Abstract
Background : Prostate cancer is the second most common cancer in the male that affects the health, social and economic life of person. Different compounds such as Wharton’s jelly, have been used to treat prostate cancer. Wharton’s jelly is a tissue rich in cells with mesenchymal morphology. Wharton’s jelly compound inhibited the growth of various cancer cells, including ovarian, osteosarcoma, breast, and prostate cancers, and also reduced the expression of CXCR4 and VLA-4 genes involved in the metastasis process.
Materials and Methods: To do this research, Wharton’s jelly stem cells and DU145 cancer cell line were cultured. After cell culture, the effect of Wharton’s jelly on this cell line was evaluated by scratching and MTT assay. The expression of CXCR4 and VLA-4 genes was also evaluated by Real-time PCR.
Results: The results of MTT and Scratching tests showed that Wharton’s jelly inhibited the growth of DU145 cancer cells and also decreased the expression level of CXCR4 and VLA-4 genes.
Conclusion: The results of this study showed that Wharton’s jelly can be considered as an effective compound for decreasing metastasis of prostate cancer.
Key Words: Prostate cancer, Wharton jelly, CXCR4 gene, VLA-4 gene
Introduction
In developed countries, prostate cancer is the second most common (after skin cancer) and the most deadly cancer (after lung cancer) in men. One in six men has this type of cancer. Epidemiological studies have demonstrated that 10% of prostate cancer are due to genetic factors 1,2 . One of the important abilities of tumor cells is to invade and metastasize. Metastasis is the spread of cancer from early tumors and the development of new tumors in distant organs. Metastasis to distant parts of the body involves several successive steps such as the entry of tumor cells from the primary tissue into the blood vessels, survival in the bloodstream, migration to secondary organs, and the proliferation of cancer cells in the target tissue3. Circulatory cancer cells often produce new tumors from the originated tissue. But blood circulation alone does not result in all cases of tumor spread. Clinical evidence suggests that an Implantation mechanism is responsible for some metastatic cases. For example, prostate and breast cancer often spread to the bone first, and lung cancer often leads to new tumors in the adrenal glands. This junction phenomenon may be related to the identification of the exit site of the circulation by the tumor cell as well as the identification of the location of the new tissue. An example of a nesting phenomenon at the molecular level involves a substance called CXCL12, which is secreted by stromal cells. It absorbs cells that express receptors called CXCR4, which are found in certain types of cancer cells such as breast cancer cells or chronic myelogenous leukemia 4 . Recent studies show that chemokines and their receptors (C-X-C chemokine receptor type 4) play a vital role in the metastasis process and their misstatement can lead to cancer5. The dependence of cancer cells on CXCR4 expression at CXCL12-secreting tissues leads to the movement of cancer cells from their main sites of formation 4 .
CXCR4 expression significantly increases in breast cancer. Studies have shown that CXCR4 plays an important role in cell survival, migration, proliferation, and metastasis of several types of cancer, including breast cancer. The binding of CXCR4 to its ligand (SDF-1) leads to the circulation of cancer cells in the blood, their entry into other tissues, including bone, liver and lung, and the formation of invasive tumors 6 .
The integrin family, which includes cell surface receptors for extracellular matrix compounds, is one of the receptors involved in various aspects of adherent leukocytes. VLA family is one of the integrin-related antigens involved in extracellular matrix adhesion and acts as a receptor for fibronectin as well as cell-to-cell adhesion receptors7.
Various anticancer drugs and compounds that can stimulate tumor-specific immune responses have been studied. There are several reports that confirm hWJSCs have strong tumor-suppressing effects on a variety of cancers such as mammalian adenocarcinoma, ovarian, osteosarcoma, cholangiocarcinoma, bladder, and lymphoma cancer8-19. HWJSCs suppress cancer cells by inducing apoptosis, proliferation and cell cycle inhibition, and the PI3K / Akt signaling pathway inhibition 8, 9, 18 . Numerous studies have shown that hWJSC {[hWJSC-cell lysate (hWJSC-CL) or hWJSC-conditioned medium (hWJSC-CM)] prevents breast cancer and osteosarcoma in vitro and in vivo. Interestingly, hWJSCs, unlike hBMMSCs, do not convert to TAF 8, 20 . HWJSC-CM and hWJSC-CL also inhibit cancer cell growth, so inhibitory mechanisms are inhibited not only by cell-to-cell a but also by hWJSCs 15, 17 .
Materials and methods
Human Wharton’s jelly stem cells
The fetal umbilical cord was prepared under sterile conditions and in physiological serum from Tabriz International Hospital and then washed with 70% alcohol. Afterwards, the umbilical cord was crushed into small 2cm pieces and re-washed using PBS and HBSS buffer. The small pieces were then cut down into smaller segments, and the Wharton jelly was extracted. Du145 Prostate cancer cell line, DMEM Low Glucose, FBS, and Pen-Strip (0.25%) were used to culture the Du145 prostate cancer cell line.
Induction of cancer cells by Wharton jelly stem cells
To investigate the apoptosis of induced cells, 15% and 30% doses of Wharton’s jelly were added to the Du145 cell culture medium. Apoptosis of induced cells was studied under the microscope, and the cell count was performed. In some induced cells, the cells were frozen before apoptosis, and then RNA was extracted.
Investigation of cell migration
Scratching test was used to determine the cell migration. DU145 cancer cells were scraped diagonally from the middle, and then were treated with 15 and 30% doses of Wharton’s jelly. Cell migration was measured at 6, 12, 24, and 48 hours with imaging under the microscope.
Study of gene expression
RNA was extracted from induced cells with 15 and 30% doses of Wharton’s jelly. The expression of CXCR4 and VLA-4 genes was evaluated using real-time PCR. The B2M gene was considered to be the internal control gene. The primers used are presented in Table 1.
Table1.
CXCR4, VLA-4 and Β2M primers
| gene | primers | Length |
|---|---|---|
| CXCR4 | F 5’ CGCCACCAACAGTCAGAG 3’ R 5’ AACACAACCACCCACAAGTC 3’ |
177bp |
| VLA-4 | F 5’ CAAGAATCCAAACTACGGAC 3’ R 5’ TTGCATTCAGTGTTGTGGGA 3’ |
145bp |
| Β2M | F 5’GAGAAGTATGACAACAGCCTC 3’ R 5’ TGAGTCCTTCCACGATACC 3’ |
112bp |
Statistical analysis
Statistical data were analyzed using SPSS PASW Statistics software (version 18). All graphs were drawn using GraphPad PRISM version 6.01. The statistical significance was considered with p < 0.05.
Results
Cell viability
To evaluate the effect of Wharton’s jelly on DU145 cells and the inhibitory concentration of IC50, cancer cells were treated with different doses of Wharton’s jelly at 24 and 48h. The cell viability was decreased at the high concentration of Wharton’s jelly and in longer times (Figure 1) (Table 2).
Figure1.
Statistical analyzes showed a significant difference in the cell growth of Wharton’s jelly-treated cells compared to the control group at 30M and 15M concentrations. This indicated inhibition of DU145 cell growth by Wharton’s jelly (p value <0.05).
Table 2.
Inhibitory effect of Wharton’s jelly on 145 DU cell in prostate cancer
| Cell | IC 50 at 24h | IC 50 at 48h |
|---|---|---|
| DU145 | 30µM | 15µM |
Cell migration
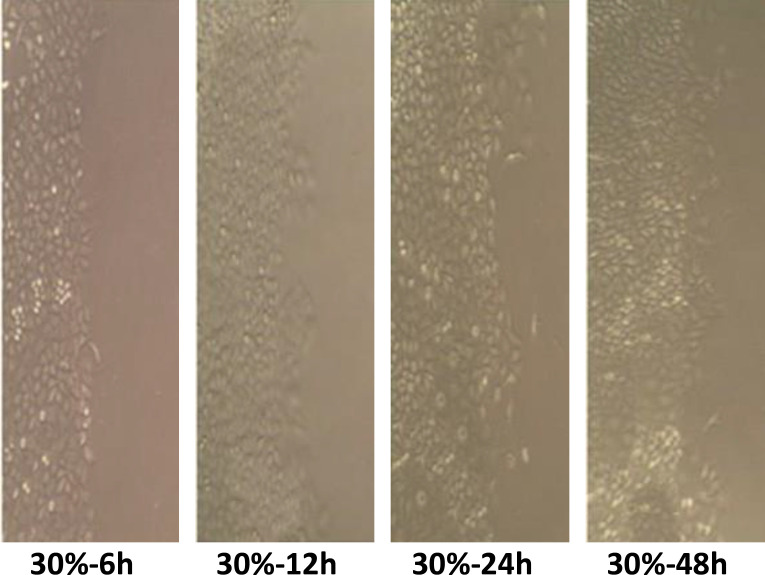
Statistical analyses related to the scratching test showed that cell migration decreased compared to the control group at 15 and 30% concentration of Wharton’s jelly after 12 hours, while at 24 and 48 hours an increase in migration was observed (Figure 2-4).
Figure 2.
Cell migration at different doses of wharton’ s jelly (P <0.001)
Figure 4.
The effect of 30% Wharton’s jelly on cancer cell migration (* 400)
Figure 3.
The effect of 15%Wharton’s jelly on cancer cell migration (* 400)
The apoptotic effect of Wharton’s jelly on Du145 cell line
Microscopic images of DU145 cells apoptotic with Wharton jelly are shown in Figure 5. According to the picture, the cancer cells underwent apoptosis after induction with Wharton jelly.
Figure 5.
Cell morphology. a: Wharton’s jelly-induced cells with apoptosis. b: DU145 cancer cells without induction. c: Wharton’s jelly stem cells (* 1000).
Expression of CXCR-4 and VLA-4 genes in DU145 cell line
The expression of CXCR4 and VLA-4 genes was decreased significantly after treatment of the DU145 cell line with 15 and 30% concentrations of Wharton’s jelly (P-value <0.001). The expression of these genes was decreased in a dose-dependent manner of Wharton’s jelly; therefore, a decrease in expression was significant only at 30% concentrations of Wharton’s jelly, and no significant decrease was observed at 15%. These results indicated that at higher concentrations of Wharton’s jelly, the expression of CXCR4 and VLA-4 genes decreased (Figure 6).
Figure 6.
The expression of CXCR-4 and VLA-4 genes in the DU145 cell after treatment with Wharton ‘s jelly.
Discussion
In our study, DU145 cells growth decreased at 15 and 30% concentrations Wharton’s jelly, which indicated that Wharton’s jelly has a high inhibitory effect on the growth and proliferation of cancer cells. DU145 cell growth was reduced in a dose- and time-dependent manner that demonstrated the anti-tumor effect of Wharton’s jelly.
We also showed that CXCR4 gene expression significantly decreased at a 30% concentration of Wharton’s jelly, which indicated that CXCR4 expression is dependent on Wharton’s jelly high doses. CXCR4 is activated in the metastatic pathway, and according to our results, it can be said that Wharton’s jelly can reduce cell metastasis in a dose-dependent manner. VLA-4 expression in the DU145 cell line was decreased after treatment with 30%Wharton’s jelly, which indicated that VLA-4 is decreased at high doses of Wharton’s jelly.
We also studied DU145 cell apoptosis after treatment with Wharton’s jelly by microscope witch. DU145 cells were affected by apoptosis with different doses of Wharton’s jelly stem cells.
Numerous studies on chemokines and their receptors have indicated their role in cancer cell metastasis and tumor spread. CXCR4 plays a vital role in cell survival, proliferation, migration, and metastasis 5,21. Thus, CXCR4 antagonists can be considered as a significant factor in the prevention and treatment of prostate cancer. Today, various CXCR4 inhibitors have been reported, including Wharton’s jelly. Wharton’s jelly has an anti-tumor effect, and various studies have reported that Wharton’s jelly has a role in different messaging mechanisms, including initiation of transcription, differential expression of functional genes, and reprogramming specific cell types 22 . VLA-4, a receptor for fibronectin and cell-to-cell binding, is involved in extracellular matrix adhesion. It is one of the genes involved in the humming process, and its decreased expression leads to reduce metastasis23. According to our study, the appropriate dose for the reduction of the VLA-4 expression was 30% Wharton’s jelly, so it can be said that a 30% dose of Wharton’s jelly inhibits the metastasis in prostate cancer. Various studies have been performed on the role of VLA-4, CXCR4, and Wharton’s jelly in cancer.
CXCR4 antagonists inhibit the growth and spread of cancer. Numerous studies showed that CXCR4 receptors are essential for invasion, proliferation, and metastasis in breast cancer. Therefore, CXCR4 is considered as a diagnostic marker and an important therapeutic target 24 . Studies have shown that the SDF-1 / CXCR-4 signaling pathway is active in most cancer cells 25 . Depending on our results, Wharton’s gel inactivates the SDF-1 / CXCR4 pathway by decreasing CXCR-4 expression, which leads to apoptosis in prostate cancer. Studies showed that VLA-4 expression has significantly increased in tumor cells 26, 27 . Diao et al. reported the role of VLA-4 metastasis in 2016. Diao et al. reported that activation of the P38MPK and NF-β pathway leads to metastasis by positively regulating VCAM-1 / VLA-4 expression 28 . Kalamegam et al. demonstrated that Wharton’s jelly inhibits breast cancer and displays anti-cancer effects in other cancers, including ovarian cancer and osteosarcoma. An interesting point in their studies was that the anti-cancer effect varied between cell lines, which was more severe on osteosarcoma and less on ovarian cancer 22 . Evidence have shown that human Bone Marrow MSCs and other types of MSCs inhibit tumor growth in vitro and in vivo. Khakoo et al. showed that injection of Wharton’s jelly-derived hBMMSC into Kaposi’s sarcoma mouse model inhibited tumor growth in a dose-dependent manner 29 . Zhang et al. studied the effect of fetal umbilical cord stem cells on the MDA-MB-231 cancer cells and showed that fetal umbilical cord cells are involved in inducing apoptosis 30 .
CONCLUSION
The study aimed to assess the antitumor effect of Wharton’s jelly on prostate cancer. Our results showed that different concentrations of Wharton’s jelly might change the cell migration and dysregulate the expression of CXCR4 and VLA-4 in a time- dependent manner. Wharton’s jelly at high doses significantly decreases the migration of DU145 cells and the expression of CXCR4 and VLA-4. Considering that CXCR4 and VLA-4 are involved in the metastasis pathway, it can be said that Wharton’s jelly prevents tumor metastasis. Although the results of the present study and previous studies suggest that Wharton’s jelly may be considered as a treatment option for invasive prostate cancer, more studies are needed to understand its anti-metastatic effects.
References
- 1.Jemal A, Murray t, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Carter H, Partin A, Retik A, et al. Diagnosis and staging of prostate cancer Campbell, s urology. Sydney: Elsevier science publishers; 2002. [Google Scholar]
- 3.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer . 2002;2(8):563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 4. Available from: https://www.britannica.com/science/metastasis.
- 5.Xu C, Zhao H, Chen H, et al. CXCR4 in breast cancer: oncogenic role and therapeutic targeting. Drug Des Devel Ther. 2015;9:4953–64. doi: 10.2147/DDDT.S84932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 7.Larson RS, Corbi AL, Berman L, et al. Primary structure of the leukocyte function-associated molecule-1 alpha subunit: an integrin with an embedded domain defining a protein superfamily. J Cell Biol. 1989;108(2):703–12. doi: 10.1083/jcb.108.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong CY, Subramanian A, Gauthaman K, et al. Human umbilical cord Wharton’s jelly stem cells undergo enhanced chondrogenic differentiation when grown on nanofibrous scaffolds and in a sequential two-stage culture medium environment. Stem Cell Rev Rep. 2012;8(1):195–209. doi: 10.1007/s12015-011-9289-8. [DOI] [PubMed] [Google Scholar]
- 9.Lin HD, Bongso A, Gauthaman K, et al. Human Wharton’s jelly stem cell conditioned medium enhances freeze-thaw survival and expansion of cryopreserved CD34+ cells. Stem Cell Rev Rep. 2013;9(2):172–83. doi: 10.1007/s12015-013-9426-7. [DOI] [PubMed] [Google Scholar]
- 10.Rachakatla RS, Marini F, Weiss ML, et al. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007;14(10):828–35. doi: 10.1038/sj.cgt.7701077. [DOI] [PubMed] [Google Scholar]
- 11.Ayuzawa R, Doi C, Rachakatla RS, et al. Naive human umbilical cord matrix derived stem cells significantly attenuate growth of human breast cancer cells in vitro and in vivo. Cancer Lett. 2009;280(1):31–7. doi: 10.1016/j.canlet.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura M, Kawabata A, Ohta N, et al. Wharton's jelly stem cells as agents for cancer therapy. Tissue Eng Regen Med . 2011;4:39–47. [Google Scholar]
- 13.Maurya DK, Doi C, Kawabata A, et al. Therapy with un-engineered naive rat umbilical cord matrix stem cells markedly inhibits growth of murine lung adenocarcinoma. BMC Cancer. 2010;10:590. doi: 10.1186/1471-2407-10-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao KC, Yang HT, Chen MW. Human umbilical cord mesenchymal stem cells suppress breast cancer tumourigenesis through direct cell–cell contact and internalization. J Cell Mol Med . 2012;16(8):1803. doi: 10.1111/j.1582-4934.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Han G, Liu H, et al. Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling. PLoS One. 2013;8(4):e62844. doi: 10.1371/journal.pone.0062844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y, Hao X, Zhang S, et al. The in vitro and in vivo effects of human umbilical cord mesenchymal stem cells on the growth of breast cancer cells. Breast Cancer Res Treat. 2012;133(2):473–85. doi: 10.1007/s10549-011-1774-x. [DOI] [PubMed] [Google Scholar]
- 17.Wu S, Ju GQ, Du T, et al. Microvesicles derived from human umbilical cord Wharton’s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS One. 2013;8(4):e61366. doi: 10.1371/journal.pone.0061366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin HD, Fong CY, Biswas A, et al. Human Wharton's jelly stem cells, its conditioned medium and cell-free lysate inhibit the growth of human lymphoma cells. Stem Cell Rev Rep. 2014;10(4):573–86. doi: 10.1007/s12015-014-9514-3. [DOI] [PubMed] [Google Scholar]
- 19.Kawabata A, Ohta N, Seiler G, et al. Naive rat umbilical cord matrix stem cells significantly attenuate mammary tumor growth through modulation of endogenous immune responses. Cytotherapy. 2013;15(5):586–97. doi: 10.1016/j.jcyt.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian A, Shu‐Uin G, Kae‐Siang N, et al. Human umbilical cord wharton's jelly mesenchymal stem cells do not transform to tumor‐associated fibroblasts in the presence of breast and ovarian cancer cells unlike bone marrow mesenchymal stem cells. J Cell Biochem. 2012;113(6):1886–95. doi: 10.1002/jcb.24057. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Lee HJ, Song YS. Stem cell based gene therapy in prostate cancer. Biomed Res Int. 2014;2014:549136. doi: 10.1155/2014/549136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gauthaman K, Yee FC, Cheyyatraivendran S, et al. Human umbilical cord Wharton's jelly stem cell (hWJSC) extracts inhibit cancer cell growth in vitro. J Cell Biochem. 2012;113(6):2027–39. doi: 10.1002/jcb.24073. [DOI] [PubMed] [Google Scholar]
- 23.Abe M, Hiura K, Ozaki S, et al. Vicious cycle between myeloma cell binding to bone marrow stromal cells via VLA-4–VCAM-1 adhesion and macrophage inflammatory protein-1α and MIP-1β production. J Bone Miner Metab. 2009;27(1):16–23. doi: 10.1007/s00774-008-0012-z. [DOI] [PubMed] [Google Scholar]
- 24.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14(3):171–9. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Libura J, Drukala J, Majka M, et al. CXCR4–SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood. 2002;100(7):2597–606. doi: 10.1182/blood-2002-01-0031. [DOI] [PubMed] [Google Scholar]
- 26.Honn KV, Tang DG. Adhesion molecules and tumor cell interaction with endothelium and subendothelial matrix. Cancer Metastasis Rev. 1992;11(3-4):353–75. doi: 10.1007/BF01307187. [DOI] [PubMed] [Google Scholar]
- 27.Albelda S. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993;68(1):4–17. [PubMed] [Google Scholar]
- 28.Diao XW, Feng JY, Wang QW, et al. SDF-1/CXCR4 axis promotes prostate cancer cell invasion and bone metastasis through p38, NF-κB and HIF-1α pathways. Int J Clin Exp Pathol. 2016;9(2):2706–17. [Google Scholar]
- 29.Khakoo AY, Pati S, Anderson SA, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203(5):1235–47. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C, Yang SJ, Wen Q, et al. Human-derived normal mesenchymal stem/stromal cells in anticancer therapies. J Cancer. 2017;8(1):85–96. doi: 10.7150/jca.16792. [DOI] [PMC free article] [PubMed] [Google Scholar]