Abstract
OBJECTIVES
Coronary transfer remains the most crucial part of the arterial switch operation (ASO); yet, certain coronary anatomies prohibit the use of button or trap-door transfer techniques. In the rare setting of ‘non-separable’ single sinus coronary arteries with intramural course, the modified Yacoub aortocoronary flap technique is a viable option. The aim of this study is to describe this operative technique and review its early- and mid-term outcomes.
METHODS
This retrospective analysis included all cases with ‘non-separable’ single sinus coronary arteries with intramural course where the modified Yacoub aortocoronary flap technique served as a bail-out option.
RESULTS
Of 516 patients who underwent ASO at our institution between January 1977 and April 2022, 14 underwent the modified Yacoub aortocoronary flap technique. The median age at ASO was 10 (interquartile range 7–19) days. Hospital mortality occurred in 3 patients (21.4%), all being related to coronary complications. All hospital survivors were still alive at a median of 9.1 (interquartile range 4.2–18.3) years after the ASO. None of them developed complaints of ischaemia, ventricular arrhythmias, ventricular dysfunction or exercise intolerance. Surveillance computed tomography angiography showed stable aortocoronary relationships free from stenosis, compression and kinking. No reoperations for coronary artery problems and/or neoaortic valve or root problems were needed.
CONCLUSIONS
Although close monitoring of early coronary events seems crucial to prevent perioperative mortality, the modified Yacoub aortocoronary flap technique may serve as a viable bail-out option in patients with ‘non-separable’ single sinus coronary anatomy with intramural course, with excellent results among hospital survivors.
Keywords: Arterial switch operation, Congenital heart disease, Single coronary artery, Intramural coronary artery, Transposition of the great arteries
Outcomes after the arterial switch operation (ASO) for transposition of the great arteries (TGA) have improved significantly; yet, coronary transfer remains the most crucial part of the ASO.
INTRODUCTION
Outcomes after the arterial switch operation (ASO) for transposition of the great arteries (TGA) have improved significantly; yet, coronary transfer remains the most crucial part of the ASO. Coronary artery anomalies are present in ∼20% of all patients with TGA and even more frequently in certain subgroups such as Taussig–Bing anomaly [1]. Unusual coronary patterns have been associated with increased risk of adverse postoperative outcomes [2], and some of these patterns have specific implications for management [1, 3]. Certain coronary anatomies prohibit the use of the button or trap-door transfer techniques; this is usually the case when the coronary ostia are so close together that they cannot be separated or rotated, often in association with intramural coronary artery course (similar to Yacoub type B and C coronary anatomy) [4]. In this rare setting of ‘non-separable’ single sinus coronary arteries with intramural course, the aortocoronary flap technique—first developed by Yacoub in the late 1970s and later modified by others—can be used as an alternative to the button transfer and trap-door techniques [4]. Outcome data about these coronary artery variants corrected with the (modified) Yacoub technique are limited and are specifically addressed in only few studies [5–10]. Therefore, the purpose of this study was to evaluate the early- and mid-term outcomes of the modified Yacoub aortocoronary flap technique as a bail-out option in the rare setting ‘non-separable’ single sinus coronary arteries with intramural course in neonatal ASO.
PATIENTS AND METHODS
Study design and definitions
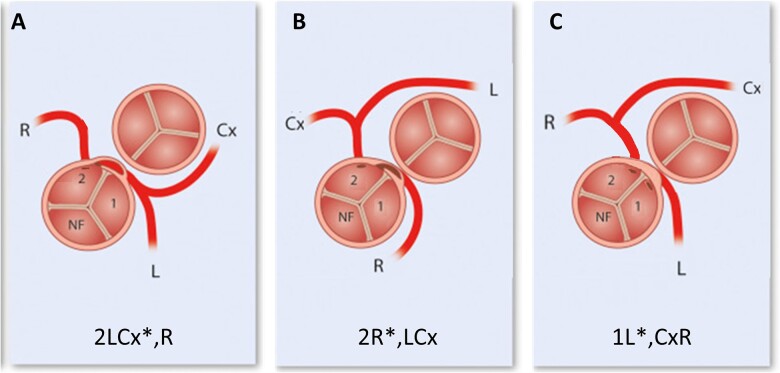
All patients with TGA who underwent ASO with modified Yacoub aortocoronary flap technique for ‘non-separable’ single sinus coronary arteries with intramural course in a single centre between January 1977 and April 2022 were identified from the local database. Data on demographics, morphologic and surgical details, postoperative course and details of follow-up [i.e. need for reoperations, catheter interventions, occurrence of ischaemia or arrhythmias and latest assessment of cardiac function and coronary patency based on echocardiographic and computed tomography angiography (CTA) images] of these patients were collected using hospital and outpatient records. Early mortality was defined as mortality within 30 days or before hospital discharge after ASO repair; other deaths were classified as late mortality. Coronary artery anatomy was classified according to the Leiden Convention coronary coding system [11] (Fig. 1). Three-dimensional coronary artery lumen and large vessel lumen (neo-aorta and neo-pulmonary arteries) segmentation was acquired from CTAs performed for coronary artery surveillance (Vitrea Advanced Visualization Software, version 7.12, Vital Images Inc., Minnetonka, USA). On these images, the aortocoronary flap and coronary arteries were assessed for the presence of coronary ostial stenosis, coronary artery angulation and external compression.
Figure 1:
Major coronary patterns involving ‘non-separable’ single sinus coronary artery with intramural course in transposition of the great arteries anatomy. According to the Leiden Convention coronary coding system (figures derived from and modified after ref [11]). (A) 2LCx*,R = separate coronary ostia from sinus 2 with intramural (*) LCA; 2LCx*R = common coronary ostium from sinus 2 with intramural (*) LCA. (B) 2R*,LCx = separate coronary ostia from sinus 2 with intramural (*) RCA. (C) 1L*,2CxR = 2 ostia located very close to each other, each on 1 side of the commissure between the 2 facing sinuses. Note that, in reality, the ostia would lie closer to each other than might be suggested based on the diagrams. LCA: left coronary artery; RCA: right coronary artery.
Ethics statement
The study was approved by the local scientific board and the Medical Ethical Committee waived the need for individual, separate informed consent.
Surgical procedure
For every variation in coronary anatomy, multiple alternative surgical approaches have been proposed. In the setting of single sinus coronary arteries with intramural course, some centres advocate a direct implantation of the coronary button after having unroofed the entire intramural segment of the vessel [12, 13], while other centres favour an aortopulmonary fenestration with an aortic wall flap or pericardial baffle [14, 15] or the creation of an aortic sinus pouch [16, 17]. In most cases, the intramural coronary artery has its connection to the opposite sinus, with either a single coronary ostium or 2 ostia lying close together, often located close to the posterior aortic commissure. At our institution, when the coronary arteries are deemed ‘non-separable’ (and thus conventional button transfer is not deemed to be feasible), a modified version of the aortocoronary flap technique according to Yacoub has been used as a bail-out option [4] (Fig. 2). In short, the surgical creation of the modified Yacoub aortopulmonary flap during ASO is as follows: the coronary cuff is detached from the aortic sinus and rotated to align the ostia with the pulmonary trunk (i.e. neo-aorta) in horizontal plane (at a maximum of 45°). The upper border of the coronary cuff is then anastomosed to the basis of the transected pulmonary trunk (i.e. neo-aorta) (Fig. 2A and B). Thereafter, the distal aorta is connected to the pulmonary trunk (i.e. neo-aorta) and the coronary cuff, thus achieving arterial switch and creating the aortocoronary flap. In contrast to the original description by Yacoub [4] where the lower border of the coronary cuff is anastomosed directly to the distal aorta, at our institution, we perform a longitudinal aortic incision and use a pericardial hood as a roof patch to connect the lower border of the coronary cuff to the distal aorta (Fig. 2C and D). This modification leads to the creation of a ‘pouch’, thus augmenting the effective area for flow from the neo-aorta into the coronary ostia and minimizing the risk of myocardial ischaemia from external compression on the aortocoronary flap. Finally, the neo-pulmonary trunk is reconstructed using a patch of fresh autologous pericardium and the posterior commissure is reattached if necessary.
Figure 2:
Schematic stepwise representation of the modified Yacoub aortocoronary flap technique. Right anterior-related position of the aorta compared to the pulmonary artery with aligned commissures of the semilunar valves and coronary artery with intramural course. A surgical incision is made around both coronary ostia to create a coronary flap (A). Rotation of the the coronary flap to align the ostia with the neo-aorta in the horizontal plane (maximum 45°) with anastomosis of the basis of the coronary artery button to the neo-aorta (B). The aorta is positioned above the pulmonary valve and pulmonary trunks and a small longitudinal aortic incision has been made (C) for the creation of the aortocoronary flap using a pericardial hood to connect the coronary flap to the neo-aorta (D) and to accomplish the arterial switch (E).
Using the (modified) Yacoub aortocoronary flap technique, the original direction and course of the proximal coronary arteries are not altered, and the risk of coronary kinking is avoided. Sometimes the coronary artery cannot be detached from the aortic sinus without removal of part of the posterior aortic commissure. In addition, when 2 ostia of the coronary arteries connecting to the same sinus are located very close to each other, we prefer not to divide them into 2 coronary buttons—but to detach them together as a single cuff for the formation of the aortocoronary flap—to reduce the risk of ostial stenosis after reimplantation. Depending on the relationship of the great arteries and the position of the aortocoronary flap, a Lecompte manoeuvre can or cannot be performed.
Analysis
Continuous variables were assessed for normality using the Shapiro–Wilk test and are expressed as mean (standard deviation) or median [interquartile range (IQR)], as appropriate. Categorical variables are expressed as counts and frequencies (%). All analyses were performed with R Statistical Software (version 4.2.1; Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient characteristics
Between January 1977 and April 2022, 516 patients underwent ASO for TGA at our institution. TGA morphology in these patients was as follows: TGA with intact ventricular septum (IVS) in 311 (60.3%), TGA with ventricular septal defect (VSD) in 151 (29.2%) and double outlet right ventricle with overriding pulmonary trunk and subpulmonary VSD (i.e. Taussig–Bing anomaly) in 54 (10.5%) patients. Fourteen patients (2.7% of the entire population) underwent ASO with the modified Yacoub aortocoronary flap technique for ‘non-separable’ single sinus coronary arteries with intramural course. Baseline patient characteristics of these patients are shown in Table 1.
Table 1:
Baseline characteristics and cardiac anatomy
| Nr. | Sex | Year of ASO | Age at ASO (days) | Weight at ASO (kg) | Diagnosis | Coronary anatomya | Intramural coronary | Position of great arteries |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 1985 | 21 | 3.4 | TGA-VSD | 2LCx,Rb | Unknown | Ao right anterior to PA |
| 2 | Male | 1985 | 9 | 1.8 | TGA-IVS | 2LCx*,R | LCA | Ao anterior |
| 3 | Male | 1996 | 113c | 4.8 | TGA-IVS | 2LCx*Rb | LCA | Ao right anterior to PA |
| 4 | Male | 2005 | 49 | 3.6 | Taussig–Bing | 2LCx*R | LCA | Ao right anterior to PA |
| 5 | Female | 2007 | 12 | 3.1 | TGA-VSD | 2LCxR | Unknown | Ao left anterior to PA |
| 6 | Male | 2008 | 57 | 3.4 | Taussig–Bing with interrupted aortic arch | 2R*,LCx | RCA | Ao left anterior to PA |
| 7 | Male | 2011 | 11 | 3.9 | TGA-VSD | 2LCx*,R | LCA | Ao left anterior to PA |
| 8 | Male | 2014 | 6 | 3.5 | TGA-IVS | 2LCx*,R | LCA | Ao right anterior to PA |
| 9 | Male | 2015 | 8 | 4.0 | TGA-IVS | 1L*,2CxR | LAD | Ao right anterior to PA |
| 10 | Male | 2015 | 9 | 2.9 | TGA-IVS | 2LCx*,R | LCA | Ao left anterior to PA |
| 11 | Male | 2017 | 13 | 3.4 | TGA-VSD | 2LCx*,R | LCA | Ao anterior |
| 12 | Male | 2018 | 6 | 3.4 | TGA-IVS | 2LCx*,R | LCA | Ao anterior |
| 13 | Male | 2020 | 6 | 3.4 | TGA-VSD | 2LCx*,R | LCA | Ao anterior |
| 14 | Male | 2022 | 7 | 3.2 | TGA-IVS | 2R*,LCx | RCA | Ao right anterior to PA |
According to the Leiden Convention coronary coding system.
Ostia directly above posterior commissure.
Two-stage strategy: modified Blalock–Taussig shunt and pulmonary artery banding, followed by late arterial switch operation.
Ao: aorta; ASO: arterial switch operation; Cx: circumflex; IVS: intact ventricular septum; LCA: left coronary artery; PA: pulmonary artery; RCA: right coronary artery; TGA: transposition of the great arteries; VSD: ventricular septal defect.
Of the 14 identified patients, 7 had TGA-IVS (50%), 5 had TGA-VSD (35.7%) and 2 had Taussig–Bing anomaly (14.3%) of which one had an associated interrupted aortic arch type B. Two major coronary artery patterns were present (Fig. 1), both corresponding to Yacoub type B coronary anatomy: 2LCx*R with common or separate coronary ostia (i.e. 2LCx*R or 2LCx*,R) in 11 patients (of note, intramural course could not be verified from surgical reports but was expected to be present in 2 of these 11 patients) and 2R*,LCx in 2 patients. In addition, 1 patient had a pattern that resembled Yacoub type C coronary anatomy: 1L*,2CxR (2 ostia located very close to each other, each on 1 side of the commissure between the 2 facing sinuses). While the latter does not strictly contain a single coronary ostium, it exhibits similar complex coronary artery morphology requiring the same surgical approach.
The median age at ASO was 10 (IQR 7–19) days. The Lecompte manoeuvre was not feasible in 5 (37.5%) patients because of potential compression of the coronary button by the neo-pulmonary trunk and pulmonary artery branches. The median cardiopulmonary bypass time was 196 (IQR 174–227) min and the median aortic cross-clamp time was 116 (IQR 107–151) min; antegrade cerebral perfusion was necessary in 1 patient during aortic arch repair. The sternum was left open in 8 patients (57.1%). Since 2005 (4th case), all patients received lifelong platelet aggregation therapy (aspirin) postoperatively.
Early outcomes
Hospital mortality occurred in 3 patients (21.4%). Causes of early death were all related to coronary complications. Three patients (21.4%) necessitated extracorporeal membrane oxygenation (ECMO) support following immediate coronary revision directly after surgery for severe ventricular dysfunction; 2 of these patients died (at days 2 and 12 post-ASO) and 1 patient showed full recovery. One patient could not be weaned from bypass and died in the operation room after coronary revision (before the ECMO era). Patients who did not survive the ASO (nos. 4, 8 and 12 in Table 1) are described in more detail:
Patient no. 4 with Taussig–Bing anomaly (coronary anatomy 2LCx*R; no arch abnormality) underwent ASO with VSD closure (via aortic valve and small right ventriculotomy) and Lecompte manoeuvre at the age of 7 weeks. This patient could not be weaned from bypass due to ischaemia of the left ventricular anterior wall and septum and died in the operation room after coronary revision (before the ECMO era).
Patient no. 8 with TGA-IVS (coronary anatomy 2LCx*,R) underwent ASO without Lecompte manoeuvre at day 6. After uneventful weaning from cardiopulmonary bypass, the postoperative course was complicated by a thrombus in the coronary anastomosis during transport from the operation room to the intensive care unit (ICU) with the total obstruction of both coronary arteries. In the second instance, revision of the coronary anastomosis, removal of the thrombus and connection to ECMO support were applied. Mitral valve plasty was performed on postoperative day 10 while still on ECMO. The patient died on postoperative day 12 from ECMO-related severe bleeding complications.
Patient no. 12 with TGA-IVS (coronary anatomy 2LCx*,R) underwent the ASO with Lecompte manoeuvre at day 6. After initial adequate systolic cardiac function while on bypass, ischaemia and poor systolic left ventricular function occurred during rewarming and weaning from bypass. To exclude traction of the pericardial hood patch on the coronary button, the patch was enlarged distally with the 2nd autologous pericardial patch and the pulmonary artery base was subsequently enlarged by a short segment Contegra 14-mm interponate graft. ECMO support was started to recover from potential myocardial stunning. The patient died at day 2 post-ASO due to a persistent asystole without development of any cardiac electrical activity and poor systolic cardiac function.
Of the hospital survivors, the median hospital length of stay was 18.0 (IQR 12.8–24.3) days with a median ICU length of stay of 10.5 (IQR 6.5–14.0) days. The ventilation time of these patients was at a median of 3.0 (IQR 1.8–6.0) days.
Late follow-up
One patient was lost to follow-up abroad. For the 10 remaining hospital survivors, follow-up was available for a median of 9.1 (IQR 4.2–18.3) years post-ASO. The oldest patient in the series was 36.7 years old. None of the patients developed complaints of ischaemia or ischaemia-related ventricular arrhythmias at latest follow-up. Systolic cardiac function was preserved in all hospital survivors without any wall motion abnormalities. Exercise testing was performed in all patients older than 8 years of age (n = 5) and revealed normal results.
Surveillance coronary CTA for early detection of coronary artery problems (i.e. coronary artery stenosis, compression or kinking) was performed in 9 of 10 patients at a median age of 7.1 (IQR 3.6–15.9) years post-ASO. One patient (no. 14) underwent the ASO with a modified Yacoub aortocoronary flap technique <6 months ago and, therefore, has not yet received a surveillance coronary CTA. No coronary artery stenosis was observed in any of the patients. One patient (no. 2) with coronary anatomy 2LCx*,R showed a sharp angulation of the left coronary artery and right coronary artery from the mildly dilated neoaortic root (34.5 mm, average of cusp-to-commissure measurements) at the age of 23.7 years post-ASO: the left coronary artery originated from its ostium at an angle of 50°; the right coronary artery originated from its ostium at an angle of 30° and showed proximal tailoring at the level of the intramural course. Subsequent myocardial perfusion magnetic resonance scintigraphy revealed no signs of ischaemia. Coronary CTA was repeated at the age of 35.9 years post-ASO with similar results in line with the previous CT examination with a mildly dilated neoaortic root diameter (39.0 mm) (Fig. 3A). Other representative CTA images and CTA-based 3D reconstructions are presented in Fig. 3B–E, showing stable aortocoronary relationships free from stenosis, compression and kinking during follow-up.
Figure 3:
Representative examples of conventional coronary CTA images and CTA-based 3D reconstructions showing the neo-aorta with modified Yacoub aortocoronary flap and coronary arteries. (A) Axial view CTA of patient Nr. 2 (2LCx*,R coronary anatomy) at the 35.9 post-ASO. (B) Sagittal view CTA of patient Nr. 7 (2LCx*,R coronary anatomy) at 2.7 years post-ASO. (C) CTA-based 3D reconstruction of patient Nr. 9 (1L*,2CxR coronary anatomy) at 7.0 years post-ASO. (D) CTA-based 3D reconstruction of patient Nr. 13 (2LCx*,R coronary anatomy) at 2 months post-ASO. (E) CTA-based 3D reconstruction of patient Nr. 2 (2LCx*,R coronary anatomy) at the 35.9 post-ASO. ACF: modified Yacoub aortocoronary flap; Ao: neo-aorta; ASO: arterial switch operation; CTA: computerized tomography angiography; Cx: circumflex coronary artery; L: left coronary artery; PT: neo-pulmonary trunk; R: right coronary artery.
No reoperations for coronary artery problems and/or neoaortic valve or root problems were needed. Three patients (nos. 5–7) were reoperated for right ventricular outflow tract obstruction; 1 was reoperated twice for this problem. All of them had pulmonary artery reconstruction without Lecompte manoeuvre during the ASO. Patient no. 5 (TGA-VSD) underwent relief of a supravalvular pulmonary stenosis including a resection of a related neo-pulmonary artery patch aneurysm at the age of 1.7 years. Patient no. 6 (Taussig–Bing anomaly with interrupted aortic arch type B) had a small (6 mm) bicuspid neo-pulmonary valve and underwent relief for right ventricular outflow tract obstruction with a transannular patch at the age of 0.7 years. Patient no. 7 (TGA-VSD) was reoperated twice at the age of 1.4 and 2.4 years to relief a supravalvular pulmonary stenosis: an augmentation plasty of the pulmonary trunk was performed along with a stent implantation in the right pulmonary artery intraoperatively.
DISCUSSION
Coronary artery anomalies are present in ∼20% of all patients with TGA [1]. Among these unusual anatomical variants, those with ‘non-separable’ single sinus coronary ostia (Yacoub type B coronary anatomy; variations in 2LCx*R or 2R*LCx) or 2 coronary ostia lying close to one another at both sides of a commissure (Yacoub type C coronary anatomy; variations of 1L*,2CxR), especially when associated with intramural course of a coronary artery, represent a particular challenge for coronary transfer during the ASO. The coronary ostia cannot be separated without causing injury, and the coronary arteries cannot be mobilized or rotated without provoking kinking. In the series of 18 patients based on which Yacoub et al. developed their classification system, 2 had a type B and 1 had a type C coronary anatomy [4]. From the experience at our centre, we could estimate the prevalence of type B and C coronary anatomies at 2.5% (13/516) and 0.2% (1/516), respectively. The results of our present study demonstrate that in these selected cases, the modified Yacoub aortocoronary flap technique may be a viable alternative that allows for effective coronary transfer.
The current knowledge about efficacy and safety of the modified Yacoub aortocoronary flap technique has been based on a limited number of studies, most of which discussed the technique only as part of their overall experience with the ASO. The study by Choi et al. included 9 patients who underwent coronary transfer using the aortocoronary flap technique, 1 of whom required coronary revision on postoperative day 3 due to myocardial ischaemia [7]. In their experience with 8 patients, Sung et al. reported failure to wean from bypass due to myocardial failure and subsequent death in 1 patient; the authors hypothesized that coronary artery injury during mobilization related to inexperience with the technique as well as long ischaemic times might have led to the outcome [5]. They also reported coronary injury in 1 other patient which could be successfully repaired and revision due to bleeding from the aortocoronary flap in 1 patient. The 7 hospital survivors demonstrated excellent myocardial function up to 63 months of follow-up. Among the 8 patients in the case series by Kim et al. [10], coronary injury occurred during division of coronary artery from aortic wall and required coronary artery bypass grafting in 2 patients. In another patient, coronary revision with switch to individual coronary button technique was required on postoperative day 2 because of myocardial ischaemia. For hospital survivors, no coronary stenoses were observed on CTA up to 10 years post-ASO. Jung et al. [9] reported on 3 patients undergoing coronary transfer using the modified Yacoub technique, 1 of whom required coronary revision for initial failure of cardiopulmonary bypass weaning and subsequently died on postoperative day 1 and the 2 others of whom required reoperations due to severe left main coronary artery stenosis (at 8.2 and 90.4 months post-ASO). In contrast, Merino et al. [6] and Fricke et al. [18] reported on 2 and 3 patients, respectively, with no complications and excellent ventricular function at follow-up. Finally, Chegondi et al. [8] reported on a patient who required ECMO due to biventricular dysfunction during rewarming but who could eventually be weaned successfully and at follow-up CTA at 6 months of age showed unobstructed flow in the coronary arteries.
Our present study, including 14 patients, is the largest to date to report on early- and mid-term outcomes of the modified Yacoub aortocoronary flap technique. In agreement with previous studies, our results demonstrate that the mortality rate associated with this technique (3/14, 21.4%) is not negligible. Furthermore, postoperative ventricular dysfunction, failure to wean from cardiopulmonary bypass and need for ECMO are common, and sudden ischaemia or infarction may occur during ICU stay. Indeed, several aspects of the technique may lead to an elevated risk of coronary events. First, the abnormal location of the coronary arteries following this technique represents a potential nidus for coronary stenosis or kinking. Accurate placement of the flap is therefore of key importance because even a small derangement of the flap can cause distortion of 1 or both coronary arteries. However, even then it should be considered that significant coronary ostial stenosis may coexist with intramural coronary anatomy. Failure to address such stenosis may result in ischaemia and may potentially be fatal. Although the presence of coronary ostial stenosis could not be verified in this series, it is possible that the death of 3 of these patients was related to ischaemia from such underlying stenosis. It should be considered that some of the patients might nowadays have been a candidate for other techniques which are able to address coronary ostial stenosis, such as coronary unroofing and/or splitting. Second, the pericardial tissue used to create the ‘patch’ is prone to thrombosis, which may lead to extensive myocardial ischaemia or infarction because both coronary arteries are critically dependent on flow through the same anastomosis with the aorta. For this reason, all of our patients from 2004 onwards (4th patient) received aspirin for life to prevent the formation of thrombi in the aortocoronary flap. Finally, the aortocoronary flap can be compressed between the neo-aorta and neo-pulmonary arteries, potentially resulting in myocardial ischaemia or infarction during the operation or in the early postoperative period. Indeed, Thrupp et al. [19] reported on a patient who developed ischaemic changes on the electrocardiogram when weaning from bypass, resulting from dynamic compression of the pericardial hood by the neo-pulmonary artery. Surgical revision was performed and the issue could be resolved by elongation of the neo-pulmonary artery, plication of the posterior pericardial patch of the neo-aorta and placement of anterior plication sutures on the aorta to change the angulation of the pericardial hood. For all of the above reasons, the aortocoronary flap technique is likely to be highly operator dependent. Excellent outcomes may be achieved, however, only in experienced hands and when intensive monitoring is performed in the perioperative period. Regardless, this technique should likely be reserved for cases where the coronary ostia are really deemed ‘non-separable’ (and thus conventional techniques are not considered to be feasible), because splitting of the common button with/without unroofing is currently being performed in an increasing number of patients who share a similar coronary pattern.
In agreement with previous data [5, 8, 10], our study found excellent prognosis for hospital survivors following ASO with the aortocoronary flap technique. At a median of 9.1 years post-ASO, none of them developed complaints of ischaemia, ventricular arrhythmias, ventricular dysfunction or exercise intolerance. No reoperations for coronary artery problems and/or neoaortic valve or root problems were needed. Furthermore, surveillance CTA showed stable aortocoronary relationships free from stenosis, compression and kinking. It could therefore be questioned whether routine CTA in patients during follow-up in childhood may actually be useful in asymptomatic survivors; future studies may help to further elucidate this.
Limitations
The findings of the present study are limited by its small sample size, retrospective nature, heterogeneity of patients and wide span of surgical eras. As already suggested above, some of the patients in this series might not have undergone the Yacoub aortocoronary flap technique in the contemporary era, given the availability of various alternative techniques (such as coronary unroofing and splitting). Judicious and selective use is therefore advised.
CONCLUSION
In conclusion, the Yacoub aortocoronary flap technique may serve as a viable alternative to ‘conventional’ coronary transfer techniques in selected cases with ‘non-separable’ single sinus coronary anatomy with intramural course. Although close monitoring is needed to detect and manage early postoperative coronary events, follow-up among hospital survivors is excellent.
Glossary
ABBREVIATIONS
- ASO
Arterial switch operation
- CTA
Computed tomography angiography
- ECMO
Extracorporeal membrane oxygenation
- ICU
Intensive care unit
- IVS
Intact ventricular septum
- IQR
Interquartile range
- TGA
Transposition of the great arteries
- VSD
Ventricular septal defect
Contributor Information
Jef Van den Eynde, Department of Cardiothoracic Surgery, Leiden University Medical Center, Leiden, Netherlands; Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium.
Roel L F van der Palen, Department of Pediatrics, Division of Pediatric Cardiology, Leiden University Medical Center, Leiden, Netherlands.
Ingmar Knobbe, Department of Pediatrics, Division of Pediatric Cardiology, Amsterdam UMC, Amsterdam, Netherlands.
Bart Straver, Department of Pediatrics, Division of Pediatric Cardiology, Amsterdam UMC, Amsterdam, Netherlands.
Lauran Stöger, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands.
Gabriella Ricciardi, Department of Cardiac Surgery, University of Lille, Lille University Hospital, Lille, France.
Thelma C Konings, Department of Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands.
Monique R M Jongbloed, Department of Cardiology, Leiden University Medical Center, Leiden, Netherlands; Department of Anatomy & Embryology, Leiden University Medical Center, Leiden, Netherlands.
Mark G Hazekamp, Department of Cardiothoracic Surgery, Leiden University Medical Center, Leiden, Netherlands.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest: none declared.
DATA AVAILABILITY
Databases and code will be made available from the corresponding author upon reasonable request.
Author contributions
Jef Van den Eynde: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Roel L.F. van der Palen: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Ingmar Knobbe: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing. Bart Straver: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing. Lauran Stöger: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing. Gabriella Ricciardi: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing. Thelma C. Konings: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing. Monique R.M. Jongbloed: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing. Mark G. Hazekamp: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks René Prêtre, André Rüffer, Rajesh Sharma and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Presented at the 36th EACTS Annual Meeting, Milan, Italy, 6 October 2022.
REFERENCES
- 1. Moll M, Michalak KW, Sobczak-Budlewska K, Moll JA, Kopala M, Szymczyk K. et al. Coronary artery anomalies in patients with transposition of the great arteries and their impact on postoperative outcomes. Ann Thorac Surg 2017;104:1620–8. [DOI] [PubMed] [Google Scholar]
- 2. Pasquali SK, Hasselblad V, Li JS, Kong DF, Sanders SP.. Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries: a meta-analysis. Circulation 2002;106:2575–80. [DOI] [PubMed] [Google Scholar]
- 3. Angeli E, Formigari R, Pace Napoleone C, Oppido G, Ragni L, Picchio FM. et al. Long-term coronary artery outcome after arterial switch operation for transposition of the great arteries. Eur J Cardiothorac Surg 2010;38:714–20. [DOI] [PubMed] [Google Scholar]
- 4. Yacoub MH, Radley-Smith R.. Anatomy of the coronary arteries in transposition of the great arteries and methods for their transfer in anatomical correction. Thorax 1978;33:418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sung SC, Chang YH, Lee HD, Kim S, Woo JS, Lee YS.. Arterial switch operation for transposition of the great arteries with coronary arteries from a single aortic sinus. Ann Thorac Surg 2005;80:636–41. [DOI] [PubMed] [Google Scholar]
- 6. Merino CM, Casares J, Mataro MJ, Avalos R, Conejero MT, Gomez E. et al. Arterial switch operation with separate coronary arteries arising from a single aortic sinus. Rev Esp Cardiol 2008;61:1338–41. [PubMed] [Google Scholar]
- 7. Choi KH, Sung SC, Kim H, Lee HD, Ko H, Byun JH.. The coronary reimplantation after neoaortic reconstruction technique can make a difference in arterial switch operation. J Cardiothorac Surg 2019;14:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chegondi M, Ricci M, Ashwath RC.. Case report: challenging perioperative decision-making in a neonate with transposition of great arteries and novel coronary anatomy. Front Pediatr 2022;10:900142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jung JC, Kwak JG, Kim ER, Bang JH, Min J, Lim JH. et al. Reoperation for coronary artery stenosis after arterial switch operation. Interact CardioVasc Thorac Surg 2018;27:169–76. [DOI] [PubMed] [Google Scholar]
- 10. Kim H, Sung SC, Kim SH, Chang YH, Ahn HY, Lee HD.. Arterial switch operation in patients with intramural coronary artery: early and mid-term results. Korean J Thorac Cardiovasc Surg 2011;44:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gittenberger-de Groot AC, Koenraadt WMC, Bartelings MM, Bokenkamp R, DeRuiter MC, Hazekamp MG. et al. Coding of coronary arterial origin and branching in congenital heart disease: the modified Leiden Convention. J Thorac Cardiovasc Surg 2018;156:2260–9. [DOI] [PubMed] [Google Scholar]
- 12. Asou T, Karl TR, Pawade A, Mee RB.. Arterial switch: translocation of the intramural coronary artery. Ann Thorac Surg 1994;57:461–5. [DOI] [PubMed] [Google Scholar]
- 13. Padalino MA, Ohye RG, Devaney EJ, Bove EL.. Double intramural coronary arteries in D-transposition of the great arteries. Ann Thorac Surg 2004;78:2181–3. [DOI] [PubMed] [Google Scholar]
- 14. Aubert J, Pannetier A, Couvelly JP, Unal D, Rouault F, Delarue A.. Transposition of the great arteries. New technique for anatomical correction. Br Heart J 1978;40:204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moat NE, Pawade A, Lamb RK.. Complex coronary arterial anatomy in transposition of the great arteries. Arterial switch procedure without coronary relocation. J Thorac Cardiovasc Surg 1992;103:872–6. [PubMed] [Google Scholar]
- 16. Ko Y, Nomura K, Kinami H, Kawamura R.. Aortic sinus pouch technique for transposition of the great arteries with intramural coronary artery. J Thorac Cardiovasc Surg 2018;155:e127–e9. [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto Y, Nomura K, Isobe S, Murayama F.. Aortic sinus pouch technique for dextro-transposition of the great arteries with a single or intramural coronary artery. Oper Tech Thorac Cardiovasc Surg 2021;26:616–28. [Google Scholar]
- 18. Fricke TA, Bulstra AE, Naimo PS, Bullock A, Robertson T, d'Udekem Y. et al. Excellent long-term outcomes of the arterial switch operation in patients with intramural coronary arteries. Ann Thorac Surg 2016;101:725–9. [DOI] [PubMed] [Google Scholar]
- 19. Thrupp SF, Gentles TL, Kerr AR, Finucane K.. Arterial switch operation: early and late outcome for intramural coronary arteries. Ann Thorac Surg 2012;94:2084–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Databases and code will be made available from the corresponding author upon reasonable request.
Author contributions
Jef Van den Eynde: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Roel L.F. van der Palen: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Ingmar Knobbe: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing. Bart Straver: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing. Lauran Stöger: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing. Gabriella Ricciardi: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing. Thelma C. Konings: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing. Monique R.M. Jongbloed: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing. Mark G. Hazekamp: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks René Prêtre, André Rüffer, Rajesh Sharma and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.