Abstract
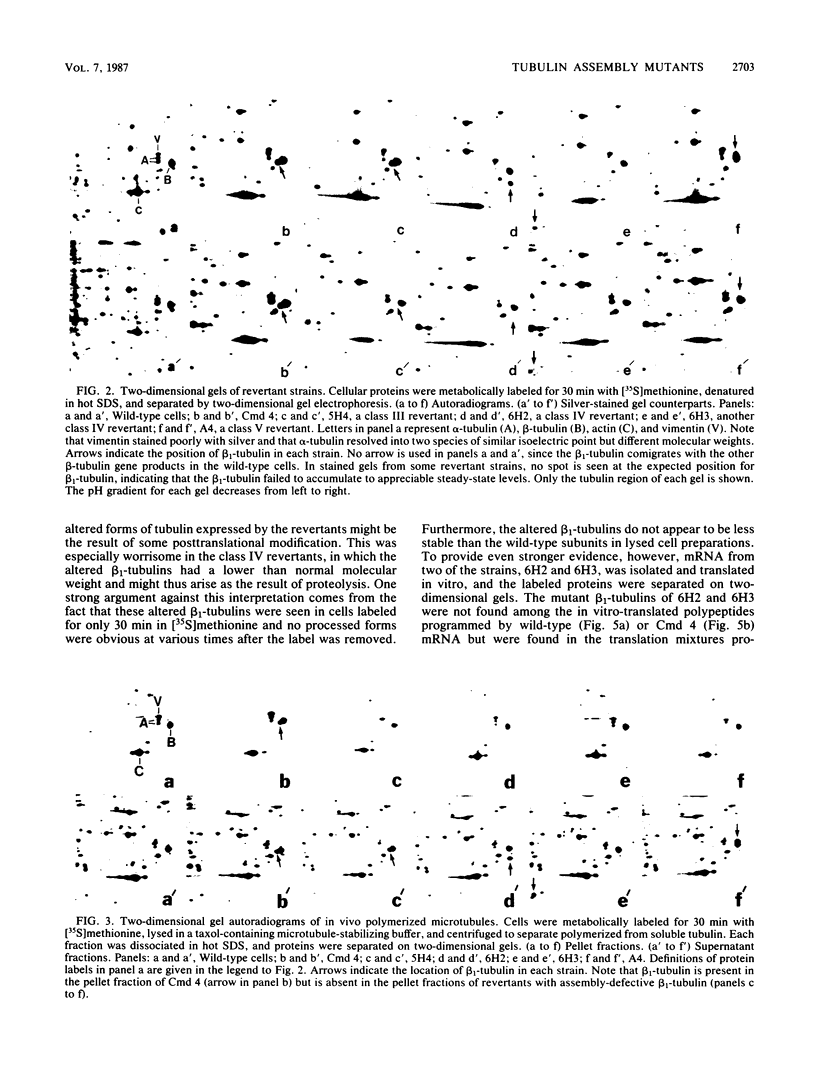
Eight strains of Chinese hamster ovary (CHO) cells having an assembly-defective beta-tubulin were found among revertants of strain Cmd 4, a mutant with a conditional lethal mutation in a beta-tubulin gene (F. Cabral, M. E. Sobel, and M. M. Gottesman, Cell 20:29-36, 1980). The altered beta-tubulins in these strains have electrophoretically silent alterations or, in some cases, an increase or a decrease in apparent molecular weight based on their migration in two-dimensional gels. The identity of these variant proteins as beta-tubulin was confirmed by peptide mapping, which also revealed the loss of distinct methionine-containing peptides in the assembly-defective beta-tubulins of lower apparent molecular weight. The altered mobility of these beta-tubulin polypeptides was not the result of a posttranslational modification, since the altered species could be labeled in very short incubations with [35S]methionine and were found among in vitro-translated polypeptides by using purified mRNA. In at least one strain, an altered DNA restriction fragment could be demonstrated, suggesting that an alteration occurred in one of the structural genes for beta-tubulin. Assembly-defective beta-tubulin was unstable and turned over with a half-life of only 1 to 2 h in exponentially growing cells. This rapid degradation of a tubulin gene product resulted in approximately 30% lower steady-state levels of both alpha- and beta-tubulin yet did not affect the growth rate of the cells or the distribution of the microtubules as judged by immunofluorescence microscopy. These results argue that CHO cells possess a beta-tubulin gene product that is not essential for survival.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cabral F., Abraham I., Gottesman M. M. Revertants of a Chinese hamster ovary cell mutant with an altered beta-tubulin: evidence that the altered tubulin confers both colcemid resistance and temperature sensitivity on the cell. Mol Cell Biol. 1982 Jun;2(6):720–729. doi: 10.1128/mcb.2.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Schatz G. High resolution one- and two- dimensional electrophoretic analysis of mitochondrial membrane polypeptides. Methods Enzymol. 1979;56:602–613. doi: 10.1016/0076-6879(79)56057-1. [DOI] [PubMed] [Google Scholar]
- Cabral F., Sobel M. E., Gottesman M. M. CHO mutants resistant to colchicine, colcemid or griseofulvin have an altered beta-tubulin. Cell. 1980 May;20(1):29–36. doi: 10.1016/0092-8674(80)90231-7. [DOI] [PubMed] [Google Scholar]
- Caron J. M., Jones A. L., Kirschner M. W. Autoregulation of tubulin synthesis in hepatocytes and fibroblasts. J Cell Biol. 1985 Nov;101(5 Pt 1):1763–1772. doi: 10.1083/jcb.101.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Sullivan K. F. Molecular biology and genetics of tubulin. Annu Rev Biochem. 1985;54:331–365. doi: 10.1146/annurev.bi.54.070185.001555. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., LeCam A., Bukowski M., Pastan I. Isolation of multiple classes of mutants of CHO cells resistant to cyclic AMP. Somatic Cell Genet. 1980 Jan;6(1):45–61. doi: 10.1007/BF01538695. [DOI] [PubMed] [Google Scholar]
- Gundersen G. G., Kalnoski M. H., Bulinski J. C. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984 Oct;38(3):779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Venner T. J., Chopra A. Genetic and biochemical studies with mutants of mammalian cells affected in microtubule-related proteins other than tubulin: mitochondrial localization of a microtubule-related protein. Can J Biochem Cell Biol. 1985 Jun;63(6):489–502. doi: 10.1139/o85-068. [DOI] [PubMed] [Google Scholar]
- Hall J. L., Dudley L., Dobner P. R., Lewis S. A., Cowan N. J. Identification of two human beta-tubulin isotypes. Mol Cell Biol. 1983 May;3(5):854–862. doi: 10.1128/mcb.3.5.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the major outer membrane proteins of Escherichia coli. Annu Rev Genet. 1981;15:91–142. doi: 10.1146/annurev.ge.15.120181.000515. [DOI] [PubMed] [Google Scholar]
- Kemphues K. J., Kaufman T. C., Raff R. A., Raff E. C. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982 Dec;31(3 Pt 2):655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Little M., Ludueña R. F. Structural differences between brain beta 1- and beta 2-tubulins: implications for microtubule assembly and colchicine binding. EMBO J. 1985 Jan;4(1):51–56. doi: 10.1002/j.1460-2075.1985.tb02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla A. E., MacDonald R. J., Harding J. D., Pictet R. L., Rutter W. J. Accumulation of the predominant pancreatic mRNAs during embryonic development. J Biol Chem. 1979 Mar 25;254(6):2154–2159. [PubMed] [Google Scholar]
- Saxton W. M., Stemple D. L., Leslie R. J., Salmon E. D., Zavortink M., McIntosh J. R. Tubulin dynamics in cultured mammalian cells. J Cell Biol. 1984 Dec;99(6):2175–2186. doi: 10.1083/jcb.99.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. Microtubule dynamics in interphase cells. J Cell Biol. 1986 Mar;102(3):1020–1031. doi: 10.1083/jcb.102.3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Williams D. R., Weeks M. O., Maryak J. M., Vass W. C., Scolnick E. M. Comparison of the genomic organization of Kirsten and Harvey sarcoma viruses. J Virol. 1978 Jul;27(1):45–55. doi: 10.1128/jvi.27.1.45-55.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Penningroth S. M., Kirschner M. W. Turnover of tubulin and the N site GTP in Chinese hamster ovary cells. Cell. 1977 Nov;12(3):587–600. doi: 10.1016/0092-8674(77)90259-8. [DOI] [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Whitfield C., Abraham I., Ascherman D., Gottesman M. M. Transfer and amplification of a mutant beta-tubulin gene results in colcemid dependence: use of the transformant to demonstrate regulation of beta-tubulin subunit levels by protein degradation. Mol Cell Biol. 1986 May;6(5):1422–1429. doi: 10.1128/mcb.6.5.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yoffe G., Spitzer G., Boggs B. A., McCredie K. B., Stass S. A., Chinault A. C. Determination of clonality in acute nonlymphocytic leukemia by restriction fragment length polymorphism and methylation analysis. Leukemia. 1987 Mar;1(3):226–230. [PubMed] [Google Scholar]