Abstract
Objective:
Given estrogen’s role in HIV disease progression and the higher rates of neurocognitive decline in affected women, the purpose of this study was to assess whether the relationship of white matter features and reproductive hormone levels differed between men versus women (sex as a moderator), controlling for selected cardiometabolic risk factors, HIV-related health indicators, and demographics in an aging population of persons living with HIV (PLWH).
Methods:
Older PLWH (ages 50 years and older; 44 women and 35 men; mean age 59.8±0.6 years, 55.7% women, 72.2% non-Hispanic black) participated in a cross-sectional study involving a fasting blood draw and a demographic survey (visit 1) and an MRI scan (visit 2) to determine white matter volume and white matter hyperintensity volume (WMH). Associations between reproductive hormones (FSH, E2, T, DHEA-S) and white matter features were assessed in linear regression models. Covariates were age, BMI, hypertension, diabetes, dyslipidemia, current smoking status, CD4 count, and cranial size.
Results:
For white matter volume, a sexually-dimorphic interaction was seen for DHEA-S (B=21.23; p-value = 0.012) and observed for FSH (B=−22.97, p-value=0.08) with a trend for significance after controlling for risk factors. In women, higher white matter volume was associated with higher DHEAS (B=13.89, p-value=0.017) and lower FSH (B=23.58, p-value=0.01). No hormone associations were shown in men for white matter volume. For WMH volume, no significant interaction effects between sex and reproductive hormones were identified. For WMH, sex did not predict associations with reproductive hormones after controlling for risk factors.
Conclusions:
Although sexually-dimorphic interactions of reproductive hormones and total white matter volume were demonstrated, our study findings do not support a role for sex-based differences in reproductive hormones as predictive correlates of WMH in a small sample of older PLWH.
Keywords: HIV, sex differences, reproductive hormones, white matter disease, Alzheimer’s Disease
INTRODUCTION
As PLWH in the US are aging, the clinical manifestations of accelerated brain deterioration are being seen. Despite some limited study of white matter hyperintensities (WMH) among PLWH showing a link with aging and longer duration of HIV infection, sex differences have not been examined.1 This lack of attention is notable given evidence of sexual dimorphism in HIV infection and viremia due to anatomical, immunological, and hormonal distinctions between men and women,2 with women generally having lower viral loads and greater immune activation in response to HIV compared with men.3 Moreover, sex differences have been consistently found in WMH features among non-HIV populations.4–6
Reproductive hormones, especially estrogens, play a crucial role in HIV disease progression and latency. Estradiol (E2) reduces the susceptibility of target cells like CD-4 T-cells and macrophages to HIV infection,7 and may regulate HIV-1 transcription in both sexes.8–10 While the extent of sex differences in HIV infection and comorbidity are not fully understood, women living with HIV have a heightened risk of cerebrovascular disease compared to men living with HIV.2,11
White matter lesions commonly observed as incidental hyperintensities (WMH), in the magnetic resonance imaging (MRI) scans of older adult brains4 are considered a biomarker of cerebral small vessel disease including stroke and dementia.12,13 Evidence obtained from normal aging populations suggests that there are significant sex differences in features of WMH.4,5 Despite higher prevalence of risk factors for cerebrovascular disease among men, such as hypertension and diabetes, WMH is consistently found at higher volumes as a proportion of total white matter volume in women compared to men, the basis of which is not understood.4–6
Further, there is epidemiologic evidence to support the protective effects of estrogen on cerebrovascular diseases, such as stroke and dementia, via a reduction in vascular reactivity and an increase in blood flow. Nonetheless, most of the limited research on the relationship between reproductive hormones and WMH has focused on menopausal women – a time of estrogen decline. In two major randomized, placebo-controlled trials that included elderly women in the first14 and younger, recently menopausal women in the second15, the use of menopause hormone therapy (MHT) as a predictor of WMH was assessed. In the Women’s Health Initiative Memory Study (WHIMS), no evidence was found to support that MHT exposure increased ischemic lesions, which included white matter lesions.14 In the Kronos Early Estrogen Prevention (KEEPS) trial, 48 months of MHT with either transdermal estradiol or oral conjugated estrogens (both pulsed with progesterone) failed to prevent increases in WMH vs placebo.15 However, in both hormone-treated groups, less adverse changes in WMH (vs. placebo) were associated with differential responses in FSH and estrogen compared to baseline. Changes in LH were not associated with changes in WMH.15 Other studies examining post-menopausal women have reported that hormone treatment reduces the number and size of WMH except in women over age 70.16,17 In the MsFlash study assessing the relationship between hot flashes and brain health among midlife women without cardiovascular disease (CVD), more hot flashes during sleep were associated with greater WMH, controlling for age, race, and body mass index (β [SE] = 0.0002 [0.0001], P = 0.03] although confirmatory hormones measures were not assessed.18 Results suggest that the relationship between estrogen deficiency symptoms and adverse health effects observed in the periphery may extend to the brain.18
Similarly, testosterone may be associated with cardiovascular and cerebrovascular degeneration in older adults.19 Studies of the role of endogenous testosterone in ischemic cerebrovascular disease, have revealed mixed findings. In a Honolulu-Asia Aging Study involving a cohort of 2197 men aged 71 to 93 years free of stroke, coronary heart disease, and cancer, those with elevated 17β estradiol values in the top quintile experienced a twofold excess risk of stroke compared to men in lower quintiles, whereas no risk of stroke was related to testosterone in this population.20 In an analysis of data from the Atherosclerosis Risk in Communities (ARIC) study, a prospective cohort study of men and women aged 45 to 64 years, researchers obtained a sample size of 1,558 male participants and assessed the relationship between endogenous testosterone and cerebrovascular events operationalized via measurement of plasma testosterone and percent WMH.21 Among men in the study without previous cardiac disease, stroke or androgen exposure, researchers observed a significant association between lower testosterone and incident stroke events and percent WMH; however, after multivariable adjustment, the association was no longer significant.21 Similarly, a cross-sectional retrospective cohort study that assessed the medical records of 342 Korean male patients aged 50 and older showed no significant relationship between total testosterone and WMH.19
Despite the growing but still sparse literature on the relationship between reproductive hormones and WMH, there is little study of these factors in persons living with HIV (PLWH). Investigating the endogenous reproductive hormone milieu as a predictor of differences in white matter abnormalities among aging HIV-infected individuals may provide insight into possible sex-based disparities in the development or progression of cerebrovascular diseases. Therefore, the goal of this study was to assess whether the relationship of white matter features and reproductive hormone levels in an aging population living with HIV differed by sex (men versus women; moderator) controlling for selected cardiometabolic risk factors, HIV-related health indicators, and other covariates We hypothesized that due to known sex differences in the aging reproductive hormone milieu and HIV immunopathology, older women living with HIV would demonstrate stronger relationships between measures of white matter disease and reproductive hormone levels when compared to their male HIV-positive counterparts controlling for covariates including age, cardiometabolic risk factors, and HIV-related health indicators.
METHODS
Study participants were recruited from a research volunteer registry comprising individuals who had participated in the VIP-HANA (Video Information Provider – HIV-Associated Non-AIDS) study (ClinicalTrials.gov Identifier: NCT03182738) and consented to be reached for future studies. Study flyers were also posted at HIV-related community organizations and clinics across New York City, allowing for snowball sampling as enrolled participants distributed flyers among their peers. Informed consent was obtained from all participants and all study protocols were reviewed and approved by the Institutional Review Board of Columbia University Irving Medical Center. Data were collected between January 2019 and July 2019.
Inclusion and exclusion criteria
Participants were enrolled if they were HIV-positive, 50 years or older currently taking antiretroviral therapy, able to safely undergo MRI, comfortable using a tablet to complete a survey, and able to communicate and read in English. To assess capacity to consent during screening, participants must have also received a total error score of 20 or less on the Short Orientation Memory Concentration Test (SOMCT). Participants were excluded if they were pregnant or still menstruating in the last 12 months, currently taking exogenous hormones, did not meet eligibility criteria for MRI screening, or scored greater than 20 on the SOMCT.
Procedures
Eligible volunteers participated in a cross-sectional study consisting of two in-person study visits; one visit included a fasting blood draw, and a demographic survey; the MRI scan was performed during a separate appointment. Both visits were scheduled about a week apart and each study volunteer provided written informed consent at their initial visit. Participant demographic data, vascular risk factors (Diabetes mellitus, hypertension, and dyslipidemia), and history of CD4 count and viral load were self-reported on the questionnaire and verified by chart review if local medical records were available. All viral load data was verified by chart review. A subset of the participants (N=32) cardiovascular risk factors were available for verification via the medical record. As sex steroids decline markedly in both men and women with aging, we measured estradiol and testosterone in all individuals as well as dehydroepiandrosterone sulphate (DHEA-S), their adrenal androgen precursor. FSH was measured as the primary biomarker of menopause. Total white matter volume and WMH were determined from MRI data. Further details on the sample collection procedures are detailed elsewhere.1
Study Instruments
Magnetic Resonance Imaging
All MRI scans were carried out at the Columbia University Zuckerman Institute on a 3T scanner with a 64-channel coil (Siemens MAGNETOM Prisma). Imaging consisted of 3D-T1 MPRAGE (voxel size 1×1×1mm, isometric, TR/TE (ms) 2300/2.26, field of view 256 mm, echo spacing 6.8 ms) and T2-weighted FLAIR (voxel size 0.45×0.45×0.90 mm, TR/TE (ms) 5000/387, field of view 230mm, echo spacing 3.62 ms). To quantify white matter volume, images were skull stripped, and a Gaussian curve was fit to map voxel intensity values22To quantify WMH volume, voxel intensity values were mapped by fitting a Gaussian distribution and all voxels 1.5 standard deviations above the mean image intensity were labeled22. False positives were edited manually, and the remaining voxels were classified as WMH. The number of labeled voxels was summed and multiplied by voxel dimensions to yield total WMH volume measures in cm^3.23
Blood Collection and Hormone Analysis
Blood samples obtained by venipuncture were collected in vacutainer gold top serum separator tubes and serum was separated and stored at −80°C until analyses. E2 and FSH immunoassays were performed on the Roche modular systems (Basel, Switzerland) in a CLIA-waived clinical research laboratory at NewYork Presbyterian Hospital.. Testosterone and DHEA-S were measured using an automated analyzer, Immulite 1000 from Siemens (Munich, Germany) in the Columbia University biomarker Core laboratory. The lower limits of detection of each assay were: 5 pg/ml for E2, 0.1 mIU/ml for FSH, 20 ng/dL for testosterone, and 15 μg/dL for DHEA-S. In blinded quality control samples, DHEA-S median for females was 170 μg/dL (range 35–430) for females and median was 280 for males (range 80–560). In blinded quality control samples, FSH median values for post-menopausal females was 26μg/dL (range: not detectable-43) and median was 317 for males over 50 years of age (range 129–767).
Statistical Analysis
We used chi-squared or Mann-Whitney tests to compare risk factors and biomarkers between women and men. We assessed the bivariate relationship between WMH and each risk factor variable stratified by sex using Spearman correlation, biserial correlation, or ranked biserial correlation coefficients depending on the distribution of the variables. We then used multivariable linear regression models to assess whether sex moderates the relationship between reproductive hormones and white matter features (volume and WMH volume) controlling for risk factors. Risk factors including participants’ age, BMI, hypertension, diabetes, dyslipidemia, current smoking status, and CD4 count were selected into the multivariable regression models if p-values of bivariate relationships between the risk factors and the corresponding outcome were lower than .20.24 Cranial size was included in final models regardless of its p-values in bivariate associations due to its clinical importance. We checked multicollinearity of variables included in the final models. As the reproductive hormone ratio variables (Ratio FSH/E2, Ratio T/E2, and Ratio DHEA-S/T) were derived from reproductive hormone values, they were removed from the final multivariable regression analyses due to high multi-collinearity (variance inflation factor>5.0). In multivariable analyses, non-normal positively-skewed continuous variables were transformed with base 2 logarithm including WMH volume, and all hormone measures. To assess whether sex moderated the relationship between hormone measures and white matter features (white matter volume and WMH volume), we first created an interaction term for each hormone measure and sex. Next, we examined the associations of white matter features with reproductive hormones by sex to determine the moderation effect of sex. Data analyses were performed with IBM SPSS Statistics version 26 or SAS version 9.4. SAS macro %biserial was used to estimate bivariate relationship between a binary variable and a ordinal or continuous variable.25 Based on the common rule, a bivariate correlation coefficient of 0.3 to 0,7 indicated a moderate relationship.26
RESULTS
Data were collected from 85 volunteers who completed all study activities. After excluding exogenous hormone users (n=6), we analyzed data from 44 women and 35 men age 50 or older and living with HIV (mean age 59.8±0.6 years, 55.7% women, 72.2% non-Hispanic black). Demographic characteristics, cardiovascular risk factors, reproductive hormone values, and white matter measures in women and men are compared in Table 1.
Table 1:
Mann-Whitney and chi-squared test results for sex differences in risk factors, reproductive hormones, and white matter pathologies.
| Women (n = 44) | Men (n = 35) | p -Value | |
|---|---|---|---|
| Demographic and risk factors | |||
| Age, y, mean (SD) | 59.7 (6.0) | 59.8 (6.3) | 0.98 |
| BMI, kg/m2, mean (SD) | 29.0 (5.9) | 27.7 (4.4) | 0.39 |
| Race (Black) n% | 34(77) | 27(77) | 0.60 |
| Hispanic n% | 9 (20) | 8(23) | 0.80 |
| ε4 carrier, n (%) | 17 (39) | 10 (29) | 0.21 |
| Current smoker, n (%) | 17 (39) | 21 (60) | 0.06b |
| Diabetes mellitus, n (%) | 5 (11) | 8 (23) | 0.17 |
| Hypertension, n (%) | 30 (68) | 21 (60) | 0.45 |
| Dyslipidemia, n (%) | 17 (39) | 12 (34) | 0.69 |
| CD4 count, cells/mm3, mean (SD) | 671.5 (361.3) | 590.3 (367.2) | 0.12 |
| Viral load, copies/Ml, mean (SD) | 6,542.2 (19,310.6) | 8,487.4 (32,081.7) | 0.99 |
| Viral load suppressed (<200 copies/Ml), n (%) | 34(77) | 26(74) | 0.76 |
| Some college education or higher, n (%) | 24 (55) | 16 (46) | 0.44 |
| Total Cranial size(cm3) | 1,356,369 (146,802) | 1,577,528(89,660) | <.0001 a |
| Alcohol Use (AUDIT-C) | 11(25) | 10(29) | 0.72 |
| Illegal substance use in the past three months: | |||
| Cannabis | 15(34) | 9(26) | 0.33 |
| Cocaine | 5(11) | 8(23) | 0.28 |
| Street opioids | 1(2) | 1(3) | 0.33 |
| Prescription opioids | 2(5) | 3(9) | 0.38 |
| Sex hormones | |||
| Estradiol (E2), pg/mL, mean (SD) | 12.2 (16.3) | 28.3 (12.7) | <0.001a |
| FSH, mIU/mL, mean (SD) | 71.3 (30.1) | 9.8 (10.4) | <0.001 a |
| Testosterone (T), ng/dL, mean (SD) | 41.6 (52.7) | 675.7 (420.9) | <0.001 a |
| DHEA-S, μg/dL, mean (SD) | 50.9 (39.9) | 82.2 (59.7) | 0.01 a |
| FSH:E2 ratio, mean (SD) | 10.6 (5.9) | 0.5 (0.8) | <0.001 a |
| T:E2 ratio, mean (SD) | 4.9 (3.4) | 23.3 (11.9) | <0.001 a |
| DHEA-S:T ratio, mean (SD) | 1.7 (1.4) | 0.4 (1.2) | <0.001 a |
| White matter measure | |||
| Total white matter volume, cm3, mean (SD) | 393.9 (51.5) | 446.9 (56.1) | <0.001 a |
| WMH volume, cm3,mean (SD) | 7.3 (7.7) | 4.9 (3.7) | 0.37 |
Note..
Identifies statistical significance with a p-value <0.05
Identifies an existing trend with a p-value <0.10
- SD =Standard deviation
- BMI = Body Mass Index
- AUDIT-C = Alcohol Use Disorders Identification Test—Consumption
- FSH = Follicle-Stimulating Hormone
- DHEA-S = Dehydroepiandrosterone Sulfate
- E2 = Estradiol
- T = Testosterone
- WMH = White Matter Hyperintensity
Most participants reported a history of hypertension (64.6%) which was verified through chart review, when the data was available. Mean ± SE CD4 count and viral load were 636±42 copies/mL and 6,592±2,880 cells/mm3, respectively. Approximately half of the sample had at least some college education, nearly half of the sample (48.1%) were current smokers, 36.7% had dyslipidemia, and 16.5% had type 2 diabetes. There were no differences in demographics or risk factors between women and men. As expected, all plasma hormone measures and hormone ratios differed by sex (p<0.01), with women exhibiting E2 and FSH profiles typical of post-menopause, and men demonstrating androgenic profiles typical of aging. Men had greater total white matter volume compared to women (p<0.001), although no significant sex differences were observed in WMH volume.
In our preliminary analysis, we used Spearman correlation and ranked biserial correlation coefficients to assess bivariate relationships between white matter features (volume and WMH volume) with cardiovascular risk factors, HIV-related indicators, and reproductive hormone values according to sex (Table 2). White matter volume was higher in those men with at least some college education (r=0.42, p-value<0.05), while the relationship was weak in women (r=0.03, p-value>0.05). Men with diabetes had lower white matter volume than men without diabetes (r=−0.48, p-value<0.01), whereas white matter volume did not differ in women with and without diabetes (r=0.11, p-value>0.05).. White matter volume was positively correlated with DHEA-S hormone levels among women (r=0.45; p<0.01) with trends for significance for both FSH/E2 and DHEA/T. No significant relationships or trends between hormones and white matter volume were observed among men.
Table 2:
Spearman correlation and Biserial correlation results for risk factors and reproductive hormone values with measures of white matter volume among older Women vs Men living with HIV (n=79)
| White matter volume, cm3 | WMH volume, cm3 | |||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Age, y | −0.29a | 0.05 | 0.40b | −0.02 |
| BMI, kg/m2 | 0.13 | −0.1 | −0.04 | −0.01 |
| Total Cranial size | 0.45b | 0.68d | −0.14 | 0.21 |
| Current smoker | −0.02 | −0.16 | −0.15 | 0.14 |
| Diabetes mellitus | 0.11 | −0.48c | −0.26 | −0.17 |
| Hypertension | −0.05 | 0.01 | −0.16 | 0.29a |
| Dyslipidemia | −0.10 | 0.07 | 0.39b | 0.30a |
| CD4 count, cells/mm3 | 0.15 | −0.01 | 0.08 | 0.18 |
| Viral load, copies/mL | −0.17 | 0.11 | −0.05 | 0.11 |
| Some college education or higher | 0.03 | 0.42b | −0.08 | −0.02 |
| E2, pg/mL | 0.19 | 0.11 | −0.2 | 0.07 |
| FSH, mIU/mL | −0.15 | 0.18 | −0.12 | 0.26 |
| T, ng/dL | 0.29a | 0.19 | −0.14 | −0.08 |
| DHEA-S, μg/dL | 0.45c | −0.22 | −0.15 | 0.09 |
| Ratio FSH/E2 | −0.26a | 0.07 | −0.08 | 0.1 |
| Ratio T/E2 | 0.12 | 0.17 | 0.12 | −0.09 |
| Ratio DHEA-S/T | 0.28a | −0.22 | −0.1 | 0.15 |
Identifies an existing trend with a p-value <0.10
Identifies statistical significance with a p-value <0.05
Identifies statistical significance with a p-value <0.01
Identifies statistical significance with a p-value <0.001
Notes: Spearman correlation coefficients were calculated if both variables are continuous or ordinal. (Ranked) biserial correlation coefficients were calculated if one variable is binary.
- HIV = Human Immunodeficiency Virus
- WMH = White Matter Hyperintensity
- BMI = Body Mass Index
- E2 = Estradiol
- FSH = Follicle-Stimulating Hormone
- T = Testosterone
- DHEA-S = Dehydroepiandrosterone Sulfate
For WMH volume, a positive correlation was demonstrated among women for age (r=0.40, p-value<0.05) and dyslipidemia (r = 0.39, p value <0.05). In men, a trend for an association with dyslipidemia (r = .30, p=< 0.10) and hypertension (r = .29, p = <0.10) was observed. No significant bivariate associations or trends were observed between WMH and any of the hormone measures in either sex.
Final multivariable linear regression models evaluating reproductive hormones as factors of white matter features (volume and WMH volume) are presented in Table 3. After preliminary model variable selection, the final models included age, cranial size, education, and diabetes for white matter volume, and age, hypertension, dyslipidemia, and cranial size for WMH volume. Controlling for these factors, there was a significant interaction effect between sex and DHEA-S (B= 21.13, p-value=0.012; Figure 1 depicts the interaction effect) on white matter volume; there was a trend for significant interaction effect between sex and FSH (B=− 22.97, p-value=0.08); there was no significant interaction effect between sex and E2 and T (for full model, see Table, Supplemental Digital Content 1). Finally, we examined the results by sex to determine how sex moderates the relationship between hormone measures and white matter volume (Table 3). Controlling for risk factors, in women, higher white matter volume was associated with higher DHEA-S (B=13.89, p-value=0.017), and lower FSH (B= −23.58, p-value=0.022).However, in men, associations of white matter volume with DHEA-S and FSH were not significant. For WMH volume, no significant interaction effects between sex and reproductive hormones were identified.
Table 3.
Multivariable liner regression models assessing the relationship between reproductive hormones with white matter volume and WMH volume by sex.
| white matter volume | ||||||||
|---|---|---|---|---|---|---|---|---|
| Women (n=44) | Men(n=35) | |||||||
| Hormone | B | 95% Confidence Intervals | P_value | B | 95% Confidence Intervals | P_value | ||
| E 2 | −4.72 | −18.56 | 9.11 | 0.50 | 6.18 | −21.80 | 34.16 | 0.67 |
| FSH | −23.58 | −43.76 | −3.39 | 0.022a | −0.61 | −15.83 | 14.60 | 0.94 |
| T | 14.33 | −2.39 | 31.05 | 0.093b | 8.06 | −9.32 | 25.45 | 0.36 |
| DHEA-S | 13.89 | 2.50 | 25.27 | 0.017a | −7.24 | −19.48 | 4.99 | 0.25 |
| WMH Volume | ||||||||
| Women (n=44) | Men(n=35) | |||||||
| E 2 | −0.21 | −0.68 | 0.26 | 0.38 | 0.02 | −0.97 | 1.01 | 0.97 |
| FSH | −0.27 | −1.00 | 0.47 | 0.47 | 0.38 | −0.16 | 0.92 | 0.16 |
| T | 0.08 | −0.51 | 0.66 | 0.80 | 0.08 | −0.47 | 0.62 | 0.78 |
| DHEA-S | −0.15 | −0.52 | 0.22 | 0.42 | 0.28 | −0.14 | 0.71 | 0.19 |
p-value = <0.05
p-value = <0.10
WMH = white matter hyperintensity; E2= estradiol; FSH = follicle stimulating hormone; T = testosterone; DHEA-S = dehydroepiandrosterone sulphate; WMH and sex hormone values were log 2 base transformed.
Figure #1:
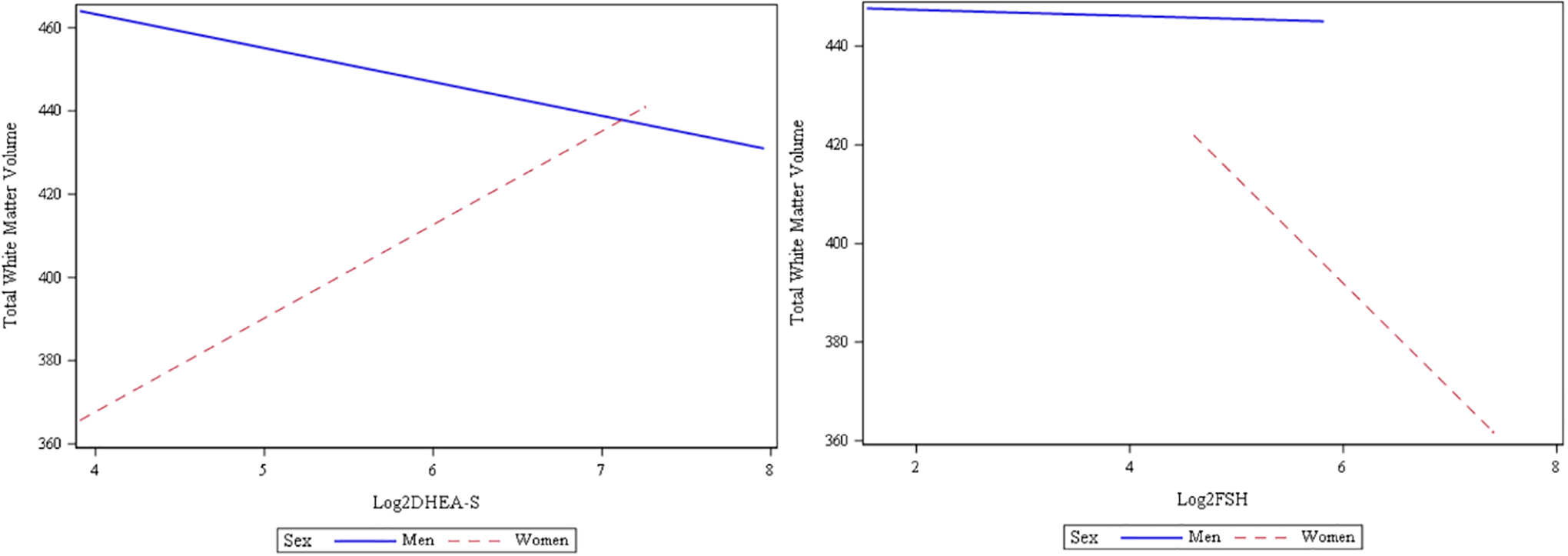
relationship between reproductive hormones (Log2 transformed DHEA-S and FSH) and total white matter volumes by sex
DISCUSSION
Although WMH are imaging biomarkers of covert cerebrovascular disease frequently studied among PLWH and associated with aging and longer duration of HIV infection,27 sex differences have been underexplored in this populatoion.1 This pilot study expands on the existing literature by investigating the role of reproductive hormones and other risk factors as predictors of white matter disease as a function of sex within the context of HIV infection. Characterizing sex differences among a diverse and aging cohort of PLWH has diagnostic and clinical implications for white matter disease burden and HIV care among an aging population. This is of particular importance as researchers work to better describe the biomarker profile of HIV-associated neurocognitive disorders (HAND), which is occasionally confused with other cognitive disorders and clinical conditions by patients and providers.28,29
In this pilot study, the relationship between total white matter volume and peripheral concentrations of reproductive hormones, controlling for HIV related indicators and vascular risk differed between men and women. These findings suggest that biological sex within the aging HIV-positive population may contribute to differing risk profiles for white matter disease among PLWH.30,31 When analyzing data from men and women separately, we observed sex differences in the preliminary bivariate relationships between white matter measures and selected variables related to aging, vascular risk, and hormone levels. There was a significant relationship between white matter volume and DHEA-S levels in women but not men, supporting the view that its presumed role in neurocognitive function may possibly be moderated by underlying sex differences. While previous research has demonstrated positive correlations between DHEA-S and white matter volume, there are few data on how sex may influence this relationship.32 We also found in women, there was a significant association between FSH and white matter volume, but no such relationship in men. This finding is also supported by a previous report of elderly women experiencing a greater burden of white matter features related to aging when compared to men and after adjusting for midlife vascular risk factors.
Given the mixed findings associated with reproductive hormones and WMH in the general population and the dearth of evidence in PLWH, further study is warranted to understand the relationship between factors associated with dementia and non-estrogen hormones of the hypothalamic-pituitary-gonadal axis.33,34 Recent animal and human research suggests that both androgens - dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA-S) - play a role in promoting neuronal growth and reducing cognitive impairment.32,35 In the KEEPS trial, neither LH nor FSH which rises throughout the menopausal transition due to estrogen loss, were associated at baseline with WMH volume among recently menopausal women (mean age of 53 years)15, which is consistent with the findings in our study. Whether sample size may have played a role in these results in both studies is unclear, but future longitudinal data is needed.
Our study has several limitations. Since the goal of the parent study did not focus on sex differences or the menopause transition, we did not exclude women with a history of a hysterectomy or bilateral oophorectomy. The small data set may have contributed to over-fitting making study findings less generalizable. Further, there may be sampling bias in our sample which was largely comprised of PLWH from racial and ethnic minority groups who were overweight, and current smokers. We also failed to collect data on luteinizing hormone (LH), progesterone, prolactin, thyroid hormones and cortisol which are known to influence neurocognition and change with aging. We also did not obtain depression scores from study participants and depressive symptoms and apathy have been associated with higher WMH volumes.36,37 We also failed to assess for a history of trauma and sexual assault which may be especially high in this population and has recently been shown to be associated with higher WMH volumes in uninfected midlife women.38 Further, as this study used a cross-sectional design, we were unable to draw conclusions that consider the causality of white matter disease in this population. Future studies should include longitudinal MRI data to examine if risk factors of interest are associated with the extent of white matter disease progression over time.
CONCLUSION
Although sexually -dimorphic interactions of reproductive hormones and total white matter volume were demonstrated in a small sample of older PLWH, sex-based differences in reproductive hormones as predictive correlates of WMH was not supported. Future studies are needed in larger and more generalizable samples of PLWH to better understand these findings.
Supplementary Material
Sources of funding:
This research is a result of grant funding from the National Institutes of Health (NIH) (grant #s: K24NR018621, R01NR015737 and UL1TR001873). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Financial disclosures/conflicts of interest: Nancy Reame helped develop a perimenopause questionnaire survey for NextGenJane.com in 2020, served as a one-time consultant with Merk Pharmaceuticals regarding future trends in menopause research for a one-time presentation in 2022, and was reimbursed for travel expenses during 2019 and 2020 as a member of the National Academy of Medicine’s Committee to Assess the Utility of Compounded Bioidential Hormone Therapy. The other authors have nothing to disclose.
The data used in this manuscript were presented at the poster session of the 2021 NAMS Annual Meeting in Chicago, Illinois.
SUPPLEMENTAL DIGITAL CONTENT
• Supplemental Digital Content 1. Tables that demonstrate the full models assessing whether sex moderates the associations of white matter features with reproductive hormones (n=79) controlling for covariates. PDF
REFERENCES
- 1.Gutierrez J, Porras TN, Yoo-Jeong M, et al. Cerebrovascular Contributions to Neurocognitive Disorders in People Living With HIV. J Acquir Immune Defic Syndr. Sep 1 2021;88(1):79–85. doi: 10.1097/qai.0000000000002729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scully EP. Sex Differences in HIV Infection. Current HIV/AIDS reports. 2018;15(2):136–146. doi: 10.1007/s11904-018-0383-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griesbeck M, Scully E, Altfeld M. Sex and gender differences in HIV-1 infection. Clinical science. 2016;130(16):1435–1451. doi: 10.1042/CS20160112 [DOI] [PubMed] [Google Scholar]
- 4.Alqarni A, Jiang J, Crawford JD, et al. Sex differences in risk factors for white matter hyperintensities in non-demented older individuals. Neurobiology of Aging. 2021;98:197–204. doi: 10.1016/j.neurobiolaging.2020.11.001 [DOI] [PubMed] [Google Scholar]
- 5.Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiology of Aging. 2009;30(6):946–956. doi: 10.1016/j.neurobiolaging.2007.08.023 [DOI] [PubMed] [Google Scholar]
- 6.Fatemi F, Kantarci K, Graff-Radford J, et al. Sex differences in cerebrovascular pathologies on FLAIR in cognitively unimpaired elderly. Neurology. Feb 6 2018;90(6):e466–e473. doi: 10.1212/wnl.0000000000004913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Garcia M, Biswas N, Patel MV, et al. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV-infection. PloS one. 2013;8(4):e62069. doi: 10.1371/journal.pone.0062069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asin SN, Heimberg AM, Eszterhas SK, Rollenhagen C, Howell AL. Estradiol and progesterone regulate HIV type 1 replication in peripheral blood cells. AIDS research and human retroviruses. 2008;24(5):701–716. doi: 10.1089/aid.2007.0108 [DOI] [PubMed] [Google Scholar]
- 9.Szotek EL, Narasipura SD, Al-Harthi L. 17β-Estradiol inhibits HIV-1 by inducing a complex formation between β-catenin and estrogen receptor α on the HIV promoter to suppress HIV transcription. Virology. 2013;443(2):375–383. doi: 10.1016/j.virol.2013.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rechtien A, Altfeld M. Sexual dimorphism in HIV-1 infection. Seminars in immunopathology. 2019;41(2):195–202. doi: 10.1007/s00281-018-0704-y [DOI] [PubMed] [Google Scholar]
- 11.Chow FC, Regan S, Zanni MV, et al. Elevated ischemic stroke risk among women living with HIV infection. AIDS. 2018;32(1):59–67. doi: 10.1097/QAD.0000000000001650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Jin A, Fu Y, et al. Heterogeneity of White Matter Hyperintensities in Cognitively Impaired Patients With Cerebral Small Vessel Disease. Original Research. Frontiers in Immunology. 2021-December-09 2021;12doi: 10.3389/fimmu.2021.803504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin J, Wang D, Lan L, Fan Y. Multiple Factors Involved in the Pathogenesis of White Matter Lesions. Biomed Res Int. 2017;2017:9372050. doi: 10.1155/2017/9372050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coker LH, Hogan PE, Bryan NR, et al. Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology. Jan 13 2009;72(2):125–34. doi: 10.1212/01.wnl.0000339036.88842.9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kling JM, Miller VM, Tosakulwong N, Lesnick T, Kantarci K. Associations of pituitary-ovarian hormones and white matter hyperintensities in recently menopausal women using hormone therapy. Menopause. Aug 2020;27(8):872–878. doi: 10.1097/gme.0000000000001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook IA, Morgan ML, Dunkin JJ, et al. Estrogen replacement therapy is associated with less progression of subclinical structural brain disease in normal elderly women: a pilot study. International Journal of Geriatric Psychiatry. 2002;17(7):610–618. doi: 10.1002/gps.644 [DOI] [PubMed] [Google Scholar]
- 17.Liu Y-Y, Hu L, Ji C, et al. Effects of hormone replacement therapy on magnetic resonance imaging of brain parenchyma hyperintensities in postmenopausal women. Acta Pharmacologica Sinica. 2009;30(7):1065–1070. doi: 10.1038/aps.2009.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thurston RC, Aizenstein HJ, Derby CA, Sejdić E, Maki PM. Menopausal hot flashes and white matter hyperintensities. Menopause. Jan 2016;23(1):27–32. doi: 10.1097/gme.0000000000000481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park BJ, Shim JY, Lee YJ, Lee JH, Lee HR. Association between sex hormone levels and leukoaraiosis (LA) in older Korean men. Arch Gerontol Geriatr. Mar-Apr 2012;54(2):e73–6. doi: 10.1016/j.archger.2011.06.035 [DOI] [PubMed] [Google Scholar]
- 20.Abbott RD, Launer LJ, Rodriguez BL, et al. Serum estradiol and risk of stroke in elderly men. Neurology. 2007;68(8):563–568. doi: 10.1212/01.wnl.0000254473.88647.ca [DOI] [PubMed] [Google Scholar]
- 21.Srinath R, Gottesman RF, Hill Golden S, Carson KA, Dobs A. Association Between Endogenous Testosterone and Cerebrovascular Disease in the ARIC Study (Atherosclerosis Risk in Communities). Stroke. 2016;47(11):2682–2688. doi: 10.1161/strokeaha.116.014088 [DOI] [PubMed] [Google Scholar]
- 22.Brickman AM, Provenzano FA, Muraskin J, et al. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch Neurol. Dec 2012;69(12):1621–7. doi: 10.1001/archneurol.2012.1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brickman AM, Sneed JR, Provenzano FA, et al. Quantitative approaches for assessment of white matter hyperintensities in elderly populations. Psychiatry research. 2011;193(2):101–106. doi: 10.1016/j.pscychresns.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenland S, Pearce N. Statistical foundations for model-based adjustments. Annu Rev Public Health. Mar 18 2015;36:89–108. doi: 10.1146/annurev-publhealth-031914-122559 [DOI] [PubMed] [Google Scholar]
- 25.Duling D, Thompson W, Schubert S. SAS Institute, Inc. 2003;
- 26.Schober P, Boer C, Schwarte LA. Correlation Coefficients: Appropriate Use and Interpretation. Anesth Analg. May 2018;126(5):1763–1768. doi: 10.1213/ane.0000000000002864 [DOI] [PubMed] [Google Scholar]
- 27.Mina Y, Wu T, Hsieh HC, et al. Association of White Matter Hyperintensities With HIV Status and Vascular Risk Factors. Neurology. Apr 6 2021;96(14):e1823–e1834. doi: 10.1212/wnl.0000000000011702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liboro RM, Ibañez-Carrasco F, Rourke SB, et al. Barriers to addressing HIV-Associated neurocognitive disorder (HAND): Community-based service provider perspectives. Journal of HIV/AIDS & Social Services. 2018;17(3):209–223. doi: 10.1080/15381501.2018.1431168 [DOI] [Google Scholar]
- 29.Sundermann EE, Bondi MW, Campbell LM, et al. Distinguishing Amnestic Mild Cognitive Impairment From HIV-Associated Neurocognitive Disorders. The Journal of Infectious Diseases. 2020;doi: 10.1093/infdis/jiaa760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallart-Palau X, Lee BS, Adav SS, et al. Gender differences in white matter pathology and mitochondrial dysfunction in Alzheimer’s disease with cerebrovascular disease. Molecular brain. 2016;9:27. doi: 10.1186/s13041-016-0205-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman A, Jackson H, Hristov H, et al. Sex and Gender Driven Modifiers of Alzheimer’s: The Role for Estrogenic Control Across Age, Race, Medical, and Lifestyle Risks. Frontiers in aging neuroscience. 2019;11:315. doi: 10.3389/fnagi.2019.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samaras N, Samaras D, Frangos E, Forster A, Philippe J. A review of age-related dehydroepiandrosterone decline and its association with well-known geriatric syndromes: is treatment beneficial? Rejuvenation research. 2013;16(4):285–294. doi: 10.1089/rej.2013.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blair JA, McGee H, Bhatta S, Palm R, Casadesus G. Hypothalamic-pituitary-gonadal axis involvement in learning and memory and Alzheimer’s disease: more than “just” estrogen. Frontiers in endocrinology. 2015;6(45)doi: 10.3389/fendo.2015.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Chadwick W, Park SS, et al. Gonadotropin-releasing hormone receptor system: modulatory role in aging and neurodegeneration. CNS & neurological disorders drug targets. 2010;9(5):651–660. doi: 10.2174/187152710793361559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrne ML, Whittle S, Vijayakumar N, Dennison M, Simmons JG, Allen NB. A systematic review of adrenarche as a sensitive period in neurobiological development and mental health. Developmental cognitive neuroscience. 2017;25:12–28. doi: 10.1016/j.dcn.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oudega ML, Siddiqui A, Wattjes MP, et al. Are Apathy and Depressive Symptoms Related to Vascular White Matter Hyperintensities in Severe Late Life Depression? J Geriatr Psychiatry Neurol. Jan 2021;34(1):21–28. doi: 10.1177/0891988720901783 [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Leonards CO, Sterzer P, Ebinger M. White matter lesions and depression: A systematic review and meta-analysis. Journal of Psychiatric Research. 2014/September/01/ 2014;56:56–64. doi: 10.1016/j.jpsychires.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 38.Thurston RC, Jakubowski KP, Wu M, et al. Sexual assault and white matter hyperintensities among midlife women. Brain Imaging Behav. Apr 2022;16(2):773–780. doi: 10.1007/s11682-021-00536-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


