Abstract
Background
Minimally invasive endoscopic technique is an important component of Enhanced Recovery After Surgery (ERAS) protocol for neurosurgery. In recent years, unilateral biportal endoscopic lumbar interbody fusion (ULIF) has been used in the treatment of lumbar degenerative diseases (LDD). This study aims to investigate whether ULIF could enhance the recovery of patients with LDD compared with the conventional minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) or posterior lumbar interbody fusion (PLIF).
Methods
A comprehensive literature search was performed for relevant studies in PubMed, EMBASE, Web of Science, Cochrane Library database, China National Knowledge Internet, and Wanfang database. Surgical data, clinical outcomes, radiographic outcomes, and surgical complications were compared between patients with LDD who underwent ULIF and those who underwent conventional MI-TLIF or PLIF.
Results
Notably, 12 studies, comprising 981 patients with LDD, were included. Of these patients, 449 underwent ULIF and 532 patients (355 MI-TLIF and 177 PLIF) were treated with conventional procedures. There was no significant difference in the fusion rate, cage subsidence rate, and surgical complications between the ULIF group and the MI-TLIF or PLIF group. Compared with MI-TLIF, the ULIF group presented a significantly reduced estimated blood loss (EBL) (WMD, −106.00; 95% CI −140.99 to −71.10, P < 0.001) and shorter length of hospital stay (LOS) (WMD, −1.27; 95% CI −1.88 to −0.66, P < 0.001); better short-term improvement in ODI (WMD, −2.12; 95% CI −3.53 to −0.72, P = 0.003) and VAS score for back pain (VAS-BP) (WMD, −0.86; 95% CI −1.15 to −0.58, P < 0.001) at 1 month post-operatively. Compared with PLIF, the ULIF group presented a significantly reduced EBL (WMD, −149.22; 95% CI −284.98 to −13.47, P = 0.031) and shorter LOS (WMD, −4.40; 95% CI −8.04 to −0.75, P = 0.018); better short-term improvement in VAS-BP (WMD, −1.07; 95% CI −1.77 to −0.38, P = 0.002) and VAS score for leg pain (VAS-LP) (WMD, −0.40; 95% CI −0.72 to −0.08, P = 0.014) at 1–2 week post-operatively; enhanced short- and long-term improvement in ODI at 1 month post-operatively (WMD, −3.12; 95% CI −5.72 to −0.53, P = 0.018) and the final follow-up (WMD, −1.97; 95% CI −3.32 to −0.62, P = 0.004), respectively.
Conclusion
Compared with conventional MI-TLIF and PLIF, ULIF was associated with reduced EBL, shorter LOS, and comparable fusion rate as well as complication management. Compared with MI-TLIF, a better short-term improvement in VAS-BP and ODI was achieved by ULIF; compared with open PLIF, additional enhanced short-term improvement in VAS-LP and long-term improvement in ODI were observed in ULIF. ULIF could enhance the recovery of patients with LDD compared with conventional posterior procedures.
Systematic trial registration
https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=230695, CRD42021230695.
Keywords: unilateral biportal endoscopic lumbar interbody fusion, minimally-invasive transforaminal lumbar interbody fusion, posterior lumbar interbody fusion, lumbar degenerative disease, Enhanced Recovery After Surgery, neurosurgery
Introduction
Lumbar degenerative disease (LDD), including lumbar spinal stenosis (LSS), lumbar disc herniation (LDH), and degenerative or isthmic lumbar spondylolisthesis (LS), has been one of the most prevalent and disabling spinal disorders that cause low back and leg pain, disability, and poor quality of life (1, 2). As a result, evolutions in both non-surgical and surgical treatment of LDD continue through the present day.
Among surgical procedures, lumbar interbody fusion is the gold standard for stabilizing spinal instability and decompressing neural elements (3). The most commonly used surgical approach is the posterior approach. Conventional posterior procedures include minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) through microscopic tubular technique and open posterior lumbar interbody fusion (PLIF) (4). However, lumbar interbody fusion has been rated as one of the most painful procedures (5, 6). The main disadvantage of the conventional MI-TLIF or PLIF is the extensive paraspinal iatrogenic damage caused by dissection and retraction, which would induce the risk of chronic pain and delay patients' post-operative recovery and mobilization, placing a substantial economic burden on the public healthcare systems (7, 8). Therefore, there is a significant clinical and economic rationale for improving the management and outcomes of these conditions (9).
The concept of “fast-track” surgery was initiated by Kehlet in the 1990s and further developed as Enhanced Recovery After Surgery (ERAS) (10, 11). ERAS is a multidisciplinary and multimodal perioperative management approach that aims to improve surgical outcomes, reduce complications, and shorten the length of the hospital stay (12, 13). With the increasing application of ERAS protocols in neurosurgery, minimally invasive uniportal endoscopic technique has gained popularity as a key component for the management of lumbar interbody fusion (14–17). However, this technique was restricted by its vision and specific instruments. In recent years, the biportal endoscopic system and unilateral biportal endoscopic lumbar interbody fusion (ULIF) were developed to combine the advantages of conventional and endoscopic surgery (18–20). Through independent viewing and working channels, unrestricted vision, and ample operation space could be obtained while the posterior structure could be preserved. ULIF has been used to treat LDD; however, whether ULIF could enhance recovery compared with conventional procedures remains controversial.
The purpose of this systematic review and meta-analysis was to compare the surgical data, clinical outcomes, laboratory outcomes, radiographic outcomes, and surgical complications between ULIF and conventional MI-TLIF or PLIF for the treatment of LDD.
Materials and methods
This study was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and registered with PROSPERO (ID: CRD42021230695) (21, 22).
Search strategy
PubMed, EMBASE, Web of Science, Cochrane Library database, China National Knowledge Internet, and Wanfang database were searched using the following terms: (fusion) AND [(((((UBE) OR (biportal endoscopic)) OR (unilateral biportal endoscopic)) OR (biportal endoscopic spinal surgery)) OR (unilateral laminotomy bilateral decompression)) OR (biportal endoscopy)].
The literature search was updated on 30 October 2022. Two reviewers (H.Y. and F.C.) independently screened the titles and abstracts, and any differences were settled by a discussion with a third reviewer (Y.L.).
Surgical technique of ULIF
Under general anesthesia, the patients were placed in a prone position. C-arm fluoroscopy was performed to confirm the surgical level. The surface projection of the target bilateral pedicles and intervertebral space was marked on the skin. Two longitudinal skin incisions were made. Both the portals were 1.0 cm long, 3.0 cm apart from each other, and located 0.5 cm lateral to the ipsilateral spinous process. After the channel expanded through serial dilators, independent viewing and working channels were placed, and the submuscular operation space was formed on the surface of the lamina. A continuous fluid irrigation system with constant outflow was used. The paraspinal muscle attached to the lamina and articular process was detached by a stripper. Bipolar radiofrequency ablation could be applied for bleeding control. Laminectomy from the inferior edge of the cranial lamina to the superior edge of the caudal lamina and facetectomy for the medial edge of the articular process was performed using a power burr and gun forceps. Then, a flavectomy was performed to decompress the lumbar spinal canal and nerve root canals. The discectomy was operated under direct vision. After the endplate preparation, bone grafts and an interbody fusion cage were inserted. At last, bilateral percutaneous pedicle screw fixation was performed prior to the incision closure.
Inclusion and exclusion criteria
The inclusion criteria are as follows: (1) patients diagnosed with lumbar degenerative diseases, including LSS, LDD, and LS of Meyerding grades I-II; (2) studies in which the intervention was ULIF; (3) studies comparing patients who underwent conventional MI-TLIF or PLIF; and (4) studies with the following outcomes: surgical data, clinical outcomes, laboratory outcomes, radiographic outcomes, and surgical complications.
The exclusion criteria are as follows: (1) studies that included patients with spinal tumors or infection; (2) studies that reported the outcomes of ULIF without comparison groups; (3) reviews, case reports, biomechanical analysis, and cadaveric research; (4) studies with no available full text; (5) duplicate publications; and (6) articles not published in English or Chinese.
Assessment of study quality
Study quality was assessed independently by two reviewers (YH and AP) using the Newcastle-Ottawa scale (NOS) recommended by Cochrane Handbook version 5.2.0 (23). The level of evidence rating was assigned according to the published guidelines (24).
Outcomes
Surgical data included estimated blood loss (EBL), operating time (ORT), length of hospital stay (LOS), and post-operative drainage. Clinical outcomes were Oswestry Disability Index (ODI) as well as visual analog scale (VAS) score for back pain (VAS-BP) and leg pain (VAS-LP) assessed at baseline, post-operatively, and the final follow-up. The excellent/good rate of surgical therapy according to the modified Macnab criteria was also evaluated at the final follow-up. Laboratory outcomes indicated serum creatine phosphokinase (CPK) and C-reactive protein (CRP) measured at baseline and 2 or 3 days post-operatively. Radiographic outcomes included cage subsidence rate and fusion rate at the final follow-up. Fusion was defined as the presence of bridging interbody trabecular bone using computed tomography scans or radiographs (25). Unplanned return to the operating room (OR) and surgical complications, including epidural hematoma, dural tear, surgical site infection, and neurologic deficits, were assessed during the perioperative period.
Data extraction
Data extraction was performed independently by two reviewers (HY and FC). Demographic information, including age, sex, body mass index (BMI), diagnosis, operative level, and follow-up duration, was recorded. The data for 14 variables were extracted for analysis. Continuous outcomes included EBL, ORT, LOS, post-operative drainage, ODI, VAS-BP, VAS-LP, CPK, and CRP. Dichotomous outcomes included excellent/good rate of surgical therapy, cage subsidence rate, fusion rate, unplanned return to OR, and surgical complications.
Data analysis
All statistical analyses were performed using the Stata version 15.1. Outcomes reported in at least two studies would be analyzed. For continuous outcomes, the weighted mean difference (WMD) or standard mean difference (SMD) was used to estimate the effect. The effect measure of dichotomous outcomes is displayed as a risk ratio (RR). The mean and standard deviation values of continuous outcomes or the counts and percentages of dichotomous outcomes for comparisons of data points are also displayed. The statistical heterogeneity among studies was evaluated using the I-square test and Cochran's Q-test. If the I2-value was <50% and the P-value was >0.10, a fixed-effects model was used. If the I2-value was >50% or the P-value was <0.10, a sensitivity analysis was applied to assess the impact of each study. If a source of potential heterogeneity could not be found, a random-effects model was used.
Assessment of publication bias
Potential publication bias was assessed by applying Egger's test at a P < 0.10 level of significance (26). If publication bias was indicated, we further evaluated the number of missing studies by applying the “trim and fill” method and recalculated the pooled WMD, SME, or RR with the addition of those missing studies (27).
Results
Study selection
The systematic search yielded 455 articles, of which 310 were duplicates, 114 were excluded by screening the title and abstract, and 19 were considered improper after full-text review. Eventually, 12 studies were included in this systematic review and meta-analysis (Figure 1) (28–39).
Figure 1.
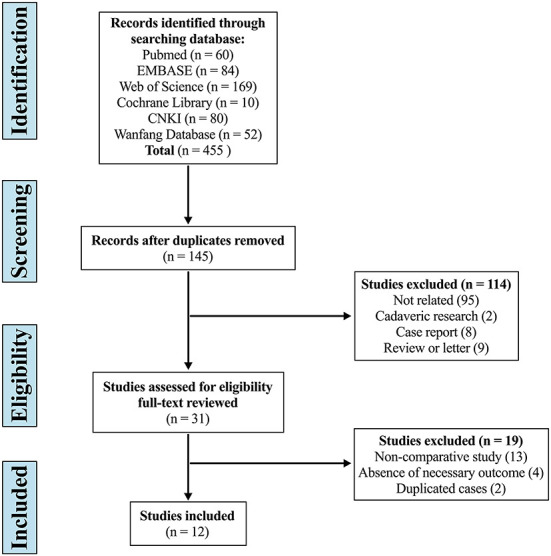
Flow diagram depicting the literature review, search strategy, and selection process.
Assessment of study quality and publication bias
The quality of the included studies was assessed using the Newcastle-Ottawa Scale (Table 1). Of the 12 studies included, eight were of high quality with scores of 8–9, and four were of moderate quality with scores of 7. The level of evidence was III for nine studies and IV for three studies. Publication bias was not detected for any variable.
Table 1.
Quality assessment of studies according to Newcastle-Ottawa Scale (NOS).
| References | Year | Selection | Comparability | Exposure | Total score |
|---|---|---|---|---|---|
| Heo and Park (33) | 2019 | 3 | 2 | 3 | 8 |
| Park et al. (38) | 2019 | 4 | 2 | 3 | 9 |
| Zhu et al. (31) | 2021 | 4 | 2 | 2 | 8 |
| Zhang et al. (37) | 2021 | 3 | 2 | 2 | 7 |
| Zhang et al. (36) | 2021 | 3 | 2 | 2 | 7 |
| Kim et al. (35) | 2021 | 3 | 2 | 3 | 8 |
| Kang et al. (34) | 2021 | 4 | 2 | 3 | 9 |
| Gatam et al. (32) | 2021 | 4 | 2 | 2 | 8 |
| Ma et al. (30) | 2022 | 3 | 2 | 2 | 7 |
| Liu et al. (39) | 2022 | 4 | 2 | 3 | 9 |
| Kong et al. (29) | 2022 | 4 | 2 | 2 | 8 |
| Jiang et al. (28) | 2022 | 3 | 2 | 2 | 7 |
Characteristics of included studies
Twelve studies, comprising 981 patients with LDD, were included. Of these patients, 449 underwent ULIF and 532 patients (355 MI-TLIF and 177 PLIF) were treated with conventional procedures. Characteristics of the included studies and patients are presented in Table 2. There were no significant differences at baseline between the ULIF group and MI-TLIF group in the patient's age (60.26 ± 9.26 years vs. 59.49 ± 9.90 years, P = 0.114), male-to-female ratio (0.75 vs. 0.86, P = 0.379), BMI (24.94 ± 3.24 kg/m2 vs. 25.32 ± 3.01 kg/m2, P = 0.111), diagnosis (P = 0.745), operative level (P = 0.382), ODI (58.75 ± 9.02 vs. 59.63 ± 8.21, P = 0.464), VAS-BP (6.43 ± 1.31 vs. 6.48 ± 1.25, P = 0.436), and VAS-LP (6.83 ± 1.85 vs. 6.79 ± 1.87, P = 0.246), CPK (P = 0.892), and CRP (P = 0.934). The duration of follow-up was 12.65 ± 2.74 months in the ULIF group and 13.25 ± 3.41 months in the MI-TLIF group (P = 0.098). Moreover, there were no significant differences at baseline between the ULIF group and PLIF group in the patient's age (60.50 ± 8.12 years vs. 61.58 ± 9.06 years, P = 0.574), male-to-female ratio (0.66 vs. 0.89, P = 0.500), BMI (24.56 ± 4.03 kg/m2 vs. 23.94 ± 3.83 kg/m2, P = 0.163), diagnosis (P = 0.521), operative level (P = 0.460), ODI (49.16 ± 9.10 vs. 45.95 ± 9.67, P = 0.129), VAS-BP (6.39 ± 1.29 vs. 6.50 ± 1.45, P = 0.076), and VAS-LP (5.98 ± 1.45 vs. 6.08 ± 1.41, P = 0.104). The duration of follow-up was 11.74 ± 3.49 months in the ULIF group and 12.63 ± 3.25 months in the PLIF group (P = 0.210).
Table 2.
Characteristics of the included studies.
| References | Year | Design | Level of evidence | Group | Sample size | Age | Sex (M/F) | Diagnosis | Operative level | BMI (kg/m2) | FU (month) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Heo and Park (33) | 2019 | Retrospective | III | ULIF | 23 | 61.4 ± 9.4 | 7/16 | LSS LS |
L3/4 (3) L4/5 (17) L5/S1 (3) |
NA | 13.4 ± 2.5 |
| MI-TLIF | 46 | 63.5 ± 10.5 | 19/27 | L3/4 (4) L4/5 (29) L5/S1 (13) |
NA | ||||||
| Zhu et al. (31) | 2021 | Retrospective | III | ULIF | 35 | 50.94 ± 12.12 | 16/19 | LSS (19) LDH (7) LS (9) |
L3/4 (0) L4/5 (28) L5/S1 (7) |
NA | 15.29 ± 1.98 |
| MI-TLIF | 41 | 53.44 ± 14.37 | 19/22 | LSS (21) LDH (13) LS (7) |
L3/4 (2) L4/5 (25) L5/S1 (14) |
NA | 16.12 ± 2.59 | ||||
| Kim et al. (35) | 2021 | Retrospective | III | ULIF | 32 | 70.5 ± 8.26 | 17/15 | LS (32) | L2/3 (1) L3/4 (3) L4/5 (20) L5/S1 (8) |
NA | 27.2 ± 5.4 |
| MI-TLIF | 55 | 67.3 ± 10.7 | 25/30 | LS (55) | L2/3 (0) L3/4 (2) L4/5 (46) L5/S1 (7) |
NA | 31.5 ± 7.3 | ||||
| Kang et al. (34) | 2021 | Retrospective | IV | ULIF | 47 | 66.87 ± 10.41 | 17/30 | LSS LS |
L2/3 (4) L3/4 (7) L4/5 (34) L5/S1 (20) |
25.32 ± 3.15 | 14.5 ± 2.3 |
| MI-TLIF | 32 | 66.38 ± 9.45 | 17/15 | L2/3 (1) L3/4 (9) L4/5 (22) L5/S1 (11) |
26.23 ± 3.26 | 15.78 ± 3.16 | |||||
| Gatam et al. (32) | 2021 | Retrospective | III | ULIF | 72 | 55.1 ± 5.12 | 26/46 | LS (72) | L3/4 (8) L4/5 (56) L5/S1 (8) |
23.6 ± 3.67 | ≥ 12 |
| MI-TLIF | 73 | 52.3 ± 6.13 | 28/45 | LS (73) | L3/4 (10) L4/5 (48) L5/S1 (15) |
24.8 ± 3.42 | |||||
| Ma et al. (30) | 2022 | Retrospective | III | ULIF | 32 | 58.81 ± 12.49 | 19/13 | LSS (32) | L3/4 (1) L4/5 (23) L5/S1 (8) |
24.96 ± 4.34 | 8.2 ± 1.5 |
| MI-TLIF | 43 | 57.42 ± 9.67 | 26/17 | LSS (32) | L3/4 (2) L4/5 (29) L5/S1 (12) |
24.23 ± 3.37 | |||||
| Kong et al. (29) | 2022 | Retrospective | III | ULIF | 35 | 55.10 ± 7.75 | 13/22 | LSS (12) LDH (15) LS (8) |
L2/3 (1) L3/4 (5) L4/5 (17) L5/S1 (10) L4/S1 (2) |
25.80 ± 1.80 | ≥ 6 |
| MI-TLIF | 40 | 56.00 ± 8.00 | 18/22 | LSS (15) LDH (9) LS (16) |
L1/2 (1) L2/3 (4) L3/4 (7) L4/5 (15) L5/S1 (12) L4/S1 (1) |
26.00 ± 2.00 | |||||
| Jiang et al. (28) | 2022 | Retrospective | IV | ULIF | 25 | 63.28 ± 8.51 | 9/16 | LSS (25) | L4/5 (24) L5-S1 (1) |
NA | ≥ 3 |
| MI-TLIF | 25 | 59.68 ± 10.38 | 8/17 | LSS (25) | L4/5 (23) L5-S1 (2) |
NA | |||||
| Park et al. (38) | 2019 | Retrospective | III | ULIF | 71 | 68.00 ± 8.00 | 26/45 | LSS (7) LDH (2) LS (62) |
L3/4 (13) L4/5 (50) L5/S1 (8) |
NA | 17.1 ± 4.9 |
| PLIF | 70 | 66.00 ± 9.00 | 20/50 | LSS (11) LDH (2) LS (57) |
L3/4 (8) L4/5 (56) L5/S1 (6) |
NA | 20.4 ± 7.2 | ||||
| Zhang et al. (37) | 2021 | Retrospective | IV | ULIF | 21 | 58.90 ± 9.20 | 14/7 | LSS LDS |
NA | 22.70 ± 5.90 | ≥ 6 |
| PLIF | 35 | 62.80 ± 10.40 | 18/17 | 23.90 ± 6.20 | |||||||
| Zhang et al. (36) | 2021 | Retrospective | III | ULIF | 29 | 51.14 ± 6.85 | 17/12 | LDH (29) | NA | 24.69 ± 3.16 | ≥ 12 |
| PLIF | 39 | 53.92 ± 7.16 | 26/13 | LDH (39) | 23.84 ± 2.97 | ||||||
| Liu et al. (39) | 2022 | Prospective | III | ULIF | 27 | 63.89 ± 8.44 | 12/15 | LSS (7) LDH (14) LS (6) |
L3/4 (4) L4/5 (18) L5/S1 (5) |
24.91 ± 3.03 | 11.67 ± 5.05 |
| PLIF | 33 | 63.70 ± 9.69 | 13/20 | LSS (9) LDH (17) LS (7) |
L3/4 (7) L4/5 (20) L5/S1 (6) |
24.02 ± 2.32 | 12.15 ± 4.18 |
ULIF indicates unilateral biportal endoscopic lumbar interbody fusion; MI-TLIF, minimally invasive transforaminal lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; LSS, lumbar spinal stenosis; LDH, lumbar disc herniation; LS, lumbar spondylolisthesis; BMI, body mass index; FU, follow-up; NA, not available.
Surgical data
Estimated blood loss
ULIF vs. MI-TLIF
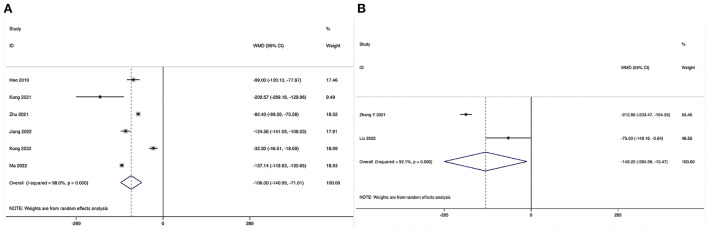
Estimated blood loss could be obtained in six studies (28–31, 33, 34), and significant heterogeneity was detected (I2 = 98.0%, P < 0.001). The pooled results revealed significantly reduced EBL in the ULIF group compared with that in the MI-TLIF group (WMD, −106.00; 95% CI −140.99 to −71.10, P < 0.001; Table 3, Figure 2A).
Table 3.
The pooled outcomes between ULIF and MI-TLIF group.
| Outcomes | Included studies | ULIF* | MI-TLIF* | WMD/SMD or RR | 95% CI | P Effect | Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|---|
| I 2 | P | ||||||||
| Surgical data | |||||||||
| EBL | 6 | 197 | 227 | −106.00 | −140.99 | −71.10 | <0.001 | 98.0% | <0.001 |
| ORT | 7 | 229 | 282 | 22.91 | 10.60 | 35.23 | <0.001 | 92.8% | <0.001 |
| LOS | 6 | 206 | 236 | −1.27 | −1.88 | −0.66 | <0.001 | 66.4% | 0.011 |
| Post-operative drainage | 3 | 104 | 100 | −47.98 | −68.15 | −27.81 | <0.001 | 89.2% | <0.001 |
| Clinical outcomes | |||||||||
| ODI | |||||||||
| ODI at 1–2 week post-op | 4 | 164 | 194 | −4.70 | −9.13 | −0.27 | 0.038 | 92.2% | <0.001 |
| ODI at 1 month post-op | 3 | 107 | 97 | −2.12 | −3.53 | −0.72 | 0.003 | 40.1% | 0.188 |
| ODI at 3 month post-op | 5 | 196 | 237 | −1.49 | −2.77 | −0.22 | 0.022 | 43.7% | 0.130 |
| ODI at 6 month post-op | 3 | 151 | 148 | −1.07 | −4.00 | 1.86 | 0.473 | 70.2% | 0.035 |
| ODI at finial follow-up | 8 | 301 | 355 | −0.23 | −0.69 | 0.24 | 0.346 | 11.2% | 0.343 |
| VAS-BP | |||||||||
| VAS-BP at 1–2 day post-op | 2 | 95 | 119 | −1.22 | −1.30 | −1.13 | <0.001 | 33.0% | 0.222 |
| VAS-BP at 1–2 week post-op | 2 | 67 | 96 | −1.08 | −1.50 | −0.65 | <0.001 | 0.0% | 0.893 |
| VAS-BP at 1 month post-op | 4 | 149 | 168 | −0.86 | −1.15 | −0.58 | <0.001 | 36.5% | 0.193 |
| VAS-BP at 6 month post-op | 2 | 119 | 105 | −0.03 | −0.37 | 0.30 | 0.853 | 0.0% | 0.383 |
| VAS-BP at finial follow-up | 6 | 244 | 287 | −0.12 | −0.25 | 0.01 | 0.069 | 0.0% | 0.995 |
| VAS-LP | |||||||||
| VAS-LP at 1–2 week post-op | 2 | 67 | 96 | −0.20 | −0.56 | 0.16 | 0.281 | 0.0% | 1.000 |
| VAS-LP at 1 month post-op | 4 | 149 | 168 | −0.15 | −0.34 | 0.03 | 0.100 | 0.0% | 0.592 |
| VAS-LP at 6 month post-op | 2 | 119 | 105 | 0.49 | −0.02 | 1.00 | 0.059 | 0.0% | 0.710 |
| VAS-LP at finial follow-up | 6 | 244 | 287 | −0.02 | −0.17 | 0.13 | 0.843 | 0.0% | 0.563 |
| Excellent/good rate | 3 | 86.9% (86/99) | 86.3% (120/139) | 1.00 | 0.91 | 1.11 | 0.951 | 0.0% | 0.856 |
| Laboratory outcomes | |||||||||
| CPK | 2 | 72 | 57 | −1.15 | −1.86 | −0.45 | 0.001 | 69.0% | 0.057 |
| CRP | 2 | 72 | 57 | −1.21 | −1.59 | −0.83 | <0.001 | 42.3% | 0.188 |
| Radiographic outcomes | |||||||||
| Fusion rate | 7 | 89.8% (264/294) | 87.7% (299/341) | 1.02 | 0.96 | 1.07 | 0.545 | 0.0% | 0.973 |
| Cage subsidence | 3 | 0.8% (1/127) | 4.3% (7/162) | 0.34 | 0.08 | 1.46 | 0.146 | 0.0% | 0.498 |
| Unplanned return to OR | 4 | 1.1% (2/174) | 1.9% (4/206) | 0.76 | 0.20 | 2.93 | 0.687 | 0.0% | 0.689 |
| Surgical complications | |||||||||
| Overall | 7 | 6.9% (19/276) | 6.7% (22/330) | 0.96 | 0.53 | 1.74 | 0.896 | 0.0% | 0.992 |
| Epidural hematoma | 5 | 2.9% (5/172) | 2.3% (5/214) | 1.19 | 0.37 | 3.83 | 0.775 | 0.0% | 0.951 |
| Dural tear | 5 | 4.8% (10/209) | 2.1% (5/234) | 2.08 | 0.76 | 5.74 | 0.156 | 0.0% | 0.850 |
| Surgical site infection | 5 | 0.0% (0/209) | 2.6% (6/234) | 0.34 | 0.09 | 1.33 | 0.120 | 0.0% | 0.985 |
| Neurologic deficits | 3 | 3.5% (4/114) | 4.7% (6/128) | 0.74 | 0.22 | 2.50 | 0.632 | 0.0% | 0.721 |
n or incidence (events/total).
ULIF, unilateral biportal endoscopic lumbar interbody fusion; MI-TLIF, minimally invasive transforaminal lumbar interbody fusion; WMD, weighted mean difference; SMD, standard mean difference; RR, risk ratio; CI, confidence interval; EBL, estimated blood loss; ORT, operating time; LOS, length of hospital stay; ODI, Oswestry Disability Index; VAS-BP, Visual Analog Scale score for back pain; VAS-LP, Visual Analog Scale score for leg pain; CPK, creatine phosphokinase; CRP, C-reactive protein; OR, operating room.
Figure 2.
Forest plot of the estimated blood loss. (A) ULIF vs. MI-TLIF; (B) ULIF vs. PLIF.
ULIF vs. PLIF
Estimated blood loss could be obtained in two studies (36, 39), and significant heterogeneity was detected (I2 = 92.1%, P < 0.001). The pooled results revealed significantly reduced EBL in the ULIF group compared with that in the PLIF group (WMD, −149.22; 95% CI −284.98 to −13.47, P = 0.031; Table 4, Figure 2B).
Table 4.
The pooled outcomes between ULIF and PLIF group.
| Outcomes | Included studies | ULIF* | PLIF* | WMD/ SMD or RR | 95% CI | P effect | Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|---|
| I 2 | P | ||||||||
| Surgical data | |||||||||
| EBL | 2 | 56 | 72 | −149.22 | −284.98 | −13.47 | 0.031 | 92.1% | <0.001 |
| ORT | 4 | 148 | 177 | 48.30 | 26.07 | 70.54 | <0.001 | 94.7% | <0.001 |
| LOS | 3 | 77 | 107 | −4.40 | −8.04 | −0.75 | 0.018 | 96.9% | <0.001 |
| Post-operative drainage | 3 | 77 | 107 | −139.84 | −216.22 | −63.47 | <0.001 | 95.5% | <0.001 |
| Clinical outcomes | |||||||||
| ODI | |||||||||
| ODI at 1–2 week post-op | 2 | 56 | 72 | −3.40 | −4.02 | −2.78 | <0.001 | 9.6% | 0.293 |
| ODI at 1 month post-op | 2 | 48 | 68 | −3.12 | −5.72 | −0.53 | 0.018 | 0.0% | 0.551 |
| ODI at finial follow-up | 4 | 138 | 177 | −1.97 | −3.32 | −0.62 | 0.004 | 37.7% | 0.186 |
| VAS-BP | |||||||||
| VAS-BP at 1–2 week post-op | 2 | 88 | 103 | −1.07 | −1.77 | −0.38 | 0.002 | 78.9% | 0.030 |
| VAS-BP at finial follow-up | 3 | 117 | 142 | −0.17 | −0.37 | 0.04 | 0.114 | 0.0% | 0.574 |
| VAS-LP | |||||||||
| VAS-LP at 1–2 week post-op | 2 | 88 | 103 | −0.40 | −0.72 | −0.08 | 0.014 | 0.0% | 0.465 |
| VAS-LP at finial follow-up | 3 | 117 | 142 | 0.01 | −0.20 | 0.22 | 0.937 | 29.9% | 0.240 |
| Excellent/good rate | 2 | 83.3% (40/48) | 85.3% (58/68) | 0.97 | 0.82 | 1.14 | 0.709 | 0.0% | 0.561 |
| Radiographic outcomes | |||||||||
| Fusion rate | 2 | 94.3% (83/88) | 90.3% (93/103) | 1.04 | 0.96 | 1.13 | 0.296 | 0.0% | 0.690 |
| Surgical complications | |||||||||
| Overall | 4 | 6.8% (10/148) | 5.1% (9/177) | 1.29 | 0.56 | 2.95 | 0.553 | 0.0% | 0.429 |
| Dural tear | 4 | 4.7% (7/148) | 2.8% (5/177) | 1.68 | 0.57 | 4.92 | 0.344 | 0.0% | 0.762 |
| Neurologic deficits | 2 | 1.1% (1/92) | 1.0% (1/105) | 1.25 | 0.20 | 7.64 | 0.811 | 28.5% | 0.237 |
n or incidence (events/total).
ULIF, unilateral biportal endoscopic lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; WMD, weighted mean difference; SMD, standard mean difference; RR, risk ratio; CI, confidence interval; EBL, estimated blood loss; ORT, operating time; LOS, length of hospital stay; ODI, Oswestry Disability Index; VAS-BP, Visual Analog Scale score for back pain; VAS-LP, Visual Analog Scale score for leg pain.
Operating time
ULIF vs. MI-TLIF
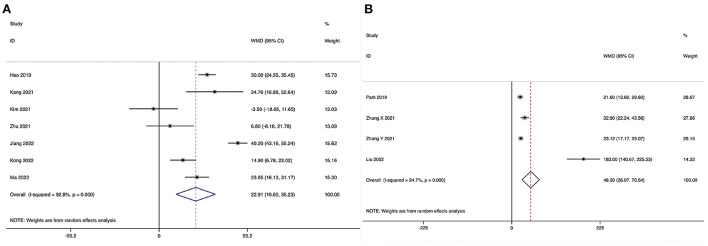
Operating time could be obtained in seven studies (28–31, 33–35), and significant heterogeneity was detected (I2 = 92.8%, P < 0.001). The pooled results revealed significantly prolonged ORT in the ULIF group compared with that in the MI-TLIF group (WMD, 22.91; 95% CI 10.60–35.23, P < 0.001; Figure 3A).
Figure 3.
Forest plot of the operating time. (A) ULIF vs. MI-TLIF; (B) ULIF vs. PLIF.
ULIF vs. PLIF
Operating time could be obtained in four studies (36–39), and significant heterogeneity was detected (I2 = 94.7%, P < 0.001). The pooled results revealed significantly prolonged ORT in the ULIF group compared with that in the PLIF group (WMD, 48.30; 95% CI 26.07–70.54, P < 0.001; Figure 3B).
Length of hospital stay
ULIF vs. MI-TLIF
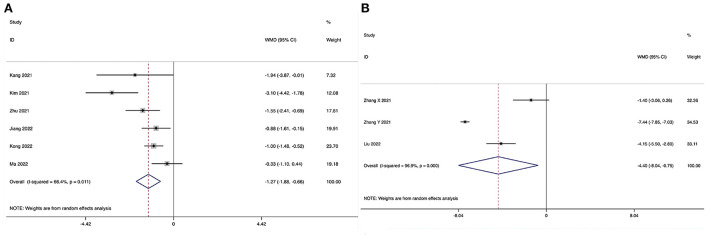
The length of hospital stay could be obtained in six studies (28–31, 34, 35), and significant heterogeneity was detected (I2 = 66.4%, P = 0.011). The pooled results revealed significantly reduced LOS in the ULIF group compared with that in the MI-TLIF group (WMD, −1.27; 95% CI −1.88 to −0.66, P < 0.001; Figure 4A).
Figure 4.
Forest plot of the length of hospital stay. (A) ULIF vs. MI-TLIF; (B) ULIF vs. PLIF.
ULIF vs. PLIF
The length of hospital stay could be obtained in three studies (36, 37, 39), and significant heterogeneity was detected (I2 = 96.9%, P < 0.001). The pooled results revealed significantly reduced LOS in the ULIF group compared with that in the PLIF group (WMD, −4.40; 95% CI −8.04 to −0.75, P = 0.018; Figure 4B).
Post-operative drainage
ULIF vs. MI-TLIF
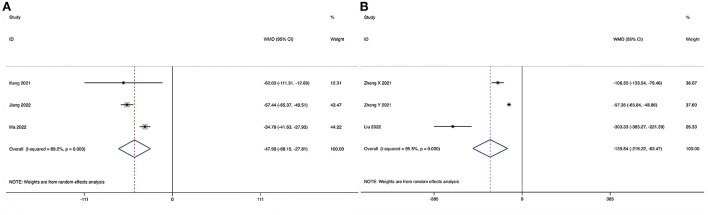
Post-operative drainage could be obtained in three studies (28, 30, 34), and significant heterogeneity was detected (I2 = 89.2%, P < 0.001). The pooled results revealed significantly reduced post-operative drainage in the ULIF group compared with that in the MI-TLIF group (WMD, −47.98; 95% CI −68.15 to −27.81, P < 0.001; Figure 5A).
Figure 5.
Forest plot of the post-operative drainage. (A) ULIF vs. MI-TLIF; (B) ULIF vs. PLIF.
ULIF vs. PLIF
Post-operative drainage could be obtained in three studies (36, 37, 39), and significant heterogeneity was detected (I2 = 95.5%, P < 0.001). The pooled results revealed significantly reduced post-operative drainage in the ULIF group compared with that in the PLIF group (WMD, −139.84; 95% CI −216.22 to −63.47, P < 0.001; Figure 5B).
Clinical outcomes
Oswestry disability index
One to two weeks post-operatively
ULIF vs. MI-TLIF.
The Oswestry Disability Index at 1–2 weeks post-operatively could be obtained in four studies (28, 31, 32, 35), and significant heterogeneity was detected (I2 = 92.2%, P < 0.001). The pooled results revealed significantly lower ODI at 1–2 weeks post-operatively in the ULIF group compared with that in the MI-TLIF group (WMD, −4.70; 95% CI −9.13 to −0.27, P = 0.038).
ULIF vs. PLIF.
The Oswestry Disability Index at 1–2 weeks post-operatively could be obtained in two studies (36, 39), and no significant heterogeneity was detected (I2 = 9.6%, P = 0.293). The pooled results revealed significantly lower ODI at 1–2 weeks post-operatively in the ULIF group compared with that in the PLIF group (WMD, −3.40; 95% CI −4.02 to −2.78, P < 0.001).
One month post-operatively
ULIF vs. MI-TLIF.
The Oswestry Disability Index at 1 month post-operatively could be obtained in three studies (28, 29, 34), and no significant heterogeneity was detected (I2 = 40.1%, P = 0.188). The pooled results revealed significantly lower ODI at 1 month post-operatively in the ULIF group compared with that in the MI-TLIF group (WMD, −2.12; 95% CI −3.53 to −0.72, P = 0.003).
ULIF vs. PLIF.
The Oswestry Disability Index at 1 month post-operatively could be obtained in two studies (37, 39), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.551). The pooled results revealed significantly lower ODI at 1 month post-operatively in the ULIF group compared with that in the PLIF group (WMD, −3.12; 95% CI −5.72 to −0.53, P = 0.018).
The third month post-operatively
ULIF vs. MI-TLIF.
The Oswestry Disability Index at 3 month post-operatively could be obtained in five studies (28, 30–32, 35), and no significant heterogeneity was detected (I2 = 43.7%, P = 0.130). The pooled results revealed significantly lower ODI at 3 month post-operatively in the ULIF group compared with that in the MI-TLIF group (WMD, −1.49; 95% CI −2.77 to −0.22, P = 0.022).
The sixth month post-operatively
ULIF vs. MI-TLIF.
The Oswestry Disability Index at 6 month post-operatively could be obtained in three studies (30, 32, 34), and significant heterogeneity was detected (I2 = 70.2%, P = 0.035). The pooled results revealed no significant difference in ODI at 6 month post-operatively between the ULIF group and the MI-TLIF group (WMD, −1.07; 95% CI −4.00–1.86, P = 0.473).
Final follow-up
ULIF vs. MI-TLIF.
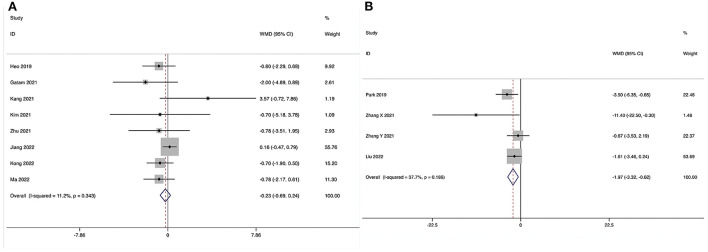
The Oswestry Disability Index at the final follow-up could be obtained in eight studies (28–35), and no significant heterogeneity was detected (I2 = 11.2%, P = 0.343). The pooled results revealed no significant difference in ODI at the final follow-up between the ULIF group and the MI-TLIF group (WMD, −0.23; 95% CI −0.69–0.24, P = 0.346; Figure 6A).
Figure 6.
Forest plot of the Oswestry Disability Index at the final follow-up. (A) ULIF vs. MI-TLIF; (B) ULIF vs. PLIF.
ULIF vs. PLIF.
The Oswestry Disability Index at the final follow-up could be obtained in four studies (36–39), and no significant heterogeneity was detected (I2 = 37.7%, P = 0.186). The pooled results revealed significantly lower ODI at the final follow-up in the ULIF group compared with that in the PLIF group (WMD, −1.97; 95% CI −3.32 to −0.62, P = 0.004; Figure 6B).
Visual Analog Scale score for back pain
One to two days post-operatively
ULIF vs. MI-TLIF
The Visual Analog Scale score for back pain at 1–2 days post-operatively could be obtained in two studies (32, 33), and no significant heterogeneity was detected (I2 = 33.0%, P = 0.222). The pooled results revealed significantly lower VAS-BP at 1–2 days post-operatively in the ULIF group compared with that in the MI-TLIF group (WMD, −1.22; 95% CI −1.30 to −1.13, P < 0.001).
One to two weeks post-operatively
ULIF vs. MI-TLIF
The Visual Analog Scale score for back pain at 1–2 weeks post-operatively could be obtained in two studies (31, 35), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.893). The pooled results revealed significantly lower VAS-BP at 1–2 weeks post-operatively in the ULIF group compared with that in the MI-TLIF group (WMD, −1.08; 95% CI −1.50 to −0.65, P < 0.001).
ULIF vs. PLIF
The Visual Analog Scale score for back pain at 1–2 weeks post-operatively could be obtained in two studies (38, 39), and significant heterogeneity was detected (I2 = 78.9%, P = 0.030). The pooled results revealed significantly lower VAS-BP at 1–2 weeks post-operatively in the ULIF group compared with that in the PLIF group (WMD, −1.07; 95% CI −1.77 to −0.38, P = 0.002).
One month post-operatively
ULIF vs. MI-TLIF
The Visual Analog Scale score for back pain at 1 month post-operatively could be obtained in four studies (29, 31, 34, 35), and no significant heterogeneity was detected (I2 = 36.5%, P = 0.193). The pooled results revealed significantly lower VAS-BP at 1 month post-operatively in the ULIF group compared with that in the MI-TLIF group (WMD, −0.86; 95% CI −1.15 to −0.58, P < 0.001).
The sixth month post-operatively
ULIF vs. MI-TLIF
The Visual Analog Scale score for back pain at 6 month post-operatively could be obtained in two studies (32, 34), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.383). The pooled results revealed no significant difference in VAS-BP at 6 month post-operatively between the ULIF group and the MI-TLIF group (WMD, −0.03; 95% CI −0.37–0.30, P = 0.853).
Final follow-up
ULIF vs. MI-TLIF
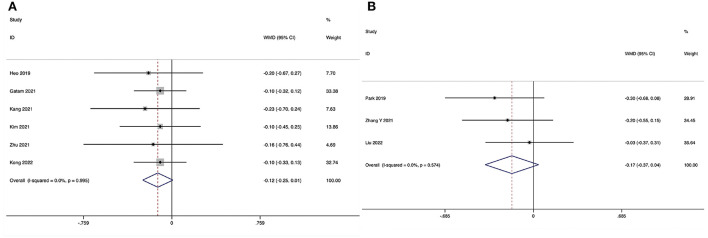
The Visual Analog Scale score for back pain at the final follow-up could be obtained in six studies (29, 31–35), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.995). The pooled results revealed no significant difference in VAS-BP at the final follow-up between the ULIF group and the MI-TLIF group (WMD, −0.12; 95% CI −0.25–0.01, P = 0.069; Figure 7A).
Figure 7.
Forest plot of the Visual Analog Scale score for back pain at the final follow-up. (A) ULIF vs. MI-TLIF; (B) ULIF vs. PLIF.
ULIF vs. PLIF
The Visual Analog Scale score for back pain at the final follow-up could be obtained in three studies (36, 38, 39), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.574). The pooled results revealed no significant difference in VAS-BP at the final follow-up between the ULIF group and the PLIF group (WMD, −0.17; 95% CI −0.37–0.04, P = 0.114; Figure 7B).
Visual Analog Scale score for leg pain
One to two weeks post-operatively
ULIF vs. MI-TLIF
The Visual Analog Scale score for leg pain at 1–2 weeks post-operatively could be obtained in two studies (31, 35), and no substantial heterogeneity was detected (I2 = 0.0%, P = 1.000). The pooled results revealed no significant difference in VAS-LP at 1–2 weeks post-operatively between the ULIF group and the MI-TLIF group (WMD, −0.20; 95% CI −0.56–0.16, P = 0.281).
ULIF vs. PLIF
The Visual Analog Scale score for leg pain at 1–2 weeks post-operatively could be obtained in two studies (38, 39), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.465). The pooled results revealed significantly lower VAS-LP at 1–2 weeks post-operatively in the ULIF group compared with that in the PLIF group (WMD, −0.40; 95% CI −0.72 to −0.08, P = 0.014).
One month post-operatively
ULIF vs. MI-TLIF
The Visual Analog Scale score for leg pain at 1 month post-operatively could be obtained in four studies (29, 31, 34, 35), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.592). The pooled results revealed no significant difference in VAS-LP at 1 month post-operatively between the ULIF group and the MI-TLIF group (WMD, −0.15; 95% CI −0.34–0.03, P = 0.100).
The sixth month post-operatively
ULIF vs. MI-TLIF
The Visual Analog Scale score for leg pain at 6 month post-operatively could be obtained in two studies (32, 34), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.710). The pooled results revealed no significant difference in VAS-LP at 6 month post-operatively between the ULIF group and the MI-TLIF group (WMD, 0.49; 95% CI −0.02–1.00, P = 0.059).
Final follow-up
ULIF vs. MI-TLIF
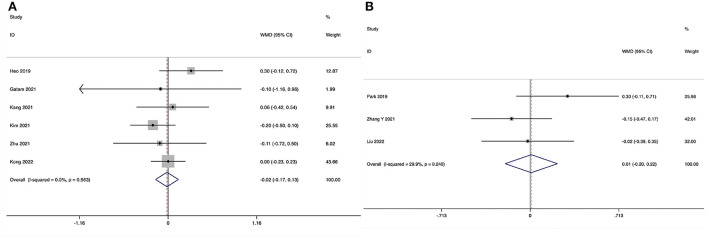
The Visual Analog Scale score for leg pain at the final follow-up could be obtained in six studies (29, 31–35), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.563). The pooled results revealed no significant difference in VAS-LP at the final follow-up between the ULIF group and the MI-TLIF group (WMD, −0.02; 95% CI −0.17–0.13, P = 0.843; Figure 8A).
Figure 8.
Forest plot of the Visual Analog Scale score for leg pain at the final follow-up. (A) ULIF vs. MI-TLIF; (B) ULIF vs. PLIF.
ULIF vs. PLIF
The VAS-BP at the final follow-up could be obtained in three studies (36, 38, 39), and no significant heterogeneity was detected (I2 = 29.9%, P = 0.240). The pooled results revealed no significant difference in VAS-LP at the final follow-up between the ULIF group and the PLIF group (WMD, 0.01; 95% CI −0.20–0.22, P = 0.937; Figure 8B).
Excellent/good rate of modified Macnab criteria
ULIF vs. MI-TLIF
The excellent/good rate of surgical therapy according to the modified Macnab criteria at the final follow-up could be obtained in three studies (30, 31, 35), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.856). The pooled results revealed no significant difference in the excellent/good rate of modified Macnab criteria between the ULIF group and the MI-TLIF group (RR, 1.00; 95% CI 0.91–1.11, P = 0.951).
ULIF vs. PLIF
The excellent/good rate of surgical therapy according to the modified Macnab criteria at the final follow-up could be obtained in two studies (37, 39), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.561). The pooled results revealed no significant difference in the excellent/good rate of modified Macnab criteria between the ULIF group and the PLIF group (RR, 0.97; 95% CI 0.82–1.14, P = 0.709).
Laboratory outcomes
Creatine phosphokinase
ULIF vs. MI-TLIF
Post-operative CPK could be obtained in two studies (28, 34), and significant heterogeneity was detected (I2 = 69.0%, P = 0.057). The pooled results revealed significantly lower post-operative CPK in the ULIF group compared with that in the MI-TLIF group (SMD, −1.15; 95% CI −1.86 to −0.45, P = 0.001).
C-reactive protein
ULIF vs. MI-TLIF
Post-operative CRP could be obtained in two studies (28, 34), and no significant heterogeneity was detected (I2 = 42.3%, P = 0.188). The pooled results revealed significantly lower post-operative CRP in the ULIF group compared with that in the MI-TLIF group (SMD, −1.21; 95% CI −1.59 to −0.83, P < 0.001).
Radiographic outcomes
Fusion rate
ULIF vs. MI-TLIF
The fusion rate at the final follow-up could be obtained in seven studies (29–35), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.973). The pooled results revealed no significant difference in fusion rate between the ULIF group and the MI-LIF group (RR, 1.02; 95% CI 0.96–1.07, P = 0.545; Figure 9A).
Figure 9.
Forest plot of the fusion rate. (A) ULIF vs. MI-TLIF; (B) ULIF vs. PLIF.
ULIF vs. PLIF
The fusion rate at the final follow-up could be obtained in two studies (38, 39), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.690). The pooled results revealed no significant difference in fusion rate between the ULIF group and the PLIF group (RR, 1.04; 95% CI 0.96–1.13, P = 0.296; Figure 9B).
Cage subsidence
ULIF vs. MI-TLIF
The incidence of cage subsidence at the final follow-up could be obtained in three studies (30, 32, 33), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.498). The pooled results revealed no significant difference in the incidence of cage subsidence between the ULIF group and the MI-TLIF group (RR, 0.34; 95% CI 0.08–1.46, P = 0.146).
Unplanned return to the operating room
ULIF vs. MI-TLIF
The incidence of unplanned return to OR could be obtained in four studies (32–35), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.689). The pooled results revealed no significant difference in the incidence of unplanned return to OR between the ULIF group and the MI-TLIF group (RR, 0.76; 95% CI 0.20–2.93, P = 0.687).
Surgical complications
Overall
ULIF vs. MI-TLIF
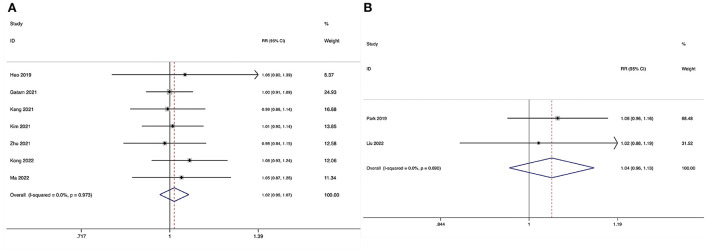
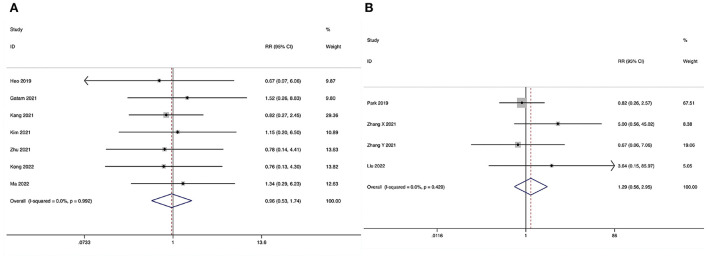
The overall surgical complication rate during the perioperative period could be obtained in seven studies (29–35), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.992). The pooled results revealed no significant difference in the overall surgical complication rate between the ULIF group and the MI-TLIF group (RR, 0.96; 95% CI 0.53–1.74, P = 0.896; Figure 10A).
Figure 10.
Forest plot of the overall surgical complication rate. (A) ULIF vs. MI-TLIF; (B) ULIF vs. PLIF.
ULIF vs. PLIF
The overall surgical complication rate during the perioperative period could be obtained in four studies (36–39), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.429). The pooled results revealed no significant difference in the overall surgical complication rate between the ULIF group and the PLIF group (RR, 1.29; 95% CI 0.56–2.95, P = 0.553; Figure 10B).
Epidural hematoma
ULIF vs. MI-TLIF
The incidence of epidural hematoma could be obtained in five studies (29, 31, 33–35), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.951). The pooled results revealed no significant difference in the incidence of an epidural hematoma between the ULIF group and the MI-TLIF group (RR, 1.19; 95% CI 0.37–3.83, P = 0.775).
Dural tear
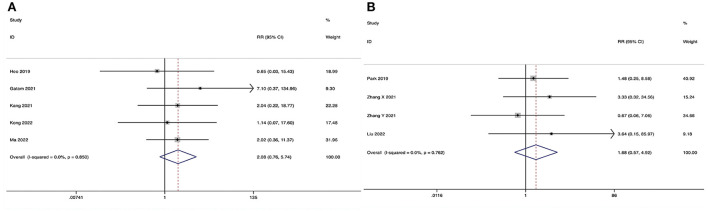
ULIF vs. MI-TLIF
The incidence of dural tear could be obtained in five studies (29, 30, 32–34), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.850). The pooled results revealed no significant difference in the incidence of dural tear between the ULIF group and the MI-TLIF group (RR, 2.08; 95% CI 0.76–5.74, P = 0.156; Figure 11A).
Figure 11.
Forest plot of the incidence of dural tear. (A) ULIF vs. MI-TLIF; (B) ULIF vs. PLIF.
ULIF vs. PLIF
The incidence of dural tear could be obtained in four studies (36–39), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.762). The pooled results revealed no significant difference in the incidence of dural tear between the ULIF group and the PLIF group (RR, 1.68; 95% CI 0.57–4.92, P = 0.344; Figure 11B).
Surgical site infection
ULIF vs. MI-TLIF
The incidence of surgical site infection could be obtained in five studies (29, 30, 32–34), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.985). The pooled results revealed no significant difference in the incidence of surgical site infection between the ULIF group and the MI-TLIF group (RR, 0.34; 95% CI 0.09–1.33, P = 0.120).
Neurologic deficits
ULIF vs. MI-TLIF
The incidence of neurologic deficits could be obtained in three studies (31, 34, 35), and no substantial heterogeneity was detected (I2 = 0.0%, P = 0.721). The pooled results revealed no significant difference in the incidence of neurologic deficits between the ULIF group and the MI-TLIF group (RR, 0.74; 95% CI 0.22–2.50, P = 0.632).
ULIF vs. PLIF
The incidence of neurologic deficits could be obtained in two studies (37, 38), and no significant heterogeneity was detected (I2 = 28.5%, P = 0.237). The pooled results revealed no significant difference in the incidence of neurologic deficits between the ULIF group and the PLIF group (RR, 1.25; 95% CI 0.20–7.64, P = 0.811).
Sensitivity analyses
The sensitivity analyses indicated that the additional omission of any study would not significantly affect the results, which verified the stability of the data and rationality of the analyses.
Discussion
Enhanced Recovery After Surgery is a multidisciplinary perioperative care pathway designed to achieve early recovery for patients undergoing major surgery. The three phases of ERAS protocol are pre-operative, intraoperative, and post-operative periods, and the key components include optimization of nutrition, emotional support, multimodal opioid-sparing analgesia, antimicrobial prophylaxis, appropriate surgical procedure, and early mobilization (40). Since the first publication of the ERAS consensus statement in 2005, the ERAS Society has now published guidelines in more than 20 surgical specialties, including colorectal surgery (11), pancreatoduodenectomy (41), radical cystectomy (42), gastrectomy (43), bariatric surgery (44), liver surgery (45), lung surgery (46), and cardiac surgery (47). For spine surgery, some cohort studies and a meta-analysis suggested that improved outcomes could be obtained through the implementation of ERAS protocols during the perioperative period (48–51). In 2021, an evidence-based recommendation for lumbar fusion surgery was developed by the ERAS Society (52). Although surgical techniques should be decided on a case-by-case basis, the minimally invasive technique achieved a strong recommendation grade because is paramount for post-operative recovery (52, 53).
Conventional MI-TLIF and open PLIF were effective surgical procedures of lumbar interbody fusion for treating LDD, but the paraspinal muscle damage and blood loss may delay pain relief and functional recovery. With the advancement of optical technologies, water-based endoscopic procedures have gained popularity (54–57). ULIF, combining the endoscope and the minimally invasive spine instruments, has been increasingly used as an alternative to conventional lumbar interbody fusion techniques (18, 58). This systematic review and meta-analysis directly compared the outcomes and complications of ULIF to conventional MI-TLIF or PLIF for LDD. Different from the previous meta-analysis by Lin et al. the current study did not merge the patients who underwent MI-TLIF or PLIF in a single group because these two posterior procedures had very different paraspinal muscle injury levels (59). The results revealed that there was no significant difference in the radiographic outcomes and complications between the ULIF group and MI-TLIF or PLIF group. Nevertheless, enhanced recovery was observed through superior clinical outcomes, surgical data, and laboratory outcomes in patients receiving ULIF.
The LOS was shortened by 1.27 days (P < 0.001) and 4.40 days (P = 0.018) in the ULIF group compared with the MI-TLIF group and the PLIF group, respectively. This effect was associated with enhanced pain relief and function recovery by ULIF. The current study suggests that ULIF has a significantly better short-term improvement in VAS-BP and ODI than both MI-TLIF and PLIF groups. In addition, an enhanced short-term improvement in VAS-LP and long-term improvement in ODI were noted in the ULIF group compared with the PLIF group. These findings may be attributed to the reduced atrophy, denervation, and ischemic paraspinal muscle damage caused by dissection and retraction (60, 61). Furthermore, the endoscope provides a clear and magnified view, allowing more precise manipulation for the decompression of the central canal, lateral recess, and bilateral nerve roots (32). The study by Kim et al. reported that there was no significant difference in the early and final ODI between unilateral biportal endoscopic and open microscopic techniques for lumbar discectomy (61). Therefore, preserving paraspinal muscle and posterior soft tissue may benefit lumbar interbody fusion more than sole discectomy, which requires less muscle retraction. Inevitable systemic inflammatory response due to iatrogenic muscle injury is associated with post-operative pain and disability (34, 62). Thus, effective alleviation or suppression of the inflammatory response is essential for the enhanced recovery of patients. CPK and CRP were presentative biomarkers, which peeked on post-operative 2–3 days, recovering to the normal range weeks after the surgery (39, 63). In the current study, the peek of both CPK and CRP was significantly lower in the ULIF group, indicating that the biportal endoscopic technique produces less systemic inflammatory response than conventional procedures. This advantage may also relate to favorable pain relief, function improvement, and LOS.
Like conventional procedures, ULIF is also frequently accompanied by substantial surgical blood loss, especially when resecting ligamentum flavum and superior articular process, which would postpone the recovery and induce complications (63, 64). In this study, we found that the EBL and post-operative drainage in the ULIF group were significantly reduced than both the MI-TLIF group and the PLIF group. Continuous fluid irrigation played a vital role in controlling epidural and bone surface hemorrhage. However, the pressure of irrigation and constant outflow should be noted to prevent post-operative neck pain and seizures caused by increased intracranial pressure (65). In addition, rather than electrocautery, bipolar radiofrequency ablation could be applied to obtain effective microvascular coagulation around the dural sac (34, 38, 66). Therefore, better bleeding control leads to less post-operative drainage and early mobilization in patients who underwent ULIF.
Evaluating the fusion rate is of paramount importance for patients who underwent lumbar interbody fusion, as failed solid fusion could jeopardize the surgical effect and quality of life (67). This study yielded similar fusion rates between ULIF and conventional procedures (ULIF vs. MI-TLIF, 89.8 vs. 87.7%; ULIF vs. PLIF, 94.3 vs. 90.3%). Some advantages of the biportal endoscopic system might facilitate the fusion rate of ULIF. Meticulous endplate preparation could be performed under the clean and magnified real-time surgical visualization, offering a favorable fusion environment by completely removing the cartilaginous portion (35, 68). Continuous fluid irrigation may disperse the thermal energy, which could induce necrosis of the endplate and further cage subsidence (34, 69). Moreover, unlike the uniportal endoscopic system which only allows small-sized cages to pass the cannula, large-sized or expandable cages could be used in an independent working portal, which obtained a favorable fusion rate as conventional procedures.
Although there are various advantages, longer ORT and a steep learning curve are the potential drawbacks of ULIF. In the current study, the ORT of the ULIF group was 179.63 ± 29.34 min in MI-TLIF studies and 184.43 ± 41.50 min in PLIF studies, which were significantly longer than the 148.01 ± 24.17 min in the MI-TLIF group and 130.87 ± 23.22 min of the PLIF group. The biportal endoscopic technique is just like arthroscopy. During the initial stages of the learning curve, single-handed instrument handling and identification of anatomical landmarks may be factors that increase the ORT for ULIF (38). For less-experienced surgeons, delicate decompression manipulations may become complex and easily induce complications. Although the surgical complication rate was similar between ULIF and conventional procedures (ULIF vs. MI-TLIF, 6.9 vs. 6.7%; ULIF vs. PLIF, 6.8% vs. 5.1%), the slightly higher incidence of dural tear in the ULIF group should be noted (ULIF vs. MI-TLIF, 4.8% vs. 2.1%; ULIF vs. PLIF, 4.7% vs. 2.8%). Therefore, ULIF is recommended for surgeons who have performed at least 54 cases of biportal endoscopic decompression (70). Another complication was the epidural hematoma, most likely due to oozing from the bone trapped under the intact posterior tension band, which usually could be resolved by itself (32). The incidence of this complication was low (ULIF vs. MI-TLIF, 2.9 vs. 2.3%). The incidence of surgical site infection was slightly lower in the ULIF group than in the MI-TLIF group (0.0 vs. 2.6%). This finding may be attributed to the reduced surgical smoke and wound contamination by the bipolar radiofrequency ablation.
Limitations
This study has several limitations. First, the impact of smoking was not considered due to the missing data, which could overestimate the fusion rate and cage subsidence rate (71). Second, most studies lacked data on comorbidities, which could have influenced some of the outcomes analyzed. Third, the heterogeneity of the included patients should be acknowledged because they had various LDD, including LSS, LDH, and LS. Fourth, the number of research studies focused on the comparison of ULIF and conventional procedures is still limited. Therefore, some pooled outcomes may not be reliable when more results were reported in future studies. Additionally, no randomized controlled study was included at a higher level of methodological quality. Further multicenter randomized controlled trials with longer follow-up periods should be performed to obtain more convincing conclusions.
Conclusion
Compared with conventional MI-TLIF and PLIF, ULIF was associated with reduced EBL, shorter LOS, alleviated inflammatory response, and comparable fusion rate as well as complication management. Compared with MI-TLIF, a better short-term improvement in VAS-BP and ODI was achieved by ULIF; compared with open PLIF, additional enhanced short-term improvement in VAS-LP and long-term improvement in ODI were observed in ULIF. ULIF could enhance the recovery of patients with LDD compared with conventional posterior procedures.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HY, YL, and AP contributed to the study concept and design, revised, and edited the manuscript. HY, FC, and YL took part in the initial literature search and assessed the eligibilities of feasible studies. HY and FC interpreted the findings and wrote the first draft of the manuscript. HY, FC, and AP prepared the figures and tables. All authors approved the final version of the manuscript, contributed to the article, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Reid PC, Morr S, Kaiser MG. State of the union: a review of lumbar fusion indications and techniques for degenerative spine disease. J Neurosurg Spine. (2019) 31:1–14. 10.3171/2019.4.SPINE18915 [DOI] [PubMed] [Google Scholar]
- 2.Zhang C, Li Z, Yu K, Wang Y. A postoperative phenomenon of percutaneous endoscopic lumbar discectomy: rebound pain. Orthop Surg. (2021) 13:2196–205. 10.1111/os.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including Plif, Tlif, Mi-Tlif, Olif/Atp, llif and alif. J Spine Surg. (2015) 1:2–18. 10.3978/j.issn.2414-469X.2015.10.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hey HW, Hee HT. Lumbar degenerative spinal deformity: surgical options of Plif, Tlif and Mi-Tlif. Indian J Orthop. (2010) 44:159–62. 10.4103/0019-5413.62066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. (2013) 118:934–44. 10.1097/ALN.0b013e31828866b3 [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Yang H, Hai Y, Cheng Y. Perioperative low-dose ketamine for postoperative pain management in spine surgery: a systematic review and meta-analysis of randomized controlled trials. Pain Res Manag. (2022) 2022:1507097. 10.1155/2022/1507097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan SW, Hu ZJ, Fang XQ, Zhao FD, Huang Y, Yu HJ. Comparison of paraspinal muscle injury in one-level lumbar posterior inter-body fusion: modified minimally invasive and traditional open approaches. Orthop Surg. (2010) 2:194–200. 10.1111/j.1757-7861.2010.00086.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortesi PA, Assietti R, Cuzzocrea F, Prestamburgo D, Pluderi M, Cozzolino P, et al. Epidemiologic and economic burden attributable to first spinal fusion surgery: analysis from an italian administrative database. Spine. (2017) 42:1398–404. 10.1097/BRS.0000000000002118 [DOI] [PubMed] [Google Scholar]
- 9.Resnick DK, Tosteson AN, Groman RF, Ghogawala Z. Setting the equation: establishing value in spine care. Spine. (2014) 39 (22 Suppl. 1):S43–50. 10.1097/BRS.0000000000000581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. (1997) 78:606–17. 10.1093/bja/78.5.606 [DOI] [PubMed] [Google Scholar]
- 11.Fearon KC, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CH, Lassen K, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. (2005) 24:466–77. 10.1016/j.clnu.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 12.Corniola MV, Debono B, Joswig H, Lemée JM, Tessitore E. Enhanced recovery after spine surgery: review of the literature. Neurosurg Focus. (2019) 46:E2. 10.3171/2019.1.FOCUS18657 [DOI] [PubMed] [Google Scholar]
- 13.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. (2017) 152:292–8. 10.1001/jamasurg.2016.4952 [DOI] [PubMed] [Google Scholar]
- 14.Wang MY, Chang PY, Grossman J. Development of an enhanced recovery after surgery (Eras) approach for lumbar spinal fusion. J Neurosurg Spine (2017) 26:411–8. 10.3171/2016.9.SPINE16375 [DOI] [PubMed] [Google Scholar]
- 15.Duojun W, Hui Z, Zaijun L, Yuxiang G, Haihong C. Enhanced recovery after surgery pathway reduces the length of hospital stay without additional complications in lumbar disc herniation treated by percutaneous endoscopic transforaminal discectomy. J Orthop Surg Res. (2021) 16:461. 10.1186/s13018-021-02606-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang MY, Chang HK, Grossman J. Reduced acute care costs with the eras® minimally invasive transforaminal lumbar interbody fusion compared with conventional minimally invasive transforaminal lumbar interbody fusion. Neurosurgery. (2018) 83:827–34. 10.1093/neuros/nyx400 [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Zhang C, Chen W, Li S, Yu B, Zhao H, et al. Percutaneous endoscopic transforaminal discectomy versus conventional open lumbar discectomy for upper lumbar disc herniation: a comparative cohort study. Biomed Res Int. (2020) 2020:1852070. 10.1155/2020/1852070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwa Eum J, Hwa Heo D, Son SK, Park CK. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine. (2016) 24:602–7. 10.3171/2015.7.SPINE15304 [DOI] [PubMed] [Google Scholar]
- 19.Soliman HM. Irrigation endoscopic decompressive laminotomy. A new endoscopic approach for spinal stenosis decompression. Spine J. (2015) 15:2282–9. 10.1016/j.spinee.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 20.Heo DH, Son SK, Eum JH, Park CK. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: technical note and preliminary clinical results. Neurosurg Focus. (2017) 43:E8. 10.3171/2017.5.FOCUS17146 [DOI] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies S. The importance of prospero to the national institute for health research. Syst Rev. (2012) 1:5. 10.1186/2046-4053-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 24.Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. (2003) 85:1–3. 10.2106/00004623-200301000-00001 [DOI] [PubMed] [Google Scholar]
- 25.Williams AL, Gornet MF, Burkus JK. Ct evaluation of lumbar interbody fusion: current concepts. AJNR Am J Neuroradiol. (2005) 26:2057–66. [PMC free article] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 28.Jiang C, Huang YH, Zuo H, Sun Y, Sun JF. [Clinical effect of unilateral biportal endoscopic lumbar interbody fusion and minimally invasive transforaminal lumbar interbody fusion on single-segment lumbar stenosis with instability]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. (2022) 44:563–9. 10.3881/j.issn.1000-503X.14549 [DOI] [PubMed] [Google Scholar]
- 29.Kong F, Zhou Q, Qiao Y, Wang W, Zhang C, Pan Q, et al. [Comparison of unilateral biportal endoscopic transforaminal lumbar interbody fusion versus minimally invasive tubular transforaminal lumbar interbody fusion for lumbar degenerative disease]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2022) 36:592–9. 10.7507/1002-1892.202201005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma ML, Ma ZJ, Wang YX, Feng XM, He YL, Li SX, et al. [Percutaneous transforaminal endoscopic decompression combined with unilateral biportal endoscopy lumbar interbody fusion in treatment of lumbar stenosis combined with lumbar spine instability]. Chin J Bone Joint Injury. (2022) 37:686–90. 10.7531/j.issn.1672-9935.2022.07.004 [DOI] [Google Scholar]
- 31.Zhu J, Gao YJ, Ren ZN, Zhu GD, Yu L, Zhang PK, et al. [Preliminary study of unilateral biportal endoscopic lumbar interbody fusion for the treatment of de-generative spinal disease]. Chin J Spine Spinal Cord. (2021) 31:1026–33. 10.3969/j.issn.1004-406X.2021.11.09 [DOI] [Google Scholar]
- 32.Gatam AR, Gatam L, Mahadhipta H, Ajiantoro A, Luthfi O, Aprilya D. Unilateral biportal endoscopic lumbar interbody fusion: a technical note and an outcome comparison with the conventional minimally invasive fusion. Orthop Res Rev. (2021) 13:229–39. 10.2147/ORR.S336479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heo DH, Park CK. Clinical results of percutaneous biportal endoscopic lumbar interbody fusion with application of enhanced recovery after surgery. Neurosurg Focus. (2019) 46:E18. 10.3171/2019.1.FOCUS18695 [DOI] [PubMed] [Google Scholar]
- 34.Kang MS, You KH, Choi JY, Heo DH, Chung HJ, Park HJ. Minimally invasive transforaminal lumbar interbody fusion using the biportal endoscopic techniques versus microscopic tubular technique. Spine J. (2021) 21:2066–77. 10.1016/j.spinee.2021.06.013 [DOI] [PubMed] [Google Scholar]
- 35.Kim JE, Yoo HS, Choi DJ, Park EJ, Jee SM. Comparison of minimal invasive versus biportal endoscopic transforaminal lumbar interbody fusion for single-level lumbar disease. Clin Spine Surg. (2021) 34:E64–71. 10.1097/BSD.0000000000001024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Zhu Y, Li Y, Lu WL, Fan XH, Li GL, et al. [Clinical study of unilateral biportal endoscopic in the treatment of 68 cases of lumbar intervertebral disc herniation]. Chin J Exp Surg. (2021) 38:2262–5. 10.3760/cma.j.cn421213-20210313-01092 [DOI] [Google Scholar]
- 37.Zhang XQ, Fan J, Tian DS, Peng QH, Hong SH, Ma DN, et al. [Comparison of short-term efficacy between unilateral biportal endoscopy and open surgery for posterior lumbar decompression and fusion]. J Nanjing Med Univer. (2021) 41:1503–8. 10.7655/NYDXBNS20211014 [DOI] [Google Scholar]
- 38.Park MK, Park SA, Son SK, Park WW, Choi SH. Clinical and radiological outcomes of unilateral biportal endoscopic lumbar interbody fusion (Ulif) compared with conventional posterior lumbar interbody fusion (Plif): 1-year follow-up. Neurosurg Rev. (2019) 42:753–61. 10.1007/s10143-019-01114-3 [DOI] [PubMed] [Google Scholar]
- 39.Liu G, Liu W, Jin D, Yan P, Yang Z, Liu R. Clinical outcomes of unilateral biportal endoscopic lumbar interbody fusion (Ulif) compared with conventional posterior lumbar interbody fusion (Plif). Spine J. (2022). 10.1016/j.spinee.2022.10.001 [DOI] [PubMed] [Google Scholar]
- 40.Kaye AD, Urman RD, Cornett EM, Hart BM, Chami A, Gayle JA, et al. Enhanced recovery pathways in orthopedic surgery. J Anaesthesiol Clin Pharmacol. (2019) 35 (Suppl. 1):S35–9. 10.4103/joacp.JOACP_35_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer M, et al. Guidelines for perioperative care for pancreaticoduodenectomy: enhanced recovery after surgery (Eras®) society recommendations. Clin Nutr. (2012) 31:817–30. 10.1016/j.clnu.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 42.Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O, Hubner M, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: enhanced recovery after surgery (Eras(®)) society recommendations. Clin Nutr. (2013) 32:879–87. 10.1016/j.clnu.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 43.Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, et al. Consensus guidelines for enhanced recovery after gastrectomy: enhanced recovery after surgery (Eras®) society recommendations. Br J Surg. (2014) 101:1209–29. 10.1002/bjs.9582 [DOI] [PubMed] [Google Scholar]
- 44.Thorell A, MacCormick AD, Awad S, Reynolds N, Roulin D, Demartines N, et al. Guidelines for perioperative care in bariatric surgery: enhanced recovery after surgery (Eras) society recommendations. World J Surg. (2016) 40:2065–83. 10.1007/s00268-016-3492-3 [DOI] [PubMed] [Google Scholar]
- 45.Melloul E, Hübner M, Scott M, Snowden C, Prentis J, Dejong CH, et al. Guidelines for perioperative care for liver surgery: enhanced recovery after surgery (Eras) society recommendations. World J Surg. (2016) 40:2425–40. 10.1007/s00268-016-3700-1 [DOI] [PubMed] [Google Scholar]
- 46.Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the enhanced recovery after surgery (Eras®) society and the european society of thoracic surgeons (Ests). Eur J Cardiothorac Surg. (2019) 55:91–115. 10.1093/ejcts/ezy301 [DOI] [PubMed] [Google Scholar]
- 47.Engelman DT, Ben Ali W, Williams JB, Perrault LP, Reddy VS, Arora RC, et al. Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg. (2019) 154:755–66. 10.1001/jamasurg.2019.1153 [DOI] [PubMed] [Google Scholar]
- 48.Pennington Z, Cottrill E, Lubelski D, Ehresman J, Theodore N, Sciubba DM. Systematic review and meta-analysis of the clinical utility of enhanced recovery after surgery pathways in adult spine surgery. J Neurosurg Spine. (2020) 34:1–23. 10.3171/2020.6.SPINE20795 [DOI] [PubMed] [Google Scholar]
- 49.Brusko GD, Kolcun JPG, Heger JA, Levi AD, Manzano GR, Madhavan K, et al. Reductions in length of stay, narcotics use, and pain following implementation of an enhanced recovery after surgery program for 1- to 3-level lumbar fusion surgery. Neurosurg Focus. (2019) 46:E4. 10.3171/2019.1.FOCUS18692 [DOI] [PubMed] [Google Scholar]
- 50.Debono B, Corniola MV, Pietton R, Sabatier P, Hamel O, Tessitore E. Benefits of enhanced recovery after surgery for fusion in degenerative spine surgery: impact on outcome, length of stay, and patient satisfaction. Neurosurg Focus. (2019) 46:E6. 10.3171/2019.1.FOCUS18669 [DOI] [PubMed] [Google Scholar]
- 51.Staartjes VE, de Wispelaere MP, Schröder ML. Improving recovery after elective degenerative spine surgery: 5-year experience with an enhanced recovery after surgery (Eras) protocol. Neurosurg Focus. (2019) 46:E7. 10.3171/2019.1.FOCUS18646 [DOI] [PubMed] [Google Scholar]
- 52.Debono B, Wainwright TW, Wang MY, Sigmundsson FG, Yang MMH, Smid-Nanninga H, et al. Consensus statement for perioperative care in lumbar spinal fusion: enhanced recovery after surgery (Eras®) society recommendations. Spine J. (2021) 21:729–52. 10.1016/j.spinee.2021.01.001 [DOI] [PubMed] [Google Scholar]
- 53.Elsarrag M, Soldozy S, Patel P, Norat P, Sokolowski JD, Park MS, et al. Enhanced recovery after spine surgery: a systematic review. Neurosurg Focus. (2019) 46:E3. 10.3171/2019.1.FOCUS18700 [DOI] [PubMed] [Google Scholar]
- 54.Gadjradj PS, Harhangi BS, Amelink J, van Susante J, Kamper S, van Tulder M, et al. Percutaneous transforaminal endoscopic discectomy versus open microdiscectomy for lumbar disc herniation: a systematic review and meta-analysis. Spine. (2021) 46:538–49. 10.1097/BRS.0000000000003843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou C, Zhang G, Panchal RR, Ren X, Xiang H, Xuexiao M, et al. Unique complications of percutaneous endoscopic lumbar discectomy and percutaneous endoscopic interlaminar discectomy. Pain Phys. (2018) 21:E105–12. 10.36076/ppj.2018.2.E105 [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Liu C, Hai Y, Yin P, Zhou L, Zhang Y, et al. Percutaneous endoscopic transforaminal lumbar interbody fusion for the treatment of lumbar spinal stenosis: preliminary report of seven cases with 12-month follow-up. Biomed Res Int. (2019) 2019:3091459. 10.1155/2019/3091459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu J, Liu H, Ao S, Zheng W, Li C, Li H, et al. Percutaneous endoscopic lumbar interbody fusion: technical note and preliminary clinical experience with 2-year follow-up. Biomed Res Int. (2018) 2018:5806037. 10.1155/2018/5806037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang MS, Heo DH, Kim HB, Chung HT. Biportal endoscopic technique for transforaminal lumbar interbody fusion: review of current research. Int J Spine Surg. (2021) 15 (Suppl. 3):S84–s92. 10.14444/8167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin GX, Yao ZK, Zhang X, Chen CM, Rui G, Hu BS. Evaluation of the outcomes of biportal endoscopic lumbar interbody fusion compared with conventional fusion operations: a systematic review and meta-analysis. World Neurosurg. (2022) 160:55–66. 10.1016/j.wneu.2022.01.071 [DOI] [PubMed] [Google Scholar]
- 60.He K, Head J, Mouchtouris N, Hines K, Shea P, Schmidt R, et al. The implications of paraspinal muscle atrophy in low back pain, thoracolumbar pathology, and clinical outcomes after spine surgery: a review of the literature. Global Spine J. (2020) 10:657–66. 10.1177/2192568219879087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu ZJ, Zhang JF, Xu WB, Zhao FD, Wang JY, Fan SW, et al. Effect of pure muscle retraction on multifidus injury and atrophy after posterior lumbar spine surgery with 24 weeks observation in a rabbit model. Eur Spine J. (2017) 26:210–20. 10.1007/s00586-015-4247-9 [DOI] [PubMed] [Google Scholar]
- 62.Firidin MN, Akyüz ME. Preoperative and postoperative diagnostic efficiency of multi-inflammatory index on pain scoring of degenerated intervertebral disc. Adv Clin Exp Med. (2022) 31:947–52. 10.17219/acem/149336 [DOI] [PubMed] [Google Scholar]
- 63.Sasaoka R, Nakamura H, Konishi S, Nagayama R, Suzuki E, Terai H, et al. Objective assessment of reduced invasiveness in med. Compared with conventional one-level laminotomy. Eur Spine J. (2006) 15:577–82. 10.1007/s00586-005-0912-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang YH, Ou CY. Significant blood loss in lumbar fusion surgery for degenerative spine. World Neurosurg. (2015) 84:780–5. 10.1016/j.wneu.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 65.Kang T, Park SY, Lee SH, Park JH, Suh SW. Assessing changes in cervical epidural pressure during biportal endoscopic lumbar discectomy. J Neurosurg Spine. (2020) 34:1–7. 10.3171/2020.6.SPINE20586 [DOI] [PubMed] [Google Scholar]
- 66.Groetz SF, Birnbaum K, Meyer C, Strunk H, Schild HH, Wilhelm KE. Thermometry during coblation and radiofrequency ablation of vertebral metastases: a cadaver study. Eur Spine J. (2013) 22:1389–93. 10.1007/s00586-012-2647-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang H, Liu J, Hai Y. Is instrumented lateral lumbar interbody fusion superior to stand-alone lateral lumbar interbody fusion for the treatment of lumbar degenerative disease? A meta-analysis. J Clin Neurosci. (2021) 92:136–46. 10.1016/j.jocn.2021.08.002 [DOI] [PubMed] [Google Scholar]
- 68.Kim JE, Choi DJ. Biportal endoscopic transforaminal lumbar interbody fusion with arthroscopy. Clin Orthop Surg. (2018) 10:248–52. 10.4055/cios.2018.10.2.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pisano AJ, Fredericks DR, Steelman T, Riccio C, Helgeson MD, Wagner SC. Lumbar disc height and vertebral hounsfield units: association with interbody cage subsidence. Neurosurg Focus. (2020) 49:E9. 10.3171/2020.4.FOCUS20286 [DOI] [PubMed] [Google Scholar]
- 70.Xu J, Wang D, Liu J, Zhu C, Bao J, Gao W, et al. Learning curve and complications of unilateral biportal endoscopy: cumulative sum and risk-adjusted cumulative sum analysis. Neurospine. (2022) 19:792–804. 10.14245/ns.2143116.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bartels RH, Donk RD, Feuth T. Subsidence of stand-alone cervical carbon fiber cages. Neurosurgery. (2006) 58:502–8; discussion −8. 10.1227/01.NEU.0000197258.30821.50 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.