Abstract
Microbial growth requires energy for maintaining the existing cells and producing components for the new ones. Microbes therefore invest a considerable amount of their resources into proteins needed for energy harvesting. Growth in different environments is associated with different energy demands for growth of yeast Saccharomyces cerevisiae, although the cross-condition differences remain poorly characterized. Furthermore, a direct comparison of the energy costs for the biosynthesis of the new biomass across conditions is not feasible experimentally; computational models, on the contrary, allow comparing the optimal metabolic strategies and quantify the respective costs of energy and nutrients. Thus in this study, we used a resource allocation model of S. cerevisiae to compare the optimal metabolic strategies between different conditions. We found that S. cerevisiae with respiratory-impaired mitochondria required additional energetic investments for growth, while growth on amino acid-rich media was not affected. Amino acid supplementation in anaerobic conditions also was predicted to rescue the growth reduction in mitochondrial respiratory shuttle-deficient mutants of S. cerevisiae. Collectively, these results point to elevated costs of resolving the redox imbalance caused by de novo biosynthesis of amino acids in mitochondria. To sum up, our study provides an example of how resource allocation modeling can be used to address and suggest explanations to open questions in microbial physiology.
Keywords: energy requirements, metabolic modeling, anaerobic growth, resource allocation, mitochondria
In the article, the authors blended computational modeling with experimental measurements to identify additional energy costs for growth of Saccharomyces cerevisiaewhen it cannot use respiration for energy generation.
Introduction
Energy turnover is central to life as we know it: living cells use energy for many of their functions, such as to maintain chemical gradients across membranes, to polymerize macromolecules (Verduyn et al. 1990a, Lahtvee et al. 2017), to perform mechanical work, or to condition unfavorable environments (e.g. to neutralize toxic compounds). As a result, cells invest a substantial amount of available resources into energy-harvesting pathways: quantitative proteomics measurements of budding yeast Saccharomyces cerevisiae grown in aerobic, glucose-limited chemostats suggested that up to 1/3 of the total cell proteome is allocated to energy generation pathways (Elsemman et al. 2022).
The steady-state energy turnover is described as an equal rate of sum energy extraction from nutrients and investment into growth. S. cerevisiae intensively ferments glucose into ethanol in glucose-excess conditions (Blank and Sauer 2004), yet, fluctuations of nutrient levels are a frequent phenomenon in both natural and biotechnologically-relevant environments (Haringa et al. 2016). There, oscillations of glucose availability, for instance, are handled by anticipatory protein expression (van den Brink et al. 2008, Grigaitis and Teusink 2022). Thus the cells in glucose-scarce conditions are indeed primed to consume the incoming flux of nutrients and store the released energy in the high-energy bonds of the ATP molecule.
The other side of the balance, ATP investment into the biosynthesis of new cell components, tends to be more dynamic. First, the cell composition of S. cerevisiae can greatly vary across conditions, even for the same growth-limiting substrate as its availability changes (Lange and Heijnen 2001, Canelas et al. 2011). Second, external factors (e.g. presence of oxygen) can dictate the availability of some of the biosynthesis routes.
Costs of producing new cell biomass from nutrients can be computed from the biosynthesis routes of major cell components: proteins, nucleic acids, lipids, and carbohydrates. Computational models, namely, genome-scale metabolic models, can be of great help in this endeavor. A genome-scale metabolic model (GEM) is a compendium of the reactions that can happen in an organism based on its genome sequence (Lu et al. 2019). To the day, GEMs are the most extensive tools to approximate biochemistry in a fine-grained manner at large-scale and have a diverse range of applications (Somerville et al. 2022). Extensions of genome-scale models, so-called protein-constrained GEMs, encompass (i) fine-grained descriptions of protein turnover, and (ii) a set of constraints which describe the protein capacity of different cellular compartments. The interplay between investments and yields in protein-constrained models therefore aids identification of the most optimal metabolic strategies at genome-scale in terms of allocation of cellular resources (Elsemman et al. 2022).
Prediction of condition-dependent metabolic strategies and quantification of energy- and nutrient costs for biomass production can explain metabolic phenotypes and offer targets to optimize industrial processes. In this study, we present an updated resource allocation model of S. cerevisiae, which we used to identify and metabolic strategies of yeast across conditions and genotypes. We found that growth with impaired- or inactive mitochondria in chemically defined minimal media requires additional energetic and proteomic investments. The model predictions pointed to additional costs of resolving the surplus of mitochondrial redox equivalents accumulated due to biosynthetic processes in mitochondria. Overall, we argue that the computational analysis of microbial metabolic strategies is a powerful approach to deepen understanding of microbial physiology.
Results
The updated proteome-constrained model of S. Cerevisiae, pcYeast8
We improved our previously published version of the protein constrained model of yeast (pcYeast7.6; (Elsemman et al. 2022)) and created an updated version, pcYeast8. The pcYeast8 model was created based on the Yeast8 metabolic model (Lu et al. 2019), which was a major upgrade of the yeast GEM from the version Yeast7.6. We first performed some manual curation of the Yeast8 model, e.g. we have reconstructed some previously missing pathways related to lipoic acid synthesis; curated the glycerol: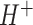 symport reaction, to name a few (for more information, see Supplementary Notes). Then we used custom code, which we first reported for the proteome-constrained model of fission yeast Schizosaccharomyces pombe (Grigaitis et al. 2022a) to develop the pcYeast8.
symport reaction, to name a few (for more information, see Supplementary Notes). Then we used custom code, which we first reported for the proteome-constrained model of fission yeast Schizosaccharomyces pombe (Grigaitis et al. 2022a) to develop the pcYeast8.
We first used the model to revisit our earlier analysis of growth in aerobic glucose-limited chemostats (Fig. 1A-C). Contrary to the pcYeast7.6, pcYeast8 predicted glycerol excretion in batch cultures (Fig. 1A), which constitutes a more accurate representation of resolving redox balance at high specific growth rates. Other than that, specific consumption and excretion fluxes (Fig. 1A), the respiratory quotient ( ), which represents the ratio of flux through respiration versus fermentation (Fig. 1B), and the biomass yield of glucose (
), which represents the ratio of flux through respiration versus fermentation (Fig. 1B), and the biomass yield of glucose ( ) (Fig. 1C) were all in good agreement with both experimental data and pcYeast7.6 predictions.
) (Fig. 1C) were all in good agreement with both experimental data and pcYeast7.6 predictions.
Figure 1.

Predicted and measured physiological response of S. cerevisiae CEN.PK as a function of the specific growth rate in glucose limited aerobic chemostats. (A). Specific uptake and excretion rates as function of the specific growth rate. (B). The respiratory quotient (RQ), the ratio between specific exchange rates of carbon dioxide and oxygen. (C). Biomass yield on glucose. (D). Expressions of the model constraints. If the constraint expression equals 1, it is considered active. (E). Proteome fractions allocated to main pathways in yeast metabolism. The shading in the panels corresponds to the active constraint in Fig. 1D. Proteome annotations taken from (Elsemman et al. 2022). Data from glucose-limited chemostat cultures (circles), trehalose- or glucose excess cultures (triangles), and glucose-excess cultures from the control experiment of cycloheximide treatment (squares) from (Elsemman et al. 2022).
At every simulation step, the model predicts which proteome constraints limit growth (shading of panels in Fig. 1 represent different proteome constraints actively limiting growth). The sequence of constraints hit as a function of specific growth rate was consistent with the pcYeast7.6 (Elsemman et al. 2022). At low specific growth rates, and before the critical dilution rate, glucose transport capacity actively limited growth, to be followed by the mitochondrial capacity constraint, and after 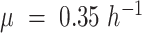 , limitation in the cytosolic proteome capacity (Fig. 1D). At maximal specific growth rate (glucose excess conditions), the only active proteome constraint was the cytosolic proteome capacity.
, limitation in the cytosolic proteome capacity (Fig. 1D). At maximal specific growth rate (glucose excess conditions), the only active proteome constraint was the cytosolic proteome capacity.
To further validate the model predictions, we compared the predicted proteome fractions allocated to specific pathways with quantitative proteomics data (Fig. 1E). Notably, the model predicts minimal required proteome fractions ( = assuming that proteins operate at  ). The predicted proteome fractions of TCA cycle and oxidative phosphorylation proteins were, similarly to the pcYeast7.6 predictions, in quantitative agreement with the experimental measurements, and predictions captured the qualitative trend for, e.g. amino acid biosynthesis proteins. The predicted proteome fractions of protein translation-related proteins (translation initiation and elongation factors) were in line with experimental measurements (Supplementary Fig. 1) and predicted more accurately than by pcYeast7.6. With this we conclude that the updated proteome constrained model of S. cerevisiae can give us improved insight in the physiology and proteome composition of S. cerevisiae grown in aerobic, glucose limited chemostat cultures.
). The predicted proteome fractions of TCA cycle and oxidative phosphorylation proteins were, similarly to the pcYeast7.6 predictions, in quantitative agreement with the experimental measurements, and predictions captured the qualitative trend for, e.g. amino acid biosynthesis proteins. The predicted proteome fractions of protein translation-related proteins (translation initiation and elongation factors) were in line with experimental measurements (Supplementary Fig. 1) and predicted more accurately than by pcYeast7.6. With this we conclude that the updated proteome constrained model of S. cerevisiae can give us improved insight in the physiology and proteome composition of S. cerevisiae grown in aerobic, glucose limited chemostat cultures.
Reduced mitochondrial respiration leads to increased energy costs for growth
With the pcYeast8 model fully parametrized for glucose-limited aerobic chemostat cultures, we aimed to characterize the physiology of S. cerevisiae in energy-perturbed states. Mitochondria are central to energy harvesting in eukaryal cells: these are specialized organelles, which host the TCA cycle proteins and the oxidative phosphorylation system. In yeast, the biosynthesis of mitochondria is mainly regulated by the transcription factor Hap4 (Winde and Grivell 1995, Maris et al. 2001). We thus asked whether we can predict changes in yeast physiology due to modulation of Hap4 expression levels.
We mimicked overexpression and deletion of Hap4 by adjusting the mitochondrial proteome capacity in glucose-limited chemostats (Fig. 2A and B). For the overexpression of Hap4 (Fig. 2A), we needed to increase the mitochondrial capacity to 125% of the wild-type value (Fig. 1) to find good agreement in terms of the shifted critical dilution rate 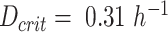 . The subsequent model predicted exchange (uptake and excretion) fluxes across different dilution rates were very close to the observed fluxes.
. The subsequent model predicted exchange (uptake and excretion) fluxes across different dilution rates were very close to the observed fluxes.
Figure 2.
Specific uptake and excretion fluxes as a function of specific growth rate in glucose-limited chemostat cultures. (A-B) Aerobic growth of the strains with perturbed Hap4 expression: (A) Hap4 overexpression strain; (B) Hap4 deletion mutant ( ). (C). Anaerobic growth in glucose-limited chemostats. Points are experimental measurements, lines are model predictions. Shading of the panels corresponds to active proteome constraints at different simulation points as represented in Fig. 1. The thicker and thinner lines in b-c represent simulations with the growth associated ATP maintenance (GAM) set to 40 and 24
). (C). Anaerobic growth in glucose-limited chemostats. Points are experimental measurements, lines are model predictions. Shading of the panels corresponds to active proteome constraints at different simulation points as represented in Fig. 1. The thicker and thinner lines in b-c represent simulations with the growth associated ATP maintenance (GAM) set to 40 and 24  , respectively. Data for a from (Maris et al. 2001), for b from (Raghevendran et al. 2006), for c, two different strains: CEN.PK (circles) from (Tai et al. 2005, 2007, Jewett et al. 2013, Björkeroth et al. 2020), CBS8066 (triangles) from (Nissen et al. 1997).
, respectively. Data for a from (Maris et al. 2001), for b from (Raghevendran et al. 2006), for c, two different strains: CEN.PK (circles) from (Tai et al. 2005, 2007, Jewett et al. 2013, Björkeroth et al. 2020), CBS8066 (triangles) from (Nissen et al. 1997).
Less straightforward, however, were the results from an analogous experiment of the Hap4 deletion mutant (Fig. 2B). We had to reduce the mitochondrial proteome capacity to 28% of the WT value to capture the  of the
of the 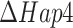 strain (Raghevendran et al. 2006). Then, we observed that predicted fluxes were lower than measured flux values at the glucose excess conditions (thinner lines in the Fig. 2B). This observation called to reassess the ATP maintenance parameters for the
strain (Raghevendran et al. 2006). Then, we observed that predicted fluxes were lower than measured flux values at the glucose excess conditions (thinner lines in the Fig. 2B). This observation called to reassess the ATP maintenance parameters for the  strain, the non-growth-associated ATP maintenance (NGAM) and/or the growth-associated ATP maintenance (GAM). Increased NGAM value did not result in more accurate predictions (data not shown), and we found that a substantial increase of GAM to ca. 160% of the initial (naïve) value (40 vs. 24
strain, the non-growth-associated ATP maintenance (NGAM) and/or the growth-associated ATP maintenance (GAM). Increased NGAM value did not result in more accurate predictions (data not shown), and we found that a substantial increase of GAM to ca. 160% of the initial (naïve) value (40 vs. 24 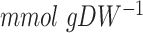 ) did so (thicker lines of the Fig. 2B). With the increased GAM, we then had to readjust the mitochondrial proteome capacity to get to the critical dilution rate and arrived at 32% of the wild-type proteome capacity. Yet, the ratio of ethanol produced per glucose consumed
) did so (thicker lines of the Fig. 2B). With the increased GAM, we then had to readjust the mitochondrial proteome capacity to get to the critical dilution rate and arrived at 32% of the wild-type proteome capacity. Yet, the ratio of ethanol produced per glucose consumed 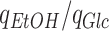 remained largely unaltered because of the changed GAM value (Fig. S3).
remained largely unaltered because of the changed GAM value (Fig. S3).
Based on our observations, here we conclude that growth with reduced mitochondrial respiration leads to additional energy expenditures, which are currently not captured by the pcYeast8 model. The improved predictions were achieved by changing global model parameters, in this case, growth-associated ATP maintenance (GAM).
Additional energetic and proteome investments in anaerobic growth
We wondered if the increased energy costs for growth was specific for Hap4 deletion under glucose excess only or was more generic for loss in respiratory capacity. We therefore used our model to analyze experimental data of anaerobic glucose-limited chemostats (Fig. 2C). As with the 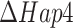 strain, we tested growth with both the naïve (24) and increased (40
strain, we tested growth with both the naïve (24) and increased (40 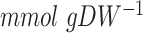 ) GAM values. We observed that the same increase in the GAM value, 24–40
) GAM values. We observed that the same increase in the GAM value, 24–40  , was needed to capture the exchange fluxes in anaerobic glucose-limited chemostats (see thicker vs. thinner lines in the Fig. 2C). However, we observed that the in silico maximal specific growth rate (of
, was needed to capture the exchange fluxes in anaerobic glucose-limited chemostats (see thicker vs. thinner lines in the Fig. 2C). However, we observed that the in silico maximal specific growth rate (of  ) largely exceeded the experimental value (
) largely exceeded the experimental value ( 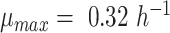 , (Björkeroth et al. 2020)) for the CEN.PK strain—even with the GAM value of 40
, (Björkeroth et al. 2020)) for the CEN.PK strain—even with the GAM value of 40  (Fig. S4). We looked at the predicted active constraints for anaerobic growth and identified that to arrive to the maximal specific growth rate, characteristic to the CEN.PK strain, the minimal level of the ‘unspecified protein’ (UP) had to be increased from the initial value of 0.22 to 0.32
(Fig. S4). We looked at the predicted active constraints for anaerobic growth and identified that to arrive to the maximal specific growth rate, characteristic to the CEN.PK strain, the minimal level of the ‘unspecified protein’ (UP) had to be increased from the initial value of 0.22 to 0.32 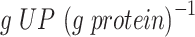 .
.
The UP is an artificial protein of average composition and length, and its expression represents all proteins that do not contribute actively to biomass production (i.e. metabolically inactive proteins). The minimal UP mass fraction is, therefore, a proxy for total cytosolic proteome capacity, which becomes an active constraint when the UP fraction in the proteome reaches this preset minimal value. At that point, each protein in the cytosol contributes directly to growth, and investment in one protein has to come at the expense of another. Unlike the increased energy demand, the increase in the UP value is only relevant for anaerobic growth, and suggests lower ‘usable’ proteome capacity even at glucose excess.
Overall, the combination of the model parameters we had to change explicitly to capture the anaerobic physiology of S. cerevisiae suggests the presence of additional energy and proteomic demands associated with growth when no or a very limited amount of oxygen can be used for oxidative phosphorylation.
Predicting growth of S. cerevisiae in rich media
The growth-associated ATP maintenance and the minimal UP level that we needed to adjust to capture anaerobic growth, are global parameters, estimated from available data but under uncertainty and assumption. We thus wanted to test whether the parameter values we adopted lead to a reasonable prediction of growth under different growth conditions, for which we studied a nutritional upshift.
A major contributor to the energy and proteome costs for growth in minimal, chemically-defined, media is the biosynthesis of amino acids. Later polymerized into proteins, these are the most abundant constituent of yeast dry biomass (between 35 and 50% of the total dry mass, (Canelas et al. 2011)) and protein turnover is a major consumer of ATP in the cell (Lahtvee et al. 2017). It is thus unsurprising that, compared to minimal media, S. cerevisiae exhibits a ca. 20% higher specific growth rate in rich media, such as YPD or SC (Metzl-Raz et al. 2017).
We analyzed the experiment performed by (Björkeroth et al. 2020): growing S. cerevisiae in either minimal Verduyn medium, or medium supplemented with amino acids (rich medium), in both aerobic and anaerobic conditions. We estimated the transporter capacity and uptake of amino acids for the rich medium as detailed in Methods, and predicted batch growth in these four conditions (Fig. 3).
Figure 3.
Overview of the predicted specific growth rates and proteome composition in batch cultures with glucose as the main carbon source. (A). Predicted specific growth rates for batch cultures in minimal and amino acid-rich media. (B-C) Protein abundance in different conditions (mass fractions 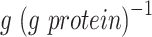 ): (B). translation-related proteins, (C). clusters of energy- and amino acid metabolism. Blue and orange points/bars represent minimal and rich media, respectively. Points in (B-C) are experimental measurements, bars are model predictions. Proteome annotations taken from (Elsemman et al. 2022). Data from (Björkeroth et al. 2020).
): (B). translation-related proteins, (C). clusters of energy- and amino acid metabolism. Blue and orange points/bars represent minimal and rich media, respectively. Points in (B-C) are experimental measurements, bars are model predictions. Proteome annotations taken from (Elsemman et al. 2022). Data from (Björkeroth et al. 2020).
We first simulated growth using the GAM values as determined previously: 24  for aerobic and 40
for aerobic and 40 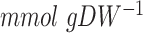 for anaerobic growth. The predicted batch growth rates were similar to the experimentally measured values (Fig. 3A) for 3 out of 4 conditions, with the exception of the underpredicted anaerobic growth rate on the rich medium. For this condition, we reversed the GAM value to 24
for anaerobic growth. The predicted batch growth rates were similar to the experimentally measured values (Fig. 3A) for 3 out of 4 conditions, with the exception of the underpredicted anaerobic growth rate on the rich medium. For this condition, we reversed the GAM value to 24  and observed an increased predicted growth rate which was more consistent to the experimental measurements. Consequently, we have adopted the GAM value of 40
and observed an increased predicted growth rate which was more consistent to the experimental measurements. Consequently, we have adopted the GAM value of 40  for anaerobic growth on minimal Verduyn medium only.
for anaerobic growth on minimal Verduyn medium only.
In the proteome predictions, we have observed a specific growth rate-dependent increase in the proteome fraction, allocated to ribosomes (Fig. 3B). The predictions were in agreement with previous reports (Metzl-Raz et al. 2017, Elsemman et al. 2022), however, the absolute ribosomal proteome fraction in aerobic conditions in the data set of (Björkeroth et al. 2020) was considerably higher as reported of (Metzl-Raz et al. 2017, Elsemman et al. 2022). Nonetheless, the relative upshift of ribosomes in amino acid-supplemented media was predicted correctly (Fig. S5).
Other translation-related non-ribosomal proteins (translation factors etc.) exhibit a specific growth rate-dependent increase as well (Fig. 3B, similar to the increase due to glucose availability, Fig. S1). A notable exception between data and predictions was the reported fraction of translation elongation factors, which was ca. 3-fold higher than both the model predictions (Fig. 3B), and our previously published data from aerobic batch cultures in minimal media (Fig. S1). We do not have explanations for the high abundance of translation elongation factors and ribosomes in the particular data set of (Björkeroth et al. 2020), and we speculate that this could be perhaps dictated by technical bias.
In glucose batch conditions, where the cytosolic proteome constraint is active, the increase in the proteome spent on protein translation has to be accompanied by a decrease in other proteome clusters. The major, and the most obvious change for growth on rich medium was the ca. 2-fold decrease in proteins associated with amino acid biosynthesis, as well as TCA cycle proteins (Fig. 3C). Some other proteins relevant to the biosynthesis of amino acids, such as pentose phosphate pathway, were also less expressed in rich media. Contrary to that, as expected, the abundance of glycolysis proteins did not significantly vary because of amino acid supplementation.
To sum up, here we challenged the pcYeast8 model to rich, amino acid-supplemented media in both aerobic and anaerobic conditions. The current estimates of the GAM and minimal UP values for growth in both aerobic- and anaerobic conditions allowed us to successfully predict physiology and proteomes for growth in rich media, using an independent data set. The predicted additional energy investment, needed for anaerobic growth on a minimal medium, was alleviated by supplementation of amino acids to the medium. Together with the previous observations in the aerobic  cells, these results suggest that the additional costs of growth in anaerobic conditions might stem from the requirement of functional mitochondria for the de novo biosynthesis of amino acids.
cells, these results suggest that the additional costs of growth in anaerobic conditions might stem from the requirement of functional mitochondria for the de novo biosynthesis of amino acids.
Redox shuttling via acetaldehyde-ethanol shuttle involves additional costs
Since the GAM value is a global parameter with no mechanistic role in the pcYeast8 model, we next wanted to explore possible origins of increased cost of generating new biomass under anaerobic conditions—new amino acids for proteins, to be specific. One specific challenge in anaerobic/respiratory-deficient growth is maintaining redox balance in cell compartments. Synthesis of cellular components creates a surplus of reduced electron carriers, such as NAD(P)H, in both cytosol and mitochondria. Aerobically, cytosolic redox equivalents are shuttled into mitochondria and fed into the electron transport chain. Additionally, in fast aerobic growth (Fig. 1A), some of the NADH is reoxidized in the glycerol shunt (production of glycerol from dihydroxyacetone phosphate).
Yet in anaerobic conditions, the only available option to resolve redox imbalances is via glycerol production in the cytosol (Fig. 2C, (Nissen et al. 1997)), and shuttling of redox equivalents out of mitochondria becomes essential for growth. There are three main redox shuttles connecting cytosolic and mitochondrial NAD+/NADH pools in S. cerevisiae (Bakker et al. 2001); two of them are active in aerobic conditions, and the acetaldehyde-ethanol shuttle (operated by the mitochondrial alcohol dehydrogenase Adh3) is also present anaerobically (Bakker et al. 2000) (Fig. 4B). Notably, the pcYeast8 model predicted no additional costs linked to the shift from oxygen-dependent shuttles to acetaldehyde-ethanol shuttle (Fig. 2C, thin lines). So we asked how the predicted physiology changes depending on the shuttle used.
Figure 4.
Specific uptake and excretion fluxes as a function of specific growth rate in anaerobic glucose-limited chemostat cultures with different redox shuttles in mitochondria. (A-B) Physiology of wild-type cultures with the parameters changed (see Fig. 2C). In the absence of oxygen, model predicts the acetaldehyde-ethanol shuttle (B) to be active. (C-D) Physiology of the deletion mutant of the mitochondrial alcohol dehydrogenase Adh3, where oxaloacetate-malate shuttle (D) is predicted to shuttle NADH from mitochondria to cytosol. Points in (A) and (C) are experimental measurements (see Fig. 2C for legend and sources), lines are model predictions. The following parameter values were used for A and C, respectively: GAM, 40 and 24  ; UP, 0.32 and 0.22
; UP, 0.32 and 0.22 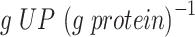 .
.
As introduced previously, for cells using the acetaldehyde-ethanol shuttle, we needed to set additional energy and proteome costs order to tailor the flux predictions to experimental observations (Fig. 2C, reproduced in Fig. 4A). The activity of the shuttle does not result in transfer of additional metabolites (such as protons) across the mitochondrial membrane (Fig. 4B). However, it is known that high concentration of alcohols results in significant increase in proton permeability of membranes (Leão and Van Uden 1984). Yet the magnitude of the ethanol-induced permeability (i.e. stoichiometry of protons crossing the membrane per cycle of acetaldehyde-ethanol shuttle) is not known (and likely hard to measure experimentally). We have estimated that import of 3 protons/cycle to mitochondria could eventually explain the increase in energy demand (data not shown), but we believe such a high ratio is unlikely and thus reject this being the only factor linked to these costs. Moreover, the specific excretion rate of ethanol between aerobic vs. anaerobic glucose batch (Fig. 1A vs. Fig. 2C) varies within 30% range, and so permeabilization would also lead to additional energetic costs in aerobic batch cultures, although we have no supporting data (e.g. need to change model parameters) to confirm this.
Since operating the acetaldehyde-ethanol shuttle seems to be ‘too cheap’ in the model, are there any alternatives? The cells depleted of Adh3 use another oxygen-independent option for redox balancing, the malate-oxaloacetate shuttle ((Bakker et al. 2001), Fig. 4D). In fact, it is an energy-costly option since it involves oxaloacetate-proton symport to mitochondria: the imported protons must be pumped out back to the cytosol by the mitochondrial ATPase at the cost of 1 ATP per proton. Surprisingly, use of the malate-oxaloacetate shuttle does not require any changes to the global parameters (GAM and minimal UP value) to predict both the fluxes and the maximal specific growth rate in glucose excess within correct range (Fig. 4B).
Existing experimental data, however, highlights the role of the acetaldehyde-ethanol shuttle in anaerobically growing cells ((Bakker et al. 2000) and discussed below). Based on this, we cannot assume that the  flux profile (use of an alternative shuttle with extra costs) corresponds to the actual wild-type behavior. There might be multiple explanations and assumptions we could undertake (see Discussion). However, in sake of consistency, in the current study we will further consider the case with the acetaldehyde-ethanol shuttle present and model parameters adjusted as the wild-type profile.
flux profile (use of an alternative shuttle with extra costs) corresponds to the actual wild-type behavior. There might be multiple explanations and assumptions we could undertake (see Discussion). However, in sake of consistency, in the current study we will further consider the case with the acetaldehyde-ethanol shuttle present and model parameters adjusted as the wild-type profile.
Effective redox shuttling in and out of mitochondria is required for fast anaerobic growth
We have previously observed that redox shuttling from mitochondria to cytosol is key for anaerobic growth, where mitochondria serve mainly biosynthetic functions. Moreover, some of the biosynthetic processes in mitochondria use NADPH, the majority of which is supplied by the malic enzyme Mae1 in anaerobically-growing cells (Boles et al. 1998). We thus wanted to stratify the importance of these two systems in anaerobic, glucose-limited growth, and analyzed the predicted impact of deletion of Mae1 and Adh3 on the physiology of the anaerobic-growing S. cerevisiae (Fig. 5).
Figure 5.
Physiological parameters and predicted intracellular fluxes for anaerobically growing deletion strains and wild-type strain in nitrogen-limited chemostats. (A-C) Deletion strains of the malic enzyme 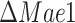 and mitochondrial redox shuttle
and mitochondrial redox shuttle  . (A) Predicted maximal specific growth rate in anaerobic batch cultures. (B) Predicted yield on glucose in anaerobic glucose-limited chemostats at
. (A) Predicted maximal specific growth rate in anaerobic batch cultures. (B) Predicted yield on glucose in anaerobic glucose-limited chemostats at 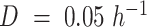 ,
, 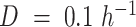 and batch cultures. (C-D) Comparison of the predicted flux profiles in the central carbon metabolism. Line width in (D) represent relative flux value (maximal thickness 2.5-fold of the wild-type value), with the wild-type batch (C) as reference. Highlighted in orange or green are fluxes which differ by at least 30% from the wild-type value, and grey lines indicate inactive fluxes. Data for (A-B) from (Boles et al. 1998, Bakker et al. 2000, Björkeroth et al. 2020).
and batch cultures. (C-D) Comparison of the predicted flux profiles in the central carbon metabolism. Line width in (D) represent relative flux value (maximal thickness 2.5-fold of the wild-type value), with the wild-type batch (C) as reference. Highlighted in orange or green are fluxes which differ by at least 30% from the wild-type value, and grey lines indicate inactive fluxes. Data for (A-B) from (Boles et al. 1998, Bakker et al. 2000, Björkeroth et al. 2020).
It should be noted that in the pcYeast8 model, the glycerol 3-phosphate shuttle (Gpd2) is respiration-independent and has to be knocked-out in silico together with Adh3 for predicting the phenotype of experimental  mutants (model predictions further called just
mutants (model predictions further called just  instead of
instead of 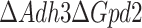 ). Both Mae1 and Adh3 deletion were reported to have very little impact on the physiology of glucose-limited chemostats at low dilution rates (Boles et al. 1998, Bakker et al. 2000), and this claim is supported by our model predictions (Fig. S6). Thus we focused on the batch growth of the
). Both Mae1 and Adh3 deletion were reported to have very little impact on the physiology of glucose-limited chemostats at low dilution rates (Boles et al. 1998, Bakker et al. 2000), and this claim is supported by our model predictions (Fig. S6). Thus we focused on the batch growth of the 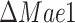 and
and 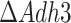 mutants instead.
mutants instead.
First, we looked at the predicted maximal growth rate (Fig. 5A) and yield on glucose (Fig. 5B) in anaerobic cultures of  and
and 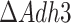 mutants. The batch growth rate for the
mutants. The batch growth rate for the 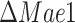 mutant was slightly reduced compared to the wild-type strain. Meanwhile, the
mutant was slightly reduced compared to the wild-type strain. Meanwhile, the  strain showed a ca. 1/3 reduction of the batch growth rate, consistent with experimental reports (Bakker et al. 2000). On the contrary to the drastic decrease in the specific growth rate in batch of the
strain showed a ca. 1/3 reduction of the batch growth rate, consistent with experimental reports (Bakker et al. 2000). On the contrary to the drastic decrease in the specific growth rate in batch of the  strain, the predicted growth yields on glucose hardly changed (Fig. 5B). We believe that these, albeit minor, variations are likely to be model artifacts and are still within agreement with experimentally reported ‘little-to-no impact on the physiology by the Mae1 deletion’ (Boles et al. 1998) and the measured yield of the
strain, the predicted growth yields on glucose hardly changed (Fig. 5B). We believe that these, albeit minor, variations are likely to be model artifacts and are still within agreement with experimentally reported ‘little-to-no impact on the physiology by the Mae1 deletion’ (Boles et al. 1998) and the measured yield of the  mutant (Fig. 5B).
mutant (Fig. 5B).
To understand the differences in observed specific growth rates of 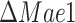 and
and  strains, we looked at the predicted intracellular fluxes (Fig. 5C and D), with a focus to the central carbon metabolism. The main difference between the predicted flux distributions of the wild-type and mutant strains is that the mutant strains generate a lot more (ca. 20
strains, we looked at the predicted intracellular fluxes (Fig. 5C and D), with a focus to the central carbon metabolism. The main difference between the predicted flux distributions of the wild-type and mutant strains is that the mutant strains generate a lot more (ca. 20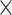 the WT flux) mitochondrial ATP using one of the TCA cycle reactions, succinyl-CoA ligase (succinyl-CoA + ADP + Pi ↔ succinate + CoA + ATP). For running this ATP generation route, the coenzyme A moiety is transferred from the acetyl-CoA to succinate (succinate + acetyl-CoA ↔ succinyl-CoA + acetate), which is then regenerated in the succinyl-CoA reaction. What differs between the mitochondrial ATP generation in
the WT flux) mitochondrial ATP using one of the TCA cycle reactions, succinyl-CoA ligase (succinyl-CoA + ADP + Pi ↔ succinate + CoA + ATP). For running this ATP generation route, the coenzyme A moiety is transferred from the acetyl-CoA to succinate (succinate + acetyl-CoA ↔ succinyl-CoA + acetate), which is then regenerated in the succinyl-CoA reaction. What differs between the mitochondrial ATP generation in 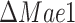 and
and 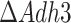 strains is the predicted source of mitochondrial acetyl-CoA: the
strains is the predicted source of mitochondrial acetyl-CoA: the 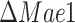 mutant supplies the acetyl-CoA via decarboxylation in the mitochondria by pyruvate dehydrogenase complex, while
mutant supplies the acetyl-CoA via decarboxylation in the mitochondria by pyruvate dehydrogenase complex, while  uses cytosolic acetyl-CoA, shuttled to mitochondria using the carnitine shuttle.
uses cytosolic acetyl-CoA, shuttled to mitochondria using the carnitine shuttle.
Normally, acetyl-CoA is produced in the cytosol in low amounts for the biosynthesis of fatty acids. In the  strain, cytosolic acetyl-CoA production is increased multiple-fold, even though this is an energy-spilling route: for one ATP generated in mitochondria, 2 equivalents of ATP are consumed in cytosol (acetate + CoA + ATP
strain, cytosolic acetyl-CoA production is increased multiple-fold, even though this is an energy-spilling route: for one ATP generated in mitochondria, 2 equivalents of ATP are consumed in cytosol (acetate + CoA + ATP 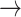 acetyl-CoA + AMP + PPi). However, the reduced NADH (gained through the aldehyde dehydrogenase reaction, acetaldehyde + NAD+
acetyl-CoA + AMP + PPi). However, the reduced NADH (gained through the aldehyde dehydrogenase reaction, acetaldehyde + NAD+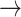 acetate + NADH + H+) remains in the cytosol, and a proton (because respective molecule of pyruvate is not imported into mitochondria) is not imported to mitochondria. The amount of excess NADH produced scales with the specific growth rate, as indicated by decreasing NAD/NADH ratio as the specific growth rate increases (Canelas et al. 2011), and the high additional demand for energy to resolve the redox imbalances could explain the lower batch growth rate of the
acetate + NADH + H+) remains in the cytosol, and a proton (because respective molecule of pyruvate is not imported into mitochondria) is not imported to mitochondria. The amount of excess NADH produced scales with the specific growth rate, as indicated by decreasing NAD/NADH ratio as the specific growth rate increases (Canelas et al. 2011), and the high additional demand for energy to resolve the redox imbalances could explain the lower batch growth rate of the  strain. This, however, stands true only to growth on minimal medium: the predicted batch growth rate of the
strain. This, however, stands true only to growth on minimal medium: the predicted batch growth rate of the  mutant on a rich medium was only mildly lower than the predicted wild-type rate (Fig. S7).
mutant on a rich medium was only mildly lower than the predicted wild-type rate (Fig. S7).
Discussion
In this study, we presented the updated proteome-constrained model of the budding yeast Saccharomyces cerevisiae, pcYeast8 (Fig. 1). We have built a proteome-constrained model on the basis of the genome-scale model (GEM) Yeast8 (Lu et al. 2019), inspired by our recently published model pcYeast7.6 (Elsemman et al. 2022). We manually curated some of the model reactions (Supplementary Notes) which allowed correct prediction of some growth strategies, e.g. glycerol excretion in aerobic, glucose-excess conditions (Fig. 1), which were not properly captured by the pcYeast7.6 yet.
The focus of our study was to identify condition-dependent patterns of yeast physiology, with extra attention to the energy expenditures for growth. We first modulated the respiratory capacity of S. cerevisiae, and tested scenarios of increased, restricted, or completely blocked respiration (Fig. 2). Here we identified two model parameters that must be tweaked in order to capture the uptake and excretion fluxes in respiration-deficient ( ) mutants, or anaerobically grown cells (Fig. 2B and C): the growth-associated ATP maintenance (GAM) and the minimal fraction of the proteome, to be occupied by the so-called unspecified protein (UP). In fact, despite the size and extent of the pcYeast8 model, only a handful of global model parameters can be effectively used for fitting: both in the previous model iteration (Elsemman et al. 2022), and in our current study, we addressed three global parameters: the uptake of carbon source (see Methods), the minimal UP fraction in proteome, and growth-associated ATP maintenance. We have validated the choice of the parameter values for the minimal medium in an experiment of growth in rich medium (Fig. 3), where we successfully predicted the batch growth rates and proteome compositions across conditions.
) mutants, or anaerobically grown cells (Fig. 2B and C): the growth-associated ATP maintenance (GAM) and the minimal fraction of the proteome, to be occupied by the so-called unspecified protein (UP). In fact, despite the size and extent of the pcYeast8 model, only a handful of global model parameters can be effectively used for fitting: both in the previous model iteration (Elsemman et al. 2022), and in our current study, we addressed three global parameters: the uptake of carbon source (see Methods), the minimal UP fraction in proteome, and growth-associated ATP maintenance. We have validated the choice of the parameter values for the minimal medium in an experiment of growth in rich medium (Fig. 3), where we successfully predicted the batch growth rates and proteome compositions across conditions.
Both aerobic  , and anaerobic S. cerevisiae exhibit reduced mitochondrial content (Fig. S2); however, mitochondria remain important—or even essential—for some growth processes. The significant reduction in the biomass yield per mol ATP produced (
, and anaerobic S. cerevisiae exhibit reduced mitochondrial content (Fig. S2); however, mitochondria remain important—or even essential—for some growth processes. The significant reduction in the biomass yield per mol ATP produced ( ), comparing anaerobic glucose-limited chemostats vs. aerobic batch cultures was already observed by (Verduyn et al. 1990b), especially at higher dilution rates in anaerobic chemostats. The additional energy expenses for growth on minimal medium, however, are very high (an increase in the GAM value by
), comparing anaerobic glucose-limited chemostats vs. aerobic batch cultures was already observed by (Verduyn et al. 1990b), especially at higher dilution rates in anaerobic chemostats. The additional energy expenses for growth on minimal medium, however, are very high (an increase in the GAM value by  , or a 60% increase of the naïve value. Our model predicted that supplementation of amino acids rescued the extra energy requirement in anaerobically-grown S. cerevisiae (Fig. 3A). Inhibited respiration in fission yeast Schizosaccharomyces pombe resulted in reduced proliferation and decreased levels of 4 amino acids (Arg, Lys, Glu, Gln) (Malecki et al. 2020); supplementation of Arg to the medium rescued the growth reduction. Our modeling results are in line with these observations, since the increase of GAM from 24 to 40
, or a 60% increase of the naïve value. Our model predicted that supplementation of amino acids rescued the extra energy requirement in anaerobically-grown S. cerevisiae (Fig. 3A). Inhibited respiration in fission yeast Schizosaccharomyces pombe resulted in reduced proliferation and decreased levels of 4 amino acids (Arg, Lys, Glu, Gln) (Malecki et al. 2020); supplementation of Arg to the medium rescued the growth reduction. Our modeling results are in line with these observations, since the increase of GAM from 24 to 40 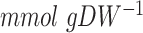 was not needed for correctly predicting the physiology of anaerobically-grown S. cerevisiae in rich medium (Fig. 3A).
was not needed for correctly predicting the physiology of anaerobically-grown S. cerevisiae in rich medium (Fig. 3A).
A combination of several factors, e.g. increased futile cycling or decoupling of energy generation from biomass formation, were proposed to contribute to additional costs, usually without clear mechanistic insight. Some studies highlighted the function of mitochondria in biosynthesis (Visser et al. 1994) and lipid metabolism (Reiner et al. 2006) and suggested that maintaining functional mitochondria in anaerobic conditions is an energy-costly process (Drgoň et al. 1991, Visser et al. 1994). Moreover, recent reports allow us to speculate that maintenance of proton-motive force across cell membranes could be one of the factors leading to extra costs in anaerobic growth ((Malina et al. 2021, Terradot et al. 2021), T. Pilizota, personal communication). Aerobically, the proton-motive force is generated by the electron transport chain in the mitochondrial inner membrane, and some of it is used for, e.g. import of metabolites and proteins into mitochondria. In anaerobic settings, not only the proton-motive force is not maintained by the electron transport chain, but also can be eradicated due to increased permeability of membranes due to increased intracellular ethanol concentration (Leão and Van Uden 1984).
We speculated that these energy costs might be associated with impaired redox shuttling in mitochondria. Redox equivalents can be shuttled using two (acetaldehyde-ethanol and malate-oxaloacetate) shuttles in anaerobic growth. Yet the simulations of the preferred (acetaldehyde-ethanol) shuttle were contradictory to experimental data as we had to change global model parameters to capture experimental data (Figs 2C and 4A). Meanwhile, use of alternative redox shuttle led into predictions consistent with experimental measurements (Fig. 4C). This contradiction could be linked to different putative roles of the preferred redox shuttle, e.g. potential moonlighting roles of Adh3 enzyme, or simultaneous shuttling through both acetaldehyde-ethanol and malate-oxaloacetate shuttles—all of which are still open for further investigation. For instance, a characterization of a double knock-out ( + some part of the malate-oxaloacetate shuttle) would either support or rule out the option of the 2-system redox shuttling from mitochondria.
+ some part of the malate-oxaloacetate shuttle) would either support or rule out the option of the 2-system redox shuttling from mitochondria.
We did scenario testing with respect to the maintenance of redox balance in anaerobic mitochondria (Fig. 5). We have simulated batch growth of a mitochondrial redox shuttle-deficient yeast and obtained predictions of batch growth rate in agreement with experimental data (Fig. 5A, (Bakker et al. 2000)). We suggest that the increased cytosolic decarboxylation of pyruvate at the expense of ATP (Fig. 5C, D) can sustain biosynthesis and partially alleviate the redox imbalance in mitochondria, but not at high specific growth rates, when the glycolytic flux is very high. Predicted batch growth rate of  mutant in rich medium was very comparable to the wild-type (Fig. S7), and, again, pointed out to the reduction of growth being related to amino acid biosynthesis. Yet, the exact mechanisms of the energy-consuming processes, characteristic to low- or non-respiring mitochondria, are not clear to date. Experimental validation of the
mutant in rich medium was very comparable to the wild-type (Fig. S7), and, again, pointed out to the reduction of growth being related to amino acid biosynthesis. Yet, the exact mechanisms of the energy-consuming processes, characteristic to low- or non-respiring mitochondria, are not clear to date. Experimental validation of the 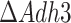 mutant growing at a comparable specific growth rate to the WT strain in rich medium under anaerobic conditions would strongly support our hypothesis on the costly redox shuttling.
mutant growing at a comparable specific growth rate to the WT strain in rich medium under anaerobic conditions would strongly support our hypothesis on the costly redox shuttling.
The additional cost of proteome maintenance in anaerobic conditions (Fig. 2C) is also an important consideration. In our model, we express the maximal capacity of ‘useful’ ( = involved in biomass production) proteome as the minimal proteome fraction of the ‘dummy’ protein, UP. We had to increase the minimal fraction of UP in order to observe a batch growth rate, corresponding to the experimentally observed growth of S. cerevisiae CEN.PK strain.
We speculate the additional proteome space is allocated to anticipatory protein expression, mainly, proteins needed for growth in the presence of oxygen. For instance, a substantial overexpression of the oxidative phosphorylation enzymes was observed in microaerobic conditions (Rintala et al. 2009). In the lab settings, unsaturated fatty acid- or ergosterol are supplemented to anaerobic cultures of S. cerevisiae to override the need for oxygen-requiring biosynthesis steps. Anticipation of presence of oxygen might also explain why S. cerevisiae can grow anaerobically, albeit slowly, without unsaturated fatty acid- or ergosterol supplementation, which is a common technique in laboratory cultivation (Dekker et al. 2019). In general, cells show anticipatory expression of metabolic enzymes when the selection pressure for the transient specific growth rate is absent: we have recently suggested a similar phenomenon for glycolytic enzymes for aerobic glucose-limited cultures (Grigaitis and Teusink 2022). Also, glycolytic enzymes show similar levels in both minimal and rich media despite the differences in glycolytic flux (Björkeroth et al. 2020).
To conclude, in this study we have used the proteome-constrained model of yeast metabolism to explore the metabolic strategies of S. cerevisiae across different growth conditions. We have identified condition- and mutant-specific costs of growth, which we associated with impaired mitochondrial respiration and redox shuttling. Some of these costs have direct mechanistic explanations, coming out of the predictions of the pcYeast8 model; for some, the exact underpinnings are still open for future studies. In the end, we hope that our modeling work will open some new avenues for the research of yeast metabolic- and resource allocation strategies.
Methods
Kinetics and proteome data for the pcYeast8
The detailed description of reconstruction of the proteome-constrained model of S. cerevisiae is provided in the Supplementary Notes. 5’-UTR sequences and proteome annotations (composition of macromolecular complexes, Gene Ontology terms etc.) were collected from Saccharomyces Genome Database (SGD, (Cherry et al. 2012)).
We used the reference proteome of S. cerevisiae from UniProt (The UniProt Consortium et al. 2021). The kinetic data (enzyme turnover values) were collected from the BRENDA database (Chang et al. 2021). For every enzymatic complex with an Enzyme Commission (EC) number, we queried the BRENDA database for a value from the wild-type enzymes. When available, values from S. cerevisiae were preferentially selected. Otherwise, the highest value for a wild-type enzyme in mesophilic (and close to growth conditions of S. cerevisiae) conditions was taken. When no 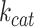 value was available, we assumed
value was available, we assumed  as a default value (Nilsson and Nielsen 2016). If the experimentally determined
as a default value (Nilsson and Nielsen 2016). If the experimentally determined  value was lower than
value was lower than 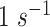 , we set this value.
, we set this value.
Model simulations
Unless stated otherwise, all simulated media were Verduyn minimal medium (Verduyn et al. 1992). Growth in a specific medium was modeled by closing all reverse exchange (‘uptake’) reactions, except for those nutrients which are present in the medium (the upper bound of the exchange reaction equals 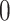 ). In the case of anaerobic growth, minute amounts of ergosta-5,7,22,24(28)-tetraen-3beta-ol, oleate, and palmitoleate were also provided for the biosynthesis of unsaturated fatty acids (mimicking the addition of ergosterol and Tween 80 in respective experiments).
). In the case of anaerobic growth, minute amounts of ergosta-5,7,22,24(28)-tetraen-3beta-ol, oleate, and palmitoleate were also provided for the biosynthesis of unsaturated fatty acids (mimicking the addition of ergosterol and Tween 80 in respective experiments).
Glucose limitation was simulated by altering the saturation of respective plasma membrane transporters, 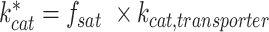 . To prevent the plasma membrane from being fully filled with transporters (effectively no nutrient limitation), we have computationally estimated the available fraction of the total membrane area that these nutrient transporters can occupy (Table 1). We based the value for C-transporters on the maximal glucose uptake rate in minimal media under aerobic conditions (Blank and Sauer 2004), the rest of the values were computationally inferred ad hoc.
. To prevent the plasma membrane from being fully filled with transporters (effectively no nutrient limitation), we have computationally estimated the available fraction of the total membrane area that these nutrient transporters can occupy (Table 1). We based the value for C-transporters on the maximal glucose uptake rate in minimal media under aerobic conditions (Blank and Sauer 2004), the rest of the values were computationally inferred ad hoc.
Table 1.
Plasma membrane transporter area for different growth conditions.
| Condition | Carbon transporters, % | Nitrogen transporters, % |
|---|---|---|
| Aerobic, minimal medium | 11.0 | 4.5 |
| Aerobic, rich medium | 13.0 | 5.5 |
| Anaerobic | 13.0 | 4.5 |
The definition for the rich medium (Verduyn + amino acids) was determined on the basis of data from batch cultures (Björkeroth et al. 2020). There, the time evolution of biomass concentration in the growth medium ( ) and amino acid abundance (
) and amino acid abundance ( ) was reported. We computed the slope of depletion of every amino acid in the media ( = uptake by cells) and multiplied by the specific growth rate
) was reported. We computed the slope of depletion of every amino acid in the media ( = uptake by cells) and multiplied by the specific growth rate 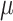 to determine the upper flux bound for each amino acid quantified. When the slope was nonnegative (no uptake in essence), we set the upper flux to zero.
to determine the upper flux bound for each amino acid quantified. When the slope was nonnegative (no uptake in essence), we set the upper flux to zero.
Software
The pcYeast8 model was simulated using the CBMPy package (version 0.8.1) (Olivier et al. 2021) in a Python 3.6 environment with the IBM ILOG CPLEX Optimization Studio (version 12.10.0) and SoPlex (version 5.0.2) (Gleixner et al. 2018) as the low- and high-precision LP solver, respectively. In SoPlex, the primal (−f flag) and dual (−o flag) feasibility tolerance was set to 10−16. R (version 3.6.3) and Python (version 3.10) were used for further data analysis and derivation of relations between the cell size and biomass composition as a function of the growth rate.
CRediT Author Role Statement
Pranas Grigaitis: conceptualization, methodology, software, validation, formal analysis, investigation, data curation, writing—original draft, writing—review & editing, visualization, supervision; Samira L van den Bogaard: methodology, validation, formal analysis, investigation, writing—review & editing; Bas Teusink: supervision, project administration, funding acquisition, writing—review & editing.
Supplementary Material
Acknowledgements
We thank Frank J. Bruggeman and Jack Pronk for discussions, and Ursula Kummer for her comments on the draft version of the manuscript.
Contributor Information
Pranas Grigaitis, Systems Biology Lab, A-Life/AIMMS, Vrije Universiteit Amsterdam, De Boelelaan 1085, 1081HV Amsterdam, the Netherlands.
Samira L van den Bogaard, Systems Biology Lab, A-Life/AIMMS, Vrije Universiteit Amsterdam, De Boelelaan 1085, 1081HV Amsterdam, the Netherlands.
Bas Teusink, Systems Biology Lab, A-Life/AIMMS, Vrije Universiteit Amsterdam, De Boelelaan 1085, 1081HV Amsterdam, the Netherlands.
Competing interests statement
None declared.
Funding
PG and BT acknowledge the funding by Marie Skłodowska-Curie Actions ITN ‘SynCrop’ (grant agreement No 764591). We thank SURFsara for the HPC resources through access to the Lisa Compute Cluster.
References
- Bakker BM, Bro C, Kötter Pet al. The mitochondrial alcohol dehydrogenase Adh3p is involved in a redox shuttle in Saccharomyces cerevisiae. J Bacteriol. 2000;182:4730–7. 10.1128/JB.182.17.4730-4737.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker BM, Overkamp KM, van Maris AJAet al. Stoichiometry and compartmentation of NADH metabolism in Saccharomyces cerevisiae. FEMS Microbiol Rev. 2001;25:15–37. 10.1111/j.1574-6976.2001.tb00570.x [DOI] [PubMed] [Google Scholar]
- Björkeroth J, Campbell K, Malina Cet al. Proteome reallocation from amino acid biosynthesis to ribosomes enables yeast to grow faster in rich media. Proc Natl Acad Sci. 2020;117:21804–12. 10.1073/pnas.1921890117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank LM, Sauer U. TCA cycle activity in Saccharomyces cerevisiae is a function of the environmentally determined specific growth and glucose uptake rates. Microbiology. 2004;150:1085–93. 10.1099/mic.0.26845-0 [DOI] [PubMed] [Google Scholar]
- Boles E, de Jong-Gubbels P, Pronk JT. Identification and characterization of MAE1, the Saccharomyces cerevisiae structural gene encoding mitochondrial malic enzyme. J Bacteriol. 1998;180:2875–82. 10.1128/JB.180.11.2875-2882.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canelas AB, Ras C, ten Pierick Aet al. An in vivo data-driven framework for classification and quantification of enzyme kinetics and determination of apparent thermodynamic data. Metab Eng. 2011;13:294–306. 10.1016/j.ymben.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Chang A, Jeske L, Ulbrich Set al. BRENDA, the ELIXIR core data resource in 2021: new developments and updates. Nucleic Acids Res. 2021;49:D498–508. 10.1093/nar/gkaa1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JM, Hong EL, Amundsen Cet al. Saccharomyces Genome Database: the genomics resource of budding yeast. Nucleic Acids Res. 2012;40:D700–705. 10.1093/nar/gkr1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker WJC, Wiersma SJ, Bouwknegt Jet al. Anaerobic growth of Saccharomyces cerevisiae CEN.PK113-7D does not depend on synthesis or supplementation of unsaturated fatty acids. FEMS Yeast Res. 2019;19:foz060. 10.1093/femsyr/foz060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgoň T, Šabová L, Nelson Net al. ADP/ATP translocator is essential only for anaerobic growth of yeast Saccharomyces cerevisiae. FEBS Lett. 1991;289:159–62. 10.1016/0014-5793(91)81059-H [DOI] [PubMed] [Google Scholar]
- Elsemman IE, Rodriguez Prado A, Grigaitis Pet al. Whole-cell modeling in yeast predicts compartment-specific proteome constraints that drive metabolic strategies. Nat Commun. 2022;13:801. 10.1038/s41467-022-28467-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleixner A, Bastubbe M, Eifler Let al. The SCIP Optimization Suite 6.0 (No. 18–26). ZIB, Takustr. 2018;7:14195.Berlin. 10.1128/msystems.00423-22 [DOI] [Google Scholar]
- Grigaitis P, Grundel DAJ, van Pelt-KleinJan Eet al. A computational toolbox to investigate the metabolic potential and resource allocation in fission yeast. Msystems. 2022a;7:e00423–22. 10.1002/1873-3468.14484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigaitis P, Teusink B. An excess of glycolytic enzymes under glucose-limited conditions may enable Saccharomyces cerevisiae to adapt to nutrient availability. FEBS Lett. 2022;596:1873–3468.14484. 10.1002/1873-3468.14484 [DOI] [PubMed] [Google Scholar]
- Grigaitis P, van den Bogaard SL, Teusink B. 2022b. https://zenodo.org/record/7322850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haringa C, Tang W, Deshmukh ATet al. Euler-Lagrange computational fluid dynamics for (bio)reactor scale down: an analysis of organism lifelines. Eng Life Sci. 2016;16:652–63. 10.1002/elsc.201600061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett MC, Workman CT, Nookaew Iet al. Mapping condition-dependent regulation of lipid metabolism in Saccharomyces cerevisiae. G3 GenesGenomesGenetics. 2013;3:1979–95. 10.1534/g3.113.006601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahtvee P-J, Sánchez BJ, Smialowska Aet al. Absolute quantification of protein and mRNA abundances demonstrate variability in gene-specific translation efficiency in yeast. Cell Syst. 2017;4:495–504.e5. 10.1016/j.cels.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Lange HC, Heijnen JJ. Statistical reconciliation of the elemental and molecular biomass composition of Saccharomyces cerevisiae. Biotechnol Bioeng. 2001;75:334–44. 10.1002/bit.10054 [DOI] [PubMed] [Google Scholar]
- Leão C, Van Uden N. Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1984;774:43–48. 10.1016/0005-2736(84)90272-4 [DOI] [PubMed] [Google Scholar]
- Lu H, Li F, Sánchez BJet al. A consensus S. Cerevisiae metabolic model Yeast8 and its ecosystem for comprehensively probing cellular metabolism. Nat Commun. 2019;10:3586. 10.1038/s41467-019-11581-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecki M, Kamrad S, Ralser Met al. Mitochondrial respiration is required to provide amino acids during fermentative proliferation of fission yeast. EMBO Rep. 2020;21. 10.15252/embr.202050845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malina C, Di Bartolomeo F, Kerkhoven EJet al. Constraint-based modeling of yeast mitochondria reveals the dynamics of protein import and iron-sulfur cluster biogenesis. Iscience. 2021;24:103294. 10.1016/j.isci.2021.103294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris AJA, Bakker BM, Brandt Met al. Modulating the distribution of fluxes among respiration and fermentation by overexpression of HAP4 in Saccharomyces cerevisiae. FEMS Yeast Res. 2001;1:139–49. 10.1111/j.1567-1364.2001.tb00025.x [DOI] [PubMed] [Google Scholar]
- Metzl-Raz E, Kafri M, Yaakov Get al. Principles of cellular resource allocation revealed by condition-dependent proteome profiling. Elife. 2017;6:e28034. 10.7554/eLife.28034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson A, Nielsen J. Metabolic trade-offs in yeast are caused by F1F0-ATP synthase. Sci Rep. 2016;6:22264. 10.1038/srep22264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen TL, Schulze U, Nielsen Jet al. Flux distributions in Anaerobic, glucose-limited continuous cultures of Saccharomyces cerevisiae. Microbiology. 1997;143:203–18. 10.1099/00221287-143-1-203 [DOI] [PubMed] [Google Scholar]
- Olivier B, Gottstein W, Molenaar Det al. CBMPy release 0.8.2. Zenodo. 2021. 10.5281/ZENODO.5546608, https://zenodo.org/record/5546608. [DOI] [Google Scholar]
- Raghevendran V, Patil KR, Olsson Let al. Hap4 Is not essential for activation of respiration at low specific growth rates in Saccharomyces cerevisiae. J Biol Chem. 2006;281:12308–14. 10.1074/jbc.M512972200 [DOI] [PubMed] [Google Scholar]
- Reiner S, Micolod D, Zellnig Get al. A genomewide screen reveals a role of mitochondria in Anaerobic uptake of sterols in yeast. Mol Biol Cell. 2006;17:90–103. 10.1091/mbc.e05-06-0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintala E, Toivari M, Pitkänen J-Pet al. Low oxygen levels as a trigger for enhancement of respiratory metabolism in Saccharomyces cerevisiae. Bmc Genomics [Electronic Resource]. 2009;10:461. 10.1186/1471-2164-10-461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville V, Grigaitis P, Battjes Jet al. Use and limitations of genome-scale metabolic models in food microbiology. Curr Opin Food Sci. 2022;43:225–31. 10.1016/j.cofs.2021.12.010 [DOI] [Google Scholar]
- Tai SL, Boer VM, Daran-Lapujade Pet al. Two-dimensional transcriptome analysis in chemostat cultures. J Biol Chem. 2005;280:437–47. 10.1074/jbc.M410573200 [DOI] [PubMed] [Google Scholar]
- Tai SL, Daran-Lapujade P, Luttik MAHet al. Control of the glycolytic flux in Saccharomyces cerevisiae grown at low temperature. J Biol Chem. 2007;282:10243–51. 10.1074/jbc.M610845200 [DOI] [PubMed] [Google Scholar]
- Terradot G, Krasnopeeva E, Swain PSet al. The proton motive force determines Escherichia coli ’s robustness to extracellular pH (preprint). Microbiology. 2021. 10.1101/2021.11.19.469321. [DOI] [Google Scholar]
- The UniProt Consortium, Bateman A, Martin M-J, Orchard Set al. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49:D480–9. 10.1093/nar/gkaa1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink J, Canelas AB, van Gulik WMet al. Dynamics of glycolytic regulation during adaptation of Saccharomyces cerevisiae to fermentative metabolism. Appl Environ Microbiol. 2008;74:5710–23. 10.1128/AEM.01121-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verduyn C, Postma E, Scheffers WAet al. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8:501–17. 10.1002/yea.320080703 [DOI] [PubMed] [Google Scholar]
- Verduyn C, Postma E, Scheffers WAet al. Energetics of Saccharomyces cerevisiae in Anaerobic glucose-limited chemostat cultures. J Gen Microbiol. 1990a;136:405–12. 10.1099/00221287-136-3-405 [DOI] [PubMed] [Google Scholar]
- Verduyn C, Postma E, Scheffers WAet al. Physiology of Saccharomyces cerevisiae in Anaerobic glucose-limited chemostat culturesx. J Gen Microbiol. 1990b;136:395–403. 10.1099/00221287-136-3-395 [DOI] [PubMed] [Google Scholar]
- Visser W, van der Baan AA, Batenburg-van der Vegte Wet al. Involvement of mitochondria in the assimilatory metabolism of anaerobic Saccharomyces cerevisiaecultures. Microbiology. 1994;140:3039–46. 10.1099/13500872-140-11-3039 [DOI] [PubMed] [Google Scholar]
- Winde JH, Grivell LA. Regulation of mitochondrial biogenesis in Saccharomyces cerevisiae. Intricate interplay between general and specific transcription factors in the promoter of the QCR8 gene. Eur J Biochem. 1995;233:200–8. 10.1111/j.1432-1033.1995.200_1.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






