe cases, by initial viral pneumonia with fever, cough, dyspnea, and hypoxemia followed by acute respiratory failure consistent with the acute respiratory distress syndrome.2 The COVID-19 pandemic was the cause of >1.8 million deaths globally in 2020 alone.1 The development of effective vaccines against the SARS-CoV-2 virus has been a major turning point for infection rate and associated severity, although more recent viral variants, such as Delta (late 2021) and Omicron (early 2022) remain associated with high severity and mortality.1 In addition, a large number of patients who were infected with SARS-CoV-2 continue to experience symptoms after the initial acute phase, and “Long COVID” has been defined as a post-acute sequela associated with this viral infection.3 Despite the global impact that COVID-19 had and the severity of immediate and long-term symptoms, a large number of individuals remain at elevated risk of infection and severe outcomes related to poor lifestyle behaviors and/or associated comorbidities. In this state-of-the-art narrative review, we will provide an update on the risk factors that may negatively contribute to worse outcomes in patients infected by SARS-CoV-2 and provide potential strategies to minimize this augmented risk (Table 1 ).
Table 1.
The effect of modifiable lifestyle factors and predisposing factors on the risk of COVID-19 infection, symptomatology, severity, ICU/hospital admission, and mortality.
| Infection | Symptomatology | Severity | ICU/Hospital Admission | Mortality | ||
|---|---|---|---|---|---|---|
| Modifiable Lifestyle Factors | Physical Inactivity | ↑ | ↑ | |||
| Cigarette Use–- Current | ? | ↑ | ↑ | ↑ | ||
| Cigarette Use–- Former | ↑ | ↑ | ||||
| E-Cigarettes | ? | |||||
| Dual Users Cigarettes & E-Cigarettes |
↑ | ↑ | ||||
| Heavy Alcohol Consumption | ↑ | ↑ | ||||
| Optimal Nutrition Status & a High Quality Diet | ↓ | ↓ | ||||
| Predisposing Factors | Hypertension | ↑ | ↑ | ↑ | ||
| Obesity | ↑ | ↑ | ↑ | |||
| Type 2 Diabetes Mellitus | ↑ | ↑ | ↑ | |||
| Renal Diseases | ↑ | ↑ | ||||
| Respiratory Diseases | ↑ | ↑ | ↑ | ↑ |
↑ = Increased risk of outcome; ↓ = Decreased risk of outcome;? = Limited evidence available as to the effect on the outcome. Renal diseases include the effects of chronic kidney disease. Respiratory diseases include the effects of chronic obstructive pulmonary disease, interstitial lung disease, and lung cancer.
Lifestyle factors
Physical activity (PA)
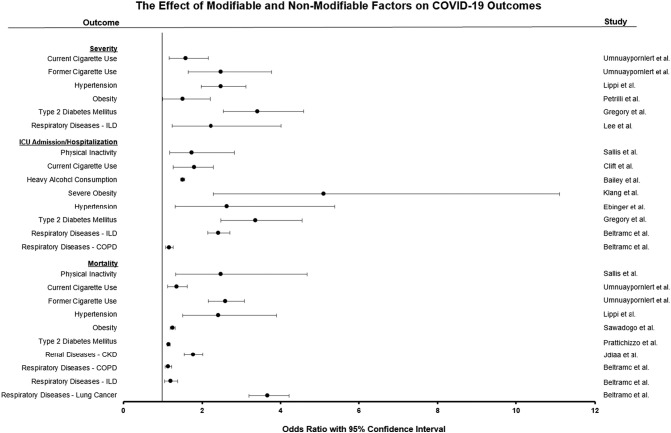
The Department of Health and Human Services established its guidelines for PA for United States (U.S.) adults as 150 min per week of moderate-intensity aerobic PA and at least two days of muscle-strengthening activities per week.4 Only one in four U.S. adults meet PA recommendations5; it appears sedentary behaviors (SB) have increased further during the COVID-19 pandemic.6 Taken together, these data raised concerns related to the physical inactivity (PI) and SB epidemic, ultimately resulting in a cascade of negative health effects.5 This is especially concerning given the link between PI and COVID-19 complications as outlined below7, 8, 9 (Fig. 1 ).
Fig. 1.
The effect of modifiable and not-modifiable factors on COVID-19 outcomes.
All values expressed as statistically significant odds ratios (OR). Information has been compiled from statistically significant ORs from the corresponding study. Severe obesity: individuals with a body mass index ≥ 40 kg/m2. CKD: Chronic kidney disease; COPD: Chronic obstructive pulmonary disease; ILD: Interstitial lung disease.
Clearly, PA is associated with a decreased risk of chronic non-communicable diseases such as dementia, depression, type 2 diabetes mellitus (T2DM), cardiovascular (CV) disease (CVD), and some types of cancer.5 , 6 Thus, it is not a surprise that sedentary individuals have more adverse health events compared to those who are physically active,10 even related to the risk of a more severe clinical course with SARS-CoV-2 infection.11 Current data support that patients with COVID-19 who did not meet the recommended weekly aerobic PA level exhibited an increased risk of hospitalization, ICU admission, and death related to COVID-19 complications.9 These findings are supported by a large meta-analysis including >5000 individuals showing that physical inactivity is associated with increased risk for COVID-19-related hospitalizations.7 To further stress the importance of PA on COVID-19 outcomes, a recent study found that, besides age and having a history of organ transplant, PI is the strongest risk factor for severe COVID-19 compared to other risk factors, such as smoking and more typical comorbidities.9 This is on par with other evidence supporting that regular PA is associated with increased resistance to infectious diseases.11 Specifically, higher levels of habitual PA were associated with a 31% and 37% relative risk reduction of community-acquired infectious disease and infectious disease mortality, respectively.11
Despite the positive effects of PA, the mechanisms behind the protection against severe COVID-19 outcomes are still unknown. A potential explanation is related to the multiple benefits that regular exercise confers to the CV12, 13, 14 and immune15, 16, 17 systems. For example, exercise protects CV health and reduces the development of endothelial dysfunction, characterized by diminished vasodilation and an enhanced prothrombotic and proinflammatory state.13 , 18 Notably, endothelial dysfunction is a key feature of CVD and is linked to unfavorable COVID-19 outcomes.19 Regarding immune modulation, moderate-intensity PA promotes the release of natural killer cells and viral-specific T-cells15 leading to decreased susceptibility to SARS-CoV2 infection.20 COVID-19 severity and mortality have also been correlated to significantly elevated levels of proinflammatory cytokines,20 which moderate-intensity PA can decrease.17 , 21 Additionally, physical activity has been shown to enhance vaccine responses, reduce pro-inflammatory mediators, increase the immune system, and decrease oxidative stress,21 all factors clearly involved in COVID-19 severity.20 , 22
Given the link between PA and COVID-19 risk, it is imperative that policymakers and health professionals emphasize the benefits associated with PA as an affordable, accessible, safe, and adaptable preventive strategy that can minimize the risk of COVID-19 clinical severity and mortality.
Cigarettes and E-cigarettes usage
The detrimental long-term health effects of cigarette smoking are well documented, including an increased risk for CVD and lung disease, various cancers, and diabetes.23 Despite this information being public knowledge, smoking is still common with 12.5% of individuals over 18 years old in the U.S. being frequent users.24 Given the global COVID-19 pandemic and the prevalence of cigarette smoking, the question of how cigarette usage alters susceptibility and related outcomes of COVID-19 is of critical importance.
The current literature presents overwhelming evidence that the use of conventional cigarettes is associated with COVID-19 risk of infection25 , 26 progression,27 , 28 , 29 severity,28 , 30 , 31 and mortality25 , 28 , 30 , 31 (Fig. 1). For example, smokers had 90% increased risk of COVID-19 severity progression.27 Furthermore, prevailing data found that current smokers have an increased risk of hospitalization25 , 30 and were 140% more likely to be admitted to the ICU when compared to those who never smoked.30
The evidence regarding the effect of cigarette smoking on the pathogenesis of those individuals diagnosed with COVID-19 leaves little room for debate, clearly highlighting smoking as negative prognosticator. However, there is still controversy regarding the risk of initial infection in smokers. Recent data supports that cigarette smoking is a significant risk factor for COVID-19 infection.25 , 26 On the other hand, a controversial hypothesis termed the “smokers' paradox” supports that, despite an increased severity of disease after infection with SARS-CoV-2, smoking cigarettes may reduce the risk of initial infection.32, 33, 34 Of note, the overwhelming scientific outcry has cautioned against the claim of this paradox.25 , 26 , 28 , 35 , 36
Apart from current smokers, past smoking history has also been associated with an increased COVID-19 severity risk and mortality. A large meta-analysis evaluating 40 studies identified that former smokers have a 150% greater risk of disease severity when compared to never-smokers. Moreover, former smokers exhibited 2.6 times increased risk of COVID-19-related mortality.31 Overall, it appears that both current and former smokers are at an increased risk of COVID-19 mortality and should be informed about this risk.
The mechanisms driving cigarette smoking to increased COVID-19 severity, hospitalization, and mortality remain largely unexplored. Current literature suggests that cigarettes cause upregulation of the angiotensin-converting enzyme 2 (ACE2) receptor, the same receptor that SARS-CoV-2 uses to infect host cells ultimately leading smokers to have increased viral loads and, therefore, worse outcomes.26 , 28 , 37 Although more research is needed, there is also evidence of the negative effects of cigarettes on the immune system.38 Examples include decreased levels of immunoglobulin (Ig) G in smokers when compared to non-smokers which could be classified as a moderate secondary immunodeficiency.39 Additionally, long-term nicotine exposure is known to suppress B lymphocyte development and proliferation, possibly explaining the decreased levels of IgG.39 Furthermore, nicotine has been shown to increase cytosolic calcium levels,40 with calcium playing a key role in the replication of viruses41 and SARS-CoV-2 host cell entry.42 The evidence that cigarettes impact the immune system is ample, however, more research is needed to determine the exact mechanisms causing the clinical outcomes seen for COVID-19/ in smokers.
Apart from traditional tobacco, it is important to discuss the role of e-cigarettes which have been rising in popularity in the last decade, particularly, among adolescents.43 Indeed, an alarming 11% of high school students and 3% of middle school students reported past 30-day e-cigarette use, representing ∼2.4 million adolescents in the U.S..44 Additionally, 14% of undergraduate students have used an e-cigarette in the 30-days prior to their survey, and close to 5% are daily users.45 However, despite their popularity, there is a paucity of data related to the long-term effects of e-cigarettes on health, with several studies currently ongoing to answer this important scientific question. Recent evidence suggests that e-cigarette use is associated with an increased risk of contracting SARS-CoV-2 infection,37 , 46 although conflicting results have also been published.47 , 48 A recent study found that e-cigarette users who are 13 to 24 years of age were five times more likely to be diagnosed with COVID-19 than never-users.46 Among the potential explanations behind those results are that e-cigarette users are less likely to adhere to social distancing practices49 or the possibility for the e-liquid to increase ACE2 expression and SARS-CoV-2 spike protein binding.37 Unlike traditional cigarette use, there is also little research investigating COVID-19 hospitalization and e-cigarette use. A lack of evidence should be approached with caution, as the demographics of cigarette versus e-cigarette users most notably diverge based on age, with e-cigarette users being substantially younger than cigarette users.24 , 43 Of note, e-cigarette use has been associated with a greater prevalence of asthma, increased susceptibility to pulmonary infections, and early indicators of CVD, all of which have the potential to affect COVID-19 outcomes.50
Finally, it is worthwhile to address how dual cigarette and e-cigarette users are affected by COVID-19. A recent study found that past 30-day dual-users were 4.7 times more likely to display symptoms of COVID-19 compared to never-users.46 Furthermore, it was found that both ever and past 30-day dual users were 7 times more likely to be infected with COVID-19 than never users.46 Similar to exclusive e-cigarette use, dual users were less likely to self-report adhering to social distancing practices compared to never-users.49 Although cigarettes and e-cigarettes pose their own unique risks regarding COVID-19, the above evidence suggests that their dual use may produce a negative synergistic effect on COVID-19 outcomes.
Before the COVID-19 pandemic, cigarette smoking was already responsible for >480,000 deaths annually in the U.S.23 Given the above evidence and the increased risk of negative COVID-19 outcomes with smoking, this number is expected to increase if more robust smoking cessation efforts are not conducted.27 , 30 , 51 , 52 Additionally, information regarding the possibility of increased risk among dual cigarette and e-cigarette users should be more widely disseminated,46 and adolescents and young adults should be further cautioned about the potential risk associated with e-cigarette usage.53
Alcohol consumption
While low-to-moderate alcohol consumption seems to have a neutral effect or even some potential protective health effect in some exceptional cases, heavy alcohol consumption exerts a well-known harmful effect.54 , 55 With regards to its relationship with COVID-19, certain types of alcohol (i.e., red wine, white wine, champagne) in moderate consumption have been associated with a lower risk for COVID-19 infection and hospitalization.56 , 57 Specifically, recent information from a meta-analysis evaluating >400,000 cases from the United Kingdom (UK) described that the consumption of red wine was associated with a lower risk for COVID-19.58 Similar associations were identified with white wine and champagne, regardless of frequency or amount.58 These potential effects have been attributed to the polyphenolic content presented in wine, which exerts cardiovascular beneficial properties that are independent of the presence of alcohol59 and minimize the risks for different viral infections such as influenza virus, enterovirus, or another coronavirus.60, 61, 62 In this line, a randomized, placebo-controlled, pilot study has described that the use of resveratrol, one of the primary polyphenols identified in wine, was associated with a lower incidence of COVID-19 as well as reduced risk of hospitalization when compared to placebo.63 However, it is important to emphasize that, consuming the proposed content of polyphenols through wine will require a very large amount of product and therefore, alcohol,64 which could mitigate any potential beneficial effect, so caution needs to be exerted when interpreting these results.
On the other hand, the adverse effects of heavy alcohol consumption have been largely documented. Related to COVID-19, it has been described that heavy alcohol consumption presents with a higher risk of infection, hospitalization, and poor prognosis58 , 65 , 66 (Fig. 1). Specifically, those consuming spirits more than five times per week seem to present the highest risk.58 It is well known that excessive alcohol consumption suppresses the immune system and increases the susceptibility to bacterial and viral infections.67 In addition, spirits present with the highest alcohol concentrations and the lowest polyphenolic content, minimizing the potential benefits attributed to other types of alcoholic beverages when consumed in moderation. Similarly, beer also presents with low concentrations of polyphenols,68 which has been suggested to be the primary reason behind the increased risk for COVID-19 infection among those that consume beer as opposed to red wine.58 These results contrast with the previous notion supporting the positive effects of moderate consumption of beer on CV and overall health.69
Although preclinical and epidemiological investigations suggest a potential cardioprotective effect of moderate wine consumption, with heavy alcohol consumption being associated with worse outcomes, the evidence related to the role of alcohol in COVID-19 remains preliminary, clearly requiring well-designed prospective randomized trials that will allow drawing meaningful conclusions. In the meantime, alcohol consumption should not be recommended to prevent or to treat COVID-19.
Nutrition status, dietary intervention, and dietary supplements
Nutrition status, dietary intake, and dietary intervention play a crucial role in the development and progression of several cardiometabolic diseases; however, their role in the development of COVID-19 or the progression of the disease has been minimally investigated.70 The limited evidence available, however, suggest that nutritional status and diet quality might play a role in the development of COVID-19, while the use of dietary supplements has resulted in disappointing results.
With regards to nutritional status, individuals with frailty, but not those with malnutrition and sarcopenia, present a ∼ 7-fold greater risk to develop COVID-19.71 Among the different domains of frailty, reduced PA was the strongest predictor of the risk of COVID-19,71 which is in line with what we have described earlier in this review. However, in patients diagnosed with COVID-19, patients diagnosed with malnutrition present a worse prognosis compared to those without malnutrition, in both children and adults.72 This suggests that although the presence of malnutrition has not been associated with the risk of COVID-19, maintaining an optimal nutritional status has the potential to prevent severe disease progression in those diagnosed with COVID-19. Despite this initial evidence that nutritional status might influence the risk and progression of COVID-19, prospective trials aimed at its modulation are urgently needed to determine whether worse nutritional status may play a causative role in the development and progression of COVID-19 or whether it is a mere marker of disease severity.
With regard to diet, the role of dietary patterns has also been investigated in a few studies. In an analysis of 592,571 individuals enrolled in the COVID-19 Symptom Study, individuals with a pre-pandemic diet with a higher quality analyzed with food frequency questionnaires presented a 9% lower relative risk reduction to develop COVID-19 in the first place as well as a 41% lower relative risk reduction to develop severe COVID-19 when COVID-19 was diagnosed. Similarly, individuals with a greater adherence to a Mediterranean dietary pattern presented a lower risk for the development of COVID-19 (OR = 0.64, 95% CI: 0.42–0.98),73 with no individual components of the Mediterranean diet having an independent role, suggesting that the overall adherence to the Mediterranean dietary pattern is required to potentially exert its protective effects. Similarly, other studies have also found a greater adherence to the Mediterranean diet to be associated with a lower risk of COVID-19.74 , 75 Although we and others have hypothesized that a diet able to activate a pro-inflammatory response might increase the risk for COVID-19 and its related progression,74 to date, no studies have prospectively investigated whether a diet rich in nutrients with anti-inflammatory properties can modulate the history of the disease.
With regards to dietary intervention and dietary supplementation strategies, a focus has been placed on dietary supplementation, particularly on vitamin C and vitamin D. Of note, appropriately powered randomized trials with a dietary intervention using food or nutrition support (i.e., enteral and parenteral nutrition) have not been conducted to date. A few studies have, however, investigated the role of vitamin C and of vitamin D in randomized controlled studies. While initial findings suggested that individuals with the lower plasma level of vitamin D and C presented a greater risk for COVID-19 and COVID-19-related progression,76 , 77 interventions aimed at supplementing such nutrients did not show clinical benefits. For vitamin C, a meta-analysis of 6 randomized controlled trials found that the intervention did not improve survival nor the length of stay when admitted to the hospital with COVID-19, independent of whether vitamin C was administered orally or intravenously.78 Similarly, a meta-analysis of randomized controlled trials investigating the role of vitamin D in patients with COVID-19 found no benefits in this population.79 Taken together, the available evidence does not support the use of vitamin C or vitamin D to prevent and treat COVID-19 nor of any other dietary supplements for which adequately powered randomized trials should be conducted.70
Predisposing factors
Hypertension
Multiple reports have supported that people living with hypertension are at higher risk of severe COVID-19 and associated mortality80 , 81 (Fig. 1), even after full vaccination.82 Indeed, the presence of high blood pressure has been associated with a nearly 2.5-fold higher risk of severe COVID-19, hospitalization, and mortality.83 In a recent comparative analysis of over 900,000 patients with COVID-19 from the U.S., 26% of hospitalizations were attributed to prevailing hypertension.84 Thus, supporting evidence indicates that patients with a history of hypertension are at higher risk of developing severe outcomes related to COVID-19. To note, the elevated risk is still presented with different SARS-CoV-2 variants or even after vaccination.85 However, contradictory data is also available, suggesting that age, rather than hypertension per se, is driving prevailing observations.86 Along this line, a large population-based study involving over 17 million patients from the UK reported a lack of association between hypertension, COVID-19, and mortality risk,80 while identifying that age plays a significant role in the association between COVID-19 infection and worse outcomes.80 Similar results have been also reported in other studies in smaller sample size,86 questioning if elevated blood pressure was independently associated with adverse prognosis when COVID-19 infection was presented, or if age mediates such relationship. Considering that mean blood pressure increases with age and the greatest burden of hypertension is in individuals that are 60 years or older,87 differentiating the effects of each other is difficult, however, if interventions aimed at reducing blood pressure improved COVID-19 related outcomes, it would suggest an independent role of hypertension. Of note, such studies are lacking.
Looking at the mechanisms of infection of the virus, as described above, the SARS-CoV-2 binds to the ACE2 receptors that allow the entry of the virus into the respiratory tract system.88 ACE2 is a well-known regulator of blood pressure homeostasis by modulating the renin-angiotensin system (RAS).89 , 90 Certain hypertension treatments with RAS-blockers increase the expression of ACE2, which initially was hypothesized as a potentially greater risk of COVID-19.91 In addition, different populations with CVD present with an elevated expression of ACE2, which has been proposed as an increased risk for the severe condition if infected by SARS-CoV2.92 However, very limited data is still available to support this hypothesis,93, 94, 95 and discontinuation of these drugs has not been strongly discouraged by the scientific community.96 Indeed, several reports have described beneficial effects related to the use of RAS inhibitors and COVID-19 clinical outcomes in patients with hypertension97, 98, 99, 100 including lower viral load,97 lower risk of severe COVID-19,98 and better overall clinical outcomes.93 , 98 Even after adjusting for potential confounding factors, results support lower COVID-19-related mortality in patients who received RAS inhibitor therapies.98 Two potential hypotheses have been suggested to explain this potential protective effect. One is related to a reduced pro-inflammatory response associated with the use of RAS inhibitors.97 The second suggests an upregulating of the immune system through an increased epithelial-immune cell interaction after RAS treatments.101 Thus, blood pressure-lowering drugs may provide a dual benefit by inhibiting the inflammatory response and by upregulating the immune system in response to SARS-CoV-2 infection.
Considering that hypertension is the world's leading risk factor for CVD, strategies aimed at minimizing the risk related to COVID-19 infection when high blood pressure is present is an urgent need. Changes in lifestyle factors including a greater diet quality with strategies proven to improve blood pressure such as controlling sodium intake, in association with increased daily PA and treating excess adiposity associated with elevated blood pressure (i.e., obesity) are all key determinants and could play a major role in reducing COVID-19-related severity in individuals with hypertension.
Obesity and T2DM
Obesity and T2DM have been consistently associated with a greater risk for COVID-19-related hospitalization as well as worse prognosis, as previously described elsewhere.102, 103, 104, 105
Patients with obesity are approximately three times more likely to be hospitalized for COVID-19 and to be diagnosed with severe COVID-19 (Fig. 1). A recent meta-analysis of 208 studies with >3 million individuals from >32 countries found that obesity was associated with a 72% and 25% relative risk increase for COVID-19-related hospitalization and death, respectively.102 Particularly, in individuals who are 50 years of age or younger, class III or severe obesity (body mass index ≥ 40 kg/m2) is an independent risk factor for COVID-19-related in-hospital mortality (OR = 5.1; 95% CI: 2.3–11.1),106 further emphasizing the crucial role that obesity places in the progression of COVID-19, even in younger individuals. These results highlight the importance of preventing obesity in the first place, but also, placing greater effort into preventive strategies for COVID-19 in patients with obesity considering their augmented risk for worse outcomes when COVID-19 is diagnosed.
Similar to obesity, T2DM and glycemic control are also independent risk factors for worsening COVID-19107 (Fig. 1). In 174 prospective patients with COVID-19, those with T2DM had a greater risk for severe pneumonia and they presented greater markers of systemic inflammation, both strong prognosticators with negative clinical implications in COVID-19. In addition to the presence of T2DM, even among those with T2DM, worse glycemic control is associated with a worse prognosis in patients with established COVID-19. Great glycemic control measured as glycated hemoglobin measured prior to a COVID-19 diagnosis has been associated with a 15% relative risk increase for COVID-19-related mortality.108 Moreover, patients with COVID-19 with a lower glycemic variability measured with a continuous glucose monitoring device during hospitalization, presented a more favorable survival with an 84% relative risk reduction for all-cause mortality compared to those with greater glycemic variability.109
Taken together, these results suggest that metabolic diseases such as obesity and T2DM are strong predictors for worse outcomes in patients with COVID-19. These results also suggest that treating obesity and obesity-related metabolic abnormalities and improving glycemic control in patients with COVID-19 represent potential beneficial therapeutic strategies in this population. Ultimately, randomized controlled trials targeting obesity and/or T2DM with pharmacologic as well as nonpharmacologic approaches may have the potential to improve outcomes in patients diagnosed with COVID-19.
Renal diseases
It is estimated that 37 million people are at an increased risk of COVID-19 progression and mortality due to renal disorders such as chronic kidney disease (CKD)110 (Fig. 1). A recent population study in England evaluating 17 million people suggested that CKD, secondary to age,111 was the strongest risk factor for severe COVID-19.80 Indeed, CKD may contribute to 4% of the 22% of the world's population that is at risk for severe COVID-19.111 , 112 Considering the severity of CKD and that its incidence increases with age,110 it is not surprising that CKD is associated with a higher risk of COVID-19 progression113 and represents an independent risk factor for COVID-19-related mortality.80 In addition, those with more severe CKD, as evaluated by reduced estimated glomerular filtration rate (eGFR), are also at higher risk of severe COVID-19.114 For example, compared to those without CKD, moderately decreased eGFR and severely decreased eGFR were associated with 1.33 and 2.52 increased risk of COVID-19 mortality, respectively.80 Similar information has been identified in moderate to severe CKD stages with 1.46 and 2.84 increased risk of COVID-19 mortality, respectively.114 In addition, data from 68 hospitals across the U.S. described that patients with COVID-19 and concomitant CKD requiring dialysis had a median time from symptom onset to intensive care unit (ICU) admission of 4 days, compared to 7 days for those non-dialysis-dependent.113 Furthermore, 50% of patients with CKD and COVID-19 died within 28 days of ICU admission, compared to 35% of patients without CKD.113 These findings emphasize that patients with CKD have a greater risk for severe COVID-19 than the general population and that the severity of the disease further correlates with worsening COVID-19 progression and outcome, although the underlying mechanisms that link both conditions are still unclear. Several reports have suggested that uremia may impair the immune system and subsequently, reduce the ability to fight infections.113 , 115 The high expression of the ACE2 receptor in the kidney116 as well as the cytokine-storm associated with SARS-CoV-2 infection117 have been also proposed as mechanisms linking both conditions. In addition, the high prevalence of comorbidities presented in patients with CKD may also increase the risk for COVID-19 poor outcomes.115 Indeed, CKD and concomitant CVD-associated mortality accounts for ∼40% of all deaths of patients with advanced CKD118. As explained before, CVD also increases the risk for severe outcomes when infected by SARS-CoV-2.119 Thus, it is not surprising that managing risk factors that contribute to those conditions (i.e. high blood pressure or blood sugar) have been emphasized as a key strategy to protect the health of patients with CKD.110
Considering that CKD is a major risk factor for severe COVID-19 and mortality, further studies are needed to determine the mechanisms linking both conditions to reduce the risk and increased treatment options. In addition, emphasizing the role that lifestyle factors and other comorbidities play in the development of this disease seems to be critical to reducing their risk for severe outcomes if infected by SARS-CoV-2.
Respiratory diseases
The presence of lung disorders is frequently associated with overall worse outcomes as well as with other comorbidities, including CVD120. Thus, it is not surprising that respiratory diseases are associated with worse COVID-19 outcomes and increased risk of hospitalization121 (Fig. 1). Even after controlling for multiple demographic factors including age, sex, body mass, smoking status, and other comorbidities, individuals with pre-existing lung disorders were at increased risk of hospitalization.122
When evaluating specific pulmonary disorders, patients with chronic obstructive pulmonary disease (COPD) present with a higher risk of severe disease,123 hospitalization 124 , and death due to COVID-19.121 Among the potential factors increasing the risk, smoking, which represents the most important risk factor for COPD, is a well-established risk for COVID-19 infection, as previously described.125 Other risk factors that may also play a role in increasing the risk of COVID-19 in patients with COPD are prior poor adherence to therapy,126 limited access to medical care during pandemic,127 , 128 greater ACE2 expression in bronchial epithelial cells126 or worse lung function and therefore, associated lower oxygen levels.127 , 128 Individuals with COPD are also at higher risk of presenting with elevated systemic inflammation, which could provide a lung environment more prone to SARS-CoV-2 severe infection.129
Similarly, other lung disorders including patients with preexisting interstitial lung disease (ILD), also exhibit a higher risk of both acquiring and having worse outcomes related to COVID-19 disease.130 , 131 Patients with ILD infected with SARS-CoV2 required more often oxygen therapy, have higher ICU admissions, need mechanical ventilation, and have higher mortality.130 Possible explanations proposed for the increased risk have been impaired lung function, high risk to develop pulmonary exacerbations, or medication that interacts with viral clearance.132 , 133
On the other hand, contradictory information has been reported for other lung disorders such as asthma, a highly prevalent lung disease with >300 million cases globally.134 , 135 At the beginning of the pandemic, there were multiple concerns that individuals with asthma had a greater vulnerability to SARS-CoV-2 infection.135 However, several meta-analyses evaluating large sample sizes from different regions in the world have confirmed that asthma was not associated with greater COVID-19 risk, severity, or worse prognosis when compared to individuals without asthma.136 , 137
In the case of lung cancer, patients are at twice the risk of hospital admission than people without lung cancer and their associated risk of death was 77% higher than the general population.121 An increased risk has been proposed to be associated with lung cancer treatments including immunotherapy, chemotherapy, or radiation therapy that may increase the inflammatory response and reduce the immune response to SARS-CoV-2 infection.138 Other possible explanations have been related to possible delays in cancer care with longer quarantine recommendations for immunocompromised individuals and longer waiting times for treatments.139
In summary, most people with lung disease present with a higher risk for severe COVID-19. Patients with different respiratory disorders should follow basic infection-control measures to prevent SARS-CoV-2 infection, as emphasized by four major lung health medical societies in the U.S..140 In addition, improving lifestyle behaviors seems to be critical for reducing COVID-19 risks in individuals with lung disorders. As previously explained, emphasizing the risks associated with smoking is crucial, as well as encouraging patients to be physically active through pulmonary rehabilitation programs126 or through the use of virtual technology to maintain patients active even when at home141 and minimize COVID-19 risk and severity.
Conclusions
The COVID-19 pandemic has exerted major social, economic, and health impacts and placed society at its highest limits. With the larger number of global casualties related to SARS-CoV-2 infection and severe symptomatology that, in some cases, leave significant sequelae in the form of Long-COVID syndrome, it is imperative to minimize the burden associated with this infection. Beyond the well-known social distance and masking, different preventive strategies have been proposed aimed at reducing the risk or the severity of this viral infection. Maintaining an active lifestyle, minimizing the consumption of tobacco products, and maintaining an adequate nutrition status are some of the key factors that, in an affordable and accessible way, have the potential to improve health and minimize COVID-19 impact. In addition, bringing awareness of the higher risks and poor prognosis of COVID-19 when other conditions are present seems to be essential to protect those individuals that present with the highest risk for severe outcomes.
Disclosures
No conflicts of interest are declared by the authors.
Grants
P.R.-M. is supported by an American Heart Association Career Development Award (18CDA34110323), by a Rapid Response Project Awarded via NIDA and FDA Center for Tobacco Products (U54DA036105), by the Clinical and Translational Science Awards Program from NIH to Virginia Commonwealth University (UL1TR002649), and by the Child Health Research Institute of Children's Hospital of Richmond at Virginia Commonwealth University.
A.H. is supported by the Virginia Commonwealth University School of Medicine Dean's Summer Fellowship, under P.R.-M. mentorship.
S.C. is supported by an American Heart Association Career Development Award (19CDA34660318) and by the Clinical and Translational Science Awards Program from NIH to Virginia Commonwealth University (UL1TR002649).
Declaration of Competing Interest
None.
References
- 1.Organization WH . 2022. WHO Coronavirus Dashboard. [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michelen M., Manoharan L., Elkheir N., et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021:6. doi: 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Services DoHaH . 2nd ed. 2023. Physical Activity Guidelines for Americans - Executive Summary; pp. 1–7. [Google Scholar]
- 5.Prevention CfDCa . Vol. 2022. U.S. Department of Health & Human Services; 2022. Physical Activity and COVID-19. [Google Scholar]
- 6.Amini H., Habibi S., Islamoglu A.H., Isanejad E., Uz C., Daniyari H. COVID-19 pandemic-induced physical inactivity: the necessity of updating the global action plan on physical activity 2018-2030. Environ Health Prev Med. 2021;26:32. doi: 10.1186/s12199-021-00955-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill A.L., Whitfield G., Morford M., et al. 2023. Brief Summary of Findings on the Association Between Physical Inactivity and Severe COVID-19 Outcomes; p. 63. [Google Scholar]
- 8.Tavakol Z., Ghannadi S., Tabesh M.R., et al. Relationship between physical activity, healthy lifestyle and COVID-19 disease severity; a cross-sectional study. Z Gesundh Wiss. 2021:1–9. doi: 10.1007/s10389-020-01468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallis R., Young D.R., Tartof S.Y., et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br J Sports Med. 2021;55:1099–1105. doi: 10.1136/bjsports-2021-104080. [DOI] [PubMed] [Google Scholar]
- 10.Park J.H., Moon J.H., Kim H.J., Kong M.H., Oh Y.H. Sedentary lifestyle: overview of updated evidence of potential health risks. Korean J Fam Med. 2020;41:365–373. doi: 10.4082/kjfm.20.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chastin S.F.M., Abaraogu U., Bourgois J.G., et al. Effects of regular physical activity on the immune system, vaccination and risk of community-acquired infectious disease in the general population: systematic review and Meta-analysis. Sports Med. 2021;51:1673–1686. doi: 10.1007/s40279-021-01466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crisafulli A., Pagliaro P. Physical activity/inactivity and COVID-19. Eur J Prev Cardiol. 2021:28. doi: 10.1177/2047487320927597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Francescomarino S., Sciartilli A., Di Valerio V., Di Baldassarre A., Gallina S. The effect of physical exercise on endothelial function. Sports Med. 2009;39:797–812. doi: 10.2165/11317750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Nystoriak M.A., Bhatnagar A. Cardiovascular effects and benefits of exercise. Front Cardiovasc Med. 2018;5:135. doi: 10.3389/fcvm.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duggal N.A., Niemiro G., Harridge S.D.R., Simpson R.J., Lord J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. 2019;19:563–572. doi: 10.1038/s41577-019-0177-9. [DOI] [PubMed] [Google Scholar]
- 16.da Silveira M.P., da Silva Fagundes K.K., Bizuti M.R., et al. Physical exercise as a tool to help the immune system against COVID-19: an integrative review of the current literature. Clin Exp Med. 2021;21:15–28. doi: 10.1007/s10238-020-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieman D.C. Coronavirus disease-2019: a tocsin to our aging, unfit, corpulent, and immunodeficient society. J Sport Health Sci. 2020;9:293–301. doi: 10.1016/j.jshs.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajendran P., Rengarajan T., Thangavel J., et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavriilaki E., Anyfanti P., Gavriilaki M., Lazaridis A., Douma S., Gkaliagkousi E. Endothelial dysfunction in COVID-19: lessons learned from coronaviruses. Curr Hypertens Rep. 2020;22:63. doi: 10.1007/s11906-020-01078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohn M.K., Hall A., Sepiashvili L., Jung B., Steele S., Adeli K. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology (Bethesda) 2020;35:288–301. doi: 10.1152/physiol.00019.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieman D.C., Wentz L.M. The compelling link between physical activity and the body’s defense system. J Sport Health Sci. 2019;8:201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietrobon A.J., Teixeira F.M.E., Sato M.N. I mmunosenescence and Inflammaging: risk factors of severe COVID-19 in older people. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.579220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prevention CfDCa . Vol. 2022. U.S. Department of Health & Human Services; 2022. Fast Facts. [Google Scholar]
- 24.Prevention CfDCa . Vol. 2022. U.S. Department of Health & Human Services; 2022. Burden of Cigarette Use in the U.S. [Google Scholar]
- 25.Clift A.K., von Ende A., Tan P.S., et al. Smoking and COVID-19 outcomes: an observational and Mendelian randomisation study using the UK biobank cohort. Thorax. 2022;77:65–73. doi: 10.1136/thoraxjnl-2021-217080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haddad C., Bou Malhab S., Sacre H., Salameh P. Smoking and COVID-19: a scoping review. Tob Use Insights. 2021;14:1–9. doi: 10.1177/1179173X21994612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patanavanich R., Glantz S.A. Smoking is associated with COVID-19 progression: a Meta-analysis. Nicotine Tob Res. 2020;22:1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy R.K., Charles W.N., Sklavounos A., Dutt A., Seed P.T., Khajuria A. The effect of smoking on COVID-19 severity: a systematic review and meta-analysis. J Med Virol. 2020;93:1045–1056. doi: 10.1002/jmv.26389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu W., Tao Z.W., Wang L., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta A.K., Nethan S.T., Mehrotra R. Tobacco use as a well-recognized cause of severe COVID-19 manifestations. Respir Med. 2021;176 doi: 10.1016/j.rmed.2020.106233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umnuaypornlert A., Kanchanasurakit S., Lucero-Prisno D.E.I., Saokaew S. Smoking and risk of negative outcomes among COVID-19 patients: a systematic review and meta-analysis. Tob Induc Dis. 2021;19:09. doi: 10.18332/tid/132411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hippisley-Cox J., Young D., Coupland C., et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106:1503–1511. doi: 10.1136/heartjnl-2020-317393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paleiron N., Mayet A., Marbac V., et al. Impact of tobacco smoking on the risk of COVID-19: a large scale retrospective cohort study. Nicotine Tob Res. 2021;23:1398–1404. doi: 10.1093/ntr/ntab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prinelli F., Bianchi F., Drago G., et al. Association between smoking and SARS-CoV-2 infection: cross-sectional study of the EPICOVID19 internet-based survey. JMIR Public Health Surveill. 2021;7 doi: 10.2196/27091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usman M.S., Siddiqi T.J., Khan M.S., et al. Is there a smoker’s paradox in COVID-19? BMJ Evid Based Med. 2021;26:279–284. doi: 10.1136/bmjebm-2020-111492. [DOI] [PubMed] [Google Scholar]
- 36.Wenzl T. Smoking and COVID-19: did we overlook representativeness? Tob Induc Dis. 2020;18:89. doi: 10.18332/tid/129584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh A., Girish V., Yuan M.L., et al. Combustible and electronic cigarette exposures increase ACE2 activity and SARS-CoV-2 spike binding. Am J Respir Crit Care Med. 2022;205:129–133. doi: 10.1164/rccm.202106-1377LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lugg S.T., Scott A., Parekh D., Naidu B., Thickett D.R. Cigarette smoke exposure and alveolar macrophages: mechanisms for lung disease. Thorax. 2022;77:94–101. doi: 10.1136/thoraxjnl-2020-216296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarbiah N., Todd I., Tighe P.J., Fairclough L.C. Cigarette smoking differentially affects immunoglobulin class levels in serum and saliva: an investigation and review. Basic Clin Pharmacol Toxicol. 2019;125:474–483. doi: 10.1111/bcpt.13278. [DOI] [PubMed] [Google Scholar]
- 40.Zia S., Ndoye A., Nguyen V.T., Grando S.A. Nicotine enhances expression of the alpha 3, alpha 4, alpha 5, and alpha 7 nicotinic receptors modulating calcium metabolism and regulating adhesion and motility of respiratory epithelial cells. Res Commun Mol Pathol Pharmacol. 1997;97:243–262. [PubMed] [Google Scholar]
- 41.Zhou Y., Frey T.K., Yang J.J. Viral calciomics: interplays between Ca2+ and virus. Cell Calcium. 2009;46:1–17. doi: 10.1016/j.ceca.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai A.L., Freed J.H. SARS-CoV-2 fusion peptide has a greater membrane Perturbating effect than SARS-CoV with highly specific dependence on Ca. J Mol Biol. 2021;433 doi: 10.1016/j.jmb.2021.166946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sapru S., Vardhan M., Li Q., Guo Y., Li X., Saxena D. E-cigarettes use in the United States: reasons for use, perceptions, and effects on health. BMC Public Health. 2020;20:1518. doi: 10.1186/s12889-020-09572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gentzke A.S., Wang T.W., Cornelius M., et al. Tobacco product use and associated factors among middle and high school students — National Youth Tobacco Survey, United States, 2021. MMWR Surveil Summ Cent Disase Contr Prev. 2022:1–30. doi: 10.15585/mmwr.ss7105a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Association ACH . Silver Spring; 2019. American College Health Association-National College Health Assessment II: Undergraduate Student Executive Summary Spring; pp. 1–19. MD2019. [Google Scholar]
- 46.Gaiha S.M., Cheng J., Halpern-Felsher B. Association between youth smoking, electronic cigarette use, and COVID-19. J Adolesc Health. 2020;67:519–523. doi: 10.1016/j.jadohealth.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benowitz N.L., Goniewicz M.L., Halpern-Felsher B., et al. Tobacco product use and the risks of SARS-CoV-2 infection and COVID-19: current understanding and recommendations for future research. Lancet Respir Med. 2022;10:900–915. doi: 10.1016/S2213-2600(22)00182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burnett-Hartman A.N., Goldberg Scott S., Powers J.D., et al. The Association of Electronic Cigarette use with SARS-CoV-2 infection and COVID-19 disease severity. Tob Use Insights. 2022;15 doi: 10.1177/1179173X221096638. 1179173X221096638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen D.T., Kyriakos C.N. Cigarette and E-cigarettes dual users, exclusive users and COVID-19: findings from four UK birth cohort studies. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18083935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wold L.E., Tarran R., Crotty Alexander L.E., et al. Cardiopulmonary consequences of vaping in adolescents: a scientific statement from the American Heart Association. Circ Res. 2022;131:e70–e82. doi: 10.1161/RES.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 51.Alqahtani J.S., Oyelade T., Aldhahir A.M., et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and Meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alqahtani J.S., Aldhahir A.M., Oyelade T., Alghamdi S.M., Almamary A.S. Smoking cessation during COVID-19: the top to-do list. NPJ Prim Care Respir Med. 2021;31:22. doi: 10.1038/s41533-021-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merianos A.L., Russell A.M., Mahabee-Gittens E.M., Barry A.E., Yang M., Lin H.C. Assessment of exclusive, dual, and Polytobacco E-cigarette use and COVID-19 outcomes among college students. Am J Health Promot. 2022;36:421–428. doi: 10.1177/08901171211055904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukamal K.J., Conigrave K.M., Mittleman M.A., et al. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. 2003;348:109–118. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]
- 55.Ronksley P.E., Brien S.E., Turner B.J., Mukamal K.J., Ghali W.A. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342 doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang B.H., Inan-Eroglu E., Shaban R.Z., Hamer M., Britton A., Stamatakis E. Alcohol intake and mortality risk of COVID-19, pneumonia, and other infectious diseases: an analysis of 437191 UK biobank participants. Prev Med Rep. 2022;26 doi: 10.1016/j.pmedr.2022.101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamer M., Kivimaki M., Gale C.R., Batty G.D. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai X.J., Tan L., Ren L., Shao Y., Tao W., Wang Y. COVID-19 risk appears to vary across different alcoholic beverages. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.772700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruf J.C. Overview of epidemiological studies on wine, health and mortality. Drugs Exp Clin Res. 2003;29:173–179. [PubMed] [Google Scholar]
- 60.Annunziata G., Maisto M., Schisano C., et al. Resveratrol as a novel anti-herpes simplex virus nutraceutical agent: an overview. Viruses. 2018:10. doi: 10.3390/v10090473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faith S.A., Sweet T.J., Bailey E., Booth T., Docherty J.J. Resveratrol suppresses nuclear factor-kappaB in herpes simplex virus infected cells. Antiviral Res. 2006;72:242–251. doi: 10.1016/j.antiviral.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Lin S.C., Ho C.T., Chuo W.H., Li S., Wang T.T., Lin C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17:144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCreary M.R., Schnell P.M., Rhoda D.A. Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19) Sci Rep. 2022;12:10978. doi: 10.1038/s41598-022-13920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiskirchen S., Weiskirchen R. Resveratrol: how much wine do you have to drink to stay healthy? Adv Nutr. 2016;7:706–718. doi: 10.3945/an.115.011627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lassen M.C.H., Skaarup K.G., Sengelov M., et al. Alcohol consumption and the risk of acute respiratory distress syndrome in COVID-19. Ann Am Thorac Soc. 2021;18:1074–1076. doi: 10.1513/AnnalsATS.202008-988RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bailey K.L., Sayles H., Campbell J., et al. COVID-19 patients with documented alcohol use disorder or alcohol-related complications are more likely to be hospitalized and have higher all-cause mortality. Alcohol Clin Exp Res. 2022;46:1023–1035. doi: 10.1111/acer.14838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szabo G., Saha B. Alcohol’s effect on host defense. Alcohol Res. 2015;37:159–170. [PMC free article] [PubMed] [Google Scholar]
- 68.Radonjic S., Maras V., Raicevic J., Kosmerl T. Wine or beer? Comparison, changes and improvement of polyphenolic compounds during technological phases. Molecules. 2020;25 doi: 10.3390/molecules25214960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spaggiari G., Cignarelli A., Sansone A., Baldi M., Santi D. To beer or not to beer: a meta-analysis of the effects of beer consumption on cardiovascular health. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mechanick J.I., Carbone S., Dickerson R.N., et al. Clinical nutrition research and the COVID-19 pandemic: a scoping review of the ASPEN COVID-19 task force on nutrition research. JPEN J Parenter Enteral Nutr. 2021;45:13–31. doi: 10.1002/jpen.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lengele L., Locquet M., Moutschen M., et al. Frailty but not sarcopenia nor malnutrition increases the risk of developing COVID-19 in older community-dwelling adults. Aging Clin Exp Res. 2022;34:223–234. doi: 10.1007/s40520-021-01991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurtz A., Grant K., Marano R., et al. Long-term effects of malnutrition on severity of COVID-19. Sci Rep. 2021;11:14974. doi: 10.1038/s41598-021-94138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez-Araluce R., Martinez-Gonzalez M.A., Gea A., Carlos S. Components of the Mediterranean diet and risk of COVID-19. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.805533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arena R., Bond S., Calvo I.R., et al. Shelter from the cytokine storm: healthy living is a vital preventative strategy in the COVID-19 era. Prog Cardiovasc Dis. 2022;73:56–60. doi: 10.1016/j.pcad.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perez-Araluce R., Martinez-Gonzalez M.A., Fernandez-Lazaro C.I., Bes-Rastrollo M., Gea A., Carlos S. Mediterranean diet and the risk of COVID-19 in the “Seguimiento Universidad de Navarra” cohort. Clin Nutr. 2022;41(12):3061–3068. doi: 10.1016/j.clnu.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teshome A., Adane A., Girma B., Mekonnen Z.A. The impact of vitamin D level on COVID-19 infection: systematic review and Meta-analysis. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.624559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tomasa-Irriguible T.M., Bielsa-Berrocal L. COVID-19: up to 82% critically ill patients had low vitamin C values. Nutr J. 2021;20:66. doi: 10.1186/s12937-021-00727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rawat D., Roy A., Maitra S., Gulati A., Khanna P., Baidya D.K. Vitamin C and COVID-19 treatment: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. 2021;15 doi: 10.1016/j.dsx.2021.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hosseini B., El Abd A., Ducharme F.M. Effects of vitamin D supplementation on COVID-19 related outcomes: a systematic review and Meta-analysis. Nutrients. 2022;14 doi: 10.3390/nu14102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim S.W., Kim S.M., Kim Y.K., et al. Clinical characteristics and outcomes of COVID-19 cohort patients in Daegu Metropolitan City outbreak in 2020. J Korean Med Sci. 2021;36 doi: 10.3346/jkms.2021.36.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kabia A.U., Li P., Jin Z., et al. The effects of hypertension on the prognosis of coronavirus disease 2019: a systematic review and meta-analysis on the interactions with age and antihypertensive treatment. J Hypertens. 2022;40(12):2323–2336. doi: 10.1097/HJH.0000000000003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lippi G., Wong J., Henry B.M. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130:304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 84.O’Hearn M., Liu J., Cudhea F., Micha R., Mozaffarian D. Coronavirus disease 2019 hospitalizations attributable to Cardiometabolic conditions in the United States: a comparative risk assessment analysis. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ebinger J., Driver M., Joung S., et al. Hypertension and excess risk for severe COVID-19 illness despite booster vaccination. Hypertension. 2022;79:e132–e134. doi: 10.1161/HYPERTENSIONAHA.122.19694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hosseinzadeh R., Goharrizi M., Bahardoust M., et al. Should all patients with hypertension be worried about developing severe coronavirus disease 2019 (COVID-19)? Clin Hypertens. 2021;27:3. doi: 10.1186/s40885-021-00161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and Management of High Blood Pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 88.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(271–280) doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li J., Wang X., Chen J., Zhang H., Deng A. Association of Renin-Angiotensin System Inhibitors with Severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5:825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reynolds H.R., Adhikari S., Pulgarin C., et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Savoia C., Volpe M., Kreutz R. Hypertension, a moving target in COVID-19: current views and perspectives. Circ Res. 2021;128:1062–1079. doi: 10.1161/CIRCRESAHA.121.318054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(281–292) doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shang J., Ye G., Shi K., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sriram K., Insel P.A. Risks of ACE inhibitor and ARB usage in COVID-19: evaluating the evidence. Clin Pharmacol Ther. 2020;108:236–241. doi: 10.1002/cpt.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meng J., Xiao G., Zhang J., et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yahyavi A., Hemmati N., Derakhshan P., et al. Angiotensin enzyme inhibitors and angiotensin receptor blockers as protective factors in COVID-19 mortality: a retrospective cohort study. Intern Emerg Med. 2021;16:883–893. doi: 10.1007/s11739-020-02523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang P., Zhu L., Cai J., et al. Association of Inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Almeida-Pititto B., Dualib P.M., Zajdenverg L., et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr. 2020;12:75. doi: 10.1186/s13098-020-00586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trump S., Lukassen S., Anker M.S., et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat Biotechnol. 2021;39:705–716. doi: 10.1038/s41587-020-00796-1. [DOI] [PubMed] [Google Scholar]
- 102.Sawadogo W., Tsegaye M., Gizaw A., Adera T. Overweight and obesity as risk factors for COVID-19-associated hospitalisations and death: systematic review and meta-analysis. BMJ Nutr Prev Health. 2022;5:10–18. doi: 10.1136/bmjnph-2021-000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yehya A., Carbone S. Managing type 2 diabetes mellitus during COVID-19 pandemic: the bittersweet. Diabetes Metab Res Rev. 2021;37 doi: 10.1002/dmrr.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou Y., Chi J., Lv W., Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19) Diabetes Metab Res Rev. 2021;37 doi: 10.1002/dmrr.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in new York City: prospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klang E., Kassim G., Soffer S., Freeman R., Levin M.A., Reich D.L. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring) 2020;28:1595–1599. doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gregory J.M., Slaughter J.C., Duffus S.H., et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the Pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526–532. doi: 10.2337/dc20-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prattichizzo F., de Candia P., Nicolucci A., Ceriello A. Elevated HbA1c levels in pre-Covid-19 infection increases the risk of mortality: a sistematic review and meta-analysis. Diabetes Metab Res Rev. 2022;38 doi: 10.1002/dmrr.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu L., She Z.G., Cheng X., et al. Association of Blood Glucose Control and Outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(1068–1077) doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prevention CfDCa . In: Chronic Kidney Disease in the United States. GudoHaHs A., editor. US Department of Health and Human Services; Atlanta: 2021. [Google Scholar]
- 111.Council E-E, Group EW Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021;36:87–94. doi: 10.1093/ndt/gfaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clark A., Jit M., Warren-Gash C., et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8:e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Flythe J.E., Assimon M.M., Tugman M.J., et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis. 2021;77:190–203.e191. doi: 10.1053/j.ajkd.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jdiaa S.S., Mansour R., El Alayli A., Gautam A., Thomas P., Mustafa R.A. COVID-19 and chronic kidney disease: an updated overview of reviews. J Nephrol. 2022;35:69–85. doi: 10.1007/s40620-021-01206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Appelman B., Oppelaar J.J., Broeders L., et al. Mortality and readmission rates among hospitalized COVID-19 patients with varying stages of chronic kidney disease: a multicenter retrospective cohort. Sci Rep. 2022;12:2258. doi: 10.1038/s41598-022-06276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hardenberg J.B., Luft F.C. Covid-19, ACE2 and the kidney. Acta Physiol (Oxf) 2020;230 doi: 10.1111/apha.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jankowski J., Floege J., Fliser D., Bohm M., Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bae S., Kim S.R., Kim M.N., Shim W.J., Park S.M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart. 2021;107:373–380. doi: 10.1136/heartjnl-2020-317901. [DOI] [PubMed] [Google Scholar]
- 120.Carter P., Lagan J., Fortune C., et al. Association of Cardiovascular Disease with Respiratory Disease. J Am Coll Cardiol. 2019;73:2166–2177. doi: 10.1016/j.jacc.2018.11.063. [DOI] [PubMed] [Google Scholar]
- 121.Aveyard P., Gao M., Lindson N., et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study. Lancet Respir Med. 2021;9:909–923. doi: 10.1016/S2213-2600(21)00095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beltramo G., Cottenet J., Mariet A.S., et al. Chronic respiratory diseases are predictors of severe outcome in COVID-19 hospitalised patients: a nationwide study. Eur Respir J. 2021;58 doi: 10.1183/13993003.04474-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanchez-Ramirez D.C., Mackey D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID-19 outcomes: a systematic review and meta-analysis. Respir Med. 2020;171 doi: 10.1016/j.rmed.2020.106096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meza D., Khuder B., Bailey J.I., Rosenberg S.R., Kalhan R., Reyfman P.A. Mortality from COVID-19 in patients with COPD: a US study in the N3C data enclave. Int J Chron Obstruct Pulmon Dis. 2021;16:2323–2326. doi: 10.2147/COPD.S318000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao Q., Meng M., Kumar R., et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol. 2020;92:1915–1921. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Halpin D.M.G., Criner G.J., Papi A., et al. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;(203):24–36. doi: 10.1164/rccm.202009-3533SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elbeddini A., Tayefehchamani Y. Amid COVID-19 pandemic: challenges with access to care for COPD patients. Res Social Adm Pharm. 2021;17:1934–1937. doi: 10.1016/j.sapharm.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Press V.G., Gershon A.S., Sciurba F.C., Blagev D.P. Concerns about coronavirus disease-related collateral damage for patients with COPD. Chest. 2020;158:866–868. doi: 10.1016/j.chest.2020.05.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zheng Y., Liu X., Le W., et al. A human circulating immune cell landscape in aging and COVID-19. Protein Cell. 2020;11:740–770. doi: 10.1007/s13238-020-00762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee H., Choi H., Yang B., et al. Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur Respir J. 2021;58 doi: 10.1183/13993003.04125-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gallay L., Uzunhan Y., Borie R., et al. Risk factors for mortality after COVID-19 in patients with preexisting interstitial lung disease. Am J Respir Crit Care Med. 2021;203:245–249. doi: 10.1164/rccm.202007-2638LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Esposito A.J., Menon A.A., Ghosh A.J., et al. Increased odds of death for patients with interstitial lung disease and COVID-19: a case-control study. Am J Respir Crit Care Med. 2020;202:1710–1713. doi: 10.1164/rccm.202006-2441LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Drake T.M., Docherty A.B., Harrison E.M., et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An international multicenter study. Am J Respir Crit Care Med. 2020;202:1656–1665. doi: 10.1164/rccm.202007-2794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dharmage S.C., Perret J.L., Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246. doi: 10.3389/fped.2019.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eger K., Bel E.H. Asthma and COVID-19: do we finally have answers? Eur Respir J. 2021;57 doi: 10.1183/13993003.04451-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu S., Cao Y., Du T., Zhi Y. Prevalence of comorbid asthma and related outcomes in COVID-19: a systematic review and Meta-analysis. J Allergy Clin Immunol Pract. 2021;9:693–701. doi: 10.1016/j.jaip.2020.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sunjaya A.P., Allida S.M., Di Tanna G.L., Jenkins C.R. Asthma and COVID-19 risk: a systematic review and meta-analysis. Eur Respir J. 2022;59 doi: 10.1183/13993003.01209-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garassino M.C., Whisenant J.G., Huang L.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Passaro A., Addeo A., Von Garnier C., et al. ESMO management and treatment adapted recommendations in the COVID-19 era: lung cancer. ESMO Open. 2020:5. doi: 10.1136/esmoopen-2020-000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Physicians ACoC, Association AL, Society AT, Foundation C . 2020. Joint Statement on Importance of Patients with Chronic Lung Disease Wearing Facial Coverings During COVID-19 Pandemic. [Google Scholar]
- 141.Demeyer H., Louvaris Z., Frei A., et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax. 2017;72:415–423. doi: 10.1136/thoraxjnl-2016-209026. [DOI] [PMC free article] [PubMed] [Google Scholar]