Abstract
Background:
mTOR inhibitors such as everolimus may cause oral stomatitis, often a dose-limiting toxicity. Prior clinical research has suggested that a dexamethasone mouth rinse might help prevent and/or treat this.
Methods:
Alliance A221701 was a randomized phase III trial of patients initiating 10 mg daily oral everolimus that compared dexamethasone mouthwash taken preventively (initial dexamethasone group) vs. therapeutically (initial placebo group) to assess two co-primary endpoints: the incidence of mTOR inhibitor-associated stomatitis (mIAS), and the area under the curve (AUC) of mIAS-associated pain over an eight-week treatment period. A Fisher’s Exact test was used to compare the incidences while a Wilcoxon rank-sum test was used to compare the AUCs. In addition, we performed an exploratory analysis of the association of everolimus trough concentrations and toxicity using a Mann-Whitney U test.
Results:
Due to slow accrual, this study closed after 39 patients were randomized (19 to upfront placebo and 20 to upfront dexamethasone). There were no significant differences between groups seen in either of the co-primary endpoints; furthermore, we found no association between whole blood everolimus trough concentrations and toxicity.
Conclusions:
Although limited by poor enrollment, the results of this study do not suggest that prophylactic dexamethasone mouthwash is superior to therapeutic dexamethasone mouthwash (initiated at the first sign of mouth pain) for reducing the incidence or severity of mIAS from everolimus.
Keywords: everolimus, mucositis, mTOR inhibitors, dexamethasone, mouth rinse
Background
Mammalian target of rapamycin (mTOR) inhibitors, including everolimus, cause stomatitis, which can significantly impair quality of life in patients with cancer. In the BOLERO-2 study, there was a 59% rate of stomatitis and a 30% rate of grade 2 or 3 stomatitis in patients with breast cancer who received everolimus and exemestane.[1] In a meta-analysis of BOLERO-2, RECORD-2 (for renal cell carcinoma), RADIANT-2 (for carcinoid), RADIANT-3 (for pancreatic neuroendocrine tumors), and EXIST-1 and 2 (for tuberous sclerosis complex), the rate of everolimus-induced stomatitis was 67%, while the rate of grade 3 or 4 stomatitis was 9%. It was noted that 89% of these events occurred within 8 weeks of starting everolimus. [2]
Topical corticosteroids were proposed as a means of reducing stomatitis in this setting based on their efficacy for treating recurrent aphthous ulcers and Behcet’s Syndrome.[3–6] The proposed mechanism for corticosteroids in this setting was a decreased production of lymphocytes and other cytokines involved in an inflammatory response.[7] Given that the underlying pathogenesis of stomatitis due to mTOR inhibitors appears to be inflammatory, like aphthous ulcers, topical corticosteroids were studied as a treatment for mTOR inhibitor-associated stomatitis (mIAS), with non-randomized studies suggesting substantial benefit.[8, 9]
This My Individualized Stomatitis Treatment (MIST) trial was designed to assess whether a preventative strategy would be superior to a reactive strategy when dexamethasone mouthwash was used during everolimus therapy for cancer.
Methods
This multi-center, randomized, double-blind, placebo-controlled phase III clinical trial enrolled patients who were preparing to start everolimus 10 mg orally daily for cancer, not concurrently on chemotherapy, not suffering from stomatitis/mucositis or mouth ulcers, and not already receiving a corticosteroid or any other agent considered to be a treatment for stomatitis. A history of oral Candida infection (thrush) within the last 3 months, hemoglobin A1C greater than 8%, current pregnancy or lactation, non-English literacy, Eastern Cooperative Oncology Group (ECOG) Performance Status > 2, and age <18 years were all exclusion criteria. After providing IRB-approved protocol-specific written informed consent, participants were randomized in a 1:1 fashion to one of two treatment arms: 1) dexamethasone mouthwash; 2) placebo mouthwash that consisted of ORA-Sweet®, which contains purified water, sucrose, glycerin, and sorbitol, buffered with citric acid and sodium phosphate, and preserved with methylparaben and potassium sorbate. Randomization was done using Pocock-Simon dynamic allocation, stratified by age (<50 vs. 50-65 vs >65) and cancer type (breast vs other). Regardless of the arm, participants were instructed to swish 10 mL of their assigned mouthwash for 2 minutes then spit it out, four times per day, for 8 weeks. This schedule was based on what had been used in previous small clinical trials with promising results.[10] Patients were told that if they developed any mouth pain related to mouth sores, they were to fill a prescription for dexamethasone oral solution, stop their study drug, and instead initiate open label swish-and-spit use of dexamethasone four times daily for 2 minutes each time until 8 weeks from the start of treatment.
There were two co-primary endpoints in this study. Patient-reported mouth pain was measured by a simple 11-item response scale [0, 1, 2, …, 10] with zero indicating “no pain” and ten indicating “pain as bad as can be”, obtained at baseline (within seven days prior to the start of treatment) and then daily for 8 weeks. The first co-primary endpoint was the binary outcome mouth pain (yes; no) defined as a patient reporting at least one serially measured mouth pain score greater than zero during the eight-week study period. The second co-primary endpoint was the area under the curve (AUC) summary measure calculated for each patient based on the patient’s serially measured patient-reported mouth pain scores; the AUC calculated for each patient was scaled according to the number of assessable patient-reported mouth pain scores to obtain a transformed AUC score on a scale of 0-100 with higher scores corresponding to increased pain. We hypothesized that there would be a lower incidence rate of mIAS-associated mouth pain in the dexamethasone arm, and that the average AUC of the mouth pain scores would be less in the dexamethasone arm. The secondary endpoint safety analysis population included all patients who started at least one cycle of treatment (regardless of the availability of post-baseline mouth pain scores).
To achieve the study’s co-primary objectives, the planned sample size was 254 patients or 127 patients per arm. However, due to slow accrual between 2/15/2019 (accrual start date) and 11/1/2020, the study closed to accrual on 11/2/2020 after 39 patients were randomized (19 to upfront placebo and 20 to upfront dexamethasone). The analyses presented herein, therefore, are explorative in nature. For the first co-primary endpoint, we compared the incidence rate of mIAS-associated mouth pain between the two arms using a two-sided Fisher’s exact test.[11] For the second co-primary endpoint, we compared the average AUC of the serially measured mIAS-associated mouth pain scores between the two arms using a two-sided Wilcoxon rank-sum test.[12] The co-primary analyses were based on the full analysis set defined as all randomized patients with at least one post-baseline mIAS-associated mouth pain measurement. In three sensitivity analyses, the co-primary analyses were repeated: 1) excluding the patients with baseline mIAS-associated mouth pain > 0; 2) including only those patients who completed at least 50% of their post-baseline pain reports; and 3) including only those patients who completed at least 80% of their pain reports. Everolimus blood trough concentrations at week 4 and week 8 were quantitated by LC-MS/MS with an assay utilizing 20 μL whole blood, [13C2D4]-everolimus as internal standard, and a protein precipitation with ammonium bicarbonate, zinc sulfate, and acetonitrile; the standard curve was linear over the range of 2-100 ng/mL. Everolimus trough concentrations were corrected for exact sampling time to the 24 h concentration based on the average everolimus terminal half-life of 30 hours in whole blood as previously described, [13, 14] and corrected values were compared between patients with or without filling of the dexamethasone prescription, and with or without mouth sores, mouth pain, grade ≥2 mIAS, or any of these events by Mann-Whitney U test (exact significance).[11] Data were collected by the Alliance Statistics and Data Management Center (SDMC). Statistical analyses were performed by the SDMC using SAS, version 9.4 on a database frozen on January 8, 2021. Data quality was ensured by review of data by the SDMC and by the study chairperson following Alliance policies. Statistical significance was assessed at the nominal 5% significance level for both primary endpoints, with no adjustment for multiple testing.
Results
Thirty-nine patients (19 placebo-Arm A; 20 dexamethasone-Arm B) were randomized. Thirty-three patients were evaluable for the co-primary endpoints: 17 in Arm A (1 patient did not start treatment, 1 patient did not provide any post-baseline pain scores) and 16 in Arm B (3 patients did not start treatment, 1 patient did not provide any post-baseline pain scores).
The number of evaluable patients for toxicity was 18 in Arm A (1 patient did not start treatment) and 17 for Arm B (3 patients did not start treatment). All grade 3 and above adverse events deemed at least possibly related to treatment were counted. Two patients (11.1%) on the placebo arm reported at least one grade 3 non-hematologic adverse event (lower gastrointestinal hemorrhage, and oral mucositis), and three patients (17.6%) on the dexamethasone arm reported at least one grade 3 non-hematologic adverse event (mucositis, enterocolitis, pneumonitis). No grade 4 or 5 adverse events were reported. The number of evaluable patients for the co-primary endpoints was 17 in Arm A and 16 in Arm B (one patient on each arm was excluded because they did not provide any post-baseline pain scores).
Baseline characteristics were clinically balanced across the two arms in the cohort in whom the co-primary endpoints were assessed (Table 1). More than 2/3 of these participants had breast cancer. The incidence of mouth pain was 52.9% [95% Confidence Interval: 0.278, 0.770] in the placebo arm and 56.3% [95% Confidence Interval: 0.299, 0.802] in the dexamethasone arm, p-value=0.999. Furthermore, the median AUC was 0.7 in the placebo arm and 5.5 in the dexamethasone arm (p-value=0.335). Sensitivity analyses similarly revealed no significant difference between the arms in either co-primary endpoint. Of the patients evaluable for the co-primary endpoints, the percent of patients who filled the prescription dexamethasone script based on weekly phone calls with the nurses was 35.3% in the placebo arm and 31.3% in the dexamethasone arm. Additionally, of those evaluable for the co-primary endpoints, the median number of Numerical Analogue Mouth Pain Scale forms with at least one pain score provided was nine in both arms (Arm A had a lower quartile of 7 and an upper quartile of 9; Arm B had a lower quartile of 5.5 and an upper quartile of 9). No significant difference in everolimus blood levels was detectable between patients with or without side effects or with or without a fill of a dexamethasone script (Figure 1).
Table 1:
Baseline characteristics of patients with at least one post-baseline pain score available
| Arm | |||
|---|---|---|---|
| A: Placebo (N=17) |
B: Dexamethasone (N=16) |
Total (N=33) |
|
| Age (in years) | |||
|
| |||
| N | 17 | 16 | 33 |
|
| |||
| Mean | 65.3 | 65.3 | 65.3 |
|
| |||
| SD | 8.46 | 10.71 | 9.46 |
|
| |||
|
| |||
| Age group, n (%) | |||
|
| |||
| <50 | 1 (5.9%) | 1 (6.3%) | 2 (6.1%) |
|
| |||
| 50 – 65 | 7 (41.2%) | 8 (50.0%) | 15 (45.5%) |
|
| |||
| >65 | 9 (52.9%) | 7 (43.8%) | 16 (48.5%) |
|
| |||
|
| |||
| Cancer type, n (%) | |||
|
| |||
| Breast | 12 (70.6%) | 11 (68.8%) | 23 (69.7%) |
|
| |||
| Other | 5 (29.4%) | 5 (31.3%) | 10 (30.3%) |
|
| |||
|
| |||
| Sex, n (%) | |||
|
| |||
| Female | 15 (88.2%) | 12 (75.0%) | 27 (81.8%) |
|
| |||
| Male | 2 (11.8%) | 4 (25.0%) | 6 (18.2%) |
|
| |||
|
| |||
| ECOG performance status, n (%) | |||
|
| |||
| 0 | 7 (41.2%) | 8 (50.0%) | 15 (45.5%) |
|
| |||
| 1 | 10 (58.8%) | 7 (43.8%) | 17 (51.5%) |
|
| |||
| 2 | 0 (0.0%) | 1 (6.3%) | 1 (3.0%) |
ECOG: Eastern Cooperative Oncology Group
Figure 1.
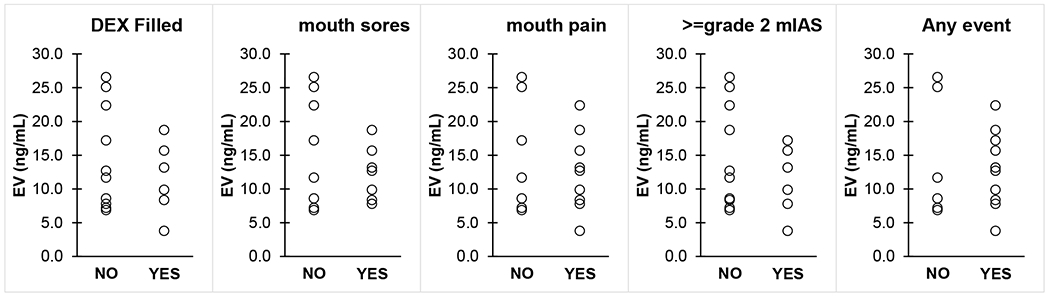
Whole blood everolimus (EV) trough concentrations in patients with or without EV side effects and who did or did not fill a commercial dexamethasone (DEX) prescription. No statistical significance was detected through Mann-Whitney U test.
Discussion
We did not observe a significant difference between the two arms in either co-primary endpoint. While these negative results could have been the consequence of our inability to accrue even 20% of the desired 279 total (and 254 evaluable) patients, it is sobering that in our patient population, there was no hint of less stomatitis in the prophylactic dexamethasone arm of this trial (if anything, we might have found less in the placebo arm had we enrolled more patients). This suggests that prophylactic use of dexamethasone mouthwash before initiation of everolimus is not superior to reactive use of dexamethasone mouthwash if mouth pain develops. This trial (MIST) differed from the SWISH trial not only in that it randomized to a reactive vs. prophylactic strategy and used a placebo control to blind participants and providers from the treatment, but also in its patient population and primary endpoint. In the SWISH trial, only patients initiating everolimus and exemestane for advanced breast cancer were enrolled,[10] and clinician grading of adverse events (using CTCAE version 4.0) was the primary endpoint, with grade 1 stomatitis found in 19% and grade 2 in 2%, totalling 21% (n=18, 95% CI 13.06-31.39). Also, SWISH did not evaluate the frequency of mouthwash use or if a delayed start of dexamethasone at the time of development of stomatitis would have been as effective as its use in the preventive setting. It is important to note that very few grade 3 adverse events were observed in the present trial (and all were more likely due to everolimus than to topical corticosteroid therapy), similar to other studies of corticosteroid mouth rinses in this setting.[15] In light of previous studies reporting low rates of severe mucositis when dexamethasone mouthwash is used during everolimus therapy, reactive use of dexamethasone mouthwash (initiated if mouth pain develops) may be beneficial in this setting.
Funding
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health: Alliance for Clinical Trials in Oncology Operation [Award Number U10 CA180821], Statistics and Data Management Center for the Alliance for Clinical Trials in Oncology [Award Number: U10 CA180882], Alliance NCORP Research Base [Award Number UG1 CA189823], and [Award Numbers: UG1 CA233323, UG1 CA233337, and UG1 CA232760], as described in https://acknowledgments.alliancefound.org. This project used the UPMC Hillman Cancer Center’s Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and was supported in part by National Cancer Institute [Award P30 CA47904] and [Award: R50 CA211241]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Interests:
Hope S. Rugo, MD, declares research grant support from Pfizer, Merck, Novartis, Lilly, Roche, Daiichi, Seattle Genetics, Macrogenics, Sermonix, Boehringer Ingelheim, Polyphor, AstraZeneca, Ayala, Astellas and Gilead and honoraria from Puma, Samsung, and NAPO. Charles Loprinzi, M.D. reports personal fees from PledPharma, Disarm Therapeutics, Asahi Kasei, Metys Pharmaceuticals, OnQuality, Mitsubishi Tanabe, NKMax, Novartis, HengRui, Nuro Bio, Osmol Therapeutics, Inc., and Grunenthal outside the submitted work. Lionel D. Lewis MA., MBBCh., MD., FRCP (London), FBPhS, declares consultation to G1 Therapeutics and to 7 Hills Pharma LLC and research grant support for Clinical Trials from Bayer Pharmaceuticals, ER Squibb, AbbVie & Astra Zeneca. All other authors declare no relevant conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier: NCT03839940
References
- 1.Rugo HS, Pritchard KI, Gnant M, et al. Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: insights from BOLERO-2. Annals of oncology : official journal of the European Society for Medical Oncology. 2014;25(4):808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rugo HS, Hortobagyi GN, Yao J, et al. Meta-analysis of stomatitis in clinical studies of everolimus: incidence and relationship with efficacy. Annals of oncology : official journal of the European Society for Medical Oncology. 2016;27(3):519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altenburg A, El-Haj N, Micheli C, et al. The treatment of chronic recurrent oral aphthous ulcers. Dtsch Arztebl Int. 2014;111(40):665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26(8):728–32. [DOI] [PubMed] [Google Scholar]
- 5.Femiano F, Buonaiuto C, Gombos F, Lanza A, Cirillo N. Pilot study on recurrent aphthous stomatitis (RAS): a randomized placebo-controlled trial for the comparative therapeutic effects of systemic prednisone and systemic montelukast in subjects unresponsive to topical therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(3):402–7. [DOI] [PubMed] [Google Scholar]
- 6.Fani MM, Ebrahimi H, Pourshahidi S, Aflaki E, Shafiee Sarvestani S. Comparing the Effect of Phenytoin Syrup and Triamcinolone Acetonide Ointment on Aphthous Ulcers in Patients with Behcet’s Syndrome. Iran Red Crescent Med J. 2012;14(2):75–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent SD, Lilly GE. Clinical, historic, and therapeutic features of aphthous stomatitis. Literature review and open clinical trial employing steroids. Oral Surg Oral Med Oral Pathol. 1992;74(1):79–86. [DOI] [PubMed] [Google Scholar]
- 8.de Oliveira MA, Martins EMF, Wang Q, et al. Clinical presentation and management of mTOR inhibitor-associated stomatitis. Oral Oncol. 2011;47(10):998–1003. [DOI] [PubMed] [Google Scholar]
- 9.Nicolatou-Galitis O, Nikolaidi A, Athanassiadis I, Papadopoulou E, Sonis S. Oral ulcers in patients with advanced breast cancer receiving everolimus: a case series report on clinical presentation and management. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 116(2):e110–6. [DOI] [PubMed] [Google Scholar]
- 10.Rugo HS, Seneviratne L, Beck JT, et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol. 2017;18(5):654–62. [DOI] [PubMed] [Google Scholar]
- 11.Conover W Practical Nonparametric Statistics. New York: John Wiley and Sons; 1980. [Google Scholar]
- 12.Moses LE, Emerson JD, Hosseini H. Analyzing data from ordered categories. N Engl J Med. 1984;311(7):442–8. [DOI] [PubMed] [Google Scholar]
- 13.Shipkova M, Hesselink DA, Holt DW, et al. Therapeutic Drug Monitoring of Everolimus: A Consensus Report. Ther Drug Monit. 2016;38(2): 143–69. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Chia YL, Nedelman J, et al. A therapeutic drug monitoring algorithm for refining the imatinib trough level obtained at different sampling times. Ther Drug Monit. 2009;31(5):579–84. [DOI] [PubMed] [Google Scholar]
- 15.Jones VE, McIntyre KJ, Paul D, et al. Evaluation of Miracle Mouthwash plus Hydrocortisone Versus Prednisolone Mouth Rinses as Prophylaxis for Everolimus-Associated Stomatitis: A Randomized Phase II Study. Oncologist. 2019;24(9): 1153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


