Abstract
Introduction:
The objective of the current study was to develop a human treated dentin matrix (hTDM) hydrogel for use as a scaffold to allow the controlled release of an antimicrobial agent for regenerative endodontics.
Materials and Methods:
Human extracted teeth were treated via chemical demineralization using ethylene diamine tetra-acetic acid solution to produce hTDM powder. Fourier transform infrared spectroscopy (FTIR) was conducted to determine the functional groups of hTDM, scanning electron microscopy (SEM) was used to define the morphology/particle size of hTDM, and energy dispersive X-ray analysis was performed to identify the superficial apatite groups. Prepared hTDM powder was added to the amoxicillin-clavulanate mixture with a mass ratio of 1:1. Then, the combination was dripped into a 5% (w/v) calcium chloride solution. Antibiotic release profiles were evaluated for 14 days via high performance liquid chromatography (HPLC). Hydrogel degradation properties were studied for 14 days using 10 mL of phosphate buffered saline (PBS). Encapsulation efficiency was determined by HPLC, while minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of amoxicillin-clavulanate were determined against Enterococcus faecalis (E. faecalis). The antibacterial activity of amoxicillin-clavulanate against E. faecalis was investigated for 14 days via agar diffusion test. Statistical analysis was performed with the Shapiro-Wilk test (P=0.05).
Results:
hTDM showed statistically a significant difference for percentage weight change (P=0.1). The encapsulation efficiencies for hTDM hydrogel with antibiotic and hydrogel with antibiotic was 96.08%±0.02 and 94.62%±0.11, respectively. MIC and MBC values of amoxicillin-clavulanate against E. faecalis were 2.4 µg/mL and 9.6 µg/mL, respectively. The antibacterial activity of antibiotic loaded hTDM hydrogels was significantly greater than loaded hydrogels alone by 31% after 4 days and 100% at 14 days, respectively (P≤0.001).
Conclusions:
This in vitro study showed antibiotic-loaded injectable hTDM hydrogel could be an alternative system to transfer antibiotic-based intracanal medicaments for use in regenerative endodontics.
Key Words: Demineralized Dentin Matrix, Drug Delivery Systems, Hydrogels, Regenerative Endodontics
Introduction
Endodontic-related treatments, e.g. vital pulp therapy (VPT) modalities (i.e. direct/indirect pulp capping, partial/miniature/full pulpotomy) and regenerative endodontic therapy, necessitate the use of suitable carriers for the controlled release of disinfectants and bioactive molecules for pulp regeneration [1]. Controlled release drug delivery products are designed to release the active ingredients in a predictable pattern over an extended period [2].
Most medications can be administered/delivered by a variety of routes, which can be divided into two categories: systemic and local. The conventional systemic drug delivery forms include simple oral, topical, inhaled, or injection methods. Both injection and oral administration are routinely used; however, they cannot ensure a sufficient concentration of active drugs at the affected site due to the poor penetration into the targeted tissue and can even cause systemic toxicity [3]. Local drug delivery is considered a promising alternative to oral administration and injection, which allows better drug potency, controls drug release, provides greater safety, and targets a higher drug concentration specifically to the desired tissue. Moreover, the side effects and risk of overdose are limited by the absorption, distribution, and metabolism of a drug compound [4].
Indeed, infection eradication and maintaining the viability of periapical tissues are considered important and deliberated as the key factors for success of regenerative endodontics [5]. For these purposes, it has been of great significance to optimize disinfection protocols, a trend which has acted as a momentum for the development of various local drug delivery mechanisms to safely disinfect the root canals during regenerative endodontic therapy while maintaining stem cell viability [1].
A potential alternative to the oral and parenteral administration of antibiotic formulations, such as amoxicillin-clavulanate (AmC) mixtures which are effective in the case of endodontic infections [5, 6], has been investigated; i.e. the local delivery.
High biocompatibility, tissue incorporation, controlled biodegradability, and antimicrobial properties should be exhibited in ideal regenerative endodontic biomaterials [6, 7]. Several products, e.g. biopolymers, bioceramics, complex biocomposites, and nanohydroxyapatite, have been tested for local antibiotic and antimicrobial administrations [8]. An injectable drug delivery system using nanohydroxyapatite microspheres with drug-releasing capability for regenerative therapy was synthesized by Ferraz et al. [9]. An electrospun nanocomposite fibrous scaffold was developed by Bottino et al. [10] to serve as a matrix for regenerative endodontics, and simultaneously act as a local drug delivery system to help in root maturogenesis and pulp-dentin complex regeneration [10]. A recent experimental in vitro study introduced an innovative local delivery system of bioceramic microparticles made of hydroxyapatite and tricalcium phosphate coated with a biopolymer, namely polylactic coglycolic acid (PLGA), to transfer loaded antibiotics to the root canal system and remove dominant intracanal microorganisms, with positive results [11].
Treated dentin matrix (TDM) is considered a novel biomaterial with valuable therapeutic uses; including sealing of perforations, reconstruction of furcation involvement, pulp capping and stimulation of dentin/bone regeneration [12, 13]. Moreover, it can be used as a scaffold or a source of growth factors; e.g. dentin sialoprotein (DSP), β-transforming growth factor, decorin, biglycan, dentin matrix protein (DMP-1) involved in cell proliferation control and odontogenic differentiation [12]. However, the plasticity of TDM is not optimal, rendering pulp capping not convenient enough. The inclusion of excipients, e.g. alginate as a hydrogel former, seems to boost the handling properties of the material without compromising its bioactivity [12, 13]. Hydrogels are polymeric three-dimensional network structures with high water content. Hydrogels are classed based on their polymer composition, which can range from natural to synthetic [14]. Hydrogels are considered ideal tissue engineering drug delivery vectors capable of mimicking the extracellular topography of the matrix and delivering the requisite bioactive agents that facilitate tissue regeneration [15]. Alginate hydrogels are highly versatile and adaptable biomaterials, with great potential for use in biomedical applications. Their extracellular matrix-like features have been elaborated as key factors for their choice as vehicles for cell delivery strategies aimed at tissue repair. Alginates are anionic polymers typically extracted from brown seaweeds and unbranched polysaccharides consisting of 1,4-linked β-D mannuronic acid (M) and α-L-guluronic acid (G) units, depending on the alginate source. Alginate is gaining importance, particularly in bone tissue engineering due to its biocompatibility and gel forming properties. Additionally, alginate composites have shown enhanced biochemical significance in terms of porosity, mechanical strength, cell adhesion, biocompatibility, cell proliferation, excellent mineralization, and osteogenic differentiation [16].
The present study aimed to develop a sodium alginate/human treated dentin matrix (SA/hTDM) hydrogel, loaded with AmC, as a potential drug delivery scaffold for regenerative endodontics with enhanced efficacy and extended drug release. The proposed null hypothesis was that the use of hTDM with sodium alginate loaded with AmC would not enhance the release profile or antimicrobial properties of the loaded antimicrobial agent compared to sodium alginate alone loaded with AmC.
Materials and Methods
This study was approved by the institutional review board of the Faculty of Dentistry, Alexandria University 00010556-IORG 0008839 (April 2018).
Fabrication of hTDM
Forty freshly extracted sound mandibular and maxillary first and second molars were collected from the out-patient clinic of the Oral and Maxillofacial Surgery Department, Faculty of Dentistry, Alexandria University, Egypt, following patients’ consent. The crown and cementum were cautiously removed using a high-speed handpiece and diamond bur (Dentsply Maillefer, Ballaigues, Switzerland) underwater spray according to Tabatabaei et al. [17, 18]. The radicular pulp tissues were completely extirpated using endodontic files and the roots were perforated using a size 4 round diamond bur to adequately perfuse the ethylene diamine tetra-acetic acid (EDTA) solution (Prevest DenoPro, Jammu, India). The teeth were successively treated with decreasing EDTA concentrations; i.e. 17%, 10%, and 5% for 10, 10, and 5 min, respectively; to gradually demineralize and efficiently preserve proteins and functional factors [12, 17].
Then, the teeth were immersed in isopropanol (New Pharmachemical Co., Egypt) for 2 h to remove organic components followed by being ground with a homogenizer (HG-15D; Wise Tis, Wonju, Korea). The resultant powder was sieved (vibratory sieve, GILSON CO.WORTHINGTON, USA) to obtain particle-sized powder (120 µm-180 µm) [10, 17]. The obtained powder was sterilized with 25 KGy of Cobalt radiation (Teratron 780, Nordion, Canada) and stored at 4°C until use [19].
Characterization of hTDM
Scanning electron microscopy (JSM 5600LV; JEOL, Tokyo, Japan) at an accelerating voltage of 20 kV, after the gold coating of specimens, was used to determine the morphology and particle size of hTDM [12, 17]. Fourier transform infrared spectroscopy (FTIR) spectra were recorded in the range of 400-4000 cm−1 on an FTIR spectrophotometer (Perkin-Elmer, Baden Seewerk, Germany), after being mulled with dry potassium bromide pellets at room temperature to determine the surface functional groups of hTDM [17]. Energy dispersive X-ray analysis (EDXA) (Oxford Instruments, Tokyo, Japan) was performed to identify the superficial apatite groups [17, 19].
Development of biodegradable hydrogel matrix as a carrier for antibiotics
Four hydrogel formulae; i.e. A1 (SA/hTDM hydrogel with antibiotic), B1 (SA hydrogel with antibiotic), A2 (SA/hTDM blank hydrogel without antibiotics), and B2 (SA blank hydrogel without antibiotics), were prepared (Table 1). SA/hTDM hydrogel loaded with AmC (Magnibiotic; GlaxoSmithKline, Cairo, Egypt) (A1) was prepared by dispersing 0.125 gm of sodium alginate(SA) (viscosity ≥ 2000 cps, Sigma Chemical Co., St. Louis, USA) in 2.5 mL of AmC mixture (4 mg/mL) to produce 5% (w/w) SA/AmC solution. Magnibiotic powder was reconstituted in sterile distilled water (10 mL) to prepare a stock
Table 1.
The composition of the different studied groups A1, B1, A2, and B2
| Group | Composition |
|---|---|
| A1 | SA/hTDM hydrogel with AmC |
| B1 | SA hydrogel with AmC |
| A2 | SA/hTDM hydrogel |
| B2 | SA hydrogel |
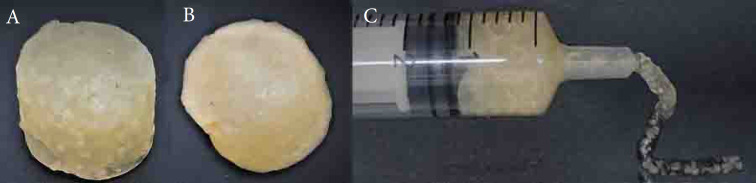
solution of 600 mg/10mL which was used to prepare AmC (4 mg/mL) prior to filtration through a 0.22 µm syringe filter. Then, it was filtered using a 0.22 µm syringe filter for sterilization Next, sterile hTDM powder was dispersed in the sterile SA/AmC solution to produce a SA:hTDM mass ratio of 1:1. The hydrogel was then formed by dripping the solution into a sterile 5% CaCl2 (w/v) solution (Sigma-Aldrich, St. Louis, MO, USA) [10, 12, 20]. The prepared hydrogel (A1) was then transferred into a single syringe to remove excess unused solution and obtain a uniform injectable mass (Figure 1) [12, 20]. A positive control hydrogel (B1); composed of sodium alginate (SA) without hTDM was prepared with the same procedure mentioned above.
Figure 1.
The different groups of prepared hydrogels, A) SA/ hTDM hydrogel loaded with AmC (A1); B) SA hydrogel loaded with AmC (B1); C) Injectable SA/hTDM hydrogel loaded with AmC; (SA; sodium alginate, hTDM; human treated dentin matrix, AmC: amoxicillin-clavulanate mixture)
Two blank hydrogels without antibiotics serving as controls (A2 and B2 representing hTDM and SA, respectively) were also prepared using the same procedure mentioned above.
Characterization of the prepared hydrogel
Degradation behavior of blank hydrogels
After the complete swelling of blank hydrogels (A2 and B2) for 24 h in a refrigerator at 4-10 °C; the degradation of blank hydrogels (A2 and B2) was studied using phosphate buffered saline (PBS) (Sigma Chemical Co, St. Louis, USA) with pH of 7.4 under incubation for 14 days. Hydrogels were weighed by an electronic balance (RADWAG, AS 220.R2, Poland) before being incubated in 50 mL of PBS. On daily basis, the hydrogels were reweighed after removing excess surface solution by a filter paper (n=3, analyzed in triplicate). The percent weight change of hydrogels concerning time was determined by using the following equation:
Where Q is the percentage weight change, W0 is the initial weight of gel before degradation and W1 is the weight of gel after degradation [21].
Encapsulation efficiency of drug-loaded hydrogels
After the complete swelling and hydration of A1and B1 hydrogels for 24 h in a refrigerator at 4-10 °C, the excess unused solution from the hydrogel was separated by applying uniform manual pressure on the syringe plunger. The volume of the obtained solution was measured and antibiotic content was determined via high performance liquid chromatography (HPLC). The drug content in the excess unused solution was then subtracted from theoretical content to determine the actual drug content of the hydrogels (n=3, analyzed in triplicate).
The system of HPLC (G1329B – 1260 Agilent, Germany) consisted of a Symmetry C18 Agilent column (4 mm × 30 cm) equipped with a UV detector at λ max = 220 nm. The mobile phase is composed of methanol and monobasic sodium phosphate buffer (1:19) under a flow rate of 1 mL/min and an injection volume of 20 µL. Encapsulation efficiency (EE) was then calculated using the following equation [22, 23]:
In vitro cumulative drug release
In vitro antibiotic release from the drug-loaded hydrogels (A1 and B1) was performed using a membrane-less method at 37°C± 0.5°C. An accurately weighed amount of each hydrogel (1gm) was added to 10 mL simulated body fluid (SBF) prepared as described in Kokubo protocol [24], with a pH of 7.4. Next, the tubes were incubated at 37 C and were sampled at different time intervals after 1, 4, 7, 10, and 14 days (n=3, each sample was analyzed in triplicates). At each sampling time, an aliquot of 5 mL was withdrawn and replaced with a fresh medium, at 37°C then filtered through a 0.22 μm syringe filter [9, 23, 25]. The concentration of the released AmC mixture was determined by the HPLC assay technique. The cumulative antibiotic released (%) was determined using the following equation:
where “Rt” and “I” represent the cumulative amount of AmC released at the time of “t” and the initial amount (μg/mL), respectively [26].
Bioassay of antibacterial activity
Enterococcus (E.) faecalis (ATCC 10541, USA) was selected as the experimental bacterium in the current study. The concentration of the experimental bacterium was prepared using a spectrophotometer (Perkin-Elmer, Germany) by adjusting 0.5 McFarland suspension (108 CFU/mL). An aliquot of 1.5 mL of the adjusted bacterial suspension was incorporated into 250 mL of sterilized Muller Hinton agar (Himedia, Mumbai, India). The agar was poured into sterile plates and left at room temperature to solidify. A uniform well of 8-mm diameter for each test material was punched in agar using a sterile cork borer. Cavities were filled with 100 μL of specimens withdrawn after different time intervals (1, 4, 7, 10, and 14 days). All assays were carried out in triplicates under aseptic conditions. The plates were incubated at 37°C for 16-24 h. After incubation, the diameters of the bacterial growth inhibition zones formed around the wells were measured in millimeters with the aid of a zone reader [9].
Determination of the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC)
The broth microdilution method using 96 well microtiter plates was applied to determine the MIC and MBC of AmC against E. faecalis (ATCC 10541) [27]. Two-fold serial dilutions of AmC ranging from 0.6 to 10,000 µg/mL were prepared using Muller Hinton broth (Himedia, Mumbai, India). Each well received 25 µL of E. faecalis (106 CFU/mL) and 25 µL of Muller Hinton broth containing various concentrations of AmC. Three wells did not receive AmC and another three wells contained only Muller Hinton broth to be used as control. The plates were incubated at 37°C for 24 h and the lowest concentration of AmC that completely inhibited the visible growth of E. faecalis was recorded as the MIC. From each well showing no visual growth, an inoculum was cultured onto Muller Hinton agar (Himedia, Mumbai, India) and incubated at 37°C for 72 h to determine the MBC. The lowest concentration of AmC that completely prevented the growth of E. faecalis on Muller Hinton agar was recorded as MBC.
Sample size estimation
The minimal sample size was calculated based on a study aimed to determine the effectiveness of several antibiotic-loaded hydrogel scaffolds against E. faecalis [28]. A total sample size of 60 specimens was divided into 4 groups with a sample size of 15 specimens per group. Samples taken at each time interval were analyzed in triplicate (n=3) [29].
Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (IBM Corp, Armonk, NY, USA). For continuous data, they were tested for normality by the Shapiro-Wilk test. Distributed data were expressed as a range (minimum and maximum), mean, standard deviation, and median. A student t-test was used to compare two groups for normally distributed quantitative variables while. The significance of the obtained results was judged at the (P=0.05).
Results
Surface characterization of prepared dentin powder
Examination under SEM demonstrated that dentinal tubules were exposed (Figure 2A). SEM confirmed the particle size of human dentin ranged from 120 µm to 180 µm (Figure 2B) and showed that SA/hTDM hydrogels had a dense morphology (Figure 2C).
Figure 2.
A) Scanning electron microscopic characterization of hTDM showing dentinal tubules sufficiently exposed and loosened fiber bundles of intertubular and peritubular dentin after specific EDTA treatment at X10.000; B) Particle size range of prepared hTDM powder (120µm -180µm); C) The dense morphology of SA/hTDM hydrogels; (hTDM; human treated dentin matrix, EDTA; Ethylenediaminetetraacetic acid SA; sodium alginate)
EDXA (Energy Dispersive X-ray Analysis)
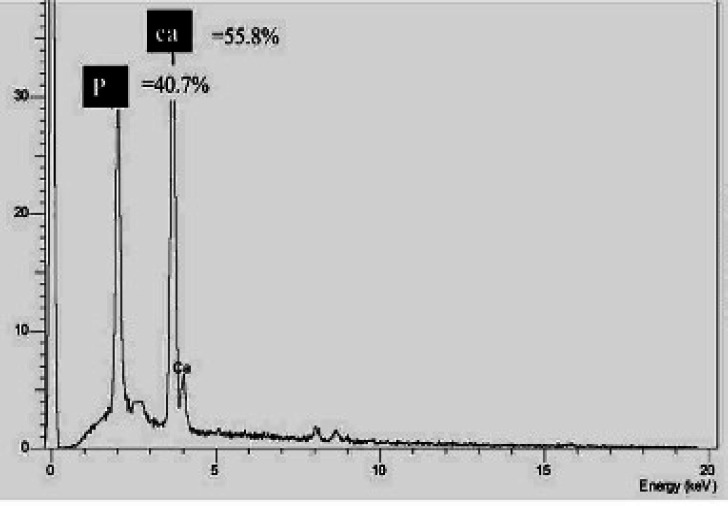
EDXA was performed for the analysis of inorganic (mineral) components and showed a total weight percent of calcium and phosphorus equivalent to 55.8% and 40.7%, respectively (Figure 3). The analysis of inorganic components of hTDM by using EDXA is shown in the supplementary appendix Table 2.
Figure 3.
Energy Dispersive X-ray analysis results, EDX showed the total percentage of calcium at 55.8% and phosphorus at 40.7%; (EDX; Energy dispersive X-ray)
Table 2.
Comparison between the two studied groups (A1 and B1) according to encapsulation efficiency (n = 3)
| Encapsulation efficiency | A1 | B1 | T | P-value |
|---|---|---|---|---|
| Mean (SD) | 96.08 (0.02) | 94.62 (0.11) | 22.740* | <0.001* |
SD: Standard deviation; t: Student t-test; p: p-value for comparing the studied groups; *: Statistically significant at P≤0.05; A1: SA/hTDM hydrogel with AmC; B1: SA hydrogel with AmC
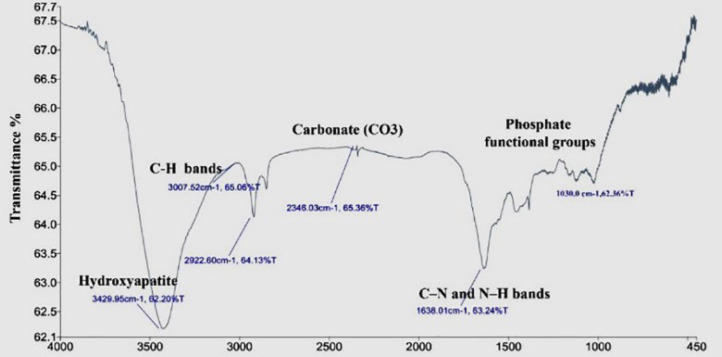
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR analysis of hTDM is shown in (Figure 4). FTIR absorptions at 1030 cm-1 belonged to phosphate functional groups (PO4)−3. The peaks present at 1638 cm−1 represented carbonate (CO3) and C–N and N–H bands. Small peaks around 2346 cm−1 corresponded to the CO3 compound. The peak seen in the region of 3429.95 cm−1 can be attributed to the presence of hydroxyapatite, as well as amide N–H.
Figure 4.
Fourier transform infrared spectroscopy analysis of hTDM. FTIR absorptions at1030 cm-1 belonged to phosphate functional groups (PO4)3. The peaks present at 1638 cm-1 represented carbonate (CO3) and C–N and N–H bands. Small peaks around 2346 cm-1 corresponded to the CO3 compound. The peak seen in the region of 3429.95 cm-1 can be attributed to the presence of hydroxyapatite, as well as amide N–H; (FTIR; Fourier transform infrared spectroscopy)
Characterization of the prepared hydrogel
Degradation behavior of blank hydrogel
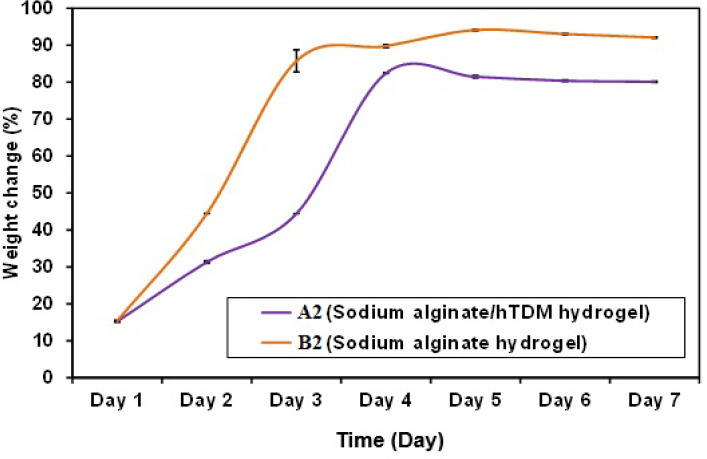
The effect of hTDM on the degradation behavior of blank hydrogels
in PBS at pH 7.4 is presented in (Figure 5). The Statistical analysis of the weight variation results showed a statistically significant difference for percentage weight change with (P=0.1).
Figure 5.
Comparison between the two studied groups A2 and B2 according to the percentage of weight change
Encapsulation efficiency (EE)
The percentage of entrapment efficiency for the drug-loaded hydrogels was 96.08±0.02 and 94.62±0.11 for A1 and B1 respectively (Table 3). The entrapment efficiency of A1 hydrogel showed a statistically significant difference (P=0.1) compared to B1 hydrogel.
Table 3.
The analysis of inorganic components of hTDM by using EDXA. PKa is the phosphorus component with a total pea percentage of 40.7%, and CaKa represents calcium with a total percentage of 55.8 and 3.5% at the CaKa and CaKb energy levels, respectively
| Label | Range (KeV) | Gross | Net | % Total |
|---|---|---|---|---|
| PKa | 1.888 to 2.148 | 13386 | 7660 | 40.7 |
| CaKa | 3.547 to 3.828 | 16080 | 10500 | 55.8 |
| CaKb | 3.868 to 4.168 | 3538 | 658 | 3.5 |
Determination of the MIC and MBC of AmC
The MIC and MBC values of AmC against E. faecalis were 2.4 and 9.6 µg/mL, respectively. Turbidity was observed in the wells that did not receive AmC while no turbidity was observed in the wells that contained only Muller Hinton broth.
Drug release profile
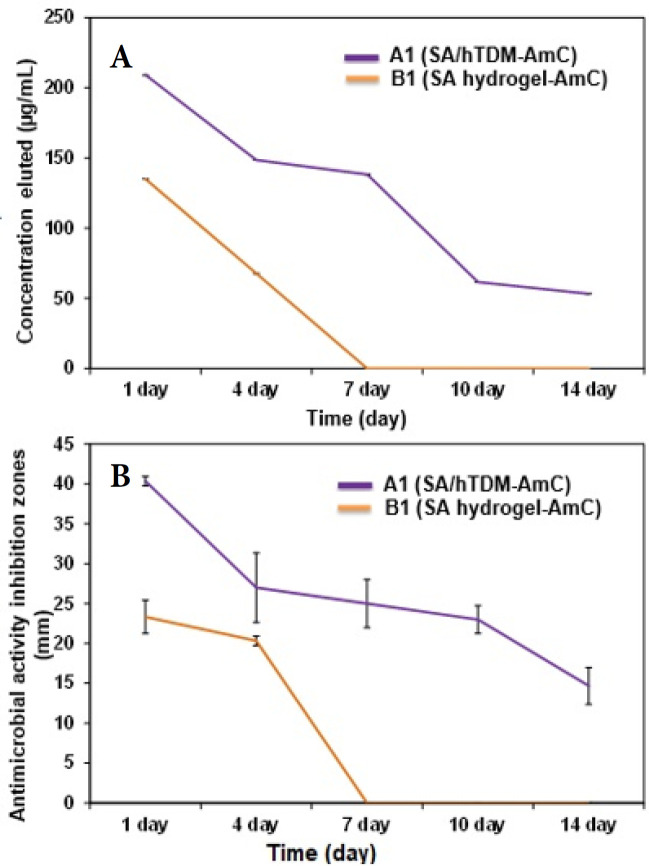
Drug release data (concentration; μg/mL) from both A1 (SA/hTDM with AmC) and B1 (SA with AmC) hydrogels are shown in Table 4 and Figure 6A. A1 showed an initial burst release (209.05 µg/mL) during the first day followed by a prolonged slow decrease in the sustained release rate (up to 53.2 µg/mL) on day14. On the other hand, for B1, the entire entrapped drug was released within the first four days. The comparison between A1 and B1 concerning the reductions in concentration eluted during the mentioned period is presented in Table 5 (P≤0.001).
Table 4.
Comparison between the different studied groups A1 and B1 concerning the antimicrobial activity inhibition zones, cumulative release %, and concentration eluted (µg/mL) (n=3)
| Antimicrobial activity inhibition zones (mm) | Cumulative release (%) | Concentration eluted (µg/mL) | ||||
|---|---|---|---|---|---|---|
| A1 | B1 | A1 | B1 | A1 | B1 | |
| Day 1 | ||||||
| Mean (SD) | 40.33 (0.58) | 23.33 (2.08) | 22.92 (0.02) | 19.10 (0.10) | 209.05 (0.03) | 134.93 (0.01) |
| t (p) | 13.630* (<0.001*) | 64.719* (<0.001*) | 4059.720* (<0.001*) | |||
| Day 4 | ||||||
| Mean (SD) | 27.0 (4.36) | 20.33 (0.58) | 24.10 (0.36) | 8.80 (0.10) | 148.5 (0.03) | 67.6 (0.01) |
| t (p) | 2.626 (0.115) | 105.996* (<0.001*) | 4358.123* (<0.001*) | |||
| Day 7 | ||||||
| Mean (SD) | 25.0 (3.0) | – | 25.68 (0.41) | 3.10 (0.10) | 138.3 (0.10) | – |
| t (p) | – | 206.604* (<0.001*) | – | |||
| Day 10 | ||||||
| Mean (SD) | 23.0 (1.73) | – | 17.70 (0.17) | – | 61.55 (0.01) | – |
| Day 14 | ||||||
| Mean (SD) | 14.67 (2.31) | – | 12.11 (0.11) | – | 53.22 (0.01) | – |
SD: Standard deviation; t: Student t-test; p: p value for comparing the studied groups; *: Statistically significant at p ≤ 0.05; A1: SA/hTDM hydrogel with AmC; B1: SA hydrogel with AmC
Figure 6.
A) The mean concentration of released antibiotic as functions of release time of A1 and B1; B) The diameters of inhibition zones of A1 and B1
Table 5.
Comparison between the two studied groups A1 and B1 concerning the reductions in concentration eluted during the period (n=3)
| Reductions in concentration eluted/Time (µg/mL) | A1 | B1 | t | p |
|---|---|---|---|---|
| Mean (SD) | 155.8 (0.04) | 134.9 ( 0.01) | 967.482* | <0.001* |
SD: Standard deviation; t: Student t-test; p: p value for comparing the studied groups; *: Statistically significant at p ≤ 0.05; A1: SA/hTDM hydrogel with AmC; B1: SA hydrogel with AmC
Bioassay of Antibacterial Activity
The data presented in Table 4 and Figure 6B show that the biodegradable SA/hTDM loaded with AmC (A1) exhibited bacterial growth inhibition zones of 40.3, 27, 25, 23, 14.6 mm on days 1, 4, 7, 10, and 14, respectively. However, the diameters of the inhibition zone of AmC released from B1 hydrogel were 23.3 mm, and 20.3 mm on days 1 and 4, respectively. Nonetheless, on days 7, 10, and 14, no inhibition zones were observed. Moreover, the released concentration was within the minimal inhibitory concentration (MIC) of E. faecalis only for the first four days (P≤0.001). The diameters of the inhibition zones recorded by the SA/hTDM hydrogel with AmC were greater than the SA hydrogel with AmC by 31% and 100% after 4 and 14 days, respectively. The blank groups that received no AMC (A2 and B2) demonstrated zone diameters of 0.00 mm for all time intervals.
Discussion
In the current study, a novel scaffold made from human-treated dentin matrix/sodium alginate hydrogel with the capability of controlled drug release was experimentally prepared and characterized for potential use in regenerative endodontic procedures, which are primarily performed in immature necrotic permanent teeth. In such situations, not only would stem cells, scaffolds, and growth factors are included in the triad to ensure regeneration, but it would intertwine with the critical need to obtain a well-disinfected root canal space where tissue regeneration could take place [28, 30]. Additionally, for the present investigation, E. faecalis was chosen as an experimental strain because it represents one of the most prevalent bacteria associated with chronic endodontic infections. It has been demonstrated that E. faecalis can endure intracanal procedures and systemic antibiotics, even under stressful ecological conditions [6, 31].
For the current analysis, amoxicillin-clavulanate (AmC) mixture was chosen because it is one of the most powerful antibiotics against E. faecalis and could be a promising candidate to be loaded on local drug delivery systems [6]. Augmentin was used by Nosrat et al. [32] for 5 weeks as an intracanal medication; resulting in excellent infection management, full osseous healing of periapical lesions, and root apex formation. In addition, Ferraz et al. [9] reported that there was a rise in the number of cells (MG63 human osteoblast cells) relative to equivalent non-AmC cultured cells, suggesting that the presence of this antibiotic may have a beneficial impact on the proliferation of cells [9]. However, amoxicillin showed no cytotoxic effects on the primary human osteoblast (PHO), MG63 osteosarcoma cell line, HeLa epithelial cell line, nor on any mitochondrial control [33]. In comparison, unlike other antibiotics targeting bacterial proteins or DNA synthesis, Augmentin inhibits the formation of bacterial cell walls; affecting bacteria since human cells lack the corresponding wall [34]. On the other hand, Alguilan et al. [35] found regeneration protocols using a triple antibiotic paste, double antibiotic paste, and calcium hydroxide with significant reductions in dental pulp stem cell proliferation on dentin.
Partial demineralization of hTDM was a key step in the present study, as was shown by the partially demineralized dentin matrix [13, 17]. EDXA showed a total percent of calcium and phosphorus equivalent to 13.95 M, and 13.14 M, respectively (the ratio between Ca and P in demineralized hTDM 1.06 in comparison to ratio between Ca and P in hydroxyapatite 1.67) and FTIR absorbance peaks could be attributed to the presence of hydroxyapatite. As a result, the partial demineralization process of hTDM could be confirmed by EDXA and FTIR data, indicating that the demineralization procedure used did not completely remove the minerals.
Degradation behavior for both hydrogels (A2; SA/hTDM hydrogel and B2; SA hydrogel) showed that the combination of hTDM and SA (A2) was able to slow down the hydrogel degradation rate. That is probably due to the stronger gel matrix increased cross-linkage [36, 37], which retained its shape well up to 14 days compared to SA hydrogel (B2). Indicating that hTDM behaved as a barrier to prevent water leakage from the hydrogel during the period of the study. This may have allowed a more prolonged drug release [36].
The encapsulation effectiveness of A1 (SA/hTDM hydrogel with AmC) was found to be greater than that of B1 (SA hydrogel with AmC). This could be due to the inclusion of hTDM particles (inorganic filler) act as AmC carriers, which can improve the encapsulation effectiveness of cross-linked SA hydrogels without hTDM [38]. This is in agreement with Gabriel et al. [39], who found that the encapsulation efficacy of amoxicillin-loaded membranes with four bacterial strains was dependent on both the drug and nanoHAp concentration.
In the current study, the size of hTDM particles ranged from 120 μm to 180 μm. As reported by Kim et al. [18], dentin granules should be between 75 and 500 m in size for osteogenic effects. Additionally, Koga et al. [40] found that the bigger the particle size (1000 μm) of hTDM, the greater the regeneration of bone. On the other hand, Chen et al. [12] used hTDM as a paste for pulp capping with smaller sized particles < 76 μm and showed that TDM paste could (i) achieve both dentin regeneration and vital pulp conservation, and (ii) serve as a convenient substitute for calcium hydroxide in the reparative procedure of dental pulp.
The drug release profile is determined by the particle size of the drug carrier as bigger particles have less packing efficiency and more rapid release of the drug [41, 42]. Ferraz et al. (8) demonstrated this by testing the drug-releasing ability and antibacterial activity of nanohydroxyapatite microspheres against E. faecalis. They discovered that hydroxyapatite granules were inadequate compared to nanohydroxyapatite microsphere they loaded since released all antibiotics during the first hours. The release profiles of effective antibiotic dosages, on the other hand, were achieved using nanohydroxyapatite microspheres (fast initial release followed by a long-term sustained release for 28 days). As a result, the range of 120-180 μm granules was chosen for the current analysis to ensure the maintenance of osteogenic/dentinogenic effects while also seeking a sustained drug release profile. This would improve the capacity to fill defect spaces while maximizing the amount of antibiotics that will be released [9, 41]. It can also help with improved handling, sealing, and adaptability [43].
In the current study, the amounts of antibiotics extracted from the drug-loaded hydrogels were measured using HPLC. HPLC is one of the most common techniques used to evaluate drug release with great precision [10, 26]. The antibacterial activity of the released antibiotic was tested against E. faecalis using the agar well diffusion method following protocols presented by Ferraz et al. [9], Ueng et al. [25], and Sara Borrego et al. [7]. hTDM hydrogel provided a considerable release of AmC over 14 days. Furthermore, the MIC and MBC values of released AmC against E. faecalis were determined using the broth microdilution method and were found to be 2.4 µg/mL (1:4096) and 9.6 µg/mL (1:1024), respectively. Our results are in agreement with Seguel N et al. [6, 44] and Barbosa-Ribeiro M et al. [6, 44]. They determined the MIC for AmC against different strains of E. faecalis by an Epsilometer test. The results showed that all strains were susceptible to the amoxicillin/clavulanic acid combination (MIC, 6 - 0.025 µg/mL), (MIC, 0.09–2.67 µg/mL), respectively. The released concentration of AmC from SA/hTDM hydrogel was above the MIC and MBC of AmC against E. faecalis. This may be due to the presence of hTDM acting as an inorganic cross-linker to restrict the movability of SA polymer chains and slow down their swelling/dissolution rates, which could be the fundamental cause for better drug loading and controlled release behavior. This finding agrees with Zhang et al. [26] who demonstrated that in the nanocomposite beads, HA microparticles served as inorganic fillers, reducing the mobility of the SA polymer chains and decreasing their dissolving rates. Considering the above-mentioned release profiles, the hTDM hydrogel may have met a crucial characteristic of an ideal drug delivery system where initial large dosages of the selected drug are required for an effective attack on bacteria, followed by a continuous release. In contrast, AmC was released in a burst from SA hydrogel alone in the first few days. Because of the faster release, the SA hydrogels without the inorganic filler (hTDM) have higher water sorption [40]. Absorbed water can diffuse more easily inside the SA matrix alone, and it can act as an AmC carrier from inside the polymer matrix.
To the best of our knowledge, this may be the first study that used the SA/hTDM hydrogels with an antimicrobial agent as a drug delivery scaffold aimed at regenerative endodontic therapy. The physicochemical properties, together with the antibacterial efficacy suggest that this biomaterial could be employed as a potential scaffold for regenerative endodontic applications. Several limitations cannot be overlooked, especially since the antimicrobial properties of this formulation were tested against E. faecalis only. Furthermore, the biocompatibility and physical properties of this formulation still require assessment in an in vivo animal model which is potentially contaminated.
The release profile and antibacterial capabilities of hTDM with SA loaded with AmC were significantly different from sodium alginate alone loaded with AmC (P≤0.001) throughout the study period.
Conclusions
Antibiotic loaded injectable hTDM hydrogel provided sustained release of the loaded drug, enhancing antibacterial activity. The capability of sustained antibiotic release with the bioactive molecules present within the hTDM matrix. In addition to the resorbability of novel hTDM hydrogel and its potential use as an easily applicable pulp capping material. These advantages may provide a four-fold beneficial effect of hTDM hydrogel as a potential scaffold for regenerative endodontic applications.
Conflict of Interest:
‘None declared’.
References
- 1.Parhizkar A, Asgary S. Local Drug Delivery Systems for Vital Pulp Therapy: A New Hope. Int J Biomater. 15;2021:5584268. doi: 10.1155/2021/5584268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva JS, Marques-da-Silva D, Lagoa R. Towards the Development of Delivery Systems of Bioactive Compounds With Eyes Set on Pharmacokinetics. Modeling and Control of Drug Delivery Systems. Elsevier; 2021. pp. 125–144. [Google Scholar]
- 3.Mueller B, Treccani L, Rezwan K. Antibacterial active open-porous hydroxyapatite/lysozyme scaffolds suitable as bone graft and depot for localized drug delivery. J. Biomater. 2017;31(8):1123–34. doi: 10.1177/0885328216688074. [DOI] [PubMed] [Google Scholar]
- 4.Shahri F, Parhizkar A. Pivotal local drug delivery systems in endodontics; A review of the literature. Iran Endod J. 2020;15(2):65–78. doi: 10.22037/iej.v15i2.30374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soriano-Souza CA, Rossi AL, Mavropoulos E, Hausen MA, Tanaka MN, Calasans-Maia MD, et al. Chlorhexidine-loaded hydroxyapatite microspheres as an antimicrobial delivery system and its effect on in vivo osteoconductive properties. J. Mater. Sci.: Mater. Med. 2015;26(4):166. doi: 10.1007/s10856-015-5505-4. [DOI] [PubMed] [Google Scholar]
- 6.Barbosa-Ribeiro M, De-Jesus-Soares A, Zaia AA, Ferraz CC, Almeida JF, Gomes BP. Antimicrobial susceptibility and characterization of virulence genes of Enterococcus faecalis isolates from teeth with failure of the endodontic treatment. J. Endod. 2016;42(7):1022–8. doi: 10.1016/j.joen.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Borrego-González S, Romero-Sánchez LB, Blazquez J, Diaz-Cuenca A. Nanostructured hybrid device mimicking bone extracellular matrix as local and sustained antibiotic delivery system. Micropor. Mesopor. Mat. 2018;256:165–76. [Google Scholar]
- 8.Chen I-H, Lee T-M, Huang C-L. Biopolymers hybrid particles used in dentistry. Gels. 2021;7(1):31. doi: 10.3390/gels7010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferraz M, Mateus A, Sousa J, Monteiro F. Nanohydroxyapatite microspheres as delivery system for antibiotics: release kinetics, antimicrobial activity, and interaction with osteoblasts. J. Biomed. Mater. Res. A. 2007;81(4):994–1004. doi: 10.1002/jbm.a.31151. [DOI] [PubMed] [Google Scholar]
- 10.Bottino M, Kamocki K, Yassen G, Platt J, Vail M, Ehrlich Y, et al. Bioactive nanofibrous scaffolds for regenerative endodontics. J. Dent. Res. 2013;92(11):963–9. doi: 10.1177/0022034513505770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parhizkar A, Nojehdehian H, Tabatabaei F, Asgary S. An innovative drug delivery system loaded with a modified combination of triple antibiotics for use in endodontic applications. Int. J. Dent. 2020:2020. doi: 10.1155/2020/8859566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Cui C, Qiao X, Yang B, Yu M, Guo W, et al. Treated dentin matrix paste as a novel pulp capping agent for dentin regeneration. JTERM. 2017;11(12):3428–36. doi: 10.1002/term.2256. [DOI] [PubMed] [Google Scholar]
- 13.Holiel AA, Mahmoud EM, Abdel-Fattah WM, Kawana KY. Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping. Clin Oral Investig. 2021;25(4):2101–12. doi: 10.1007/s00784-020-03521-z. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Huang Y. Rational design of smart hydrogels for biomedical applications. Front. Chem. 2021:1288. doi: 10.3389/fchem.2020.615665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen B, Huang Y, Qiu T, Huo F, Xie L, Liao L, et al. Reparative Dentin Formation by Dentin Matrix Proteins and Small Extracellular Vesicles. J. Endod. 2021;47(2):253–62. doi: 10.1016/j.joen.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Venkatesan J, Bhatnagar! Manivasagan P, Kang KH, Kim SK. Alginate composites for bone tis sue engineering: a review. Int J Biol Macromol. 2015;72:269–81. doi: 10.1016/j.ijbiomac.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Tabatabaei FS, Tatari S, Samadi R, Torshabi M. Surface characterization and biological properties of regular dentin, demineralized dentin, and deproteinized dentin. J. Mater. Sci.: Mater. Med. . 2016;27(11):1–11. doi: 10.1007/s10856-016-5780-8. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y-K, Lee J, Um I-W, Kim K-W, Murata M, Akazawa T, et al. Tooth-derived bone graft material. J Korean Assoc Oral Maxillofac Surg. 2013;39(3):103–11. doi: 10.5125/jkaoms.2013.39.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran HLB, Nguyen MTN, Doan VN. Fabrication and evaluation of human dentin as scaffold for dental pulp stem cells. JTERM. 2015;12(4):222–30. [Google Scholar]
- 20.Espona-Noguera A, Ciriza J, Cañibano-Hernández A, Fernandez L, Ochoa I, Del Burgo LS, et al. Tunable injectable alginate-based hydrogel for cell therapy in Type 1 Diabetes Mellitus. Int. J. Biol. Macromol. 2018;107:1261–9. doi: 10.1016/j.ijbiomac.2017.09.103. [DOI] [PubMed] [Google Scholar]
- 21.Moshaverinia A, Chen C, Akiyama K, Ansari S, Xu X, Chee WW, et al. Alginate hydrogel as a promising scaffold for dental-derived stem cells: an in vitro study. J. Mater. Sci.: Mater. Med. . 2012;23(12):3041–51. doi: 10.1007/s10856-012-4759-3. [DOI] [PubMed] [Google Scholar]
- 22.Rivas M, Pelechà M, Franco L, Turon P, Alemán C, Del Valle LJ, et al. Incorporation of chloramphenicol loaded hydroxyapatite nanoparticles into polylactide. Int. J. Mol. Sci. 2019;20(20):5056. doi: 10.3390/ijms20205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Şanlı O, Ay N, Işıklan N. Release characteristics of diclofenac sodium from poly (vinyl alcohol)/sodium alginate and poly (vinyl alcohol)-grafted-poly (acrylamide)/sodium alginate blend beads. Eur J Pharm Biopharm. 2007;65(2):204–14. doi: 10.1016/j.ejpb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface‐structure changes in bioactive glass‐ceramic A‐W3. J. Biomed. Mater. Res. 1990;24(6):721–34. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 25.Ueng SW, Lee MS, Lin SS, Chan EC, Liu SJ. Development of a biodegradable alginate carrier system for antibiotics and bone cells. J. Orthop. Res. 2007;25(1):62–72. doi: 10.1002/jor.20286. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Wang Q, Wang A. In situ generation of sodium alginate/hydroxyapatite nanocomposite beads as drug-controlled release matrices. Acta Biomater. 2010;6(2):445–54. doi: 10.1016/j.actbio.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Wayne P. Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing. 2011. Report No.: M43-A. [PubMed] [Google Scholar]
- 28.Aksel H, Mahjour F, Bosaid F, Calamak S, Azim AA. Antimicrobial activity and biocompatibility of antibiotic-loaded chitosan hydrogels as a potential scaffold in regenerative endodontic treatment. J. Endod. . 2020;46(12):1867–75. doi: 10.1016/j.joen.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Hertzog MA. Considerations in determining sample size for pilot studies. Research in nursing & health. 2008;31(2):180–91. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 30.Saberi EA, Karkehabadi H, Mollashahi NF. Cytotoxicity of various endodontic materials on stem cells of human apical papilla. Iran Endod J. 2016;11(1):17. doi: 10.7508/iej.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seneviratne CJ, Suriyanarayanan T, Swarup S, Chia KHB, Nagarajan N, Zhang C. Transcriptomics analysis reveals putative genes involved in biofilm formation and biofilm-associated drug resistance of Enterococcus faecalis. J. Endod. 2017;43(6):949–55. doi: 10.1016/j.joen.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Nosrat A, Li KL, Vir K, Hicks ML, Fouad AF. Is pulp regeneration necessary for root maturation? J. Endod. 2013;39(10):1291–5. doi: 10.1016/j.joen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Duewelhenke N, Krut O, Eysel P. Influence on mitochondria and cytotoxicity of different antibiotics administered in high concentrations on primary human osteoblasts and cell lines. Antimicrob. Agents Chemother. 2007;51(1):54–63. doi: 10.1128/AAC.00729-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S, Malek M, Sigurdsson A, Lin L, Kahler B. Regenerative endodontics: a comprehensive review. Int. Endod. J. 2018;51(12):1367–88. doi: 10.1111/iej.12954. [DOI] [PubMed] [Google Scholar]
- 35.Alghilan M, Windsor LJ, Palasuk J, Yassen GH. Attachment and proliferation of dental pulp stem cells on dentine treated with different regenerative endodontic protocols. Int. Endod. J. 2017;50(7):667–75. doi: 10.1111/iej.12669. [DOI] [PubMed] [Google Scholar]
- 36.Hua S, Ma H, Li X, Yang H, Wang A. pH-sensitive sodium alginate/poly (vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010;46(5):517–23. doi: 10.1016/j.ijbiomac.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Baysal K, Aroguz AZ, Adiguzel Z, Baysal BM. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int. J. Biol. Macromol. 2013;59:342–8. doi: 10.1016/j.ijbiomac.2013.04.073. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Liu X, Deng T, Yao P, Song H, Zhou S, et al. Development of Drug Loaded Nanoparticles Binding to Hydroxyapatite Based on a Bisphosphonate Modified Nonionic Surfactant. J. Nanomater. 2015;2015:393968. [Google Scholar]
- 39.Furtos G, Rivero G, Rapuntean S, Abraham GA. Amoxicillin‐loaded electrospun nanocomposite membranes for dental applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017;105(5):966–76. doi: 10.1002/jbm.b.33629. [DOI] [PubMed] [Google Scholar]
- 40.Koga T, Minamizato T, Kawai Y, Miura K-i, I T, Nakatani Y, et al. Bone regeneration using dentin matrix depends on the degree of demineralization and particle size. PloS one. 2016;11(1):e0147235. doi: 10.1371/journal.pone.0147235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandra A, Yadav RK, Shakya VK, Luqman S, Yadav S. Antimicrobial efficacy of silver nanoparticles with and without different antimicrobial agents against Enterococcus faecalis and Candida albicans. Dent. Hypotheses. 2017;8(4):94. [Google Scholar]
- 42.Sasikumar S. Effect of particle size of calcium phosphate based bioceramic drug delivery carrier on the release kinetics of ciprofloxacin hydrochloride: An in vitro study. Front. Mater. Sci. 2013;7(3):261–8. [Google Scholar]
- 43.Soheilipour E, Kheirieh S, Madani M, Baghban AA, Asgary S. Particle size of a new endodontic cement compared to Root MTA and calcium hydroxide. Iran Endod J. 2009;4(3):112. [PMC free article] [PubMed] [Google Scholar]
- 44.Seguel N, Quezada-Aguiluz M, González-Rocha G, Bello-Toledo H, Sánchez-Sanhueza G, SEGUEL N. Antibiotic resistance of Enterococcus faecalis from persistent endodontic infections. Int j odontostomatol. 2020:448–56. [Google Scholar]