Abstract
Myxopapillary ependymoma (MPE) is a primary tumor of the central nervous system (CNS), characteristically an indolent malignancy involving the spinal conus medullaris, Filum terminale or cauda equina. We present a rare case of MPE, recurrent in the pelvic soft tissue with eventual pleural and intra-pulmonary metastasis. Refractory to repeated gross resection, adjuvant radiotherapy, platinum-based chemotherapy and temozolomide exploitation of mutant somatic BRCA1 status with the addition of a poly (ADP-ribose); polymerase inhibitor (PARPi) in a novel combination regimen with olaparib-temozolomide (OT) has achieved stable radiological disease after 10 cycles.
Keywords: Oncology, ependymoma, PARP inhibitor
Introduction
Myxopapillary ependymoma (MPE) is a primary tumor of the central nervous system (CNS), characteristically an indolent malignancy involving the spinal conus medullaris, Filum terminale or cauda equina. We present a rare case of late, distant recurrent MPE with a Pathological somatic BRCA1 truncation. Refractory to repeated gross Resection, adjuvant radiotherapy, platinum-based chemotherapy and single agent Temozolomide, clinical stability was obtained by the use of a combination of poly (ADP Ribose) polymerase inhibitor (PARPi) olaparib in combination with temozolomide.
Case presentation
A 48-year-old female with a history of WHO Grade I pelvic MPE and Eastern Cooperative Oncology Group (ECOG) performance status 0, presented to secondary care in January 2019 with acute shortness of breath, Type 1 respiratory failure and massive right-sided pleural effusion. 20 years prior, she had been diagnosed with pelvic MPE involving the intradural filum terminale, with no other evidence of CNS infiltration on magnetic resonance imaging (MRI), and was treated with radical surgical resection. Three further isolated intra-pelvic recurrences were treated with repeated gross resection and adjuvant radiotherapy prior to the current presentation (Figure 1).
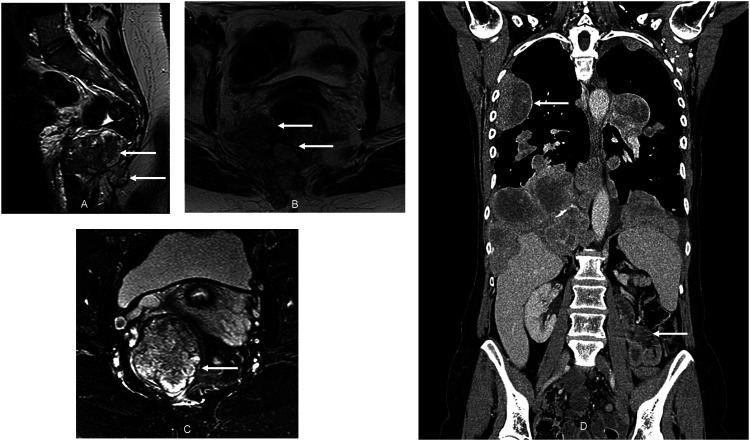
Figure 1.
A rare presentation of metastatic myxopapillary ependymoma. Magnetic Resonance Imaging (a and b) demonstrating a rare pelvic presentation of metastatic myxopapillary ependymoma. First presentation of pelvic disease is demonstrated in (a) Pelvic MRI (b) demonstrates disease progression after a 24-month period of disease stabilization with adjuvant radiotherapy, prior to final resection. There is evident a complex lobulated mass centered at the level of the coccyx and extending inferiorly and predominantly to the right of the midline, invading the posterior rectal wall and threatening the sciatic nerve, craniocaudal extent 8.9 cm. Status of pelvic disease, recurrent after multiple resections, at the time of presentation with metastatic disease to the thorax is shown in (c) Computed Tomography (d) demonstrating rare presentation of thoracic disease in metastatic myxopapillary ependymoma. Disease is shown at commencement of olaparib-temozolomide, where there is evident extensive intrathoracic involvement (intrapulmonary and pleural), in addition to small-volume pelvic (inguinal) nodal and hepatic disease (latter not shown).
Presentation with respiratory failure followed 3 years of radiological surveillance, free of disease recurrence. Thoracic CT Pulmonary Angiography now demonstrated massive right-sided pleural effusion, lung collapse and consolidation, multiple bilateral pleural mass lesions, intrapulmonary nodules and hilar lymphadenopathy. Staging confirmed left inguinal pelvic disease, small-volume hepatic involvement, but critically and unusually, sparing of the CNS.
Pleural histopathology confirmed infiltration by malignant tumor with rosetting architecture composed of mainly cubo-columnar cells with fibrillary matrix, consistent alongside methylation array sequencing with the rare diagnosis of metastatic extra-CNS MPE (Figure 2). Tumor cells were characteristically positive for CD56, CD99, negative for cytokeratin and thyroid transcription factor −1 (TTF-1) and strongly positive for glial fibrillary acidic protein (GFAP). Methylome analysis of tumor DNA confirmed the diagnosis as myxopapillary ependymoma.
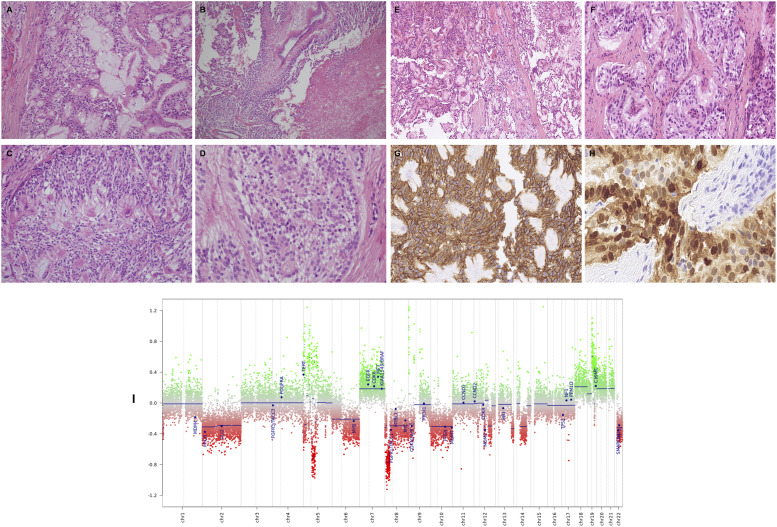
Figure 2.
Histological and mutational characteristics of pleural disease. Histologically, this tumor comprises a mixture of papillary, solid and glandular architectures (A–D). The cells are arranged around variably well-formed fibrovascular cores (A and B), with prominent areas of myxoid stroma (A and C). Focal tumor necrosis is present (B). At higher magnification (D) the tumor is composed of cells with relatively small, bland, ovoid, vesicular nuclei with abundant fibrillary or focally clear cytoplasm. No pleomorphism or mitotic activity is discernible (hematoxylin and eosin). At low power (E), there is prominent papillary architecture with stromal cores being partly myxoid. A separate area (F) shows organoid nests of mildly atypical cells. Tumor cells stain diffusely for CD56 (G). Most tumor cells stain diffusely for S-100 (G). M-ethylation array profiling of pleural tissue (I) using the Illumina Infinium 850k EPIC array analysed as per the DKFZ Heidelberg algorithm, confirming myxopapillary ependymoma (MPE).
Next generation sequencing of pleural tissue (FoundationOne CDx) revealed a low tumour mutational burden (1 mutation per megabase) and microsatellite stability (MSS-stable). A splice variant of PTCH1 (splice site 946-2A>C) of uncertain significance, and a likely deleterious pathogenic truncation of BRCA1 (G817fs*29) was identified. Confirmatory germline testing using the Invitae panel did not uncover any aberrations, suggesting the BRCA mutation was acquired somatically.
Systemic anti-cancer therapy was initiated firstly with carboplatin-etoposide, followed by oral temozolomide, with progressive disease observed as the best response for both. Given the PTCH1 splice variant of unknown significance, a trial of the Hedgehog-inhibitor vismodegib was attempted with again minimal radiological benefit and progression after six cycles.
Longitudinal extensive review of interval CT-imaging suggested that the slowest pace of disease progression occurred with temozolomide therapy, and this together with the genomic finding of the somatic BRCA1 truncation suggested that a PARP inhibitor could be used in combination to restore temozolomide sensitivity. The patient was commenced on the combination of olaparib and temozolomide as per the recommended Phase 2 dose determined in the OPARATIC trial (temozolomide 75 mg/m2 once daily with concomitant olaparib 200 mg twice daily on days 1–7 of each 21-days cycle).1 Treatment continued to best response of stable disease (by RECIST and Choi criteria) for 12 cycles (Figure 3), and was then stopped due to declining performance status, specifically low appetite, fatigue and shortness of breath. The patient remained off treatment for 15 months until their death, which was likely caused by Covid-19 infection. Imaging in this period showed relatively indolent and slow growth. Treatment with dose-dense temozolomide and lapatinib was considered given positive results in recurrent CNS-only ependymoma.2 However, treatment was not administered given a performance status of 2 and patient choice. During the period off treatment the patient developed pulmonary hypertension requiring long term oxygen therapy and nocturnal BiPAP which was likely secondary to hilar tumour burden.
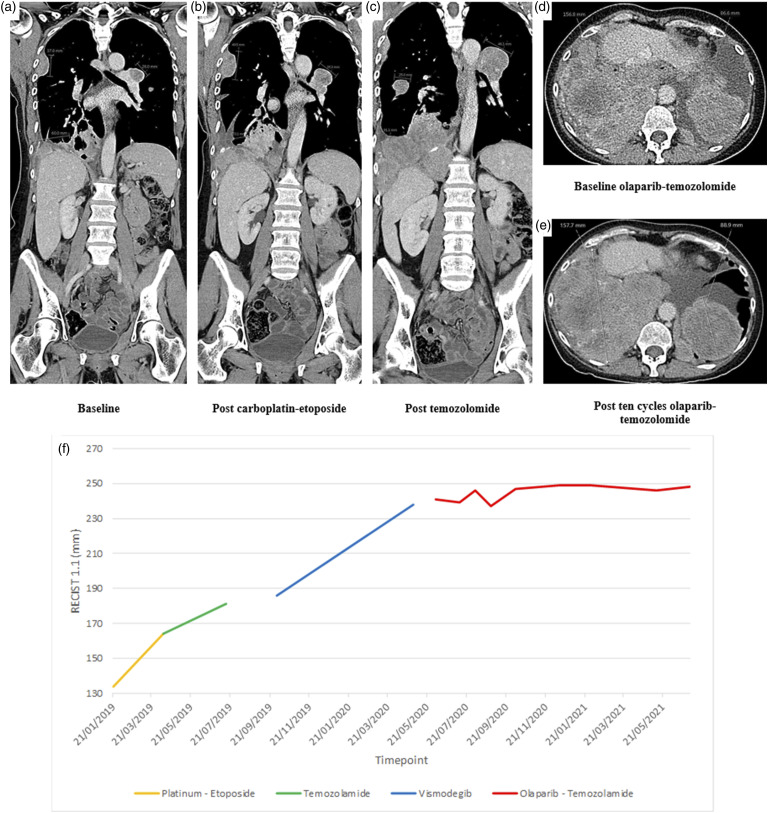
Figure 3.
Treatment response. (1) Carboplatin-etoposide. Disease progression (b) after three cycles of platinum-based chemotherapy is demonstrated, compared to baseline (a) (2) Temozolomide monotherapy. Disease progression (c) after six cycles of temozolomide is demonstrated, compared to pre-treatment (b). (3) Olaparib-Temozolomide combination therapy. Stable bulky thoracic disease (e) after 10 cycles of olaparib-temozolomide is demonstrated, compared to pre-treatment (d). (f) Plateau in rate of growth of target lesions by RECISIT 1.1 after commencement of olaparib-temozolomide.
Discussion
Myxopapillary ependymoma
Myxopapillary ependymoma (MPE) is a rare, WHO Grade 1-classified subtype of ependymoma, central nervous system malignancy arising from neuroepithelial glial tissue. MPE is typically indolent and predominantly involves the intramedullary spinal conus medullaris, cauda equina or filum terminale, accounting for 13% of all spinal ependymomas. The incidence of MPE in the USA is 0.05–0.08 per 100,000 persons per year, with the disease manifesting primarily in young adult and pediatric populations.3
Primary extradural disease or recurrence outside of the thecal sac is rare, as is cerebrospinal or distant dissemination. Extradural MPE has most commonly been observed in the pelvic pre-sacral space or sacrococcygeal soft tissues, and appears to have a greater predilection for metastatic spread than primary CNS disease. Extra-neural metastasis occurs in 6% of cases of all spinal ependymomas, including high-grade variants.3,4 The rarity of distant metastatic spread is particularly evident for MPE with less than 10 published cases since 1950 including pulmonary disease.5,6
Clinicopathological characteristics of MPE
The appearance of MPE on MRI is that of a well-circumscribed, gadolinium-enhancing lesion. Microscopic examination of MPE demonstrates circumscribed, commonly non-infiltrative neoplastic growth of cuboidal tumor cells, with the presence of perivascular pseudorosettes, papillae formation and evidence of mucin production. Cells demonstrate GTPase-activating protein (GAP) expression and lack cytokeratin, forming a mucoid matrix with overall low mitotic activity.3
Conventional histopathological classification of ependymoma has been superseded by the advent of methylation array sequencing and molecular profiling of disease tissue, leading to the proposition of nine molecular disease subgroups, further delineating genetic heterogeneity not identifiable by microscopic assessment. Molecular subgrouping is now advocated as superior to conventional categorization, and aligns more closely with observed phenotypic differences in disease behavior.7 Despite having a demonstrably distinct molecular signature from other ependymoma subtypes, notable targetable mutations for MPE have so far not been described.
Treatment
10-years survival rates for MPE exceed 90% for published series. Maximal surgical gross total resection (GTR) is recommended as the primary intervention at diagnosis and recurrence; it remains the only intervention associated with significant improvement in progression free (PFS) and overall survival (OS).8,9 The use of adjuvant radiotherapy (RT) remains variable in cases of radiologically effective GTR, but is presumed necessary in cases of subtotal resection and early recurrence, with large series disagreeing on the magnitude of benefit.8,9
Data addressing the role of systemic anti-cancer therapy in ependymoma management are limited, with chemotherapy reserved for clinical trials and salvage therapy in recurrent disease.10 Platinum-based regimens are favored as first-line and demonstrate a higher proportion of observed responses; however no robust data exist to show significant improvement in PFS or OS with the use of chemotherapy, across all ependymomas.11
Temozolomide in refractory disease
Temozolomide is an oral-alkylating agent, now used as standard of care in malignant gliomas, with retrospective small series also demonstrating an improved PFS and OS in intracranial ependymal tumors. However, both primary or secondary acquired resistance to Temozolomide is a significant limitation to its therapeutic efficacy.12
Poly (ADP-ribose) polymerase inhibitors (PARPi) are a family of agents developed to exert an inhibitory effect on PARP enzymes (notably PARP1 and 2) at DNA damage sites to achieve cytotoxicity. Currently approved for use in ovarian and breast cancer, they are being evaluated in clinical trials as single agent therapy or in combination with conventional DNA-damaging cytotoxic agents in several advanced malignancies.13 PARP inhibition has offered an opportunity to potentiate the effects of other DNA-damaging agents, with notable specific use in disease demonstrating somatic or germline loss of function mutations affecting DNA repair, such as BRCA1.14
PARPi exert their effect on germline BRCA1/2 mutated malignancy by augmentation of defective DNA repair through synthetic lethality, where a combination of PARP1 inhibition by PARPi, disrupting the repair of single-strand DNA breaks, and defective homologous recombination (HR) as a result of BRCA1/2 tumor suppressor protein loss, synergistically leads to cell death.15
Early understanding of the mechanism of PARPi activity focused on catalytic inhibition of PARP1, but has since developed to include the concept of PARP1 ‘trapping’ at DNA repair sites. The existence of two such mechanisms is thought to underpin variable activity observed by different PARPi, and those with greater PARP-trapping activity have been shown to have a greater cytotoxic effect on HR-defective (e.g. BRCA1/2 mutated) cancer.16
Temozolomide cytotoxicity is enacted through DNA disruption, where aberrant cycles of mismatch repair as a consequence of base damage, lead to an accumulation of single strand breaks (SSBs) and eventual apoptosis.17 Mechanisms of inherent and acquired temozolomide resistance include high O-6-Methylguanine-DNA Methyltransferase (MGMT) expression and mismatch repair protein (MMR) deficiency, which facilitate more effective DNA repair.16,17 The addition of PARPi, particularly those with significant PARP-trapping activity, leads to the formation of PARP-DNA complexes at sites of temozolomide-induced SSBs, blocking base excision DNA repair mechanisms and potentiating cytotoxicity.
Pre-clinical evidence has demonstrated robust sensitization of temozolomide by PARPi, as well as synthetic lethality in homologous repair (HR) deficient cancers.18 The chemo-potentiating effects of PARPi on temozolomide led to early clinical trials of such combinations, with positive results in patients with a range of tumor types.12,18,19 Olaparib is a PARPi approved for use in germline or somatic BRCA-mutated metastatic ovarian, fallopian tube, peritoneal, human epidermal growth factor receptor-2 (HER2)-negative breast and pancreatic cancer, as well as prostate cancer with HR repair (HR)-mutated status. The selection of combination olaparib and temozolomide in our patient was supported by evidence of superior in vitro activity of this combination in comparison to other temozolomide-PARPi alternatives, alongside evidence of its safety and tolerability in patients with malignant glioblastoma.1,20
Future considerations; precision management
In an era of more widespread genomic and molecular profiling with next generation sequencing technology, this case emphasizes the growing potential of proactive identification of somatic mutations that may suggest HR DNA damage repair deficiency, meriting the use of PARP inhibitors either as monotherapy or in combination for clinical benefit, particularly with regard to rare and refractory cancers.
Acknowledgements
The authors acknowledge research support from: Ms Hannah Holmes, Library Services at The Royal Marsden Hospital, London; Library services, Institute of Cancer Research, London; The National Institute of Health Research (NHS NIHR) funding to the Royal Marsden Hospital Biomedical Research Centre.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.C. reports grants from the NIHR, MedImmune/AstraZeneca, Clovis, Eli Lilly, 4SC, Bayer, Celgene, Roche and Leap Therapeutics, and he is on the scientific advisory board of OVIBIO. I.C. reports grants from Eli Lilly and Janssen-Cilag; he is on the scientific advisory board of Eli Lilly, Bristol Meyers Squibb, MSD, Bayer, Roche, Merck-Serono, Five Prime Therapeutics, Astra-Zeneca, OncXerna, Pierre Fabre, Boehringer Ingelheim, Incyte, Astella, GSK, Sotio, Eisai and Daiichi-Sankyo; and he receives honoraria from Eli Lilly and Servier. The remaining authors report no conflicts of interest. The views expressed are those of the authors and not necessarily those of the UK NIHR or the Department of Health and Social Care.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NHS National Institute of Health and Research (NIHR) funding to The Royal Marsden Hospital and The Institute of Cancer Research Biomedical Research Centre (BRC).
Ethical approval: The Royal Marsden NHS Foundation Trust does not require ethical approval for reporting individual cases or case series.
Informed consent: The authors/investigators confirm that they have obtained informed consent to publish information and images pertaining to the participant discussed in this report.
Contributorship: PM wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iD
Sam Smith https://orcid.org/0000-0002-7654-9732
References
- 1.Hanna C., Kurian KM, Williams K, et al. Pharmacokinetics, safety, and tolerability of olaparib and temozolomide for recurrent glioblastoma: results of the phase I OPARATIC trial. Neuro Oncol 2020; 22(12): 1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert M.R., Yuan Y, Wu J, et al. A phase II study of dose-dense temozolomide and lapatinib for recurrent low-grade and anaplastic supratentorial, infratentorial, and spinal cord ependymoma. Neuro Oncol 2021; 23(3): 468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reni M., Gatta G., Mazza E., et al. Ependymoma. Crit Rev Oncol Hematol 2007; 63: 81–89. [DOI] [PubMed] [Google Scholar]
- 4.Bates J. E., Choi G., Milano MT. Myxopapillary ependymoma: a SEER analysis of epidemiology and outcomes. J Neurooncol 2016; 129: 251–258. [DOI] [PubMed] [Google Scholar]
- 5.Fujimori T., Iwasaki M, Nagamoto Y, et al. Extraneural metastasis of ependymoma in the cauda equina. Glob Spine J 2013; 3: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batich K. A., Riedel RF, Kirkpatrick JP, et al. Recurrent extradural myxopapillary ependymoma with oligometastatic spread. Front Oncol 2019; 9: 1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pajtler K. W., Witt H, Sill M, et al. Molecular classification of ependymal tumors across all cns compartments, histopathological grades, and age groups. Cancer Cell 2015; 27: 728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pica A., Miller R, VillA S, et al. The results of surgery, with or without radiotherapy, for primary spinal myxopapillary ependymoma: a retrospective study from the rare cancer network. Int J Radiat Oncol 2009; 74: 1114–1120. [DOI] [PubMed] [Google Scholar]
- 9.Weber D. C., Wang Y, Miller R, et al. Long-term outcome of patients with spinal myxopapillary ependymoma: treatment results from the MD anderson cancer center and institutions from the rare cancer network. Neuro Oncol 2015; 17: 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J., Armstrong T. S., Gilbert M. R. Biology and management of ependymomas. Neuro Oncol 2016; 18: 902–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudà R., Bosa C, Magistrello M, et al. Temozolomide as salvage treatment for recurrent intracranial ependymomas of the adult: a retrospective study. Neuro Oncol 2016; 18: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiapaer S., Furuta T., Tanaka S., et al. Potential strategies overcoming the temozolomide resistance for glioblastoma. Neurol Med Chir (Tokyo) 2018; 58: 405–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mateo J., Lord C, Serra V, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol 2019; 30: 1437–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plummer R., Lorigan P., Steven N., et al. A phase II study of the potent PARP inhibitor, rucaparib (PF-01367338, AG014699), with temozolomide in patients with metastatic melanoma demonstrating evidence of chemopotentiation. Cancer Chemother Pharmacol 2013; 71: 1191–1199. [DOI] [PubMed] [Google Scholar]
- 15.Farmer H., McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005; 434: 917–921. [DOI] [PubMed] [Google Scholar]
- 16.Murai J., Zhang Y, Morris J, et al. Rationale for poly(ADP-ribose) polymerase (PARP) inhibitors in combination therapy with camptothecins or temozolomide based on PARP trapping versus catalytic inhibition. J Pharmacol Exp Ther 2014; 349: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newlands E. S., Stevens M. F. G., Wedge S. R., et al. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev 1997; 23: 35–61. [DOI] [PubMed] [Google Scholar]
- 18.Plummer R., Jones C, Middleton M, et al. Phase i study of the poly(ADP-Ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res 2008; 14: 7917–7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman B., Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015; 33: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valiakhmetova A., Gorelyshev S., Konovalov A., et al. Treatment of pediatric glioblastoma with combination olaparib and temozolomide demonstrates 2-year durable response. Oncologist 2020; 25: e198–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]