IMPORTANCE:
At present, it is not clear if critically ill COVID-19 survivors have different needs in terms of follow-up compared with other critically ill survivors, and thus if duplicated post-ICU trajectories are mandatory.
OBJECTIVES:
To compare the post-intensive care syndrome (PICS) of COVID-19 acute respiratory distress syndrome and non-COVID-19 (NC) survivors referred to a follow-up clinic at 3 months (M3) after ICU discharge.
DESIGN, SETTING, AND PARTICIPANTS:
Adults who survived an ICU stay greater than or equal to 7 days and attended the M3 consultation were included in this observational study performed in a post-ICU follow-up clinic of a single tertiary hospital.
MAIN OUTCOMES AND MEASURES:
Patients underwent a standardized assessment, addressing health-related quality of life (3-level version of EQ-5D), sleep disorders (Pittsburgh Sleep Quality Index [PSQI]), physical status (Barthel index, handgrip and quadriceps strengths), mental health disorders (Hospital Anxiety and Depression Scale and Impact of Event Scale-Revised [IES-R]), and cognitive impairment (Montreal Cognitive Assessment [MoCA]).
RESULTS:
A total of 143 survivors (86 COVID and 57 NC) attended the M3 consultation. Their median age and severity scores were similar. NC patients had a shorter ICU stay (10 d [8–17.2 d]) compared with COVID group (18 d [10.8–30 d]) (p = 0.001). M3 outcomes were similar in the two groups, except for a higher PSQI (p = 0.038) in the COVID group (6 [3–9.5]) versus NC group (4 [2–7]), and a slightly lower Barthel index in the NC group (100 [100–100]) than in the COVID group (100 [85–100]) (p = 0.026). However, the proportion of patients with abnormal values at each score was similar in the two groups. Health-related quality of life was similar in the two groups. The three MoCA (≥ 26), IES-R (<33), and Barthel (=100) were normal in 58 of 143 patients (40.6%). In contrast, 68.5% (98/143) had not returned to their baseline level of daily activities.
CONCLUSIONS AND RELEVANCE:
In our follow-up clinic at 3 months after discharge, the proportion of patients presenting alterations in the main PICS domains was similar whether they survived a COVID-19 or another critical illness, despite longer ICU stay in COVID group. Cognition and sleep were the two most affected PICS domains.
Keywords: COVID-19, critical illness, post-intensive care syndrome, survivors, outcome assessment
KEY POINTS
Question: Is there a difference in post-intensive care syndrome (PICS) presentation in patients referred to follow-up clinic after a COVID-19 acute respiratory distress syndrome compared with other critical illnesses?
Finding: In this observational study, the proportion of patients presenting alterations in the main PICS domains was similar whether they survived a COVID-19 or another critical illness, despite longer ICU stay in COVID group. Cognition and sleep were the two most affected PICS domains.
Meaning: PICS is and will continue to be a public health concern, independently of the initial critical entity.
The improvement in the management of critically ill patients over the last decades and the subsequent increase in short-term survival rates led to the emergence of mid- and long-term morbidities related to the critical illness itself, the required support, and the environment. The basic categories of disorders following an ICU stay include new or worsened physical (neuromuscular weakness and reduced autonomy for activities of daily living [ADL]), mental (anxiety, depression, post-traumatic stress disorder [PTSD]), and neurocognitive disorders. Since 2012, the term “post-intensive care syndrome (PICS)” define these disorders negatively affecting daily functioning and quality of life in survivors of critical illness (1). An expanded definition has recently been suggested, including additional factors, such as osteopenia, metabolic disorders, endocrine dysfunction, vulnerability, sleep disorders, chronic pain, and fatigue (2). Notably, ICU survivors have a poorer quality of life compared with matched controls (3).
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can induce acute respiratory distress syndrome (ARDS). The management of COVID-19 ARDS improved after the first wave of the pandemic, and the number of ICU survivors increased significantly (4). In the same way, the COVID-19 epidemic is likely to generate a secondary epidemic of PICS among COVID-19 patients who survived a long ICU stay. An increasing number of cohort studies report a significant burden of COVID-19 ARDS among ICU survivors, from ICU discharge up to 12 months after critical illness (5–12). These survivors, in variable proportions, seem to present respiratory impairments related to the primary disease, as well as impairments in one or more PICS domain. However, these data were mostly derived from survivors of the first COVID-19 wave (13). Less is known about outcomes of survivors of the following waves, given the changes in treatment of the COVID-19 ARDS (4).
In different hospitals, multidisciplinary follow-up clinics have been created to detect PICS and to address the clinical needs of ICU survivors. Such strategies have been debated for several years (14–18). Since the beginning of the pandemic, the need of follow-up clinics to facilitate COVID-19 survivors recovery and to understand the long-term course of the disease have been established worldwide (19, 20). At present, it is not clear if critically ill COVID-19 survivors have different needs in terms of follow-up compared with non-COVID-19 (NC) ICU survivors (21), and thus if duplicated post-ICU trajectories are mandatory.
The aim of this single center cohort study was to compare the mid-term PICS of COVID-19 and NC survivors referred to a face-to-face consultation in our post-ICU follow-up clinic at 3 months (M3) following a prolonged ICU stay. The secondary aim was to analyze the same outcomes separately in COVID-19 patients of the first wave of the pandemic and in those of the following waves.
METHODS
Participants—Data Sources
Patients surviving an ICU stay greater than or equal to 7 days are routinely invited to our post-intensive care follow-up clinic at 1, 3, and 12 months following ICU discharge. Patients do not enter the post-ICU trajectory of our follow-up clinic if they are unable to communicate in French, the local language, if they have been transferred to another hospital, if we are unable to give them information about the post-ICU follow-up clinic. The scheduled face-to-face consultation is generally canceled if they are still hospitalized in an acute care facility or in an inpatient rehabilitation facility or if they refuse it. The follow-up is standardized, addressing physical status and functional performances, nutritional status and body composition, bone health, mental health disorders, cognitive impairment, sleep disorders, and health-related quality of life (HRQoL). A blood analysis focuses on inflammation and metabolic biomarkers. Organ-specific assessment is not managed by the follow-up clinic but rather by the referring specialists.
All consecutive critically ill patients surviving an ICU stay greater than or equal to 7 days for COVID-19 ARDS from March 1, 2020, to December 1, 2021 (COVID cohort) and for any other NC critical illness meanwhile (NC cohort), were invited to attend the 3-month post-ICU consultation, if they met the inclusion criteria of our post-ICU follow-up clinic. Patients who attended M3 follow-up were separated into two groups: 1) patients who survived a COVID-19 ARDS (COVID group) and 2) patients who survived a NC critical illness (NC group). The COVID group has been further divided into two subgroups, including patients of the first wave of the pandemic, admitted to ICU between March 1, 2020, and July 17, 2020 (W1 subgroup), and those of the following waves, admitted to ICU between August 1, 2020, and December 1, 2021 (Wx subgroup). The waves were defined according to the treatment strategies employed (4) rather than the viral strains.
This observational study was conducted according to the guidelines of the Declaration of Helsinki (1964) and its later amendments. In accordance with Belgian law, informed consent was not required because the study did not modify patients’ management and the data were anonymously collected. This interpretation was confirmed by the Ethics Committee of the University Hospital of Liege (local reference 2020/424, Chairperson Pr Vincent Seutin, February 2, 2021).
Clinical Variables
Global cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) (22). Mental health status was assessed using the Hospital Anxiety and Depression Scale and the Impact of Event Scale-Revised (IES-R) (23, 24). The Pittsburgh Sleep Quality Index (PSQI) is a validated tool used to obtain self-reported sleep quality (25). ADL were assessed using Barthel Index (26). Peripheral muscle strength was determined by using handgrip and quadriceps dynamometry (27, 28). HRQoL was measured using the 3-level version of EQ-5D (EQ-5D-3L) (29). Description and scoring of each test are detailed in the Supplementary Material (http://links.lww.com/CCX/B133).
Finally, patients were questioned about their living condition, and their return to previous level of activities (employment or leisure activities in unemployed patients).
Demographic data and data related to the ICU stay were collected from the medical charts. In the COVID group, d-dimers plasmatic concentration was systematically assessed during the first 24 hours following ICU admission during the pandemic and was also collected.
Biological Variables
The biological data were generated from one single laboratory (Unilab, CHU de Liège) accredited for ISO 15,189 Guideline. The normal range for d-dimers is less than 500 µg/L (Innovance d-dimer assay; Siemens, Erlangen, Germany). The normal range for C-reactive protein (CRP) is 0–5 mg/L (Alinity C; Abbott, Chicago, IL).
Statistical Analyses
Given the descriptive setting, no a priori sample size was calculated. Statistical analysis was performed using Graphpad Prism (Version 9.0 for Mac OSX; Graphpad, San Diego, CA). Quantitative variables are expressed as median and interquartile range, and qualitative variables are described as count and percentage. Nonparametric tests were used as some datasets did not pass the normality test. Comparisons between groups were made using Fisher exact test for categorical variables and using Mann-Whitney U test for continuous variables. A p value of less than 0.05 was considered statistically significant.
RESULTS
Description of the Cohorts
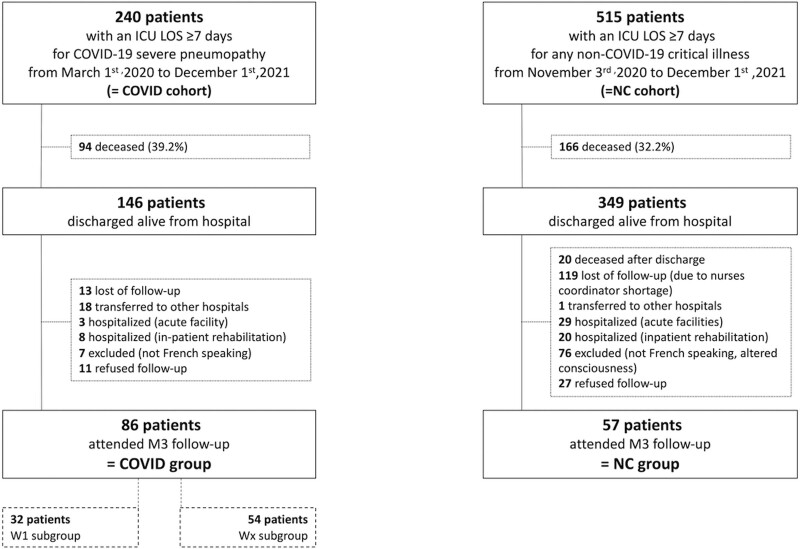
There were 240 and 515 patients in the initial COVID and NC cohorts, respectively. The demographics of the two cohorts are detailed in Supplemental Table 1 (http://links.lww.com/CCX/B133). From these cohorts, 86 and 57 patients attended the M3 follow-up and were included in the COVID group and NC group, respectively (Fig. 1).
Figure 1.
Flow chart. LOS = length of stay, M3 = 3 mo, NC = non-COVID-19.
The characteristics of the patients attending the M3 follow-up in the COVID and NC groups are detailed in Table 1. Their age, gender ratio, and severity scores at ICU admission were similar. NC patients had a lower body mass index (BMI) than COVID patients (p < 0.001). COVID patients had a longer ICU length of stay (LOS) than NC patients (p = 0.001). However, the hospital LOS was similar in the two groups. The proportion of patients who required mechanical ventilation and vasopressor support was similar in the two groups, but the duration of mechanical ventilation was longer in the COVID group (p < 0.001). Midazolam was administered more frequently in COVID patients (p = 0.016), and for a longer period (p = 0.001), in the COVID group compared with NC group. The proportion of patients who have received steroids was higher in the COVID group compared with NC group (p < 0.001). Finally, the pre-ICU conditions were similar in the two groups, except sleeping pills more frequently used in NC group compared with COVID group (p = 0.049).
TABLE 1.
Demographics in the COVID and Non-COVID-19 Groups
| Data | COVID Group (n = 86) | Non-COVID-19 Group (n = 57) | p |
|---|---|---|---|
| Age, yr | 61 (53–68) | 64.5 (50.7–73) | 0.186 |
| Male, n (%) | 53 (61.6) | 37 (64.5) | 0.726 |
| Weight, kg | 90 (77.3–102.7) | 77 (69.9–89.7) | 0.001 |
| Body mass index, kg/m2 | 30.9 (26.9–34.5) | 26.5 (23.8–29.6) | < 0.001 |
| Comorbidities | |||
| Diabetes | 30 (34.8) | 10 (17.5) | |
| Hypertension | 36 (41.9) | 22 (38.6) | |
| Cardiaca | 18 (20.9) | 10 (17.5) | |
| Respiratoryb | 14 (16.3) | 2 (3.5) | |
| Chronic kidney disease | 7 (8.1) | 4 (7) | |
| Immunosuppression | 5 (5.8) | 3 (5.3) | |
| Employment before ICU admission, n (%) | |||
| Employed | 42 (48.8) | 22 (38.8) | |
| Unemployed | 1 (1.2) | 1 (1.4) | |
| Disabled | 8 (9.3) | 8 (14.2) | |
| Retired | 35 (40.7) | 26 (45.6) | |
| Admission type, n (%) | |||
| Medical | 86 (100) | 27 (47.4) | |
| Surgical | N/A | 30 (52.6) | |
| Admission failure, n (%) | |||
| Cardiovascular | N/A | 26 (45.6) | |
| Pulmonary | 86 (100) | 6 (10.5) | |
| Neurologic | N/A | 14 (24.6) | |
| Other | N/A | 7 (19.3) | |
| Sequential Organ Failure Assessment at admission | 4 (3–7) | 5 (3–8) | 0.850 |
| Simplified Acute Physiology Score II | 31.5 (25.2–41.7) | 32 (27.5–48.8) | 0.415 |
| d-dimers, µg/L | 1,051 (639–2,133) | ||
| Mechanical ventilation, n (%) | 58 (67.4) | 38 (66.7) | > 0.999 |
| Duration of mechanical ventilation, d | 17.5 (11–27) | 5 (2–15.2) | < 0.001 |
| Vasopressive support, n (%) | 49 (57) | 33 (57.9) | > 0.999 |
| Duration of norepinephrine administration, d | 4 (2–7.5) | 3 (2–5.5) | 0.748 |
| Midazolam, n (%) | 57 (66.3) | 26 (45.6) | 0.016 |
| Duration of midazolam sedation, d | 7 (2–12) | 2 (1–4.2) | 0.01 |
| Corticosteroids, n (%) | 73 (84.9) | 23 (40.4) | < 0.001 |
| Renal replacement therapy, n (%) | 4 (4.7) | 3 (5.3) | > 0.999 |
| Duration of renal replacement therapy, d | 17.5 (16–19.8) | 4 (4–7) | 0.029 |
| Extracorporeal membrane oxygenation, n (%) | 5 (5.8) | 4 (7) | > 0.999 |
| Duration of extracorporeal membrane oxygenation, d | 12 (8–42) | 25 (2.7–45) | 0.778 |
| ICU LOS, d | 18 (10.8–30) | 10 (8–17.2) | 0.001 |
| Hospital LOS, d | 35 (22–54) | 32 (20.7–49.5) | 0.826 |
| Destination at hospital discharge, n (%) | |||
| Home | 64 (77.4) | 46 (80.7) | 0.001 |
| Rehabilitation facility | 22 (25.6) | 4 (7.1) | |
| Nursing home | 0 | 5 (8.7) | |
| Other | 0 | 2 (3.5) | |
| n = 38 | n = 50 | ||
| Preexisting use of, n (%) | |||
| Anxiolytics | 3 (7.9) | 6 (12) | 0.735 |
| Antidepressants | 2 (5.2) | 7 (14) | 0.289 |
| Sleeping pills | 3 (7.9) | 13 (26) | 0.049 |
| Preexisting memory complaints, n (%) | 1 (2.6) | 3 (6) | 0.631 |
| Preexisting follow-up for, n (%) | |||
| Psychologic disorders | 4 (10.5) | 12 (24) | 0.162 |
| Chronic pain | 6 (15.8) | 9 (18) | > 0.999 |
| Sleep disorders | 2 (5.2) | 7 (14) | 0.289 |
LOS = length of stay.
Includes ischemic heart disease, valvular disease, cardiomyopathies, and chronic heart disease.
Includes asthma, chronic obstructive pulmonary disease, and interstitial lung disease.
Data are presented as median (interquartile range) unless otherwise specified.
The characteristics of patients who were discharged alive from hospital but who did not attend the M3 consultation are detailed in Supplemental Table 2 (http://links.lww.com/CCX/B133). Compared with M3 COVID group, patients of COVID alive cohort (n = 60) had lower weight and BMI (p = 0.029 and p = 0.004, respectively). Other demographics characteristics were similar. Compared with the M3 NC group, patients of the NC alive cohort (n = 292) had a slightly longer duration of ICU stay (p = 0.013), but required less mechanical ventilation, less midazolam-based sedation, and less noradrenaline support (p = 0.004, p = 0.011, and p = 0.019, respectively). Their severity scores were similar.
Outcomes at M3
The M3 visit occurred 96.5 days (91–109 d) and 96.5 days (86–107 d) after ICU discharge in COVID group and NC group, respectively (p = 0.433). In COVID and NC groups at M3, 83 of 86 (96.5%) and 49 of 57 (86%) patients had returned home, respectively (p = 0.027). Most of the patients did not return to previous level of activity, either employment for previously active patients or leisure for unemployed or retired patients: 54 of 70 (77.1%) and 44 of 57 (77.2%) in COVID and NC groups, respectively (p > 0.999).
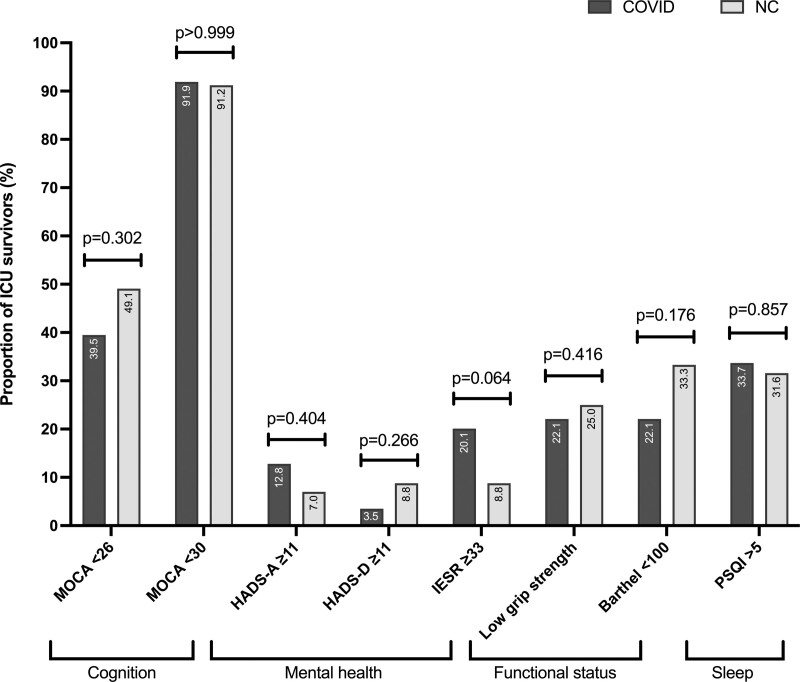
The M3 assessment is detailed in Table 2. Scores for questionnaires and tests were similar in the two groups, except for PSQI which was higher in the COVID group (p = 0.038) and Barthel index which was slightly lower in the NC group (p = 0.026). The proportion of patients with abnormal results to the questionnaires and tests are shown in Figure 2. These proportions were similar in the two groups for all the questionnaires and tests, including for sleep disorder and dependency. In the two groups, about 40% of the patients were considered as having fully recovered (i.e., presenting simultaneously normal scores at MoCA [≥ 26], IES-R [< 33], and Barthel index [=100]: 34/86 [43.8%] and 24/57 [42.1%] in COVID and NC group, respectively [p = 0.862]). In contrast, most of the patients experiencing post-ICU sequelae had partial PICS. An association of cognitive impairment, PTSD and decreased autonomy was observed in only five of 86 (5.8%) and three of 57 (5.3%) in COVID and NC groups, respectively (p > 0.999). About a fifth of patients (16/86 and 13/54 in COVID and NC groups, respectively) demonstrated a persistent inflammation based on CRP blood level (p = 0.518). In patients with persistent inflammation, CRP reached 12.6 mg/L (8.2–32.4 mg/L) and 12.7 mg/L (8.5–22.1 mg/L) in COVID and NC groups, respectively (p = 0.871).
TABLE 2.
Three Months Assessment in the COVID and Non-COVID-19 Groups
| Data | COVID Group (n = 86) | Non-COVID-19 Group (n = 57) | p |
|---|---|---|---|
| Montreal Cognitive Assessment | 27 (25–28) | 26 (23.5–28) | 0.102 |
| Hospital Anxiety and Depression Scale-Anxiety | 4 (1–8) | 3 (1–7) | 0.461 |
| Hospital Anxiety and Depression Scale-Depression | 1 (0–3) | 1 (0–6) | 0.697 |
| Impact of Event Scale-Revised | 9 (4–26) | 6 (1.5–19) | 0.054 |
| Pittsburgh Sleep Quality Index | 6 (4–9.5) | 4 (2–7) | 0.038 |
| EQ-5D-3L score | 7 (6–8) | 7 (5.2–8.7) | 0.58 |
| EQ-5D-3L visual analogic scale | 70 (65–80) | 72.5 (60–83.7) | 0.859 |
| Barthel index | 100 (100–100) | 100 (85–100) | 0.026 |
| Handgrip strength (kg) | 28 (18–38) | 25 (19.5–38) | 0.824 |
| Quadriceps strength (N/kg) | 2.7 (2.1–3.3) | 2.3 (1.8–3.2) | 0.188 |
| C-reactive protein (mg/L) | 2.5 (1.6–4.6) | 2.4 (1–7.2) | 0.518 |
Data are presented as median and interquartile range.
Figure 2.
Proportion of survivors with abnormal results to the questionnaires. HADS-A = Hospital Anxiety and Depression Scale-Anxiety, HADS-D = Hospital Anxiety and Depression Scale-Depression, IES-R = Impact of Event Scale-Revised, MoCA = Montreal Cognitive Assessment, NC = non-COVID-19, PSQI = Pittsburgh Sleep Quality Index.
Subgroup Analysis in the COVID Group
Demographics and M3 assessment in the two subgroups are presented in Supplemental Table 3 (http://links.lww.com/CCX/B133). In W1 subgroup, patients had a longer ICU stay (p = 0.018) and required more mechanical ventilation (p = 0.001), more midazolam-based sedation (p = 0.02) and more renal replacement therapy (p = 0.006) than in the Wx subgroup. However, their Sequential Organ Failure Assessment score was slightly lower than in the Wx subgroup (p = 0.013). Their hospital LOS was similar in the two subgroups. Despite these differences, the results for the M3 assessment scores and tests were similar in the two subgroups.
DISCUSSION
In both COVID-19 and NC critically ill survivors who attended a face-to-face 3-month follow-up consultation, we observed similar proportions of patients presenting alterations in the studied PICS domains (cognition, mental health, functional status, sleep, and HRQoL). According to a recent publication, similar findings were also observed later in the post-ICU trajectory during a remote telephone assessment that did not include muscle strength measurement or sleep quality evaluation. At 6 months after discharge, there was no difference in new disabilities in COVID-19 and non-COVID survivors who required mechanical ventilation during the ICU stay (30).
We observed a lower level of independency for ADL in the NC group, corresponding with a lower proportion of patients who have returned home at M3 in this group. We also observed a lower reported sleep quality in the COVID group. Greater use of mechanical ventilation, or midazolam, and ICU environment, as observed in the COVID group, could explain this observation. However, the role of these in-ICU factors on sleep disturbance after critical illness are still unclear (31). Furthermore, they may not be the only one explanation. According to a recent published study, sleep architecture seems to be altered 1 month after a confirmed COVID-19 (32). Exact causes of such findings are still unknown. SARS-CoV-2 is thought to preferentially affect central nervous areas that are known to be implicated in sleep pathways (33). Furthermore, brain hypometabolism has been demonstrated using 18F-fluorodeoxyglucose-positron emission tomography, suggesting functional alterations of the CNS. From another perspective, some data also indicates abnormal patterns at polysomnography after hospital discharge in critical illness survivors (31). In the present study, sleep quality was assessed using only a subjective scale. Whether the observed difference in reported sleep quality between COVID-19 and NC survivors persists objectively on a polysomnography requires further comparative investigations.
For the intensive care community, PICS has been an increasing concern in the recent years. However, the rapid accumulation of survivors after a severe COVID-19 pneumopathy has alerted population health authorities about the risk of having to deal with a huge number of patients presenting mid- and long-term sequela of this critical illness. It has been anticipated that COVID-19 ARDS could lead to more serious PICS, due to prolonged ICU stay and potential need for deep sedation to avoid patient-ventilator dyssynchrony and counteract high respiratory drive (6, 34). Furthermore, the neurotropism of SARS-CoV-2 was supposed to be a rationale for psychologic and cognitive sequelae through direct viral infection of the CNS or indirectly via the cytokine storm and the subsequent neuroinflammation (35–37). Finally, quarantines and lockdowns have been associated with negative psychologic effects in the general population, including frustration, anxiety and PTSD, possibly exacerbated by social restrictions (38). This context was the rationale for the creation of follow-up systems worldwide dedicated to COVID-19 ICU survivors (13). However, in the present study, the concerns related to COVID-19 sequelae did not prove to be true, as the proportion of affected survivors was not different in these survivors. It is still unclear how the metabolic alterations observed in patients affected by the COVID-19 disease (39, 40) (and potentially translated into altered BMI) could explain the similarity in mid-term outcomes compared with NC survivors. Of note, a previous analysis already demonstrated that long-term outcomes after ARDS did not differ from findings in patients surviving other forms of critical illness (41). Based on these observations, patients should not be treated separately if they survived a COVID-19 ARDS because they face the same problems as other critically ill survivors. PICS is, and will continue to be, a public health concern, independently of the initial critical entity. COVID-19 pandemic could be an incentive for health authorities and hospitals to set up unique post-intensive care trajectory, helping to diagnose and manage post-ICU conditions for all survivors, whatever the initial critical illness.
Despite a drastic change in clinical management of COVID-19 ARDS after the first wave including systematic dexamethasone administration (4), and a subsequent reduction in ICU LOS and need for mechanical ventilation, PICS features were not affected by a wave effect in the present cohort. This may be explained by the multifactorial nature of PICS. Occurrence of PICS is not only the consequence of ICU factors but also involves patient factors and systemic factors that are not necessarily closely linked to the primary critical illness, such as age, past medical history, pre-ICU functional status or social support and access to rehabilitation services (42). ICU factors are risk factors, not causal factors in a mathematical relationship. Equally, inflammation, and its persistence, is an important potential cause of post-ICU morbidities (43–45). As inflammation is a common characteristic in all critically ill patients, independent of ICU LOS, it could explain the similar PICS features observed in the present study.
A huge heterogeneity in PICS presentation was observed among all studied survivors in the present study. Once again, this highlights the need for an individualized follow-up, as already claimed by other authors who observed similar findings (46).
Some limitations need to be acknowledged. First, the samples from a unique center were small, potentially limiting the external validation of the results. However, ICU treatments followed international guidelines, and post-ICU outcomes were assessed using a standardized protocol including validated questionnaires and tests. We can thus consider these results would be similar to other follow-up clinics. Second, several survivors did not attend the M3 consultation, mostly in the NC group. This difference is partly due to a higher number of patients presenting exclusion criteria for entering the post-ICU trajectory of our follow-up clinic. In addition, a number of patients were lost to follow-up, mainly due to reduced human resources in our follow-up clinic. Further, patients who refused to attend the consultation could have been either patients without any complaints or, in contrast, bedridden patients. However, these two categories of survivors are probably those who would benefit the least from a follow-up clinic. Altogether, these issues could have led to an unwanted selection bias. This bias is inherent in follow-up studies, as seen in previously published studies (30). Face-to-face follow-up allows a more comprehensive assessment, but is clearly onerous for patients, requiring them to make the trip to hospital. In this context, a greater extent of dropout could be expected. However, the aim of the present study was not to examine the prevalence of post-ICU disorders in ICU survivors but to depict the clinical situation of patients attending a post-ICU follow-up clinic in a “real life mode.” Third, the two M3 groups were not exactly comparable in terms of organ support, ICU stay duration or post-ICU trajectory. Impact of these characteristics on PICS elements is still poorly understood. Fourth, the present analysis is focused on mid-term outcomes and does not include the other time points planned in our post-ICU follow-up. Unfortunately, not all patients attended the three scheduled face-to-face consultations during the year of follow-up, thus limiting a potential longitudinal analysis. Finally, this study lacks precise assessment of baseline clinical status. It is a common issue with many studies assessing long-term outcomes in ICU survivors and is related to the unpredictable characteristic of ICU admissions. This pitfall can lead to misinterpretation of what is considered as post-intensive care sequelae.
CONCLUSIONS
In our follow-up clinic at M3 after discharge, the proportion of patients presenting alterations in the main PICS domains was similar whether they survived a COVID-19 or another critical illness, despite longer organ supports and ICU stay in COVID group. Cognition and sleep were the two most affected PICS domains. The burden of the critical illness and/or the ICU stay was substantial: at least one PICS domain was affected in more than half of the studied survivors, and about two thirds of them did not return their baseline level of daily activities.
ACKNOWLEDGMENTS
We would like to thank our entire team who is working so hard to provide excellent clinical care since the beginning of the pandemic and who contributed to the smooth operation of the post-ICU follow-up clinic: the medical team, the nurses, the physiotherapists, the dieticians, the psychologists, and the secretaries of our intensive care department. We would also like to thank J. Geoffrey Chase (Department of Mechanical Engineering, Centre for Bio‐Engineering, University of Canterbury, Christchurch, New Zealand) for the valuable English editing of our article.
Supplementary Material
Footnotes
In accordance with Belgian law, informed consent was not required because the study did not modify patients’ management and the data were anonymously collected. This was confirmed by the Ethics Committee of the University Hospital of Liege.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Dr. Rousseau designed the research. Dr. Rousseau, Ms. Colson, Ms. Minguet, Dr. Kellens, Ms. Collard, and Ms Vancraybex conducted the research. Dr. Rousseau, Pr. Lambermont, and Pr. Misset analyzed the data. Dr. Rousseau wrote the article. Ms. Colson, Dr. Guiot, Pr. Lambermont, and Pr. Misset critically reviewed the article. All authors approved the final article.
The authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Needham DM, Davidson J, Cohen H, et al. : Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit Care Med 2012; 40:502–509 [DOI] [PubMed] [Google Scholar]
- 2.Rousseau AF, Prescott HC, Brett SJ, et al. : Long-term outcomes after critical illness: Recent insights. Crit Care 2021; 25:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuthbertson BH, Roughton S, Jenkinson D, et al. : Quality of life in the five years after intensive care: A cohort study. Crit Care 2010; 14:R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambermont B, Rousseau AF, Seidel L, et al. : Outcome improvement between the first two waves of the coronavirus disease 2019 pandemic in a single tertiary-care hospital in Belgium. Crit Care Explor 2021; 3:e0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martillo MA, Dangayach NS, Tabacof L, et al. : Postintensive care syndrome in survivors of critical illness related to coronavirus disease 2019: Cohort study from a New York City critical care recovery clinic. Crit Care Med 2021; 49:1427–1438 [DOI] [PubMed] [Google Scholar]
- 6.Van Aerde N, Van den Berghe G, Hermans G: Weakness in the ICU: The right weight on the right scale. Intensive Care Med 2021; 47:137–138 [DOI] [PubMed] [Google Scholar]
- 7.Morin L, Savale L, et al. ; Writing Committee for the COMEBAC Study Group: Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021; 325:1525–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valent A, Dudoignon E, Ressaire Q, et al. : Three-month quality of life in survivors of ARDS due to COVID-19: A preliminary report from a French academic centre. Anaesth Crit Care Pain Med 2020; 39:740–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamberini L, Mazzoli CA, Sintonen H, et al. : Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Qual Life Res 2021; 30:2805–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Huang L, Wang Y, et al. : 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021; 397:220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latronico N, Peli E, Calza S, et al. : Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Thorax 2022; 77:300–303 [DOI] [PubMed] [Google Scholar]
- 12.Darcis G, Bouquegneau A, Maes N, et al. : Long-term clinical follow-up of patients suffering from moderate-to-severe COVID-19 infection: A monocentric prospective observational cohort study. Int J Infect Dis 2021; 109:209–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakanishi N, Liu K, Kawakami D, et al. : Post-intensive care syndrome and its new challenges in coronavirus disease 2019 (COVID-19) pandemic: A review of recent advances and perspectives. J Clin Med 2021; 10:3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijayaraghavan BKT, Willaert X, Cuthbertson BH: Should ICU clinicians follow patients after ICU discharge? No. Intensive Care Med 2018; 44:1542–1544 [DOI] [PubMed] [Google Scholar]
- 15.Meyer J, Brett SJ, Waldmann C: Should ICU clinicians follow patients after ICU discharge? Yes. Intensive Care Med 2018; 44:1539–1541 [DOI] [PubMed] [Google Scholar]
- 16.Rosa RG, Ferreira GE, Viola TW, et al. : Effects of post-ICU follow-up on subject outcomes: A systematic review and meta-analysis. J Crit Care 2019; 52:115–125 [DOI] [PubMed] [Google Scholar]
- 17.Rousseau AF, Preiser JC: To critically ill survivors: LIFE-UP!. J Crit Care 2021; 64:139–140 [DOI] [PubMed] [Google Scholar]
- 18.Van Der Schaaf M, Bakhshi-Raiez F, Van Der Steen M, et al. : Recommendations for intensive care follow-up clinics; report from a survey and conference of Dutch intensive cares. Minerva Anestesiol 2015; 81:135–144 [PubMed] [Google Scholar]
- 19.Rovere Querini P, De Lorenzo R, Conte C, et al. : Post-COVID-19 follow-up clinic: Depicting chronicity of a new disease. Acta Biomed 2020; 91:22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter K: An inside look at a post-COVID-19 clinic. JAMA 2021; 325:2036–2037 [DOI] [PubMed] [Google Scholar]
- 21.Rasulo FA, Piva S, Latronico N: Long-term complications of COVID-19 in ICU survivors: What do we know? Minerva Anestesiol 2022; 88:72–79 [DOI] [PubMed] [Google Scholar]
- 22.Nasreddine ZS, Phillips NA, Bedirian V, et al. : The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699 [DOI] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP: The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67:361–370 [DOI] [PubMed] [Google Scholar]
- 24.Weiss D, Marmar C: The impact of event scale - revised. In: Assessing Pschycological Trauma and PTSD. Wilson J, Keane T. (Eds). New York, NY, Guilford, 1997, pp 399–411 [Google Scholar]
- 25.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. : The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989; 28:193–213 [DOI] [PubMed] [Google Scholar]
- 26.Mahoney FI, Barthel DW: Functional evaluation: The Barthel index. Md State Med J 1965; 14:61–65 [PubMed] [Google Scholar]
- 27.Spies C, Krampe H, Paul N, et al. : Instruments to measure outcomes of post-intensive care syndrome in outpatient care settings – results of an expert consensus and feasibility field test. J Intensice Care Soc 2021; 22:159–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rousseau AF, Kellens I, Freycenon G, et al. : Highly standardized quadriceps dynamometry of critically ill adults at bedside: A step towards individualized rehabilitation. Acta Anaesthesiol Belg 2018; 69:159–164 [Google Scholar]
- 29.Rabin R, de Charro F: EQ-5D: A measure of health status from the EuroQol group. Ann Med 2001; 33:337–343 [DOI] [PubMed] [Google Scholar]
- 30.Hodgson CL, Higgins AM, Bailey MJ, et al. : Comparison of 6-month outcomes of survivors of COVID-19 versus non-COVID-19 critical illness. Am J Respir Crit Care Med 2022; 205:1159–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altman MT, Knauert MP, Pisani MA: Sleep disturbance after hospitalization and critical illness: A systematic review. Ann Am Thorac Soc 2017; 14:1457–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goyal A, Saxena K, Kar A, et al. : Sleep EEG signatures in COVID-19 survivors. Sleep Vigil 2021; 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee D, Viswanath B: Neuropsychiatric manifestations of COVID-19 and possible pathogenic mechanisms: Insights from other coronaviruses. Asian J Psychiatr 2020; 54:102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambermont B, Davenne E, Maclot F, et al. : SARS-CoV-2 in carotid body. Intensive Care Med 2021; 47:342–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazza MG, De Lorenzo R, Conte C, et al. : Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun 2020; 89:594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta P, McAuley DF, Brown M, et al. : COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matschke J, Lutgehetmann M, Hagel C, et al. : Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol 2020; 19:919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooks SK, Webster RK, Smith LE, et al. : The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020; 395:912–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen B, Yi X, Sun Y, et al. : Proteomic and metabolomic characterization of COVID-19 patient sera. Cell 2020; 182:59–72.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorente JA, Nin N, Villa P, et al. : Metabolomic diferences between COVID-19 and H1N1 influenza induced ARDS. Crit Care 2021; 25:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bein T, Weber-Carstens S, Apfelbacher C: Long-term outcome after the acute respiratory distress syndrome: Different from general critical illness? Curr Opin Crit Care 2018; 24:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jutte JE, Erb CT, Jackson JC: Physical, cognitive, and psychological disability following critical illness: What is the risk? Semin Respir Crit Care Med 2015; 36:943–958 [DOI] [PubMed] [Google Scholar]
- 43.Herridge MS, Batt J, Hopkins RO: The pathophysiology of long-term neuromuscular and cognitive outcomes following critical illness. Crit Care Clin 2008; 24:179–199 [DOI] [PubMed] [Google Scholar]
- 44.Griffith DM, Lewis S, Rossi AG, et al. : Systemic inflammation after critical illness: Relationship with physical recovery and exploration of potential mechanisms. Thorax 2016; 71:820–829 [DOI] [PubMed] [Google Scholar]
- 45.Griffith DM, Vale ME, Campbell C, et al. : Persistent inflammation and recovery after intensive care: A systematic review. J Crit Care 2016; 33:192–199 [DOI] [PubMed] [Google Scholar]
- 46.Geense WW, Zegers M, Peters MAA, et al. : New physical, mental, and cognitive problems 1 year after ICU admission: A prospective multicenter study. Am J Respir Crit Care Med 2021; 203:1512–1521 [DOI] [PubMed] [Google Scholar]