Abstract
Chiral γ-amino alcohols are the prevalent structural motifs and building blocks in pharmaceuticals and bioactive molecules. Enantioselective hydrogenation of β-amino ketones provides a straightforward and powerful tool for the synthesis of chiral γ-amino alcohols, but the asymmetric transformation is synthetically challenging. Here, a series of tridentate ferrocene-based phosphine ligands bearing modular and tunable unsymmetrical vicinal diamine scaffolds were designed, synthesized, and evaluated in the iridium-catalyzed asymmetric hydrogenation of β-amino ketones. The system was greatly effective to substrates with flexible structure and functionality, and diverse β-tertiary-amino ketones and β-secondary-amino ketones were hydrogenated smoothly. The excellent reactivities and enantioselectivities were achieved in the asymmetric delivery of various chiral γ-amino alcohols with up to 99% yields, >99% ee values, and turnover number (TON) of 48,500. The gram-scale reactions with low catalyst loading showed the potential application in industrial synthesis of chiral drugs, such as (S)-duloxetine, (R)-fluoxetine, and (R)-atomoxetine.
Subject terms: Asymmetric catalysis, Synthetic chemistry methodology, Asymmetric synthesis
Enantioselective hydrogenation of β-amino ketones is a powerful tool to produce bioactive molecules, but their asymmetric transformation is synthetically challenging. Here, an iridium-catalysed system with tridentate ferrocene-based phosphine ligands bearing unsymmetrical vicinal diamine scaffolds is developed for the efficient asymmetric synthesis of diverse γtertiary-amino and γ-secondary-amino alcohols, including intermediates of (S)-duloxetine, (R)-fluoxetine and (R)-atomoxetine.
Introduction
Enantiomerically enriched γ-amino alcohols, a class of prevalent building blocks, play a vital role as indispensable intermediates in organic transformation and pharmaceutical production of the antidepressant drugs such as (S)-duloxetine, (R)-fluoxetine, and (R)-atomoxetine, as well as the potential chiral drugs (Fig. 1a)1–6. A multitude of processes have been developed for the synthesis of chiral γ-amino alcohols, for example, the nucleophilic substitution of the chiral alcohol bearing the leaving group in the γ-position with the amine (Fig. 1b, path 1)7–10, the Ru-catalyzed asymmetric hydroamination of the unsaturated alcohol and the amine (Fig. 1b, path 2)11,12, the Michael addition–asymmetric transfer hydrogenation of the unsaturated ketone and the amine (Fig. 1b, path 3)13, as well as the asymmetric hydrogenation of the β-amino ketone (Fig. 1b, path 4)14–22. The transition-metal-catalyzed asymmetric hydrogenation of prochiral β-amino ketones is doubtlessly regarded as one of the most promising and favorable methods due to the perfect atom economy and environmental benignity.
Fig. 1. The designed ligand for asymmetric hydrogenation of β-amino ketones.
a Drugs and potential analgesic agent containing γ-amino alcohol units. b Methods for the synthesis of chiral γ-amino alcohols. c The designed ligand for asymmetric hydrogenation of β-amino ketones.
Enantioselective hydrogenation of β-amino ketones catalyzed by chiral Ru– and Rh–phosphine complexes to afford optically active amino alcohols has been extensively studied during the past decades, promoting the generation of chiral drugs to a certain extent. In 1991, Achiwa developed the (2S,4S)-MCCPM–Rh catalyst in the successful synthesis of (R)-fluoxetine by enantioselective reduction of 3-(methylbenzylamino)-1-phenyl-1-propanone hydrochloride with 90.8% ee and substrate to catalyst molar ratio (S/C) of 100014. But the lower enantioselectivity of 79.8% ee was observed for 3-(methylamino)-1-phenyl-1-propanone hydrochloride. Subsequently, Noyori and co-workers reported efficient protocols for the asymmetric synthesis of γ-tertiary-amino alcohols using chiral [RuCl2(diphosphine)(1,2-diamine)] catalysts with excellent outcomes15–17. In 2005, Zhang and co-workers reported the enantioselective hydrogenation of β-secondary-amino ketones by Rh–duanphos catalyst in 90–93% yields with 93–99% ee and up to 4500 TON18. Moreover, Zhang applied RuPHOX–Ru complex and P-chiral Rh–bisphosphine complex in the asymmetric reduction of β-amino ketones providing the corresponding products with satisfactory results19–22.
Although the chiral Rh catalysts and Ru catalysts with varied phosphine ligands predominated in the asymmetric hydrogenation of β-amino ketones, the requirement of high catalyst loading and long reaction time, the poor stability, narrow substrate scope, as well as low reactivity and enantioselectivity of the reported catalysts could not be ignored. Therefore, the development of efficient and practical chiral catalysts is remarkably significant and dramatically desirable for the asymmetric access to γ-amino alcohols, albeit with the great challenge in finding novel and robust ligands. In addition to the Rh and Ru catalysts, the Ir catalysts were also widely employed in the asymmetric hydrogenation, and exhibited excellent reactivity and enantioselectivity for the reduction of various unsaturated compounds containing C═C23–32, C═O33–41, and C═N42–48, but the Ir-catalyzed enantioselective hydrogenation of challenging β-amino ketones was less studied. In 2011, Zhou presented excellent SpiroPAP-Ir catalyst for enantioselective hydrogenation of simple ketones, showing a dramatically high TON and promoting the development and application of Ir catalysts in the reduction of ketones33. In 2018, Zhang39 and Zhong40 reported the asymmetric reduction of simple ketones using Ir–PNN complexes containing C2 symmetric ethylenediamine and cyclohexanediamine scaffolds, respectively. The symmetric vicinal diamines were limited, while unsymmetrical vicinal diamines, such as those from amino acids were versatile, modular, and tunable. We envisioned that this type of ligand bearing diverse unsymmetrical diamines from amino acids might provide a great opportunity to systematically assess the catalytic performance of corresponding Ir catalysts for asymmetric hydrogenation through fine-tuning of electronic effect and steric hindrance, owing to the easy synthesis, multiple tuning sites, low cost, and high robustness. Herein, we reported a series of Ir catalysts with chiral ferrocene-based phosphine ligands bearing unsymmetrical vicinal diamine scaffolds and their successful application in asymmetric hydrogenation of β-amino ketones for the generation of various chiral γ-amino alcohols, including diverse γ-tertiary-amino and γ-secondary-amino alcohol intermediates of (S)-duloxetine, (R)-fluoxetine, and (R)-atomoxetine.
Results
Reaction optimization
The chiral tridentate ferrocene-based phosphine ligands (RC,SP,RC)-L1–L7 bearing monosulfonyl unsymmetrical vicinal diamine scaffolds were easily accessed via efficient coupling of acetate derived from commercial (R)-Ugi’s amine and unsymmetrical vicinal diamines from common chiral amino alcohols (see 2.1 in Supplementary Information). With these stable ligands in hand, we investigated the catalytic performance in terms of reactivity and enantioselectivity with the catalysts prepared in situ by mixing the [Ir(COD)Cl]2 with ligands (RC,SP,RC)-L1–L7 in iPrOH under 30 atm of H2 in the presence of LiOtBu. N-Boc-3-(methylamino)-1-(2-thienyl)-1-propanone 1a was tested as the model substrate, which was relatively challenging due to the additional coordination ability of the sulfur atom. For example, the aryl-substituted β-amino ketones could be hydrogenated smoothly by RuPHOX–Ru catalyst, while the reduction of thienyl-substituted substrates was failed19. As shown in Table 1, alkyl and aryl substituents in the unsymmetrical vicinal diamine scaffolds of ligands were proved to have an important impact on the catalytic behavior. The Me and iPr as the R groups promoted the transformation to proceed smoothly in >99% conversions and with 87% and 45% ee, respectively (entries 1–2), while the tBu led to the significant loss of the product (entry 3). The reaction performances deteriorated successively in the order of Me > iPr > tBu, suggesting that the steric hindrance of R group was crucial. When the phenyl or benzyl was on the α-position of sulfonamide group, 99% conversions were obtained with 76% and 93% ee, respectively (entries 4–5). Moreover, the significant effects on enantioselectivity were exerted by the steric hindrances of sulfonamide groups of ligands. The (RC,SP,RC)-L6 with the replacement of p-toluenesulfonyl in (RC,SP,RC)-L1 with 2,4,6-trimethylbenzenesulfonyl resulted in the obviously increased enantioselectivity of 97% ee, but Ir–(RC,SP,RC)-L7 catalyst gave the similar catalytic outcomes with Ir–(RC,SP,RC)-L5 catalyst (entries 6–7). X-ray crystallography determined the absolute configuration of 2a to be S (see Supplementary Fig. 276 and Supplementary Data 1).
Table 1.
Optimization of reaction conditions for asymmetric hydrogenation of 1a.
 | ||||
|---|---|---|---|---|
| Entrya | Ligand | Base | Conv. [%]b | ee [%]c |
| 1 | (RC,SP,RC)-L1 | LiOtBu | >99 | 87 |
| 2 | (RC,SP,RC)-L2 | LiOtBu | >99 | 45 |
| 3 | (RC,SP,RC)-L3 | LiOtBu | NR | ND |
| 4 | (RC,SP,RC)-L4 | LiOtBu | >99 | 76 |
| 5 | (RC,SP,RC)-L5 | LiOtBu | >99 | 93 |
| 6 | (RC,SP,RC)-L6 | LiOtBu | >99 | 97 |
| 7 | (RC,SP,RC)-L7 | LiOtBu | >99 | 93 |
| 8 | (RC,SP,RC)-L6 | NaOtBu | >99 | 98 |
| 9 | (RC,SP,RC)-L6 | KOtBu | >99 | 93 |
| 10 | (RC,SP,RC)-L6 | LiOH | >99 | 97 |
| 11 | (RC,SP,RC)-L6 | NaOH | >99 | 97 |
| 12 | (RC,SP,RC)-L6 | KOH | >99 | 94 |
| 13 | (RC,SP,RC)-L6 | K2CO3 | NR | ND |
| 14 | (RC,SP,RC)-L6 | Na2CO3 | NR | ND |
| 15d | (RC,SP,RC)-L6 | NaOtBu | NR | ND |
| 16e | (RC,SP,RC)-L6 | NaOtBu | >99 | 99 |
| 17f | (RC,SP,RC)-L6 | NaOtBu | >99 | 98 |
| 18g | (RC,SP,RC)-L6 | NaOtBu | >99 | 99 |
| 19h | (RC,SP,RC)-L6 | NaOtBu | >99 | 99 |
aReaction conditions: 0.4 mmol scale, 0.05 mol% [Ir(COD)Cl]2, 0.105 mol% Ligand, 5 mol% base, 2.0 mL solvent, room temperature (25–30 °C). bDetermined by 1H NMR analysis. cDetermined by HPLC analysis. d2 mL MeOH as solvent. e2 mL hexane as solvent. f2 mL THF as solvent. g2 mL CH2Cl2 as solvent. h2 mL toluene as solvent.
Bases have shown extremely discrepant results in asymmetric hydrogenation. The mechanism research on the asymmetric reduction of acetophenone using [RuX2(diphosphine)(1,2-diamine)] catalysts showed the promptly accelerated effect for H-H bond cleavage and relatively lower barriers for hydride transfer in the presence of KOtBu49. Thus, the base effect was evaluated in Ir–(RC,SP,RC)-L6-catalyzed asymmetric hydrogenation of 1a in iPrOH. LiOH and NaOH presented quantitative conversions and equal ee values of 97%, as the same with LiOtBu (entries 10–11), while NaOtBu slightly increased the enantioselectivity to 98% ee (entry 8). Moreover, >99% conversions were observed in the presence of KOtBu or KOH with 93% or 94% ee, respectively (entries 9 and 12). It seemed that the K+ caused the loss of enantioselectivity in contrast with the cases of Li+ and Na+, probably due to the electronegativity difference of alkali metal cations. Ulteriorly, the enantioselective reduction was adversely affected by the weaker bases including K2CO3 and Na2CO3, and no desired product was detected (entries 13–14). Thereby, NaOtBu was identified to be the optimal base in this procedure. Next, we turned our concern into the influence of solvents. Unfortunately, the replacement of iPrOH with MeOH resulted in the lack of target product (entry 15). Subsequently, aprotic solvents were further explored. To our delight, hexane, CH2Cl2, or toluene as solvent all provided 2a in >99% conversions and 99% ee values (entries 16 and 18–19). Moreover, THF also exhibited excellent performance in >99% conversion and 98% ee, suggesting the aprotic solvents benefited the Ir–(RC,SP,RC)-L6-catalyzed asymmetric hydrogenation of β-amino ketones (entry 17). Taking the consideration of the solubility, especially the toxicity and volatility of CH2Cl2, toluene was selected for further utilization, which was frequently used in industry.
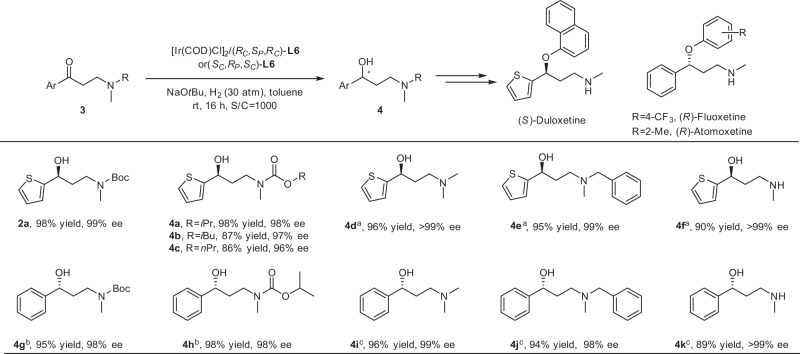
Substrate scope
With the optimized conditions of asymmetric hydrogenation in hand, the reduction of diverse N-Boc substituted substrates was performed, which could allow easy access to plentiful important γ-secondary-amino alcohols (Fig. 2). A variety of heteroaromatic and aromatic β-amino ketones were confirmed to be compatible with the system, affording the corresponding chiral γ-amino alcohols in excellent yields and tremendously high ee values. N-Boc-3-(methylamino)-1-(3-thienyl)-1-propanone 1b, which was different in the substitution site on thienyl with model substrate, was examined, and 93% yield and 98% ee were obtained. The substrates bearing substituents in the ortho- or meta-position of sulfur atom on the thienyl ring provided the corresponding products with extremely high enantioselectivities and admirable yields (2c–2e). The thienyl-substituted substrate with multiple electron-withdrawing groups, like di-Cl groups, provided 2f in 87% yield and 98% ee. Moreover, the fused ring benzothienyl-substituted ketone 1g was hydrogenated smoothly and delivered the chiral alcohol 2g with 99% yield and 99% ee. The outcomes of varied heteroaromatic substrates with substituted thienyl groups suggested that the sulfur atom had no influence on the catalytic capability of the Ir–(RC,SP,RC)-L6 catalyst, though it usually poisoned catalysts in transition-metal-catalyzed reactions50–53. In addition, the aromatic β-amino ketones without substituent (1h) or bearing varied electron-withdrawing/electron-donating substituent groups formed admirable enantioselectivities of 97–99% ee and good to excellent yields of 77–98%, regardless in the meta- or para-position on the phenyl ring (2h–2u). The satisfactory results showed that electrical properties of substituents exerted no implication for the enantioselectivities. While the ortho-substituted substrate (1v) gave the product with a slightly lower ee, suggesting that substituent on the benzene ring adjacent to the carbonyl had a little influence on the enantioselectivity. Meanwhile, the π-π-conjugated biphenyl and disubstituted aryl β-amino ketones triumphantly participated in the reaction with excellent outcomes (2w–2aa). Replacement of the phenyl skeleton by fused rings, such as 1-naphthyl, 2-naphthyl, or 2-fluorenyl led to superb results with 96–98% ee and 93–96% yields (2ab–2ad).
Fig. 2. Asymmetric hydrogenation of various N-Boc-β-amino ketones with Ir–(RC,SP,RC)-L6 catalyst.
Reaction conditions: 0.4 mmol scale, 0.05 mol% [Ir(COD)Cl]2, 0.105 mol% (RC,SP,RC)-L6, 5 mol% NaOtBu, 2.0 mL toluene, room temperature (25–30 °C). Isolated yields. The ee determined by HPLC. aat 60 °C.
More broadly, the scope of amino groups was then evaluated (Fig. 2). Alkyl substituents with different steric hindrances on the nitrogen atom of N-Boc-3-(alkylamino)-1-(2-thienyl)-1-propanone, even 2-adamantyl, were all compatible. The corresponding chiral products were obtained successfully in excellent yields of 98–99% and enantioselectivities of 98– > 99% ee (2ae–2ah). Substrates with benzyl or benzyl groups bearing substituents of different electronic properties on the nitrogen atom were also hydrogenated smoothly giving satisfactory outcomes (2ai–2al). The configuration of optically pure α-methyl benzylamino in 1am could remain in the transformation, and 2am was obtained with > 99:1 dr. The hydrogenation of the α-phenyl substituted benzylamino substrate provided 2an in 99% yield and > 99% ee. In addition, N-Boc-3-(isopropylamino)-1-phenyl-1-propanone was hydrogenated to deliver 2ao in 95% yield and 99% ee, and the case of N-Boc-3-(benzylamino)-1-phenyl-1-propanone for the synthesis of 2ap was 99% yield and 98% ee.
This asymmetric method provided an efficient approach for the delivery of a diverse array of chiral γ-secondary-amino alcohols often encountered in bioactive molecules. On the basis of the extensive substrate compatibility, the β-amino ketones with other N-substituted groups were tested in Fig. 3. To our delight, all kinds of common intermediates of (S)-duloxetine, (R)-fluoxetine, and (R)-atomoxetine containing N-alkoxycarbonyl β-amino ketones, β-tertiary-amino ketones, and β-secondary-amino ketones underwent asymmetric hydrogenation smoothly. Diverse alkoxycarbonylamino groups, such as isopropoxycarbonylamino, isobutoxycarbonylamino, and n-propoxycarbonylamino substituted β-amino ketones, which were easily synthesized on a large scale, were hydrogenated successfully in Ir–(RC,SP,RC)-L6-catalyzed asymmetric hydrogenation with 86–98% yields and 96–98% ee (4a–4c). Moreover, 3-(dimethylamino)-1-(2-thienyl)-1-propanone hydrochloride (3d·HCl) and 3-(methylbenzylamino)-1-(2-thienyl)-1-propanone hydrochloride (3e·HCl) also performed well in asymmetric reduction and afforded the corresponding chiral alcohol products with excellent results (>99% and 99% ee values, 96% and 95% yields, respectively). Most notably, when the β-secondary-amino ketone hydrochloride (3f·HCl) in a more stable form was used, the high enantioselectivity of >99% ee and 90% yield were accessed. The hydrochlorides could ensure substrates stable in an alkaline environment and reduce the side reactions. In this way, a series of chiral γ-amino alcohols covering γ-secondary-amino alcohol and γ-tertiary-amino alcohol intermediates of (S)-duloxetine were obtained by Ir–(RC,SP,RC)-L6 catalyst in high yields and enantioselectivities. It is worth mentioning that the catalysts that can exert great performance for the asymmetric hydrogenation of both heteroaromatic β-tertiary-amino ketones and β-secondary-amino ketones have not been reported. Further, the ligand (SC,RP,SC)-L6, the enantiomer of (RC,SP,RC)-L6, was synthesized and used in the asymmetric delivery of familiar γ-amino alcohol intermediates of (R)-fluoxetine and (R)-atomoxetine, which were contrary to the intermediates of (S)-duloxetine in configuration. The phenyl-substituted β-methyl alkoxycarbonylamino ketones and phenyl-substituted β-dimethylamino, β-methylbenzylamino, and β-monomethylamino ketone hydrochlorides underwent asymmetric reduction smoothly in 89–98% yields and 98– > 99% ee values (4g–4k). These dramatic results showed that this Ir-catalyzed system could provide efficiently both (S)- and (R)-configurations of various γ-amino alcohols.
Fig. 3. Asymmetric synthesis of chiral intermediates of (S)-duloxetine, (R)-fluoxetine, and (R)-atomoxetine.
Reaction conditions: 0.4 mmol scale, 0.05 mol% [Ir(COD)Cl]2, 0.105 mol% (RC,SP,RC)-L6, 5 mol% NaOtBu, 2.0 mL toluene, room temperature (25–30 °C). Isolated yields. The ee determined by HPLC. a0.4 mmol 3·HCl, 0.44 mmol NaOtBu. bIr–(SC,RP,SC)-L6 as catalyst. c0.4 mmol 3·HCl, 0.44 mmol NaOtBu, Ir–(SC,RP,SC)-L6 as catalyst.
The development of efficient method for asymmetric catalysis of β-dialkyl amino ketones was also highly desired, because different γ-dialkyl amino alcohols were involved in many important potential drugs54–58. Besides the Rh–MCCPM catalyst and Ru complexes14–17,19, Genov59 and Huang60 also respectively reported RuII–((R,R)-bicp) complex and [RuCl2(BIDN)(DPPF)] complex for the reduction of 3-(dimethylamino)-1-(2-thienyl)-1-propanone to obtain the corresponding product with 96% and 94% ee, respectively. This type of substrate was very different from simple ketones in terms of stability and was inclined to undergo facile elimination to produce a large number of by-products in alkaline environment, which made the asymmetric transformation extremely challenging. To our delight, the Ir-catalyzed system was dramatically amenable to β-dialkyl amino ketones with excellent reactivity and enantioselectivity. As shown in Fig. 4, free amino ketone or its hydrochloride underwent smooth hydrogenation with excellent results (4d). Diverse aromatic β-dimethylamino ketone hydrochlorides with halogen atoms on the ortho-, meta-, or para-position as well as tBu or phenyl on the para-position of phenyl ring were tolerated and the corresponding products were provided in 88–97% yields with extremely high enantioselectivities of 96– > 99% ee values (6a–6h). The asymmetric reduction of fused ring aryl and heteroaryl substrates also proceeded smoothly (6i–6j). The furanyl-substituted ketone gave 6k in 90% yield and 93% ee. A vast of substrates with various functionalized amino groups worked well in this transformation. Cyclic amino groups such as tetrahydropyrrolidinyl, piperidinyl, morpholinyl, and tetrahydroisoquinolinyl substituted substrates provided chiral alcohols in 89–97% yields with 99– > 99% ee values (6l–6o). The acyclic amino-substituted product 6p was formed in 95% yield and 98% ee. Gratifyingly, 6q with 4-hydroxypiperidinyl substituent was also obtained in 97% yield and >99% ee, indicating the hydroxyl had no effect on the catalytic behaviors. In addition, the piperazinyl-substituted substrates were also investigated to further certify the tolerance of functional groups. Varied substituents including cycloalkyl, benzoyl, Boc, aryl, heteroaryl, or benzyl on the nitrogen atom of piperazinyl were compatible, and the corresponding alcohols were afforded in 86–99% yields with 99– > 99% ee values (6r–6y, 6aa–6ad). Thereinto, 6ac, and 6ad could be converted to potential analgesic agent and antidepressant agent, respectively5,54. Furthermore, the substrate bearing spirocyclic amino group offered the chiral product 6z in 92% yield and >99% ee.
Fig. 4. Asymmetric hydrogenation of various β-tertiary-amino ketones with Ir-(RC,SP,RC)-L6 catalyst.
aCondition A: 0.4 mmol 5·HCl, 0.05 mol% [Ir(COD)Cl]2, 0.105 mol% (RC,SP,RC)-L6, 0.44 mmol NaOtBu, 3.0 mL toluene, room temperature (25–30 °C). bCondition B: 0.4 mmol 5, 0.05 mol% [Ir(COD)Cl]2, 0.105 mol% (RC,SP,RC)-L6, 5 mol% NaOtBu, 3.0 mL toluene, room temperature (25–30 °C). Isolated yields. The ee determined by HPLC.
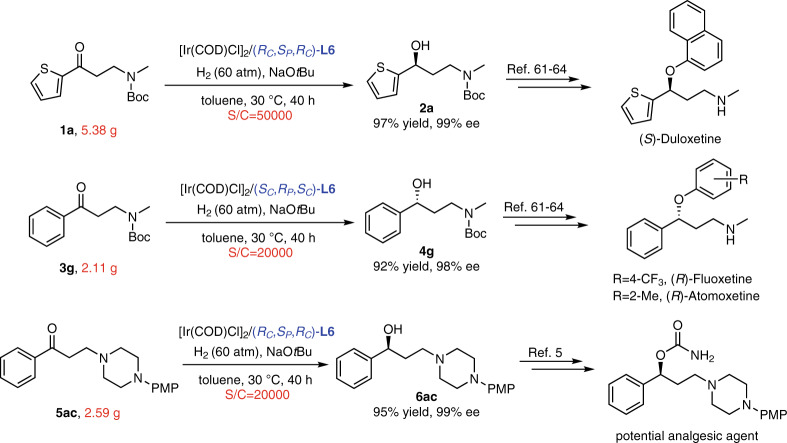
Gram-scale reactions
In order to demonstrate the potential utility of the protocol for the generation of important chiral drugs, gram-scale reactions were conducted under high S/C (Fig. 5). 1a was hydrogenated smoothly by Ir–(RC,SP,RC)-L6 catalyst with S/C of 50000, and the excellent performances remained (97% yield and 99% ee). Moreover, the Ir–(SC,RP,SC)-L6 catalyst was used in the asymmetric transformation of 3g accomplishing the 20000 S/C with 92% yield and 98% ee, and the case of Ir–(RC,SP,RC)-L6 catalyst for 5ac was 95% yield and 99% ee. The corresponding products could be further converted to the antidepressant drugs61–64 or potential analgesic agents5. It indicated that this Ir-catalyzed asymmetric transformation had great potential in industrial applications.
Fig. 5. Gram-scale reactions.
The asymmetric hydrogenation of 1a, 3g, and 5ac with high S/C.
Discussion
In summary, we developed an efficient catalytic system using iridium catalysts with chiral tridentate ferrocene-based phosphine ligands bearing unsymmetrical vicinal diamine scaffolds for the asymmetric hydrogenation of diverse β-amino ketones. This method allowed the access to all kinds of chiral γ-amino alcohols including γ-tertiary-amino alcohols and γ-secondary-amino alcohols with up to 99% yields, up to >99% ee values, and up to 48500 TON, providing an efficient approach for the synthesis of bioactive compounds. The intermediates of (S)-duloxetine, (R)-fluoxetine, and (R)-atomoxetine were obtained at gram scale with extremely low catalyst loading, indicating the method was of great significance in the industrial synthesis of chiral pharmaceutical drugs.
Methods
General procedure for the asymmetric hydrogenation of 1 (S/C = 1000)
Under nitrogen atmosphere, [Ir(COD)Cl]2 (1.4 mg, 0.002 mmol), (RC,SP,RC)-L6 (2.8 mg, 0.0042 mmol), and anhydrous iPrOH (1 mL) were added to an oven-dried 10 mL schlenk tube. The mixture was stirred at room temperature for 2.5 h to give an orange solution. A part of catalyst solution (100 μL, 0.0004 mmol) was transferred into a 10 mL vial containing N-Boc-β-amino ketone 1 (0.4 mmol), NaOtBu (1.9 mg, 0.02 mmol), and anhydrous toluene (2.0 mL). The vials were transferred to an autoclave, and the autoclave was purged with nitrogen and hydrogen three times in sequence, then charged with 30 atm of H2 and stirred for 16 h at room temperature. After hydrogen pressure was slowly released, the solvent was evaporated and the residue was purified by silica gel column chromatography to give the corresponding hydrogenation product 2. Then the enantiomeric excesses were determined by HPLC analysis.
General procedure for the asymmetric hydrogenation of 3 (S/C = 1000)
Under nitrogen atmosphere, [Ir(COD)Cl]2 (1.4 mg, 0.002 mmol), (RC,SP,RC)-L6 or (SC,RP,SC)-L6 (2.8 mg, 0.0042 mmol), and anhydrous iPrOH (1 mL) were added to an oven-dried 10 mL schlenk tube. The mixture was stirred at room temperature for 2.5 h to give an orange solution. A part of catalyst solution (100 μL, 0.0004 mmol) was transferred into a 10 mL vial containing ketone 3 or 3·HCl (0.4 mmol), NaOtBu (1.9 mg for 3a–3c and 3g–3h, 42.2 mg for 3d–3f·HCl and 3i–3k·HCl), and anhydrous toluene (2.0 mL). The vials were transferred to an autoclave, and the autoclave was purged with nitrogen and hydrogen three times in sequence, then charged with 30 atm of H2 and stirred for 16 h at room temperature. After hydrogen pressure was slowly released, the solvent was evaporated and the residue was purified by silica gel column chromatography to give the corresponding hydrogenation product 4. Then the enantiomeric excesses were determined by HPLC analysis.
General procedure for the asymmetric hydrogenation of 5 (S/C = 1000)
Under nitrogen atmosphere, [Ir(COD)Cl]2 (1.4 mg, 0.002 mmol), (RC,SP,RC)-L6 (2.8 mg, 0.0042 mmol), and anhydrous iPrOH (1 mL) were added to an oven-dried 10 mL schlenk tube. The mixture was stirred at room temperature for 2.5 h to give an orange solution. A part of catalyst solution (100 μL, 0.0004 mmol) was transferred into a 10 mL vial containing β-tertiary-amino ketone 5 or 5·HCl (0.4 mmol), NaOtBu (42.2 mg for 5a–5p·HCl, 1.9 mg for 5q–5ad), and anhydrous toluene (3.0 mL). The vials were transferred to an autoclave, and the autoclave was purged with nitrogen and hydrogen three times in sequence, then charged with 35 atm of H2 and stirred for 16 h at room temperature. After hydrogen pressure was slowly released, the solvent was evaporated and the residue was purified by silica gel column chromatography to give the corresponding hydrogenation product 6. Then the enantiomeric excesses were determined by HPLC analysis.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22071056).
Author contributions
C.L. performed most of experiments and wrote the manuscript. L.Z. performed part of experiments. L.C., Y.X., and Y.M. participated in the discussion and revised the manuscript. R.C. and J.Y. designed and supervised the project.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information. For experimental details and compound characterization data see Supplementary Notes 1, 2. For 1H NMR13,C NMR, and 31P NMR spectra see Supplementary Figs. 1–192 and HPLC spectra see Supplementary Figs. 193–275. The X-ray crystallographic data for 2a could be obtained free of charge from The Cambridge Crystallographic Data Centre with the accession code CCDC 2116544 via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ruihua Cheng, Email: rhcheng@gdut.edu.cn.
Jinxing Ye, Email: yejx@ecust.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-022-00678-4.
References
- 1.Robertson DW, Krushinski JH, Fuller RW, Leander JD. The absolute configurations and pharmacological activities of the optical isomers of fluoxetine, a selective serotonin-uptake inhibitor. J. Med. Chem. 1988;31:1412–1417. doi: 10.1021/jm00402a027. [DOI] [PubMed] [Google Scholar]
- 2.Chumpradit S, et al. Iodinated tomoxetine derivatives as selective ligands for serotonin and norepinephrine uptake sites. J. Med. Chem. 1992;35:4492–4497. doi: 10.1021/jm00101a029. [DOI] [PubMed] [Google Scholar]
- 3.Nowakowska E, Kus K, Chodera A. Pharmacological activity of fluoxetine. Acta Physiol. Hung. 1996;84:445–447. [PubMed] [Google Scholar]
- 4.Kirwin JL, Goren JL. Duloxetine: A dual serotonin-norepinephrine reuptake inhibitor for treatment of major depressive disorder. Pharmacotherapy. 2005;25:396–410. doi: 10.1592/phco.25.3.396.61600. [DOI] [PubMed] [Google Scholar]
- 5.Chae E, et al. Synthesis and pharmacological evaluation of carbamic acid 1-phenyl-3-(4-phenyl-piperazine-1-yl)-propyl ester derivatives as new analgesic agents. Bioorg. Med. Chem. Lett. 2012;22:2434–2439. doi: 10.1016/j.bmcl.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Spänkuch B, et al. Drugs by numbers: reaction-driven de novo design of potent and selective anticancer leads. Angew. Chem. Int. Ed. 2013;52:4676–4681. doi: 10.1002/anie.201206897. [DOI] [PubMed] [Google Scholar]
- 7.Ali IS, Sudalai A. Pd-catalyzed kinetic resolution of benzylic alcohols: a practical synthesis of (R)-tomoxetine and (S)-fluoxetine hydrochlorides. Tetrahedron Lett. 2002;43:5435–5436. doi: 10.1016/S0040-4039(02)01073-0. [DOI] [Google Scholar]
- 8.Ratovelomanana-Vidal V, et al. Enantioselective hydrogenation of β-keto esters using chiral diphosphine-ruthenium complexes: optimization for academic and industrial purposes and synthetic applications. Adv. Synth. Catal. 2003;345:261–274. doi: 10.1002/adsc.200390021. [DOI] [Google Scholar]
- 9.Carato P, Graulich A, Jensen N, Roth BL, Liegeois JF. Synthesis and in vitro binding studies of substituted piperidine naphthamides. Part I: Influence of the substitution on the basic nitrogen and the position of the amide on the affinity for D2L, D4.2, and 5-HT2A receptors. Bioorg. Med. Chem. Lett. 2007;17:1565–1569. doi: 10.1016/j.bmcl.2006.12.096. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed-Omer B, Sanderson AJ. Preparation of fluoxetine by multiple flow processing steps. Org. Biomol. Chem. 2011;9:3854–3862. doi: 10.1039/c0ob00906g. [DOI] [PubMed] [Google Scholar]
- 11.Pan Y, et al. Asymmetric synthesis of γ-secondary amino alcohols via a borrowing-hydrogen cascade. Org. Lett. 2020;22:7278–7283. doi: 10.1021/acs.orglett.0c02614. [DOI] [PubMed] [Google Scholar]
- 12.Xu R, et al. Anti-Markovnikov hydroamination of racemic allylic alcohols to access chiral γ-amino alcohols. Angew. Chem. Int. Ed. 2020;59:21959–21964. doi: 10.1002/anie.202009754. [DOI] [PubMed] [Google Scholar]
- 13.Wu L, et al. A Michael addition–asymmetric transfer hydrogenation one-pot enantioselective tandem process for syntheses of chiral γ-secondary amino alcohols. Org. Lett. 2017;19:3047–3050. doi: 10.1021/acs.orglett.7b00823. [DOI] [PubMed] [Google Scholar]
- 14.Sakuraba S, Achiwa K. Practical asymmetric synthesis of (R)-Fluoxetine hydrochloride catalyzed by (2S,4S)-4-dicyclohexylphosphino-2-diphenylphosphinomethyl-1-(N-methylcarbamoyl)pyrrolidine-rhodium complex. Synlett. 1991;10:689–690. doi: 10.1055/s-1991-34784. [DOI] [Google Scholar]
- 15.Ohkuma T, Ishii D, Takeno H, Noyori R. Asymmetric hydrogenation of amino ketones using chiral RuCl2(diphophine)(1,2-diamine) complexes. J. Am. Chem. Soc. 2000;122:6510–6511. doi: 10.1021/ja001098k. [DOI] [Google Scholar]
- 16.Ohkuma T, Koizumi M, Yoshida M, Noyori R. General asymmetric hydrogenation of hetero-aromatic ketones. Org. Lett. 2000;2:1749–1751. doi: 10.1021/ol0000814. [DOI] [PubMed] [Google Scholar]
- 17.Ohkuma T, et al. trans-RuH(η1-BH4)(binap)(1,2-diamine): A catalyst for asymmetric hydrogenation of simple ketones under base-free conditions. J. Am. Chem. Soc. 2002;124:6508–6509. doi: 10.1021/ja026136+. [DOI] [PubMed] [Google Scholar]
- 18.Liu D, Gao W, Wang C, Zhang X. Practical synthesis of enantiopure γ-amino alcohols by rhodium-catalyzed asymmetric hydrogenation of β-secondary-amino ketones. Angew. Chem. Int. Ed. 2005;44:1687–1689. doi: 10.1002/anie.200462178. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Liu D, Liu Y, Zhang W. Asymmetric hydrogenation of β-amino ketones with the bimetallic complex RuPHOX-Ru as the chiral catalyst. Org. Biomol. Chem. 2013;11:3855–3861. doi: 10.1039/c3ob40135a. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Wang J, Liu D, Zhang W. Synthesis of chiral γ-amino alcohols via a RuPHOX-Ru catalyzed asymmetric hydrogenation of β-imide ketones. Chin. J. Org. Chem. 2014;34:1766–1772. doi: 10.6023/cjoc201404035. [DOI] [Google Scholar]
- 21.Wang J, Wang Y, Liu D, Zhang W. Asymmetric hydrogenation of β-secondary amino ketones catalyzed by a ruthenocenyl phosphino-oxazoline-ruthenium complex (RuPHOX-Ru): the synthesis of γ-secondary amino alcohols. Adv. Synth. Catal. 2015;357:3262–3272. doi: 10.1002/adsc.201500653. [DOI] [Google Scholar]
- 22.Hu Q, Zhang Z, Liu Y, Imamoto T, Zhang W. ZnCl2-promoted asymmetric hydrogenation of β-secondary-amino ketones catalyzed by a P-chiral Rh–bisphosphine complex. Angew. Chem. Int. Ed. 2015;54:2260–2264. doi: 10.1002/anie.201411384. [DOI] [PubMed] [Google Scholar]
- 23.Tang W, Wang W, Zhang X. Phospholane–oxazoline ligands for Ir-catalyzed asymmetric hydrogenation. Angew. Chem. Int. Ed. 2003;42:943–946. doi: 10.1002/anie.200390251. [DOI] [PubMed] [Google Scholar]
- 24.Källström K, Hedberg C, Brandt P, Bayer A, Andersson PG. Rationally designed ligands for asymmetric iridium-catalyzed hydrogenation of olefins. J. Am. Chem. Soc. 2004;126:14308–14309. doi: 10.1021/ja0464241. [DOI] [PubMed] [Google Scholar]
- 25.Bell S, et al. Asymmetric hydrogenation of unfunctionalized, purely alkyl-substituted olefins. Science. 2006;311:642–644. doi: 10.1126/science.1121977. [DOI] [PubMed] [Google Scholar]
- 26.Schrems MG, Neumann E, Pfaltz A. Iridium-catalyzed asymmetric hydrogenation of unfunctionalized tetrasubstituted olefins. Angew. Chem. Int. Ed. 2007;46:8274–8276. doi: 10.1002/anie.200702555. [DOI] [PubMed] [Google Scholar]
- 27.Zhu S-F, Yu Y-B, Li S, Wang L-X, Zhou Q-L. Enantioselective hydrogenation of α-substituted acrylic acids catalyzed by iridium complexes with chiral spiro aminophosphine ligands. Angew. Chem. Int. Ed. 2012;51:8872–8875. doi: 10.1002/anie.201204363. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Han Z, Wang Z, Ding K. SpinPhox/iridium(I)-catalyzed asymmetric hydrogenation of cyclic α-alkylidene carbonyl compounds. Angew. Chem. Int. Ed. 2014;53:1978–1982. doi: 10.1002/anie.201309521. [DOI] [PubMed] [Google Scholar]
- 29.Zhu S-F, Zhou Q-L. Iridium-catalyzed asymmetric hydrogenation of unsaturated carboxylic acids. Acc. Chem. Res. 2017;50:988–1001. doi: 10.1021/acs.accounts.7b00007. [DOI] [PubMed] [Google Scholar]
- 30.Biosca M, Magre M, Pàmies O, Diéguez M. Asymmetric hydrogenation of disubstituted, trisubstituted, and tetrasubstituted minimally functionalized olefins and cyclic β-enamides with easily accessible Ir–P, oxazoline catalysts. ACS Catal. 2018;8:10316–10320. doi: 10.1021/acscatal.8b03170. [DOI] [Google Scholar]
- 31.Liu J, Krajangsri S, Yang J, Li J-Q, Andersson PG. Iridium-catalysed asymmetric hydrogenation of allylic alcohols via dynamic kinetic resolution. Nat. Catal. 2018;1:438–443. doi: 10.1038/s41929-018-0070-0. [DOI] [Google Scholar]
- 32.Ge Y, Han Z, Wang Z, Ding K. Ir-catalyzed double asymmetric hydrogenation of 3,6-dialkylidene-2,5-diketopiperazines for enantioselective synthesis of cyclic dipeptides. J. Am. Chem. Soc. 2019;141:8981–8988. doi: 10.1021/jacs.9b02920. [DOI] [PubMed] [Google Scholar]
- 33.Xie J-H, Liu X-Y, Xie J-B, Wang L-X, Zhou Q-L. An additional coordination group leads to extremely efficient chiral iridium catalysts for asymmetric hydrogenation of ketones. Angew. Chem. Int. Ed. 2011;50:7329–7332. doi: 10.1002/anie.201102710. [DOI] [PubMed] [Google Scholar]
- 34.Nie H, Zhou G, Wang Q, Chen W, Zhang S. Asymmetric hydrogenation of aromatic ketones using an iridium(I) catalyst containing ferrocene-based P–N–N tridentate ligands. Tetrahedron.: Asymmetry. 2013;24:1567–1571. doi: 10.1016/j.tetasy.2013.10.012. [DOI] [Google Scholar]
- 35.Bao D-H, Wu H-L, Liu C-L, Xie J-H, Zhou Q-L. Development of chiral Spiro P-N-S ligands for iridium-catalyzed asymmetric hydrogenation of β-alkyl-β-ketoesters. Angew. Chem. Int. Ed. 2015;54:8791–8794. doi: 10.1002/anie.201502860. [DOI] [PubMed] [Google Scholar]
- 36.Hou C-J, Hu X-P. Sterically hindered chiral ferrocenyl P,N,N-ligands for highly diastereo-/enantioselective Ir-catalyzed hydrogenation of α-alkyl-β-ketoesters via dynamic kinetic resolution. Org. Lett. 2016;18:5592–5595. doi: 10.1021/acs.orglett.6b02828. [DOI] [PubMed] [Google Scholar]
- 37.Wu W, et al. Iridium catalysts with f-amphox ligands: asymmetric hydrogenation of simple ketones. Org. Lett. 2016;18:2938–2941. doi: 10.1021/acs.orglett.6b01290. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Yang G, Xie F, Zhang W. A ferrocene-based NH-free phosphine-oxazoline ligand for iridium-catalyzed asymmetric hydrogenation of ketones. Org. Lett. 2018;20:6135–6139. doi: 10.1021/acs.orglett.8b02591. [DOI] [PubMed] [Google Scholar]
- 39.Liang Z, Yang T, Gu G, Dang L, Zhang X. Scope and mechanism on iridium-f-amphamide catalyzed asymmetric hydrogenation of ketones. Chin. J. Chem. 2018;36:851–856. doi: 10.1002/cjoc.201800129. [DOI] [Google Scholar]
- 40.Ling F, et al. Development of ferrocene-based diamine-phosphine-sulfonamide ligands for iridium-catalyzed asymmetric hydrogenation of ketones. J. Org. Chem. 2018;83:10749–10761. doi: 10.1021/acs.joc.8b01276. [DOI] [PubMed] [Google Scholar]
- 41.Zhang F-H, Zhang F-J, Li M-L, Xie J-H, Zhou Q-L. Enantioselective hydrogenation of dialkyl ketones. Nat. Catal. 2020;3:621–627. doi: 10.1038/s41929-020-0474-5. [DOI] [Google Scholar]
- 42.Mršić N, Minnaard AJ, Feringa BL, de Vries JG. Iridium/monodentate phosphoramidite catalyzed asymmetric hydrogenation of N-aryl imines. J. Am. Chem. Soc. 2009;131:8358–8359. doi: 10.1021/ja901961y. [DOI] [PubMed] [Google Scholar]
- 43.Hou G, et al. Enantioselective hydrogenation of N−H imines. J. Am. Chem. Soc. 2009;131:9882–9883. doi: 10.1021/ja903319r. [DOI] [PubMed] [Google Scholar]
- 44.Han Z, Wang Z, Zhang X, Ding K. Spiro[4,4]-1,6-nonadiene-based phosphine-oxazoline ligands for iridium-catalyzed enantioselective hydrogenation of ketimines. Angew. Chem. Int. Ed. 2009;48:5345–5349. doi: 10.1002/anie.200901630. [DOI] [PubMed] [Google Scholar]
- 45.Hou G, Tao R, Sun Y, Zhang X, Gosselin F. Iridium-monodentate phosphoramidite-catalyzed asymmetric hydrogenation of substituted benzophenone N−H imines. J. Am. Chem. Soc. 2010;132:2124–2125. doi: 10.1021/ja909583s. [DOI] [PubMed] [Google Scholar]
- 46.Gao K, Yu C-B, Wang D-S, Zhou Y-G. Iridium-catalyzed asymmetric hydrogenation of 3-substituted 2H-1,4-benzoxazines. Adv. Synth. Catal. 2012;354:483–488. doi: 10.1002/adsc.201100568. [DOI] [Google Scholar]
- 47.Nagano T, et al. Additive effects of amines on asymmetric hydrogenation of quinoxalines catalyzed by chiral iridium complexes. Chem. Eur. J. 2012;18:11578–11592. doi: 10.1002/chem.201201366. [DOI] [PubMed] [Google Scholar]
- 48.Cartigny D, et al. General asymmetric hydrogenation of 2-alkyl- and 2-aryl-substituted quinoxaline derivatives catalyzed by iridium-difluorphos: unusual halide effect and synthetic application. J. Org. Chem. 2012;77:4544–4556. doi: 10.1021/jo300455y. [DOI] [PubMed] [Google Scholar]
- 49.Dub PA, Henson NJ, Martin RL, Gordon JC. Unravelling the mechanism of the asymmetric hydrogenation of acetophenone by [RuX2(diphosphine)(1,2-diamine)] catalysts. J. Am. Chem. Soc. 2014;136:3505–3521. doi: 10.1021/ja411374j. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez JA, Hrbek J. Interaction of sulfur with well-defined metal and oxide surfaces: unraveling the mysteries behind catalyst poisoning and desulfurization. Acc. Chem. Res. 1999;32:719–728. doi: 10.1021/ar9801191. [DOI] [Google Scholar]
- 51.Argyle M, Bartholomew C. Heterogeneous catalyst deactivation and regeneration: a review. Catalysts. 2015;5:145–269. doi: 10.3390/catal5010145. [DOI] [Google Scholar]
- 52.Miao B, Ma SSK, Wang X, Su H, Chan SH. Catalysis mechanisms of CO2 and CO methanation. Catal. Sci. Technol. 2016;6:4048–4058. doi: 10.1039/C6CY00478D. [DOI] [Google Scholar]
- 53.Nirmal Kumar S, Appari S, Kuncharam BVR. Techniques for overcoming sulfur poisoning of catalyst employed in hydrocarbon reforming. Catal. Surv. Asia. 2021;25:362–388. doi: 10.1007/s10563-021-09340-w. [DOI] [Google Scholar]
- 54.Martínez-Esparza J, et al. New 1-aryl-3-(4-arylpiperazin-1-yl)propane derivatives, with dual action at 5-HT1A serotonin receptors and serotonin transporter, as a new class of antidepressants. J. Med. Chem. 2001;44:418–428. doi: 10.1021/jm001059j. [DOI] [PubMed] [Google Scholar]
- 55.Köksal M, Bilge SS. Synthesis and antidepressant-like profile of novel 1-aryl-3-[(4-benzyl)piperidine-1-yl]propane derivatives. Arch. Pharm. Pharm. Med. Chem. 2007;340:299–303. doi: 10.1002/ardp.200700028. [DOI] [PubMed] [Google Scholar]
- 56.Weber KC, da Silva ABF. A chemometric study of the 5-HT1A receptor affinities presented by arylpiperazine compounds. Eur. J. Med. Chem. 2008;43:364–372. doi: 10.1016/j.ejmech.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 57.Berrade L, et al. Novel benzo[b]thiophene derivatives as new potential antidepressants with rapid onset of action. J. Med. Chem. 2011;54:3086–3090. doi: 10.1021/jm2000773. [DOI] [PubMed] [Google Scholar]
- 58.Moreno-Viguri E, et al. In vitro and in vivo anti-Trypanosoma cruzi activity of new arylamine mannich base-type derivatives. J. Med. Chem. 2016;59:10929–10945. doi: 10.1021/acs.jmedchem.6b00784. [DOI] [PubMed] [Google Scholar]
- 59.Genov DG, Ager DJ. Asymmetric hydrogenation of ketones catalyzed by RuII–bicp complexes. Angew. Chem. Int. Ed. 2004;43:2816–2819. doi: 10.1002/anie.200353441. [DOI] [PubMed] [Google Scholar]
- 60.Zhu Q, Shi D, Xia C, Huang H. Ruthenium catalysts containing rigid chiral diamines and achiral diphosphanes for highly enantioselective hydrogenation of aromatic ketones. Chem. Eur. J. 2011;17:7760–7763. doi: 10.1002/chem.201100820. [DOI] [PubMed] [Google Scholar]
- 61.Gao Y, Sharpless KB. Asymmetric synthesis of both enantiomers of tomoxetine and fluoxetine. Selective reduction of 2,3-epoxycinnamyl alcohol with Red-Al. J. Org. Chem. 1988;53:4081–4084. doi: 10.1021/jo00252a036. [DOI] [Google Scholar]
- 62.Kamal A, Khanna GBR, Ramu R, Krishnaji T. Chemoenzymatic synthesis of duloxetine and its enantiomer: lipase-catalyzed resolution of 3-hydroxy-3-(2-thienyl) propanenitrile. Tetrahedron Lett. 2003;44:4783–4787. doi: 10.1016/S0040-4039(03)00945-6. [DOI] [Google Scholar]
- 63.Träff A, Lihammar R, Bäckvall JE. A chemoenzymatic dynamic kinetic resolution approach to enantiomerically pure (R)- and (S)-duloxetine. J. Org. Chem. 2011;76:3917–3921. doi: 10.1021/jo2003665. [DOI] [PubMed] [Google Scholar]
- 64.Zhou J-N, et al. Copper(II)-catalyzed enantioselective hydrosilylation of halo-substituted alkyl aryl and heteroaryl ketones: asymmetric synthesis of (R)-fluoxetine and (S)-duloxetine. Org. Biomol. Chem. 2014;12:1009–1017. doi: 10.1039/c3ob42214c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information. For experimental details and compound characterization data see Supplementary Notes 1, 2. For 1H NMR13,C NMR, and 31P NMR spectra see Supplementary Figs. 1–192 and HPLC spectra see Supplementary Figs. 193–275. The X-ray crystallographic data for 2a could be obtained free of charge from The Cambridge Crystallographic Data Centre with the accession code CCDC 2116544 via www.ccdc.cam.ac.uk/data_request/cif.