ABSTRACT
Combination therapy with ampicillin plus ceftriaxone (AMP+CRO) is the first-line therapy for treating severe infections due to Enterococcus faecalis. However, the pharmacokinetic/pharmacodynamic (PK/PD) index linked to the in vivo efficacy of the combination is not yet defined, hindering dose optimization in the clinic. Because classical PK/PD indices are not directly applicable to antimicrobial combinations, two novel indices were tested in the optimized murine model of infection by E. faecalis to delineate the potentiation of AMP by CRO: the time above the CRO threshold (T>threshold) and the time above the AMP instantaneous MIC (T>MICi). The potential clinical relevance was evaluated by simulating human doses of AMP and CRO. Hill’s equation fitted well the exposure-response data in terms of T>threshold, with a CRO threshold of 1 mg/L. The required exposures were 46%, 49%, and 52% for stasis and 1- and 2-log10 killing, respectively. Human ceftriaxone doses of 2 g every 12 h (q12h) would reach the target in >90% of strains with thresholds ≤64 mg/L. The AMP T>MICi index also fitted well, and the required exposures were 37%, 41%, and 46% for stasis and 1- and 2-log10 killing, respectively. In humans, the addition of CRO would allow use of lower AMP doses to reach the same T>MICi and to treat strains with higher MICs. This is the first report of the PK/PD indices and required magnitudes linked to AMP+CRO against E. faecalis; these results can be used as the basis to guide the design of clinical trials to improve combined therapy against enterococci.
KEYWORDS: PK/PD index, antibiotic combination, Enterococcus, antimicrobial combinations, pharmacodynamics, pharmacokinetics, synergism
INTRODUCTION
Animal infection models are considered critical tools in preclinical antimicrobial pharmacokinetic/pharmacodynamic (PK/PD) research (1, 2). Specifically, preclinical evaluation of antimicrobials can define the PK/PD driver and the target magnitude, guide dosing regimen design, and forecast the likelihood of success at the infection site (2, 3). This approach has been successful in the case of single antibiotics but not in the context of combinations, where several challenges remain: the interactions between drug combinations have not been well understood, the traditional PK/PD index methodology cannot be applied straightforwardly (4, 5), and there is unclear evidence whether in vitro synergy testing results correlate with patient outcomes (6, 7).
An initial approach for antimicrobial combination would be to use the optimal dose of each antibiotic administered separately (5). However, considering that PK/PD modeling has relied on a static parameter, the MIC (8), it does not perform well when one drug affects the MIC of the other and when this effect varies over time, for example, with β-lactam–β-lactamase inhibitor combinations (βL/βLI), where the inhibitor reduces the MIC of the partner β-lactam (9). A more difficult task is to define an approach to assess and predict the outcome of antimicrobial combinations in terms of synergism, indifference, or antagonism. In most in vivo studies, these terms are used inconsistently with different definitions and methods (10). The experiments are commonly designed using point dose-effect estimates with one or two dose levels instead of the whole dose-response curve, leading to potentially discordant interpretations (6).
A novel approach for in vivo evaluation of antibiotic combinations has been proposed that relies on estimating the complete dose-response relationship and defines the type of interaction according to the changes in the PD parameters Emax (synergism or antagonism) and ED50 (potentiation). This method demonstrated that the in vivo effect of the combination of ampicillin and ceftriaxone (AMP+CRO), considered synergistic against Enterococcus faecalis, is a case of potentiation in the murine thigh infection model, and the actual antibacterial effect observed will depend on the dose location in the exposure-response curve (10).
It was also found that the potentiation of AMP by CRO exhibited a quantal (all-or-none) nature, with doses of CRO of ≥25 mg/kg of body weight/day displaying the same effect (10), suggesting that there is a threshold concentration or exposure that, once reached, yields the maximal response. Thus, the PK/PD index T>threshold, which has been successfully used with βL/βLI inhibitor combinations such as piperacillin-tazobactam (PIP/TAZ) (11), could also be used to explain the AMP+CRO combination. In both cases there is a MIC reduction, by inhibition of beta-lactamase with tazobactam and by complete saturation of penicillin-binding proteins (PBPs) with CRO (12). On the other hand, taking into account that in vivo the concentration of CRO is continuously changing and thus the MIC of AMP changes as well, another PK/PD index that can capture this fluctuating susceptibility is the time above the instantaneous MIC (MICi), described initially for imipenem-relebactam (9). The current study aimed to determine if these novel PK/PD indices drive the efficacy of AMP+CRO against E. faecalis and can be used to translate the results to humans and optimize dosing regimens.
RESULTS
Susceptibility and in vitro antibiotic synergy testing.
E. faecalis ATCC 29212 was AMP susceptible and CRO resistant; the modal MICs were 1 and 128 mg/L, respectively. Ceftriaxone at 1 mg/L reduced the AMP MIC 4-fold and 8-fold at 4 mg/L (Table 1). According to the checkerboard assay, synergy was observed with fractional inhibitory concentration (FIC) index values from 0.26 when AMP is combined with CRO at ≥1 mg/L. For lower ceftriaxone concentrations, the effect was indifferent.
TABLE 1.
Synergistic testing of AMP+CRO combination by the checkerboard method against E. faecalis ATCC 29212a
| A (mg/L) | FICA | B (mg/L) | FICB | Interaction |
|
|---|---|---|---|---|---|
| FIC index | Interpretation | ||||
| 1 | 0 | MICAMP | |||
| 1 | 1.000 | 0.125 | 0.001 | 1.001 | I |
| 0.5 | 0.500 | 0.25 | 0.002 | 0.502 | I |
| 0.5 | 0.500 | 0.5 | 0.004 | 0.504 | I |
| 0.25 | 0.250 | 1 | 0.008 | 0.258 | S |
| 0.25 | 0.250 | 2 | 0.016 | 0.266 | S |
| 0.125 | 0.125 | 4 | 0.031 | 0.156 | S |
| 0.125 | 0.125 | 8 | 0.063 | 0.188 | S |
| 0.063 | 0.063 | 16 | 0.125 | 0.188 | S |
| 0.063 | 0.063 | 32 | 0.250 | 0.313 | S |
| 0 | 128 | MICCRO | |||
A, MIC of AMP in combination with CRO; FICA, fractional inhibitory concentration of AMP; B, MIC of CRO in combination with AMP; FICB, fractional inhibitory concentration of CRO; FIC index (FICA + FICB); I, indifferent effect; S, synergistic effect.
Effect of CRO on the MIC of AMP.
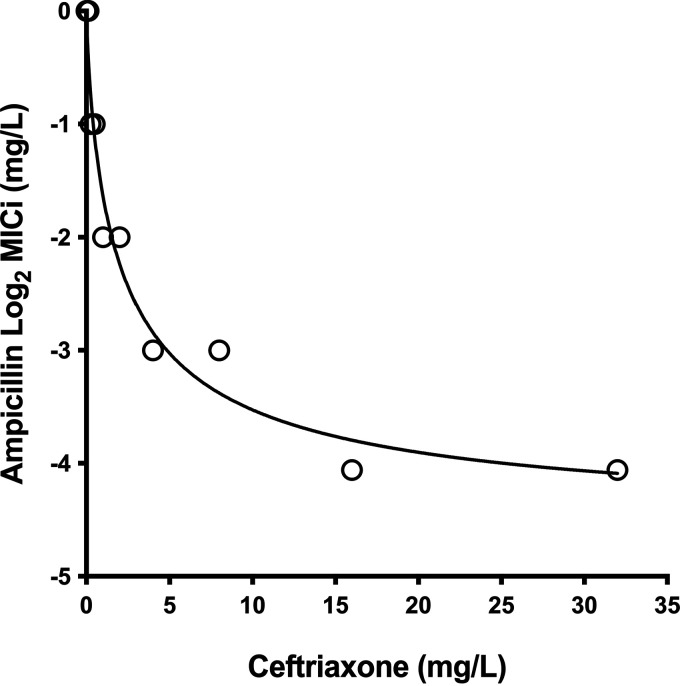
The sigmoid inhibitory Emax model characterized well the relationship between AMP susceptibility and the cephalosporin concentration (adjusted R2 = 0.92). The best-fitting parameter estimates were a MIC0 of 0.04 log2 mg/L, Emax of −4.58 log2 mg/L, Sigmoidicity coefficient (H) of 0.78, and a CRO concentration for 50% of the maximum effect (I50) of 2.13 log2 mg/L. As seen in Fig. 1, without CRO, the AMP MIC was 1 mg/L, and with the addition of CRO, it decreased gradually and reached 0.0625 mg/L with CRO at 32 mg/L (a 16-fold reduction).
FIG 1.
AMP instantaneous MIC profile. Model fitting ampicillin MIC data for E. faecalis ATCC 29212 in the presence of escalating ceftriaxone concentrations (R2 = 0.96). Open circles indicate experimental data, and the line represents the predicted MIC.
Single-dose serum PK modeling of CRO in infected mice.
The one-compartment model with linear elimination and first-order absorption described the kinetics of ceftriaxone. Table 2 presents the values of the population parameters, and Fig. S1 and S2 in the supplemental material display the time-concentration profile and observed-predicted plots. The individual and population fits were very good, with R2 of 1 and 0.91, respectively.
TABLE 2.
Parameter estimates of CRO for the final one-compartment pharmacokinetic model
| Parameter | Mean | SD | CV (%)a | Median | % shrinkage |
|---|---|---|---|---|---|
| kel (h−1) | 0.98 | 0.36 | 36.9 | 1.06 | 1.9e−11 |
| Vc (L) | 0.02 | 0.00 | 22.6 | 0.02 | 5.5e−15 |
| Ka (h−1) | 12.1 | 4.51 | 37.3 | 11.9 | 3.1e−10 |
CV, coefficient of variation.
PK/PD analysis.
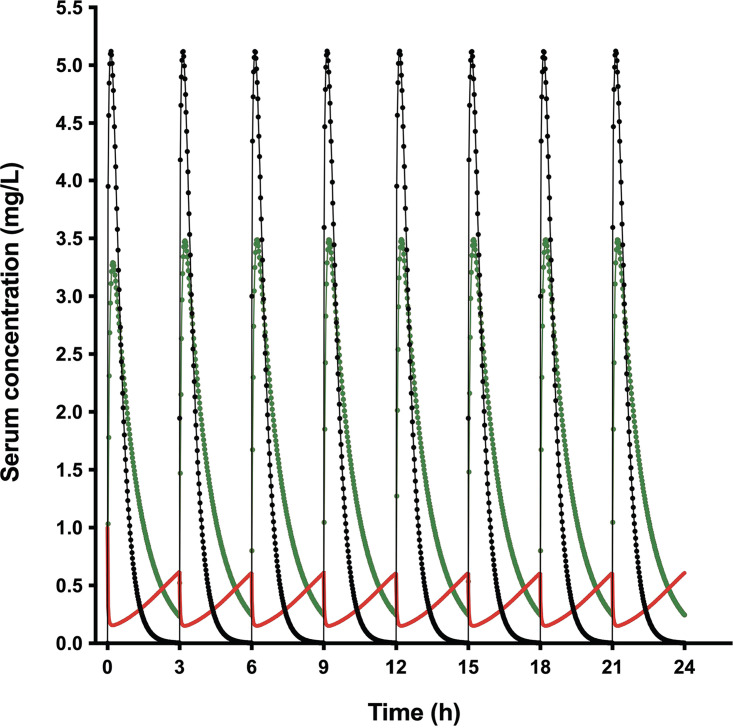
(i) MICi in vivo and T>MICi. By using the AMP and CRO pharmacokinetic models and the instantaneous MIC (MICi) Emax model, the in vivo AMP MICi profile along 24 h of treatment was simulated for all the AMP-CRO combinations, as well as the percentage of time that AMP concentration was above MICi (T>MICi) (Table S2). Figure 2 shows the pharmacokinetics and MICi profile for AMP at 150 mg/kg/day plus CRO at 25 mg/kg/day every 3 h (q3h). It can be seen that in the absence of CRO, the MIC of AMP is 1 mg/L, when CRO is at its peak, the MIC of AMP goes down to 0.22 mg/L, and when CRO is at its trough, the MIC increases to 0.73 mg/L.
FIG 2.
Simulation of murine AMP and CRO pharmacokinetics and ampicillin MICi profile. The ampicillin concentration-time profile at 150 mg/kg/day q3h (black line), ceftriaxone concentration-time profile at 25 mg/kg/day q3h (green line), and AMP MICi profile (red line) are shown.
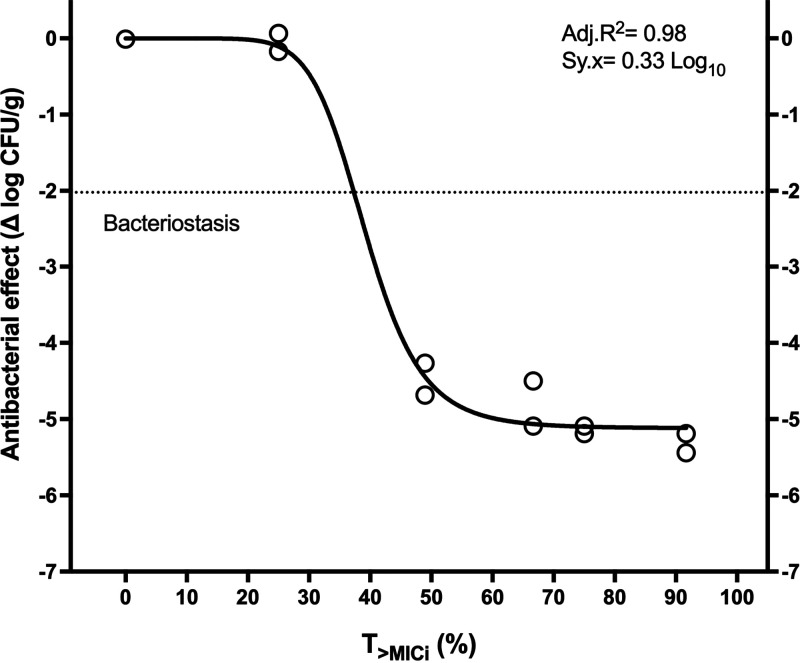
Regarding the exposure-response relationship, Hill’s model fitted well to the data and yielded significant parameters. The ampicillin T>MICi magnitudes required were 37.3% for stasis, 40.9% for 1-log10 killing, and 45.7% for 2-log10 killing (Table 3 and Fig. 3). The analysis with ceftriaxone MICi did not yield a valid regression (Fig. S3).
TABLE 3.
In vivo pharmacodynamics of AMP+CRO against E. faecalis ATCC 29212 in terms of T>MICi
| Parameter | Value (mean ± standard error [SE]) |
|---|---|
| Emax (log10 CFU/g) | 5.12 ± 0.15 |
| Exposure for stasis (%) | 37.3 ± 4.11 |
| Exposure for 1-log10 killing (%) | 40.9 ± 3.22 |
| Exposure for 2-log10 killing (%) | 45.7 ± 2.35 |
FIG 3.
In vivo pharmacodynamics of ampicillin plus ceftriaxone in terms of T>MICi against E. faecalis ATCC 29212. The exposures required were 37.3% for stasis, 40.9% for 1-log10 killing, and 45.7% for 2-log10 killing.
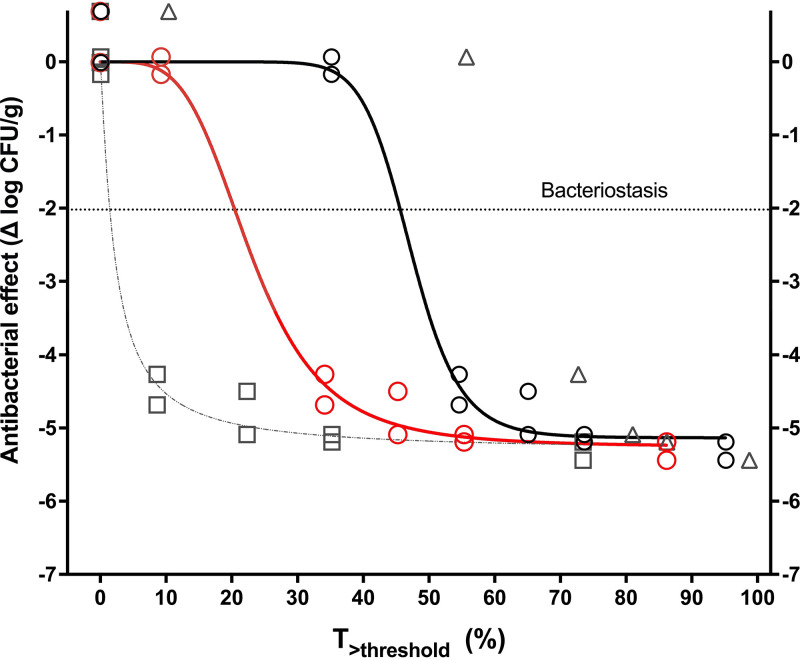
(ii) T>threshold. The times above threshold (T>threshold) of each CRO dose are shown in Table S3. Figure 4 displays the nonlinear regressions of the exposure-response relationship in terms of total T>threshold for CRO thresholds of 0.5, 1, 2, and 4 mg/L (parameter estimates and diagnostics are presented in Table 4). At a threshold of 0.5 mg/L, Hill’s equation did not fit the data. At thresholds of 1 and 2 mg/L, the regression fitted well and yielded significant parameters. At the threshold of 4 mg/L, despite a high adjusted R2, the regression was invalid, with parameters not significantly different from zero. Thus, the lowest CRO concentration with a valid regression was chosen as the threshold (1 mg/L), and the magnitudes required were 45.6% for stasis, 48.5% for 1-log10 killing, and 52.2% for 2-log10 killing. None of the regressions using the free (unbound) CRO concentrations fitted the data.
FIG 4.
Pharmacodynamics of AMP+CRO in terms of T>threshold against E. faecalis ATCC 29212. The solid lines represent a significant and valid regression with CRO thresholds of 1 mg/L (black curve and circles) and 2 mg/L (red curve and circles); the CRO threshold of 4 mg/L yielded invalid parameters (gray dotted line and squares), and Hill’s equation did not fit the CRO threshold of 0.5 mg/L (gray triangles, no curve). The chosen threshold was 1 mg/L.
TABLE 4.
Pharmacodynamics of AMP+CRO against E. faecalis ATCC 29212 in terms of T>thresholda
| CRO concn (mg/L) for T>threshold | Adj. R2 | Sy.x | Emax (log10 CFU/g) | ED50 | Required exposure (%) for: |
||
|---|---|---|---|---|---|---|---|
| Stasis | 1-log10 killing | 2-log10 killing | |||||
| 1 | 0.98 | 0.33 | 5.13 ± 0.15 | 47.2 ± 2.61 | 45.6 ± 2.30 | 48.5 ± 2.29 | 52.3 ± 1.68 |
| 2 | 0.98 | 0.31 | 5.25 ± 0.19 | 22.9 ± 3.20 | 20.4 ± 3.54 | 24.6 ± 3.00 | 30.4 ± 2.43 |
| 4 | 0.98 | 0.32 | 5.32 ± 0.24 | IP | IP | 2.79 ± 1.23 | 5.86 ± 1.64 |
Adj. R2, adjusted coefficient of determination; Sy.x, standard error of the estimate; Emax and ED50, primary pharmacodynamic parameters from Hill’s equation. T>threshold, percentage of time that CRO concentration is above a critical level or threshold; IP, invalid parameter (not different from zero). All values are presented as means and standard errors.
Human CRO pharmacokinetic analysis, Monte Carlo simulation, and probability of target attainment (PTA).
The fitting of 1- and 2-compartment models with linear elimination was compared (Table S4), with the latter exhibiting the lowest −2 log-likelihood, Akaike information criterion (AIC), and Bayesian information criteria (BIC). The medians of the parameters were as follows: elimination rate constant (kel), 0.268 h−1; volume of the central compartment (Vc), 4.022 L; transfer rate constant from the central to the peripheral compartment (Kcp), 4.465 h−1; and transfer rate constant from the peripheral to the central compartment (Kpc), 4.774 h−1. The goodness of fit and model diagnostics with individual and population observed-predicted plots are shown in Fig. S4.
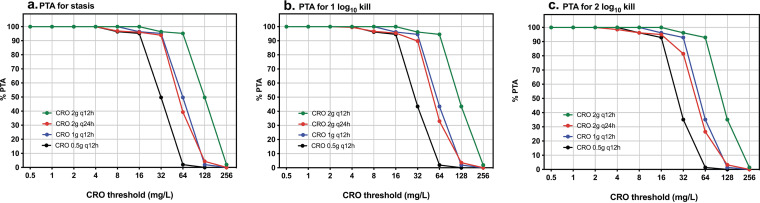
Figure 5 shows the PTA for four different CRO dosing regimens (0.5 g q12h, 1 g q12h, 2 g q12h, and 2 g q24h) for a range of thresholds from 0.5 to 256 mg/L. For stasis and 1-log10 killing, CRO at 0.5 g q12h reached a PTA of ≥90% for thresholds of ≤16 mg/L, 1 g q12h reached this PTA at ≤32 mg/L, and 2 g q12h reached it at ≤64 mg/L (Fig. 5a and b). For 2-log10 killing, the 0.5-g-q12h and 2-g-q24h doses reached the target in ≥90% of the population at thresholds of ≤16 mg/L, 1 g q12h at ≤32 mg/L, and 2 g q12h at ≤64 mg/L (Fig. 5c).
FIG 5.
Probability of target attainment of AMP+CRO against E. faecalis ATCC 29212 at CRO doses of 0.5 g q12h (black), 1 g q12h (blue), 2 g q12 h (green), and 2 g q24 h (red) for targets in terms of T>thresholds necessary for stasis (a), 1-log10 killing (b), and 2-log10 killing (c) with a range of CRO thresholds from 0.5 to 256 mg/L.
Human AMP pharmacokinetic model, MICi and fT>MICi.
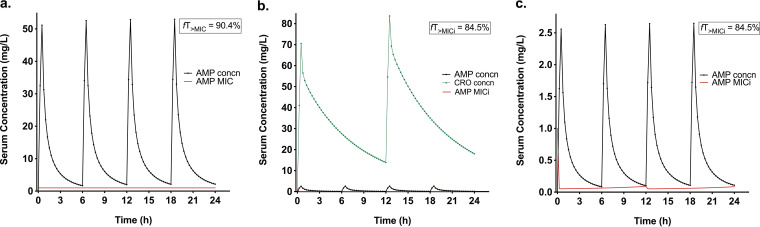
The fitting of 1- and 2-compartment models was compared (Table S5), with the latter exhibiting the lowest −2LL, AIC, and BIC. The medians of the parameters were as follows: clearance (CL), 8.17 L/h; Vc 5.77 L; intercompartmental clearance (Q), 3.8 L/h; and volume of the peripheral compartment (Vp), 4.76 L. The goodness of fit and model diagnostics with individual and population observed-predicted plots are shown in Fig. S5. The human AMP MICi profile was simulated along 24 h of treatment for AMP and AMP+CRO. The time during which the free concentration was above the instantaneous MIC (fT>MICi) for AMP at 500 mg q6h was 90.4%, and that for AMP at 25 mg q6h plus CRO at 500 mg q12h was 85.8%. These magnitudes are very close, indicating that in combination, a smaller dose of AMP could achieve the same exposure. Figure 6 shows the human AMP pharmacokinetics and MICi profile with and without CRO. In the absence of CRO, the MICi of AMP is 1 mg/L all the time (Fig. 6a), and when CRO is present, the MIC of AMP is reduced to 0.05 to 0.09 mg/L along the 24-h treatment (Fig. 6b and c).
FIG 6.
Simulation of human ampicillin total concentration profile (black lines), ceftriaxone total concentration profile (green lines), and ampicillin MICi (red line). (a) Ampicillin at 500 mg q6h; (b and c) ampicillin at 25 mg q6h + CRO at 500 mg q12h. Panel c is a closeup of panel b in the 0-to-2.5-mg/L concentration range for better visualization.
DISCUSSION
Little is known about AMP+CRO PK/PD relationships, and consequently, dose optimization remains a challenge, because traditional PK/PD indices are not directly applicable to antimicrobial combinations. To identify the PK/PD index that drives the efficacy of antibiotic combinations, it is necessary first to determine their in vivo effect and to characterize the interaction. Our laboratory previously showed that the AMP+CRO combination against E. faecalis is a case of potentiation (10). Extending from this framework and going beyond dose-response characterization, the objective of this study was to integrate pharmacokinetic information with the exposure-response relationship in terms of two novel PK/PD indices: T>threshold and T>MICi.
CRO increases AMP activity against E. faecalis as a susceptibility-modifying agent by lowering its MIC (13, 14), as in the case of βL/βLI combinations. To define the pharmacodynamics of βL/βLI, a new pharmacodynamic index was introduced based on the notion of a threshold or critical concentration (CT) (15). This value represents the lowest βLI concentration required to inhibit β-lactamases and restore the antibacterial activity of the partner β-lactam (14), with the index T>threshold driving the efficacy of several βL/βLI combinations (16–18). A PK/PD in vitro study with ceftolozane plus tazobactam found that the time above threshold (T>threshold) was the best index to predict the efficacy compared to area under the concentration-time curve (AUC) and maximum concentration (Cmax) (17). Louie et al. (19) reported that in a hollow-fiber model, T>threshold rather than AUC/threshold is the critical PD variable dynamically linked to cell killing and resistance suppression with ceftaroline plus avibactam against Enterobacter cloacae and Klebsiella pneumoniae. Furthermore, Rodriguez et al. (11), using the neutropenic mouse thigh infection model, found that the PK/PD index that best correlates with the piperacillin-tazobactam effect was the time the free inhibitor concentration was above the threshold (fT>threshold).
In the current study, the total and unbound CRO concentrations (protein binding is ~95% in humans and 90% in mice) (20) were used to analyze the threshold exposure-response relationships, and only the total concentration yielded significant results. Although it is recognized that the free fraction of an antibiotic is the one responsible for the antibacterial activity, the bound fraction in the case of CRO also contributes to it (20). Elkhaili et al. (21) described two binding sites for CRO in albumin, one with high affinity and low capacity and the other with low affinity and high capacity. The latter is labile and allows CRO to easily unbind and interact with PBPs, for which it has higher affinity.
To our knowledge, this is the first in vivo report of the PK/PD index T>threshold for the AMP+CRO combination against E. faecalis and the required magnitudes for bacteriostasis, 1-log10 killing, and 2-log10 killing. To put these results in a clinical context, the CRO standard human dose of 1 g q12h would attain the PD target for 2-log10 killing in combination with AMP in >90% of patients when the infection is due to strains with a CRO threshold of ≤32 mg/L, and the dose of 2 g q12h would attain it for thresholds of ≤64 mg/L (Fig. 5c), not different from a continuous infusion of 4 g/24 h (Fig. S6). Once-daily dosing (2 g q24h), a commonly used interval, reached the target only for thresholds of ≤16 mg/L (Fig. 5c). It should be noted that the potentiation by CRO occurs in the middle region of the AMP dose-response curve (10) and the cephalosporin would not add to the antibacterial effect if AMP is maximally effective in monotherapy, as would happen at the clinical doses up to 2,000 mg q4h for susceptible strains (MIC ≤ 4 mg/L, 98% of isolates according to the EUCAST distribution) (22). However, the addition of CRO at a dose based on the strain threshold would allow the use of smaller doses of AMP for susceptible isolates or treatment of resistant strains with MICs up to 64 mg/L with AMP at the maximal dose (10) (Table S2).
Another PD approach evaluated here attempted to reconstruct the AMP MIC as an instantaneous MIC dependent on CRO concentration, based on the modeling framework proposed initially by Bhagunde et al. (9) for relebactam in combination with imipenem and then extended to ceftazidime-avibactam and piperacillin-tazobactam. The model showed that intermittent dosing with the βLI results in a fluctuating MIC over time of the partner βL, and the efficacy of the combination was linked to the time above the instantaneous MIC (T>MICi) (9, 23, 24). Although the mechanism by which βLI reduce the MIC is different from that described for CRO (saturation of multiple PBPs: AMP binds to PBPs 4 and 5 and CRO to PBPs 2 and 3, leading to increased effect) (12), there is a similar trend of AMP susceptibility fluctuation in vivo in the presence of CRO (Fig. 2). This is the first report showing that the index T>MICi drives the efficacy of the AMP+CRO combination and that the required exposures for net bacterial stasis and 1- and 2-log10 killing are approximately 37%, 41%, and 45%, respectively. These magnitudes are very similar to those obtained with AMP monotherapy in terms of T>MIC (10) (Fig. S7), confirming that the necessary duration of AMP exposure above the MIC is the same when the MIC is fixed (as in monotherapy) or variable (when combined with CRO); however, in combination, a lower dose of AMP is required to reach the same exposure (10). In the clinical context, the standard AMP dose of 500 mg q6h yields a fT>MIC of 90% against strains with an MIC of 1 mg/L, leading to maximal efficacy; in combination with CRO at 500 mg q12h, the same exposure and effect could be obtained with a smaller AMP dose of 25 mg q6h, illustrating the potential of the combination to attain the pharmacodynamic targets with strains exhibiting higher AMP MICs.
There are some limitations to our study. First, the practical application in clinical microbiology laboratories is difficult, because the estimation of the instantaneous MIC requires a checkerboard assay, which is technically complex and not routinely available. On the other hand, determining the threshold involves experiments with multiple doses, feasible only in research laboratories. Second, only one strain of E. faecalis was tested, and the inclusion of additional strains with different AMP and CRO susceptibilities is warranted to expand the results in future studies. Third, considering that the AMP+CRO combination is the standard treatment for E. faecalis infective endocarditis, this PK/PD approach would merit testing in an endocarditis animal model. Fourth, the maximum dose of CRO tested in the mouse model (200 mg/kg/day) was ineffective in monotherapy (time above the CRO MIC of 128 mg/L of 0%); however, for a human CRO dose of 2 g q12h, the T>MIC would be 47.6%, achieving antimicrobial effect and potentially changing the type of interaction with AMP. This hypothesis warrants studies with higher doses of CRO. Finally, the estimated exposures and effects were derived from the 24-h mouse thigh infection model; although this model has been shown to accurately predict the therapeutic response in humans (3), the specific results in E. faecalis infections require further validation in the clinical setting.
In conclusion, we demonstrated that the AMP+CRO combination effect is linked to the pharmacodynamic indices T>threshold and T>MICi. These results can be used as the basis to design clinical trials of combination therapy, especially in nonendocardial enterococcal infections due to strains with higher MICs.
MATERIALS AND METHODS
Bacterial strains, antibiotics, and susceptibility testing.
Enterococcus faecalis ATCC 29212 was used. The experiments were done with AMP (Ampicilina; Genfar, Colombia) and CRO (Rocephin; Roche, Switzerland). As an AMP innovator is not available in Colombia, the in vivo activity of the generic Genfar product was compared to innovator (brand name) ampicillin-sulbactam (Unasyn, Pfizer, Switzerland) against β-lactamase negative enterococci and found to be equivalent in efficacy and potency (10). The MICs of AMP and CRO were determined by broth microdilution in duplicate, and determinations were repeated independently three times following CLSI methods, testing AMP from 0.125 to 64 mg/L and CRO from 4 to 2054 mg/L (25).
In vitro antibiotic synergy testing.
The susceptibility of E. faecalis to AMP was assessed in the presence of escalating concentrations of CRO. The checkerboard assay was used to evaluate the in vitro pharmacodynamic interactions following the CLSI recommendations (26). The assays were performed in duplicate in a 96-well plate; AMP at concentrations of 0, 0.031, 0.063, 0.125, 0.25, 0.5, 1, and 2 mg/L were tested with CRO at concentrations of 0, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, and 32 mg/L, generating 80 combinations. The interactions were defined by the FIC index according to the following equation:
| (1) |
where FIC is the fractional inhibitory concentration, A is the MIC of drug A in combination with B, MICA is the MIC of drug A alone, B is the MIC of drug B in combination with A, and MICB is the MIC of drug B alone. The combination is synergistic when the FIC index is ≤0.5, indifferent when it is >0.5 to <2, and antagonistic when it is ≥2 (27).
CRO microbiological assay.
A glass plate of 36 by 36 cm was used to allow the simultaneous run of all the samples (28). Difco antibiotic medium no. 1 (Becton Dickinson, USA) was seeded with Staphylococcus aureus ATCC 6538P (29). The standard curve consisted of 8 concentrations of CRO serially diluted 1:2 in neutropenic mouse serum (from 2 to 256 mg/L). CRO concentrations plotted against the diameters of their respective inhibition zones produced standard curves by linear regression (Prism 7; GraphPad Software, Inc., USA).
Mice.
For pharmacokinetics studies, murine-pathogen-free Swiss albino mice of the strain Udea:ICR(CD-2) were used. Animals were maintained in the pathogen-free vivarium of the University of Antioquia and housed in a one-cage system (Lab Products, USA). They were fed and watered ad libitum and kept under controlled temperature (20°C and 25°C) and lighting (12-h day-night cycles) conditions. Animals were allocated to treatment or control groups. When an animal reached the inability to obtain food or water or showed no response to gentle stimuli or a moribund state before the end of the experiment, it was sacrificed immediately under isoflurane sedation. This study was approved by the University of Antioquia Animal Experimentation Ethics Committee (9 July 2015 session). Additionally, we followed the national guidelines for biomedical research (resolution 008430 of 1993, articles 87 to 93 by the Colombian Health Minister), the ARRIVE guidelines, and the International Guiding Principles for Biomedical Research involving animals.
Single-dose serum PK of CRO in infected mice.
Two hours after infection with E. faecalis ATCC 29212, two groups of 16 neutropenic mice received a single subcutaneous injection (0.2 mL) containing a dose of CRO of 25 or 100 mg/kg. Two mice from each group were bled by a submandibular puncture at 5, 15, 30, 45, 60, 120, and 150 min postdose (30). Serum was obtained by blood centrifugation at 13,000 × g for 10 min, and serum samples of 10 μL were plated immediately in the agar plate in duplicate for the microbiological assay. It was stored at 4°C for 2 h (prediffusion), followed by incubation under an air atmosphere at 35°C for 18 h. Then, inhibition zones were measured with electronic calipers (Mitutoyo Corp., Japan). Inhibition zones were interpolated by linear regression against the standard curve to determine the CRO concentration (Prism 7; GraphPad Software, Inc., USA). The primary PK parameters were computed by the nonparametric adaptive grid (NPAG) algorithm fitting a one-compartment model (Pmetrics; Laboratory of Applied Pharmacokinetics and Bioinformatics, University of Southern California, USA) (31).
For AMP, a two-compartment pharmacokinetic model in infected mice estimated by nonparametric techniques was used (32). The medians of the parameters were as follows: kel, 20.4 h−1; Vc, 0.007 L; Kcp 78.2 h−1; Kpc, 15.0 h−1; and absorption rate constant (Ka), 6.45 h−1 (10, 32).
In vivo pharmacodynamic data.
Previously we obtained the dose-response relationship of AMP+CRO against E. faecalis in the optimized neutropenic murine thigh infection model. AMP doses from 9.375 to 2,400 mg/kg/day combined with fixed CRO doses from 3.125 to 200 mg/kg/day, divided and given q3h, were tested (10). From the data set, the following AMP+CRO doses (in milligrams per kilogram per day) ranging from minimal to maximal effects were used to obtain a sigmoid curve: AMP 2,400 + CRO 200, AMP 1,200 + CRO 50, AMP 600 + CRO 37.5, AMP 150 + CRO 25, AMP 37.5 + CRO 12.5, and AMP 9.375 + CRO 3.125. Table S1 shows the AMP+CRO combinations and their corresponding effects.
PK/PD analysis.
(i) MICi. The AMP MIC in the presence of changing CRO concentrations was characterized using a modified sigmoid maximum effect (Emax)-type model (9), as follows:
| (2) |
where MIC is the AMP MIC in the presence of CRO, MIC0 is the intrinsic AMP MIC, I is the CRO concentration, Emax is the maximum CRO effect, H is the sigmoidicity coefficient, and I50 is the CRO concentration for 50% of the maximum effect (9). The best-fit parameter estimates were then used to simulate the MICi profile as a function of the CRO concentration.
(ii) T>MICi. For each AMP+CRO combination, the time the total concentration of ampicillin was above the MICi (AMP T>MICi) was determined using the mouse AMP and CRO PK models and the MICi Emax model. The CRO T>MICi was also estimated. The total AMP concentration was used considering that the protein binding in mice is negligible, only 3% (32, 33). Finally, Hill’s model was fitted to the data to estimate Emax, ED50, N, and the exposures required for stasis, 1-log10 killing, and 2-log10 killing (Prism 7; GraphPad Software, Inc., USA).
(iii) T>threshold. For each dose of CRO, the time the total and free antibiotic concentrations (protein binding in mice is 90%) (34, 35) were above a range of thresholds from 0.25 to 16 mg/L (CRO T>threshold) was calculated using the mouse PK model in Pmetrics. Finally, Hill’s model was fitted to the exposure-response data to estimate the primary and secondary PD parameters. The best-fitting regression that yielded significant parameters with the lowest CRO concentration was used to select the threshold (Prism 7; GraphPad Software, Inc., USA).
Human AMP and CRO pharmacokinetics, Monte Carlo simulation, PTA and MICi.
To put the animal data in a clinical context, we developed nonparametric pharmacokinetic models for CRO and AMP in humans with published time-concentration profiles (36–38). CRO doses of 500 mg q12h, 1,000 mg q12h, 2,000 mg q12h, and 2,000 mg q24h (as 30-min intravenous infusions), and 2,000 and 4,000 mg/day in continuous infusion were simulated, and the probability of T>threshold target attainment (PTA) for stasis, 1-log10 killing, and 2-log10 killing was estimated for thresholds from 0.25 to 254 mg/L in a 5,000-patient population. For AMP, doses of 25 mg q6h and 500 mg q6h as 30-min intravenous infusions were simulated to obtain the human AMP MICi profile and the fT>MICi (protein binding of 20%) in monotherapy or combined with CRO at 500 mg q12h.
ACKNOWLEDGMENTS
We thank the Infectious Diseases Problems Research Group (GRIPE) for their participation during the experimental procedures, data analysis, and discussion. Also, we thank the Integrated Laboratory of Specialized Medicine (LIME) and Corporación Ciencias Básicas Biomédicas of the University of Antioquia for their academic support.
This project was funded by Minciencias 785 National Ph.D. Scholarship 2017 (I.J.-T.) (www.minciencias.gov.co); Sistema General de Regalías, project BPIN: 2020000100152; and the University of Antioquia (www.udea.edu.co). The funders had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
C.A.R. has received honoraria for lectures from Allergan, Biosidus, Novartis and Pfizer, unrelated to this research project. A.F.Z. has received honoraria for advisory boards and lectures not related to the content of this paper from Allergan, Amgen, Janssen, Lilly, Merck, Novartis, Novo Nordisk, Pfizer, Roche, and Sanofi. None of these companies or any other were involved in the design, execution, or publication of this study. I.J.-T., J.D.O., H.P.-M., and O.V. have declared that no conflicts of interest exist.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Andes DR, Lepak AJ. 2017. In vivo infection models in the pre-clinical pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Curr Opin Pharmacol 36:94–99. 10.1016/j.coph.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents 19:261–268. 10.1016/S0924-8579(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Antimicrobial resistance: pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis 44:79–86. 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- 4.Brill MJE, Kristoffersson AN, Zhao C, Nielsen EI, Friberg LE. 2018. Semi-mechanistic pharmacokinetic-pharmacodynamic modelling of antibiotic drug combinations. Clin Microbiol Infect 24:697–706. 10.1016/j.cmi.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Couet W. 2018. Pharmacokinetics/pharmacodynamics characterization of combined antimicrobial agents: a real challenge and an urgent need. Clin Microbiol Infect 24:687–688. 10.1016/j.cmi.2018.03.047. [DOI] [PubMed] [Google Scholar]
- 6.Foucquier J, Guedj M. 2015. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect 3:e00149. 10.1002/prp2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thieme L, Hartung A, Makarewicz O, Pletz MW. 2020. In vivo synergism of ampicillin, gentamicin, ceftaroline and ceftriaxone against Enterococcus faecalis assessed in the Galleria mellonella infection model. J Antimicrob Chemother 75:2173–2181. 10.1093/jac/dkaa129. [DOI] [PubMed] [Google Scholar]
- 8.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 9.Bhagunde P, Chang KT, Hirsch EB, Ledesma KR, Nikolaou M, Tam VH. 2012. Novel modeling framework to guide design of optimal dosing strategies for beta-lactamase inhibitors. Antimicrob Agents Chemother 56:2237–2240. 10.1128/AAC.06113-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jimenez-Toro I, Rodriguez CA, Zuluaga AF, Otalvaro JD, Vesga O. 2020. A new pharmacodynamic approach to study antibiotic combinations against enterococci in vivo: application to ampicillin plus ceftriaxone. PLoS One 15:e0243365. 10.1371/journal.pone.0243365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez CA, Agudelo M, Zuluaga AF, Vesga O. 2017. In vivo pharmacodynamics of piperacillin/tazobactam: implications for antimicrobial efficacy and resistance suppression with innovator and generic products. Int J Antimicrob Agents 49:189–197. 10.1016/j.ijantimicag.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Mainardi JL, Gutmann L, Acar JF, Goldstein FW. 1995. Synergistic effect of amoxicillin and cefotaxime against Enterococcus faecalis. Antimicrob Agents Chemother 39:1984–1987. 10.1128/AAC.39.9.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beganovic M, Luther MK, Rice LB, Arias CA, Rybak MJ, LaPlante KL. 2018. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin Infect Dis 67:303–309. 10.1093/cid/ciy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman K, Levasseur P, Girard AM, Borgonovi M, Miossec C, Merdjan H, Drusano G, Shlaes D, Nichols WW. 2014. Activities of ceftazidime and avibactam against beta-lactamase-producing Enterobacteriaceae in a hollow-fiber pharmacodynamic model. Antimicrob Agents Chemother 58:3366–3372. 10.1128/AAC.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sy SKB, Derendorf H. 2018. Experimental design and modelling approach to evaluate efficacy of beta-lactam/beta-lactamase inhibitor combinations. Clin Microbiol Infect 24:707–715. 10.1016/j.cmi.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Berkhout J, Melchers MJ, van Mil AC, Seyedmousavi S, Lagarde CM, Schuck VJ, Nichols WW, Mouton JW. 2016. Pharmacodynamics of ceftazidime and avibactam in neutropenic mice with thigh or lung infection. Antimicrob Agents Chemother 60:368–375. 10.1128/AAC.01269-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanScoy B, Mendes RE, Nicasio AM, Castanheira M, Bulik CC, Okusanya OO, Bhavnani SM, Forrest A, Jones RN, Friedrich LV, Steenbergen JN, Ambrose PG. 2013. Pharmacokinetics-pharmacodynamics of tazobactam in combination with ceftolozane in an in vitro infection model. Antimicrob Agents Chemother 57:2809–2814. 10.1128/AAC.02513-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh R, Kim A, Tanudra MA, Harris JJ, McLaughlin RE, Patey S, O’Donnell JP, Bradford PA, Eakin AE. 2015. Pharmacokinetics/pharmacodynamics of a beta-lactam and beta-lactamase inhibitor combination: a novel approach for aztreonam/avibactam. J Antimicrob Chemother 70:2618–2626. 10.1093/jac/dkv132. [DOI] [PubMed] [Google Scholar]
- 19.Louie A, Castanheira M, Liu W, Grasso C, Jones RN, Williams G, Critchley I, Thye D, Brown D, Vanscoy B, Kulawy R, Drusano GL. 2012. Pharmacodynamics of beta-lactamase inhibition by NXL104 in combination with ceftaroline: examining organisms with multiple types of beta-lactamases. Antimicrob Agents Chemother 56:258–270. 10.1128/AAC.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garot D, Respaud R, Lanotte P, Simon N, Mercier E, Ehrmann S, Perrotin D, Dequin PF, Le Guellec C. 2011. Population pharmacokinetics of ceftriaxone in critically ill septic patients: a reappraisal. Br J Clin Pharmacol 72:758–767. 10.1111/j.1365-2125.2011.04005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkhaili H, Zachary P, Linger L, Gallion C, Monteil H, Jehl F. 2001. Bactericidal activity of bound ceftriaxone against Streptococcus pneumoniae. Med Mal Infect 31:410–416. 10.1016/S0399-077X(01)00212-8. [DOI] [Google Scholar]
- 22.EUCAST. Antimicrobial wild type distributions of microorganisms. https://mic.eucast.org/. Accessed 5 November 2022.
- 23.Zidaru A, Eales BM, Wang W, Merlau PR, Lasco TM, Sofjan AK, Tam VH. 2020. MIC profiling of ceftazidime/avibactam against two carbapenemase-producing Klebsiella pneumoniae isolates. J Glob Antimicrob Resist 23:385–387. 10.1016/j.jgar.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abodakpi H, Chang KT, Gao S, Sanchez-Diaz AM, Canton R, Tam VH. 2019. Optimal piperacillin-tazobactam dosing strategies against extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 63:e01906-18. 10.1128/AAC.01906-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CLSI. 2022. Performance standards for antimicrobial susceptibility testing. 32nd ed. CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.CLSI. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. CLSI document M26-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 27.Jiménez-Toro I, Rodriguez CA, Zuluaga AF. 2019. Eficacia de las combinaciones antibióticas para el tratamiento de infecciones enterocócicas: una revisión crítica. Rev Chilena Infectol 36:556–564. 10.4067/S0716-10182019000500556. [DOI] [PubMed] [Google Scholar]
- 28.Zuluaga AF, Agudelo M, Rodriguez CA, Vesga O. 2009. Application of microbiological assay to determine pharmaceutical equivalence of generic intravenous antibiotics. BMC Clin Pharmacol 9:1. 10.1186/1472-6904-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manfio M, Agarrayua D, Machado J, Schmidt C. 2013. A fully validated microbiological assay to evaluate the potency of ceftriaxone sodium. Braz J Pharmaceut Sci 49:753–762. 10.1590/S1984-82502013000400015. [DOI] [Google Scholar]
- 30.Golde WT, Gollobin P, Rodriguez LL. 2005. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 34:39–43. 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 31.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez CA, Agudelo M, Gonzalez JM, Vesga O, Zuluaga AF. 2015. An optimized mouse thigh infection model for enterococci and its impact on antimicrobial pharmacodynamics. Antimicrob Agents Chemother 59:233–238. 10.1128/AAC.02352-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Ogtrop ML, Mattie H, Sekh BR, van Strijen E, van Furth R. 1992. Comparison of the antibacterial efficacies of ampicillin and ciprofloxacin against experimental infections with Listeria monocytogenes in hydrocortisone-treated mice. Antimicrob Agents Chemother 36:2375–2380. 10.1128/AAC.36.11.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frimodt-Moller N, Bentzon MW, Thomsen VF. 1987. Experimental pneumococcus infection in mice: comparative in vitro and in vivo effect of cefuroxime, cefotaxime and ceftriaxone. Acta Pathol Microbiol Immunol Scand B 95:261–267. 10.1111/j.1699-0463.1987.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 35.Moine P, Vallee E, Azoulay-Dupuis E, Bourget P, Bedos JP, Bauchet J, Pocidalo JJ. 1994. In vivo efficacy of a broad-spectrum cephalosporin, ceftriaxone, against penicillin-susceptible and -resistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob Agents Chemother 38:1953–1958. 10.1128/AAC.38.9.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel IH, Chen S, Parsonnet M, Hackman MR, Brooks MA, Konikoff J, Kaplan SA. 1981. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother 20:634–641. 10.1128/AAC.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergan T. 1978. Pharmacokinetic comparison of oral bacampicillin and parenteral ampicillin. Antimicrob Agents Chemother 13:971–974. 10.1128/AAC.13.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyers BR, Wilkinson P, Mendelson MH, Walsh S, Bournazos C, Hirschman SZ. 1991. Pharmacokinetics of ampicillin-sulbactam in healthy elderly and young volunteers. Antimicrob Agents Chemother 35:2098–2101. 10.1128/AAC.35.10.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.00966-22-s0001.pdf, PDF file, 1.0 MB (1MB, pdf)