Abstract
The chloroplast ATP synthase produces the ATP needed for photosynthesis and plant growth. The trans-membrane flow of protons through the ATP synthase rotates an oligomeric assembly of c subunits, the c-ring. The ion-to-ATP ratio in rotary F1F0-ATP synthases is defined by the number of c-subunits in the rotor c-ring. Engineering the c-ring stoichiometry is, therefore, a possible route to manipulate ATP synthesis by the ATP synthase and hence photosynthetic efficiency in plants. Here, we describe the construction of a tobacco (Nicotiana tabacum) chloroplast atpH (chloroplastic ATP synthase subunit c gene) mutant in which the c-ring stoichiometry was increased from 14 to 15 c-subunits. Although the abundance of the ATP synthase was decreased to 25% of wild-type (WT) levels, the mutant lines grew as well as WT plants and photosynthetic electron transport remained unaffected. To synthesize the necessary ATP for growth, we found that the contribution of the membrane potential to the proton motive force was enhanced to ensure a higher proton flux via the c15-ring without unwanted low pH-induced feedback inhibition of electron transport. Our work opens avenues to manipulate plant ion-to-ATP ratios with potentially beneficial consequences for photosynthesis.
Transplastomic tobacco plants with the c15-ring in the chloroplast ATP synthase maintain the normal photosynthetic growth by increasing the magnitude of proton motive force.
Introduction
In the light reactions of photosynthesis, electron transport is coupled to proton translocation across the thylakoid membrane. The resulting electrochemical proton gradient (ΔµH+) which is dependent on the membrane potential (Δψ) and proton concentration gradient (ΔpH) gives rise to the proton motive force (pmf) that drives ATP synthesis (Bailleul et al. 2010; Davis et al. 2017). The energy budget in chloroplasts depends on the ratio of protons taken up by the thylakoid membrane to electrons transferred from water to NADP+. For linear electron transport it is generally accepted that the ratio of H+/e− is fixed at a value of 3 (Kobayashi et al. 1995; Sacksteder et al. 2000).
The chloroplast F1F0-ATP synthase is a large ∼550 kDa multisubunit complex which produces ATP using ΔµH+ as the energy source (Abrahams et al. 1994; Boyer 1997; Noji et al. 1997; Hahn et al. 2018). The trans-membrane flow of H+ through the complex drives the rotation of an oligomeric assembly of c-subunits, also called the c-ring, resembling an hourglass-shaped cylinder with a central, lipid filled pore (Meier et al. 2001, 2005). The c-ring stoichiometry in ATP synthases defines the so-called ion-to-ATP ratio, a bioenergetic parameter that defines the number of translocated protons required to produce 1 ATP molecule. The c-ring stoichiometries have been investigated in a variety of bacterial, animal, and photosynthetic organisms, giving a range from c8 in animal mitochondria (Watt et al. 2010) to a c15-ring in the cyanobacterium Spirulina platensis (Pogoryelov et al. 2005) and in one case, in the bacterium Burkholderia pseudomallei, a c17-membered ring (Schulz et al. 2017). The ion-to-ATP ratio hence can vary between 2.7 and 5.3. The c-subunit sequence defines the c-ring stoichiometry so while the stoichiometry is variable between species it is constant within a given species (Meier et al. 2011; Cheuk and Meier 2021).
In plant chloroplasts with a c14-ring (Seelert et al. 2000) the given fixed ratios would mean that 14 H+ are required for one complete rotation of the c-ring and the production of 3 molecules of ATP (ion-to-ATP ratio = 4.6). Given that linear photosynthetic electron transport translocates 12 H+ across the thylakoid membrane per 2 molecules of NADPH produced, and assuming a ratio of H+/e− of 3, this would mean that the ratio of ATP/NADPH is only 1.29 and consequently insufficient to satisfy the ATP demands of the Calvin–Benson cycle (Allen 2002).
Cyclic electron transport (CET) around photosystem I (PSI) recycles electrons from ferredoxin to plastoquinone and generates ΔµH+ without net accumulation of NADPH (Yamori and Shikanai 2016). The additional ATP required by the Calvin–Benson cycle is mainly supplied by CET. In angiosperms, CET consists of 2 pathways (Munekage et al. 2004). The main pathway is sensitive to antimycin A and depends on the PROTON GRADENT REGULATION 5 protein (Tagawa et al. 1963; Munekage et al. 2002; Sugimoto et al. 2013). The minor pathway depends on the chloroplast NADH dehydrogenase-like complex (Burrows et al. 1998; Shikanai et al. 1998; Hashimoto et al. 2003).
Besides contributing to the pmf needed for ATP synthesis, the ΔpH component of pmf downregulates the efficiency of light energy utilization via the acidification of the thylakoid lumen (Shikanai and Yamamoto 2017). Luminal acidification is sensed by PsbS and violaxanthin de-epoxidase and induces the thermal dissipation of excess absorbed light energy from the PSII antennae (Ruban and Wilson 2021). The process is conveniently monitored by analysis of the nonphotochemical quenching (NPQ) of chlorophyll fluorescence. Low luminal pH also downregulates the rate of electron transport through the cytochrome (Cyt) b6f complex to restrict the electron transport toward PSI (Rumberg and Siggel 1969; Tikhonov et al. 1981; Nishio and Whitmarsh 1993; Malone et al. 2021). This regulation is called “photosynthetic control” and is essential for the photoprotection of PSI especially under conditions of fluctuating light intensity (Suorsa et al. 2012; Yamamoto and Shikanai 2019).
Since ΔµH+ contributes to both ATP synthesis and the downregulation of electron transport, its size should be optimized in response to fluctuations in light intensity. The tradeoff between ATP synthesis and the downregulation of electron transport is optimized by altering the ratio of ΔpH and Δψ. K+ EXPORTING ANTIPORTER 3 functions to substitute ΔpH by Δψ thereby reducing the low pH-induced inhibition of electron transport at low light, especially after a shift from high light (Armbruster et al. 2014; Wang et al. 2017). Since the ATP synthase is a large consumer of ΔµH+, its activity also substantially affects the extent of downregulation (Kanazawa and Kramer 2002; Rott et al. 2011; Takagi et al. 2017; Galvis et al. 2020). In mutant plants expressing reduced levels of ATP synthase, the ATP synthase activity was unable to relax ΔpH sufficiently and consequently electron transport was arrested because of enhanced photosynthetic control rather than the low Calvin–Benson cycle activity due to reduced ATP levels (Kramer and Evans 2011; Rott et al. 2011).
These findings underline the key importance of the c-ring stoichiometry for bioenergetics and why it might vary between different species (Davis and Kramer 2020; Cheuk and Meier 2021). For instance, the relatively large c13-ring is critical for growth of the alkaliphile Bacillus pseudofirmus OF4 at pH > 10, as it allows the ATP synthase to operate at a lower overall pmf, while growth stalled in a mutant with a smaller c12-ring (Preiss et al. 2013). Along the same lines, but in the opposite direction (c11 to c12), an enlarged c-ring stoichiometry in the ATP synthase of the bacterium Ilyobacter tartaricus led to a situation where the enzyme required more protons per ATP synthesized, but started operating at a lower overall pmf (Pogoryelov et al. 2012). Conversely, reducing the stoichiometry of the c-ring could improve the efficiency of ATP synthesis if a suitable high pmf is maintained. However, predicting the effects of re-engineering the size of the c-ring in chloroplasts is complicated by the important role luminal acidification plays in downregulating electron transport and in photoprotection (Davis and Kramer 2020).
The structure of the c-ring shows a series of conserved glycine repeats (the GxGxGxG motif) located in the N-terminal α-helix of the c-subunit hairpin. This motif has been shown to establish a very tight α-helical packing within a given c-ring (Vonck et al. 2002). The previous work showing how the c-ring stoichiometry in bacteria can be modified by introducing mutations into this motif to cause stoichiometry changes (Pogoryelov et al. 2012; Preiss et al. 2013) encouraged us to specifically alter the stoichiometry of the c-ring in the chloroplast ATP synthase and to analyze its impact on the proton budget in photosynthesis.
Results
Engineering tobacco plants with a c15-ring
In tobacco (Nicotiana tabacum), the c-subunit of the ATP synthase is encoded by the plastid atpH gene and is part of the atpI-H-F-A operon (Fig. 1A). To introduce mutations into the atpH gene via chloroplast transformation technology, the aadA cassette conferring spectinomycin resistance was inserted between the atpH and atpF genes. To reduce possible effects on the expression of downstream genes, the psbA terminator was removed from the aadA cassette, as previously reported (Yamamoto et al. 2021). The upstream sequence used for homologous recombination into the plastid genome included mutations in 5 codons so that the gene encoded a protein with the same amino-acid sequence surrounding the glycine-repeat motif as that found in the cyanobacterium S. platensis which is known to have a c15-ring (Pogoryelov et al. 2005) in its ATP synthase (Fig. 1A and Supplemental Fig. S1).
Figure 1.
Construction of c15 tobacco plants by plastid transformation. A) The gene structure of atpI operon in the WT and transplastomic plants. The terminator (T)-less aadA cassette was inserted between atpH and atpF genes for the tobacco-platensis and control lines. The substituted nucleotides and amino acids in the platensis lines are indicated in bold. The MboI-restriction site and Gly-repeat sequences are underlined. For the ΔatpH lines, the coding sequence of atpH was replaced by the aadA cassette. B) RT-PCR analysis of atpH RNA. Total RNA was prepared from leaves and used for the cDNA synthesis. The sequence containing the atpH open reading frame was amplified and the resulting RT-PCR products were digested by MboI(+).
In angiosperms, including tobacco, the sequence surrounding the glycine repeats (underlined) is conserved GLAVGLASIGPGVGQGT (Supplemental Fig. S1). To increase the c-stoichiometry to 15, the sequence was converted to that of S. platensis (aLAVGigSIGPGlGQGq), where the mismatches are indicated in lower case (Pogoryelov et al. 2009). As a control, the aadA cassette was inserted into the same site without modifying the atpH gene. To confirm the impact of knocking out the atpH gene, additional mutants were made in which the entire coding region of the atpH gene was replaced by the aadA cassette (Fig. 1A, ΔatpH).
We also introduced sequences derived from the c-subunit of Synechococcus elongatus SAG89.79 (GLAVGLgSIiPGiGQGs) which is known to harbor a c13-ring (Pogoryelov et al. 2007). However, the tobacco-SAG lines were albino-like the atpH knockout lines (Supplemental Fig. S2A). The c-ring with the SAG mutations was probably unstable in chloroplasts and so these tobacco-SAG lines were not studied further.
In contrast, the plants with the S. platensis sequence (denoted here as tobacco-platensis lines) grew like the wild-type (WT) tobacco plants and control lines on soil (Supplemental Fig. S2B). For further detailed analyses, we focused on 2 independent WT control lines (#9 and #10) containing the aadA cassette and 3 independent lines of tobacco-platensis (#1, #3, and #6).
The sequence surrounding the glycine repeat motif was amplified and the resulting PCR products were sequenced to confirm the mutations (Supplemental Fig. S3). To verify that all copies of the chloroplast genome carried the mutated sequence in the tobacco-platensis lines (and so were homoplasmic), the PCR products were digested by the restriction enzyme MboI, which cleaves the mutated sequence but not the WT sequence (Fig. 1A). In all the tobacco lines, we found that a small proportion of the PCR fragment was resistant to MboI digestion (Supplemental Fig. S4). This is probably because a related DNA sequence has been transferred from the chloroplast genome to the other genomes (Maliga and Nixon 1998). Because promiscuous DNA is unlikely to be transcribed in these genomes, we also amplified the RT-PCR products, which were subjected to MboI digestion. The products were completely digested, indicating that the tobacco-platensis lines were homoplasmic and did not have any functional WT copies of the atpH gene (Fig. 1B).
Reduced accumulation of the ATP synthase in the tobacco-platensis lines
To analyze the impact of the platensis mutations, thylakoid membrane proteins were solubilized in 1% (w/v) n-dodecyl-β-D-maltoside detergent and separated on a large-pore blue-native polyacrylamide gel (Supplemental Fig. S5). The protein profiles were highly similar in the WT, the 2 control lines (#9 and #10) and the 3 tobacco-platensis lines (#1, #3, and #6), indicating that the composition of the thylakoid membrane proteins was not drastically affected in the tobacco-platensis lines.
For a more quantitative analysis, thylakoid proteins were subjected to SDS–PAGE and immunoblotted using specific antibodies (Fig. 2A). The levels of diagnostic subunits of PSI (PsaA), PSII (PsbC), and the Cyt b6f complex (Cyt f) were unaffected in the control or tobacco-platensis lines, although the level of PsaD (PSI) was slightly lower. However, the levels of ATP synthase subunits, AtpH (c), AtpF (b), AtpA (α), and AtpB (β) were decreased to less than 50% of the WT and control plant levels. To quantify the level of ATP synthase more precisely, the immunoblot analysis was repeated using a more extensive dilution series of the WT protein and an antibody specific for AtpB (Fig. 2B). The level of ATP synthase in the tobacco-platensis lines was estimated to be reduced to ∼25% of WT levels.
Figure 2.
Analysis of thylakoid protein. A) Chloroplast membrane protein of tobacco-platensis and control lines was separated by SDS–PAGE, as well as a dilution series of WT protein. The membrane protein (100%) corresponding to 1 µg (for AtpH detection) or 3 µg chlorophyll was loaded per lane. The blots were probed with specific antibodies raised against AtpH (c), AtpF (b), AtpA (α), and AtpB (β), PsbC (PSII), PsaA, and PsaD (PSI), Cyt f (Cyt b6f) and PsbS. B) The tobacco-platensis protein was loaded with a more diluted series of WT protein and probed by the AtpB antibody. This is the representative result of 3 independent experiments.
The c-ring consists of 15 c-subunits in the tobacco-platensis lines
To confirm the number of c-subunits in the c-ring, we attempted to use cryo-electron microscopy. However, it was not feasible to purify a homogeneous population of c-rings from the tobacco-platensis plants. Previous work has shown that c-rings are often resistant to SDS solubilization and can be visualized as complete c-ring complexes by SDS–PAGE (Meier and Dimroth 2002). Consequently, as an alternative approach, we isolated samples of oligomeric c-rings with known stoichiometry from I. tartaricus (c11), Spinacia oleracea (c14), Synechococcus sp. PCC 7002 (c14), and S. platensis (c15) (Meier et al. 2005; Pogoryelov et al. 2007, 2009) and analyzed them by SDS–PAGE alongside c-ring samples isolated from control WT tobacco plants and tobacco-platensis lines (Fig. 3A). To validate and confirm that the isolated proteins are c-rings, rather than an unrelated protein, we treated the samples with trichloroacetic acid (TCA), which dissociates the c-rings into monomeric c subunits (Fig. 3B). We used the known association between electrophoretic mobility and stoichiometries of isolated c-rings (Pogoryelov et al. 2007) to estimate a c-ring stoichiometry of 14 for WT tobacco and 15 for the tobacco-platensis line (Supplemental Fig. S6). In the gel, the c-ring isolated from tobacco-platensis lines (X) migrated slightly faster than the c-ring isolated from S. platensis (c15) (Supplemental Fig. S6A). This can be explained by the difference in the theoretical molecular weight, which is 120.47 in tobacco-platensis lines compared with 122.72 in S. platensis (Supplemental Fig. S6A). It was also observed that in the tobacco-platensis lines the c15-ring complex was less resistant to SDS solubilization, with signs of degradation of the complex (Fig. 3A). This observation would suggest that the non-native, c15-ring does not display the same structural stability as the WT tobacco c-ring and the native, endogenous c-ring complexes studied previously in cyanobacteria (Pogoryelov et al. 2007), consistent with the decrease in ATP synthase levels observed in vivo in the tobacco-platensis lines (Fig. 2).
Figure 3.
SDS–PAGE gel sizing of purified ATP synthase c-rings. A) The c-rings from I.t: Ilyobacter tartaricus c11, S. 7002: Synechococcus sp. PCC 7002 c14, S.o: Spinacia oleracea c14, N.t: Nicotiana tabacum WT c14, N.t-pl: tobacco-platensis c15, S.p: Spirulina platensis c15 were purified and compared on the same gel. Example stoichiometries from c11 to c15 are shown. All c-rings used are highly SDS stable, except tobacco-platensis c15-ring, which shows signs of degradation (e.g. protein bands at and below 25 kDa level). B) TCA-treated samples of each c-ring preparation showing monomeric c subunits only. The TCA-treated samples are in the same order as in A). The gels contained 13.4% (w/w) acrylamide were stained with silver. Molecular weight markers are indicated on both sides of the gels. The c-rings and the monomeric forms of the c subunits are indicated. The signal of TCA-treated samples stained with silver is not quantitative. All gels contained 13.4% polyacrylamide. For details see “Materials and methods.” This is the representative result of at least 10 independent experiments including Supplemental Fig. S6.
The c15-ring does not affect electron transport in the tobacco-platensis lines
To assess the impact of expressing an ATP synthase with a c15-ring on photosynthesis, the photon flux density (PFD)-dependence of chlorophyll fluorescence parameters associated with PSII function and photosynthetic activity were compared between the WT, the WT control, and the tobacco-platensis plants. Consistent with the observed normal growth in soil, the quantum yield of PSII photochemistry, Y(II), was similar in the tobacco-platensis and control lines at all PFDs (Supplemental Fig. S7A). Although the level of NPQ in control line #9 was slightly higher than that in the other lines at low PFD, NPQ was unaffected in the tobacco-platensis lines (Supplemental Fig. S7B). Overall, the decreased level of ATP synthase containing the c15-ring did not have dramatic effects on the chlorophyll fluorescence parameters.
We also analyzed the PFD-dependence of P700 parameters. Y(I) represents the fraction of reduced P700 that is oxidizable by a saturating light pulse and is used to estimate the quantum yield of PSI (Klughammer and Schreiber 1994). Consistent with the normal Y(II) parameter, the level of Y(I) was similar in the tobacco-platensis and control lines at all PFDs (Supplemental Fig. S8A). Y(NA) represents the proportion of reduced P700 that is not oxidized by a saturating light pulse and so an indicator of the acceptor limitation of PSI (Klughammer and Schreiber 1994). The levels of Y(NA) were slightly higher in the tobacco-platensis lines at low to moderate PFDs (51 to 319 µmol photons m−2 s−1), although the difference was statistically significant only between the control lines and tobacco-platensis lines at 191 µmol photons m−2 s−1 (Supplemental Fig. S8C). Y(ND) represents the proportion of oxidized P700 (P700+) and is used to monitor the operation of photosynthetic control at the Cyt b6f complex (Klughammer and Schreiber 1994). The difference in Y(NA) was accompanied by a slightly lower Y(ND) value in the tobacco-platensis lines than in the control lines (Supplemental Fig. S8B). However, the difference was minor and the PSI parameters in all genotypes were unaffected at higher PFDs. Collectively, these data (Supplemental Figs. S7 and S8) indicated that the decreased level of ATP synthase with the c15-ring did not affect electron transport, in line with the normal growth of the transgenic lines in soil (Supplemental Fig. S2B).
The thylakoid membrane relaxes pmf more rapidly in the tobacco-platensis lines than in WT plants
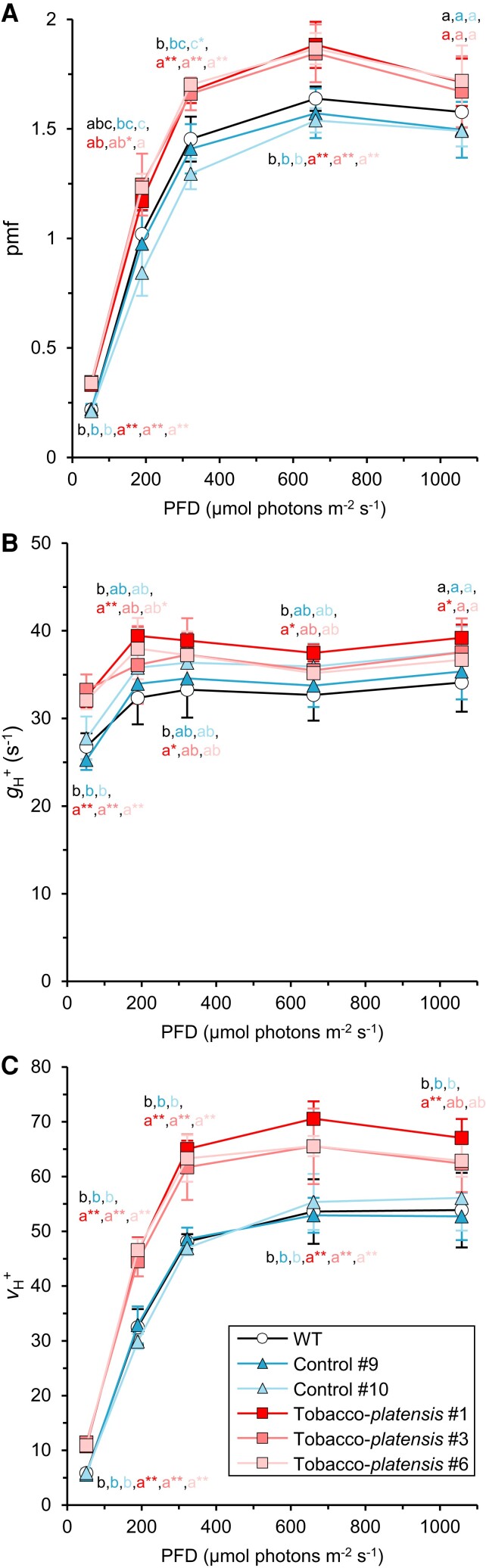
The activity of the chloroplast ATP synthase can be monitored by the fast relaxation kinetics of the electrochromic shift (ECS) after a light-to-dark transition. The ECSt parameter is the light–dark difference of the ECS signal and is used to estimate the size of the pmf formed in the light (Cruz et al. 2001; Klughammer et al. 2013). The ECSt level was standardized against the 515 nm absorbance change induced by a single-turnover flash (ECSST). The size of pmf was larger in the tobacco-platensis lines than in the WT and control lines, although the difference was less significant at 189 µmol photon m−2 s−1 (Fig. 4A). The gH+ parameter represents the proton conductivity of the thylakoid membrane and is mainly affected by the ATP synthase activity (Kanazawa and Kramer 2002). As reported in other works (Kanazawa and Kramer 2002; Rott et al. 2011; Takagi et al. 2017; Galvis et al. 2020), a drastic decline in ATP synthase activity decreases gH+ and leads to an elevated pmf. This is because the level of ATP synthase limits the rate of proton efflux. However, we found that the level of gH+ was higher in the tobacco-platensis lines than that in the WT and control lines at 52 µmol photons m−2 s−1, despite the reduced levels of ATP synthase (Fig. 4B). This similar tendency was observed at higher PFDs, although the statistical difference was less significant. The magnitude of the pmf was significantly larger in the tobacco-platensis lines than in the WT plants at 52 µmol photons m−2 s−1 and at PFDs greater than 322 µmol photons m−2 s−1 (Fig. 4A). Consequently, the vH+ parameter calculated as pmf × gH+, which represents the proton flux through the ATP synthase (Baker et al. 2007), was elevated over the entire range of PFD examined (Fig. 4C). The ECS signals depend on the content of electrochromically sensitive carotenoid pigments, but we did not observe any alteration in the protein composition of the thylakoid membrane (Fig. 2 and Supplemental Fig. S5). The ECS analysis suggests that the c15-ring did not limit ATP synthesis even when the level was lowered to 25% of the WT level. The higher vH+ suggests that the thylakoid membrane relaxed pmf more rapidly in the tobacco-plantensis lines than in the WT plants. This could be due to a combination of a larger c-ring plus a larger pmf at PFDs greater than 322 µmol photons m−2 s−1 (Fig. 4). Possibly, the reduced level of ATP synthase masked the increase in gH+ by the larger c-ring. However, the rapid rotation of c15-ring is mainly ascribed to a larger pmf rather than a larger gH+ at PFDs greater than 322 µmol photons m−2 s−1 (Fig. 4).
Figure 4.
ECS analysis. A) The total magnitude of pmf was estimated by monitoring the light–dark difference of ECS signals (ECSt). The ECSt level was standardized by the ECS signal by a single-turnover flash (ECSST). B) The gH+ parameter represents the proton conductivity of the thylakoid membrane and was calculated by the rapid decay kinetics of the ECS signal (See Methods). C) The vH+ parameter represents the steady-state proton flux to the stroma and was calculated as pmf × gH+. Data represent means ± SD (n = 4 to 5 biological replicates). Different letters indicate statistically significant differences by the Tukey–Kramer test (P < 0.05). Asterisks indicate a statistically significant difference from WT (*P < 0.05, **P < 0.01), confirmed by the Dunnett test. PFD, photon flux density; pmf, proton motive force; gH+, proton conductivity of the thylakoid membrane; vH+, steady-state rate of proton flux.
Despite the elevated size of the pmf (Fig. 4A), the ΔpH-dependent downregulation of electron transport, measured as NPQ, and Y(ND) (photosynthetic control) were not enhanced in the tobacco-platensis lines (Supplemental Figs. S7B and S8B). We, therefore, analyzed the partitioning of pmf into the proton concentration gradient (ΔpH) and the membrane potential (ΔΨ). The ΔpH component estimated by ECSinv can be distinguished from ΔΨ based on its relatively rapid relaxation in the dark within several seconds (Cruz et al. 2005). The remaining pmf is attributed to ΔΨ (ECSt-inv). Because this fraction is influenced by NPQ-related absorbance changes (Johnson and Ruban 2014; Wilson et al. 2021), we analyzed the levels of xanthophyll carotenoids by monitoring the ratio of absorbance at 450 nm between vioaxanthin and chlorophyll a in dark-adapted leaves (Supplemental Fig. S9) and the abundance of PsbS (Fig. 2A). Consistent with the normal PFD-dependence of NPQ (Supplemental Fig. S8B), their levels were unaffected in the tobacco-platensis lines.
We found that ECSinv (ΔpH) was the main contributor to pmf in the WT and control lines, especially at low PFDs (Fig. 5A), but was significantly reduced in the tobacco-platensis line to less than 85% of the total pmf at 52 µmol photons m−2 s−1 and less than 75% at 322 to 661 µmol photons m−2 s−1. Although the size of the pmf was larger in the tobacco-platensis lines (Fig. 4A), the absolute value of ΔpH was similar to that found in the WT and control plants although the contribution of ΔpH to pmf decreased at PFDs greater than 189 µmol photons m−2 s−1 (Fig. 5, A and B). Unlike Arabidopsis (Arabidopsis thaliana) (Hashimoto et al. 2003; Basso et al. 2022), the contribution of ECSt-inv was low especially at 52 µmol photons m−2 s−1 in the WT tobacco (Fig. 5C). The absolute size of ECSt-inv was higher in the tobacco-platensis lines than in the WT and control lines over the entire range of PFD (Fig. 5C).
Figure 5.
Analysis of pmf components. A) The ratio of ΔpH (ECSinv) per pmf (ECSt). B) The size of ΔpH (ECSinv/ESCST). C) The size of Δψ (ECSt-inv/ECSST). Data represent means ± SD (n = 8 to 9 biological replicates). Different letters indicate statistically significant differences by the Tukey–Kramer test (P < 0.05).
Discussion
We have succeeded in re-engineering the c-ring stoichiometry of the chloroplast ATP synthase from 14 to 15 in tobacco. However, the abundance of the variant ATP synthase was decreased to ∼25% of that in the WT plants because of the platensis mutations created in the c subunit. This means that a careful evaluation of the mutant phenotype requires consideration of both decreased expression of the ATP synthase and the formation of a larger c-ring in the F0-complex of the ATP synthase.
From a structural point of view, the accommodation of a larger (c15) ring within a c14-ring containing ATP synthase seems entirely feasible: On the stromal side of the membrane, the γ- and ε-subunits establish a number of high-affinity interactions with the c-ring, notably a conserved ε-subunit histidine and 2 γ-subunit glutamates with arginine and glutamine residues in the highly conserved loop region (RQPE) of the c-subunit (Pogoryelov et al. 2008). Further, it has been shown that the γ- and ε-subunits are highly flexible in accommodating a c15-ring to an ATP synthase rotor that has a c11-ring in the WT (Pogoryelov et al. 2012). From the side of F0, a-subunit, the motor geometry at the a-subunit half channels and the distances between c-ring ion-binding sites are conserved among a large variety of ATP synthases, with an edge-to-edge distance between access and release channels of ∼6 Å (Kühlbrandt 2019), and an ion-binding site distance of ∼10.6 Å for both the chloroplast c14- and the Spirulina c15-rings (Pogoryelov et al. 2009; Vlasov et al. 2019). However, despite this remarkably high structural conservation, we cannot a priori exclude effects on proton affinity or ion specificity, as the 2 ion-binding pockets differ by an additional glutamine residue seen in the S. platensis c15-ring (Krah et al. 2010). We also do not eliminate the possibility that H+ translocation is partially uncoupled from ATP synthesis in the mutant enzyme. Biochemical analysis of the purified complex is necessary for further discussion.
Previous work has already analyzed the effect of reducing the expression of the tobacco ATP synthase to 25% of WT levels. This was achieved by either mutating the atpB gene promoter (line aadA-atpB) or changing the translational start codon from ATG to TTG (line TTG-atpB) (Rott et al. 2011). Although the growth of the TTG-atpB line was slightly retarded, both lines failed to show a dramatic impact on photosynthetic performance, including proton conductance through the ATP synthase as determined by the decay kinetics of the ECS signal. A decrease in levels of the WT ATP synthase to ∼25% is therefore unlikely to limit photosynthesis.
However, a reduction in the levels of ATP synthase to 10% to 25% in an antisense knock-down line of AtpC and to less than 10% in a GTG-atpB line did retard plant growth especially in the GTG-atpB line (Rott et al. 2011). The level of proton conductance (gH+) was decreased in these lines, indicating that the reduced level of ATP synthase now restricted the relaxation of the pmf. Consequently, the pmf increased in magnitude with the result that the thylakoid lumen became more acidified, leading to enhanced induction of NPQ, greater photosynthetic control, and an overall downregulation of electron transport. This is a typical impact of a decrease in the ATP synthase level below a threshold of about 25% of WT levels as similar phenotypes have also been reported for Arabidopsis mutants (Kanazawa and Kramer 2002; Takagi et al. 2017; Galvis et al. 2020).
In the tobacco-platensis lines, neither light-induced chlorophyll fluorescence nor P700+ absorbance kinetics were affected (Supplemental Figs. S7 and S8), suggesting that the ATP synthase with an engineered c15-ring did not restrict photosynthetic electron transport, despite decreased accumulation to ∼25% of the WT level. Clear differences were, however, observed from the analysis of the ECS. The gH+ parameter represents the proton conductivity of the thylakoid membrane which mainly depends on the activity of the ATP synthase. The level of gH+ was significantly higher in the tobacco-platensis lines than in the WT plants at 52 µmol photons m−2 s−1 (Fig. 4B), which suggests that the ATP synthase with the larger c-ring may start operating at a lower pmf, as observed previously for a variant ATP synthase of I. tartaricus with a c12 rather than a c11-ring (Pogoryelov et al. 2012). However, over the entire range of PFDs examined, the size of the thylakoid pmf was elevated in the tobacco-platensis lines, which would also contribute to a higher proton flux through the ATP synthase as represented by vH+ (Fig. 4A).
Importantly, the enhancement in pmf was due to an increase in ΔΨ (ECSt-int) not ΔpH (Fig. 5). A similar phenotype has been observed in the Arabidopsis best1 (bestrophin-like protein 1) mutant defective in the chloride (Cl−) channel, VCCN1, localized to the thylakoid membrane (Duan et al. 2016). The enhanced contribution of ΔΨ to the pmf in the tobacco-platensis lines might therefore reflect changes in ion permeability of the thylakoid membrane through altered regulation of the activity of channels or transporters. In WT plants, counter-ion movements across the thylakoid in the light, either cations leaking to the stroma or anions to the lumen (Li et al. 2021), transiently decrease ΔΨ to fine-tune the downregulation of electron transport by luminal acidification (Cruz et al. 2001; Davis et al. 2017).
To maintain the size of pmf, it is necessary to upregulate the rate of light-driven translocation of protons into the lumen to balance the higher rate of proton efflux through the ATP synthase. Most probably this is achieved by activation of CET around PSI. Although the yield of PSI monitored by Y(I) was slightly higher in the tobacco-platensis lines at high PFDs than in other genotypes, the difference was not statistically significant (Supplemental Figs. S8). Theoretically, a 7% increase in Y(I) is sufficient to provide the necessary pmf required to drive the c15-ring, which may be too small to be detected by the P700 analysis. Consequently, we cannot yet exclude the possibility that rotation of the c15-ring is driven by enhanced CET.
In summary, we have discovered a surprising flexibility in the synthesis of ATP in the chloroplast. Rotation of the larger c15-ring was mainly energized by a larger pmf. This is unexpected because in principle a larger c-ring stoichiometry would allow the F0 motor to operate at a lower pmf (Pogoryelov et al. 2007). We could not find solid evidence for the activation of CET around PSI to generate the higher pmf. Instead, we found that the larger pmf preferentially depended on an increase in ΔΨ, with the added benefit that the rate of electron transport was not downregulated further by a larger contribution of ΔpH to the pmf. Although the molecular mechanism is unclear, the regulation of ion permeability through the thylakoid membrane likely plays a critical role in the flexibility of the proton budget in chloroplasts. Overall, our results provide routes for specifically engineering the plant chloroplast ATP synthase leading to altered and perhaps beneficial properties that can be exploited in challenging photosynthetic situations.
Materials and methods
Vector construction
The tobacco (N. tabacum) plastid DNA sequence covering the atpI operon (Fig. 1A) was amplified using primers, 5′-TCTTAGTTGGTATTCAAAATATCCGATTC-3′ and 5′-GTATCTGAGCAATTCTTCCCGTTGC-3′ and was cloned into the pCR4Blunt-TOPO (Thermo Fisher Scientific, Burlington, MA). The atpH sequence was replaced by that with the platensis mutations (5′-GCTTCCGTTATTGCTGCTGctTTGGCCGTAGGGaTTGgaTCTATTGGACCCGGAtTaGGTCAAGGGcaaGCaGCGGGTCAAGCTGTAGAG-3′, the mutations in the forward primer are indicated in lower case) using the QuikChange Site-Directed Mutagenesis Kit (Agilent). The SAG mutations (5′-GATTGGCCGTAGGGCTTGgaTCTATTattCCCGGAaTTGGTCAAGGGtCTGCaaCtGGTggtGCTGTAGAGGGTATCGCG-3′) were also introduced with the same method. The terminator-less aadA cassette was amplified by PCR with primers, 5′-cgctttcatccttccTCTAGTTGGATTTGCTCCCCCGCCG-3′ and 5′-cttggtctatgaacGTTATTTGCCAACTACCTTAGTGATC-3′ (lower cases indicate the sequences for the infusion cloning) using the pLD6 vector (GenBank accession No. CS165374) (Yamamoto et al. 2006) as a template. The site for the aadA cassette insertion was created between the atpH and atpF gene (Fig. 1A) by an inverse PCR using primers, 5′-GGAAGGATGAAAGCGAGTCAGTATGC-3′ and 5′-CGTTCATAGACCAAGGGAAACTCTTTTTAG-3′. The terminator-less aadA cassette was inserted to the site using the In-Fusion HD cloning kit (Clontech, CA, USA). The vector with the WT atpH sequence was also used for the insertion of the terminator-less aadA cassette to construct the control vector. To knockout the atpH gene (ΔatpH), the sequence for the atpH gene was removed from the vector by an inverse PCR using primers, 5′-GATAAGTTCCTCGTACCAAAAAAAAG-3′ and 5′-TCTTAGCTTAGAAATATGAAAAATAAATAC-3′. The full length of the aadA cassette was amplified using primers, 5′-tacgaggaacttatcTCTAGTTGGATTTGCTCCCCCGCCG-3′ and 5′-atttctaagctaagaAAGCTTCGAATATAGCTCTTCTTTC-3′ (lower cases indicate the sequences for the infusion cloning) and inserted into the position of the atpH gene.
Plastid transformation
Tobacco plastid transformation was performed by biolistic bombardment as described previously (Svab and Maliga 1993). To obtain homoplasmic plants, several independent transplastomic plants were subjected to 3 rounds of regeneration on the RMOP medium containing 500 μg mL−1 spectinomycin. Homoplastomic plants were used for physiological and biochemical analyses. To confirm the homoplasmy of the tobacco-platensis lines, the region including the mutation sites was amplified using primers, 5′-CTTCCGGCCCCTTGTGACTGTGAATTG-3′ and 5′-GAGTCAGTATGCTAATTCCTCATCCGC-3′ with genomic DNA or using primers, 5′-ATGAATCCACTGATTTCTGCCGCTTCCG-3′ and 5′-CTAAGCTAAGATTAAACAAAAGGATTCGC-3′ with cDNA as templates. The PCR product was digested by MboI. Except for Fig. 3, tobacco plants were cultured in a growth chamber at 250 µmol photons m−2 s−1 (12-h light/12-h dark) at 25°C with 60% humidity.
Analysis of thylakoid proteins
Intact chloroplasts were purified from leaves as previously described (Munekage et al. 2002). The purified chloroplasts were ruptured in 20 mM HEPES-KOH (pH 7.6) containing 5 mM MgCl2 and 2.5 mM EDTA. The insoluble fraction containing thylakoids and envelopes was separated from the soluble fraction by centrifugation for 10 min at 15,000×g. The concentration of chlorophyll was determined as described previously (Porra et al. 1989). To solubilize the c-ring completely, the samples were heated at 65°C for 20 min in the SDS sample buffer (Fig. 2). Proteins separated by 12.5% (w/v) SDS–PAGE were electrotransferred onto polyvinylidene fluoride membranes. The antibodies were added, and the protein–antibody complexes were labeled using an ECL Prime Western blotting detection system (GE Healthcare, MA, USA). The chemiluminescence was detected with a lumino-image analyzer LAS3000 (FUJIFILM, Tokyo, Japan) and analyzed by Multi Gauge Version 3.0 software (FUJIFILM, Tokyo, Japan).
Large-pore blue-native–PAGE analysis
The large-pore blue-native (lpBN)–PAGE analysis was performed as described (Järvi et al. 2011). Solubilized thylakoid membranes with 1% (w/v) n-dodecyl-β-D-maltoside corresponding to 10 μg chlorophyll were subjected to 3.5% to 12% (w/v) lpBN–PAGE. The images of gels were captured with an image scanner, and gels were stained by Coomassie Brilliant Blue.
Isolation of Spinacia oleracea and N. tabacum thylakoid membranes
Thylakoid membranes were prepared from Spinacia oleracea (spinach), WT N. tabacum (tobacco), and tobacco-platensis plants. Spinach leaves were purchased from local supermarkets, stored at 4°C overnight and washed thoroughly before use. Tobacco WT and tobacco-platensis plants were grown from seeds in long-day conditions (16 h light at 22°C, 8 h dark at 20°C) in specialized growth rooms (Fig. 3). Tobacco leaves were collected, then stored at 4°C overnight for thylakoid membrane isolation the following day. Thylakoid membranes containing ATP synthase were isolated from leaves as described (Varco-Mertha et al. 2008) with the following alterations. All steps were performed on ice unless otherwise stated. Approximately 50 g of leaf material was homogenized in 200 mL homogenization buffer (0.4 M sucrose, 100 mM Tricine-NaOH pH 8.0, 2 mM MgCl2) in a 1 L household blender with 10-s low-speed bursts. The homogenate was filtered through 4 layers of Miracloth (Merck Millipore, Burlington, MA) and the resulting filtrate was centrifuged at 15,000 × g for 25 min at 4°C. The supernatant was discarded, and the resulting green pellet resuspended in wash buffer 1 (10 mM Tris–HCl pH 8.0, 0.5 mM MgCl2) and homogenized in a Dounce homogenizer (Pyrex). The homogenate was then centrifuged at 15,000 × g for 25 min at 4°C. The supernatant was discarded, and pellet resuspended in wash buffer 2 (0.4 M sucrose, 10 mM Tris–HCl pH 8.0, 150 mM NaCl, 0.5 mM MgCl2) and homogenized in a Dounce homogenizer. The homogenate was once again centrifuged at 15,000 × g for 25 min at 4°C, supernatant discarded and pellet resuspended in resuspension buffer (0.4 M sucrose, 50 mM Tricine-NaOH pH 8.0, 2 mM MgCl2). The chlorophyll concentration of the sample was determined as described (Wellburn and Lichtenthaler 1984) and the suspension diluted to 5 mg/mL with resuspension buffer before being snap frozen in liquid nitrogen and stored at −80°C.
Isolation of Synechococcus sp. PCC 7002 thylakoid membranes
Cells were grown in 10 L of modified A-D7 media (Włodarczyk et al. 2020) at 30°C, under constant light and bubbling with air. Cells were harvested at OD730 = 0.8 by centrifugation at 3,000 × g for 20 min at 4°C. The cell pellet was resuspended in ∼50 mL of Buffer A [40 mM KH2PO4 (pH 8.0), 100 mM NaCl, 2 mM MgCl2] and lysed with 3 passes through a cell disruptor (Constant Systems) at 27 kpsi at 4°C. The lysate was centrifuged at 16,000 × g for 20 min at 4°C and the pellet was discarded. The supernatant was ultracentrifuged at 200,000 × g for 1 h at 4°C (Ti45 rotor). Thylakoid membranes in the pellet were resuspended in Buffer B [50 mM Tricine-NaOH (pH 8.0), 2 mM MgCl2, 100 mM NaCl] to a final chlorophyll a concentration of 0.5 mg/mL, measured as described (Wellburn and Lichtenthaler 1984).
Preparation of oligomeric c-ring samples
Oligomeric c-rings from cyanobacteria and plants were prepared using slightly modified protocols from Meier et al. (2003) as indicated. Cyanobacterial and chloroplast thylakoid membranes were isolated by solubilization in 1% (w/v) N-lauroylsarcosine and ammonium sulfate precipitation as described in (Pogoryelov et al. 2005). After overnight dialysis against 10 mM Tris–HCl buffer (pH 8.0), the samples were concentrated by ultrafiltration (Amicon Ultra-15, 100k MWCO). For the tobacco-platensis c15 sample, the dialysis stage was performed at 4°C for 16 h to slow degradation. The c-rings from I. tartaricus and S. platensis were purified according to the previously described methods (Meier et al. 2005; Pogoryelov et al. 2005).
Trichloroacetic acid treatment of c-ring samples
Oligomeric c-ring samples, were treated with 5× starting volume of 15% (v/v) TCA acid for 5 min at 4°C. The sample was then spun at 15,000 × g for 5 min and the supernatant removed. The remaining pellet was resuspended in 1× SDS-loading buffer and analyzed by SDS–PAGE alongside untreated c-ring samples.
Other biochemical methods
SDS–PAGE and subsequent silver staining were performed as described (Schägger and Jagow 1987; Nesterenko et al. 1994). Bicinchoninic acid method (Pierce) was used for protein concentration determination with bovine serum albumin as a protein standard.
In vivo analyses of chlorophyll fluorescence and P700+ absorbance change
Chlorophyll fluorescence and P700+ absorption change were simultaneously measured using a DUAL-pulse-amplitude modulation portable chlorophyll fluorometer, DUAL-PAM-100 MODULAR Version equipped with a P700 dual wavelength emitter at 830 and 870 nm (Walz). Plants were adapted to the dark for 20 min and then detached leaves were used for the measurement. Minimal fluorescence in the dark (F0 was excited by a measuring light (620 nm) at a PFD of 0.05 to 0.1 µmol photons m−2 s−1. A saturating pulse of light (SP, 300 ms, 20,000 µmol photons m−2 s−1) was applied to determine the maximal fluorescence in the dark (Fm) and during actinic (AL) illumination (Fm′). The steady-state fluorescence level (Fs) was recorded during AL illumination (635 nm). Y(II) (quantum yield of PSII) and NPQ were calculated as (Fm − Fs)/Fm′ and (Fm − Fm′)/Fm′, respectively. The redox change of P700 was assessed by monitoring the absorbance changes of transmission light at 830 and 875 nm. The maximal level of P700+ (oxidized P700) in the dark (Pm) was determined by the application of an SP in the presence of far-red light (720 nm). The maximal P700+ level during AL illumination (Pm′) was determined by applying an SP. The steady-state P700+ level (P) was recorded just before an SP application. Y(I) was calculated as (Pm − P)/Pm. Y(NA) representing the acceptor-side limitation of PSI was calculated as (Pm − Pm′)/Pm. Y(ND) representing the donor-side limitation of PSI was calculated as P/Pm. Because P700 takes 1 of 3 states, Y(I) + Y(NA) + Y(ND) = 1 (Klughammer and Schreiber 1994).
ECS measurements
The ECS measurements were carried out using a Dual-PAM-100 equipped with a P515/535 module (Walz). Plants were adapted to the dark for 20 min and then detached leaves were analyzed. The ECS signal was obtained after 3 min of illumination at different AL intensities, and then AL was turned off for 1 min to record ECSt and to chase the decay curve in the dark. ECSt represents the size of the light-induced pmf and was estimated from the total amplitude of the rapid decay of the ECS signal in the dark. ECSt levels were standardized against a 515-nm absorbance change induced by a single-turnover flash (ECSST), as measured in dark-adapted leaves before analysis. gH+ was estimated by fitting the first 300 ms of the decay curve with a first-order exponential decay kinetic as the inverse of the decay time constant, and νH+ was calculated as pmf × gH+, as described (Avenson et al. 2005). The relative partitioning of pmf into ΔpH (ECSinv) and Δψ (ECSt-inv) was analyzed as previously described (Cruz et al. 2005).
Carotenoid analysis
Tobacco plants (3 WT plants and a plant of tobacco-planteasis lines, #1, #3, and #6) were dark-adapted for 30 min and their leaves were used for the isolation of thylakoid membranes. The thylakoid samples were diluted to 5 mg/mL chlorophyll before snap freezing and storage. Acetone (300 µL, 80%) was added to the 20 µL of samples for the pigment extraction. After filtration the samples were applied to HPLC, as described (Zab and Hussain 2020). Six peaks were detected by monitoring the absorbance change at 450 nm. The violaxanthin content was calculated as a ratio to chlorophyll a by comparing the absorbance at 450 nm.
Statistical analyses
Tukey–Kramer test and the Dunnett's test were performed for the statistical analyses.
Accession numbers
Sequence data from this article can be found in the data libraries under accession numbers gsl2909 (Gloeobacter violaceus PCC 7421, Cyanobase), D5A0Q7 (S. platensis, UniProtKB), (NP_054483.1 (N. tabacum, NCBI), YP_358659 (N. sylvestris, NCBI), NP_054919 (S. oleracea, NCBI), NP_051046 (A. thaliana, NCBI), YP_003587563 (Pisum sativum, NCBI), NP_043020 (Zea mays, NCBI), WP_010871366.1 (Synechocystis sp. PCC 6803, NCBI), Q31RF5 (S. elongatus sp. PCC 7942, UniProtKB), P12409 (Anabaena sp. PCC7120, UniProtKB), and Q05366 (Synechococcus sp. PCC 6716, UniProtKB).
Supplementary Material
Contributor Information
Hiroshi Yamamoto, Department of Botany, Graduate School of Science, Kyoto University, Kyoto 606-8502, Japan.
Anthony Cheuk, Department of Life Sciences, Sir Ernst Chain Building-Wolfson Laboratories, Imperial College London, S. Kensington Campus, London SW7 2AZ, UK.
Julia Shearman, Department of Life Sciences, Sir Ernst Chain Building-Wolfson Laboratories, Imperial College London, S. Kensington Campus, London SW7 2AZ, UK.
Peter J Nixon, Department of Life Sciences, Sir Ernst Chain Building-Wolfson Laboratories, Imperial College London, S. Kensington Campus, London SW7 2AZ, UK.
Thomas Meier, Department of Life Sciences, Sir Ernst Chain Building-Wolfson Laboratories, Imperial College London, S. Kensington Campus, London SW7 2AZ, UK.
Toshiharu Shikanai, Department of Botany, Graduate School of Science, Kyoto University, Kyoto 606-8502, Japan.
Author contributions
H.Y. and T.S. designed the research; H.Y. produced and analyzed transgenic plants; A.C. and J.S. grew sample plants (seeds provided by H.Y. and T.S.) and bacteria control strains, and purified and analyzed the c-rings used in this work. All authors designed the experiments, interpreted results. H.Y., P.J.N., T.M., and T.S. wrote the manuscript.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. An alignment of c subunit sequences.
Supplemental Figure S2. Growth of transgenic plants.
Supplemental Figure S3. Nucleotide sequences for the region including the glycine repeat.
Supplemental Figure S4. Genome analysis of the transgenic lines.
Supplemental Figure S5. Large-pore blue-native gel analysis of thylakoid proteins.
Supplemental Figure S6. Estimation of the molecular mass of the c-ring of tobacco-platensis plants from its relative mobility on SDS–PAGE.
Supplemental Figure S7. Photon flux density (PFD)-dependence of chlorophyll fluorescence parameters.
Supplemental Figure S8. Photon flux density (PFD)-dependence of P700 parameters.
Supplemental Figure S9. HPLC analysis of carotenoids.
Funding
This work was supported by the Japanese Society for the Promotion of Science (16H06555, 19H00992 and 21K19258) to T.S. This work was funded in part by the Wellcome Trust (WT110068/Z/15/Z) to T.M. and the BBSRC Doctoral Training Program (BB/M011178/1).
References
- Abrahams JP, Leslie AGW, Lutter R, Walker JE. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994:370(6491):621–628. 10.1038/370621a0 [DOI] [PubMed] [Google Scholar]
- Allen JF. Photosynthesis of ATP-electrons, proton pumps, rotors, and poise. Cell. 2002:110(3):273–276. 10.1016/S0092-8674(02)00870-X [DOI] [PubMed] [Google Scholar]
- Armbruster U, Carrillo LR, Venema K, Pavlovic L, Schmidtmann E, Kornfeld A, Jahns P, Berry JA, Kramer DM, Jonikas MC. Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat Commun. 2014:5(1):5439. 10.1038/ncomms6439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenson TJ, Cruz JA, Kanazawa A, Kramer DM. Regulating the proton budget of higher plant photosynthesis. Proc Natl Acad Sci USA. 2005:102(27):9709–9713. 10.1073/pnas.0503952102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul B, Cardol P, Breyton C, Finazzi G. Electrochromism: a useful probe to study algal photosynthesis. Photosynth Res. 2010:106(1–2):179–189. 10.1007/s11120-010-9579-z [DOI] [PubMed] [Google Scholar]
- Baker NB, Harbinson J, Kramer DM. Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ. 2007:30(9):1107–1125. 10.1111/j.1365-3040.2007.01680.x [DOI] [PubMed] [Google Scholar]
- Basso L, Sakoda K, Kobayashi R, Yamori W, Shikanai T. Flavodiiron proteins enhance the rate of CO2 assimilation in Arabidopsis under fluctuating light intensity. Plant Physiol. 2022:189(1):375–387. 10.1093/plphys/kiac064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer PD. The ATP synthase—a splendid molecular machine. Annu Rev Biochem. 1997:66(1):717–749. 10.1146/annurev.biochem.66.1.717 [DOI] [PubMed] [Google Scholar]
- Burrows PA, Sazanov LA, Svab Z, Maliga P, Nixon PJ. Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J. 1998:17(4):868–876. 10.1093/emboj/17.4.868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuk A, Meier T. Rotor subunits adaptations in ATP synthases from photosynthetic organisms. Biochem Soc Trans. 2021:49(2):541–550. 10.1042/BST20190936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JA, Kanazawa A, Treff N, Kramer DM. Storage of light-driven transthylakoid proton motive force as an electric field (Δψ) under steady-state conditions in intact cells of Chlamydomonas reinhardtii. Photosynth Res. 2005:85(2):221–233. 10.1007/s11120-005-4731-x [DOI] [PubMed] [Google Scholar]
- Cruz JA, Sacksteder CA, Kanazawa A, Kramer DM. Contribution of electric field (Δψ) to steady-state transthylakoid proton motive force (pmf) in vitro and in vivo. Control of pmf parsing into Δψ and ΔpH by ionic strength. Biochemistry. 2001:40(5):1226–1237. 10.1021/bi0018741 [DOI] [PubMed] [Google Scholar]
- Davis GA, Kramer DM. Optimization of ATP synthase c-rings for oxygenic photosynthesis. Front Plant Sci. 2020:10:1778. 10.3389/fpls.2019.01778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GA, Rutherford AW, Kramer DM. Hacking the thylakoid proton motive force for improved photosynthesis: modulating ion flux rates that control proton motive force partitioning into Δψ and ΔpH. Philos Trans R Soc Lond B Biol Sci. 2017:372(1730):20160381. 10.1098/rstb.2016.0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z, Kong F, Zhang L, Li W, Zhang J, Peng L. A bestrophin-like protein modulates the proton motive force across the thylakoid membrane in Arabidopsis. J Integr Plant Biol. 2016:58(10):848–858. 10.1111/jipb.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvis VC, Strand DD, Messer M, Thiele W, Bethmann S, Hübner D, Uflewski M, Kaiser E, Siemiatkowska B, Morris BA, et al. H+ transport by K+ EXCHANGE ANTIPORTER3 promotes photosynthesis and growth in chloroplast ATP synthase mutants. Plant Physiol. 2020:182(4):2126–2142. 10.1104/pp.19.01561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Vonck J, Mills DJ, Meier T, Kühlbrandt W. Structure, mechanism, and regulation of the chloroplast ATP synthase. Science. 2018:360(6389):eaat4318. 10.1126/science.aat4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Endo T, Peltier G, Tasaka M, Shikanai T. A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J. 2003:36(4):541–549. 10.1046/j.1365-313X.2003.01900.x [DOI] [PubMed] [Google Scholar]
- Järvi S, Suorsa M, Paakkarinen V, Aro E-M. Optimized native gel systems for separation of thylakoid protein complexes: novel super- and mega-complexes. Biochem J. 2011:439(2):207–214. 10.1042/BJ20102155 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Ruban AV. Rethinking the existence of a steady-state Δψ component of the proton motive force across plant thylakoid membranes. Photosynth Res. 2014:119(1–2):233–242. 10.1007/s11120-013-9817-2 [DOI] [PubMed] [Google Scholar]
- Kanazawa A, Kramer DM. In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc Natl Acad Sci USA. 2002:99(20):12789–12794. 10.1073/pnas.182427499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U. An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P7001-absorbance changes at 830 nm. Planta. 1994:192(2):261–268. 10.1007/BF01089043 [DOI] [Google Scholar]
- Klughammer C, Siebke K, Schreiber U. Continuous ECS-indicated recording of the proton-motive charge flux in leaves. Photosynth Res. 2013:117(1–3):471–487. 10.1007/s11120-013-9884-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Neimanis S, Heber U. Coupling ratios H+/e=3 versus H+/e=2 in chloroplasts and quantum requirements of net oxygen exchange during the reduction of nitrite, ferricyanide or methylviologen. Plant Cell Physiol. 1995:36:1613–1620. 10.1093/oxfordjournals.pcp.a078928 [DOI] [Google Scholar]
- Krah A, Pogoryelov D, Meier T, Faraldo-Gómez JD. On the structure of the proton-binding site in the Fo rotor of chloroplast ATP synthases. J Mol Biol. 2010:395(1):20–27. 10.1016/j.jmb.2009.10.059 [DOI] [PubMed] [Google Scholar]
- Kramer DM, Evans JR. The importance of energy balance in improving photosynthetic productivity. Plant Physiol. 2011:155(1):70–78. 10.1104/pp.110.166652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühlbrandt W. Structure and mechanisms of F-type ATP synthases. Annu Rev Biochem. 2019:88(1):515–549. 10.1146/annurev-biochem-013118-110903 [DOI] [PubMed] [Google Scholar]
- Li M, Svoboda V, Davis G, Kramer D, Kunz H-H, Kirchhoff H. Impact of ion fluxes across thylakoid membranes on photosynthetic electron transport and photoprotection. Nat Plants. 2021:7(7):979–988. 10.1038/s41477-021-00947-5 [DOI] [PubMed] [Google Scholar]
- Maliga P, Nixon P. Judging the homoplastomic state of plastid transformants. Trends Plant Sci. 1998:3(10):367–377. 10.1016/S1360-1385(98)01314-4 [DOI] [Google Scholar]
- Malone LA, Proctor MS, Hitchcock A, Hunter CN, Johnson MP. Cytochrome b6f—orchestrator of photosynthetic electron transfer. Biochim Biophys Acta – Bioenerg. 2021:1862(5):148380. 10.1016/j.bbabio.2021.148380 [DOI] [PubMed] [Google Scholar]
- Meier T, Dimroth P. Intersubunit bridging by Na+ ions as a rationale for the unusual stability of the c-rings of Na+-translocating F1F0 ATP synthases. EMBO Rep. 2002:3(11):1094–1098. 10.1093/embo-reports/kvf216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier T, Faraldo-Gómez JD, Börsch M. “ATP synthase: a paradigmatic molecular machine” In: Frank J, editor. Molecular machines in biology. Cambridge University Press; 2011 [Google Scholar]
- Meier T, Matthey U, Henzen F, Dimroth P, Müller DJ. The central plug in the reconstituted undecameric c cylinder of a bacterial ATP synthase consists of phospholipids. FEBS Lett. 2001:505(3):353–356. 10.1016/S0014-5793(01)02837-X [DOI] [PubMed] [Google Scholar]
- Meier T, Matthey U, von Ballmoos C, Vonck J, von Nidda TK, Kühlbrandt W, Dimroth P. Evidence for structural integrity in the undecameric c-rings isolated from sodium ATP synthases. J Mol Biol. 2003:325(2):389–397. 10.1016/S0022-2836(02)01204-4 [DOI] [PubMed] [Google Scholar]
- Meier T, Polzer P, Diederichs K, Welte W, Dimroth P. Structure of the rotor ring of F-type Na+-ATPase from Ilyobacter tartaricus. Science. 2005:308(5722):659–662. 10.1126/science.1111199 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T. Cyclic electron flow around photosystem I is essential for photosynthesis. Nature. 2004:429(6991):579–582. 10.1038/nature02598 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T. PGR5 Is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell. 2002:110(3):361–371. 10.1016/S0092-8674(02)00867-X [DOI] [PubMed] [Google Scholar]
- Nesterenko MV, Nesterenko M, Upton SJ. A simple modification of Blum's Silver stain method allows for 30 minute detection of proteins in polyacrylamide gels. J Biochem Biophys Methods. 1994:28(3):239–242. 10.1016/0165-022X(94)90020-5 [DOI] [PubMed] [Google Scholar]
- Nishio JN, Whitmarsh J. Dissipation of the proton electrochemical potential in intact chloroplasts (II. The pH gradient monitored by cytochrome f reduction kinetics). Plant Physiol. 1993:101(1):89–96. 10.1104/pp.101.1.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji H, Yasuda R, Yoshida M, Kinosita Jr K. Direct observation of the rotation of F1-ATPase. Nature. 1997:386(6622):299–302. 10.1038/386299a0 [DOI] [PubMed] [Google Scholar]
- Pogoryelov D, Klyszejko AL, Krasnoselska GO, Heller EM, Leone V, Langer JD, Vonck J, Müller DJ, Faraldo-Gómez JD, Meier T. Engineering rotor ring stoichiometries in the ATP synthase. Proc Natl Acad Sci USA. 2012:109(25):E1599–E1608. 10.1073/pnas.1120027109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoryelov D, Nikolaev Y, Schlattner U, Pervushin K, Dimroth P, Meier T. Probing the rotor subunit interface of the ATP synthase from Ilyobacter tartaricus. FEBS J. 2008:275(19):4850–4862. 10.1111/j.1742-4658.2008.06623.x [DOI] [PubMed] [Google Scholar]
- Pogoryelov D, Reichen C, Klyszejko AL, Brunisholz R, Muller DJ, Dimroth P, Meier T. The oligomeric state of c rings from cyanobacterial F-ATP synthases varies from 13 to 15. J Bacteriol. 2007:189(16):5895–5902. 10.1128/JB.00581-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoryelov D, Yildiz Ö, Faraldo-Gómez JD, Meier T. High-resolution structure of the rotor ring of a proton-dependent ATP synthase. Nat Struct Mol Biol. 2009:16(10):1068–1073. 10.1038/nsmb.1678 [DOI] [PubMed] [Google Scholar]
- Pogoryelov D, Yu J, Meier T, Vonck J, Dimroth P, Muller DJ. The c15 ring of the Spirulina platensis F-ATP synthase: F1/F0 symmetry mismatch is not obligatory. EMBO Rep. 2005:6(11):1040–1044. 10.1038/sj.embor.7400517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophylls standards by atomic absorption spectroscopy. Biochim Biophys Acta. 1989:975(3):384–394. 10.1016/S0005-2728(89)80347-0 [DOI] [Google Scholar]
- Preiss L, Klyszejko AL, Hicks DB, Liu J, Fackelmayer OJ, Yildiz Ö, Krulwich TA, Meier T. The c-ring stoichiometry of ATP synthase is adapted to cell physiological requirements of alkaliphilic Bacillus pseudofirmus OF4. Proc Natl Acad Sci USA. 2013:110(19):7874–7879. 10.1073/pnas.1303333110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott M, Martins NF, Thiele W, Lein W, Bock R, Kramer DM, Schöttler MA. ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell. 2011:23(1):304–321. 10.1105/tpc.110.079111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Wilson S. The mechanism of non-photochemical quenching in plants: localization and driving forces. Plant Cell Physiol. 2021:62(7):1063–1072. 10.1093/pcp/pcaa155 [DOI] [PubMed] [Google Scholar]
- Rumberg B, Siggel U. pH changes in the inner phase of the thylakoids during photosynthesis. Naturwissenschaften. 1969:56(3):130–132. 10.1007/BF00601025 [DOI] [PubMed] [Google Scholar]
- Sacksteder CA, Kanazawa A, Jacoby ME, Kramer DM. The proton to electron stoichiometry of steady-state photosynthesis in living plants: a proton-pumping Q cycle is continuously engaged. Proc Natl Acad Sci USA. 2000:97(26):14283–14288. 10.1073/pnas.97.26.14283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H, Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987:166(2):368–379. 10.1016/0003-2697(87)90587-2 [DOI] [PubMed] [Google Scholar]
- Schulz S, Wilkes M, Mills DJ, Kühlbrandt W, Meier T. Molecular architecture of the N-type ATPase rotor ring from Burkholderia pseudomallei. EMBO Rep. 2017:18(4):526–535. 10.15252/embr.201643374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelert H, Poetsch A, Dencher NA, Engel A, Stahlberg H, Müller DJ. Structural biology. Proton-powered turbine of a plant motor. Nature. 2000:405(6785):418–419. 10.1038/35013148 [DOI] [PubMed] [Google Scholar]
- Shikanai T, Endo T, Hashimot T, Yamada Y, Asada K, Yokota A. Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proc Natl Acad Sci USA. 1998:95(16): 9705–9709. 10.1073/pnas.95.16.9705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T, Yamamoto H. Contribution of cyclic and pseudo-cyclic electron transport to the formation of proton motive force in chloroplasts. Mol Plant. 2017:10(1):20–29. 10.1016/j.molp.2016.08.004 [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Okegawa Y, Tohri A, Long TA, Sarah FS, Hisabori T, Shikanai T. A single amino acid alteration in PGR5 confers resistance to antimycin A in cyclic electron transport around PSI. Plant Cell Physiol. 2013:54(9):1525–1534. 10.1093/pcp/pct098 [DOI] [PubMed] [Google Scholar]
- Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell. 2012:24(7):2934–2948. 10.1105/tpc.112.097162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z, Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA. 1993:90(3):913–917. 10.1073/pnas.90.3.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa K, Tsujimoto HY, Arnon DI. Role of chloroplast ferredoxin in the energy conversion process of photosynthesis. Proc Natl Acad Sci USA. 1963:49(4):567–572. 10.1073/pnas.49.4.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi D, Amako K, Hashiguchi M, Fukaki H, Ishizaki K, Goh T, Fukao Y, Sano R, Kurata T, Demura T, et al. Chloroplastic ATP synthase builds up a proton motive force preventing production of reactive oxygen species in photosystem I. Plant J. 2017:91(2):306–324. 10.1111/tpj.13566 [DOI] [PubMed] [Google Scholar]
- Tikhonov AN, Khomutov GB, Ruuge EK, Blumenfeld LA. Electron transport control in chloroplasts. Effects of photosynthetic control monitored by the intrathylakoid pH. Biochim Biophys Acta. 1981:637(2):321–333. 10.1016/0005-2728(81)90171-7 [DOI] [Google Scholar]
- Varco-Mertha B, Frommea R, Wang M, Fromme P. Crystallization of the c14-rotor of the chloroplast ATP synthase reveals that it contains pigments. Biochim Biophys Acta–Bioenerg. 2008:1777(7–8):605–612. 10.1016/j.bbabio.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasov AV, Kovalev KV, Marx SH, Round ES, Gushchin IY, Polovinkin VA, Tsoy NM, Okhrimenko IS, Borshchevskiy VI, Büldt GD, et al. Unusual features of the c-ring of F1FO ATP synthases. Sci Rep. 2019:9(1):18547–18547. 10.1038/s41598-019-55092-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonck J, von Nidda TK, Meier T, Matthey U, Mills DJ, Kühlbrandt W, Dimroth P. Molecular architecture of the undecameric rotor of a bacterial Na+-ATP synthase. J Mol Biol. 2002:321(2):307–316. 10.1016/S0022-2836(02)00597-1 [DOI] [PubMed] [Google Scholar]
- Wang C, Yamamoto H, Narumiya F, Munekage YN, Finazzi G, Szabo I, Shikanai T. Fine-tuned regulation of the K+/H+ antiporter KEA3 is required to optimize photosynthesis during induction. Plant J. 2017:89(3):540–553. 10.1111/tpj.13405 [DOI] [PubMed] [Google Scholar]
- Watt IN, Montgomery MG, Runswick MJ, Leslie AGW, Walker JE. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc Natl Acad Sci USA. 2010:107(39):16823–16827. 10.1073/pnas.1011099107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellburn AR, Lichtenthaler H.. Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In: Sybesma C, editor, Advances in Photosynthesis Research, Proceedings of the VIth International Congress on Photosynthesis, Volume 2. Dordrecht (Netherlands: ): Springer; 1984 [Google Scholar]
- Wilson S, Johnson MP, Ruban AV. Proton motive force in plant photosynthesis dominated by ΔpH in both low and high light. Plant Physiol. 2021:187(1):263–275. 10.1093/plphys/kiab270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Włodarczyk A, Selão TT, Norling B, Nixon PJ. Newly discovered Synechococcus sp. PCC 11901 is a robust cyanobacterial strain for high biomass production. Commun Biol. 2020:3(1):215. 10.1038/s42003-020-0910-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kato H, Shinzaki S, Horiguchi S, Shikanai T, Hase T, Endo T, Nishioka M, Makino A, Tomizawa KI, et al. Ferredoxin limits cyclic electron flow around PSI (CEF-PSI) in higher plants—stimulation of CEF-PSI enhances non-photochemical quenching of Chl fluorescence in transplastomic tobacco. Plant Cell Physiol. 2006:47(10):1355–1371. 10.1093/pcp/pcl005 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sato N, Shikanai T. Critical role of NdhA in the incorporation of the peripheral arm into the membrane-embedded part of the chloroplast NADH dehydrogenase-like complex. Plant Cell Physiol. 2021:62(7):1131–1145. 10.1093/pcp/pcaa143 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Shikanai T. PGR5-dependent cyclic electron flow protects PSI under fluctuating light at donor and acceptor sides. Plant Physiol. 2019:179(2):588–600. 10.1104/pp.18.01343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori W, Shikanai T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu Rev Plant Biol. 2016:67(1):81–106. 10.1146/annurev-arplant-043015-112002 [DOI] [PubMed] [Google Scholar]
- Zeb A, Hussain A. Chemo-metric analysis of carotenoids, chlorophylls, and antioxidant activity of Trifolium hybridum. Heliyon. 2020:6:e03195. 10.1016/j.heliyon.2020.e03195 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.