Abstract
Precision nanomedicine can be employed as an alternative to chemo- or radiotherapy to overcome challenges associated with the often narrow therapeutic window of traditional treatment approaches, while safely inducing effective, targeted anti-tumor responses. Herein, we report the formulation of a therapeutic nanocomposite comprising of a hyaluronic acid (HA)-coated gold nanoframework (AuNF) delivery system and encapsulated IT848, a small molecule with potent anti-lymphoma and -myeloma properties that targets the transcriptional activity of nuclear factor kappa B (NF-κB). The porous AuNFs fabricated via a liposome-templated approach were loaded with IT848 and surface functionalized with HA to formulate the nanotherapeutics that were able to efficiently deliver the payload with high specificity to myeloma and lymphoma cell lines in vitro. In vivo studies characterized biodistribution, pharmacokinetics and safety of HA-AuNFs, and we demonstrated superior efficacy of HA-AuNF formulated IT848 versus free IT848 in lymphoma mouse models. Both in vitro and in vivo results affirm that the AuNF system can be adopted for targeted cancer therapy, improving the drug safety profile, and enhancing its efficacy with minimal dosing. HA-AuNF-formulated IT848 therefore has strong potential for clinical translation.
Keywords: Lymphoma, Nuclear factor-κB inhibitor, gold nanoparticles, targeted drug delivery, CD44, hyaluronic acid
Graphical Abstract

1. INTRODUCTION
Hematologic malignancies, comprising of approximately one-third of all cancers worldwide, originate from abnormal differentiation or mutations of hematopoietic stem cells in bone marrow1–2. Radiation, chemotherapy and/or stem cell transplantation remain the mainstay of blood cancer treatment. However, these treatment modalities are often characterized by low therapeutic indices, poor bioavailability, high-dose requirements, and significant healthy tissue toxicity2–4. To overcome these drawbacks, molecularly targeted drugs have emerged as an appealing alternative. Targeted cancer therapeutics are vastly classified into immunotherapy with macromolecule (monoclonal antibodies5 or effector cells such as CAR T cells6–7) and small molecule agents8–9. Compared to monoclonal antibodies (~150kDa) that typically target only cell surface antigens, small molecules (≤500Da) can readily translocate through the plasma membrane and potentially bind to various extracellular and intracellular targets10–11. Small molecules have shown great potentials to mediate specific inhibition of oncogenic signaling pathways targeting serine/threonine/tyrosine kinases, proteosomes, matrix metalloproteinases (MMPs), heat shock proteins (HSPs), and anti-apoptotic proteins9, 11. Nuclear factor-κB (NF-κB) is a family of transcription factors that regulate various genes contributing to normal cellular activities (cell propagation, differentiation, and survival)12 as well as pathogenesis of numerous diseases such as cancer, inflammation and autoimmunity13–15. There is a wide variety of clinically approved biologics, macromolecules and small molecules that are modulating the NF-κB signaling cascades (often discovered later, after development and marketing of the drugs)13, 16. The novel small molecule NF-κB inhibitor IT848 ((9-chloro-8-(hexyloxy)-2H-chromeno[2,3-d]pyrimidine-2,4(3H)-dione)), currently in preclinical development17, is a promising candidate for targeted treatment of hematologic malignancies. We have recently shown that IT848 can specifically inhibit NF-κB transcriptional activity to induce anti-tumor functions against multiple myeloma cell lines and patient cells in vitro and reduce the tumor burden in mouse models of multiple myeloma18.
In recent years, rapid developments in the field of nanotechnology have fueled the evolution of nanomedicine for cancer therapy2, 19–20. As recognized, nanomaterial/nanoparticle-based systems provide an opportunity for multimodal and site-specific delivery of therapeutics to target tissues with improved pharmacokinetics and stability, reduced non-specificity, and enhanced permeability and retention3–4, 21–22. As a matter of fact, neoplastic diseases, especially disseminated hematological malignancies (such as lymphoma, leukemia, and multiple myeloma), can benefit immensely from the broad panorama of nanoparticles providing innovative non-invasive approaches for diagnostics, treatment and theranostics20, 23–26. Among a plethora of functionalized nanomaterials (carbon nanotubes, inorganic mesoporous silica, quantum dots, peptide nanostructures, liposomes, polymeric nanoparticles, etc.)19, 27 used in drug delivery, disease diagnostics and therapy, gold-based nanoparticles display excellent efficacy as drug carriers, photothermal agents, contrast agents and radiosensitizers4, 22, 28, owed to their versatility with regard to chemical modifications, high biocompatibility, and strong localized surface plasmon resonance (LSPR)4, 29–30. Porous gold nanoframeworks (AuNFs) with a large interior surface present a unique opportunity to easily accommodate therapeutic molecules within the well-defined mesopores and then, in contrast to more rapidly degradable polymeric nanocarriers, delay their elimination by the kidney or liver for a prolonged therapeutic period. In addition, the intensified electromagnetic (EM) field on AuNFs from the LSPR effect also enables surface-enhanced Raman scattering (SERS)31 for noninvasively tracking extrinsic molecules and monitoring intracellular changes32–33. Taking advantage of the abovementioned features, AuNFs successfully exemplified their capabilities for targeted delivery of doxorubicin to solid breast tumors in a xenograft model22. Preferential tumor accumulation and increased cellular uptake of AuNF nanocarriers can be achieved by exploiting the binding interaction between the cell-surface transmembrane glycoprotein CD44, overexpressed on tumor cells, and hyaluronic acid (HA)34. This HA-CD44 ligand-receptor based active targeting approach improves the drug carrier stability/pharmacokinetics and reduces adverse effects34. However, it still remains to be shown whether AuNF-based targeted drug delivery can be applied to hematological malignancies to achieve similar therapeutic benefits as in solid tumors. Furthermore, the green synthesis route and apt control over particle size, mesopores, and surface modification make these particles ideal vehicles for therapeutics such as IT848 that require customized formulations to minimize the risk for adverse effects such as dose-limiting cumulative toxicity, which was identified in mouse studies using frequent intraperitoneal (i.p.) administration of cyclodextrin-encapsulated IT84818. We therefore developed a mesoporous AuNF-based IT848 formulation and evaluated intravenous (i.v.) weekly delivery of IT848 for targeted lymphoma therapy as illustrated in Figure 1. Our study demonstrates that our AuNF-based targeted delivery system was better tolerated by mice and afforded superior anti-lymphoma activity compared to cyclodextrin-encapsulated IT848. Although in this study only the drug delivery capabilities of AuNFs were explored, further efforts will be made to utilize the photothermal effect of AuNFs for photo-switchable release of the payload and their surface plasmonic effect for enhanced Raman imaging to intracellularly monitor molecular alterations.
Figure 1.

Engineering and pharmacodynamics of IT848-loaded mesoporous gold nanoframeworks (AuNFs). A) Depiction of the expected biodistribution of IT8484-AuNFs in the tumor microenvironment and major organs (liver, spleen, kidneys, lungs, bone marrow and heart) after intravenous injection into the lymphoma-bearing mice, along with selective target/uptake by HA receptor (CD44)-overexpressing cancer cells. Upon uptake by target cells, IT848 released from AuNFs will mediate the inhibition of NF-kB transcriptional activity; B) Schematic illustration of preparation of IT848-HA-AuNFs following 1) AuNF fabrication via the liposome templating approach, 2) IT848 encapsulation and 3) HA surface functionalization of porous AuNFs. The chemical structure of IT848 is also shown.
2. MATERIALS AND METHODS
2.1. Chemicals.
Dipalmitoyl phosphatidylcholine (DPPC) and cholesterol were purchased from Fisher Scientific International, Inc. (Waltham, MA). Tetrachloroauric acid trihydrate (HAuCl4·3H2O), polyethylene glycol (PEG) 400, ascorbic acid (AA), thiol-PEG-amine (SH-PEG-NH2, average MW= 5,000 kDa), hyaluronic acid (HA, average MW=10k and 1000k), sodium carbonate, chloroform and 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) were acquired from Sigma-Aldrich (St. Louis, MO). Carboxylic acid Cyanine-7 (Cy7) was purchased from Lumiprobe (Regus, MD). IT848 was kindly provided by ImmuneTarget Inc (San Diego, CA). All chemicals were used as received without additional purification. Milli-Q deionized (D.I.) water (>18.2 MΩ cm) was used in synthesis and other processing. All other reagents were obtained from the Fisher Scientific other than indicated.
2.2. Preparation of free IT848 formulation.
Free IT848 solution (1mg/mL) was prepared by dissolving the small molecule drug in phosphate buffered saline (PBS, pH 7.4) containing 30 % (w/v) cyclodextrin (2-Hydroxypropyl-beta-cyclodextrin obtained from abcam, UK)) and 5 % (w/v) PEG 1000.
2.3. Synthesis of liposome templates.
Liposomes were synthesized based on the thin film hydration method previously established in our lab22, 35. Typically, a mixture of DPPC and cholesterol at a molar ratio of 55:45 was dissolved in 10 mL chloroform in a 50-mL round bottom flask and placed in a high vacuum rotary evaporator at 55 °C to remove the residual organic solvent. This was followed by hydration of the thin lipid film with 10 mL of freshly prepared ascorbic acid (AA) solution (300 mM) via sonication at room temperature. The unilamellar liposomes were separated by centrifugation at 13,000 rpm for 15 min at 15 °C. The supernatant liposomes containing AA reducing agents were collected for use in the synthesis of AuNFs.
2.4. Fabrication of porous AuNFs and IT848-loaded AuNFs.
Porous AuNFs were synthesized following a modified liposome-templated approach22. Briefly, 0.4 mL of HAuCl4·3H2O solution (8 mM) was added dropwise into the liposome solutions (1 mL) and gently stirred for 1 h. During the reaction, color of the solution rapidly changed from pale blue into blue gray. The AuNFs were washed with D.I. water three times, then centrifugated at 10,000 rpm for 10 min to obtain the pellets for further use. To assure the complete removal of liposome templates from AuNFs, the synthesized AuNFs were sonicated after each D.I. water washing step.
To load IT848 into AuNFs, 3.0 mg of bulk IT848 was firstly mixed with 150 μL of PEG 400 by vortexing to obtain a homogenous suspension. Then, 250 μL of 1N solution Na2CO3 was added into the above mixture to get a clear orange/red solution. Next, this solution was mixed with AuNFs (2 mg/mL). After stirring for 12 h, SH-PEG-NH2 (10 mg) was added into the above solution and stirred for another 6 h at room temperature. Then, EDC (20 mg) and HA (20 mg) were added into the solution and stirred for 12 h at room temperature to immobilize HA onto AuNFs. IT848 remaining in the loading solution was determined measuring UV absorbance at 392nm and then calculated with the established standard curve. The % IT848 loading capacity into AuNFs was defined as “(Amount of initial IT848 – Amount of supernatant IT848)/Amount of AuNFs”. The excessive unbounded IT848 was removed by centrifugation at 10,000 rpm for 10 min and rinsing with D.I. water several times. To fluorescently label the AuNFs with Cy7, Cy7-COOH (25 μg/mL) was immobilized onto NH2-PEG-AuNFs (250 μg/mL) via EDC coupling prior to HA conjugation.
2.5. Characterization of AuNFs and IT848-loaded AuNFs.
SEM and TEM images of AuNFs were acquired using a scanning electron microscope (SEM, Carl Zeiss SMT Inc., Peabody, MA) and a transmission electron microscope (TEM, Titan Themis 200). The size of AuNFs was determined by dynamic light scattering (DLS) with a Nano-ZS Zetasizer (Malvern Instruments Ltd., Malvern, UK). Chemical modifications and HA conjugation were confirmed by Fourier transform infrared (FTIR) and zeta-potential measurement. UV-vis absorption spectra of empty and IT848-loaded AuNFs were recorded with a UV-vis spectrophotometer (Synergy HT, Bio Tek Instruments, Inc., Winooski, VT).
To evaluate the stability upon oxidative degradation, IT848-HA-AuNFs were incubated with or without H2O2 (1%, v/v) for 18 h. H2O2 treated free IT848 was included as a control. All groups contained an equivalent amount of IT848 (0.33 mg) for better comparison. To measure the remaining intact IT848, IT848-HA-AuNFs were sonicated to force the release of IT848. The samples were centrifuged and the supernatant containing IT848 was collected to record the UV spectra using a microplate reader (Synergy HT, BioTek Instruments, Inc., Winooski, VT).
Release of IT848 from IT848-HA-AuNFs was investigated using a dialysis method. Briefly, 1 mL of IT848-HA-AuNFs (2 mg/mL) was placed in a dialysis tube (MWCO = 14 kDa) at 37 °C under gentle stirring. At designated time points (0, 2, 4, 6, 8, 10, 12, 24, 36, 48, 60, 72, 84, 96, 108, 120 h), IT848 released into the surrounding solutions was measured using a UV spectrophotometry to establish the release profiles.
2.6. Measurement of CD44 expression levels and cellular uptake.
Cell lines (EL4 and MM.1S) originally obtained from the American Type Culture Collection (ATCC, Manassas, VA) were maintained and propagated according to the ATCC recommendations and were validated negative for mycoplasma. Hematological cancer cells (5 × 105 cells/well), including mouse T cell lymphoma cells (EL4) and human multiple myeloma cells (MM.1S) along with normal mouse splenocytes were collected, stained with mouse/human anti-CD44 antibody (IM7, conjugated with Alexa-Flour 700, BioLegend, San Diego, CA, 1:400)) for 20 min at 4℃ in PBS with 0.5% (w/v) bovine serum albumin (PBS/BSA), fixed in 2% (w/v) paraformaldehyde (PFA) in PBS for 15 min, washed, and resuspended in PBS/BSA prior to analysis of surface markers. The levels of CD44 expression on cells were measured by flow cytometry (FACS Fortessa, BD Biosciences, CA, USA) and expressed as the mean fluorescence intensity (MFI), calculated using the Flowjo software (FlowJo, LLC, Ashland, OR).
Furthermore, Cy-7 conjugated AuNFs were employed as model particles for cellular uptake study. The EL4, MM.1S and splenocytes were seeded into a 12-well plate (5 × 105 cells/well) and treated for 3 h with Cy-7 labelled AuNFs with varying surface functionalization/HA molecular weights (HA-1000k, HA-10k, PEG-coated and non-functionalized control particles). The cells were then collected, centrifuged, and washed to remove free AuNFs, suspended in 500 μL PBS, and immediately subjected to flow cytometric analyses and presented as MFIs and scatter plots. All cells were cultured in RPMI medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin antibiotics at 37 °C and 5% humidity.
2.7. In vitro efficacy testing of IT848-loaded AuNFs.
To evaluate their efficacy in inhibiting EL4 cell growth, IT848-loaded AuNFs (at a concentration of 2 μM and 4 μM) were added into the culture media and time-dependent cell proliferation was evaluated over 96 h by MTS assay per manufacturer’s instructions (Promega, Madison, WI). Control conditions included cultures without IT848 treatment (empty control AuNFs) or with free IT848 (2 and 4 μM).
Furthermore, the effectiveness of treatment was also quantified indirectly by luciferase assay of firefly luciferase transduced EL4 cells. At the indicated time points over 72 h, luminescence intensity measurements were obtained by adding firefly luciferin (1 μL of a 15mg/mL solution per 100 μl of media) (Goldbio, St Louis, MO) and measuring luminescence intensity with a Tecan M Plex Pro 200 plate reader.
EL4 cells receiving different treatments were also quantitatively analyzed for apoptosis. Briefly, EL4 cells incubated with free or AuNF-loaded IT848 (2, 4 or 6 μM) or empty vehicle or control particles for 24 h were washed with PBS, resuspended in Annexin V binding buffer, stained with Annexin V-FITC and 7-aminoactinomycin D (7-AAD) (BioLegend), and analyzed by flow cytometry within 1 h. All the quantitative results were obtained from at least three independent runs of three technical replicates each.
An NF-κB/Jurkat/GFP transcriptional reporter cell line36 was purchased from System Biosciences (Mountain View, CA). Cells were stimulated with human TNF-alpha (10ng/mL) (Gemini Bioproducts, West Sacramento, CA) and incubated with empty vehicle or 2, 4 and 6 μM of free IT848 or equivalent amounts of IT848-loaded AuNFs for up to 96 h. NF-κB transcriptional activity was analyzed by measuring the GFP florescence at 6, 24, 30, 48, 72 and 96 h and by flow cytometry at 24 and 48 h timepoints.
2.8. Animal models.
All animals were maintained at the Center for Discovery and Innovation in accordance with the Institutional Animal Care and Use Committee (IACUC) Standards. Standard food and filtered water were available ad libitum. For the subcutaneous model, EL4 mouse lymphoma cells were transduced with tdTomato-firefly luciferase and 2×106 cells in Matrigel® were inoculated into the left flank of B6 mice (males and females) at 5 mice/group. After randomization, the IT848 treatment or Cy7-labelled AuNFs (for biodistribution) were administered between day 7–9. The tumor volumes were determined by caliper measurements.
The orthotopic lymphoma model was developed via tail vein injection of EL4-tdtom-luciferase cells (2 × 106 cells) into 6 to 8-week-old B6 females. Anti-lymphoma treatment was initiated on day 9 post the tumor injection and animals were monitored for survival (with hind-leg paralysis as the endpoint) and longitudinal tumor progression. Mice received either the AuNF formulation or the IT848 solution retro-orbitally at 5mg/kg body weight (once a week). The tumor progression was monitored biweekly by in vivo bioluminescence imaging (BLI). Briefly, mice were anesthetized using isoflurane inhalant anesthetic and administered 3 mg/kg firefly luciferin intraperitoneally and imaged using the IVIS Lumina X5 optical imaging platform. We superimposed pseudocolor images showing the whole-body distribution of bioluminescent signal intensity on grayscale photographs and quantified total flux (photons s−1) for individual mice using Living Image 4.7.3 software (Perkin Elmer, Waltham, MA).
2.9. Fluorescence imaging and biodistribution studies.
The subcutaneously implanted EL4 mice were administered with a single dose of Cy7-labeled AuNFs (control and HA coated) in 0.1 mL PBS retro-orbitally. In vivo fluorescence imaging was carried out after 10 min, 3 h, 6 h and 9 h. This was followed by euthanizing the mice and performing ex vivo imaging at 12 h and 24 h and collecting the major organs and the tumor nodules (at 12, 24, 48, 96 and 168 h) to quantify the Cy-7 distribution via fluorescence intensities of the regions of interest (ROI) using Living image software. The harvested organs and tumor blocks were further analyzed using inductively coupled plasma (ICP) measurements to detect the trace amounts of gold metals from the nanoparticles. To quantify the gold element content in each tissue, the samples were subjected to digestion by soaking in aqua regia at a ratio of approximately 0.1 mg sample/1 mL aqua regia. After 24 h, completely digested mixtures were filtered through a 0.45 μm filter and the final volume was adjusted to a total of 5 mL. The concentration of gold element was then measured with ICP-OES (Agilent, Santa Clara, CA).
2.10. Pharmacokinetic (PK) studies.
Pharmacokinetics of HA-AuNFs were studied in healthy B6 mice (n=4) by collecting whole blood 15 min, 30 min, 1 h, 4 h, 12 h and 24 h post AuNF treatment. Blood samples were digested, and the concentration of gold element was evaluated similarly to the above-mentioned process for inductively coupled plasma - optical emission spectrometry (ICP-OES) measurements.
2.11. Hemocompatibility assay.
Fresh mouse whole blood was collected in the heparin-coated tubes and healthy RBCs were isolated by centrifugation at 2,000 rpm for 10 min and purified by three successive washings with PBS. Thereafter, the RBCs were diluted ten folds with PBS. The diluted RBC suspension (10 μL) was added to 90 μL of water (positive control), 90 μL of PBS (negative control) and 90 μL of AuNF/PBS with three different concentrations (30, 62, 100 μg/mL). After gentle mixing, the mixtures were kept undisturbed for 2 h at room temperature, followed by 1 min centrifugation at 10,000 rpm. Photos were taken to record the hemolysis and absorbance of the supernatants (hemoglobin) was measured at 541 nm using the Tecan M plex pro 200 plate reader.
2.12. Toxicology assessment.
Healthy B6 mice were randomized into 2 groups and treated with either PBS (as control) or IT848-loaded AuNFs (5 mg of IT848/kg) to perform the biochemical and hematological blood analysis. These mice were individually identified and weighed biweekly over 60 days post treatment. For the complete blood count (CBC) analysis approximately 100 μL of fresh whole blood was collected every two weeks post-treatment via a retro-orbital bleeding with a heparin-coated microhematocrit tube into a Microtainer® tube containing lyophilized K2EDTA (BD Diagnostics, Franklin Lakes, NJ). An in-house hematology analyzer Heska Element HT5 system was used to analyze the WBCs, Neutrophils, Lymphocytes, RBCs and platelet counts.
For Blood biochemistry, the mice were bled retro-orbitally at 4 week and 8-week timepoints post-treatment. Samples were allowed to clot at room temperature for 1 h prior to centrifugation at 14,000 rpm for 10 min. The upper layers were carefully transferred into a fresh Eppendorf tube to obtain around 160 μL of serum, and the frozen samples were shipped to In Vivo Research Services at Rutgers School of Public Health (Piscataway, NJ) for the assay. The comprehensive panel for serum analysis was run on Heska Element DC5X, and the Albumin, Blood Urea Nitrogen (BUN), Creatinine, total Bilirubin and ALT (GPT) levels were tested.
Immediately after euthanasia at 8 weeks post-treatment, all mice were necropsied, and selected organs were carefully dissected and processed for histology. Thin sections of major organs were stained with hematoxylin and eosin (H&E) for histological analysis.
2.13. Histopathology of lymphoma nodules.
The subcutaneous lymphoma nodules were collected at the end point of experiments and stained with H&E to analyze the tumor histology. TUNEL Assay Kit - HRP-DAB (ab206386, Abcam) was used, according to the manufacturer’s instructions, to assess the level of apoptosis in paraffin-embedded tissue sections. 3 randomly selected fields (10× magnification) of each specimen were taken and apoptotic cells (brown) and total cells were recorded. Representative images of each group as well as quantitative data (expressed as the percentage of TUNEL positive cells per total cells present in each counted microscopic field) are presented.
2.14. Statistical analysis.
Error bars in the presented data the standard error of the arithmetic mean (SEM). Survival data were analyzed with the Kaplan Meier method and Log-Rank test. Analysis of longitudinal tumor growth was performed by nonparametric ANOVA and Mann-Whitney test for pairwise comparisons. In the event of incomplete follow-up data we used a more powerful repeated measures model designed for longitudinal tumor studies37. For non-survival pointwise analyses, nonparametric Mann-Whitney test was used for non-Gaussian distributions for comparison between two groups. A p value of less than 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism 5 (La Jolla, CA).
3. RESULTS AND DISCUSSION
3.1. Mesoporous AuNF platform for targeted delivery of small molecule therapeutics.
We accomplished template-assisted synthesis of porous gold nanoparticles named AuNFs22, 38 using liposomes (40 nm in diameter) as the template to guide the formation of AuNFs with 40 nm mesopores, which is large enough to accommodate a variety of therapeutic molecules (even large monoclonal antibodies)5. In this study, IT848 was loaded into porous AuNFs and then sealed with the CD44 ligand of hyaluronic acid (HA) using an SH-PEG-NH2 linker (Figure 1A) to prevent rapid release of the payload and to provide molecular targeting through CD44 binding. Figure 1B depicts our study design, utilizing HA-AuNFs for targeted cancer therapy in a mouse model of T cell lymphoma.
Morphological characterization and presence of mesopores were confirmed by both scanning (SEM) and transmission (TEM) electron microscopy. Interestingly, the mesopores were distributed not only on the surface (Figure 2A) but throughout the entire nanoparticle and interconnected to form a bicontinuous structure (Figure 2B), providing a potent interface for drug loading. Dynamic light scattering measurement indicated that the template-guided synthesis yielded AuNFs with relatively uniform hydrodynamic diameters (148±17 nm, Figure 2C), suggesting the efficiency of such a templating synthesis method. Although the functionalization density of PEG-NH2 and HA on the AuNF surface remains to be determined, excessive PEG-NH2 and HA were used in this study to assure sufficient immobilization of HA onto AuNFs. The surface functionalization and chemical modifications with PEG and HA were confirmed via zeta potential measurements showing the alterations in surface charges aligned with the spectral data from FTIR (Figure 2D–E). Absorbances at 3350 cm−1 and 1085 cm−1 were attributed to the stretching of primary amine N-H and C-O of the PEG linker molecule. Aliphatic C-S stretching was also found between 710 cm−1 and 685 cm−1. Conjugation of HA onto AuNFs also contributed to the strong enhancement of absorbance between 2840 cm−1 to 3000 cm−1 due to abundant C-H bonding in the HA chain.
Figure 2.
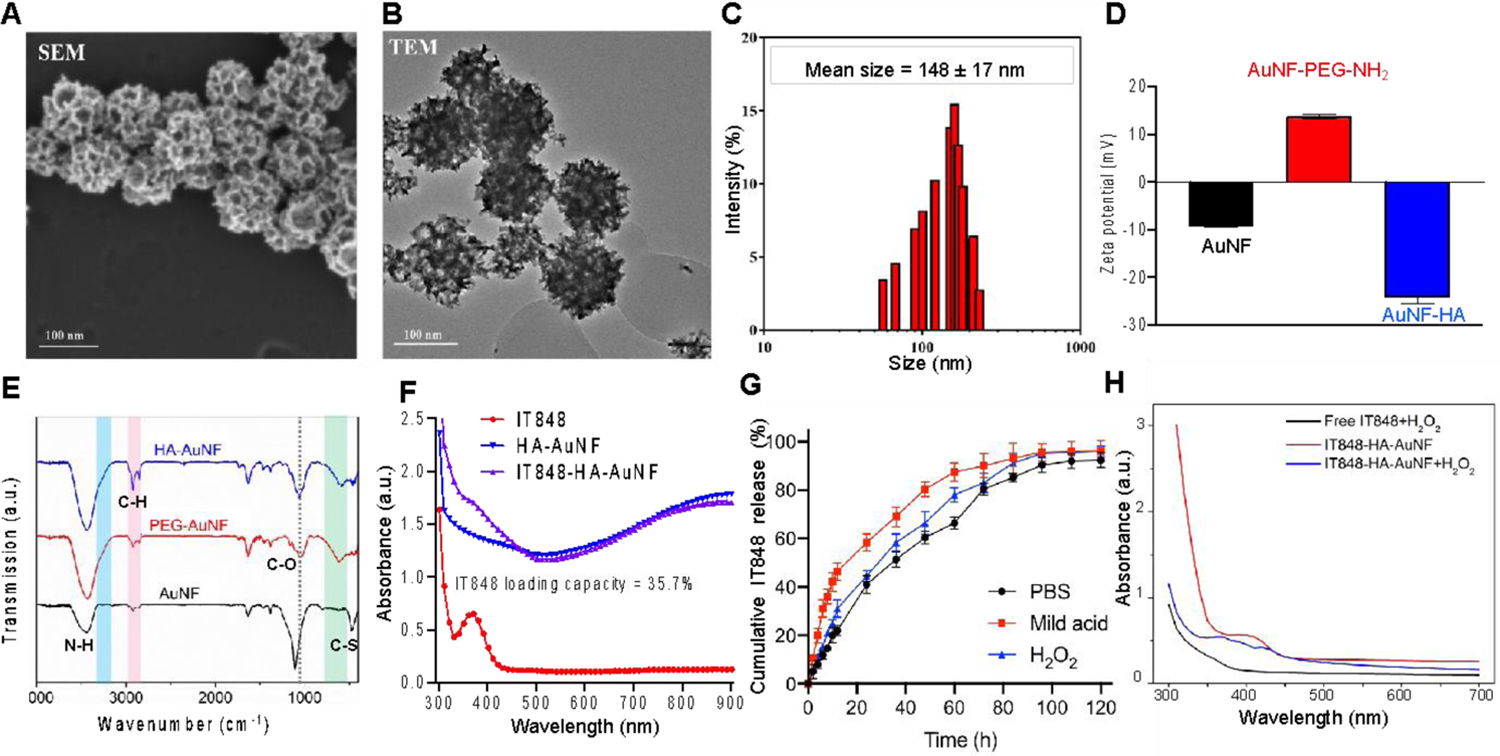
In vitro characterization of AuNFs and IT848-HA-AuNFs. A and B) SEM and TEM images of HA-AuNFs with large mesopores for drug loading; C and D) Size distribution (hydrodynamic radius) (C) and zeta potential (D) of AuNFs determined by DLS; E) Chemical modifications of AuNFs confirmed by FTIR; F) IT848 loading capacity (35.7%) into AuNFs measured using UV spectrometry; G) Cumulative release of IT848 from IT848-HA-AuNFs for 5 days in PBS, pH=6 acidic solution or PBS containing 100 μM H2O2. H) Peroxide-based oxidative degradation of IT848-HA-AuNFs measured by UV-vis absorption spectra as described in the M&M section.
To evaluate the drug-loading capacity, HA-functionalized AuNFs were incubated with the small molecule NF-κB inhibitor IT848, and excessive drug amounts were removed via centrifugal filtration. The UV-absorbance of IT848 at 392 nm allowed us to easily detect the remaining IT848 in the loading solution and calculate the payload of IT848 in the AuNFs. As shown in Figure 2F, a loading capacity (the weight of IT848 loaded into the unit weight of AuNFs) of 35.7% (WIT848/WAuNFs) was achieved with our approach. Such a relatively high loading capacity is essential to reduce the amount of AuNFs needed for delivery of sufficient therapeutic molecules to the target cells. The IT848 loading efficiency (i.e., the percentage of IT484, which was loaded into AuNFs) was calculated to be 23.3%. The satisfactory loading capacity and loading efficiency most likely resulted from the presence of mesopores throughout the AuNFs, which significantly increase the surface area available for loading of drug molecules. Since the size of liposome templates can be tuned, the mesopore size could also be modulated accordingly to further increase the surface area. However, the mesopores need to meet a threshold (i.e., pore/drug size ratio >1)39 allowing the therapeutic molecules (e.g., IT848) to readily reach the interior surface.
The release of IT848 from the AuNFs was characterized by incubating the IT848-loaded AuNFs in PBS (pH7.4), mild acidic (pH6) solution, and H2O2 (100 μM)-containing PBS (pH7.4), respectively. Continuous release of IT848 was observed in all three conditions [up to 95.87% (PBS), 98.17% (acidic), 97.79% (H2O2) of the loaded amount] for up to 5 days (Figure 2G), confirming the suitability of AuNFs as carriers for sustained IT848 release and the efficiency AuNF surface-immobilized of HA to retain IT848. In contrast to neutral PBS, slightly higher drug release rates were observed with mild acidic conditions or in the presence of H2O2, which can be attributed to acceleration the hydrolysis of HA under mild acidic conditions40, and to increased oxidative degradation of HA by H2O241. According to the overall release profile in Figure 2G, about 40 to 60 % of the loaded IT848 was released within the first 24 h, followed by a nearly linear release up to 94% at 96 h and then slow and yet continuous release for the remaining time. Surface modification of AuNFs with hydrophilic HA molecules via the thiol-PEG-amine linker provided the barrier for gradient-driven sustained release of IT848, facilitating prolonged therapeutic efficacy of the molecule and consequently reducing the frequency of administration.
The bio/cyto-compatibility of empty HA-AuNFs was confirmed by measuring the metabolic viability of EL4 lymphoma cells and healthy splenocytes via MTS assay after incubation for 24 h (Figure S1), showing negligible differences compared with untreated control cells. Stability testing of IT848 itself revealed that IT848 had good photostability and only minor degradation was observed after exposure to high temperature (60 °C) and/or high humidity (75% relative humidity). The highest levels of degradation were seen with the exposure to acidic conditions (0.1 N HCl, pH 1) and oxidation (3% H2O2). Interestingly, encapsulation of IT848 within HA-AuNFs could partially protect IT848 from oxidative degradation (Figure 2H), indicating the HA-AuNF formulation of IT848 not only facilitates targeted drug delivery but also improves preservation of the chemical integrity of the IT848 molecule.
3.2. In vitro cellular targeting, uptake, and selective cytotoxicity of IT848-loaded AuNFs.
HA, an essential ubiquitous natural anionic biopolymer of the extra cellular matrix42, is widely adopted for biomedical applications owing to its biodegradability, biocompatibility, non-immunogenicity, ease of chemical modification and specific receptor-binding42–44. Additional theoretical benefits of HA include its ability to provide stealth against unspecific protein absorption onto AuNFs and allow for opsonization elution and evasion of complement activation45–46, and furthermore, its ability to easily de-crosslink and destabilize under cytoplasmic/enzymatic reductive conditions can be utilized to enhance the anti-tumor efficiency of nanoparticles with minimal side effects47. The receptor specific to HA is CD44, a molecule that is frequently overexpressed in malignant cells and serves as a selective therapeutic target against various cancers, including lung cancer48–49, breast cancer47, ovarian cancer42, colorectal cancer50, glioblastomas51 and some multidrug-resistant carcinomas42, 48, 52–54. We performed a flow cytometric analysis of CD44 expression in multiple myeloma and lymphoma cell lines and healthy control cells. We found high levels of CD44 on lymphoma/myeloma cell lines (16 to 24-fold increase compared to healthy splenocyte control cells) (Figure 3A), suggesting that CD44 expression can be exploited for lymphoma cell targeting by coating AuNFs with the non-immunogenic HA55. Figures 3B+C show that compared to uncoated or PEG-coated AuNFs, HA-coated AuNFs (fluorescently labelled with Cy-7) were preferentially taken up by lymphoma and myeloma cells and HA coating enhanced the cellular uptake of AuNFs in a molecular weight dependent fashion, consistent with previous findings that high molecular weight HA (e.g., 1000kD) forms more irreversible bonds with CD44 compared with oligoHA (e.g., 10kD)56. These results suggest that HA-AuNFs can be used as vehicles for targeted delivery of IT848 to tumor cells with high CD44 expression, and we therefore used high molecular weight HA-coated IT848-loaded AuNF for subsequent in vitro and in vivo studies. Moreover, comparisons of equivalent doses of free IT848 with HA-AuNF-formulated IT848 revealed that the HA-AuNF formulation was superior in both inhibition of NF-κB transcriptional activity (Figures 3D+E) and anti-tumor activity against EL4 lymphoma cells as determined by Annexin V assay (Figure 3F), luciferase assay (Figure 3G) and MTS assay (Figure 3H). Importantly, the viability of healthy cells was not affected by IT848-HA-AuNF treatment (Figure S2).
Figure 3.

HA-AuNF formulation enhances the specificity and potency of IT848 delivery to target cells in vitro. A) CD44 expression levels in EL4 cells, MM.1S cells and control splenocytes from B6 mice were measured by flow cytometry and are presented as mean florescence intensity (MFI), B+C) Selective cellular uptake of Cy7-AuNFs without coating or coated with polyethylene glycol (PEG), hyaluronic acid (HA) (MW 10K) or HA (MW 1000K). AuNFs were incubated for 4 h with EL4 cells, MM.1S cells, and control splenocytes and AuNF cellular uptake was analyzed by flow cytometry. Representative scatter plots (B) and the Mean ± SEM of Cy7 MFI (C) are presented, D +E) Jurkat/GFP/NF-κB transcriptional reporter cells were stimulated with TNF-α and incubated in presence of empty vehicle or 2, 4 and 6 μM of free IT848 (Free-IT848) and equivalent amounts of IT848-loaded AuNFs for up to 96 h. NF-κB transcriptional activity was analyzed at 6, 24, 30, 48, 72 and 96 h by measuring GFP florescence (D) and by flow cytometric analysis of GFP negative cells at 24 and 48 h timepoints (E). Data presented as Mean ± SEM were normalized as percentage of the vehicle treated baseline activity. One of two independent experiments is shown. F-H) Luciferase-expressing EL4 cells were incubated with free or AuNF-encapsulated IT848 (2, 4 μM) or empty vehicle or control particles; F) apoptosis of EL4 cells after 24 h was analyzed by Annexin V/7AAD staining, G) EL4 growth over 72 h was quantified by luciferase assay, H) EL4 metabolic viability for up to 96 h was measured by MTS assay. Data were presented as Mean ± SEM.
3.3. Biodistribution of HA-AuNFs in lymphoma-bearing mice.
To assess the biodistribution of HA-AuNFs and verify HA-mediated lymphoma targeting in vivo, we utilized the syngeneic EL4 T cell lymphoma model57–58 to establish subcutaneous lymphoma nodules in mice, while keeping in mind that the HA receptor CD44 is expressed on a wide range of healthy cell types, even though at a much lower level than cancer cells59–60. Moreover, recent studies on subcutaneous tumor-induced liver injury caused by accumulation of myeloid-derived suppressor cells (MDSC) in livers of tumor-bearing mice also inspired our interest in comparing the livers from EL4 tumor bearers with those from naïve mice61–62. As expected, lymphoma establishment was associated with a significant increase in the frequency of polymorphonuclear (PMN)-MDSC (CD11b+Ly6G+Ly6C–) in the livers of C57BL/6 mice (Figure 4A) when compared to naïve control mice with no tumors. Consistent with an acute liver process, we also found an increased expression of CD44 in the hepatic hematopoietic cells (CD45+) of tumor-bearing mice, while CD44 expression in the hepatic epithelial cells (EpCAM+) was not altered by the tumor-induced liver injury (Figure 4B). For real-time assessment of AuNF biodistribution, we conjugated Cy7 to the surface of AuNFs, enabling in vivo near-infrared (NIR) fluorescence imaging. These studies revealed that HA coating resulted in enhanced Cy7 florescence in the liver (Figure 4C), indicating that the tumor-induced high levels of hepatic CD44 expression indeed increased liver uptake of HA-coated AuNFs in comparison to the non-coated control particles. Maximum AuNF accumulation was observed during the first 9 h post-injection and the fluorescence signal intensity diminished after 24 h in vivo, most likely due to nanoparticle clearance by the reticuloendothelial system (RES)63–64. Quantitative analyses of the ex vivo fluorescence of major organs excised at 12 h post AuNF administration confirmed that HA-AuNF uptake was significantly enhanced (vs. control AuNFs) in livers, kidneys, lungs, and spleens of mice. Of note, several previous studies have shown that systemically delivered AuNFs typically accumulate in the spleen (more specifically in the B cells), which is the second largest lymphoid organ65–67. This effect promotes passive targeting of AuNFs to malignant B cells and facilitates spleen-directed anti-lymphoma responses, thus providing an additional rationale for the development of AuNF-based therapeutics for B cell lymphomas.
Figure 4.

HA coating enhances the uptake of AuNFs by liver and lymphoma cells. A+B) B6 naïve mice or mice bearing EL4 subcutaneous tumors were sacrificed and hepatic cells were analyzed by flow cytometry (n=3–5), A) Monocytic (M)-MDSC (CD11b+Ly6G-Ly6C+) and polymorphonuclear (PMN)-MDSC (CD11b+Ly6G+Ly6C-) frequencies of hepatic CD45+ cells are presented (Mean ± SEM); B) Expression of CD44 in the hepatic hematopoietic cells (CD45+) and epithelial cells (EpCAM+) was determined via flow cytometry; C-H) Subcutaneous EL4 lymphoma nodules were established in B6 mice and groups of four mice each were treated with Cy7-labled control AuNFs or HA-coated AuNFs. C) Biodistribution of Cy-AuNFs and Cy7-HA-AuNFs was analyzed at different timepoints (10 min, 3 h, 6 h, 9 h and 24 h) by in vivo NIR fluorescence imaging; D) Ex vivo fluorescence imaging-based quantitative analysis of various organs at 12 h and 24 h post i.v. injection of control and HA-coated AuNFs; E) ICP-OES analysis to detect the trace amounts of gold nanoparticles in explanted organs at 12 h and 24 h post-AuNF injection. Data is presented as Mean ± SEM; F) The normalized HA-AuNF quantity in the whole blood of mice (percentage of the injected dose (ID) remaining in the blood) at various time points after HA-AuNF injection (15 min, 30 min, 1 h, 4 h, 12 h, 1 day, 2 day, 4 day, 7 day) was used to determine the pharmacokinetics of intravenously administered HA-AuNFs in mice (n=5); G) Co-localization of fluorescence signal showing that HA-coated AuNFs (red) accumulate in EL4 tumor (blue) nodules in vivo 9 h after i.v. HA-AuNF administration; an image of one representative mouse is presented; H) AuNFs in EL4 lymphoma nodules were quantified via ICP-OES for up to 7 days post i.v. injection of control and HA-coated AuNFs. Nonlinear regression curves and Mean ± SEM of individual measurements are presented.
The difference in fluorescence intensity between HA-coated and non-coated AuNFs was still prominent in the lungs and spleens at the 24 h timepoint (Figure 4 D). ICP-OES detection of trace amounts of gold from AuNFs in explanted organs (calculated as the percentage of injected dose (ID) per gram) at 12 h and 24 h post-AuNF injection further reiterated these findings (Figure 4E). HA-promoted accumulation in the spleen and liver can be ascribed to HA-binding to CD44 homologs such as hyaluronan receptor for endocytosis (HARE)68 and lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) on lymphatic endothelial cells which are abundant in spleen69. Moreover, the mucoadhesive properties of HA may promote interactions between HA-AuNFs and mucus, which might lead to delayed mucociliary clearance of HA-AuNFs from the lungs.
In addition to the distribution to those major organs, we also monitored the levels of HA-AuNFs in the blood of mice for up to seven days, revealing that blood levels of HA-AuNFs decreased rapidly within 1 h after i.v. administration (Figure 4F). However, after the initial decrease, the level remained steady at around 9 % of the injected dose for 24 h, and then decreased further to 1.9 % by day 7 (Figure 4F). Most importantly, the intravenously injected AuNFs were effectively taken up by lymphoma cells. A representative image of co-localization of fluorescence signal showed that HA-AuNFs (red) accumulate in EL4 tumor (blue) nodules in vivo 9 h after i.v. HA-AuNF administration (Figure 4G). Moreover, ICP-OES data further validated that HA-AuNFs yielded a significantly higher tumor uptake compared to the noncoated control AuNFs, persistently up to 1 week post administration (Figure 4H), which agreed well with a HA-CD44 mediated active targeting effect.
3.4. Toxicological evaluation of IT848-loaded AuNFs.
Blood compatibility and functional assessments of vital organs are the prerequisite for pre-clinical testing of nanoparticle-based delivery systems. We therefore evaluated the hemolytic activity of control and IT848-loaded HA-AuNFs following a 2-h incubation at room temperature with freshly drawn human blood, with PBS as the negative control and double distilled water as the positive control. Figures 5A+B demonstrate the absence of hemoglobin release suggestive of excellent hemocompatibility of IT848-loaded HA-AuNFs. The hemolytic activity was well below the standard level of 5 % conforming to the national biological safety requirements70. In addition, AuNF administration caused no weight loss (Figure 5C) in mice. Complete blood count (CBC) analysis of mouse blood measured at 2-, 4-, 6-, and 8-weeks post-injection showed no significant changes in white blood cells, neutrophils, lymphocytes, red blood cell and platelet populations between control (PBS) and the AuNF-injected mice (Figure 5D). Serum chemistry analysis after 4- and 8-weeks post-injection revealed no significant increase in urea (BUN) and creatinine levels that typically indicate compromised renal functions or nephrotoxicity, and no significant changes in serum marker levels indicative of hepatotoxicity (albumin, total bilirubin, and serum alanine transaminase) (Figures 5E+F). Furthermore, histological evaluations of H&E-stained major organs showed no tissue damage or residual particles, even in spleen and liver, where the AuNFs mostly accumulated (Figure 5G). These findings confirm the minimal toxicity of both HA-AuNF and IT848-loaded HA-AuNFs. Our observations are consistent with our previously reported preclinical results with free IT84818, in which a narrow therapeutic window of i.p. administered free IT848 was noticed (some in vivo efficacy but at the expense of toxicity), but no toxicity was observed when limiting the cumulative IT848 dose to less than 50mg/kg body weight18. On the other hand, our new strategy, i.e., utilizing HA-AuNFs to formulate i.v. administered IT848, is well tolerated and characterized by a significantly improved therapeutic window, providing efficacy with cumulative IT848 doses as low as 15–20mg/kg. Taken together, while additional toxicological testing is warranted prior to any first applications to humans, our biosafety studies to date did not reveal any evidence of IT848-HA-AuNF-mediated organ toxicities.
Figure 5.

In vitro and in vivo toxicology testing of IT848-loaded AuNFs. A+B) Human PBMCs were resuspended in 100 mL of a hypotonic solution (water) or an isotonic solution (PBS). PBS samples were supplemented with empty HA-AuNFs or IT848-loaded HA-AuNFs (30, 62, 100 mg/mL) or no HA-AuNFs (control). Samples were incubated at room temperature for 4 h. Photographs showing the presence or absence of hemolysis are presented in panel A. Panel B shows the quantitative analysis of the presence or absence of hemolysis by measuring absorbance at 541 nm using a microplate spectrophotometer. Data is presented as Mean ± SEM. C-F) B6 mice were injected with 100 mL of PBS (control) or IT848-loaded HA-AuNFs (5 mg of IT848/kg) via retroorbital sinus injection (n=5). C) Time-dependent body weight changes are presented as Mean ± SEM; D) Complete blood counts (CBC) were measured 2-, 4-, 6-, and 8-weeks post-injection. White blood cell counts (WBC), neutrophils, lymphocytes, red blood cells (RBC) and platelets are presented as Mean ± SEM; E+F) Serum samples were collected from control and IT848-AuNF-injected mice at week 4 and 8 post-injection and levels of blood urea nitrogen (BUN), Creatinine, Albumin, total Bilirubin and Alanine Transaminase (ALT) were measured. Data is presented as Mean ± SEM; G) Histological images of the major organs of the control and IT848 treated mice, 8 weeks post-injection with HA-AuNFs. Images were acquired at 10× magnification.
3.5. In vivo efficacy of IT848-HA-AuNF monotherapy in subcutaneous and orthotopic lymphoma models.
We previously reported that IT848 treatment of mice resulted in decreased NF-κB transcriptional activity in lymphoma cells in vivo18. With this in mind, we investigated the in vivo efficacy of IT848-loaded AuNFs in the above-described subcutaneous EL4 lymphoma model (Figure 6A). Weekly i.v. administration of a low dose of free IT848 (5 mg/kg) was compared to an equivalent dose of IT848-loaded HA-AuNFs, with empty HA-AuNFs as controls. The experiment was carried out for 3 weeks, and the tumor volume was measured twice a week with an electronic caliper. As seen in Figure 6B, tumor growth was significantly suppressed by IT848-HA-AuNFs compared to the control treatment (empty HA-AuNFs) (p = 0.027). Free IT848 administration also showed some tumor retardation but the effect was much less significant (p = 0.6) than the anti-lymphoma activity of the IT848-HA-AuNF formulation. Histological examinations including H&E staining and TUNEL assay of explanted tumors revealed that IT848 treatment not only decreased the tumor size but also the lymphoma tissue density (Figure S3), and IT848-HA-AuNFs induced significantly more apoptosis in the subcutaneous lymphoma nodules than control AuNF particles (p=0.002) or free IT848 (p=0.04) (Figure 6C+D). We next evaluated the microenvironment of explanted lymphoma nodules by flow cytometric analysis of immunosuppressive cell populations. Consistent with our previous findings in a multiple myeloma mouse model18, we observed a significant decrease in lymphoma infiltrating regulatory T cells (Tregs) in IT848-treated mice (Figure 6E), suggesting therapeutically beneficial immunomodulatory effects of NF-κB inhibition on the tumor microenvironment.
Figure 6.

HA-AuNFs loaded with IT848 inhibit lymphoma progression with infrequent intravenous dosing in subcutaneous and orthotopic immunocompetent mouse models. B6 mice received 2×106 EL4 lymphoma cell via subcutaneous injection. One week after lymphoma cell inoculation, mice were assigned to treatment (free IT848 5mg/kg i.v. once a week; IT848-HA-AuNF 5mg/kg i.v. once a week) and control (empty HA-AuNF i.v. once a week) groups. A) Schematic representation of subcutaneous EL4 tumor inoculation followed by IT848-AuNF intervention once a week; B) Mean and SEM of longitudinal tumor volume measurements are presented (n=5). The inset shows representative tumor images at the end of treatment (day 20); C-E) Lymphoma nodules on day 20 post-treatment were harvested for histological and flow cytometric studies. C) Representative images of TUNEL- labeled tumor sections of different treatment groups. Apoptotic cell nuclei were stained dark brown by TUNEL assay and the sections were counter-stained with methyl green. D) Semi-quantification of TUNEL-positive cells out of the total cells. Values are expressed as mean ± SEM. E) Homogenized lymphoma nodules were analyzed via multiparameter flow cytometry for the presence of regulatory T cells (Tregs). Mean and SEM of the Treg frequency of CD45+ cells in the tumor microenvironment are presented (n=5). F) Dissemination patterns of lymphoma progression after intravenous injection of 2 × 106 luciferase-expressing EL4 cells was analyzed longitudinally by in vivo bioluminescence imaging at the indicated time points. Pseudocolor images of a representative mouse superimposed on conventional photographs is presented. G) Schematic representation of intravenous EL4 tumor inoculation followed by IT848-AuNF administration once a week; H) The curves show the probability of overall survival curve in the orthotopic lymphoma model and the comparison between the following groups: control (empty AuNFs i.v. once a week); free IT848 5mg/kg i.v. once a week; IT848-AuNF (5mg/kg) i.v. once a week. Differences between groups were analyzed by Log-rank test. Combined data from three independent experiments are presented (n=10–20).
We finally studied the efficacy of IT848-HA-AuNF monotherapy following i.v. administration of luciferase-transduced EL4 cells in a more clinically relevant orthotopic lymphoma model (Figures 6F+G). Successful lymphoma engraftment was confirmed by in vivo BLI 10 days post-inoculation of EL4 cells, and the mice were randomly assigned to the same three groups as described above (empty AuNFs; free IT848 5mg/kg; IT848-HA-AuNFs 5mg/kg) for weekly i.v. treatments. Survival was monitored daily over a 5-week period (Figure 6H). We found that, similar to our results in subcutaneous lymphoma studies, weekly administration of free IT848 had a minor effect on survival and was inferior to AuNF-formulated IT848, which significantly (p=0.005) extended the median survival time of mice compared with the control (empty AuNFs) group. These results underscore the potential of our strategy to serve as an easily adaptable platform for targeted cancer therapy and to inspire the development of innovative, well-tolerated and effective protocols for the treatment of aggressive hematologic malignancies.
4. CONCLUSIONS
We adopted HA-coated gold nanoframeworks as an integrative platform for CD44-targeted delivery of a small molecule NF-κB inhibitor (IT848), displaying potent anti-tumor effects towards CD44-positive lymphoma cells in vitro and in vivo. HA-functionalized and IT848-loaded AuNFs were synthesized via a simple, reproducible, and inexpensive liposome-assisted technique that can easily be scaled up, a critical feature for clinical relevance. Our results indicate that active targeted delivery of IT848 using AuNFs via HA-CD44 ligand-receptor mediated endocytosis is a promising strategy to augment IT848 uptake by CD44-overexpressing tumor cells, mitigate rapid clearance of nanoparticles from the body and yield a highly desirable and sustained (as compared to free IT848) anti-tumor therapeutic effect. Our HA-coated, AuNF-based drug delivery approach offers infrequent (weekly) low dose administration and robust efficacy with minimal toxicity risk. In fact, weekly i.v. administration of IT848-loaded HA-AuNFs was not associated with any signs of toxicity, while intraperitoneal administration of free IT848 for more than 2 weeks typically leads to ascites development in up to 50% of the recipients18. Collectively, our AuNF system facilitates targeted drug delivery, improving the drug safety profile and enhancing therapeutic efficacy. Of note, we observed significant in vivo efficacy with HA-IT848-AuNF monotherapy, even though low dose, single agent molecular therapy such as HA-IT848-AuNF treatment is not expected to induce durable responses against aggressive cancers such as the EL4 lymphoma model. The remarkable efficacy of HA-IT848-AuNF monotherapy is most likely related to the immunomodulatory potency of IT848 therapy, reducing immunosuppression of the tumor microenvironment in addition to direct anti-tumor effects resulting from NF-κB inhibition in lymphoma cells. Additional studies are needed to develop curative treatment regimens based on synergistic, highly active, and well-tolerated combinatorial approaches with other targeted agents (such as immune checkpoint inhibitors, hypomethylating agents, or CAR-T cells), and to exploit the full advantages of AuNF-based theranostics in the setting of lymphoma therapy, such as surface enhanced Raman imaging to intracellularly monitor molecular alterations.
Supplementary Material
Figure S1: HA-AuNF incubation does not impact cell survival (PDF).
Figure S2: IT848-HA-AuNF treatment does not compromise the viability of healthy cells (PDF).
Figure S3: IT848 treatment decreases the density of lymphoma cells in a subcutaneous lymphoma model (PDF).
ACKNOWLEDGMENT
JLZ received the grant support from the National Cancer Institute (NCI 1R37CA250661-01A1), the Children’s Leukemia Research Association, the American Society of Hematology, the International Myeloma Society, the Hackensack Meridian School of Medicine, the HMH MSK Immunology Collaboration, and the HMH Foundation/Tackle Kids Cancer. HW received the grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (award number 1R01AR067859).
ABBREVIATIONS
- AuNFs
Gold nanoframeworks
- HA
Hyaluronic Acid
- FTIR
Fourier-transform infrared spectroscopy
- NF-κB
Nuclear factor-κB
- ICP-OES
Inductively coupled plasma - optical emission spectrometry
- NIR
near infrared
REFERENCES
- (1).Samal P; Begum S Drug Loaded Nanomaterials for Hematological Malignancies Diagnosis and Enhanced Targeted Therapy. In Advanced Nanomaterials for Point of Care Diagnosis and Therapy; Elsevier: 2022; pp 383–398. [Google Scholar]
- (2).Powsner EH; Harris JC; Day ES Biomimetic Nanoparticles for the Treatment of Hematologic Malignancies. Advanced NanoBiomed Research 2021, 1 (4), 2000047. [Google Scholar]
- (3).Bromma K; Chithrani DB Advances in Gold Nanoparticle-based Combined Cancer Therapy. Nanomaterials 2020, 10 (9), 1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Jain S; Hirst D; O’Sullivan J Gold Nanoparticles as Novel Agents for Cancer Therapy. The British Journal of Radiology 2012, 85 (1010), 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Scott AM; Allison JP; Wolchok JD Monoclonal Antibodies in Cancer Therapy. Cancer Immunity Archive 2012, 12 (1), 14–22. [PMC free article] [PubMed] [Google Scholar]
- (6).Muhammad N; Mao Q; Xia H CAR T-cells for Cancer Therapy. Biotechnology and Genetic Engineering Reviews 2017, 33 (2), 190–226. [DOI] [PubMed] [Google Scholar]
- (7).June CH; O’Connor RS; Kawalekar OU; Ghassemi S; Milone MC CAR T Cell Immunotherapy for Human Cancer. Science 2018, 359 (6382), 1361–1365. [DOI] [PubMed] [Google Scholar]
- (8).Zhong L; Li Y; Xiong L; Wang W; Wu M; Yuan T; Yang W; Tian C; Miao Z; Wang T Small Molecules in Targeted Cancer Therapy: Advances, Challenges, and Future Perspectives. Signal Transduction and Targeted Therapy 2021, 6 (1), 1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Sun G; Rong D; Li Z; Sun G; Wu F; Li X; Cao H; Cheng Y; Tang W; Sun Y Role of Small Molecule Targeted Compounds in Cancer: Progress, Opportunities, and Challenges. Frontiers in Cell and Developmental Biology 2021, 2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bedard PL; Hyman DM; Davids MS; Siu LL Small Molecules, Big Impact: 20 Years of Targeted Therapy in Oncology. The Lancet 2020, 395 (10229), 1078–1088. [DOI] [PubMed] [Google Scholar]
- (11).Lavanya V; Adil M; Ahmed N; Rishi A; Jamal S Small Molecule Inhibitors as Emerging Cancer Therapeutics. Integrative Cancer Science and Therapeutics 2014, 1 (3), 39–46. [Google Scholar]
- (12).Tilstra JS; Clauson CL; Niedernhofer LJ; Robbins PD NF-κB in Aging and Disease. Aging and Disease 2011, 2 (6), 449. [PMC free article] [PubMed] [Google Scholar]
- (13).Zhang L; Shi L; Soars SM; Kamps J; Yin H Discovery of Novel Small-Molecule Inhibitors of NF- κB Signaling with Antiinflammatory and Anticancer Properties. Journal of Medicinal Chemistry 2018, 61 (14), 5881–5899. [DOI] [PubMed] [Google Scholar]
- (14).Ruan Q; Chen YH Nuclear Factor-KappaB in Immunity and Inflammation: The Treg and Th17 Connection. Advances in Experimental Medicine and Biology 2012, 946, 207–21. [DOI] [PubMed] [Google Scholar]
- (15).Puar YR; Shanmugam MK; Fan L; Arfuso F; Sethi G; Tergaonkar V Evidence for the Involvement of the Master Transcription Factor NF- κB in Cancer Initiation and Progression. Biomedicines 2018, 6 (3), 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Ramadass V; Vaiyapuri T; Tergaonkar V Small molecule NF-κB Pathway Inhibitors in Clinic. International Journal of Molecular Sciences 2020, 21 (14), 5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Ouk S; Liou H-C, Methods of Treating Cancer with Small Molecule NF-κB Inhibitors. US Patent App. 16/020,688: 2019.
- (18).Bariana M; Cassella E; Rateshwar J; Ouk S; Liou HC; Heller C; Colorado I; Feinman R; Makhdoom A; Siegel DS; Heller G; Tuckett A; Mondello P; Zakrzewski JL Inhibition of NF-κB DNA Binding Suppresses Myeloma Growth via Intracellular Redox and Tumor Microenvironment Modulation. Molecular Cancer Therapeutics 2022, 21(12):1798–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lim EK; Kim T; Paik S; Haam S; Huh YM; Lee K Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chemical Reviews 2015, 115 (1), 327–94, DOI: 10.1021/cr300213b. [DOI] [PubMed] [Google Scholar]
- (20).Gu W; Qu R; Meng F; Cornelissen J; Zhong Z Polymeric Nanomedicines Targeting Hematological Malignancies. Journal of Controlled Release 2021, 337, 571–588, DOI: 10.1016/j.jconrel.2021.08.001. [DOI] [PubMed] [Google Scholar]
- (21).Senapati S; Mahanta AK; Kumar S; Maiti P Controlled Drug Delivery Vehicles for Cancer Treatment and their Performance. Signal transduction and targeted therapy 2018, 3 (1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wang J; Sun J; Wang Y; Chou T; Zhang Q; Zhang B; Ren L; Wang H Gold Nanoframeworks with Mesopores for Raman–Photoacoustic Imaging and Photo-chemo Tumor Therapy in the Second Near-Infrared Biowindow. Advanced Functional Katerials 2020, 30 (9), 1908825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Karthik S; Puvvada N; Kumar BP; Rajput S; Pathak A; Mandal M; Singh NP Photoresponsive Coumarin-tethered Multifunctional Magnetic Nanoparticles for Release of Anticancer Drug. ACS Applied Materials & Interfaces 2013, 5 (11), 5232–5238. [DOI] [PubMed] [Google Scholar]
- (24).Vinhas R; Mendes R; Fernandes AR; Baptista PV Nanoparticles—Emerging Potential for Managing Leukemia and Lymphoma. Frontiers in Bioengineering and Biotechnology 2017, 5, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Etrych T; Daumová L; Pokorná E; Tušková D; Lidický O; Kolářová V; Pankrác J; Šefc L; Chytil P; Klener P Effective Doxorubicin-based Nano-therapeutics for Simultaneous Malignant Lymphoma Treatment and Lymphoma Growth Imaging. Journal of Controlled Release 2018, 289, 44–55. [DOI] [PubMed] [Google Scholar]
- (26).Zhong Y; Meng F; Deng C; Mao X; Zhong Z Targeted Inhibition of Human Hematological Cancers In Vivo by Doxorubicin Encapsulated in Smart Lipoic Acid-crosslinked Hyaluronic Acid Nanoparticles. Drug Delivery 2017, 24 (1), 1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Mitchell MJ; Billingsley MM; Haley RM; Wechsler ME; Peppas NA; Langer R Engineering Precision Nanoparticles for Drug Delivery. Nature Reviews Drug Discovery 2021, 20 (2), 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Dreaden EC; Austin LA; Mackey MA; El-Sayed MA Size Matters: Gold Nanoparticles in Targeted Cancer Drug Delivery. Therapeutic Delivery 2012, 3 (4), 457–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Lim Z-ZJ; Li J-EJ; Ng C-T; Yung L-YL; Bay B-H Gold Nanoparticles in Cancer Therapy. Acta Pharmacologica Sinica 2011, 32 (8), 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Yang Y; Gao N; Hu Y; Jia C; Chou T; Du H; Wang H Gold Nanoparticle-enhanced Photodynamic Therapy: Effects of Surface Charge and Mitochondrial Targeting. Therapeutic Delivery 2015, 6 (3), 307–321. [DOI] [PubMed] [Google Scholar]
- (31).Saha K; Agasti SS; Kim C; Li X; Rotello VM Gold Nanoparticles in Chemical and Biological Sensing. Chemical Reviews 2012, 112 (5), 2739–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Pal S; Ray A; Andreou C; Zhou Y; Rakshit T; Wlodarczyk M; Maeda M; Toledo-Crow R; Berisha N; Yang J; Hsu HT; Oseledchyk A; Mondal J; Zou S; Kircher MF DNA-enabled Rational Design of Fluorescence-Raman Bimodal Nanoprobes for Cancer Imaging and Therapy. Nature Communications 2019, 10 (1), 1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Andreou C; Neuschmelting V; Tschaharganeh DF; Huang CH; Oseledchyk A; Iacono P; Karabeber H; Colen RR; Mannelli L; Lowe SW; Kircher MF Imaging of Liver Tumors using Surface-Enhanced Raman Scattering Nanoparticles. ACS Nano 2016, 10 (5), 5015–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Yu T; Li Y; Gu X; Li Q Development of a Hyaluronic Acid-based Nanocarrier Incorporating Doxorubicin and Cisplatin as a pH-Sensitive and CD44-Targeted Anti-breast Cancer Drug Delivery System. Frontiers in Pharmacology 2020, 11, 532457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Chen K-J; Chaung E-Y; Wey S-P; Lin K-J; Cheng F; Lin C-C; Liu H-L; Tseng H-W; Liu C-P; Wei M-C Hyperthermia-mediated Local Drug Delivery by a Bubble-generating Liposomal System for Tumor-Specific Chemotherapy. ACS nano 2014, 8 (5), 5105–5115. [DOI] [PubMed] [Google Scholar]
- (36).Egan LJ; Toruner M NF-KappaB Signaling: Pros and Cons of Altering NF-KappaB as a Therapeutic Approach. Annals of the New York Academy of Sciences 2006, 1072, 114–122. [DOI] [PubMed] [Google Scholar]
- (37).Tan M; Fang HB; Tian GL; Houghton PJ Small-sample Inference for Incomplete Longitudinal Data with Truncation and Censoring in Tumor Xenograft Models. Biometrics 2002, 58 (3), 612–620. [DOI] [PubMed] [Google Scholar]
- (38).Wang YC; Rheaume E; Lesage F; Kakkar A Synthetic Methodologies to Gold Nanoshells: An Overview. Molecules 2018, 23 (11), 2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Vallet-Regí M; Balas F; Arcos D Mesoporous Materials for Drug Delivery. Angewandte Chemie International Edition 2007, 46 (40), 7548–7558. [DOI] [PubMed] [Google Scholar]
- (40).Tømmeraas K; Melander C Kinetics of Hyaluronan Hydrolysis in Acidic Solution at Various pH Values. Biomacromolecules 2008, 9 (6), 1535–40. [DOI] [PubMed] [Google Scholar]
- (41).Choi KY; Han HS; Lee ES; Shin JM; Almquist BD; Lee DS; Park JH Hyaluronic Acid-based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: Beyond CD44-Mediated Drug Delivery. Advanced Materials 2019, 31 (34), e1803549. [DOI] [PubMed] [Google Scholar]
- (42).Yang X; Singh A; Choy E; Hornicek FJ; Amiji MM; Duan Z MDR1 siRNA Loaded Hyaluronic Acid-based CD44 Targeted Nanoparticle Systems Circumvent Paclitaxel Resistance in Ovarian Cancer. Scientific Reports 2015, 5 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Lee WH; Rho JG; Han HS; Kweon S; Park JH; Kim W Self-assembled Hyaluronic Acid Nanoparticle Suppresses Fat Accumulation via CD44 in Diet-Induced Obese Mice. Carbohydrate Polymers 2020, 237, 116161. [DOI] [PubMed] [Google Scholar]
- (44).Chiesa E; Riva F; Dorati R; Greco A; Ricci S; Pisani S; Patrini M; Modena T; Conti B; Genta I On-Chip Synthesis of Hyaluronic Acid-based Nanoparticles for Selective Inhibition of CD44+ Human Mesenchymal Stem Cell Proliferation. Pharmaceutics 2020, 12 (3), 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Nasti A; Zaki NM; De Leonardis P; Ungphaiboon S; Sansongsak P; Rimoli MG; Tirelli N Chitosan/TPP and Chitosan/TPP-Hyaluronic Acid Nanoparticles: Systematic Optimisation of the Preparative Process and Preliminary Biological Evaluation. Pharmaceutical Research 2009, 26 (8), 1918–1930. [DOI] [PubMed] [Google Scholar]
- (46).Spadea A; Rios de la Rosa JM; Tirella A; Ashford MB; Williams KJ; Stratford IJ; Tirelli N; Mehibel M Evaluating the Efficiency of Hyaluronic Acid for Tumor Targeting via CD44. Molecular pharmaceutics 2019, 16 (6), 2481–2493. [DOI] [PubMed] [Google Scholar]
- (47).Zhong Y; Zhang J; Cheng R; Deng C; Meng F; Xie F; Zhong Z Reversibly Crosslinked Hyaluronic Acid Nanoparticles for Active Targeting and Intelligent Delivery of Doxorubicin to Drug Resistant CD44+ Human Breast Tumor Xenografts. Journal of controlled release 2015, 205, 144–154. [DOI] [PubMed] [Google Scholar]
- (48).Zhang W; Xu W; Lan Y; He X; Liu K; Liang Y Antitumor Effect of Hyaluronic-Acid-modified Chitosan Nanoparticles Loaded with siRNA for Targeted Therapy for Non-Small Cell Lung Cancer. International Journal of Nanomedicine 2019, 14, 5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Kapanadze T; Gamrekelashvili J; Ma C; Chan C; Zhao F; Hewitt S; Zender L; Kapoor V; Felsher DW; Manns MP Regulation of Accumulation and Function of Myeloid Derived Suppressor Cells in Different Murine Models of Hepatocellular Carcinoma. Journal of Hepatology 2013, 59 (5), 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Wang C; Xie J; Guo J; Manning HC; Gore JC; Guo N Evaluation of CD44 and CD133 as Cancer Stem Cell Markers for Colorectal Cancer. Oncology Reports 2012, 28 (4), 1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Hayward SL; Wilson CL; Kidambi S Hyaluronic Acid-conjugated Liposome Nanoparticles for Targeted Delivery to CD44 Overexpressing Glioblastoma Cells. Oncotarget 2016, 7 (23), 34158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Liang J; Zeng F; Zhang M; Pan Z; Chen Y; Zeng Y; Xu Y; Xu Q; Huang Y Green Synthesis of Hyaluronic Acid-based Silver Nanoparticles and their Enhanced Delivery to CD44+ Cancer Cells. RSC Advances 2015, 5 (54), 43733–43740. [Google Scholar]
- (53).Chen C; Zhao S; Karnad A; Freeman JW The Biology and Role of CD44 in Cancer Progression: Therapeutic Implications. Journal of Hematology & Oncology 2018, 11 (1), 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Fang XJ; Jiang H; Zhu YQ; Zhang LY; Fan QH; Tian Y Doxorubicin Induces Drug Resistance and Expression of the Novel CD44 via NF- κB in Human Breast Cancer MCF-7 Cells. Oncology Reports 2014, 31 (6), 2735–2742. [DOI] [PubMed] [Google Scholar]
- (55).Eliaz RE; Szoka FC Liposome-encapsulated Doxorubicin Targeted to CD44: A Strategy to Kill CD44-overexpressing Tumor Cells. Cancer Research 2001, 61 (6), 2592–2601. [PubMed] [Google Scholar]
- (56).Tavianatou AG; Caon I; Franchi M; Piperigkou Z; Galesso D; Karamanos NK Hyaluronan: Molecular Size-dependent Signaling and Biological Functions in Inflammation and Cancer. FEBS J 2019, 286 (15), 2883–2908. [DOI] [PubMed] [Google Scholar]
- (57).Matsumoto T; Suetsugu A; Shibata Y; Nakamura N; Aoki H; Kunisada T; Tsurumi H; Shimizu M; Hoffman RM A Color-coded Imageable Syngeneic Mouse Model of Stromal-Cell Recruitment by Metastatic Lymphoma. Anticancer Research 2015, 35 (9), 4647–4654. [PubMed] [Google Scholar]
- (58).Di Rosso ME; Sterle HA; Cremaschi GA; Genaro AM Beneficial Effect of Fluoxetine and Sertraline on Chronic Stress-induced Tumor Growth and Cell Dissemination in a Mouse Model of Lymphoma: Crucial Role of Antitumor Immunity. Frontiers in immunology 2018, 9, 1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Sneath R; Mangham D The Normal Structure and Function of CD44 and its Role in Neoplasia. Molecular Pathology 1998, 51 (4), 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Ponta H; Sherman L; Herrlich PA CD44: From Adhesion Molecules to Signaling Regulators. Nature Reviews Molecular Cell Biology 2003, 4 (1), 33–45. [DOI] [PubMed] [Google Scholar]
- (61).Eggert T; Medina-Echeverz J; Kapanadze T; Kruhlak MJ; Korangy F; Greten TF Tumor Induced Hepatic Myeloid Derived Suppressor Cells can cause Moderate Liver Damage. PLoS One 2014, 9 (11), e112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Ilkovitch D; Lopez DM The Liver Is a Site for Tumor-induced Myeloid-derived Suppressor Cell Accumulation and Immunosuppression. Cancer Research 2009, 69 (13), 5514–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Chanda N; Kattumuri V; Shukla R; Zambre A; Katti K; Upendran A; Kulkarni RR; Kan P; Fent GM; Casteel SW Bombesin Functionalized Gold Nanoparticles Show In Vitro and In Vivo Cancer Receptor Specificity. Proceedings of the National Academy of Sciences 2010, 107 (19), 8760–8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Rattan R; Bhattacharjee S; Zong H; Swain C; Siddiqui MA; Visovatti SH; Kanthi Y; Desai S; Pinsky DJ; Goonewardena SN Nanoparticle-macrophage Interactions: A Balance between Clearance and Cell-Specific Targeting. Bioorganic & Medicinal Chemistry 2017, 25 (16), 4487–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Almeida JPM; Lin AY; Langsner RJ; Eckels P; Foster AE; Drezek RA In Vivo Immune Cell Distribution of Gold Nanoparticles in Naive and Tumor Bearing Mice. Small 2014, 10 (4), 812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Jasinski DL; Li H; Guo P The Effect of Size and Shape of RNA Nanoparticles on Biodistribution. Molecular Therapy 2018, 26 (3), 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Lin AY; Rink JS; Karmali R; Xu J; Kocherginsky M; Thaxton CS; Gordon LI Tri-Ethylene Glycol Modified Class B and Class C Cpg Conjugated Gold Nanoparticles for the Treatment of Lymphoma. Nanomedicine: Nanotechnology, Biology and Medicine 2020, 30, 102290. [DOI] [PubMed] [Google Scholar]
- (68).Pandey MS; Weigel PH A Hyaluronan Receptor for Endocytosis (HARE) Link Domain N-Glycan is Required for Extracellular Signal-regulated Kinase (ERK) and Nuclear Factor-κB (NF-κB) Signaling in Response to the Uptake of Hyaluronan but not Heparin, Dermatan Sulfate, or Acetylated Low Density Lipoprotein (LDL). Journal of Biological Chemistry 2014, 289 (32), 21807–21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Banerji S; Ni J; Wang S-X; Clasper S; Su J; Tammi R; Jones M; Jackson DG LYVE-1, a New Homologue of the CD44 Glycoprotein, is a Lymph-Specific Receptor for Hyaluronan. The Journal of Cell Biology 1999, 144 (4), 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Jesus S; Marques AP; Duarte A; Soares E; Costa JP; Colaço M; Schmutz M; Som C; Borchard G; Wick P Chitosan Nanoparticles: Shedding Light on Immunotoxicity and Hemocompatibility. Frontiers in Bioengineering and Biotechnology 2020, 8, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: HA-AuNF incubation does not impact cell survival (PDF).
Figure S2: IT848-HA-AuNF treatment does not compromise the viability of healthy cells (PDF).
Figure S3: IT848 treatment decreases the density of lymphoma cells in a subcutaneous lymphoma model (PDF).


