Abstract
Colorectal cancer (CRC) is the third leading cause of cancer deaths worldwide and is characterised by frequently mutated genes, such as APC, TP53, KRAS and BRAF. The current treatment options of chemotherapy, radiation therapy and surgery are met with challenges such as cancer recurrence, drug resistance, and overt toxicity. CRC therapies exert their efficacy against cancer cells by activating biological pathways that contribute to various forms of regulated cell death (RCD). In 2012, ferroptosis was discovered as an iron-dependent and lipid peroxide-driven form of RCD. Recent studies suggest that therapies which target ferroptosis are promising treatment strategies for CRC. However, a greater understanding of the mechanisms of ferroptosis initiation, propagation, and resistance in CRC is needed. This review provides an overview of recent research in ferroptosis and its potential role as a therapeutic target in CRC. We also propose future research directions that could help to enhance our understanding of ferroptosis in CRC.
Subject terms: Colon cancer, Cancer metabolism
Introduction
Worldwide, colorectal cancer (CRC) is a leading cause of mortality and morbidity and the third most common cause of death from cancer [1]. For 2022, the estimated number of newly diagnosed CRC cases was 151,030 [2]. The aetiology of CRC is complex, however, its development is likely due to a combination of environmental influences (such as, dietary exposures, smoking and alcohol use) and the presence and interplay of pathogenic microbiota, genetic susceptibility, and immune response mechanisms [3–6]. Current treatments for CRC include surgery, radiation, and chemotherapies. Present chemotherapies used to treat CRC include (1) bevacizumab, an anti-vascular endothelial growth factor-A (anti-VEGF-A) antibody, (2) aflibercept, a VEGF-A, VEGF-B, and placental growth factor inhibitor, (3) regorafenib, a multi-kinase inhibitor and (4) cetuximab and panitumumab, anti-epidermal growth factor receptor (anti-EGFR) antibodies [7]. However, not all patients respond to these therapies. For example, patients with tumours that present on the right side of the colon have little benefit from panitumumab therapy [8], and cetuximab and panitumumab do not work as well for tumours with RAS mutations [9, 10].
Ferroptosis is a nonapoptotic and iron-dependent form of cell death that was discovered in 2012 [11]. Since its discovery, three hallmarks of ferroptosis have been established: (1) oxidation of polyunsaturated fatty acid (PUFA)-containing phospholipids, (2) iron dependency and (3) the loss of lipid peroxide repair mechanisms [12]. In addition to its potential roles in CRC, ferroptosis has been linked to the development of other cancers: breast, cervical, gastric, hepatocellular, lung, ovarian, prostate and renal carcinomas, as well as lymphoma and melanoma [13]. It has also been linked to mechanisms of lung fibrosis, stroke, traumatic brain injury, Alzheimer’s disease and Huntington’s disease [14]. Numerous studies have shown that modulating the ferroptotic cell death pathway can enhance the antitumor effects of drugs for CRC treatment. Similarly, in vitro and in in vivo experiments have revealed that the use of pharmacologics that trigger ferroptosis-induced cancer cell death (Erastin and Ras-selected lethal 3 (RSL3)) have promise in CRC [15, 16]. Yet there is still an urgent need to understand the mechanisms of how ferroptosis is regulated in CRC, and how these pathways and pharmacologics can be used for potential clinical translation.
This review aims to discuss the mechanisms of ferroptosis and its resistance pathways in cancer. We summarise what is currently known about ferroptosis in CRC tumorigenesis and metabolism and the use of ferroptosis inducers as treatments for CRC. We also offer concluding thoughts about the therapeutical potential of targeting ferroptotic pathways.
Mechanisms of ferroptosis induction
Ferroptosis is distinct from other types of RCD as its mechanism is dependent on active ferrous ions and the accumulation of phospholipid hydroperoxides (PLOOHs) [17]. It is also dependent on important proteins that play various roles in normal cellular homeostasis, helping to regulate lipid recycling, iron metabolism, and to maintain redox status [18]. Table 1 illustrates the main molecular contributors to ferroptosis [19–38]. Mechanisms of ferroptosis which are tightly associated with CRC will be discussed below: iron metabolism, lipid peroxidation, redox status, lipid reprogramming and amino acid metabolism.
Table 1.
Main molecular regulators of ferroptosis.
| Gene | Name | Function | Reference |
|---|---|---|---|
| Cystine uptake | |||
| SLC7A11 | Solute carrier family 7 member 11 cystine/glutamate antiporter xCT | Cystine uptake in exchange for glutamate | [19, 148] |
| SLC3A2 | Solute carrier family 3 member 2 | Maintains SLC7A11 stability | [149, 150] |
| ATF3 | Activating transcription factor 3 | Suppresses xCT by binding to the SLC7A11 promoter | [151] |
| OTUB1 | Ovarian tumour family member | Stabilises SLC7A11 expression | [152, 153] |
| Redox homoeostasis regulators | |||
| GPX4 | Glutathione peroxidase 4 | Prevents lipid hydroperoxide formation | [154–156] |
| NRF2 | Nuclear factor erythroid 2-related factor 2 | Regulates antioxidant response by increasing target gene transcription | [29] |
| Lipid metabolism | |||
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 | Converts AA/AdA into AA CoA/AdA CoA | [26, 31, 157] |
| LPCAT3 | Lysophosphatidylcholine acyltransferase 3 | Esterifies AA CoA/ AdA CoA into PEs | [31] |
| LOXs | Lipoxygenases | Generate lipid ROS by oxidising AA-PE and AdA-PE | [59, 158] |
| FSP1 | Ferroptosis suppressor protein | Reduces CoQ10 to ubiquinol | [159, 160] |
| SCD1 | Stearoyl-CoA desaturase | Involved in fatty acid biosynthesis | [161] |
| POR | Cytochrome P450 oxidoreductase | Promotes PUFA peroxidation | [162] |
| SQLE | Squalene epoxidase | Causes squalene accumulation | [74] |
| SCF2 | Sterol carrier protein 2 | Mediates trafficking of lipid ROS to mitochondria | [28] |
| GCH1 | Cyclohydrolase-1 | Increases antioxidant BH4 and CoQ10 abundance | [163] |
| Iron metabolism | |||
| TFR1 | Transferrin receptor | Imports iron into cells | [39, 115, 164] |
| HSPB1 | Heat-shock 27-kDa protein 1 | Reduces cellular iron uptake | [165] |
| IREB2 | Iron response element binding protein 2 | Limits iron uptake | [166] |
| NFS1 | Nitrogen fixation 1 | Supplies sulfur for iron–sulfur cluster synthesis | [167] |
| STEAP3 | Six-transmembrane epithelial antigen of prostate 3 | Converts Fe3+ to Fe2+ | [168] |
| DMT1 | Divalent metal transporter 1 | Mediates the release of Fe2+ into a labile iron pool | [169, 170] |
| FTH1 | Ferritin heavy chain 1 | Stores intracellular iron | [167, 171] |
| Energy metabolism | |||
| SLC1A5 | Solute carrier family 1 member 5 | Controls glutamine uptake | [115] |
| GLS2 | Mitochondrial glutaminase | Converts glutamine to glutamate | [172] |
| GOT1 | Glutamic-oxaloacetic transaminase 1 | Blocks the synthesis of a-ketoglutarate | [173] |
Iron metabolism
The connection between iron metabolism and ferroptosis was discovered through the observation that deferoxamine (DFO), an iron chelator, inhibits Erastin- and RSL3-induced cell death [39]. Iron levels are carefully regulated through coordinated processes such as iron storage, import, export, and recycling. Iron increases lipid peroxidation through two mechanisms. First, it can directly oxidise lipids via the Fenton reaction, in which the reaction between ferrous (Fe2+) or ferric (Fe3+) iron and hydrogen peroxide (H2O2) are involved (Fe2+ +H2O2 = Fe3+ + OH- + HO·, Fe3++H2O2 = Fe2+ + HOO· + H+) [11]. Iron also contributes to lipid peroxidation indirectly by serving as a cofactor for enzymes such as the lipoxygenases (LOXs) that directly oxidise lipids [40, 41]. Therefore, increased levels of labile Fe2+ enhance the sensitivity of cells to ferroptosis, whereas Fe3+ is incorporated into proteins or stored by ferritin, reducing ferroptosis sensitivity. Ferritin is composed of 24 polypeptides that are either ferritin light chain (FTL) or ferritin heavy chain (FTH1) [42]. FTH1 is the main regulator of ferritin activity and controls the oxidation of Fe2+ to Fe3+. In RAS-mutant cells, it has been shown that decreased ferritin expression contributes to ferroptosis sensitivity by increasing iron uptake and decreasing iron storage [39]. Ferritinophagy, mediated by nuclear receptor coactivator 4 (NCOA4), is the process by which ferritin is degraded and Fe2+ is released into the labile iron pool. Increased ferritinophagy has been shown to promote ferroptosis.
Transcription factors also regulate ferroptosis by controlling the expression of genes involved in iron-related metabolism. Among them is nuclear factor erythroid 2-like 2 (NFE2L2/NRF2), a key regulator of the cellular antioxidant response. NRF2 controls several aspects of antioxidant, iron and cell metabolic status, including regulation of the cystine-glutamate antiporter (xCT: SLC7A11) and glutathione peroxidase 4 (GPX4), which are two critical targets for the induction of ferroptosis.
It is also likely that iron regulates ferroptosis through its roles in hypoxia and DNA methylation. Hypoxia modulates ferroptosis via hypoxia-inducible factors (HIFs) and their interactions with NRF2 [43]. HIF prolyl hydroxylases are sensitive to iron concentrations in addition to hypoxia [44]. Increased NRF2 activity under hypoxia facilitates haem oxygenase-1 (HO-1) expression, protecting cells from ferroptosis [45, 46]. Expression of proteins such as Stearoyl-CoA desaturase 1 (SCD1) also increases under hypoxia. SCD1 has ferroxidase activity, which could potentially attenuate ferroptosis by limiting intracellular Fe2+. Lastly, NCOA4 expression decreases under hypoxia, inhibiting ferritinophagy and protecting cells from ferroptosis [47, 48].
Epigenetic regulators such as DNA methylation, histone acetylation and miRNA actively regulate cellular iron homoeostasis. Several studies have reported significant DNA methylation alterations upon increases in intracellular reactive oxygen species (ROS) and iron [49–51]. Remarkably, methylcytosine dioxygenase Tet1 was first identified as an iron-dependent regulator of multiple downstream target genes through its ability to oxidise and demethylate 5-methylcytosines in DNA, and ubiquitinate ferroportin [52]. In addition, DNA methylation changes in ferroptotic myeloma cells also demonstrated enrichment of CpG probes located in genes associated with cell cycle progression and senescence via increased iron levels, such as nuclear receptor subfamily 4 group A member 2 (NR4A2) [53]. More recently, Fe2+ stress was found to induce DNA methylation and genomic instability [54]. Future studies that continue to explore the relationship between DNA methylation, hypoxia and iron metabolism might provide important insight into ferroptosis biology. In terms of treatments that could target iron metabolism, DFO and deferiprone are iron chelators that are undergoing trials for treating conditions associated with increased inflammation, including Parkinson’s disease. Whether these iron chelators play a direct role in ferroptosis and how they could be implemented in CRC warrants investigation [55].
Lipid peroxidation
The role of lipid peroxidation in ferroptosis has been reviewed thoroughly elsewhere [56–58]. However, we emphasise here that lipid peroxidation is an important hallmark of ferroptosis, and regulation of lipid peroxidation by LOXs and Acyl-CoA Synthetase Long-Chain Family Member 4 (ACSL4) present as potential therapeutic targets, especially in CRC (Fig. 1).
Fig. 1. The regulation and targeting of ferroptosis in CRC.
The following nodes are proposed as potential therapeutic targets for CRC: ① p53: p53 negatively regulates ferroptosis by inhibiting DPP4 in CRC cells. ② ACSL4: ACSL4 plays a crucial role in the induction of ferroptosis in KRAS mutant CRC. ③ GPX4: Several molecules have been reported to induce ferroptosis by targeting GPX4 in CRC. ④ SLC7A11: As a subunit of system xCT, inhibited can induce ferroptosis. ⑤ AA: AA metabolism might be a potential target in CRC. LOXs also oxidise AA at different carbon sites and regulate cellular redox homoeostasis. Evidence showed that LOXs are important regulatory mechanisms of ferroptosis and its inhibitors such as the vitamin E family (tocopherols and tocotrienols) are effective in preventing ferroptotic death. ACSL4 acyl coenzyme A synthetase long-chain family member 4, AA arachidonic acid, BH2 dihydrobiopterin, BH4 tetrahydrobiopterin, CoA coenzyme A, DHFR dihydrofolate reductase, DHCR7 7-dehydrocholesterol reductase, Fe iron, FSP1 ferroptosis suppressor protein 1, FPP farnesyl pyrophosphate, GSH glutathione, GSSG glutathione disulfide, GCH1 GTP cyclohydrolase-1, GCS glutamylcysteine synthetase, GLS glutaminase, GPX4 glutathione peroxidase 4, GPP geranyl pyrophosphate, HMG-CoA 3-hydroxy-3-methylglutaryl CoA, HETE hydroxyeicosatetraenoic acids, HPETE hydroperoxyeicosatetraenoic acid, IPP Isopentenyl pyrophosphate, LPCAT3 lysophosphatidylcholine acyltransferase 3, LPCAT lysophosphatidylcholine acyltransferase, LTA4 leukotriene A4, LTB4 leukotriene B4, LTC4 leukotriene C4, NRF2, nuclear factor E2-related factor 2; OOH, hydroperoxide; PE-PUFA, phosphatidylethanolamine polyunsaturated fatty acid, PUFA polyunsaturated fatty acid, PGD2 prostaglandin D2, PGE2 prostaglandin E2, PGF2α prostaglandin F2α, PGF2β prostaglandin F2β, SAM S-Adenosylmethionine, SAH S-Adenosylhomocysteine, TCA cycle tricarboxylic acid cycle, TFRC transferrin receptor, TSP pathway transsulfuration pathway, γ-GC γ-glutamylcysteine.
The mechanism of how the oxidation of polyunsaturated fatty acids (PUFAs) to PUFA hydroperoxides (PUFA-OOHs) triggers ferroptosis is under debate. LOX, particularly 12/15-LOX (i.e., ALOX15), has been repeatedly suggested to play a central role in lipid peroxidation and ferroptosis [28]. Yang et al. observed that PUFA oxidation by LOX via a Phosphorylase Kinase Catalytic Subunit Gamma 2 (PHKG2)-dependent iron pool is necessary for ferroptosis, and the covalent inhibition of the catalytic selenocysteine in GPX4 prevents elimination of PLOOHs [28]. Furthermore, it has been indicated that the initial accumulation of PLOOH regardless of the contribution of LOX is the actual driver of ferroptosis [59]. Recent evidence has revealed that ACSL4 contributes to ferroptosis by upregulating the production of PUFAs and specifically aiding the incorporation of PUFAs into the cell membrane. In 2017, ACSL4 was reported to drive ferroptosis via the accumulation of oxidised cellular membrane phospholipids [26]. These oxidised cellular membrane phospholipids were subsequently identified as oxidised phosphatidylethanolamines (PEs), which are produced via ACSL4 activity [60]. ACSL4 catalyzes the ligation of an arachidonoyl or adrenoyl (AdA) moiety to produce arachidonoyl and AdA acyl-CoA derivatives, respectively. These are then esterified into arachidonoyl-PEs and AdA-PEs by lysophosphatidylcholine acyltransferase 3 (LPCAT3). Subsequently, arachidonoyl-PE and AdA-PE are oxidised by 15-LOX to generate lipid hydroperoxides, the proximate executors of ferroptosis. Supporting this, it has been suggested that lipid peroxidation at the cell membrane is a common requirement for both Erastin and RSL3-induced ferroptosis. ACSL4, however, is only required for RSL3-induced ferroptosis [61]. Why ACSL4 is dispensable for Erastin-induced ferroptosis and whether it is necessary for other modes of ferroptosis induction are important questions and areas for future research.
Another key question in ferroptosis biology is where is the precise location of lethal lipid peroxidation in the cell? While the endoplasmic reticulum, lysosome, and mitochondrial membrane are plausible suggestions, current evidence suggests that the cell membrane is the most probable. It is plausible that extensive lipid peroxidation, resulting from the accumulation of free PUFAs, might alter the chemical make-up of the lipid bilayer. This can compromise membrane integrity and affect barrier function. Attacks on PUFA-containing phospholipids (PUFA-PLs) at their bis-allylic sites by LOXs, radicals and oxygen, drive cell death. This later phenomenon is partly reconcilable with the prior hypothesis because it suggests that free PUFAs must be incorporated into phospholipids in a reaction catalysed by ACSL4, a protein involved in lipid biosynthesis [14, 62].
Redox status, lipid metabolism and amino acid metabolism
Redox status, lipid metabolism, and amino acid metabolism are also important in the regulation of ferroptosis, and we briefly introduce them here. The thioredoxin system is essential for redox homoeostasis, which can protect DNA from lipid peroxidation and oxidative stress-related damage [63]. One recent study suggested that Auranofin/buthionine sulfoxime and Erastin/buthionine sulfoxime cotreatment alters redox homoeostasis by increasing levels of NRF2 and HO-1, and by decreasing GPX4 levels to induce ferroptosis [64].
As aforementioned, lipid peroxidation occurs in PUFAs on specific phospholipids, and various lipid metabolic pathways are involved in lipid peroxidation and ferroptosis. One of the core questions remaining is how the cells acquire and maintain fatty acid levels in cells? While monounsaturated fatty acids and saturated fatty acids can be generated from acetyl-CoA in cells, long-chain PUFAs such as arachidonic acid (AA) and AdA can be synthesised from dietary essential fatty acids such as linoleic acid in cells. In addition, long-chain PUFAs can be obtained extracellularly. Further, fatty acid uptake and release are tightly regulated by cellular states [65]. Together, these factors determine the levels of fatty acid in cells, which ultimately affect ferroptosis sensitivity [65].
Evidence has demonstrated that amino acid metabolism is critical for ferroptosis; for example, the metabolism of cyst(e)ine, glutamine, branched-chain amino acids (including leucine, valine and isoleucine), tryptophan, and other amino acids (such as arginine, serine, glycine, lysine, etc.). As basic nutrients and energy sources, amino acids contribute to homoeostasis and tumour proliferation significantly as intermediates in glucose, lipid, and nucleotide metabolic pathways. Although amino acid metabolism has been a recent target for newer cancer treatment strategies, a full understanding of amino acid metabolism and ferroptosis in cancer is needed which will help guide drug development and cancer therapy.
Mechanisms of ferroptosis suppression
Since the initial identification of GPX4 as the covalent target for RSL3, the study of suppression systems in ferroptosis has continued to expand. As this point in time, the four key suppression systems that neutralise lipid peroxides include (1) the GPX4-glutathione (GSH) system, (2) the ferroptosis suppressor protein 1 (FSP1)-ubiquinol (CoQH2) system, (3) the dihydroorotate dehydrogenase (DHODH)-CoQH2 system and (4) the GTP cyclohydrolase I (GCH1)-tetrahydrobiopterin (BH4) system. These four systems have been reviewed in detail elsewhere [66]. Here, we discuss the potential ferroptosis-suppressing functions of two new emerging systems, the transsulfuration pathway (TSP) and mevalonate pathway.
Transsulfuration pathway
Cellular cysteine is a critical metabolite for ferroptosis. It is primarily acquired through the xCT, which consists of subunits SLC7A11 and SLC3A2 and imports cystine in exchange for glutamate. Intracellularly, cystine is reduced to cysteine, which is used for the synthesis of GSH and detoxification of ROS [67]. In addition to extracellular uptake of cystine, another source of cysteine is the TSP, in which cysteine is synthesised and regulated by cystathionine-B-synthase (CBS) and cystathionine-γ-lyase (CGL) [68]; methionine is used as a sulfur donor and converted into cysteine through homocysteine and cystathionine intermediates (Fig. 1). The TSP thus provides a compensatory source of cysteine when extracellular uptake through xCT system is inhibited [69]. Ferroptosis inducers, that block cystine uptake during glutamate exchange, such as erastin, might be lethal to cancer cells with a defective TSP [70].
Mevalonate pathway
The mevalonate pathway, which regulates the generation of isopentenyl pyrophosphate (IPP), CoQ10, squalene, and cholesterol, impacts ferroptosis in several ways (Fig. 1). IPP modulates selenocysteine (Sec) transfer RNA during the process of GPX maturation [71, 72]. Blocking the rate-limiting enzyme of the mevalonate pathway 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase with statins compromises the efficient translation of GPX4 and consequently sensitises cells to ferroptosis [73]. Squalene has been implicated to act as an anti-ferroptotic agent in a subset of cancer cells. Cells devoid of squalene monooxygenase activity show squalene accumulation and resistance to ferroptotic inducers, which disappears when squalene synthase (SQS) is disrupted [74].
Both 7-dehydrocholesterol (7-DHC) and cholesterol are potential modulators of lipid peroxidation and ferroptosis [75]. RSL3-triggered ferroptosis was shown to accumulate exogenous PUFAs in a cholesterol-independent manner [28]. Further studies are needed to explore the relationship between the mevalonate pathway, cholesterol metabolism and ferroptosis induction.
Ferroptosis in CRC tumorigenesis
CRCs harbour a high frequency of mutations, and tumours between patients are highly heterogenous in immune responses and metabolism [76]. The most common CRC activation pathway is triggered by the inactivation of the tumour suppressor gene adenomatous polyposis coli (APC), which promotes adenoma formation. Subsequent mutations to Kirsten rat sarcoma virus (KRAS) and TP53 promote the formation of carcinomas [77]. Here, we discuss how each of these events might impact ferroptosis sensitivity in CRC.
Mutations in APC, a member of the destruction complex consisting of GSK-3β and Axin, occur in about 80% of all sporadic CRCs [78]. APC is a negative regulator of Wnt/β-catenin signalling, and biallelic loss of APC causes an initiated epithelium that is “over-charged” and progresses to the adenoma stage. Elevated β-catenin levels enhance transcriptional activity of proto-oncogenes such as MYC and cell cycle markers, including cyclin D1 [79, 80]. There is currently a dearth of information on the functional significance of APC loss in ferroptotic cell death. However, pre-treatment of HeLa cells with a GSK-3β inhibitor prevented erastin-induced ferroptosis cell death [81], and it is possible that loss of APC elicits a similar effect. It is also likely that the effect of APC mutations on ferroptosis sensitivity will depend on the overall metabolic changes that accompany such mutations. These are intriguing questions that warrant further laboratory experimentation.
Subsequent activation of oncogenes such as KRAS occurs in about 30–40% of colon adenomas and further enables progression to carcinoma [82]. The KRAS oncogene is one of the most frequent mutations found in CRCs, and tumours harbouring these mutations are also the most difficult to treat. Emerging evidence suggests that mutant KRAS protects cancer cells from ferroptosis through several mechanisms. In CRC, KRAS mutations are associated with increased expression of FSP1 and relative ferroptosis resistance [83]. Notably, FSP1 aids in cellular transformation and tumour initiation, and inhibiting FSP1 sensitised KRAS-mutant cells to ferroptosis. In lung cancer, mutant KRAS was also found to regulate the synthesis of ferroptosis-suppressing monounsaturated fatty acids, establishing a targetable vulnerability in those cells [84]. It has also been suggested that KRAS could regulate glutaminolysis, mitochondrial ROS production, and glucose uptake in CRC [85], and all three of these processes have indirect effects on ferroptosis.
Given an increased role for ferroptosis in anticancer immune responses, there is also a possible connection between pro-inflammatory metabolites, ferroptosis and immune modulation caused by KRAS that leads to immune escape in CRC. KRAS can induce chemokines, cytokines and signalling pathways that promote tumorigenesis in CRC [86]. Knockdown of mutant KRAS in a poorly immunogenic CRC mouse cell line led to an improved immune response and tumour regression [87]. KRAS might also sustain high iron levels for CRC growth by regulation of HIF-2α and Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway signalling, which promotes tumour growth through HIF-2α-mediated iron import via divalent metal transporter 1 (DMT1) [88, 89]. Analysis of data from The Cancer Genome Atlas (TCGA) revealed that KRAS potentiates the expression of iron importers in CRC [88].
As described previously, RSL3 is a potent small-molecule compound that induce ferroptosis. RSL3 has been shown to exert cytotoxicity on RAS-mutant cancer cell lines (such as KRAS-mutant HCT116 cell lines) and relies on the cellular iron pool for this process [90]. These findings explain the rationale for a recent study that explored the therapeutic efficacy of cetuximab in combination with RSL3 in cancer cell lines. Yang and colleagues showed that cetuximab administration to KRAS-mutant cell lines is insufficient to increase lipid peroxides or trigger ferroptosis [91]. However, when cetuximab was used in combination with RSL3, it enhanced the cytotoxic effect of RSL3 by upregulating ROS production and malondialdehyde (MDA), a lipid peroxide adduct [91]. Similarly, cetuximab increased tumour MDA levels in a KRAS-mutant CRC xenograft mouse model, suggesting that this phenomenon is reproducible in vivo [91]. These findings further strengthen the clinical potential of ferroptosis inducers as part of combination therapies to target tumour antioxidant status and treat CRC. Given that metastatic RAS-mutant CRC is typically unresponsive to cetuximab and particularly difficult to treat, novel combination strategies are urgently needed.
Following APC and KRAS mutations, the last step in CRC malignant transformation is often the loss of the tumour suppressor protein function of p53 [92]. To date, p53 has been implicated as both a positive and negative modulator of ferroptosis [93–98]. It was shown that p53 promotes ferroptosis by repressing the expression of SLC7A11 and inhibiting cystine uptake [99]. Strikingly, a mutant form of p53 that retains the ability to repress SLC7A11 expression but is unable to halt cell cycle progression and induce apoptosis, prevents tumour growth comparably to wild-type p53. On the contrary, overexpression of SLC7A11 in cancer cells eliminated p53-mediated tumour suppression. It was further demonstrated that ALOX12 is critical for p53-mediated ferroptosis and inhibition of cancer growth in a CRC cell line (HCT116) and other human cancer cell lines [93]. With this in mind, ALOX12 expression may be regarded as a prognostic signature of ferroptosis; a recent analysis of gene expression data from two publicly available databases (TCGA and NCBI GEO) identified ALOX12 as a prognosis-related gene that correlated ferroptosis with survival in colon cancer patients [100]. Conversely, p53 has also been shown to have opposite effects on ferroptosis. In human CRC cells, p53 constrained erastin-stimulated ferroptosis through the inhibition of dipeptidyl-peptidase-4 (DPP4). Silencing of p53 facilitated plasma membrane-associated DPP4-dependent lipid peroxidation and thus prevented nuclear accumulation of DPP4 (Fig. 1) [94]. Given the context-dependence of ferroptosis regulation, it remains unclear whether p53 always plays a ferroptosis-suppressing role in CRC or if its overall function is dependent on other cellular metabolic and signalling pathways.
The potential connection between ferroptosis and metabolism in CRC
Arachidonic acid metabolism
As mentioned previously, oxidation of PUFAs to PLOOHs can drive ferroptosis. AA is a key PUFA which can be metabolised enzymatically by COXs and LOXs to form eicosanoids (e.g., prostaglandins, thromboxanes, lipoxins and leukotrienes) and non-enzymatically through autooxidation to generate lipid peroxides which activate ferroptosis as described above [57, 101]. This is important to note in CRC, as COX-2 mediated AA metabolism is well-established as a having roles in CRC progression [102]. A recent report showed that the elongation of very long-chain fatty acids-like 5 (ELOVL5), a rate-limiting enzyme involved in de novo PUFA synthesis, drives sensitivity towards ferroptosis-induced cancer cell death [103]. ELOVL5 is upstream of AA and adrenic acid metabolism. Lipid profiling revealed that mesenchymal gastric cancer cells expressing high ELOVL5 levels are able to synthesise PUFAs, indicating a possible explanation for their ferroptosis sensitivity [103]. Additional reports have shown that AA can also directly induce ferroptosis in certain tumour cells [104]. Most notably, new evidence revealed that T cell-derived interferon-γ in combination with AA induces immunogenic tumour ferroptosis, serving as a mode of action for CD8 + T cell (CTL)-mediated tumour killing [105]. This, combined with previous studies that identified roles for ferroptosis in the mechanism of action of both immunotherapy and radiotherapy, suggests that ferroptosis resistance could represent a key mechanism by which CRCs proliferate despite treatment[34, 106]. Despite these findings, it is not completely understood how normal cells and cancer cells differentially regulate the intracellular pools of AA. It is possible that cancer cells might simply take up or synthesise more PUFAs at baseline (likely related to their growth needs and overall changes in lipid metabolism). Alternatively, if there are no differences in the amounts of PUFAs between normal cells and cancer cells, then there might be changes in where the PUFAs are located: e.g., cytosol, lipid droplet storage, membrane conjugated to PLs, etc. It will also be important to further characterise what other signalling molecules work in combination with AA to induce ferroptosis, as AA is insufficient to induce ferroptosis on its own at physiologic concentrations.
Under normal conditions, AA is released from intracellular phospholipids by cytosolic phospholipases (cPLA) such as cPLA2α [107]. Two studies have identified the calcium-independent phospholipase A2 (iPLA2β) as a major ferroptosis suppressor [108, 109]. Although suppressing ferroptosis by increasing AA production might appear paradoxical, iPLA2β likely accomplishes this by reducing the quantity of AA available at critical cellular membranes. Future studies, however, are required to confirm this and determine whether other phospholipases also modify ferroptosis.
A two-fold increased abundance of AA has been shown in patients with ulcerative colitis (UC) compared to healthy mucosa, suggesting a role for AA in CRC development [110]. While this finding might also appear counterintuitive, elevated AA can also promote the synthesis of eicosanoids, such as the pro-inflammatory hydroxyeicosatetraenoic acid (HETE) oxylipids 5-HETE, 11-HETE and 15-HETE, which drive cancer progression and immune evasion (Fig. 2) [102]. It is possible that during tumorigenesis, the synthesis of these mediators counteracts any ferroptosis-promoting actions of AA, whereas once tumorigenesis occurs the metabolic adaptations associated with it heighten cellular vulnerability to ferroptosis. Under certain conditions, AA metabolites might also cooperate with endogenous antioxidants to prevent ferroptosis. The hydrophilic antioxidant N-acetylcysteine (NAC), for instance, synergises with PGE2, a COX-derived prostaglandin, to confer a ferroptosis-resistant state in neuronal cells in a haemorrhagic stroke model [111]. The enhancing effects of exogenous antioxidants on ferroptosis resistance may be due to the increased activity of GPX4 [112]. This would be consistent with previous studies that showed GPX4 activity is required to suppress AA metabolism [113].
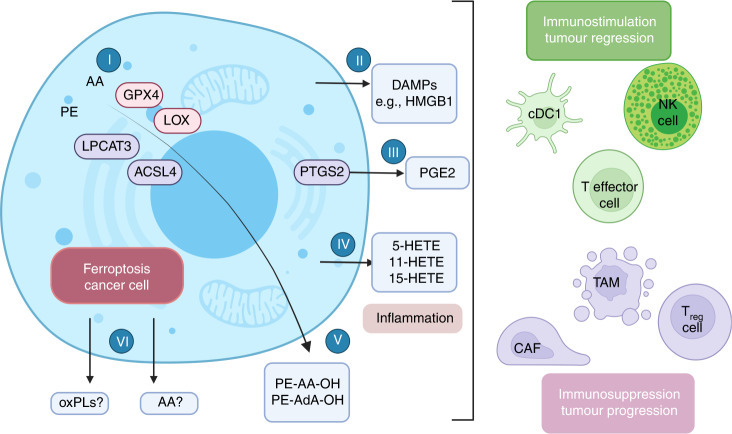
Fig. 2. Modulation of ferroptosis cancer cell immunity.
GPX4, ACSL4, LPCAT3 and LOXs (labelled (I)) mediate sensitivity to ferroptosis. ACSL4 is a key regulator of ferroptosis. It catalyzes the esterification of AA or AdA into PE, whereas LOXs can oxidise PE-AA and PE-AdA to PE-AA-OH and PE-AdA-OH (labelled (V)), further promoting ferroptosis. Ferroptosis can induce the attraction and activation of innate immune cells such as neutrophils, which are effectively engulfed by phagocytic cells. HMGB1 (labelled (II)), a damage-related molecular patterns (DAMP), has been shown to be released in response to ferroptosis [146]. It has been shown that in addition to PGE2 (labelled (III)), siderophiles also release 20-alkane compounds, such as 5-HETE, 12-HETE and 15-HETE (labelled (IV), in response to induction of GPX4 depletion [147]. ACSL4 acyl coenzyme A synthetase long-chain family member 4, AA arachidonic acid, AdA adrenic acid, CAF cancer-associated fibroblasts, CDC1 conventional type 1 dendritic cells, GPX4 glutathione peroxidase 4, HMGB1 high-mobility group box 1, LPCAT3 lysophosphatidylcholine acyltransferase 3, LOX Lipoxygenase, NK natural killer, oxPLs oxidised phospholipids, PE phosphatidylethanolamine, 5-HETE 5-hydroxy-eicosatrienoic acid, 12-HETE 12-hydroxy-eicosatrienoic acid, 15-HETE 15-hydroxy-eicosatrienoic acid, PTGS2 prostaglandin-endoperoxidase synthase 2, PGE2 prostaglandin E2, TAM tumour-associated macrophages, Treg cells regulatory T cell.
Glutaminolysis, glutamine metabolism and ferroptosis
As the most abundant amino acid in the body, glutamine supplies nitrogen for the biosynthesis of other amino acids, lipids, and TCA intermediates; glutaminases (GLS) are rate-limiting for the latter [114]. Under situations of amino acid starvation, supplementation with glutamine induces ferroptosis [115]. The induction of ferroptosis through glutamine relies on glutaminolysis, in which GLS converts glutamine to glutamate. In 2015, Gao et al. showed that GLS2, and not GLS1, is indispensable for ferroptosis even though these two enzymes both hydrolyse glutamine [115]. This discrepancy is explained by the role of increased mitochondrial ROS in ferroptosis [116]. GLS1 is a cytosolic protein, whereas GLS2 localises in the mitochondria, where it contributes to mitochondrial ROS. This is explained by later work from the same group demonstrating the crucial role of mitochondria in cysteine deprivation- and erastin-induced ferroptosis but not RSL3-induced ferroptosis [115]. Cysteine deprivation leads to mitochondrial membrane potential hyperpolarization and lipid peroxide accumulation [117]. The therapeutic importance of this was demonstrated in an ex vivo cardiac model of ischaemia/reperfusion injury-induced ferroptosis, whereby inhibition of GLS improved left ventricular developed pressure (LVDP) and reduced the number of myocardial infarcts compared to hearts treated with vehicle [115].
As discussed above, the role of the mitochondria in ferroptosis may be broadly due to its supply of TCA intermediates from glutamine but also by the tumour suppressor fumarate hydratase/fumarase (FH) [114]; renal cancer cells lacking FH were less sensitive to cysteine-deprived induced ferroptosis. Furthermore, CRISPR-Cas9-mediated knockout of GPX4 promoted ferroptotic cell death in human fibrosarcoma HT1080 cells regardless of mitochondrial depletion, indicating that the role of the mitochondria in ferroptosis is context-dependent [117]. Nonetheless, it is possible that the upstream supply of small molecules by the substrate glutamine is needed for oxidative phosphorylation and ROS production, which may influence ferroptosis.
Hypoxia-inducible factor 1-alpha (HIF-1α)
Hypoxia commonly occurs in solid tumours and regulates metabolism, redox homoeostasis and cell proliferation via the HIF-1α and HIF-2α signalling pathway [118]. Cancer cell resistance to chemotherapy is tightly linked to hypoxia, thus implicating HIF-1/2 as potential targets for therapy. Singhal et al. showed that Cre-Lox mediated activation of HIF-2α contributed to doxorubicin resistance in a manner consistent with the role of HIF signalling [119]. In contrast, chemical induction of HIF-2α sensitises intestinal cancer cells to Erastin-induced ferroptotic death [120]. In addition, the mitochondrial metabolite dimethyl fumarate (DMF) caused cell death in HIF-2α-expressing tumour enteroids and increased the abundance of mitochondrial metabolites after DMF-treatment of colon cancer cell lines. In this context, DMF serves as an analogue of fumarate, a mitochondrial TCA intermediate, thus indicating the association between mitochondrial metabolism and HIF-induced ferroptosis. As reviewed by Li et al., HIF may transcriptionally regulate a subset of genes to limit mitochondrial metabolism and oxygen consumption suggesting that HIF is an important factor for mitochondrial-related ferroptosis [118].
As previously discussed, hypoxia signalling also intersects with iron metabolism, and the specific cellular and contextual contributions of hypoxia-related signalling molecules to ferroptosis regulation require further disentangling.
Ferroptosis in CRC therapy
Regulators that induce ferroptosis can directly or indirectly affect GPX4 activity by modulating various metabolic pathways, resulting in a decrease of antioxidant capacity and accumulation of lipid ROS in cells, ultimately leading to ferroptotic cell death. Moreover, cancer cells have an increased demand of metabolic intermediates which are required for energy generation to maintain their rapid proliferation, which results in ROS production. In line with this, cancer cells respond to high ROS levels by boosting their antioxidant defence mechanism to resist cell death [121]; the successful use of chemotherapeutic agents (including oxaliplatin and 5-fluorouracil) is partly linked to their ROS-generating capacities and depletion of intracellular GSH [122–124]. It has also been shown that iron exposure activates the production of ROS and NRF2, leading to an upregulation of SLC7A11 and GPX4. This counteracts iron-induced lipid peroxidation and rescues CRC cells from ferroptosis [125]. Thus, targeting NRF2 may be a viable strategy for ferroptosis induction in CRC.
In CRC research, studies have identified that targeting ferroptosis could be a promising therapeutic opportunity. This has been shown in vitro with the use of RSL3 which inhibits GPX4 and increases ROS production in CRC cells [126]. To date, GPX4 and SLC7A11 are still the main targets for induction of ferroptosis in CRC (Fig. 1). Several ferroptosis inducers and inhibitors have been tested in various CRC studies, including RSL3, resibufogenin, bromelain, apatinib, Acyl-CoA Dehydrogenase Short/Branched Chain (ACADSB), 2-imino-6-methoxy-2H-chromene-3-carbothioamide (IMCA), and others. Table 2 summarises the results of these studies. RSL3 suppresses CRC by inhibiting GPX4 and generating ROS, thereby reducing cell growth [127]. IMCA decreases the viability of CRC cells in vitro and inhibits tumour growth in vivo. This is accomplished by decreasing expression of SLC7A11 and depleting cysteine and GSH. ACADSB negatively regulated GPX4 expression, and overexpression of ACADSB enhanced Fe2+, superoxide dismutase, and lipid peroxidation in CRC cells, which induces ferroptosis [128]. Apatinib decreased the expression of GPX4 in gastric cancer [129] and promoted ferroptosis in HCT116 cells by upregulating ACSL4 and ECOVL6 expression; this was accompanied by the downregulation of GPX4 and FTH1 expression [130]. Notably, Sorafenib, which was initially considered a ferroptosis inducer, was shown to not induce ferroptosis in a series of tumour cell lines, unlike the xCT inhibitors sulfasalazine and Erastin [131].
Table 2.
Inducers of ferroptosis in CRC.
| Compound | Model | Target | Mechanism | Ref. |
|---|---|---|---|---|
| Talaroconvolutin A | HCT116, SW480, and SW620; xenografts mice | SLC7A11 | Talaroconvolutin A induces ferroptosis by targeting ROS accumulation and SLC7A11 in CRC | [174] |
| Erastin | HT-29 | SLC7A11 | Targeting SLC7A11 specifically suppresses the progression of CRC stem cells via inducing ferroptosis | [175] |
| IMCA | DLD-1, HCT116; xenografts mice | SLC7A11 | IMCA induces ferroptosis mediates by SLC7A11 through the AMPK/mTOR pathway in CRC | [176] |
| Andrographis | HCT116, SW480; xenografts mice | β-catenin/Wnt-signalling pathways | Activation of ferroptosis and suppression of β-catenin/Wnt-signalling pathways were the key mediators for the anticancer and chemosensitizing effects of andrographis | [177] |
| ACADSB | SW620, LoVo | GPX4 | ACADSB induces ferroptosis of CRC cells by negatively regulating GPX4 | [128] |
| Apatinib | HCT116 | ELOVL6/ACSL4 | Apatinib promotes ferroptosis in CRC cells by targeting ELOVL6/ACSL4 | [130] |
| Bromelain | Kras wild-type (Caco2; NCI-H508) or mutant (HCT116, G13D; DLD-1, G12D); KRASG12D mutant heterozygous mice | ACSL4 | Bromelain effectively exerts cytotoxic effects in KRAS-mutant CRC cells by targeting ACSL4 | [135] |
| Iron | HCT116, HT-29; xenografts mice | Warburg effect, NRF2 pathway | Iron promotes Warburg Effect, induce ROS production and activate the NRF2 pathway in CRC | [178] |
| Resibufogenin | HT-29, SW480; xenografts mice | GPX4 | Resibufogenin inhibits CRC cell growth through triggering ferroptosis mediated by GPX4 inactivation | [179] |
| RSL3 | HCT116, LoVo, and HT-29 | GPX4 | RSL3-induced cell death in CRC cells | [126] |
| Vitamin C | DiFi cell; CRC organoids | ROS | Vitamin C regulates iron metabolism, increased ROS production and induce ferroptosis | [180] |
| β-elemene, cetuximab | HCT116, LoVo; xenografts mice | Epithelial–mesenchymal transformation | KRAS-mutant CRC cells are sensitive to the combination treatment of β-elemene and cetuximab by inhibiting EMT and inducing ferroptosis. | |
| Cisplatin | HCT116 | GPX4 | Depletion of reduced glutathione caused by cisplatin and the inactivation of GPX4 plays the vital role in CRC cells to induce ferroptosis | [181] |
IMCA benzopyran derivative 2-imino-6-methoxy-2H-chromene-3-carbothioamide, ACADSB acyl-CoA dehydrogenase, short/branched-chain, EMT epithelial–mesenchymal transformation.
However, a number of studies have used a combination of natural products or FDA-approved drugs to target ferroptosis in CRC. For example, treatment-triggered tumoricidal immunity was explored using a hypoxia-responsive nanoelicitor for promoting lipid peroxidation, which facilitated ferroptosis by relieving a GPX4-mediated brake [62]. Activated tumoricidal immunity was co-stimulated by chlorogenic acid (a polyphenol found in fruits, vegetables, coffee and tea) and mitoxantrone (a chemotherapeutic used to treat prostate cancer and acute nonlymphocytic leukaemia), which simultaneously inhibited the xCT system and GPX4 pathway [62]. This resulted in the accumulation of lipid peroxides, which promoted iron-initiated tumour cell damage [62]. Honokiol (HNK) was recently investigated and shown to induce ferroptosis by regulating GPX4 in a number of colon cancer cell lines [132].
Compounds that target mutant KRAS proteins have not reached the clinic yet for CRC, however, in 2021 the FDA approved a mutant KRAS (G12C) inhibitor for lung cancer [133, 134]. Studies using various drugs to target mutant KRAS in CRC have shown that they also modulate ferroptosis. For example, bromelain, a mixture of proteolytic enzymes derived from pineapple stem, suppressed mutant KRAS by stimulating ferroptosis [135]. Compared to KRAS wild-type CRC cell lines, KRAS-mutant cell lines also exhibited a strong upregulation of ACSL4 when treated with bromelain [135]. Likewise, cetuximab, a monoclonal antibody for epidermal growth factor receptor (EGFR), enhanced the induction of ferroptosis by RSL3 on KRAS-mutant CRC cells by inhibiting the NRF2/HO-1 axis through the activation of p38 MAPK [136]. Further, combination of cetuximab and β-elemene, a bioactive compound isolated from Chinese herb Curcumae Rhizoma, sensitised KRAS-mutant CRC cells by inducing ferroptosis, and was accompanied by ROS accumulation, GSH depletion, lipid peroxidation, and upregulation of HO-1 and transferrin [137]. Curcumin, a yellow pigment found primarily in turmeric, can trigger ferroptosis and suppress CRC proliferation by inhibiting PI3K/AKT/mTOR signalling pathway [138]. Collectively, these studies show that targeting EGFR, PI3K and ACSL4 could induce ferroptosis in KRAS-mutant CRCs. Conversely, Yi et al. revealed that oncogenic activation of the PI3K/AKT/mTOR pathway can confer resistance to ferroptosis [139]. In a panel of human cancer cells, it was shown that the cells which harboured PIK3CA-activating mutations had diminished sensitivity to RSL3-induced ferroptosis, moreover, pharmacological blockade of PI3K and AKT dramatically sensitised cells to ferroptosis and lipid peroxidation [139].
To date, most experiments have been performed in cell lines and xenograft models where the immune system cannot be studied in most cases. Therefore, future studies will indicate whether metabolites associated with ferroptosis (i.e., PE-AA-OH and PE-AdA-OH (labelled (V), Fig. 2) affect anti-tumour immunity. It is conceivable that potential immunomodulatory signals are AA oxidation products and oxPLs (labelled (VI), Fig. 2) that are released from ferroptotic cancer cells, although further studies are needed to confirm this. Recently, a nanovaccine was designed to inhibit tumour growth by inducing specific cytotoxic T lymphocyte (CTL) immune responses against tumour cells and fibroblast activation protein-α (FAP) + cancer-associated fibroblasts (CAFs) to reshape the tumour microenvironment (TME) in a colon cancer model. This nanovaccine could promote tumour ferroptosis by releasing interferon-gamma from CTLs and depleting FAP+CAFs [140]. In addition, expression of SLC7A11, SLC3A2 and GPX4 were decreased compared to controls, suggesting that the nanovaccine may stimulate the antitumor immunity and initiate ferroptosis in tumours [140].
While ferroptosis-targeting therapies are intended to target cancer cells, beneficial cell populations in the TME, such as immune cells, are also vulnerable to ferroptosis. For example, a recent study showed that large amounts of lipid peroxides were detectable in CD8+ T cells derived from tumours but not from lymph nodes, and identified ferroptosis as a metabolic vulnerability of tumour-specific CD8+ T cells. The killing rates and numbers of CD8+ T cells were reduced by GPX4 inhibitors that had no impact on the cancer cell survival, and it is likely that CD36-mediated PUFA uptake drives this sensitivity [141, 142]. In addition to CD8 + T cells, ferroptosis can impact macrophages, dendritic cells, and neutrophils. Inducible nitric oxide synthase (iNOS)/NO•-enrichment of activated M1 (but not alternatively activated M2) macrophages/microglia modulates susceptibility to ferroptosis [143]. Furthermore, ferroptosis of cancer cells impairs dendritic cell maturation and antigen presentation, limiting adaptive antitumor immune responses [144]. Ferroptosis of tumour infiltrating neutrophils has a similar effect, where release of oxygenated lipids impairs the tumoricidal activity of CD8 + T cells [145]. Overall, it is likely that the effects of ferroptosis inducers in the TME will depend on the overall cellular architecture of specific cancer types.
Concluding remarks and future perspectives
Accumulating evidence has demonstrated that ferroptosis could play a role in CRC therapy. Moreover, several molecular targets had been identified in models of CRC that could have potential therapeutic application such as GPX4, SLC7A11, ACSL4 and TP53 (Fig. 1). However, less is known about the role of oncogenes that commonly occur in CRC, and what their differential effects might be on ferroptosis via various signalling mechanisms. In addition, studies in other cancer types have shown that ferroptosis can modulate inflammation and immune responses, which has not yet been approached in CRC.
Our review has raised several key questions for future investigation: (1) What is the effect of genetic mutations (including KRAS) on ferroptosis sensitivity? (2) What influence does the TME have on hypoxia, nutrient availability, and ferroptosis sensitivity? (3) What is the role of AA metabolism in ferroptosis in CRC? (4) Given the context-dependency of ferroptosis regulation, are there ferroptosis induction or suppression mechanisms that are unique to CRC? (5) What is the exact cellular location of lipid peroxidation that contributes to ferroptosis? Is it on the mitochondrial membrane, endoplasmic reticulum, lysosomes or other subcellular locations?, and (6) What regulates the coupling phase of free PUFAs to phospholipids for lethal lipid peroxide formation?
With evidence mounting in the connection between ferroptosis, inflammation and immunity, PGE2, HETEs and GPX4 have received considerable attention. Undeniably, more work is warranted to understand the immunomodulatory role of ferroptotic CRC cells in antitumor immunity. Although substantial progress has been made to elucidate how oncogenic signalling drives CRC proliferation, less is known about how this drives or alters susceptibility to ferroptosis. In addition, the combined usage of novel ferroptosis drugs with other small molecules that regulate cellular and molecular pathways, including lipid peroxidation, mitochondrial respiration and redox status hold promise for future clinical testing of these drugs. Furthermore, continued research is needed to understand how KRAS mutations directly shape the metabolism of CRC and ferroptotic networks. Exploiting these networks, especially in KRAS-mutant context, could be a promising therapeutic strategy for improving treatment regimens.
Author contributions
Literature review and data collection were performed by HY and CJ. The first draft of the review was written by HY. Figures and tables were generated by HY. RT participated in editing the draft and tables. OA participated in editing the draft. MB participated in editing the draft. CJ contributed to the draft editing and language improvement. All authors approved the final manuscript.
Funding
This work was supported by funding from the American Cancer Society Research Scholar Grant 134273-RSG-20-065-01-TBE (CHJ), NIH/NCI under Award Number K12CA215110, and the NIH/NCI Yale SPORE in Skin Cancer under award number 5P50CA121974. RT is supported by NIH/NCI F30CA254246, and OA is supported by the Yale Cancer Center T32CA250803.
Data availability
No data was used for the research described in the article.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi REM, Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg. 2016;68:7–11. doi: 10.1007/s13304-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Bouvard V, Loomis D, Guyton KZ, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16:1599–1600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 4.Johnson CH, Dejea CM, Edler D, Hoang LT, Santidrian AF, Felding BH, et al. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015;21:891–7. doi: 10.1016/j.cmet.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci USA. 2014;111:18321–6. doi: 10.1073/pnas.1406199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parent ME, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012;176:751–9. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 7.Xie Y-H, Chen Y-X, Fang J-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:1–30. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boeckx N, Koukakis R, de Beeck KO, Rolfo C, Van Camp G, Siena S, et al. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: results from two randomized first-line panitumumab studies. Ann Oncol. 2017;28:1862–8. doi: 10.1093/annonc/mdx119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stintzing S, Modest DP, Rossius L, Lerch MM, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17:1426–34. doi: 10.1016/S1470-2045(16)30269-8. [DOI] [PubMed] [Google Scholar]
- 10.Hecht JR, Cohn A, Dakhil S, Saleh M, Piperdi B, Cline-Burkhardt M, et al. SPIRITT: a randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clin Colorectal Cancer. 2015;14:72–80. doi: 10.1016/j.clcc.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon SJ, Stockwell BR. The hallmarks of ferroptosis. Annu Rev Cancer Biol. 2019;3:35–54. doi: 10.1146/annurev-cancerbio-030518-055844. [DOI] [Google Scholar]
- 13.Shi Z, Zhang L, Zheng J, Sun H, Shao C. Ferroptosis: biochemistry and biology in cancers. Front Oncol. 2021;11:579286. [DOI] [PMC free article] [PubMed]
- 14.Yan H-f, Zou T, Tuo Q-z, Xu S, Li H, Belaidi AA, et al. Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther. 2021;6:1–16. doi: 10.1038/s41392-020-00428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sui X, Zhang R, Liu S, Duan T, Zhai L, Zhang M, et al. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front Pharmacol. 2018;9:1371. [DOI] [PMC free article] [PubMed]
- 16.Hu Q, Wei W, Wu D, Huang F, Li M, Li W, et al. Blockade of GCH1/BH4 axis activates ferritinophagy to mitigate the resistance of colorectal cancer to erastin-induced ferroptosis. Front Cell Dev Biol. 2022;10:810327. [DOI] [PMC free article] [PubMed]
- 17.Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21:648–57. doi: 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, An P, Xie E, Wu Q, Fang X, Gao H, et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66:449–65. doi: 10.1002/hep.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon Scott J, Lemberg Kathryn M, Lamprecht Michael R, Skouta R, Zaitsev Eleina M, Gleason, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, et al. Pharmacological inhibition of cystine–glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louandre C, Ezzoukhry Z, Godin C, Barbare J-C, Mazière J-C, Chauffert B, et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133:1732–42. doi: 10.1002/ijc.28159. [DOI] [PubMed] [Google Scholar]
- 22.Panka DJ, Wang W, Atkins MB, Mier JW. The Raf inhibitor BAY 43-9006 (Sorafenib) induces caspase-independent apoptosis in melanoma cells. Cancer Res. 2006;66:1611–9. doi: 10.1158/0008-5472.CAN-05-0808. [DOI] [PubMed] [Google Scholar]
- 23.Yang Wan S, SriRamaratnam R, Welsch Matthew E, Shimada K, Skouta R, Viswanathan Vasanthi S, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. 2015;10:1604–9. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larraufie M-H, Yang WS, Jiang E, Thomas AG, Slusher BS, Stockwell BR. Incorporation of metabolically stable ketones into a small molecule probe to increase potency and water solubility. Bioorg Med Chem Lett. 2015;25:4787–92. doi: 10.1016/j.bmcl.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–84. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaschler MM, Hu F, Feng H, Linkermann A, Min W, Stockwell BR. Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chem Biol. 2018;13:1013–20. doi: 10.1021/acschembio.8b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–92. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, et al. CD8 + T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–4. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai E, Zhang W, Cong D, Kang R, Wang J, Tang D. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem Biophy Res Commun. 2020;523:966–71. doi: 10.1016/j.bbrc.2020.01.066. [DOI] [PubMed] [Google Scholar]
- 36.Feng H, Schorpp K, Jin J, Yozwiak CE, Hoffstrom BG, Decker AM, et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020;30:3411–23.e3417. doi: 10.1016/j.celrep.2020.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–90. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun T, Chi J-T. Regulation of ferroptosis in cancer cells by YAP/TAZ and Hippo pathways: the therapeutic implications. Genes Dis. 2021;8:241–9. doi: 10.1016/j.gendis.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234–45. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li D, Li Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct Target Ther. 2020;5:1–10. doi: 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shintoku R, Takigawa Y, Yamada K, Kubota C, Yoshimoto Y, Takeuchi T, et al. Lipoxygenase‐mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017;108:2187–94. doi: 10.1111/cas.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muhoberac BB, Vidal R. Iron, ferritin, hereditary ferritinopathy, and neurodegeneration. Front Neurosci. 2019;13:1195. [DOI] [PMC free article] [PubMed]
- 43.Fratantonio D, Cimino F, Speciale A, Virgili F. Need (more than) two to Tango: multiple tools to adapt to changes in oxygen availability. Biofactors. 2018;44:207–18. doi: 10.1002/biof.1419. [DOI] [PubMed] [Google Scholar]
- 44.Mole DR. Iron homeostasis and its interaction with prolyl hydroxylases. Antioxid Redox Signal. 2010;12:445–58. doi: 10.1089/ars.2009.2790. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Zhang L, Zhou X. Activation of Nrf2 signaling protects hypoxia‐induced HTR‐8/SVneo cells against ferroptosis. J Obstet Gynaecol Res. 2021;47:3797–806. doi: 10.1111/jog.15009. [DOI] [PubMed] [Google Scholar]
- 46.Liu XJ, Lv YF, Cui WZ, Li Y, Liu Y, Xue YT, et al. Icariin inhibits hypoxia/reoxygenation‐induced ferroptosis of cardiomyocytes via regulation of the Nrf2/HO‐1 signaling pathway. FEBS Open Bio. 2021;11:2966–76. doi: 10.1002/2211-5463.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuhrmann DC, Mondorf A, Beifuß J, Jung M, Brüne B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020;36:101670. doi: 10.1016/j.redox.2020.101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni S, Yuan Y, Qian Z, Zhong Z, Lv T, Kuang Y, et al. Hypoxia inhibits RANKL-induced ferritinophagy and protects osteoclasts from ferroptosis. Free Radic Biol Med. 2021;169:271–82. doi: 10.1016/j.freeradbiomed.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 49.Kawai K, Li Y-S, Song M-F, Kasai H. DNA methylation by dimethyl sulfoxide and methionine sulfoxide triggered by hydroxyl radical and implications for epigenetic modifications. Bioorg Med Chem Lett. 2010;20:260–5. doi: 10.1016/j.bmcl.2009.10.124. [DOI] [PubMed] [Google Scholar]
- 50.Pogribny IP, Tryndyak VP, Pogribna M, Shpyleva S, Surratt G, Gamboa da Costa G, et al. Modulation of intracellular iron metabolism by iron chelation affects chromatin remodeling proteins and corresponding epigenetic modifications in breast cancer cells and increases their sensitivity to chemotherapeutic agents. Int J Oncol. 2013;42:1822–32. doi: 10.3892/ijo.2013.1855. [DOI] [PubMed] [Google Scholar]
- 51.Ye Q, Trivedi M, Zhang Y, Böhlke M, Alsulimani H, Chang J, et al. Brain iron loading impairs DNA methylation and alters GABAergic function in mice. FASEB J. 2019;33:2460–71. doi: 10.1096/fj.201801116RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang L, Wang J, Wang K, Wang H, Wu Q, Yang C, et al. RNF217 regulates iron homeostasis through its E3 ubiquitin ligase activity by modulating ferroportin degradation. Blood. 2021;138:689–705. doi: 10.1182/blood.2020008986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Logie E, Van Puyvelde B, Cuypers B, Schepers A, Berghmans H, Verdonck J, et al. Ferroptosis induction in multiple myeloma cells triggers DNA methylation and histone modification changes associated with cellular senescence. Int J Mol Sci. 2021;22:12234. doi: 10.3390/ijms222212234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.YÜKSEL EA, AYDIN M, TAŞPINAR MS, AĞAR G. Iron toxicity-induced DNA damage, DNA methylation changes, and LTR retrotransposon polymorphisms in Zea mays. Turkish J Bot. 2022;46:197–204. doi: 10.55730/1300-008X.2682. [DOI] [Google Scholar]
- 55.Guiney SJ, Adlard PA, Bush AI, Finkelstein DI, Ayton S. Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem Int. 2017;104:34–48. doi: 10.1016/j.neuint.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Latunde-Dada GO. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochimica et Biophysica Acta (BBA)-Gen Subj. 2017;1861:1893–1900. doi: 10.1016/j.bbagen.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 57.Feng H, Stockwell BR. Unsolved mysteries: how does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16:e2006203. doi: 10.1371/journal.pbio.2006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bayır H, Anthonymuthu TS, Tyurina YY, Patel SJ, Amoscato AA, Lamade AM, et al. Achieving life through death: redox biology of lipid peroxidation in ferroptosis. Cell Chem Biol. 2020;27:387–408. doi: 10.1016/j.chembiol.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah R, Shchepinov MS, Pratt DA. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent Sci. 2018;4:387–96. doi: 10.1021/acscentsci.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, St Croix C, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magtanong L, Mueller GD, Williams KJ, Billmann M, Chan K, Armenta DA, et al. Context-dependent regulation of ferroptosis sensitivity. Cell Chem Biol. 2022;29:1409–18. [DOI] [PMC free article] [PubMed]
- 62.Chen C, Du W, Jing W, Sun P, Shi C, Zhang S, et al. Leveraging tumor cell ferroptosis for colorectal cancer treatment via nanoelicitor-activated tumoricidal immunity. Chem Eng J. 2022;430:132983. doi: 10.1016/j.cej.2021.132983. [DOI] [Google Scholar]
- 63.Lee D, Xu IMJ, Chiu DKC, Leibold J, Tse APW, Bao MHR, et al. Induction of oxidative stress through inhibition of thioredoxin reductase 1 is an effective therapeutic approach for hepatocellular carcinoma. Hepatology. 2019;69:1768–86. doi: 10.1002/hep.30467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lippmann J, Petri K, Fulda S, Liese J. Redox modulation and induction of ferroptosis as a new therapeutic strategy in hepatocellular carcinoma. Transl Oncol. 2020;13:100785. doi: 10.1016/j.tranon.2020.100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee J-Y, Kim WK, Bae K-H, Lee SC, Lee E-W. Lipid metabolism and ferroptosis. Biology. 2021;10:184. doi: 10.3390/biology10030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;25:1–6. [DOI] [PMC free article] [PubMed]
- 67.Bannai S. Transport of cystine and cysteine in mammalian cells. Biochimica et Biophysica Acta (BBA)-Rev Biomembranes. 1984;779:289–306. doi: 10.1016/0304-4157(84)90014-5. [DOI] [PubMed] [Google Scholar]
- 68.Sbodio JI, Snyder SH, Paul BD. Regulators of the transsulfuration pathway. Br J Pharmacol. 2019;176:583–93. doi: 10.1111/bph.14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McBean GJ. The transsulfuration pathway: a source of cysteine for glutathione in astrocytes. Amino Acids. 2012;42:199–205. doi: 10.1007/s00726-011-0864-8. [DOI] [PubMed] [Google Scholar]
- 70.Hayano M, Yang W, Corn C, Pagano N, Stockwell B. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23:270–8. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moosmann B, Behl C. Selenoproteins, cholesterol-lowering drugs, and the consequences revisiting of the mevalonate pathway. Trends Cardiovasc Med. 2004;14:273–81. doi: 10.1016/j.tcm.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 72.Angeli JPF, Conrad M. Selenium and GPX4, a vital symbiosis. Free Radic Biol Med. 2018;127:153–9. doi: 10.1016/j.freeradbiomed.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 73.Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–7. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcia-Bermudez J, Baudrier L, Bayraktar EC, Shen Y, La K, Guarecuco R, et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature. 2019;567:118–22. doi: 10.1038/s41586-019-0945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Angeli JPF, Freitas FP, Nepachalovich P, Puentes L, Zilka O, Inague A, et al. 7-Dehydrocholesterol is an endogenous suppressor of ferroptosis. 2021. [DOI] [PubMed]
- 76.Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–6. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van den Driest L, Johnson CH, Rattray NJW, Rattray Z. Development of an accessible gene expression bioinformatics pipeline to study driver mutations of colorectal cancer. Alternatives Lab Anim. 2022;50:282–92. doi: 10.1177/02611929221107546. [DOI] [PubMed] [Google Scholar]
- 78.Yang J, Zhang W, Evans PM, Chen X, He X, Liu C. Adenomatous polyposis coli (APC) differentially regulates beta-catenin phosphorylation and ubiquitination in colon cancer cells. J Biol Chem. 2006;281:17751–7. doi: 10.1074/jbc.M600831200. [DOI] [PubMed] [Google Scholar]
- 79.Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng Y, Sakamoto N, Wu R, Liu JY, Wiese A, Green ME, et al. Tissue-specific effects of reduced beta-catenin expression on adenomatous polyposis coli mutation-instigated tumorigenesis in mouse colon and ovarian epithelium. PLoS Genet. 2015;11:e1005638. doi: 10.1371/journal.pgen.1005638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang L, Ouyang S, Li B, Wu H, Wang F. GSK-3beta manipulates ferroptosis sensitivity by dominating iron homeostasis. Cell Death Discov. 2021;7:334. doi: 10.1038/s41420-021-00726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trobridge P, Knoblaugh S, Washington MK, Munoz NM, Tsuchiya KD, Rojas A, et al. TGF-beta receptor inactivation and mutant Kras induce intestinal neoplasms in mice via a beta-catenin-independent pathway. Gastroenterology. 2009;136:1680–8.e1687. doi: 10.1053/j.gastro.2009.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Müller F, Lim JK, Bebber CM, Seidel E, Tishina S, Dahlhaus A, et al. Elevated FSP1 protects KRAS-mutated cells from ferroptosis during tumor initiation. Cell Death Differ. 2022;1–15. [DOI] [PMC free article] [PubMed]
- 84.Bartolacci C, Andreani C, Vale G, Berto S, Melegari M, Crouch AC, et al. Targeting de novo lipogenesis and the Lands cycle induces ferroptosis in KRAS-mutant lung cancer. Nat Commun. 2022;13:1–19. doi: 10.1038/s41467-022-31963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wong CC, Xu J, Bian X, Wu J-L, Kang W, Qian Y, et al. In colorectal cancer cells with mutant KRAS, SLC25A22-mediated glutaminolysis reduces DNA demethylation to increase WNT signaling, stemness, and drug resistance. Gastroenterology. 2020;159:2163–80.e2166. doi: 10.1053/j.gastro.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 86.West NR, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15:615–29. doi: 10.1038/nri3896. [DOI] [PubMed] [Google Scholar]
- 87.Smakman N, Veenendaal LM, Van Diest P, Bos R, Offringa R, Rinkes IHB, et al. Dual effect of Kras D12 knockdown on tumorigenesis: increased immune-mediated tumor clearance and abrogation of tumor malignancy. Oncogene. 2005;24:8338–42. doi: 10.1038/sj.onc.1208995. [DOI] [PubMed] [Google Scholar]
- 88.Xue X, Ramakrishnan SK, Weisz K, Triner D, Xie L, Attili D, et al. Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 2016;24:447–61. doi: 10.1016/j.cmet.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xue X, Taylor M, Anderson E, Hao C, Qu A, Greenson JK, et al. Hypoxia-inducible factor-2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 2012;72:2285–93. doi: 10.1158/0008-5472.CAN-11-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234–45. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang J, Mo J, Dai J, Ye C, Cen W, Zheng X, et al. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021;12:1079. doi: 10.1038/s41419-021-04367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol: Mech. Dis. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 93.Chu B, Kon N, Chen D, Li T, Liu T, Jiang L, et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21:579–91. doi: 10.1038/s41556-019-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20:1692–704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 95.Jennis M, Kung C-P, Basu S, Budina-Kolomets A, Julia I, Leu J, et al. An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 2016;30:918–30. doi: 10.1101/gad.275891.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162–8. doi: 10.1016/j.freeradbiomed.2018.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ou Y, Wang S-J, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci USA. 2016;113:E6806–E6812. doi: 10.1073/pnas.1607152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuganesan N, Dlamini S, Tillekeratne LV Taylor WR. Tumor suppressor p53 promotes ferroptosis in oxidative stress conditions independent of modulation of ferroptosis by p21, CDKs, RB, and E2F. J Biol Chem. 2021;6:297. [DOI] [PMC free article] [PubMed]
- 99.Jiang L, Kon N, Li T, Wang S-J, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nie J, Shan D, Li S, Zhang S, Zi X, Xing F, et al. A novel ferroptosis related gene signature for prognosis prediction in patients with colon cancer. Front Oncol. 2021;11:1442. doi: 10.3389/fonc.2021.654076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ayala A, Muñoz MF Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014:360438. [DOI] [PMC free article] [PubMed]
- 102.Dong M, Guda K, Nambiar PR, Rezaie A, Belinsky GS, Lambeau G, et al. Inverse association between phospholipase A 2 and COX-2 expression during mouse colon tumorigenesis. Carcinogenesis. 2003;24:307–15. doi: 10.1093/carcin/24.2.307. [DOI] [PubMed] [Google Scholar]
- 103.Lee JY, Nam M, Son HY, Hyun K, Jang SY, Kim JW, et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci USA. 2020;117:32433–42. doi: 10.1073/pnas.2006828117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Polavarapu S, Dwarakanath BS, Das UN. Differential action of polyunsaturated fatty acids and eicosanoids on bleomycin-induced cytotoxicity to neuroblastoma cells and lymphocytes. Arch Med Sci. 2018;14:207–29. doi: 10.5114/aoms.2018.72244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liao P, Wang W, Wang W, Kryczek I, Li X, Bian Y, et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022;40:365–78. e366. doi: 10.1016/j.ccell.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11ferroptosis connects radiotherapy and immunotherapy. Cancer Discov. 2019;9:1673–85. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dong M, Guda K, Nambiar PR, Rezaie A, Belinsky GS, Lambeau G, et al. Inverse association between phospholipase A2 and COX-2 expression during mouse colon tumorigenesis. Carcinogenesis. 2003;24:307–15. doi: 10.1093/carcin/24.2.307. [DOI] [PubMed] [Google Scholar]
- 108.Chen D, Chu B, Yang X, Liu Z, Jin Y, Kon N, et al. iPLA2β-mediated lipid detoxification controls p53-driven ferroptosis independent of GPX4. Nat Commun. 2021;12:1–15. doi: 10.1038/s41467-021-23902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun W-Y, Tyurin VA, Mikulska-Ruminska K, Shrivastava IH, Anthonymuthu TS, Zhai Y-J, et al. Phospholipase iPLA2β averts ferroptosis by eliminating a redox lipid death signal. Nat Chem Biol. 2021;17:465–76. doi: 10.1038/s41589-020-00734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nishida T, Miwa H, Shigematsu A, Yamamoto M, Iida M, Fujishima M. Increased arachidonic acid composition of phospholipids in colonic mucosa from patients with active ulcerative colitis. Gut. 1987;28:1002–7. doi: 10.1136/gut.28.8.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karuppagounder SS, Alin L, Chen Y, Brand D, Bourassa MW, Dietrich K, et al. N-acetylcysteine targets 5 lipoxygenase-derived, toxic lipids and can synergize with prostaglandin E2 to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Ann Neurol. 2018;84:854–72. doi: 10.1002/ana.25356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu Q, Zhang Y, Lou H, Ou Z, Liu J, Duan W, et al. GPX4 and vitamin E cooperatively protect hematopoietic stem and progenitor cells from lipid peroxidation and ferroptosis. Cell Death Dis. 2021;12:706. doi: 10.1038/s41419-021-04008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang H-S, Chen C-J, Suzuki H, Yamamoto S, Chang W-C. Inhibitory effect of phospholipid hydroperoxide glutathione peroxidase on the activity of lipoxygenases and cyclooxygenases☆. Prostaglandins Other Lipid Mediat. 1999;58:65–75. doi: 10.1016/S0090-6980(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 114.Wang H, Liu C, Zhao Y, Gao G. Mitochondria regulation in ferroptosis. Eur J Cell Biol. 2020;99:151058. doi: 10.1016/j.ejcb.2019.151058. [DOI] [PubMed] [Google Scholar]
- 115.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cassago A, Ferreira AP, Ferreira IM, Fornezari C, Gomes ER, Greene KS, et al. Mitochondrial localization and structure-based phosphate activation mechanism of glutaminase C with implications for cancer metabolism. Proc Natl Acad Sci USA. 2012;109:1092–7. doi: 10.1073/pnas.1112495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, et al. Role of mitochondria in ferroptosis. Mol Cell. 2019;73:354–63.e353. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li W, Xiang Z, Xing Y, Li S, Shi S. Mitochondria bridge HIF signaling and ferroptosis blockage in acute kidney injury. Cell Death Dis. 2022;13:308. doi: 10.1038/s41419-022-04770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singhal R, Mitta SR, Das NK, Kerk SA, Sajjakulnukit P, Solanki S, et al. HIF-2alpha activation potentiates oxidative cell death in colorectal cancers by increasing cellular iron. J Clin Invest. 2021;131:e143691. [DOI] [PMC free article] [PubMed]
- 120.Zhou X, Zheng Y, Sun W, Zhang Z, Liu J, Yang W, et al. D-mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a HIF-2α-dependent manner. Cell Prolif. 2021;54:e13134. doi: 10.1111/cpr.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA, et al. Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther. 2021;6:49. doi: 10.1038/s41392-020-00428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang H, Aladelokun O, Ideta T, Giardina C, Ellis LM, Rosenberg DW. Inhibition of PGE2/EP4 receptor signaling enhances oxaliplatin efficacy in resistant colon cancer cells through modulation of oxidative stress. Sci Rep. 2019;9:4954. doi: 10.1038/s41598-019-40848-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoneda J, Nishikawa S, Kurihara S. Oral administration of cystine and theanine attenuates 5-fluorouracil-induced intestinal mucositis and diarrhea by suppressing both glutathione level decrease and ROS production in the small intestine of mucositis mouse model. BMC Cancer. 2021;21:1–12. doi: 10.1186/s12885-021-09057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li D, Song C, Zhang J, Zhao X. ROS and iron homeostasis dependent ferroptosis play a vital role in 5-Fluorouracil induced cardiotoxicity in vitro and in vivo. Toxicology. 2022;468:153113. doi: 10.1016/j.tox.2022.153113. [DOI] [PubMed] [Google Scholar]
- 125.Yuan Y, Ni S, Zhuge A, Li B, Li L. Iron regulates the Warburg effect and ferroptosis in colorectal cancer. Front Oncol. 2021;11:614778. [DOI] [PMC free article] [PubMed]
- 126.Sui X, Zhang R, Liu S, Duan T, Zhai L, Zhang M, et al. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front Pharmacol. 2018;9:1371. [DOI] [PMC free article] [PubMed]
- 127.Sui X, Zhang R, Liu S, Duan T, Zhai L, Zhang M, et al. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front Pharmacol. 2018;9:1371. doi: 10.3389/fphar.2018.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu D, Yang Z, Xia Q, Gao S, Sun S, Luo X, et al. ACADSB regulates ferroptosis and affects the migration, invasion, and proliferation of colorectal cancer cells. Cell Biol Int. 2020;44:2334–43. doi: 10.1002/cbin.11443. [DOI] [PubMed] [Google Scholar]
- 129.Zhao L, Peng Y, He S, Li R, Wang Z, Huang J, et al. Apatinib induced ferroptosis by lipid peroxidation in gastric cancer. Gastric Cancer. 2021;24:642–54. doi: 10.1007/s10120-021-01159-8. [DOI] [PubMed] [Google Scholar]
- 130.Tian X, Li S, Ge G. Apatinib promotes ferroptosis in colorectal cancer cells by targeting ELOVL6/ACSL4 signaling. Cancer Manag Res. 2021;13:1333. doi: 10.2147/CMAR.S274631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zheng J, Sato M, Mishima E, Sato H, Proneth B, Conrad M. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell Death Dis. 2021;12:1–10. doi: 10.1038/s41419-021-03998-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo C, Liu P, Deng G, Han Y, Chen Y, Cai C, et al. Honokiol induces ferroptosis in colon cancer cells by regulating GPX4 activity. Am J Cancer Res. 2021;11:3039. [PMC free article] [PubMed] [Google Scholar]
- 133.Bond MJ, Chu L, Nalawansha DA, Li K, Crews CM. Targeted degradation of oncogenic KRASG12C by VHL-recruiting PROTACs. ACS Cent Sci. 2020;6:1367–75. doi: 10.1021/acscentsci.0c00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383:1207–17. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park S, Oh J, Kim M, Jin E-J. Bromelain effectively suppresses Kras-mutant colorectal cancer by stimulating ferroptosis. Anim Cells Syst. 2018;22:334–40. doi: 10.1080/19768354.2018.1512521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang J, Mo J, Dai J, Ye C, Cen W, Zheng X, et al. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021;12:1–11. doi: 10.1038/s41419-021-04367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen P, Li X, Zhang R, Liu S, Xiang Y, Zhang M, et al. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics. 2020;10:5107. doi: 10.7150/thno.44705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen M, Tan A-h, Li J. Curcumin represses colorectal cancer cell proliferation by triggering ferroptosis via PI3K/Akt/mTOR signaling. Nutr Cancer 2022;1–8. [DOI] [PubMed]
- 139.Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA. 2020;117:31189–97. doi: 10.1073/pnas.2017152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hu S, Ma J, Su C, Chen Y, Shu Y, Qi Z, et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021;135:567–81. doi: 10.1016/j.actbio.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 141.Drijvers JM, Gillis JE, Muijlwijk T, Nguyen TH, Gaudiano EF, Harris IS, et al. Pharmacologic screening identifies metabolic vulnerabilities of CD8+ T cells metabolic screening of CD8+ T cells. Cancer Immunol Res. 2021;9:184–99. doi: 10.1158/2326-6066.CIR-20-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, et al. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001–12.e1005. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kapralov AA, Yang Q, Dar HH, Tyurina YY, Anthonymuthu TS, Kim R, et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol. 2020;16:278–90. doi: 10.1038/s41589-019-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wiernicki B, Maschalidi S, Pinney J, Adjemian S, Vanden Berghe T, Ravichandran KS, et al. Cancer cells dying from ferroptosis impede dendritic cell-mediated anti-tumor immunity. Nat Commun. 2022;13:1–15. doi: 10.1038/s41467-022-31218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim R, Hashimoto A, Markosyan N, Tyurin VA, Tyurina YY, Kar G, et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022;612:338–46. [DOI] [PMC free article] [PubMed]
- 146.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–83. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 147.Angeli JPF, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018;38:1–13. doi: 10.1186/s40880-018-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018;38:12. doi: 10.1186/s40880-018-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee N, Carlisle AE, Peppers A, Park SJ, Doshi MB, Spears ME, et al. xCT-driven expression of GPX4 determines sensitivity of breast cancer cells to ferroptosis inducers. Antioxidants. 2021;10:317. doi: 10.3390/antiox10020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang L, Liu Y, Du T, Yang H, Lei L, Guo M, et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc–. Cell Death Differ. 2020;27:662–75. doi: 10.1038/s41418-019-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang H, Cheng Y, Mao C, Liu S, Xiao D, Huang J, et al. Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol Ther. 2021;29:2185–208. [DOI] [PMC free article] [PubMed]
- 153.Song J, Liu T, Yin Y, Zhao W, Lin Z, Yin Y, et al. The deubiquitinase OTUD1 enhances iron transport and potentiates host antitumor immunity. EMBO Rep. 2021;22:e51162. doi: 10.15252/embr.202051162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zou Y, Palte MJ, Deik AA, Li H, Eaton JK, Wang W, et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10:1–13. [DOI] [PMC free article] [PubMed]
- 155.Eaton JK, Furst L, Ruberto RA, Moosmayer D, Hilpmann A, Ryan MJ, et al. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat Chem Biol. 2020;16:497–506. doi: 10.1038/s41589-020-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 2018;14:507–15. doi: 10.1038/s41589-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284–99. doi: 10.1038/s41418-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–48. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 159.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–8. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 160.Santoro MM. The antioxidant role of non-mitochondrial CoQ10: mystery solved! Cell Metab. 2020;31:13–15. doi: 10.1016/j.cmet.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 161.Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478:838–44. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 162.Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W, et al. Cytochrome P450 oxidoreductase contributes tophospholipid peroxidation in ferroptosis. Nat Chem Biol. 2020;16:302–9. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gammella E, Buratti P, Cairo G, Recalcati S. The transferrin receptor: the cellular iron gate. Metallomics. 2017;9:1367–75. doi: 10.1039/C7MT00143F. [DOI] [PubMed] [Google Scholar]
- 165.Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X, et al. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34:5617–25. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Anderson GJ, Vulpe CD. Mammalian iron transport. Cell Mol Life Sci. 2009;66:3241. doi: 10.1007/s00018-009-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017;551:639–43. doi: 10.1038/nature24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Song X, Xie Y, Kang R, Hou W, Sun X, Epperly MW, et al. FANCD2 protects against bone marrow injury from ferroptosis. Biochem Biophys Res Commun. 2016;480:443–9. doi: 10.1016/j.bbrc.2016.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Galy B, Ferring-Appel D, Becker C, Gretz N, Gröne H-J, Schümann K, et al. Iron regulatory proteins control a mucosal block to intestinal iron absorption. Cell Rep. 2013;3:844–57. doi: 10.1016/j.celrep.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 170.Toyokuni S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 2009;100:9–16. doi: 10.1111/j.1349-7006.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shpyleva SI, Tryndyak VP, Kovalchuk O, Starlard-Davenport A, Chekhun VF, Beland FA, et al. Role of ferritin alterations in human breast cancer cells. Breast Cancer Res Treat. 2011;126:63–71. doi: 10.1007/s10549-010-0849-4. [DOI] [PubMed] [Google Scholar]
- 172.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 173.Zhang K, Wu L, Zhang P, Luo M, Du J, Gao T, et al. miR-9 regulates ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in melanoma. Mol Carcinog. 2018;57:1566–76. doi: 10.1002/mc.22878. [DOI] [PubMed] [Google Scholar]
- 174.Xia Y, Liu S, Li C, Ai Z, Shen W, Ren W, et al. Discovery of a novel ferroptosis inducer-talaroconvolutin A—killing colorectal cancer cells in vitro and in vivo. Cell Death Dis. 2020;11:1–18. doi: 10.1038/s41419-020-03194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xu X, Zhang X, Wei C, Zheng D, Lu X, Yang Y, et al. Targeting SLC7A11 specifically suppresses the progression of colorectal cancer stem cells via inducing ferroptosis. Eur J Pharm Sci. 2020;152:105450. doi: 10.1016/j.ejps.2020.105450. [DOI] [PubMed] [Google Scholar]
- 176.Zhang L, Liu W, Liu F, Wang Q, Song M, Yu Q, et al. IMCA induces ferroptosis mediated by SLC7A11 through the AMPK/mTOR pathway in colorectal cancer. Oxid Med Cell Longev. 2020;2020. [DOI] [PMC free article] [PubMed]
- 177.Sharma P, Shimura T, Banwait JK, Goel A. Andrographis-mediated chemosensitization through activation of ferroptosis and suppression of β-catenin/Wnt-signaling pathways in colorectal cancer. Carcinogenesis. 2020;41:1385–94. doi: 10.1093/carcin/bgaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yuan Y, Ni S, Zhuge A, Li B, Li L. Iron regulates the Warburg effect and ferroptosis in colorectal cancer. Front Oncol. 2021;11:1491. doi: 10.3389/fonc.2021.614778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shen LD, Qi WH, Bai JJ, Zuo CY, Bai DL, Gao WD, et al. Resibufogenin inhibited colorectal cancer cell growth and tumorigenesis through triggering ferroptosis and ROS production mediated by GPX4 inactivation. Anat Rec. 2021;304:313–22. doi: 10.1002/ar.24378. [DOI] [PubMed] [Google Scholar]
- 180.Lorenzato A, Magrì A, Matafora V, Audrito V, Arcella P, Lazzari L, et al. Vitamin C restricts the emergence of acquired resistance to EGFR-targeted therapies in colorectal cancer. Cancers. 2020;12:685. doi: 10.3390/cancers12030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C, et al. Ferroptosis: a novel anti-tumor action for cisplatin. Cancer Res Treat. 2018;50:445. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.