Abstract
This preliminary study investigated the interaction between working memory and social cognition in adolescents and young adults with traumatic brain injury (TBI). It was hypothesized that participants with or without TBI would better recognize social information when working memory or social cognitive load was low, and that adolescents and young adults with TBI would be more affected by increased cognitive demand than their uninjured peers. Eight adolescents and young adults with complicated mild-severe TBI (aged 14–22 years) and eight age- and sex-matched typically developing (TD) adolescents completed computer-based n-back tasks requiring recognition of either face identity or facial affect, with 0-back, 1-, and 2-back conditions. The TBI group had lower scores overall than the TD group, and scores for both groups were lower for affect recognition than identity recognition. Scores for both groups were lower in conditions with a higher working memory load. There was a significant group-by-working memory interaction, with larger group differences in high-working memory conditions. Study results and their potential implications for social outcomes are discussed.
Keywords: traumatic brain injury, adolescents, young adults, working memory, facial affect recognition
Introduction
Social interactions require us to recognize and interpret a stream of verbal, gestural, and facial cues from our communication partners, and use these cues to infer our partners’ thoughts and feelings. The ability to make sense of and use these social cues is referred to as social cognition (Kelly et al., 2017; Kennedy & Adolphs, 2012). As social interactions evolve over time, individuals must maintain and manipulate social information within working memory. For example, during a conversation, listeners must continually hold and update their mental representations based on information gleaned from their partners’ verbal and nonverbal cues and integrate their new representations with past knowledge to respond in a socially acceptable way.
As social interaction requires coordination of working memory and social cognition, it is important to understand how these two cognitive processes function together. For example, one might expect working memory capacity for social stimuli to vary as a function of working memory factors, like the number of items to be held in mind, and social factors, like whether stimuli are basic or complex emotions. Likewise, working memory for social stimuli might be predicted to engage some combination of brain regions usually activated on working memory tasks and regions specifically linked to social cognition. There is evidence to support these predictions. Neta and Whalen (2011) for example, found that typically developing young adults performed more poorly on a working memory task when they were asked to recall more complex social information (i.e., facial affect versus facial identity). Further, performance of both tasks was associated with activation in both canonical working memory regions and brain regions known to be involved in social cognition. Similarly, Smith and colleagues (2017) found that maintenance of social information, in this case others’ emotions, was associated with activation in brain regions associated with working memory and representation of emotional information. Thornton and Conway (2013) reported similar results in a study using three-dimensional faces constructed to show a continuum of trustworthiness. Participants were asked to state whether a face was either as trustworthy or in the same location as a face shown 0, 1, or 2 trials previously. Results for accuracy showed the expected working memory effect, with lower scores as working memory load increased. Participants also had lower accuracy scores on social items than non-social items, consistent with the study by Neta and Whalen (2011). Results for reaction time, however, revealed an interesting pattern: reaction times were faster for social than non-social items. The authors attributed the social speed advantage to pre-coding or “chunking” of social information, which would allow humans to be more efficient at processing social cues, suggesting that working memory for social stimuli might be more than just the product of these two cognitive functions.
While studies to date provide evidence that performance on social working memory tasks (i.e., tasks that require maintenance and manipulation of high-level social information (Meyer & Collier, 2020)) requires a combination of working memory and social cognitive skills, most research has focused on adults. Adults have an established repertoire of social knowledge and skills (Kilford et al., 2016) that can support their social performance. By contrast, adolescents are just developing social knowledge and skills (Kilford et al., 2016; Steinberg, 2010) and working memory for social stimuli may be a critical part of this development (Amadó et al., 2016; Gordon & Olson, 1998; Im-Bolter et al., 2016). Brain regions that support social cognition and working memory, particularly the prefrontal cortex, undergo major changes during adolescence and continue to develop into early adulthood (Feinberg & Campbell, 2010; Tamnes et al., 2017). Consistent with these brain changes, performance on behavioral measures of social cognition improves from childhood into early adulthood (Choudhury et al., 2006; Humphrey & Dumontheil, 2016; Lawrence et al., 2015; Thomas et al., 2007; Vetter et al., 2018). Working memory also develops through late adolescence (Embury et al., 2019; Master et al., 2020; Simmonds et al., 2017), as individuals develop metacognitive skills and improve their strategy use to manage information. Thus, while children are able to recall single units of information as well as adults, the ability to store and strategically organize this information continues to develop into adulthood (Best & Miller, 2010; Conklin et al., 2007; Master et al., 2020).
Unfortunately, adolescence also is a period of increased risk for traumatic brain injury (TBI) (Sarmiento et al., 2019; Taylor et al., 2017). TBIs result from a blow, jolt, or bump to the head and disrupt typical brain functioning (Centers for Disease Control and Prevention, 2021). Falls and motor vehicle accidents are the most common injury mechanisms among adolescents and young adults (Taylor et al., 2017). Such injuries impose rapid acceleration-deceleration forces upon the brain, resulting in a diffuse injury pattern (Bigler, 2001; Martin-Rodriguez & Leon-Carrion, 2010) that includes areas critical to both social cognition (i.e., medial prefrontal cortex, temporal poles, orbitofrontal cortex) and working memory (i.e., dorsolateral and ventrolateral prefrontal cortex, posterior parietal cortex). Further, TBI often causes diffuse axonal injuries to the white matter tracts that connect social and working memory brain networks (Ryan et al., 2017; Tuerk et al., 2020). As a result of these injuries and associated developmental disruptions, adolescents and adults with TBI have are at risk for impairments in both social cognition (Deighton et al., 2019; McDonald et al., 2013; Ryan et al., 2020; Tousignant et al., 2018) and working memory (Gorman et al., 2017; Gorman et al., 2012; Phillips et al., 2017). Adolescents and children with TBI have been reported to have impaired theory of mind (Deighton et al., 2019; Dennis et al., 2012; Ryan et al., 2017) and facial affect recognition (Babbage et al., 2011; Schmidt et al., 2010; Tonks et al., 2009; Turkstra et al., 2001) when compared with their uninjured peers. Ryan and colleagues (2020) measured social cognition using a composite test battery that required participants to infer the mental states of others from facial affect displays, speech acts, and a non-verbal false-belief scenario. In this large, international study, children and adolescents with complicated mild to severe TBI had poorer social cognition than orthopedic controls and a non-injured comparison group. There is also preliminary evidence that, as in adults, adolescents with TBI are as accurate as their peers in face identification but less accurate in facial affect recognition (Tonks et al., 2008). That is, adolescents with TBI may make more errors when a social demand is added to a general cognitive demand, and thus might have disproportionately lower scores than peers on working memory tasks with complex social stimuli.
For many individuals with TBI, social cognition and working memory impairments are associated with negative social outcomes (Milders, 2019) including impairments in social communication (McDonald et al., 2013; Rowley et al., 2017), problems in peer and family interactions (Morton & Wehman, 1995; Rogers & McKinlay, 2019; Yeates et al., 2004) and reduced social integration (Binder et al., 2019; May et al., 2017; Tousignant et al., 2018; Tousignant et al., 2017). Adolescents are at particular risk for poor social outcomes after TBI, because of the combination of high social demands during this developmental stage and potential disruption of ongoing development of brain structures and functions that are critical for social performance (Steinberg, 2010). Information about the interaction of working memory and social cognition could inform our understanding of everyday social performance in adolescents with TBI and help identify assessment and intervention tools that will improve social outcomes for those affected. As a first step, the objective of this preliminary study was to explore the effects of TBI on adolescents’ working memory for social information, specifically facial affect recognition. To achieve this objective, adolescents and young adults with TBI and uninjured peers completed n-back tasks in which we manipulated both working memory (0-, 1-, and 2-back conditions) and social cognitive (facial recognition vs. facial affect recognition) demand. Study hypotheses were as follows:
Face recognition scores would be higher than emotion recognition scores in both groups, as would scores on items with lower working memory demand.
Adolescents with TBI would have lower scores than typically developing (TD) peers on all study tasks based on evidence that working memory and social cognition may be impaired after TBI.
Between-groups differences on the experimental tasks would be disproportionately larger for affect recognition than face recognition and would increase as working memory load increased.
Methods
Participants
Participants were eight adolescents and young adults with TBI (5 males, 3 females), ages 14–22 years, and eight TD participants matched for age and sex. All were recruited through community sources (e.g., flyers, outreach to community TBI support groups and resource networks) from a mid-sized midwestern city in the US. Participant demographic characteristics are shown in Table 1. As shown in Table 2, seven participants with TBI had sustained moderate-to-severe injuries and one participant sustained three concussions over a three-year span and had documented cognitive impairments related to those injuries. All participants were injured at least six months prior to study enrollment. No participant in either group had a documented history of learning, speech, language, or psychiatric diagnosis (pre-morbidly for the TBI group). All participants were native English speakers by self-report. The two experimental groups did not differ significantly in age, t = 1.37, p = 0.19 however, the TBI group had significantly lower IQ scores, t = −2.78, p = 0.03 as measured by the Kaufman Brief Intelligence Test – Second Edition (Kaufman & Kaufman, 2004).
Table 1.
Participant demographic characteristics
| TBI (n = 8) | TD (n = 8) | |||
|---|---|---|---|---|
|
| ||||
| Median | Range | Median | Range | |
| Males:Females | 5:3 | - | 5:3 | - |
| Age (years;months) | 20;1 | 14;2–22;2 | 19;7 | 13;11–21;1 |
| Estimated IQ | 100 | 76–127 | 111 | 100–127 |
Table 2.
TBI Group Injury Characteristics
| Participant | Sex | Age at Injury Years; Months | Time Post Injury Years; Months | Severity | Injury Mechanism |
|---|---|---|---|---|---|
| 1 | Male | 12;8 | 1;6 (time post most recent injury) | Complicated Mild | fall, hit head on object; sports related injury |
| 2 | Male | 18;2 | 1;10 | Severe | MVA |
| 3 | Male | 18;3 | 1;11 | Severe | MVA |
| 4 | Male | 18;1 | 0;10 | Severe | MVA |
| 5 | Female | 19;4 | 1;2 | Severe | MVA |
| 6 | Female | 20;6 | 1;8 | Moderate-Severe | MVA |
| 7 | Male | 18;4 | 2;6 | Severe | MVA |
| 8 | Female | 13;10 | 6;1 | Severe | MVA |
Tasks
Emotion recognition and face recognition were assessed using separate computer-based n-back tasks with 0-, 1-, and 2-back conditions. Participants completed the tasks while seated at an Apple iMac computer, and tasks were programmed in PsyScope (Cohen et al., 1993). Each stimulus image appeared at the center of the screen for 800 milliseconds. Consecutive images were separated by an interstimulus interval of 1.6 seconds during which a blank white screen appeared. Stimuli were black and white photographs of 24 adults (12 females, 12 males; 16 white adults, 8 Asian adults) displaying four validated facial emotions (happy, neutral, sad, and angry) (Biehl et al., 1997; Ekman & Friesen, 1976). Photographs were all processed to the same size (300 pixels by 405 pixels), and features such as hair or shoulders were eliminated with a black filter. For both the face and emotion recognition tasks, participants were instructed, on screen, to respond “yes” if a stimulus image matched the image n-images previously presented. Participants were instructed to use the forward slash (“/”) key for “yes” if they were right-handed or the “z” key if they were left-handed and preferred to respond with their left hand. Before the start of each task, participants were trained on a basic n-back task using a paper example with a series of simple shapes. During the training task, the experimenter explained the tasks and conditions and provided feedback following each correctly identified or missed response. After the paper-based training, participants completed a 10-item computer-based practice trial, which had a 70% hit rate. No feedback was provided during the computer-based practice trial.
Emotion recognition task
In the 0-back condition, participants were instructed to press the response key each time an image of an individual demonstrating a “mad” facial expression appeared on the screen. In the 1-back condition, participants pressed the key when two consecutive faces displayed the same facial emotion, ignoring the person’s identity and attending only to the emotion displayed. Likewise, in the 2-back condition, participants pressed the key when they saw a facial affect that had been displayed two photos previously in the sequence (e.g., happy, neutral, happy). No feedback was given during the experimental tasks.
The 0-back condition included 100 images with 21 possible hits. Participants viewed 160 images in the 1-back conditions with 48 possible hits. A hit was defined as correct identification of the target emotion in the 0-back condition or accurate identification of two consecutive presentations of a given emotion in the 1-back condition. In the 2-back condition, participants viewed 250 faces with 60 possible hits (i.e., 2-back emotion matches). Matches never occurred consecutively in the 1-back condition (e.g., happy, happy, angry, angry), and no more than two matches were presented in a row in the 2-back condition (e.g., happy, angry, happy, angry).
Face recognition task
The structure of the face recognition task was identical to that of the emotion recognition task, with the exception that there were 20 rather than 21 possible hits in the 0-back condition. All faces showed neutral expressions, and participants responded based on identity of the pictured individuals rather than affective features.
The outcome measure for the two tasks was A’, which is the probability of correctly identifying targets (hit rate), calculated for each condition, as a function of the false alarm rate and hit rate (Evans et al., 2011). A’ was calculated according to Donaldson’s formula (Donaldson, 1992) used in an earlier n-back study of children with TBI (Levin et al., 2004): A’ = .5 + ([(hits – false alarms)*(1 + hits – false alarms)] / [4*(hits(1 – false alarms))). Scores could range from .50 (chance) to 1.0 (perfect target/non-target discriminability), with higher A’ values indicating better discrimination.
Working memory was measured using the Competing Language Processing Test (CLPT; (Gaulin & Campbell, 1994)). The CLPT was designed to test the ability to temporarily store verbal information in working memory while analyzing sentence meaning (Gaulin & Campbell, 1994). It is comprised of groups of one to six simple spoken sentences. Participants were asked to listen to each sentence and immediately indicate whether the sentence was true or false (e.g., after hearing the sentence “Children can play” the participant should respond, “true”). At the end of each sentence group, participants were asked to recall the final words of each sentence in that group (Gaulin & Campbell, 1994). For example, if participants heard, “Children can play. Ice is hot” they would answer, “play, hot” after the second sentence. The dependent measures for the CLPT were accuracy of true/false judgments (percent correct) and the percent of total last words recalled.
Procedures
All participants gave informed consent or assent, depending on their age, at the start of the study protocol. Participants then completed the CLPT and emotion recognition and face identity tasks. Tasks were administered in two separate two-hour sessions and order of administration was randomized across participants. Within both tasks, participants completed the 0-back condition first, then the 1-back, and finally the 2-back condition. Each participant was paid $10 per hour. All procedures were approved by the Institutional Review Board at the University of Wisconsin-Madison. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Data Analysis
Scores on the n-back tasks were compared using a three-way repeated-measures analysis of variance (ANOVA), with group as the between-subjects variable and task (emotion vs. face identity) and working memory level (0-, 1-, and 2-back) as within-subjects variables. Three main effects were predicted: a main effect of group, with higher scores in the TD group; a main effect of working memory load, with progressively lower A’ scores in the 0-, 1-, and 2-back conditions; and a main effect of task, with lower scores overall for the emotion recognition task. We also predicted an interaction of group by task, with a larger between-groups difference on the emotion recognition task than the face recognition task; and an interaction of group by working memory load, with disproportionately lower scores in the TBI group for the 1- and 2-back emotion recognition task conditions relative to the other task and condition. The criterion alpha level for these comparisons was 0.05. Planned post-hoc tests were conducted as appropriate.
CLPT scores were compared between groups to document working memory impairments in the TBI group. One-tailed t-tests were used to compare comprehension accuracy and recall. The criterion alpha level for these comparisons was set at 0.05. All analyses were conducted using STATA™ statistical software.
Results
Before analysis, we examined data for normality and sphericity of distribution. Data did not meet criteria for homoscedasticity, so we used repeated-measures ANOVA for analysis of main and interaction effects, nonparametric Kolmogorov-Smirnov tests for post-hoc tests, and Cliff’s d1 (Cliff, 1993) for effect size calculations.
Main effects
Results are shown in Figure 1 (face identity recognition) and Figure 2 (emotion recognition) and individual participant data are shown in Table 3. Results supported the first two hypotheses. Analysis of variance showed a significant effect of group, F(1, 94) = 39.37, p < .001, with significantly higher scores in the TD group than the TBI group. There was also a significant effect of task, F(1, 94) = 32.54, p < .001, with higher scores for face identity recognition than emotion recognition. There was a significant effect of working memory load, F(2, 93) = 26.91, p < .001; with significantly higher scores on 0-back trials than 2-back trials, D = .50, exact p < .001; significantly higher scores on 1-back trials than 2-back trials, D = .44, exact p = .002; and no significant difference between scores on the 0-back and 1-back trials, D = .19, exact p = .53.
Figure 1 -.
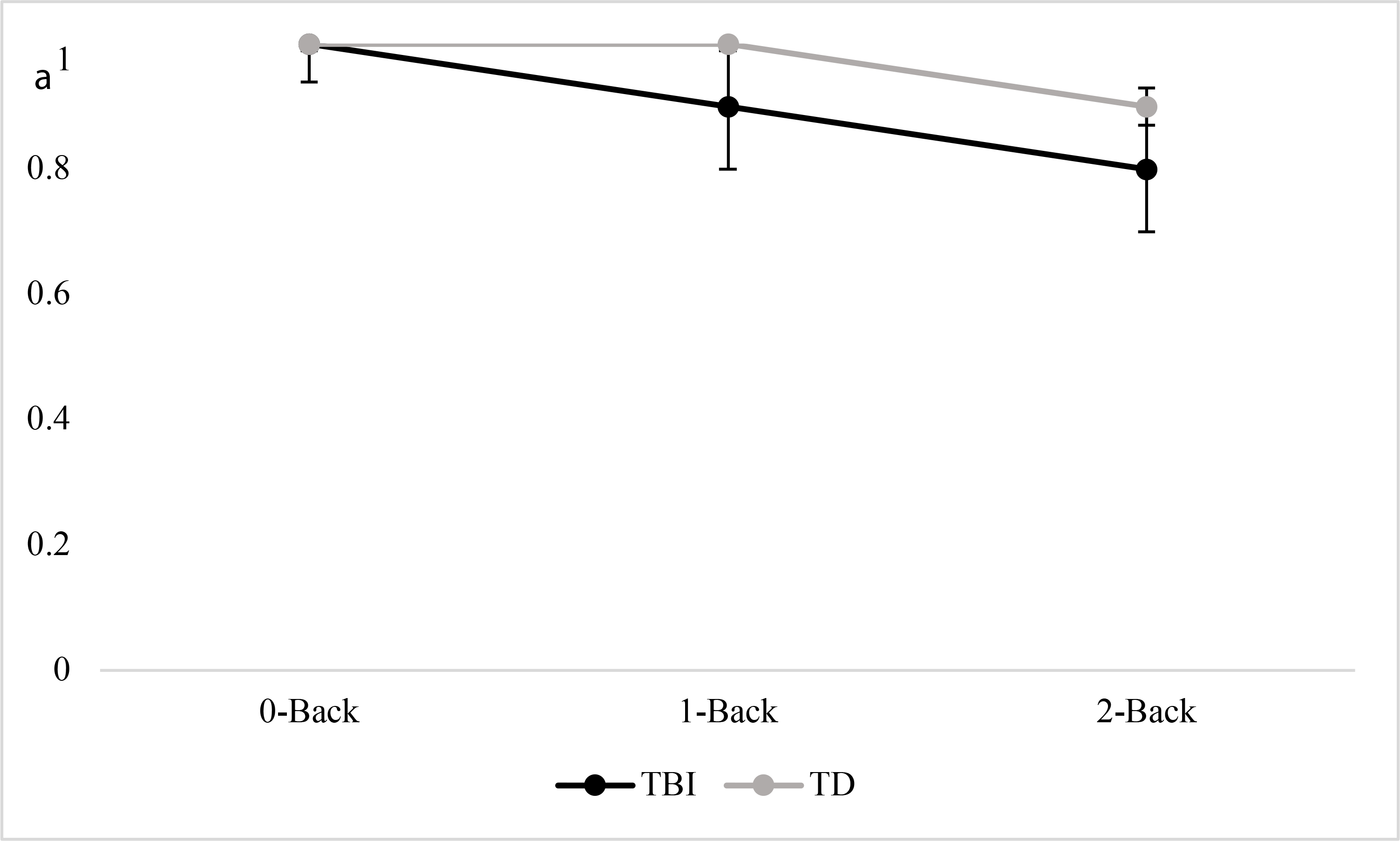
A’ data for face identify recognition
Figure 2 -.
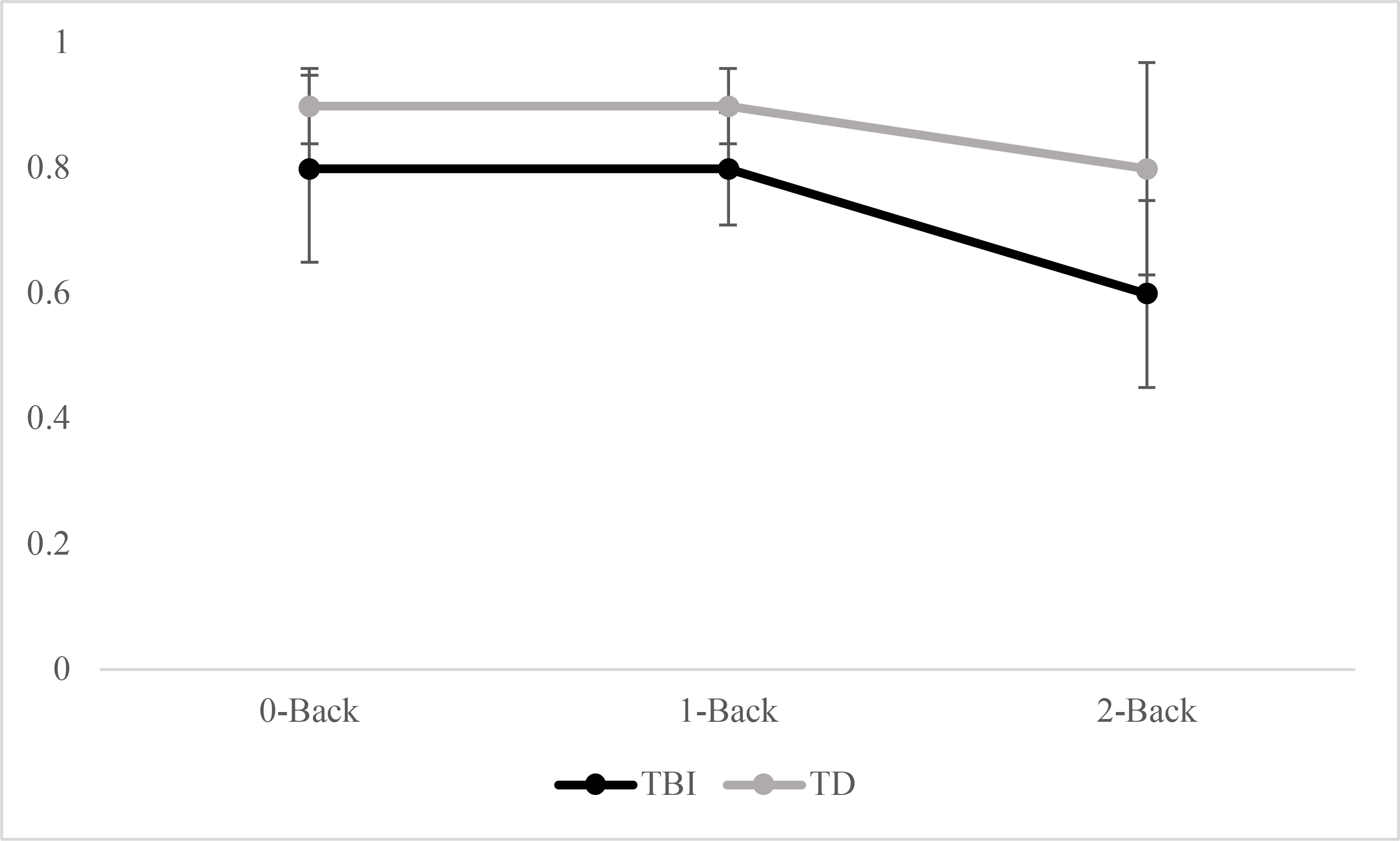
A’ data for emotion recognition
Table 3.
Average A’ Scores by Condition and Working Memory Level
| Group | Participant | Neutral Average A’ | Emotion Average A’ | ||||
|---|---|---|---|---|---|---|---|
| 0-back | 1-back | 2-back | 0-back | 1-back | 2-back | ||
| TBI | 1 | 1.00 | 0.97 | 0.88 | 0.83 | 0.89 | 0.60 |
| 2 | 0.96 | 1.00 | 0.81 | 0.83 | 0.80 | 0.62 | |
| 3 | 1.00 | 1.00 | .98 | 0.97 | 0.97 | 0.79 | |
| 4 | 0.84 | 0.86 | 0.66 | 0.83 | 0.82 | 0.42 | |
| 5 | 1.00 | 0.97 | 0.76 | 0.91 | 0.85 | 0.62 | |
| 6 | 1.00 | 0.95 | 0.80 | 0.92 | 0.85 | 0.59 | |
| 7 | 0.94 | 0.81 | 0.65 | 0.76 | 0.78 | 0.30 | |
| 8 | 0.90 | 0.72 | 0.66 | 0.50 | 0.67 | 0.47 | |
| TD | 1 | 1.00 | 0.99 | 0.93 | 0.93 | 0.94 | 0.90 |
| 2 | 1.00 | 1.00 | 0.95 | 0.96 | 0.94 | 0.82 | |
| 3 | 0.98 | 0.97 | 0.95 | 0.96 | 0.95 | 0.88 | |
| 4 | 0.96 | 0.98 | 0.92 | 0.81 | 0.91 | 0.79 | |
| 5 | 1.00 | 0.96 | 0.90 | 0.94 | 0.80 | 0.74 | |
| 6 | 0.99 | 0.97 | 0.95 | 0.96 | 0.96 | 0.93 | |
| 7 | 1.00 | 0.98 | 0.89 | 0.93 | 0.87 | 0.43 | |
| 8 | 1.00 | 1.00 | .099 | 1.00 | .097 | 0.97 | |
Interaction effects
The third hypothesis was partially supported. There was a significant interaction of group by working memory load, F(2, 84) = 5.21, p < .01. Post-hoc tests revealed that across tasks, there were significant between-groups differences that were smallest for the 0-back trials, D = .50, exact p = .02, d = .38; with a larger difference for the 1-back trials, D = .50, exact p = .02, d = .56; and the largest difference on the 2-back trials, D = .69, exact p < .001, d = .88. There was no significant interaction of group by task, F(1, 84) = 2.38, p = .13.
Working memory comparison
The TBI and TD groups did not differ significantly in accuracy judgments on the CLPT, t = 0.04, p = .97, however the TBI group had significantly poorer word recall than did the TD group, t = −2.00, p = .04.
Discussion
Evidence that working memory and social cognition are impaired in adolescents and young adults with TBI comes primarily from studies in which the two constructs were tested separately. In real-time social interactions, however, working memory and social cognition interact, thus it was of interest to conduct a preliminary examination of this interaction experimentally. We predicted that adolescents and young adults with TBI would have lower scores overall than would TD peers and would make relatively more errors as working memory load increased. We also predicted that while both groups would make more errors for affect recognition than face recognition, this effect would be greater for participants with TBI. Results supported the first two predictions and provided partial support for the third.
Regarding working memory, participants with TBI had significantly lower scores on both the CLPT, which was used to characterize the samples’ working memory, and the experimental n-back tasks. As expected, between-groups differences on the n-back tasks increased as working memory load increased and scores for the TBI group approached chance performance. These findings extend previous n-back research in children with TBI (Levin et al., 2004), and agree with previous findings in adolescents with TBI using other working memory tests (e.g., (Gorman et al., 2017; Moran & Gillon, 2004; Phillips et al., 2017)).
In regard to social cognition, results provided further evidence of impairments in emotion recognition in adolescents and young adults with TBI, which have been documented previously using varying stimuli including photographs and voice recordings (Ryan et al., 2020; Tonks et al., 2008). Social cognition results were also consistent with previous studies in children and adults with TBI using a variety of experimental media (Binder et al., 2019; Rosenberg et al., 2014; Ryan et al., 2020). Taken together, these preliminary findings are consistent with previous research indicating that adolescents’ and young adults’ risk for impaired working memory and social cognition after TBI and indicate a need for additional research with larger samples.
While the findings overall were consistent with previous research, some results were surprising. TBI and TD groups were expected to be similar in the face recognition task when the working memory demand was low and differ only as memory load increased. While our TBI and TD groups were similar in the 0-back face identification condition, the TBI group’s performance declined in the 1- and 2-back conditions. Tonks and colleagues (2008) reported similar scores for face identity matching in adolescents with versus without TBI. Likewise, a previous n-back study using face matching showed no difference between adults with severe TBI and uninjured peers, in either a 1-back or 2-back condition (Newsome et al., 2007). Further, impairments in face recognition are relatively rare after TBI (Valentine et al., 2006). There are, however, caveats to the previously reported findings. First, data from Tonks and colleagues were from seven adolescents, and while the group average score did not differ from that of typical peers, inspection of individual data revealed that four adolescents with TBI had negative z-scores, with two close to −1.0 and the others more than 1 standard deviation below zero. A fifth participant had a perinatal stroke rather than TBI. Thus, the group average in the Tonks et al. (2008) study may not represent the risk for face identification problems in adolescents with TBI. The second caveat is that studies reporting no between-groups differences were in adults, and results might not apply to adolescents. For example, a sequential-letter n-back study in adults revealed no group differences at the 0- or 1-back level (Perlstein et al., 2004), but a similar study in children with TBI revealed significant deficits even in those with mild or moderate injuries (Levin et al., 2004). In the latter study, the authors did not report data for individual n-back conditions, so results for 0- and 1-back levels could not be determined. The findings overall, however, suggest that children and adolescents might be more vulnerable than adults to working memory impairments after TBI, perhaps because working memory is still developing at this stage. The findings related to face recognition merit replication, as errors in updating face representations in working memory could have significant social consequences (Binder et al., 2019), particularly during the adolescent years when social networks are expanding (Hamm et al., 2014; Turkstra, 2000).
For the emotion recognition task, previous evidence of basic emotion recognition impairments after TBI predicted a group difference even in the 0-back condition. The task might have been made even more difficult because the target emotion was anger. Anger has been shown to be more difficult to recognize from facial expressions compared to emotions like happiness (Byom et al., 2019; Rigon et al., 2018; Rosenberg et al., 2014). A previous study of facial affect recognition in adolescents with TBI revealed disproportionate impairment in recognition of anger and sadness compared to happiness (Turkstra et al., 2001). Similarly, studies of adults with TBI have also reported impairments in anger recognition (Byom et al., 2019; Philippi et al., 2009; Rosenberg et al., 2014). Selective impairments in recognition of negative emotions such as anger also have been reported in patients with frontotemporal dementia (Lough et al., 2006), brain tumors (Mu et al., 2012), and multiple sclerosis (Krause et al., 2009), in all cases associated with lesions to the prefrontal cortex. Emotion processing problems may extend to production of emotions, as McDonald et al., (2011) showed selective impairment in facial mimicry of angry vs. happy expressions in adults with TBI.
We expected that the combination of working memory load and social stimuli would disproportionately affect participants in the TBI group, but there was no significant interaction of group by task. The lack of a significant interaction should be interpreted with caution, however, because of an apparent floor effect in the TBI group. Inspection of individual data revealed that 5 of 8 participants in the TBI group had scores that were close to chance (less than .60) versus only one participant in the TD group. Only two other A’ values in the study approached chance, both on the emotion recognition task: one in the TBI group 0-back condition and one in the TD group 2-back condition. Thus, the high-working memory social condition seemed particularly challenging for adolescents with TBI.
Implications for Social Outcome
There is evidence that social cognition and working memory impairments can affect social interactions in everyday life. In a study of adults with TBI, also using the Ekman photographs (Spikman et al., 2013), accuracy recognizing anger and sadness was negatively correlated with frequency of other-reported behavior problems in everyday life. Similarly, Binder and colleagues (2019) reported a significant correlation between facial emotion recognition and community integration post-TBI. There is also evidence that in children and adolescents with TBI, social cognition more broadly, is associated with social skill competence and behavioral problems after TBI (Ryan et al., 2020). If impaired emotion recognition affects social outcomes, it also may underlie some of the social problems experienced by adolescents with TBI. For example, if an adolescent with TBI consistently fails to recognize her friends’ facial emotions she may be perceived as indifferent or inappropriate, putting her at risk to lose important peer relationships. Injury in adolescence also may interrupt development of social cognition skills, with downstream effects on adult social performance.
Impaired working memory has also been linked to a variety of problems in social interactions, such as errors in understanding inferences and abstract language (for review see Rowley et al., 2017), impairments in comprehension of the gist of a story (Chapman et al., 2006), and acting without considering consequences of behaviors (Romer et al., 2011).
These findings and those of others (Binder et al., 2019; McDonald, 2013) suggest that individuals with TBI are at risk for acquired problems “reading” other people and that these problems can have consequences in everyday social life. While social problems are challenging at any age, they may be particularly penalizing for adolescents because of the acute focus on peers and development of interpersonal intimacy skills at this stage of development. For adolescents with TBI, who are navigating a new sense of self and relationships with others, impairments in social cognition may be particularly devastating. While the current findings need to be replicated in larger, more diverse samples, they are in line with previous TBI research supporting clinical consideration of social cognitive abilities post-TBI. Tools and resources exist to support evaluation and treatment of social cognition (for review see Neumann et al., 2017; Sohlberg et al., 2019), however the current findings also suggest the need to consider the working memory demands imposed by social cognitive assessment tools, interventions, and adolescents’ daily interactions. If replicated, they may also suggest that social functioning might be supported by environmental modifications that reduce the need to hold social information in working memory.
Limitations and Future Directions
Results from this preliminary study add additional evidence that adolescents with TBI are at risk for both working memory and facial affect recognition impairments. These results were drawn from a small and racially homogenous sample of participants, limiting generalization of findings to the general adolescent TBI population. Replication with a larger, more diverse sample is an important next step. A larger sample will also be important so that effects of injury-related factors, such as age at injury, on social working memory can be examined.
A larger sample would also allow evaluation of the relationships between IQ and social cognitive and working memory performance in adolescents with TBI. On average, our TBI group had lower estimated IQ scores than the TD group, which may have impacted performance on emotion and facial identification tasks. Performance on working memory and social cognition tasks, while psychometrically distinct from IQ, have been significantly correlated with IQ scores in studies of typically developing children and adolescents (Ackerman et al., 2005; Lawrence et al., 2015) and thus it is an important next step to examine the potentially mediating role of general cognitive ability on social working memory post-TBI.
An additional consideration for future research is the experimental tasks used to measure interacting effects of social cognition and working memory. Cognitive models of working memory (Baddeley, 2000; Cowan, 2010) informed our hypotheses and choice of social working memory tasks for this preliminary study, however future studies should include more in-depth examinations of verbal and non-verbal working memory and their potential impact on social functioning. The ecological validity of facial affect recognition tasks must also be considered in future research. By design, we focused only on recognition of a single emotion in static, experimentally simplified images of adults, which does not represent the range and timing of emotions encountered in adolescent social life. Participants were tested in a quiet room and had on- and off-line practice trials before the n-back tasks. For these reasons, the results may underestimate the effects of social cognition impairments in everyday interactions of adolescents with TBI. For other reasons, the results might have overestimated everyday problems: on the surface, n-back tasks are unlike anything a typical adolescent might encounter in daily life, although basic mechanisms underlying task performance might be similar; stimuli were presented without auditory and context cues that support social interactions in daily life. Thus, future studies that use stimuli that more closely approximate typical adolescent emotion recognition demands, could build on this study and connect task performance to measures of social outcome in everyday life.
Conclusions
Human social interaction is complex and requires an array of cognitive processes, including working memory and social cognition, which continue to develop into adolescence and can be disrupted by TBI. Findings from the current study provide further evidence that adolescents and young adults with TBI are at risk for emotion recognition and working memory deficits. Study findings also indicated that the ability to recognize social information was significantly affected by working memory demand, particularly for adolescents with TBI. An unexpected result was that adolescents with TBI performed more poorly than their typically developing peers in basic facial identification, even when working memory demands were relatively low. These findings and the negative social outcomes associated with working memory and social cognition impairments warrant continued consideration of the interplay among the cognitive processes that support social success in adolescence.
Acknowledgements
Financial Support: This study was supported by the U.S. National Institute on Deafness and Other Communication Disorders (University of Wisconsin-Madison Department of Communication Sciences and Disorders, grant number NIDCD T32DC005359), (L.B., grant number NIDCD F31DC012481) and the Walker Fund.
The authors wish to thank Drs. Julia Evans and Seth Pollak for their assistance with study methods and materials.
Footnotes
Cliff’s Delta is calculated by comparing pairs of data points between samples: d = (#(xTD>xTBI)-#(xTBI-xTD))/n; # = number of times, x = A’, and n = number of comparisons of xTDto xTBI.
Conflicts of Interest: None.
Ethical Standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Ackerman PL, Beier ME, & Boyle MO (2005). Working memory and intelligence: The same or different constructs? Psychological Bulletin, 131(1), 30. [DOI] [PubMed] [Google Scholar]
- Amadó A, Serrat E, & Vallès-Majoral E (2016, 2016-September-13). The Role of Executive Functions in Social Cognition among Children with Down Syndrome: Relationship Patterns [Original Research]. Frontiers in Psychology, 7(1363). 10.3389/fpsyg.2016.01363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbage DR, Yim J, Zupan B, Neumann D, Tomita MR, & Willer B (2011). Meta-analysis of facial affect recognition difficulties after traumatic brain injury. Neuropsychology, 25(3), 277–285. 10.1037/a0021908 [DOI] [PubMed] [Google Scholar]
- Baddeley A (2000). The episodic buffer: A new component of working memory? Trends in Cognitive Sciences, 4(11), 417–423. 10.1016/s1364-6613(00)01538-2 [DOI] [PubMed] [Google Scholar]
- Best JR, & Miller PH (2010, Nov-Dec). A developmental perspective on executive function. Child Development, 81(6), 1641–1660. 10.1111/j.1467-8624.2010.01499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehl M, Matsumoto D, Ekman P, Hearn V, Heider K, Kudoh T, & Ton V (1997). Matsumoto and Ekman’s Japanese and Caucasian Facial Expressions of Emotion (JACFEE): Reliability Data and Cross-National Differences. Journal of Nonverbal Behavior, 21(1), 3–21. [Google Scholar]
- Bigler ED (2001). The lesion(s) in traumatic brain injury: Implications for clinical neuropsychology. Archives of Clinical Neuropsychology, 16(2), 95–131. 10.1016/s0887-6177(00)00095-0 [DOI] [PubMed] [Google Scholar]
- Binder AS, Lancaster K, Lengenfelder J, Chiaravalloti ND, & Genova HM (2019). Community Integration in Traumatic Brain Injury: The Contributing Factor of Affect Recognition Deficits. Journal of the International Neuropsychological Society, 25(8), 890–895. 10.1017/S1355617719000559 [DOI] [PubMed] [Google Scholar]
- Byom L, Duff M, Mutlu B, & Turkstra L (2019). Facial emotion recognition of older adults with traumatic brain injury. Brain Injury, 33(3), 322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, N. C. f. I. P. a. C. (2010, 17, March, 2010). Injury Prevention & Control: Traumatic Brain Injury. Centers for Disease Control and Prevention. Retrieved 25, March from http://www.cdc.gov/traumaticbraininjury/statistics.html [Google Scholar]
- Chapman SB, Gamino JF, Cook LG, Hanten G, Li X, & Levin HS (2006). Impaired discourse gist and working memory in children after brain injury. Brain and Language, 97(2), 178–188. 10.1016/j.bandl.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Choudhury S, Blakemore S-J, & Charman T (2006, Dec). Social cognitive development during adolescence. Social Cognitive and Affective Neuroscience, 1(3), 165–174. 10.1093/scan/nsl024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff N (1993). Dominance Statistics: Ordinal Analyses to Answer Ordinal Questions. Psychological Bulletin, 114(3), 494–509. [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, & Provost J (1993). PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers, 25, 257–271. [Google Scholar]
- Conklin HM, Luciana M, Hooper CJ, & Yarger RS (2007). Working memory performance in typically developing children and adolescents: Behavioral evidence of protracted frontal lobe development. Developmental Neuropsychology, 31(1), 103–128. 10.1207/s15326942dn3101_6 [DOI] [PubMed] [Google Scholar]
- Cowan N (2010). The magical mystery four: How is working memory capacity limited, and why? Current Directions in Psychological Science, 19(1), 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighton S, Durish CL, Taylor HG, Rubin K, Dennis M, Bigler ED, Vannatta K, Gerhardt CA, Stancin T, & Yeates KO (2019). Theory of Mind and Parental Nurturance as Predictors of Peer Relationships After Childhood Traumatic Brain Injury: A Test of Moderated Mediation. Journal of the International Neuropsychological Society, 25(9), 931–940. 10.1017/S135561771900064X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Simic N, Gerry Taylor H, Bigler ED, Rubin K, Vannatta K, Gerhardt CA, Stancin T, Roncadin C, & Yeates KO (2012, Sep). Theory of mind in children with traumatic brain injury. Journal of the International Neuropsychological Society, 18(5), 908–916. 10.1017/S1355617712000756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson W (1992, Sep). Measuring recognition memory. Journal of Experimental Psychology General, 121(3), 275–277. http://www.ncbi.nlm.nih.gov/pubmed/1402701 [DOI] [PubMed] [Google Scholar]
- Ekman P, & Friesen WV (1976). Pictures of facial affect. Consulting Psychologists Press. [Google Scholar]
- Embury CM, Wiesman AI, Proskovec AL, Mills MS, Heinrichs-Graham E, Wang Y-P, Calhoun VD, Stephen JM, & Wilson TW (2019). Neural dynamics of verbal working memory processing in children and adolescents. NeuroImage, 185, 191–197. 10.1016/j.neuroimage.2018.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JL, Selinger C, & Pollak SD (2011). P300 as a measure of processing capacity in auditory and visual domains in specific language impairment. Brain Research, 1389, 93–102. 10.1016/j.brainres.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, & Campbell IG (2010). Sleep EEG changes during adolescence: An index of a fundamental brain reorganization. Brain and Cognition, 72(1), 56–65. 10.1016/j.bandc.2009.09.008 [DOI] [PubMed] [Google Scholar]
- Gaulin CA, & Campbell TF (1994). Procedure for assessing verbal working memory in normal school-age children: Some preliminary data. Perceptual and Motor Skills, 79(1, Pt 1), 55–64. 10.2466/pms.1994.79.1.55 [DOI] [PubMed] [Google Scholar]
- Gordon AC, & Olson DR (1998). The relation between acquisition of a theory of mind and the capacity to hold in mind. Journal of Experimental Child Psychology, 68(1), 70–83. [DOI] [PubMed] [Google Scholar]
- Gorman S, Barnes MA, Swank PR, & Ewing-Cobbs L (2017). Recovery of working memory following pediatric traumatic brain injury: a longitudinal analysis. Developmental Neuropsychology, 42(3), 127–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman S, Barnes MA, Swank PR, Prasad M, & Ewing-Cobbs L (2012, Jan). The effects of pediatric traumatic brain injury on verbal and visual-spatial working memory. Journal of the International Neuropsychological Society, 18(1), 29–38. 10.1017/S1355617711001251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JV, Farmer TW, Lambert K, & Gravelle M (2014). Enhancing peer cultures of academic effort and achievement in early adolescence: Promotive effects of the SEALS intervention. Developmental Psychology, 50(1), 216–228. 10.1037/a0032979 [DOI] [PubMed] [Google Scholar]
- Humphrey G, & Dumontheil I (2016). Development of risk-taking, perspective-taking, and inhibitory control during adolescence. Developmental Neuropsychology, 41(1–2), 59–76. [DOI] [PubMed] [Google Scholar]
- Im-Bolter N, Agostino A, & Owens-Jaffray K (2016). Theory of mind in middle childhood and early adolescence: Different from before? Journal of Experimental Child Psychology, 149, 98–115. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, & Kaufman NL (2004). K-BIT2: Kaufman Brief Intelligence Test (Second ed.). NCS Pearson, Inc. [Google Scholar]
- Kelly M, McDonald S, & Frith MHJ (2017). A Survey of Clinicians Working in Brain Injury Rehabilitation: Are Social Cognition Impairments on the Radar? The Journal of Head Trauma Rehabilitation, 32(4), E55–E65. 10.1097/htr.0000000000000269 [DOI] [PubMed] [Google Scholar]
- Kennedy DP, & Adolphs R (2012). The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences, 16(11), 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilford EJ, Garrett E, & Blakemore S-J (2016). The development of social cognition in adolescence: An integrated perspective. Neuroscience & Biobehavioral Reviews, 70, 106–120. [DOI] [PubMed] [Google Scholar]
- Krause M, Wendt J, Dressel A, Berneiser J, Kessler C, Hamm AO, & Lotze M (2009, Dec 14). Prefrontal function associated with impaired emotion recognition in patients with multiple sclerosis. Behavioral Brain Research, 205(1), 280–285. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19686782 [DOI] [PubMed] [Google Scholar]
- Lawrence K, Campbell R, & Skuse D (2015). Age, gender, and puberty influence the development of facial emotion recognition. Frontiers in Psychology, 6, 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Zhang L, Swank PR, Ewing-Cobbs L, Dennis M, Barnes MA, Max J, Schachar R, Chapman SB, & Hunter JV (2004). Changes in Working Memory After Traumatic Brain Injury in Children. Neuropsychology, 18(2), 240–247. 10.1037/0894-4105.18.2.240 [DOI] [PubMed] [Google Scholar]
- Lough S, Kipps CM, Treise C, Watson P, Blair JR, & Hodges JR (2006). Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia, 44(6), 950–958. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16198378 [DOI] [PubMed] [Google Scholar]
- Martin-Rodriguez JF, & Leon-Carrion J (2010, Apr). Theory of mind deficits in patients with acquired brain injury: a quantitative review. Neuropsychologia, 48(5), 1181–1191. https://doi.org/S0028-3932(10)00053-9 [DOI] [PubMed] [Google Scholar]
- Master SL, Eckstein MK, Gotlieb N, Dahl R, Wilbrecht L, & Collins AGE (2020, 2020/February/01/). Distentangling the systems contributing to changes in learning during adolescence. Developmental Cognitive Neuroscience, 41, 100732. 10.1016/j.dcn.2019.100732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M, Milders M, Downey B, Whyte M, Higgins V, Wojcik Z, Amin S, & O’Rourke S (2017, May). Social Behavior and Impairments in Social Cognition Following Traumatic Brain Injury. Journal of the International Neuropsychological Society, 23(5), 400–411. 10.1017/s1355617717000182 [DOI] [PubMed] [Google Scholar]
- McDonald S (2013, Mar). Impairments in social cognition following severe traumatic brain injury Journal of the International Neuropsychological Society, 19(3), 231–246. 10.1017/S1355617712001506 [DOI] [PubMed] [Google Scholar]
- McDonald S, English T, Randall R, Longman T, Togher L, & Tate RL (2013). Assessing social cognition and pragmatic language in adolescents with traumatic brain injuries. Journal of the International Neuropsychological Society, 19(5), 528–538. 10.1017/S1355617713000039 [DOI] [PubMed] [Google Scholar]
- McDonald S, Li S, De Sousa A, Rushby J, Dimoska A, James C, & Tate RL (2011). Impaired mimicry response to angry faces following severe traumatic brain injury. Journal of Clinical Experimental Neuropsychology, 33(1), 17–29. 10.1080/13803391003761967 [DOI] [PubMed] [Google Scholar]
- Meyer ML, & Collier E (2020). Theory of minds: managing mental state inferences in working memory is associated with the dorsomedial subsystem of the default network and social integration. Social Cognitive and Affective Neuroscience, 15(1), 63–73. 10.1093/scan/nsaa022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milders M (2019, 2019/January/02). Relationship between social cognition and social behaviour following traumatic brain injury. Brain Injury, 33(1), 62–68. 10.1080/02699052.2018.1531301 [DOI] [PubMed] [Google Scholar]
- Moran CA, & Gillon G (2004). Language and memory profiles of adolescents with traumatic brain injury. Brain Injury, 18(3), 273–288. 10.1080/02699050310001617415 [DOI] [PubMed] [Google Scholar]
- Morton MV, & Wehman P (1995, 1995/January/01). Psychosocial and emotional sequelae of individuals with traumatic brain injury: a literature review and recommendations. Brain Injury, 9(1), 81–92. 10.3109/02699059509004574 [DOI] [PubMed] [Google Scholar]
- Mu YG, Huang LJ, Li SY, Ke C, Chen Y, Jin Y, & Chen ZP (2012). Working memory and the identification of facial expression in patients with left frontal glioma. Neuro-oncology, 14 Suppl 4, iv81–89. 10.1093/neuonc/nos215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, & Whalen PJ (2011). Individual differences in neural activity during a facial expression vs. identity working memory task. Neuroimage, 56(3), 1685–1692. 10.1016/j.neuroimage.2011.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Westerhof-Evers HJ, Visser-Keizer AC, Fasotti L, Schönherr MC, Vink M, van der Naalt J, & Spikman JM (2017). Effectiveness of a treatment for impairments in social cognition and emotion regulation (T-ScEmo) after traumatic brain injury: a randomized controlled trial. Journal of Head Trauma Rehabilitation, 32(5), 296–307. [DOI] [PubMed] [Google Scholar]
- Newsome MR, Scheibel RS, Steinberg JL, Troyanskaya M, Sharma RG, Rauch RA, Li X, & Levin HS (2007). Working memory brain activation following severe traumatic brain injury. Cortex, 43(1), 95–111. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17334210 [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Cole MA, Demery JA, Seignourel PJ, Dixit NK, Larson MJ, & Briggs RW (2004). Parametric manipulation of working memory load in traumatic brain injury: behavioral and neural correlates. Journal of the International Neuropsychological Society, 10(5), 724–741. 10.1017/S1355617704105110 [DOI] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, & Rudrauf D (2009). Damage to association fiber tracts impairs recognition of the facial expression of emotion. Journal of Neuroscience, 29(48), 15089–15099. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19955360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips NL, Parry L, Mandalis A, & Lah S (2017). Working memory outcomes following traumatic brain injury in children: A systematic review with meta-analysis. Child Neuropsychology, 23(1), 26–66. [DOI] [PubMed] [Google Scholar]
- Rigon A, Voss MW, Turkstra LS, Mutlu B, & Duff MC (2018). Different aspects of facial affect recognition impairment following traumatic brain injury: The role of perceptual and interpretative abilities. Journal of Clinical and Experimental Neuropsychology, 40(8), 805–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, & McKinlay A (2019). The long-term effects of childhood traumatic brain injury on adulthood relationship quality. Brain Injury, 33(5), 649–656. [DOI] [PubMed] [Google Scholar]
- Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Yang W, & Hurt H (2011). Does adolescent risk taking imply weak executive function? A prospective study of relations between working memory performance, impulsivity, and risk taking in early adolescence. Developmental Science, 14(5), 1119–1133. 10.1111/j.1467-7687.2011.01061.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H, McDonald S, Dethier M, Kessels RPC, & Westbrook RF (2014). Facial Emotion Recognition Deficits following Moderate–Severe Traumatic Brain Injury (TBI): Re-examining the Valence Effect and the Role of Emotion Intensity. Journal of the International Neuropsychological Society, 20(10), 994–1003. 10.1017/S1355617714000940 [DOI] [PubMed] [Google Scholar]
- Rowley DA, Rogish M, Alexander T, & Riggs KJ (2017). Cognitive correlates of pragmatic language comprehension in adult traumatic brain injury: A systematic review and meta-analyses. Brain Injury, 31(12), 1564–1574. [DOI] [PubMed] [Google Scholar]
- Ryan NP, Anderson V, Bigler ED, Dennis M, Taylor HG, Rubin K, Vannatta K, Gerhardt CA, Stancin T, & Beauchamp MH (2020). Delineating the nature and correlates of social dysfunction after childhood traumatic brain injury using common data elements: evidence from an international multi-cohort study. Journal of Neurotrauma, 38(2), 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NP, Catroppa C, Beare R, Silk TJ, Hearps SJ, Beauchamp MH, Yeates KO, & Anderson VA (2017). Uncovering the neuroanatomical correlates of cognitive, affective and conative theory of mind in paediatric traumatic brain injury: a neural systems perspective. Social Cognitive and Affective Neuroscience, 12(9), 1414–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento K, Thomas KE, Daugherty J, Waltzman D, Haarbauer-Krupa JK, Peterson AB, Haileyesus T, & Breiding MJ (2019). Emergency department visits for sports-and recreation-related traumatic brain injuries among children—United States, 2010–2016. Morbidity and Mortality Weekly Report, 68(10), 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AT, Hanten GR, Li X, Orsten KD, & Levin HS (2010, Aug). Emotion recognition following pediatric traumatic brain injury: longitudinal analysis of emotional prosody and facial emotion recognition. Neuropsychologia, 48(10), 2869–2877. 10.1016/j.neuropsychologia.2010.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, & Luna B (2017). Protracted development of executive and mnemonic brain systems underlying working memory in adolescence: A longitudinal fMRI study. NeuroImage, 157, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Lane RD, Alkozei A, Bao J, Smith C, Sanova A, Nettles M, & Killgore WD (2017). Maintaining the feelings of others in working memory is associated with activation of the left anterior insula and left frontal-parietal control network. Social Cognitive and Affective Neuroscience, 12(5), 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlberg MM, MacDonald S, Byom L, Iwashita H, Lemoncello R, Meulenbroek P, Ness B, & O’Neil-Pirozzi TM (2019). Social communication following traumatic brain injury part I: State-of-the-art review of assessment tools. International journal of speech-language pathology, 21(2), 115–127. [DOI] [PubMed] [Google Scholar]
- Spikman JM, Milders MV, Visser-Keizer AC, Westerhof-Evers HJ, Herben-Dekker M, & van der Naalt J (2013). Deficits in facial emotion recognition indicate behavioral changes and impaired self-awareness after moderate to severe traumatic brain injury. PLoS One, 8(6), e65581. 10.1371/journal.pone.0065581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2010). Adolescence (Ninth ed.). McGraw-Hill College. [Google Scholar]
- Tamnes CK, Herting MM, Goddings A-L, Meuwese R, Blakemore S-J, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, Crone EA, & Mills KL (2017). Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness. The Journal of Neuroscience, 37(12), 3402–3412. 10.1523/jneurosci.3302-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, Bell JM, Breiding MJ, & Xu L (2017). Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths -- United States, 2007 and 2013. MMWR Surveillance Summaries, 66(9), 1–16. 10.15585/mmwr.ss6609a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LA, De Bellis MD, Graham R, & LaBar KS (2007). Development of emotional facial recognition in late childhood and adolescence. Developmental Science, 10(5), 547–558 [DOI] [PubMed] [Google Scholar]
- Thornton MA, & Conway AR (2013, Apr 15). Working memory for social information: chunking or domain-specific buffer? Neuroimage, 70, 233–239. 10.1016/j.neuroimage.2012.12.063 [DOI] [PubMed] [Google Scholar]
- Tonks J, Slater A, Frampton I, Wall SE, Yates P, & Williams WH (2009, Jan). The development of emotion and empathy skills after childhood brain injury. Dev Med Child Neurol, 51(1), 8–16. 10.1111/j.1469-8749.2008.03219.x [DOI] [PubMed] [Google Scholar]
- Tonks J, Williams WH, Frampton I, Yates P, Wall SE, & Slater A (2008, Apr). Reading emotions after childhood brain injury: case series evidence of dissociation between cognitive abilities and emotional expression processing skills. Brain Injury, 22(4), 325–332. 10.1080/02699050801968303 [DOI] [PubMed] [Google Scholar]
- Tousignant B, Jackson PL, Massicotte E, Beauchamp MH, Achim AM, Vera-Estay E, Bedell G, & Sirois K (2018, Apr). Impact of traumatic brain injury on social cognition in adolescents and contribution of other higher order cognitive functions. Neuropsychological Rehabilitation, 28(3), 429–447. 10.1080/09602011.2016.1158114 [DOI] [PubMed] [Google Scholar]
- Tousignant B, Sirois K, Achim AM, Massicotte E, & Jackson PL (2017, 2017/July/01/). A comprehensive assessment of social cognition from adolescence to adulthood. Cognitive Development, 43, 214–223. 10.1016/j.cogdev.2017.05.001 [DOI] [Google Scholar]
- Tuerk C, Dégeilh F, Catroppa C, Dooley JJ, Kean M, Anderson V, & Beauchamp MH (2020, 2020/February//). Altered resting-state functional connectivity within the developing social brain after pediatric traumatic brain injury. Human Brain Mapping, 41(2), 561–576. 10.1002/hbm.24822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkstra LS (2000). Should my shirt be tucked in or left out? The communication context of adolescence. Aphasiology, 14(4), 349–364. [Google Scholar]
- Turkstra LS, McDonald S, & DePompei R (2001, Oct). Social information processing in adolescents: data from normally developing adolescents and preliminary data from their peers with traumatic brain injury. Journal of Head Trauma Rehabilitation, 16(5), 469–483. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11574042 [DOI] [PubMed] [Google Scholar]
- Valentine T, Powell J, Davidoff J, Letson S, & Greenwood R (2006, 2006/June/01). Prevalence and correlates of face recognition impairments after acquired brain injury. Neuropsychological Rehabilitation, 16(3), 272–297. 10.1080/09602010500176443 [DOI] [PubMed] [Google Scholar]
- Vetter NC, Drauschke M, Thieme J, & Altgassen M (2018). Adolescent basic facial emotion recognition is not influenced by puberty or own-age bias. Frontiers in Psychology, 9, 956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Swift E, Taylor HG, Wade SL, Drotar D, Stancin T, & Minich N (2004, May). Short- and long-term social outcomes following pediatric traumatic brain injury. Journal of the International Neuropsychological Society, 10(3), 412–426. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15147599 [DOI] [PubMed] [Google Scholar]


