Abstract
Aim
Vitamin D deficiency is very common among children with IBD. Since there are conflicting results regarding the association of vitamin D with IBD, we conducted this systematic review to confirm the association of vitamin D with IBD.
Methods
We conducted a systematic search in Scopus, Cochrane Library, Web of Science, PubMed, and Google Scholar to find relevant studies. Articles with cross-sectional and case-control designs that reported the association between vitamin D and IBD among children were included.
Results
Eventually, 9 studies (with 16 effect sizes) reported the mean and SD or the median and the interquartile range of serum vitamin D levels in both subjects with IBD and control subjects. The random effects meta-analysis revealed that subjects with IBD had −1.159 ng/ml (95% CI: −2.783, 0.464) lower serum vitamin D concentrations compared with their healthy counterparts, but this difference was not significant. A total of 14 studies (with 18 effect sizes) with 2,602 participants provided information for the prevalence of vitamin D deficiency or insufficiency in patients with IBD as 44% (95% CI: 0.34–0.54) with significant heterogeneity noted among studies (p < 0.001; I2 = 97.31%).
Conclusion
This systematic and meta-analysis study revealed that vitamin D deficiency was associated with IBD. Longitudinal studies should be conducted in the future to confirm our findings. Large randomized controlled trials assessing the doses of supplementation of vitamin D would provide a better understanding of the association between vitamin D and IBD.
Keywords: vitamin D, inflammatory bowel disease (IBD), children, systematic review, supplement
Introduction
Inflammatory bowel disease (IBD) includes both ulcerative colitis (UC) and Crohn's disease (CD). IBD is a systemic, chronic disease. UC is confined to the rectum and/or the colon, whereas CD involves the entire gastrointestinal tract, with the most common occurrence in the ileum and the colon (1). Overall, the pediatric prevalence of IBD increased by 133% from 2007 to 2016 in the United States, and the subgroup of children aged 10–17 years was the major contributor to the rising pediatric IBD prevalence (2). IBD presents differently in adults and children. For example, CD is more prevalent in pediatrics when compared to UC. This is completely different from that of adult IBD (3). Even among the two age groups, the disease characteristics differ. The complications are more in pediatric IBD compared to adults (4).
The exact etiology of IBD may be attributed to changes in the intestinal flora, residing in urban areas, and diets having high amounts of fats and carbohydrates (5). Various studies reported the role of vitamin D in IBD (6, 7). Vitamin D deficiency may lead to a reduction in bacterial clearance in the colon (8). This vitamin changes the immune responses by influencing macrophages and T lymphocytes, hence avoiding excessive immune responses, and also repairs the intestinal mucosal barrier (9, 10).
Low vitamin D levels are more common in patients with IBD (11). However, it is not specific whether low vitamin D levels are related to malabsorption due to damage in the intestinal mucosa (12). Some observational studies reported low vitamin levels in patients with IBD (13, 14), and some studies reported a lack of decrease in vitamin D levels (15, 16). Vitamin D deficiency is very common among children with CD. Even osteoporosis and growth retardation are commonly noticed in CD when compared to UC (17). Similar to our study, a study was conducted previously by Del Pinto et al. (18) in 2015, considering all age groups. However, focusing on the age group of children and adolescents is necessary due to their critical age for growth and development. In addition, no significant results were obtained in that subgroup analysis due to the limitations of studies related to children and adolescents. Since there is no clear evidence of a direct association between vitamin D and IBD among children and adolescents, we conducted this systematic review to confirm the association of vitamin D with IBD.
Materials and methods
In this systematic review and meta-analysis, we investigated the data extracted based on components of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (19) and MOOSE (Meta-Analyses of Observational Studies in Epidemiology) checklist (20) on the relationship between serum vitamin D and inflammatory bowel disease in pediatric patients.
Search strategy and selection process
A structural and comprehensive literature was accomplished based on articles published up to 20 February (year) in the following electronic databases: Scopus, Cochrane Library, Web of Science, PubMed, and Google Scholar. The search used the terms “vitamin D,” “ergocalciferol,” “inflammatory bowel disease,” “Crohn's disease,” and “ulcerative colitis” in several combinations. The full electronic search strategy is reported in Supplementary Table 1. There were no date or language restrictions on imported articles. Moreover, all clinical trial and review article references were checked to find any relevant studies. After deleting duplicate articles, the title and abstract screening processes were performed. Subsequently, two researchers studied the full-text papers independently to find all the obligatory data for meta-analysis. The third researcher was called to resolve any discrepancies. Only articles that met the following criteria were included in this meta-analysis:
a) Observational studies—either case-control, cross-sectional, or cohort designs,
b) Studies with adolescents or children (age < 18 years),
c) Articles that showed mean and standard deviation (SD) or prevalence of insufficiency or deficiency of serum vitamin D levels in patients with IBD (Crohn's disease or ulcerative colitis).
Papers with (1) randomized clinical trial designs, (2) animal-based studies, (3) gray literature (chapters of books, abstracts in conferences, or review articles), (4) adult patients, (5) athletics, and (6) assessment of IBD in the acute phase were excluded from the analysis.
Data extraction and syntheses
Required information of the eligible papers was extracted by two observers, such as the author's last name, publication date, the study location, study design, mean age of cases and controls, the number of cases and controls, type of study population (UC or CD), mean and SD of vitamin D concentration in patients and healthy participants or prevalence of vitamin D deficiency or insufficiency status, seasonally matching or adjusting, and method of vitamin D assessment.
Quality assessments
The quality of the chosen papers in this meta-analysis was assessed using the Newcastle-Ottawa scale (21), which included 9 questions in three main sections such as selection of participants (0–4 points), comparability between groups (0–3 points), and outcome assessment (0–3 points). The quality evaluation process was reviewed by two investigators, and any differences in scoring were resolved by consensus.
Statistical analyses
All data analyses were performed using the Stata 13 software (Stata Corp., College Station, Texas, USA). Data on vitamin D levels in patients with IBD in case-control studies were reported as weighted mean differences and SD or prevalence of deficiency or insufficiency in cross-sectional articles. The studies were conducted in different populations and countries, so the random-effects analysis was used to control variation. To determine the heterogeneity, the I-square (I2) index was assessed by random-effects analysis, and high heterogeneity was described if I2 was more than 50%. A sensitivity analysis was conducted to find out which study had the highest proportion in the pool effect size. Afterward, Egger's regression model was used to examine the publication bias in the funnel plots. Due to the high heterogeneity between records, we decided to carry out the subgroup analysis to distinguish the possible reasons for high heterogeneity and the effect of variant agents on the relationship between serum levels of vitamin D and IBD. These subgroups were based on assessing vitamin D methods such as HPLC (high-performance liquid chromatography), RIA (radioimmunoassay), and chemiluminescence-based competitive protein-binding assay and evaluating matching the season to vitamin D concentration (yes, matched; NR, not reported).
Literature review
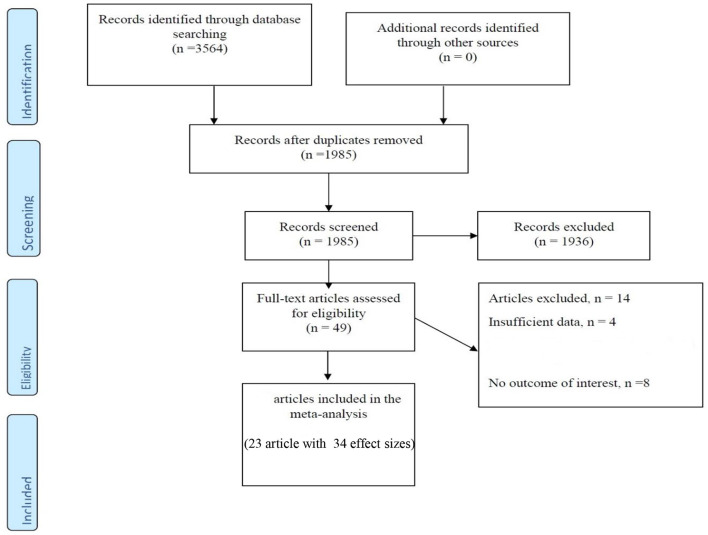
The main characteristics of case-control and cross-sectional studies are presented in Table 1 and Figure 1. Approximately 3,564 articles were searched electronically; 1,579 duplicate items were removed in the screening process, and 1,936 articles were eliminated as they had no relation to the purpose of the study. In addition, 14 articles did not meet the qualitative and quantitative criteria given in this study. Notably, a related cohort study was identified, which was omitted due to the format of the analysis, so we described it in the discussion part. Eventually, 9 articles (16 effect sizes) with a case-control design declared the concentration of vitamin D in children with a mean (SD), and 14 studies (with 18 effect sizes) declared the prevalence of vitamin D deficiency or insufficiency in pediatrics. These studies were published between 2003 and 2019, and most of the records (n = 11) were conducted in the United States (22, 25, 29–31, 33–36, 38, 39, 42, 43) and the rest in Canada (23, 40), Australia (32), Finland (24), Denmark (26), Italy (28), South Korea (27, 41), and Israel (37). In general, we entered data of 4,803 children participants with an age range of 2–18 years. Based on the methodological assay, the quality score was at least 6 points for the articles, and the majority gained high-quality points. Notably, the vitamin D measurement seasons were different in the studies, and some did not report any data, and some case-control studies presented season matching or adjustment. In addition, various measurement methods have been applied; some studies used HPLC (high-performance liquid chromatography) (24, 43) or RIA (radioimmunoassay) (29) and others applied chemiluminescence-based competitive protein-binding assays (23, 28, 30, 32, 35, 36, 39, 44) to measure the concentration of vitamin D.
Table 1.
Characteristics of studies.
| No | References | Country | Study Design | Population of study | Mean age, y | Total N | N case |
Mean/ sdcase (ng/ml) |
N control |
Mean/sd control (ng/ml) | Deficiency or insufficiency status |
Season adjust/ matching |
Season of measurement | Q | Method of vitamin D assessing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nwosu (22) | USA | Case-control | CD | 8.5 | 25 | 28.24+10.28 | 49 | 26.16 + 10.44 | NR/yes | Winter + spring,14/25 (56) | DiaSorin Liaison | |||
| 2 | El-Matary et al., (23) | Canada | Case-control | CD | 12.2 | 39 | 26.68 + 10.2 | 56 | 32.68 + 6.16 | NR/yes | NR | Protein binding assay after column chromatography (Esoterix | |||
| 3 | El-Matary et al. (23) | Canada | Case-control | UC | 12.2 | 21 | 22.76 + 8.8 | 56 | 32.68 + 6.16 | NR/yes | NR | ||||
| 4 | Laakso et al. (24) | Finland | Case-control | IBD | 14.9 | 80 | 21.6 + 25.48 | 80 | 18.5 + 19.25 | NR/ NR | NR | (HPLC) | |||
| 5 | Alkhouri et al. (15) | USA | Case-control | CD | 12.3 | 46 | 29.9 + 12.7 | 61 | 26.7 + 9.4 | NR/ NR | Is less vitamin D deficiency in the summer months as compared with | (HPLC) | |||
| 6 | Alkhouri et al. (15) | USA | Case-control | UC | 12.3 | 12 | 32 + 25.8 | 61 | 26.7 + 9.4 | NR/ NR | Spring (P0.08), autumn (P0.15), and winter (P0.08). | ||||
| 7 | Alkhouri et al. (15) | USA | Case-control | ID | 12.3 | 3 | 21.7 + 7.5 | 61 | 26.7 + 9.4 | NR/ NR | Spring (P0.08), autumn (P0.15), and winter (P0.08). | ||||
| 8 | Prosnitz (Black participants) (25 ) | USA | Case-control | CD | 13.5 | 8 | 10.5 + 4.6 | 62 | 15.8 + 7.9 | Radioimmunoassay (RIA) with I125-labeled tracer | |||||
| 9 | Prosnitz (Non-black participants) (25) | USA | Case-control | CD | 13.5 | 70 | 23.5 + 0.2 | 159 | 25.3 + 8.7 | Yes/NR | Winter | ||||
| 10 | Veit et al. (16) | USA | Case-control | IBD | 16.4 Control (14.6) | 58 | 24 + 10.24 | 116 | 24 + 10.88 | Yes/yes | Summer–fall, %:57.6 | DiaSorin Liaison | |||
| 11 | Thorsen et al. (26) | Denmark | Case-control | CD | 14 | 155 | 11.2 + 6.54 | 384 | 10.68 + 6.9 | NR/Yes | All | Liquid chromatography mass spectrometry (LC-MS) | |||
| 12 | Thorsen et al. (26) | Denmark | Case-control | UC | 15 | 210 | 10.92 + 7.08 | 384 | 10.68 + 6.9 | NR/Yes | All | ||||
| 13 | Thorsen et al. (26) | Denmark | Case-control | IC | 11 | 19 | 12.72 + 8.68 | 384 | 10.68 + 6.9 | NR/Yes | All | ||||
| 14 | Sohn et al. (27) | Korea | Case-control | CD | 14.4 | 43 | 16.3 + 9.3 | 45 | 16.5 + 5.3 | NR/NR | NR | Liquid chromatography mass spectrometry (LC-MS) | |||
| 15 | Sohn et al. (27) | Korea | Case-control | UC | 14.4 | 17 | 19.9 + 7.2 | 45 | 16.5 + 5.3 | NR/NR | NR | ||||
| 16 | Strisciuglio et al. (28) | Italy | Case-control | IBD | 11 (Control: 9.4) | 33 | 19.2 + 9.7 | 18 | 28.2 + 12.1 | NR/Yes | Winter-spring | Enzyme-linked immunosorbent | |||
| 17 | Sentongo et al. (29) | Philadelphia | Cross-sectional | CD | 15.76 | 113 | 16% < 20 ng/ml Hypovitaminosis < 38 nmol/L. | Radioimmunoassay with a radioiodinated tracer | |||||||
| 18 | Pappa et al. (30) | USA | Cross-sectional | IBD | 15.76 | 130 | 34.6% < 15 ng/ml 10.8%: 25OHD ≤ 8 | Nichols Advantage chemiluminescence-based competitive protein-binding assay (Nichols Institute Diagnostics,San Clemente, CA | |||||||
| 19 | Pappa et al. (30) | USA | Cross-sectional | CD | 15.76 | 94 | 38.3% < 15 ng/ml 12.8%: 25OHD ≤ 8 | Nichols Advantage chemiluminescence-based competitive protein-binding assay (Nichols Institute Diagnostics,San Clemente, CA | |||||||
| 20 | Pappa et al. (30) | USA | Cross-sectional | UC | 15.76 | 36 | 25% < 15 ng/ml 5.6 %: 25OHD ≤ 8 | Nichols Advantage chemiluminescence-based competitive protein-binding assay (Nichols Institute Diagnostics,San Clemente, CA | |||||||
| 21 | Salehi et al. (31) | USA | Cross-sectional | IBD | NR | 110 | 63.6% < 20 ng/ml < 50 nmol/l | NR | |||||||
| 22 | Levin et al. (32) | Australia | Cross-sectional | IBD | 12.6 | 263 | 19: 20–30 ng/ml 51–75 nmol/l | Antibody-based chemiluminescence assay | |||||||
| 23 | Levin et al. (32) | Australia | Cross-sectional | IBD | 12.6 | 263 | 38 < 20 ng/ml Defficiency < 51 nmol/l | Antibody-based chemiluminescence assay | |||||||
| 24 | Goldberg et al. (33) | USA | Cross-sectional | UC | 2-21 | 24 | 80 < 20 ng/ml Insufficiency 20–30 nmol/L | NR | |||||||
| 25 | Goldberg et al. (33) | USA | Cross-sectional | CD | 2-21 | 52 | 60 < 20 ng/ml Insufficiency 20–30 nmol/L | NR | |||||||
| 26 | Schaefer et al. (34) | USA | Cross-sectional | IBD | NR | 92 | < 30 ng/mL < 30 ng/mL | NR | |||||||
| 27 | Syed et al. (35) | USA | Cross-sectional | IBD | 5-18.9 | 69 | 77 20–30 ng/ml Insufficiency < 30 ng/mL | Automated chemiluminescent technique (Automated IDS-iSYS System, Immunodiagnostic Systems, Fountain Hills, AZ) | |||||||
| 28 | Syed et al. (36) | USA | Cross-sectional | IBD | 5-18.9 | 69 | 38 < 20 ng/ml Deficiency < 20 ng/ml | Automated chemiluminescent technique (Automated IDS-iSYS System, Immunodiagnostic Systems, Fountain Hills, AZ) | |||||||
| 29 | Brandvayman et al. (37) | Israeil | Cross-sectional | IBD | 14 | 623 | 21 < 20 ng/ml defficiency | NR | |||||||
| 30 | Winter et al. (38) | USA | Cross-sectional | IBD | 203 | 31 < 20 ng/ml LOW < 9 ng/ml | NR | ||||||||
| 31 | Sauer et al. (39) | USA | Cross-sectional | UC | 12.7 | 388 | 57 20–30 ng/ml insufficient < 30 ng/Ml | IDS-iSYS 25-Hydroxyvitamin D automated chemiluminescense immunoassay (Gaithersburg, MD) | |||||||
| 32 | Mager et al. (40) | Canada | Cross-sectional | CD | NR | 102 | 56.80% 20–30 ng/ml insufficiency < 50 nmol/l | Liquid chromatogrpahy-mass spectroscopy (LC-MS) technology | |||||||
| 33 | Mager et al. (40) | Canada | Cross-sectional | UC | NR | 63 | 43.20% 20–30 ng/ml insufficiency < 50 nmol/l | Liquid chromatogrpahy-mass spectroscopy (LC-MS) technology | |||||||
| 34 | Kim et al. (41) | Korea | Cross-sectional | IBD | 13.1 | 96 | 56.80% 20–30 ng/ml insufficiency < 50 nmol/l | Liquid chromatogrpahy-mass spectroscopy (LC-MS) technology |
Figure 1.
Flow chart of the included studies, including identification, screening, eligibility and the final sample included.
Meta-analysis
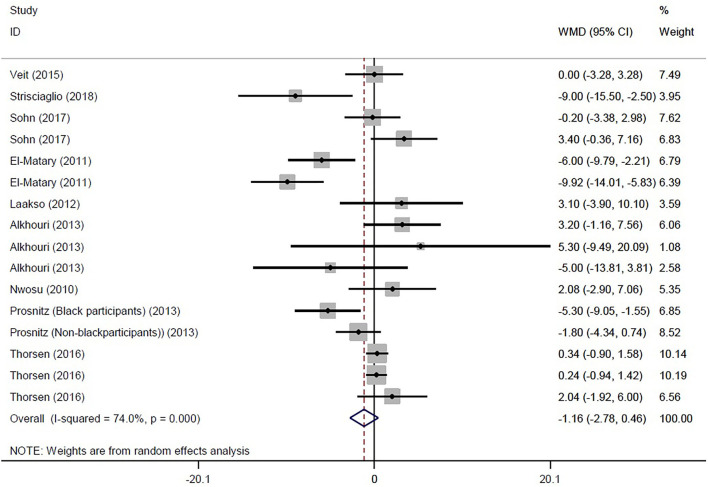
Eventually, 16 studies reported the mean and SD or median and interquartile range of serum vitamin D levels in both IBD and control subjects. The random effects meta-analysis revealed that subjects with IBD had −1.159 ng/ml (95% CI: −2.783, 0.464) lower serum vitamin D concentrations compared with their healthy counterparts, but this difference was not significant (Figure 2). However, the tests also showed that there was significant heterogeneity among the studies (I2 = 74.0%, P < 0.001). Heterogeneity in meta-analysis refers to the variation in study results between studies. To find out the source of heterogeneity, we conducted studies based on the following subgroups: type of patients (IBD, UC, CD, or IC), assessment method of 25OHD, and matching of the season for the control group. In the subgroup analysis, results remained non-significant, while the types of patients (IBD, UC, and CD) and assessment method of 25OHD were considered as possible sources of heterogeneity (Supplementary Figures 1–3).
Figure 2.
Forest plot show the WMD in serum vitamin D concentrations between participants with IBD and healthy control.
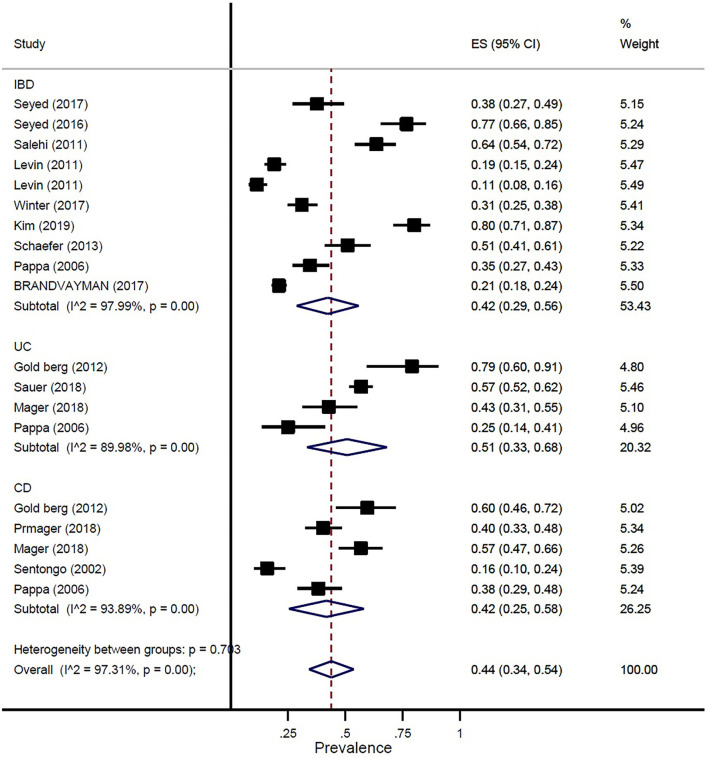
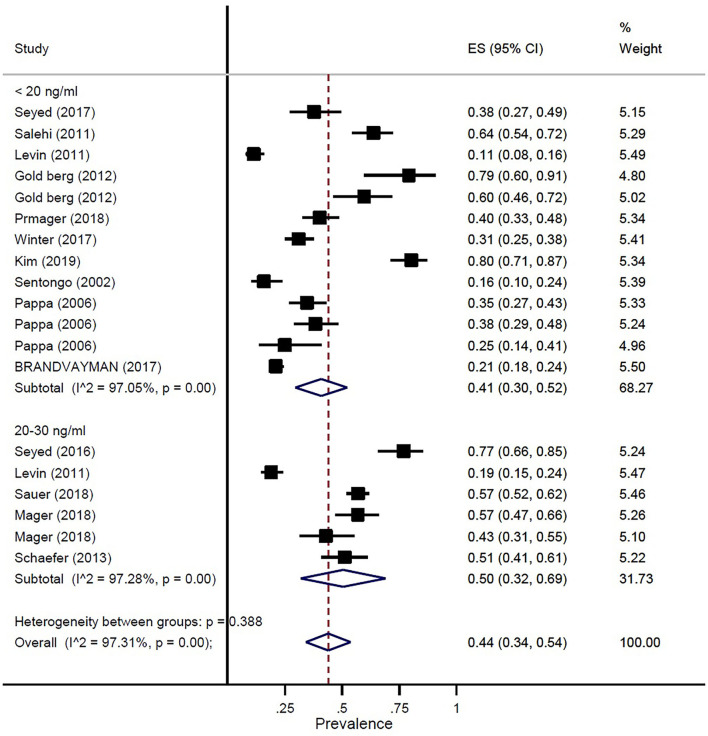
The prevalence of vitamin D deficiency or insufficiency in patients with IBD was found as 44% (95% CI: 0.34–0.54) by eighteen studies with a total of 2,602 participants, with significant heterogeneity noted among studies (p < 0.001; I2 = 97.31%). The pooled prevalence of vitamin D deficiency or insufficiency in patients with IBD was 42% (95% CI: 0.29–0.56), UC was 51% (95% CI: 0.33–0.68), and CD was 42% (95% CI: 0.25–0.58) (Figure 3). In addition, the prevalence of participants with a level of vitamin D < 20 ng/ml was 41% (95% CI: 0.30–0.52) and the level of 20–30 ng/ml was 50% (95% CI: 0.32–0.69) (Figure 4).
Figure 3.
Pooled estimate of the prevalence of vitamin D deficiency or insufficiency in children and adolescence with IBD, UC, and CD.
Figure 4.
Pooled estimate of the prevalence of vitamin D status in children and adolescence with IBD.
Publication bias and sensitivity analysis
Evaluation of publication bias by visual inspection of the funnel plot and Egger's test demonstrated the evidence for publication bias in the meta-analysis of the prevalence of vitamin D deficiency or insufficiency in patients with IBD (p = 0.05) (Supplementary Figure 4). However, the results of the meta-trim-and-fill analysis found no studies. Egger's linear regression test for case-control studies revealed no publication bias (p = 0.31) (Supplementary Figure 5). Sensitivity analysis revealed that removing some of the studies would not have a significant impact on the overall results (Supplementary Figures 6, 7).
Discussion
This systematic review and meta-analysis were conducted using 35 articles. Our study results showed that vitamin D is deficient in children with IBD when compared to the healthy control group. But the results were not statistically significant. These results were similar to another study. A meta-analysis was conducted, including 14 studies of 1,891 patients, and reported that patients with IBD had more vitamin D deficiency when compared to controls, and the results were statistically significant (18). Vitamin D regulates cytokine secretion, which is involved in the inflammatory response in immune systems (45). Many studies that were conducted previously have reported that the levels of vitamin D are lower in children with IBD (23, 46). Even there are contradictory results regarding the association of vitamin D with IBD (15). Sufficient vitamin D has been associated with clinical benefits in children with IBD. There was a reduction in disease activity in children with sufficient vitamin D (41). Guzman-Prado et al. also showed that vitamin D supplementation in patients with IBD and vitamin D deficiency is effective at correcting vitamin D levels and is associated with improvements in clinical and biochemical disease activity scores (47).
The subgroup analysis of our study did not reveal a statistically significant deficiency in vitamin D concentrations. The same results were obtained in a study conducted by Kim (41). El-Matary et al. (23) reported that the levels of vitamin D were lower in children with UC, but it was not statistically significant. Another study reported that the levels of vitamin D were higher in children with CD, and it was statistically significant (48). Sensitivity analysis revealed that removing some of the studies would not have a significant impact on the overall results. The quality of all the studies was assessed using the Newcastle-Ottawa scale.
The scientific evidence on the role of vitamin D in children with IBD was reviewed by studies conducted previously (6, 49–51). Although, the effective vitamin D supplementation in children with IBD remains controversial. Our meta-analysis evaluating vitamin D status in 2602 participants, a large cohort population, would provide consistent and useful results.
A low level of vitamin D is clinically significant. Both UC and CD are related to environmental factors (52). Hypovitaminosis D in IBD is related to malabsorption, which may be due to inflammation in the bowel or surgical resection (12); less exposure to sunlight (50); and higher intake of vitamin D by the inflammatory cells (53).
In our study, the prevalence of vitamin D deficiency or insufficiency in patients with IBD was 44%. The pooled prevalence of vitamin D deficiency or insufficiency in patients with IBD was 42%, UC was 51%, and CD was 42%. In addition, the prevalence of participants with a level of vitamin D of < 20 ng/ml was 41% and the level of vitamin D of 20–30 ng/ml was 50%. Our study results confirmed prior study findings that reported a high prevalence of vitamin D deficiency in IBD (13, 29).
There are a few major strengths of the present systematic review and meta-analysis. First, a large number of studies were included, which provided a large sample size. In addition, we successfully pooled the results of subgroup analyses, sensitivity analyses, and publication bias. Similarly, there are some limitations in our study that should be considered. First, there existed a substantial degree of heterogeneity in the designs of studies, with a wide range of methods of vitamin D assessment. Nonetheless, we performed subgroup analysis to find possible sources of heterogeneity. Another important limitation of this study is that we could not identify the relationship between the season of vitamin D level measurement and IBD because many studies did not report this variable well.
Conclusion
This systematic review and meta-analysis study showed that patients with IBD were associated with vitamin D deficiency. Longitudinal studies should be conducted in the future to confirm our findings. Large randomized controlled trials assessing the doses of vitamin D supplementation would provide a better understanding of the association between vitamin D and IBD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
SF, NAly, NAlb, FM, and MS contributed to the conception, design, and statistical analysis. SF, KP, NAly, NAlb, FM, PR, NK, AH, and AS contributed to data collection and the manuscript draft. AS, AH, and AA-Z supervised the study. All authors contributed the manuscript draft, critical revision, and approved the final version of the manuscript.
Acknowledgments
We express our gratitude to the participants of this study.
Funding Statement
This article is taken from the disease registry, titled Registration of inflammatory bowel diseases in children and with code number IR.SBMU.RETECH.REC.1397.1199 from the ethics committee, which was supported by the deputy of research and technology at Shahid Beheshti University of Medical Sciences (http://dregistry.sbmu.ac.ir).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1007725/full#supplementary-material
References
- 1.Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol. (2013) 29:357–62. 10.1097/MOG.0b013e32836229fb [DOI] [PubMed] [Google Scholar]
- 2.Ye Y, Manne S, Treem WR, Bennett D. Prevalence of inflammatory bowel disease in pediatric and adult populations: recent estimates from large national databases in the United States, 2007–2016. Inflamm Bowel Dis. (2020) 26:619–25. 10.1093/ibd/izz182 [DOI] [PubMed] [Google Scholar]
- 3.Ruel J, Ruane D, Mehandru S, Gower-Rousseau C, Colombel J-F, IBD. across the age spectrum—is it the same disease? Nat Rev Gastroenterol Hepatol. (2014) 11:88–98. 10.1038/nrgastro.2013.240 [DOI] [PubMed] [Google Scholar]
- 4.Gower-Rousseau C, Vasseur F, Fumery M, Savoye G, Salleron J, Dauchet L, et al. Epidemiology of inflammatory bowel diseases: new insights from a French population-based registry (EPIMAD). Digest Liver Dis. (2013) 45:89–94. 10.1016/j.dld.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN. Environmental risk factors for inflammatory bowel diseases: a review. Dig Dis Sci. (2015) 60:290–8. 10.1007/s10620-014-3350-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1, 25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. (2000) 130:2648–52. 10.1093/jn/130.11.2648 [DOI] [PubMed] [Google Scholar]
- 7.Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, Malaguarnera L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol. (2012) 280:36–43. 10.1016/j.cellimm.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 8.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. (2010) 151:2423–32. 10.1210/en.2010-0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. (2012) 71:50–61. 10.1017/S0029665111001650 [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, et al. Protective role of 1, 25 (OH) 2vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. (2012) 12:1–14. 10.1186/1471-230X-12-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abraham BP, Prasad P, Malaty HM. Vitamin D deficiency and corticosteroid use are risk factors for low bone mineral density in inflammatory bowel disease patients. Dig Dis Sci. (2014) 59:1878–84. 10.1007/s10620-014-3102-x [DOI] [PubMed] [Google Scholar]
- 12.Farraye F, Nimitphong H, Stucchi A, Dendrinos K, Boulanger A, Vijjeswarapu A, et al. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn's disease. Inflamm Bowel Dis. (2011) 17:2116–21. 10.1002/ibd.21595 [DOI] [PubMed] [Google Scholar]
- 13.Bruyn JR, Ponsioen CY, Brink GRvd, Löwenberg M, Bredenoord AJ, et al. Vitamin D deficiency in Crohn's disease and healthy controls: a prospective case–control study in the Netherlands. J Crohn's Colitis. (2014) 8:1267–73. 10.1016/j.crohns.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen SP, Hvas CL, Agnholt J, Christensen LA, Heickendorff L, Dahlerup JF. Active Crohn's disease is associated with low vitamin D levels. J Crohn's Colitis. (2013) 7:e407–e13. 10.1016/j.crohns.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 15.Alkhouri RH, Hashmi H, Baker RD, Gelfond D, Baker SS. Vitamin and mineral status in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2013) 56:89–92. 10.1097/MPG.0b013e31826a105d [DOI] [PubMed] [Google Scholar]
- 16.Veit LE, Maranda L, Fong J, Nwosu BU. The vitamin D status in inflammatory bowel disease. PLoS ONE. (2014) 9:e101583. 10.1371/journal.pone.0101583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harries A, Brown R, Heatley R, Williams L, Woodhead S, Rhodes J. Vitamin D status in Crohn's disease: association with nutrition and disease activity. Gut. (1985) 26:1197–203. 10.1136/gut.26.11.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F. Association between inflammatory bowel disease and vitamin D deficiency: a systematic review and meta-analysis. Inflamm Bowel Dis. (2015) 21:2708–17. 10.1097/MIB.0000000000000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selçuk AA, A. guide for systematic reviews: PRISMA. Turkish Arch Otorhinolaryngol. (2019) 57:57. 10.5152/tao.2019.4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooke BS, Schwartz TA, Pawlik TM, MOOSE. reporting guidelines for meta-analyses of observational studies. JAMA Surg. (2021) 156:787–8. 10.1001/jamasurg.2021.0522 [DOI] [PubMed] [Google Scholar]
- 21.Margulis AV, Pladevall M, Riera-Guardia N, Varas-Lorenzo C, Hazell L, Berkman ND, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: The Newcastle-Ottawa scale and the RTI item bank. Clin Epidemiol. (2014) 6:359. 10.2147/CLEP.S66677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nwosu BU, Maranda L. Vitamin D status and adiposity in pediatric malabsorption syndromes. Digestion. (2010) 92:1–7. 10.1159/000381895 [DOI] [PubMed] [Google Scholar]
- 23.El-Matary W, Sikora S, Spady D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Dig Dis Sci. (2011) 56:825–9. 10.1007/s10620-010-1380-5 [DOI] [PubMed] [Google Scholar]
- 24.Laakso S, Valta H, Verkasalo M, Toiviainen-Salo S, Viljakainen H, Mäkitie O. Impaired bone health in inflammatory bowel disease: a case–control study in 80 pediatric patients. Calcif Tissue Int. (2012) 91:121–30. 10.1007/s00223-012-9617-2 [DOI] [PubMed] [Google Scholar]
- 25.Prosnitz AR, Leonard MB, Shults J, Zemel BS, Hollis BW, Denson LA, et al. Changes in vitamin D and parathyroid hormone metabolism in incident pediatric Crohn's disease. Inflamm Bowel Dis. (2013) 19:45–53. 10.1002/ibd.22969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorsen SU, Jakobsen C, Cohen A, Lundqvist M, Thygesen LC, Pipper C, et al. Perinatal vitamin D levels are not associated with later risk of developing pediatric-onset inflammatory bowel disease: a Danish case-cohort study. Scand J Gastroenterol. (2016) 51:927–33. 10.3109/00365521.2016.1144218 [DOI] [PubMed] [Google Scholar]
- 27.Sohn J, Chang EJ, Yang HR. Vitamin D status and bone mineral density in children with inflammatory bowel disease compared to those with functional abdominal pain. J Korean Med Sci. (2017) 32:961–7. 10.3346/jkms.2017.32.6.961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strisciuglio C, Cenni S, Giugliano FP, Miele E, Cirillo G, Martinelli M, et al. The role of Inflammation on Vitamin D levels in a cohort of pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2018) 67:501–6. 10.1097/MPG.0000000000002049 [DOI] [PubMed] [Google Scholar]
- 29.Sentongo TA, Semaeo EJ, Stettler N, Piccoli DA, Stallings VA, Zemel BS. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am J Clin Nutr. (2002) 76:1077–81. 10.1093/ajcn/76.5.1077 [DOI] [PubMed] [Google Scholar]
- 30.Pappa HM, Grand RJ, Gordon CM. Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis. (2006) 12:1162–74. 10.1097/01.mib.0000236929.74040.b0 [DOI] [PubMed] [Google Scholar]
- 31.Salehi V, Small L, Yau M, Sockolow R. Vitamin D deficiency and abnormal bone density in pediatric patients with Inflammatory Bowel Disease. Inflam Bowel Dis. (2011) 17:S61–S. 10.1097/00054725-201112002-00194 [DOI] [Google Scholar]
- 32.Levin AD, Wadhera V, Leach ST, Woodhead HJ, Lemberg DA, Czarina Mendoza-Cruz A, et al. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci. (2011) 56:830–6. 10.1007/s10620-010-1544-3 [DOI] [PubMed] [Google Scholar]
- 33.Goldberg L, Yau M, Salehi V, Sockolow RE, Ward MJ, Antal Z. Small bowel disease in children and adolescents with inflammatory bowel disease is associated with deteriorating vitamin D status. Gastroenterology. (2012) 142:S370–S1. 10.1016/S0016-5085(12)61399-7 [DOI] [Google Scholar]
- 34.Schaefer M, Falaiye T. Monitoring Vitamin D levels in pediatric inflammatory bowel disease patients. Inflamm Bowel Dis. (2016) 22:S73–S4. 10.1097/01.MIB.0000480336.60576.4b [DOI] [Google Scholar]
- 35.Syed S, Smith EM, Tangpricha V, Chesdachai S, Kumar A, Prince J, et al. Assessing the relationship of vitamin D with iron status in children with inflammatory bowel disease. Faseb J. (2016) 30:128–7. [Google Scholar]
- 36.Syed S, Michalski ES, Tangpricha V, Chesdachai S, Kumar A, Prince J, et al. Vitamin D status is associated with hepcidin and hemoglobin concentrations in children with inflammatory bowel disease. Inflamm Bowel Dis. (2017) 23:1650–8. 10.1097/MIB.0000000000001178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandvayman Y, Rinawi F, Shamir R, Assa A. Associations of seasonal patterns and vitamin D levels with onset and flares of pediatric inflammatory bowel disease. Minerva Pediatr. (2017) 73:42–49. 10.23736/S2724-5276.17.04847-2 [DOI] [PubMed] [Google Scholar]
- 38.Winter RW, Lucci MB, Collins E, Cao B, Carrellas M, Crowell AM, et al. Higher 25-Hydroxyvitamin D levels are associated with a greater likelihood of remission with anti-tumor necrosis factor-alpha medications among patients with inflammatory bowel diseases. Gastroenterology. (2015) 148:S249–S. 10.1016/S0016-5085(15)30819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauer CG, Loop MS, Venkateswaran S, Tangpricha V, Ziegler TR, Dhawan A, et al. Free and bioavailable 25-hydroxyvitamin D concentrations are associated with disease activity in pediatric patients with newly diagnosed treatment naive ulcerative colitis. Inflamm Bowel Dis. (2018) 24:641–50. 10.1093/ibd/izx052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mager DR, Carroll MW, Wine E, Siminoski K, MacDonald K, Kluthe CL, et al. Vitamin D status and risk for sarcopenia in youth with inflammatory bowel diseases. Eur J Clin Nutr. (2018) 72:623–6. 10.1038/s41430-018-0105-2 [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Kang Y, Park S, Koh H, Kim S. Association of Vitamin D with inflammatory bowel disease activity in pediatric patients. J Korean Med Sci. (2019) 34:e204. 10.3346/jkms.2019.34.e204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veit LE, Maranda L, Nwosu BU. The nondietary determinants of vitamin D status in pediatric inflammatory bowel disease. Nutrition. (2015) 31:994–9. 10.1016/j.nut.2015.03.010 [DOI] [PubMed] [Google Scholar]
- 43.Santucci NR, Alkhouri RH, Baker RD, Baker SS. Vitamin and zinc status pretreatment and posttreatment in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2014) 59:455–7. 10.1097/MPG.0000000000000477 [DOI] [PubMed] [Google Scholar]
- 44.Strisciuglio C, Cenni S, Giugliano FP, Erasmo M, Cirillo G, Martinelli M, et al. The role of inflammation on vitamin D axis in a cohort of pediatric patients with inflammatory bowel disease. Digestive Liver Dis. (2017) 49:E260–E. 10.1016/j.dld.2017.09.050 [DOI] [Google Scholar]
- 45.Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. J Parenteral Enteral Nutr. (2011) 35:308–16. 10.1177/0148607110381267 [DOI] [PubMed] [Google Scholar]
- 46.Bernstein CN, Blanchard JF, Leslie W, Wajda A, Yu BN. The incidence of fracture among patients with inflammatory bowel disease: a population-based cohort study. Ann Intern Med. (2000) 133:795–9. 10.7326/0003-4819-133-10-200011210-00012 [DOI] [PubMed] [Google Scholar]
- 47.Guzman-Prado Y, Samson O, Segal JP, Limdi JK, Hayee BH. Vitamin D therapy in adults with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. (2020) 26:1819–30. 10.1093/ibd/izaa087 [DOI] [PubMed] [Google Scholar]
- 48.Raffner Basson A, Swart R, Jordaan E, Mazinu M, Watermeyer G. Vitamin D deficiency increases the risk for moderate to severe disease activity in Crohn's disease patients in South Africa, measured by the Harvey Bradshaw index. J Am Coll Nutr. (2016) 35:163–74. 10.1080/07315724.2015.1039665 [DOI] [PubMed] [Google Scholar]
- 49.Mouli VP, Ananthakrishnan AN. vitamin D and inflammatory bowel diseases. Aliment Pharmacol Ther. (2014) 39:125–36. 10.1111/apt.12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer MT, Weaver CT. Linking vitamin d deficiency to inflammatory bowel disease. Inflamm Bowel Dis. (2013) 19:2245–56. 10.1097/MIB.0b013e31828a3b6f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cantorna MT. Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch Biochem Biophys. (2012) 523:103–6. 10.1016/j.abb.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molodecky N, Panaccione R, Ghosh S, Barkema H, Kaplan G. Alberta Inflammatory Bowel Disease Consortium Challenges associated with identifying the environmental determinants of the inflammatory bowel diseases. Inflamm Bowel Dis. (2011) 17:1792–9. 10.1002/ibd.21511 [DOI] [PubMed] [Google Scholar]
- 53.Abreu M, Kantorovich V, Vasiliauskas E, Gruntmanis U, Matuk R, Daigle K, et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn's disease patients with elevated 1, 25-dihydroxyvitamin D and low bone mineral density. Gut. (2004) 53:1129–36. 10.1136/gut.2003.036657 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.