Graphical Abstract

Keywords: Isoquinolines, factor XIIa, anticoagulants, clotting, enzyme inhibitors
1. MAIN COMPOUND CLASSES
2. DESCRIPTION OF THE INVENTION
The molecules of this invention are inhibitors of factor Xlla (FXIIa), and, therefore, possess several potential therapeutic uses in which FXlla is implicated. FXIIa is a serine protease that is formed from its zymogen precursor factor XII (FXII). Single-chain FXII has limited proteolytic activity which is enhanced upon interaction with negative surfaces [1]. Proteolytic cleavage of FXII to FXIIa’s light and heavy chains considerably increases its catalytic activity. FXIIa exists in two forms: αFXIIa with a full heavy chain and βFXIla with a small fragment of the heavy chain.
Structurally, FXIIa is different from many other serine proteases. For example, Tyr99 residue partially blocks the S2 pocket in the active site of FXIIa. Other serine proteases containing this Tyr99 residue, for example, tissue plasminogen activator (tPA), factor Xa (FXa), and factor IXa (FIXa), have an open S2 pocket. Furthermore, FXIIa possesses an incomplete “aromatic box” leading to a more open S4 pocket. Nevertheless, many trypsin-like serine proteases have their S4 pocket lined by an “aromatic box”, which contributes to their inhibitors’ P4-driven selectivity and activity [2–5].
FXII, in addition to high molecular weight kininogen and plasma pre-kallikrein (PK), constitutes the contact system. Several mechanisms activate this system. These include interactions with negatively charged surfaces and molecules, artificial surfaces, unfolded proteins, and foreign tissues (biological transplants such as bacteria, organ/tissue transplants, bio-prosthetic heart valves, and biological surfaces including extracellular matrix and endothelium). Additionally, the system can be activated by plasmin. Activation of the contact system results in the activation of the complement system, the kallikrein-kinin system (KKS), and the intrinsic coagulation pathway. FXIIa converts PK to plasma kallikrein (PKa), which positively feedbacks the activation of FXII to FXIla. In addition, FXIIa has several other direct and indirect substrates, including plasminogen, proteinase-activated receptors (PARs), and neuropeptide Y (NPY), which can contribute to the biological activity of FXIla. Thus, FXIIa inhibition provides therapeutic benefits by treating illnesses about these systems.
PAR2 activation by PKa precipitates neuroinflammation and may lead to neuro-inflammatory disorders such as multiple sclerosis [6]. PKa-mediated activation of PAR1 and PAR2 on vascular smooth muscle cells has also been implicated in atherosclerosis and vascular hypertrophy [7]. FXIIa-mediated activation of plasminogen contributes to fibrinolysis [8]. PKa proteolytically cleaves NPY, thus altering its binding to its receptors [9]. Inhibition of FXIIa could provide clinical benefits in diseases caused by PAR signaling, plasminogen activation, and NPY metabolism. Activation of KKS by FXIIa leads to the production of bradykinin (BK), which can mediate pain, inflammation, angioedema, vasodilation, and vascular hyperpermeability [10, 11].
CSL-312, a FXIIa antibody inhibitory, is currently in clinical trials for the treatment and prevention of normal Cl inhibitor as well as Cl inhibitor deficient hereditary angioedema (HAE), which results in intermittent swelling of face, throat, hands, gastrointestinal tract, and genitals [12]. Mutations in FXII that promote its activation to FXIIa were identified as a cause of HAE [13, 14]. Because FXIIa facilitates the generation of PKa, FXIIa inhibitors could provide protective effects against all forms of BK-mediated angioedema, including HAE and nonhereditary bradykinin-mediated angioedema (BK-nHAE) [15–18]. Specific types of BK-nHAE include nonhereditary angioedema with normal Cl Inhibitor (AE-nCl lnh), which can be drug-induced, hormonal, or environmental.
Environmental factors that cause AE-nCl Inh include air pollution [19] and silver nanoparticles, such as the nanoparticles used as antibacterial components in healthcare, biomedical, and consumer products [20]. Studies suggested a link between the contact system, bradykinin pathways, and BK-nHAE [21–24]. For example, BK-medicated angioedema can be attributed to thrombolytic therapy. Furthermore, angioedema that is induced by t-PA is a life-threatening complication following thrombolytic therapy in acute stroke victims [25–29]. It was also reported that certain drugs could cause angioedema [30, 31]. Hermanrud et al. reported recurrent angioedema associated with dipeptidyl peptidase IV inhibitors and also discussed acquired angioedema induced by angiotensin-converting enzyme inhibitors (ACEI) [32]. Kim et al. [33] reported angiotensin Il receptor blocker (ARB)-related angioedema. Reichman et al. also reported angioedema risk for patients taking ARBs, ACEIs, and β-blockers [34]. Diestro et al. also reported a possible association between certain ARBs and angioedemas [35]. Giard et al. reported that bradykinin-mediated angioedema can be precipitated by estrogen contraception, called “oestrogen associated angioedema” [36].
Activation of KKS by the contact system has also been linked to diabetic retinopathy and retinal edema [37]. FXIIa concentrations are elevated in the vitreous fluid of patients with diabetic macular edema (DME) or advanced diabetic retinopathy [38, 39]. FXIIa appears to be a mediating factor in both vascular endothelial growth factor (VEGF)-independent [40, 41] and -dependent DME [40]. FXII deficiency is protective against VEGF-induced retinal edema in mice. Thus, it has been proposed that FXIIa inhibition can provide some benefits regarding diabetic retinopathy and retinal edema caused by retinal vascular hyperpermeability, including DME, age-related macular degeneration (AMD), and retinal vein occlusion. The contact system can be activated by bacteria, and thus, FXIIa has been implicated in the treatment of bacterial sepsis [42]. Overall, FXIIa inhibitors potentially carry therapeutic benefits in treating bacterial sepsis, sepsis, and disseminated intravascular coagulation (DIC).
Activation of KKS and BK production, which are mediated by FXIIa, has been implicated in neurodegenerative diseases including epilepsy, multiple sclerosis, Alzheimer’s disease, and migraine [43–46]. Therefore, FXIIa inhibitors can provide therapeutic benefits in decreasing the clinical symptoms and the progression of the above neurodegenerative diseases. FXIIa has also been implicated in anaphylaxis; therefore, its inhibitors can be therapeutically beneficial in mitigating the incidence and clinical severity of anaphylactic reactions [47, 48].
Plasma FXIIa’s role in the coagulation process was identified more than 50 years ago [49]. Activation of factor Xl (FXI) by FXIIa stimulates the intrinsic coagulation pathway. Furthermore, FXIIa can promote coagulation in an FXI-independent fashion [50, 51]. Studies on both experimental animal models and humans have exhibited that FXII deficiency prolongs activated partial prothrombin time (APTT) without increasing bleeding [52, 53]. Pharmacological inhibition of FXIIa also prolongs APTT without adversely affecting hemostasis [54]. These data suggest that FXIIa inhibition can prevent and/or treat thrombosis without causing bleeding. FXIIa inhibitors have been proposed to treat several prothrombotic conditions, including deep vein thrombosis, cancer-associated thrombosis, pulmonary embolism, complications caused by artificial heart valves, extracorporeal membrane oxygenation, catheters, left ventricular assisted devices, cardiopulmonary bypass, dialysis, joint arthroplasty, and sickle cell disease as well as tPA-induced thrombosis, Budd-Chari syndrome, and Paget-Schroetter syndrome. FXIIa inhibitors may also be useful for treating or preventing thromboembolism by decreasing the tendency of devices that come in contact with blood to clot. Examples of such devices are stents, in-dwelling and external catheters, vascular grafts, extracorporeal circulation systems, and orthopedic and cardiac prostheses. Preclinical studies showed that FXIIa contributes to stroke and its complications [55–59]. Furthermore, FXII deficiency decreased the formation of atherosclerotic lesions in ApoE−/− mice [60]. As a result, FXIIa inhibition has been proposed to improve clinical neurological outcomes of the treatment of patients with stroke or atherosclerosis.
Several FXIIa inhibitors have been described before [61–66]. Nevertheless, there remains a need to develop new FXIIa inhibitors that will have utility to treat a wide range of disorders. The patent in this highlight claimed 6- (arylaminomethyl) isoquinolines as a new class of FXIIa inhibitors. Fig. (1) provides specific examples.
Fig. (1).
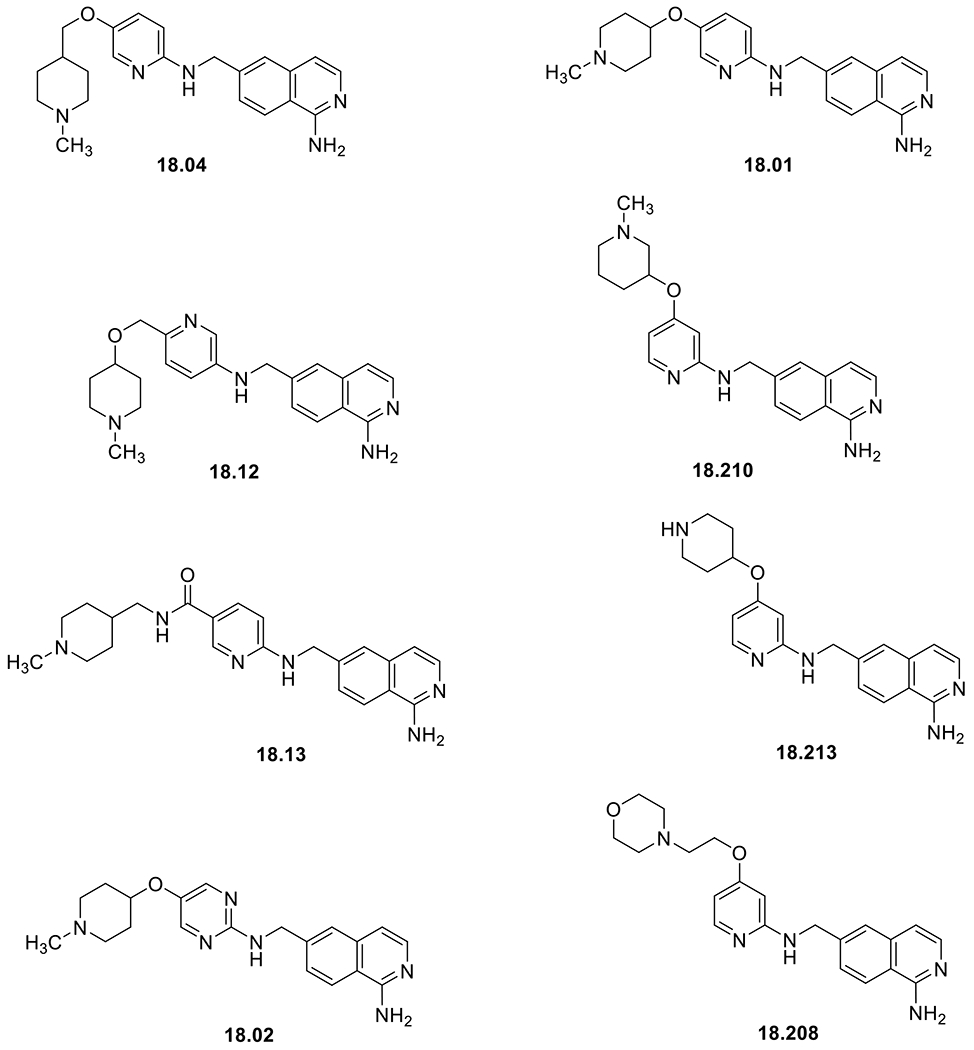
Key derivatives of 6-(Arylaminomethyl) Isoquinolines are claimed as FXIIa inhibitors in this patent.
3. DEFINITIONS
More details are in the patent.
W, X, Y, and Z can be C and N such that the ring containing W, X, Y, and Z can be benzene, pyridine, pyrimidine, pyridazine, triazine, and pyrazine
R1, R4, and R5 can be absent or selected from H, alkoxy, alkyl, -OH, CF3, halo, -CN, -COOR12, and -CONR14R15
when X is C, one of R2 and R3 is -L-V-R13, and the other of R2 and R3 can be selected from H, alkoxy, alkyl, -OH, CF3, halo, -CN, -COOR12, and -CONR14R15
when X is N, R2 is -L-V-R13, and R3 is absent
R6-10 can be selected from H, alkoxy, alkyl, -OH, CF3, halo, -CN, -COOR12, and -CONR14R15
L is selected from a bond, -C(O)-, and alkylene
V is absent or selected from NR12 and O
R12 can be selected from alkyl and H
R13 is (CH2)0-3(heterocyclyl)
alkyl is a linear saturated hydrocarbon having up to 4 carbon atoms (C1-C4) or a branched saturated hydrocarbon of 3 or 4 carbon atoms (C3-C4); alkyl can be substituted with 1 or 2 substituents selected from (C1-C3) alkoxy, -CN, halo, -OH, -NR14R15, -NHCOCH3, -COOR12, and- CONR14R15
alkyl is a linear saturated hydrocarbon having up to 4 carbon atoms (C1-C4) or a branched saturated hydrocarbon of 3 or 4 carbon atoms (C3-C4); alkyl can be substituted with 1 or 2 substituents selected from -CN, -OH, halo, and -NHCOCH3
alkylene is a bivalent linear saturated hydrocarbon having 1 to 4 carbon atoms (C1-C4) or a branched bivalent saturated hydrocarbon having 3 to 4 carbon atoms (C3-C4)
alkoxy is a linear O-Linked hydrocarbon of between 1 and 3 carbon atoms (C1-C3) or a branched O-Linked hydrocarbon of between 3 and 4 carbon atoms (C3-C4); alkoxy may optionally be substituted with 1 or 2 substituents independently selected from -OH, F, -CF3, -CN, and N(R12)2
halo is I, Br, Cl, or F
heterocyclyl is a 4-, 5-, or 6-, membered carbon-containing nonaromatic ring containing one or two ring members that are selected from N, NR16, and O; the heterocyclyl can be substituted with 1, 2, 3, or 4 substituents selected from oxo, alkyl, alkoxy, -OH, -CF3, halo, -CN, -COOR12, and -CONR14R15
R14 and R15 can be selected from alkyl and H
R16 is selected from alkyl and H
4. BIOLOGICAL ASSAY
FXIIa inhibitory activity in vitro was determined using standard published methods [67–69]. Human FXIIa was incubated at 25°C with a fluorogenic substrate and several concentrations of the test molecule. Residual enzyme activity was determined by measuring the change in absorbance at λ410 nm, and the IC50 value for the test molecule was determined. Selectivity against factor XIa was determined similarly.
5. BIOLOGICAL DATA
The potency of the inhibitors against both factor XIIa and factor XIa was provided as IC50 values. Table 1 shows representative examples:
Table 1.
Inhibition profiles of key structures against human FXIIa.
| Inhibitor | FXIIa IC50 (nM) | FXIa IC50 (nM) |
|---|---|---|
| 18.04 | < 1,000 | > 40,000 |
| 18.12 | < 1,000 | 33,1000 |
| 18.01 | 1,000-3,000 | 36,400 |
| 18.210 | 1,000-3,000 | NA |
| 18.13 | 3,000-10,000 | NA |
| 18.213 | 3,000-10,000 | NA |
| 18.02 | 10,000-40,000 | NA |
| 18.208 | 10,000-40,000 | NA |
CONCLUSION
FXIIa is being targeted for developing effective anticoagulants that are not associated with bleeding risk and many other diseases. The preparation of FXIIa inhibitors of 6-(arylaminomethyl) isoquinolines derivatives has been described. Key inhibitors were evaluated for their ability to inhibit FXIIa. The claimed inhibitors’ potential and pharmacokinetics are yet to be determined in appropriate animal models.
ACKNOWLEDGEMENTS
NIGMS of the National Institute of Health under award number to RAAH supports the author.
FUNDING
The author is supported by NIGMS of the National Institute of Health under award number SC3GM131986 to RAAH.
LIST OF ABBREVIATIONS
- ACEI
Angiotensin-Converting Enzyme Inhibitors
- AMD
Age-Related Macular Degeneration
- ARB
Angiotensin Il Receptor Blocker
- BK
Bradykinin
- DIC
Disseminated Intravascular Coagulation
- DME
Diabetic Macular Edema
- HAE
Hereditary Angioedema
- KKS
Kallikrein Kinin System
- NPY
Neuropeptide Y
- PARs
Proteinase-Activated Receptors
- tPA
Tissue Plasminogen Activator
- VEGF
Vascular Endothelial Growth Factor
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
DISCLAIMER: The above article has been published, as is, ahead-of-print, to provide early visibility but is not the final version. Major publication processes like copyediting, proofing, typesetting and further review are still to be done and may lead to changes in the final published version, if it is eventually published. All legal disclaimers that apply to the final published article also apply to this ahead-of-print version.
Patent Publication Number: WO 2021/032937 A1
Priority Application: PCT/GB2019/0523 59
Inventors: Edwards, Hannah Joy; Evans, David Michael; Mazzacani, Alessandro; Obara, Alicja Stela; Clark, David Edward; Gancia, Emanuela; Pittaway, Rachael; Wrigglesworth, Joseph William
Assignee Company: Kalvista Pharmaceuticals Limited, UK
Disease Area: Thrombosis, inflammation, and angioedema
Biological Target: Factor XIIa
REFERENCES
- [1].Ivanov I; Matafonov A; Sun M; Cheng Q; Dickeson SK; Verhamme IM; Emsley J; Gailani D Proteolytic properties of single-chain factor XII: A mechanism for triggering contact activation. Blood, 2017, 129(11), 1527–1537. 10.1182/blood-2016-10-744110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pathak M; Manna R; Li C; Kaira BG; Hamad BK; Belviso BD; Bonturi CR; Dreveny I; Fischer PM; Dekker LV; Oliva MLV; Emsley J Crystal structures of the recombinant β-factor XIIa protease with bound Thr-Arg and Pro-Arg substrate mimetics. Acta Crystallogr. D Struct. Biol, 2019, 75(6), 578–591. 10.1107/S2059798319006910 [DOI] [PubMed] [Google Scholar]
- [3].Dementiev A; Silva A; Yee C; Li Z; Flavin MT; Sham H; Partridge JR Structures of human plasma β–factor XIIa cocrystallized with potent inhibitors. Blood Adv., 2018, 2(5), 549–558. 10.1182/bloodadvances.2018016337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fischer PM Design of small-molecule active-site inhibitors of the S1A family proteases as procoagulant and anticoagulant drugs. J. Med. Chem, 2018, 61(9), 3799–3822. 10.1021/acs.jmedchem.7b00772 [DOI] [PubMed] [Google Scholar]
- [5].Hamad BK; Pathak M; Manna R; Fischer PM; Emsley J; Dekker LV Assessment of the protein interaction between coagulation factor XII and corn trypsin inhibitor by molecular docking and biochemical validation. J. Thromb. Haemost, 2017, 15(9), 1818–1828. 10.1111/jth.13773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Göbel K; Asaridou CM; Merker M; Eichler S; Herrmann AM; Geuß E; Ruck T; Schüngel L; Groeneweg L; Narayanan V; Schneider-Hohendorf T; Gross CC; Wiendl H; Kehrel BE; Kleinschnitz C; Meuth SG Plasma kallikrein modulates immune cell trafficking during neuroinflammation via PAR2 and bradykinin release. Proc. Natl. Acad. Sci. USA, 2019, 116(1), 271–276. 10.1073/pnas.1810020116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Abdallah RT; Keum JS; Lee M-H; Wang B; Gooz M; Luttrell DK; Luttrell LM; Jaffa AA; Jaffa AA Plasma kallikrein promotes epidermal growth factor receptor transactivation and signaling in vascular smooth muscle through direct activation of protease-activated receptors. J. Biol. Chem, 2010, 285(45), 35206–35215. 10.1074/jbc.M110.171769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Konings J; Hoving LR; Ariëns RS; Hethershaw EL; Ninivaggi M; Hardy LJ; de Laat B; ten Cate H; Philippou H; Govers-Riemslag JWP The role of activated coagulation factor XII in overall clot stability and fibrinolysis. Thromb. Res, 2015, 136(2), 474–480. 10.1016/j.thromres.2015.06.028 [DOI] [PubMed] [Google Scholar]
- [9].Abid K; Rochat B; Lassahn PG; Stöcklin R; Michalet S; Brakch N; Aubert JF; Vatansever B; Tella P; De Meester I; Grouzmann E Kinetic study of neuropeptide Y (NPY) proteolysis in blood and identification of NPY3-35: A new peptide generated by plasma kallikrein. J. Biol. Chem, 2009, 284(37), 24715–24724. 10.1074/jbc.M109.035253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kaplan AP; Joseph K Pathogenic mechanisms of bradykinin mediated diseases: dysregulation of an innate inflammatory pathway. Adv. Immunol, 2014, 121, 41–89. 10.1016/B978-0-12-800100-4.00002-7 [DOI] [PubMed] [Google Scholar]
- [11].Hopp S; Nolte MW; Stetter C; Kleinschnitz C; Sirén AL; Albert-Weissenberger C Alleviation of secondary brain injury, posttraumatic inflammation, and brain edema formation by inhibition of factor XIIa. J. Neuroinflammation, 2017, 14(1), 39. 10.1186/s12974-017-0815-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].A study to investigate CSL312 in Subject with Hereditary AngioedemaAvailable from: https://clinicaltrials.gov/ct2/show/NCT03712228?cond=NCT03712228&draw=1&rank=1
- [13].Björkqvist J; de Maat S; Lewandrowski U; Di Gennaro A; Oschatz C; Schönig K; Nöthen MM; Drouet C; Braley H; Nolte MW; Sickmann A; Panousis C; Maas C; Renné T Defective glycosylation of coagulation factor XII underlies hereditary angioedema type III. J. Clin. Invest, 2015, 125(8), 3132–3146. 10.1172/JCI77139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de Maat S; Björkqvist J; Suffritti C; Wiesenekker CP; Nagtegaal W; Koekman A; van Dooremalen S; Pasterkamp G; de Groot PG; Cicardi M; Renné T; Maas C Plasmin is a natural trigger for bradykinin production in patients with hereditary angioedema with factor XII mutations. J. Allergy Clin. Immunol, 2016, 138(5), 1414–1423.e9. 10.1016/j.jaci.2016.02.021 [DOI] [PubMed] [Google Scholar]
- [15].Veronez CL; Aabom A; Martin RP; Filippelli-Silva R; Gonçalves RF; Nicolicht P; Mendes AR; Da Silva J; Guilarte M; Grumach AS; Mansour E; Bygum A; Pesquero JB Genetic variation of kallikrein-kinin system and related genes in patients with hereditary angioedema. Front. Med. (Lausanne), 2019, 6, 28. 10.3389/fmed.2019.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Recke A; Massalme EG; Jappe U; Steinmüller-Magin L; Schmidt J; Hellenbroich Y; Hüning I; Gillessen-Kaesbach G; Zillikens D; Hartmann K Identification of the recently described plasminogen gene mutation p.Lys330Glu in a family from Northern Germany with hereditary angioedema. Clin. Transl. Allergy, 2019, 9(1), 9. 10.1186/s13601-019-0247-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mansi M; Zanichelli A; Coerezza A; Suffritti C; Wu MA; Vacchini R; Stieber C; Cichon S; Cicardi M Presentation, diagnosis and treatment of angioedema without wheals: a retrospective analysis of a cohort of 1058 patients. J. Intern. Med, 2015, 277(5), 585–593. 10.1111/joim.12304 [DOI] [PubMed] [Google Scholar]
- [18].de Maat S; Clark CC; Boertien M; Parr N; Sanrattana W; Hofman ZLM; Maas C Factor XII truncation accelerates activation in solution. J. Thromb. Haemost, 2019, 17(1), 183–194. 10.1111/jth.14325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kedarisetty S; Jones E; Tint D; Soliman AMS Air pollution and angioedema. Otolaryngol. Head Neck Surg, 2019, 161(3), 431–438. 10.1177/0194599819846446 [DOI] [PubMed] [Google Scholar]
- [20].Long YM; Zhao XC; Clermont AC; Zhou QF; Liu Q; Feener EP; Yan B; Jiang GB Negatively charged silver nanoparticles cause retinal vascular permeability by activating plasma contact system and disrupting adherens junction. Nanotoxicology, 2016, 10(4), 501–511. 10.3109/17435390.2015.1088589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baş M; Hoffmann TK; Kojda G Icatibant in ACE-inhibitorinduced angioedema. N. Engl. J. Med, 2015, 372(19), 1867–1868. [DOI] [PubMed] [Google Scholar]
- [22].Leibfried M; Kovary A C1 Esterase Inhibitor (Berinert) for ACE inhibitor-induced angioedema: two case reports. J. Pharm. Pract, 2017, 30(6), 668–671. 10.1177/0897190016677427 [DOI] [PubMed] [Google Scholar]
- [23].van den Elzen M; Go MFCL; Knulst AC; Blankestijn MA; van Os-Medendorp H; Otten HG Efficacy of treatment of nonhereditary angioedema. Clin. Rev. Allergy Immunol, 2018, 54(3), 412–431. 10.1007/s12016-016-8585-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Han ED; MacFarlane RC; Mulligan AN; Scafidi J; Davis AE III Increased vascular permeability in C1 inhibitor–deficient mice mediated by the bradykinin type 2 receptor. J. Clin. Invest, 2002, 109(8), 1057–1063. 10.1172/JCI200214211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Simão F; Ustunkaya T; Clermont AC; Feener EP Plasma kallikrein mediates brain hemorrhage and edema caused by tissue plasminogen activator therapy in mice after stroke. Blood, 2017, 129(16), 2280–2290. 10.1182/blood-2016-09-740670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fröhlich K; Macha K; Gerner ST; Bobinger T; Schmidt M; Dörfler A; Hilz MJ; Schwab S; Seifert F; Kallmünzer B; Winder K Angioedema in stroke patients with thrombolysis. Stroke, 2019, 50(7), 1682–1687. 10.1161/STROKEAHA.119.025260 [DOI] [PubMed] [Google Scholar]
- [27].Rathbun KM Angioedema after thrombolysis with tissue plasminogen activator: an airway emergency. Oxf. Med. Case Rep, 2019, 2019(1), omy112. 10.1093/omcr/omy112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lekoubou A; Philippeau F; Derex L; Olaru A; Gouttard M; Vieillart A; Kengne AP Audit report and systematic review of orolingual angioedema in post-acute stroke thrombolysis. Neurol. Res, 2014, 36(7), 687–694. 10.1179/1743132813Y.0000000302 [DOI] [PubMed] [Google Scholar]
- [29].Hill MD; Lye T; Moss H; Barber PA; Demchuk AM; Newcommon NJ; Green TL; Kenney C; Cole-Haskayne A; Buchan AM Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology, 2003, 60(9), 1525–1527. 10.1212/01.WNL.0000058840.66596.1A [DOI] [PubMed] [Google Scholar]
- [30].Stone C Jr; Brown NJ Angiotensin-converting enzyme inhibitor and other drug-associated angioedema. Immunol. Allergy Clin. North Am, 2017, 37(3), 483–495. 10.1016/j.iac.2017.04.006 [DOI] [PubMed] [Google Scholar]
- [31].Scott SI; Andersen MF; Aagaard L; Buchwald CV; Rasmussen ER Dipeptidyl Peptidase-4 inhibitor induced angioedema - an overlooked adverse drug reaction? Curr. Diabetes Rev, 2018, 14(4), 327–333. 10.2174/1573399813666170214113856 [DOI] [PubMed] [Google Scholar]
- [32].Hermanrud T; Bygum A; Rasmussen ER Recurrent angioedema associated with pharmacological inhibition of dipeptidyl peptidase IV. BMJ Case Rep, 2017, 2017, bcr2016217802. 10.1136/bcr-2016-217802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim H; Baik SY; Yang SJ; Kim TM; Lee SH; Cho JH; Choi IY; Kim JH; Yoon KH; Kim HS Clinical experiences and case review of angiotensin II receptor blocker-related angioedema in Korea. Basic Clin. Pharmacol. Toxicol, 2019, 124(1), 115–122. 10.1111/bcpt.13097 [DOI] [PubMed] [Google Scholar]
- [34].Reichman ME; Wernecke M; Graham DJ; Liao J; Yap J; Chillarige Y; Southworth MR; Keeton S; Goulding MR; Mott K; Kelman JA Antihypertensive drug associated angioedema: effect modification by race/ethnicity. Pharmacoepidemiol. Drug Saf, 2017, 26(10), 1190–1196. 10.1002/pds.4260 [DOI] [PubMed] [Google Scholar]
- [35].Diestro JDB; Sedano LSP; Reyes NGD; San Jose MCZ Hemilingual angioedema after thrombolysis in a patient on an angiotensin II receptor blocker. J. Stroke Cerebrovasc. Dis, 2019, 28(5), e44–e45. 10.1016/j.jstrokecerebrovasdis.2019.01.030 [DOI] [PubMed] [Google Scholar]
- [36].Giard C; Nicolie B; Drouet M; Lefebvre-Lacoeuille C; Le Sellin J; Bonneau JC; Maillard H; Rénier G; Cichon S; Ponard D; Drouet C; Martin L Angio-oedema induced by oestrogen contraceptives is mediated by bradykinin and is frequently associated with urticaria. Dermatology, 2012, 225(1), 62–69. 10.1159/000340029 [DOI] [PubMed] [Google Scholar]
- [37].Liu J; Feener EP Plasma kallikrein-kinin system and diabetic retinopathy. bchm, 2013, 394(3), 319–328. 10.1515/hsz-2012-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gao BB; Clermont A; Rook S; Fonda SJ; Srinivasan VJ; Wojtkowski M; Fujimoto JG; Avery RL; Arrigg PG; Bursell SE; Aiello LP; Feener EP Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat. Med, 2007, 13(2), 181–188. 10.1038/nm1534 [DOI] [PubMed] [Google Scholar]
- [39].Gao BB; Chen X; Timothy N; Aiello LP; Feener EP Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J. Proteome Res, 2008, 7(6), 2516–2525. 10.1021/pr800112g [DOI] [PubMed] [Google Scholar]
- [40].Kita T; Clermont AC; Murugesan N; Zhou Q; Fujisawa K; Ishibashi T; Aiello LP; Feener EP Plasma kallikrein-kinin system as a VEGF-independent mediator of diabetic macular edema. Diabetes, 2015, 64(10), 3588–3599. 10.2337/db15-0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Clermont A; Murugesan N; Zhou Q; Kita T; Robson PA; Rushbrooke LJ; Evans DM; Aiello LP; Feener EP Plasma kallikrein mediates vascular endothelial growth factor–induced retinal dysfunction and thickening. Invest. Ophthalmol. Vis. Sci, 2016, 57(6), 2390–2399. 10.1167/iovs.15-18272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Morrison DC; Cochrane CG Direct evidence for Hageman factor (factor XII) activation by bacterial lipopolysaccharides (endotoxins). J. Exp. Med, 1974, 140(3), 797–811. 10.1084/jem.140.3.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zamolodchikov D; Chen ZL; Conti BA; Renné T; Strickland S Activation of the factor XII-driven contact system in Alzheimer’s disease patient and mouse model plasma. Proc. Natl. Acad. Sci. USA, 2015, 112(13), 4068–4073. 10.1073/pnas.1423764112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Simões PSR; Zanelatto AO; Assis MC; Varella PPV; Yacubian EM; Carrete H; Centeno R; Araujo MS; Cavalheiro EA; Tersariol ILS; Motta G; Naffah-Mazzacoratti MG Plasma kallikrein-kinin system contributes to peripheral inflammation in temporal lobe epilepsy. J. Neurochem, 2019, 150(3), 296–311. 10.1111/jnc.14793 [DOI] [PubMed] [Google Scholar]
- [45].Göbel K; Pankratz S; Asaridou CM; Herrmann AM; Bittner S; Merker M; Ruck T; Glumm S; Langhauser F; Kraft P; Krug TF; Breuer J; Herold M; Gross CC; Beckmann D; Korb-Pap A; Schuhmann MK; Kuerten S; Mitroulis I; Ruppert C; Nolte MW; Panousis C; Klotz L; Kehrel B; Korn T; Langer HF; Pap T; Nieswandt B; Wiendl H; Chavakis T; Kleinschnitz C; Meuth SG Blood coagulation factor XII drives adaptive immunity during neuroinflammation via CD87-mediated modulation of dendritic cells. Nat. Commun, 2016, 7(1), 11626. 10.1038/ncomms11626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Efficacy and safety of 10NIS-PKKRx for preventive treatment of chronić migraine Available from: https://clinicaltrials.gov/ct2/show/NCT03108469?cond=NCT03108469&draw=2&rank=1
- [47].Bender L; Weidmann H; Rose-John S; Renné T; Long AT Factor XII-Driven inflammatory reactions with implications for anaphylaxis. Front. Immunol, 2017, 8, 1115. 10.3389/fimmu.2017.01115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sala-Cunill A; Björkqvist J; Senter R; Guilarte M; Cardona V; Labrador M; Nickel KF; Butler L; Luengo O; Kumar P; Labberton L; Long A; Di Gennaro A; Kenne E; Jämsä A; Krieger T; Schlüter H; Fuchs T; Flohr S; Hassiepen U; Cumin F; McCrae K; Maas C; Stavrou E; Renné T Plasma contact system activation drives anaphylaxis in severe mast cell–mediated allergic reactions. J. Allergy Clin. Immunol, 2015, 135(4), 1031–1043.e6. 10.1016/j.jaci.2014.07.057 [DOI] [PubMed] [Google Scholar]
- [49].Davie EW; Ratnoff OD Waterfall sequence for intrinsic blood clotting. Science, 1964, 145(3638), 1310–1312. 10.1126/science.145.3638.1310 [DOI] [PubMed] [Google Scholar]
- [50].Radcliffe R; Bagdasarian A; Colman R; Nemerson Y Activation of bovine factor VII by hageman factor fragments. Blood, 1977, 50(4), 611–617. 10.1182/blood.V50.4.611.611 [DOI] [PubMed] [Google Scholar]
- [51].Puy C; Tucker EI; Wong ZC; Gailani D; Smith SA; Choi SH; Morrissey JH; Gruber A; McCarty OJT Factor XII promotes blood coagulation independent of factor XI in the presence of long-chain polyphosphates. J. Thromb. Haemost, 2013, 11(7), 1341–1352. 10.1111/jth.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Renné T; Pozgajová M; Grüner S; Schuh K; Pauer HU; Burfeind P; Gailani D; Nieswandt B Defective thrombus formation in mice lacking coagulation factor XII. J. Exp. Med, 2005, 202(2), 271–281. 10.1084/jem.20050664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Simão F; Feener EP The effects of the contact activation system on hemorrhage. Front. Med. (Lausanne), 2017, 4, 121. 10.3389/fmed.2017.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Worm M; Köhler EC; Panda R; Long A; Butler LM; Stavrou EX; Nickel KF; Fuchs TA; Renné T The factor XIIa blocking antibody 3F7: a safe anticoagulant with anti-inflammatory activities. Ann. Transl. Med, 2015, 3(17), 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Barbieri CM; Wang X; Wu W; Zhou X; Ogawa AM; O’Neill K; Chu D; Castriota G; Seiffert DA; Gutstein DE; Chen Z Factor XIIa as a novel target for thrombosis: target engagement requirement and efficacy in a rabbit model of microembolic signals. J. Pharmacol. Exp. Ther, 2017, 360(3), 466–475. 10.1124/jpet.116.238493 [DOI] [PubMed] [Google Scholar]
- [56].Krupka J; May F; Weimer T; Pragst I; Kleinschnitz C; Stoll G; Panousis C; Dickneite G; Nolte MW The coagulation factor XIIa inhibitor rHA-Infestin-4 improves outcome after cerebral ischemia/reperfusion injury in rats. PLoS One, 2016, 11(1), e0146783. 10.1371/journal.pone.0146783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Leung PY; Hurst S; Berny-Lang MA; Verbout NG; Gailani D; Tucker EI; Wang RK; McCarty OJT; Gruber A Inhibition of Factor XII-mediated activation of factor XI provides protection against experimental acute ischemic stroke in mice. Transl. Stroke Res, 2012, 3(3), 381–389. 10.1007/s12975-012-0186-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gailani D Making thrombolysis safer in stroke. Blood, 2017, 129(16), 2212–2213. 10.1182/blood-2017-02-765610 [DOI] [PubMed] [Google Scholar]
- [59].Liu J; Gao BB; Clermont AC; Blair P; Chilcote TJ; Sinha S; Flaumenhaft R; Feener EP Hyperglycemia-induced cerebral hematoma expansion is mediated by plasma kallikrein. Nat. Med, 2011, 17(2), 206–210. 10.1038/nm.2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Didiasova M; Wujak L; Schaefer L; Wygrecka M Factor XII in coagulation, inflammation and beyond. Cell. Signal, 2018, 51, 257–265. 10.1016/j.cellsig.2018.08.006 [DOI] [PubMed] [Google Scholar]
- [61].Rao, Factor XIIa Inhibitors. WO2018/093695, 2020. [Google Scholar]
- [62].Hicks, Factor Xlla Inhibitors. WO2018/093716, 2019. [Google Scholar]
- [63].Breslow. Aminotriazole immunomodulators for treating autoimmune diseases. WO2017/123518, 2014. [Google Scholar]
- [64].Ponda, Aminacylindazole immunomodulators for treatment of autoimmune diseases. WO2017/205296, 2014. [Google Scholar]
- [65].Pyranopyrazole and pyrazolopyridine immunomodulators for treatment of autoimmune diseases. WO2019/108565, 2018. [Google Scholar]
- [66].Nolte, Factor XII inhibitors for the administration with medical procedures comprising contact with artificial surfaces. W02012/120128, 2015. [Google Scholar]
- [67].Shori DK; Proctor GB; Chao J; Ka-Ming C; Garrett JR New specific assays for tonin and tissue kallikrein activities in rat submandibular glands. Biochem. Pharmacol, 1992, 43(6), 1209–1217. 10.1016/0006-2952(92)90494-4 [DOI] [PubMed] [Google Scholar]
- [68].Baeriswyl V; Calzavarini S; Chen S; Zorzi A; Bologna L; Angelillo-Scherrer A; Heinis C A synthetic factor XIIa inhibitor blocks selectively intrinsic coagulation initiation. ACS Chem. Biol, 2015, 10(8), 1861–1870. 10.1021/acschembio.5b00103 [DOI] [PubMed] [Google Scholar]
- [69].Bouckaert C; Serra S; Rondelet G; Dolušić E; Wouters J; Dogné JM; Frédérick R; Pochet L Synthesis, evaluation and structure-activity relationship of new 3-carboxamide coumarins as FXIIa inhibitors. Eur. J. Med. Chem, 2016, 110, 181–194. 10.1016/j.ejmech.2016.01.023 [DOI] [PubMed] [Google Scholar]


