Abstract
Introduction
The incidence of renal tumours is increasing and anatomic imaging cannot reliably distinguish benign tumours from renal cell carcinoma. Up to 30% of renal tumours are benign, with oncocytomas the most common type. Biopsy has not been routinely adopted in many centres due to concerns surrounding non-diagnostic rate, bleeding and tumour seeding. As a result, benign masses are often unnecessarily surgically resected. 99mTc-sestamibi SPECT/CT has shown high diagnostic accuracy for benign renal oncocytomas and other oncocytic renal neoplasms of low malignant potential in single-centre studies. The primary aim of MULTI-MIBI is to assess feasibility of a multicentre study of 99mTc-sestamibi SPECT/CT against a reference standard of histopathology from surgical resection or biopsy. Secondary aims of the study include obtaining estimates of 99mTc-sestamibi SPECT/CT sensitivity and specificity and to inform the design and conduct of a future definitive trial.
Methods and analysis
A feasibility prospective multicentre study of participants with indeterminate, clinical T1 renal tumours to undergo 99mTc-sestamibi SPECT/CT (index test) compared with histopathology from biopsy or surgical resection (reference test). Interpretation of the index and reference tests will be blinded to the results of the other. Recruitment rate as well as estimates of sensitivity, specificity, positive and negative predictive value will be reported. Semistructured interviews with patients and clinicians will provide qualitative data to inform onward trial design and delivery. Training materials for 99mTc-sestamibi SPECT/CT interpretation will be developed, assessed and optimised. Early health economic modelling using a decision analytic approach for different diagnostic strategies will be performed to understand the potential cost-effectiveness of 99mTc-sestamibi SPECT/CT.
Ethics and dissemination
Ethical approval has been granted (UK HRA REC 20/YH/0279) protocol V.5.0 dated 21/6/2022. Study outputs will be presented and published nationally and internationally.
Trial registration number
ISRCTN12572202.
Keywords: Urological tumours, Nuclear radiology, HEALTH ECONOMICS
Strengths and limitations of this study.
MULTI-MIBI is the first multicentre prospective study to assess 99mTc-sestamibi SPECT/CT in the evaluation of indeterminate renal tumours.
A composite reference standard of biopsy or surgical pathology allows generalisability of results to patients unwilling or unable to undergo surgical resection.
Blinding of clinicians interpreting index and reference tests reduces risk of bias.
Possible study limitations include the risk of non-diagnostic renal tumour biopsies and tumour misclassification on biopsy.
If the primary outcome (successful recruitment) is met, this will inform a large-scale multicentre study.
Introduction
The widespread use of cross-sectional imaging has led to an increase in the incidental detection of renal tumours.1 Based on data from surgical series, it is estimated that up to 30% of renal tumours are benign,2 with an increasing prevalence of benign histology with decreasing tumour size.3 The most common type of benign tumour is the oncocytoma. Unlike renal cell carcinoma (RCC), which commonly requires treatment, renal oncocytomas can be safely managed expectantly.4–6 However, a critical challenge lies in the identification of benign renal tumours, as traditional anatomic imaging techniques such as ultrasound, CT and MRI are unable to reliably distinguish between the various renal tumour histologies. Although renal mass biopsy can help in this regards, the relatively high non-diagnostic rate (~15%) and associated risk of complications with this procedure have led to its limited adoption in clinical practice.7 8 Thus, the majority of patients presenting with an incidental renal mass undergo treatment for a presumed cancer, exposing those with benign tumours to unnecessary surgical risk while consuming significant health resources.9
Investigation of new imaging approaches to improve characterisation of incidentally detected small renal masses has been identified as a priority research need by the Renal Cancer Gap Analysis Collaborative, a group composed of clinicians, researchers, patients and caregivers.10 In recent years, 99mTc-sestamibi SPECT/CT has emerged as a promising non-invasive tool for the identification of benign renal oncocytomas. 99mTc-sestamibi is a lipophilic cationic radiopharmaceutical that readily accumulates in cells with high concentrations of mitochondria, such as renal oncocytomas.11 Conversely, most histologic subtypes of RCC are relatively devoid of mitochondria and express membrane multidrug resistance pumps, which are known to actively export 99mTc-sestamibi out of cells.11 These biological differences result in oncocytomas appearing avid, or ‘hot’ and RCCs non-avid or ‘cold’ on MIBI-kidney studies. A systematic review and meta-analysis including 117 renal lesions from single-centre studies showed pooled sensitivity and specificity of MIBI-kidney to detect renal oncocytomas versus other renal lesions was 92% (95% CI 72% to 98%) and 88% (95% CI 79% to 94%), respectively.12 No previous trials of MIBI-kidney have been conducted in the United Kingdom (UK), and there have been no multicentre trials.
One potential limitation of 99mTc-sestambi SPECT/CT imaging of renal tumours is that a subset of RCCs exhibits relatively high intracellular concentrations of mitochondria and, therefore, display uptake of the radiotracer.13–15 These tumours include the chromophobe subtype of RCC and other oncocytic/chromophobe RCC.16 It is reassuring to note that these tumours exhibit generally indolent behaviour and low metastatic potential with excellent outcomes on active surveillance.17 We, therefore, termed this group of tumours as oncocytic renal neoplasms of low malignant potential and suggest that with few exception identification of such cT1 tumours on 99mTc-sestamibi SPECT/CT should be managed similarly to that of benign renal oncocytomas.
Given the excellent performance characteristics of 99mTc-sestamibi SPECT/CT for the non-invasive identification of renal oncocytomas and oncocytic renal neoplasms of low malignant potential, there is interest in utilising this test within the UK National Health System (NHS). However, the literature on 99mTc-sestamibi SPECT/CT remains limited to single centres reporting relatively few tumours. We have recently reported on a pump-priming pilot study in the UK.18 Herein, we present the protocol for our feasibility study with the following aims1: to evaluate the feasibility of a large scale, UK-based, multicentre, clinical trial of 99mTc-sestamibi SPECT/CT in the diagnostic pathway for renal tumours and2 to obtain estimates of sensitivity and specificity with which to power a larger scale trial.
Methods and analysis
Study methods are reported with reference to Standard Protocol Items: Recommendations for Interventional Trials Checklist (SPIRIT)19 and SPIRIT-Path extension for cellular and molecular pathology content in clinical trial protocols.20
Study design
A prospective, multicentre study to assess the feasibility and diagnostic performance characteristics of 99mTC-sestamibi SPECT/CT in adults (n=50) with solid, enhancing clinical renal tumours (2–7 cm) on cross-sectional imaging. The study design is summarised in figure 1.
Figure 1.
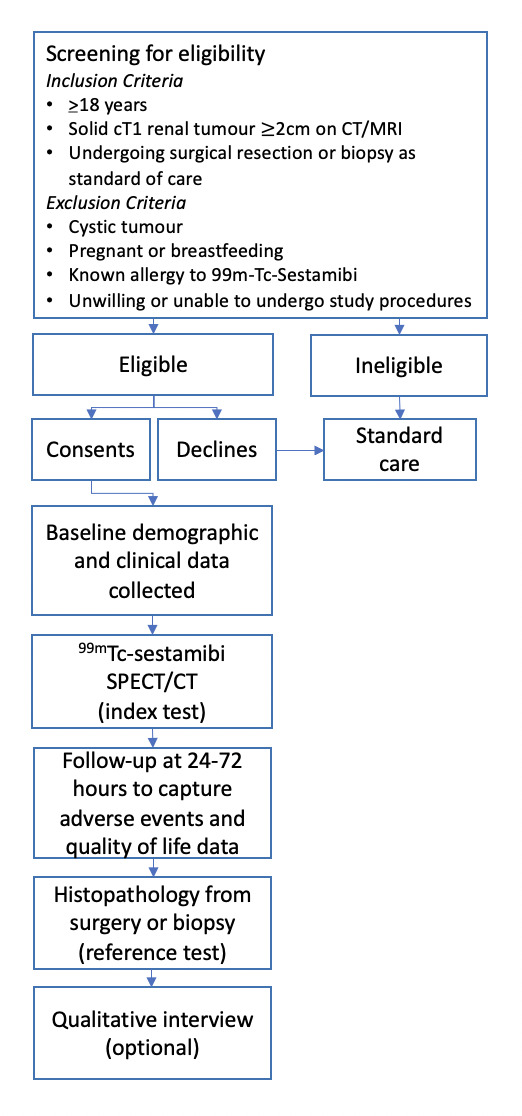
Study flow diagram.
Objectives and outcomes
The primary aim of the study is to evaluate whether a multicentre diagnostic test evaluation study of 99mTc-sestamibi SPECT/CT can recruit successfully. Secondary aims are to assess patient and clinician acceptability, refine inclusion/exclusion criteria, sample size requirements and determine clinician training needs for 99mTc-sestamibi SPECT/CT interpretation.
The study objectives are to determine:
Will patients consent to have a 99mTc-sestamibi SPECT/CT prior to surgery or biopsy, including those from under-represented and underserved groups?
What factors influence patient’s decisions to participate?
What are the perceptions of clinicians and patients of 99mTc-sestamibi SPECT/CT?
What barriers and facilitators are there for adoption of 99mTc-sestamibi SPECT/CT?
What is the potential cost-effectiveness of using 99mTc-sestamibi SPECT/CT within the NHS?
What are the minimally acceptable criteria (MAC) for the sensitivity and specificity of 99mTc-sestamibi SPECT/CT?
Is it feasible to train nuclear medicine clinicians across the UK, including those serving under-represented and underserved communities, to interpret 99mTc-sestamibi SPECT/CT?
The study outcomes are as follows:
Primary outcome
Recruitment rate.
Secondary outcomes
Sensitivity and specificity of MIBI to detect benign lesions in this study.
Define the MAC for MIBI-kidney to be adopted in clinical practice, to inform the design and parameters of the future definitive clinical trial.
Interobserver variability and training requirements in the interpretation of MIBI-kidney (local and central reports will be compared).
Patient and clinician perceptions of utility and experience of MIBI-kidney scans and training.
The evidence requirements for a cost-effectiveness analysis.
Study setting
The study will be conducted in 3–6 NHS hospitals in England.
Eligibility criteria
Consecutive patients discussed at specialist multidisciplinary team meetings will be screened for eligibility over a planned 15-month recruitment period. The inclusion criteria for entry to the study are adult patients (≥18 years) of any gender with a clinical T1 indeterminate solid renal tumour (2–7 cm) on cross-sectional imaging, willing and able to provide informed consent. Patients will be required to have surgery or renal tumour biopsy planned as part of their standard clinical care. Patients entering watchful waiting or active surveillance pathways without histologic diagnosis will be excluded. Other exclusion criteria will include cystic tumours, pregnant and breastfeeding patients, those with a known allergy to 99mTc-sestamibi and those unwilling or unable to undergo the study procedures.
Test methods
Index test
Nuclear medicine clinicians involved in the study will receive study-specific training on the interpretation of 99mTc-sestamibi SPECT/CT from international experts at the beginning of the recruitment period. The training will include a lecture on 99mTc-sestamibi SPECT/CT principles, ‘hands-on training’ supported by experienced faculty and a precourse and postcourse assessment.
900 MBq of 99mTc-sestamibi will be injected intravenously in a single bolus, 75 min before SPECT/CT acquisition of the abdomen with the superior extent of the field-of-view set to the top of the liver dome. CT and SPECT image acquisition will follow manufacturer instructions and local experience. At minimum, we suggest that participating centres have SPECT/CT systems with the following specifications: at least two-slice helical diagnostic CT scanner, available low-energy all-purpose or low-energy high-resolution collimator, gamma camera or digital detector elements appropriate for 140-kEv photopeak acquisition and manufacturer-derived iterative reconstruction that includes scatter and attenuation correction.
The reporting clinician will document a qualitative assessment of the tumour as avid, non-avid or indeterminate on reconstructed SPECT/CT images, blinded to clinical information and the result of the histopathology reference test. A spherical region of interest will be drawn to measure maximum uptake in attenuation-corrected images within (a) the tumour and (b) the ipsilateral renal parenchyma. A ratio of maximum uptake between the tumour and normal renal parenchyma will be calculated. All 99mTC-sestamibi SPECT/CT scans will be transferred for central review at the lead site (Royal Free Hospital) and discordant reports resolved by discussion and consensus. Local site clinicians will report a subset of studies a second time at the end of the recruitment period to allow assessment of intrarater reliability.
Reference test
Histopathology from the final surgical resection specimen is considered the ‘gold standard’ diagnostic test to determine renal tumour subtype. It is worth noting that although biopsy allows for histological diagnosis, questions remain about the accuracy of this technique for determining the precise histology of a renal tumour, mostly relating to an approximately 15% non-diagnostic rate of this procedure7 8 and the need for architectural findings in the tissue sample to definitively diagnose some tumour types.21 Despite this, we feel a composite reference standard of surgery and biopsy allows generalisability of the results to non-surgical populations. To maximise the accuracy of this procedure, tumour biopsy will be performed using an image-guided approach by an interventional radiologist experienced in the technique. In the case of a non-diagnostic biopsy, the patient will be offered a second attempt, according to local guidelines.
Histopathological reporting of both biopsy and surgical samples will be performed by qualified pathologists at collaborating sites in accordance with the current WHO classification system for renal tumours,16 as per standard care. Pathologists will be blinded to the 99mTc-sestamibi SPECT/CT result. Pathology slides/images will be exported for central review by a specialist uro-oncology pathologist and archiving at the lead site (Royal Free Hospital).
Sample size and recruitment
The aim of this study is to assess the feasibility of a multicentre study of 99mTC-sestamibi SPECT/CT in the diagnostic pathway for renal tumours. Data from the feasibility phase will be used to inform the design and sample size of the definitive trial. We will aim to recruit 50 patients from 3–6 centres. This sample size will allow us to assess if 80% (95% CI 70% to 90%) of approached patients agree to undergo the study scan. Additionally, this sample size will have sufficient power to detect if there is a significant difference in the estimates of sensitivity between our study population and those reported in the literature. A sample size of 40 patients would achieve 81% power to detect a sensitivity of 0.65 (representing an estimate outside the lower end of the 95% CI for sensitivity from the literature) using a two-sided binomial test at the 5% two-sided alpha level. A 20% inflation to 50 patients, will allow for possible dropouts and other methodological challenges.
Analysis
Qualitative study of feasibility and acceptability
Qualitative data obtained from semistructured interviews (conducted either by telephone or on virtual platforms for example, Microsoft Teams) with patients, carers and staff will be combined with documentary analysis (reports, meeting minutes) and will be used to inform within trial decision-making processes via a rapid feedback evaluation approach.22 Transcripts and key documents will be imported into NVivo and analysed using framework analysis.23 Data collection and analysis will be carried out in parallel and emerging findings will be shared with the trial team on a monthly basis to inform trial design and delivery.
The findings from the interviews and documentary analysis will be used to develop a discrete choice experiment to gain an understanding of preferences for trial participation and how participants trade-off different attributes of 99mTC-sestamibi SPECT/CT with other management scenarios. In addition, a survey will be conducted—informed by a rapid review of survey instruments reported in the published literature to capture the acceptability of interventions in clinical trials, to provide insights into the barriers or facilitators to patient decision-making and determine the degree of acceptability of 99mTC-sestamibi SPECT/CT.
Study of diagnostic accuracy
Diagnostic accuracy of 99mTc-sestamibi SPECT/CT will be estimated by generating 2×2 tables for both avid and non-avid qualitative assessment, and relative radiotracer uptake ratio >0.6 and ≤0.6 for external validation of a predefined threshold from the literature.24 Analysis of a range of relative uptake ratios will be explored to assess performance at different thresholds. Diagnostic accuracy of 99mTC-sestamibi SPECT/CT will be calculated in terms of sensitivity, specificity and predictive values along with their 95% CIs. The prevalence of renal oncocytoma and other histology subtypes will be calculated with a 95% CI.
Inconclusive test results will be reported.25 The proportion of participants with invalid 99mTC-sestamibi SPECT/CT results, for example, due to technical failure, will be reported. The proportion of valid but inconclusive results will also be reported, and their impact on estimates will be assessed by including them as either test positive or test negative in sensitivity analyses. This is to inform how 99mTc-sestamibi SPECT/CT might be used in the diagnostic pathway. If intended as a replacement test for histopathology, a valid but indeterminate 99mTc-sestamibi SPECT/CT would be considered non-avid to avoid misclassifying malignant tumours as benign. If, however, 99mTc-sestamibi SPECT/CT were to be used as a triage test, where avid tumours undergo confirmatory biopsy, then an indeterminate test could be considered avid to reduce the risk of surgery for benign pathology. The proportion of patients who do not complete the study schedule defined in the protocol will be calculated.
We will assess inter-rater and intrarater agreement using percentage agreement and Gwet’s first-order agreement coefficient.26
We do not anticipate the need to adjust for diagnostic drift for the reference test, given the short study duration. However, if current pathologic guidelines for renal neoplasia are updated during the course of the study archived samples will be rereviewed and reported according to the latest guidelines.
Study of health economics
Health economic modelling will be used to understand the potential cost-effectiveness of 99mTC-sestamibi SPECT/CT in the evaluation of patients presenting with an indeterminate renal mass.27 A decision analytic approach will compare the following scenarios:
Patients have empiric surgery (current standard-of-care).
Patients undergo tumour biopsy, those consistent with cancer have surgery and those with benign histology have active surveillance.
Patients undergo 99mTc-sestamibi SPECT/CT, those with a ‘cold’ scan (suggestive of cancer) have surgery and those with a ‘hot’ scan (suggestive of benign tumour) have active surveillance.
Patients undergo 99mTc-sestamibi SPECT/CT, those with a ‘cold’ scan have surgery, and those with a ‘hot’ scan have a confirmatory biopsy (MIBI would be likened to a triage test to select patients for biopsy for tissue confirmation before embarking on active surveillance).
The model will be populated with evidence from trial and published literature.28 Where data are not available, an expert elicitation approach will be employed to provide parameter values.29 The analysis will then compare the different approaches to standard-of-care by estimating the incremental cost-effectiveness ratios and assessing the uncertainty of these estimates using value of information (VOI) analysis. The VOI analysis will quantify the potential value of further research, identify areas of study with the greatest potential benefit and generate recommendations on future study designs.
Data collection
Case report forms (CRFs) in paper and electronic format will be trialled. The CRFs will not bear the participant’s name or other directly identifiable data. The participants’ study ID will be used for identification purposes. Study-related procedures will be carried out during the baseline routine clinical visit, 99mTc-sestamibi SPECT/CT visit and thereafter by telephone or email according to participant preference, as shown in table 1. CCRFs will be checked for completeness and accuracy by designated individuals against source data. Study data captured in paper format will be transcribed to an electronic database. Quality of life data will be captured using the previously validated EQ-5D-5L instrument.30 No analysis will begin until accuracy of the data has been assured. The final trial data set will be accessible to the chief investigator, statistician and health economist.
Table 1.
Visit schedule and assessments
| Procedures | Screening | Baseline | Intervention | 24–72 hour follow-up | Follow-up (standard of care) | Interview follow-up |
| Demographics | X | |||||
| Medical history | X | X | ||||
| Consent (obtained by clinician/research nurse) | X | |||||
| Imaging | X | |||||
| 99mTc-sestamibi SPECT/CT | X | |||||
| QoL questionnaire | X | X | ||||
| Adverse event reporting | X | X | ||||
| Histology test and result | X | |||||
| Semi-structured interview | X |
SPECT: Single photon emission computed tomography.
QoL: Quality of life.
A participant may withdraw their consent to participate at any time prior to the 99mTc-sestamibi SPECT/CT scan. The decision to withdraw will be recorded in the CRF and medical notes. Participants withdrawing prior to 99mTc-sestamibi SPECT/CT will be replaced. If following 99mTc-sestamibi SPECT/CT, the participant states they do not wish to participate in scheduled follow-up (EQ-5D-5L completion), or deviate from the protocol, then data already collected will be kept and analysed. These patients will not be replaced.
Baseline data items will include the following:
Baseline demographics (age, gender, ethnicity, medical and surgical history, current medication and allergies).
Baseline blood test results (full blood count, renal function, coagulation screen).
Baseline imaging (multiphase (to include non-contrast, arterial-phase, venous-phase and delayed-phase), contrast-enhanced CT or MRI of the abdomen).
Renal tumour characteristics (complexity scoring, location, number of lesions).
Quality of life questionnaire (EQ-5D-5L).
The following data on resource use will be collected at the time of the intervention
Duration of visit to nuclear medicine department.
Adverse events (AEs) during and immediately post-MIBI-kidney.
The following data will be collected at postintervention follow-up by telephone or email
AEs following MIBI-kidney.
Quality of life questionnaire (EQ-5D-5L).
After participation in the trial participants will continue follow-up as per standard care.
Patient and public involvement
Patient and public involvement (PPI) has been central to the project concept and design. A prestudy PPI focus group informed the trial protocol and plain English summary. An online PPI survey received 231 responses and indicated 90% would be willing to participate in the proposed study. In addition to the qualitative workstream, PPI representatives from Kidney Cancer UK will form a study support group, meeting at regular intervals throughout the trial to provide advice and input on any trial challenges and developing/approving dissemination materials.
Harms
99mTc-Sestamibi has been used for cardiac and parathyroid imaging globally for decades and is known to be a safe radiopharmaceutical. The radiation exposure from one MIBI-kidney scan is 14 mSv, equivalent to approximately 5 years of average UK background radiation.31 As MIBI-kidney is the only study intervention in addition to standard care, a data-monitoring committee will not be required.
All AEs, whether related or unrelated to MIBI-kidney, will be documented in the patient’s notes, study CRF and the AE log. The AE log will be sent to the Sponsor (University College London & University College London Hospitals Joint Research Office) at least once per year. Incidental clinically significant abnormalities identified on MIBI-kidney will be recorded as AEs and communicated to the referring clinician and patient. All serious AEs will be recorded on an SAE form and reported to the Sponsor and relevant REC within 15 working days of the chief investigator becoming aware of the event.
Auditing
Investigators and sites will permit trial-related monitoring, audit, REC review and regulatory inspection(s) and provide access to required data and documents.
Ethics and dissemination
Ethical approval for this study has been granted (UK HRA REC 20/YH/0279). Protocol amendments will be promptly disseminated to Sponsor, investigators and trial steering committee members. The study is recorded on the trial registration website. The trial involves the administration of unsealed radioactive substances. An Administration of Radioactive Substances Advisory Committee certificate has been granted (AA-3990).
Study outputs will be presented at national and international conferences and published in peer-reviewed journals. Patient representatives will be involved in output dissemination to the public individual trial participants via study newsletter.
Supplementary Material
Acknowledgments
We are grateful for the invaluable contribution provided by patient representatives.
HW is funded by The Urology Foundation and Pan London Cancer Alliance (Royal Marsden Partners, North Central London Cancer Alliance, North East London Cancer Alliance, South East London Cancer Alliance and the NIHR BRCs). VK receives funding from Prostate Cancer UK and the John Black Charitable Foundation. GDS and IAM are supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014) and GDS by the Cancer Research UK Cambridge Centre [C9685/A25177]. EP is supported by the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) North Thames at Bart’s Health NHS Trust. Mark Emberton receives research support from the United Kingdom’s National Institute of Health Research (NIHR) UCLH / UCL Biomedical Research Centre. He was conferred NIHR Senior Investigator Status in 2015. MGBT receives research funding from NIHR, St Peter’s Trust, Royal Free Charity, RCS, Facing up 2 Kidney Cancer and Kidney Cancer UK. The views expressed are those of the authors and not necessarily those of the funders.
Footnotes
Twitter: @hannahrwarren, @veerukasi, @mrsprostate, @andyscarsbrook, @AmmarAlanbuki, @TzeWah1, @CeciliaVindrola
Contributors: MGBT conceived the study and is the chief investigator and grant holder. HW and MGBT drafted the study protocol. HW, TW, MAG, SR, BFH, DP, SE-S, RB, PP, FM, AB, VK, CMM, NC, JC, AS, FH, TSOB, GDS, IM, SD, AA, WW, TMW, CV-P, EP, H-MD, PL, KG, ME and MGBT have contributed to study design, protocol writing and design of trial documents. SE-S was responsible for pathology content of the trial protocol. The Sponsor assisted with protocol writing and design of trial documents. The funding source was not involved in the trial design, protocol writing or design of trial documents and will not participate in its execution, analysis or interpretation. HW and MGBT drafted the manuscript and all other authors contributed to and approved the final document. Publications will be subject to the permission of the Sponsor. Responsibility to report on trial results will lie solely with the authors.
Funding: This study is funded by the NIHR Research for Patient Benefit Programme (NIHR203545). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Competing interests: GDS has received educational grants from Pfizer, AstraZeneca and Intuitive Surgical; consultancy fees from Pfizer, Merck, EUSA Pharma and CMR Surgical; Travel expenses from Pfizer and Speaker fees from Pfizer. SD provides educational consultancy for GE Healthcare, Bayer, AAA and AVAANT diagnostics.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Nayan M, Jewett MAS, Finelli A. Uncoupling diagnosis and treatment of incidentally imaged renal masses. JAMA Intern Med 2018;178:727–8. 10.1001/jamainternmed.2018.1180 [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Li S, Khandwala Y, et al. Association of prevalence of benign pathologic findings after partial nephrectomy with preoperative imaging patterns in the United States from 2007 to 2014. JAMA Surg 2019;154:225–31. 10.1001/jamasurg.2018.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernando A, Fowler S, O'Brien T, et al. Nephron-sparing surgery across a nation - outcomes from the British Association of Urological Surgeons 2012 national partial nephrectomy audit. BJU Int 2016;117:874–82. 10.1111/bju.13353 [DOI] [PubMed] [Google Scholar]
- 4.Neves J, Varley R, Agnesi S, et al. Growth and renal function dynamics of renal oncocytomas on active surveillance: a retrospective cohort analysis. BJU International 2021;128:722–7. 10.1111/bju.15499 [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Lee S, Rashid P, et al. Active surveillance is suitable for intermediate term follow-up of renal oncocytoma diagnosed by percutaneous core biopsy. BJU Int 2016;118 Suppl 3:30–4. 10.1111/bju.13538 [DOI] [PubMed] [Google Scholar]
- 6.Richard PO, Jewett MAS, Bhatt JR, et al. Active surveillance for renal neoplasms with oncocytic features is safe. J Urol 2016;195:581–7. 10.1016/j.juro.2015.09.067 [DOI] [PubMed] [Google Scholar]
- 7.Marconi L, Dabestani S, Lam TB, et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol 2016;69:660–73. 10.1016/j.eururo.2015.07.072 [DOI] [PubMed] [Google Scholar]
- 8.Patel HD, Johnson MH, Pierorazio PM, et al. Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: systematic review of the literature. J Urol 2016;195:1340–7. 10.1016/j.juro.2015.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson DC, Vukina J, Smith AB, et al. Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate. J Urol 2015;193:30–5. 10.1016/j.juro.2014.07.102 [DOI] [PubMed] [Google Scholar]
- 10.Rossi SH, Blick C, Handforth C, et al. Essential research priorities in renal cancer: a modified Delphi consensus statement. Eur Urol Focus 2020;6:991–8. 10.1016/j.euf.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 11.Rowe SP, Gorin MA, Solnes LB, et al. Correlation of 99mTc-sestamibi uptake in renal masses with mitochondrial content and multi-drug resistance pump expression. EJNMMI Res 2017;7:80. 10.1186/s13550-017-0329-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson MP, Katlariwala P, Murad MH, et al. Diagnostic accuracy of 99mTc-sestamibi SPECT/CT for detecting renal oncocytomas and other benign renal lesions: a systematic review and meta-analysis. Abdom Radiol 2020;45:2532–41. 10.1007/s00261-020-02469-8 [DOI] [PubMed] [Google Scholar]
- 13.Gorin MA, Rowe SP, Baras AS, et al. Prospective evaluation of (99m)Tc-sestamibi SPECT/CT for the diagnosis of renal oncocytomas and hybrid Oncocytic/Chromophobe tumors. Eur Urol 2016;69:413–6. 10.1016/j.eururo.2015.08.056 [DOI] [PubMed] [Google Scholar]
- 14.Asi T, Tuncali Meltem Çağlar, Tuncel M, et al. The role of Tc-99m MIBI scintigraphy in clinical T1 renal mass assessment: does it have a real benefit? Urol Oncol 2020;38:937.e11–937.e17. 10.1016/j.urolonc.2020.07.018 [DOI] [PubMed] [Google Scholar]
- 15.Sistani G, Bjazevic J, Kassam Z, et al. The value of 99mTc-sestamibi single-photon emission computed tomography-computed tomography in the evaluation and risk stratification of renal masses. Can Urol Assoc J 2020;15. 10.5489/cuaj.6708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moch H, Amin MB, Berney DM, et al. The 2022 World Health organization classification of tumours of the urinary system and male genital Organs-Part A: renal, penile, and testicular tumours. Eur Urol 2022;82:458–68. 10.1016/j.eururo.2022.06.016 [DOI] [PubMed] [Google Scholar]
- 17.Miller BL, Mankowski Gettle L, Van Roo JR, et al. Comparative analysis of surgery, thermal ablation, and active surveillance for renal oncocytic neoplasms. Urology 2018;112:92–7. 10.1016/j.urology.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 18.Warren H, Boydell A-R, Reza A, et al. 99mTc-Sestamibi SPECT/CT for indeterminate renal tumours: a pilot diagnostic accuracy study. BJUI. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kendall TJ, Robinson M, Brierley DJ, et al. Guidelines for cellular and molecular pathology content in clinical trial protocols: the SPIRIT-Path extension. Lancet Oncol 2021;22:e435–45. 10.1016/S1470-2045(21)00344-2 [DOI] [PubMed] [Google Scholar]
- 21.Gorin MA, Rowe SP, Allaf ME. Oncocytic neoplasm on renal mass biopsy: a diagnostic conundrum. Oncology 2016;30:426–35. [PubMed] [Google Scholar]
- 22.Vindrola-Padros C, Chisnall G, Cooper S, et al. Carrying out rapid qualitative research during a pandemic: emerging lessons from COVID-19. Qual Health Res 2020;30:2192–204. 10.1177/1049732320951526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gale NK, Heath G, Cameron E, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13:117. 10.1186/1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorin MA, Rowe SP, Baras AS, et al. Prospective evaluation of (99m)Tc-sestamibi SPECT/CT for the diagnosis of renal Oncocytomas and hybrid Oncocytic/Chromophobe tumors. Eur Urol 2016;69:413–6. 10.1016/j.eururo.2015.08.056 [DOI] [PubMed] [Google Scholar]
- 25.Shinkins B, Thompson M, Mallett S, et al. Diagnostic accuracy studies: how to report and analyse. BMJ 2013;2778:1–11. 10.1136/bmj.f2778 [DOI] [PubMed] [Google Scholar]
- 26.Gwet K. Handbook of inter-rater reliability. In: The definitive guide to measuring the extent of agreement amongst raters. 4th ed. Gaithersburg, MD 20886-2696: Advances Analytics, LLC, 2014. [Google Scholar]
- 27.Love-Koh J. How useful are early economic models? Comment on "Problems and promises of health technologies: the role of early health economic modelling". Int J Health Policy Manag 2020;9:215–7. 10.15171/ijhpm.2019.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su ZT, Patel HD, Huang MM, et al. Cost-effectiveness analysis of 99mTc-sestamibi SPECT/CT to Guide Management of Small Renal Masses. Eur Urol Focus 2021;7:827–34. 10.1016/j.euf.2020.02.010 [DOI] [PubMed] [Google Scholar]
- 29.Sperber D, Mortimer D, Lorgelly P, et al. An expert on every street corner? Methods for eliciting distributions in geographically dispersed opinion pools. Value Health 2013;16:434–7. 10.1016/j.jval.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 30.Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717–27. 10.1007/s11136-012-0322-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UK Health Security Agency . Ionising radiation: dose comparisons, 2011. Guidance Document. Available: https://www.gov.uk/government/publications/ionising-radiation-dose-comparisons/ionising-radiation-dose-comparisons [Accessed 24 May 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


