Abstract
Immunoassays designed to detect SARS-CoV-2 protein antigens (Ag) are commonly used to diagnose COVID-19. The most widely used tests are lateral flow assays that generate results in approximately 15 minutes for diagnosis at the point-of-care. Higher throughput, laboratory-based SARS-CoV-2 Ag assays have also been developed. The number of commercially available SARS-CoV-2 Ag detection tests has increased rapidly, as has the COVID-19 diagnostic literature. The Infectious Diseases Society of America (IDSA) convened an expert panel to perform a systematic review of the literature and develop best-practice guidance related to SARS-CoV-2 Ag testing. This guideline is an update to the third in a series of frequently updated COVID-19 diagnostic guidelines developed by the IDSA. IDSA's goal was to develop evidence-based recommendations or suggestions that assist clinicians, clinical laboratories, patients, public health authorities, administrators, and policymakers in decisions related to the optimal use of SARS-CoV-2 Ag tests in both medical and nonmedical settings. A multidisciplinary panel of infectious diseases clinicians, clinical microbiologists, and experts in systematic literature review identified and prioritized clinical questions related to the use of SARS-CoV-2 Ag tests. A review of relevant, peer-reviewed published literature was conducted through 1 April 2022. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology was used to assess the certainty of evidence and make testing recommendations. The panel made 10 diagnostic recommendations that address Ag testing in symptomatic and asymptomatic individuals and assess single versus repeat testing strategies. US Food and Drug Administration (FDA) SARS-CoV-2 Ag tests with Emergency Use Authorization (EUA) have high specificity and low to moderate sensitivity compared with nucleic acid amplification testing (NAAT). Ag test sensitivity is dependent on the presence or absence of symptoms and, in symptomatic patients, on timing of testing after symptom onset. In most cases, positive Ag results can be acted upon without confirmation. Results of point-of-care testing are comparable to those of laboratory-based testing, and observed or unobserved self-collection of specimens for testing yields similar results. Modeling suggests that repeat Ag testing increases sensitivity compared with testing once, but no empirical data were available to inform this question. Based on these observations, rapid RT-PCR or laboratory-based NAAT remain the testing methods of choice for diagnosing SARS-CoV-2 infection. However, when timely molecular testing is not readily available or is logistically infeasible, Ag testing helps identify individuals with SARS-CoV-2 infection. Data were insufficient to make a recommendation about the utility of Ag testing to guide release of patients with COVID-19 from isolation. The overall quality of available evidence supporting use of Ag testing was graded as very low to moderate.
Keywords: SARS-CoV-2, diagnostic testing, rapid antigen tests, systematic review, diagnostic test performance
EXECUTIVE SUMMARY
Diagnostic testing is an important tool to combat coronavirus disease 2019 (COVID-19). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen (Ag) tests are now widely available, which has helped expand testing to settings outside of the hospital or clinic. Most SARS-CoV-2 Ag tests in clinical use are point-of-care (POC) lateral flow devices that generate results in approximately 15 minutes. Laboratory-based Ag test platforms also exist, but experience with their performance and utility is limited. The main advantage of POC testing is the rapid availability of results, which facilitates isolation, contact tracing, quarantine, and potential treatment decisions. Given the recent expansion of the literature on diagnostic testing along with widespread adoption of Ag testing, particularly outside of healthcare settings, the Infectious Diseases Society of America (IDSA) has updated evidence-based guidelines for the use of US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) SARS-CoV-2 Ag tests.
The overall specificity of SARS-CoV-2 Ag tests was 99% or higher compared with standard nucleic acid amplification testing (NAAT; ie, rapid reverse transcriptase–polymerase chain reaction [RT-PCR] or laboratory-based NAAT) (Supplementary Figure 2B). Therefore, routine confirmation of positive Ag results by a reference molecular method is not necessary in most settings. In contrast, Ag test sensitivity was low or moderate and was dependent on the presence or absence of COVID-19 symptoms and the time of testing after symptom onset. Pooled Ag test sensitivity was 81% (95% confidence interval [CI]: 78% to 84%) for symptomatic individuals (Supplementary Figure 2A) and 89% (95% CI: 83% to 93%) if testing occurred within the first 5 days of illness (Supplementary Figure 3A); after 5 days, sensitivity fell to 54% (Supplementary Figure 4A). Testing patients within 3 days of symptom onset yielded results similar to testing within 5 days; studies reporting results of testing of patients within 1 or 2 days of symptoms were not identified. Among asymptomatic individuals, the pooled sensitivity of Ag testing was 63% (Supplementary Figure 12A). Antigen tests performed similarly in adults and children, although data on children were limited (Supplementary Figures 13A and 13B).
Despite the widespread use of Ag testing to guide individual attendance at school, work, and large social gatherings, the panel identified no clinical trials or observational studies that directly informed these testing applications, and so it was unable to make recommendations about Ag testing in these situations. Similarly, the panel found no clinical trials or observational studies that compared the risk of onward transmission of SARS-CoV2 from patients who were released from isolation based on time from symptom onset versus results of an Ag test. Therefore, the panel was unable to make a recommendation about the utility of Ag testing to guide discontinuation of isolation.
Since no empirical data were identified to inform the value of serial versus single sample testing compared with molecular testing, results of serial testing were estimated using mathematical modeling; results of this analysis suggested that repeat testing would improve sensitivity (Note: On 11 August 2022, the FDA issued recommendations for repeat Ag testing to diagnose COVID-19 in symptomatic and asymptomatic persons; https://www.fda.gov/medical-devices/safety-communications/home-covid-19-antigen-tests-take-steps-reduce-your-risk-false-negative-results-fda-safety#:∼:text=Currently%2C%20all%20at%2Dhome%20COVID,t%20have%20COVID%2D19%20symptoms. This recommendation was based on publication of a preprint that reported improved sensitivity of rapid Ag testing compared with a composite nucleic acid amplification reference standard when asymptomatic study participants tested 3 times at 48-hour intervals and symptomatic study participants tested 2 times within 48 hours; https://pubmed.ncbi.nlm.nih.gov/35982680/). Other evidence gaps included the performance of Ag tests in vaccinated individuals or those previously infected with SARS-CoV-2. Very limited data were available on the performance of Ag tests in immunocompromised patients (although the literature review excluded studies that included only immunocompromised individuals), or in individuals infected with recent SARS-CoV-2 variants. In the literature search conducted through April 2022, the panel identified only 1 study that included persons tested after November 2021, the time during which Omicron variants emerged and became dominant. All studies compared Ag with molecular test results, with none using a clinical reference standard.
Specific recommendations and comments related to the use of SARS-CoV-2 Ag tests with FDA-EUA status are summarized below. An algorithm based on these recommendations is provided to aid in decision making (Figure 1). A detailed description of background, methods, evidence summary, and rationales that support each recommendation, as well as unmet research needs, can be found online in the full text.
Figure 1.
Algorithm for antigen recommendations. aNo recommendation for or against antigen testing could be made for the specific populations of students in educational settings, employees at work, or individuals planning to attend a large social gathering (evidence gaps). bNo recommendation for or against home testing using NAAT could be made (evidence gap). cNucleic acid amplification test (NAAT) refers to rapid or laboratory-based nucleic amplification test. dFor NAAT, either rapid or standard laboratory-based testing is suggested (conditional recommendation). eFor unexposed, asymptomatic individuals undergoing procedures or planned for hospital admission, routine NAAT testing is not suggested (conditional recommendations). fFor NAAT in symptomatic individuals, the IDSA panel suggests collecting either nasopharyngeal (NP) swab, anterior nasal (AN) swab, oropharyngeal (OP) swab, midturbinate (MT) swab, saliva or mouth gargle specimens (conditional recommendation). gFor NAAT in symptomatic individuals, the IDSA panel suggests that anterior nares and midturbinate specimens can be either self-collected or collected by a healthcare provider (conditional recommendation). hEither point-of-care or laboratory-based antigen testing is suggested (conditional recommendation). iIf the specimen is self-collected, either observed or unobserved collection is suggested (conditional recommendation). jThe IDSA panel suggests against using NAAT in patients with COVID-19 to guide discontinuation of isolation or prior to a procedure or surgery (conditional recommendations). kFor guidance on the timing of repeat testing for a specific assay, please consult the respective assay package insert or the latest FDA guidance. Abbreviations: COVID-19, coronavirus disease 2019; FDA, Food and Drug Administration; IDSA, Infectious Diseases Society of America.
Briefly, an expert panel consisting of clinicians, medical microbiologists, and methodologists critically appraised the SARS-CoV-2 Ag diagnostic literature using Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology to assess the certainty of evidence. Per GRADE, recommendations are categorized as “strong” or “conditional.” The word “recommend” indicates a strong recommendation and “suggest” indicates a conditional recommendation. This guideline assumed availability of rapid Ag testing and focuses on testing for diagnosis and asymptomatic screening.
Given the superior sensitivity of molecular diagnostics, the panel suggests using standard NAAT over Ag tests if standard NAAT is available and results of testing will be timely. The panel recognizes the value of diagnosing COVID-19 quickly, since treatment options are typically approved for administration within 5 days of symptom onset. In addition, rapid isolation of contagious patients is expected to reduce SARS-CoV-2 transmission. Therefore, rapid Ag testing has value when timely NAAT is unavailable, especially when results are positive; the high specificity of Ag testing means that positive results are actionable without needing confirmation. In contrast, negative Ag results should be confirmed by standard NAAT when clinical suspicion of COVID-19 is high. Ultimately, deciding whether to use rapid Ag tests in lower-risk, nonmedical settings will depend on several factors, including the prevalence of disease in the population, combined with assessment of the value of detecting true SARS-CoV-2 infection versus the detrimental effects of erroneous results (ie, falsely negative or positive results). Feasibility of test implementation and costs of testing are other important considerations.
Recommendation 1: For symptomatic individuals suspected of having COVID-19, the IDSA panel recommends a single Ag test over no test. (strong recommendation, moderate certainty evidence)
Remarks
Symptomatic individuals were defined as those with at least 1 of the common symptoms of COVID-19.
For optimal performance, Ag tests should be performed within 5 days of symptom onset.
If clinical suspicion for COVID-19 remains high, a negative Ag result should be confirmed by standard NAAT (ie, rapid RT-PCR or laboratory-based NAAT).
A single Ag test has high specificity; a positive result can be used to guide treatment and isolation decisions without confirmation.
There were limited data regarding the analytical performance of Ag tests in children, immunocompromised or vaccinated individuals, or in those who had had prior SARS-CoV-2 infection.
The panel was unable to identify studies that compared risk of transmission among patients recovering from COVID-19 who were released from isolation based on results of Ag testing versus no testing.
Recommendation 2: For symptomatic individuals suspected of having COVID-19, the IDSA panel suggests using standard NAAT (ie, rapid RT-PCR or laboratory-based NAAT) over a rapid Ag test. (conditional recommendation, low certainty evidence)
Remarks
If standard NAAT is unavailable or results are expected to be delayed more than 1 day, the IDSA panel suggests using a rapid Ag test over standard NAAT.
For optimal performance, Ag tests should be performed within 5 days of symptom onset.
The panel was unable to identify studies comparing the risk of transmission among patients recovering from COVID-19 who were released from isolation based on results of Ag testing versus standard NAAT.
Recommendation 3: For symptomatic individuals suspected of having COVID-19, the IDSA panel suggests using a single standard NAAT (ie, rapid RT-PCR or laboratory-based NAAT) rather than a strategy of 2 consecutive rapid Ag tests. (conditional recommendation, very low certainty evidence)
Remarks
In situations where NAAT results are not available in a timely manner and a first Ag test is negative, the IDSA panel suggests repeating Ag testing.
Because of the absence of direct, empirical evidence to inform this question, the analysis done was based on modeling of diagnostic test accuracy using a repeat testing algorithm involving 2 consecutive Ag tests.
To optimize sensitivity, repeat testing should be performed within 5 days of symptom onset.
If the first Ag test is positive, there is no need for repeat testing.
Recommendation 4: For asymptomatic individuals with known exposure to SARS-CoV-2 infection, the IDSA panel suggests using a single (ie, one-time) Ag test over no testing in specific situations. (conditional recommendation, moderate certainty evidence)
Remarks
SARS-CoV-2 testing in the absence of COVID-19–like symptoms should be individualized. One-time Ag testing may be considered if the test result will impact an individual's subsequent actions. For example, a single test may be considered in situations where a positive test would lead to increased monitoring for symptoms and signs of infection in persons at high risk of serious COVID-19, or in outbreak settings where positive results would assist in decision making about isolation, quarantine, and contact tracing.
A negative Ag test result reduces the likelihood of SARS-CoV-2 infection. However, the longer the time since testing, the more this likelihood reduction wanes, especially early in infection when virus replication may be rapid. That is, a negative test result today may not reflect infection status tomorrow or on subsequent days. In contrast, a positive test result is associated with a high positive-predictive value.
The panel recognizes the lack of evidence supporting therapy in asymptomatic persons and the absence of treatment approved through FDA EUA for asymptomatic COVID-19, but acknowledges that individual clinical scenarios may lead clinicians toward testing and consideration of treatment.
Recommendation 5: For asymptomatic individuals with known exposure to SARS-CoV-2 infection, the IDSA panel suggests using a single standard NAAT (ie, rapid RT-PCR or laboratory-based NAAT) over a single rapid Ag test. (conditional recommendation, low certainty evidence)
Remarks
SARS-CoV-2 testing in the absence of COVID-19–like symptoms should be individualized. A one-time standard NAAT may be considered if the test result will impact an individual's subsequent actions. For example, a single test may be considered in situations where a positive test would lead to increased monitoring for symptoms and signs of infection for persons at high risk of severe COVID-19, or in an outbreak setting where positive results would assist in decision making about isolation, quarantine, and contact tracing.
Access to timely results of standard NAAT may be unavailable or limited in some settings; in such situations, use of an Ag test can be considered.
The panel recognizes the lack of evidence supporting COVID-19 therapy in asymptomatic persons, and the absence of treatment approved through FDA EUA for asymptomatic COVID-19 but acknowledges that individual clinical scenarios may lead clinicians toward testing and consideration of treatment.
Recommendation 6: In asymptomatic individuals with a known exposure to SARS-CoV-2, if standard NAAT testing or results are not available in a timely manner and a first Ag test is negative, the IDSA panel suggests repeat Ag testing. (conditional recommendation, very low certainty evidence)
Remarks
Because of the absence of direct, empirical evidence to inform this question, the analysis was based on modeling of diagnostic test accuracy using a repeat testing algorithm involving 2 consecutive Ag tests.
Recommendation 7: Among students in educational settings or employees in workplaces for whom SARS-CoV-2 testing is desired, the IDSA panel suggests neither for nor against 2 consecutive Ag tests over no testing for the diagnosis of SARS-CoV-2 infection. (evidence gap)
Remarks
The IDSA panel found no direct evidence comparing 2 Ag tests versus a single standard NAAT with a third reference standard in group settings such as schools, colleges, or workplaces.
Because of the absence of direct, empirical evidence to inform this question, the analysis was based on modeling of diagnostic test accuracy using a repeat testing algorithm involving 2 consecutive Ag tests.
Recommendation 8: For asymptomatic individuals planning to attend a large gathering (eg, concert, conference, party, sporting event), the IDSA panel suggests neither for nor against Ag testing over no testing. (evidence gap)
Remarks
No studies directly addressed this question.
Recommendation 9: For individuals for whom Ag testing is desired, the IDSA panel suggests either POC or laboratory-based Ag testing. (conditional recommendation, low certainty evidence).
Remarks
Although the results of test performance for POC and laboratory-based Ag testing appear to be comparable, an important limitation of the evidence is that studies did not report the relative numbers of symptomatic and asymptomatic subjects. Since Ag test sensitivity is higher in symptomatic than in asymptomatic individuals, the unknown proportions of symptomatic and asymptomatic individuals included in POC or laboratory-based studies may have influenced the results to minimize differences between the 2 testing strategies.
Recommendation 10: The IDSA panel suggests either observed or unobserved self-collection of swab specimens for Ag testing if self-collection is performed. (conditional recommendation, low certainty evidence)
Remarks
There were no studies comparing observed and unobserved specimen collection in the same patients.
Studies reported heterogeneity in the techniques used for specimen collection and in the reference standard used as the comparator.
Providing instructions for optimal specimen collection may improve the quality of self-collected specimens.
BACKGROUND
Making a rapid and accurate diagnosis of SARS-CoV-2 infection remains an essential component of comprehensive mitigation strategies aimed at curtailing COVID-19. Standard NAAT, defined throughout this document as rapid RT-PCR or laboratory-based NAATs, is considered the reference method for diagnosing symptomatic or asymptomatic SARS-CoV-2 infection. However, over the course of the pandemic, especially early on, molecular diagnostic test shortages and delayed test turnaround times plagued testing initiatives in many locations. Currently, multiple pharmacologic therapies for COVID-19 have EUA from the US FDA for use within the first 5 days of symptoms, justifying the need for rapid, accurate test results.
Commercially available, rapid Ag tests that detect SARS-CoV-2 proteins have helped to address the ongoing need for widespread access to SARS-CoV-2 testing. While Ag-based assays for respiratory viruses are generally less sensitive than reference molecular methods, Ag tests can be easier and faster to perform, and these assays are typically less expensive than NAAT. In addition, rapid Ag testing can be easily deployed outside of clinic or hospital settings, with analysis performed by nonmedical staff. Table 1 compares the advantages and limitations of Ag testing versus NAAT.
Table 1.
Comparisons Between SARS-CoV-2 Antigen and Molecular Diagnostic Tests
| Test Features | Ag Tests | Nucleic Acid Amplification Tests |
|---|---|---|
| Methods |
|
|
| Targets | Viral protein:
|
Viral RNA:
|
| Specimen typesb |
|
|
| Point-of-care use |
|
|
| Advantages |
|
|
| Limitations |
|
|
Abbreviations: Ag, antigen; COVID-19, coronavirus disease 2019; LFA, lateral flow assay; NAAT, nucleic acid amplification test; RT-PCR, reverse transcriptase–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMA, transcription-mediated amplification.
aLateral flow assays also include tests designated as chromatographic digital immunoassays.
bApproved specimen types vary by test. Alternate types require laboratory validation.
cStandard NAAT includes rapid RT-PCR and laboratory-based assays.
As of September 2022, 51 SARS-CoV-2 Ag tests have received EUA from the FDA [1]. SARS-CoV-2 Ag tests use monoclonal antibodies to capture and detect viral proteins in respiratory secretions obtained with a nasopharyngeal, midturbinate, or nasal swab. On 23 September 2021, the FDA revised the EUAs of certain Ag tests to require manufacturers to evaluate the impact of SARS-CoV-2 viral mutations on their test's performance, and to update their authorized labeling accordingly [1]. Depending on the manufacturer, Ag test swabs may either be analyzed directly or placed in an approved transport media or other fluid for testing. Currently available SARS-CoV-2 Ag tests come in a variety of formats, including rapid lateral flow assays (LFAs) and other types of immunoassays. Lateral flow assays are the most used method for SARS-CoV-2 Ag detection and are amendable to testing at the POC. In addition, several SARS-CoV-2 LFAs have received EUA designation for home testing. Lateral flow assays are configured as single-use test strips with results read either visually or by an instrument in approximately 15 minutes. Other immunoassay designs may require instrumentation or procedural steps that must be performed in a clinical laboratory by laboratory-trained staff, with results typically generated in under 1 hour of instrument run time.
Most SARS-CoV-2 Ag tests with EUA status are labeled for testing symptomatic individuals who are suspected of having COVID-19, but an increasing number of tests are labeled for postexposure screening of asymptomatic persons [1]. Most Ag tests have indications for use within the first 5, 7, 12, or 14 days of symptom onset, depending on the test. Antigen testing is also being used for surveillance purposes (ie, testing asymptomatic individuals with no known or suspected exposure to a confirmed case of SARS-CoV-2 infection). The Centers for Medicare and Medicaid Services exercised enforcement discretion to allow the use of all Ag tests in asymptomatic individuals for the duration of the COVID-19 public health emergency. Depending on the indication for testing, Ag testing may also be completed once (single test) or performed sequentially over time (repeated tests) [2].
Given the broad range of uses of Ag tests and the rapidly growing number of published studies focused on Ag testing, the IDSA convened an expert panel to systematically review the SARS-CoV-2 Ag diagnostic test literature with a focus on assays with EUA status. The panel compared pooled estimates of test accuracy to make evidence-based recommendations for best use in clinical practice. This guide assumes ongoing transmission of SARS-CoV-2 in the community and the availability of EUA-designated Ag tests but does not address use for public health surveillance.
METHODS
Panel Composition
The panel was composed of clinicians and clinical microbiologists who are members of IDSA, the American Society for Microbiology (ASM), the Society for Healthcare Epidemiology of America (SHEA), and the Pediatric Infectious Diseases Society (PIDS). They represent the disciplines of infectious diseases, pediatrics, and medical microbiology. The Evidence Foundation provided technical support and guideline methodologists for development of this guideline.
Disclosure and Management of Potential Conflicts of Interest
The conflict-of-interest (COI) review group included 2 representatives from IDSA who were responsible for reviewing, evaluating, and approving all disclosures. All members of the expert panel complied with the COI process for reviewing and managing COIs, which required disclosure of any financial, intellectual, or other interest that might be construed as constituting an actual, potential, or apparent conflict, regardless of relevancy to the guideline topic. The assessment of disclosed relationships for possible COIs was based on the relative weight of the financial relationship (ie, monetary amount) and the relevance of the relationship (ie, the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The COI review group ensured that the majority of the panel and chair were without potentially relevant conflicts (ie, those related to the topic). The chair and all members of the technical team were determined to be unconflicted.
Question Generation
Clinical questions related to the use of SARS-CoV-2 Ag tests were developed into a PICO (Population, Intervention, Comparison, Outcomes) format prior to the first panel meeting (Supplementary Table 1). Panel members prioritized questions with available evidence that met the minimum acceptable criteria (ie, the body of evidence reported on at least a case-series design; case reports were excluded)
Search Strategy
A comprehensive search of several databases from January 2019 to 1 April 2022, limited to humans and the English language, was conducted. The databases included PubMed MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials. The search strategy was designed and conducted by an experienced librarian with input from the methodology panel. Controlled vocabulary was used, supplemented with keywords to search for SARS-CoV-2, diagnosis, and Ag testing. Reference lists and literature suggested by panelists were reviewed for inclusion. Preprints were followed for final publication but were not included in the literature review unless they were published. During the evidence assessment and recommendation process, horizon scans were performed to locate additional grey literature (ie, information produced outside of traditional publishing and distribution channels), manuscript preprints, and literature published after the last search date. Reference lists and literature suggested by panelists were reviewed for inclusion. The complete search strategy is found in Supplementary Table 2.
Screening and Study Selection
Inclusion Criteria
Four reviewers (A. E. A., I. K. E. M., R. M., P. P., and F. A.) independently screened titles and abstracts and eligible full-text studies. Studies reporting on the diagnostic test accuracy of Ag testing (cohort studies, cross-sectional studies, and case-control studies) were included. We aimed to identify studies that compared the diagnostic performance of Ag testing or Ag test-based strategies with rapid RT-PCR testing or no testing using a third reference standard. When such studies were not identified, we selected studies that reported diagnostic test accuracy of Ag testing compared with rapid RT-PCR as a reference standard. We limited our inclusion to tests that had FDA EUA or Conformite Europeenne mark as of March 2022. We only included studies that used a single or multiple NAATs as reference standards. We included any study regardless of the prevalence of COVID-19. We included studies regardless of timing of symptom onset if they compared Ag testing with predefined reference standards. We only included studies that used upper respiratory tract samples (anterior nasal, midturbinate, or nasopharyngeal swabs). Reviewers extracted relevant information into a standardized data-extraction form. Studies of testing strategies were included if they reported the effect of the testing strategy on disease prevalence or outcomes.
Exclusion Criteria
We excluded studies that compared Ag with viral culture as a reference standard, studies that included fewer than 100 patients for sensitivity or specificity assessment, studies that reported either only sensitivity or specificity, tests with no FDA EUA or Ce mark, and studies that did not provide enough information to allow calculation of sensitivity and specificity. We excluded studies of pooled samples and studies that evaluated analytical sensitivity/specificity (no clinical samples). We excluded studies that included only immunocompromised individuals, as questions related to this patient population were not prioritized for the current update. We also excluded preprint studies that did not undergo the process of peer review.
Data Collection and Analysis
The review team abstracted data from the included studies. The extracted data included general study characteristics (authors, publication year, country, study design), the diagnostic index test and reference standard, the prevalence of COVID-19, and parameters to determine test accuracy (ie, sensitivity and specificity of the index test). For each test, we extracted sampling sites, sampling method (healthcare worker, self, or supervised self-collection), use of transport media (vs dry swabs or direct testing), location of sample collection (eg, ambulatory, hospital-based, field), the target Ag, and the test platform (eg, lateral flow). We also recorded whether the same specimen was used for Ag and NAAT testing; whether the same site was used for both tests (when different specimens were used); whether the specimen for 1 test was obtained before the other systematically (eg, Ag swabs always collected first); whether there was a time gap between collection of specimens (eg, a specimen for NAAT collected on admission followed by a specimen for Ag testing collected a few days later); and whether the sample was collected from the right, left, or both sides when laterality is possible (eg, nasal swabs), alongside the timing of specimen collection relative to symptom onset.
For each study, we calculated the sensitivity and specificity of the diagnostic index test and used the Clopper–Pearson method to estimate 95% CIs. We then fit the random-effects bivariate binomial model of Chu and Cole [3] to pool accuracy estimates using the glmer function of the lme4 package in R (version 4.1.2; R Foundation for Statistical Computing). To pool accuracy estimates for analyses including fewer than 5 studies, we fit a fixed-effects model as implemented in the meta package in R (version 4.1.2). We used forest plots to plot individual and summary estimates and conducted subgroup analyses to explore heterogeneity.
For repeat testing, we included studies that reported outcomes of repeat testing on people with COVID-19.
This guideline assumes the risk of acquiring SARS-CoV-2 as a result of exposure in a community, household, or facility. To determine the prevalence of infection for each PICO question, we considered published literature in consultation with clinical experts. Prevalence, as defined by the results of surveillance NAAT testing over the last 14 days in each community, has been shown to change over time. For purposes of the guideline, we applied 1%, 5%, and 10% pretest probability for asymptomatic cases and used 5%, 20%, to 50% pretest probability for symptomatic patients—that is, those with at least 1 of the common symptoms of COVID-19. These pretest probabilities were chosen based on the prevalence of SARS-COV-2 reported by the CDC and other sources at different times during the pandemic [4]. Instances of higher pretest probability include symptomatic patients, residence in a community with high prevalence, and/or a person living in a household or with continued contact with someone with confirmed COVID-19 within the antecedent 14 days. For comparative purposes, the diagnostic accuracy of rapid RT-PCR and laboratory-based NAAT from 5 studies that used a composite reference standard was used as a reference standard against which to compare the performance of Ag testing [5–9] (Supplementary Figure 10). The performance of NAAT in each of these 5 studies was compared against a composite reference standard composed of at least 2 other NAATs.
Risk of Bias and Certainty of Evidence
We conducted the risk-of-bias assessment for diagnostic test accuracy studies using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 revised tool (Supplementary Table 3) [10]. The GRADE framework was used to assess overall certainty by evaluating the evidence for each outcome on the following domains: risk of bias, imprecision, inconsistency, indirectness, and publication bias [11, 12]. Indirectness was judged to be present if there were no head-to-head comparisons of analytical performance of the testing strategies reported. For decision making, the panel considered additional factors, such as the feasibility (ie, availability, convenience) of the test, timeliness of results, cost, and prevalence. The GRADE summary of findings tables were developed using the GRADEpro Guideline Development Tool [13].
Evidence for Recommendations
The panel considered core elements of GRADE evidence in the decision process, including certainty of evidence and balance between desirable and undesirable effects. Additional domains were acknowledged, where applicable (eg, feasibility, resource use, acceptability). For all recommendations, the expert panelists reached consensus. Voting rules were agreed on prior to panel meetings for situations when consensus could not be reached.
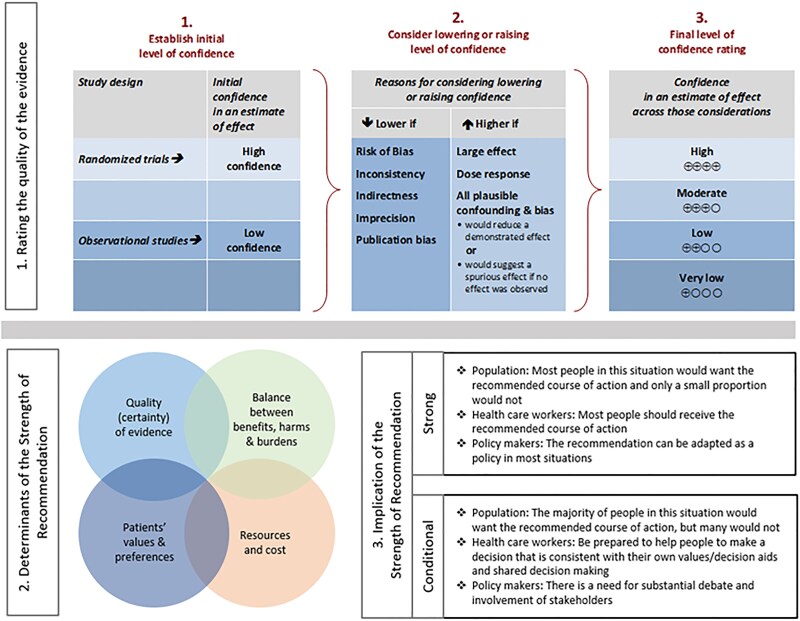
As per GRADE methodology, recommendations are labeled as “strong” or “conditional.” The words “we recommend” indicate strong recommendations, with “we suggest” indicating conditional recommendations. Figure 2 provides the suggested interpretation of strong and weak recommendations for patients, clinicians, and healthcare policymakers. Rarely, low certainty evidence may lead to strong recommendations. In those instances, we followed generally recommended approaches by the GRADE working group, which are outlined in 5 paradigmatic situations (eg, avoiding catastrophic harm) [141]. For recommendations where comparators are not formally stated, the comparison of interest is implicitly referred to as “not using the test.” Some recommendations acknowledge current “knowledge gaps” and aim at avoiding premature favorable recommendations for test use and promulgating potentially inaccurate tests.
Figure 2.
Approach and implications to rating the quality of evidence and strength of recommendations using the GRADE methodology (unrestricted use of the figure granted by the US GRADE Network). Abbreviation: GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
Revision Process
The draft guideline underwent rapid review for approval by the IDSA Board of Directors Executive Committee external to the guideline development panel. The guideline was reviewed and endorsed by ASM, SHEA, and PIDS. The IDSA Board of Directors Executive Committee reviewed and approved the guideline prior to dissemination.
Updating Process
Regular screening of the literature and the COVID-19 situation will take place to determine the need for revisions based on the likelihood that any new data will have an impact on the recommendations. If necessary, the entire expert panel will reconvene to discuss potential changes.
Search Results
A systematic review and horizon scan of the literature identified 17 334 references, 95 of which informed the evidence base for these recommendations (Supplementary Figure 1). Characteristics of the included studies can be found in Supplementary Table 4.
RESULTS
Antigen Testing Versus No Testing in Symptomatic Individuals
Recommendation 1: For symptomatic individuals suspected of having COVID-19, the IDSA panel recommends a single Ag test over no test. (strong recommendation, moderate certainty evidence)
Remarks
Symptomatic individuals were defined as those with at least 1 of the common symptoms of COVID-19.
For optimal performance, Ag tests should be used within 5 days of symptom onset; the panel identified no studies that reported Ag test performance on the first or second day of symptoms.
If clinical suspicion for COVID-19 remains high, a negative Ag result should be confirmed by standard NAAT (ie, rapid RT-PCR or laboratory-based NAAT).
A single Ag test has high specificity; a positive result can be used to help guide treatment and isolation decisions without confirmation.
There were limited data regarding the analytical performance of Ag tests in children, immunocompromised or vaccinated individuals, or in those who had had prior SARS-CoV-2 infection.
The panel was unable to identify studies that compared the risk of transmission among patients recovering from COVID-19 who were released from isolation based on results of Ag testing versus no testing.
Summary of the Evidence
We found no direct evidence that assessed patient- or population-centered outcomes of testing versus no testing in symptomatic patients. Therefore, the panel relied on diagnostic test accuracy data to inform this recommendation. The reference standard in the included studies was standard NAAT (ie, rapid RT-PCR or laboratory-based NAAT).
We identified 65 studies [15–78] that evaluated the diagnostic accuracy of Ag testing as compared with NAAT as a reference test in symptomatic individuals (Table 2). The studies included 20 272 individuals for sensitivity and 51 063 for specificity. We conducted subgroup analyses based on time since symptom onset (ie, ≤3 days vs >3 days, ≤5 days vs >5 days, and ≤7 days vs >7 days). Additional subgroup analyses were performed based on different age groups (ie, adult vs pediatric patients). Overall and subgroup test accuracy data for symptomatic patients are reported in Supplementary Figures 2–9. Pooled diagnostic test accuracy measures did not differ in any subgroup or sensitivity analysis except for assessment of time post–symptom onset, with reduced sensitivity of Ag testing after 5 or 7 days of symptoms. Studies did not separately report the effect of immunocompromised status, vaccination, or prior COVID-19 on diagnostic accuracy. We searched for studies that stated that they had included SARS-CoV-2 variants, and also attempted to infer inclusion of variants by date of specimen collection. Only 1 study was found; it reported reduced sensitivity for detection of Omicron versus Delta variants for several rapid Ag tests [79]. We were also unable to identify studies that compared the risk of transmission among patients recovering from COVID-19 who were released from isolation based on results of Ag testing versus no testing.
Table 2.
GRADE Evidence Profile of Test Accuracy Results for Prevalence/Pre-test Probability of 5%, 20%, and 50%, for Symptomatic Patients Overall with Nucleic Acid Amplification Testing as the reference standard
| Overall performance of antigen testing | |
|---|---|
| Sensitivity | .81 (95% CI: .78 to .84) |
| Specificity | 1.00 (95% CI: .99 to 1.00) |
| Outcome | No. of Studies (No. of Patients) | Study Design | Factors That May Decrease Certainty of Evidence | Effect per 1000 Patients Tested | Test Accuracy CoE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias | Pretest Probability of 5%a | Pretest Probability of 20%a | Pretest Probability of 50%a | ||||
| True positives (patients with COVID-19) | 65 Studies (20 272 patients) | Cohort and case-control–type studies | Not seriousb | Not seriousc | Seriousd | Not serious | None | 41 (39 to 42) | 162 (156 to 168) | 405 (390 to 420) | ⨁⨁⨁◯ Moderate |
| False negatives (patients incorrectly classified as not having COVID-19) | 9 (8 to 11) | 38 (32 to 44) | 95 (80 to 110) | ||||||||
| True negatives (patients without COVID-19) |
65 Studies (51 063 patients) | Cohort and case-control–type studies | Not seriousb | Not seriousc | Not serious | Not serious | None | 950 (941 to 950) | 800 (792 to 800) | 500 (495 to 500) | ⨁⨁⨁⨁ High |
| False positives (patients incorrectly classified as having COVID-19) | 0 (0 to 9) | 0 (0 to 8) | 0 (0 to 5) | ||||||||
aWe used a pretest probability of 5% to represent low community prevalence and used a 20% and 50% pretest probability for cases of medium to high community prevalence, known close contact or during outbreaks. Abbreviations: CI, confidence interval; CoE, certainty of evidence; COVID-19, coronavirus disease 2019; GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
bAlthough some of the included studies were judged to have a high or unclear risk of bias in 1 or more domains, a sensitivity analysis excluding studies with a high risk of bias did not show a difference in the effect estimate. For this reason, we did not downgrade for risk of bias.
cThere is some indirectness as the test accuracy results were to inform on patient-important outcomes.
dThere is serious unexplained inconsistency in the results despite partial explanation of having different types of tests in different studies.
We analyzed diagnostic test accuracy for specimens collected from patients before and after 3, 5, and 7 days of symptoms. Three days was chosen because of concern that Ag tests had lower sensitivity when used soon after development of symptoms; we were unable to identify studies that reported testing specimens collected only on the first or second day of symptoms. Five days was chosen because several COVID-19 treatments have EUA to begin therapy within 5 days of symptoms. Seven days was chosen because many Ag tests evaluated received EUA for use within 7 days of symptom onset.
The pooled sensitivity was 81% (95% CI: 78% to 84%) and the pooled specificity was 100% (95% CI: 100% to 100%). The certainty of the evidence was moderate for sensitivity due to unexplained inconsistency of reported test performance, even for the same Ag test, same specimen source, and similar time from symptom onset. The certainty of evidence was high for specificity.
For the subset of patients who were symptomatic for less than or equal to 5 days, 8 studies were included [31, 33, 35, 37, 54, 64, 66, 68], with 584 positive and 2092 negative results, based on standard NAAT. The pooled sensitivity for this group was 89% (95% CI: 83% to 93%) and the pooled specificity was 100% (95% CI: 99% to 100%). The certainty of the evidence was moderate for sensitivity due to unexplained inconsistency and high for specificity (Table 3). Results for the subset of patients who were symptomatic for less than or equal to 3 days were similar (ie, we did not observe a reduction in sensitivity or specificity compared with standard NAAT) (Supplementary Figure 7).
Table 3.
GRADE Evidence Profile of Test Accuracy Results for Prevalence/Pre-test Probability of 5%, 20%, and 50%, for Patients Having Symptoms for 5 or Fewer Days with Nucleic Acid Amplification Testing as the reference standard
| Performance of Antigen Testing in Patients Having Symptoms for 5 or Fewer Days | |
|---|---|
| Sensitivity | .89 (95% CI: .83 to .93) |
| Specificity | 1.00 (95% CI: .99 to 1.00) |
| Outcome | No. of Studies (No. of Patients) | Study Design | Factors That May Decrease Certainty of Evidence | Effect per 1000 Patients Tested | Test Accuracy CoE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias | Pretest Probability of 5%a | Pretest Probability of 20%a | Pretest Probability of 50%a | ||||
| True positives (patients with COVID-19) |
8 Studies (584 patients) | Cohort and case-control–type studies | Not seriousb | Not seriousc | Seriousd | Not serious | None | 45 (42 to 47) | 178 (166 to 186) | 445 (415 to 465) | ⨁⨁⨁◯ Moderate |
| False negatives (patients incorrectly classified as not having COVID-19) |
5 (3 to 8) | 22 (14 to 34) | 55 (35 to 85) | ||||||||
| True negatives (patients without COVID-19) |
8 Studies (2092 patients) | Cohort and case-control–type studies | Not seriousb | Not seriousc | Not serious | Not serious | None | 950 (941 to 950) | 800 (792 to 800) | 500 (495 to 500) | ⨁⨁⨁⨁ High |
| False positives (patients incorrectly classified as having COVID-19) |
0 (0 to 9) | 0 (0 to 8) | 0 (0 to 5) | ||||||||
aWe used a pretest probability of 5% to represent low community prevalence and used a 20% and 50% pretest probability for medium to high community prevalence, cases of known close contact or during outbreaks. Abbreviations: CI, confidence interval; CoE, certainty of evidence; COVID-19, coronavirus disease 2019; GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
bAlthough some of the included studies were judged to have a high or unclear risk of bias in 1 or more domains, a sensitivity analysis excluding studies with a high risk of bias did not show a difference in the effect estimate. For this reason, we did not downgrade for risk of bias.
cThere is some indirectness as the test accuracy results were to inform on patient-important outcomes.
dThere is serious unexplained inconsistency in the results.
For the subset of patients tested more than 5 days after symptom onset, 15 studies were included, with 1076 positive and 4933 negative patients, based on standard NAAT. The pooled sensitivity for this group was 54% (95% CI: 44% to 64%) and the pooled specificity was 100% (95% CI: 99% to 100%) (Supplementary Figures 4A). The certainty of the evidence was low for sensitivity due to unexplained inconsistency and high for specificity. Results of analysis of specimens collected more than 7 days after symptom onset were similar to results of specimens collected more than 5 days after symptom onset (Table 4).
Table 4.
GRADE Evidence Profile of Test Accuracy Results for Prevalence/Pre-test Probability of 5%, 20%, and 50%, for Patients Having Symptoms for More than 5 Days with Nucleic Acid Amplification Testing as the reference standard
| Performance of Antigen Testing in Patients Having Symptoms for More than 5 Days | |
|---|---|
| Sensitivity | .54 (95% CI: .44 to .64) |
| Specificity | 1.00 (95% CI: .99 to 1.00) |
| Outcome | No. of Studies (No. of Patients) | Study Design | Factors That May Decrease Certainty of Evidence | Effect per 1000 Patients Tested | Test Accuracy CoE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias | Pretest Probability of 10%a | Pretest Probability of 20%a | Pretest Probability of 50%a | ||||
| True positives (patients with COVID-19) | 15 Studies (1076 patients) | Cohort and case-control–type studies | Not seriousb | Not seriousc | Seriousd | Seriouse | None | 54 (44 to 64) | 108 (88 to 128) | 270 (220 to 320) | ⨁⨁◯◯ Low |
| False negatives (patients incorrectly classified as not having COVID-19) | 46 (36 to 56) | 92 (72 to 112) | 230 (180 to 280) | ||||||||
| True negatives (patients without COVID-19) |
15 Studies (4933 patients) | Cohort and case-control–type studies | Not seriousb | Not seriousc | Not serious | Not serious | None | 900 (891 to 900) | 800 (792 to 800) | 500 (495 to 500) | ⨁⨁⨁⨁ High |
| False positives (patients incorrectly classified as having COVID-19) |
0 (0 to 9) | 0 (0 to 8) | 0 (0 to 5) | ||||||||
aWe used a pretest probability of 5% to represent low community prevalence and used a 20% and 50% pretest probability for moderate to high community prevalence, cases of known close contact or during outbreaks. Abbreviations: CI, confidence interval; CoE, certainty of evidence; COVID-19, coronavirus disease 2019; GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
bAlthough some of the included studies were judged to have a high or unclear risk of bias in 1 or more domains, a sensitivity analysis excluding studies with a high risk of bias did not show a difference in the effect estimate. For this reason, we did not downgrade for risk of bias.
cThere is some indirectness as the test accuracy results were to inform on patient-important outcomes.
dThere is serious unexplained inconsistency in the results despite partial explanation of having different types of tests in different studies.
eThe false-negative range at 50% crosses the false-negative accuracy threshold of 20% (200/1000).
Benefits and Harm
The panel assumed that diagnosis of COVID-19 in symptomatic patients has benefits for both individuals and for the community. Establishing SARS-CoV-2 as the etiology of an individual's symptoms can influence decisions about initiation of therapy and isolation in those who are infected, and about contact tracing and quarantine. Sensitivity of a single Ag test is dependent on timing of testing relative to symptom onset, with higher sensitivity earlier in the course of symptomatic infection. The false-negative rate of Ag testing performed within 5 days of symptom onset ranged from 5 (range: 3 to 8) patients per 1000 patients tested at a prevalence of 5%, to 55 (range: 35 to 85) patients per 1000 patients tested at a prevalence of 50%. As noted above, results of single Ag testing within 3 days of symptom onset were similar to results of testing within 5 days of symptom onset, but the panel was unable to locate reports of testing on day 1 or 2 after symptom onset. Antigen testing of symptomatic individuals after 5 days of symptoms demonstrated a much lower sensitivity of 54% (95% CI: 44% to 64%), with almost equal numbers of true-positive and false-negative results. False-negative results can lead to failure to treat symptomatic patients in whom treatment is indicated, potentially leading to poorer patient outcomes. False-negative results can also lead to failure to isolate an infected person or to quarantine close contacts, potentially increasing the risk of onward transmission of SARS-CoV-2. Because of these potential patient harms, a negative result in someone with continued suspicion for COVID-19 should be confirmed promptly with a standard NAAT.
In contrast, specificity of Ag testing remained close to 100% regardless of time from symptom onset. Currently available therapies are recommended to be started within 5 days of symptoms. Antigen testing during this time yielded almost no false-positive results, even if the prevalence of COVID-19 was as low as 5% (0 false-positive results; range: 0 to 9 false-positive results per 1000 patients tested). This suggests that Ag testing within the first 5 days of symptom onset yields actionable results in symptomatic patients who test positive and qualify for treatment. The high specificity of Ag testing makes the risk of inappropriate treatment due to a false-positive result very low.
Few studies reported on symptomatic pediatric patients, but the available data indicated an overall sensitivity comparable to that in adults (80%; 95% CI: 74% to 86%), with overall specificity also close to 100% (95% CI: 94% to 100%). Depending on prevalence, the number of false-negative test results ranged from 10 to 100 per 1000 children tested. The panel was unable to find sufficient studies to allow for a robust comparison of test performance based on symptom duration in children.
Additional Considerations
While the IDSA panel recommends Ag testing versus no testing for patients with symptoms suggestive of COVID-19, there are a few scenarios in which testing of symptomatic individuals might be unnecessary. For example, it is plausible that a young, vaccinated, otherwise healthy, symptomatic adult who is not eligible for treatment and who chooses to isolate without a diagnostic confirmation would not need testing. The imperfect correlation between positive SARS-CoV-2 culture and Ag test results also precludes using a positive Ag test result to predict infectiousness. Still, while a negative Ag test result does not exclude infectiousness, a positive result makes infectiousness more likely.
Conclusions and Research Needs for This Recommendation
Positive Ag tests in symptomatic individuals have a high positive-predictive value for COVID-19 and can be used to help guide decision making about treatment and isolation of patients, contact tracing, and quarantine. Negative Ag tests have lower negative-predictive values to rule out COVID-19 infection. Individuals with a negative Ag test result who remain symptomatic and for whom an alternative diagnosis has not been established should undergo prompt testing for SARS-CoV-2 using standard NAAT.
Questions remain regarding the impact that variant strains, immunocompromised host status, vaccination, and/or prior COVID-19 may have on the analytical accuracy of Ag tests, including optimal specimen source (eg, anterior nares vs throat) and timing of testing (eg, sensitivity of Ag testing on day 1 or 2 of symptoms) [80]. The performance of antigen testing in very young children (eg, <6 months of age) is also poorly understood. This is especially notable since these individuals cannot mask and are not eligible for receipt of currently available COVID-19 vaccines.
The panel identified a few studies [81–84] that reported better positive percent agreement between Ag testing and viral culture than between standard NAAT and viral culture but identified no empirical evidence that informed the question of whether Ag test results predict infectiousness, as measured by transmission. Further, the IDSA panel found no empirical evidence to support the use of Ag test results to guide release of patients with COVID-19 from isolation. Given the consequences of this widespread practice, including cost, studies to identify a marker of infectivity are needed. Ensuring equal access to accurate, affordable, and timely SARS-CoV-2 diagnostic testing for underserved populations, including racial and ethnic minority groups, should be a priority [84].
Antigen Testing Versus Standard NAAT in Symptomatic Individuals
Recommendation 2: For symptomatic individuals suspected of having COVID-19, the IDSA panel suggests using standard NAAT (ie, rapid RT-PCR or laboratory-based NAAT) over a rapid Ag test. (conditional recommendation, low certainty evidence)
Remarks
If standard NAAT is not available or results are expected to be delayed more than 1 day, the IDSA panel suggests using a rapid Ag test over standard NAAT.
For optimal performance, Ag tests should be used within 5 days of symptom onset; the panel was unable to identify any study that reported results of Ag testing within 2 days of symptom onset.
The panel was unable to identify studies comparing the risk of transmission among patients recovering from COVID-19 who were released from isolation based on results of Ag testing versus results of standard NAAT.
Summary of the Evidence
Due to lack of direct evidence comparing Ag testing and standard NAAT with a third reference standard, we relied on diagnostic test accuracy data for Ag testing using standard NAAT as the reference standard. To calculate standard NAAT diagnostic test accuracy, we pooled results from 5 studies [85–89] that reported a comparison of standard NAAT results to a composite reference standard (Supplementary Figure 10). This analysis yielded a sensitivity of 97% (95% CI: 93% to 99%) and a specificity of 100% (95% CI: 96% to 100%).
We summarized the evidence for overall symptomatic (any day after symptom onset) (Table 2), less than or equal to 5 days after symptom onset (Table 3), and more than 5 days after symptom onset (Table 4). Additional subgroups included the following: less than or equal to 7 days after symptom onset (Supplementary Figure 5) and more than 7 days after symptom onset (Supplementary Figure 6). The more than 5 day cutoff was chosen because several commonly used COVID-19 therapies have EUA to begin treatment within the first 5 days of symptoms. The more than 7 day cutoff was chosen because many of the available rapid Ag tests have EUA for use within 7 days of symptom onset.
For comparative results, we included 70 studies—65 informing Ag testing [15–78, 90] and the 5 studies [85–89] discussed above that informed standard NAAT, with 20 621 positive and 51 593 negative results (Table 5). The pooled sensitivity for Ag testing was 81% (95% CI: 78% to 84%) and the pooled specificity was 100% (95% CI: 100% to 100%). This resulted in an additional 8 to 80 false-negative Ag test results, compared with NAAT, when the prevalence of SARS-CoV-2 infection ranged from 5% to 50%. The patients included in the 5 studies of standard NAAT versus a composite reference standard were different from those who participated in the 65 studies of Ag testing versus standard NAAT; hence, the comparison of standard NAAT and Ag test performance was indirect, seriously reducing confidence in the certainty of the evidence. Certainty of the evidence was therefore low for sensitivity due to indirectness and unexplained inconsistency and low for specificity due to indirectness.
Table 5.
GRADE Evidence Profile of Test Accuracy Results for Prevalence/Pre-test Probability of 5%, 20%, and 50%, for Symptomatic Patients Overall Versus Standard NAAT
| Performance Characteristics of Antigen Testing and Standard NAAT Overall | Antigen Testing | Standard NAAT |
|---|---|---|
| Sensitivity | .81 (95% CI: .78 to .84) | .97 (95% CI: .93 to .99) |
| Specificity | 1.00 (95% CI: .99 to 1.00) | 1.00 (95% CI: .96 to 1.00) |
| Outcome | No. of Studies (No. of Patients) | Study Design | Factors That May Decrease Certainty of Evidence | Effect per 1000 Patients Tested | Test Accuracy CoE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretest Probability of 5%a | Pretest Probability of 20%a | Pretest Probability of 50%a | ||||||||||||
| Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias | Antigen Testing | Standard NAAT | Antigen Testing | Standard NAAT | Antigen Testing | Standard NAAT | ||||
| True positives (patients with COVID-19) |
70 Studies (20 621 patientsb,c) | Cohort and case-control–type studies | Not seriousd | Seriouse | Seriousf | Not serious | None | 41 (39 to 42) | 49 (47 to 50) | 162 (156 to 168) | 194 (186 to 198) | 405 (390 to 420) | 485 (465 to 495) | ⨁⨁◯◯ Low |
| 8 fewer TPs in antigen testing | 32 fewer TPs in antigen testing | 80 fewer TPs in antigen testing | ||||||||||||
| False negatives (patients incorrectly classified as not having COVID-19) |
9 (8 to 11) | 1 (0 to 3) | 38 (32 to 44) | 6 (2 to 14) | 95 (80 to 110) | 15 (5 to 35) | ||||||||
| 8 more FNs in antigen testing | 32 more FNs in antigen testing | 80 more FNs in antigen testing | ||||||||||||
| True negatives (patients without COVID-19) |
70 Studies (51 593 patientsb,g) | Cohort and case-control–type studies | Not seriousd | Seriouse | Not serious | Not serious | None | 950 (941 to 950) | 950 (912 to 950) | 800 (792 to 800) | 800 (768 to 800) | 500 (495 to 500) | 500 (480 to 500) | ⨁⨁◯◯ Low |
| 0 fewer TNs in antigen testing | 0 fewer TNs in antigen testing | 0 fewer TNs in antigen testing | ||||||||||||
| False positives (patients incorrectly classified as having COVID-19) |
0 (0 to 9) | 0 (0 to 38) | 0 (0 to 8) | 0 (0 to 32) | 0 (0 to 5) | 0 (0 to 20) | ||||||||
| 0 fewer FPs in antigen testing | 0 fewer FPs in antigen testing | 0 fewer FPs in antigen testing | ||||||||||||
aWe used a pretest probability of 5% to represent low community prevalence and used a 20% and 50% pretest probability to represent moderat to high community prevalence, for cases of known close contact or during outbreaks. Abbreviations: CI, confidence interval; CoE, certainty of evidence; COVID-19, coronavirus disease 2019; FN, false negative; FP, false positive; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; NAAT, nucleic acid amplification testing; TN, true negative; TP, true positive.
bSixty-five studies assessed antigen while 5 studies assessed standard NAAT.
cA total of 20 272 patients came from studies that assessed antigen while only 349 patients came from studies that assessed a standard NAAT arm.
dAlthough some of the included studies were judged to have a high or unclear risk of bias in 1 or more domains, a sensitivity analysis excluding studies with a high risk of bias did not show a difference in the effect estimate. For this reason, we did not downgrade for risk of bias.
eThere were no studies that evaluated the accuracy of antigen and NAAT testing in the same population. Studies either evaluated the accuracy of antigen against a reference standard or NAAT against another reference standard.
fThere is serious unexplained inconsistency in the results with a sensitivity and specificity range.
gThe majority of the patients came from the antigen arm (51 063 patients) while only 530 patients were from the standard NAAT arm.
Benefits and Harm
The panel considered minimizing the number of false-negative COVID-19 diagnoses in symptomatic patients to be a priority. Standard NAAT has a higher sensitivity compared with a composite reference standard than does rapid Ag testing compared with standard NAAT. During a COVID-19 surge when SARS-CoV-2 prevalence in the community is high (ie, 50%) testing with a single Ag test resulted in 80 more false-negative results per 1000 patients tested compared with a standard NAAT overall. If the Ag test were to be performed within 5 days of onset of symptoms, false-negative results decreased to 40 per 1000 patients tested, but if performed after 7 days of onset of symptoms, false-negative results increased to 215 per 1000 patients tested. During nonsurge periods when the community prevalence among symptomatic individuals is lower, the number of false-negative results is also relatively lower. At a prevalence of 20%, there were 16 more false-negative results per 1000 persons undergoing Ag testing within 5 days of symptom onset and 86 more false-negative results if Ag testing was done after 5 days of onset of symptoms. Therefore, a single Ag test can result in more false-negative results compared with a single standard NAAT.
However, the panel also placed a high value on test availability and result timeliness. Obtaining a standard NAAT generally requires a visit to a testing site, and results may not be available for several days. This delay can push patients outside the antiviral treatment window, which is usually within 5 days of symptom onset. Long turnaround times for COVID-19 diagnostic tests can cause delays in isolation of infected patients, contact tracing, and quarantine of their close contacts, potentially allowing further COVID-19 transmission. Alternatively, long turnaround times for patients who ultimately test negative for COVID-19 may cause unnecessary home isolation and absence from work or school. In contrast to standard NAAT, Ag tests are often more available, results are reported usually within 15 minutes of testing, and Ag self-testing can be performed by patients at home. These considerations led the IDSA panel to suggest rapid Ag testing if results of standard NAAT will be delayed more than 1 day.
Antigen testing has very high specificity, and a positive result is actionable immediately. Because of lower sensitivity, a negative Ag test result should be confirmed with a standard NAAT if clinical suspicion for COVID-19 remains high. Especially in patients in whom treatment of COVID-19 would be indicated, Ag testing should be done within 5 days of symptom onset to minimize the number of false-negative results and to diagnose patients within the treatment eligibility window.
Additional Considerations
Standard NAAT (ie, rapid RT-PCR or laboratory-based NAAT) is the gold standard for diagnosis of viral respiratory infections because of the accuracy of results. However, availability and timeliness of standard NAAT for SARS-CoV-2 during the COVID-19 pandemic have often been wanting. Federal government subsidization of Ag testing has evolved during the pandemic, with the federal government sometimes providing no-cost home test kits upon request. Insurance company reimbursement for home tests has also varied over time. Uninsured individuals may be able to access free at-home Ag test kits through programs sponsored by their local or state public health departments, through community programs and nonprofit organizations, and through Medicare-certified health clinics. These programs may serve households in rural areas and individuals belonging to underserved populations who traditionally experience barriers to accessing healthcare (although access to Ag testing was not assessed by the panel). Currently, both at the national and local levels, there is a strong public health effort to ensure continued access to testing and to use Ag testing as the primary testing modality given that it can be performed at home, requires minimal technical expertise, and is relatively inexpensive compared with standard NAAT.
Conclusions and Research Needs for This Recommendation
For symptomatic patients, the IDSA panel suggests using standard NAAT over rapid Ag tests due to higher sensitivity, thus reducing the risk of missing a diagnosis of SARS-CoV-2 infection. However, regardless of the lower sensitivity of Ag tests, they will continue to be used due to their ease of use, rapid results, low cost, and availability. Testing individuals within the first 5 days of symptoms optimizes the sensitivity of Ag tests. If Ag tests are used for testing symptomatic individuals, a negative test result should be confirmed with a standard NAAT when a clinical suspicion for COVID-19 remains and no alternative diagnosis has been reached. Alternatively, given the high specificity of Ag tests, a positive test result does not require routine confirmation.
As new variants emerge, the performance of Ag tests may change. Therefore, monitoring the performance of Ag tests for diagnosis of new-variant COVID-19 is critical [80]. Research to identify epitope binding regions that can improve sensitivity while maintaining specificity is needed. Better understanding of protein-folding mutations that affect Ag testing will help test manufacturers develop more robust assays. Other factors that require investigation include optimal timing of detection of SARS-CoV-2 for different variants and in different specimen sources (eg, anterior nares vs throat) and the performance of Ag tests compared with multiplex molecular assays. Last, although difficult to design and implement, rigorously designed clinical trials comparing a single Ag test with standard NAAT to assess both treatment and transmission outcomes would provide direct evidence to guide this recommendation. Ensuring equal access to accurate, affordable, and timely SARS-CoV-2 diagnostic testing for underserved populations, including racial and ethnic minority groups, should be a priority.
Repeat Rapid Antigen Testing Versus Single Standard NAAT in Symptomatic Individuals
Recommendation 3: For symptomatic individuals suspected of having COVID-19, the IDSA panel suggests using a single standard NAAT (ie, rapid RT-PCR or laboratory-based NAAT) rather than a strategy of 2 consecutive rapid Ag tests. (conditional recommendation, very low certainty evidence)
Remarks
In situations where NAAT results are not available in a timely manner and a first Ag test is negative, the IDSA panel suggests repeating Ag testing.
Because of the absence of direct evidence to inform this question, the analysis done was based on modeling of diagnostic test accuracy using a repeat testing algorithm involving 2 consecutive Ag tests.
To optimize sensitivity, repeat testing should be performed within 5 days of symptom onset; the panel was unable to identify any study that reported results of testing within 2 days of symptom onset.
If the first Ag test is positive, there is no need for repeat testing.
Summary of the Evidence
There was no direct evidence comparing consecutive Ag testing vs standard NAAT (ie, rapid RT-PCR or laboratory-based NAAT) with a third reference standard. For this reason, modeling analysis was performed using a repeat testing algorithm. Results of the modeling analysis were compared with standard NAAT diagnostic accuracy (Supplementary Figure 11A). For all comparisons, 5%, 20%, and 50% were used for the prevalence of SARS-COV-2 infection in the symptomatic population. The modeled sensitivity and specificity for Ag testing and repeat Ag testing (total of 2 Ag tests) within the first 5 days of symptoms were estimated as 98% (95% CI: 97% to 99%) and 100% (95% CI: 99% to 100%), respectively. For standard NAAT diagnostic test accuracy data, we pooled the results from 5 studies [85–89] that reported comparison of standard NAAT results to a composite reference standard (Supplementary Figure 10). This analysis yielded a sensitivity of 97% (95% CI: 93% to 99%) and specificity of 100% (95% CI: 96% to 100%). Comparing the 2 testing strategies estimated 0 to 5 fewer false-negative results with repeat Ag testing compared with standard NAAT, depending on the disease prevalence. The modeled sensitivity and specificity for first Ag testing within the first 7 days of symptom onset and repeat testing after 7 days of symptom onset were 93% (95% CI: 89% to 96%) and 100% (95% CI: 99% to 100%), respectively. Comparing both modalities showed 2 to 20 more false-negative results per 1000 persons tested with repeat Ag testing compared with standard NAAT, depending on the prevalence of disease. The sensitivity and specificity for Ag testing and repeat Ag testing after the first 5 days of symptom onset were 75% (95% CI: 69% to 86%) and 100% (95% CI: 99% to 100%), respectively. Comparing both modalities showed 11 to 110 more false-negative results per 1000 persons tested with repeat Ag testing compared with standard NAAT, depending on the prevalence of COVID-19.
The certainty was very low and low for sensitivity and specificity, respectively, due to indirectness and inconsistency. Indirectness occurred because the results for consecutive Ag testing were based on a modeling analysis, whereas the standard NAAT results used as the comparator were based on primary patient data. Additionally, the comparison between repeat testing and standard NAAT testing was indirect due to different populations. There was serious unexplained inconsistency in the original single Ag test studies.
Benefits and Harms
Antigen test results are typically available within less than 1 hour (eg, 15 minutes), whereas the timing of availability of NAAT results may vary depending on factors such as receipt time at the site of testing, delays before testing begins, run times of individual testing instruments, and time from result availability to delivery of results. Delays in diagnosis of COVID-19 can deny affected patients with a positive test result potentially life-saving therapy and risk exposing others to SARS-CoV-2 because of delayed isolation of infected patients, contact tracing, and quarantine of close contacts. Alternatively, long turnaround times can prolong unnecessary isolation of individuals who test negative for SARS-CoV-2 infection. While repeat Ag testing is potentially a faster option, by definition it means that an initial test is negative but the person may still be infected.
Additional Considerations
In symptomatic individuals, the recommended test is NAAT. However, access to NAAT testing may be limited (eg, on weekends and holidays) and is more costly than Ag testing, and therefore Ag testing may be preferred in some scenarios. In addition, the time to results of standard NAAT may be delayed if there is not a rapid and reliable system in place to communicate results to healthcare providers and patients. In the end, the specific scenario (eg, high-risk patient, outbreak setting, long-term care facility, high clinical suspicion, COVID-19 surge, history of prior COVID-19, vaccination history) may impact whether Ag testing or standard NAAT is performed. Finally, in settings where respiratory viruses other than SARS-CoV-2 are circulating (eg, influenza, respiratory syncytial virus [RSV]), multiplex molecular respiratory pathogen testing may be warranted.
Conclusions and Research Needs for This Recommendation
While the IDSA panel suggests a single standard NAAT over 2 consecutive/serial Ag tests for diagnosis of SARS-CoV-2 infection in symptomatic individuals, published, peer-reviewed studies directly comparing 2 consecutive rapid Ag tests with a single standard NAAT in patients were lacking and are needed. Such studies should include vaccinated, boosted, and unvaccinated populations, and those with and without prior COVID-19 infection, as well as those infected with contemporary SARS-CoV-2 variants (eg, Omicron). Finally, in persons with prior COVID-19 infection, the timing between the first and potential subsequent infection bears consideration as a test could remain positive from prior infection if it occurred in the recent past and therefore not represent a new infection; the differential specificity of a standard NAAT versus Ag testing in this situation needs to be defined. The ideal time interval between the repeat Ag tests also needs definition.
Antigen Testing Versus No Testing in Asymptomatic Individuals With Known SARS-CoV-2 Exposure
Recommendation 4: For asymptomatic individuals with known exposure to SARS-CoV-2 infection, the IDSA panel suggests using a single (ie, one-time) Ag test over no testing in specific situations. (conditional recommendation, moderate certainty evidence)
Remarks
SARS-CoV-2 testing in the absence of COVID-19–like symptoms should be individualized. One-time Ag testing may be considered if the test result will impact an individual's subsequent actions. For example, a single test may be considered in situations where a positive test would lead to increased monitoring for symptoms and signs of infection in persons at high risk of serious COVID-19, or in outbreak settings where positive results would assist in decision making about isolation, quarantine, and contact tracing.
A negative Ag test result reduces the likelihood of infection. However, the longer the time since testing, the more this likelihood reduction wanes, especially early in infection when virus replication may be rapid. That is, a negative test result today may not reflect infection status tomorrow or on subsequent days. In contrast, a positive test result is associated with a high positive-predictive value.
The panel recognizes the lack of evidence supporting therapy in asymptomatic persons and the absence of treatments approved through EUA for asymptomatic COVID-19 but acknowledges that individual clinical scenarios may lead clinicians toward testing and consideration of treatment.
Summary of the Evidence
There was no direct evidence that assessed patient outcomes of testing versus no testing in asymptomatic individuals with known exposures to COVID-19. Therefore, we relied on diagnostic test accuracy data to inform this recommendation. The reference standard used in all studies included in the analysis was standard NAAT.
Fifty-nine studies were included [5–9, 16, 18–23, 25–29, 32, 34, 35, 37, 41, 42, 47, 48, 50, 52–54, 56, 58–60, 62, 64–68, 70, 71, 73, 74, 91–104], with 4553 positive and 97 541 negative patient results, based on standard NAAT testing, to inform this recommendation. The pooled sensitivity was 63% (95% CI: 56% to 69%) and the pooled specificity was 100% (95% CI: 100% to 100%) (Table 6). The IDSA panel considered 1%, 5%, and 10% as the prevalence of COVID-19 in asymptomatic patients with known exposure. In the pediatrics population, the numbers were similar, with a sensitivity of 62% (95% CI: 53% to 70%) and specificity of 99% (95% CI: 99% to 100%) (Supplementary Figure 13). The certainty of the evidence was moderate for sensitivity due to unexplained inconsistency and high for specificity. No other outcomes were reported. No information was reported on the type of exposure or timing of exposure relative to testing.
Table 6.
GRADE Evidence Profile of Test Accuracy Results for Prevalence/Pretest Probability of 1%, 5%, and 10% for Asymptomatic Patients Overall with Nucleic Acid Amplification Testing as the reference standard
| Performance of Antigen Testing Overall | |
|---|---|
| Sensitivity | .63 (95% CI: .56 to .69) |
| Specificity | 1.00 (95% CI: 1.00 to 1.00) |
| Outcome | No. of Studies (No. of Patients) | Study Design | Factors that May Decrease Certainty of Evidence | Effect per 1000 Patients Tested | Test Accuracy CoE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias | Pretest Probability of 1%a | Pretest Probability of 5%a | Pretest Probability of 10%a | ||||
| True positives (patients with COVID-19) |
59 Studies (4553 patients) | Cohort and case-control–type studies | Not seriousb | Not seriousc | Seriousd | Not serious | None | 6 (6 to 7) | 32 (28 to 34) | 63 (56 to 69) | ⨁⨁⨁◯ Moderate |
| False negatives (patients incorrectly classified as not having COVID-19) |
4 (3 to 4) | 18 (16 to 22) | 37 (31 to 44) | ||||||||
| True negatives (patients without COVID-19) |
59 Studies (97 541 patients) | Cohort and case-control–type studies | Not seriousb | Not seriousc | Not serious | Not serious | None | 990 (990 to 990) | 950 (950 to 950) | 900 (900 to 900) | ⨁⨁⨁⨁ High |
| False positives (patients incorrectly classified as having COVID-19) |
0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | ||||||||
aWe used 1%, 5%, and 10% pretest probability to mirror a range of community prevalence. Abbreviations: CI, confidence interval; CoE, certainty of evidence; COVID-19, coronavirus disease 2019; GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
bAlthough some of the included studies were judged to have a high or unclear risk of bias in 1 or more domains, a sensitivity analysis excluding studies with a high risk of bias did not show a difference in the effect estimate. For this reason, we did not downgrade for risk of bias.
cThere is some indirectness as the test accuracy results were to inform on patient-important outcomes.
dThere is serious unexplained inconsistency in the results despite partial explanation of having different types of tests in different studies.
Benefits and Harms
The panel placed high value on minimizing the number of false-negative results, especially in higher-risk healthcare settings. Although a single positive Ag test result may theoretically help reduce exposure to SARS-CoV-2 if it triggers isolation of the person who tests positive, the panel found no evidence that the use of Ag tests reduces transmission of SARS-CoV-2. Furthermore, treatment is not recommended for asymptomatic persons. A negative Ag test result may provide false assurance of non-infectiousness. Users of rapid Ag tests may not understand the limits of a negative test result. In 1 study, two-thirds of participants believed that they were noninfectious the day following a negative rapid Ag test [105]. In addition, sensitivity is linked to timing of exposure; a negative test result may convert to positive within hours early in the course of infection [81, 83].
The panel considered a range of prevalence for SARS-CoV-2 infection, using standard NAAT as the reference standard. When the prevalence of SARS CoV-2 infection was 1%, the number of true-positive Ag test results was small and approximated the number of false-negative results (ie, 6 true positives and 4 false negatives per 1000 asymptomatic individuals tested) (Table 6). When deciding on asymptomatic testing, communities and institutions should weigh the resources necessary for testing versus the benefits of detecting a few true cases of SARS CoV-2 infection, especially if infection-prevention strategies such as masking and distancing would be adhered to regardless of the test result. As the prevalence increased, so did the potential utility of testing, with 63 true positives (95% CI: 56–69) and 37 false negatives (95% CI: 31–44) detected when the prevalence of infection was 10% (Table 6). In contrast, the number of false-positive results was estimated to be 0 regardless of a true prevalence of disease of between 1% and 10%. Routine confirmation of positive Ag test results does not appear to be necessary in most cases.
Additional Considerations
The following considerations and assumptions are important to state for this PICO question:
1. There are currently no treatment options approved through FDA EUA for asymptomatic COVID-19.
2. The IDSA COVID-19 Diagnostics Panel assumed that there may be benefit in identifying asymptomatic individuals through testing.
3. The panel assumed that asymptomatic individuals are likely infectious at some point during their infection.
4. The panel found no direct evidence that testing for SARS-CoV-2 in asymptomatic individuals reduces risk of transmission.
Whether commercially available Ag tests perform comparably to one another and across SARS-CoV-2 variants has not been established. Postexposure monoclonal antibody prophylaxis may be an alternative to testing in high-risk asymptomatic individuals exposed to SARS-CoV-2, if/when EUA options exist for currently circulating variants [106]. Education of users on the interpretation of rapid Ag tests, including their limitations, is important to ensure that appropriate actions are taken after positive or negative test results.
Conclusions and Research Needs for This Recommendation
The decision to pursue rapid Ag testing versus no testing should be individualized. Given the relatively low sensitivity of Ag tests, factors to consider include the potential benefits of identifying a case of COVID-19 versus the potential harms of reporting a falsely negative result. The potential to reduce transmission by identifying asymptomatic infections should be weighed against the resources required for testing and account for changes in prevalence that arise with increased vaccine uptake or widespread adoption of effective infection-prevention measures such as masking. Antigen testing may be useful in guiding mitigation efforts during an outbreak. Further research is required to assess whether Ag screening reduces transmission in various settings, including schools and nonmedical workplaces.
Antigen Testing Versus Standard NAAT in Asymptomatic Individuals With Known Exposure to SARS-CoV-2
Recommendation 5: For asymptomatic individuals with known exposure to SARS-CoV-2 infection, the IDSA panel suggests using a single standard NAAT (ie, rapid RT-PCR or laboratory-based NAAT) over a single rapid Ag test. (conditional recommendation, low certainty evidence)
Remarks
SARS-CoV-2 testing in the absence of COVID-19–like symptoms should be individualized. A one-time standard NAAT may be considered if the test result will impact an individual's subsequent actions. For example, a single test may be considered in situations where a positive test would lead to increased monitoring for symptoms and signs of infection for persons at high risk of severe COVID-19 or in an outbreak setting where positive results would assist in decision making about isolation, contact tracing, and quarantine.
Access to timely results of standard NAAT may be unavailable or limited in some settings; in such situations, use of an Ag test can be considered.
The panel recognizes the lack of evidence supporting COVID-19 therapy in asymptomatic persons and the absence of treatments approved through FDA EUA for asymptomatic COVID-19 but acknowledges that individual clinical scenarios may lead clinicians toward testing and consideration of treatment.
Summary of the Evidence
There were no studies that reported patient- or population-based outcomes of Ag testing versus no testing in asymptomatic persons. Therefore, the panel relied on diagnostic test accuracy data to inform this recommendation. The reference standard in the studies included was standard NAAT (ie, rapid RT-PCR or laboratory-based NAAT). For calculation of the standard NAAT reference standard, we pooled results from 5 studies [85–89] that compared standard NAAT results with a composite reference standard (Supplementary Figure 10). This comparison showed a pooled sensitivity of 97% (95% CI: 93% to 99%) and specificity of 100% (95% CI: 96% to 100%). The IDSA panel considered 1%, 5%, and 10% as the prevalence of COVID-19 in asymptomatic patients with known exposure. (Table 7)
Table 7.
GRADE Evidence Profile of Test Accuracy Results for Prevalence/Pre-test Probability of 1%, 5%, and 10% for Asymptomatic Patients Overall versus Nucleic Acid Amplification Testing
| Performance of Antigen Testing Overall | Antigen Test | Standard NAAT |
|---|---|---|
| Sensitivity | .63 (95% CI: .56 to .69) | .97 (95% CI: .93 to .99) |
| Specificity | 1.00 (95% CI: 1.00 to 1.00) | 1.00 (95% CI: .96 to 1.00) |
| Outcome | No. of Studies (No. of Patients) | Study Design | Factors That May Decrease Certainty of Evidence | Effect per 1000 Patients Tested | Test Accuracy CoE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretest Probability of 1%a | Pretest Probability of 5%a | Pretest Probability of 10%a | ||||||||||||
| Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias | Antigen Test | Standard NAAT | Antigen Test | Standard NAAT | Antigen Test | Standard NAAT | ||||
| True positives (patients with COVID-19) | 64 Studies (4902 patientsb,c) | Cohort and case-control–type studies | Not seriousd | Seriouse | Seriousf | Not serious | None | 6 (6 to 7) | 10 (9 to 10) | 32 (28 to 34) | 49 (47 to 50) | 63 (56 to 69) | 97 (93 to 99) | ⨁⨁◯◯ Low |
| 4 fewer TPs in antigen test | 17 fewer TPs in antigen test | 34 fewer TPs in antigen test | ||||||||||||
| False negatives (patients incorrectly classified as not having COVID-19) |
4 (3 to 4) | 0 (0 to 1) | 18 (16 to 22) | 1 (0 to 3) | 37 (31 to 44) | 3 (1 to 7) | ||||||||
| 4 more FNs in antigen test | 17 more FNs in antigen test | 34 more FNs in antigen test | ||||||||||||
| True negatives (patients without COVID-19) |
64 Studies (98 071 patientsb,g) | Cohort and case-control–type studies | Not seriousd | Very seriouse | Not serious | Not serious | None | 990 (990 to 990) | 990 (950 to 990) | 950 (950 to 950) | 950 (912 to 950) | 900 (900 to 900) | 900 (864 to 900) | ⨁⨁◯ Low |
| 0 fewer TNs in antigen test | 0 fewer TNs in antigen test | 0 fewer TNs in antigen test | ||||||||||||
| False positives (patients incorrectly classified as having COVID-19) |
0 (0 to 0) | 0 (0 to 40) | 0 (0 to 0) | 0 (0 to 38) | 0 (0 to 0) | 0 (0 to 36) | ||||||||
| 0 fewer FPs in antigen test | 0 fewer FPs in antigen test | 0 fewer FPs in antigen test | ||||||||||||
aWe used 1%, 5%, and 10% pre-test probability to mirror a range of community prevalence. Abbreviations: CI, confidence interval; CoE, certainty of evidence; COVID-19, coronavirus disease 2019; FN, false negative; FP, false positive; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; NAAT, nucleic acid amplification testing; TN, true negative; TP, true positive.
bFifty-nine studies assessed antigen while only 5 studies assessed standard NAAT.
cThe majority of the patients came from studies that assessed antigen (4553 patients) while only 349 patients came from studies that assessed a standard NAAT arm.
dAlthough some of the included studies were judged to have a high or unclear risk of bias in 1 or more domains, a sensitivity analysis excluding studies with a high risk of bias did not show a difference in the effect estimate. For this reason, we did not downgrade for risk of bias.
eThere were no studies that evaluated the accuracy of antigen and NAAT testing in the same population. Studies either evaluated the accuracy of antigen against a reference standard or NAAT against another reference standard.
fThere is serious unexplained inconsistency in the results despite partial explanation of having different types of tests in different studies.
gThe majority of the patients came from studies that assessed antigen (97 541 patients) while only 530 patients came from studies that assessed a standard NAAT arm.
For this PICO question, we included 64 studies: 59 informing Ag testing [5–9, 16, 18–23, 25–29, 32, 34, 35, 37, 41, 42, 47, 48, 50, 52–54, 56, 58–60, 62, 64–68, 70, 71, 73, 74, 91–104] and 5 informing standard NAAT with 4902 positive and 98 071 negative patient test results [85–89]. The pooled sensitivity for Ag testing was 63% (95% CI: 56% to 69%) and the pooled specificity was 100% (95% CI: 100% to 100%) (Supplementary Figure 12). This comparison showed an additional 4 to 34 false-negative results with Ag testing when the prevalence ranged between 1% and 10%. The patients who underwent standard NAAT were different from those who underwent Ag testing; hence, comparisons were indirect, reducing confidence in the certainty of the evidence. The certainty of the evidence was very low for sensitivity due to indirectness and unexplained inconsistency and low for specificity due to indirectness.
Benefits and Harms
Antigen tests have reduced sensitivity for the detection of SARS-CoV-2 in asymptomatic individuals compared with standard NAAT, and Ag testing detects infection during a narrower window of time. In contrast, the specificity of Ag testing compared with standard NAAT is high, approaching 100% (Table 7). Therefore, the potential harm of using an Ag test instead of a standard NAAT is the potential for false-negative results. False-negative Ag test results are expected to be most harmful in high-risk settings such as healthcare settings, where failure to diagnose presymptomatic individuals before major elective surgery may increase patients’ risk of adverse events in the perioperative period [107, 108]; of note, methodologic challenges and conduct of these studies before widespread COVID-19 vaccination may limit their current relevance [109]. False-negative results of SARS-CoV-2 Ag testing might also lead to transmission of SARS-CoV-2 to other patients, residents, and staff of hospitals or long-term care facilities, especially if infection-prevention practices such as masking are dependent on test results.
Additional Considerations
The following considerations and assumptions are important to state for this question addressing asymptomatic individuals:
1. There are currently no treatment options approved through FDA EUA for asymptomatic individuals.
2. The IDSA COVID-19 Diagnostics Panel assumed that asymptomatic individuals are contagious at some point during the course of their infection.
3. The IDSA panel assumed that there may be benefit in identifying infected, asymptomatic individuals through testing.
4. The panel found no direct evidence that testing for SARS-CoV-2 in asymptomatic individuals reduces risk of transmission.
Conclusions and Research Needs for This Recommendation
A large number of individuals testing falsely negative may diminish public health efforts to contain COVID-19 outbreaks and may cause the greatest potential harm in healthcare and congregate settings, especially long-term care settings. Standard NAATs will detect the larger number of cases of SARS-CoV-2 infection and provide a greater number of opportunities to prevent transmission compared with currently available Ag tests, through targeted isolation of individuals who test positive, contact tracing, and quarantine of close contacts. The superior performance of standard NAAT is expected to have the greatest impact when the prevalence of asymptomatic infection in the community is moderate to high (ie, ≥10%). However, the use of less-sensitive rapid Ag tests may still be helpful in some lower prevalence settings when standard NAAT is not available. Antigen testing is expected to detect infection when viral load is high. Additionally, given the high specificity of Ag testing observed across studies of asymptomatic individuals, routine confirmation of positive results is not necessary in most situations. Large-scale studies evaluating the value of Ag versus RNA detection in relation to SARS-CoV-2 transmission events are needed, especially as vaccine coverage and the number of previously infected individuals increases. The development of new Ag tests with increased analytic sensitivity is of great interest.
Repeat Antigen Testing Versus Single Standard NAAT in Asymptomatic Individuals With Known Exposure to SARS-CoV-2
Recommendation 6: In asymptomatic individuals with a known exposure to SARS-CoV-2, if standard NAAT testing or results are not available in a timely manner and a first Ag test is negative, the IDSA panel suggests repeat Ag testing. (conditional recommendation, very low certainty evidence)
Remarks
Because of the absence of direct evidence to inform this question, the analysis done was based on modeling of diagnostic test accuracy using a repeat testing algorithm involving 2 consecutive Ag tests.
Summary of the Evidence
There was no direct evidence comparing 2 Ag tests vs a single standard NAAT with a third reference standard, and the data analyzed did not compare repeat Ag testing with standard NAAT in asymptomatic SARS-CoV-2–exposed individuals. For this reason, modeling analysis was performed using a repeat testing algorithm (2 consecutive tests). Results of the modeling analysis were compared with diagnostic accuracy of standard NAAT. For all comparisons, 1%, 5%, and 10% SARS-CoV-2 prevalence in an asymptomatic population was assumed. The prevalence of asymptomatic infection in an exposed individual depends, in part, on the nature of the exposure, with household contacts representing some of the highest risk settings (eg, 10% prevalence of a secondary case of asymptomatic COVID-19) [110, 111]. The sensitivity and specificity of Ag testing and repeat testing were modeled and were found to be 86% (95% CI: 80% to 90%) and 100% (95% CI: 99% to 100%), respectively. For standard NAAT diagnostic test accuracy data, we pooled the results from 5 studies [85–89] that reported comparison of NAAT results to a composite reference standard (Supplementary Figure 10). This showed a sensitivity of 97% (95% CI: 93% to 99%) and a specificity of 100% (95% CI: 96% to 100%). Comparing the 2 testing strategies, there were 1 to 11 more false-negative results per 1000 persons tested for repeat Ag testing compared with standard NAAT depending on disease prevalence.
The certainty of the evidence was very low for sensitivity and low for specificity, due to indirectness and inconsistency. Indirectness was due to the results being based on modeling analysis and not primary human testing data. Additionally, comparison between repeat testing and standard NAAT testing was indirect because the data used came from different populations. There was also serious unexplained inconsistency in the original single Ag testing results.
Benefits and Harms
A theoretical benefit of testing following an exposure in an asymptomatic individual would be to provide an early diagnosis of infection to enable early treatment; however, the IDSA panel noted that, at the current time, no specific treatment would be indicated in such a situation, as there is no FDA-approved or EUA therapy for asymptomatic COVID-19. The other theoretical benefit would be to prevent transmission of SARS-CoV-2, but we were unable to identify studies of serial testing for SARS-CoV-2 infection compared with molecular testing that included transmission as an outcome. Therefore, the analysis presented focuses on diagnostic test accuracy. The justification to perform testing of asymptomatic individuals in the general population after exposure is unclear. In congregate settings, such as nursing homes, incorporation of serial rapid Ag testing into a bundle of control measures during an outbreak may help identify individuals most likely to be contagious and guide isolation recommendations [82]. We identified one study that assessed serial testing as compared with isolation and showed noninferiority of testing for the prevention of transmission [112]. The available data did not inform the timing of NAAT or Ag testing following an exposure, or the timing of repeat Ag testing.
Additional Considerations
For the purposes of this guideline, the IDSA panel considered a SARS-CoV-2 exposure to be a close contact as defined by the CDC [113]. The IDSA panel's recommendation considered access and availability of standard NAAT testing, although arguably, in the scenario presented, timeliness of results would likely not be critical. If, for example, standard NAAT was not available on a weekend, it could be performed on a weekday if the exposed individual quarantined or took other measures to reduce the risk of onward transmission of infection while waiting to be tested. Not all exposures are the same. For example, prolonged household exposures carry more transmission risk than do shorter non-household exposures [111, 114], with transmission risk also being influenced by the level of infectiousness of the person to whom the individual is exposed, the level of immunity in the exposed person (vaccination history, prior history of COVID-19 infection, and timing thereof), and the viral variant.
The following assumptions and remarks are important to state for this question addressing asymptomatic individuals:
1. There are currently no treatment options approved through FDA EUA for asymptomatic individuals who test positive for SARS-CoV-2.
2. The IDSA COVID-19 Diagnostics Panel assumed that asymptomatic individuals are usually contagious at some point during the course of their infection.
3. The IDSA panel assumed that there may be benefit in identifying asymptomatic individuals through testing.
4. The panel found no direct evidence that testing for SARS-CoV-2 in asymptomatic reduces risk of transmission.
Conclusions and Research Needs for This Recommendation
Published, peer-reviewed studies directly comparing 2 consecutive rapid Ag tests with a single standard NAAT in asymptomatic individuals exposed to SARS-CoV-2 were lacking and are needed. Such studies should include special populations such as children, immunocompromised hosts, vaccinated, boosted, and unvaccinated populations, and those with and without prior COVID-19 infection, as well as those exposed to contemporary SARS-CoV-2 variants. Finally, in individuals with prior COVID-19 infection, the timing between the prior and subsequent infections bears consideration as a test could remain positive from the prior infection if it occurred in the recent past and therefore not represent a new infection; the differential specificity of a standard NAAT versus Ag testing in this situation needs to be defined. The ideal time interval between the repeat Ag tests also needs definition.
Repeat Antigen Testing Versus No Testing in Asymptomatic Students in Educational Settings and Employees in Workplaces
Recommendation 7: Among students in educational settings or employees in workplaces for whom SARS-CoV-2 testing is desired, the IDSA panel suggests neither for nor against 2 consecutive Ag tests over no testing for the diagnosis of SARS-CoV-2 infection. (evidence gap)
Remarks
Because of the absence of direct evidence to inform this question, the analysis done was based on modeling of diagnostic test accuracy using a repeat testing algorithm involving 2 consecutive Ag tests.
Summary of the Evidence
We identified no studies that compared serial Ag testing with no testing among students in an educational setting or employees in a workplace with an outcome of SARS-CoV-2 transmission, COVID-19 incidence, or diagnostic test accuracy. Therefore, a modeling analysis was performed using a repeat testing algorithm (2 consecutive Ag tests). Results of each test were considered to be independent, which might not be a valid assumption. For all comparisons, prevalences of 1%, 5%, and 10% SARS-CoV-2 infection were considered. The sensitivity and specificity of testing (2 consecutive repeat Ag tests) versus no testing, using standard NAAT as the reference standard, were 86% (95% CI: 80% to 90%) and 100% (95% CI: 99% to 100%), respectively. Comparing 2 repeated tests versus no testing showed 1 to 14 false-negative results per 1000 patients tested, depending on disease prevalence.
The certainty of the evidence is very low and low for sensitivity and specificity, respectively, due to indirectness and inconsistency. Indirectness was due to the fact that the results were based on a modeling analysis and not primary human testing data. Additionally, the comparison between repeat testing and no testing was indirect because the data used came from different populations.
Benefits and Harms
Theoretical benefits of serial Ag testing of asymptomatic individuals in schools, colleges, other educational settings, and workplaces include preventing transmission of SARS-CoV-2, but the IDSA panel was unable to identify any studies that directly addressed whether serial Ag testing versus no testing reduced SARS-CoV-2 transmission in these settings. Some indirect evidence was identified that suggested possible benefit of serial testing. A large, cluster-randomized trial of English secondary schools and colleges found that daily Ag testing was noninferior to self-isolation in preventing secondary cases of COVID-19, with similar numbers of contacts testing positive for SARS-CoV-2 in both study arms [112]. A retrospective cohort study of students at 18 colleges and universities in Connecticut, United States, reported that institutions that tested students more frequently detected more COVID-19 cases and prevented further spread [115]; in the fall of 2020, each additional test per student per week was associated with a decrease of 0.0014 cases per student per week (95% CI: −.0028 to −.00001).
Additional Considerations
This recommendation assumes widespread availability of Ag testing and does not take cost considerations into account. Furthermore, it is known that not all classroom or workplace settings are the same in terms of risk. Learning environments or workplace settings may range from small classrooms with young children to factory floors with closely packed, poorly ventilated workstations, to larger workplaces with distantly spaced worksites. In some workplaces, such as in the entertainment industry, there may be unique risks, such as those associated with close contact (including intimate contact) required for film/television production. The risk of exposure and viral transmission may also be related to the level of immunity in the exposed person (vaccination history, prior history of COVID-19 infection, and timing thereof), age and comorbid medical conditions, the timing of the exposure relative to disease onset in the index case, and the viral variant.
The IDSA panel recognizes that serial rapid Ag testing of students and employees is common, and that testing cadences vary, with common cadences being daily, twice weekly, or weekly Ag testing. We chose to model 2 consecutive rapid Ag tests. Performing additional rounds of testing would be expected to alter the performance characteristics of the testing strategy.
Employers may require serial testing of asymptomatic employees who decline SARS-CoV-2 vaccination. The IDSA panel found no evidence that serial testing for COVID-19 provided benefit comparable to the proven benefits of vaccination, nor that serial testing reduced the incidence of occupational transmission of COVID-19.
Conclusions and Research Needs for This Recommendation
The IDSA panel found no empirical evidence that serial Ag testing of asymptomatic students in educational settings or employees in workplaces provided benefit compared with no testing. To generate evidence to inform this recommendation, students and/or employees would need to be subjected to no testing, single Ag testing, or serial Ag testing at varying cadences. Because actions of 1 subject could impact others in the same cohort, this might best be performed as a cluster-randomized trial. Variables such as prior vaccination and/or prior COVID-19 infection would need to be accounted for, as would circulating variants and underlying risk factors in the students/employees. Outcomes of interest could include illness (including numbers of confirmed SARS-CoV-2 infections, both symptomatic and asymptomatic), time away from school or work, healthcare encounters, hospitalizations, and deaths in study subjects and their contacts.
Antigen Testing Versus No Testing in Asymptomatic Individuals Planning to Attend Large Gatherings
Recommendation 8: For asymptomatic individuals planning to attend a large gathering (eg, concert, conference, party, sporting event), the IDSA panel suggests neither for nor against Ag testing over no testing. (evidence gap)
Remarks
No studies directly addressed this question.
Summary of the Evidence
There was no direct evidence comparing Ag testing with no testing prior to attending a large gathering. For this reason, testing data were retrieved from a single study [116] of asymptomatic individuals who participated in home Ag testing, since it was assumed that, if testing were done before a large gathering, it would be done at home. There were 86 positive and 601 negative results, based on standard NAAT. The sensitivity and specificity of rapid home Ag testing of these asymptomatic individuals were 41% (95% CI: 25% to 61%) and 100% (95% CI: 97% to 100%), respectively. These sensitivity and specificity values were considered together with prevalence of COVID-19 of 1%, 5%, and 10% in an asymptomatic community population. The certainty of the evidence was very low and low for sensitivity and specificity, respectively, due to indirectness and imprecision. Indirectness occurred since patients undergoing home testing were not specifically the same population as those attending large gatherings. Imprecision was due to the low number of subjects in the study and the wide CIs.
Benefits and Harms
The theoretical benefit of Ag testing of asymptomatic individuals before a large gathering is likely less to the person with the positive test result and more so to the person who tests negative. This benefit assumes that someone with a positive Ag test would not attend the large gathering and that someone with a negative test would attend. The theoretical benefit to the population of testing before a large gathering is to reduce the risk of SARS-CoV-2 transmission from asymptomatically infected persons who might attend, particularly in settings where distancing is not possible or ventilation is poor, and community prevalence of asymptomatic infection is moderate to high (ie, >10%). However, we were unable to identify empirical evidence to support that Ag testing of asymptomatic individuals before a large gathering reduced transmission of SARS-CoV-2. Thus, this benefit remains theoretical.
Additional Considerations
Requiring those attending large gatherings (eg, weddings, graduations, sporting events, music festivals, conferences) to self-administer an Ag test prior to the gathering assumes that people will do the test in the first place, do it correctly, interpret it correctly, and act appropriately (ie, not attend the gathering if the test is positive). If the gathering requires cost or logistics to attend, or is highly desirable to an individual, not being able to attend might be an incentive to not participate in testing or reporting thereof, or to inappropriately collect a sample, compromising test performance. In addition, Ag tests would either need to be purchased by or made available to those attending the gathering, adding cost either way. If the former, there may be issues of economic hardship and inequity if testing before the gathering was required.
Conclusions and Research Needs for This Recommendation
No empirical studies directly addressed this question and thus no recommendation for or against Ag testing over no testing in asymptomatic individuals prior to attending large gatherings was made. One-time Ag testing (vs no testing) of asymptomatic individuals immediately before an event may potentially reduce transmission in settings of moderate to high community asymptomatic infection prevalence (ie, ≥10%) where distancing is not possible, attendees are unmasked, or ventilation is poor. However, there is no empirical evidence to date that Ag testing reduces the risk of transmission. The question of the possible benefit of one-time testing before a large gathering might be answered using a cluster-randomized trial. Even such a trial could yield results that vary depending on local geography, vaccine coverage (including type, timing, and boosting), and history of prior COVID-19 infection among attendees and the local population (or the population attendees will return to after the gathering), characteristics of people attending the gathering (comorbidities, age), whether masking is used and what type, whether food is consumed, whether physical distancing is in place, whether the event is indoors or outdoors, levels of ventilation (for indoor sites), and the stage in the pandemic (eg, surges, waves, variants). Finally, the specific Ag test used might impact performance based on variability in test design and potential impact of the circulating viral variants [80].
Point-of-Care Versus Laboratory-Based Antigen Testing
Recommendation 9: For individuals for whom Ag testing is desired, the IDSA panel suggests for either POC or laboratory-based Ag testing. (conditional recommendation, low certainty evidence)
Remarks
Although the results of test performance for POC and laboratory-based Ag testing appear to be comparable, an important limitation of the evidence is that available studies did not report the relative numbers of symptomatic and asymptomatic subjects. Since Ag test sensitivity is higher in symptomatic than in asymptomatic individuals, unknown proportions of symptomatic versus asymptomatic individuals included in POC versus laboratory-based studies may have influenced the results to minimize differences between the 2 testing strategies.
Summary of the Evidence
For this PICO, we identified 5 studies [117–121] that directly compared multiple laboratory-based and POC SARS-CoV-2 Ag tests, using standard NAAT as the reference standard. The outcome of interest was diagnostic test performance. The studies included a total of 2304 patients, 374 who tested positive and 1930 who tested negative based on standard NAAT (Table 8). We categorized the assays as POC versus laboratory-based assays based on the location where the test was completed, and results were interpreted. If the test was completed at the bedside immediately after specimen collection, it was considered as POC. If the test was completed after transport of a specimen to a laboratory, it was considered laboratory based.
Table 8.
GRADE Evidence Profile of Test Accuracy Results for Prevalence/Pre-test Probability of 1%, 5%, and 10% for Point-of-Care Versus Laboratory-Based Antigen Testing
| Performance of Antigen Testing | POC Antigen Testing | Laboratory-Based Antigen Testing |
|---|---|---|
| Sensitivity | .63 (95% CI: .28 to .88) | .70 (95% CI: .40 to .89) |
| Specificity | 1.00 (95% CI: .97 to 1.00) | 1.00 (95% CI: .99 to 1.00) |
| Outcome | No. of Studies (No. of Patients) | Study Design | Factors That May Decrease Certainty of Evidence | Effect per 1000 Patients Tested | Test Accuracy CoE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretest Probability of 5%* | Pretest Probability of 10%* | Pretest Probability of 20%* | ||||||||||||
| Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias | POC Antigen Testing | Laboratory-Based Antigen Testing | POC Antigen Testing | Laboratory-Based Antigen Testing | POC Antigen Testing | Laboratory-Based Antigen Testing | ||||
| True positives (patients with COVID-19) |
5 Studies (374 patients) | Cohort and case-control–type studies | Not seriousa | Seriousb | Seriousc | Not serious | None | 32 (14 to 44) | 35 (20 to 45) | 63 (28 to 88) | 70 (40 to 89) | 126 (56 to 176) | 140 (80 to 178) | ⨁⨁◯◯ Low |
| 3 fewer TPs in POC antigen testing | 7 fewer TPs in POC antigen testing | 14 fewer TPs in POC antigen testing | ||||||||||||
| False negatives (patients incorrectly classified as not having COVID-19) |
18 (6 to 36) | 15 (5 to 30) | 37 (12 to 72) | 30 (11 to 60) | 74 (24 to 144) | 60 (22 to 120) | ||||||||
| 3 more FNs in POC antigen testing | 7 more FNs in POC antigen testing | 14 more FNs in POC antigen testing | ||||||||||||
| True negatives (patients without COVID-19) |
5 Studies (1930 patients) | Cohort and case-control–type studies | Not seriousa | Seriousb | Not serious | Not serious | None | 950 (922 to 950) | 950 (941 to 950) | 900 (873 to 900) | 900 (891 to 900) | 800 (776 to 800) | 800 (792 to 800) | ⨁⨁⨁◯ Moderate |
| 0 fewer TNs in POC antigen testing | 0 fewer TNs in POC antigen testing | 0 fewer TNs in POC antigen testing | ||||||||||||
| False positives (patients incorrectly classified as having COVID-19) |
0 (0 to 28) | 0 (0 to 9) | 0 (0 to 27) | 0 (0 to 9) | 0 (0 to 24) | 0 (0 to 8) | ||||||||
| 0 fewer FPs in POC antigen testing | 0 fewer FPs in POC antigen testing | 0 fewer FPs in POC antigen testing | ||||||||||||
*We used 5%, 10%, and 20% pre-test probability to mirror a range of community prevalence. Abbreviations: CI, confidence interval; CoE, certainty of evidence; COVID-19, coronavirus disease 2019; FN, false negative; FP, false positive; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; POC, point of care; TN, true negative; TP, true positive.
aAlthough some of the included studies were judged to have a high or unclear risk of bias in 1 or more domains, a sensitivity analysis excluding studies with a high risk of bias did not show a difference in the effect estimate. For this reason, we did not downgrade for risk of bias.
bPatients from the POC arm are different than patients in the laboratory-based arm.
cThere is serious unexplained inconsistency in the results despite partial explanation of having different types of tests in different studies.
The sensitivity and specificity of POC Ag testing were 63% (95% CI: 28% to 88%) and 100% (95% CI: 97% to 100%), respectively (Supplementary Figure 14). The sensitivity and specificity of laboratory-based Ag testing were 70% (95% CI: 40% to 89%) and 100% (95% CI: 99% to 100%), respectively (Supplementary Figure 15). We considered 5%, 10%, and 20% as prevalences of SARS-CoV-2 in the overall population. Point-of-care Ag testing showed 3 to 14 more false-negatives per 1000 individuals tested compared with laboratory-based Ag testing, depending on the prevalence.
Publications were not stratified by symptom status of study participants, so we could not report results for symptomatic and asymptomatic individuals separately. Since the included studies were conducted in mixed populations, we rated the strength of evidence downward for indirectness when the evidence was used to inform decisions about testing in symptomatic versus asymptomatic individuals. Also, CIs for sensitivity were wide, and considering the lower versus the upper limits might lead to different clinical decisions. Therefore, we downgraded the certainty of evidence for imprecision. There was also unexplained inconsistency among studies informing sensitivity. The overall certainty of the evidence was low for sensitivity and moderate for specificity.
Benefits and Harms
Whether Ag testing is performed at the POC or in the laboratory will likely depend on available resources and the indication for testing. The main benefit of POC testing is rapid results, enabling decision making in near real time. Other benefits to patients include greater privacy, convenience, and control over their own health. Possible harms to patients or to the population might arise if home testing were associated with more technical errors, incorrect test interpretation, or failure to report results to public health or other relevant parties. Education of patients and the development of quick, easy ways to report results might mitigate these theoretical harms. The potential benefits and harms outlined here were not assessed in available studies.
Point-of-care Ag tests are now widely available for home or field use but testing multiple individuals simultaneously as part of large testing programs can be logistically challenging. Alternatively, several laboratory-based Ag analyzers enable testing greater numbers of samples in an automated fashion, with results potentially available within hours. This approach could be useful for situations where a clinical laboratory has the required equipment, large numbers of samples need to tested, and a same-day turnaround time to results is acceptable. Laboratory-based tests may be slightly more sensitive than POC tests, thus resulting in fewer false-negative results.
Additional Considerations
Currently, few laboratory-based Ag testing platforms have EUA for SARS-CoV-2 testing in symptomatic or asymptomatic individuals in the United States. Laboratory-based Ag tests are usually more expensive than POC Ag tests but less expensive than molecular tests, including standard NAAT.
Conclusions and Research Needs for This Recommendation
Diagnostic test accuracy of POC and laboratory-based testing is similar. Point-of-care testing has the advantage of lower cost and faster turnaround time, allowing clinical decisions to be made during a patient encounter. In contrast, because laboratory-based testing is often automated and can be batched, it may be more amenable to large-volume testing, such as might be done for some screening or surveillance programs. Whether the diagnostic test accuracy of POC versus laboratory-based testing differs for asymptomatic versus symptomatic individuals is not known. Other knowledge gaps include analytical performance of POC versus laboratory-based testing in special populations, such as immunocompromised hosts, children, vaccinated individuals, or persons infected with newer SARS-CoV-2 variants, such as Omicron.
Observed Versus Unobserved Self-Collection of Specimens for Antigen Testing
Recommendation 10: The IDSA panel suggests either observed or unobserved self-collection of swab specimens for Ag testing if self-collection is performed. (conditional recommendation, low certainty evidence)
Remarks
There were no studies comparing observed and unobserved specimen collection in the same patients.
Studies reported heterogeneity in the techniques used for specimen collection and in the reference standard used as the comparator.
Providing instructions for optimal specimen collection may improve the quality of self-collected specimens.
Summary of the Evidence
We found no direct evidence comparing observed or unobserved self-collection of specimens for Ag testing with a reference standard. For this reason, studies reporting on each technique separately were compared with standard NAAT.
Twelve studies were identified that informed this PICO question. Eleven studies [5, 9, 22, 28, 39, 58, 59, 65, 122–124] provided information on diagnostic test accuracy for observed specimen self-collection and 1 study [116] provided diagnostic test accuracy information for unobserved specimen self-collection. There were 1570 positive and 17 196 negative patient results, based on standard NAAT. Only 101 positives and 723 negatives were from the study of unobserved self-collected specimens (Table 9).
Table 9.
GRADE Evidence Profile of Test Accuracy Results for Prevalence/Pre-test Probability of 1%, 5%, and 10% for Observed Versus Unobserved Self-Collection of Swab Specimens with Nucleic Acid Amplification Testing as reference standard
| Performance of Antigen Testing | Observed Self-Collection | Unobserved Self-Collection |
|---|---|---|
| Sensitivity | .72 (95% CI: .59 to .82) | .63 (95% CI: .54 to .72) |
| Specificity | 1.00 (95% CI: .99 to 1.00) | 1.00 (95% CI: .99 to 1.00) |
| Outcome | No. of Studies (No. of Patients) | Study Design | Factors That May Decrease Certainty of Evidence | Effect per 1000 Patients Tested | Test Accuracy CoE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretest Probability of 5%a | Pretest Probability of 10%a | Pretest Probability of 20%a | ||||||||||||
| Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias | Observed Self-Collection | Unobserved Self-Collection | Observed Self-Collection | Unobserved Self-Collection | Observed Self-Collection | Unobserved Self-Collection | ||||
| True positives (patients with COVID-19) |
12 Studies (17 196 patientsb,c) | Cohort and case-control–type studies | Not serious | Seriousd | Seriouse | Not serious | None | 36 (30 to 41) | 32 (27 to 36) | 72 (59 to 82) | 63 (54 to 72) | 144 (118 to 164) | 126 (108 to 144) | ⨁⨁◯◯ Low |
| 4 more TPs in observed self-collection | 9 more TPs in observed self-collection | 18 more TPs in observed self-collection | ||||||||||||
| False negatives (patients incorrectly classified as not having COVID-19) |
14 (9 to 20) | 18 (14 to 23) | 28 (18 to 41) | 37 (28 to 46) | 56 (36 to 82) | 74 (56 to 92) | ||||||||
| 4 fewer FNs in observed self-collection | 9 fewer FNs in observed self-collection | 18 fewer FNs in observed self-collection | ||||||||||||
| True negatives (patients without COVID-19) |
12 Studies (17 196 patientsbf) | Cohort and case-control–type studies | Not serious | Seriousd | Seriouse | Not serious | None | 950 (941 to 950) | 950 (941 to 950) | 900 (891 to 900) | 900 (891 to 900) | 800 (792 to 800) | 800 (792 to 800) | ⨁⨁◯◯ Low |
| 0 fewer TNs in observed self-collection | 0 fewer TNs in observed self-collection | 0 fewer TNs in observed self-collection | ||||||||||||
| False positives (patients incorrectly classified as having COVID-19) |
0 (0 to 9) | 0 (0 to 9) | 0 (0 to 9) | 0 (0 to 9) | 0 (0 to 8) | 0 (0 to 8) | ||||||||
| 0 fewer FPs in observed self-collection | 0 fewer FPs in observed self-collection | 0 fewer FPs in observed self-collection | ||||||||||||
aWe used 5%, 10%, and 20% pretest probability to mirror a range of community prevalence. Abbreviations: CI, confidence interval; CoE, certainty of evidence; COVID-19, coronavirus disease 2019; FN, false negative; FP, false positive; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; TN, true negative; TP, true positive.
b The majority of the studies came from the observed arm (11 studies) while only 1 study came from the unobserved arm.
cThe majority of the patients came from the studies with the observed arm (1469) while only 101 patients came from the unobserved arm.
dThe comparison between observed and unobserved self-collection is indirect as the samples came from different populations.
eThere is serious unexplained inconsistency in the observed arm.
fThe majority of the patients came from the studies with the observed arm (16 473) while only 723 patients came from the unobserved arm.
The pooled sensitivity and specificity of Ag testing of observed self-collected specimens were 72% (95% CI: 59% to 82%) and 100% (95% CI: 99% to 100%), respectively (Supplementary Figure 16). The sensitivity and specificity for Ag testing of unobserved self-collected specimens from the single study of Ag testing of unobserved self-collected specimens in symptomatic patients were 63% (95% CI: 54% to 72%) and 100% (95% CI: 99% to 100%), respectively (Supplementary Figure 17). SARS-CoV-2 prevalences of 5%, 10%, and 20% were used to assess the impact of these performance characteristics in different populations of patients. Regardless of prevalence, there were more false-negative results when self-collection of specimens was unobserved compared with when it was observed.
The certainty of evidence was low for both sensitivity and specificity, due to indirectness. Indirectness was due to an absence of head-to-head comparisons of observed and unobserved specimen self-collection in symptomatic patients, which required the panel to compare observed and unobserved specimen self-collection in 2 populations of patients.
Benefits and Harms
The potential benefit of unobserved Ag testing is that tests are readily available, and testing may be more likely to be performed and performed faster, than if observed testing needed to be arranged. This may be a particular benefit to individuals in rural or other areas without convenient access to a testing facility, or to individuals who prefer to avoid healthcare facilities. Cost is another consideration; observed testing adds cost to patient care, either to the patient directly or to the healthcare system.
The potential harm of Ag testing overall is the risk of a false-negative result. This can provide false assurance as to the absence of SARS-CoV-2 infection, potentially facilitating spread of infection if an infected but undiagnosed person does not take measures to prevent transmission. If the infected person is symptomatic, a false-negative result might also result in failure to treat someone who would benefit from treatment. On the other hand, with appropriate understanding that a negative test does not rule out infection (and a recommendation for follow-up testing), such potential harms may be mitigated through the provision of detailed instructions (written materials, illustrations, videos) on specimen collection, test performance, and interpretation of results.
Additional Considerations
Availability and use of appropriate instructions for unobserved testing (eg, visual aids, videos) are likely to influence test performance but were not specifically assessed [125]. More research is needed comparing observed and unobserved Ag testing in the same individuals, with a reference NAAT collected from the same patients at the same time. The specific Ag test used might impact diagnostic sensitivity based on variability in test design and potential impact on detection of viral variants [79, 80]. Finally, the reason for doing the testing might impact sensitivity in cases of unobserved self-collection (although arguably, this could occur with observed self-collection depending on the nature of the observation); if, for example, the desired endpoint is a negative result (eg, return to work or school, participation in a preferred activity), the quality of specimen collection may be purposely compromised.
Conclusions and Research Needs for This Recommendation
Although we found no direct evidence comparing observed self-collected and unobserved self-collected Ag testing with a reference standard in symptomatic or asymptomatic individuals, the IDSA panel suggests either observed or unobserved specimen collection for testing. Ideally, a study would be performed directly comparing observed and unobserved self-collected Ag testing with reference standards of healthcare provider–collected Ag testing and healthcare provider–collected NAAT. Peer-reviewed studies assessing the performance of self-testing at home are also needed.
DISCUSSION
Universal access to accurate SARS-CoV-2 testing remains an important part of comprehensive COVID-19 mitigation strategies. The availability, simplicity, and relative low cost of rapid Ag tests have enabled expanded testing initiatives, particularly in nonmedical settings. Recent studies demonstrate that rapid SARS-CoV-2 Ag tests can be performed accurately, without the need for highly qualified laboratory personnel, in a variety of community locations such as pharmacies, nursing homes, and schools. Laboratory-based Ag testing is an alternative approach that allows for testing larger numbers of specimens at one time. However, the need for specimen transport to a centralized laboratory increases turnaround time for results. More performance data were available for rapid Ag test performance than for laboratory-based Ag tests, but the sensitivity and specificity of rapid POC versus laboratory-based Ag tests appear to be comparable (Supplementary Figures 14 and 15).
An important finding of this updated systematic review is the observation that rapid Ag tests have very high specificity. Early concerns about false-positive Ag results were not borne out in the medical literature [126]. Importantly, many of the studies included in our analysis used nonmedical staff to administer rapid Ag testing in the field. Unobserved self-collection of anterior nares specimens for testing appeared to yield comparable results to observed specimen collection, although no head-to-head comparisons of these 2 approaches were found. Whether the same accuracy can be achieved with self-testing at home, however, has yet to be definitively determined. Recent studies published after completion of the literature review for this guideline suggest that accuracy of Ag self-test interpretation may be poor but can be improved with patient education [125, 127]. Given the high specificity of EUA rapid Ag tests, routine confirmation of positive test results is not usually necessary; positive results can be used immediately to help guide treatment, isolation, and quarantine decisions. Even when the pretest probability or prevalence is low (ie, 1%), the number of false-positive Ag results is expected to be very small, on the order of 0–10 false-positive results per 1000 individuals tested (Table 6), regardless of the presence of symptoms or timing of testing relative to onset of illness. However, confirmation of positive Ag test results may be considered rarely on a case-by-case basis when the pretest probability or prevalence of infection is very low (ie, <1%) and/or if the impact of a potential false-positive result is deemed to be significant.
Current EUA SARS-CoV-2 Ag tests are less sensitive than standard NAAT. Sensitivity differences were most apparent in comparisons across groups of symptomatic versus asymptomatic individuals. The clinical sensitivity of Ag testing was highest (89%) (Supplementary Figure 2A) for symptomatic individuals tested early during the course of illness, the time when the viral load is expected to be highest. Test sensitivity dropped to 54% (Supplementary Figure 4A) after more than 5 days of symptoms. Some recent anecdotes and 1 carefully performed observational study [83] published after the literature search for this guideline was completed have reported lower Ag test sensitivity within the first day or 2 of symptoms, possibly related to specific SARS-CoV-2 variants and/or vaccination status of infected individuals. However, the IDSA panel was unable to identify studies that reported Ag test performance this early after symptom onset during the period of the literature review. The sensitivity of Ag testing within 3 days of symptoms onset was similar to sensitivity within 5 days of symptoms. Antigen test sensitivity during this time was again lower for asymptomatic individuals (63%) (Supplementary Figure 12A). Few studies reported on children with COVID-19. The overall sensitivity of Ag testing in
The isolation of replication-competent virus in culture has been used as a surrogate to infer the presence of infectious virus in a clinical sample. In the original IDSA guideline on Ag testing for the diagnosis of COVID-19, the panel analyzed the relation between Ag positivity and replication-competent SARS-CoV-2 [84]. This observation supported the assertion that Ag testing should identify most culture-positive individuals, and by inference, this would be a group who would more likely be shedding infectious virus. However, the panel noted several important caveats to this interpretation. First, while culture-positive specimens were also likely to be Ag positive, culture negativity or Ag negativity does not mean that transmission of infection is not possible. Viral culture is a relatively insensitive method that is also prone to analytical variability across laboratories. Additionally, false-negative Ag results were observed in all of the studies that used culture as a comparator (range: 3%–21% false-negative Ag tests vs culture) [64, 128–130]. It is likely that some individuals with SARS-CoV-2 infection who test negative by Ag and/or culture are contagious. While the use of Ag testing to infer contagiousness and need for isolation is common, the panel identified no studies that provided direct empirical evidence in support of this practice. Careful epidemiologic investigations in households or other high-transmission settings coupled with genomic analysis of SARS-CoV-2 are needed to determine how well Ag test results correlate with contagiousness. New tests capable of accurately predicting contagiousness are also needed.
The panel identified other notable evidence gaps. Despite the common use of single or serial Ag testing as a tool to reduce the risk of SARS-CoV-2 transmission in schools, colleges, workplaces, and before large social gatherings, we were unable to identify any empirical evidence in support of these practices. Mathematical modeling has suggested that repeated Ag testing will help overcome the sensitivity limitations of rapid Ag tests and that the frequency of testing and turnaround time to results may be just as important as test sensitivity in certain situations. Well-designed studies are needed to measure the effect of repeated testing strategies on analytical test performance and transmission events in a variety of settings. In addition, the cost-effectiveness of repeated Ag testing versus less-frequent rapid RT-PCR, or potentially no testing depending on prevalence, needs to be determined. Potential effectiveness measures should include the number of SARS-CoV-2 cases identified, the results of contact tracing around new cases, and ideally, transmission events. In addition to the price of test kits (eg, reagents and consumables), assessments of cost should also factor in the resources required to scale-up testing.
Information was also limited on the performance of Ag tests in immunocompromised persons and in individuals who had received 1 or more doses of a COVID-19 vaccine or who had had natural COVID-19 infection. Data on the performance of Ag tests in detecting contemporary SARS-CoV-2 variants, including Omicron, were also lacking. One study published after the literature search for the current systematic review was completed used deep mutational scanning to identify SARS-CoV-2 nucleocapsid escape mutations of rapid Ag tests. This report predicted that available Ag tests that target the nucleocapsid would detect current and previous SARS-CoV-2 variants [130]. Peer-reviewed studies of Ag test performance in populations infected by the newest variants are needed. Testing recommendations may change as additional data on test performance in these populations increase.
Finally, it is important to note that we included only studies of Ag tests with FDA-EUA or CE status. Non-EUA tests may perform similarly, better or worse than EUA and CE marked tests. New tests are also likely to come to market in the future and will need to be evaluated.
CONCLUSIONS
Equitable access to testing resources such as rapid Ag testing should be ensured across all communities. The ease of use and lower price per test relative to standard NAAT are attractive features of rapid Ag testing. Overall, Ag testing had a sensitivity of 80% in symptomatic individuals and 63% in asymptomatic persons, with specificities of close to 100% in both populations, compared with a single standard NAAT. Given the low sensitivity of Ag tests, standard NAAT remains the diagnostic modality of choice for detecting SARS-CoV-2 infection, especially when the pretest probability of infection is moderate to high and/or the harms of falsely negative results are significant. In situations where standard NAAT is not available, timely, or feasible, Ag testing can be used without the need to routinely confirm positive test results. However, a negative Ag test does not rule out SARS-CoV-2 infection. Ideally, negative Ag test results should be confirmed by standard NAAT if the suspicion of COVID-19 is moderate or high; repeat Ag testing may be considered when standard NAAT is not an option. Notably, a negative Ag test does not rule out SARS-CoV-2 infectiousness, although a positive Ag test makes infectiousness more likely.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Mary K Hayden, Division of Infectious Diseases, Department of Internal Medicine, Rush University Medical Center, Chicago, Illinois, USA; Department of Pathology, Rush University Medical Center, Chicago, Illinois, USA.
Kimberly E Hanson, Divisions of Infectious Diseases and Clinical Microbiology, University of Utah, Salt Lake City, Utah, USA.
Janet A Englund, Department of Pediatrics, University of Washington, Seattle Children's Research Institute, Seattle, Washington, USA.
Francesca Lee, Departments of Pathology and Internal Medicine, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Mark J Lee, Department of Pathology and Clinical Microbiology Laboratory, Duke University School of Medicine, Durham, North Carolina, USA.
Mark Loeb, Division of Pathology and Molecular Medicine, McMaster University, Hamilton, Ontario, Canada.
Daniel J Morgan, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Robin Patel, Division of Clinical Microbiology, Department of Laboratory Medicine and Pathology, and the Division of Public Health, Infectious Diseases, and Occupational Medicine, Department of Medicine, Mayo Clinic, Rochester, MN, USA.
Abdallah El Alayli, Department of Internal Medicine, Saint Louis University, St Louis, Missouri, USA.
Ibrahim K El Mikati, Outcomes and Implementation Research Unit, Department of Internal Medicine, University of Kansas Medical Center, Kansas City, Kansas, USA.
Shahnaz Sultan, Division of Gastroenterology, Hepatology, and Nutrition, University of Minnesota, Minneapolis VA Healthcare System, Minneapolis, Minnesota, USA.
Yngve Falck-Ytter, Department of Medicine, Case Western Reserve University, School of Medicine, Cleveland, Ohio, USA; VA Northeast Ohio Healthcare System, Cleveland, Ohio, USA.
Razan Mansour, Department of Internal Medicine, University of Kansas Medical Center, Kansas City, Kansas, USA.
Justin Z Amarin, Division of Pediatric Infectious Diseases, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Rebecca L Morgan, Department of Medicine, Case Western Reserve University, School of Medicine, Cleveland, Ohio, USA; Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada.
M Hassan Murad, Division of Public Health, Infectious diseases and occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Payal Patel, Department of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, Emory University, Atlanta, Georgia, USA.
Adarsh Bhimraj, Department of Infectious Diseases, Cleveland Clinic, Cleveland, Ohio, USA.
Reem A Mustafa, Division of Nephrology and Hypertension, Department of Internal Medicine, University of Kansas Medical Center, Kansas City, Kansas, USA.
Notes
Disclaimer. It is important to realize that guidelines cannot always account for individual variation among patients. They are assessments of current scientific and clinical information provided as an educational service; are not continually updated and may not reflect the most recent evidence (new evidence may emerge between the time information is developed and when it is published or read); should not be considered inclusive of all proper treatment methods of care, or as a statement of the standard of care; do not mandate any particular course of medical care; and are not intended to supplant physician judgment with respect to particular patients or special clinical situations. Whether and the extent to which to follow guidelines is voluntary, with the ultimate determination regarding their application to be made by the physician in the light of each patient's individual circumstances. While IDSA makes every effort to present accurate, complete, and reliable information, these guidelines are presented “as is” without any warranty, either expressed or implied. IDSA (and its officers, directors, members, employees, and agents) assume no responsibility for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, or consequential damages, incurred in connection with these guidelines or reliance on the information presented. The guidelines represent the proprietary and copyrighted property of IDSA. Copyright 2022 Infectious Diseases Society of America. All rights reserved. No part of these guidelines may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of IDSA. Permission is granted to physicians and healthcare providers solely to copy and use the guidelines in their professional practices and clinical decision making. No license or permission is granted to any person or entity, and prior written authorization by IDSA is required to sell, distribute, or modify the guidelines, or to make derivative works of or incorporate the guidelines into any product, including but not limited to clinical decision-support software or any other software product. Except for the permission granted above, any person or entity desiring to use the guidelines in any way must contact IDSA for approval in accordance with the terms and conditions of third-party use, in particular any use of the guidelines in any software product.
Author Contributions. Methodologists: Reem A. Mustafa (lead), Abdallah El Alayli, Ibrahim K. El Mikati, Shahnaz Sultan, Yngve Falck-Ytter, Razan Mansour, Justin Z. Amarin, Rebecca L. Morgan, M. Hassan Murad, Payal Patel. Panel members: Mary K. Hayden (lead), Kimberly E. Hanson, Adarsh Bhimraj, Janet A. Englund (Pediatric Infectious Diseases Society [PIDS] representative), Francesca Lee, Mark J. Lee, Mark Loeb (Society for Healthcare Epidemiology of America [SHEA] representative), Daniel J. Morgan, Robin Patel (American Society for Microbiology [ASM] representative).
Acknowledgments. The expert panel thanks the Infectious Diseases Society of America (IDSA) for supporting guideline development and, specifically, the Executive Committee of the IDSA Board of Directors as well as IDSA staff members Dana Wollins, Sheila Tynes, Genet Demisashi, Jon Heald, and Hannah Rehm for their continued support throughout the guideline process. The panel also expresses its appreciation to the members of SHEA, PIDS, and ASM who provided their thoughtful and comprehensive review. The methodology team thanks Farouk Alabed for his continuous support in the title and abstract screening step.
Financial support. This project was funded in part by a cooperative agreement with the Centers for Disease Control and Prevention (CDC) (grant number 6 NU50CK000477-04-01). The CDC is an agency within the Department of Health and Human Services (HHS). The contents of this guideline do not necessarily represent the policy of the CDC or HHS and should not be considered an endorsement by the federal government.
References
- 1. US Food and Drug Administration . In vitro diagnostics EUAs—antigen diagnostic tests for SARS-CoV-2. Available at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2#Revision. Accessed 25 September 2022.
- 2. Centers for Disease Control and Prevention . Using antigen tests. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html. Accessed 29 April 2021.
- 3. Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. J Clin Epidemiol 2006; 59:1331–2. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Available at: https://covid.cdc.gov/covid-data-tracker/#cases_positivity7day. Accessed 25 September 2022.
- 5. Tinker SC, Szablewski CM, Litvintseva AP, et al. Point-of-care antigen test for SARS-CoV-2 in asymptomatic college students. Emerg Infect Dis 2021; 27:2662–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peacock WF, Soto-Ruiz KM, House SL, et al. Utility of COVID-19 antigen testing in the emergency department. J Am Coll Emerg Physicians Open 2022; 3:e12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernández-Rivas G, Barallat J, Gonzalez V, et al. Analytical performance of quantitative DiaSorin liaison SARS-COV-2 antigen test for the asymptomatic population. Front Public Health 2021; 9:788581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montalvo Villalba MC, Sosa Glaria E, Rodriguez Lay LLA, et al. Performance evaluation of Elecsys SARS-CoV-2 antigen immunoassay for diagnostic of COVID-19. J Med Virol 2021; 94:1001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. García-Fiñana M, Hughes DM, Cheyne CP, et al. Performance of the Innova SARS-CoV-2 antigen rapid lateral flow test in the Liverpool asymptomatic testing pilot: population based cohort study. BMJ 2021; 374:n1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155:529–36. [DOI] [PubMed] [Google Scholar]
- 11. Schünemann HJ, Mustafa RA, Brozek J, et al. GRADE guidelines: 21 part 1. Study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. J Clin Epidemiol 2020; 122:129–41. [DOI] [PubMed] [Google Scholar]
- 12. Schünemann HJ, Mustafa RA, Brozek J, et al. GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J Clin Epidemiol 2020; 122:142–52. [DOI] [PubMed] [Google Scholar]
- 13. GRADEpro GDT . GRADEpro guideline development tool [software]. McMaster University, 2020 (developed by Evidence Prime, Inc). Available at: https://gradepro.org/. Accessed 1 May 2021.
- 14. Alexander PE, Bero L, Montori VM, et al. World Health Organization recommendations are often strong based on low confidence in effect estimates. J Clin Epidemiol 2014; 67:629–34. [DOI] [PubMed] [Google Scholar]
- 15. Leixner G, Voill-Glaninger A, Bonner E, et al. Evaluation of the AMP SARS-CoV-2 rapid antigen test in a hospital setting. Int J Infect Dis 2021; 108:353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Venekamp RP, Veldhuijzen IK, Moons KGM, et al. Detection of SARS-CoV-2 infection in the general population by three prevailing rapid antigen tests: cross-sectional diagnostic accuracy study. BMC Med 2022; 20:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van der Moeren N, Zwart VF, Lodder EB, et al. Evaluation of the test accuracy of a SARS-CoV-2 rapid antigen test in symptomatic community dwelling individuals in the Netherlands. PLoS One 2021; 16:e0250886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schuit E, Venekamp RP, Veldhuijzen IK, et al. Head-to-head comparison of the accuracy of saliva and nasal rapid antigen SARS-CoV-2 self-testing: cross-sectional study. BMC Med 2022; 20:406. 10.1186/s12916-022-02603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allan-Blitz LT, Klausner JD. A real-world comparison of SARS-CoV-2 rapid antigen testing versus PCR testing in Florida. J Clin Microbiol 2021; 59:e0110721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Almendares O, Prince-Guerra JL, Nolen LD, et al. Performance characteristics of the Abbott BinaxNOW SARS-CoV-2 antigen test in comparison to real-time reverse transcriptase PCR and viral culture in community testing sites during November 2020. J Clin Microbiol 2022; 60:e0174221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prince-Guerra JL, Almendares O, Nolen LD, et al. Evaluation of Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection at two community-based testing sites—Pima County, Arizona, November 3–17, 2020. MMWR Morb Mortal Wkly Rep 2021; 70:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ford L, Whaley MJ, Shah MM, et al. Antigen test performance among children and adults at a SARS-CoV-2 community testing site. J Pediatric Infect Dis Soc 2021; 10:1052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. James AE, Gulley T, Kothari A, Holder K, Garner K, Patil N. Performance of the BinaxNOW coronavirus disease 2019 (COVID-19) antigen card test relative to the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) real-time reverse transcriptase polymerase chain reaction (rRT-PCR) assay among symptomatic and asymptomatic healthcare employees. Infect Control Hosp Epidemiol 2022; 43:99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaikh N, Friedlander EJ, Tate PJ, et al. Performance of a rapid SARS-CoV-2 antigen detection assay in symptomatic children. Pediatrics 2021; 148:e2021050832. [DOI] [PubMed] [Google Scholar]
- 25. Pollock NR, Jacobs JR, Tran K, et al. Performance and implementation evaluation of the Abbott BinaxNOW rapid antigen test in a high-throughput drive-through community testing site in Massachusetts. J Clin Microbiol 2021; 59:e00083–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siddiqui ZK, Chaudhary M, Robinson ML, et al. Implementation and accuracy of BinaxNOW rapid antigen COVID-19 test in asymptomatic and symptomatic populations in a high-volume self-referred testing site. Microbiol Spectr 2021; 9:e0100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ollier Q, Pillet S, Mory O, et al. Prospective evaluation of the point-of-care use of a rapid antigenic SARS-CoV-2 immunochromatographic test in a pediatric emergency department. Clin Microbiol Infect 2022; 28:734.e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tonen-Wolyec S, Dupont R, Awaida N, Batina-Agasa S, Hayette MP, Bélec L. Evaluation of the practicability of Biosynex antigen self-test COVID-19 AG+ for the detection of SARS-COV-2 nucleocapsid protein from self-collected nasal mid-turbinate secretions in the general public in France. Diagnostics 2021; 11:2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fitoussi F, Tonen-Wolyec S, Awaida N, Dupont R, Bélec L. Analytical performance of the point-of-care BIOSYNEX COVID-19 Ag BSS for the detection of SARS-CoV-2 nucleocapsid protein in nasopharyngeal swabs: a prospective field evaluation during the COVID-19 third wave in France. Infection 2022; 50:625–633. 10.1007/s15010-021-01723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hagbom M, Carmona-Vicente N, Sharma S, et al. Evaluation of SARS-CoV-2 rapid antigen diagnostic tests for saliva samples. Heliyon 2022; 8:e08998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pérez-García F, Romanyk J, Gómez-Herruz P, et al. Diagnostic performance of CerTest and Panbio antigen rapid diagnostic tests to diagnose SARS-CoV-2 infection. J Clin Virol 2021; 137:104781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merino-Amador P, González-Donapetry P, Domínguez-Fernández M, et al. Clinitest rapid COVID-19 antigen test for the diagnosis of SARS-CoV-2 infection: a multicenter evaluation study. J Clin Virol 2021; 143:104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Courtellemont L, Guinard J, Guillaume C, et al. High performance of a novel antigen detection test on nasopharyngeal specimens for diagnosing SARS-CoV-2 infection. J Med Virol 2021; 93:3152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Homza M, Zelena H, Janosek J, et al. Five antigen tests for SARS-CoV-2: virus viability matters. Viruses 2021; 13:684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nörz D, Olearo F, Perisic S, et al. Multicenter evaluation of a fully automated high-throughput SARS-CoV-2 antigen immunoassay. Infect Dis Ther 2021; 10:2371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aoki K, Nagasawa T, Ishii Y, et al. Evaluation of clinical utility of novel coronavirus antigen detection reagent, Espline® SARS-CoV-2. J Infect Chemother 2021; 27:319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim D, Lee J, Bal J, et al. Development and clinical evaluation of an immunochromatography-based rapid antigen test (GenBody™ COVAG025) for COVID-19 diagnosis. Viruses 2021; 13:796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klajmon A, Olechowska-Jarząb A, Salamon D, Sroka-Oleksiak A, Brzychczy-Włoch M, Gosiewski T. Comparison of antigen tests and qPCR in rapid diagnostics of infections caused by SARS-COV-2 virus. Viruses 2022; 14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiu RYT, Kojima N, Mosley GL, et al. Evaluation of the INDICAID COVID-19 rapid antigen test in symptomatic populations and asymptomatic community testing. Microbiology Spectrum 2021; 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aoki K, Nagasawa T, Ishii Y, et al. Clinical validation of quantitative SARS-CoV-2 antigen assays to estimate SARS-CoV-2 viral loads in nasopharyngeal swabs. J Infect Chemother 2021; 27:613–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bianco G, Boattini M, Barbui AM, et al. Evaluation of an antigen-based test for hospital point-of-care diagnosis of SARS-CoV-2 infection. J Clin Virol 2021; 139:104838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leli C, Di Matteo L, Gotta F, et al. Performance of a SARS-CoV-2 antigen rapid immunoassay in patients admitted to the emergency department. Int J Infect Dis 2021; 110:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Drain PK, Ampajwala M, Chappel C, et al. A rapid, high-sensitivity SARS-CoV-2 nucleocapsid immunoassay to aid diagnosis of acute COVID-19 at the point of care: a clinical performance study. Infect Dis Ther 2021; 10:753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koskinen JM, Antikainen P, Hotakainen K, et al. Clinical validation of automated and rapid mariPOC SARS-CoV-2 antigen test. Sci Rep 2021; 11:20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Caruana G, Croxatto A, Kampouri E, et al. Implementing SARS-CoV-2 rapid antigen testing in the emergency ward of a Swiss university hospital: the INCREASE study. Microorganisms 2021; 9:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murillo-Zamora E, Trujillo X, Huerta M, Ríos-Silva M, Mendoza-Cano O. Performance of antigen-based testing as frontline diagnosis of symptomatic COVID-19. Medicina (Kaunas) 2021; 57:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brihn A, Chang J, OYong K, et al. Diagnostic performance of an antigen test with RT-PCR for the detection of SARS-CoV-2 in a hospital setting—Los Angeles county, California, June–August 2020. MMWR Morb Mortal Wkly Rep 2021; 70:702–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Regev-Yochay G, Kriger O, Mina MJ, et al. Real world performance of SARS-CoV-2 antigen rapid diagnostic tests in various clinical settings. Infect Control Hosp Epidemiol 2022:1–20. 10.1017/ice.2022.3. [DOI] [PubMed] [Google Scholar]
- 49. Leber W, Lammel O, Siebenhofer A, Redlberger-Fritz M, Panovska-Griffiths J, Czypionka T. Comparing the diagnostic accuracy of point-of-care lateral flow antigen testing for SARS-CoV-2 with RT-PCR in primary care (REAP-2). EClinicalMedicine 2021; 38:101011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ifko M, Tkalčić Švabek Ž, Friščić I, et al. Diagnostic validation of two SARS-CoV-2 immunochromatographic tests in Slovenian and Croatian hospitals. Croat Med J 2021; 62:513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Villaverde S, Domínguez-Rodríguez S, Sabrido G, et al. Diagnostic accuracy of the Panbio severe acute respiratory syndrome coronavirus 2 antigen rapid test compared with reverse-transcriptase polymerase chain reaction testing of nasopharyngeal samples in the pediatric population. J Pediatr 2021; 232:287–9, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Masiá M, Fernández-González M, Sánchez M, et al. Nasopharyngeal Panbio COVID-19 antigen performed at point-of-care has a high sensitivity in symptomatic and asymptomatic patients with higher risk for transmission and older age. Open Forum Infect Dis 2021; 8:ofab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Escribano P, Sánchez-Pulido AE, González-Leiva J, et al. Different performance of three point-of-care SARS-CoV-2 antigen detection devices in symptomatic patients and close asymptomatic contacts: a real-life study. Clin Microbiol Infect 2022; 28:865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Landaas ET, Storm ML, Tollånes MC, et al. Diagnostic performance of a SARS-CoV-2 rapid antigen test in a large, Norwegian cohort. J Clin Virol 2021; 137:104789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Albert E, Torres I, Bueno F, et al. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag rapid test device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect 2021; 27:472.e7–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bulilete O, Lorente P, Leiva A, et al. Panbio™ rapid antigen test for SARS-CoV-2 has acceptable accuracy in symptomatic patients in primary health care. J Infect 2021; 82:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alqahtani M, Abdulrahman A, Mustafa F, Alawadhi AI, Alalawi B, Mallah SI. Evaluation of rapid antigen tests using nasal samples to diagnose SARS-CoV-2 in symptomatic patients. Front Public Health 2021; 9:728969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Krüger LJ, Gaeddert M, Tobian F, et al. The Abbott PanBio WHO emergency use listed, rapid, antigen-detecting point-of-care diagnostic test for SARS-CoV-2—evaluation of the accuracy and ease-of-use. PLoS One 2021; 16:e0247918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Klein JAF, Krüger LJ, Tobian F, et al. Head-to-head performance comparison of self-collected nasal versus professional-collected nasopharyngeal swab for a WHO-listed SARS-CoV-2 antigen-detecting rapid diagnostic test. Med Microbiol Immunol 2021; 210:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Merino P, Guinea J, Muñoz-Gallego I, et al. Multicenter evaluation of the Panbio COVID-19 rapid antigen-detection test for the diagnosis of SARS-CoV-2 infection. Clin Microbiol Infect 2021; 27:758–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mboumba Bouassa RS, Veyer D, Péré H, Bélec L. Analytical performances of the point-of-care SIENNA™ COVID-19 antigen rapid test for the detection of SARS-CoV-2 nucleocapsid protein in nasopharyngeal swabs: a prospective evaluation during the COVID-19 second wave in France. Int J Infect Dis 2021; 106:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smith RD, Johnson JK, Clay C, et al. Clinical evaluation of Sofia rapid antigen assay for detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among emergency department to hospital admissions. Infect Control Hosp Epidemiol 2021:1–6. 10.1017/ice.2021.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Beck ET, Paar W, Fojut L, Serwe J, Jahnke RR. Comparison of the Quidel Sofia SARS FIA test to the Hologic Aptima SARS-CoV-2 TMA test for diagnosis of COVID-19 in symptomatic outpatients. J Clin Microbiol 2021; 59:e02727–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pray IW, Ford L, Cole D, et al. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses—Wisconsin, September–October 2020. MMWR Morb Mortal Wkly Rep 2021; 69:1642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Harris DT, Badowski M, Jernigan B, et al. SARS-CoV-2 rapid antigen testing of symptomatic and asymptomatic individuals on the university of Arizona campus. Biomedicines 2021; 9:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mitchell SL, Orris S, Freeman T, et al. Performance of SARS-CoV-2 antigen testing in symptomatic and asymptomatic adults: a single-center evaluation. BMC Infect Dis 2021; 21:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kahn M, Schuierer L, Bartenschlager C, et al. Performance of antigen testing for diagnosis of COVID-19: a direct comparison of a lateral flow device to nucleic acid amplification based tests. BMC Infect Dis 2021; 21:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim HW, Park M, Lee JH. Clinical evaluation of the rapid STANDARD Q COVID-19 Ag test for the screening of severe acute respiratory syndrome coronavirus 2. Ann Lab Med 2022; 42:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Holzner C, Pabst D, Anastasiou OE, et al. SARS-CoV-2 rapid antigen test: fast-safe or dangerous? An analysis in the emergency department of a university hospital. J Med Virol 2021; 93:5323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jakobsen KK, Jensen JS, Todsen T, et al. Accuracy and cost description of rapid antigen test compared with reverse transcriptase-polymerase chain reaction for SARS-CoV-2 detection. Dan Med J 2021; 68:A03210217. [PubMed] [Google Scholar]
- 71. Amer RM, Samir M, Gaber OA, et al. Diagnostic performance of rapid antigen test for COVID-19 and the effect of viral load, sampling time, subject's clinical and laboratory parameters on test accuracy. J Infect Public Health 2021; 14:1446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rahman MM, Hoque AF, Karim Y, et al. Clinical evaluation of SARS-CoV-2 antigen-based rapid diagnostic test kit for detection of COVID-19 cases in Bangladesh. Heliyon 2021; 7:e08455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Turcato G, Zaboli A, Pfeifer N, et al. Rapid antigen test to identify COVID-19 infected patients with and without symptoms admitted to the emergency department. Am J Emerg Med 2022; 51:92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kernéis S, Elie C, Fourgeaud J, et al. Accuracy of saliva and nasopharyngeal sampling for detection of SARS-CoV-2 in community screening: a multicentric cohort study. Eur J Clin Microbiol Infect Dis 2021; 40:2379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fourati S, Soulier A, Gourgeon A, et al. Performance of a high-throughput, automated enzyme immunoassay for the detection of SARS-CoV-2 antigen, including in viral “variants of concern”: implications for clinical use. J Clin Virol 2022; 146:105048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Carbonell-Sahuquillo S, Lázaro-Carreño MI, Camacho J, et al. Evaluation of a rapid antigen detection test (Panbio™ COVID-19 Ag rapid test device) as a point-of-care diagnostic tool for COVID-19 in a pediatric emergency department. J Med Virol 2021; 93:6803–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gonzalez-Donapetry P, García-Clemente P, Bloise I, et al. Think of the children: evaluation of SARS-CoV-2 rapid antigen test in pediatric population. Pediatr Infect Dis J 2021; 40:385–8. [DOI] [PubMed] [Google Scholar]
- 78. Kolwijck E, Brouwers-Boers M, Broertjes J, et al. Validation and implementation of the Panbio COVID-19 Ag rapid test for the diagnosis of SARS-CoV-2 infection in symptomatic hospital healthcare workers. Infect Prev Pract 2021; 3:100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Osterman A, Badell I, Basara E, et al. Impaired detection of Omicron by SARS-CoV-2 rapid antigen tests. Med Microbiol Immunol 2022; 211(2–3):105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. US Food and Drug Administration . SARS-CoV-2 viral mutations: impact on COVID-19 tests. Available at: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests. Accessed 29 October 2022.
- 81. Killingley B, Mann AJ, Kalinova M, et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med 2022; 28:1031–41. [DOI] [PubMed] [Google Scholar]
- 82. McKay SL, Tobolowsky FA, Moritz ED, et al. Performance evaluation of serial SARS-CoV-2 rapid antigen testing during a nursing home outbreak. Ann Intern Med 2021; 174:945–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hakki S, Zhou J, Jonnerby J, et al. Onset and window of SARS-CoV-2 infectiousness and temporal correlation with symptom onset: a prospective, longitudinal, community cohort study. Lancet Respir Med 2022; 10:1061–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hanson KE, Altayar O, Caliendo AM, et al. The Infectious Diseases Society of America guidelines on the diagnosis of COVID-19: antigen testing (June 2021). Clin Infect Dis 2024; 78:e208–e229. [DOI] [PubMed] [Google Scholar]
- 85. Altamimi AM, Obeid DA, Alaifan TA, et al. Assessment of 12 qualitative RT-PCR commercial kits for the detection of SARS-CoV-2. J Med Virol 2021; 93:3219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kim HN, Yoon SY, Lim CS, Yoon J. Comparison of three molecular diagnostic assays for SARS-CoV-2 detection: evaluation of analytical sensitivity and clinical performance. J Clin Lab Anal 2022; 36:e24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lephart PR, Bachman MA, LeBar W, et al. Comparative study of four SARS-CoV-2 nucleic acid amplification test (NAAT) platforms demonstrates that ID NOW performance is impaired substantially by patient and specimen type. Diagn Microbiol Infect Dis 2021; 99:115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yun J, Park JH, Kim N, et al. Evaluation of three Multiplex real-time reverse transcription PCR assays for simultaneous detection of SARS-CoV-2, influenza A/B, and respiratory syncytial virus in nasopharyngeal swabs. J Korean Med Sci 2021; 36:e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Smith E, Zhen W, Manji R, Schron D, Duong S, Berry GJ. Analytical and clinical comparison of three nucleic acid amplification tests for SARS-CoV-2 detection. J Clin Microbiol 2020; 58:e01134–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fourati S, Langendorf C, Audureau E, et al. Performance of six rapid diagnostic tests for SARS-CoV-2 antigen detection and implications for practical use. J Clin Virol 2021; 142:104930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gili A, Paggi R, Russo C, et al. Evaluation of Lumipulse® G SARS-CoV-2 antigen assay automated test for detecting SARS-CoV-2 nucleocapsid protein (NP) in nasopharyngeal swabs for community and population screening. Int J Infect Dis 2021; 105:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dierks S, Bader O, Schwanbeck J, et al. Diagnosing SARS-COV-2 with antigen testing, transcription-mediated amplification and real-time PCR. J Clin Med 2021; 10:2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Drain P, Sulaiman R, Hoppers M, Lindner NM, Lawson V, Ellis JE. Performance of the LumiraDx microfluidic immunofluorescence point-of-care SARS-CoV-2 antigen test in asymptomatic adults and children. Am J Clin Pathol 2021; 157:602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ferté T, Ramel V, Cazanave C, et al. Accuracy of COVID-19 rapid antigenic tests compared to RT-PCR in a student population: the StudyCov study. J Clin Virol 2021; 141:104878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Baro B, Rodo P, Ouchi D, et al. Performance characteristics of five antigen-detecting rapid diagnostic test (Ag-RDT) for SARS-CoV-2 asymptomatic infection: a head-to-head benchmark comparison. J Infect 2021; 82:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Di Domenico M, De Rosa A, Di Gaudio F. Diagnostic accuracy of a new antigen test for SARS-CoV-2 detection. Int J Environ Res Public Health 2021; 18:6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Aranaz-Andrés JM, Chávez ACF, Laso AM, et al. Analysis of the diagnostic accuracy of rapid antigenic tests for detection of SARS-CoV-2 in hospital outbreak situation. Eur J Clin Microbiol Infect Dis 2022; 41:305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Winkel B, Schram E, Gremmels H, et al. Screening for SARS-CoV-2 infection in asymptomatic individuals using the Panbio COVID-19 antigen rapid test (Abbott) compared with RT-PCR: a prospective cohort study. BMJ Open 2021; 11:e048206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. von Ahnen T, von Ahnen M, Wirth U, Schardey HM, Herdtle S. Evaluation of a rapid-antigen test for COVID-19 in an asymptomatic collective: a prospective study. Wien Med Wochenschr 2022; 172(3–4):70–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fernandez-Montero A, Argemi J, Rodríguez JA, Ariño AH, Moreno-Galarraga L. Validation of a rapid antigen test as a screening tool for SARS-CoV-2 infection in asymptomatic populations. Sensitivity, specificity and predictive values. EClinicalMedicine 2021; 37:100954. 10.1016/j.eclinm.2021.100954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Peña MÁ, Ampuero M, Garcés C. Performance of SARS-CoV-2 rapid antigen test compared with real-time RT-PCR in asymptomatic individuals. Int J Infect Dis 2021; 107:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mungomklang A, Trichaisri N, Jirachewee J, Sukprasert J, Tulalamba W, Viprakasit V. Limited sensitivity of a rapid SARS-CoV-2 antigen detection assay for surveillance of asymptomatic individuals in Thailand. Am J Trop Med Hyg 2021; 105:1505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kumar KK, Sampritha UC, Maganty V. Pre-operative SARS CoV-2 rapid antigen test and reverse transcription polymerase chain reaction: a conundrum in surgical decision making. Indian J Ophthalmol 2021; 69:1560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wachinger J, Olaru ID, Horner S, Schnitzler P, Heeg K, Denkinger CM. The potential of SARS-CoV-2 antigen-detection tests in the screening of asymptomatic persons. Clin Microbiol Infect 2021; 27:1700.e1–.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Betsch C, Sprengholz P, Siegers R, et al. Empirical evidence to understand the human factor for effective rapid testing against SARS-CoV-2. Proc Natl Acad Sci USA 2021; 118:e2107179118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. COVID-19 Treatment Guidelines Panel . Coronavirus disease 2019 (COVID-19) treatment guidelines, anti-SARS-CoV-2 monoclonal antibodies. National Institutes of Health. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed 25 November 2022.
- 107. Deng JZ, Chan JS, Potter AL, et al. The risk of postoperative complications after major elective surgery in active or resolved COVID-19 in the United States. Ann Surg 2022; 275:242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. COVIDSurg Collaborative; GlobalSurg Collaborative . Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia 2021; 76:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Le ST, Kipnis P, Cohn B, Liu VX. COVID-19 vaccination and the timing of surgery following COVID-19 infection. Ann Surg 2022; 276:e265–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Baker JM, Nakayama JY, O'Hegarty M, et al. SARS-CoV-2 B.1.1.529 (Omicron) variant transmission within households—four U.S. jurisdictions. MMWR Morb Mortal Wkly Rep 2022; 71:341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household secondary attack rates of SARS-CoV-2 by variant and vaccination status: an updated systematic review and meta-analysis. JAMA Netw Open 2022; 5:e229317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Young BC, Eyre DW, Kendrick S, et al. Daily testing for contacts of individuals with SARS-CoV-2 infection and attendance and SARS-CoV-2 transmission in English secondary schools and colleges: an open-label, cluster-randomised trial. Lancet 2021; 398:1217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Centers for Disease Control and Prevention . Contact tracing—Interim guidance on developing a COVID-19 case investigating & contract tracing plan: Overview—appendices. Available at: https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/appendix.html. Accessed 25 September 2022.
- 114. Lyngse FP, Mortensen LH, Denwood MJ, et al. Household transmission of the SARS-CoV-2 Omicron variant in Denmark. Nat Commun 2022; 13(1):5573. 10.1038/s41467-022-33328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schultes O, Clarke V, Paltiel AD, Cartter M, Sosa L, Crawford FW. COVID-19 testing and case rates and social contact among residential college students in Connecticut during the 2020–2021 academic year. JAMA Netw Open 2021; 4:e2140602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Møller IJB, Utke AR, Rysgaard UK, Østergaard LJ, Jespersen S. Diagnostic performance, user acceptability, and safety of unsupervised SARS-CoV-2 rapid antigen-detecting tests performed at home. Int J Infect Dis 2022; 116:358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Alghounaim M, Bastaki H, Bin Essa F, Motlagh H, Al-Sabah S. The performance of two rapid antigen tests during population-level screening for SARS-CoV-2 infection. Front Med (Lausanne) 2021; 8:797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ishii T, Sasaki M, Yamada K, et al. Immunochromatography and chemiluminescent enzyme immunoassay for COVID-19 diagnosis. J Infect Chemother 2021; 27:915–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Osterman A, Iglhaut M, Lehner A, et al. Comparison of four commercial, automated antigen tests to detect SARS-CoV-2 variants of concern. Med Microbiol Immunol 2021; 210(5–6):263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Paul D, Gupta A, Rooge S, Gupta E. Performance evaluation of automated chemiluminescence immunoassay based antigen detection—moving towards more reliable ways to predict SARS-CoV-2 infection. J Virol Methods 2021; 298:114299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Petonnet D, Marot S, Leroy I, et al. Comparison of rapid and automated antigen detection tests for the diagnosis of SARS-CoV-2 infection. Diagnostics (Basel) 2022; 12:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nikolai O, Rohardt C, Tobian F, et al. Anterior nasal versus nasal mid-turbinate sampling for a SARS-CoV-2 antigen-detecting rapid test: does localisation or professional collection matter? Infect Dis (Lond) 2021; 53:947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Okoye NC, Barker AP, Curtis K, et al. Performance characteristics of BinaxNOW COVID-19 antigen card for screening asymptomatic individuals in a university setting. J Clin Microbiol 2021; 59:e03282–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Currie DW, Shah MM, Salvatore PP, et al. Relationship of SARS-CoV-2 antigen and reverse transcription PCR positivity for viral cultures. Emerg Infect Dis 2022; 28:717–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Denford S, Towler L, Ali B, et al. Feasibility and acceptability of daily testing at school as an alternative to self-isolation following close contact with a confirmed case of COVID-19: a qualitative analysis. BMC Public Health 2022; 22:742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. US Food and Drug Administration . Potential for false positive results with antigen tests for rapid detection of SARS-CoV-2—letter to clinical laboratory staff and health care providers. Available at: https://www.fda.gov/medical-devices/letters-health-care-providers/potential-false-positive-results-antigen-tests-rapid-detection-sars-cov-2-letter-clinical-laboratory. Accessed 17 May 2021.
- 127. Papenburg J, Campbell JR, Caya C, et al. Adequacy of serial self-performed SARS-CoV-2 rapid antigen detection testing for longitudinal mass screening in the workplace. JAMA Netw Open 2022; 5:e2210559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kohmer N, Toptan T, Pallas C, et al. The comparative clinical performance of four SARS-CoV-2 rapid antigen tests and their correlation to infectivity in vitro. J Clin Med 2021; 10:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pekosz A, Parvu V, Li M, et al. Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin Infect Dis 2021; 73:e2861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Frank F, Keen MM, Rao A, et al. Deep mutational scanning identifies SARS-CoV-2 nucleocapsid escape mutations of currently available rapid antigen tests. Cell 2022; 185:3603–16, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.