Abstract
Hexahydromethanocarbazole is a privileged scaffold in the discovery of new drugs and photoactive organic materials due to its good balance between structural complexity and minimized entropy penalty upon receptor binding. To address the difficulty of synthesizing this highly desirable bridged polycyclic scaffold, we designed a convenient multicomponent reaction cascade as intercepted Heck addition/C-H activation/C-palladacycle formation/electrophilic attack of ANP/N-palladacycle formation/Buchwald amination. A distinguishing feature of this sophisticated strategy is the successive generation of two key phenylnorbornyl palladium species to control the reaction flow towards desired products. DFT calculations further reveal the crucial roles of Cs2CO3 and 5,6-diester substitutions on the norbornene reactant in preventing multiple side-reactions. This innovative method exhibits a broad scope with good yields, and therefore will enable the construction of natural-product-like compound libraries based on hexahydromethanocarbazole.
Subject terms: Synthetic chemistry methodology, Heterogeneous catalysis, Drug discovery and development
Hexahydromethanocarbazole is a bridged polycyclic scaffold present in drugs and photoactive organic materials, however the efficient synthesis of this scaffold remains challenging. Here, the authors develop a multicomponent reaction cascade via phenylnorbornyl palladium species to generate hexahydromethanocarbazole-based libraries with CYP11B1 inhibition activity.
Introduction
In contrast to the immense chemical space, only limited molecular structural scaffolds have been explored for drug discovery until now. These scaffolds are largely characterized by flat conformations1,2, which, unfortunately, are frequently associated with attritions in the subsequent stages of drug development3. To solve this problem, increasing the number of sp3-hybridized carbons within a molecule has been recommended4. Through such a strategy, the resulting compounds are more “natural-product-like” and exhibit increased topological diversity and structural complexity, and hence could enable the probe of deeper and wider chemical space for biologically relevant molecular designs. Moreover, the abundance of sp3-hybridized carbons could lead to increased aqueous solubility via interfering crystal packing, reduced plasma protein binding, and thus could potentially improve the pharmacokinetic properties of drug candidates5. However, sp3-hybridized carbons tend to increase open-chained moieties’ flexibilities, which usually account for the entropy penalty upon binding of small-molecule ligands to their protein targets6. To counter this, cyclization of open-chained moieties will lead to saturated polycyclic skeletons with limited bond rotations; while insertion of an additional bridge will further rigidify molecular configurations, and thus mitigate the loss in entropy and binding affinity. The bridged aza bi- or tri-cycloakane scaffolds, in particular hexahydromethanocarbazole (Fig. 1), are examples of a good balance between structural complexity arising from the abundance of sp3-hybridized carbons and minimized entropy penalty upon binding, and are therefore potential privileged structures in drug discovery. Unfortunately, the syntheses of such bridged polycyclic scaffolds frequently suffer from lengthy synthetic routes, complex or harsh reaction conditions and mixtures of stereoisomers as a result.
Fig. 1. Presence of Hexahydromethanocarbazole.
Hexahydromethanocarbazole moieties present in natural products, materials, solar cells, and potential therapeutic compounds.
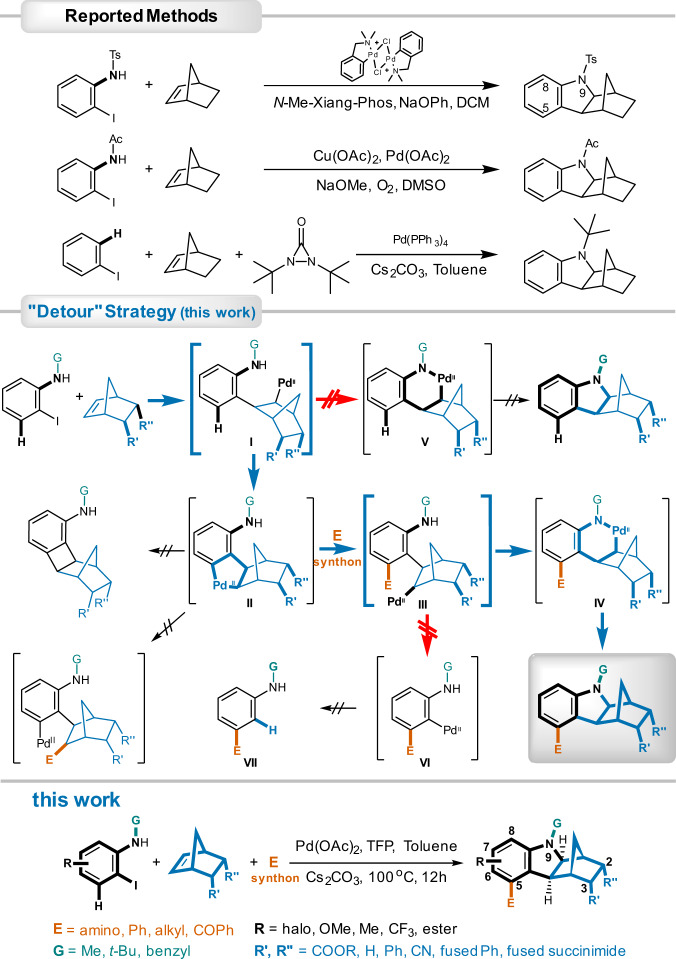
Hexahydromethanocarbazole is present in natural products including pleiomutinune7, and has been exploited as an essential pharmacophore in drug discovery against hyperlipidemia (PCSK9 inhibitors)8 (Fig. 1). Beyond that, due to its electron-donating nature, hexahydromethanocarbazole is also used in constructing D–π–A dyes as sensitizers for photodynamic anti-cancer therapies9 and solar cells10. Furthermore, based on the norbornene moiety of hexahydromethanocarbazole, it has the potential to be used in high-density fuels11, photoclick hydrogels for tissue engineering12, and membranes for targeted gas separations13. However, this highly desirable bridged aza-polycyclic core is far from being fully exploited as a privileged scaffold in these fields, partly due to its poor synthetic accessibility, especially when multi-substituted. In previously reported methods, two strategies have been employed for the synthesis of hexahydro-1H-1,4-methanocarbazole (Fig. 2), either by a palladium-catalyzed direct condensation between 2-iodoaniline and norbornene (NBE)14–16; or via an ortho C-H activation followed by an electrophilic attack by an external amine surrogate17–19. Unfortunately, these methods are not effective with regard to the synthesis of compounds with structural complexity, in particular 5-substituted analogs, with the 5-substituents mostly being the simple methyl group among the few examples that have been reported. This is because when adopting the existing synthetic methods, such a substituent would have to be pre-installed ortho to iodine in the starting materials, which are usually difficult to obtain both commercially and through in-house synthesis. Amine surrogates like diaziridinones are not readily available either. More importantly, there are potential adverse impacts on the oxidative addition of iodide to Pd(0) and NBE migratory insertion, as well as the risk of inducing NBE extrusion, if the pre-installed ortho-substituents are bulky. Strong bases like NaOPh and NaOMe that are necessary in most cases may not be compatible with sensitive groups as well.
Fig. 2. Strategies and methods in synthesizing hexahydro-1,4-methanocarbazole ring.
Traditional methods involved palladium-catalyzed direct condensation, or electrophilic insertion by external amine surrogates. In contrast, “detour” strategy in this work employed successive generation of two key phenylnorbornyl palladium species to construct the hexahydro-1,4-methanocarbazole core and introduce 5-substitution in tandem. Ligands to PdII, and electrophile-PdIV complexes were omitted when illustrating for the sake of clarity.
We encountered the aforementioned difficulties and problems in our pursuit of the 5-substituted hexahydro-1H-1,4-methanocarbazoles as CYP11B1 inhibitors20. Since CYP11B1 is a crucial enzyme in the biosynthesis of the glucocorticoid cortisol, such an inhibition would reduce abnormally high levels of cortisol in plasma and tissues, and thus constituted a promising therapy for related severe diseases such as Cushing’s syndrome and diabetic foot ulcer. To address the synthetic problems, we resolved to develop a novel strategy to effectively synthesize the target molecules through manipulating phenylnorbornyl palladium species in a multicomponent reaction approach. Herein, we report the convenient synthesis of 52 of such natural-product-like molecules with structural complexity and diversity using this new strategy. We further report mechanistic insights into how the desired main synthetic route competes with possible side reactions based on density functional theory (DFT)-based calculations. This innovative method exhibits a broad scope to various reactants and will enable a wide range of multi-substituted analogs of hexahydromethanocarbazole to be explored for drug design, therapeutic interventions, and the development of advanced energy materials.
Results
Design concept
Our synthetic strategy was inspired by the intriguing reactive properties of the phenylnorbornyl palladium species exhibited in the Catellani-Lautens reactions21–25, where it mediated the ortho-substitution via activating the adjacent C(sp2)-H bonds. The subsequent elimination of the NBE moiety facilitated a nucleophilic ipso-substitution. With such a superiority of disubstitution in one-pot, the Catellani-Lautens reactions were widely employed in the synthesis of various type of chemicals26–30, in particular, natural products31–33. The use of analogous norbornadiene, in contrast, triggered retro-Diels-Alder and thus led to convenient synthesis of indoles34.
In the current work, two phenylnorbornyl palladium species (I & III) generated successively were exploited as switches to control the reaction flow towards the desired destiny via C- and N- aryl-NBE palladacycle (ANP) II and IV, respectively (Fig. 2). The initial key intermediate I was obtained from the migratory insertion by a modified NBE to the oxidative adduct of Pd(0) and an easily available iodoaniline as a staring material. Rather than forming the six-membered N-PdII coordinating palladacycle V (a possible mechanistic route of the reported direct condensation methods), the phenylnorbornyl palladium complex I was modulated into C-ANP II via ortho C-H activation. Subsequent oxidative addition and reductive elimination upon the ANP II by a proper electrophile agent not only introduced an ortho substituent into the molecule, but also brought the intermediate back to a second phenylnorbornyl palladium species III, which enabled the options of NBE elimination or sustainment within the compound. By avoiding NBE extrusion (a unique feature of the Catellani-Lautens reaction) from complex III, the reaction was guided into the construction of the ortho substituted six-membered N-PdII coordinating palladacycle IV. Consequent reductive elimination of the palladacycle IV yielded the ultimate product 5-substituted hexahydro-1H-1,4-methanocarbazoles. Apparently, competitions at each diverging point (i.e., phenylnorbornyl palladium species I & III) determined the destiny of this reaction. Some potential side reactions may occur, such as prematurely elimination of the ANP II to norbornyl benzocyclobutene, as well as migration of the electrophile to the norbornyl instead of the phenyl (Fig. 2). However, despite of these challenges, we achieved this sophisticated synthesis of 5-substituted hexahydro-1H-1,4-methanocarbazoles by means of modifications on NBE reactants, deliberate choices of proper electrophile and extensive optimizations of reaction conditions.
Initial attempts and optimizations
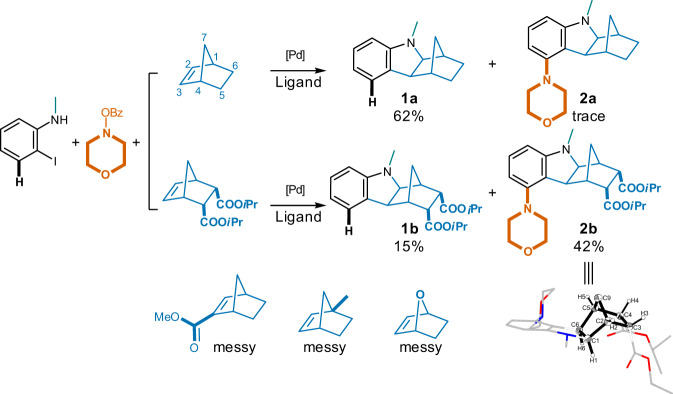
Since modifications on NBE would significantly influence its reactive and catalytic behavior35, five NBE analogs with distinct substitution patterns (Fig. 3) were first attempted. 2-Iodo-N-methylaniline (1 eq.) and morpholino benzoate (1.3 eq.) were employed as the reactants in the presence of Pd(OAc)2 (5% eq.), PPh3 (10% eq.) and NaOtBu (2.5 eq.). It turned out that unmodified NBE yielded predominately unsubstituted methanocarbazole 1a (62%); while analogs with 7-oxa, or groups on the bridge-head (1-Me) or the double bond (2-COOMe) led to messy results (Fig. 3). In contrast, NBE with 5,6-di(isopropyl carboxylate) substitution36 gave the desired 5-morpholino hexahydro-1,4-methanocarbazole 2b in a moderate yield (42%, Fig. 3) together with minor amount of unsubstituted methanocarbazole (1b, 15%). Intriguingly, compound 2b was obtained as a single stereoisomer, and its crystal structure (CCDC 2121980 (Deposition Number CCDC 2121980 contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service), Supplementary Data 1, Fig. 3) indicated an exo- cis- configuration for its rigid and somewhat strained scaffold. This is probably caused by the fact that the Re face is sterically much less hindered compare to the opposite Si face, and the reacting partners therefore approach predominantly from the Re face37.
Fig. 3. Initial investigations on NBE analogs capable of ortho C(sp2)-H activation to synthesize the 5-substituted hexahydro-1H-1,4-methanocarbazoles.
Reaction conditions: 2-iodo-N-methylaniline (1.0 eq.), NBE analog (1.0 eq.), morpholino benzoate (1.3 eq.), Pd(OAc)2 (5% eq.), PPh3 (10% eq.), NaOtBu (2.5 eq.), acetonitrile, 100 oC, 12 h.
Encouraged by this observation, the reaction conditions were further optimized using 5,6-diCOOiPr NBE as the template as well as various Pd (pre)catalysts, ligands, bases and solvents (Table 1). Replacement of NaOtBu with a milder base Cs2CO3 gave a similar yield of the desired 2a but suppressed the production of the unsubstituted methanocarbazole by approximately fivefold, while simultaneous alteration of the solvent from acetonitrile to toluene increased the yield of the 5-morpholino product to 59% (Table 1, entries 1 & 2). However, other bases such as KOAc and K3PO4 did not exhibit many advantages in distinguishing 5-morpholino and the unsubstituted products. Moreover, the dicyclohexylphosphino ligands such as RuPhos, DavePhos, and XPhos were ineffective in constructing the methanocarbazole core, whereas tri(o-furyl)phosphine (TFP) doubled the yield of the 5-morpholino compound 2b to 93% when compared to PPh3, and gave only trace of the unsubstituted by-product 1b (Table 1, entry 5). When Pd2(dba)3, Pd(PPh3)4 or Pd(dppf)Cl2 was employed instead of the precatalyst Pd(OAc)2 or PdCl2, similar amounts of 5-morpholino and the unsubstituted compounds were formed in yields of ~35%. Finally, a significant influence of the solvent used was observed in that THF led to low production of the methanocarbazole scaffold, in contrast to ~70% yields of the 5-morpholino analog 2b accompanied by a trace of the by-product in DMF or DME (Table 1, entries 10–12). Taking all aspects investigated into consideration, the optimal synthetic condition was determined to be Pd(OAc)2 (5% eq.), TFP (10% eq.) and Cs2CO3 (2.5 eq.) in toluene heating at 100 oC for 12 h.
Table 1.
Selected attempts in condition optimizationsa.
| No. | [Pd] | Ligand | Base | Solvent | Yield 2b (%) | Yield 1b (%) |
|---|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | PPh3 | Cs2CO3 | MeCN | 48 | 3 |
| 2 | Pd(OAc)2 | PPh3 | Cs2CO3 | Toluene | 59 | 6 |
| 3 | Pd(OAc)2 | PPh3 | KOAC | Toluene | 37 | 36 |
| 4 | Pd(OAc)2 | PPh3 | K3PO4 | Toluene | 45 | 16 |
| 5 | Pd(OAc)2 | TFP | Cs2CO3 | Toluene | 93 | Trace |
| 6 | Pd(OAc)2 | RuPhos | Cs2CO3 | Toluene | 9 | 28 |
| 7 | Pd2(dba)3 | TFP | Cs2CO3 | Toluene | 35 | 31 |
| 8 | Pd(PPh3)4 | — | Cs2CO3 | Toluene | 32 | 38 |
| 9 | Pd(dppf)Cl2 | — | Cs2CO3 | Toluene | 37 | 24 |
| 10 | Pd(OAc)2 | TFP | Cs2CO3 | THF | 19 | 23 |
| 11 | Pd(OAc)2 | TFP | Cs2CO3 | DMF | 69 | Trace |
| 12 | Pd(OAc)2 | TFP | Cs2CO3 | DME | 75 | Trace |
aUnless indicated otherwise, 2-iodo-N-methylaniline (1.0 eq.), NBE-5,6-diCOOiPr (1.0 eq.), morpholino benzoate (1.3 eq.), Pd (pre)catalyst (5% eq.), ligand (10% eq.), base (2.5 eq.), 100 oC, 12 h.
Reaction scopes
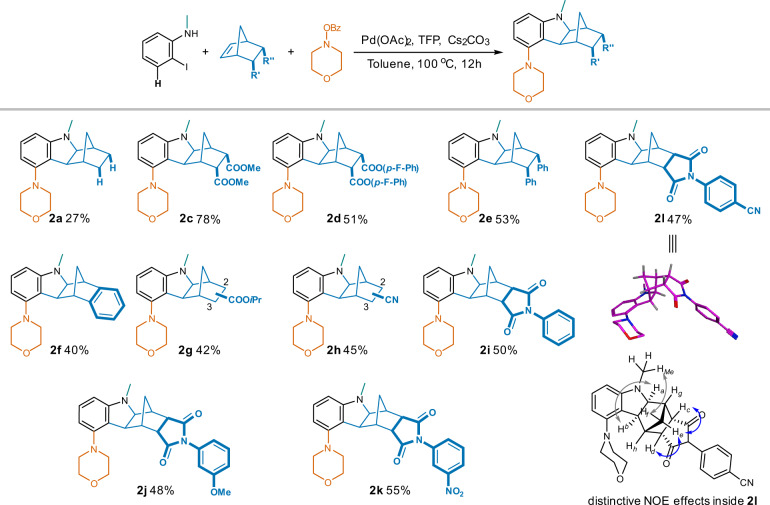
Under this optimal condition, unsubstituted NBE gave the desired 5-morpholino compound 2a in a significantly elevated yield of 27% compared to a trace under the initial condition, and the formation of 1a (without 5-insertion) was suppressed by half to 35% at the meantime (Fig. 4). In contrast to the low yield from the unsubstituted NBE, the 5,6-dicarboxylic ester substitutions led to good to excellent yields of the 5-morpholino products (93% for isopropyl ester 2b, 78% for methyl ester 2c, and 51% for the much bulkier 4-fluorophenyl ester 2d). Replacement of the carboxylic esters by 5,6-diphenyl (2e) or fused phenyl (2f) sustained adequate production of the desired 5-substituted analogs, indicating a broader scope of substitution choices in addition to the carbonyl containing groups in 2b, 2c, and 2d. Different from the 5,6-disubstitution, the mono-substituted NBE starting materials resulted in mixtures of 2- and 3-substituted 5-morpholino hexahydro-1H-1,4- methanocarbazoles (2g and 2h). The fused N-phenyl succinimides also led to their corresponding 5-morpholino products (2i-2l) in yields of ~50% regardless of the distinct stereoelectronic properties of substituents on N-phenyl. NOE effects among aliphatic protons in compound 2l revealed an exo-configuration between the indoline and NBE moieties (in accordance with the crystal data of compound 2b), however, an endo-configuration between the NBE and succinimide moieties (for configuration determination of compound 2l using 2D-NMR spectra, see Supplementary Note 1).
Fig. 4. Influences of NBE substitutions in synthesizing the corresponding 5-substituted hexahydro-1H-1,4-methanocarbazoles.
Reaction conditions: 2-iodo-N-methylaniline (1.0 eq.), NBE analog (1.0 eq.), morpholino benzoate (1.3 eq.), Pd(OAc)2 (5% eq.), TFP (10% eq.), Cs2CO3 (2.5 eq.), toluene, 100 oC, 12 h.
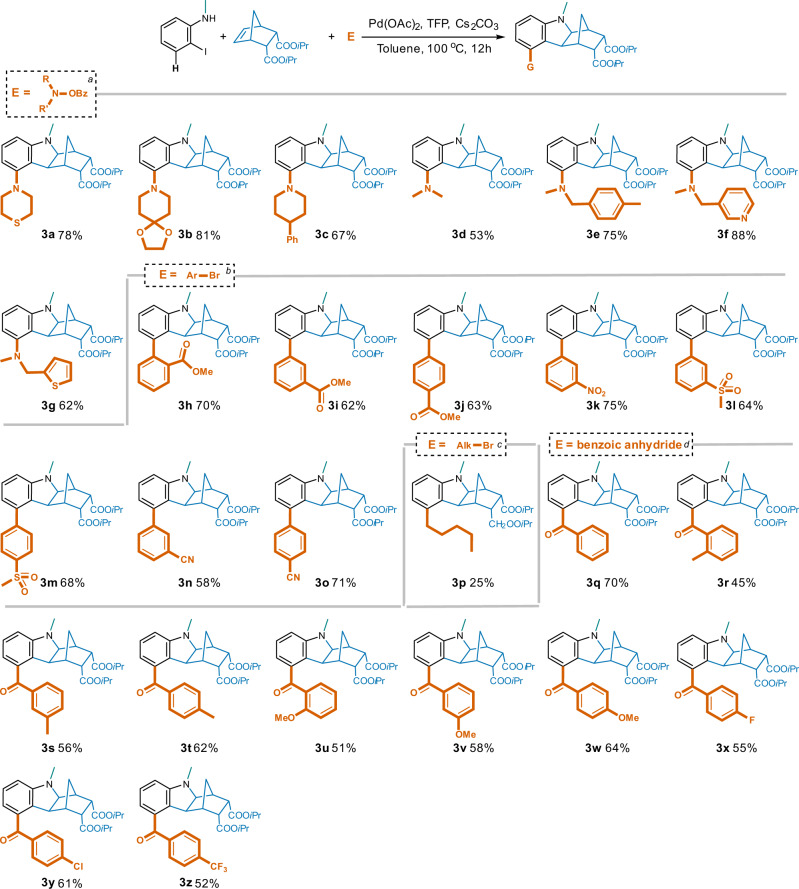
To further investigate the scope of this reaction, various types of electrophiles were employed, and broad tolerance was demonstrated (Fig. 5). When using N-benzoyloxyamines to introduce amino groups (amination)38,39 at the 5-position, morpholino (2b), thiomorpholino (3a), and the simpler dimethylamino (3d), as well as the more structurally complex and bulkier analogs (3b, 3d and 3f–3g) were all successful with good to excellent yields. Similarly, aryl bromides bearing electron- withdrawn groups like cyano, nitro, methoxycarbonyl, and methylsulfonyl led to the desired 5-aryl hexahydro-1H-1,4-methanocarbazoles (3h–3o) in polar solvent DMF with good yields. Potential steric hindrance induced by ortho-substitution was overcome as well (3 h, o-COOMe, 70% yield). By contrast, the introduction of an alkyl group or an aryl with electron-donating moiety was more challenging typically resulting in only a trace of the desired products. After further condition optimizations, 5-n- pentyl compound (3p) was isolated in 25% yield from DMF with PPh3 as the ligand. Nevertheless, introduction of the 5-benzoyl moieties (acylation)40,41 (3q–3z) using their corresponding benzoic anhydrides took place smoothly irrespective of the electronic properties and substitution patterns in good yields ranging between 45% and 70% catalyzed by PdCl2 in DME.
Fig. 5. Generation of 5-substituted hexahydro-1H-1,4-methanocarbazoles with various electrophiles.
Reaction conditions: [a]2-iodo-N-methylaniline (1.0 eq.), NBE-5,6-diCOOiPr (1.0 eq.), N-benzoyloxyamine (1.3 eq.), Pd(OAc)2 (5% eq.), TFP (10% eq.), Cs2CO3 (2.5 eq.), toluene, 100 oC, 12 h; [b]arylbromide (1.3 eq.), and DMF were used instead; [c]n-pentylbromide (1.3 eq.), PPh3 (10% eq.), and DMF were used instead; [d] benzoic anhydride (1.3 eq.), PdCl2 (5% eq.), and DME were used instead.
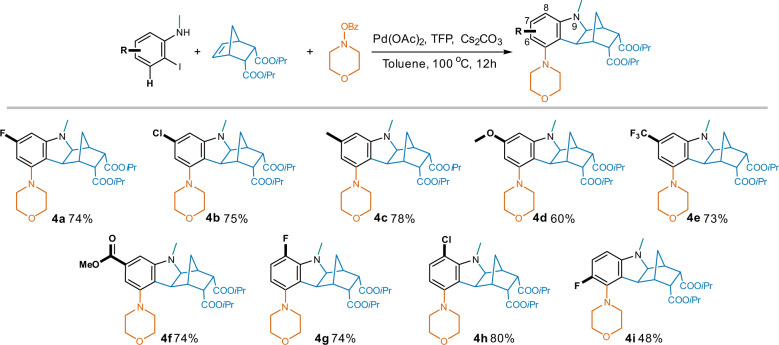
The high tolerance of this reaction to groups on 2-iodo-N-methylaniline reactants was shown in Fig. 6. Both electron-withdrawn (halogen, CF3 and COOMe) and electron-donating (Me and OMe) groups at the 7-position (meta- to morpholino) led to yields in the range of 60% to 78% (compounds 4a–4f). There is a slight decrease in yield for the 6-F (ortho- to morpholino) analog 4i (48%), probably due to a potential steric hindrance; while the 8-halo (para- to morpholino) compounds 4g and 4h were obtained in good yields (74% and 80%, respectively).
Fig. 6. Compatibility of substituents on 2-iodo-N-methylaniline.
Reaction conditions: substituted 2-iodo-N-methylaniline (1.0 eq.), NBE-5,6-diCOOiPr (1.0 eq.), morpholino benzoate (1.3 eq.), Pd(OAc)2 (5% eq.), TFP (10% eq.), Cs2CO3 (2.5 eq.), toluene, 100 oC, 12 h.
The high tolerance of this reaction to groups on 2-iodo-N-methylaniline reactants was shown in Fig. 6. Both electron-withdrawn (halogen, CF3 and COOMe) and electron-donating (Me and OMe) groups at the 7-position (meta- to morpholino) led to yields in the range of 60% to 78% (compounds 4a–4f). There is a slight decrease in yield for the 6-F (ortho- to morpholino) analog 4i (48%), probably due to a potential steric hindrance; while the 8-halo (para- to morpholino) compounds 4g and 4h were obtained in good yields (74% and 80%, respectively).
Different N-substituents were also demonstrated to be compatible within this transformation (Fig. 7). Notwithstanding a reduction in yield to 55% after enlarging the N-group from Me to tBu (5a), bulky phenyl with various substituents (H, F, OMe) at different positions achieved the corresponding analogs (5b-5e) with good yield of around 80%. Pyridyl methyl substituted aniline also resulted in the desired compound (5f) in 69% yield.
Fig. 7. Influence of N-substituents on the production of 5-substituted hexahydro-1H-1,4-methanocarbazoles.
Reaction conditions: N-substituted 2-iodoaniline (1.0 eq.), NBE-5,6-diCOOiPr (1.0 eq.), morpholino benzoate (1.3 eq.), Pd(OAc)2 (5% eq.), TFP (10% eq.), Cs2CO3 (2.5 eq.), toluene, 100 oC, 12 h.
This three-component reaction was subsequently successfully applied in synthesizing compound 3f in 2g scale with a yield of 85% using commercially available starting materials in one step (Supplementary Fig. 1), which has been identified as a potent CYP11B1 inhibitor with an IC50 of 255 nM. In contrast, a 5-step synthesis of the iodoaniline starting material was necessary if following the direct condensation method and only a moderate yield of 42% was observed in the core construction step. The method of external amine integration produced worse results as the desired compound could barely be obtained. These facts clearly demonstrated the superiority of our method regarding convenience and efficiency in constructing the 5-substituted hexahydro-1H-1,4-methanocarbazole privileged scaffold.
As manifested above, a broad scope was demonstrated for this multicomponent reaction with respect to various types of electrophiles, as well as distinct substituents at almost every topological position of each reactant. This advantage not only guarantees fast access of target molecules from easily available starting materials, but also provides the possibility to build “natural-product-like” compound libraries with high structural complexity and diversity within a short space of time via, e.g., a combinatory chemistry approach. The 52 compounds synthesized in this manuscript (see Supplementary Method 1 for synthetic details and characterization data, and see Supplementary Data 2 for spectra of 1H-, 13C-, and 19F-NMR as well as HRMS) comprised a small focused compound library and were evaluated for their inhibition of CYP11B1 (see Supplementary Method 2 for inhibitory assay conditions). Most compounds exhibited moderate to potent inhibition (Supplementary Table 1) and hence were considered as lead compounds for further drug discovery in treating diabetic foot and hypercortisolism.
Computational investigations on mechanisms
Furthermore, we employed DFT-based calculations to provide some mechanistic insights into the reaction pathways to understand how the desired main synthetic route competes with possible side reactions at the two diverging points—the phenylnorbornyl palladium species I and III. The DFT optimizations, potential energy scan and frequency calculations were performed using B3LYP-D3 level of theory with additional M06-2X single point calculations carried out using the DFT optimized geometry. The free energy profiles that we used in the discussion are the sum of the M06-2X potential energy and thermal corrections to free energy from the B3LYP-D3 frequency calculations (see Supplementary Method 3 for full details of the computational methods, and see Supplementary Data 3 for Cartesian coordinates of all the structures reported and their absolute energies in Hartree).
| 1 |
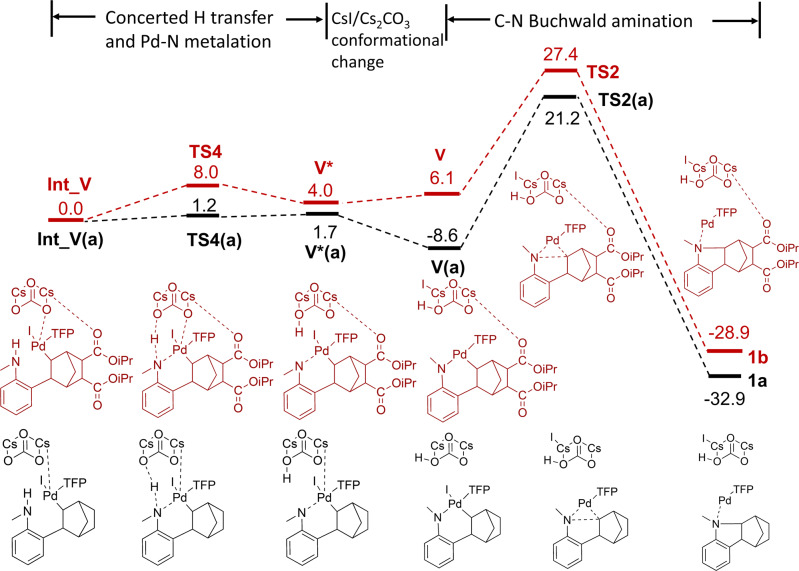
The initial phenylnorbornyl palladium intermediate I is flexible and can either rotate for the reaction to flow towards Int_I → II or to follow the direction of V to by-product 1b. A previous computational study34 demonstrates that the energy associated with the rotation of I towards the two competing reaction pathways is nearly identical and is only 2–3 kcal/mol. Therefore, we assumed this step was unlikely to provide significant mechanistic insights and first calculated the energy profile related to the Pd-N metalation and intramolecular C(sp3)-N Buchwald amination to yield methanocarbazoles (by-product 1b) for 5,6-diCOOiPr NBE. We manually placed the base Cs2CO3 in Intermediate I in a similar position as seen in the previous computational study34 and optimized the system without any constraints. This resulted in a conformation where the distance between O in Cs2CO3 and H in the amine group is 1.73 Å, the distance between another O in Cs2CO3 and Pd is 2.1 Å and the distance between N in the amine group and Pd is 3.18 Å. It is noteworthy that the two Cs form interactions with the two carbonyl O in the 5,6-DiCOOiPr substitution on NBE (3.16 Å and 3.19 Å, respectively) (Supplementary Fig. 2). It seems Cs2CO3 forms multiple interactions with the intermediate to hold it in balance to form Int_V. The subsequent reaction involved two steps—Step 1 is the concerted hydrogen transfer from the amine group to the base Cs2CO3 and Pd-N metalation with a moderate reaction barrier of 8.0 kcal/mol and an overall reaction energy of 4.0 kcal/mol. Along the reaction pathway, the Pd-N distance is decreased from 3.18 Å to 2.14 Å while the O(Cs2CO3)-Pd distance is increased from 2.1 Å to 3.4 Å as the base is moving away from the reaction center upon protonation. Release of CsI from the reaction center is associated with a 2.1 kcal/mol increase in energy to form V. This is then followed by Step 2, the formation of the C–N bond to close the five-membered ring to form 1b. The energy barrier for the C–N bond formation is 21.3 kcal/mol with an energy of reaction of –35.0 kcal/mol for 5,6-diCOOiPr NBE (Fig. 8).
Fig. 8. Computed free energy profile of Int_V → V → by-product 1a and 1b.
Intermediates and transit states with 5,6-diCOOiPr were illustrated in dark red, while unsubstituted ones were illustrated in black. The energies are given in kcal/mol.
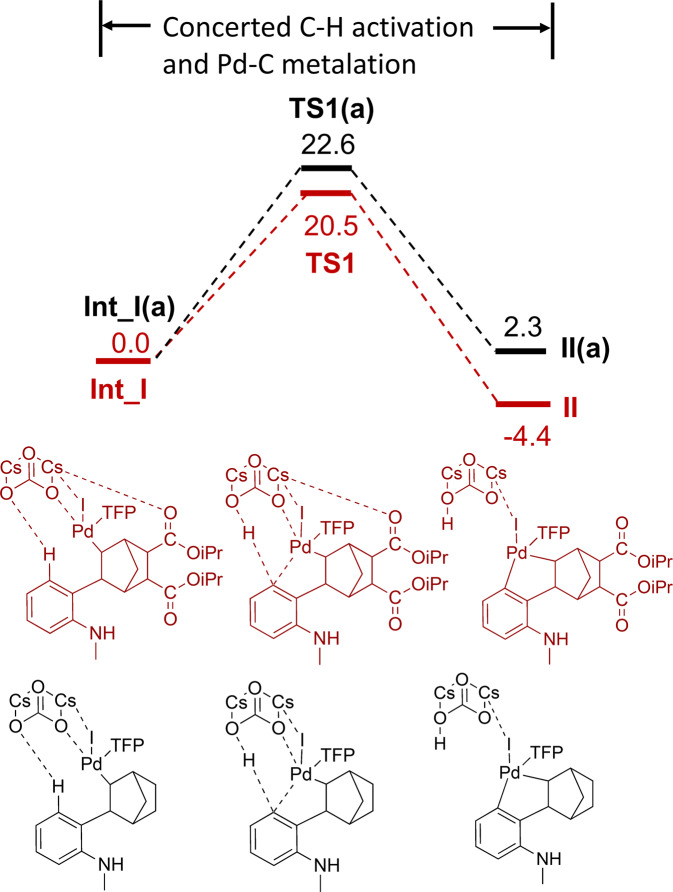
We then calculated the energy profile of generating II for 5,6-diCOOiPr NBE, which is the key intermediate step leading to the formation of the desired product 2b. Similar to the aforementioned approach, Intermediate I was rotated to a conformation suitable for C-H activation and Cs2CO3 was placed in a position close to the reaction center. After optimization, Cs2CO3 interacts with H in the C-H activation moiety, Pd and the carbonyl O in the 5,6-DiCOOiPr substitution to form Int_I (distances 2.16 Å, 2.09 Å, and 3.16 Å, respectively, Supplementary Fig. 3). This reaction is a concerted metalation–deprotonation process of the C-H activation and formation of the Pd–C bond, similar to what was observed in a previous study42.
The reaction barrier height was calculated to be 20.5 kcal/mol and an energy of reaction –4.4 kcal/mol for this step (Fig. 9). The Pd-C distance is reduced from 3.16 Å to 2.03 Å along the reaction pathway. It is clear that for 5,6-diCOOiPr NBE, the C-H activation direction of rection (I → II) is more kinetically and thermodynamically favored compared to the overall barrier of 27.4 kcal/mol for the Pd-N metalation/C-N Buchwald amination steps (I → V) (Fig. 8). This would explain why 2b usually exhibited a higher yield than 1b in both Fig. 3 and Table 1, strongly indicating that at the first diverging point, the reaction favors the direction of I → II.
Fig. 9. Computed free energy profile of Int_I → TS1 → II.
Intermediates and transit states with 5,6-diCOOiPr were illustrated in dark red, while unsubstituted ones were illustrated in black. The energies are given in kcal/mol.
Additionally, when TFP is replaced by DavePhos in Int_I, O in Cs2CO3 is further away from the hydrogen for C-H activation at 3.81 Å (Supplementary Fig. 3). This longer hydrogen transfer distance is expected to give rise to a higher energy barrier for the C-H activation with DavePhos, consistent with the experimental observation that bulky ligands such as RuPhos, DavePhos, and XPhos were ineffective in constructing the methanocarbazole core.
| 2 |
The reaction mechanism of converting III to IV is different to what has been discussed for Int_V → V → by-product. After introducing a substitution group at the 5-position using morpholino in III, the concerted hydrogen transfer from the amine group to the base Cs2CO3 and Pd-N metalation occurs simultaneously along the optimization without a clear barrier. The energy barrier for the C-N formation is lowered to 17.0 kcal/mol and the energy of reaction is –43.0 kcal/mol, both lower compared to V → by-product 1b (Supplementary Fig. 4). Consistent with the study by Zhang et al.34, the barrier for the C-N Buchwald amination decreased significantly, which may be due to conjugation with the secondary amine group. It is noteworthy that the mechanism of this C-N Buchwald amination is similar to the DFT study reported by McMullin et al.43, but different from the study by Zhang et al.34, in which the bulkier tert-butyl (tBu) and tert-butyloxycarbonyl (tBOC) groups were used on the nitrogen. In that case, the steric hindrance from the bulkier groups prevents Pd from attacking the nitrogen directly and the hydrogen transfer from the amine would occur with a considerable barrier (~16 kcal/mol), followed by Pd-N metalation also with a lower barrier (~9 kcal/mol) (15.5 kcal/mol if the hydrogen transfer and Pd-N metalation are concerted). In our DFT study, the smaller methyl group on the nitrogen enabled simultaneous hydrogen transfer and Pd-N metalation, leading to the rapid formation of IV. It is plausible that this introduces a catalytic advantage for the main reaction pathway (III–IV) over the side reaction of NBE extrusion in the Catellani-Lanterns reaction (III–VI). Consistent with insights from the DFT calculations, we observed a reduction in yield from 78% to 55% experimentally after enlarging the N-group from Me to tBu (5a) (Fig. 7), which may be the result of an increased side reaction of III → VI. Interestingly, the steric hindrance of the N-groups seems to be most pronounced in the position right adjacent to the nitrogen, as various bulky benzyl analogs (a slim methylene presents in between) achieved good yields (Fig. 7).
| 3 |
The effect of NBE substitutions was studied by comparing unsubstituted NBE with 5,6-diCOOiPr NBE in Int_I → II and Int_V → V → 1a/1b, respectively, at the first diverging point. Although the C-H activation in transforming from Int_I to II is the well-studied concerted metalation–deprotonation process, 5,6-diCOOiPr NBE exhibits a slightly lower reaction barrier (20.5 kcal/mol) compared to that of the unsubstituted NBE (22.6 kcal/mol) (Fig. 9) and the transition state structures of these two are also noticeably different. The transition state structure of unsubstituted NBE from our calculation is similar to a methyl analog reported in a previous DFT study using the same level of theory43. However, in the transition state structure of 5,6-diCOOiPr NBE, the Pd ligand TFP is rotated substantially because of the steric hindrance caused by the substitution, and a Cs atom from the base Cs2CO3 forms a coordination with a carbonyl in the NBE substitution (Fig. 10). We tried to delete the 5,6-diCOOiPr and re-optimize the complex, but it did not give a valid structure, implying that the NBE substitutions along with Cs2CO3 may facilitate the C-H activation through an altered conformation in comparison to the unsubstituted NBE.
Fig. 10. Transition state structures TS1 and TS1(a).
Transition state structures of the Cs2CO3-induced C-H activation through a concerted metalation–deprotonation process to result in II and II(a). A With 5,6-diCOOiPr NBE (barrier height 20.5 kcal/mol), where a coordination between Cs and carbonyl O was observed, and B With the unsubstituted NBE (barrier height 22.6 kcal/mol). Different orientations of TFP were noted.
Along the Int_V(a)→V(a)→by-product 1a pathway, the distance between O in Cs2CO3 and H in the amine group is 1.60 Å, poised for H transfer and the distance between N in the amine group and Pd is 2.24 Å in Int_Va, significantly shorter than the 3.18 Å seen in Int_V. In addition, no coordination is formed between O in Cs2CO3 and Pd (Supplementary Fig. 2). Consequently, the concerted hydrogen transfer from the amine group to the base Cs2CO3 and Pd-N metalation occurs with a negligible 1.2 kcal/mol barrier and 1.7 kcal/mol for the energy of reaction to form V(a). The barrier for the C–N bond formation from V to 1a is 29.8 kcal/mol for the unsubstituted NBE. Comparison with unsubstituted and substituted NBE indicates that Cs2CO3 plays multiple roles in the substituted case-forming coordination with Pd, interacting with the 5,6-DiCOOiPr substitution along with H abstraction, which raises the barrier in going from Int_V to V and pushes the reaction flow towards the main reaction pathway consequently. This indicated that interactions between Cs and the substitutions on NBE may modulate the reaction barriers. Taken together, the unsubstituted NBE is more likely to proceed through the C-N Buchwald amination from I. This is consistent with our experiment that the yield of 1a (62%) was significantly higher than that of 1b (15%) in Fig. 3. It is worth noting that some calculated barriers are sensitive to the level of DFT method used, in particular when they are associated with hydrogen transfer and the position of Cs2CO3 (Supplementary Table 2). This is due to factors such as the intrinsic errors of DFT methods and the limits of implicit solvent model and that energetics are derived from potential energy scan, which restricts conformational sampling. Nonetheless, the order of barriers from the current study is in line with previous computational studies where energy differences between different reaction pathways can be also rather small. More importantly, our DFT calculations are consistent with our experimental data and provide additional mechanistic insights, which reflects the strength of a combined experimental and computational approach.
Discussions
The sophisticated but convenient reaction accomplished in our multicomponent strategy was a cascade consisted of intercepted Heck addition/C(sp2)-H activation/C-palladacycle (ANP) formation/electrophilic oxidative addition and reductive elimination/N-palladacycle formation/intramolecular Buchwald amination (Fig. 2). In contrast, the previously reported palladium-catalyzed direct condensation involved only the commencing and consequent stages, in which the initial phenylnorbornyl palladium species I was generated by intercepting a Heck reaction and then underwent an immediate Buchwald amination to yield the final compound. Compared to direct condensation, a detour was deliberately introduced in our approach, in which the initial phenylnorbornyl palladium species I was driven into an ANP II via ortho C(sp2)-H activation and a more complex phenylnorbornyl palladium species III for the final Buchwald amination was subsequently regenerated from an electrophilic attack of the ANP. Obviously, such a regeneration of reactive species provided the possibilities of introducing further structural complexity in a single reaction.
The first part of this method was alike to that of the Catellani-Lautens reaction in mechanism. However, a Catellani-Lautens reaction would not take place when a secondary amino group furnished ortho to the carbon being palladated. An intramolecular Buchwald amination would occur instead after the migratory insertion of NBE (as seen in method of direct condensation)44,45. Our study provided an unusual example of transforming a phenylnorbornyl palladium species into an ANP in the presence of an adjacent amino group. Moreover, a distinct divergence occurred upon the regenerated phenylnorbornyl palladium species III, which underwent a Buchwald amination to form the methanocarbazole core in our method, but would eliminate the NBE moiety resulting in a phenyl palladium complex VI in a Catellani-Lautens reaction. Nevertheless, as minor amount of NBE extruding product VII (<5%) was observed in some cases, it would be possible to achieve Catellani-Lautens reactions with suitable nucleophiles after additional modifications of NBE, N-substituents and reacting conditions. Apparently, the initial and regenerated phenylnorbornyl palladium species (I & III) served as switches and these two diverging points determined the outcomes of this complex reaction comprising of multiple concomitant electrophiles and nucleophiles.
In this study, the initial phenylnorbornyl palladium species I was generated via the migratory insertion of a modified NBE to a aryl-Pd(II) intermediate, which was yielded from the oxidative addition of an aryl halide (e.g., aminophenyl iodide) to a Pd(0) catalyst. Notably, such an aryl-Pd(II) intermediate could also be obtained by direct electrophilic palladation of heterocycles like thiophene46 and indole47,48. These heterocycles could be explored as the substrates, therefore further expanding the scope of this method. Similarly, other bridged bicyclic olefins as bioisosteres of NBE, such as 7-azanorbornene, vince lactam, and camphene, are likely to produce analogous aryl-bicycloalkyl palladium species for subsequent reaction cascade due to their strained and tethered conformations. These potential substrates could further increase the structural diversity of the end products when employing our method to build a natural-product-like compound library.
Furthermore, the phenylnorbornyl palladium species exploited in our study are a special case of σ-alkyl palladium complexes, which present in various C-H and C-C activations as reactive intermediates49. In most reports, only a single σ-alkyl palladium species was generated in situ and subsequently trapped by a reaction partner to terminate the reaction. However, besides interception of an Heck reaction, the σ-alkyl palladium complex could be generated in several ways, including directed C(sp3)−H activation assisted with coordinating auxiliaries and electrophilic attacks of certain palladacycles. Moreover, these σ-alkyl palladium species could also be captured in multiple approaches, such as reacting with various nucleophiles (e.g., boronic acids), and coupling with benzyne. Given these facts, the “detour by regeneration” strategy in exploiting phenylnorbornyl palladium species manifested in the current study could be feasible for other σ-alkyl palladium complexes, and therefore would facilitate their applications in synthesizing highly structurally complex molecules via multicomponent cascades within a single reaction.
Conclusion
In conclusion, we have accomplished a convenient multicomponent construction of 5-substituted hexahydro-1H-1,4-methanocarbazoles as single stereoisomers, which were otherwise difficult to access. This was achieved through manipulation of two phenylnorbornyl palladium species generated successively by modifying the NBE reactant with distinct substituents and careful alterations of the reaction conditions. This method exhibited a broad scope to various reactants and would facilitate the construction of natural-product-like compound libraries with high structural complexity and diversity. Such a “detour by regeneration” concept in exploiting phenylnorbornyl palladium species could be feasible for the application of other σ-alkyl palladium complexes in sophisticated organic synthesis. We believe this innovative synthesis strategy will promote the implementation of hexahydromethanocarbazole as a privileged scaffold for the development of new drugs, materials and biomedical probes.
Methods
A mixture of the corresponding substituted 2-iodo-N-substituted aniline (0.43 mmol), substituted norbornene derivatives (0.65 mmol), a proper electrophile reagent (such as amino benzoate, arylbromide, or benzoic anhydride, 0.86 mmol), Cs2CO3 (224 mg, 1.60 mmol), Pd(OAC)2 (9 mg, 0.04 mmol), and TFP (24 mg, 0.08 mmol) was stirred in toluene (2 mL) at 100 °C under an inert atmosphere for 12 h. After the reaction mixture was cooled down to room temperature, it was filtrated and extracted with ethyl acetate/brine for three times. The combined organic layers were dried over anhydrous Na2SO4 and concentrated to give the crude product, which was subsequently purified by column chromatography (petroleum ether/ethyl acetate) to yield the desired product.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This publication was supported by the National Natural Science Foundation of China (Grant No. 81872739), the Zhujiang Distinguish Professorship of Guangdong Province, China (2018), the International Scientific Collaboration Program of Guangdong Province, China (Grant No. 2020A0505100053), the Key Research and Development Program of Guangdong Province, China (Grant No. 2019B02021002) and the Key Research Program of Guangdong Provincial Bureau of Education, China (Grant No. 2019KZDXM057).
Author contributions
Q.H. and L.Y. conceived and designed the study; T.G., J.C., D.P., J.L., J.H., J.W., X.C., T.Y., X.H., and Y.H. carried out the chemical synthesis and biological tests; J.P. conducted the computational research; Q.H., J.P., and L.Y. wrote the manuscript.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
Methods and experimental procedures of chemical synthesis, CYP11B1 inhibitory assay and DFT calculations, characterization of final compounds, as well as energy profiles and illustrations of key intermediates are available in the Supplementary Information. The X-ray crystallographic coordinates for structures reported in this Article have been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition number CCDC 2121980. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif. Supplementary Data 1: crystal data of compound 2b. Supplementary Data 2: The 1H-, 13C-, and 19F-NMR as well as HRMS spectra of final compounds. (10.6084/m9.figshare.21252192.v1). Supplementary Data 3: Cartesian coordinates of all the structures reported and their absolute energies in Hartree (10.6084/m9.figshare.21252711.v1).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ting Guan, Jie Cheng, Dongchao Pan, Jinyang Lu.
Contributor Information
Lina Yin, Email: linayin@gzucm.edu.cn.
Jiayun Pang, Email: j.pang@gre.ac.uk.
Qingzhong Hu, Email: huqqzh@gzucm.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-022-00759-4.
References
- 1.Shelat AA, Guy RK. Scaffold composition and biological relevance of screening libraries. Nat. Chem. Biol. 2007;3:442–446. doi: 10.1038/nchembio0807-442. [DOI] [PubMed] [Google Scholar]
- 2.Clemons PA, et al. Quantifying structure and performance diversity for sets of small molecules comprising small-molecule screening collections. Proc. Natl Acad. Sci. USA. 2011;108:6817–6822. doi: 10.1073/pnas.1015024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovering F, Bikker J, Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 4.Wei WX, Cherukupalli S, Jing LL, Liu XY, Zhan P. Fsp(3): a new parameter for drug-likeness. Drug Discov. Today. 2020;25:1839–1845. doi: 10.1016/j.drudis.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Yang YD, Engkvist O, Llinas A, Chen HM. Beyond size, ionization state, and lipophilicity: influence of molecular topology on absorption, distribution, metabolism, excretion, and toxicity for druglike compounds. J. Med. Chem. 2012;55:3667–3677. doi: 10.1021/jm201548z. [DOI] [PubMed] [Google Scholar]
- 6.Chang CEA, Chen W, Gilson MK. Ligand configurational entropy and protein binding. Proc. Natl Acad. Sci. USA. 2007;104:1534–1539. doi: 10.1073/pnas.0610494104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ndongo JT, et al. Carbazole-, aspidofractinine-, and aspidocarpamine-type alkaloids from Pleiocarpa pycnantha. J. Nat. Prod. 2018;81:1193–1202. doi: 10.1021/acs.jnatprod.7b00958. [DOI] [PubMed] [Google Scholar]
- 8.Fazio S., Tavori, H. & Wu, J. Methods of inhibiting PCSK9. Patent WO2018129205A1 (2018).
- 9.Fuse S, et al. Thiophene-based organic D-pi-A dyes as potent sensitizers for photodynamic therapy. Eur. J. Org. Chem. 2017;2017:5170–5177. doi: 10.1002/ejoc.201701019. [DOI] [Google Scholar]
- 10.Gudim, N. S. et al. Monitoring the dependence of the photovoltaic properties of dye-sensitized solar cells from the structure of D-A-pi-A-type sensitizers with a 9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazole donor building block. Mol. Syst. Des. Eng. 10.1039/d1032me00025c (2022).
- 11.Zhang, C. Q., Zhang, X. W., Zou, J. J. & Li, G. Z. Catalytic dimerization of norbornadiene and norbornene into hydrocarbons with multiple bridge rings for potential high-density fuels. Coordin. Chem. Rev. 436, 10.1016/j.ccr.2021.213779 (2021).
- 12.Lin, C. C., Ki, C. S. & Shih, H. Thiol-norbornene photoclick hydrogels for tissue engineering applications. J. Appl. Polym. Sci. 132, 10.1002/app.41563 (2015). [DOI] [PMC free article] [PubMed]
- 13.Wang XY, et al. Substituted polynorbornene membranes: a modular template for targeted gas separations. Polym. Chem. 2021;12:2947–2977. doi: 10.1039/D1PY00278C. [DOI] [Google Scholar]
- 14.Catellani M, Del Rio A. Catalytic arylation of carbon-carbon double bond followed by N- or O-cyclization. Russ. Chem. Bull. 1998;47:928–931. doi: 10.1007/BF02498163. [DOI] [Google Scholar]
- 15.Dolja E, Funken N, Slak D, Schnakenburg G, Gansauer A. A Divergent duo: palladium catalyzed carboamination in enantioselective desymmetrization and regiodivergent catalysis. Chemcatchem. 2019;11:5421–5424. doi: 10.1002/cctc.201901784. [DOI] [Google Scholar]
- 16.Tao MN, Li WB, Zhang JL. Pd/Xiang-Phos-catalyzed enantioselective intermolecular carboheterofunctionalization of norbornene and norbornadiene. Chem. Commun. 2020;56:13125–13128. doi: 10.1039/D0CC04996D. [DOI] [PubMed] [Google Scholar]
- 17.Zheng HJ, Zhu YG, Shi Y. Palladium(0)-catalyzed heck reaction/C-H activation/amination sequence with diaziridinone: a facile approach to indolines. Angew. Chem. Int. Ed. 2014;53:11280–11284. doi: 10.1002/anie.201405365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jafarpour F, Jalalimanesh N, Teimouri M, Shamsianpour M. Palladium/norbornene chemistry: an unexpected route to methanocarbazole derivatives via three Csp(3)-Csp(2)/Csp(3)-N/Csp(2)-N bond formations in a single synthetic sequence. Chem. Commun. 2015;51:225–228. doi: 10.1039/C4CC06789D. [DOI] [PubMed] [Google Scholar]
- 19.Ghasemi M, Jafarpour F, Habibi A. Palladium/norbornene chemistry in the synthesis of polycyclic indolines with simple nitrogen sources. Synthesis (Stuttg.) 2020;52:2092–2098. doi: 10.1055/s-0039-1707988. [DOI] [Google Scholar]
- 20.Yin L, et al. Design, synthesis, and biological evaluations of pyridyl 4,5,6,7-tetrahydro-4,7-methanobenzo[d]isoxazoles as potent and selective inhibitors of 11beta-hydroxylase. J. Med. Chem. 2022;65:11876–11888. doi: 10.1021/acs.jmedchem.2c01037. [DOI] [PubMed] [Google Scholar]
- 21.Wang JC, Dong GB. Palladium/norbornene cooperative catalysis. Chem. Rev. 2019;119:7478–7528. doi: 10.1021/acs.chemrev.9b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Della CaN, Fontana M, Motti E, Catellani M. Pd/Norbornene: a winning combination for selective aromatic functionalization via C-H bond activation. Acc. Chem. Res. 2016;49:1389–1400. doi: 10.1021/acs.accounts.6b00165. [DOI] [PubMed] [Google Scholar]
- 23.Lautens M, Piguel S. A new route to fused aromatic compounds by using a palladium-catalyzed alkylation-alkenylation sequence. Angew. Chem. Int. Ed. 2000;39:1045–1046. doi: 10.1002/(SICI)1521-3773(20000317)39:6<1045::AID-ANIE1045>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Ye J, Lautens M. Palladium-catalysed norbornene-mediated C-H functionalization of arenes. Nat. Chem. 2015;7:863–870. doi: 10.1038/nchem.2372. [DOI] [PubMed] [Google Scholar]
- 25.Wu Z, Xu X, Wang J, Dong G. Carbonyl 1,2-transposition through triflate-mediated alpha-amination. Science. 2021;374:734–740. doi: 10.1126/science.abl7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Wang J, Dong G. Modular entry to functionalized tetrahydrobenzo[b]azepines via the palladium/norbornene cooperative catalysis enabled by a C7-modified norbornene. J. Am. Chem. Soc. 2021;143:9991–10004. doi: 10.1021/jacs.1c04575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Dong Z, Yang C, Dong G. Modular and regioselective synthesis of all-carbon tetrasubstituted olefins enabled by an alkenyl Catellani reaction. Nat. Chem. 2019;11:1106–1112. doi: 10.1038/s41557-019-0358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong Y, Liu R, Wang W. Catalytic asymmetric Catellani-type reaction: a powerful tool for axial chirality construction. Green. Syn. Catal. 2020;1:83–85. doi: 10.1016/j.gresc.2020.09.002. [DOI] [Google Scholar]
- 29.Ding L, Sui X, Gu Z. Enantioselective synthesis of biaryl atropisomers via Pd/norbornene-catalyzed three-component cross-couplings. Acs Catal. 2018;8:5630–5635. doi: 10.1021/acscatal.8b01037. [DOI] [Google Scholar]
- 30.Liu ZS, et al. Construction of axial chirality via palladium/chiral norbornene cooperative catalysis. Nat. Catal. 2020;3:727–733. doi: 10.1038/s41929-020-0494-1. [DOI] [Google Scholar]
- 31.Sui X, Zhu R, Li G, Ma X, Gu Z. Pd-catalyzed chemoselective Catellani ortho-arylation of iodopyrroles: rapid total synthesis of rhazinal. J. Am. Chem. Soc. 2013;135:9318–9321. doi: 10.1021/ja404494u. [DOI] [PubMed] [Google Scholar]
- 32.Richardson, A. D., Vogel, T. R., Traficante, E. F., Glover, K. J. & Schindler C. S. Total synthesis of (+)-cochlearol B by an APproach Based on A Catellani Reaction and Visible-light-enabled [2+2] cycloaddition. Angew. Chem. Int. Ed. 61, 10.1002/anie.202201213 (2022). [DOI] [PMC free article] [PubMed]
- 33.Weinstabl H, Suhartono M, Qureshi Z, Lautens M. Total synthesis of (+)-linoxepin by utilizing the Catellani reaction. Angew. Chem. Int. Ed. 2013;52:5305–5308. doi: 10.1002/anie.201302327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang BS, et al. Synthesis of C4-aminated indoles via a Catellani and retro-diels-alder strategy. J. Am. Chem. Soc. 2019;141:9731–9738. doi: 10.1021/jacs.9b05009. [DOI] [PubMed] [Google Scholar]
- 35.Li RH, Dong GB. Structurally modified norbornenes: a key factor to modulate reaction selectivity in the palladium/norbornene cooperative catalysis. J. Am. Chem. Soc. 2020;142:17859–17875. doi: 10.1021/jacs.0c09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, et al. Pd(II)/Norbornene-catalyzed Meta-C-H alkylation of nosyl-protected phenylalanines. J. Org. Chem. 2018;83:13211–13216. doi: 10.1021/acs.joc.8b01933. [DOI] [PubMed] [Google Scholar]
- 37.Li CF, Liu L, Fu XG, Huang JH. Norbornene in organic synthesis. Synthesis (Stuttg.) 2018;50:2799–2823. doi: 10.1055/s-0037-1610143. [DOI] [Google Scholar]
- 38.Dong Z, Dong G. Ortho vs ipso: site-selective Pd and norbornene-catalyzed arene C-H amination using aryl halides. J. Am. Chem. Soc. 2013;135:18350–18353. doi: 10.1021/ja410823e. [DOI] [PubMed] [Google Scholar]
- 39.Shi H, Babinski DJ, Ritter T. Modular C-H functionalization cascade of aryl iodides. J. Am. Chem. Soc. 2015;137:3775–3778. doi: 10.1021/jacs.5b01082. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Zhu R, Zhao K, Gu Z. Palladium-catalyzed catellani ortho-acylation reaction: an efficient and regiospecific synthesis of diaryl ketones. Angew. Chem. Int. Ed. 2015;54:12669–12672. doi: 10.1002/anie.201506446. [DOI] [PubMed] [Google Scholar]
- 41.Dong Z, Wang J, Ren Z, Dong G, Ortho C-H. Acylation of aryl iodides by palladium/norbornene catalysis. Angew. Chem. Int. Ed. 2015;54:12664–12668. doi: 10.1002/anie.201506397. [DOI] [PubMed] [Google Scholar]
- 42.Liang YJ, Jiang YY, Liu YX, Bi SW. Mechanism of Pd-catalyzed acylation/alkenylation of aryl iodide: a DFT study. Org. Biomol. Chem. 2017;15:6147–6156. doi: 10.1039/C7OB01021D. [DOI] [PubMed] [Google Scholar]
- 43.McMullin CL, et al. Computational study of (PBu3)-Bu-t as ligand in the palladium-catalysed amination of phenylbromide with morpholine. J. Mol. Catal. A. 2010;324:48–55. doi: 10.1016/j.molcata.2010.02.030. [DOI] [Google Scholar]
- 44.Qi XT, Wang JC, Dong Z, Dong GB, Liu P. Compatibility score for rational electrophile selection in Pd/NBE cooperative catalysis. Chem. 2020;6:2810–2825. doi: 10.1016/j.chempr.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lautens M, PaquinJ F, Piguel S, Dahlmann M. Palladium-catalyzed sequential alkylation−alkenylation reactions and their application to the synthesis of fused aromatic rings. J. Org. Chem. 2001;66:8127–8134. doi: 10.1021/jo0107296. [DOI] [PubMed] [Google Scholar]
- 46.Li RH, Zhou Y, Xu XL, Dong GB. Direct vicinal difunctionalization of thiophenes enabled by the palladium/norbornene cooperative catalysis. J. Am. Chem. Soc. 2019;141:18958–18963. doi: 10.1021/jacs.9b10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capito, E., Brown, J. M., & Ricci A. Directed palladation: fine tuning permits the catalytic 2-alkenylation of indoles. Chem. Commun. 14, 1854–1856 (2005). [DOI] [PubMed]
- 48.Ferreira EM, Stoltz BM. Catalytic C - H bond functionalization with palladium(II): aerobic oxidative annulations of indoles. J. Am. Chem. Soc. 2003;125:9578–9579. doi: 10.1021/ja035054y. [DOI] [PubMed] [Google Scholar]
- 49.He J, Wasa M, Chan KSL, Shao O, Yu JQ. Palladium-catalyzed transformations of alkyl C-H bonds. Chem. Rev. 2017;117:8754–8786. doi: 10.1021/acs.chemrev.6b00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Methods and experimental procedures of chemical synthesis, CYP11B1 inhibitory assay and DFT calculations, characterization of final compounds, as well as energy profiles and illustrations of key intermediates are available in the Supplementary Information. The X-ray crystallographic coordinates for structures reported in this Article have been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition number CCDC 2121980. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif. Supplementary Data 1: crystal data of compound 2b. Supplementary Data 2: The 1H-, 13C-, and 19F-NMR as well as HRMS spectra of final compounds. (10.6084/m9.figshare.21252192.v1). Supplementary Data 3: Cartesian coordinates of all the structures reported and their absolute energies in Hartree (10.6084/m9.figshare.21252711.v1).