Abstract
Background
Growing evidence suggests an association between the vitamin D levels and respiratory outcomes of preterm infants. The objective of this systematic review and meta-analysis was to explore whether premature neonates with a vitamin D deficiency have an increased risk of respiratory distress syndrome (RDS).
Methods
We searched PubMed, EMBASE, and the Cochrane Library up through July 20, 2021. The search terms were ‘premature infant’, ‘vitamin D’, and ‘respiratory distress syndrome’. We retrieved randomized controlled trials and cohort and case-control studies. For statistical analysis, we employed the random-effects model in Comprehensive Meta-Analysis Software ver. 3.3. We employed the Newcastle-Ottawa Scales for quality assessment of the included studies.
Results
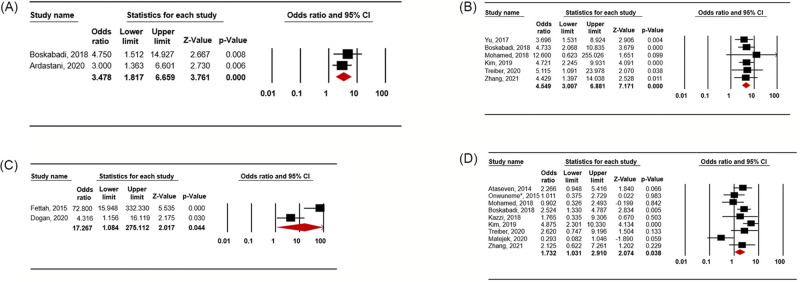
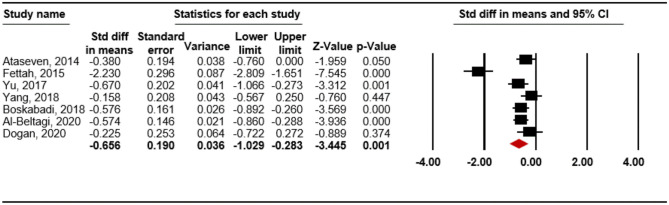
A total of 121 potentially relevant studies were found, of which 15 (12 cohort studies and 3 case-control studies) met the inclusion criteria; the studies included 2,051 preterm infants. We found significant associations between RDS development in such infants and vitamin D deficiency within 24 h of birth based on various criteria, thus vitamin D levels < 30 ng/mL (OR 3.478; 95% CI 1.817–6.659; p < 0.001), < 20 ng/mL (OR 4.549; 95% CI 3.007–6.881; p < 0.001), < 15 ng/mL (OR 17.267; 95% CI 1.084–275.112; p = 0.044), and < 10 ng/ml (OR 1.732; 95% CI 1.031–2.910; p = 0.038), and an even lower level of vitamin D (SMD = –0.656; 95% CI –1.029 to –0.283; p = 0.001).
Conclusion
Although the vitamin D deficiency definitions varied and different methods were used to measure vitamin D levels, vitamin D deficiency or lower levels of vitamin D within 24 h of birth were always associated with RDS development. Monitoring of neonatal vitamin D levels or the maintenance of adequate levels may reduce the risk of RDS.
Introduction
Vitamin D participates in mineral-ion homeostasis (e.g., calcium and phosphorus) and bone metabolism [1]. Apart from these classical functions, a role for vitamin D in lung development has been demonstrated by several studies (including animal works) [1–12]. Vitamin D regulates cell proliferation/differentiation, apoptosis, and angiogenesis in the lungs [6] and elsewhere in the body [1]. A recent meta-analysis found an association between the vitamin D level within 24 h of birth and the risk of bronchopulmonary dysplasia (BPD) in premature neonates [13].
Vitamin D receptors are expressed mainly in the lung during late pregnancy [2]; vitamin D thus affects lung functional and anatomical development, including cell differentiation and surfactant synthesis/secretion [3, 5, 14]. Animal studies performed by Nguyen et al. [7–9] and Marin et al. [2, 10] using rat models revealed high-level expression of vitamin D receptor on type II alveolar cells at the end of gestation, when alveolar cell differentiation and surfactant synthesis commence [2, 10]. Rehan et al. [3] reported that the active form of vitamin D enhanced surfactant synthesis by type II pulmonary cells. Surfactant phospholipid and protein B synthesis increased in human pulmonary cancer cell lines. Such laboratory-based work raised the question of whether the respiratory distress syndrome (RDS) risk is increased in preterm neonates with vitamin D deficiencies in clinical settings.
Several clinical studies have described low vitamin D levels in preterm infants who suffered from RDS [15, 16]. However, Matejek et al. [17] found no association between vitamin D deficiency and RDS. Given such conflicting evidence, we performed a systematic review and meta-analysis to explore whether vitamin D deficiency or the vitamin D level measured within 24 h of birth was associated with an increased risk of RDS.
Methods
Search strategy and study selection
This meta-analysis was reported based the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [18, 19] (S1 Table). We searched PubMed, EMBASE, and the Cochrane Library using the following search terms: [‘premature infant’ or preterm or newborn or neonate or ‘low birth weight infant’ or ‘very low birth weight infant’ or ‘extremely low birth weight infant’] and [‘Vitamin D’ or ‘25-hydroxyvitamin D’ or 25-hydroxyergocalciferol or ergocalciferol or cholecalciferol or hydroxycholecalciferol or calcifediol or dihydroxycholecalciferol or 25(OH)D or 1,25(OH)2-vitD], and [‘respiratory distress syndrome’ or RDS or ‘hyaline membrane disease’ or ‘transient tachypnea’ or ‘pulmonary surfactants’]. Additionally, we manually checked all reference lists in an effort to identify additional relevant studies. The last search was performed on July 20, 2021. We did not place any restrictions, including on language.
We initially reviewed the titles and abstracts of the articles, and then reviewed the full-text articles. The reviews were performed independently by two reviewers (HW Park and Y Kim) using criteria that we determined before the review process for inclusion in the meta-analysis. Any disagreement during the process was resolved by the third author (R Lee).
Inclusion and exclusion criteria
We chose to include studies that satisfied the following criteria. Study design: A randomized controlled trial, or a cohort study (prospective or retrospective), or a case-control study; Patients and interventions (exposures): Newborns for whom vitamin D levels were known; Outcome: RDS. We excluded case reports, case series, editorials, review articles, and letters. Articles with inadequate or irrelevant data for analysis were also excluded after reading the full text.
Outcomes
The primary outcome was neonatal RDS diagnosed via chest radiographic findings and clinical presentations (e.g., tachypnea, nasal flaring, chest retraction, and cyanosis) within hours of birth.
Data extraction
We extracted the data from all included studies via full text review. Two authors (Y Kim and HW Park) reviewed and extracted data using a pre-prepared form. The data were the first author, publication year, study period, study design, study location, study population, sample size, method of vitamin D measurement, definition of vitamin D deficiency, diagnostic criteria for RDS, sample size, number of patients diagnosed with RDS or vitamin deficiency, and vitamin D levels or odds ratios of RDS if vitamin D was deficient for RDS, when possible. If there were any discrepancies during the review process with two authors (YJ Kim and HW Park), we discussed these with the third reviewer (R Lee) and we reviewed the study again.
Study quality assessment
We (Y Kim and HW Park) also separately assessed the quality of included studies using the Newcastle–Ottawa Scale (NOS) [20]. Disparities in the assessment were resolved through discussion with the third author (R Lee). This scale is composed of three domains that explore selection, comparability, and outcome. The three domains contain a total of eight items. We gave one star to an item that met the criteria, except for comparability (two stars). The score range is 0–9, and the total score indicates the methodological quality of the study; ≤ 3 is low, 4–5 is moderate, and ≥ 6 is high. The scores of each study for each items of NOS are provided in the S2 Table.
Data synthesis and statistical analyses
We performed this meta-analysis to calculate a pooled estimate of odds ratio (OR) for the association between vitamin D deficiency and the occurrence of RDS. We performed the analysis separately when studies reported more than one result based on different definitions of vitamin D deficiency; we thus kept the statistical assumption of the independence of an effect.
We used the I2 statistic to evaluate statistical heterogeneity; the value is expressed as the percentage of total variation across studies. If the I2 value is greater than 50%, this indicates the presence of significant heterogeneity across the studies. We performed our analysis conservatively; based on estimation of the between-study variations in effect size, we used a random-effects model, which yields wider CIs than a fixed-effects model [21]. We also conducted sensitivity analyses to evaluate the effect of each study on the robustness of the combined estimates and contribution to the pooled OR. A cumulative analysis was conducted by adding one study at a time, by year of publication, to evaluate temporal trends.
To detect publication bias, the Begg and Mazumdar rank-correlation test and Egger’s regression test were used. Publication bias was also evaluated based on the distribution of the effect sizes against the standard errors on a graphically displayed funnel plot. We detected publication bias by inspecting the funnel plot. Asymmetry of the funnel plot or a P-value < 0.05 in the Begg and Mazumdar rank-correlation test or Egger’s regression test were taken to indicate the presence of publication bias. The current meta-analysis was conducted using Comprehensive Meta-Analysis software version 3.3 (Biostat Inc., Englewood, NJ, USA).
Results
Literature search and study selection
We show the flow of study selection and exclusion in Fig 1. In total, 38 duplicates were removed from the 121 studies retrieved in the initial search, including the manual search. During review of abstracts and titles, 48 studies were removed, leaving 35 studies. After full-text review, 20 studies were excluded for the reasons described in Fig 1, leaving 15 studies for meta-analysis.
Fig 1. Flow chart of study selection process.
Characteristics of the included studies
The characteristics of the 15 studies [5, 11, 15–17, 22–31] included in this meta-analysis are shown in Table 1. When evaluating the association between vitamin D deficiency and RDS, 2,051 infants were included. The mean birth weight of the study population in the 15 included studies was 1,833.2 g (standard error 143.91), and the mean gestational age of the infants at birth was 32.0 weeks (standard error 0.90); one study [26] did not describe gestational age at birth.
Table 1. Characteristics of studies included in the meta-analysis.
| Studies | Study design | Nation | Study population | Time of vitamin D measurement | Methods of measurement of vitamin D | Definition of vitamin D deficiency (serum 25(OH)D level) | NOS |
|---|---|---|---|---|---|---|---|
| Ataseven et al. [22] | prospective cohort | Turkey | GA 29–35 weeks | < 24 h after birth | LC-MS | severe: <10 ng/mL moderate: 10–20 ng/mL mild: 20–30 ng/mL |
8 |
| Fettah et al. [23] | prospective cohort | Turkey | GA < 32 weeks | soon after birth (cord) | ELISA | <15 ng/mL | 7 |
| Onwuneme et al. [24] | prospective cohort | Ireland | GA <32 weeks or BW <1,500g | < 24 h after birth | chemiluminescence | <12 ng/mL | 5 |
| Yu et al. [15] | retrospective cohort | China | GA<32 weeks | <24 h after birth | chemiluminescence | <20 ng/ml | 8 |
| Mohamed et al. [25] | prospective case–control | Egypt | GA <34 weeks | < 24 h after birth | ELISA | <20 ng/ml | 5 |
| Yang et al. [26] | retrospective cohort | China | GA <37 weeks | 1st sampling after birth | automatic biochemical analyzer | measured value* | 4 |
| Boskabadi et al. [27] | prospective case-control | Iran | GA < 34 weeks and BW < 2,000g | NA | ELISA | severe: <10 ng/mL moderate: 10–20 ng/mL mild: 20–30 ng/mL |
7† |
| Kazzi et al. [28] | prospective cohort | USA | Preterm infants and BW ≤1,250 g | < 24 h after birth | LC-MS | ≤10 ng/mL | 5 |
| Kim et al. [29] | retrospective cohort | Korea | BW <1,500 g | < 24 h after birth | LC-MS and chemiluminescence | severe: <10 ng/mL deficiency: 10–20 ng/mL insufficiency: 20–30 ng/mL |
8 |
| Treiber et al. [30] | prospective cohort | Slovenia | newborn | soon after birth (cord) | chemiluminescence | severe deficiency: <10ng/mL deficiency: 10–20 ng/ml insufficiency: 20–30 ng/ml |
4 |
| Ardastani et al. [5] | prospective cohort | Iran | GA 28–37 weeks and BW ≥1,000 g | soon after birth (cord) or <24h after birth | ELISA | severe deficiency: <10ng/mL deficiency: 10–20 ng/ml inadequate: 20–30 ng/ml |
7 |
| Matejek et al. [17] | prospective cohort | Czech | BW <1,500 g | soon after birth (cord) | LC-MS | <10 ng/mL | 7 |
| Al-Beltagi et al. [31] | prospective case-control | Egypt | Preterm infants | soon after birth (cord) | ELISA | measured value* | 5† |
| Dogan et al. [16] | prospective cohort | Turkey | GA ≤32 weeks | < 6 h after birth | LC-MS | severe: <5 ng/mL moderate: 5–15 ng/mL mild:15–30 ng/mL |
7 |
| Zhang et al. [11] | prospective cohort | China | GA< 32 week | < 24 h after birth | chemiluminescence | severe deficiency: <10ng/mL deficiency: 10–20 ng/ml insufficiency: 20–30 ng/ml |
7 |
* The measured value of vitamin D was used in the analysis
† The two case-control studies were assessed using the Newcastle-Ottawa Scale for quality assessment of case-control studies; the other studies were assessed using the Newcastle-Ottawa Scale for the assessment of cohort studies
Abbreviations: GA, gestational age at birth; BW, birth weight; g, gram; NOS, Newcastle–Ottawa Scale, LC-MS, liquid chromatography-tandem mass spectrometry; ELISA, enzyme linked immunosorbent assay
The definition of vitamin D deficiency in each study is shown in Table 1. The occurrence rates of RDS are presented by reference to the severity of vitamin D deficiency. Thus, we used the various levels in meta-analysis: a vitamin D level below 30 ng/ml in two studies [5, 27], 20 ng/ml in six studies [11, 15, 25, 27, 29, 30], 15 ng/ml in two studies [16, 23], and 10 ng/ml in nine studies [11, 17, 22, 24, 25, 28–30, 32].
The scores on the Newcastle–Ottawa Scale for quality assessment of all studies are shown in Table 1.
Pooled meta-analysis results
There were significant associations between vitamin D deficiency and RDS, regardless of the definition of vitamin D deficiency; thus at cut off values of 30 ng/ml, 20 ng/ml, 15 ng/ml, and 10 ng/ml.
Vitamin D deficiency defined using a cut off value of 30 ng/ml was associated with RDS (OR 3.478; 95% CI 1.817–6.659; p < 0.001; Fig 2A) in the random-effects model analysis. Among the included studies, there was no significant heterogeneity (p = 0.517; I2 = 0%). No single study affected the pooled result on sensitivity analysis (S1-1 Fig in S1 Fig) or cumulative analysis (S1-2 Fig in S1 Fig). Publication bias was not assessed since only two studies were included.
Fig 2. Meta-analysis for the association between vitamin D deficiency and respiratory distress syndrome according to the definition of vitamin D deficiency.
(A) Vitamin D deficiency < 30 ng/ml, (B) Vitamin D deficiency < 20 ng/ml, (C) Vitamin D deficiency < 15 ng/ml, (D) Vitamin D deficiency < 10 ng/ml.
Vitamin D deficiency defined as a vitamin D level of less than 20 ng/ml was also associated with RDS (OR 4.549; 95% CI 3.007–6.881; p < 0.001; Fig 2B) in the random-effects model analysis. There was no significant heterogeneity (p = 0.983; I2 = 0%) among the included studies. No single study affected the result of the sensitivity analysis (S2-1 Fig in S2 Fig) or cumulative analysis (S2-2 Fig in S2 Fig). In the process of funnel plot inspection (S2-3 Fig in S2 Fig), asymmetry was detected, but there was no evidence of publication bias in either the Begg and Mazumdar rank-correlation test (p = 0.452) or Egger’s regression test (p = 0.131).
Vitamin D deficiency defined as a vitamin D level of less than 15 ng/ml was associated with RDS (OR 17.267; 95% CI 1.084–275.112; p = 0.044; Fig 2C) in the random-effects model analysis. There was significant heterogeneity (p = 0.006; I2 = 86.8%) between the included studies (n = 2). We performed sensitivity analysis and cumulative analysis, but only two studies were included. We could not assess publication bias because of the small sample size.
Vitamin D deficiency defined as a vitamin D level of less than 10 ng/ml was also associated with RDS (OR 1.732; 95% CI 1.031–2.910; p = 0.038; Fig 2D) in the random-effects model analysis. There was heterogeneity (p = 0.014; I2 = 58.12%) among the included studies, and some studies affected the results of sensitivity analysis (S3-1 Fig in S3 Fig) and cumulative analysis (S3-2 Fig in S3 Fig). In the process of funnel-plot inspection, the presence of publication bias was not clear (S3-3 Fig in S3 Fig). In both the Begg and Mazumdar rank-correlation test (p = 0.118) and Egger’s regression test (p = 0.156), there was no evidence of publication bias.
A lower level of vitamin D was also associated with RDS (SMD = -0.656; 95% CI -1.029 to -0.283; p = 0.001; Fig 3) in the random-effects model. On assessment of heterogeneity, there was significant heterogeneity among studies (p < 0.001; I2 = 83.50%), but the result of sensitivity analysis (S4-1 Fig in S4 Fig) or cumulative analysis (S4-2 Fig in S4 Fig) showed no significant change in the pooled results. On inspection of the funnel plot, we could not distinguish the presence of publication bias (S4-3 Fig in S4 Fig). Thus, we performed the Begg and Mazumdar rank-correlation test (p = 0.368) and Egger’s regression test (p = 0.202), which showed no publication bias.
Fig 3. Meta-analysis for the association between vitamin D level and respiratory distress syndrome.
Discussion
We found that vitamin D deficiency (defined using all existing criteria) was significantly associated with RDS development. Moreover, a lower level of vitamin D within 24 h of birth was associated with RDS. Thus, vitamin D deficiency may be a risk factor for RDS.
RDS reflects impaired or delayed surfactant synthesis and secretion, triggering pulmonary atelectasis and respiratory distress in preterm infants. The incidence of RDS decreases with increasing gestational age at birth, from 97% at 23 weeks of gestation to 65% at 28 weeks [33], 10.5% at 34 weeks, and 0.3% at 38 weeks [34]. The administration of antenatal corticosteroids to the mother and exogenous instillation of surfactant soon after birth are established preventative strategies for RDS [35]. The methods and timing of surfactant therapy have changed over the past decade. A thin catheter is used for prophylactic treatment of babies at high risk of RDS. Rescue therapy increasingly features early, nasal, continuous positive airway pressure and antenatal steroids [36].
In the clinical setting, vitamin D deficiency is frequently observed in pregnant women and infants [37–39]. Vitamin D deficiency in pregnant women is associated with preterm birth [40–42]. Moreover, vitamin D levels are lower in preterm infants than in term infants because less stored vitamin D is transferred transplacentally, and preterm infants have higher vitamin requirements [43]. Vitamin D levels at birth were lower in neonates born at less than 28 weeks of gestation [39], and vitamin D deficiency was more prevalent in neonates born at less than 32 weeks of gestation [38] than in more mature neonates. The vitamin D level of the fetus depends entirely on maternal circulation of vitamin D, and the level of 25(OH)D in the fetus is about two-thirds to three-quarters of the maternal level [44]. Levels measured within 24 h after birth (before supplementation), or in cord blood, correlate well with the levels in the mother and the fetus. The concentration of 25-(OH)D in blood is widely used as a biomarker reflecting vitamin D status because of the long half-life of 25-(OH)D [1, 45]. Most of the studies in this meta-analysis obtained vitamin D levels on the first day of life, but two studies did not describe the exact times of vitamin D measurements [26, 27].
No consensus definition of vitamin D deficiency has been established; studies have used different cutoff values when defining vitamin D deficiency [46]. The Institute of Medicine Committee and the American Academy of Pediatrics [47, 48] recommend that vitamin D levels should be maintained above 12 ng/ml (close to 20 ng/ml) for normal bone accretion in infants less than 1 year of age. In the study by Holick [1], a vitamin D level above 20 ng/ml was recommended.
Various hormones, including corticosteroids and thyroid hormones, are involved in surfactant synthesis [23]. Vitamin D, a steroid hormone, plays roles in surfactant synthesis and lung maturation [2, 3, 5, 11, 12]. Moreover, vitamin D deficiency itself has been associated with an increased need for assisted ventilation and a longer duration of ventilator support in preterm infants [24], and with development of BPD [13] as well as RDS. An adequate vitamin level may help to reduce the risk of RDS, BPD, and the need for ventilator support.
Limitation of the study
There are several limitations to our study. First, each study used different diagnostic criteria when defining vitamin D deficiency, and small numbers of studies were thus included in the meta-analyses for the different cutoff values. This may have influenced the pooled effect. We were not able to assess the publication bias of studies with cutoff values of less than 30 ng/ml in terms of vitamin D deficiency. Second, various methods of measuring vitamin D levels in the blood have been used. Immunoassays such as enzyme-linked immunosorbent assays (ELISAs) and chemiluminescence assays, and liquid chromatography tandem mass spectrometry (LC-MS), were used to assess vitamin D status (Table 1). LC-MS is the gold standard method for 25-(OH)D measurement [43, 49]. Immunoassays yield higher vitamin D levels [43], and the performance thereof is not as good as that of LC-MS, especially at low vitamin D concentrations (< 8 ng/mL) [50]. The variabilities of vitamin D levels in this range seldom affect the identification of a vitamin D deficiency. Third, we could not control for any effect(s) of gestational age and/or the use of antenatal corticosteroids on RDS development; we could work only with the data from the included studies. Lastly, we found both heterogeneity and effects of individual studies on the results of sensitivity analysis and cumulative analysis for vitamin D deficiency < 10 ng/ml. Neonates included in the group with vitamin D >10 ng/ml could also have been affected by vitamin D deficiency (< 30 ng/ml or < 20 ng/ml). The use of different measuring methods for vitamin D could be why heterogeneity was present and the robustness of the sensitivity and cumulative analyses varied.
Conclusion
This meta-analysis demonstrated an association between vitamin D deficiency or the vitamin D level within 24 h after birth, and the risk of RDS. In this meta-analysis, the included studies described the risk of RDS at various levels of vitamin D, thus vitamin D levels below 30 ng/ml, 20 ng/ml, 15 ng/ml, and 10 ng/ml respectively. Monitoring of neonatal vitamin D levels soon after birth and of maternal vitamin D levels during pregnancy, and maintaining adequate vitamin D levels, may help to reduce the risk of respiratory morbidities including RDS and BPD [13] in preterm infants. Although, there is no consensus regarding what constitutes vitamin D deficiency or good vitamin D supplementation, vitamin D deficiency based on a level below 30 ng/ml showed an increased risk of RDS development in this meta-analysis. Thus, we cautiously assume that the optimal level of 25(OH)D should be higher than 30 ng/ml.
Supporting information
(DOC)
(DOCX)
(ZIP)
(ZIP)
(ZIP)
(ZIP)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
All authors received no specific funding for this work.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553 . [DOI] [PubMed] [Google Scholar]
- 2.Marin L, Dufour ME, Tordet C, Nguyen M. 1,25(OH)2D3 stimulates phospholipid biosynthesis and surfactant release in fetal rat lung explants. Biol Neonate. 1990;57(3–4):257–60. doi: 10.1159/000243200 . [DOI] [PubMed] [Google Scholar]
- 3.Rehan VK, Torday JS, Peleg S, Gennaro L, Vouros P, Padbury J, et al. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxy vitamin D3: production and biological activity studies in pulmonary alveolar type II cells. Mol Genet Metab. 2002;76(1):46–56. doi: 10.1016/s1096-7192(02)00022-7 . [DOI] [PubMed] [Google Scholar]
- 4.Lykkedegn S, Sorensen GL, Beck-Nielsen SS, Christesen HT. The impact of vitamin D on fetal and neonatal lung maturation. A systematic review. Am J Physiol Lung Cell Mol Physiol. 2015;308(7):L587–602. doi: 10.1152/ajplung.00117.2014 . [DOI] [PubMed] [Google Scholar]
- 5.Ardastani AG, Hashemi E, Beheshtinejad M, Dorostkar R. Comparison of 25- hydroxy Vitamin D levels in premature infants with and without respiratory distress. Iran J Neonatol. 2020;11(3):109–14. doi: 10.22038/ijn.2020.42523.1705 [DOI] [Google Scholar]
- 6.Sakurai R, Shin E, Fonseca S, Sakurai T, Litonjua AA, Weiss ST, et al. 1alpha,25(OH)2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. Am J Physiol Lung Cell Mol Physiol. 2009;297(3):L496–505. doi: 10.1152/ajplung.90539.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen TM, Guillozo H, Marin L, Tordet C, Koite S, Garabedian M. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. Am J Physiol. 1996;271(3 Pt 1):L392–9. doi: 10.1152/ajplung.1996.271.3.L392 . [DOI] [PubMed] [Google Scholar]
- 8.Nguyen TM, Guillozo H, Marin L, Dufour ME, Tordet C, Pike JW, et al. 1,25-dihydroxyvitamin D3 receptors in rat lung during the perinatal period: regulation and immunohistochemical localization. Endocrinology. 1990;127(4):1755–62. doi: 10.1210/endo-127-4-1755 . [DOI] [PubMed] [Google Scholar]
- 9.Nguyen M, Trubert CL, Rizk-Rabin M, Rehan VK, Besançon F, Cayre YE, et al. 1,25-Dihydroxyvitamin D3 and fetal lung maturation: immunogold detection of VDR expression in pneumocytes type II cells and effect on fructose 1,6 bisphosphatase. J Steroid Biochem Mol Biol. 2004;89-90(1–5):93–7. doi: 10.1016/j.jsbmb.2004.03.054 . [DOI] [PubMed] [Google Scholar]
- 10.Marin L, Dufour M, Nguyen T, Tordet C, Garabedian M. Maturational changes induced by 1 alpha, 25-dihydroxyvitamin D3 in type II cells from fetal rat lung explants. Am J Physiol. 1993;265(1):L45–L52. doi: 10.1152/ajplung.1993.265.1.L45 . [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Luo K, He X, Chen P. Association of vitamin D status at birth with pulmonary disease morbidity in very preterm infants. Pediatr Pulmonol. 2021;56(5):1215–20. doi: 10.1002/ppul.25233 . [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Wilson R, Bennett E, Zosky GR. Identification of vitamin D sensitive pathways during lung development. Respir Res. 2016;17:47. doi: 10.1186/s12931-016-0362-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HW, Lim G, Park YM, Chang M, Son JS, Lee R. Association between vitamin D level and bronchopulmonary dysplasia: A systematic review and meta-analysis. doi: 10.1371/journal.pone.0235332 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phokela SS, Peleg S, Moya FR, Alcorn JL. Regulation of human pulmonary surfactant protein gene expression by 1alpha,25-dihydroxyvitamin D3. Am J Physiol Lung Cell Mol Physiol. 2005;289(4):L617–26. doi: 10.1152/ajplung.00129.2004 . [DOI] [PubMed] [Google Scholar]
- 15.Yu R-Q, Chen D-Z, Hao X-Q, Jiang S-H, Fang G-D, Zhou Q. Relationship between serum 25 (OH) D levels at birth and respiratory distress syndrome in preterm infants. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19(11):1134–7. doi: 10.7499/j.issn.1008-8830.2017.11.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dogan P, Ozkan H, Koksal N, Bagci O, Varal IG. Vitamin D deficiency and its effect on respiratory distress syndrome in premature infants: results from a prospective study in a tertiary care centre. Afr Health Sci. 2020;20(1):437–43. doi: 10.4314/ahs.v20i1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matejek T, Zemankova J, Malakova J, Cermakova E, Skalova S, Palicka V. Severe vitamin D deficiency in preterm infants: possibly no association with clinical outcomes? J Matern Fetal Neonatal Med. 2020:1–9. doi: 10.1080/14767058.2020.1762560 . [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008 . [DOI] [PubMed] [Google Scholar]
- 20.GA Wells BS, D O’Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 21.Kontopantelis E, Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: a simulation study. Stat Methods Med Res. 2012;21(4):409–26. doi: 10.1177/0962280210392008 [DOI] [PubMed] [Google Scholar]
- 22.Ataseven F, Aygün C, Okuyucu A, Bedir A, Kücük Y, Kücüködük S. Is vitamin D deficiency a risk factor for respiratory distress syndrome? Int J Vitam Nutr Res. 2014;83(4):232–7. doi: 10.1024/0300-9831/a000165 . [DOI] [PubMed] [Google Scholar]
- 23.Fettah ND, Zenciroğlu A, Dilli D, Beken S, Okumuş N. Is higher 25-hydroxyvitamin D level preventive for respiratory distress syndrome in preterm infants? Am J Perinatol. 2015;32(3):247–50. doi: 10.1055/s-0034-1383849 . [DOI] [PubMed] [Google Scholar]
- 24.Onwuneme C, Martin F, McCarthy R, Carroll A, Segurado R, Murphy J, et al. The association of vitamin D status with acute respiratory morbidity in preterm infants. J Pediatr. 2015;166(5):1175–80.e1. doi: 10.1016/j.jpeds.2015.01.055 [DOI] [PubMed] [Google Scholar]
- 25.Mohamed Hegazy A, Mohamed Shinkar D, Refaat Mohamed N, Abdalla Gaber H. Association between serum 25 (OH) vitamin D level at birth and respiratory morbidities among preterm neonates. J Matern Fetal Neonatal Med. 2018;31(20):2649–55. doi: 10.1080/14767058.2017.1350162 . [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Feng Y, Chen X, Mao XN, Zhang JH, Zhao L, et al. Is there a potential link between vitamin D and pulmonary morbidities in preterm infants? J Chin Med Assoc. 2018;81(5):482–6. doi: 10.1016/j.jcma.2017.07.011 . [DOI] [PubMed] [Google Scholar]
- 27.Boskabadi H, Mamoori G, Khatami SF, Faramarzi R. Serum level of vitamin D in preterm infants and its association with premature-related respiratory complications: a case-control study. Electronic physician. 2018;10(1):6208–14. doi: 10.19082/6208 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kazzi SNJ, Karnati S, Puthuraya S, Thomas R. Vitamin D deficiency and respiratory morbidity among African American very low birth weight infants. Early Hum Dev. 2018;119:19–24. doi: 10.1016/j.earlhumdev.2018.02.013 . [DOI] [PubMed] [Google Scholar]
- 29.Kim I, Kim SS, Song JI, Yoon SH, Park GY, Lee YW. Association between vitamin D level at birth and respiratory morbidities in very-low-birth-weight infants. Korean J Pediatr. 2019;62(5):166–72. doi: 10.3345/kjp.2018.06632 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treiber M, Mujezinović F, Pečovnik Balon B, Gorenjak M, Maver U, Dovnik A. Association between umbilical cord vitamin D levels and adverse neonatal outcomes. J Int Med Res. 2020;48(10):300060520955001. doi: 10.1177/0300060520955001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Beltagi M, Rowiesha M, Elmashad A, Elrifaey SM, Elhorany H, Koura HG. Vitamin D status in preterm neonates and the effects of its supplementation on respiratory distress syndrome. Pediatr Pulmonol. 2020;55(1):108–15. doi: 10.1002/ppul.24552 . [DOI] [PubMed] [Google Scholar]
- 32.Boskabadi H, Maamouri G, Kalani-Moghaddam F, Ataee Nakhaei MH, Zakerihamidi M, Rakhshanizadeh F. Comparison of umbilical cord serum vitamin D levels between infants with transient tachypnea of the newborn and those without respiratory distress. Arch Iran Med. 2020;23(8):530–5. doi: 10.34172/aim.2020.55 . [DOI] [PubMed] [Google Scholar]
- 33.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–56. doi: 10.1542/peds.2009-2959 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Consortium on Safe Labor; Hibbard JU, Wilkins I, Sun L, Gregory K, Haberman S, Hoffman M, et al. Respiratory Morbidity in Late Preterm Births. JAMA. 2010;304(4):419–25. doi: 10.1001/jama.2010.1015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of respiratory distress syndrome—2016 Update. Neonatology. 2017;111(2):107–25. doi: 10.1159/000448985 . [DOI] [PubMed] [Google Scholar]
- 36.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te Pas A, et al. European consensus guidelines on the management of respiratory distress syndrome—2019 Update. Neonatology. 2019;115(4):432–50. doi: 10.1159/000499361 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saraf R, Morton SM, Camargo CA Jr., Grant CC. Global summary of maternal and newborn vitamin D status—a systematic review. Matern Child Nutr. 2016;12(4):647–68. doi: 10.1111/mcn.12210 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burris HH, Van Marter LJ, McElrath TF, Tabatabai P, Litonjua AA, Weiss ST, et al. Vitamin D status among preterm and full-term infants at birth. Pediatr Res. 2014;75(1–1):75–80. doi: 10.1038/pr.2013.174 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monangi N, Slaughter JL, Dawodu A, Smith C, Akinbi HT. Vitamin D status of early preterm infants and the effects of vitamin D intake during hospital stay. Arch Dis Child Fetal Neonatal Ed. 2014;99(2):F166–F8. doi: 10.1136/archdischild-2013-303999 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou SS, Tao YH, Huang K, Zhu BB, Tao FB. Vitamin D and risk of preterm birth: Up-to-date meta-analysis of randomized controlled trials and observational studies. J Obstet Gynecol Res. 2017;43(2):247–56. doi: 10.1111/jog.13239 . [DOI] [PubMed] [Google Scholar]
- 41.Chen Y-H, Fu L, Hao J-H, Wang H, Zhang C, Tao F-B, et al. Influent factors of gestational vitamin D deficiency and its relation to an increased risk of preterm delivery in Chinese population. Sci Rep. 2018;8(1):3608. doi: 10.1038/s41598-018-21944-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodnar LM, Platt RW, Simhan HN. Early-pregnancy vitamin D deficiency and risk of preterm birth subtypes. Obstet Gynecol. 2015;125(2):439–47. doi: 10.1097/AOG.0000000000000621 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894 . [DOI] [PubMed] [Google Scholar]
- 44.Weisman Y. Maternal, fetal and neonatal vitamin D and calcium metabolism during pregnancy and lactation. Endocr Dev. 2003;6:34–49. doi: 10.1159/000072768 . [DOI] [PubMed] [Google Scholar]
- 45.National Institute of Health. Dietary Supplement Fact Sheet: Vitamin D. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- 46.Gatera VA, Abdulah R, Musfiroh I, Judistiani RTD, Setiabudiawan B. Updates on the status of vitamin D as a risk factor for respiratory distress syndrome. Adv Pharmacol Sci. 2018;2018:8494816. doi: 10.1155/2018/8494816 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross AC TC, Yaktine AL. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Washington (DC): National Academies Press (US); 2011. The National Academies Collection: Reports funded by National Institutes of Health. [PubMed]
- 48.The American Academy of Pediatrics Statement of endorsement. Dietary reference intakes for calcium and vitamin D. Pediatrics. 2012;130(5):e1424–e. doi: 10.1542/peds.2012-2590 [DOI] [Google Scholar]
- 49.Altieri B, Cavalier E, Bhattoa Harjit P, Pérez-López FR, López-Baena MT, Pérez-Roncero GR, et al. Vitamin D testing: advantages and limits of the current assays. Eur J Clin Nutr. 2020;74(2):231–47. doi: 10.1038/s41430-019-0553-3 . [DOI] [PubMed] [Google Scholar]
- 50.Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem. 2012;58(3):531–42. doi: 10.1373/clinchem.2011.172155 . [DOI] [PubMed] [Google Scholar]