Abstract
T-cell/natural killer cell lymphoproliferative disorders are rare, associated with poor overall survival, and have limited treatment options. We report a case of a patient who developed hydroa vacciniforme-like lymphoma (HVLL, an EBV-peripheral T-cell lymphoma), refractory to multiple lines of systemic therapy including methotrexate, mycophenolate mofetil, dapsone, thalidomide, prednisone, and romidepsin. We conducted morphoproteomic analysis of the patient’s tumor which provided important biological insights. Histopathology showed primarily lymphohistiocytic infiltrates strongly positive EBV expression with a Ki-67 of >50% in the pretreatment biopsy and approximately 90% in the post-treatment biopsy, strong expression of Enhancer of Zester Homolog 2 (EZH2), a constitutively active mTOR pathway, 50% cytoplasmic BCL-2 expression; largely negative PD-1 positive CD8 T-cells. Based on this morphoproteomic analysis and published literature, we postulated that novel agents, including venetoclax, tazemetostat, and other agents may provide a targeted approach for treating HVLL. This case illustrates the use of morphoproteomic analysis to better understand the biology of tumors.
Key Words: Hydroa vacciniforme-like lymphoma (HVLL), Epstein-Barr virus, T-cell lymphoproliferative disorders, Resistance mechanisms, Morphoproteomics
Introduction
Epstein-Barr Virus (EBV) has been implicated in the pathogenesis and development of multiple B-cell lymphoproliferative disorders such as Burkitt’s lymphoma, Hodgkin’s lymphoma, post-transplant and HIV-associated lymphoproliferative disorders. Reported cases of T-cell lymphoproliferative disorders are rare and include peripheral T-cell lymphomas, angioimmunoblastic T-cell lymphoma, and extranodal nasal type natural killer/T-cell lymphoma1. Hydroa vacciniforme–like lymphoma (HVLL) is an EBV-positive T-cell lymphoproliferative disorder that mainly affects children in Central and South America and Asia. It can present with a broad clinical spectrum, prolonged clinical course, and risk of progression to systemic disease. Clinically, these patients present with skin-lesions primarily involving sun-exposed areas, hepatosplenomegaly, lymphadenopathy, and fever. Hydroa vacciniforme (HV) is usually characterized by light-induced vesicles that crust and subsequently leave behind varicelliform scars after they heal. Generally, systemic symptoms are rarely observed and the disease usually remits spontaneously in adolescence or young adulthood2. In recent years; however, a unique group of seemingly more aggressive disease similar to HV was recognized in children of Asian, Peruvian, and Mexican heritage3,4. Initially, this disease process was termed “severe” HV or edematous scarring vasculitic panniculitis (ESVP) to differentiate it from classic HV. Studies ultimately showed this disease process to be associated with EBV infection and monoclonal rearrangements of the T-cell receptor (TCR) genes were often noted. As a result, the term “HV-like lymphoma” was coined.
HV-like lymphoma and systemic EBV-positive T-cell lymphoproliferative disorders of childhood are both tumors in the subgroup of EBV-positive T-cell LPD of childhood. HVLL is recognized as a cutaneous EBV-positive T-cell lymphoma that tends to occur in children and occasionally in adolescents4. The cell origin is most commonly CD8+ phenotype though a few cases of natural killer (NK+) cell phenotype have been reported4,5. Regardless of cell-type origin, cell markers like granzyme-B and T-cell intracellular antigen 1 (TIA-1) are positive.4 CD30+ cases have also been reported in the literature3.
Case presentation
We present a case of a 28-year-old Hispanic man from Guatemala with a history of recurring blistering skin lesions involving the back since childhood. Six years prior to presentation to our institution he developed recurrent necrotic blisters and papules involving the face, trunk, and extremities that erupted symmetrically. The erythematous macules progressed to tender papules, vesicles, and, finally, crusted over. The patient described a several year history of worsening of the skin lesions upon sun exposure consistent with positive photo provocation.
When he was first evaluated in March 2015, he was on methotrexate 2.5 mg orally twice daily. Over the next several months he received multiple antimicrobial therapies both topical and systemic for “acne” including clindamycin, trimethoprim-sulfamethoxazole, cefalexin. A left forearm punch biopsy while being off methotrexate showed lichenoid type interface dermatitis. Repeat skin punch biopsies taken over the next two months showed identical findings. Antimicrobial therapy during this time showed no improvement of the skin lesions. Peripheral blood analysis using flow cytometry did not reveal any aberrant B- or T-cells. Computed tomogram (CT) scan of the chest, abdomen, and pelvis showed normal sized hyper enhancing reniform lymph nodes in the axilla, largest 2.4 x 0.9 cm and mild splenomegaly (15 x 11.7 x 9 cm). Positron emission tomography/computed tomography (PET/CT) showed multiple cutaneous and subcutaneous hypermetabolic foci in the chest wall the largest of which being in the left anterolateral chest (SUVmax 6.7). Additionally, it showed skin lesions on the extensor surfaces of the elbows largest on the right elbow (SUVmax13.1) and several lower extremity cutaneous nodules notably in the posteromedial left calf (SUVmax 6.8) and dorsum of the left foot (SUVmax 14), an FDG avid right inguinal lymph node 1.0 cm (SUVmax 3) reactive sub centimeter bilateral axillary lymph nodes (SUVmax 4.4) and inguinal lymph nodes largest being 1.2 cm (SUVmax 3). In July 2016, he was started on methotrexate 7.5 mg orally once a week which was gradually increased to 20 mg weekly with the addition of prednisone 40 mg daily. He had the best clinical response while on methotrexate and prednisone, with subjective improvement and a reduction in both the size and number of skin lesions. In February 2017 he began having frequent fevers and new skin lesions concerning for progression and therapy was switched to mycophenolate mofetil (MMF) 500 mg twice daily. The best response to MMF was stable disease and in May 2017 he had clinical progression and was switched to dapsone 50 mg and the dose was gradually increased to 150 mg daily with resolution of his fevers. Dapsone was discontinued after 4 months due to pancytopenia. A left forearm biopsy in September 2017 was consistent with HVLL. In October 2017 methotrexate 7.5 mg weekly was restarted with improvement in skin lesions however, discontinued after 5 months due to worsening liver function. In February 2018, thalidomide and prednisone were started and discontinued after two months due to the development of new skin lesions. Romidepsin was subsequently started, and he received 3 doses in
addition to methotrexate 15 mg per week and prednisone 20 mg daily. However, he developed grade 3 and 4 diarrhea, nausea, vomiting and weakness and romidepsin was discontinued. He was admitted to the hospital in August 2018 and started on CHOEP. PET/CT in September 2018 showed interval progression of lymphoproliferative disease with hepatosplenomegaly and innumerable cutaneous and subcutaneous nodules all over the head, neck, trunk, extremities with the largest lesion measuring 5.9 x 3.8 cm (max SUV 17.2).
At this point in September 2018, morphoproteomic analysis from a skin lesion was obtained to define the tumor biology. Detailed results are described in Table 1.
Table 1.
Morphoproteomic analysis findings from a skin biopsy of a patient with Hydroa-vacinee like lymphoma
| Molecular Pathway | Patient’s Tumor |
Microanatomic Region | Implication/Figure | Figure |
|---|---|---|---|---|
|
Nuclear Cell Cycle-
Related Analyte Ki-67 (G1, S, G2 and M phases of the cell cycle) |
N | Lymphomonocytic cells | >50% expression in the pre-treatment biopsy and 90% in the post-treatment biopsy [2A] |
|
|
Antiapoptotic/Tumorigeni
c Factors |
||||
| Bcl-2 | C | Perivascular and periadnexal regions |
Bcl-2 expression was 50% prior to therapy with methotrexate, mmf, dapsone, thalidomide, prednisone, and romidepsin. Bcl-2, through its interactions with Bax and Bak, is a known anti-apoptotic protein. |
2B |
| mTOR | P, 3+ N | The presence of 3+ phosphorylated mTOR in the nuclear compartment indicated constitutively activated mTORC2 pathway.6 This activation implies tumorigenic activity.6 |
2C | |
|
Enhancer of Zester
homolog 2 (EZH2) |
3+ N | Perivascular and periadnexal regions |
EZH2 is tumorigenic through histone H3 methylation on gene promoters, which represses genes that induce stem cell differentiation. This allows for an embryonic stem cell signature 7,8, which promotes proliferation 7,8. EZH2 has also been associated with disease progression in Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative 7. |
2D |
| CD8+/PD-1 | PD-1 1-3+, PL | The lack of PD-1, programmed cell-death receptor protein 1, demonstrates the disease process is comprised primarily of PD-1 negative CD8 T-cells. The activity of these T-cells is unlikely to be affected by the PD-1/PD-L1 apoptotic pathway. |
2E-G | |
| CD163 | Expressed in 50% of lesional cells. In the context of cutaneous T-cell lymphomas, M2 macrophage polarization has been associated with disease progression. The conversion of M2 to M1 macrophages can act as a potential therapeutic target.9 |
2H |
The nuclear cell cycle analytes revealed BCL-2 expression in approximately 50% of the lesional cells. A Ki-67 expression of ~90% in tumor cells up from >50% in pretreatment sample from 9/25/2017. EZH2 showed variable expression at up to 3+ of the perivascular and periadnexal lesional nuclei including those with karyomegaly. CD8 expression was seen on up to 90% of the lesional cells 1-3+ on their plasmalemma. However, <10% of them showed PD-1 expression. CD 163 expression was seen on up to 50% of the lesional cells in the tissue sample. Morphogenomically the nuclei of the tumor cells in the lesional lymphocytes showed strong positivity for EBER-1, an Epstein - Barr virus encoded RNA. In this case there was a delay in diagnosis from the initial biopsy taken in 2016 until he was diagnosed in 2017. Initial biopsies were inconclusive. The patient developed more systemic symptoms including weight loss, fevers, and had laboratory findings of neutropenia and elevated liver enzymes then biopsy in 2017 revealed a T cell lymphoproliferative disorder with a cytotoxic phenotype and EBV was found to be positive.
Discussion
The pathogenesis and mechanisms of EBV-induced T-cell lymphoproliferative disorders remain largely unknown. There is evidence that EBV infects T/NK cells during the primary infection. and may be able to infect common progenitor cells which can differentiate into mature T and NK cells.10 EBV T/NK lymphoproliferative disease seems to be a result of the accumulation of genetic mutations that drive lymphomagenesis with recurrent mutations in DDX3X, TP53, STAT3, and BCOR1, especially in extranodal nasal NK/T cell lymphoma.11 Environmental factors have also been implicated one case-control study suggested a correlation between pesticides and solvents and the development of extranodal nasal T/NK LPD12.
Current therapies for the treatment of EBV T/NK lymphoproliferative disorders include proteasome inhibitors, janus kinase (JAK) inhibitors, histone deacetylase inhibitors (HDAC) and immune checkpoint inhibitors13. Recently, the U.S. Food and Drug Administration approved romidepsin and belinostat, histone deacetylase inhibitors, for the treatment of cutaneous T-cell lymphoma and relapsed, refractory peripheral T-cell lymphoma as well as brentuximab vendotin, an anti-CD30 antibody-drug conjugate in combination with cyclophosphamide, doxorubicin, and prednisone for the treatment of CD30-expressing peripheral T-cell lymphomas14. The expression of the multi-drug resistant p-glycoprotein has also made NK/T LPDs resistant to standard chemotherapy15.
Morphoproteomic analysis as shown here can aid in molecular profiling of the tumor, with respect to protein analytes incorporating immunohistochemical assessment, quantification of their intensity of expression, state of activation, including phosphorylation, compartmental translocation and functional grouping. It can be especially useful in patients who have failed conventional therapy, or do not have standard approved treatments, or have rare tumor subtypes.
Morphoproteomic data of our patient’s tumor demonstrated 50% expression of BCL-2 in the cytoplasmic periadnexal and perivascular space. BCL-2 has now been successfully targeted in multiple hematological malignancies, including acute myeloid leukemia, chronic lymphocytic leukemia, multiple myeloma, and others. Reports of efficacy of venetoclax in tumors with high BCL-2 expression by IHC, e.g. blastic plasmacytoid dendritic cell neoplasm, early-T precursor acute lymphoblastic lymphoma provide a precedence for use of venetoclax-based regimens for refractory HVLL and provide a rationale for prospective clinical trials for such patients15,16.
Our patient also had strong expression of EZH2 which has been associated with the repression of genes that induce stem cell differentiation and noted to be associated with aggressive disease.7 Tazemetostat, an EZH2 inhibitor was recently approved for relapsed/refractory follicular lymphoma and an EZH2 mutation. Other inhibitors of EZH2 have also shown pre-clinical activity against diffuse large B cell lymphomas8. Strong EZH2 activity noted in our patient’s tumor provide rationale for testing for EZH2 mutation in such patients and determination if such patients may be candidates for inclusion in umbrella trials of EZH2 inhibitors.
Pembrolizumab, a PD-1 inhibitor has shown preliminary activity in refractory NK/T cell lymphomas and in one study 3 out of 4 patients with strong PD-L1 expression achieved complete remission and one patient with weak PD-L1 expression had partial remission. However, there have been anecdotal reports of rapid progression of virally driven T-cell neoplasms with anti-PD-L1 agents18. Our patient had weak expression of PD-1 and anti-PD-1/L1 based approaches may be areas of future investigation for such patients13.
STAT3 is another potential target due to constitutive activation in chronic active EBV infection (CAEBV). JAK1 and JAK2, which phosphorylate and activate STAT3, are inhibited by ruxolitinib which has shown pre-clinical activity in cell lines and primary samples from patients with CAEBV19. Tofacitinib, a selective JAK3 inhibitor which decreases phospho-STAT5 has also shown activity in pre-clinical models. These findings suggest that JAK inhibitors may be useful in EBV-associated T/NK cell lymphomas and warrant further investigation 20.
Unfortunately, in our patient, the disease progression, multiple toxicities developed from multiple lines of systemic therapy, and worsening clinical status altered the patient’s goal. He declined treatment with novel agents, and he elected to return to his home country, precluding application of these morphoproteomic insights to achieve disease control. However, these novel findings described here can potentially help future exploratory research and potential application or clinical development of any of these agents for patients with relapsed or refractory HVLL or EBV-associated T-cell LPD.
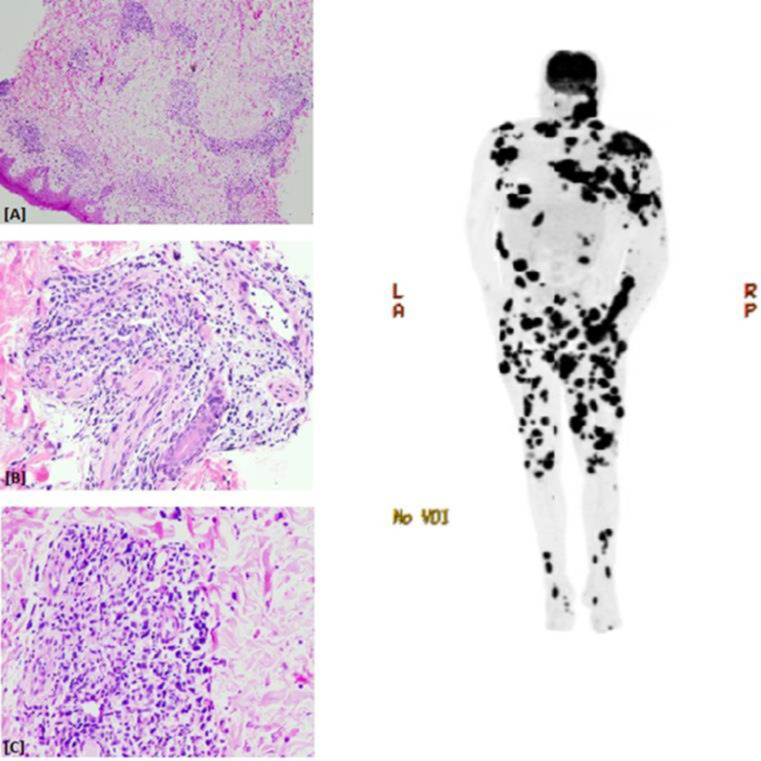
Figure 1.
The H&E stained sections of the immediate pretreatment specimen revealed a histopathology comprised mainly of a lymphohistiocytic infiltrate in a perivascular and periadnexal distribution (Figure 1a, 1b, 1c). The nuclei of the tumor cells were strongly positive for EBER-1. The serum EBV DNA level in the post-treatment sera of this patient was recorded at >1,000,000(original magnification for frames A, B and C, x40, x200 and x200, respectively) and Maximum Intensity Projection (MIP) PET Image. A post therapy scan showing innumerable metabolically active cutaneous and subcutaneous nodules and masses throughout the head/neck, extremities, and trunk, a 5.9 x 3.8 cm soft tissue mass extending from the right zygomatic arch, multiple metabolically active cervical lymph nodes, and scattered deeper intermuscular metabolically active soft tissue lesions in the lower extremities
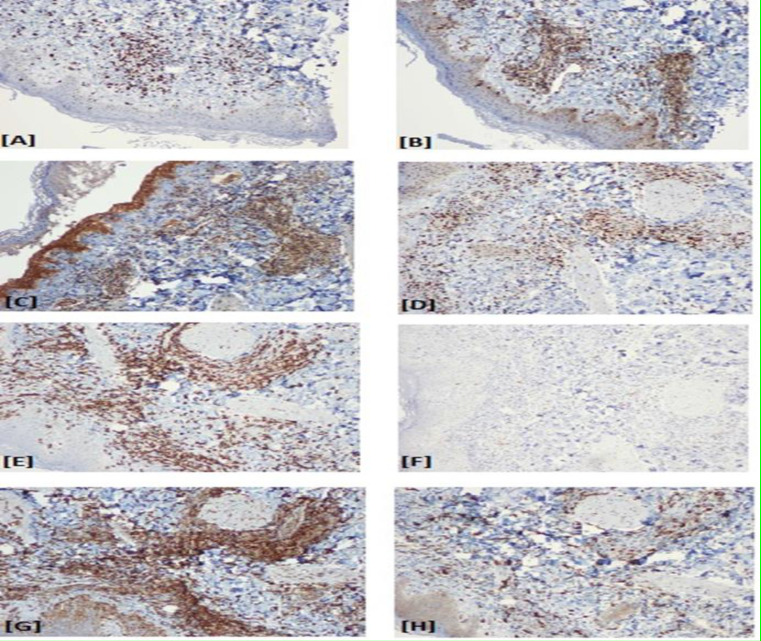
Figure 2.
[A] Ki-67 expressed in the nuclei of lesional lymphomonocytic cells in the immediate pretreatment biopsy at >50% and at ~90% of the nuclei in the post-treatment biopsy. [B] Bcl-2,in the pretreatment specimen shows, cytoplasmic expression in ~50% of the lesional cells in the perivascular and periadnexal locations. [C] (mTOR) pathway is constitutively activated in the lesional cells in this immediate pretreatment specimen phosphorylated (p) –mTOR at up to 3+ in the nuclear compartment (see arrow). [D] EZH2, is variably expressed at up to 3+ of the perivascular and periadnexal lesional nuclei in this specimen. [E] CD8 is expressed on up to 90 % of the lesional cells at 1-3+ on their plasmalemmal aspect including those cells with karyomegaly. [F] Less than 10% of programmed cell death receptor protein 1, PD-1 expression exhibited on this same cell population [G] Expression of PD-L1 by the M2 macrophage/histiocytic component of the infiltrate and the patient’s EBV+CD8+ lymphoproliferative disorder in this immediate pretreatment specimen. [H] CD163 expression up to 50% of the lesional cells in this specimen(original magnification of frame C x400 and for A, B, D, E, F, G and H x100).
References
- 1.Carbone A, Gloghini A, Dotti G. EBV-associated lymphoproliferative disorders: classification and treatment. Oncologist. 2008;13(5):577–85. doi: 10.1634/theoncologist.2008-0036. [DOI] [PubMed] [Google Scholar]
- 2.Gupta G, Man I, Kemmett D. Hydroa vacciniforme: A clinical and follow-up study of 17 cases. J Am Acad Dermatol. 2000;42(2 Pt 1):208–13. doi: 10.1016/S0190-9622(00)90127-0. [DOI] [PubMed] [Google Scholar]
- 3.Magaña M, Sangüeza P, Gil-Beristain J, et al. Angiocentric cutaneous T-cell lymphoma of childhood (hydroa-like lymphoma): a distinctive type of cutaneous T-cell lymphoma. J Am Acad Dermatol. 1998;38(4):574–9. doi: 10.1016/s0190-9622(98)70120-3. [DOI] [PubMed] [Google Scholar]
- 4.Barrionuevo C, Anderson VM, Zevallos-Giampietri E, et al. Hydroa-like cutaneous T-cell lymphoma: a clinicopathologic and molecular genetic study of 16 pediatric cases from Peru. Appl Immunohistochem Mol Morphol. 2002;10(1):7–14. doi: 10.1097/00129039-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Doeden K, Molina-Kirsch H, Perez E, et al. Hydroa-like lymphoma with CD56 expression. J Cutan Pathol. 2008;35(5):488–94. doi: 10.1111/j.1600-0560.2007.00836.x. [DOI] [PubMed] [Google Scholar]
- 6.Quesada AE, Nguyen ND, Rios A, et al. Morphoproteomics identifies constitutive activation of the mTORC2/Akt and NF-kappaB pathways and expressions of IGF-1R, Sirt1, COX-2, and FASN in peripheral T-cell lymphomas: pathogenetic implications and therapeutic options. Int J Clin Exp Pathol. 2014;7(12):8732–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Ciarapica R, Miele L, Giordano A, et al. Enhancer of zeste homolog 2 (EZH2) in pediatric soft tissue sarcomas: first implications. BMC Med. 2011;9:63. doi: 10.1186/1741-7015-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCabe MT, Ott HM, Ganji G, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492(7427):108–12. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Smith AJ, Lott MC, et al. Sulforaphane can protect lens cells against oxidative stress: implications for cataract prevention. Invest Ophthalmol Vis Sci. 2013;54(8):5236–48. doi: 10.1167/iovs.13-11664. [DOI] [PubMed] [Google Scholar]
- 10.Kimura H. EBV in T-/NK-Cell Tumorigenesis. Adv Exp Med Biol. 2018;1045:459–475. doi: 10.1007/978-981-10-7230-7_21. [DOI] [PubMed] [Google Scholar]
- 11.Dobashi A, Tsuyama N, Asaka R, et al. Frequent BCOR aberrations in extranodal NK/T-Cell lymphoma, nasal type. Genes Chromosomes Cancer. 2016;55(5):460–71. doi: 10.1002/gcc.22348. [DOI] [PubMed] [Google Scholar]
- 12.Aozasa K, Takakuwa T, Hongyo T, et al. Nasal NK/T-cell lymphoma: epidemiology and pathogenesis. Int J Hematol. 2008;87(2):110–117. doi: 10.1007/s12185-008-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129(17):2437–2442. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]
- 14.Broccoli A, Argnani L, Zinzani PL. Peripheral T-cell lymphomas: Focusing on novel agents in relapsed and refractory disease. Cancer Treat Rev. 2017;60:120–129. doi: 10.1016/j.ctrv.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimori M, Takada H, Imadome K, et al. P-glycoprotein is expressed and causes resistance to chemotherapy in EBV-positive T-cell lymphoproliferative diseases. Cancer Med. 2015;4(10):1494–504. doi: 10.1002/cam4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agha ME, Monaghan SA, Swerdlow SH. Venetoclax in a Patient with a Blastic Plasmacytoid Dendritic-Cell Neoplasm. N Engl J Med. 2018;379(15):1479–1481. doi: 10.1056/NEJMc1808354. [DOI] [PubMed] [Google Scholar]
- 17.Boidol B, Kornauth C, van der Kouwe E, et al. First-in-human response of BCL-2 inhibitor venetoclax in T-cell prolymphocytic leukemia. Blood. 2017;130(23):2499–2503. doi: 10.1182/blood-2017-05-785683. [DOI] [PubMed] [Google Scholar]
- 18.Ratner L, Waldmann TA, Janakiram M, et al. Rapid Progression of Adult T-Cell Leukemia-Lymphoma after PD-1 Inhibitor Therapy. N Engl J Med. 2018;378(20):1947–1948. doi: 10.1056/NEJMc1803181. [DOI] [PubMed] [Google Scholar]
- 19.Onozawa E, Shibayama H, Takada H, et al. STAT3 is constitutively activated in chronic active Epstein-Barr virus infection and can be a therapeutic target. Oncotarget. 2018;9(57):31077–31089. doi: 10.18632/oncotarget.25780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ando S, Kawada JI, Watanabe T, et al. Tofacitinib induces G1 cell-cycle arrest and inhibits tumor growth in Epstein-Barr virus-associated T and natural killer cell lymphoma cells. Oncotarget. 2016;7(47):76793–7805. doi: 10.18632/oncotarget.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]