IMPORTANCE:
Early risk assessment of functional decline in patients with sepsis is clinically challenging. Recently, there is increasing interest in the nonvolitional evaluation of skeletal muscle quality.
OBJECTIVES:
The aim of this study was to assess the relationship between skeletal muscle quality and functional decline after intensive care.
DESIGN, SETTING, AND PARTICIPANTS:
This pilot study was a single-center prospective observational study conducted from March 2021 to February 2022. We included consecutive patients with sepsis who were admitted to our ICU.
MAIN OUTCOMES AND MEASURES:
The primary outcome was hospital-acquired disability (HAD), which is defined as a decrease in the Barthel index score of at least 5 points from pre-hospital to hospital discharge. Muscle quality was assessed by: 1) muscle echogenicity with ultrasound and 2) phase angle (PhA) with bioelectrical impedance analysis, both of which were measured on ICU days less than 3, 3–5, 5–7, 7–10, and 10–14. We compared longitudinal changes in muscle echogenicity and PhA between the HAD and non-HAD groups using two-way repeated measures analysis of variance with mixed models.
RESULTS:
Among the 22 patients, 7 (31.8%) had HAD. Muscle echogenicity was higher in the HAD group than in the non-HAD group (p < 0.001); however, no interaction effects were found between the two groups (p = 0.189). PhA showed a main effect on each evaluation day in patients (p = 0.040) and a significant interaction effect between the groups, including an early decreased pattern in the HAD group (p = 0.036).
CONCLUSIONS AND RELEVANCE:
Higher muscle echogenicity and a decreased PhA pattern are related to HAD. Noninvasive assessment of muscle quality using ultrasound and bioelectrical impedance analysis may be useful in predicting the functional prognosis of patients with sepsis.
Keywords: activities of daily living, bioelectrical impedance analysis, hospital-acquired disability, muscle ultrasound, phase angle, sepsis
KEY POINTS
Question: Early screening methods for functional decline in patients with sepsis have not yet been established, and the feasibility of skeletal muscle quality assessment using muscle ultrasound and bioelectrical impedance analysis (BIA) was investigated.
Findings: This prospective observational study showed that muscle echogenicity on muscle ultrasound was significantly higher (worse) in patients with functional decline than in those without functional decline and that the phase angle measured by BIA showed a decreasing (worsening) pattern in the early phase of ICU admission in patients with functional decline.
Meaning: Noninvasive assessment of muscle quality may be useful in predicting the functional prognosis of patients with sepsis.
Advances in intensive care have improved the survival of patients with sepsis admitted to the ICU (1, 2). However, many patients discharged from the ICU experience a decline in physical function, called post-intensive care syndrome (3). Post-intensive care syndrome is a concept that refers to long-term physical, cognitive, and mental impairment that extends beyond hospital discharge. Recently, hospital-acquired disability (HAD) has been used to describe the decline in the performance of activities of daily living (ADLs) at hospital discharge compared with pre-hospitalization (4). Our previous study has shown that HAD occurs in 42.5% of patients with sepsis (5), and HAD is associated with an increased risk of adverse outcomes, including nursing home admission (6, 7), sustained functional decline (8), and death (9). The concept of HAD is considered part of post-intensive care syndrome, and prevention of HAD during hospitalization is important for improving long-term health problems such as post-intensive care syndrome.
Recently, muscular ultrasound and bioelectrical impedance analysis (BIA) have been widely accepted as noninvasive and nonvolitional methods to assess skeletal muscle mass and quality in ICU settings (10, 11). Although most of the published studies on muscle ultrasound and BIA have focused primarily on muscle mass, few papers have assessed muscle quality using muscle echogenicity with ultrasound or phase angle (PhA) with BIA as the key parameter of interest (12, 13). Muscle echogenicity reflects myofiber necrosis and fascial inflammation (14), while PhA represents cell quality, including that of muscle cells (15); several studies have shown that these parameters worsen during critical illness (16, 17). Additionally, deterioration in muscle echogenicity and PhA were related to poor clinical outcomes, such as ICU-acquired weakness and prolonged hospital stay (18, 19), indicating that muscle quality measurements could be used for early risk assessment to predict HAD. Furthermore, muscular ultrasound and BIA are known to be affected by tissue edema; however, the influence of abnormal fluid distribution and increased extracellular water (ECW) on assessments of muscle quality have not been well addressed in previous studies (13, 16).
The aim of this pilot study was to assess the relationship between skeletal muscle quality and HAD in patients with sepsis with consideration to abnormal fluid distribution based on BIA analysis.
METHODS
Study Design and Participants
We conducted a single-center prospective observational study at St. Luke’s International Hospital, a 520-bed teaching hospital in Tokyo, between March 2021 and February 2022.
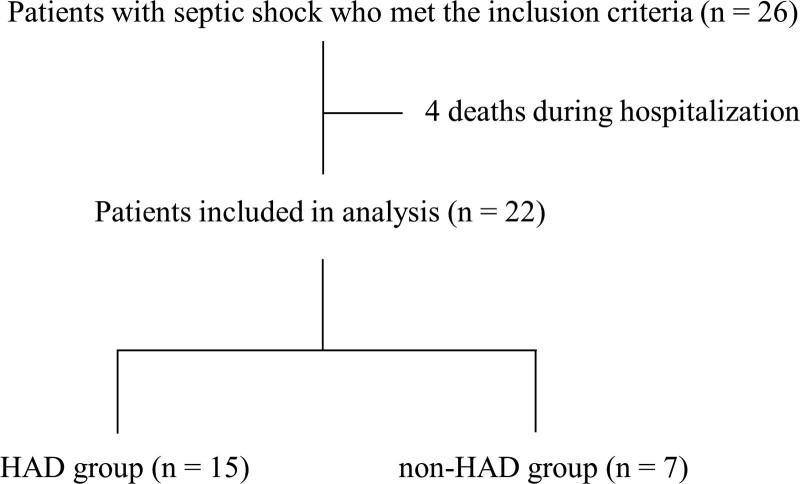
We included consecutive patients diagnosed with septic shock who were admitted to the ICU for greater than or equal to 48 hours and received rehabilitation. The diagnosis of septic shock was based on the criteria described in the Surviving Sepsis Campaign guidelines for the management of sepsis (20). Excluded patients were those less than 18 years old, patients who had died, patients who had been in the general ward for more than a week before being admitted to the ICU, and patients who had been unable to walk with or without assistance before hospital admission. A total of 26 patients were eligible for inclusion in this study; four patients who died during hospitalization were excluded. Of the 22 patients, 7 (31.8%) developed HAD at hospital discharge and were classified into the HAD group, and the remaining 15 patients (68.1%) did not develop HAD and were classified into the non-HAD group (Fig. 1).
Figure 1.
Flow chart of patient selection process. HAD = hospital-acquired disability.
This study was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines and conducted with the approval of the Ethics Committee of St. Luke’s International Hospital (date of approval: November 12, 2020, approval number: 20-R155, study title: Activities of daily living in patients with sepsis). The need for informed consent was waived by the Ethics Committee. All procedures were performed in accordance with the Declaration of Helsinki.
Data Definition and Collection
The primary outcome was HAD, which was defined as a decrease in at least 5 points on the total Barthel index score from pre-hospital to hospital discharge based on a previous study (4, 5, 9). The Barthel index is a widely and commonly used scale for assessing ADLs, and its reliability is well established (21, 22). The Barthel index scores prior to hospital admission were obtained from the patients or their relatives.
Patient data, including clinical history, laboratory results, vital signs, clinical treatment, and rehabilitation outcomes, were collected from the electronic medical records of our institution. Skeletal muscle ultrasound, BIA, and physical function measurements were taken both in the ICU and in the general ward after discharge from the ICU. These measurements were repeated on ICU days less than 3, 3–5, 5–7, 7–10, and 10–14 by the same staff (muscular ultrasound and physical function [Y.T.], BIA measurement [Y.T., N.M.]).
Onset of delirium was identified by the Confusion Assessment Method for the ICU (23). The time to start rehabilitation was calculated from the date of ICU admission to the first day of rehabilitation by a physical therapist. The time to initial ambulation was defined as the period between the ICU admission date and the date of the first trial of ambulation with or without assistance during a physical therapy session. The Acute Physiology and Chronic Health Evaluation II (24) score was calculated at the initial examination in the ICU, and the Sequential Organ Failure Assessment (25) score was calculated both at the initial examination and during ICU. The clinical frailty scale was used to assess frailty before hospital admission (26), and the Charlson Comorbidity Index was used to assess comorbid conditions (27). Infection sites at the final diagnosis included the lungs, abdomen, urinary tract, soft tissue, and bone. Discharge disposition was categorized as home or transfer to a rehabilitation hospital or a nursing home. Physical function, including the Medical Research Council Sum Score (MRC-SS) (28), Functional Status Score for the ICU (FSS-ICU) (29), and ICU Mobility Scale (IMS) (30) were repeatedly measured with muscular ultrasound and BIA.
Muscular Ultrasound and BIA
The right rectus femoris muscle was assessed for echogenicity at 2/3 distance from the anterior superior iliac spine to the superior patella border (18). We used a portable ultrasound (iViz air ver.4, Fujifilm, Tokyo, Japan) with a 5–10-MHz linear transducer. The initial settings (as contrast) were maintained during all examinations excluding the depth, which was altered individually to visualize the muscle. Subjects were assessed in the supine position with their knees in passive extension and neutral rotation. Generous amounts of contact gel were applied to avoid compression of the muscles by the transducer. To enable replication of image location on repeat ultrasound assessments, a mark was drawn on the subject’s legs. Images were saved onto the ultrasound hard drive and transferred for further analysis onto a computer using ImageJ software (National Institutes of Health, Bethesda, MD). Muscle echogenicity was measured by grayscale analysis on Image J, and the square technique was chosen to determine the region of interest based on a previous study (16). The methods for image acquisition and analysis of echogenicity were previously reported and have good reliability (31). In this study, inter-observer reliability was confirmed between the ICU physiotherapist (Y.T.) and the ICU physician (H.O.) for 10 images, and excellent intra-class correlation coefficient (0.998) was obtained.
BIA measurements were performed by a multifrequency bioimpedance device (seca mBCA525, seca, Hamburg, Germany) that uses four pairs of electrodes (eight electrodes in total) (32). Patient information, including age, sex, body weight measured using a bed scale, and height measured by supine body length, were entered into the BIA device. Patients underwent BIA in the supine position with the legs and arms away from the body. Eight BIA electrodes with disposable electrocardiogram electrodes were placed on the dorsal surface of each hand and foot at predetermined anatomical sites. After electrical currents were applied through the electrodes, total body water (TBW), ECW, ECW/TBW, resistance and reactance, and PhA as the arctangent of reactance/resistance were calculated. ECW/TBW is frequently used as a parameter of body water distribution and edema and correlate with the prognosis of patients with sepsis (33).
Rehabilitation Program
Rehabilitation was performed according to our institutional protocol and criteria based on a previous study (34, 35). The physical therapy sessions were conducted for 20–40 minutes a day and were administered for 5 days per week. The rehabilitation program was also conducted in the general ward according to the patient’s functional level and was continued until hospital discharge.
Statistical Analysis
Continuous variables were expressed as mean (sd) or median (interquartile range) for data with normal and non-normal distributions, respectively, while categorical variables were described as numbers (percentages). The Mann-Whitney U test and chi-square test were used to compare continuous and categorical variables between the two groups. The two-way repeated measures analysis of variance with mixed models was used to compare longitudinal changes of muscle echogenicity and PhA between the HAD and non-HAD groups. A mixed model was applied to treat longitudinal data properly with missing values. Spearman rank correlation coefficient was used to examine the relationships among measured variables. Due to the exploratory research, the sample size was not calculated a priori but determined upon feasible size. All reported p values were two-sided, and p values of less than 0.05 were regarded as statistically significant. All analyses were conducted using SPSS (Version 24.0 for Microsoft Windows; SPSS, Chicago, IL).
RESULTS
The HAD group had longer lengths of hospital (p = 0.009) and ICU (p = 0.047) stays, longer rehabilitation days (p = 0.009), longer time to initial ambulation (p = 0.047), and lower proportion of patients who were discharged home (p = 0.021) compared with the non-HAD group (Table 1).
TABLE 1.
Characteristics of the Study Population
| Variables | HAD (n = 7) | Non-HAD (n = 15) | p |
|---|---|---|---|
| Age, yr | 76 (73–84) | 74 (69–81) | 0.332 |
| Male | 3 (42.9) | 10 (66.7) | 0.376 |
| Body mass index, kg/m2 | 21.8 (20.4–24.9) | 23.8 (20.5–26.1) | 1.000 |
| Length of hospital stay, d | 66.0 (42.0–84.5) | 14.5 (12.3–18.5) | 0.003 |
| Length of ICU stay, d | 10 (7.0–15.5) | 5.0 (4.0–6.0) | 0.047 |
| Surgical patients | 3 (42.9) | 1 (6.6) | 0.077 |
| Mechanical ventilator use | 4 (57.1) | 5 (33.3) | 0.376 |
| Renal replacement therapy | 1 (14.3) | 0 (0) | 0.318 |
| Noradrenalin use | 7 (100) | 15 (100) | 1.000 |
| Fluid balance until first evaluation, mL | 2,445.2 (2,250–5,597.3) | 1,484.0 (1,128.4–3,294.6) | 0.056 |
| Delirium incidence | 6 (85.7) | 8 (53.3) | 0.193 |
| Rehabilitation days, d | 64.5 (38.0–88.8) | 11.0 (8.5–15.8) | 0.009 |
| Time to start rehabilitation, d | 1.0 (1.0–1.5) | 1.0 (1.0–1.0) | 1.000 |
| Time to initial ambulation, d | 7.0 (5.5–19.5) | 4.0 (3.0–6.0) | 0.047 |
| Barthel index before admission | 100.0 (87.5–100.0) | 100 (100–100) | 0.535 |
| Acute Physiology and Chronic Health Evaluation II at ICU admission | 17.0 (11.5–27.0) | 16.0 (13.0–17.0) | 0.643 |
| SOFA score at ICU admission | 10.0 (8.0–10.5) | 8.0 (6.5–10.0) | 0.351 |
| Maximum SOFA score in ICU | 10.0 (8.5–12.0) | 8.0 (8.0–11.0) | 0.311 |
| Clinical frail scale | 5.0 (4.5–5.5) | 3.0 (3.0–4.0) | 0.056 |
| Charlson Comorbidity Index | 2.0 (0–2.5) | 1.0 (0.5–2.5) | 1.000 |
| Site of infection | |||
| Lungs | 1 (14.3) | 2 (13.3) | 1.000 |
| Abdomen | 3 (42.9) | 8 (53.3) | |
| Urinary tract | 2 (28.6) | 4 (26.7) | |
| Soft tissue and bone | 1 (14.3) | 1 (6.7) | |
| Disposition | |||
| Home | 3 | 14 | 0.021 |
| Transfer | 4 | 1 |
HAD = hospital-acquired disability, SOFA = Sequential Organ Failure Assessment.
Values are expressed as median (interquartile range) or n (%).
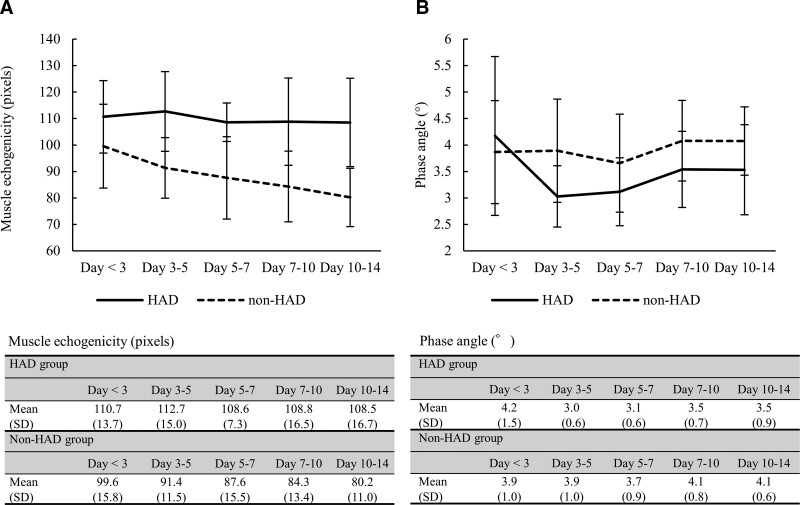
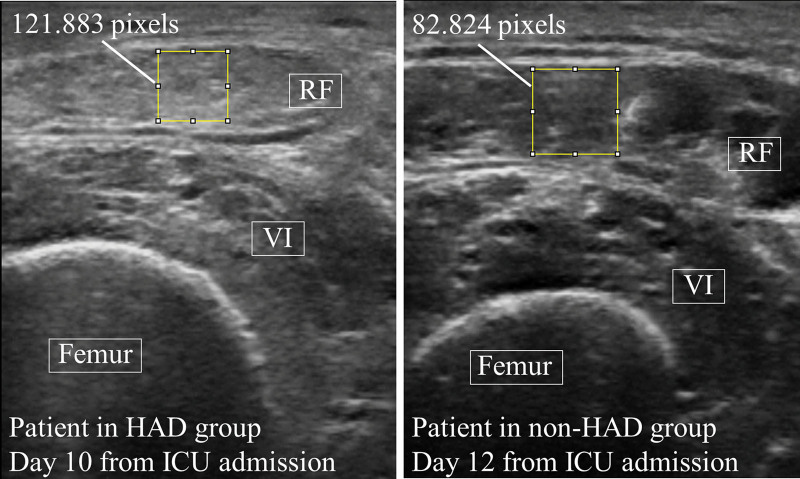
Muscle echogenicity between the two groups showed no significant interaction effects (p = 0.189, F = 1.590); that is, there was no difference in the changes of muscle echogenicity over time between the HAD and non-HAD groups (Fig. 2). However, a main effect was identified between the two groups, and the echogenicity in the HAD group was higher than that in the non-HAD group (p < 0.001, F = 27.418). No main effect was found in each evaluation day (p = 0.139, F = 1.808). In Figure 3, representative muscle ultrasound images are shown for patients in the HAD and non-HAD groups. PhA showed an interaction effect between the two groups; that is, there was a difference in the changes in PhA over time between the HAD and non-HAD groups, with an early decreased pattern in the HAD group (p = 0.036, F = 2.746). A main effect was identified in each evaluation day in all patients (p = 0.040, F = 2.683); however, there was no significant main effect between the two groups (p = 0.140, F = 2.377).
Figure 2.
Comparison of the longitudinal change of muscle echogenicity and phase angle in each group. Values are expressed as average (sd). A, Longitudinal change in muscle echogenicity at each time point. B, Longitudinal change in phase angle at each time point. HAD = hospital-acquired disability.
Figure 3.
Muscle echogenicity analysis images and muscle echogenicity values in patients in the hospital-acquired disability (HAD) and non-HAD groups. RF = rectus femoris, VI = vastus intermedius.
There was a moderate correlation between muscle echogenicity and ECW/TBW at days less than 3 (r = 0.45, p = 0.036), 3–5 (r = 0.61, p = 0.005), and 5–7 (r = 0.56, p = 0.021), and between muscle echogenicity and fluid balance at days 7–10 (r = 0.70, p = 0.016) (Table 2). Regarding PhA, it was strongly correlated with the ECW/TBW at days less than 3 (r = –0.72, p < 0.001), 3–5 (r = –0.73, p < 0.001), 5–7 (r = –0.48, p = 0.050), 7–10 (r = –0.73, p = 0.003), and 10–14 (r = –0.77, p = 0.001). A moderate correlation was also observed between PhA and ECW% at days 3–5 (r = –0.54, p = 0.017), 5–7 (r = –0.82, p < 0.001), 7–10 (r = –0.55, p = 0.040), and 10–14 (r = –0.53, p = 0.047), between PhA and TBW% at days 5–7 (r = –0.71, p = 0.001), and between PhA and fluid balance at days 5–7 (r = –0.48, p = 0.048).
TABLE 2.
Correlation Between Muscle Quality Monitoring and Fluid Distribution on Each Evaluation Day
| Muscle Quality | Comparator | Day < 3 | Days 3–5 | Days 5–7 | Days 7–10 | Days 10–14 |
|---|---|---|---|---|---|---|
| Echogenicity | TBW% | –0.35 | –0.19 | –0.03 | 0.29 | 0.06 |
| 0.119 | 0.420 | 0.905 | 0.334 | 0.831 | ||
| ECW% | –0.12 | 0.09 | 0.04 | 0.35 | –0.07 | |
| 0.577 | 0.694 | 0.863 | 0.234 | 0.802 | ||
| ECW/TBW | 0.45a | 0.61a | 0.56a | –0.05 | –0.04 | |
| 0.036 | 0.005 | 0.021 | 0.872 | 0.887 | ||
| Fluid balance | –0.09 | 0.16 | –0.07 | 0.70a | –0.22 | |
| 0.676 | 0.475 | 0.795 | 0.016 | 0.533 | ||
| Phase angle | TBW% | 0.15 | –0.17 | –0.71a | –0.47 | –0.15 |
| 0.508 | 0.485 | 0.001 | 0.089 | 0.597 | ||
| ECW% | –0.32 | –0.54a | –0.82a | –0.55a | –0.53a | |
| 0.148 | 0.017 | < 0.001 | 0.040 | 0.047 | ||
| ECW/TBW | –0.72a | –0.73a | –0.48a | –0.73a | –0.77a | |
| < 0.001 | < 0.001 | 0.050 | 0.003 | 0.001 | ||
| Fluid balance | –0.16 | –0.31 | –0.48a | –0.26 | –0.38 | |
| 0.464 | 0.171 | 0.048 | 0.431 | 0.238 |
ECW = extracellular water, TBW = total body water.
Significant results, p < 0.05.
Spearman correlation coefficient was used to determine correlations. Upper value is r and lower value is p.
There was a moderate to strong correlation between muscle echogenicity and MRC-SS at days 5–7 (r = –0.59, p = 0.012), 7–10 (r = –0.57, p = 0.033), and 7–10 (r = –0.56, p = 0.045), between muscle echogenicity and FSS-ICU at days 7–10 (r = –0.78, p = 0.001), between muscle echogenicity and Barthel index at days 7–10 (r = –0.64, p = 0.013), and between muscle echogenicity and IMS at days 7–10 (r = –0.79, p = 0.001), and 10–14 (r = –0.59, p = 0.032) (Table 3). Regarding PhA, it was moderately correlated with MRC-SS at days 10–14 (r = 0.64, p = 0.012).
TABLE 3.
Correlation Between Muscle Quality Monitoring and Physical Function on Each Evaluation Day
| Muscle Quality | Comparator | Day < 3 | Days 3–5 | Days 5–7 | Days 7–10 | Days 10–14 |
|---|---|---|---|---|---|---|
| Echogenicity | MRC-SS | –0.16 | –0.06 | –0.59a | –0.57a | –0.56a |
| 0.496 | 0.779 | 0.012 | 0.033 | 0.045 | ||
| FSS-ICU | –0.01 | –0.04 | –0.47 | –0.78a | –0.54 | |
| 0.971 | 0.835 | 0.053 | 0.001 | 0.055 | ||
| Barthel index | 0.05 | 0.07 | –0.31 | –0.64a | –0.52 | |
| 0.817 | 0.751 | 0.225 | 0.013 | 0.064 | ||
| IMS | 0.07 | 0.08 | –0.40 | 0.79a | –0.59a | |
| 0.746 | 0.705 | 0.105 | 0.001 | 0.032 | ||
| Phase angle | MRC-SS | 0.13 | 0.21 | 0.09 | 0.37 | 0.64a |
| 0.582 | 0.340 | 0.704 | 0.167 | 0.012 | ||
| FSS-ICU | 0.01 | 0.15 | –0.13 | 0.34 | 0.48 | |
| 0.964 | 0.507 | 0.588 | 0.208 | 0.076 | ||
| Barthel index | –0.08 | 0.19 | –0.12 | 0.34 | 0.43 | |
| 0.705 | 0.395 | 0.632 | 0.211 | 0.117 | ||
| IMS | 0.19 | 0.06 | –0.07 | 0.20 | 0.50 | |
| 0.934 | 0.768 | 0.755 | 0.472 | 0.068 |
FSS-ICU = Functional Status Score for ICU, IMS = ICU Mobility Scale, MRC-SS = Medical Research Council Sum Score.
Significant results, p < 0.05.
Spearman correlation coefficient was used to determine correlations. Upper value is r and lower value is p.
DISCUSSION
This study showed the clinical possibility of early screening for HAD using ultrasound and BIA in patients with sepsis. Previous studies have reported an association between muscle echogenicity and motor function during ICU stay (16) and ICU-acquired weakness (18). However, this is the first study to show an association between muscle echogenicity during the acute phase of sepsis and ability to perform ADLs at hospital discharge; muscle echogenicity was higher in the HAD group than in the non-HAD group. On the other hand, the PhA was not significantly different between the two groups and was not associated with the concurrent physical function. However, there was a significant interaction effect for PhA between the two groups, which may indicate that longitudinal change is more meaningful than cross-sectional assessment for predicting functional decline.
Previous studies investigating longitudinal changes in muscle echogenicity in ICU patients have all reported a similar pattern of increased muscle echogenicity (12, 16, 18, 36). In this study, muscle echogenicity did not increase over time. However, muscle echogenicity showed that the HAD group maintained significantly higher values compared with the non-HAD group, and a trend of decreasing echogenicity in the non-HAD group was found. Parry et al (16) provided details with individual echogenicity data on 22 patients on mechanical ventilation and reported that the average value of muscle echogenicity in all subjects increased from ICU day 3 to day 10; on the other hand, there were some cases wherein the echogenicity did not change or decreased. While they did not state the underlying cause for the unchanged or decreased muscle echogenicity, this study similarly showed that even in critically ill patients, not all patients showed an increase in muscle echogenicity. Additionally, Umbrello et al (37) showed that in patients who contracted COVID-19, the muscle echogenicity of the rectus femoris significantly increased in the nonsurvivor group, while the echogenicity increased only slightly in the survivor group. Meanwhile, this study included only surviving patients, which may be the reason for the different results from previous studies. To the best of our knowledge, there are only two reports that investigated the association between muscle echogenicity and motor function in critically ill patients. One study showed an association between muscle echogenicity and contemporaneous muscle strength and physical function, which was in line with our results (16). The other is probably the only study to report an association between muscle echogenicity during ICU stay and functional outcomes after intensive care, showing that increased muscle echogenicity is a predictor of ICU-acquired weakness (18). However, this was the first study to show the relationship between recovery of basic ADLs and muscle echogenicity, suggesting the potential role of muscle echogenicity to predict the ability to perform ADLs.
Regarding changes in PhA, it is known that patients whose PhA increases from ICU admission to discharge have a better prognosis and that a decrease in PhA is associated with a longer hospital stay (17, 19). We found different patterns of changes in PhA over time between the HAD and non-HAD groups, with the HAD group showing a pattern of early decline of PhA, which may suggest some association between PhA and functional decline. On the other hand, we found no significant difference in PhA between the two groups, indicating that the absolute value of PhA is not likely to discriminate between functional outcomes. It is known that PhA is lower in healthy elderly people with lower motor function (38); however, the association between PhA and motor function has infrequently been reported in critically ill patients in the ICU. Although Baldwin et al (13) recently found an association between PhA and muscle strength in a multicenter study of ICU patients, its association with functional outcome requires further investigation.
Increased muscle echogenicity in critically ill patients may represent fat, fibrosis, or necrosis of muscle tissue (14, 39). Hence, its interpretation should consider the confounding effects of fluid overload and edema because changes in superficial tissue edema may affect muscle ultrasound images. A key previous study regarding muscle biopsy in critically ill patients revealed processes leading to muscle necrosis by myobiopsy in critically ill patients (14). They reported sequential changes with edema, neutrophil polymorphs, and fibrin occurring in the early phase (ICU days 1–3) and that muscle necrosis follows in the late phase (ICU days 7–10). Thus, we attempted to measure the BIA along with muscle echogenicity as noninvasive evaluation of edema and abnormal fluid distribution to properly interpret changes in muscle echogenicity. As a result, muscle echogenicity and ECW/TBW were positively correlated on days less than 3, 3–5, and 5–7, suggesting that muscle echogenicity in the early stages of sepsis should be interpreted while considering the influence of tissue edema. On the contrary, after day 7, the association between muscle echogenicity and ECW/TBW disappeared, implying that genuine muscle impairment had occurred; however, the lack of histological data does not allow us to be certain. The mechanisms underlying the pattern of early PhA decline in the HAD group can be explained by the strong correlation between PhA and ECW/TBW. Since PhA is calculated by the reactance of the high-frequency current passing through the cell wall and the resistance of the low-frequency current passing through the intercellular fluid, an increase in ECW decreases PhA (11). The HAD group showed a more positive fluid balance on ICU days less than 3 compared with the non-HAD group, which may have led to a significant decrease in PhA. PhA results during treatment of septic shock with extensive infusion is strongly influenced by the increase in extracellular fluid and is unlikely to reflect the quality of the cells, including the muscles, which may be the reason why PhA was not associated with contemporaneous motor function.
Our sample size is relatively small, which may limit its generalization. However, unlike previous studies that included the entire ICU patient population, this study included only patients with sepsis, which is expected to result in less variation in disease and treatment. Furthermore, we did not control the potential confounders by multivariate analysis. In particular, illness severity and associated tissue edema, which may affect both muscle quality and HAD, were not adjusted in this study. Thus, although we carefully interpreted the confounding of severity and edema through the investigation of the association between edema (ECW/TBW) and muscle quality, further analysis by larger studies is needed.
In conclusion, this pilot study indicated that muscle echogenicity was associated with contemporaneous and future muscle strength and ADL performance inpatient with sepsis, while PhA had a less ability to directly reflect motor function. Routine assessment of muscle echogenicity using muscle ultrasound may accurately evaluate skeletal muscle function. Further research is needed to determine whether combining BIA with muscle ultrasound measurements will result in more accurate makes skeletal muscle assessment.
ACKNOWLEDGMENTS
We would like to thank the all-ICU staff at St. Luke’s International Hospital.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Kaukonen KM, Bailey M, Suzuki S, et al. : Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014; 311:1308–1316 [DOI] [PubMed] [Google Scholar]
- 2.Levy MM, Dellinger RP, Townsend SR, et al. ; Surviving Sepsis Campaign: The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374 [DOI] [PubMed] [Google Scholar]
- 3.Needham DM, Davidson J, Cohen H, et al. : Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit Care Med 2012; 40:502–509 [DOI] [PubMed] [Google Scholar]
- 4.Loyd C, Markland AD, Zhang Y, et al. : Prevalence of hospital-associated disability in older adults: A meta-analysis. J Am Med Dir Assoc 2020; 21:455–461.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi Y, Morisawa T, Okamoto H, et al. : Prevalence and predictors of hospital-acquired functional decline in patients with sepsis admitted to the intensive care unit. Int J Rehabil Res 2021; 44:307–313 [DOI] [PubMed] [Google Scholar]
- 6.Fortinsky RH, Covinsky KE, Palmer RM, et al. : Effects of functional status changes before and during hospitalization on nursing home admission of older adults. J Gerontol A Biol Sci Med Sci 1999; 54:M521–M526 [DOI] [PubMed] [Google Scholar]
- 7.Mahoney JE, Sager MA, Jalaluddin M: New walking dependence associated with hospitalization for acute medical illness: Incidence and significance. J Gerontol A Biol Sci Med Sci 1998; 53:M307–M312 [DOI] [PubMed] [Google Scholar]
- 8.Boyd CM, Landefeld CS, Counsell SR, et al. : Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc 2008; 56:2171–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sleiman I, Rozzini R, Barbisoni P, et al. : Functional trajectories during hospitalization: A prognostic sign for elderly patients. J Gerontol A Biol Sci Med Sci 2009; 64:659–663 [DOI] [PubMed] [Google Scholar]
- 10.Formenti P, Umbrello M, Coppola S, et al. : Clinical review: Peripheral muscular ultrasound in the ICU. Ann Intensive Care 2019; 9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moonen HPFX, Van Zanten ARH: Bioelectric impedance analysis for body composition measurement and other potential clinical applications in critical illness. Curr Opin Crit Care 2021; 27:344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimm A, Teschner U, Porzelius C, et al. : Muscle ultrasound for early assessment of critical illness neuromyopathy in severe sepsis. Crit Care 2013; 17:R227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldwin CE, Fetterplace K, Beach L, et al. : Early detection of muscle weakness and functional limitations in the critically ill a retrospective evaluation of bioimpedance spectroscopy. JPEN J Parenter Enteral Nutr 2020; 44:837–848 [DOI] [PubMed] [Google Scholar]
- 14.Puthucheary ZA, Phadke R, Rawal J, et al. : Qualitative ultrasound in acute critical illness muscle wasting. Crit Care Med 2015; 43:1603–1611 [DOI] [PubMed] [Google Scholar]
- 15.Looijaard WGPM, Stapel SN, Dekker IM, et al. : Identifying critically ill patients with low muscle mass: Agreement between bioelectrical impedance analysis and computed tomography. Clin Nutr 2020; 39:1809–1817 [DOI] [PubMed] [Google Scholar]
- 16.Parry SM, El-Ansary D, Cartwright MS, et al. : Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care 1151; 2015:e9–e14 [DOI] [PubMed] [Google Scholar]
- 17.Ellegård LH, Petersen P, Öhrn L, et al. : Longitudinal changes in phase angle by bioimpedance in intensive care patients differ between survivors and non-survivors. Clin Nutr ESPEN 2018; 24:170–172 [DOI] [PubMed] [Google Scholar]
- 18.Mayer KP, Thompson Bastin ML, Montgomery-Yates AA, et al. : Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit Care 2020; 24:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen AK, Gattermann T, da Silva Fink J, et al. : Low standardized phase angle predicts prolonged hospitalization in critically ill patients. Clin Nutr ESPEN 2019; 34:68–72 [DOI] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahoney FI, Barthel DW: Functional evaluation: The Barthel index. Md State Med J 1965; 14:61–65 [PubMed] [Google Scholar]
- 22.Collin C, Wade DT, Davies S, et al. : The Barthel ADL index: A reliability study. Int Disabil Stud 1988; 10:61–63 [DOI] [PubMed] [Google Scholar]
- 23.Ely EW, Margolin R, Francis J, et al. : Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001; 29:1370–1379 [DOI] [PubMed] [Google Scholar]
- 24.Knaus WA, Draper EA, Wagner DP, et al. : APACHE II: A severity of disease classification system. Crit Care Med 1985; 13:818–829 [PubMed] [Google Scholar]
- 25.Vincent JL, de Mendonga A, Cantraine F, et al. : Use of the SOFA score to assess the incidence of organ dysfunction failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998; 26:1793–1800 [DOI] [PubMed] [Google Scholar]
- 26.Morley JE, Vellas B, van Kan GA, et al. : Frailty consensus: A call to action. J Am Med Dir Assoc 2013; 14:392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40:373–383 [DOI] [PubMed] [Google Scholar]
- 28.Vanpee G, Hermans G, Segers J, et al. : Assessment of limb muscle strength in critically ill patients: A systematic review. Crit Care Med 2014; 42:701–711 [DOI] [PubMed] [Google Scholar]
- 29.Thrush A, Rozek M, Dekerlegand JL: The clinical utility of the Functional Status Score for the Intensive Care Unit (FSS-ICU) at a long-term acute care hospital: A prospective cohort study. Phys Ther 2012; 92:1536–1545 [DOI] [PubMed] [Google Scholar]
- 30.Hodgson C, Needham D, Haines K, et al. : Feasibility and inter-rater reliability of the ICU mobility scale. Heart Lung 2014; 43:19–24 [DOI] [PubMed] [Google Scholar]
- 31.Sarwal A, Parry SM, Berry MJ, et al. : Interobserver reliability of quantitative muscle sonographic analysis in the critically ill population. J Ultrasound Med 2015; 34:1191–1200 [DOI] [PubMed] [Google Scholar]
- 32.Jensen B, Moritoyo T, Kaufer-Horwitz M, et al. : Ethnic differences in fat and muscle mass and their implication for interpretation of bioelectrical impedance vector analysis. Appl Physiol Nutr Metab 2019; 44:619–626 [DOI] [PubMed] [Google Scholar]
- 33.Park I, Lee JH, Jang DH, et al. : Assessment of body water distribution in patients with sepsis during fluid resuscitation using multi-frequency direct segmental bioelectrical impedance analysis. Clin Nutr 2020; 39:1826–1831 [DOI] [PubMed] [Google Scholar]
- 34.Morris PE, Goad A, Thompson C, et al. : Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med 2008; 36:2238–2243 [DOI] [PubMed] [Google Scholar]
- 35.Takahashi T, Morisawa T, Saitoh M, et al. : Current status and future development of acute and cardiac physiotherapies in Japan. Phys Ther Res 2020; 23:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cartwright MS, Kwayisi G, Griffin LP, et al. : Quantitative neuromuscular ultrasound in the intensive care unit. Muscle Nerve 2013; 47:255–259 [DOI] [PubMed] [Google Scholar]
- 37.Umbrello M, Gugliemetti L, Formenti P, et al. : Qualitative and quantitative muscle ultrasound changes in patients with COVID-19-related ARDS. Nutrition 2021; 91-92:111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matias CN, Nunes CL, Francisco S, et al. : Phase angle predicts physical function in older adults. Arch Gerontol Geriatr 2020; 90:104151. [DOI] [PubMed] [Google Scholar]
- 39.Pereira AZ, Uezima CB, Zanella MT, et al. : Muscle echogenicity and changes related to age and body mass index. JPEN J Parenter Enteral Nutr 2021; 45:1591–1596 [DOI] [PubMed] [Google Scholar]