Abstract
Introduction:
Identifying individuals who are most likely to accumulate tau and exhibit cognitive decline is critical for Alzheimer’s disease (AD) clinical trials.
Methods:
Participants (N= 235) who were cognitively normal or with mild cognitive impairment from the Alzheimer’s Disease Neuroimaging Initiative were stratified by a cutoff on the polygenic hazard score (PHS) at 65th percentile (above as high-risk group and below as low-risk group). We evaluated the associations between the PHS risk groups and tau positron emission tomography and cognitive decline, respectively. Power analyses estimated the sample size needed for clinical trials to detect differences in tau accumulation or cognitive change.
Results:
The high-risk group showed faster tau accumulation and cognitive decline. Clinical trials using the high-risk group would require a fraction of the sample size as trials without this inclusion criterion.
Discussion:
Incorporating a PHS inclusion criterion represents a low-cost and accessible way to identify potential participants for AD clinical trials.
Keywords: Alzheimer’s disease, clinical trials, polygenic hazard score, tau positron emission tomography
1 |. INTRODUCTION
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that causes cognitive decline and gradual loss of independence in daily life. Before cognitive symptoms become apparent, pathological changes have already been present in the brain for at least a decade. Most disease-modifying treatments for AD that target specific molecular subtypes have failed because AD shows extensive clinical heterogeneity, making it difficult to select patients with underlying AD pathology.1 Genetic risk has been shown to be a strong determinant of who develops AD, and at what age symptoms start, offering a potential strategy to identify the most appropriate candidates for more efficient clinical trials.2,3
Pathological tau (abbreviated to tau) and amyloid beta (abbreviated to amyloid) deposition are considered pathological hallmarks of AD. Due to the failure of the recent amyloid-targeting therapies for effective AD treatments, and evidence that tau pathology is more closely associated with AD clinical symptoms than amyloid both temporally and topologically, tau is recognized as a promising target for AD prevention and treatment, especially prior to significant clinical decline, early in the disease process.4–8 The presence and accumulation of tau is also frequently used as an outcome of clinical trials.9,10 Positron emission tomography (PET) imaging enables in vivo detection of regional tau burden in the human brain. Tau PET is tightly yoked to cognitive symptoms and superior to magnetic resonance imaging (MRI) and amyloid PET in predicting cognitive decline, which thus has the potential to identify participants who are at risk of faster cognitive decline and enrich the participants with preclinical or prodromal AD for clinical trials.8,11–13 However, PET imaging is expensive, time consuming, and not available widely. Identifying a low-cost, less-invasive way to limit participation in trials to those who are most likely to accumulate tau and show cognitive decline was a central goal of this study.
The polygenic hazard score (PHS) is used to predict the AD risk by evaluating genetic factors associated with the age-at-onset of AD.14,15 Individuals with high PHS are more likely to develop AD at a younger age and have higher yearly AD incidence proportions.2 This sets it apart from the polygenic risk score, which does not consider age-dependent incidence risk. PHS is based on germline genotypes, regardless of the source of the samples. PHS has been reported to be associated with amyloid PET, cerebrospinal fluid tau, and cognitive change.3,16,17 The association between the PHS and tau PET has not yet been reported.
In the current study, our objective was to test the hypothesis that a simple PHS inclusion criterion could enrich for individuals who are likely to have higher tau accumulation rates and cognitive decline rates in preclinical and prodromal AD. Given that cognitive outcomes are a ubiquitous component of AD trials and required by several regulatory agencies,18,19 we also wanted to determine how our PHS inclusion criterion impacted the number of participants needed to perform a well-powered clinical trial using tau PET or cognitive measures as outcomes. Further, given known sex differences in tau accumulation20,21 and the influence of genetic risks,15 we also assessed the impact of sex on the performance of PHS inclusion criteria. The results of this study have important implications for improving recruitment for AD clinical trials, hastening the process, and reducing the inherent costs.
2 |. METHODS
2.1 |. Data source
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD.
2.2 |. Participants
We included participants (N = 235) from ADNI who were cognitively normal (CN; N = 142) or had a diagnosis of MCI (N = 93) at their baseline 18F-Flortaucipir (FTP) PET visit and had available calculated PHS by the Desikan lab at the University of California San Francisco. Among these, 112 participants (CN: N = 63, MCI: N = 49) who had more than one FTP PET scan were used for longitudinal analysis. For the secondary analysis, we included participants from ADNI with Clinical Dementia Rating Scale Sum of Boxes (CDR-SOB), Mini-Mental State Examination (MMSE), and Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) data, a required endpoint in clinical trials.19 Given the important effects of non-European ancestral genetics,22,23 we only included participants who self-identified as White.
2.3 |. Imaging data
FTP regional summary data were downloaded from ADNI. The detailed method of data processing and calculation has been described previously.24 The regional standardized uptake value Ratio (SUVR) in the dataset was intensity normalized by the inferior cerebellum gray matter as the reference region. The regions of interest (ROIs) were the regions showing early signs of tau accumulation: entorhinal cortex and meta temporal region, which is composed of bilateral entorhinal, amygdala, fusiform, and inferior and middle temporal cortices.8,10
Amyloid PET (18F-florbetapir [FBP] or 18F-florbetaben [FBB]) data were also directly downloaded from ADNI. Standard thresholds (FBP: 1.11 and FBB: 1.08) were applied to the summary cortical SUVR normalized by the whole cerebellum to determine the amyloid positivity.25 We extracted the closest amyloid PET scan to the baseline FTP PET scan for each subject as their baseline amyloid positivity status. The mean interval between the baseline FTP PET scan and the closest amyloid PET scan was 0.25 ± 0.41 years.
2.4 |. PHS determination
PHS was calculated as previously described.14,15 PHS percentiles were calculated based on the European reference panel from 1000 Genomes Project phase 3 data. In the current study, we tested the 50th, 55th, 60th, 65th, and 70th percentile of PHS as cutoffs to investigate and found using a minimum cutoff of the 65th percentile, there were significant differences in tau deposition in the entorhinal cortex between the high- and low-risk groups. Therefore, we used the 65th percentile (PHS value of 0.84) as the cutoff for the following analyses in this paper: above 65th percentile as high-risk group and below as low-risk group.
2.5 |. Cognitive measures
The cognitive outcome measures CDR-SOB, MMSE score, and ADAS-Cog data were downloaded from ADNI.
2.6 |. Statistical analyses
Age and education differences between the PHS stratified high- and low-risk groups were compared using independent t tests. Pearson’s chi-squared tests were used to detect the PHS risk group differences in sex, baseline amyloid positivity, and apolipoprotein E (APOE) ε4 carriership.
For cross-sectional analyses, we used analysis of covariance (ANCOVA) to assess the differences in baseline tau in ROIs between the high- and low-risk groups adjusting for age, sex, education, and amyloid status in CN and MCI groups separately. For the longitudinal analyses, linear mixed effects (LME) models with interaction (time by PHS risk groups) assessed the effects of PHS levels on tau accumulation over time in the ROIs adjusting for sex, education, baseline age, amyloid status, and clinical diagnosis. To interpret the significant interaction results, we fitted the LME model to assess the regional tau accumulation over time in high- and low-risk groups separately, adjusting for sex, education, baseline age, amyloid status, and diagnosis. Given the small sample size of our cohort, the LME model for longitudinal tau was applied in the combined CN and MCI groups. To control for multiple comparisons of tau deposition and accumulation in two ROIs, the significance threshold was set as 0.025 after Bonferroni correction (0.05/2).
Lastly, in the combined CN and MCI group, power analyses calculated the required sample size for clinical trials designed to detect a 25% difference in tau PET accumulation within the meta temporal region (two-arm trial of 1 year or 2 years with annual observations, a type I error rate of 5%, power of ≥80%, and equal allocation to arms). Power calculations used mean change from baseline and the residual covariance structure from mixed model repeated measures (MMRM) fitting to the combined data,17 adjusting for sex, education, baseline age, amyloid status, and clinical diagnosis. Two sample sizes were calculated, one estimating the sample size required for a trial with unrestricted inclusion, and one for a trial restricting enrollment to the PHS high-risk group.
For the secondary analysis, linear regression models assessed the associations between the rates of cognitive decline and baseline regional tau in high-risk and low-risk groups separately, adjusting for age, sex, education, and amyloid status. The effects of PHS levels over time on cognitive change were analyzed using LME with interaction (time by PHS risk group), adjusting for baseline age, sex, and education. We also did power analyses in the combined CN and MCI group to estimate the sample sizes for clinical trials using cognitive measures as the primary outcome, as described for the longitudinal tau outcome above. Power calculations assumed an MMRM analysis adjusting for sex, education, baseline age, and diagnosis.
The analyses were performed in R (version 4.0.4). The R package nlme was used for LME and MMRM analyses, and pwr was used for power analyses.
3 |. RESULTS
3.1 |. Cross-sectional tau PET
3.1.1 |. Participants
Demographics are presented in Table 1. In CN, the high-risk group was on average younger than the low-risk group. In addition, among MCI, there was a higher percentage of amyloid-positive individuals in the high-risk group than the low-risk group. In either CN or MCI, ≈85% of subjects in the high-risk group were APOE ε4 carriers.
TABLE 1.
Participant characteristics for cross-sectional tau positron emission tomography analysis
| CN (N= 142) | MCI (N= 93) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| PHS low risk (N = 88) | PHS high risk (N = 54) | P | PHS low risk (N = 57) | PHS high risk (N = 36) | P | |
| Age, mean (SD) | 78.75 (6.35) | 75.58 (7.41) | 0.011* | 79.10 (6.91) | 76.17 (7.40) | 0.098 |
| Education, mean (SD) | 16.71 (2.44) | 17.0 (2.40) | 0.483 | 16.50 (2.81) | 15.83 (3.27) | 0.329 |
| Female, N (%) | 44 (50) | 27 (50) | 1.0 | 16 (29) | 13 (36) | 0.558 |
| Amyloid_positivity, N (%) | 29 (33) | 25 (46) | 0.16 | 19 (33) | 26 (72) | <0.001** |
| APOE ε4_carrier, N (%) | 0 (0) | 46 (85) | <0.001** | 1 (2) | 31 (86) | <0.001** |
Abbreviations: APOE, apolipoprotein E; CN, cognitively normal; MCI, mild cognitive impairment; PET, positron emission tomography; PHS, polygenic hazard score; SD, standard deviation.
P < 0.025.
P < 0.005.
3.1.2 |. Baseline tau deposition difference between PHS high- and low-risk groups
First, we observed that higher raw PHS score was associated with elevated baseline tau in the entorhinal cortex, not in the meta temporal region (Table S1 in supporting information). After stratifying participants with PHS at 65th percentile, in CN, high-risk participants showed elevated baseline tau in the entorhinal cortex compared to the low-risk group (P = 0.016; Table 2 and Figure S1A in supporting information). The difference was more significant among MCI (P < 0.001; Table 2 and Figure S1A). However, we did not detect significant differences in the meta temporal region in either CN or MCI group (Table 2 and Figure S1B). Similar results were also found from the partial volume corrected (PVC) data (Table S2 and Figure S2 in supporting information).
TABLE 2.
Difference table of tau positron emission tomography deposition between polygenic hazard score risk groups
| CN (N= 142) | MCI (N= 93) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Tau PET in ROIs | PHS low risk (N = 88) | PHS high risk (N = 54) | P | PHS low risk (N = 57) | PHS high risk (N = 36) | P |
| Entorhinal_SUVR, mean (SD) | 1.144 (0.129) | 1.205 (0.160) | 0.016* | 1.143 (0.141) | 1.333 (0.218) | <0.001** |
| Meta_Temporal_SUVR, mean (SD) | 1.202 (0.122) | 1.221 (0.109) | 0.498 | 1.224 (0.161) | 1.328 (0.197) | 0.105 |
Abbreviations: CN, cognitively normal; MCI, mild cognitive impairment; PET, positron emission tomography; PHS, polygenic hazard score; ROIs, regions of interest; SD, standard deviation; SUVR, standardized uptake value ratio.
P < 0.025.
P < 0.005.
Using PVC tau PET data, we found sex differences in baseline regional tau showing that women accumulate more tau than men in both entorhinal cortex and meta temporal region (Figures S3C and S3D in supporting information). Using non-PVC data, the significant sex difference was only in the meta temporal region, but not in the entorhinal cortex (Figures S3A and S3B). In addition, we did not detect the significant two-way interaction effects of PHS risk groups and sex on the regional baseline tau in either CN or MCI group (Table S3 in supporting information).
3.2 |. Longitudinal tau PET
3.2.1 |. Participants
112 participants (63 CN and 49 MCI) had more than one FTP PET scans. Table S4 in supporting information displays the characteristics of these participants. As expected, there were also more amyloid-positive participants in the high-risk group than the low-risk group. In the high-risk group, 92% of participants were APOE ε4 carriers.
3.2.2 |. Tau accumulation rates differ between PHS high- and low-risk groups
We tested the hypothesis that the tau accumulation rates in ROIs would be greater in the high-risk group than the low-risk group. First, LME with interaction term revealed that there were significant interaction effects of time and PHS risk groups on tau PET accumulation in the meta temporal region (P = 0.002; Table 3). No significant time and PHS risk groups interaction was found in the entorhinal cortex. Results were very similar using PVC data (Table S5 in supporting information). Given the significant interaction effects, we assessed the longitudinal tau PET accumulation in the meta temporal region over time among high-risk group and low-risk group separately and found significant tau accumulation in the meta temporal region only among the high-risk group, but not in the low-risk group (Table 4 and Table S6 in supporting information). Additionally, regardless of PHS, there were significant interaction effects of time and sex on longitudinal tau PET accumulation in the meta temporal region (Table S7 in supporting information). However, we did not detect time × PHS risk groups × sex three-way interaction on either non-PVC/PVC entorhinal or meta temporal tau PET accumulation (Table S8 in supporting information).
TABLE 3.
Results of linear mixed effects models with interaction term (Time by polygenic hazard score_Risk_Group) for tau positron emission tomography accumulation
| Entorhinal_SUVR | Meta_Temporal_SUVR | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Predictors | Estimates | 95% CI | P | Estimates | 95% CI | P |
| Intercept | 0.841 | 0.471–1.210 | <0.001** | 0.944 | 0.606–1.281 | <0.001** |
| Time | −0.004 | −0.013–0.006 | 0.446 | 0.001 | −0.008–0.010 | 0.836 |
| PHS_Risk_Group | 0.104 | 0.049–0.159 | <0.001** | 0.032 | −0.017–0.082 | 0.217 |
| Sex (women) | 0.049 | −0.004–0.102 | 0.077 | 0.045 | −0.003–0.094 | 0.071 |
| Education | −0.006 | −0.016–0.004 | 0.239 | −0.002 | −0.011 –0.007 | 0.691 |
| Baseline_Age | 0.004 | −0.000–0.008 | 0.061 | 0.003 | −0.001–0.006 | 0.142 |
| Baseline_Amyloid_Status | 0.085 | 0.031–0.139 | 0.003** | 0.058 | 0.007–0.108 | 0.027 |
| Baseline_Diagnosis (MCI) | 0.041 | −0.010 –0.093 | 0.126 | 0.052 | 0.005–0.098 | 0.035 |
| Time × PHS_Risk_Group (high risk) | 0.018 | 0.002–0.033 | 0.027 | 0.023 | 0.009–0.037 | 0.002** |
Abbreviations: CI, confidence interval; MCI, mild cognitive impairment; PHS, polygenic hazard score; SUVR, standardized uptake value ratio.
P < 0.025.
P < 0.005.
TABLE 4.
Results of linear mixed effects models of regional tau positron emission tomography accumulation over time in high and low polygenic hazard score risk strata
| Meta_Temporal_SUVR | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| High risk | Low risk | |||||
|
|
|
|||||
| Predictors | Estimates | 95% CI | P | Estimates | 95% CI | P |
| Intercept | 0.992 | 0.551–1.430 | <0.001** | 1.032 | 0.563–1.503 | <0.001** |
| Time | 0.023 | 0.011–0.036 | <0.001** | 0.001 | −0.007–0.009 | 0.742 |
| Sex (women) | 0.038 | −0.028–0.106 | 0.281 | 0.043 | −0.021–0.107 | 0.197 |
| Education | −0.017 | −0.030–(−0.003) | 0.023* | 0.008 | −0.004–0.020 | 0.190 |
| Baseline_Age | 0.006 | 0.001–0.010 | 0.019* | −0.000 | −0.005–0.004 | 0.862 |
| Baseline_Amyloid_Status | 0.080 | 0.004–0.156 | 0.050 | 0.034 | −0.019–0.106 | 0.320 |
| Baseline_Diagnosis (MCI) | 0.037 | −0.031–0.106 | 0.300 | 0.044 | −0.030–0.098 | 0.185 |
Abbreviations: CI, confidence interval; MCI, mild cognitive impairment; SUVR, standardized uptake value ratio.
P < 0.025.
P < 0.005.
3.3 |. Trial enrichment through high genetic risk inclusion criteria
We estimated the sample size required to power a clinical trial using tau PET accumulation in the meta temporal region as the primary outcome measure as informed by the pattern of progression observed above. For a two-arm clinical trial, a trial restricting to high-risk enrollees would require ≈70% to 80% fewer samples than a trial without the high PHS inclusion criterion (1 year: required N = 915 vs. 4371, 2 years: required N = 329 vs. 1157; Table 5).
TABLE 5.
Sample size needed for tau positron emission tomography in the hypothetical clinical trial
| Number needed in each group | |||
|---|---|---|---|
|
|
|||
| Test | CN + MCI | 1 year | 2 years |
| Tau SUVR in meta temporal region | Full sample High RISK |
4371 915 |
1157 329 |
Note: Full sample: Participants without PHS cut off stratification.
Abbreviations: CN, cognitively normal; MCI, mild cognitive impairment; PHS, polygenic hazard score; SUVR, standardized uptake value ratio.
3.4 |. Secondary analysis
3.4.1 |. Regional tau deposition predicts cognition change
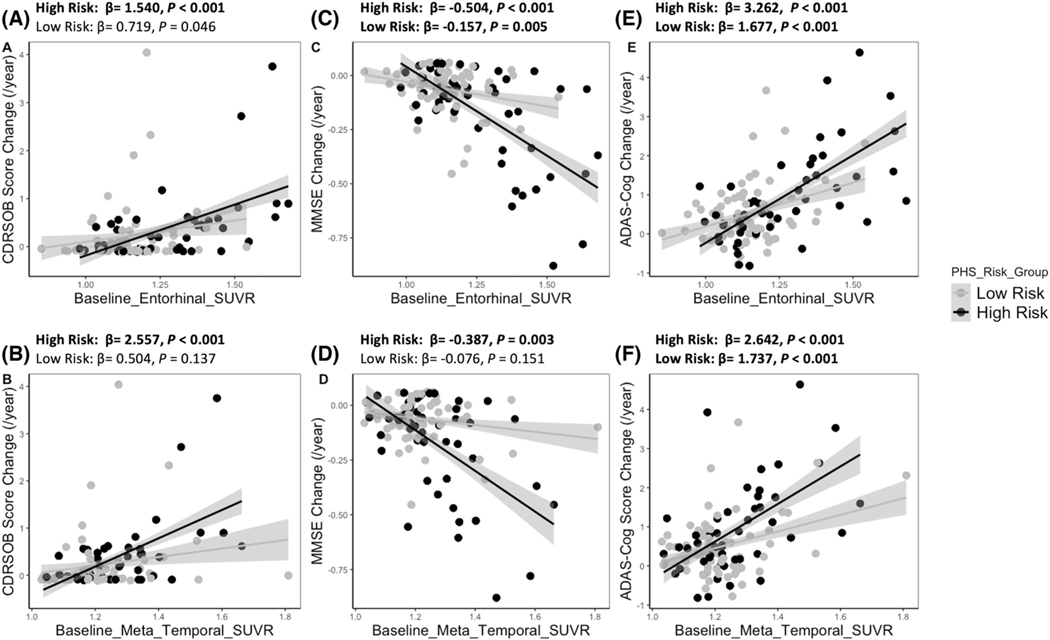
Several studies have shown that baseline regional tau PET could precisely predict the cognitive decline in preclinical and prodromal AD,8,26 which is consistent with our findings that individuals with high baseline tau in the entorhinal cortex and meta temporal region exhibited faster rates of worsening on several common outcome measures, specifically CDR-SOB increase, MMSE score decrease, and ADAS-Cog increase. Importantly, these associations between the regional tau PET and cognitive decline were mediated by the PHS risk groups (Figure 1 and Table S9 in supporting information). For CDR-SOB measure, the significant positive associations between the regional tau and CDR-SOB change rates only existed in the high-risk group (Figures 1A and 1B). For MMSE measure, we observed a significant interaction effect of PHS risk groups and the baseline tau burden in two ROIs on MMSE decline rates (Table S9). Baseline tau in the meta temporal region was significantly associated with MMSE decline rates only in the high-risk group (Figure 1D). Although there were significant associations between the baseline tau PET in the entorhinal cortex and MMSE change rates in both high-risk and low-risk groups, the effect (β estimate) of high-risk group was larger than the effect of low-risk group (Figure 1C). For ADAS-Cog, the associations between the baseline regional tau burden and ADAS-Cog change rates in PHS high risk were also stronger than the associations in the low-risk group (Figures 1E and 1F).
FIGURE 1.
PHS risk groups by tau PET in ROIs interaction on cognitive decline rates. PET, positron emission tomography; PHS, polygenic hazard score; ROIs, regions of interest; SUVR, standardized uptake volume ratio
3.4.2 |. PHS also predicts cognition change
We further tested whether the PHS risk groups could predict the change in cognition and functioning. To increase the sample size and accuracy of the results, we included participants who have undergone longitudinal CDR-SOB, MMSE, and ADAS-Cog measurements (CN: N = 305, MCI: N = 448). Table S10 in supporting information shows the characteristics of the participants. The high-risk participants were younger than the low-risk participants. However, baseline cognition of the high-risk group was worse compared to the low-risk group in MCI (Table S10). Furthermore, for longitudinal analysis of cognitive performance, the LME model with interaction term showed that there were significant interaction effects between time and PHS risk groups on CDR-SOB, MMSE, and ADAS-Cog in MCI (Table 6). Figure S4 in supporting information reveals that the PHS high-risk group showed faster cognition decline than the low-risk group within the same clinical diagnosis provided at the initial visit.
TABLE 6 |.
Results of linear mixed effects models with interaction term for cognition change
| Time × PHS_Risk_Group (high risk) | Estimates | 95% CI | P |
|---|---|---|---|
| CDR-SOB | |||
| CN (N = 305) | 0.038 | −0.000–0.076 | 0.05 |
| MCI (N = 448) | 0.289 | 0.138–0.440 | <0.001** |
| MMSE | |||
| CN (N = 305) | −0.026 | −0.118–0.065 | 0.576 |
| MCI (N = 448) | −0.279 | −0.490–(−0.007) | 0.009* |
| ADAS-Cog | |||
| CN (N = 305) | 0.293 | −0.058–0.644 | 0.103 |
| MCI (N = 448) | 0.923 | 0.458–1.387 | <0.001** |
Abbreviations: ADAS-Cog, Alzheimer’s Disease Assessment Scale–Cognitive Subscale; CDR-SOB, Clinical Dementia Rating Scale Sum of Boxes; CI, confidence interval; CN, cognitively normal; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; PHS, polygenic hazard score.
P < 0.025.
P < 0.005.
3.4.3 |. Improvement in differentiating longitudinal cognitive outcomes
Table 7 shows the sample size needed for each cognitive outcome in each arm of a two-arm hypothetical trial with combined CN and MCI participants. At each time point, for these three cognitive measures, the sample sizes needed in PHS high-risk group were all smaller than the group without the PHS inclusion criterion. At 2 years, the high-risk group required ≈48.7% fewer samples in CDR-SOB, 66.4% fewer samples in MMSE, and 84.6% fewer samples in ADAS-Cog than a clinical trial without PHS inclusion criterion. MMSE required the smallest sample size comparing CDR-SOB and ADAS-Cog at each trial length for either with or without PHS inclusion criterion.
TABLE 7.
Sample size needed for cognitive measures in the hypothetical clinical trial
| Number needed in each group | |||
|---|---|---|---|
|
|
|||
| Test | CN + MCI | 1 year | 2 years |
| CDR-SOB | Full sample | 32004 | 2663 |
| High risk | 8107 | 1366 | |
| MMSE | Full sample | 5222 | 2785 |
| High risk | 2340 | 935 | |
| ADAS-Cog | Full sample | 216203 | 11131 |
| High risk | 16268 | 1718 | |
Note: Full sample: Participants without PHS cutoff stratification.
Abbreviations: ADAS-Cog, Alzheimer’s Disease Assessment Scale–Cognitive Subscale; CDR-SOB, Clinical Dementia Rating Scale Sum of Boxes; CN, cognitively normal; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; PHS, polygenic hazard score.
4 |. DISCUSSION
Higher PHS is associated with elevated tau PET among CN and MCI. We used a simple cutoff (the PHS corresponding to 65th percentile of PHS of a 1000 Genomes Project reference panel) to define the high and low genetic risk groups of our sample of ADNI CN and MCI participants. The high-risk group showed faster tau accumulation rates and faster cognitive decline rates. To detect significant differences in tau accumulation and cognitive decline, power analyses showed the sample sizes needed with PHS high-risk group were a fraction of sample sizes needed without PHS high-risk restriction. Our findings suggested the high-risk group who are at risk of AD could enrich the participants with preclinical or prodromal AD for clinical trials.
The genetic variants of APOE, especially APOE ε4, are strong genetic risk factors for tau spreading in AD.27 Recent PET studies have reported APOE ε4 carriers showed higher regional tau load and faster regional tau accumulation rates using different cohorts ranging from CN to fully developed AD.28,29 However, in our study among CN and MCI, we did not detect significant interaction effects between time and APOE ε4 carriership on tau PET in the meta temporal ROI in the longitudinal analysis (Table S11 in supporting information). It suggested that tau has polygenic determinants and the PHS stratification outperforms APOE ε4 to predict tau accumulation. The prediction of PHS in this study is not solely explained by the influence of APOE. Other genes, such as BIN1, CR1, and CLU, which have been reported to interact with tau,30–32 are all integrated into the PHS. Women exhibited greater tau along the AD continuum.21,33 Consistently, in this study, when using partial-volume–corrected data, women accumulated more baseline tau than men. It is curious that this did not occur in the entorhinal cortex of non-corrected data (Figure S2) and is perhaps consistent with women and men showing different rates of atrophy.34 We also detected two-way significant interaction between time and sex on longitudinal tau in the meta temporal region (Table S7). Previous studies have reported sex could modulate the effect of APOE ε4 on regional tau PET in CN and MCI cohorts, respectively.20,35 However, we did not detect significant three-way interactions (time × PHS risk groups × sex) on tau accumulation. The possible explanation is that PHS incorporates 31 genetic variants some of which have different sex-specific effects on AD,15 thus might decrease the sex-bias effects on tau compared to APOE ε4 only. Alternatively, we may have insufficient participants to detect a three-way interaction.
The relationship between amyloid and tau is still a debated topic. Initially, amyloid was widely presumed to be the upstream initiator of the tau in AD pathogenesis.36,37 More recently however, evidence suggesting that amyloid toxicity is tau dependent has been reported.38,39 In our study, all of the analyses involving tau were adjusted for amyloid positivity, which indicated the PHS prediction on tau PET in this paper is independent of amyloid. This suggested that PHS, which can be collected with a cheek swab, is sufficient to predict individuals at risk of accelerated tau accumulation, and amyloid testing (which, even in plasma, remains expensive) is not necessary for the prediction.
Our study has limitations. One limitation of this study is the small sample size, which did not allow longitudinal analyses in CN and MCI groups separately. Regarding the sample size calculations, the CN and MCI participants with tau PET are likely not exactly representative of subjects that will be approached for recruitment into future clinical trials. The relative efficiency of future trials with and without the high PHS inclusion criterion will depend on the ratio of CN to MCI subjects recruited. In addition, there is no suitable, currently available alternative cohort to provide independent validation in this study although as more data become available this will be an essential next step. Our analysis nonetheless confirms that a PHS inclusion criterion can substantively improve the efficiency of AD clinical trials. Future studies representative of different recruitment pools would more definitively validate these findings and describe the extent of this improvement. Moreover, in the ROIs of the study, we did not include the hippocampus, as it is known to be contaminated by off-target binding in the choroid plexus using FTP PET.24,40 As an important region of the early stages of tau, hippocampus exclusion might decrease the significance of our results. Furthermore, the participants of our study were selfi-dentified as White and thus likely mostly of European ancestry. This is a huge limitation and one that needs to be countered in the future, in part by adapting design and recruitment methods in studies to be more inclusive of other races and ethnicities.41
Collectively, our study suggests that the PHS stratification method, a quick and low-cost method, can contribute to efficient clinical trial design for AD therapeutics.
Supplementary Material
RESEARCHINCONTEXT.
1. SystematicReview:
The literature review was performed on PubMed. Tau positron emission tomography (PET) is an efficient outcome measure in disease-modifying clinical trials for Alzheimer’s disease (AD). However, PET imaging is expensive, invasive, and time consuming. We lack a simple and cost-effective method to identify participants who should receive clinical trials for new interventions aimed at treating early-stage AD.
2. Interpretation:
We stratified participants in preclinical and prodromal stages of AD with a cutoff on the polygenic hazard score (PHS) and found that participants with high PHS are more likely to accumulate tau and exhibit cognitive decline. These findings suggest participants with high PHS might be the most appropriate candidates for clinical trials in AD.
3. FutureDirections:
Our work provides a potential method to identify participants who would benefit from the efficiency of clinical trials in early-stage AD. We look forward that this strategy could be replicated in other cohorts and applied in prospective clinical trials.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (grant numbers 1R01AG066088. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI; National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12–2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie; Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Funding information
National Institutes of Health, Grant/Award Numbers: 1R01AG066088, U01 AG024904
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
CONFLICTS OF INTEREST
Xin Wang, Iris Broce, Yuqi Qiu, Kacie D. Deters, Chun Chieh Fan, Anders M. Dale, Steven D. Edland, and Sarah J. Banks have no relevant disclosures for this paper.
DATA AVAILABILITY STATEMENT
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
REFERENCES
- 1.Yiannopoulou KG, Anastasiou AI, Zachariou V, Pelidou SH. Reasons for failed trials of disease-modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines. 2019;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan CH, Hyman BT, Tan JJX, et al. Polygenic hazard scores in preclinical Alzheimer disease. Ann Neurol. 2017;82:484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan CH, Fan CC, Mormino EC, et al. Polygenic hazard score: an enrichment marker for Alzheimer’s associated amyloid and tau deposition. Acta Neuropathol. 2018;135:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kametani F, Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front Neurosci. 2018;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2018;14:399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. [DOI] [PubMed] [Google Scholar]
- 7.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ossenkoppele R, Smith R, Mattsson-Carlgren N, et al. Accuracy of tau positron emission tomography as a prognostic marker in preclinical and prodromal Alzheimer disease: a head-to-head comparison against amyloid positron emission tomography and magnetic resonance imaging. JAMA Neurol. 2021;78:961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panza F, Solfrizzi V, Seripa D, et al. Tau-centric targets and drugs in clinical development for the treatment of Alzheimer’s disease. Biomed Res Int. 2016;2016:3245935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack CR Jr., Wiste HJ, Schwarz CG, et al. Longitudinal tau PET in ageing and Alzheimer’s disease. Brain. 2018;141:1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biel D, Brendel M, Rubinski A, et al. Tau-PET and in vivo Braak-staging as prognostic markers of future cognitive decline in cognitively normal to demented individuals. Alzheimers Res Ther. 2021;13:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng E, Manser PT, Sanabria Bohorquez S, et al. Baseline [(18)F]GTP1 tau PET imaging is associated with subsequent cognitive decline in Alzheimer’s disease. Alzheimers Res Ther. 2021;13:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Digma LA, Madsen JR, Reas ET, et al. Tau and atrophy: domain-specific relationships with cognition. Alzheimers Res Ther. 2019;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desikan RS, Fan CC, Wang Y, et al. Genetic assessment of age-associated Alzheimer disease risk: development and validation of a polygenic hazard score. PLoS Med. 2017;14:e1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan CC, Banks SJ, Thompson WK, et al. Sex-dependent autosomal effects on clinical progression of Alzheimer’s disease. Brain. 2020;143:2272–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan CH, Bonham LW, Fan CC, et al. Polygenic hazard score, amyloid deposition and Alzheimer’s neurodegeneration. Brain. 2019;142:460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banks SJ, Qiu Y, Fan CC, et al. Enriching the design of Alzheimer’s disease clinical trials: application of the polygenic hazard score and composite outcome measures. Alzheimers Dement (N Y). 2020;6:e12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold M, Amatniek J, Carrillo MC, et al. Digital technologies as biomarkers, clinical outcomes assessment, and recruitment tools in Alzheimer’s disease clinical trials. Alzheimers Dement (N Y). 2018;4:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabbagh MN, Hendrix S, Harrison JE. FDA position statement “early Alzheimer’s disease: developing drugs for treatment, guidance for industry”. Alzheimers Dement (N Y). 2019;5:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019;76:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith R, Strandberg O, Mattsson-Carlgren N, et al. The accumulation rate of tau aggregates is higher in females and younger amyloid-positive subjects. Brain. 2020;143:3805–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deters KD, Mormino EC, Yu L, Lutz MW, Bennett DA, Barnes LL. TOMM40-APOE haplotypes are associated with cognitive decline in non-demented Blacks. Alzheimers Dement. 2021;17:1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunkle BW, Schmidt M, Klein HU, et al. Novel Alzheimer disease risk loci and pathways in African American individuals using the African genome resources panel: a meta-analysis. JAMA Neurol. 2021;78:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker SL, Maass A, Jagust WJ. Considerations and code for partial volume correcting [(18)F]-AV-1451 tau PET data. Data Brief. 2017;15:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Royse SK, Minhas DS, Lopresti BJ, et al. Validation of amyloid PET positivity thresholds in centiloids: a multisite PET study approach. Alzheimers Res Ther. 2021;13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Cassady KE, Adams JN, Harrison TM, Baker SL, Jagust WJ. Regional Tau effects on prospective cognitive change in cognitively normal older adults. J Neurosci. 2021;41:366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Y, Yamada K, Liddelow SA, et al. ApoE4 markedly exacerbates taumediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therriault J, Benedet AL, Pascoal TA, et al. Association of apolipoprotein E epsilon4 with medial temporal Tau independent of amyloid-beta. JAMA Neurol. 2020;77:470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baek MS, Cho H, Lee HS, Lee JH, Ryu YH, Lyoo CH. Effect of APOE epsilon4 genotype on amyloid-beta and tau accumulation in Alzheimer’s disease. Alzheimers Res Ther. 2020;12:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franzmeier N, Rubinski A, Neitzel J, Ewers M; Alzheimer’s Disease Neuroimaging Initiative (ADNI). The BIN1 rs744373 SNP is associated with increased tau-PET levels and impaired memory. Nat Commun. 2019;10:1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Killick R, Hughes TR, Morgan BP, Lovestone S. Deletion of Crry, the murine ortholog of the sporadic Alzheimer’s disease risk gene CR1, impacts tau phosphorylation and brain CFH. Neurosci Lett. 2013;533:96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avila J, Gomez-Ramos A, Bolos M. AD genetic risk factors and tau spreading. Front Aging Neurosci. 2015;7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundermann EE, Panizzon MS, Chen X, et al. Sex differences in Alzheimer’s-related tau biomarkers and a mediating effect of testosterone. Biol Sex Differ. 2020;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ardekani BA, Convit A, Bachman AH. Analysis of the MIRIAD data shows sex differences in hippocampal atrophy progression. J Alzheimers Dis. 2016;50:847–857. [DOI] [PubMed] [Google Scholar]
- 35.Liu M, Paranjpe MD, Zhou X, et al. Sex modulates the ApoE epsilon4 effect on brain tau deposition measured by (18)F-AV-1451 PET in individuals with mild cognitive impairment. Theranostics. 2019;9:4959–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003;24:1063–1070. [DOI] [PubMed] [Google Scholar]
- 37.Stancu IC, Vasconcelos B, Terwel D, Dewachter I. Models of betaamyloid induced Tau-pathology: the long and “folded” road to understand the mechanism. Mol Neurodegener. 2014;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. [DOI] [PubMed] [Google Scholar]
- 39.Hurtado DE, Molina-Porcel L, Iba M, et al. A{beta} accelerates the spatiotemporal progression of tau pathology and augments tau amyloidosis in an Alzheimer mouse model. Am J Pathol. 2010;177:1977–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikonomovic MD, Abrahamson EE, Price JC, Mathis CA, Klunk WE. [F-18]AV-1451 positron emission tomography retention in choroid plexus: more than “off-target” binding. Ann Neurol. 2016;80:307–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill CV, Perez-Stable EJ, Anderson NA, Bernard MA. The National Institute on aging health disparities research framework. Ethn Dis. 2015;25:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf