Abstract
Background
The burden of noncardiovascular conditions is becoming increasingly prevalent in patients with heart failure (HF). We aimed to identify novel phenogroups incorporating noncardiovascular conditions to facilitate understanding and risk stratification in elderly patients with HF.
Methods and Results
Data from a total of 1881 (61.2%) patients aged ≥65 years were extracted from a prospective multicenter registry of patients hospitalized for acute HF (N=3072). We constructed subgroups of patients with HF with preserved ejection fraction (HFpEF; N=826, 43.9%) and those with non‐HFpEF (N=1055, 56.1%). Latent class analysis was performed in each subgroup using 17 variables focused on noncardiovascular conditions (including comorbidities, Clinical Frailty Scale, and Geriatric Nutritional Risk Index). The latent class analysis revealed 3 distinct clinical phenogroups in both HFpEF and non‐HFpEF subgroups: (1) robust physical and nutritional status (Group 1: HFpEF, 41.2%; non‐HFpEF, 46.0%); (2) multimorbid patients with renal impairment (Group 2: HFpEF, 40.8%; non‐HFpEF, 41.9%); and (3) malnourished patients (Group 3: HFpEF, 18.0%; non‐HFpEF, 12.1%). After multivariable adjustment, compared with Group 1, patients in Groups 2 and 3 had a higher risk for all‐cause death over the 1‐year postdischarge period (hazard ratio [HR], 2.79 [95% CI, 1.64–4.81] and HR, 2.73 [95% CI, 1.39–5.35] in HFpEF; HR, 1.96 [95% CI, 1.22–3.14] and HR, 2.97 [95% CI, 1.64–5.38] in non‐HFpEF; respectively).
Conclusions
In elderly patients with HF, the phenomapping focused on incorporating noncardiovascular conditions identified 3 phenogroups, each representing distinct clinical outcomes, and the discrimination pattern was similar for both patients with HFpEF and non‐HFpEF. This classification provides novel risk stratification and may aid in clinical decision making.
Keywords: elderly patients, heart failure, noncardiovascular conditions, phenotyping
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- BIC
Bayesian information criterion
- CFS
Clinical Frailty Scale
- GNRI
Geriatric Nutritional Risk Index
- HFpEF
heart failure with preserved ejection fraction
- LCA
latent class analysis
Clinical Perspective.
What Is New?
The present phenomapping focused on incorporating noncardiovascular conditions such as comorbidity, physical function, and nutritional status. It identified 3 phenogroups with distinct clinical prognoses (ie, robust, systemic impairment, and cachexia groups) in elderly patients with heart failure.
The discrimination pattern was similar for both patients with heart failure with preserved ejection fraction and non–heart failure with preserved ejection fraction.
This suggests that noncardiovascular conditions play a significant role in pathophysiology and prognosis among elderly patients with heart failure.
What Are the Clinical Implications?
The clinically recognizable classification reflecting elderly‐inherent phenotypes provides not only risk stratifications but also crucial information for a better understanding of the complicated pathophysiology of elderly patients with heart failure.
Because a similar discrimination pattern was observed in patients with both heart failure with preserved ejection fraction and non–heart failure with preserved ejection fraction, the present classification may apply to a broad range of elderly patients with heart failure.
Heart failure (HF) has become the leading cause of death in most developed countries. 1 , 2 , 3 In particular, elderly patients with HF have a high incidence of adverse clinical events, including both cardiovascular‐related and noncardiovascular events. 4 , 5 , 6 The exponential rise in the prevalence of coexisting noncardiovascular conditions, such as comorbidity, frailty, and malnutrition with aging, is thought to contribute significantly to HF in elderly patients. 6 , 7 , 8 , 9 , 10 , 11 , 12
The complex interplay of cardiovascular and noncardiovascular conditions complicates the underlying pathophysiology 10 and results in challenging clinical decision making for these patients. 13 The current diagnosis of HF focuses primarily on cardiovascular conditions, and homogeneous phenotypic classification based on abnormalities in cardiac function may not fully encompass the diversity of pathophysiology. 14 In addition, previous phenomapping studies have included both young and elderly populations, which differ substantially in terms of long‐term prognosis and treatment application. 8 , 15 , 16 , 17 Furthermore, the assessment of individualized noncardiovascular conditions, as performed in previous observational studies, may not have adequately captured the burden of noncardiovascular conditions. 9 , 12 , 18
Latent class analysis (LCA) has received growing attention in recent epidemiological literature. LCA was originally developed to isolate specific groups of individuals with similar characteristics based on probability calculations and offers a strategy to identify subgroups of patients with HF who are more susceptible to adverse clinical events. 19 Furthermore, previous studies have demonstrated the feasibility of LCA for phenomapping in HF with preserved ejection fraction (HFpEF) with heterogeneous pathophysiologies. 16 , 17 , 20 We hypothesized that phenomapping focused on incorporating noncardiovascular conditions could identify the novel elderly‐inherent phenotypic subgroups with distinct clinical prognoses. To facilitate use of the complexity of noncardiovascular clinical variables, we aimed to perform the LCA in a cohort of elderly patients hospitalized for acute HF, with a particular focus on conditions such as multimorbidity, physical frailty, and malnutrition.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data Sources
This study is a nonprespecified post hoc analysis of the WET‐HF (West Tokyo Heart Failure Registry), which was a prospective multicenter cohort registry designed to collect data on clinical backgrounds and outcomes from consecutive patients with acute HF who were hospitalized for requiring urgent treatment. 21 , 22 The WET‐HF registry has provided insights on the national current status of clinical outcomes 23 in patients with HF, as well as in international collaborative projects. 24 , 25 , 26 Individuals with acute HF were diagnosed by a cardiologists at each institution based on the Framingham criteria. 27 Between January 2006 and December 2017, patients with HF were registered at 6 tertiary hospitals in the Tokyo area. We added 2 further institutions in April 2018 (WET‐HF2 Registry) with an update of the collected variables, including the Clinical Frailty Scale (CFS) at the time of discharge. 28
Patient data were entered into an electronic data capture system with a robust data query engine and system validations for data quality. The principal investigators (Y.S. and S.K.) conducted periodic queries to verify the reporting quality at least once a year. Patients who refused to participate in the study or presented with concurrent HF and acute coronary syndrome were excluded from registration. As for the end points, all death‐related events were reviewed by the investigators and classified into the following groups: those requiring adjudication and those with a clearly defined mode of death. Subsequently, central committee members (Y.S., S.K., and T.Y.) reviewed the abstracted records and adjudicated the modes of death.
Before the launch of this registry, information on the present study's objectives and social significance, as well as an abstract, were provided for clinical trial registration to the University Hospital Medical Information Network of Japan (UMIN000001171). The institutional review boards at each site approved the study protocol, and the research was conducted according to the Declaration of Helsinki. Written or oral informed consent was obtained from each patient before the study.
Study Population
A total of 3072 consecutive patients with HF were registered in the WET‐HF2. We excluded patients aged <65 years (n=566 [18.4%]), those who died during the index hospitalization (n=155 [5.0%]), those who were not followed up on (n=370), and those without left ventricular ejection fraction (LVEF) data (n=100 [3.3%]). The remaining 1881 (61.2%) patients aged ≥65 years were included in the present study. We then categorized the participants into 2 groups based on their LVEF status: non‐HFpEF (n=1055 [56.1%]), LVEF <50%, HF with mildly reduced ejection fraction and HF with reduced ejection fraction; and HFpEF (n=826 [43.9%], LVEF≥50%) (Figure 1). During the index hospitalization after the HF signs and symptoms stabilized, board‐certified physicians or physiology technicians assessed LVEF on echocardiography using the modified Simpson method.
Figure 1. Study flowchart.
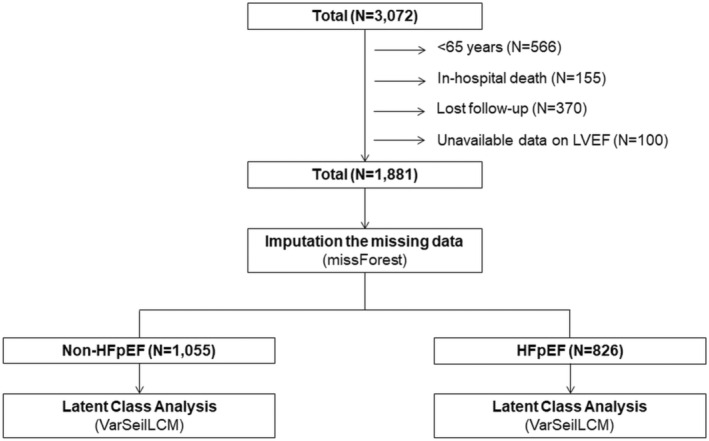
HFpEF indicates heart failure with preserved ejection fraction; and LVEF, left ventricular ejection fraction.
Definition of Comorbidity, Malnutrition, and Physical Frailty
The following comorbidities were defined as having a history of the following diagnoses. Particularly, atrial fibrillation was defined as having a history on an electrocardiogram; anemia was defined according to the World Health Organization criteria (hemoglobin at discharge <13 g/dL for men and <12 g/dL for women 29 ); chronic kidney disease was defined as an estimated glomerular filtration rate (eGFR) at discharge <60 mL/min per 1.73 m2 (the eGFR was calculated using the Modification of Diet in Renal Disease Equation for Japanese Patients, proposed by the Japanese Society of Nephrology 30 ); and hyperuricemia was defined as serum uric acid at discharge ≥8.0 mg/dL. Given the extremely high risk for adverse clinical events in patients with HF with ≥3 comorbidities in our previous research, 21 we defined them as having multimorbidity in the present study. Physical frailty was defined as a CFS score ≥5; the CFS is a simple screening tool for the identification of frailty. 31 Furthermore, based on previous findings, 31 , 32 , 33 malnutrition and physical frailty were defined as a geriatric Nutritional Risk Index (GNRI) <82 at the time of discharge; the GNRI is a simple formula that has been demonstrated to be clinically useful among patients with various medical conditions.32
Candidate Variables for LCA
We selected the following 19 available variables a priori as candidates for the LCA in terms of comorbidities, physical function, cognitive status, nutritional status, and social environment, which were essential domains when considering multidimensional assessments for elderly patients with HF, based on previous literature 10 , 34 : (1) demographic data: age, sex, body mass index, and smoking status; (2) comorbidities: hypertension, diabetes, atrial fibrillation, dyslipidemia, hyperuricemia, previous stroke, chronic obstructive pulmonary disease/asthma, malignancy, renal function assessed via eGFR, anemia assessed via hemoglobin level; (3) physical function: CFS and Barthel Index at the time of discharge (assessed by the attending physician); (4) cognitive status: dementia or lack thereof; (5) nutrition status: GNRI; and (6) social environment: living alone or not. All of these variables had <10% missing data (Table S1). Variables with a correlation coefficient >0.6 were excluded, thus keeping only the clinically meaningful variables; the Barthel Index and body mass index were highly associated with the CFS and GNRI, respectively. Therefore, they were excluded from the LCA. Consequently, a total of 17 continuous and categorical variables were used in the final analysis (Table S2).
Latent Class Analysis
LCA is a statistical approach of unsupervised clustering to identify phenogroups defined by specific combinations of variables and assumes that observed patterns result from a finite mixture of underlying clusters. 35 , 36 Clusters are assigned through iterative generation of an estimated probability of membership in each of a prespecified number of clusters via mixture modeling. Particularly, LCA is thought to have the 2 following advantages: the classifications are not predefined or limited by current conceptual frameworks, given that grouping originated from the data, 19 and that it allows for loss of information minimization by directly modeling the inherent nature of continuous and categorical data. 16 , 17 , 37
The VarSelLCM package in R software implements a modified expectation–maximization algorithm, which performs feature selection using Bayesian information criterion (BIC) and maximum likelihood inference simultaneously for a fixed number of clusters. 38 Furthermore, it also implements a penalized complete‐data log‐likelihood function to identify relevant variables for better discrimination. 39
We conducted the LCA using the VarSelLCM package (2–8 clusters) for variable selection in each subset of the non‐HFpEF and HFpEF categories (Figure 1). Each model was estimated with the maximum 1000 iterations for the expectation–maximization algorithm to accomplish the stable model. To identify the optimal number of phenogroups, we used the first minimum of the BIC. 40 The BIC is suggested to provide for even the most parsimonious model selection and is recommended in LCA. 16 , 17 Particularly, a prior study demonstrated that the BIC worked best at identifying the correct number of classes in data with both categorical and continuous variables. 41 Following confirmation of the optimal number of phenogroups, the probability of each patient belonging to each subgroup was calculated, and each patient was assigned to a subgroup with the highest likelihood. 19 Finally, we assessed the reproducibility between grouping in the full 17‐variable model, as well as that with variable selection (the top numbers of discriminatory variables) using the Cohen κ statistic.
Before clustering, we performed imputation of the missing data using the missForest package, considering the benefits of random forest imputation compared with those of multiple imputation by chained equations. 42
Clinical Outcomes
The primary outcome of this study was a composite of all‐cause death and HF readmission over the 1‐year postdischarge period. Treating physicians at each participating hospital identified HF rehospitalizations according to standard definitions. 22 HF death, sudden cardiac death, and other cardiovascular deaths, including acute coronary syndrome, acute aortic syndrome, intracranial hemorrhage, and stroke, were considered cardiovascular deaths. Noncardiovascular deaths were all other causes of death.
Statistical Analysis
We first divided the patients into non‐HFpEF and HFpEF groups and compared the patient characteristics among the 3 phenogroups within each of these groups. Parametric and nonparametric variables, as well as their respective differences, were assessed using a 1‐way ANOVA or the Kruskal‐Wallis test. Significant differences between the independent categorical variables were assessed using the χ2 test. The incidence of composite events or all‐cause death was estimated using the Kaplan‐Meier survival function and compared among the 3 phenogroups using the log‐rank test. We also compared the incidence of composite events in patients who received both β‐blockers and renin‐angiotensin system inhibitors at the time of discharge and those who did not, in each of the non‐HFpEF phenogroup, using the log‐rank test. The incidence of each mode of death was estimated, accounting for competing risks. For this, cardiovascular deaths and noncardiovascular deaths were considered competing events.
Cox‐proportional hazard analyses were used to evaluate the risk for all‐cause death among phenogroups. The covariates included in the multivariable model were LVEF, Get With The Guidelines‐Heart Failure risk score at discharge, and New York Heart Association functional class ≥III at discharge. Furthermore, when analyzing patients with non‐HFpEF, we added the prescription of β‐blockers or angiotensin coenzyme inhibitor/angiotensin II receptor blockers as covariates. In addition, we conducted a weighted Cox proportional hazard model using odds ratio based on the probability for membership in each of the 3 phenogroups as a sensitivity analysis. Statistical significance was set at P<0.05. All statistical analyses were performed using R software (version 4.1.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
The baseline characteristics of the overall study population (n=1881) are described in Tables S1–S5. Overall, the mean age (SD) was 80.7 (7.8) years, and 817 (43.4%) were women. Multimorbidity (≥3 comorbidities), physical frailty (CFS score ≥5), and malnutrition (GNRI <82) were identified in 79.7%, 37.2%, and 23.7% of the analyzed patients, respectively (Figure 2).
Figure 2. Proportion of multimorbidity, physical frailty, and malnutrition.
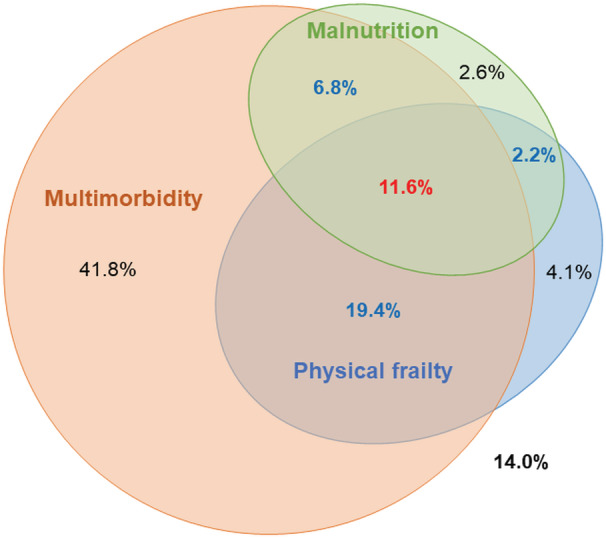
The percentage is in reference to the total cohort (N=1881).
Classification of Elderly Patients With HF
The optimal number of classified phenogroups (based on the BIC values) was 3 in both the non‐HFpEF and HFpEF subsets (Table S3). In addition, the optimal numbers of discriminatory variables to predict the phenogroups in each subset of non‐HFpEF and HFpEF were 10 and 5, respectively (Table S4). The κ coefficients for the full 17‐variable model were 0.94 in non‐HFpEF and 0.82 in HFpEF, with high reproducibility. Hemoglobin level at discharge was the most discriminatory variable for the phenogroups, regardless of LVEF categories.
The comparisons of patient characteristics among the 3 phenogroups in each subset of non‐HFpEF and HFpEF are summarized in Tables 1 and 2, respectively. In both LVEF categories, patients within each phenogroup were characterized as follows: Group 1 (46.0% in non‐HFpEF and 41.2% in HFpEF) consisted of more men, the youngest age, lowest burden of physical frailty, and highest GNRI level (well nourished). In contrast, patients in Group 2 (40.8% in non‐HFpEF and 41.9% in HFpEF) had the oldest age, highest burden of multimorbidity, and lowest eGFR and hemoglobin levels. Lastly, patients in Group 3 (18.0% in non‐HFpEF and 12.1% in HFpEF) had the lowest GNRI level, lowest burden of multimorbidity, and highest eGFR level.
Table 1.
Patient Characteristics at Discharge in Patients With Non‐HFpEF
| Characteristic | Phenogroup 1, N=485, robust | Phenogroup 2, N=442, systemic impairment | Phenogroup 3, N=128, cachexia | P value |
|---|---|---|---|---|
| Age, y | 76.0 (6.8) | 82.5 (7.3) | 80.4 (8.2) | <0.001 |
| Men | 73.4% | 57.2% | 50.8% | <0.001 |
| Body mass index, kg/m2 | 22.1 (3.6) | 19.9 (2.8) | 18.0 (2.8) | <0.001 |
| Living alone | 26.2% | 17.6% | 11.7% | <0.001 |
| Smoking | 16.1% | 4.8% | 8.6% | <0.001 |
| Clinical frailty scale | 3 (3–4) | 4 (4–6) | 5 (4–7) | <0.001 |
| Physical frailty (Clinical Frailty Scale ≥5) | 15.1% | 48.6% | 58.6% | <0.001 |
| Barthel Index | 100 (90–100) | 85 (55–100) | 65 (45–100) | <0.001 |
| GNRI | 96.0 (8.4) | 86.0 (7.9) | 78.6 (8.4) | <0.001 |
| Malnutrition, GNRI <82 | 2.9% | 30.3% | 63.3% | <0.001 |
| Systolic blood pressure, mm Hg | 112.2 (16.7) | 113.6 (18.5) | 110.3 (15.8) | 0.133 |
| Diastolic blood pressure, mm Hg | 65.2 (12.6) | 61.0 (12.0) | 62.2 (13.0) | <0.001 |
| NYHA functional class ≥III | 6.0% | 13.4% | 11.8% | <0.001 |
| GWTG‐Heart Failure risk score | 40.9 (7.2) | 44.0 (7.1) | 43.7 (6.7) | <0.001 |
| Comorbidities | ||||
| No. of morbidities | 3 (2–4) | 4 (3–5) | 2 (2–4) | <0.001 |
| Multimorbidity | 71.3% | 93.2% | 50.0% | <0.001 |
| Atrial fibrillation | 45.6% | 34.4% | 33.0% | <0.001 |
| Hypertension | 65.6% | 69.7% | 50.0% | <0.001 |
| Diabetes | 32.6% | 34.6% | 21.1% | 0.015 |
| Dyslipidemia | 43.1% | 40.7% | 25.8% | 0.002 |
| Hyperuricemia | 26.6% | 35.7% | 0.8% | <0.001 |
| Previous stroke | 11.3% | 14.0% | 12.5% | 0.468 |
| Anemia | 26.8% | 91.9% | 75.0% | <0.001 |
| Chronic kidney disease | 75.9% | 99.3% | 22.7% | <0.001 |
| COPD/asthma | 4.7% | 3.6% | 3.9% | 0.686 |
| Malignant tumor | 1.4% | 2.3% | 0.8% | 0.434 |
| Dementia | 5.4% | 12.0% | 21.1% | <0.001 |
| Echocardiography | ||||
| LVDD, mm | 56.0 (9.0) | 53.8 (8.1) | 50.2 (8.3) | <0.001 |
| LVESD, mm | 46.5 (10.0) | 44.3 (8.9) | 40.9 (9.5) | <0.001 |
| Left ventricular ejection fraction, % | 34.4 (9.3) | 35.0 (8.8) | 35.6 (8.2) | 0.339 |
| Left atrium diameter, mm | 45.2 (8.0) | 42.6 (8.2) | 40.3 (9.2) | <0.001 |
| Cause | ||||
| Ischemic cardiomyopathy | 33.4% | 43.0% | 34.4% | <0.001 |
| Dilated cardiomyopathy | 21.9% | 12.4% | 9.4% | |
| Hypertrophic cardiomyopathy | 2.1% | 0.9% | 0 | |
| Valvular heart disease | 14.4% | 21.3% | 23.4% | |
| Others | 28.2% | 22.4% | 32.8% | |
| Examinations at discharge | ||||
| Hemoglobin, g/dL | 13.6 (1.7) | 10.6 (1.2) | 11.5 (1.4) | <0.001 |
| eGFR, mL/min per 1.73 m2 | 50.7 (15.4) | 34.2 (13.5) | 75.7 (19.5) | <0.001 |
| Serum sodium, mEq/L | 138.9 (3.2) | 138.6 (3.9) | 137.9 (4.0) | 0.012 |
| Serum potassium, mEq/L | 4.4 (0.4) | 4.4 (0.5) | 4.2 (0.5) | 0.003 |
| BNP, pg/mL | 240 (125–413) | 429 (208–802) | 302 (184–560) | <0.001 |
| NT‐proBNP, pg/mL | 1716 (823.5–3071) | 4158 (1564–8239) | 4451 (1553–11 373) | <0.001 |
| Examinations, 1 y after discharge | ||||
| BNP, pg/mL | 160 (67–357) | 280 (116–603) | 141 (85–315) | <0.001 |
| NT‐proBNP, pg/mL | 1532 (761–2915) | 3864 (1510–7744) | 2218 (1151–7951) | <0.001 |
| Medications, discharge, % | ||||
| β‐Blocker | 86.6% | 78.3% | 78.9% | 0.002 |
| ACE‐I/ARB | 72.0% | 54.3% | 57.0% | <0.001 |
| Mineralocorticoid receptor antagonist | 49.2% | 36.7% | 43.0% | 0.001 |
| SGLT‐2 inhibitor | 12.8% | 5.2% | 2.3% | <0.001 |
| Loop diuretic | 87.6% | 89.1% | 83.6% | 0.239 |
| Thiazide diuretic | 2.9% | 4.5% | 0.8% | 0.088 |
| Digoxin | 5.8% | 3.2% | 5.5% | 0.152 |
| Oral anticoagulant | 65.8% | 46.2% | 56.2% | <0.001 |
| Antiplatelet agent | 41.0% | 51.4% | 41.4% | 0.004 |
| Statin | 50.5% | 50.7% | 34.4% | 0.003 |
| Antihyperuricemic | 31.8% | 33.9% | 8.6% | <0.001 |
| Medications, 1 y after discharge, % | ||||
| β‐Blocker | 87.2% | 77.6% | 77.5% | 0.004 |
| ACE‐I/ARB | 69.5% | 52.0% | 58.6% | <0.001 |
| Mineralocorticoid receptor antagonist | 48.6% | 34.4% | 48.5% | 0.001 |
| SGLT‐2 inhibitor | 16.7% | 11.0% | 1.4% | 0.001 |
| Loop diuretic | 80.6% | 80.9% | 74.3% | 0.441 |
| Thiazide diuretic | 1.6% | 5.3% | 0 | 0.009 |
Values are expressed as mean (SD) or median (interquartile range). ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; GNRI, Geriatric Nutritional Risk Index; GWTG, Get With The Guidelines; HFpEF, heart failure with preserved ejection fraction; LVDD, left ventricular end‐diastolic dimension; LVESD, left ventricular end‐systolic dimension; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; and SGLT‐2, sodium‐glucose transport protein 2.
Table 2.
Patient Characteristics at Discharge in Patients With HFpEF
| Characteristic | Phenogroup 1, N=340, robust | Phenogroup 2, N=337, systemic impairment | Phenogroup 3, N=149, cachexia | P value |
|---|---|---|---|---|
| Age, y | 80.5 (7.0) | 84.8 (6.6) | 82.1 (7.9) | <0.001 |
| Men | 53.5% | 42.7% | 43.0% | <0.001 |
| Body mass index, kg/m2 | 22.5 (4.0) | 20.4 (3.3) | 18.6 (2.9) | <0.001 |
| Living alone | 23.5% | 17.8% | 16.1% | <0.001 |
| Smoking | 7.9% | 4.7% | 6.0% | <0.001 |
| Clinical Frailty Scale | 4 (3–5) | 5 (4–6) | 5 (4–6) | <0.001 |
| Physical frailty (Clinical Frailty Scale ≥5) | 25.3% | 52.5% | 51.7% | <0.001 |
| Barthel Index | 100 (80–100) | 80 (60–100) | 85 (50–100) | <0.001 |
| GNRI | 96.8 (9.9) | 86.4 (8.7) | 81.5 (8.6) | <0.001 |
| Malnutrition, GNRI <82 | 5.9% | 32.9% | 51.7% | <0.001 |
| Systolic blood pressure, mm Hg | 115.6 (17.1) | 119.9 (18.4) | 115.6 (17.8) | 0.003 |
| Diastolic blood pressure, mm Hg | 64.7 (12.7) | 60.7 (11.4) | 62.2 (11.8) | <0.001 |
| NYHA functional class ≥III | 7.1% | 13.7% | 8.8% | 0.015 |
| GWTG‐Heart Failure risk score | 40.5 (7.7) | 42.0 (6.9) | 41.5 (7.9) | 0.03 |
| Comorbidities | ||||
| No. of morbidities | 4 (3–5) | 4 (4–5) | 3 (2–4) | <0.001 |
| Multimorbidity | 77.1% | 95.0% | 63.1% | <0.001 |
| Atrial fibrillation | 55.9% | 47.8% | 43.6% | 0.021 |
| Hypertension | 55.9% | 47.8% | 43.6% | 0.101 |
| Diabetes | 26.8% | 27.3% | 21.5% | 0.371 |
| Dyslipidemia | 33.5% | 32.9% | 27.5% | 0.395 |
| Hyperuricemia | 28.8% | 31.8% | 3.4% | <0.001 |
| Previous stroke | 12.6% | 17.2% | 12.1% | 0.161 |
| Anemia | 43.8% | 99.4% | 91.3% | <0.001 |
| Chronic kidney disease | 77.6% | 98.5% | 16.1% | <0.001 |
| COPD/asthma | 5.9% | 5.0% | 7.4% | 0.595 |
| Malignant tumor | 0.9% | 1.8% | 2.0% | 0.509 |
| Dementia | 7.6% | 12.2% | 15.4% | 0.024 |
| Echocardiography | ||||
| LVDD, mm | 45.0 (6.9) | 45.6 (7.1) | 43.4 (6.5) | 0.005 |
| LVESD, mm | 30.4 (6.1) | 30.2 (6.5) | 28.4 (5.8) | 0.004 |
| Left ventricular ejection fraction, % | 59.9 (6.9) | 61.3 (7.2) | 61.5 (8.1) | 0.02 |
| Left atrium diameter, mm | 45.4 (9.1) | 44.9 (10.0) | 41.0 (8.9) | <0.001 |
| Cause | ||||
| Ischemic cardiomyopathy | 11.5% | 15.4% | 10.7% | <0.001 |
| Dilated cardiomyopathy | 1.5% | 0.6% | 1.3% | |
| Hypertrophic cardiomyopathy | 5.0% | 1.5% | 2.0% | |
| Valvular heart disease | 34.1% | 40.4% | 45.6% | |
| Others | 47.9% | 42.1% | 40.3% | |
| Examinations at discharge | ||||
| Hemoglobin, g/dL | 12.8 (1.5) | 9.9 (1.0) | 10.6 (1.3) | <0.001 |
| eGFR, mL/min per 1.73 m2 | 48.7 (14.5) | 33.4 (13.7) | 76.3 (21.2) | <0.001 |
| Serum sodium, mEq/L | 139.4 (3.0) | 138.7 (3.6) | 137.8 (4.4) | <0.001 |
| Serum potassium, mEq/L | 4.3 (0.5) | 4.4 (0.5) | 4.2 (0.5) | 0.017 |
| BNP, pg/mL | 205 (95–362) | 297 (140–548) | 224 (147–362) | 0.014 |
| NT‐proBNP, pg/mL | 1060 (668–1694) | 2957 (1273–4912) | 2240 (941–3902) | <0.001 |
| Examinations, 1 y after discharge | ||||
| BNP, pg/mL | 214 (112–479) | 167 (92–321) | 145 (85–309) | 0.032 |
| NT‐proBNP, pg/mL | 1707 (851–2917) | 3005 (1372–6293) | 906 (479–1977) | <0.001 |
| Medications, discharge, % | ||||
| β‐Blocker | 67.9% | 51.0% | 56.4% | <0.001 |
| ACE‐I/ARB | 51.5% | 43.3% | 46.3% | 0.102 |
| Mineralocorticoid receptor antagonist | 33.5% | 29.1% | 36.2% | 0.235 |
| SGLT‐2 inhibitor | 6.8% | 1.5% | 3.4% | 0.002 |
| Loop diuretic | 80.9% | 82.5% | 74.5% | 0.118 |
| Thiazide diuretic | 2.6% | 3.6% | 1.3% | 0.383 |
| Digoxin | 5.8% | 3.2% | 5.5% | 0.152 |
| Oral anticoagulant | 71.5% | 52.8% | 55.0% | <0.001 |
| Antiplatelet agent | 26.8% | 35.9% | 39.6% | 0.006 |
| Statin | 36.5% | 40.1% | 33.6% | 0.353 |
| Antihyperuricemic | 27.9% | 32.9% | 7.4% | <0.001 |
| Medications, 1 y after discharge, % | ||||
| β‐Blocker | 68.0% | 45.8% | 61.5% | <0.001 |
| ACE‐I/ARB | 50.5% | 39.2% | 50.0% | 0.074 |
| Mineralocorticoid receptor antagonist | 34.3% | 27.1% | 32.1% | 0.345 |
| SGLT‐2 inhibitor | 7.9% | 3.6% | 3.8% | 0.168 |
| Loop diuretic | 75.6% | 77.6% | 69.2% | 0.366 |
| Thiazide diuretic | 3.6% | 4.8% | 3.8% | 0.833 |
Values are expressed as mean (SD) or median (interquartile range). ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; GNRI, Geriatric Nutritional Risk Index; GWTG, Get With The Guidelines; HFpEF, heart failure with preserved ejection fraction; LVDD, left ventricular end‐diastolic dimension; LVESD, left ventricular end‐systolic dimension; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; and SGLT‐2, sodium‐glucose transport protein 2.
Based on the above clinical characteristics, these phenogroups were labeled as robust (Group 1), systemic impairment (Group 2), and cachexia (Group 3). The phenotypic characteristics based on each variable's transformed Z scores are shown in a heatmap in Figure 3A (non‐HFpEF) and Figure 3B (HFpEF). In addition, in the non‐HFpEF subset, patients within Group 1 were more likely to receive β‐blockers, angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers, and mineralocorticoid receptor antagonists (Table 1).
Figure 3. Heat map of the phenotypic characteristics across the 3 phenogroups.
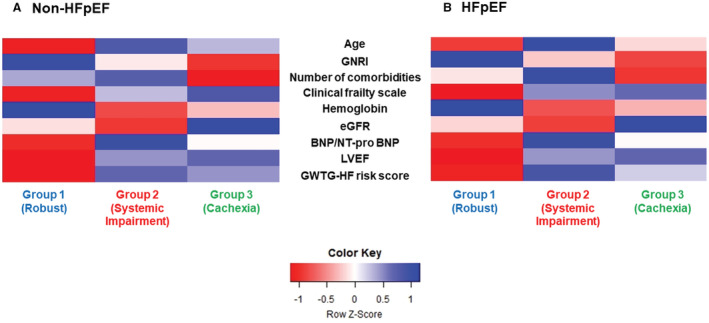
A, Non‐HFpEF. B, HFpEF. BNP indicates brain natriuretic peptide; eGFR, estimated glomerular filtration rate; GNRI, Geriatric Nutritional Risk Index; GWTG, Get With The Guidelines; HFpEF, heart failure with preserved ejection fraction; LVEF, left ventricular ejection fraction; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Clinical Outcomes After Discharge
In the non‐HFpEF subset, the robust group had a lower incidence of composite events and all‐cause deaths over the 1‐year postdischarge period than the systemic impairment or cachexia groups (composite events: 22.1%, 30.8%, and 31.3% [Figure 4A]; all‐cause death: 5.6%, 13.3%, and 17.2% [Figure 4B], respectively; both log‐rank P<0.001). In the HFpEF subset, the cumulative incidence of the composite events was highest in the systemic impairment group, followed by that in the robust and cachexia groups (systemic impairment, 30.9%; robust, 21.5%; cachexia, 16.8%; log‐rank P<0.001) (Figure 4C). However, in this subset, the incidence of all‐cause death was lowest in the robust group (robust, 4.3%; systemic impairment, 15.1%; cachexia, 12.1%; log‐rank P<0.001) (Figure 4D). In addition, we found no significant difference in the crude incidence of composite events between patients who received both β‐blockers and renin‐angiotensin system inhibitors and those without across the phenogroups (Figures S1–S3).
Figure 4. Cumulative incidence of clinical outcomes among the 3 phenogroups.
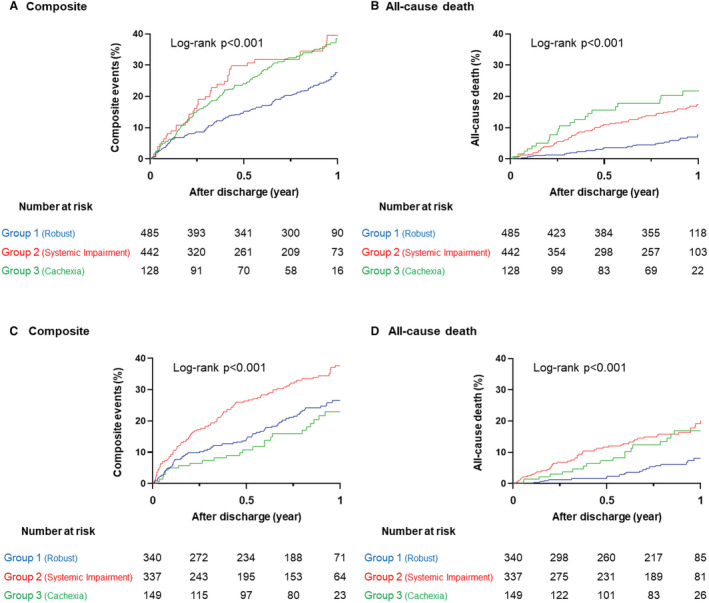
A, Composite events. B, All‐cause death in non‐HFpEF. C, Composite events. D, All‐cause death in HFpEF. The composite event was defined as all‐cause death and heart failure readmission. HFpEF indicates heart failure with preserved ejection fraction.
After adjustment for cardiovascular conditions using multivariable Cox models, the systemic impairment and cachexia groups remained at a higher risk for both composite events and all‐cause deaths than the robust group in the non‐HFpEF subset (Figure 5A). In the HFpEF subset, the systemic impairment group also had a higher risk for both clinical outcomes than the robust group. However, the cachexia group had a higher risk of all‐cause death than the robust group, and there was no significant difference in composite events between them (Figure 5B). Furthermore, these trends were robust in weighted Cox models based on the probability for membership in each of the 3 phenogroups (Table S5).
Figure 5. Associations between phenogroups and clinical outcomes.
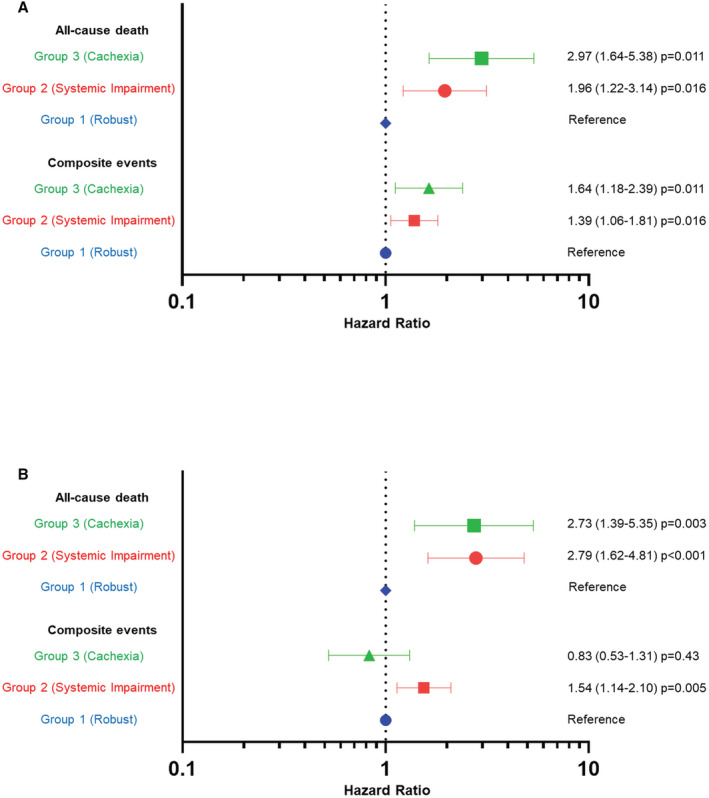
A, Non‐HFpEF. B, HFpEF. Forest plots show hazard ratios for clinical outcomes in Group 2 (systemic impairment) and Group 3 (cachexia), compared with Group 1 (robust).
The crude cardiovascular deaths and noncardiovascular deaths in each subset of patients with non‐HFpEF and HFpEF are presented in Figures 6A and 6B, respectively. The proportion of cardiovascular deaths was consistently higher in patients with non‐HFpEF than in those with HFpEF. Patients in the systemic impairment group had the highest proportion of cardiovascular death, followed by those in the cachexia and robust groups, regardless of the LVEF category (robust versus systemic impairment versus cachexia: non‐HFpEF, 46.4% versus 62.7% versus 59.1%; HFpEF, 31.6% versus 51.0% versus 38.9%).
Figure 6. Incidence of CVD and non‐CVD.
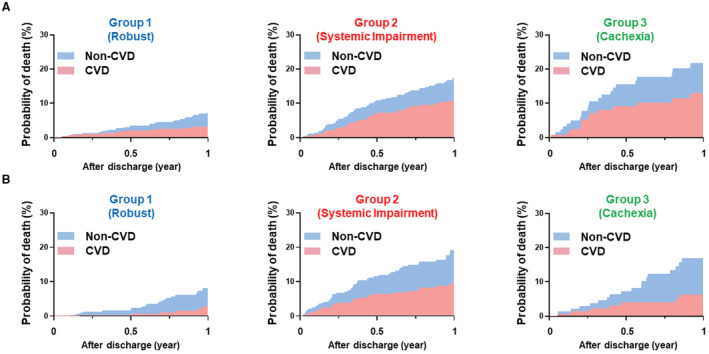
A, Non‐HFpEF. B, HFpEF. The cumulative incidence was estimated by the Fine‐Gray model. CVD indicates cardiovascular death.
Discussion
This study performed clustering analyses focused on noncardiovascular conditions in elderly patients with HF to facilitate the understanding of phenotypic diversity. These patients were successfully classified into 3 phenogroups: robust, systemic impairment, and cachexia, regardless of the systolic function status. We found that the prescription patterns of HF‐specific medications varied among the 3 phenogroups in patients with non‐HFpEF. Both the systemic impairment and cachexia groups had similarly poorer prognoses than the robust group. Furthermore, patients in the cachexia group died more often from noncardiovascular causes.
Although the importance of a multidimensional and integrated clinical assessment is advocated for the optimal management of elderly patients with HF, 10 , 43 it is often difficult for clinicians to comprehend the individual pathophysiology in routine care. This is because of the complex interactions of multiple coexisting noncardiovascular disorders. Therefore, it is crucial to comprehend the overlapping of noncardiovascular conditions and its influences on clinical prognosis, particularly in elderly patients. In this context, the present phenomapping focused on incorporating noncardiovascular conditions following clinical implications. First, we successfully identified novel and clinically recognizable phenogroups, which differed from previous clustering analyses. 8 , 15 , 16 , 17 Notably, although patients in the systemic impairment group were expected to have the poorest prognosis simply based on biomarker assessments (eg, natriuretic peptide), patients assigned to the cachexia group had equally poor prognosis compared with those in the systemic impairment group. These findings suggest that our phenomapping offers novel risk stratification, which cannot be yielded by existing clustering analyses, for elderly patients with HF. Second, despite the distinct pathophysiology between non‐HFpEF and HFpEF, similar phenogroups were identified in both LVEF categories. These findings indicate that noncardiovascular conditions play a significant role in the pathophysiology and prognosis of elderly patients with HF, regardless of systolic function status. Thus, the novel classifications may apply to a wide range of elderly patients and assist in more tailored clinical decision making, as well as the design of more targeted future trials. Furthermore, we suggest that future clinical trials for elderly patients should consider noncardiovascular conditions. Hereafter, the detailed characteristics of each phenogroup are described.
Patients in the systemic impairment group had a higher risk of cardiovascular events than the other groups, in the setting of considerable renal dysfunction, high prevalence of multimorbidity, and elevated natriuretic peptide levels. Therefore, developing optimal treatment strategies for these would remain challenging in both LVEF categories. The efficacy of HF‐specific pharmacotherapies in patients with coexisting renal impairment is uncertain 44 ; as such, patients have traditionally been recruited less in randomized controlled trials. This would lower the likelihood of initiating pharmacotherapy and promote poorer prognoses in this group. Additionally, the high prevalence of multimorbidity in conjunction with renal impairment induces chronic systemic inflammation and endothelial dysfunction, resulting in HFpEF worsening. 45 Therefore, integrated management focused on comorbid conditions is crucial for patients with this phenogroup. For instance, the management of anemia would be a modifiable factor toward improving clinical outcomes. 43 , 44 Particularly, the administration of intravenous ferric carboxymaltose for patients with systolic dysfunction and iron deficiency has resulted in decreased hospitalization for HF, as well as improved quality of life. 46 These findings indicated the importance of anemia screening as a routine baseline assessment.
Patients in the cachexia group had a high incidence of all‐cause death in the setting of low prevalence of diabetes (21.5%) and renal dysfunction, but a high prevalence of malnutrition and physical frailty, regardless of LVEF categories. Considering that previous clustering analyses have not identified such a phenotype, 8 , 15 , 16 , 17 this cachexia phenotype group reflects elderly‐inherent characteristics via phenomapping focused on incorporating noncardiovascular conditions. Notably, the causes of poor prognosis in this group would be explained by not only poor use of HF‐specific medical therapies but also the increased burden of noncardiovascular rather than cardiovascular events. Furthermore, the burden of noncardiovascular rather than cardiovascular events could explain the reasons for paradoxical results, in which patients with HFpEF in the cachexia group had a lower incidence of HF readmissions. These results emphasize that simply applying interventions targeting cardiovascular conditions is insufficient; instead, it is important to design integrated management protocols that intensively target noncardiovascular conditions. A recent clinical trial has demonstrated that, compared with a control group, individual nutrition support to reach energy, protein, and micronutrient goals reduced mortality, especially in patients with high nutritional risk. 18 Furthermore, the Rehabilitation Therapy in Older Acute Heart Failure Patients trial revealed that a tailored rehabilitation intervention resulted in physical function improvement in elderly patients with HF, especially those with coexisting physical frailty. 7 These interventions would be rather suitable options for patients in the cachexia group.
Finally, among the 3 groups, patients in the robust group were prevalently men and the youngest. These patients may suitably represent the profile of those who had been included in previous clinical trials for HF and account for less than half of the overall elderly patient population in real‐world clinical settings. We found that angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, and mineralocorticoid receptor antagonist therapies were underused in patients with non‐HFpEF in the robust group. With recently rising concerns on doubts about the benefit of HF‐specific medical therapies in patients with frailty, stratified analyses using frailty status were often conducted as subanalyses of clinical trials. Most of these analyses demonstrated no interactions of efficacy in pharmacotherapy (eg, angiotensin receptor‐neprilysin inhibitors or sodium‐glucose cotransporter 2 inhibitors) across the frailty status. 47 , 48 These findings suggest that attending physicians should persistently ensure that HF‐specific medication doses are increased to target as tolerated.
Our research has several limitations. First, validation analyses for the classification system could not be performed. Particularly, given the inherent characteristics of the LCA, the number of phenogroups may vary according to each analytic cohort. 19 Therefore, we could not exclude the possibility of representing different patterns of phenogroups when analyzing different cohorts. However, as previously mentioned, similar patterns of phenogroups were identified across LVEF categories; this would partially relieve this major concern. Second, the participants in this study were mostly Japanese patients. The identified phenogroups, especially the cachexia group with extremely low body mass index levels, may not apply to patients in Western countries. Therefore, further validation analyses are required. Third, as we performed the LCA using 17 variables focused on noncardiovascular conditions, the variables were fewer than those in previous investigations. 15 , 16 , 17 , 49 , 50 However, considering that using numerous variables may inhibit the application for clinical settings, this simple phenomapping, focused on noncardiovascular conditions, would provide important clinical benefits in terms of addressing the diversity of elderly patients. Fourth, we did not account for several essential factors of elderly patients with HF (eg, mental health or financial status). 10 Thus, we could not exclude that phenomapping would differ if more complete data were available to us on noncardiovascular conditions. Fifth, this was a multicenter observational study. Because the treatment strategy for HF was not predetermined, it varied according to the physicians' and medical centers' discretion and protocols. Finally, similar to previous reports, this study only included patients who could be followed up on, potentially leading to selection bias.
Conclusions
In elderly patients who were hospitalized for acute HF, LCA identified 3 phenogroups, each representing distinct clinical outcomes, with the incorporation of noncardiovascular conditions. These findings highlight the heterogeneity of the elderly population with HF and show that such classification may be useful for a better understanding of tailored treatment in these patients.
Sources of Funding
This study was supported by a Grant‐in‐Aid for Young Scientists (Japan Society for the Promotion of Science [KAKANHI] 18K15860), a Grant‐in‐Aid for Scientific Research (KAKENHI 21K08142, 21K08064, 20K08408, 18K08056, 17K09526, 16KK0186, and 16H05215), a Health Labour Sciences Research Grant (14528506), the Sakakibara Clinical Research Grant for Promotion of Sciences (2012 to 2020), and a grant from the Japan Agency for Medical Research and Development (201439013C).
Disclosures
Dr Shiraishi is affiliated with an endowed department by Nippon Shinyaku, Medtronic Japan, and BIOTRONIK JAPAN. Dr Shiraishi also received research grants from the SECOM Science and Technology Foundation and the Uehara Memorial Foundation, and honoraria from Otsuka Pharmaceutical and Ono Pharmaceutical. Dr Kohsaka received an unrestricted research grant from Novartis Pharmaceutical and honoraria from Bristol‐Myer Squibb. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S5
Figures S1–S3
Acknowledgments
The authors are grateful to the investigators of the WET‐HF. The English language editing (Editage, https://www.editage.jp) and article processing charge in this study were supported by a Grant‐in‐Aid for Young Scientists (Japan Society for the Promotion of Science KAKENHI; 22K16067).
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027689
For Sources of Funding and Disclosures, see page 13.
References
- 1. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015;17:884–892. doi: 10.1002/ejhf.319 [DOI] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 3. Abbafati C, Abbas KM, Abbasi‐Kangevari M, Abd‐Allah F, Abdelalim A, Abdollahi M, Abdollahpour I, Abegaz KH, Abolhassani H, Aboyans V, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ueda T, Kawakami R, Horii M, Sugawara Y, Matsumoto T, Okada S, Nishida T, Soeda T, Okayama S, Somekawa S, et al. Noncardiovascular death, especially infection, is a significant cause of death in elderly patients with acutely decompensated heart failure. J Card Fail. 2014;20:174–180. doi: 10.1016/j.cardfail.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 5. Tromp J, Shen L, Jhund PS, Anand IS, Carson PE, Desai AS, Granger CB, Komajda M, McKelvie RS, Pfeffer MA, et al. Age‐related characteristics and outcomes of patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2019;74:601–612. doi: 10.1016/j.jacc.2019.05.052 [DOI] [PubMed] [Google Scholar]
- 6. Nakamaru R, Shiraishi Y, Sandhu AT, Heidenreich PA, Shoji S, Kohno T, Takei M, Nagatomo Y, Nakano S, Kohsaka S, et al. Cardiovascular vs. non‐cardiovascular deaths after heart failure hospitalization in young, older, and very old patients. ESC Heart Fail. 2022. Nov 27. doi: 10.1002/ehf2.14245 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kitzman DW, Whellan DJ, Duncan P, Pastva AM, Mentz RJ, Reeves GR, Nelson MB, Chen H, Upadhya B, Reed SD, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385:203–216. doi: 10.1056/NEJMoa2026141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tromp J, Tay WT, Ouwerkerk W, Teng THK, Yap J, MacDonald MR, Leineweber K, McMurray JJV, Zile MR, Anand IS, et al. Multimorbidity in patients with heart failure from 11 Asian regions: a prospective cohort study using the ASIAN‐HF registry. PLoS Med. 2018;15:e1002541. doi: 10.1371/journal.pmed.1002541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XHT, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gorodeski EZ, Goyal P, Hummel SL, Krishnaswami A, Goodlin SJ, Hart LL, Forman DE, Wenger NK, Kirkpatrick JN, Alexander KP. Domain management approach to heart failure in the geriatric patient: present and future. J Am Coll Cardiol. 2018;71:1921–1936. doi: 10.1016/j.jacc.2018.02.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Driggin E, Cohen LP, Gallagher D, Karmally W, Maddox T, Hummel SL, Carbone S, Maurer MS. Nutrition assessment and dietary interventions in heart failure: JACC review topic of the week. J Am Coll Cardiol. 2022;79:1623–1635. doi: 10.1016/j.jacc.2022.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsue Y, Kamiya K, Saito H, Saito K, Ogasahara Y, Maekawa E, Konishi M, Kitai T, Iwata K, Jujo K, et al. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE‐HF cohort study. Eur J Heart Fail. 2020;22:2112–2119. doi: 10.1002/ejhf.1926 [DOI] [PubMed] [Google Scholar]
- 13. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol. 2018;72:351–366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 14. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition. J Card Fail. 2021;27:387–413. doi: 10.1016/j.cardfail.2021.01.022 [DOI] [PubMed] [Google Scholar]
- 15. Ahmad T, Lund LH, Rao P, Ghosh R, Warier P, Vaccaro B, Dahlström U, O'Connor CM, Michael Felker G, Desai NR. Machine learning methods improve prognostication, identify clinically distinct phenotypes, and detect heterogeneity in response to therapy in a large cohort of heart failure patients. J Am Heart Assoc. 2018;7:e008081. doi: 10.1161/JAHA.117.008081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Segar MW, Patel KV, Ayers C, Basit M, Tang WHW, Willett D, Berry J, Grodin JL, Pandey A. Phenomapping of patients with heart failure with preserved ejection fraction using machine learning‐based unsupervised cluster analysis. Eur J Heart Fail. 2020;22:148–158. doi: 10.1002/ejhf.1621 [DOI] [PubMed] [Google Scholar]
- 17. Sotomi Y, Hikoso S, Komukai S, Sato T, Oeun B, Kitamura T, Nakagawa A, Nakatani D, Mizuno H, Okada K, et al. Phenotyping of acute decompensated heart failure with preserved ejection fraction. Heart. 2022;108:1553–1561. doi: 10.1136/heartjnl-2021-320270 [DOI] [PubMed] [Google Scholar]
- 18. Minamisawa M, Seidelmann SB, Claggett B, Hegde SM, Shah AM, Desai AS, Lewis EF, Shah SJ, Sweitzer NK, Fang JC, et al. Impact of malnutrition using geriatric nutritional risk index in heart failure with preserved ejection fraction. JACC Heart Fail. 2019;7:664–675. doi: 10.1016/j.jchf.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 19. Mori M, Krumholz HMAH. Using latent class analysis to identify hidden clinical phenotypes. JAMA. 2020;324:700–701. doi: 10.1001/jama.2020.2278 [DOI] [PubMed] [Google Scholar]
- 20. Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, Komajda M, McMurray JJ, Lindenfeld JA. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail. 2015;17:925–935. doi: 10.1002/ejhf.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takeuchi S, Kohno T, Goda A, Shiraishi Y, Kawana M, Saji M, Nagatomo Y, Nishihata Y, Takei M, Nakano S, et al. Multimorbidity, guideline‐directed medical therapies, and associated outcomes among hospitalized heart failure patients. ESC Heart Fail. 2022;9:2500–2510. doi: 10.1002/ehf2.13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kitakata H, Kohno T, Kohsaka S, Shiraishi Y, Parizo JT, Niimi N, Goda A, Nishihata Y, Heidenreich PA, Yoshikawa T. Prognostic implications of early and midrange readmissions after acute heart failure hospitalizations: a report from a Japanese multicenter registry. J Am Heart Assoc. 2020;9:e014949. doi: 10.1161/JAHA.119.014949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shiraishi Y, Kohsaka S, Sato N, Takano T, Kitai T, Yoshikawa T, Matsue Y. 9‐year trend in the management of acute heart failure in Japan: a report from the national consortium of acute heart failure registries. J Am Heart Assoc. 2018;18:e008687.doi: 10.1161/JAHA.118.008687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagai T, Sundaram V, Shoaib A, Shiraishi Y, Kohsaka S, Rothnie KJ, Piper S, McDonagh TA, Hardman SMC, Goda A, et al. Validation of U.S. mortality prediction models for hospitalized heart failure in the United Kingdom and Japan. Eur J Heart Fail. 2018;20:1179–1190. doi: 10.1002/ejhf.1210 [DOI] [PubMed] [Google Scholar]
- 25. Shiraishi Y, Nagai T, Kohsaka S, Goda A, Nagatomo Y, Mizuno A, Kohno T, Rigby A, Fukuda K, Yoshikawa T, et al. Outcome of hospitalised heart failure in Japan and the United Kingdom stratified by plasma N‐terminal pro‐B‐type natriuretic peptide. Clin Res Cardiol. 2018;107:1103–1110. doi: 10.1007/s00392-018-1283-6 [DOI] [PubMed] [Google Scholar]
- 26. Fukuoka R, Kohno T, Kohsaka S, Shiraishi Y, Sawano M, Abe T, Nagatomo Y, Goda A, Mizuno A, Fukuda K, et al. Prediction of sudden cardiac death in Japanese heart failure patients: international validation of the Seattle Proportional Risk Model. Europace. 2020;22:588–597. doi: 10.1093/europace/euaa002 [DOI] [PubMed] [Google Scholar]
- 27. Ho KKL, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.CIR.88.1.107 [DOI] [PubMed] [Google Scholar]
- 28. Yamazaki Y, Shiraishi Y, Kohsaka S, Nagatomo Y, Fukuda K, Kohno T, Yoshikawa T. Temporal trends in tolvaptan use after revision of national heart failure guidelines in Japan. Sci Rep. 2021;11:19360. doi: 10.1038/s41598-021-98173-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, Masoudi FA, et al. Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77:772–810. doi: 10.1016/j.jacc.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 30. Nutritional anaemias: report of a WHO group of experts. World Health Organ Tech Rep Ser. 1972;503:1–29. [PubMed] [Google Scholar]
- 31. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 32. Bouillanne O, Morineau G, Dupant C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric nutritional risk index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr. 2005;82:777–783. doi: 10.1093/ajcn/82.4.777 [DOI] [PubMed] [Google Scholar]
- 33. Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Identification of frailty in chronic heart failure. JACC Hear Fail. 2019;7:291–302. doi: 10.1016/j.jchf.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 34. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sinha P, Calfee CS, Delucchi KL. Practitioner's guide to latent class analysis: methodological considerations and common pitfalls. Crit Care Med. 2021;49:e63–e79. doi: 10.1097/CCM.0000000000004710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mathew R, Fernando S, Hu K, Parlow S, Di Santo P, Brodie D, Hibbert B. Optimal perfusion targets in cardiogenic shock. JACC Adv. 2022;1:1–14. doi: 10.1016/j.jacadv.2022.100034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Waljee AK, Mukherjee A, Singal AG, Zhang Y, Warren J, Balis U, Marrero J, Zhu J, Higgins PDR. Comparison of imputation methods for missing laboratory data in medicine. BMJ Open. 2013;3:e002847. doi: 10.1136/bmjopen-2013-002847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marbac M, Sedki M. VarSelLCM: An R/C++ package for variable selection in model‐based clustering of mixed‐data with missing values. Bioinformatics. 2019;35:1255–1257. doi: 10.1093/bioinformatics/bty786 [DOI] [PubMed] [Google Scholar]
- 39. Marbac M, Sedki M. Variable selection for model‐based clustering using the integrated complete‐data likelihood. Stat Comput. 2017;27:1049–1063. doi: 10.1007/s11222-016-9670-1 [DOI] [Google Scholar]
- 40. Marbac M, Biernacki C, Vandewalle V. Model‐based clustering of Gaussian copulas for mixed data. Commun Stat – Theory Methods. 2017;46:11635–11656. doi: 10.1080/03610926.2016.1277753 [DOI] [Google Scholar]
- 41. Morgan GB. Mixed mode latent class analysis: an examination of fit index performance for classification. Struct Equ Modeling. 2015;22:76–86. doi: 10.1080/10705511.2014.935751 [DOI] [Google Scholar]
- 42. Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–466. doi: 10.1214/aos/1176344136 [DOI] [Google Scholar]
- 43. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 44. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 45. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 46. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, Lüscher TF, Arutyunov GP, Motro M, Mori C, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail. 2018;20:125–133. doi: 10.1002/ejhf.823 [DOI] [PubMed] [Google Scholar]
- 47. Butt JH, Dewan P, Merkely B, Belohlávek J, Drożdż J, Kitakaze M, Inzucchi SE, Kosiborod MN, Martinez FA, Tereshchenko S, et al. Efficacy and safety of dapagliflozin according to frailty in heart failure with reduced ejection fraction: a post hoc analysis of the DAPA‐HF trial. Ann Intern Med. 2022;175:820–830. doi: 10.7326/M21-4776 [DOI] [PubMed] [Google Scholar]
- 48. Dewan P, Jackson A, Jhund PS, Shen L, Ferreira JP, Petrie MC, Abraham WT, Desai AS, Dickstein K, Køber L, et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction—an analysis of PARADIGM‐HF and ATMOSPHERE. Eur J Heart Fail. 2020;22:2123–2133. doi: 10.1002/ejhf.1832 [DOI] [PubMed] [Google Scholar]
- 49. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahmad T, Pencina MJ, Schulte PJ, O'Brien E, Whellan DJ, Piña IL, Kitzman DW, Lee KL, O'Connor CM, Felker GM. Clinical implications of chronic heart failure phenotypes defined by cluster analysis. J Am Coll Cardiol. 2014;64:1765–1774. doi: 10.1016/j.jacc.2014.07.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figures S1–S3


