Abstract
Objective: Dysphagia rehabilitation is an important area in geriatric nutrition due to the commonality of sarcopenic dysphagia in older adults. However, there have been no reports on the efficacy of treatment by board-certified physiatrists (BCP) in patients with sarcopenic dysphagia. This study therefore aimed to investigate whether intervention by board-certified physiatrists affects the functional prognosis of patients with sarcopenic dysphagia.
Materials and Methods: Of 467 patients enrolled in the Japanese Sarcopenic Dysphagia database between November 2019 and March 2021, 433 met the study eligibility criteria. The patients were divided into two groups based on whether or not they received intervention by a BCP. The clinical characteristics and outcomes of the two groups were compared. Statistical analyses were performed by inverse probability weighting (IPW).
Results: BCPs were involved in the management of 57.0% of patients with sarcopenic dysphagia. These patients had a significantly higher increase in the Barthel index both before and after IPW correction than those not managed by a BCP (P=0.001 and P=0.016, respectively). However, sarcopenic dysphagia significantly improved in the non-BCP group before IPW correction (P<0.001), although there was no significant difference after IPW correction (P=0.301).
Conclusion: BCP management was significantly associated with higher activities of daily living (ADL), but not with an improvement in sarcopenic dysphagia. To provide and manage effective rehabilitation, it is necessary to familiarize patients with the management and training of sarcopenic dysphagia rehabilitation for BCP in order to cope in regions with few rehabilitation units.
Keywords: board-certified physiatrists, dysphagia, functional prognosis
Introduction
Dysphagia rehabilitation is an important area of geriatric nutrition due to the commonality of dysphagia in older people. Dysphagia has many causes, including organic diseases such as laryngeal cancer, inflammation and sarcopenia1, 2), and functional causes such as cerebrovascular diseases, neuromuscular diseases (e.g., Parkinson’s disease3, 4)), and psychogenic diseases such as depression and anorexia5). Dysphagia is a common cause of aspiration pneumonia in frail elderly individuals in Japan6,7,8). This condition can be diagnosed by either observation or screening involving instrumental swallowing examinations, such as videofluoroscopy and video-endoscopy, the latter of which is further performed to determine the severity of functional abnormalities and treatment prognosis9, 10). The effectiveness of rehabilitation for dysphagia with direct and indirect therapies is generally observed sporadically with and without food, respectively. Swallowing rehabilitation has been shown to be effective and can be used in conjunction with compensatory techniques11, 12).
Rehabilitation for sarcopenic dysphagia should involve treatments for both dysphagia and sarcopenia. Therefore, rehabilitation for sarcopenic dysphagia should be performed by an interdisciplinary team. Within this team, the involvement of a board-certified physiatrist (BCP) led to improvements in some rehabilitation outcomes, although the effect of their involvement in patients with dysphagia has not yet been evaluated. BCPs are certified by a governance board after verification of their knowledge and skills in rehabilitation medicine. In Japan, BCP is one of the basic domain specialties established by the Japanese Association of Rehabilitation Medicine. To be certified as a BCP, the doctor needs to have completed training over three years, given two presentations at the society congress, be in charge of rehabilitation of 100 cases, and have prepared 30 case reports. They also needed to pass the examination conducted by the Japanese Association of Rehabilitation Medicine13). The role of the BCP is to improve functional prognosis, plan rehabilitation programs, assess risks, and set goals for therapists. BCP involvement increases the efficiency of a team approach, and has the benefits of improving the quality of clinical practice, ensuring the appropriate management of comorbidities, safe management of rehabilitation, and better functional outcomes.
Kinoshita et al.14) and Momosaki et al.15) previously analyzed the Japan Rehabilitation Database and showed that BCP involvement was an effective management strategy. The involvement of a BCP led to an improvement in activities of daily living (ADL), shortened the hospitalization period, and improved the rate of discharge of patients to their homes. A study using data from the traumatic brain injury model system in the United States further reported that the ADL of patients with brain trauma was improved by the involvement of a BCP16). Kato et al. evaluated the association between the number of BCP and the volume of rehabilitation, and identified regional disparities in the number of BCPs, as well as a correlation between the number of BCPs and the provision of rehabilitation units17). However, as yet there have been no reports on the efficacy of BCP in patients with sarcopenic dysphagia. Therefore, this study aimed to investigate the association between BCP involvement and rehabilitation outcomes, such as ADL and swallowing function, in patients with sarcopenic dysphagia.
Material and Methods
Study design and participants
This retrospective, observational cohort study analyzed the Japanese Sarcopenic Dysphagia Database (JSDD) constructed by the Rehabilitation Nutrition Database Committee of the Japanese Association of Rehabilitation Nutrition and the Japanese Working Group on Sarcopenic Dysphagia18). The data were registered between November 2019 and March 2021. Sarcopenic dysphagia patients aged 20 years and older with available data on the Food Intake Level Scale (FILS) were included in the study. FILS is a method used to evaluate dysphagia with the patients’ level of food intake observed and divided into the following 10 categories: levels 1–3, no oral intake; levels 4–6, oral intake and alternative nutrition; levels 7–9, only by ingestion; and level 10, normal19). Patients with levels <8 were included in the study database. The inclusion criteria were registration in the JSDD. The only exclusion criterion was loss of follow-up data due to death or other reasons.
All potential care settings were included in the study, including acute care, rehabilitation or long-term care hospitals, other facilities, and home-visit rehabilitation. The database included data on basic patient information, ADL assessment, evaluation of dysphagia, information on facilities, and whether a BPC was involved in patient management. The database was constructed by the Research Electronic Date Capture (RED cap) using free electronic data capture20). The patients were divided into two groups (BCP and non-BCP) according to BCP involvement. Informed consent was obtained using a consent/explanation document approved by the institutional ethics committee, and after confirming that the patient fully understood the contents, consent to participate was obtained in writing. The ethics committee of Nihon University Hospital approved this study (approval number: 20190907).
Measurements
Variables and outcomes
The following information was extracted from the registry: age, sex, comorbidity severity (Charlson comorbidity index [CCI]), nutritional status (Global Leadership Initiative on Malnutrition [GLIM] criteria), FILS, Barthel index (BI), physical findings (body mass index [BMI], hand grip strength, and calf circumference), cause of dysphagia, dwelling (acute care hospital, rehabilitation hospital, long-term care hospital, others), and staff involvement (physical therapist, occupational therapist, speech-language pathologist, pharmacist, nurse, registered dietitian, dental hygienist, dentist, or physician other than a BCP).
Comorbidities were assessed using the CCI21), an indicator of the presence of multiple comorbidities, including diabetes with chronic complications, heart failure, kidney disease, liver disease, chronic lung disease, dementia, hemiplegia or paraplegia, malignancy, and acquired immunodeficiency syndrome/human immunodeficiency virus infection. This index classifies patients according to the presence of one, two, three, four, five, or ≥ five comorbidities. The GLIM is a diagnostic criterion for undernutrition constructed in 2018 by representatives of four major international academic societies on nutrition22). The GLIM assesses phenotypic criteria, including body weight change, thinness (low BMI), and reduced muscle mass, as well as etiologic criteria, including poor nutritional intake and disease burden. The causes of disease were classified as cancer, dementia, Parkinson’s disease, cerebral hemorrhage, subarachnoid hemorrhage, stroke, and sarcopenic dysphagia.
Sarcopenic dysphagia was diagnosed using a reliable and validated diagnostic algorithm that divided patients into three categories: probable, possible, or no sarcopenic dysphagia23). To assess improvements in dysphagia, Shimizu et al. used an increase in FILS of ≥2 points to indicate that swallowing function had improved24). The current study also defined improved swallowing function as an increase in FILS of ≥ two points. The ability to perform ADL was evaluated using the BI, which comprises 10 items with the sum of their scores, producing a possible total of 100 points. These ten items were eating, moving, grooming, toilet use, bathing, walking, going upstairs and downstairs, changing clothes, bowel control, and bladder control. An increase in BI was defined as the difference in total BI between admission and discharge25). The primary outcome of the study was an increase in BI, and the secondary outcome was the FILS score at the end of follow-up.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 23 (IBM Corporation, Armonk, NY, USA). Normally distributed variables are expressed as the mean ± standard deviation (SD), and variables with a skewed distribution are expressed as the median and interquartile range. Inverse probability weighting (IPW) was performed to minimize the effect of selection bias26). Propensity scores were estimated by logistic regression analysis using the baseline covariates of age, sex, BMI, FILS on admission, CCI, dwelling, BI at admission, number of drugs, cause of dysphagia, staff involvement, and BI on admission. IPW uses propensity scores to calculate the weighting of data. The effect of BCP involvement on the outcomes was analyzed using a generalized linear model after adjusting for the IPW propensity score. A BCP-group and non-BCP groups with no differences in baseline covariates in IPW were generated. The two groups were subsequently compared using Student’s t-test and χ2-test. The BCP group was weighted by the inverse of the propensity score, whereas the non-BCP group was weighted by the inverse of 1 minus the propensity score. All patients were evaluated with or without IPW adjustment of data. Generalized estimating equations (GEE) were used to estimate the propensity scores to compensate for differences between institutions27). Statistical significance was set at P<0.05.
Results
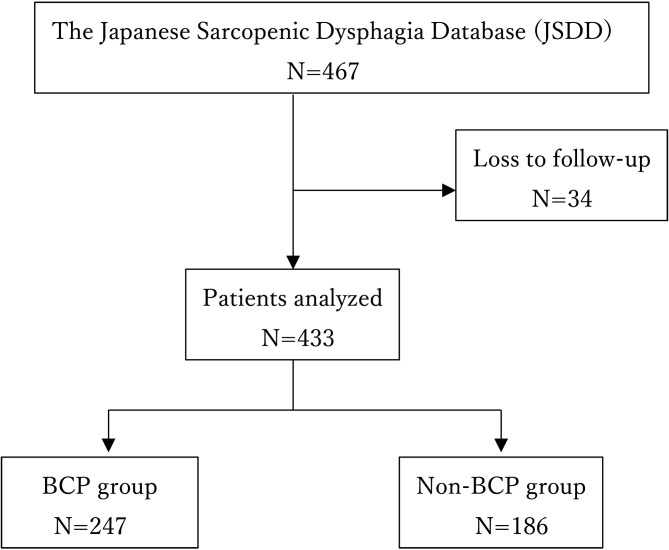
Of the 467 patients in the JSDD group, 433 met the eligibility criteria (Fig. 1). Table 1 shows the demographic and clinical characteristics of the eligible subjects and the data adjusted by IPW. Of the 433 remaining patients, 247 (52.9%) were managed using BCP. The mean age of the patients was >80 years, and 48.7 were female. At admission, the median BI was 25, and the median FILS score was 7. The unadjusted results showed that the FILS score was significantly higher in the BCP group (P<0.001), and the χ2 test showed significant differences (P<0.001) between the two groups in terms of dwelling and CCI. After adjustment of the data using IPW, the demographic and clinical characteristics were matched between the BCP and non-BCP groups.
Figure 1.
Flowchart of the study participant selection process.
BCP: board-certified physiatrists.
Table 1. Demographic and clinical characteristics of all the subjects and data adjusted by IPW.
| Total (n=433) | All subjects | Data adjusted by IPW | ||||||
|---|---|---|---|---|---|---|---|---|
| BCP (n=247) | Non-BCP (n=186) | P-value | BCP (n=247) | Non-BCP (n=186) | P-value | |||
| Mean age ± SD, y | 80.3 ± 11.3 | 80.3 ± 11.1 | 80.4 ± 11.7 | 0.933 a | 81.8 ± 11.9 | 80.7 ± 8.1 | 0.701a | |
| Sex, female, (%) | 48.7 | 44.5 | 54.3 | 0.044 b | 52.7 | 47.3 | 0.499b | |
| BMI ± SD, (kg/m2) | 20.3 ± 3.8 | 20.3 ± 3.7 | 20.3 ± 3.9 | 0.917 a | 20.3 ± 3.3 | 19.1 ± 2.7 | 0.079a | |
| CCI, (%) | <0.001 b | 0.147b | ||||||
| 0 | 25.6 | 32.0 | 17.2 | 20.1 | 12.4 | |||
| 1 | 7.2 | 8.5 | 5.4 | 4.6 | 2.4 | |||
| 2 | 29.3 | 32.4 | 25.3 | 36.2 | 10.1 | |||
| 3 | 12.5 | 8.5 | 17.7 | 5.9 | 9.5 | |||
| >4 | 25.4 | 18.6 | 34.4 | 33.1 | 65.6 | |||
| Nutrition status | ||||||||
| GLIM malnutrition, (%) | 64 | 63.2 | 65.1 | 0.906 a | 68.3 | 84.5 | 0.199 a | |
| Number of drugs on admission, [IQR] | 6 [4,8] | 6 [4,9] | 6 [3,8] | 0.022 a | 6 [4,8] | 5 [3,8] | 0.880 a | |
| Hand grip strength, kg | 13.3 ± 9.3 | 13.6 ± 9.2 | 12.8 ± 9.4 | 0.363 a | 12.4 ± 9.2 | 11.6 ± 6.4 | 0.540 a | |
| CC, cm, | 28.4 ± 4.0 | 28.7 ± 4.1 | 28.0 ± 4.0 | 0.076 a | 28.0 ± 4.1 | 28.3 ± 2.6 | 0.693 a | |
| Sarcopenia, (%) | 87.1 | 87.4 | 86.6 | 0.786 b | 84.1 | 94.5 | 0.118 b | |
| Cause of dysphagia, (%) | 0.001 b | 0.275 b | ||||||
| Cancer | 1.6 | 0.4 | 3.2 | 5.7 | 1.0 | |||
| Dementia | 2.8 | 2.8 | 2.7 | 2.7 | 1.2 | |||
| Parkinson’s disease | 3.2 | 4.0 | 2.2 | 4.1 | 2.7 | |||
| Cerebral hemorrhage | 4.4 | 5.7 | 2.7 | 3.3 | 0.7 | |||
| Subarachnoid hemorrhage | 2.1 | 2.8 | 1.1 | 1.7 | 0.3 | |||
| Cerebral infarction | 16.9 | 17.0 | 16.7 | 11.7 | 6.4 | |||
| Sarcopenic dysphagia | 61.4 | 64.0 | 58.1 | 69.4 | 83.1 | |||
| Others | 7.6 | 3.2 | 13.4 | 2.3 | 6.4 | |||
| Barthel index on admission, [IQR] | 25 [25,50] | 30 [10,50] | 20 [5,50] | 0.605 a | 15 [0,45] | 20 [5,50] | 0.051 a | |
| FILS score on admission, [IQR] | 7 [3,8] | 7 [7,8] | 6 [1,7] | <0.001 a | 7 [3,7] | 7 [4,8] | 0.201 a | |
| Dwelling, (%) | <0.001 b | 0.816 b | ||||||
| Acute care hospital | 43.0 | 21.9 | 71.0 | 41.3 | 33.1 | |||
| Rehabilitation hospital | 46.9 | 77.7 | 5.9 | 58.5 | 3.7 | |||
| Long term care hospital | 9.5 | 0 | 22.0 | 0 | 10.8 | |||
| Others | 0.7 | 0.4 | 1.6 | 0.2 | 52.4 | |||
IPW: inverse probability weighting; BCP: board-certificated physiatrists; SD: standard deviation; BMI: body mass index; CCI: Charlson comorbidity index; GLIM: global leadership initiative on malnutrition; IQR: interquartile range; CC: calf circumference, FILS: food intake level scale. a: *Definition?*, b: *Definition?*.
The percentage of staff involvement in the BCP and non-BCP groups is shown in Table 2. In the unadjusted results, the involvement of speech-language pathologists, registered dietitians, and physicians other than a BCP was significantly higher in the non-BCP group (P<0.001, P=0.002, and P<0.001, respectively), while the involvement of pharmacists, dentists, and occlusion therapists was significantly higher in the BCP group (P<0.001, P<0.001, and P=0.012, respectively). The percentage of staff involvement between the two groups was adjusted after the IPW.
Table 2. Percentage of staff involvement in the BCP and non-BCP groups.
| Total (n=433) | All subjects | Date adjusted by IPW | ||||||
|---|---|---|---|---|---|---|---|---|
| BCP (n=247) | Non-BCP (n=186) | P-value | BCP (n=247) | Non-BCP (n=186) | P-value | |||
| Staff involvement, (%) | ||||||||
| Physical therapist | 97.5 | 98.0 | 96.8 | 0.313 a | 98.2 | 96.5 | 0.238 a | |
| Occupational therapist | 75.5 | 79.8 | 69.9 | 0.012 a | 59.1 | 33.7 | 0.268 a | |
| Speech-language pathologist | 70.4 | 62.3 | 81.2 | <0.001 a | 77.1 | 89.2 | 0.191 a | |
| Pharmacist | 82.4 | 89.9 | 72.6 | <0.001 a | 87.0 | 34.3 | 0.176 a | |
| Nurse | 97.0 | 96.4 | 97.8 | 0.411 a | 92.5 | 98.0 | 0.220 a | |
| Registered dietitian | 91.2 | 87.4 | 96.2 | 0.002 a | 87.0 | 43.6 | 0.050 a | |
| Dental hygienist | 56.6 | 56.7 | 56.5 | 0.520 a | 44.9 | 25.3 | 0.301 a | |
| Dentist | 53.8 | 61.1 | 44.1 | <0.001 a | 37.4 | 22.2 | 0.338 a | |
| Physicians other than BCP | 85.7 | 75.7 | 98.9 | <0.001 a | 88.1 | 46.0 | 0.062 a | |
BCP: board-certificated physiatrists; IPW: inverse probability weighting. aUnpaired t-test.
The outcomes of the BCP and non-BCP groups are presented in Table 3. The median increase in BI was higher in the BCP group before (P=0.001) and after (P=0.016) IPW adjustment. At the end of the follow-up, the median FILS of the unadjusted data was significantly higher in the BCP group (P=0.001). However, the median FILS showed no significant difference between the BCP and non-BCP groups after adjusting for IPW (P=0.791).
Table 3. Outcomes in the BCP and non-BCP groups.
| BCP (n=247) | Non-BCP (n=186) | P-value | |||
|---|---|---|---|---|---|
| Unadjusted data (n=433) | |||||
| Barthel index score, [IQR] | |||||
| At the end of follow-up | 55 [30,80] | 45 [10,70] | 0.026 a | ||
| Barthel index increase | 20 [5,35] | 5 [0,25] | 0.001 a | ||
| FILS score, [IQR] | |||||
| At the end of follow-up | 8 [7,8] | 7 [6,8] | 0.001 a | ||
| Improvement in FILS ≥2 points, (%) | 28.7 | 46.8 | <0.001 a | ||
| Data adjusted by IPW (n=433) | |||||
| Barthel index score, [IQR] | |||||
| At the end of follow-up | 35 [20,80] | 35 [20,80] | 0.562 a | ||
| Barthel index increase | 20 [5,25] | 20 [0,25] | 0.016 a | ||
| FILS score, [IQR] | |||||
| At the end of follow-up | 7 [6,8] | 6 [7,8] | 0.791 a | ||
| Improvement in FILS ≥2 points, (%) | 41.3 | 33.7 | 0.301 a | ||
BCP: board-certificated physiatrists; IQR: interquartile range; FILS: food intake level scale; IPW: inverse probability weighting. aUnpaired t-test.
Discussion
The three major findings of this study confirmed the efficacy of BCP in patients with sarcopenic dysphagia. First, the improvement in ADL was significantly higher when BCP was involved in the management of such patients; second, there was no difference in the improvement of swallowing function between patients with and without BCP involvement; third, 43% of patients with sarcopenic dysphagia were managed without involvement of a BCP. To the best of our knowledge, this is the first study to demonstrate a relationship between BCP involvement and functional prognosis in patients with dysphagia.
There is evidence to suggest that ADLs in patients with dysphagia can be significantly improved by BCP. Greiss et al. described the effectiveness of BCP involvement using data from 148 cases in a traumatic brain injury model system and suggested that early involvement of BCP was effective at improving ADL16). Similarly, Kinoshita et al. verified the effectiveness of BCP involvement in acute stroke patients and found that it shortened the length of hospital stay and improved ADL14). In our study, several different institutions were included, with different rehabilitation outcomes and clustering observed between the institutions. Following adjustment of the data using IPW propensity scores calculated using generalized estimating equations, ADL in the BCP group was found to be significantly improved. The reason for this improvement was that BCP involvement increased the quality of rehabilitation and risk assessment, while appropriate and high rehabilitation goal setting and a team approach led to improvements in ADL. Momosaki et al. previously described how the involvement of a BCP improved ADL, and suggested that an efficient team approach, safety management, and management of comorbidities maintained good physical conditions and rehabilitation15). The same reason may explain why the involvement and characteristics of the BCPs in the current study may have influenced ADL improvement in patients with dysphagia.
We found no difference in the improvement in swallowing function between patients with and without BCP involvement. Sura et al. emphasized the importance of team events in swallowing management and stated that the involvement of many professionals may contribute to the management of dysphagia symptoms in a given patient28). Yoshimura et al. previously reported the prevalence of sarcopenia and its association with ADL and dysphagia and showed that ADL and dysphagia were independently associated with sarcopenia29). It has also been suggested that sarcopenia with disabilities should be assessed in all patients in rehabilitation settings30). In our study, sarcopenic dysphagia was observed in 61.4% of patients before the data were adjusted using IPW. Interdisciplinary rehabilitation nutrition aimed at improving malnutrition, sarcopenia, and function may all be useful in treating sarcopenic dysphagia31). Therefore, BCPs should consider the diagnosis and treatment of sarcopenia and implement rehabilitation nutrition during their daily clinical practice.
Our study showed that a BCP was not involved in the management of 43% of patients with sarcopenic dysphagia. It is estimated that approximately 4,000 BCPs are required in Japan; however, the current number remains only around 2,00032). Kato et al. previously referred to the regional disparities in the number of BCPs in Japan. The level of rehabilitation provided correlates with the number of BCPs, which indicates that more BCP need to be trained17). In addition, there are insufficient numbers of BCPs and educational opportunities for this clinical discipline. Martin et al. previously stated that a significant number of problems warranting critical self-analysis of all aspects of this educational endeavor existed, and increased productivity was required because of the shortage of BCPs in this specialty33). These results suggest that BCPs should be trained in the management of rehabilitation nutrition for sarcopenic dysphagia, by providing appropriate diagnosis, goal setting, and interventions.
Overall, the results of this study suggest that it is necessary to familiarize BCPs with the management and training of dysphagia rehabilitation to allow them to cope in regions with few rehabilitation units.
This study has several limitations which should be mentioned. First, it is unclear how BCPs performed dysphagia rehabilitation and ADL improvement at different hospitals. Second, we did not evaluate any rehabilitation program for patients with sarcopenic dysphagia. Further detailed studies are required to assess the effectiveness of BCP involvement and rehabilitation programs.
Conclusion
The improvement in ADL in patients with sarcopenic dysphagia was significantly higher when a BCP was involved in their management. However, we found no difference in the improvement of swallowing function between patients with and without BCP involvement. BCPs were not involved in 43% of patients with sarcopenic dysphagia. Engaging in rehabilitation nutrition with the aim of improving malnutrition, sarcopenia symptoms, and function using a team approach including a BCP may improve the swallowing function in patients with sarcopenic dysphagia.
Ethics
This study was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its amendments and was approved by the ethics committee of Nihon University Hospital (approval number: 20190907).
Conflict of interest
Hidetaka Wakabayashi was funded by a grant from the Japan Society for the Promotion of Science (grant number19H03979). Takako Nagai, Shinta Nishioka, and Ryo Momosaki have no conflicts of interest to disclose.
Acknowledgments
We would like to acknowledge the Rehabilitation Nutrition Database Committee of the Japanese Association of Rehabilitation Nutrition and the Japanese Working Group on Sarcopenic Dysphagia, which constructed the Japanese sarcopenic dysphagia database used in this study.
References
- 1.King SN, Dunlap NE, Tennant PA, et al. Pathophysiology of radiation-induced dysphagia in head and neck cancer. Dysphagia 2016; 31: 339–351. doi: 10.1007/s00455-016-9710-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huh G, Ahn SH, Suk JG, et al. Severe late dysphagia after multimodal treatment of stage III/IV laryngeal and hypopharyngeal cancer. Jpn J Clin Oncol 2020; 50: 185–192. doi: 10.1093/jjco/hyz158 [DOI] [PubMed] [Google Scholar]
- 3.Umemoto G, Furuya H. Management of dysphagia in patients with Parkinson’s disease and related disorders. Intern Med 2020; 59: 7–14. doi: 10.2169/internalmedicine.2373-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Hooren MR, Baijens LW, Voskuilen S, et al. Treatment effects for dysphagia in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord 2014; 20: 800–807. doi: 10.1016/j.parkreldis.2014.03.026 [DOI] [PubMed] [Google Scholar]
- 5.Gao J, Zhang HJ. Effects of chin tuck against resistance exercise versus Shaker exercise on dysphagia and psychological state after cerebral infarction. Eur J Phys Rehabil Med 2017; 53: 426–432. doi: 10.23736/S1973-9087.16.04346-X [DOI] [PubMed] [Google Scholar]
- 6.Takizawa C, Gemmell E, Kenworthy J, et al. A systematic review of the prevalence of oropharyngeal dysphagia in stroke, Parkinson’s disease, Alzheimer’s disease, head injury, and pneumonia. Dysphagia 2016; 31: 434–441. doi: 10.1007/s00455-016-9695-9 [DOI] [PubMed] [Google Scholar]
- 7.Ebihara S, Sekiya H, Miyagi M, et al. Dysphagia, dystussia, and aspiration pneumonia in elderly people. J Thorac Dis 2016; 8: 632–639. doi: 10.21037/jtd.2016.02.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Momosaki R. Rehabilitative management for aspiration pneumonia in elderly patients. J Gen Fam Med 2017; 18: 12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Re GL, Vernuccio F, Di Vittorio ML, et al. Swallowing evaluation with videofluoroscopy in the paediatric population. Acta Otorhinolaryngol Ital 2019; 39: 279–288. doi: 10.14639/0392-100X-1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pirana S, Oliveira M, Pissini F, et al. Swallowing in patients with mental disability—analysis of 189 swallowing video endoscopies. Int Arch Otorhinolaryngol 2019; 23: 25–30. doi: 10.1055/s-0038-1660775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balou M, Herzberg EG, Kamelhar D, et al. An intensive swallowing exercise protocol for improving swallowing physiology in older adults with radiographically confirmed dysphagia. Clin Interv Aging 2019; 14: 283–288. doi: 10.2147/CIA.S194723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakabayashi H, Matsushima M, Momosaki R, et al. The effects of resistance training of swallowing muscles on dysphagia in older people: A cluster, randomized, controlled trial. Nutrition 2018; 48: 111–116. doi: 10.1016/j.nut.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 13.Izumi S, Saitoh E. A brief history and international perspective of the Japanese Association of Rehabilitation Medicine: the 50th anniversary in 2013. PM R 2014; 6: 1044–1047. doi: 10.1016/j.pmrj.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita S, Kakuda W, Momosaki R, et al. Clinical management provided by board-certificated physiatrists in early rehabilitation is a significant determinant of functional improvement in acute stroke patients: a retrospective analysis of Japan rehabilitation database. J Stroke Cerebrovasc Dis 2015; 24: 1019–1024. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.026 [DOI] [PubMed] [Google Scholar]
- 15.Momosaki R, Kakuda W, Yamada N, et al. Impact of board-certificated physiatrists on rehabilitation outcomes in elderly patients after hip fracture: An observational study using the Japan Rehabilitation Database. Geriatr Gerontol Int 2016; 16: 963–968. doi: 10.1111/ggi.12582 [DOI] [PubMed] [Google Scholar]
- 16.Greiss C, Yonclas PP, Jasey N, et al. Presence of a dedicated trauma center physiatrist improves functional outcomes following traumatic brain injury. J Trauma Acute Care Surg 2016; 80: 70–75. doi: 10.1097/TA.0000000000000890 [DOI] [PubMed] [Google Scholar]
- 17.Kato Y, Shimizu M, Hori S, et al. Association between the number of board-certified physiatrists and volume of rehabilitation provided in Japan: an ecological study. J Rural Med 2022; 17: 73–78. doi: 10.2185/jrm.2021-054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizuno S, Wakabayashi H, Fujishima I, et al. Construction and quality evaluation of the Japanese Sarcopenic Dysphagia Database. J Nutr Health Aging 2021; 25: 926–932. doi: 10.1007/s12603-021-1646-y [DOI] [PubMed] [Google Scholar]
- 19.Kunieda K, Ohno T, Fujishima I, et al. Reliability and validity of a tool to measure the severity of dysphagia: the Food Intake LEVEL Scale. J Pain Symptom Manage 2013; 46: 201–206. doi: 10.1016/j.jpainsymman.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med 1993; 32: 382–387. doi: 10.1055/s-0038-1634956 [DOI] [PubMed] [Google Scholar]
- 22.Cederholm T, Jensen GL, Correia MITD, et al. GLIM Core Leadership Committee GLIM Working Group. GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. Clin Nutr 2019; 38: 1–9. doi: 10.1016/j.clnu.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 23.Wakabayashi H, Kishima M, Itoda M, et al. Japanese Working Group on Sarcopenic Dysphagia. Diagnosis and treatment of sarcopenic dysphagia: a scoping review. Dysphagia 2021; 36: 523–531. doi: 10.1007/s00455-021-10266-8 [DOI] [PubMed] [Google Scholar]
- 24.Shimizu A, Fujishima I, Maeda K, et al. The Japanese Working Group on Sarcopenic Dysphagia. Nutritional management enhances the recovery of swallowing ability in older patients with sarcopenic cysphagia. Nutrients 2021; 13: 596. doi: 10.3390/nu13020596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collin C, Wade DT, Davies S, et al. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988; 10: 61–63. doi: 10.3109/09638288809164103 [DOI] [PubMed] [Google Scholar]
- 26.Matsouaka RA, Atem FD. Regression with a right-censored predictor using inverse probability weighting methods. Stat Med 2020; 39: 4001–4015. doi: 10.1002/sim.8704 [DOI] [PubMed] [Google Scholar]
- 27.Huang S, Fiero MH, Bell ML. Generalized estimating equations in cluster randomized trials with a small number of clusters: review of practice and simulation study. Clin Trials 2016; 13: 445–449. doi: 10.1177/1740774516643498 [DOI] [PubMed] [Google Scholar]
- 28.Sura L, Madhavan A, Carnaby G, et al. Dysphagia in the elderly: management and nutritional considerations. Clin Interv Aging 2012; 7: 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimura Y, Wakabayashi H, Bise T, et al. Prevalence of sarcopenia and its association with activities of daily living and dysphagia in convalescent rehabilitation ward inpatients. Clin Nutr 2018; 37(6 Pt A): 2022–2028. doi: 10.1016/j.clnu.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 30.Kokura Y, Wakabayashi H, Maeda K, et al. Impact of a multidisciplinary rehabilitation nutrition team on evaluating sarcopenia, cachexia and practice of rehabilitation nutrition. J Med Invest 2017; 64: 140–145. doi: 10.2152/jmi.64.140 [DOI] [PubMed] [Google Scholar]
- 31.Wakabayashi H, Sakuma K. Rehabilitation nutrition for sarcopenia with disability: a combination of both rehabilitation and nutrition care management. J Cachexia Sarcopenia Muscle 2014; 5: 269–277. doi: 10.1007/s13539-014-0162-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanayama K. Rehabilitation teamwork in Japan. Jpn J Rehabil Med 2015; 52: 117–120. [Google Scholar]
- 33.Kinney CL, Raddatz MM, Sliwa JA, et al. Association of participation in the American Board of Physical Medicine and Rehabilitation Maintenance of Certification Program and physician disciplinary actions. Am J Phys Med Rehabil 2020; 99: 325–329. doi: 10.1097/PHM.0000000000001331 [DOI] [PubMed] [Google Scholar]