Abstract
Macrophages are large highly motile phagocytic leukocytes that appear early during embryonic development and have been conserved during evolution. The developmental roles of macrophages were first described nearly a century ago, at about the time these cells were being identified as central effectors in phagocytosis and elimination of microbes. Since then, we have made considerable progress in understanding the development of various subsets of macrophages and the diverse roles these cells play in both physiology and disease. This article reviews the phylogeny and the ontogeny of macrophages with a particular focus on the gastrointestinal tract, and the role of these mucosal macrophages in immune surveillance, innate immunity, homeostasis, tissue remodeling, angiogenesis, and repair of damaged tissues. We also discuss the importance of these macrophages in the inflammatory changes in neonatal necrotizing enterocolitis (NEC). This article presents a combination of our own peer-reviewed clinical and preclinical studies, with an extensive review of the literature using the databases PubMed, EMBASE, and Scopus.
Keywords: Blood counts, Inflammation, Macrophages, Monocytes, Organ injury, Signaling
Evolution of Macrophages
Macrophages have Developed in a Conserved Process through Evolution
Macrophages were first described at the beginning of the previous century by Paul Ehrlich and Ilya Metchnikoff as important mediators of innate immunity.1 The name “macrophages” or “big eaters” came from the Greek words, “makros” or large, and “phagein” or eat.2 Macrophages are large cells with an irregular cell shape, oval- or kidney-shaped nucleus, cytoplasmic vesicles, central nucleus, and high cytoplasm-to-nucleus ratio.3 These cells are highly phagocytic and motile, and modulate immune responses by releasing various mediators.4 The term mononuclear phagocyte includes lineage-committed bone marrow precursors, circulating monocytes, resident macrophages, and dendritic cells (DCs).5 In this review, we have focused on the macrophage lineage as it expands and matures first, in utero, and plays an important role in the innate immune responses of newborn infants.
Macrophages can phagocytose smaller organisms, foreign materials, and cellular debris.6,7 This ability to ingest particles larger than 0.4 μm in diameter has followed a recognizable pattern across evolution, be it in unicellular Protists such as the soil-living amoebae Dictyostelium discoideum and protozoans such as Trichomonas vaginalis, or in macrophages in multicellular eukaryotes.8,9 Phagocytic cells in invertebrate species are known by other names such as amebocytes, celomocytes, or hemocytes, but each shows morphological similarities with the macrophages of vertebrates (Flowchart 1).10,11 These similarities extend to the molecular level with the expression of proteins containing the scavenger receptor cysteine-rich (SRCR) domains.6,12
Flowchart 1:
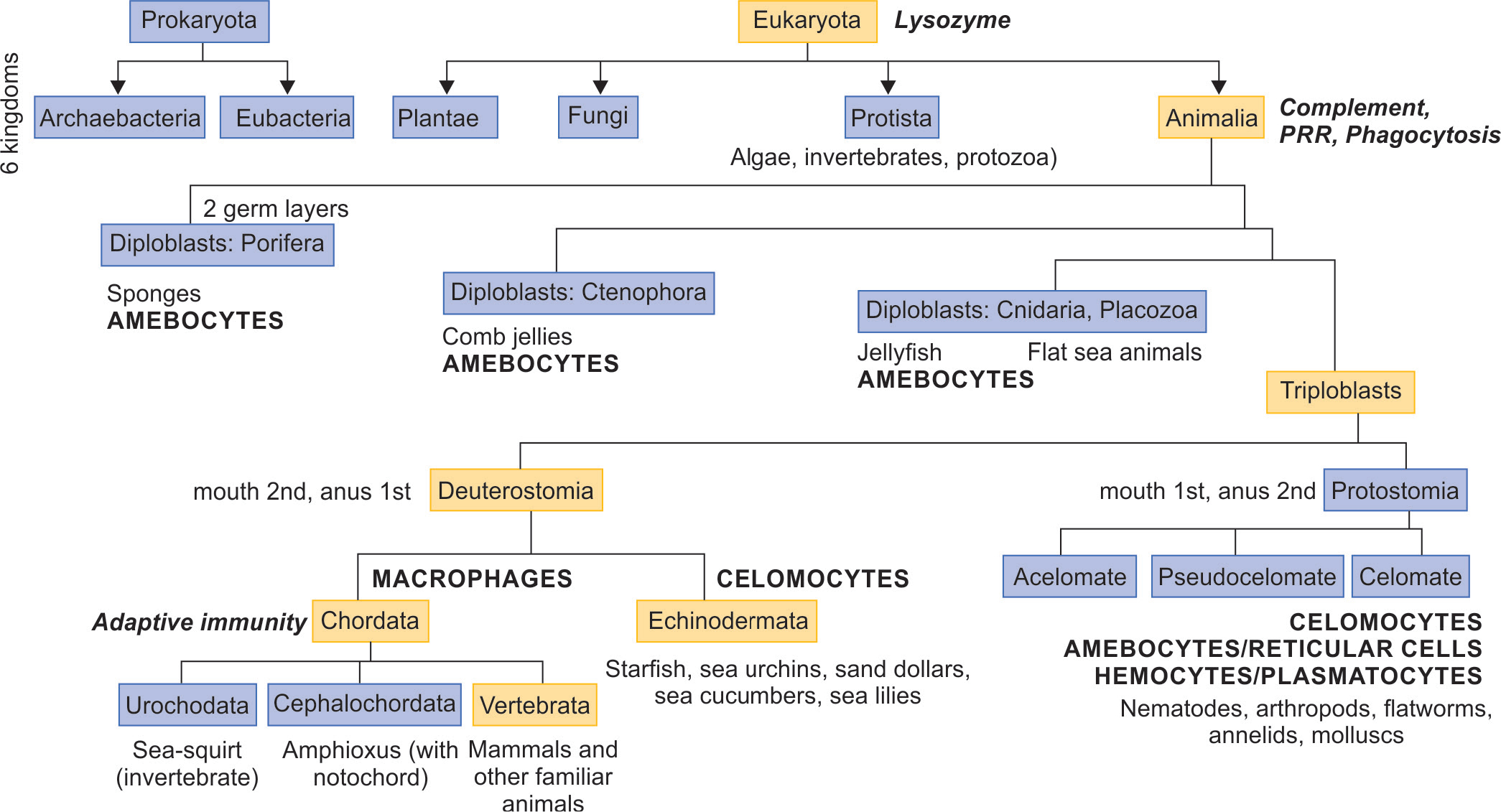
Phylogeny of macrophages. Schematic figure shows the development of macrophage-like cells and macrophages across evolution. The orange-colored boxes with eukaryota, animalia, triploblasts, deuterostomia, chordata, and vertebrata traces the evolution of vertebrate animals such as humans. The green font indicates key events in the development of immunity. Upper-case, red-font labels show the evolution of phagocytes. For each category of animals, one or more representative organisms are listed below in black font
In diploblastic animals, the cellular endo- and ectoderm are separated by a gelatinous matrix (mesoglea) that contains motile amebocytes, which ingest and digest food caught by enterocytes and then transport the nutrients to the other cells.8 These cells also promote innate immunity with pattern-recognition receptors (PRRs) and pore-forming proteins such as the macrophage-expressed gene 1 protein (Mpegl).13
In triploblasts, which represent the next stage in evolution, the middle layer is composed of mesodermal cells instead of the mesoglea but it contains similar phagocytes.14 The two subclasses, the Protostomia and the Deuterostomia, are named based on sequence of the development of the gastrointestinal tract openings.15 Vertebrates are a subclass of this group.16
Macrophage-like Cells in Triploblastic Prostomes with a Celomatic Cavity
In celomatic animals, an inner mesenteric layer holds the gut in the central cavity, and this promotes somatic growth.10 The circulatory system further helps in increasing body size through efficient diffusion of gases and nutrients, and the removal of metabolic waste products.17 This vascular system contains circulating macrophage-like defense cells known as the celomocytes/hemocytes. Similar cells, the plasmatocytes, have been identified in the corresponding ontogenic stage in developing fruit flies (Drosophila melanogaster).18
Blood vascular systems may be structured in one of two principal designs: open or closed.17 In the open circulatory system of arthropods and noncephalopod molluscs, the circulating hemolymph empties from a contractile heart and major supply vessels into the hemocele body cavity where it directly bathes the organs. Annelids like the earthworm, cephalopods, and nonvertebrate chordates have a closed circulatory system, where the intestinal surface is in contact with soil microorganisms.19 The are two freely circulating subpopulations of phagocytes, the autofluorescent eleocytes and amebocytes celomocytes.20 Amebocytes, not eleocytes, express PRRs and the Toll-like receptor (TLR) signaling pathway.21 These cells also express the oxidative stress-related superoxide dismutase and the antimicrobials lysozyme and lumbricin.22
Macrophage-like Cells in Deuterostomes such as the Chordates and its Constituent Vertebrates
Deuterostomes include the subclasses Chordata and Echinodermata (Flowchart 1).23 One of the chordate subphyla is comprised of the vertebrates. In the vertebrate intestine, the mononuclear phagocyte system has three cellular lineages: monocytes, macrophages, and DCs,24 that are released from the bone marrow.25,26 The other phylum, Echinodermata, also contains circulating macrophage-like phagocytes.27–31
In zebrafish, embryonic macrophages migrate from the mesoderm into the spleen and gut.32,33 The macrophages express tumor necrosis factor (TNF) to regulate the expression of mediators such as interferon regulatory factor 8 (IRF8), the complement Clq genes, and the G-protein-coupled receptor 35 and shape the gut microbiota. Macrophages also promote intestinal lymphangiogenesis thorough the vascular endothelial growth factors.
Amphibian macrophages promote immune defenses, homeostasis, and tissue remodeling.34 Chemokines such as the CXC ligand 12 (CXCL12) stimulate granulocyte/macrophage precursors to migrate from the liver to the bone marrow.35 Macrophage differentiation is controlled through binding of the colony-stimulating factor-1 (CSF-l) to its cognate CSF-l receptor (CSF-1R) on committed macrophage precursors. IL-34 also binds CSF-lR and promotes differentiation into morphologically and functionally distinct macrophages.36
In reptiles, macrophages are seen in the lamina propria but can migrate into the subepithelial lymphoid aggregates.10 Birds also have a well-developed mucosal immune system.37 The gut lamina propria contains innate immune cells such as macrophages, although the differences with DCs have not been reported in detail. These cells express many PRRs. Early-life microbial colonization is critical for immunological maturation. Macrophages are involved in antigen uptake and protect against invading pathogens.38,39
Mammals show macrophage maturation in patterns that resemble those in humans (as described below). Obviously, there are important species-specific differences in the timing in gestation (day of pregnancy) and the relative importance of specific genes or genetic isoforms.
Development of Macrophages in Humans
Macrophage development has three different phases during the embryonic, fetal, and neonatal period (Flowchart 2).
Flowchart 2:
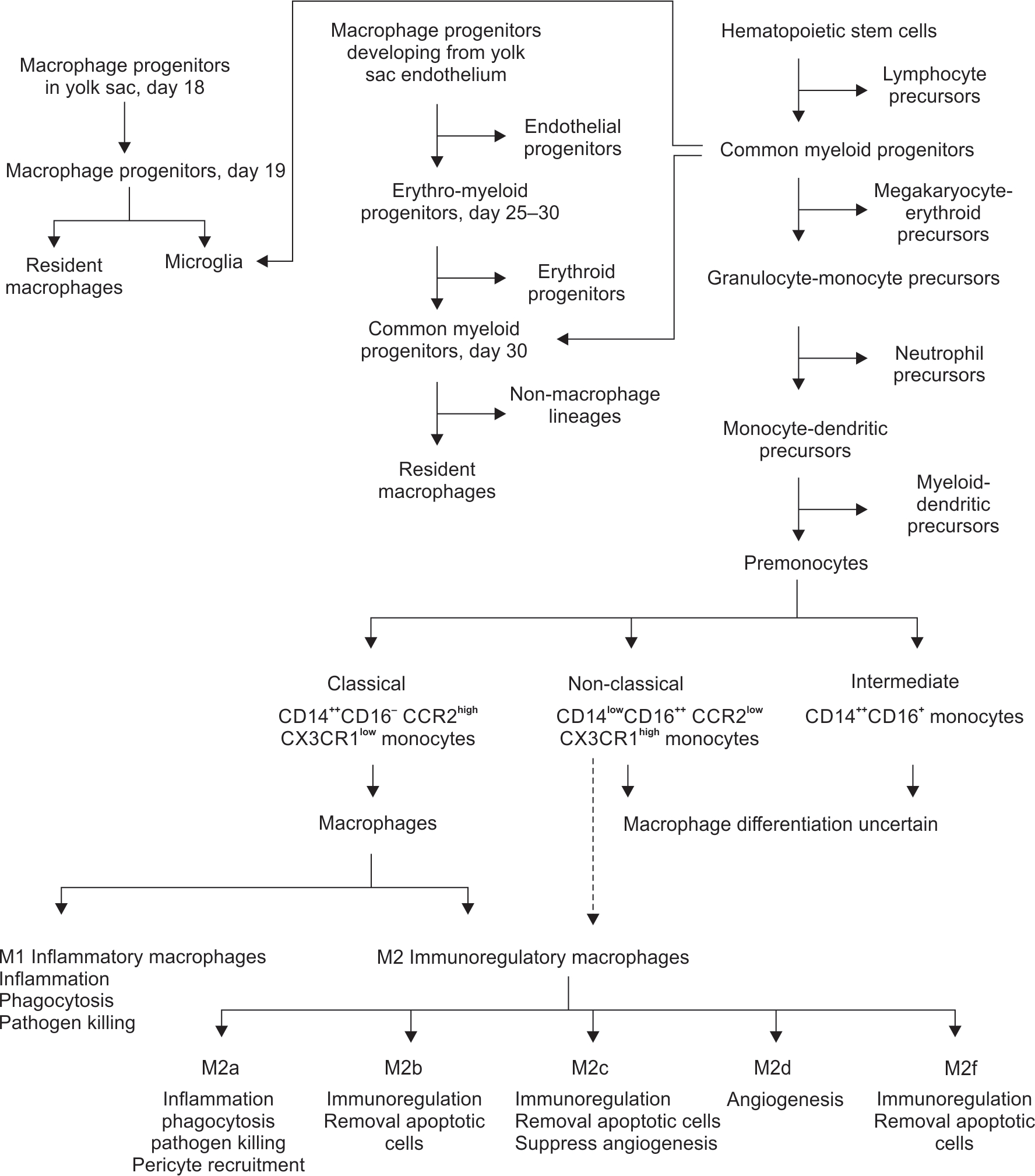
Ontogeny of gut macrophages. Schematic figure shows the development of the three major categories of macrophages in the human embryo, from progenitors in the yolk sac (YS), from the YS endothelium, and from the hematopoietic stem cells (HSCs). A subset of the HSCs evolves into monocytes, and current understanding suggests the classical CD14++ monocytes differentiate into the M1 inflammatory and M2 immunoregulatory macrophages. There is a possibility that the CD14lowCD16 monocytes may (also) evolve into the immunoregulatory macrophages, which is shown as a broken arrow. Increasing information suggests that the M2 macrophages may be a heterogenous group comprised of multiple subsets
Macrophage Differentiation in the Embryo
Lineage-restricted progenitors in the yolk sac (YS): Hemocytoblasts with myeloid characteristics are first seen in blood sinuses in the secondary YS on day 18.40 On day 19, some large-sized histiocytes, which is a term for tissue-resident macrophages, can be seen.41–44 Two distinct macrophage lineages appear at 5 weeks; a larger MHC II-neg fraction appears first in the YS, mesenchyme and the fetal liver, and then in the thymic cortex, lymph nodes, splenic red pulp, and the bone marrow.45 A minor population of MHC II+ cells can be seen in the liver at 7–8 weeks, lymph nodes at 11–13 weeks, the thymic medulla at 16 weeks, and then in the gastrointestinal tract. These MHC II+ cells then expand gradually.45,46 Yolk sac-derived macrophages are independent of the transcriptional factor c-Myb during development, but depend on the transcriptional factor PU.1.47
Erythro-myeloid progenitors (EMPs): On day 25, the YS and the embryo show EMPs developing from the capillary endothelium.48 These cells proliferate and differentiate into macrophages by day 30 and then migrate to all embryonic organs except the central nervous system (CNS).49
Endothelial precursors in the aorta-gonad-mesonephros (AGM) zone: The vascular endothelium in this zone produces CD34+ CD45+ hematopoietic stem cells (HSCs),50 which differentiate into common myeloid progenitors (CMPs) and then into tissue macrophages either directly or via a monocyte stage. These macrophages migrate to all embryonic organs, except the CNS.49
Microglial precursors: Some cluster of differentiation (CD) 45+ CD34+ myeloid cells located near the dorsal aorta migrate around day 30 into the CNS and differentiate into microglial precursors.49,51,52
Most macrophages in fetal organs develop from EMP and AGM progenitors.51,52 Some EMPs in the liver differentiate directly into Kupffer cells without passing through a monocyte stage.53 However, both these EMP-derived lineages are eventually replaced by BM-derived macrophages.
Macrophage Differentiation in the Liver
On day 32, some CD45+ CD34+ HSCs migrate from the AGM zone to the liver51 and then differentiate into monocytes and macrophage precursors in the 8–20 weeks period. These cells comprise nearly 70% of all hematopoietic cells in the fetal liver, but then involute during the 20–23 weeks period when the erythroid cell pool begin to expand.54 Some of these macrophages may arise from EMPs or from the CD34+ CD45− hemogenic endothelial cells that produce CD33+ macrophage precursors.
Monocyte development in the bone marrow followed by tissue migration and macrophage differentiation: While some CD34+ CD45+ HSCs migrate from the AGM zone into the liver, others migrate on the same day into the bone marrow. These cells remain detectable at 1 in 60 CD34+ CD45− cells even at 24 weeks’ gestation.54 In the monocyte–macrophage lineage, the differentiation shows several discernible stages, including the CMPs, granulocyte–monocyte precursors (GMP), common monocyte and DC precursors (MDP), premonocytes (committed monocyte progenitors), and then monocytes.55 These are all noticeable by the 7th week of gestation. After birth, the HSCs migrate from the liver to the bone marrow and mature as part of “definitive” hematopoiesis.56
There are three subsets of differentiating monocytes, a classification recognized by the nomenclature committee of the International Union of Immunologic Societies:
Classical, CD14++ CD16− cells (80–90%), which express CCR2, CD64, and CD62L. These show strong phagocytic activity, rapid responses to TLR ligation, and express inflammatory cytokines and reactive oxygen species (ROS) to recruit other leukocytes.57
In mice, classical monocytes (Ly6C++) differentiate into macrophages in most organs58 except in the gut, which has its own precursor cells.3 However, these cells are uniform precursors of inflammatory macrophages in disease states in all organs. Some Ly6C++ “tissue monocytes” in nonlymphoid organs may serve as effectors without differentiating into macrophages or DC, and present antigens to T-cells.59 A subgroup of Ly6C++ monocytes released from the marrow may also serve in a diurnal “anticipatory inflammation” regulated by the circadian gene Brain and Muscle ARNT-like 1 (BMAL1), as an innate response to frequently occurring environmental challenges.60
Non-classical, CD14lowCD16+ cells (10%) that lack CCR2, but express the Fc γ receptors CD64 and CD32.61 These monocytes patrol the blood vessels and respond via a TLR7-triggered pathway to remove senescent endothelial cells.62 Some cells extravasate to promote tissue healing, but show limited phagocytic activity and inflammatory responses to bacterial products.61,63
Murine studies show the corresponding Ly6C− cells to be terminally differentiated.58 These cells are regulated by the transcription factor Nuclear Receptor Subfamily 4 Group A Member 1 (NR4A1).64 Some of these Ly6C− monocytes might directly develop from MDPs in the marrow.65 Ly6C− monocytes exhibit a longer half-life of 5–7 days, longer than the 8-hour lifespan of Ly6C+ cells.66,67 Ly6C− monocytes may be also be seen as terminally differentiated resident macrophages in blood or the “vasculature macrophages,” rather than bona fide monocytes.68 Indeed, the primary function of these cells seems to be to patrol the vascular endothelium and monitor its integrity.
Intermediate cells that express both CD14 and CD16.68 A variable number of such cells express MHC-II, present antigens, and activate T lymphocytes. The exact role of these cells is not clear.
Monocytes are limited to blood, bone marrow, and spleen. These cells first appear in blood during the 5th month of gestation,54 but comprise only a small proportion of the cellular lineages until the bone marrow becomes the predominant site of hematopoiesis.69 Monocyte concentrations in the blood rise between 22 and 42 weeks; these cells comprise 3–7% of hematopoietic cells at 30 weeks70 and there is a relative monocytosis at term with counts between 300 and 3300/μL (median 1400/μL). After birth, absolute monocyte counts rise during the first 2 weeks and then begin to drop in the 3rd week.71–73 After leaving the bone marrow, monocytes circulate for 1–3 days and then move into tissues to differentiate into macrophages or into myeloid dendritic cells (mDCs). Both monocyte-derived macrophages (MDMs) and mDCs are involved in a variety of immune functions such as phagocytosis, antigen presentation and cytokine production.74 During the fetal/neonatal period, monocytes frequently develop epigenetic changes such as DNA methylation, microRNA expression, or histone modifications, that alter the immune pathways.75 For instance, decreased trimethylation of lysine 4 on histone 3 (H3K4me3) alters the maturation of neonatal monocytes.76
In infants, monocytes are important determinants of innate immunity. There are several aspects to consider:
Maturity in movement: By term gestation, monocytes attain considerable maturity in movement needed in trans-endothelial migration, chemotaxis, phagocytosis, and respiratory burst. Unlike neutrophils, cord blood monocytes show adherence, random migration, chemotaxis, phagocytosis, bactericidal activity.77
Maturity in pathogen elimination: Neonatal monocytes generate superoxide anion (O-2) and hydrogen peroxide at levels comparable to those from adults.78–80 Fetal and neonatal monocytes can kill pathogens such as S. aureus, S. epidermidis, E. coli, and C. albicans similar to those from adults.78,81
Maturity in inflammatory responses: Neonatal monocytes produce cytokines such IL-1β, IFN-α and TNF at levels comparable to adults, but not IFN-γ, IL-8, IL-10, and granulocyte–macrophage colony-stimulating factor (GM-CSF). These cells also produce less extracellular proteins such as fibronectin, and bioreactive lipids like leukotriene B4.82–86
As in amphibians and other phyla, monocyte differentiation into macrophages is controlled through binding of the colony-stimulating factor-1 (CSF-l) to its cognate CSF-l receptor (CSF1-R) on committed monocytes.87 IL-34, which is expressed mainly in the skin and the CNS, also binds CSF-lR.88 These ligands promote the differentiation of myeloid precursors into morphologically and functionally distinct macrophages.
Classification of MDMs
Monocyte-derived macrophages are comprised of two major phenotypes, the classically activated M1 and an alternatively activated, immunoregulatory M2 phenotypes (Flowchart 2).89 The M1 and M2 subpopulations could represent distinct differentiation paths of a common precursor, or could represent a maturational pathway where the M1 phenotype transitions to M2 with loss of CD14 and increased expression of CD16 and other markers. Based on murine models with progressive loss of Ly6C, the latter possibility seems more likely.90 The two subgroups show different surface markers, tissue localization, function, and intracellular signaling.
-
M1 macrophages respond to cytokines such as TNF and interferon-γ, bacterial lipopolysaccharide (LPS), and the granulocyte–macrophage colony-stimulating factor (GM-CSF). These cells express CD54, CD80, CD86, and CD197.91,92 In most situations, M1 macrophages are more efficient at phagocytosis and bactericidal functions;93 in vitro, macrophages are activated toward an M1 functional program by infectious microorganism-related molecules and by inflammation-related cytokines TNF or IFN-γ.90 M1 macrophages are characterized in vitro by an IL-12hiIL-23hiIL-10lo phenotype; are efficient producers of toxic effector molecules (ROS and NO) and inflammatory cytokines (IL-1β, TNF, IL-6); participate as inducers and effectors in polarized Th1 responses.90 These cells show high level expression of the CC chemokine receptor 2 (CCR2) and low levels of the CX3C-chemokine receptor 1 (CX3CR1).90 Accordingly, the chemokine CCL2 recruits CD14+ human/Ly6C+ murine monocytes to inflammatory sites.94
Most M1 macrophages die, killed by their own NO production.95 In an experimental acute lung injury model, these cells undergo Fas-mediated death, while the resident alveolar cells persist. M1 is likely a terminal differentiation phenotype, but some can undergo phenotype conversion to become tissue-resident macrophages. Macrophage polarization may be both transient and plastic.96 The patrolling monocytes respond to the CX3C-chemokine ligand 1 (CX3CL1; human fractalkine/murine neurotactin), a chemokine present both in soluble and membrane-bound forms expressed on endothelial cells and in tissues.97
M2 macrophages may respond more strongly to IL-4, IL-10, IL-13, and IL-21, and to glucocorticoids, and express high levels of surface scavenger receptors such as CD163, CD204, and the mannose receptor, CD206.91,92 Accordingly, these cells show low-level expression of the CC chemokine receptor 2 (CCR2) and high levels of the CX3C-chemokine receptor 1 (CX3CR1).98
M2 macrophages are active in immunoregulation, maintain tissue integrity following injuries and in chronic infections, and promote angiogenesis.93 The M2 macrophages are a relatively heterogeneous group, comprised of at least 5 sub-categories (M2a, M2b, M2c, M2d, and M2f).4,99
In vitro, M2 polarization has been noted in response to the concomitant activation of Fcγ receptors and TLRs, and to exposure to immune complexes and to anti-inflammatory molecules such as IL-10, TGF-β, and glucocorticoids.89 M2 cells are characterized by an IL-12loIL-23loIL-10hiTGF-βhi phenotype and generally have high levels of scavenger, mannose, and galactose-type receptors.58,100 In general, these macrophages take part in polarized Th2 responses, dampening of inflammation, tissue remodeling, angiogenesis, and immunoregulation.101
Tissue-resident macrophages are maintained locally by proliferative self-renewal, and retain an M2-like phenotype, for example, in the peritoneal cavity, brain, and lung.102 The proliferation rates are low in steady-state conditions, but increase under inflammatory challenges.103 Monocyte-derived macrophages may have fates similar to tissue-resident macrophages with maintenance of M2-like phenotypes and a low self-renewal capacity.58 A number of cells probably die during inflammation, where the extent of survival possibly depends on the nature and magnitude of the insult.58
Generally, monocyte/macrophage development, differentiation, and proliferation are driven by macrophage colony-stimulating factor (CSF-1), GM-CSF, and cytokines such as IL-4 and IL-34.104 CSF-1 is constitutively produced by mesenchymal cells, and it promotes the maturation of Ly6C+ monocytes to Ly6C−.105 CSF-1 also increases macrophage proliferation with a negative feedback loop via macrophage production of CCL2.58 It helps maintain the macrophage pool in the gut, kidney, peritoneal cavity, BM and hence in circulation, but not in the liver. It promotes M2 polarization. During inflammation, GM-CSF is the primary driver of hematopoiesis and it promotes the proliferation of M1-polarized MDMs.106
Dendritic cells (DCs) are a leukocyte subset specialized for antigen-presenting function. Similar to macrophages, DCs also originate early from a common granulocyte–monocyte–dendritic cell progenitor107 in the YS, mesenchyme, and the liver at 4–6 weeks of age.108 DC precursors differentiate into:109,110 (1) myeloid DCs (or mDCs), CD11c+ cells that express myeloid markers such as CD13, CD33, CD1a-d, and CD11b; and (2) plasmacytoid DCs (or pDCs), CD11c− cells with a plasmacytoid morphology with well-developed rough endoplasmic reticulum and Golgi apparatus.111 However, DCs mature most after birth112 Neonatal DCs comprise about 0.3% of all mononuclear cells.113 Compared to adults, neonatal pDCs exhibit low expression of ICAM-1 and MHC antigens and of co-stimulatory molecules CD40, CD80 or CD86, show a less efficient maturational response and immunostimulatory function,114,115 Neonatal DCs express, and are less efficient in lymphocyte activation than those from adults.114
Determinants of Macrophage Polarization
In inflamed tissues, different subtypes of macrophages can be seen. The mechanisms are unclear, but such variability in polarization may arise from the presence of conserved lineages (rooted phenotypes), plasticity (possible to shape/mold), or flexibility (possible inter-convertibility). Here are these three possibilities:
Conserved lineages: resident macrophages derived from YS or hepatic precursors are hypo-inflammatory and retain tissue-protective and reparative functions, whereas MDMs may evolve into different lineages.5,116 For instance, human CD14++ inflammatory monocytes and murine Ly6C+ monocytes may differentiate into M1 macrophages.117 Human CD16+ or murine Ly6C− monocytes may differentiate into resident tissue cells (if not from transdifferentiation of M1 macrophages, vide infra).58,118
Plasticity related to phases of inflammation/specific tissues: monocytes recruited soon after the onset of inflammation may differentiate into M1 macrophages, and those recruited during disease resolution may develop M2 characteristics.119,120 In some tissues such as the intestine, extracellular matrix (ECM) contents such as transforming growth factor (TGF)-β and other mediators such as IL-10 can convert monocytes into hypo- or noninflammatory macrophages.121
Flexibility related to phases of inflammation/microenvironment: macrophages may retain the ability to switch from one phenotype to the other depending on the microenvironmental stimuli.122 M2 macrophages can be activated into M1 following exposure to TLR ligands or IFN-γ.122 M1 macrophages might acquire M2 properties during resolving inflammation with increased sensitivity to ECM components such as TGF-β, although further evidence is needed that these changes do not get interrupted by nitric oxide.123 Another example of such a phenotypic switch is with repeated exposures to LPS, which can induce endotoxin tolerance with a global switch in gene expression program.123
Monocytes/Macrophages Can Carry and Present Antigens in Lymphoid Tissue
Some monocytes that enter inflamed tissues do not differentiate into macrophages, but are able to phagocytose antigen(s) and carry those to naïve T-cells in lymph nodes. Tissue macrophages can also present antigens to the maturing adaptive immune system. M1 MDMs may present antigens and activate/polarize effector Th1 and Th17 cells upon production of IL-12 and IL-23, respectively. The TNF superfamily and the TNF receptor superfamily are likely involved, but not co-stimulators such as CD80, CD86, and CD28. Similarly, M2-like tissue macrophages, which produce TGF-β and express the αVβ8 integrin may be involved in the polarization of regulatory T cells.
Innate Immune Memory124–130
Tissue-resident macrophages and MDMs can recognize microbial or damage-associated molecules for enhanced recruitment and differentiation of circulating monocytes into M1 macrophages.
Tissue monocytes are recently described cells that can take up antigens in the tissue and move to lymph nodes, where they are able to present antigens to naive T-cells.
After resolution of an acute inflammatory illness, memory macrophages or monocytes may be retained. These cells are functionally programed by a previously stimulus for either altered cytokine production to optimize the immune response depending on the type/concentration of the immune stimulus.
Gut Macrophages in the Developing Intestine are a Specialized, Hyper-inflammatory Cellular Population
The gastrointestinal tract contains the largest reservoir of macrophages in the body.131 Macrophages appear in the fetal intestine at 11–12 weeks’ gestation, increase rapidly during 12–22 weeks, and then at a slower pace through early childhood.44,132–137 These cellular groups are broadly similar in the small intestine and colon,10 and form a critical part of the innate immune system to encounter luminal bacteria that may breach the epithelium to enter the lamina propria112,138,139 Gut macrophages also promote peristaltic movements and promote tolerance for antigens derived from diet and commensal microbiota.
In the neonatal intestine, there are two distinct pools of macrophages, one comprised of mature macrophages that might have been derived from YS precursors, and another that could represent MDMs. During inflammation, the MDM pool enlarges and newly recruited monocytes are seen (Fig. 1). The numerical development of the macrophage pool, unlike that of mucosal lymphoid aggregates, is programmed in the fetal intestine and does not require the presence of dietary or microbial antigens.132,140 The gut macrophage pool in the fetus contrasts with the macrophages in the lung; there are very few alveolar macrophages in the fetus and this population expands after birth.141–152 However, the functional maturation of gut macrophages continues during early infancy and is influenced by antigens in ingested food and microbiota.
Figs 1A and B:
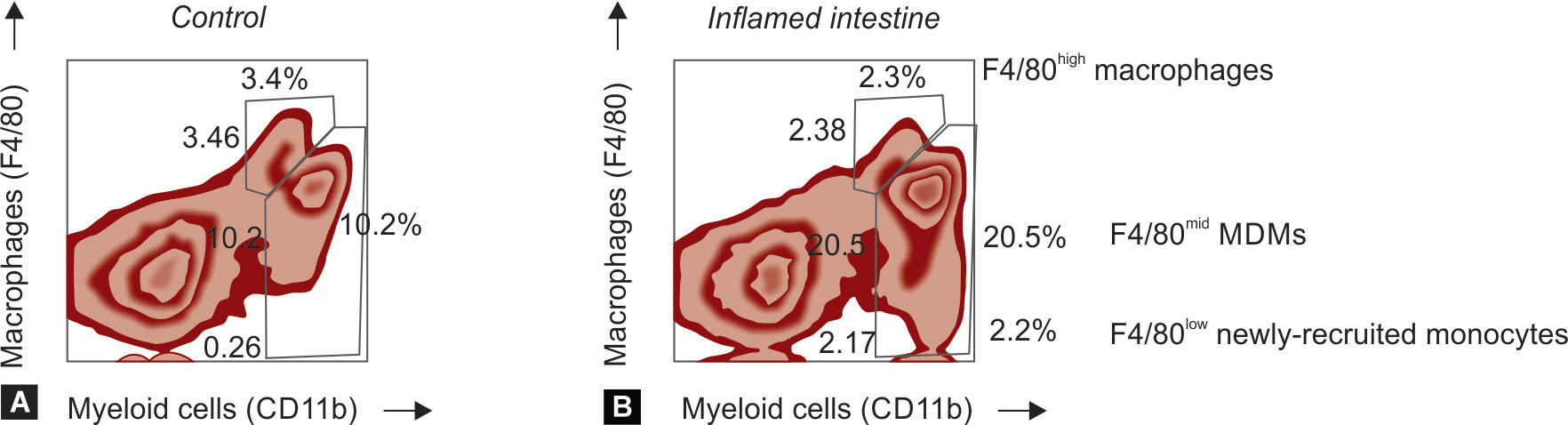
Intestinal macrophage populations in neonatal mice. We used flow cytometry to examine intestinal lysates from mouse pups with normal intestine and from others with intestinal inflammation. Macrophages were identified as the cells expressing the myeloid marker CD11b and the macrophage marker, F4/80. The normal intestine showed two distinct pools of macrophages, one of mature macrophages that expressed F4/80 at high levels and likely represents YS-derived cells. There was a second F4/80mid subset consistent with MDMs. During inflammation, the MDM pool got enlarged. Some newly recruited F4/80low monocytes were also seen (expressed high levels of the monocyte marker Ly6C; not shown)
Unlike many other organs, intestinal macrophages have very limited ability to undergo clonal expansion.138 The primary mechanism for maintaining the gut macrophage pool is through the recruitment and differentiation of blood monocytes.138,139,153 In adults, interleukin-8/CXC ligand 8 (IL-8/CXCL8) and transforming growth factor-beta (TGF-β) recruit monocytes to the intestinal mucosa.138 However, developmental limitations in the fetal intestine may preclude these two cytokine systems in effective recruitment of monocytes as macrophage precursors. In the fetus, IL-8 is expressed primarily as a longer, less-potent 77-amino acid isoform unlike the shorter 72-amino acid isomer in the adult.154 Similarly, TGF-β bioactivity is low in the early-/mid-gestation fetal intestine.155 Finally, macrophage populations begin to expand in the fetal intestine several weeks before lymphocytes or neutrophils,132,133,135 suggesting that the recruitment of monocytes as macrophage precursors may occur via specific chemoattractant(s) other than IL-8/CXCL8, which recruits both neutrophil and macrophage precursors,138,156 or TGF-β, which also mobilizes T-lymphocytes.138,157
We have reported that chemerin (the retinoic acid receptor responder-2/RARRES2) might be a key chemoattractant for monocytes in the normally developing fetal intestine. Chemerin is a 16 kDa heparin-binding molecule158,159 expressed in fetal IECs beginning at 10–14 weeks with a peak at 20–32 weeks and then gradual diminution to minimal levels towards term.160,161 The chemerin promoter contains several CpG islands located in close vicinity of retinoic acid receptor (RAR)-β binding sites;162,163 RAR-β is now recognized as an epigenetic regulator with multiple variants, the expression of which is regulated through alternate promoter usage and differential splicing.164 Interestingly, only monocytes, not neutrophils or lymphocytes, express the chemerin receptor, the chemokine-like receptor-1 (CMKLR1).165,166 The developmental importance of chemerin as a monocyte chemoattractant in the intestine is also related to the fact that it is broken by cysteine proteases into fragments that inhibit the responses of inflammatory responses of these cells to bacterial products.167 In the injured neonatal intestine, macrophage-rich infiltrates are prominent, which contrasts with the pleomorphic leukocyte infiltrates in inflammatory bowel disease or gut inflammation models in adult mice.168–173 The chemokine CXCL5 is an important chemoattractant for macrophage precursors to the neonatal intestine.168
Intestinal macrophages in mammals share some common features. Unlike macrophages in other organs, human gut macrophages express CD14 and CD16 at very low levels.174 Murine gut macrophages can be identified by the markers F4/80175 and the fractalkine receptor CX3C receptor 1 (CX3CR1).176 Gut macrophages typically express CD64 Fc-gamma receptor 1,177 CD163 (scavenger receptor for free hemoglobin or the hemoglobin–haptoglobin complex),178 and the myeloid-epithelial-reproductive tyrosine kinase (MerTK).3 In adults, most gut macrophages except those at immune inductive sites such as Peyer’s patches (PPs) typically display anti-inflammatory characteristics.139 These properties are still maturing in neonates.139 There are four main categories of gut macrophages (Flowchart 3).
Flowchart 3:
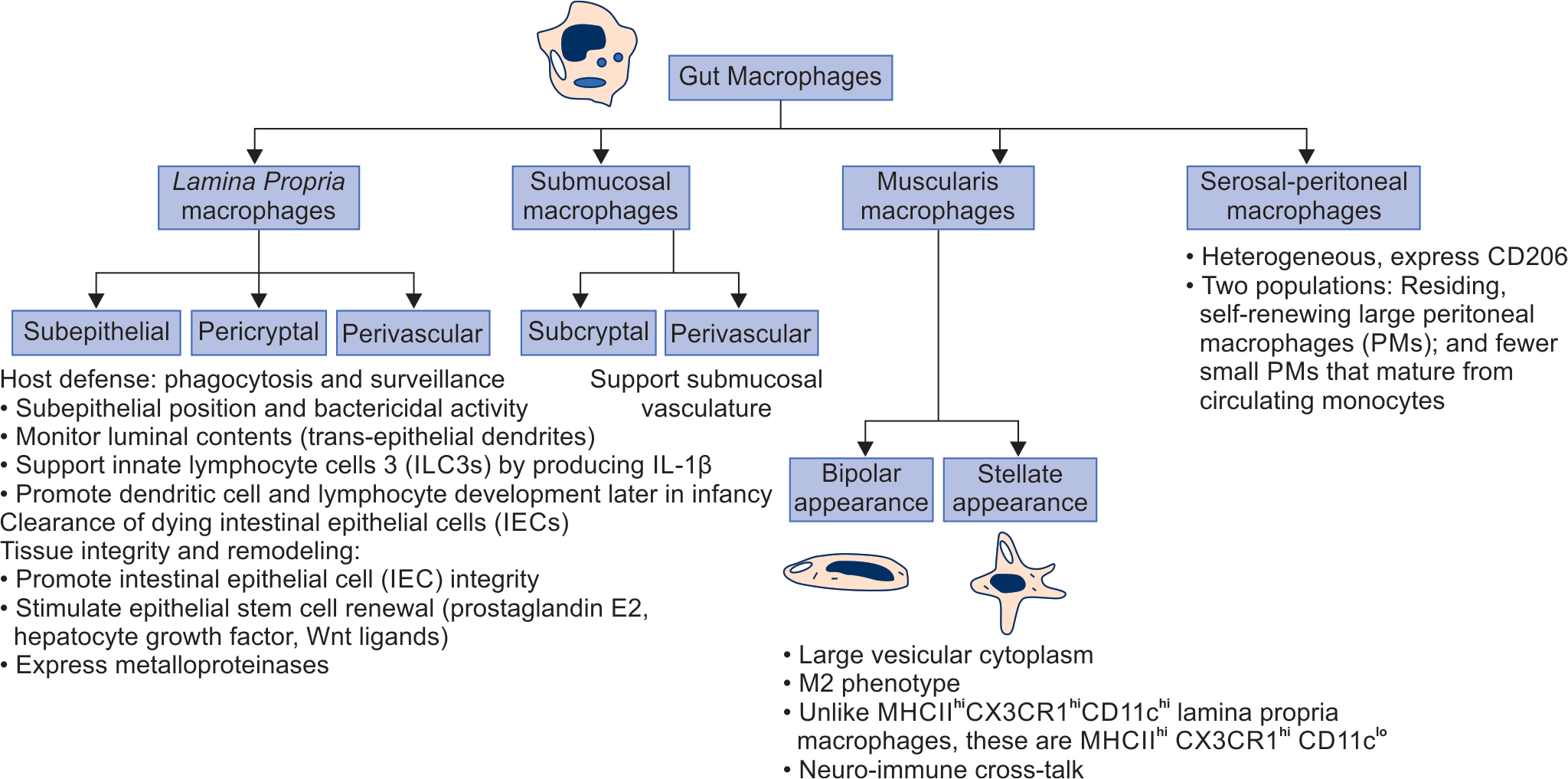
Classification of gut macrophages by location. Schematic shows the location, classification by location or shape, and the best-known function
-
Lamina propria macrophages (LPMs): These cells are located below the epithelium, around crypts, and near blood vessels in both the small intestine and the colon.3,44,168,169,172,173 The primary functions are in host defense, clearance of dead cells, and maintenance of tissue integrity.3 Mucosal perivascular macrophages also form tight interdigitating connections around the vasculature to prevent bacterial translocation into the blood circulation. These cells express angiogenesis-related genes necessary for the repair and strengthening of the vasculature.
LPMs can be derived from the primordial myeloid precursors developing in the intestine, those immigrating from the liver, or from the blood monocytes.179 The maturation of Ly6c1 lymphocyte antigen 6 complex, locus C1 (Ly6C)hi CC receptor 2 (CCR2)hi CX3CR1int monocytes into Ly6Clo CCR2lo CX3CRlhi macrophages is driven by the microbiota and by the transcription factor nuclear receptor subfamily 4, group A, member 1 (NR4Al).180 The fractalkine receptor CX3C receptor 1 (CX3CR1) promotes dendrite formation and luminal migration.97 At the base of SI and colonic crypts, some LPMs that express sialoadhesion (CD169)+ are closely associated with the stem cell niche.181 In the small intestine, these LPMs promote Paneth cell differentiation, maintain the LGR5+ stem cell pool, and promote epithelial proliferation. The analogous subset in the colon is not essential to maintain the stem cell niche, but may promote regenerative responses after injury.
Submucosal macrophages: These help maintain the submucosal vasculature. LPMs and submucosal macrophages differ in life span, transcriptional programs, and function. These cells are either self-maintaining or are derived are from regular differentiation from monocytes.3,181 Some submucosal macrophages resemble those in the muscularis externa and are located in close proximity to the neurons and vasculature. These cells comprise a long-lived, self-maintained subset of macrophages.181 Considering the location away from the gut lumen, this pool of macrophages does not seem to need the stimulation from the microbiota and dietary antigens for replenishment by circulating monocytes.
-
Muscularis macrophages are located underneath the submucosal region between circular and longitudinal muscle layers, and are therefore, relatively distant from luminal stimuli. These cells may show either a bipolar (associated with the circular muscles and the deep muscular plexus) or a stellate (in the serosal and myenteric plexus) shape.182 These are associated with the muscularis externa and associated enteric neurons, distant from any luminal stimulation.3,183
The development of muscularis macrophages is ensured by CSF-l produced by the enteric neurons, and possibly also by endothelial cells or interstitial cells of Cajal.184 The gene expression profile of these macrophages suggests a role in tissue protection, neuronal development, and intestinal peristalsis. The bone morphogenetic protein (BMP) 2 and prostaglandin E2 (PGE2), which act on enteric neurons and smooth muscles, respectively, are also expressed.182 Muscularis macrophages may also serve in a neuroprotective role by limiting infection-induced neuronal loss through the adrenergic/arginase 1/polyamines axis185 and the norepinephrine signaling via β2 adrenergic receptors (β2ARs).186
Serosal macrophages: These cells may be derived from precursors arising in the muscularis, or from peritoneal macrophages adherent to the serosa.187 The surface markers and properties vary with the degree of inflammation in the peritoneal cavity.
Inflammatory Characteristics of Macrophages in the Developing Intestine
In the adult human intestine, newly recruited monocytes differentiating into macrophages retain avid phagocytic and bactericidal activity but develop inflammatory anergy and tolerance to bacterial products.139 These macrophages lose innate response receptors such as CD14, Fcα (CD89), Fcγ (CD64, CD32, CD16), the integrin lymphocyte function-associated antigen 1 (LFA-1; CD11a/CD18); and the complement receptors (CR) 3 (CD11b/CD18) and CR4 (CD11c/CD18). These cells no longer produce inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-12, CC receptor ligand 5 (CCL5), and the TNF. This inflammatory downregulation occurs due to the exposure to extracellular matrix (ECM) factors such as TGF-β.
Unlike the gut macrophages in adults, those in the fetus/premature infants are yet to develop complete tolerance to bacterial products and display inflammatory responses upon stimulation. These cells express many of the inflammatory markers listed above (Fig. 2). There are three main reasons:
Fig. 2:
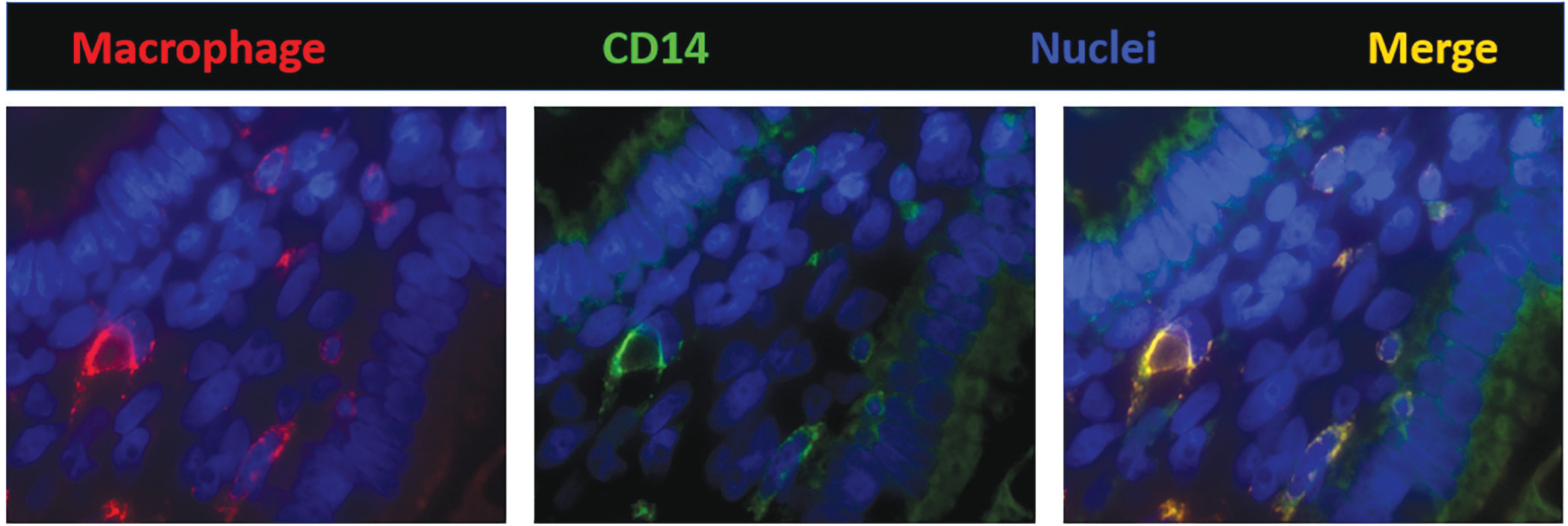
Fluorescence photomicrograph (1000×) of a villus in preterm (26 weeks) human intestine shows that macrophages (HAM56, red) express CD14 (green). CD14 is an important mediator in the bacterial lipopolysaccharide (LPS)-stimulated signaling pathways. These findings are important because macrophages in the adult human intestine do not express CD14 and are unresponsive to LPS. Nuclei are stained blue (4′,6-diamidino-2-phenylindole, or DAPI, is a fluorescent stain that binds adenine–thymine-rich regions in DNA)
The developing intestine has a deficiency of TGF-β, particularly its TGF-β2 isoform.121 During NEC, TGF-β expression and bioactivity are further reduced to levels that are even lower than in a normal fetus of a similar gestational age.
Macrophages in the premature intestine are resistant to TGF-β and are therefore, intrinsically hyper-inflammatory because of high expression of an inhibitor of TGF-β signaling, the Smad7.169 The abbreviation Smad refers to the homologies in this protein to the “Caenorhabditis elegans” SMA, the “small” worm phenotype and the MAD, the “Mothers against Decapentaplegicc family of genes in Drosophila. Bacterial products further induce Smad7 expression in neonatal, but not adult, gut macrophages.169 Smad7 increases inflammatory activity by augmenting LPS-induced NF-κB activation; it activates the inhibitor of nuclear factor kappa-B kinase subunit beta (IKK-β) expression by attaching to two smad-binding elements in the IKK-β promoter, and consequent acetylation of Lys12 residue on histone 4 (H4K12).188 Increased Smad7 expression in the developing intestine may be due to a developmental deficiency of the SKI (Sloan-Kettering Institute)-like proto-oncogene (SKIL) oncoprotein, which is a physiological repressor of the Smad7 promoter.173,189 Our findings showed Smad7 to be an important negative regulator of TGF-β signaling in the gastrointestinal tract.190 Smad7 can also suppress TGF-β signaling by competing with the activating Smads, increasing the degradation of the TGF-β receptors, and through epigenetic mechanisms by interacting with histone deacetylases.191–193 IKK-β is an essential catalytic subunit of the IKK complex, which includes another catalytic subunit, IKK-α, and a regulatory subunit, IKK-γ.194 During inflammation, cytokines and bacterial products promote phosphorylation of IKK-β, which in turn, activates the IKK complex. Activated IKK phosphorylates the IκBs, triggering their degradation and thereby releasing the NF-κB dimers for nuclear translocation. In our study, Smad7 activation of the IKK-β promoter was associated with increased H4K12 acetylation, an epigenetic marker of euchromatin,195 on the IKK-β nucleosome. H4K12 acetylation may neutralize its electrical charge, leading to structural changes that promote DNA accessibility and interactions with the H2A–H2B dimer in neighboring histones.196
Human milk contains substantial amounts of TGF-β, particularly the isoform TGF-β2, but most of it is in a latent, inactive form.197 A substantial proportion of milk-borne TGF-β2 is also inactive because it is bound to chondroitin sulfate-containing proteoglycan(s) such as biglycan.198
Macrophages in immune inductive sites in the developing intestine show some unique features.199 Peyer’s patches first appear at 11 weeks and develop during mid-late gestation.200 At birth, these lymphoid aggregates are structurally complete but “naive”, as the germinal centers take a few weeks to develop. The number of PPs in the ileum increases as a function of gestational maturation, and premature infants born prior to 32 weeks’ gestation may have only half as many PPs than those born at full term. Lymphoid aggregates in the vermiform appendix may develop only after birth following postnatal bacterial colonization.200
After birth, macrophages in PPs are exposed to more antigens than those in the Lamina propria.201 These cells do not express the typical macrophage markers such as F4/80 (a glycoprotein marker seen on mature macrophages) and CD64 (IgG Fc Receptor I) in mice and CD163 (high affinity scavenger receptor for the hemoglobin–haptoglobin complex) in humans,202 but most display the apoptotic receptor myeloid-epithelial-reproductive tyrosine kinase (MerTK) and the fractalkine receptor CX3CRl.3 In view of high-level expression of lysozyme, these macrophages labeled as lysozyme-expressing macrophages (LysoMacs).203 The muscularis and serosal macrophages located below the follicles express CD169 (sialoadhesin, a cell adhesion molecule), unlike those in the PP.10 The follicular LysoMacs express the phosphatidylserine receptor T-cell membrane protein 4 (TIM-4), whereas subepithelial and upper follicular LysoMacs do not.10 These factors could comprise one possible mechanistic explanation for the known regional specialization of macrophages inside the PPs.204
Macrophages outside the immune inductive sites such as in the small intestinal Lamina propria, both in terms of the number and functional characteristics, might be influenced by dietary factors.205 The impact on the Janus kinases (JAK)-signal transducer and activator of transcription proteins (STAT) pathway, expression of CCL2, and inflammasome activation with increased intestinal permeability, glucose metabolism, and insulin sensitivity may be important.206 Saturated fatty acids, derived from the metabolism of w-3 PUFAs, activate inflammatory responses through the TLR4–NF-κB pathway.207 Some macrophages acquire properties that promote the resolution of inflammation induced by dietary antigens.126
Colon is functionally important for the absorption of electrolytes and water, and for the management of undigested foodstuffs.208 In the distal colon, macrophages insert balloon-like protrusions between epithelial cells to sense their microenvironment, although these extensions do not quite extend into the lumen as seen in the small intestine.209,210 These protrusions sample the fluids absorbed by epithelial cells to detect toxins and protect by regulating absorption of potentially toxic luminal contents.211
The macrophages in the colonic lamina propria are continuously exposed to anti-inflammatory microbial metabolites and short-chain fatty acids (SCFAs) such as butyrate,212 and produce antimicrobials such as lysozyme, calprotectin, and ROS.213 Butyrate is known to suppress inflammatory signaling by inhibiting histone deacetylase 3.214 It also stimulates the goblet cells to produce more organized mucus by changing glycosylation-related gene expression.215 These improved mucus layers reduce bacterial translocation and induce physico-chemical changes in the oxygen and pH levels; this feedback loop regulates the inflammatory activity by altering the number and functional diversification of macrophages.215–218 Colonic macrophage functioning is also altered by TGF-β and IL-10 signaling. These pathways promote the interaction of Wiskott-Aldrich syndrome protein (WASP) and dedicator of cytokinesis (DOCK) 8, leading to the phosphorylation of STAT3 and the anti-inflammatory polarization of these macrophages.219 Dectin-1 activates colonic macrophages, resulting in inflammasome-dependent IL-1β secretion and monocyte recruitment to the inflamed colon.220
Gut Macrophages are Key Mediators of Intestinal Inflammation in NEC
Necrotizing enterocolitis is an inflammatory bowel necrosis seen in up to 10% of very-low-birth-weight (VLBW) premature infants born prior to 32 weeks’ gestation.221 The mortality rates of NEC continue to be between 20 and 30% all over the world.222 The pathogenesis of NEC is still unclear, but many risk factors have been identified such as chorioamnionitis, perinatal asphyxia, indomethacin therapy in neonates, formula feedings, use of human milk fortifiers, viral infections, use of feeding thickeners, and severe anemia; anemia may either be a risk factor in itself, or may predispose to NEC following “corrective” red blood cell transfusions.171
Nearly 80% of all intestinal tissue specimens that have been resected for NEC showed inflammatory changes with leukocyte infiltration and edema.223–225 Interestingly, despite the rapid progression of intestinal injury, the leukocyte populations were largely comprised of macrophages, not neutrophils.224,226 This prominence of macrophages in NEC lesions could be a tissue-specific finding, but also possibly related to the relative immaturity of neutrophils compared to macrophages in premature infants. A few eosinophils were seen in some lesions. The number of lymphocytes did not differ between control areas and NEC lesions, although many recent reports suggest that NEC may alter lymphocyte subsets. The severity of inflammation in NEC is usually graded by the depth of changes, beginning at the mucosa and outward progression, and by the density of leukocyte infiltration. More than half of all NEC lesions show leukocyte infiltrates extending into the submucosa or beyond.
Inflammatory infiltrates can be seen in 70–80% of resected tissue samples.168–170,172,200,226,227 These infiltrates were typically comprised of macrophages (70–80% of the leukocytes/high power microscopic field/HPF) and neutrophils (15–20% of the leukocytes/hpf).168,224 There was a modest increase in neutrophils (37.9 ± 5.8 cells in NEC vs. 7.7 ± 1.7/high-power field in control tissues). The number of lymphocytes usually does not change significantly. A few eosinophils may be seen in the involved areas, and in some infants, inflammatory pseudomembranes and crypt abscesses can also be seen. Pender et al.228 previously described similar inflammatory changes with macrophage infiltration with NEC. We have described how the pro-inflammatory characteristics of still-maturing intestinal macrophages in the preterm intestine can increase the risk of NEC and refer the reader to the articles listed in the references.121,168–173,200,226,229–237 Several genetic and epigenetic factors may also alter the susceptibility to NEC and/or its severity, its clinical and histopathological manifestations, and if surgery is needed, of an adverse postoperative course and outcome.227,238
Conclusions
Recent years have brought considerable progress in our understanding of the heterogeneity and inflammatory immaturity of gut macrophages in premature and full-term newborn infants; particularly in the context of diseases such as NEC. There is a need for continued efforts to understand the maturation of macrophages, and the mechanisms that might derail this process, and the points where timely identification and therapeutic interventions might be of translational importance. There is hope.
Key Points.
Macrophages are large highly motile leukocytes with important roles in innate immunity.
During evolution, many precursors to macrophages, such as amebocytes, coelomocytes, and hemocytes, have been identified.
In the developing human embryo, macrophage precursors are identified in the yolk sac, the pools of erythro-myeloid progenitors, endothelial precursors, and later in hematopoietic stem cells.
Macrophages play major roles in tissue homeostasis, innate immunity, inflammation, tissue repair, angiogenesis, and apoptosis of various cellular lineages.
The differentiation of monocytes into inflammatory M1 and immunoregulatory M2 macrophages is a subject of active investigation.
The gastrointestinal tract contains the largest reservoir of specialized macrophages. Small intestine and colon contain multiple, distinct macrophage subsets.
Recognizing the heterogeneity of gut macrophages in premature and term infants raises an exciting possibility that targeted augmentation or depletion of subsets might be therapeutically useful.
Source of support:
National Institutes of Health awards HL124078 and HL133022 to AM.
Footnotes
Conflict of interest: None
REFERENCES
- 1.Cavaillon J-M. The historical milestones in the understanding of leukocyte biology initiated by Elie Metchnikoff. J Leukoc Biol 2011;90(3):413–424. DOI: 10.1189/jlb.0211094. [DOI] [PubMed] [Google Scholar]
- 2.Weigert A, Olesch C, Brune B. Sphingosine-1-phosphate and macrophage biology – How the sphinx tames the big eater. Front Immunol 2019;10:1706. DOI: 10.3389/fimmu.2019.01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bain CC, Schridde A. Origin, differentiation, and function of intestinal macrophages. Front Immunol 2018;9:2733. DOI: 10.3389/fimmu.2018.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao Y, Xu XH, Jin L. Macrophage polarization in physiological and pathological pregnancy. Front Immunol 2019;10:792. DOI: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science 2010;327(5966):656–661. DOI: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaumouille V, Waterman CM. Physical constraints and forces involved in phagocytosis. Front Immunol 2020;11:1097. DOI: 10.3389/fimmu.2020.01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suckale J, Sim RB, Dodds AW. Evolution of innate immune systems. Biochem Mol Biol Educ 2005;33(3):177–183. DOI: 10.1002/bmb.2005.494033032466. [DOI] [PubMed] [Google Scholar]
- 8.Richards DM, Endres RG. The mechanism of phagocytosis: Two stages of engulfment. Biophys J 2014;107(7):1542–1553. DOI: 10.1016/j.bpj.2014.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuart LM, Ezekowitz RA. Phagocytosis and comparative innate immunity: Learning on the fly. Nat Rev Immunol 2008;8(2):131–141. DOI: 10.1038/nri2240. [DOI] [PubMed] [Google Scholar]
- 10.Arroyo Portilla C, Tomas J, Gorvel J-P, et al. From species to regional and local specialization of intestinal macrophages. Front Cell Dev Biol 2020;8:624213. DOI: 10.3389/fcell.2020.624213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanna E Evo-devo of non-bilaterian animals. Genet Mol Biol 2015;38(3):284–300. DOI: 10.1590/S1415-475738320150005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yap NV, Whelan FJ, Bowdish DM, et al. The evolution of the scavenger receptor cysteine-rich domain of the class A scavenger receptors. Front Immunol 2015;6:342. DOI: 10.3389/fimmu.2015.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benard EL, Racz PI, Rougeot J, et al. Macrophage-expressed perforins mpeg1 and mpeg1.2 have an anti-bacterial function in zebrafish. J Innate Immun 2015;7:136–152. DOI: 10.1159/000366103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salinas-Saavedra M, Rock AQ, Martindale MQ. Germ layer-specific regulation of cell polarity and adhesion gives insight into the evolution of mesoderm. Elife 2018;7:e36740. DOI: 10.7554/eLife.36740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller WE, Schwertner H, Muller IM. Porifera a reference phylum for evolution and bioprospecting: The power of marine genomics. Keio J Med 2004;53(3):159–165. DOI: 10.2302/kjm.53.159. [DOI] [PubMed] [Google Scholar]
- 16.Martindale MQ, Pang K, Finnerty JR. Investigating the origins of triploblasty: ‘Mesodermal’ gene expression in a diploblastic animal, the sea anemone Nematostella vectensis (phylum, Cnidaria; class, Anthozoa). Development 2004;131(10):2463–2474. DOI: 10.1242/dev.01119. [DOI] [PubMed] [Google Scholar]
- 17.Monahan-Earley R, Dvorak AM, Aird WC. Evolutionary origins of the blood vascular system and endothelium. J Thromb Haemost 2013;11(Suppl 1):46–66. DOI: 10.1111/jth.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf MJ, Rockman HA. Drosophila, genetic screens, and cardiac function. Circ Res 2011;109(7):794–806. DOI: 10.1161/CIRCRESAHA.111.244897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prochazkova P, Roubalova R, Dvorak J, et al. Pattern recognition receptors in annelids. Dev Comp Immunol 2020;102:103493. DOI: 10.1016/j.dci.2019.103493. [DOI] [PubMed] [Google Scholar]
- 20.Engelmann P, Hayashi Y, Bodo K, et al. Phenotypic and functional characterization of earthworm coelomocyte subsets: Linking light scatter-based cell typing and imaging of the sorted populations. Dev Comp Immunol 2016;65:41–52. DOI: 10.1016/j.dci.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Buchmann K Evolution of innate immunity: Clues from invertebrates via fish to mammals. Front Immunol 2014;5:459. DOI: 10.3389/fimmu.2014.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dvorak J, Roubalová R, Procházková P, et al. Sensing microorganisms in the gut triggers the immune response in Eisenia andrei earthworms. Dev Comp Immunol 2016;57:67–74. DOI: 10.1016/j.dci.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Cameron CB, Garey JR, Swalla BJ. Evolution of the chordate body plan: New insights from phylogenetic analyses of deuterostome phyla. Proc Natl Acad Sci USA 2000;97(9):4469–4474. DOI: 10.1073/pnas.97.9.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guilliams M, Mildner A, Yona S. Developmental and functional heterogeneity of monocytes. Immunity 2018;49(4):595–613. DOI: 10.1016/j.immuni.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell 2006;124(4):815–822. DOI: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392(6673):245–252. DOI: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 27.Golconda P, Buckley KM, Reynolds CR, et al. The axial organ and the pharynx are sites of hematopoiesis in the Sea Urchin. Front Immunol 2019;10:870. DOI: 10.3389/fimmu.2019.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ch Ho E, Buckley KM, Schrankel CS, et al. Perturbation of gut bacteria induces a coordinated cellular immune response in the purple sea urchin larva. Immunol Cell Biol 2016;94(9):861–874. DOI: 10.1038/icb.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckley KM, Rast JP. Immune activity at the gut epithelium in the larval sea urchin. Cell Tissue Res 2019;377(3):469–474. DOI: 10.1007/s00441-019-03095-7. [DOI] [PubMed] [Google Scholar]
- 30.Coates CJ, McCulloch C, Betts J, et al. Echinochrome A release by red spherule cells is an iron-withholding strategy of sea urchin innate immunity. J Innate Immun 2018;10(2):119–130. DOI: 10.1159/000484722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckley KM, Ho ECH, Hibino T, et al. IL17 factors are early regulators in the gut epithelium during inflammatory response to Vibrio in the sea urchin larva. Elife 2017;6:e23481. DOI: 10.7554/eLife.23481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stachura DL, Svoboda O, Lau RP, et al. Clonal analysis of hematopoietic progenitor cells in the zebrafish. Blood 2011;118(5):1274–1282. DOI: 10.1182/blood-2011-01-331199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wittamer V, Bertrand JY, Gutschow PW, et al. Characterization of the mononuclear phagocyte system in zebrafish. Blood 2011;117(26):7126–7135. DOI: 10.1182/blood-2010-11-321448. [DOI] [PubMed] [Google Scholar]
- 34.Grayfer L, Robert J. Amphibian macrophage development and antiviral defenses. Dev Comp Immunol 2016;58:60–67. DOI: 10.1016/j.dci.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaparla A, Reeves P, Grayfer L. Myelopoiesis of the amphibian Xenopus laevis is segregated to the bone marrow, away from their hematopoietic peripheral liver. Front Immunol 2019;10:3015. DOI: 10.3389/fimmu.2019.03015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chin KN, Wong WC. Some ultrastructural observations on the intestinal mucosa of the toad (Bufo melanostictus). J Anat 1977;123 (Pt 2):331–339. [PMC free article] [PubMed] [Google Scholar]
- 37.Nochi T, Jansen CA, Toyomizu M, et al. The well-developed mucosal immune systems of birds and mammals allow for similar approaches of mucosal vaccination in both types of animals. Front Nutr 2018;5:60. DOI: 10.3389/fnut.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Geus ED, Vervelde L. Regulation of macrophage and dendritic cell function by pathogens and through immunomodulation in the avian mucosa. Dev Comp Immunol 2013;41(3):341–351. DOI: 10.1016/j.dci.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Wigley P Salmonella enterica in the chicken: How it has helped our understanding of immunology in a non-biomedical model species. Front Immunol 2014;5:482. DOI: 10.3389/fimmu.2014.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stremmel C, Schuchert R, Wagner F, et al. Yolk sac macrophage progenitors traffic to the embryo during defined stages of development. Nat Commun 2018;9:75. DOI: 10.1038/s41467-017-02492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takashina T Haemopoiesis in the human yolk sac. J Anat 1987;151:125–135. [PMC free article] [PubMed] [Google Scholar]
- 42.Smythies LE, Maheshwari A, Clements R, et al. Mucosal IL-8 and TGF-beta recruit blood monocytes: Evidence for cross-talk between the lamina propria stroma and myeloid cells. J Leukoc Biol 2006;80(3):492–499. DOI: 10.1189/jlb.1005566. [DOI] [PubMed] [Google Scholar]
- 43.Shepard JL, Zon LI. Developmental derivation of embryonic and adult macrophages. Curr Opin Hematol 2000;7(1):3–8. DOI: 10.1097/00062752-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Maheshwari A, Kurundkar AR, Shaik SS, et al. Epithelial cells in fetal intestine produce chemerin to recruit macrophages. Am J Physiol Gastrointest Liver Physiol 2009;297(1):G1–G10. DOI: 10.1152/ajpgi.90730.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janossy G, Bofill M, Poulter LW, et al. Separate ontogeny of two macrophage-like accessory cell populations in the human fetus. J Immunol 1986;136(12):4354–4361. [PubMed] [Google Scholar]
- 46.MacDonald TT, Weinel A, Spencer J. HLA-DR expression in human fetal intestinal epithelium. Gut 1988;29(10):1342–1348. DOI: 10.1136/gut.29.10.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Celada A, Borràs FE, Soler C, et al. The transcription factor PU.1 is involved in macrophage proliferation. J Exp Med 1996;184(1):61–69. DOI: 10.1084/jem.184.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasaai B, Caolo V, Peacock HM, et al. Erythro-myeloid progenitors can differentiate from endothelial cells and modulate embryonic vascular remodeling. Sci Rep 2017;7:43817. DOI: 10.1038/srep43817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinka L, Biasch K, Khazaal I, et al. Angiotensin-converting enzyme (CD143) specifies emerging lympho-hematopoietic progenitors in the human embryo. Blood 2012;119(16):3712–3723. DOI: 10.1182/blood-2010-11-314781. [DOI] [PubMed] [Google Scholar]
- 50.Mariani SA, Li Z, Rice S, et al. Pro-inflammatory aorta-associated macrophages are involved in embryonic development of hematopoietic stem cells. Immunity 2019;50(6):1439–1452.e5. DOI: 10.1016/j.immuni.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGrath KE, Frame JM, Fegan KH, et al. Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep 2015;11(12):1892–1904. DOI: 10.1016/j.celrep.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010;330(6005):841–845. DOI: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elchaninov AV, Fatkhudinov TKh, Vishnyakova PA, et al. Phenotypical and functional polymorphism of liver resident macrophages. Cells 2019;8(9):1032. DOI: 10.3390/cells8091032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelemen E, Janossa M. Macrophages are the first differentiated blood cells formed in human embryonic liver. Exp Hematol 1980;8(8):996–1000. [PubMed] [Google Scholar]
- 55.Zhu YP, Thomas GD, Hedrick CC. 2014 Jeffrey M. Hoeg Award Lecture: Transcriptional control of monocyte development. Arterioscler Thromb Vasc Biol 2016;36(9):1722–1733. DOI: 10.1161/ATVBAHA.116.304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palis J, Yoder MC. Yolk-sac hematopoiesis: The first blood cells of mouse and man. Exp Hematol 2001;29(8):927–936. DOI: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- 57.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol 2016;17(1):2–8. DOI: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front Immunol 2014;5:514. DOI: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jakubzick C, Gautier EL, Gibbings SL, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 2013;39(3):599–610. DOI: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen KD, Fenstress SJ, Qiu Y, et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 2013;341(6153):1483–1488. DOI: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003;19(1):71–82. DOI: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 62.Prata L, Ovsyannikova IG, Tchkonia T, et al. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin Immunol 2018;40:101275. DOI: 10.1016/j.smim.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol 2001;69(1):11–20. [PubMed] [Google Scholar]
- 64.Zhang C, Zhang B, Zhang X, et al. Targeting orphan nuclear receptors NR4As for energy homeostasis and diabetes. Front Pharmacol 2020;11:587457. DOI: 10.3389/fphar.2020.587457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamura A, Hirai H, Yokota A, et al. C/EBPbeta is required for survival of Ly6C(−) monocytes. Blood 2017;130(16):1809–1818. DOI: 10.1182/blood-2017-03-772962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007;317(5838):666–670. DOI: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 67.Garré JM, Yang G. Contributions of monocytes to nervous system disorders. J Mol Med (Berl) 2018;96(9):873–883. DOI: 10.1007/s00109-018-1672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yona S, Kim K-W, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013;38(1):79–91. DOI: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Porcellini A, Manna A, Manna M, et al. Ontogeny of granulocyte-macrophage progenitor cells in the human fetus. Int J Cell Cloning 1983;1(2):92–104. DOI : 10.1002/stem.5530010204. [DOI] [PubMed] [Google Scholar]
- 70.Linch DC, Knott LJ, Rodeck CH, et al. Studies of circulating hemopoietic progenitor cells in human fetal blood. Blood 1982;59(5):976–979. [PubMed] [Google Scholar]
- 71.Christensen RD, Jensen J, Maheshwari A, et al. Reference ranges for blood concentrations of eosinophils and monocytes during the neonatal period defined from over 63,000 records in a multihospital health-care system. J Perinatol 2010;30(8):540–545. DOI: 10.1038/jp.2009.196. [DOI] [PubMed] [Google Scholar]
- 72.Xanthou M Leucocyte blood picture in healthy full-term and premature babies during neonatal period. Arch Dis Child 1970;45(240):242–249. DOI: 10.1136/adc.45.240.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weinberg AG, Rosenfeld CR, Manroe BL, et al. Neonatal blood cell count in health and disease. II. Values for lymphocytes, monocytes, and eosinophils. J Pediatr 1985;106(3):462–466. DOI: 10.1016/s0022-3476(85)80681-8. [DOI] [PubMed] [Google Scholar]
- 74.Germic N, Frangez Z, Yousefi S, et al. Regulation of the innate immune system by autophagy: Monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ 2019;26(4):715–727. DOI: 10.1038/s41418-019-0297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martino DJ, Tulic MK, Gordon L, et al. Evidence for age-related and individual-specific changes in DNA methylation profile of mononuclear cells during early immune development in humans. Epigenetics 2011;6(9):1085–1094. DOI: 10.4161/epi.6.9.16401. [DOI] [PubMed] [Google Scholar]
- 76.Bermick JR, Lambrecht NJ, denDekker AD, et al. Neonatal monocytes exhibit a unique histone modification landscape. Clin Epigenetics 2016;8:99. DOI: 10.1186/s13148-016-0265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kobayashi SD, DeLeo FR. Role of neutrophils in innate immunity: A systems biology-level approach. Wiley Interdiscip Rev Syst Biol Med 2009;1(3):309–333. DOI: 10.1002/wsbm.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Speer CP, Ambruso DR, Grimsley J, et al. Oxidative metabolism in cord blood monocytes and monocyte-derived macrophages. Infect Immun 1985;50(3):919–921. DOI: 10.1128/iai.50.3.919-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Speer CP, Wieland M, Ulbrich R, et al. Phagocytic activities in neonatal monocytes. Eur J Pediatr 1986;145(5):418–421. DOI: 10.1007/BF00439252. [DOI] [PubMed] [Google Scholar]
- 80.Weston WL, Carson BS, Barkin RM, et al. Monocyte-macrophage function in the newborn. Am J Dis Child 1977;131(11):1241–1242. DOI: 10.1001/archpedi.1977.02120240059011. [DOI] [PubMed] [Google Scholar]
- 81.D’Ambola JB, Sherman MP, Tashkin DP, et al. Human and rabbit newborn lung macrophages have reduced anti-Candida activity. Pediatr Res 1988;24(3):285–290. DOI: 10.1203/00006450-198809000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Bryson YJ, Winter HS, Gard SE, et al. Deficiency of immune interferon production by leukocytes of normal newborns. Cell Immunol 1980;55(1):191–200. DOI: 10.1016/0008-8749(80)90150-1. [DOI] [PubMed] [Google Scholar]
- 83.Weatherstone KB, Rich EA. Tumor necrosis factor/cachectin and interleukin-1 secretion by cord blood monocytes from premature and term neonates. Pediatr Res 1989;25(4):342–346. DOI: 10.1203/00006450-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 84.Wilson CB. Immunologic basis for increased susceptibility of the neonate to infection. J Pediatr 1986;108(1):1–12. DOI: 10.1016/s0022-3476(86)80761-2. [DOI] [PubMed] [Google Scholar]
- 85.Bessler H, Sirota L, Dulitzky F, et al. Production of interleukin-1 by mononuclear cells of newborns and their mothers. Clin Exp Immunol 1987;68(3):655–661. [PMC free article] [PubMed] [Google Scholar]
- 86.Kesson AM, Bryson YJ. Induction of interferon-gamma by cord blood mononuclear cells is calcium dependent. Cell Immunol 1991;133(1):138–146. DOI: 10.1016/0008-8749(91)90186-f. [DOI] [PubMed] [Google Scholar]
- 87.Bencheikh L, Diop MK, Rivière J, et al. Dynamic gene regulation by nuclear colony-stimulating factor 1 receptor in human monocytes and macrophages. Nat Commun 2019;10(1):1935. DOI: 10.1038/s41467-019-09970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Colonna M. Interkeukin-34, a cytokine crucial for the differentiation and maintenance of tissue resident macrophages and Langerhans cells. Eur J Immunol 2014;44(6):1575–1581. DOI: 10.1002/eji.201344365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orecchioni M, Ghosheh Y, Pramod AB, et al. Macrophage polarization: Different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front Immunol 2019;10:1084. DOI: 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kapellos TS, Bonaguro L, Gemünd I, et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol 2019;10:2035. DOI: 10.3389/fimmu.2019.02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014;6:13. DOI: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front Immunol 2014;5:614. DOI: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mills CD, Ley K. M1 and M2 macrophages: The chicken and the egg of immunity. J Innate Immun 2014;6(6):716–726. DOI: 10.1159/000364945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gschwandtner M, Derler R, Midwood KS. More than just attractive: How CCL2 influences myeloid cell behavior beyond chemotaxis. Front Immunol 2019;10:2759. DOI: 10.3389/fimmu.2019.02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mills CD. Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: a life or death issue. Crit Rev Immunol 2001;21(5):399–425. [PubMed] [Google Scholar]
- 96.Liu SX, Gustafson HH, Jackson DL, et al. Trajectory analysis quantifies transcriptional plasticity during macrophage polarization. Sci Rep 2020;10(1):12273. DOI: 10.1038/s41598-020-68766-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee M, Lee Y, Song J, et al. Tissue-specific role of CX3CR1 expressing immune cells and their relationships with human disease. Immune Netw 2018;18(1):e5. DOI: 10.4110/in.2018.18.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang J, Patel L, Pienta KJ. Targeting chemokine (C-C motif) ligand 2 (CCL2) as an example of translation of cancer molecular biology to the clinic. Prog Mol Biol Transl Sci 2010;95:31–53. DOI: 10.1016/B978-0-12-385071-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Graney PL, Ben-Shaul S, Landau S, et al. Macrophages of diverse phenotypes drive vascularization of engineered tissues. Sci Adv 2020;6(18):eaay6391. DOI: 10.1126/sciadv.aay6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roszer T Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015;2015:816460. DOI: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muraille E, Leo O, Moser M. TH1/TH2 paradigm extended: Macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front Immunol 2014;5:603. DOI: 10.3389/fimmu.2014.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013;38(4):792–804. DOI: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davies LC, Jenkins SJ, Allen JE, et al. Tissue-resident macrophages. Nat Immunol 2013;14(10):986–995. DOI: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boulakirba S, Pfeifer A, Mhaidly R, et al. IL-34 and CSF-1 display an equivalent macrophage differentiation ability but a different polarization potential. Sci Rep 2018;8(1):256. DOI: 10.1038/s41598-017-18433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rettenmier CW, Roussel MF, Sherr CJ. The colony-stimulating factor 1 (CSF-1) receptor (c-fms proto-oncogene product) and its ligand. J Cell Sci Suppl 1988;9:27–44. DOI: 10.1242/jcs.1988.supplement_9.2. [DOI] [PubMed] [Google Scholar]
- 106.Jaguin M, Houlbert N, Fardel O, et al. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol 2013;281(1):51–61. DOI: 10.1016/j.cellimm.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 107.Jaffe R Review of human dendritic cells: Isolation and culture from precursors. Pediatr Pathol 1993;13:821–837. DOI: 10.3109/15513819309048268. [DOI] [PubMed] [Google Scholar]
- 108.Foster CA, Holbrook KA, Farr AG. Ontogeny of langerhans cells in human embryonic and fetal skin: Expression of HLA-DR and OKT-6 determinants. J Invest Dermatol 1986;86(3):240–243. DOI: 10.1111/1523-1747.ep12285201. [DOI] [PubMed] [Google Scholar]
- 109.Grouard G, Rissoan MC, Filgueira L, et al. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med 1997;185(6):1101–1111. DOI: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.O’Doherty U, Peng M, Gezelter S, et al. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology 1994;82(3):487–493. [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y-J, Kanzler H, Soumelis V, et al. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol 2001;2(7):585–589. DOI: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 112.Denning TL, Wang YC, Patel SR, et al. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol 2007;8(10):1086–1094. DOI: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 113.Velilla PA, Rugeles MT, Chougnet CA. Defective antigen-presenting cell function in human neonates. Clin Immunol 2006;121(3):251–259. DOI: 10.1016/j.clim.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Petty RE, Hunt DW. Neonatal dendritic cells. Vaccine 1998;16 (14–15):1378–1382. DOI: 10.1016/s0264-410x(98)00095-4. [DOI] [PubMed] [Google Scholar]
- 115.Bondada S, Wu H, Robertson DA. Accessory cell defect in unresponsiveness of neonates and aged to polysaccharide vaccines. Vaccine 2000;19(4–5):557–565. DOI: 10.1016/S0264-410X(00)00161-4. [DOI] [PubMed] [Google Scholar]
- 116.Guilliams M, Svedberg FR. Does tissue imprinting restrict macrophage plasticity? Nat Immunol 2021;22:118–127. DOI: 10.1038/s41590-020-00849-2. [DOI] [PubMed] [Google Scholar]
- 117.Yang J, Zhang L, Yu C, et al. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2014;2:1. DOI: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007;204(12):3037–3047. DOI: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Das A, Sinha M, Datta S, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol 2015;185(10):2596–2606. DOI: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Avraham-Davidi I, Yona S, Grunewald M, et al. On-site education of VEGF-recruited monocytes improves their performance as angiogenic and arteriogenic accessory cells. J Exp Med 2013;210(12):2611–2625. DOI: 10.1084/jem.20120690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maheshwari A, Kelly DR, Nicola T, et al. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 2011;140(1):242–253. DOI: 10.1053/j.gastro.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8(12):958–969. DOI: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Parisi L, Gini E, Baci D, et al. Macrophage polarization in chronic inflammatory diseases: Killers or builders? J Immunol Res 2018;2018:8917804. DOI: 10.1155/2018/8917804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Van Belleghem JD, Bollyky PL. Macrophages and innate immune memory against Staphylococcus skin infections. Proc Natl Acad Sci USA 2018;115(47):11865–11867. DOI: 10.1073/pnas.1816935115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hampton HR, Chtanova T. Lymphatic migration of immune cells. Front Immunol 2019;10:1168. DOI: 10.3389/fimmu.2019.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Watanabe S, Alexander M, Misharin AV. The role of macrophages in the resolution of inflammation. J Clin Invest 2019;129(7):2619–2628. DOI: 10.1172/JCI124615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bezsonov EE, Gratchev A, Orekhov AN. Macrophages in health and non-infectious disease. Biomedicines 2021;9(5):460. DOI: 10.3390/biomedicines9050460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Italiani P, Della Camera G, Boraschi D. Induction of innate immune memory by engineered nanoparticles in monocytes/macrophages: From hypothesis to reality. Front Immunol 2020;11:566309. DOI: 10.3389/fimmu.2020.566309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol 2017;17(6):349–362. DOI: 10.1038/nri.2017.28. [DOI] [PubMed] [Google Scholar]
- 130.Netea MG, Domínguez-Andrés J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol 2020;20(6):375–388. DOI: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee SH, Starkey PM, Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med 1985;161(3):475–489. DOI: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maheshwari A, Zemlin M. Ontogeny of the intestinal immune system. Haematol Rep 2006;10:18–26. [Google Scholar]
- 133.MacDonald TT, Spencer J. Ontogeny of the mucosal immune response. Springer Semin Immunopathol 1990;12:129–137. DOI: 10.1007/BF00197501. [DOI] [PubMed] [Google Scholar]
- 134.Rognum TO, Thrane S, Stoltenberg L, et al. Development of intestinal mucosal immunity in fetal life and the first postnatal months. Pediatr Res 1992;32(2):145–149. DOI: 10.1203/00006450-199208000-00003. [DOI] [PubMed] [Google Scholar]
- 135.Braegger CP, Spencer J, MacDonald TT. Ontogenetic aspects of the intestinal immune system in man. Int J Clin Lab Res 1992;22(1):1–4. [PubMed] [Google Scholar]
- 136.Harvey J, Jones DB, Wright DH. Differential expression of MHC- and macrophage-associated antigens in human fetal and postnatal small intestine. Immunology 1990;69(3):409–415. [PMC free article] [PubMed] [Google Scholar]
- 137.Spencer J, MacDonald TT, Isaacson PG. Heterogeneity of non-lymphoid cells expressing HLA-D region antigens in human fetal gut. Clin Exp Immunol 1987;67(2):415–424. [PMC free article] [PubMed] [Google Scholar]
- 138.Smythies LE, Maheshwari A, Clements R, et al. Mucosal IL-8 and TGF-beta recruit blood monocytes: Evidence for cross-talk between the lamina propria stroma and myeloid cells. J Leukoc Biol 2006;80(3):492–499. DOI: 10.1189/jlb.1005566. [DOI] [PubMed] [Google Scholar]
- 139.Smythies LE, Sellers M, Clements RH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 2005;115(1):66–75. DOI: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mayrhofer G, Pugh CW, Barclay AN. The distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol 1983;13(2):112–122. DOI: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- 141.Alenghat E, Esterly JR. Alveolar macrophages in perinatal infants. Pediatrics 1984;74(2):221–223. [PubMed] [Google Scholar]
- 142.Jacobs RF, Wilson CB, Palmer S, et al. Factors related to the appearance of alveolar macrophages in the developing lung. Am Rev Respir Dis 1985;131(4):548–553. DOI: 10.1164/arrd.1985.131.4.548. [DOI] [PubMed] [Google Scholar]
- 143.Kurland G, Cheung ATW, Miller ME, et al. The ontogeny of pulmonary defenses: Alveolar macrophage function in neonatal and juvenile rhesus monkeys. Pediatr Res 1988;23:293–297. DOI: 10.1203/00006450-198803000-00013. [DOI] [PubMed] [Google Scholar]
- 144.Johnston RB Jr. Current concepts: Immunology. Monocytes macrophages. N Engl J Med 1988;318(12):747–752. DOI: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- 145.Yoder MC, Lanker TA, Engle WA. Culture medium oxygen tension affects fibronectin production in human adult and cord blood macrophages. Immunol Lett 1988;19(1):1–6. DOI: 10.1016/0165-2478(88)90111-3. [DOI] [PubMed] [Google Scholar]
- 146.Santiago-Schwarz F, Fleit HB. Identification of nonadherent mononuclear cells in human cord blood that differentiate into macrophages. J Leukoc Biol 1988;43(1):51–59. DOI: 10.1002/jlb.43.1.51. [DOI] [PubMed] [Google Scholar]
- 147.Stiehm ER, Sztein MB, Steeg PS, et al. Deficient DR antigen expression on human cord blood monocytes: Reversal with lymphokines. Clin Immunol Immunopathol 1984;30(3):430–436. DOI: 10.1016/0090-1229(84)90028-x. [DOI] [PubMed] [Google Scholar]
- 148.Morris RB, Nichols BA, Bainton DF. Ultrastructure and peroxidase cytochemistry of normal human leukocytes at birth. Dev Biol 1975;44(2):223–238. DOI: 10.1016/0012-1606(75)90394-2. [DOI] [PubMed] [Google Scholar]
- 149.Bhoopat L, Taylor CR, Hofman FM. The differentiation antigens of macrophages in human fetal liver. Clin Immunol Immunopathol 1986;41(2):184–192. DOI: 10.1016/0090-1229(86)90102-9. [DOI] [PubMed] [Google Scholar]
- 150.Bulmer JN, Morrison L, Smith JC. Expression of class II MHC gene products by macrophages in human uteroplacental tissue. Immunology 1988;63(4):707–714. [PMC free article] [PubMed] [Google Scholar]
- 151.Glover DM, Brownstein D, Burchett S, et al. Expression of HLA class II antigens and secretion of interleukin-1 by monocytes and macrophages from adults and neonates. Immunology 1987;61(2):195–201. [PMC free article] [PubMed] [Google Scholar]
- 152.Stiehm ER, Sztein MB, Steeg PS, et al. Deficient DR antigen expression on human neonatal monocytes: Reversal with lymphokines. Birth Defects Orig Artic Ser 1983;19:295–298. [PubMed] [Google Scholar]
- 153.Kelsall B Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages. Mucosal Immunol 2008;1(6):460–469. DOI: 10.1038/mi.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maheshwari A, Voitenok NN, Akalovich S, et al. Developmental changes in circulating IL-8/CXCL8 isoforms in neonates. Cytokine 2009;46(1):12–16. DOI: 10.1016/j.cyto.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maheshwari A, Kelly DR, Nicola T, et al. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 2011;140(1):242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fox SE, Lu W, Maheshwari A, et al. The effects and comparative differences of neutrophil specific chemokines on neutrophil chemotaxis of the neonate. Cytokine 2005;29(3):135–140. DOI: 10.1016/j.cyto.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 157.Adams DH, Hathaway M, Shaw J, et al. Transforming growth factor-beta induces human T lymphocyte migration in vitro. J Immunol 1991;147(2):609–612. [PubMed] [Google Scholar]
- 158.Kitagawa Y, Sano Y, Ueda M, et al. N-glycosylation of erythropoietin is critical for apical secretion by Madin-Darby canine kidney cells. Exp Cell Res 1994;213(2):449–457. DOI: 10.1006/excr.1994.1222. [DOI] [PubMed] [Google Scholar]
- 159.Vagin O, Kraut JA, Sachs G. The role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am J Physiol Renal Physiol 2009;296(3):F459–F469. DOI: 10.1152/ajprenal.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol 1993;9:129–161. DOI: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 161.Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev 1999;79(1):73–98. DOI: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- 162.Murakami K, Kojima T, Sakaki Y. Assessment of clusters of transcription factor binding sites in relationship to human promoter, CpG islands and gene expression. BMC Genomics 2004;5(1):16. DOI: 10.1186/1471-2164-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chandraratna RA. Tazarotene--first of a new generation of receptor-selective retinoids. Br J Dermatol 1996;135(Suppl 49):18–25. DOI: 10.1111/j.1365-2133.1996.tb15662.x. [DOI] [PubMed] [Google Scholar]
- 164.Swift ME, Wallden B, Wayner EA, et al. Truncated RAR beta isoform enhances proliferation and retinoid resistance. J Cell Physiol 2006;209(3):718–725. DOI: 10.1002/jcp.20788. [DOI] [PubMed] [Google Scholar]
- 165.Kutzleb C, Busmann A, Wendland M, et al. Discovery of novel regulatory peptides by reverse pharmacology: Spotlight on chemerin and the RF-amide peptides metastin and QRFP. Curr Protein Pept Sci 2005;6(3):265–278. DOI: 0.2174/1389203054065419. [DOI] [PubMed] [Google Scholar]
- 166.Wittamer V, Franssen J-D, Vulcano M, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med 2003;198(7):977–985. DOI: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Miller MD, Krangel MS. Biology and biochemistry of the chemokines: A family of chemotactic and inflammatory cytokines. Crit Rev Immunol 1992;12(1–2):17–46. [PubMed] [Google Scholar]
- 168.MohanKumar K, Kaza N, Jagadeeswaran R, et al. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: Evidence from an animal model. Am J Physiol Gastrointest Liver Physiol 2012;303(1):G93–G102. DOI: 10.1152/ajpgi.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.MohanKumar K, Namachivayam K, Chapalamadugu KC, et al. Smad7 interrupts TGF-β signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis. Pediatr Res 2016;79(6):951–961. DOI: 10.1038/pr.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.MohanKumar K, Namachivayam K, Cheng F, et al. Trinitrobenzene sulfonic acid-induced intestinal injury in neonatal mice activates transcriptional networks similar to those seen in human necrotizing enterocolitis. Pediatr Res 2016;81(1–1):99–112. DOI: 10.1038/pr.2016.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.MohanKumar K, Namachivayam K, Ho TTB, et al. Cytokines and growth factors in the developing intestine and during necrotizing enterocolitis. Semin Perinatol 2017;41(1):52–60. DOI: 10.1053/j.semperi.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.MohanKumar K, Namachivayam K, Song T, et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun 2019;10(1):3494. DOI: 10.1038/s41467-019-11199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Namachivayam K, Blanco CL, MohanKumar K, et al. Smad7 inhibits autocrine expression of TGF-beta2 in intestinal epithelial cells in baboon necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2013;304(2):G167–G180. DOI: 10.1152/ajpgi.00141.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Smith PD, Smythies LE, Mosteller-Barnum M, et al. Intestinal macrophages lack CD14 and CD89 and consequently are downregulated for LPS- and IgA-mediated activities. J Immunol 2001;167(5):2651–2656. DOI: 10.4049/jimmunol.167.5.2651. [DOI] [PubMed] [Google Scholar]
- 175.Waddell LA, Lefevre L, Bush SJ, et al. ADGRE1 (EMR1, F4/80) is a rapidly-evolving gene expressed in mammalian monocyte-macrophages. Front Immunol 2018;9:2246. DOI: 10.3389/fimmu.2018.02246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Medina-Contreras O, Geem D, Laur O, et al. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest 2011;121(12):4787–4795. DOI: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.De Calisto J, Villablanca EJ, Mora JR. FcgammaRI (CD64): An identity card for intestinal macrophages. Eur J Immunol 2012;42(12):3136–3140. DOI: 10.1002/eji.201243061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bain CC, Scott CL, Uronen-Hansson H, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 2013;6(3):498–510. DOI: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Grainger JR, Konkel JE, Zangerle-Murray T, et al. Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch 2017;469(3–4):527–539. DOI: 10.1007/s00424-017-1958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Honda M, Surewaard BGJ, Watanabe M, et al. Perivascular localization of macrophages in the intestinal mucosa is regulated by Nr4a1 and the microbiome. Nat Commun 2020;11(1):1329. DOI: 10.1038/s41467-020-15068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Viola MF, Boeckxstaens G. Niche-specific functional heterogeneity of intestinal resident macrophages. Gut 2021;70(7):1383–1395. DOI: 10.1136/gutjnl-2020-323121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Viola MF, Boeckxstaens G. Intestinal resident macrophages: Multitaskers of the gut. Neurogastroenterol Motil 2020;32(8):e13843. DOI: 10.1111/nmo.13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.De Schepper S, Stakenborg N, Matteoli G, et al. Muscularis macrophages: Key players in intestinal homeostasis and disease. Cell Immunol 2018;330:142–150. DOI: 10.1016/j.cellimm.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ji S, Traini C, Mischopoulou M, et al. Muscularis macrophages establish cell-to-cell contacts with telocytes/PDGFRalpha-positive cells and smooth muscle cells in the human and mouse gastrointestinal tract. Neurogastroenterol Motil 2021;33(3):e13993. DOI: 10.1111/nmo.13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Matheis F, Muller PA, Graves CL, et al. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell 2020;180(1):64–78.e16. DOI: 10.1016/j.cell.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gabanyi I, Muller PA, Feighery L, et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 2016;164(3):378–391. DOI: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bain CC, Jenkins SJ. The biology of serous cavity macrophages. Cell Immunol 2018;330:126–135. DOI: 10.1016/j.cellimm.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 188.Aung HT, Schroder K, Himes SR, et al. LPS regulates proinflammatory gene expression in macrophages by altering histone deacetylase expression. FASEB J 2006;20(9):1315–1327. DOI: 10.1096/fj.05-5360com. [DOI] [PubMed] [Google Scholar]
- 189.Briones-Orta MA, Sosa-Garrocho M, Moreno-Alvarez P, et al. SnoN co-repressor binds and represses smad7 gene promoter. Biochem Biophys Res Commun 2006;341(3):889–894. DOI: 10.1016/j.bbrc.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 190.Monteleone G, Pallone F, MacDonald TT. Smad7 in TGF-beta-mediated negative regulation of gut inflammation. Trends Immunol 2004;25(10):513–517. DOI: 10.1016/j.it.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 191.Yan X, Lin Z, Chen F, et al. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-beta signaling. J Biol Chem 2009;284(44):30097–30104. DOI: 10.1074/jbc.M109.049304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang S, Fei T, Zhang L, et al. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol 2007;27(12):4488–4499. DOI: 10.1128/MCB.01636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ovcharenko I, Loots GG, Giardine BM, et al. Mulan:Multiple-sequence local alignment and visualization for studying function and evolution. Genome Res 2005;15(1):184–194. DOI: 10.1101/gr.3007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Israel A The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol 2010;2(3):a000158. DOI: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sasaki K, Ito T, Nishino N, et al. Real-time imaging of histone H4 hyperacetylation in living cells. Proc Natl Acad Sci USA 2009;106(38):16257–16262. DOI: 10.1073/pnas.0902150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ausio J, van Holde KE. Histone hyperacetylation: Its effects on nucleosome conformation and stability. Biochemistry 1986;25(6):1421–1428. DOI: 10.1021/bi00354a035. [DOI] [PubMed] [Google Scholar]
- 197.Namachivayam K, Blanco CL, Frost BL, et al. Preterm human milk contains a large pool of latent TGF-beta, which can be activated by exogenous neuraminidase. Am J Physiol Gastrointest Liver Physiol 2013;304(12):G1055–G1065. DOI: 10.1152/ajpgi.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Namachivayam K, Coffing HP, Sankaranarayanan NV, et al. Transforming growth factor-beta2 is sequestered in preterm human milk by chondroitin sulfate proteoglycans. Am J Physiol Gastrointest Liver Physiol 2015;309(3):G171–G180. DOI: 10.1152/ajpgi.00126.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lakhdari O, Yamamura A, Hernandez GE, et al. Differential immune activation in fetal macrophage populations. Sci Rep 2019;9:7677. DOI: 10.1038/s41598-019-44181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Maheshwari A Immunologic and hematological abnormalities in necrotizing enterocolitis. Clin Perinatol 2015;42(3):567–585. DOI: 10.1016/j.clp.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bjerke K, Halstensen TS, Jahnsen F, et al. Distribution of macrophages and granulocytes expressing L1 protein (calprotectin) in human Peyer’s patches compared with normal ileal lamina propria and mesenteric lymph nodes. Gut 1993;34(10):1357–1363. DOI: 10.1136/gut.34.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yang H, Wang H, Levine YA, et al. Identification of CD163 as an antiinflammatory receptor for HMGB1-haptoglobin complexes. JCI Insight 2016;1(7). DOI: 10.1172/jci.insight.85375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mercado-Lubo R, McCormick BA. A unique subset of Peyer’s patches express lysozyme. Gastroenterology 2010;138(1):36–39. DOI: 10.1053/j.gastro.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Da Silva C, Wagner C, Bonnardel J, et al. The Peyer’s patch mononuclear phagocyte system at steady state and during infection. Front Immunol 2017;8:1254. DOI: 10.3389/fimmu.2017.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang S, Ye Q, Zeng X, et al. Functions of macrophages in the maintenance of intestinal homeostasis. J Immunol Res 2019;2019:1512969. DOI: 10.1155/2019/1512969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kawano Y, Nakae J, Watanabe N, et al. Colonic pro-inflammatory macrophages cause insulin resistance in an intestinal Ccl2/Ccr2-dependent manner. Cell Metab 2016;24(2):295–310. DOI: 10.1016/j.cmet.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 207.Rogero MM, Calder PC. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients 2018;10(4):432. DOI: 10.3390/nu10040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Al-Bahrani AZ, Darwish A, Hamza N, et al. Gut barrier dysfunction in critically ill surgical patients with abdominal compartment syndrome. Pancreas 2010;39(7):1064–1069. DOI: 10.1097/MPA.0b013e3181da8d51. [DOI] [PubMed] [Google Scholar]
- 209.Chikina AS, Nadalin F, Maurin M, et al. Macrophages maintain epithelium integrity by limiting fungal product absorption. Cell 2020;183(2):411–428.e16. DOI: 10.1016/j.cell.2020.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vallon-Eberhard A, Landsman L, Yogev N, et al. Transepithelial pathogen uptake into the small intestinal lamina propria. J Immunol 2006;176(4):2465–2469. DOI: 10.4049/jimmunol.176.4.2465. [DOI] [PubMed] [Google Scholar]
- 211.Mowat AM, Bain CC. Guardians of the epithelium: Macrophages protect against toxic fungal derivatives. Mucosal Immunol 2021;14(3):542–543. DOI: 10.1038/s41385-020-00369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hamer HM, De Preter V, Windey K, et al. Functional analysis of colonic bacterial metabolism: Relevant to health? Am J Physiol Gastrointest Liver Physiol 2012;302(1):G1–G9. DOI: 10.1152/ajpgi.00048.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Parada Venegas D, La Fuente MKD, Landskron G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 2019;10:277. DOI: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chang PV, Hao L, Offermanns S, et al. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA 2014;111(6):2247–2252. DOI: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jiminez JA, Uwiera TC, Abbott DW, et al. Butyrate supplementation at high concentrations alters enteric bacterial communities and reduces intestinal inflammation in mice infected with Citrobacter rodentium. mSphere 2017;2(4):e00243–17. DOI: 10.1128/mSphere.00243-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pelaseyed T, Bergström JH, Gustafsson JK, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev 2014;260(1):8–20. DOI: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hillman ET, Lu H, Yao T, et al. Microbial ecology along the gastrointestinal tract. Microbes Environ 2017;32(4):300–313. DOI: 10.1264/jsme2.ME17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bain CC, Bravo-Blas A, Scott CL, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 2014;15(10):929–937. DOI: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Biswas A, Shouval DS, Griffith A, et al. WASP-mediated regulation of anti-inflammatory macrophages is IL-10 dependent and is critical for intestinal homeostasis. Nat Commun 2018;9(1):1779. DOI: 10.1038/s41467-018-03670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rahabi M, Jacquemin G, Prat M, et al. Divergent roles for macrophage C-type lectin receptors, dectin-1 and mannose receptors, in the intestinal inflammatory response. Cell Rep 2020;30(13):4386–4398. e5. DOI: 10.1016/j.celrep.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 221.Gephart SM, Gordon PV, Penn AH, et al. Changing the paradigm of defining, detecting, and diagnosing NEC: Perspectives on Bell’s stages and biomarkers for NEC. Semin Pediatr Surg 2018;27(1):3–10. DOI: 10.1053/j.sempedsurg.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 222.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126(3):443–456. DOI: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Remon JI, Amin SC, Mehendale SR, et al. Depth of bacterial invasion in resected intestinal tissue predicts mortality in surgical necrotizing enterocolitis. J Perinatol 2015;35(9):755–762. DOI: 10.1038/jp.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Garg PM, Bernieh A, Hitt MM, et al. Incomplete resection of necrotic bowel may increase mortality in infants with necrotizing enterocolitis. Pediatr Res 2021;89(1):163–170. DOI: 10.1038/s41390-020-0975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Garg PM, Hitt MM, Blackshear C, et al. Clinical determinants of postoperative outcomes in surgical necrotizing enterocolitis. J Perinatol 2020;40(11):1671–1678. DOI: 10.1038/s41372-020-0728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.MohanKumar K, Namachivayam K, Siva Kumar N, et al. Severe neonatal anemia increases intestinal permeability by disrupting epithelial adherens junctions. Am J Physiol Gastrointest Liver Physiol 2020;318(4):G705–G716. DOI: 10.1152/ajpgi.00324.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Maheshwari A, Traub TM, Garg PM, et al. Necrotizing Enterocolitis: Clinical Features, Histopathological Characteristics, and Genetic Associations. Curr Pediatr Rev 2022;18(3):210–225. DOI: 10.2174/1573396318666220204113858. [DOI] [PubMed] [Google Scholar]
- 228.Pender SLF, Braegger C, Gunther U, et al. Matrix metalloproteinases in necrotising enterocolitis. Pediatr Res 2003;54(2):160–164. DOI: 10.1203/01.PDR.0000072326.23442.C3. [DOI] [PubMed] [Google Scholar]
- 229.Maheshwari A, Christensen RD, Calhoun DA. ELR+ CXC chemokines in human milk. Cytokine 2003;24(3):91–102. DOI: 10.1016/j.cyto.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 230.Maheshwari A, Christensen RD, Calhoun DA, et al. Circulating CXC-chemokine concentrations in a murine intestinal ischemia-reperfusion model. Fetal Pediatr Pathol 2004;23(2–3):145–157. DOI: 10.1080/15227950490523781. [DOI] [PubMed] [Google Scholar]
- 231.Maheshwari A, Corbin L, Schelonka RL. Neonatal Necrotizing Enterocolitis. Res Rep Neonatol 2011:39–53. DOI: 10.2147/RRN.S23459. [DOI] [Google Scholar]
- 232.Maheshwari A, Schelonka RL, Dimmitt RA, et al. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res 2014;76(1):100–108. DOI: 10.1038/pr.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Maheshwari A, Voitenok NN, Akalovich S, et al. Developmental changes in circulating IL-8/CXCL8 isoforms in neonates. Cytokine 2009;46(1):12–16. DOI: 10.1016/j.cyto.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.McILwain RB, Timpa JG, Kurundkar AR, et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab Invest 2010;90(1):128–139. DOI: 10.1038/labinvest.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mezu-Ndubuisi OJ, Maheshwari A. Role of macrophages in fetal development and perinatal disorders. Pediatr Res 2021;90(3):513–523. DOI: 10.1038/s41390-020-01209-4. [DOI] [PubMed] [Google Scholar]
- 236.Namachivayam K, MohanKumar K, Garg L, et al. Neonatal mice with necrotizing enterocolitis-like injury develop thrombocytopenia despite increased megakaryopoiesis. Pediatr Res 2017;81(5):817–824. DOI: 10.1038/pr.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Reeves AA, Johnson MC, Vasquez MM, et al. TGF-beta2, a protective intestinal cytokine, is abundant in maternal human milk and human-derived fortifiers but not in donor human milk. Breastfeed Med 2013;8(6):496–502. DOI: 10.1089/bfm.2013.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chen G, Li Y, Su Y, et al. Identification of candidate genes for necrotizing enterocolitis based on microarray data. Gene 2018;661:152–159. DOI: 10.1016/j.gene.2018.03.088. [DOI] [PubMed] [Google Scholar]


