Abstract
Context
Reaction time (RT) is a critical element of return to participation (RTP), and impairments have been linked to subsequent injury after a concussion. Current RT assessments have limitations in clinical feasibility and in the identification of subtle deficits after concussion symptom resolution.
Objectives
To examine the utility of RT measurements (clinical drop stick, simple stimulus-response, single-task Stroop, and dual-task Stroop) to differentiate between adolescents with concussion and uninjured control individuals at initial assessment and RTP.
Design
Prospective cohort study.
Setting
A pediatric sports medicine center associated with a regional tertiary care hospital.
Patients or Other Participants
Twenty-seven adolescents with a concussion (mean age = 14.8 ± 2.1 years; 52% female; tested 7.0 ± 3.3 days postconcussion) and 21 uninjured control individuals (mean age = 15.5 ± 1.6 years; 48% female).
Main Outcome Measure(s)
Participants completed the Post-Concussion Symptoms Inventory (PCSI) and a battery of RT tests: clinical drop stick, simple stimulus-response, single-task Stroop, and dual-task Stroop.
Results
The concussion group demonstrated slower clinical drop stick (β = 58.8; 95% CI = 29.2, 88.3; P < .001) and dual-task Stroop (β = 464.2; 95% CI = 318.4, 610.0; P < .001) RT measures at the initial assessment than the uninjured control group. At 1-month follow up, the concussion group displayed slower clinical drop stick (238.9 ± 25.9 versus 188.1 ± 21.7 milliseconds; P < .001; d = 2.10), single-task Stroop (1527.8 ± 204.5 versus 1319.8 ± 133.5 milliseconds; P = .001; d = 1.20), and dual-task Stroop (1549.9 ± 264.7 versus 1341.5 ± 114.7 milliseconds; P = .002; d = 1.04) RT than the control group, respectively, while symptom severity was similar between groups (7.4 ± 11.2 versus 5.3 ± 6.5; P = .44; d = 0.24). Classification accuracy and area under the curve (AUC) values were highest for the clinical drop stick (85.1% accuracy, AUC = 0.86, P < .001) and dual-task Stroop (87.2% accuracy, AUC = 0.92, P < .002) RT variables at initial evaluation.
Conclusions
Adolescents recovering from concussion may have initial RT deficits that persist despite symptom recovery. The clinical drop stick and dual-task Stroop RT measures demonstrated high clinical utility given high classification accuracy, sensitivity, and specificity to detect postconcussion RT deficits and may be considered for initial and RTP assessment.
Keywords: adolescent athletes, mild traumatic brain injuries, sports, return to participation
Key Points
Adolescent athletes recovering from a concussion may have persistent deficits in reaction time at return-to-participation clearance despite symptom recovery.
Reaction time measures such as the clinical drop stick and dual-task Stroop test should be considered for the initial and return-to-play assessments to detect lingering deficits.
Quantifying reaction time in both static and dynamic positions may allow clinicians to understand how patients perform in sport-like conditions, when their attention must be divided across motor and cognitive domains.
Concussion, broadly defined as a traumatic brain injury that may or may not involve a loss of consciousness, may result in a variety of neurologic problems including cervical spine, neuromuscular, vestibular, oculomotor, and autonomic dysfunction.1–4 Given the variability in postconcussion clinical presentation and lack of objective measures available for routine concussion assessments, it can be difficult for clinicians to quantify the deficits that exist after injury and their effects on patient functioning.5–7 An understanding of which clinical measures can accurately identify individuals at risk for poor outcomes after a concussion is important for determining appropriate clinical pathways.
After a concussion, patients may experience impairments in neuromuscular control, static or dynamic balance, and dual-task performance.6,7 These concussion-related impairments of physical function have been theorized to be associated with an increased likelihood of musculoskeletal (MSK) injury in the first year after a concussion.6,8,9 Currently available clinical assessments may not be sufficient to identify subtle deficits after the resolution of concussion symptoms, most notably at time points closer to return to participation (RTP), or to identify those at risk for subsequent injuries.8 Although other objective measures such as neuroimaging, blood-based biomarkers, and gait analysis have shown utility in detecting persistent deficits beyond symptom recovery, they may not be feasible to implement across most clinical practice settings.6,10–12 More clinically feasible measures (eg, the Standardized Assessment of Concussion, Balance Error Scoring System, or neurocognitive tests) may not possess sufficient sensitivity to identify persistent deficits.8 Reaction time (RT) is a practical measure that is clinically feasible for health care providers7 and encompasses the neurocognitive and motor tasks required for adolescents to safely participate in sport.7,13 As RT is a critical element of readiness for return to sport,5 impaired RT that persists beyond concussion recovery may result in vulnerability to further injury.1,5,13 Current RT measurements, such as those performed during computerized neurocognitive testing, have high sensitivity when used acutely postconcussion, but sensitivity decreases within 1 week after injury.6,7 In addition, neurocognitive testing may be limited by the lack of access to computers, software, or time.6,7,13 Thus, clinically feasible methods of quantifying RT across treatment settings and recovery need to be identified. Furthermore, while dual-task gait deficits persist despite improvement in subjective symptom reports,14,15 quantifying RT in both static and dynamic positions may allow clinicians to understand how patients perform in sport-like conditions, when their attention must be divided across motor and cognitive domains.
A variety of testing techniques exist to assess RT after a concussion.5 Simple RT measures involve a response to a single stimulus, such as a clinical drop stick RT test or a simple stimulus-response task.7,15,16 Procedural RT measures involve multiple stimuli and responses, such as a standing auditory Stroop test in which responses to stimuli are recorded repeatedly over a period of time.15 Dual-task RT measures, such as a dual-task gait assessment in which an individual is asked to walk while completing a concurrent mental task, offer insight into motor-cognitive functioning.15 Given the many options for evaluating RT, investigating a multifaceted battery of RT assessments in a sample of injured and uninjured individuals may help clinicians understand the potential strengths and limitations of different approaches. Therefore, the primary purpose of our study was to examine the utility of 4 RT measurement approaches (clinical drop stick, simple stimulus-response, single-task Stroop, and dual-task Stroop) to differentiate between adolescents tested within 2 weeks of concussion and uninjured control adolescents. By determining sensitivity, specificity, and cut point values, we aimed to provide context to the clinical interpretation of each measurement approach. Secondarily, we examined whether between-groups differences in each RT measurement were present approximately 1 month after the initial assessment at RTP clearance. Sport-related concussion clinical symptoms typically resolve spontaneously within 2 to 4 weeks; however, researchers have shown that deficits in neuromuscular control and attention may affect the injury risk as players return to athletic activities.4,6,17–19
METHODS
Study Design and Participants
We conducted a prospective investigation of adolescent athletes who sustained a concussion and uninjured control individuals. The participants with concussion were recruited from a pediatric sports medicine center associated with a regional tertiary care hospital, where they were receiving outpatient treatment after their concussion. Recruits were included if they were between 12 and 18 years of age. Recruits with concussion were included if they were diagnosed with a concussion by a Board-certified sports medicine physician,20 seen within 14 days of injury, and reported a Post-Concussion Symptom Inventory (PCSI) score ≥9 during the initial assessment, ensuring that they were symptomatic at that time.5 We selected a PCSI score ≥9 as a minimal standard to be certain that participants had not experienced spontaneous recovery within the first 2 weeks after injury and before enrollment.21 Control participants were recruited from the surrounding community (ie, local high school athletes) to match the general characteristics of the concussion group (Table 1). We excluded recruits with coexisting lower extremity injuries affecting balance, a concussion within the past year (other than the current concussion for the concussion group), self-reported preexisting learning disability, documented neuroimaging of a structural brain injury, or a concussion sustained during a high-velocity impact (eg, motor vehicle collision).5 Volunteers were also excluded if they did not intend to return to sport after medical clearance.5 The local institutional review board approved the study protocol before data collection. All participants and their legal guardians (for participants <18 years of age) provided written informed consent or assent to engage in the study.
Table 1.
Participant Demographics, Medical History, and Injury Characteristics
| Concussion Group (n = 27) | Control Group (n = 21) | ||
|---|---|---|---|
| Variable | No. (%) | P Value | |
| Sex, female | 14 (52) | 10 (48) | .77 |
| Sport-related concussion? | 23 (85) | ||
| History | |||
| Concussion | 11 (41) | 3 (14) | .06 |
| Musculoskeletal injury | 16 (59) | 10 (48) | .42 |
| Headache disorder | 9 (33) | 1 (5) | .02a |
| Migraines | 6 (22) | 0 (0) | .02a |
| Anxiety | 3 (11) | 2 (10) | .86 |
| Depression | 4 (15) | 1 (5) | .26 |
| Attention-deficit or attention-deficit/hyperactivity disorder | 6 (22) | 5 (24) | .90 |
| Mean ± SD | |||
| Age, y | 14.8 ± 2.1 | 15.5 ± 1.6 | .23 |
| Height, cm | 167.2 ± 10.2 | 166.7 ± 10.7 | .88 |
| Weight, kg | 61.0 ± 14.5 | 59.5 ± 14.1 | .73 |
| Post-Concussion Symptoms Inventory score | |||
| Initial | 30.0 ± 15.3 | 7.0 ± 8.2 | <.001a |
| Final | 7.4 ± 11.2 | 5.3 ± 6.5 | .44 |
| Time, d | |||
| Initial test postconcussion | 7.0 ± 3.3 | NA | NA |
| Follow-up test after initial test | 28.7 ± 10.4 | 27.3 ± 0.7 | .53 |
| Symptom resolution postconcussion | 34.3 ± 21.2 | ||
Abbreviation: NA, not applicable.
P < .05.
Participants with a concussion were assessed by a trained member of the study team within 14 days of their concussion and retested approximately 28 days later. This time frame was used for the control group as well (initial test with a follow-up test approximately 28 days later) and was selected a priori to reflect the typical RTP clearance window for adolescents after a concussion.4,22 In this observational study, we did not make any recovery diagnosis at the 28-day follow-up time point but performed only the evaluations needed for the procedures outlined in the following section.
Outcome Variables
Clinical Drop Stick RT
As in previous studies, a 1.3-m measuring stick was coated with high-friction tape, marked every 1 cm, and attached to a hockey puck.13,23,24 Participants were seated with their dominant forearm resting horizontally on a height-adjusted table so that the elbow was comfortable in 90° of flexion. The test administrator placed the drop stick device vertically between the participant’s thumb and index finger. Individuals were instructed to catch the stick as quickly as possible after it was dropped at random intervals of time. Each person was allotted 2 practice trials followed by 8 test trials. The distance the puck traveled was measured in centimeters. The average distance of the 8 trials was recorded and used for analysis. Distance was converted to RT (milliseconds) using the equation5,7:
 |
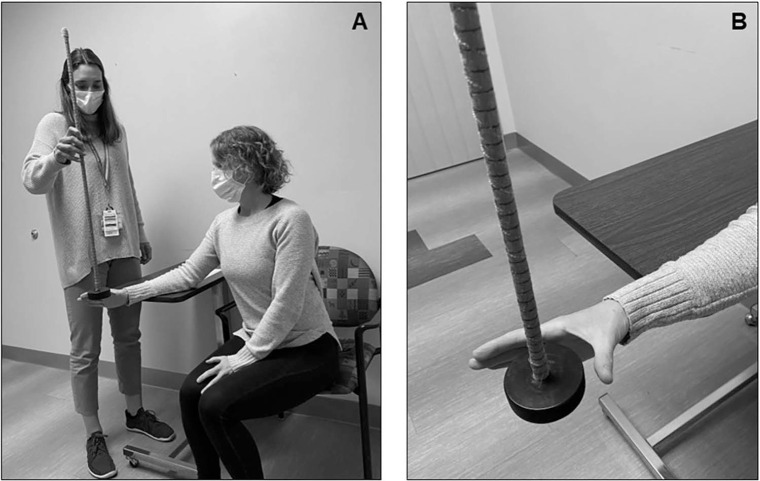
The clinical drop stick RT test has been established and validated in several studies of RT in athletes (Figure 1).7,13,23,25
Figure 1.
Representative photograph of the clinical drop stick reaction time (A) test setup and (B) starting position. The image emphasizes participant hand placement in an “L” versus a “C” for the starting position.
Simple RT (Smartphone)
Simple RT data was gathered using an RT test on a smartphone (model Galaxy S8; Samsung Electronics Co, Ltd) with the Reaction Time Tester Pro application, described earlier in a feasibility trial examining clinical RT assessment tools.16 We placed the phone flat on a table at a 2-cm standardized distance in front of the participant’s self-selected dominant hand.16 A red dot appeared in the middle of the phone screen, and at random intervals, the dot turned green. The participant was instructed to tap the dot as quickly as possible once the color changed to green and return to the resting position (ie, finger on the table) between trials. Response time was recorded in the application as the time from the dot color changing to green to the participant tapping the screen. Average response time (milliseconds) was calculated from 30 successful trials.16
Stroop RT (Single Task and Dual Task)
During the single-task Stroop, participants were instructed to stand in place with feet together and eyes open for 30 seconds while responding to a series of auditory Stroop tasks.14 A computer-recorded voice spoke the word “high” or “low” in either a high or low pitch, with word and pitch combinations selected at random. Through headphones attached to the smartphone, individuals were instructed to audibly identify the pitch of the word, not the word itself, throughout each 30-second trial.15 A smartphone application (IMPROVE; Control One LLC) was used to elicit the Stroop task audio recordings and calculate the response time to detect the response accuracy as high, low, or unknown.15,16 The smartphone recorded the time in milliseconds between each auditory cue and oral response. Reaction time was calculated by taking the difference between stimulus onset and oral response onset. Subsequently, the IMPROVE application classified each response as correct, incorrect, or unanswered. Four trials were completed per condition, and averages were obtained across the 4 trials.
Dual-task RT was assessed using a similar protocol as for the single-task Stroop, with the addition of a simultaneous walking task. Participants were instructed to walk at a self-selected pace along a 10-m pathway toward an object placed on the ground while simultaneously responding to the Stroop stimuli. On reaching the object, the person was asked to walk around it and return to the initial position.15 During the trial, the smartphone application recorded gait velocity, cognitive Stroop response accuracy, and response time. Four trials were completed, and averages were obtained across the 4 trials.
Symptom Severity (PCSI)
During initial and follow-up assessments, participants also completed the PCSI to report their current symptom severity. The PCSI is a patient-reported inventory with 22 questions measuring the perceived severity of postconcussion symptoms. Common concussion symptoms are rated from 0 (none) to 6 (severe), and the summed responses provide an overall symptom score.14,26 Total scores range from 0 to 132. The reliability and validity of the PCSI are well documented.21
Statistical Analysis
Data are presented as mean ± SD for continuous variables and the number included and corresponding percentage for categorical variables. We first compared participant demographics, medical history, and injury characteristics using independent-samples t tests and χ2 or Fisher exact (for cell sizes < 10) analyses. Characteristics that were different between groups (P < .05) were added as covariates in adjusted regression models.
To address our primary purpose, we compared the 4 RT measurements between the concussion and control groups using independent-samples t tests and calculated effect sizes (Cohen d), mean differences, and 95% CIs for the mean difference and area under the curve (AUC) values from a receiver operating characteristic analysis. For effect sizes, we interpreted effects as d > 0.8, large; 0.5 to 0.79, medium; 0.2 to 0.49, small; and <0.2, none.27 Area under the curve values were interpreted as >0.9 = outstanding, 0.8 to 0.89 = excellent, 0.7 to 0.89 = acceptable, and <0.7 = poor discrimination. From the AUC analysis, optimal cut points were identified that described the RT value that best differentiated between the concussion and control groups, along with the corresponding classification accuracy, sensitivity, and specificity at that level. In addition, we constructed multiple linear regression models to adjust for potential confounders; the predictor variable was group (concussion versus control), the outcome variable was the RT measurement, and the covariates were demographic or injury history variables that differed between groups.
For our secondary analysis, we compared groups at the follow-up time point using independent-samples t tests and calculated Cohen d effect sizes and mean differences. Due to loss to follow up and our intent to understand between-groups (not within-group) differences at that assessment, we elected to analyze individuals who returned for testing independently from our primary (initial assessment) analysis. All statistical tests were evaluated for statistical significance at α = .05, 2 sided, and performed using Stata statistical software (version 16; StataCorp LLC).
RESULTS
A total of 48 participants (27 adolescents with a concussion and 21 uninjured control adolescents) enrolled in the study and underwent initial RT assessment. Of those, 41 (85% retention; n = 23 in the concussion group and n = 18 in the control group) returned for the follow-up test and completed the multifaceted RT assessment at both time points. The 2 groups had similar demographic characteristics, although a greater proportion of the concussion group reported a preconcussion history of headache disorders and migraine than the control group (Table 1). In addition, the concussion group reported a higher concussion symptom burden on the PCSI than the control group during the initial assessment (Table 1). Thus, preinjury headache disorder, migraine, and PCSI score were included as covariates in multiple linear regression modeling.
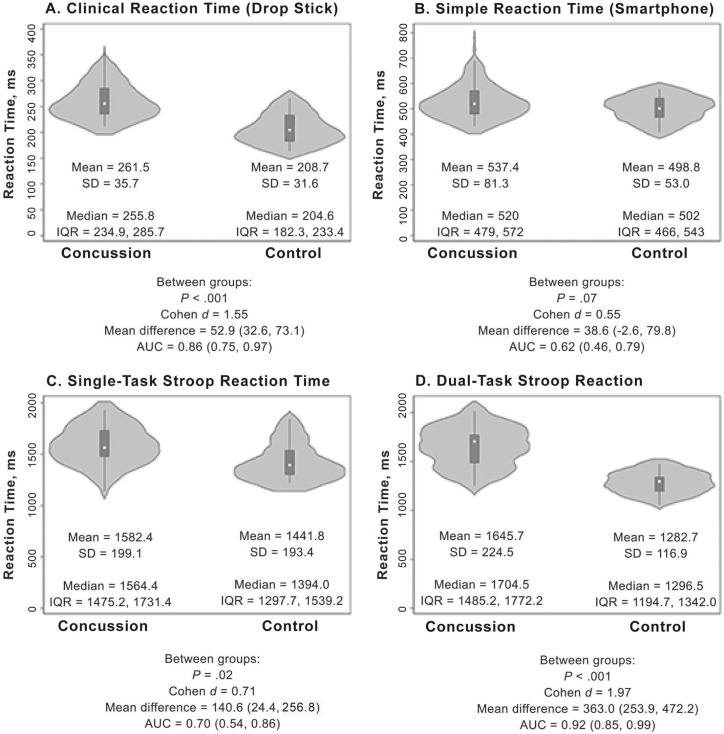
During the initial evaluation, the concussion group demonstrated slower clinical drop stick (Figure 2A), single-task Stroop (Figure 2C), and dual-task Stroop (Figure 2D) RT. The AUC was outstanding for the dual-task Stroop RT, excellent for the clinical drop stick RT, and acceptable for the single-task Stroop RT (Figure 2). We observed a medium effect size but nonsignificant difference between groups for the simple RT measurement (Figure 2B). Classification accuracy was highest for the dual-task Stroop and clinical drop stick RT measurements (Table 2). After adjusting for preinjury headache disorder, migraine, and initial symptom burden, the concussion group had slower clinical drop stick (β = 58.8; 95% CI = 29.2, 88.3; P < .001) and dual-task Stroop (β = 464.2; 95% CI = 318.4, 610.0; P < .001) RTs at the initial visit than the control group. Simple (β = −3.54; 95% CI = −58.3, 51.2; P = .90) and single-task Stroop (β = 32.8; 95% CI = −124.6, 190.2; P = .68) RT results were not associated with group.
Figure 2.
Reaction time performance characteristics and statistical test results between the concussion and control groups across the 4 methods. Violin plots are presented as median (center dot) and interquartile range (IQR; box around the median). The shaded area represents the probability density of data at each level of the scale, smoothed using a kernel density estimator. Abbreviation: AUC, area under the curve.
Table 2.
Cut Points With the Highest Classification Accuracy for Distinguishing Between the Concussion and Control Groups Within 14 Days of Concussion Across the 4 Reaction Time Measurements
| Reaction Time | Cut Point, ms | Percentage |
||
|---|---|---|---|---|
| Classification Accuracy | Sensitivity | Specificity | ||
| Clinical | 220 | 85.1 | 96.3 | 85.1 |
| Simple | 505 | 60.4 | 63.0 | 57.1 |
| Single-task (standing) Stroop | 1398 | 74.5 | 84.6 | 74.5 |
| Dual-task (walking) Stroop | 1485 | 87.2 | 76.9 | 100.0 |
At 1-month follow-up testing, differences were present between groups with large effect sizes for clinical, single-task Stroop, and dual-task Stroop RT and small or nonsignificant effects between groups for simple RT and symptom burden (Table 3). For participants with a concussion, this follow-up visit was associated with RTP clearance: patients were deemed clinically recovered from concussive symptoms.
Table 3.
Reaction Time Performance Characteristics Between the Concussion and Control Groups at the 1-Month Follow-Up Testa
| Reaction Timeb | Group, Mean ± SD |
P Value | Cohen d | Mean Difference, 95% CI | |
|---|---|---|---|---|---|
| Concussion | Control | ||||
| Clinical | 238.9 ± 25.9 | 188.1 ± 21.7 | <.001 | 2.10 | 50.8 (35.4, 66.2) |
| Simple | 525.6 ± 95.1 | 509.4 ± 59.1 | .50 | 0.20 | 16.2 (–32.5, 64.9) |
| Single-task (standing) Stroop | 1527.8 ± 204.5 | 1319.8 ± 133.5 | .001 | 1.20 | 208.0 (94.4, 321.7) |
| Dual-task (walking) Stroop | 1549.9 ± 264.7 | 1341.5 ± 114.7 | .002 | 1.04 | 208.4 (80.0, 336.8) |
| Symptom severity, Post-Concussion Symptom Inventory score | 7.4 ± 11.2 | 5.3 ± 6.5 | .44 | 0.24 | 2.2 (–3.4, 7.8) |
N = 41 participants (85% of the sample, n = 23 concussion and n = 18 control) returned for follow-up testing at approximately 1 month after the initial test.
All units were ms except for the symptom severity score.
DISCUSSION
The clinical drop stick and dual-task Stroop RT tests provided high clinical utility and may be useful during the initial and RTP concussion assessments. These approaches have high classification accuracy, sensitivity, and specificity to detect postconcussion RT deficits within the first 2 weeks postconcussion and may be useful for clinicians assessing postconcussion RT impairments as part of a multifaceted evaluation. Additionally, at 1-month follow-up testing, differences were found between groups for clinical, single-task Stroop, and dual-task Stroop RT. The RT assessments we selected are readily accessible to health care providers in a variety of settings and do not require laboratory or computerized testing to identify deficits, although some technological elements may still be required for the simple (eg, a smartphone) and Stroop RT assessments. They also are quick to perform and may offer insight into athletes’ motor function as they return to highly complex motor-cognitive tasks during sports.6,16 The existing literature, including the 2017 Concussion in Sport Group consensus statement and the 2020 Concussion Clinical Practice Guideline, recommends including RT measurement in concussion evaluations2,20 as an objective measure to monitor recovery beyond subjective symptom reporting.2 Our work extends observations that RT assessments can supply objective assessment of persisting deficits after symptom resolution and highlights the need for continued rehabilitation before RTP.
Both the clinical drop stick and dual-task Stroop RT findings were slower among injured athletes than uninjured control participants within the first 14 days of concussion. These tests involve upper extremity function (clinical drop stick RT), whole-body movement (dual-task Stroop RT), and simultaneous cognitive and motor tasks (dual-task Stroop RT), each of which is relevant to the demands of sports and may be overlooked in other RT assessments. Simple and single-task Stroop RT did not identify differences between the concussion and control groups at initial assessment, which, along with lower sensitivity and specificity, indicates that these assessments may not be ideal for evaluating RT in the first 2 weeks after concussion. Furthermore, after adjusting for potential confounding variables, we noted that the univariable difference between groups for the single-task Stroop RT was no longer significant, suggesting that a history of a headache disorder, a history of migraines, and the initial symptom burden are possible modifying factors in the relationship between RT and concussion.
At follow-up assessment, which corresponded with the time of clearance for the participants with concussion, both the clinical drop stick and dual-task Stroop RT tests detected differences between adolescents with concussion and uninjured control adolescents, despite no difference in symptom burden. Prior authors determined that athletes were at an increased risk of MSK injury after concussion compared with a healthy cohort, which may be due in part to persisting impairments in dual-task cognitive processing, neuromuscular control, or both.8,14,16–19 Although commonly used concussion assessments and mental health evaluations have not predicted subsequent MSK injuries postconcussion,8 the RT deficits noted in our study may represent an additional metric to consider given the statistically and clinically significant between-groups differences both initially postinjury and after symptom resolution. Moreover, testing RT under dual-task conditions may better reflect sport-specific demands.6 Clinically, our results have potential implications for the postconcussion injury risk. Reaction time may represent an interventional target, given that rehabilitation professionals can perform neuromuscular-training programs with athletes returning to sport after a concussion,16 with demonstrated effectiveness in reducing subsequent injuries.28 Incorporating RT training into these programs with a rehabilitation specialist may be another avenue for addressing remaining deficits, thereby reducing the likelihood of subsequent injury.
Our study had several limitations that are important to consider when interpreting findings and generalizing to additional populations. Participants were seen at a specialty pediatric sports medicine clinic, 12 to 18 years old, and evaluated initially within 14 days of concussion. Those who had recovered by the time of the first assessment (PCSI score ≤ 9) were excluded; thus, our sample had a relatively similar profile upon enrollment, but this did not account for the variation in recovery within the first 2 to 4 weeks after concussion. Therefore, our findings may not be generalizable to patients seen in other care settings, such as emergency departments or on field, or to patients seen sooner or later postinjury (ie, within hours or >2 weeks). Also, the performance improvements in the control group may reflect a practice effect for the clinical drop stick RT assessment, indicating that healthy participants improve with repeat administration of the test. These practice effects may also exist for patients with concussion, independent of improvements in neuromuscular function as part of concussion recovery. Additionally, the time frame of follow-up was selected based on previous studies generalizing that concussion RTP typically occurs around 1 month postinjury.13 We acknowledge that the concussion recovery timeline varies and that athletes may recover in as few as 7 days or have lingering symptoms past our time frame for follow-up. Further, whether patients participated in formalized rehabilitation programs or received a home program focusing on addressing RT deficits was unknown. Future authors should examine whether dual-task RT deficits that persist after clearance to resume sport participation are related to a higher incidence of subsequent MSK injury. Moreover, future researchers should identify normative values and normative-based cutoff scores for each of the RT tests and provide minimal clinically important difference values or reliable change indices to determine a clinically meaningful threshold as it relates to postinjury assessment.
In conclusion, the clinical drop stick and dual-task Stroop RT tests are assessments to consider when quantifying concussion deficits after adolescent concussion. Dual-task Stroop RT testing may be the optimal test to incorporate because clinical RT performance improvements may be seen due to practice effects. Such approaches are available in many different clinical settings and may reduce the reliance on time-consuming or cost-prohibitive approaches.
ACKNOWLEDGMENTS
Research reported in this work was supported by the National Institute of Neurological Disorders and Stroke (Award No. R43NS108823), the Children’s Hospital Colorado Research Institute, and the Tai Foundation. Unrelated to this study, Dr Howell has received research support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (Award Nos. R03HD094560, R01HD108133), the National Institute of Neurological Disorders and Stroke (Award Nos. R01NS100952, R43NS108823), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Award No. 1R13AR080451), MINDSOURCE Brain Injury Network, the Tai Foundation, and the Colorado Clinical and Translational Sciences Institute (Award No. UL1 TR002535‐05), and he serves on the Scientific/Medical Advisory Board of Synaptek, LLC.
REFERENCES
- 1. Howell DR, Lugade V, Potter MN, Walker G, Wilson JC. A multifaceted and clinically viable paradigm to quantify postural control impairments among adolescents with concussion Physiol Meas 2019. 40 (8) 084006. 10.1088/1361-6579/ab3552 [DOI] [PubMed] [Google Scholar]
- 2. Quatman-Yates CC, Hunter-Giordano A, Shimamura KK, et al. Physical therapy evaluation and treatment after concussion/mild traumatic brain injury J Orthop Sports Phys Ther 2020. 50 (4) CPG1– CPG73 10.2519/jospt.2020.0301 [DOI] [PubMed] [Google Scholar]
- 3. Leddy JJ, Haider MN, Ellis M, Willer BS. Exercise is medicine for concussion Curr Sports Med Rep 2018. 17 (8) 262– 270 10.1249/JSR.0000000000000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport Br J Sports Med 2019. 53 (4) 213– 225 10.1136/bjsports-2018-100338 [DOI] [PubMed] [Google Scholar]
- 5. Reinking S, Seehusen CN, Walker GA, Wilson JC, Howell DR. Transitory kinesiophobia after sport-related concussion and its correlation with reaction time J Sci Med Sport 2022. 25 (1) 20– 24 10.1016/j.jsams.2021.07.010 [DOI] [PubMed] [Google Scholar]
- 6. Howell DR, Lynall RC, Buckley TA, Herman DC. Neuromuscular control deficits and the risk of subsequent injury after a concussion: a scoping review Sports Med 2018. 48 (5) 1097– 1115 10.1007/s40279-018-0871-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eckner JT, Kutcher JS, Richardson JK. Effect of concussion on clinically measured reaction time in nine NCAA Division I collegiate athletes: a preliminary study PM R 2011. 3 (3) 212– 218 10.1016/j.pmrj.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buckley TA, Howard CM, Oldham JR, Lynall RC, Swanik CB, Getchell N. No clinical predictors of postconcussion musculoskeletal injury in college athletes Med Sci Sports Exerc 2020. 52 (6) 1256– 1262 10.1249/MSS.0000000000002269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oldham JR, Howell DR, Knight CA, Crenshaw JR, Buckley TA. Gait performance is associated with subsequent lower extremity injury following concussion Med Sci Sports Exerc 2020. 52 (11) 2279– 2285 10.1249/MSS.0000000000002385 [DOI] [PubMed] [Google Scholar]
- 10. Büttner F, Howell DR, Ardern CL, et al. Concussed athletes walk slower than non-concussed athletes during cognitive-motor dual-task assessments but not during single-task assessments 2 months after sports concussion: a systematic review and meta-analysis using individual participant data Br J Sports Med 2020. 54 (2) 94– 101 10.1136/bjsports-2018-100164 [DOI] [PubMed] [Google Scholar]
- 11. Kamins J, Bigler E, Covassin T, et al. What is the physiological time to recovery after concussion? A systematic review Br J Sports Med 2017. 51 (12) 935– 940 10.1136/bjsports-2016-097464 [DOI] [PubMed] [Google Scholar]
- 12. Koerte IK, Schultz V, Sydnor VJ, et al. Sex-related differences in the effects of sports-related concussion: a review J Neuroimaging 2020. 30 (4) 387– 409 10.1111/jon.12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lempke LB, Howell DR, Eckner JT, Lynall RC. Examination of reaction time deficits following concussion: a systematic review and meta-analysis Sports Med 2020. 50 (7) 1341– 1359 10.1007/s40279-020-01281-0 [DOI] [PubMed] [Google Scholar]
- 14. Howell DR, Bonnette S, Diekfuss JA, et al. Dual-task gait stability after concussion and subsequent injury: an exploratory investigation Sensors (Basel) 2020. 20 (21) 6297. 10.3390/s20216297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Howell DR, Seehusen CN, Wingerson MJ, Wilson JC, Lynall RC, Lugade V. Reliability and minimal detectable change for a smartphone-based motor-cognitive assessment: implications for concussion management J Appl Biomech 2021. 37 (4) 380– 387 10.1123/jab.2020-0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howell DR, Seehusen CN, Walker GA, Reinking S, Wilson JC. Neuromuscular training after concussion to improve motor and psychosocial outcomes: a feasibility trial Phys Ther Sport 2021. 52: 132– 139 10.1016/j.ptsp.2021.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lynall RC, Mauntel TC, Padua DA, Mihalik JP. Acute lower extremity injury rates increase after concussion in college athletes Med Sci Sports Exerc 2015. 47 (12) 2487– 2492 10.1249/MSS.0000000000000716 [DOI] [PubMed] [Google Scholar]
- 18. Brooks MA, Peterson K, Biese K, Sanfilippo J, Heiderscheit BC, Bell DR. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes Am J Sports Med 2016. 44 (3) 742– 747 10.1177/0363546515622387 [DOI] [PubMed] [Google Scholar]
- 19. Gilbert FC, Burdette GT, Joyner AB, Llewellyn TA, Buckley TA. Association between concussion and lower extremity injuries in collegiate athletes Sports Health 2016. 8 (6) 561– 567 10.1177/1941738116666509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCrory P, Meeuwisse W, Dvořák J, et al. Consensus statement on concussion in sport—the 5th International Conference on Concussion in Sport held in Berlin, October 2016 Br J Sports Med 2017. 51 (11) 838– 847 10.1136/bjsports-2017-097699 [DOI] [PubMed] [Google Scholar]
- 21. Lovell MR, Iverson GL, Collins MW, et al. Measurement of symptoms following sports-related concussion: reliability and normative data for the Post-Concussion Scale Appl Neuropsychol 2006. 13 (3) 166– 174 10.1207/s15324826an1303_4 [DOI] [PubMed] [Google Scholar]
- 22. Howell DR, Zemek R, Brilliant AN, Mannix RC, Master CL, Meehan WP., 3rd. Identifying persistent postconcussion symptom risk in a pediatric sports medicine clinic Am J Sports Med 2018. 46 (13) 3254– 3261 10.1177/0363546518796830 [DOI] [PubMed] [Google Scholar]
- 23. Eckner JT, Richardson JK, Kim H, Joshi MS, Oh YK, Ashton-Miller JA. Reliability and criterion validity of a novel clinical test of simple and complex reaction time in athletes Percept Mot Skills 2015. 120 (3) 841– 859 10.2466/25.15.PMS.120v19x6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eckner JT, Kutcher JS, Broglio SP, Richardson JK. Effect of sport related concussion on clinically measured simple reaction time. Br J Sports Med. 2014;48((2)) doi: 10.1136/bjsports-2012-091579. 10.1136/bjsports-2012-091579. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eckner JT, Richardson JK, Kim H, Lipps DB, Ashton-Miller JA. A novel clinical test of recognition reaction time in healthy adults Psychol Assess 2012. 24 (1) 249– 254 10.1037/a0025042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the Post-Concussion Symptom Scale: baseline and postconcussion factors Am J Sports Med 2012. 40 (10) 2375– 2384 10.1177/0363546512455400 [DOI] [PubMed] [Google Scholar]
- 27. Cohen J. Statistical Power Analysis for the Behavioral Sciences Academic Press; 2013. [Google Scholar]
- 28. Howell DR, Seehusen CN, Carry PM, Walker GA, Reinking SE, Wilson JC. An 8-week neuromuscular training program after concussion reduces 1-year subsequent injury risk: a randomized clinical trial Am J Sports Med 2022. 50 (4) 1120– 1129 10.1177/03635465211069372 [DOI] [PubMed] [Google Scholar]