Abstract
Objective: We performed a case-control study to investigate the correlation between DNA methylation and mRNA expression of the glutathione S-transferase alpha 4 (GSTA4) gene and the risk of intracranial aneurysm (IA) in the Chinese Han population.
Methods: After propensity score matching, 44 pairs of cases and controls were collected in this study. Fasting blood samples were collected for DNA and RNA extraction within 24 h of admission. Nine CpG dinucleotides were selected from the GSTA4 promoter region for DNA methylation pyrosequencing. mRNA expression of GSTA4 was measured by quantitative real-time polymerase chain reaction (RT-qPCR). In vitro cell experiments were conducted to verify the association between 5-aza-2′-deoxycytidine induced DNA hypomethylation and GSTA4 mRNA expression.
Results: The mean methylation level of GSTA4 was much lower in IA patients, especially in IA patients, especially in unruptured IA (UIA), than that in controls (IA vs. Control, p < .001; ruptured IA (RIA) vs. Control, p = .005; UIA vs. Control, p < .001). With sex stratification, we further found that the association between GSTA4 methylation and IA risk presented only in women (mean methylation level: IA vs. Control, p < .001; RIA vs. Control, p = .009; UIA vs. Control, p < .001). GSTA4 mRNA expression was significantly higher in the IA group than in the control group (p < .01) and negatively correlated with DNA methylation in all individuals (r = −.746, p < .001). DNA hypomethylation can increase GSTA4 mRNA expression in human primary artery smooth muscle cells. The receiver operating characteristic (ROC) curve showed that GSTA4 mean methylation (AUC = .80, p < .001) was a reliable predictor of women intracranial aneurysm, among which CpG 1 exhibited the best predictive value (AUC = .89, p < .001). In addition, GSTA4 expression levels could also predict the risk of IA in women (AUC = .87, p = .005).
Conclusion: Decreased DNA methylation and increased mRNA expression of the GSTA4 gene are associated with the risk of IA in women.
Keywords: intracranial aneurysm, DNA methylation, mRNA expression, 5-aza-2'-deoxycytidine, glutathione S-tranferase alpha 4
Introduction
Intracranial aneurysm (IA) is a common cerebrovascular disorder that presents as abnormal enlargement of the local cerebral arterial cavity. Rupture of the IA can develop into subarachnoid hemorrhage (SAH), which is a fatal acute event (Etminan and Rinkel, 2016). Epidemiological surveys have shown that IA occurs in one in 20–30 adults, and one in four IAs will rupture during the patient’s lifetime (Korja and Kaprio, 2016; Chen et al., 2018). Congenital cerebral vascular dysplasia is a vital cause of IA. Acquired risk factors, including atherosclerosis, infection, and trauma, aggravate the pathological process (Jin et al., 2019; Etminan et al., 2020). Genome-wide association studies (GWAS) have confirmed that many genes are involved in the pathophysiology of IA (Bakker et al., 2020; Bakker and Ruigrok, 2021). However, the molecular mechanisms underlying IA formation are not yet fully understood.
DNA methylation is the main form of epigenetic modification that can alter gene expression without changing the DNA sequence. DNA methylation in gene promoter regions is closely related to reduced mRNA expression and protein levels (Palou-Marquez et al., 2021). DNA methylation can serve as a bridge between genetic and environmental factors in cerebrovascular diseases (Yu et al., 2017; Law and Holland, 2019). Wang et al. reported that long-term cigarette smoking could increase DNA methylation levels of gene-encoding polypyrimidine tract-binding protein 1 (PTBP1), which further reduces PTBP1 expression and contributes to the formation of IA (Wang et al., 2021). Sex plays a vital role in DNA methylation. Liu et al. (2020) observed significant associations between phospholipase A2 group VII (PLA2G7) methylation and the risk of developing IA in women.
Literature data showed that the glutathione system might contribute to the formation of aneurysms through mechanisms related to increased oxidative stress, and a variety of glutathione S-transferases (GSTs) may play a role in protecting the cell from oxidative damage (Jeong et al., 2021; Perez-Torres et al., 2022). Glutathione S-transferase alpha 4 (GSTA4) is a phase 2 detoxifying enzyme, a member of the α-class of GSTs (Carlstrom et al., 2020). By catalyzing conjugation reactions of metabolites of lipid peroxidation to glutathione (GSH), GSTA4 participates in regulating cell proliferation and apoptosis (Laborde, 2010). GSTA4 is expressed in the cerebral cortex and brainstem and is involved in the development of gliomas (Cheng et al., 2022). Overexpression of human GSTA4 in carotid arteries can reduce neointimal formation after carotid allografts (Xu et al., 2010). In addition, GSTA4 has been reported to be critical for the phenotypic transition of vascular smooth muscle cells (SMCs). Lou et al. reported that GSTA4 overexpression inhibits SMC proliferation and activation (Luo et al., 2018). SMCs and the collagen fibers they secrete provide structural support to the cerebral arteries. Disruption of the balance between SMC proliferation and apoptosis contributes to IA formation (Frosen, 2014; Kataoka et al., 2020). In this study, we hypothesized that GSTA4 methylation is involved in the pathological process of IA. In this case-control study, we investigated the association between DNA methylation and mRNA expression of the GSTA4 gene with the risk of IA in the Chinese Han population.
Materials and methods
Participants
This was a single-center case-control study. The clinical sample collection has been described in previously published study (Wang et al., 2021). Briefly, Patients with IA and age-/sex-matched controls were recruited from Ningbo First Hospital. All IA patients were diagnosed using computed tomography angiography (CTA) or digital subtraction angiography (DSA). Healthy controls were excluded if they had cerebrovascular, cardiovascular, or other serious disorders. Fasting blood samples were obtained from participants within 24 h of hospital admission. Plasma levels of triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were measured using an automatic biochemical analyzer (Olympus AU2700, Tokyo, Japan). After propensity score matching, 44 pairs of cases and controls were included in the following GSTA4 methylation analysis. There were 22 patients with a ruptured IA (RIA) and 22 with an unruptured IA (UIA) in the case group.
Pyrosequencing assay
Genomic DNA was extracted from human peripheral blood using an automatic nucleic acid extraction apparatus (LAB-AID 820, Xiamen, China). GSTA4 is located on Chr6:52,977,953-52,995,284 (GRCh38/hg38). Nine CpG dinucleotides were selected from the promoter region for DNA methylation pyrosequencing. The EpiTect Fast Bisulfite Conversion Kit (Qiagen, Hilden, Germany) was used for bisulfite conversion. DNA methylation levels were measured using PyroMark Q24 (Qiagen). Polymerase chain reaction primers were designed using PyroMark Assay Design software (Qiagen). Primer sequences for the nine CpG regions of GSTA4 were as follows: forward primer, 5′-AGGGAAAAAAAAGAAGAGAGAAAGG-3′; reverse primer, 5′-biotin-TTTAACACTATCCAAAATACCTTACAAA-3′; and sequencing primer, 5′-GGAGATAGATTTGGAGTTTA-3′.
Quantitative real-time polymerase chain reactions (RT-qPCR)
A total of 40 gender-age-matched participants (including 20 IAs and 20 controls) were collected for mRNA detection. The TRIzol-based method was used for DNA-free RNA extraction from blood samples (Meng and Feldman, 2010). The SYBR Green Kit (TaKaRa, Dalian, China) and LightCycler 480 system (Roche, Mannheim, Germany) were used for RT-qPCR. β-actin was used as an internal reference, whose primer sequences were described in previous study (Zhang et al., 2018). qPCR primers for GSTA4 were as follows: forward primer, 5′-GTGAGAACCGTCTACAACAT-30; reverse primer, 5′- TGAACTGGCTACAGGACAT-3′.
Cell culture and 5-aza-2′-deoxycytidine (AZA) treatment
Human brain vascular smooth muscle cells (HBVSMCs) (Catalog #1100, ScienCell, Carlsbad, Canada) were used in this study. Cells were cultured in six-well plates at a density of 1 × 106 cells/well in smooth muscle cell medium (SMCM) (ScienCell, Carlsbad, Canada). AZA (Sigma-Aldrich; Darmstadt, Germany) is a DNA methyltransferase (DNMT) inhibitor typically used to induce DNA hypomethylation. Cells were treated with three different concentrations of AZA (5, 15, and 25 µM) for 3 days to assess the regulatory role of DNA methylation in GSTA4 gene expression. Total RNA was extracted from the cells for RT-qPCR analysis.
Statistical analyses
SPSS (v.23.0; SPSS Inc., Chicago, United States) and GraphPad Prism v.7.0 (GraphPad Software, CA, United States) were used to conduct statistical analyses in this study. Propensity score matching analysis was used to control for confounders on the results. For continuous variables, Student’s t-test was used for group comparison. For non-normal continuous variables, the Mann–Whitney U-test was used. Associations between GSTA4 methylation and clinical characteristics were assessed using Pearson or Spearman correlation coefficient analysis. The predictive value of GSTA4 methylation levels for the risk of IA was evaluated using the receiver operating characteristic (ROC) curve. Statistical significance was set at p values <.05.
Results
The clinical baseline and other characteristics (such as age, hypertension, diabetes, alcohol drinking, cigarette smoking status, plasma levels of TG, TC, HDL, and LDL, etc.) were not statistically different between IA and control groups (p > .05, Table 1). The nine dinucleotide loci (CpG1, CpG2, CpG3, CpG4, CpG5, CpG6, CpG7, CpG8, CpG9) in the GSTA4 promoter region (Chr6:52,977,953-52,995,284, GRCh38/hg38) were selected for DNA methylation pyrosequencing (Figure 1). Figure 2 shows significant correlations involving the methylation levels of all nine CpGs (p < .05). However, age and blood lipids, including TG, TC, LDL, and HDL, were not associated with GSTA4 methylation in men and women.
TABLE 1.
The clinical characteristics of participants after propensity score matching.
| Character | IA (n = 44) | Control (n = 44) | t/x | p |
|---|---|---|---|---|
| Age (year) | 47.73 ± 5.74 | 46.30 ± 6.02 | 1.14 | .257 |
| Sex (Male, n) | 22 | 22 | .03 | .980 |
| Hypertension (n) | 8 | 3 | 1.22 | .206 |
| Diabetes (n) | 1 | 4 | .88 | .362 |
| Drinking (n) | 10 | 5 | 1.43 | .273 |
| Smoking (n) | 12 | 9 | .35 | .631 |
| TG (mmol/L) | 1.53 ± .87 | 1.61 ± 1.41 | .31 | .754 |
| TC (mmol/L) | 4.96 ± .81 | 4.80 ± .88 | .89 | .372 |
| HDL (mmol/L) | 1.19 ± .23 | 1.27 ± .26 | 1.48 | .140 |
| LDL (mmol/L) | 3.05 ± .68 | 2.94 ± .62 | .77 | .441 |
TG, Triglycerides; TC, Total cholesterol; HDL, High-density lipoprotein; LDL, Low-density lipoprotein.
FIGURE 1.
Locations and analysis of nine CpGs in GSTA4. (A) The locations of the nine CpGs in the GSTA4 gene. (B) Representative sequencing analysis of nine methylation sites.
FIGURE 2.
Pearson correlation analysis involving GSTA4 methylation status and clinical characters. (A) In men and women: methylation levels of all nine CpGs were significantly correlated. (B) In men: methylation levels of all nine CpGs were significantly correlated. (C) In women: methylation levels of all nine CpGs were significantly correlated.
The DNA methylation levels of GSTA4 in the IAs were lower than those in the controls. As shown in Table 2, the significant differences were found in CpG1 (IA vs. Control, p = .001; RIA vs. Control, p = .033; UIA vs. Control, p = .001), CpG2 (IA vs. Control, p = .002; RIA vs. Control, p = .035; UIA vs. Control, p = .002), CpG3 (IA vs. Control, p < .001; UIA vs. Control, p = .001; RIA vs. Control, p = .001), CpG4 (IA vs. Control, p < .001; RIA vs. Control, p = .003), CpG5 (IA vs. Control, p = .024; RIA vs. Control, p = .043; UIA vs. Control, p = .001), CpG6 (IA vs. Control, p = .001; RIA vs. Control, p = .008; UIA vs. Control, p < .001), CpG7 (IA vs. Control, p = .001; RIA vs. Control, p = .023; UIA vs. Control, p < .001), CpG8 (IA vs. Control, p < .001; RIA vs. Control, p = .011; UIA vs. Control, p = .029), CpG9 (IA vs. Control, p = .034; UIA vs. Control, p = .001), Mean methylation levels of the nine CpG sites (IA vs. Control, p < .001; RIA vs. Control, p = .005; UIA vs. Control, p < .001).
TABLE 2.
DNA methylation differences of nine CpGs dinucleotides of GSTA4 gene between IAs and controls.
| Character | Control (n = 44) | IA (n = 44) | RIA (n = 22) | UIA (n = 22) | p IA vs. control | p RIA vs. control | p UIA vs. control |
|---|---|---|---|---|---|---|---|
| CpG1 (%) | 12.84 ± 3.23 | 10.33 ± 3.55 | 10.91 ± 3.71 | 9.75 ± 3.36 | .001 | .033 | .001 |
| CpG2 (%) | 10.28 ± 3.73 | 7.99 ± 2.95 | 8.26 ± 3.28 | 7.72 ± 2.64 | .002 | .035 | .002 |
| CpG3 (%) | 7.13 ± 2.42 | 5.49 ± 1.36 | 5.52 ± 1.26 | 5.45 ± 1.48 | <.001 | .001 | .001 |
| CpG4 (%) | 6.73 ± 2.37 | 5.09 ± 1.26 | 5.31 ± 1.41 | 4.88 ± 1.08 | <.001 | .003 | .074 |
| CpG5 (%) | 11.37 ± 3.51 | 9.73 ± 3.19 | 9.81 ± 2.55 | 9.66 ± 3.79 | .024 | .043 | .001 |
| CpG6 (%) | 5.68 ± 1.71 | 4.55 ± 1.20 | 4.62 ± 1.31 | 4.47 ± 1.10 | .001 | .008 | <.001 |
| CpG7 (%) | 7.30 ± 2.07 | 6.04 ± 1.30 | 6.22 ± 1.59 | 5.87 ± .92 | .001 | .023 | <.001 |
| CpG8 (%) | 7.27 ± 1.80 | 6.05 ± 1.16 | 6.30 ± 1.16 | 5.80 ± 1.13 | <.001 | .011 | .029 |
| CpG9 (%) | 6.71 ± 2.02 | 5.94 ± 1.25 | 6.10 ± 1.15 | 5.77 ± 1.34 | .034 | .127 | .001 |
| Mean (%) | 8.37 ± 2.30 | 6.80 ± 1.52 | 7.01 ± 1.50 | 6.60 ± 1.55 | <.001 | .005 | <.001 |
Abbreviation: GSTA4, Glutathione S-transferase alpha 4; IA, intracranial aneurysm; RIA, ruptured intracranial aneurysm; UIA, unruptured intracranial aneurysm. Data were presented as mean ± SD. The t-test was used for group comparison. The p-value less than or equal to .05 is in bold.
Sex is a crucial factor that influences DNA methylation levels. With sex stratification, we further found that the association between GSAT4 methylation and IA risk was present only in women. As shown in Table 3, the significant differences were found in CpG1 levels among women (IA vs. Control, p < .001; RIA vs. Control, p = .001; UIA vs. Control, p < .001), CpG2 (IA vs. Control, p = .005; UIA vs. Control, p = .006), CpG3 (IA vs. Control, p = .001; RIA vs. Control, p = .002; UIA vs. Control, p = .011), CpG4 (IA vs. Control, p < .001; RIA vs. Control, p = .020; UIA vs. Control, p = .002), CpG5 (IA vs. Control, p = .026; RIA vs. Control, p = .039), CpG6 (IA vs. Control, p = .002; RIA vs. Control, p = .039; UIA vs. Control, p = .002), CpG7 (IA vs. Control, p = .003; UIA vs. Control, p < .001), CpG8 (IA vs. Control, p = .002; UIA vs. Control, p < .001), CpG9 (IA vs. Control, p = .001; RIA vs. Control, p = .04; UIA vs. Control, p = .003), Mean methylation levels of the nine CpG sites (IA vs. Control, p < .001; RIA vs. Control, p = .009; UIA vs. Control, p < .001). However, no significant differences were found in mean methylation level in men (p > .05, Table 4). Subsequent sex comparison analysis showed that compared to that in healthy men, healthy women had higher GSTA4 methylation levels CpG1 (p = .004, Table 5), CpG2 (p = .024), CpG3 (p = .042), CpG4 (p = .041), CpG6 (p = .038), CpG8 (p = .035), CpG9 (p = .02), mean methylation levels of the nine CpG sites (p = .019). However, only CpG1 presented a significant difference between women and man IA (p = .013).
TABLE 3.
DNA methylation differences of nine CpGs dinucleotides of GSTA4 gene between IA and control in women.
| Character | Control (n = 22) | IA (n = 22) | RIA (n = 11) | UIA (n = 11) | p IA vs. control | p RIA vs. control | p UIA vs. control |
|---|---|---|---|---|---|---|---|
| CpG1 (%) | 14.19 ± 3.24 | 9.03 ± 3.19 | 9.31 ± 3.91 | 8.74 ± 2.44 | <.001 | .001 | <.001 |
| CpG2 (%) | 11.53 ± 3.65 | 8.31 ± 3.54 | 8.83 ± 4.07 | 7.78 ± 3.04 | .005 | .063 | .006 |
| CpG3 (%) | 7.86 ± 2.35 | 5.71 ± 1.49 | 5.70 ± 1.39 | 5.42 ± 1.72 | .001 | .002 | .011 |
| CpG4 (%) | 7.46 ± 2.47 | 5.11 ± 1.35 | 5.42 ± 1.72 | 4.80 ± .82 | <.001 | .020 | .002 |
| CpG5 (%) | 12.20 ± 3.83 | 9.69 ± 3.36 | 9.79 ± 2.52 | 9.60 ± 4.16 | .026 | .039 | .084 |
| CpG6 (%) | 6.21 ± 1.80 | 4.61 ± 1.43 | 4.81 ± 1.68 | 4.41 ± 1.17 | .002 | .039 | .002 |
| CpG7 (%) | 7.83 ± 1.97 | 6.16 ± 1.59 | 6.62 ± 1.96 | 5.70 ± .98 | .003 | .106 | <.001 |
| CpG8 (%) | 7.83 ± 1.77 | 6.28 ± 1.23 | 6.69 ± 1.31 | 5.86 ± 1.02 | .002 | .070 | <.001 |
| CpG9 (%) | 7.41 ± 1.71 | 5.80 ± 1.34 | 6.13 ± 1.33 | 5.47 ± 1.31 | .001 | .040 | .003 |
| Mean (%) | 9.17 ± 2.25 | 6.74 ± 1.55 | 7.03 ± 1.69 | 6.45 ± 1.43 | <.001 | .009 | <.001 |
Abbreviation: GSTA4, Glutathione S-transferase alpha 4; IA, intracranial aneurysm; RIA, ruptured intracranial aneurysm; UIA, unruptured intracranial aneurysm. Data were presented as mean ± SD. The t-test was used for group comparison. The p-value less than or equal to .05 is in bold.
TABLE 4.
DNA methylation differences of nine CpGs dinucleotides of GSTA4 gene between IA and control in men.
| Character | Control (n = 22) | IA (n = 22) | RIA (n = 11) | UIA (n = 11) | p IA vs. control | p RIA vs. control | p UIA vs. control |
|---|---|---|---|---|---|---|---|
| CpG1 (%) | 11.50 ± 2.66 | 11.64 ± 3.47 | 12.51 ± 2.83 | 10.77 ± 2.94 | .879 | .322 | .536 |
| CpG2 (%) | 9.02 ± 3.45 | 7.68 ± 2.25 | 7.69 ± 2.31 | 7.66 ± 2.31 | .134 | .258 | .124 |
| CpG3 (%) | 6.39 ± 2.31 | 5.27 ± 1.22 | 5.35 ± 1.15 | 5.20 ± 1.33 | .053 | .17 | .07 |
| CpG4 (%) | 6.00 ± 2.08 | 5.08 ± 1.19 | 5.19 ± 1.09 | 4.96 ± 1.33 | .078 | .152 | .141 |
| CpG5 (%) | 10.55 ± 3.02 | 9.78 ± 3.09 | 9.82 ± 2.71 | 9.73 ± 3.57 | .405 | .503 | .494 |
| CpG6 (%) | 5.15 ± 1.47 | 4.48 ± .94 | 4.44 ± .85 | 4.53 ± 1.06 | .083 | .151 | .224 |
| CpG7 (%) | 6.77 ± 2.07 | 5.93 ± .95 | 5.82 ± 1.05 | 6.03 ± .86 | .093 | .09 | .161 |
| CpG8 (%) | 6.70 ± 1.67 | 5.83 ± 1.06 | 5.91 ± .86 | 5.74 ± 1.27 | .046 | .083 | .104 |
| CpG9 (%) | 6.00 ± 2.10 | 6.07 ± 1.16 | 6.07 ± .99 | 6.07 ± 1.37 | .903 | .928 | .927 |
| Mean (%) | 7.57 ± 2.11 | 6.86 ± 1.53 | 6.98 ± 1.37 | 6.74 ± 1.73 | .211 | .409 | .273 |
Abbreviation: GSTA4, Glutathione S-transferase alpha 4; IA, intracranial aneurysm. Data were presented as mean ± SD. The t-test was used for group comparison. The p-value less than or equal to .05 is in bold.
TABLE 5.
The gender differences in DNA methylation of nine CpGs of GSTA4 gene in IAs and controls respectively.
| Character | Men | Women | t | p |
|---|---|---|---|---|
| All | (n = 44) | (n = 44) | ||
| CpG1 (%) | 11.57 ± 3.06 | 11.61 ± 4.11 | .05 | .962 |
| CpG2 (%) | 8.35 ± 2.96 | 9.92 ± 3.91 | 2.12 | .037 |
| CpG3 (%) | 5.83 ± 1.91 | 6.78 ± 2.23 | 2.15 | .034 |
| CpG4 (%) | 5.54 ± 1.74 | 6.29 ± 2.30 | 1.72 | .090 |
| CpG5 (%) | 10.16 ± 3.05 | 10.95 ± 3.78 | 1.07 | .287 |
| CpG6 (%) | 4.82 ± 1.26 | 5.41 ± 1.80 | 1.79 | .078 |
| CpG7 (%) | 6.35 ± 1.65 | 6.99 ± 1.96 | 1.66 | .100 |
| CpG8 (%) | 6.26 ± 1.45 | 7.05 ± 1.70 | 2.35 | .021 |
| CpG9 (%) | 6.04 ± 1.68 | 6.60 ± 1.72 | 1.56 | .121 |
| Mean (%) | 7.21 ± 1.86 | 7.96 ± 2.27 | 1.68 | .096 |
| IA | (n = 22) | (n = 22) | ||
| CpG1 (%) | 11.64 ± 3.47 | 9.03 ± 3.19 | 2.61 | .013 |
| CpG2 (%) | 7.68 ± 2.25 | 8.31 ± 3.55 | .7 | .487 |
| CpG3 (%) | 5.27 ± 1.22 | 5.71 ± 1.49 | 1.06 | .297 |
| CpG4 (%) | 5.07 ± 1.19 | 5.11 ± 1.35 | .09 | .925 |
| CpG5 (%) | 9.78 ± 3.09 | 9.69 ± 3.36 | .08 | .935 |
| CpG6 (%) | 4.48 ± .94 | 4.61 ± 1.43 | .34 | .735 |
| CpG7 (%) | 5.93 ± .95 | 6.16 ± 1.59 | .58 | .561 |
| CpG8 (%) | 5.83 ± 1.06 | 6.28 ± 1.23 | 1.31 | .199 |
| CpG9 (%) | 6.07 ± 1.16 | 5.80 ± 1.34 | .702 | .487 |
| Mean (%) | 6.86 ± 1.53 | 6.74 ± 1.55 | .25 | .803 |
| Control | (n = 22) | (n = 22) | ||
| CpG1 (%) | 11.50 ± 2.66 | 14.19 ± 3.24 | 3 | .004 |
| CpG2 (%) | 9.02 ± 3.45 | 11.53 ± 3.65 | 2.35 | .024 |
| CpG3 (%) | 6.39 ± 2.31 | 7.86 ± 2.35 | 2.1 | .042 |
| CpG4 (%) | 6.00 ± 2.08 | 7.46 ± 2.47 | 2.11 | .041 |
| CpG5 (%) | 10.55 ± 3.02 | 12.20 ± 3.83 | 1.59 | .121 |
| CpG6 (%) | 5.15 ± 1.47 | 6.21 ± 1.79 | 2.14 | .038 |
| CpG7 (%) | 6.77 ± 2.08 | 7.83 ± 1.97 | 1.73 | .091 |
| CpG8 (%) | 6.70 ± 1.67 | 7.83 ± 1.77 | 2.18 | .035 |
| CpG9 (%) | 6.01 ± 2.10 | 7.41 ± 1.71 | 2.42 | .020 |
| Mean (%) | 7.57 ± 2.11 | 9.17 ± 2.25 | 2.44 | .019 |
Abbreviation: GSTA4, Glutathione S-transferase alpha 4; IA, intracranial aneurysm; Data were presented as mean ± SD. The t-test was used for group comparison. The p-value less than or equal to .05 is in bold.
GSTA4 mRNA expression was significantly higher in the IA group than in the control group (p < .01, Figure 3A). Sex group analysis showed that GSTA4 mRNA expression was significantly higher in the women IA group (p < .01, Figure 3B), but not in men IA group (p > .05, Figure 3C). In addition, spearman correlation analysis indicated that GSTA4 mRNA expression was negatively correlated with DNA methylation levels in all individuals (r = −.746, p < .001, Figure 3D). In vitro experiments also demonstrated that DNA hypomethylation induced by AZA could increase GSTA4 mRNA expression in HBVSMCs (p < .01, Figure 3E).
FIGURE 3.
Significant association between GSTA4 mRNA expression and DNA methylation. (A) mRNA expression levels in the IA group were higher than that in controls. (B) mRNA expression levels in women IAs were higher than that in women controls. (C) No statistical significance was observed between men IAs and men controls. (D) GSTA4 mRNA expression was correlated with its DNA methylation levels in all participants. (E) DNA hypomethylation induced by AZA increased the mRNA expression of the GSTA4 gene in HBVSMCs. (F,G) GSTA4 methylation levels in between smoker and non-smoker group. (H) GSTA4 mRNA expression between smoker and non-smoker groups.
It is well known that cigarette smoking influences DNA methylation levels, contributing to the formation of IA. Therefore, we performed subgroup analysis stratified by smoker or non-smoker in all participants. No significant differences were found in GSTA4 methylation between smoker and non-smoker group (p > .05, Figure 3F). The GSTA4 methylation in non-smoker IAs were much lower than that in the smoker controls and non-smoker controls (p < .05), but no statistical differences were found in smoker IAs (p > .05, Figure 3G). A similar result was also shown in GSTA4 mRNA expression between smoker and non-smoker groups (p > .05, Figure 3H).
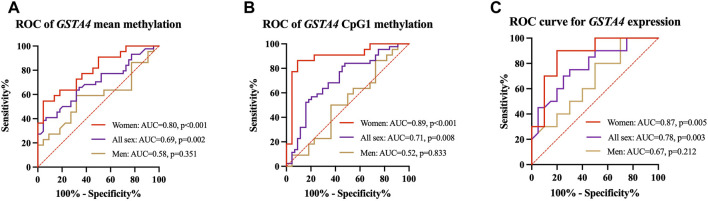
With subgroup comparisons, we discovered that decreased DNA methylation and increased mRNA expression of GSTA4 were associated with the risk of IA in women but not in men. The predictive power of GSTA4 methylation status on IA was further tested using ROC curve analysis. We found that GSTA4 mean methylation (AUC = .80, p < .001, Figure 4A) was a reliable predictor of women IA, of which CpG1 exhibited the best predictive value (AUC = .89, p < .001, Figure 4B). In addition, GSTA4 expression levels could also predict the risk of IA in women (AUC = .87, p = .005, Figure 4C).
FIGURE 4.
ROC curves for prediction of IAs in men and women. (A) GSTA4 mean methylation is a predictor of women IA. (B) GSTA4 CpG1 methylation is a predictor of women IA. (C) GSTA4 expression is a predictor of women IA.
Discussion
This case-control study investigated the association between GSTA4 methylation and expression and IA risk. We found that DNA methylation of GSTA4 negatively correlated with its mRNA expression. Patients exhibited decreased methylation and increased expression of GSTA4 compared to that in controls. GSTA4 methylation and expression are reliable predictors of IA in women.
GSTA4 is a phase 2 detoxifying enzyme that modulates cell proliferation and apoptosis by regulating lipid peroxidation. Previous studies have reported that decreased expression of GSTA4 is associated with positive outcomes in glioma models (Cheng et al., 2022), whereas increased expression of GSTA4 promotes the malignant progression of liver cancer (Liu et al., 2017). Notably, GSTA4 is involved in SMC proliferation (Luo et al., 2018) and may thus play a vital role in the pathological process of IA. As a member of the human α-class of GSTs, GSTA4 is the only one that has a gene promoter CpG island (Peng et al., 2008). Promoter DNA methylation usually downregulates mRNA levels and, consequently, protein expression of target genes (Moore et al., 2013). Our data also indicated that decreased GSTA4 promoter methylation is associated with increased GSTA4 mRNA expression.
The main finding of this study was that patients have lower GSTA4 methylation and higher GSTA4 expression than that in healthy controls. The pathological formation of IA is influenced by complex interactions between genetic and environmental risk factors such as congenital dysgenesis and acquired atherosclerosis of the cerebral arteries (Lawton et al., 2015; Zhong et al., 2021). A previous study reported that overexpressing GSTA4 can prevent neointima formation in carotid arteries and suppress SMC activation and proliferation (Xu et al., 2010; Luo et al., 2018). SMCs and the collagen fibers they secrete provide structural support to cerebral arteries. Disruption of the balance between SMC proliferation and apoptosis potentially contributes to IA formation (Frosen, 2014; Kataoka et al., 2020). This may partly explain why patients with IA tended to have lower GSTA4 methylation and higher GSTA4 expression. In addition, as an epigenetic modification, DNA methylation can be involved in disease progression and serve as a marker of environmental exposures, such as tobacco smoking, lipid peroxidation, and inflammation (Gao et al., 2019; Christiansen et al., 2021). Consistent with this hypothesis, DNA methylation of the GSTA4 promoter, which contains antioxidant response elements, may act as an adaptive mechanism through which they are transcriptionally activated during exposure to lipid peroxidation of the vascular wall in IA patients (Benes et al., 2013). Therefore, lower levels of GSTA4 methylation may serve as a biomarker of vascular impairment in patients with IA.
There are sex-based differences in morbidity, rupture risk, and prognosis in patients (Korja et al., 2014; Hu et al., 2019; Ogilvy et al., 2020). DNA methylation reportedly plays a vital role in sex-specific characteristics in the human brain (Xu et al., 2014; Xia et al., 2021). In addition, GSTA4 expression has sexually differential characteristics that are probably regulated by female hormones (Faustino et al., 2012; Tierbach et al., 2018). In this study, we observed that healthy women had higher GSTA4 methylation levels than healthy men. Notably, GSTA4 methylation and expression are reliable predictors of IA risk in women but not in men. Recent studies have reported that GSTA4 knockout results in more severe hearing loss in female mice than in male mice in a cisplatin toxicity experiment (Park et al., 2019). Collectively, these results suggest that interactions between estrogen and GSTA4 methylation may affect IA progression.
Our study had certain limitations. First, GSTA4 methylation levels in UIA patients were slightly lower than those in RIA patients. However, this was not a statistically significant difference, which may be attributed to the relatively small sample size. Second, our results showed that GSTA4 methylation was not significantly associated with smoking, which may be due to the fact that too few smoking patients were included in the analysis. Therefore, external validation trials with larger cohorts are essential. Third, in vivo animal experiments are needed to verify the effects of GSTA4 overexpression or suppression on the SMC phenotypic transition and IA formation. In future studies, we will further supplement this finding.
In conclusion, we established that IA patients had lower levels of GSTA4 promoter methylation and higher GSTA4 expression than controls. GSTA4 methylation and mRNA expression may be reliable predictive markers of the occurrence of women IA.
Funding Statement
This study was supported by the grants from the Zhejiang Provincial Natural Science Foundation of China (LY22H090001), Medicine and health science and technology projects of Zhejiang province (2022KY305, 2022KY322), Ningbo medical and health brand discipline (PPXK 2018-04) and Ningbo Science and Technology Innovation 2025 Major Project (2022Z134), Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province (2022E10026).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants and all study protocols were reviewed and approved by the Ethics Committee of Ningbo First Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JS, XG, and YH contributed to the conception and design of the study. TX, XY, SZ, YWW, YFW, SN, and CZ organized the database and experiments. XY and SW performed the statistical analysis. TX and YH wrote the first draft of the manuscript. YH carried out the review and revision of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Bakker M. K., Ruigrok Y. M. (2021). Genetics of intracranial aneurysms. Stroke 52, 3004–3012. 10.1161/STROKEAHA.120.032621 [DOI] [PubMed] [Google Scholar]
- Bakker M. K., van der Spek R., van Rheenen W., Morel S., Bourcier R., Hostettler I. C., et al. (2020). Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat. Genet. 52, 1303–1313. 10.1038/s41588-020-00725-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes H., Vuong M. K., Boerma M., McElhanon K. E., Siegel E. R., Singh S. P. (2013). Protection from oxidative and electrophilic stress in the gsta4-null mouse heart. Cardiovasc Toxicol. 13, 347–356. 10.1007/s12012-013-9215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstrom K. E., Zhu K., Ewing E., Krabbendam I. E., Harris R. A., Falcao A. M., et al. (2020). Gsta4 controls apoptosis of differentiating adult oligodendrocytes during homeostasis and remyelination via the mitochondria-associated fas-casp8-bid-axis. Nat. Commun. 11, 4071. 10.1038/s41467-020-17871-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Liu J., Zhang Y., Tian Z., Wang K., Zhang Y., et al. (2018). China intracranial aneurysm project (ciap): Protocol for a registry study on a multidimensional prediction model for rupture risk of unruptured intracranial aneurysms. J. Transl. Med. 16, 263. 10.1186/s12967-018-1641-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B., Hong X., Wang L., Cao Y., Qin D., Zhou H., et al. (2022). Curzerene suppresses progression of human glioblastoma through inhibition of glutathione s‐transferase a4. Cns Neurosci. Ther. 28, 690–702. 10.1111/cns.13800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen C., Castillo-Fernandez J. E., Domingo-Relloso A., Zhao W., El-Sayed M. J., Tsai P. C., et al. (2021). Novel dna methylation signatures of tobacco smoking with trans-ethnic effects. Clin. Epigenetics 13, 36. 10.1186/s13148-021-01018-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etminan N., Dorfler A., Steinmetz H. (2020). Unruptured intracranial aneurysms-pathogenesis and individualized management. Dtsch. Arztebl Int. 117, 235–242. 10.3238/arztebl.2020.0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etminan N., Rinkel G. J. (2016). Unruptured intracranial aneurysms: Development, rupture and preventive management. Nat. Rev. Neurol. 12, 699–713. 10.1038/nrneurol.2016.150 [DOI] [PubMed] [Google Scholar]
- Faustino L. C., Almeida N. A., Pereira G. F., Ramos R. G., Soares R. M., Morales M. M., et al. (2012). Thyroid hormone and estradiol have overlapping effects on kidney glutathione s-transferase-alpha gene expression. Am. J. Physiol. Endocrinol. Metab. 303, E787–E797. 10.1152/ajpendo.00223.2012 [DOI] [PubMed] [Google Scholar]
- Frosen J. (2014). Smooth muscle cells and the formation, degeneration, and rupture of saccular intracranial aneurysm wall--a review of current pathophysiological knowledge. Transl. Stroke Res. 5, 347–356. 10.1007/s12975-014-0340-3 [DOI] [PubMed] [Google Scholar]
- Gao X., Zhang Y., Burwinkel B., Xuan Y., Holleczek B., Brenner H., et al. (2019). The associations of dna methylation alterations in oxidative stress-related genes with cancer incidence and mortality outcomes: A population-based cohort study. Clin. Epigenetics 11, 14. 10.1186/s13148-018-0604-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Yu N., Li Y., Hao Z., Liu Z., Li M. H. (2019). A meta-analysis of risk factors for the formation of de novo intracranial aneurysms. Neurosurgery 85, 454–465. 10.1093/neuros/nyy332 [DOI] [PubMed] [Google Scholar]
- Jeong S. J., Park J. G., Oh G. T. (2021). Peroxiredoxins as potential targets for cardiovascular disease. Antioxidants (Basel) 10, 1244. 10.3390/antiox10081244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D., Song C., Leng X., Han P. (2019). A systematic review and meta-analysis of risk factors for unruptured intracranial aneurysm growth. Int. J. Surg. 69, 68–76. 10.1016/j.ijsu.2019.07.023 [DOI] [PubMed] [Google Scholar]
- Kataoka H., Yagi T., Ikedo T., Imai H., Kawamura K., Yoshida K., et al. (2020). Hemodynamic and histopathological changes in the early phase of the development of an intracranial aneurysm. Neurol. Med. Chir. (Tokyo) 60, 319–328. 10.2176/nmc.st.2020-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korja M., Kaprio J. (2016). Controversies in epidemiology of intracranial aneurysms and sah. Nat. Rev. Neurol. 12, 50–55. 10.1038/nrneurol.2015.228 [DOI] [PubMed] [Google Scholar]
- Korja M., Lehto H., Juvela S. (2014). Lifelong rupture risk of intracranial aneurysms depends on risk factors: A prospective Finnish cohort study. Stroke 45, 1958–1963. 10.1161/STROKEAHA.114.005318 [DOI] [PubMed] [Google Scholar]
- Laborde E. (2010). Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ. 17, 1373–1380. 10.1038/cdd.2010.80 [DOI] [PubMed] [Google Scholar]
- Law P. P., Holland M. L. (2019). Dna methylation at the crossroads of gene and environment interactions. Essays Biochem. 63, 717–726. 10.1042/EBC20190031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. T., Rutledge W. C., Kim H., Stapf C., Whitehead K. J., Li D. Y., et al. (2015). Brain arteriovenous malformations. Nat. Rev. Dis. Prim. 1, 15008. 10.1038/nrdp.2015.8 [DOI] [PubMed] [Google Scholar]
- Liu C. J., Yang J. H., Huang F. Z., Nie W. P., Liu C. P., Mao X. H., et al. (2017). Glutathione-s-transferase a 4 (gsta4) suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting akt pathway. Am. J. Transl. Res. 9, 301–315. [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wu X., Nie S., Zhou S., Xiao S., Gao X., et al. (2020). Methylation of phospholipase a2 group vii gene is associated with brain arteriovenous malformations in han Chinese populations. J. Mol. Neurosci. 70, 1056–1063. 10.1007/s12031-020-01508-9 [DOI] [PubMed] [Google Scholar]
- Luo J., Chen G., Liang M., Xie A., Li Q., Guo Q., et al. (2018). Reduced expression of glutathione s-transferase alpha 4 promotes vascular neointimal hyperplasia in ckd. J. Am. Soc. Nephrol. 29, 505–517. 10.1681/ASN.2017030290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L., Feldman L. (2010). A rapid trizol-based two-step method for dna-free rna extraction from arabidopsis siliques and dry seeds. Biotechnol. J. 5, 183–186. 10.1002/biot.200900211 [DOI] [PubMed] [Google Scholar]
- Moore L. D., Le T., Fan G. (2013). Dna methylation and its basic function. Neuropsychopharmacol 38, 23–38. 10.1038/npp.2012.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvy C. S., Gomez-Paz S., Kicielinski K. P., Salem M. M., Maragkos G. A., Lee M., et al. (2020). Women with first-hand tobacco smoke exposure have a higher likelihood of having an unruptured intracranial aneurysm than nonsmokers: A nested case-control study. Neurosurgery 87, 1191–1198. 10.1093/neuros/nyaa227 [DOI] [PubMed] [Google Scholar]
- Palou-Marquez G., Subirana I., Nonell L., Fernandez-Sanles A., Elosua R. (2021). Dna methylation and gene expression integration in cardiovascular disease. Clin. Epigenetics 13, 75. 10.1186/s13148-021-01064-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Kim M., Rothenberger C., Kumar A., Sampson E. M., Ding D., et al. (2019). Gsta4 mediates reduction of cisplatin ototoxicity in female mice. Nat. Commun. 10, 4150. 10.1038/s41467-019-12073-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D. F., Razvi M., Chen H., Washington K., Roessner A., Schneider-Stock R., et al. (2008). Dna hypermethylation regulates the expression of members of the mu-class glutathione s-transferases and glutathione peroxidases in barrett's adenocarcinoma. Gut 58, 5–15. 10.1136/gut.2007.146290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Torres I., Soto M. E., Manzano-Pech L., Diaz-Diaz E., Soria-Castro E., Rubio-Ruiz M. E., et al. (2022). Oxidative stress in plasma from patients with marfan syndrome is modulated by deodorized garlic preliminary findings. Oxid. Med. Cell Longev. 2022, 5492127. 10.1155/2022/5492127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierbach A., Groh K. J., Schonenberger R., Schirmer K., Suter M. J. (2018). Glutathione s-transferase protein expression in different life stages of zebrafish (danio rerio). Toxicol. Sci. 162, 702–712. 10.1093/toxsci/kfx293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhou S., Zhao J., Nie S., Sun J., Gao X., et al. (2021). Tobacco smoking increases methylation of polypyrimidine tract binding protein 1 promoter in intracranial aneurysms. Front. Aging Neurosci. 13, 688179. 10.3389/fnagi.2021.688179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Dai R., Wang K., Jiao C., Zhang C., Xu Y., et al. (2021). Sex-differential dna methylation and associated regulation networks in human brain implicated in the sex-biased risks of psychiatric disorders. Mol. Psychiatr. 26, 835–848. 10.1038/s41380-019-0416-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Wang F., Liu Y., Yu Y., Gelernter J., Zhang H. (2014). Sex-biased methylome and transcriptome in human prefrontal cortex. Hum. Mol. Genet. 23, 1260–1270. 10.1093/hmg/ddt516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Gong B., Yang Y., Awasthi Y. C., Boor P. J. (2010). Adenovirus-mediated overexpression of glutathione-s-transferase mitigates transplant arteriosclerosis in rabbit carotid allografts. Transplantation 89, 409–416. 10.1097/TP.0b013e3181c69838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Wang J., Wang S., Zhang D., Zhao Y., Wang R., et al. (2017). Dna methylation regulates gene expression in intracranial aneurysms. World Neurosurg. 105, 28–36. 10.1016/j.wneu.2017.04.064 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang L., Meng L., Cao G. K., Zhao Y. L., Wu Y. (2018). Expression changes of autophagy-related proteins in aki patients treated with crrt and their effects on prognosis of adult and elderly patients. Immun. Ageing 15, 23. 10.1186/s12979-018-0128-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W., Su W., Li T., Tan X., Chen C., Wang Q., et al. (2021). Aneurysm wall enhancement in unruptured intracranial aneurysms: A histopathological evaluation. J. Am. Heart Assoc. 10, e018633. 10.1161/JAHA.120.018633 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.