Abstract
The parasitoid Leptopilina heterotoma has been used as a model system for more than 70 years, contributing greatly to diverse research areas in ecology and evolution. Here, we synthesized the large body of work on L. heterotoma with the aim to identify new research avenues that could be of interest also for researchers studying other parasitoids and insects. We start our review with a description of typical L. heterotoma characteristics, as well as that of the higher taxonomic groups to which this species belongs. We then continue discussing host suitability and immunity, foraging behaviors, as well as fat accumulation and life histories. We subsequently shift our focus towards parasitoid‐parasitoid interactions, including L. heterotoma coexistence within the larger guild of Drosophila parasitoids, chemical communication, as well as mating and population structuring. We conclude our review by highlighting the assets of L. heterotoma as a model system, including its intermediate life history syndromes, the ease of observing and collecting natural hosts and wasps, as well as recent genomic advances.
Keywords: associative learning, endosymbiont, fitness, host‐parasitoid community, lipids, sex pheromones, virulence
The parasitoid Leptopilina heterotoma has been used as a model system in biology for more than 70 years. This review aims to provide a broad and detailed synthesis of the work performed on this system, including immunity, behavioral ecology, endosymbiotic and trophic interactions, as well as physiology. Overall, the scientific literature on L. heterotoma unites research based on field observations and experiments, as well as laboratory studies, highlighting the versatility of this model system.

1. INTRODUCTION
The parasitoid Leptopilina heterotoma is a wasp species that has long captivated biologists, with the earliest reports in the scientific literature dating back to the 1950s (Figure 1; Jenni, 1951). During the early days of scientific reporting, the species was often referred to as Pseudeucoila bochei (Weld, 1944), although L. heterotoma (Thomson, 1862) was the first recorded name for the species. This was highlighted by Nordlander (1980) in his comprehensive paper on the Leptopilina genus, which was recently updated by Lue et al. (2016) (including all other known synonyms of L. heterotoma; Table 1). L. heterotoma belongs to the cynipoid wasps (superfamily: Cynipoidea), a group that contains parasitoids (i.e., insects that develop and feed on another insect; Godfray, 1994), but also includes phytophagous gall inducers and inquilines (i.e., inhabiting the galls of others). The wide diversity of feeding habits and life histories within the cynipoids has led to several hypotheses regarding the early evolution of the group. Ronquist (1995, 1999) hypothesized that the first cynipoids were endoparasitoids of wood‐, stem‐ or cone‐boring insect larvae. In a recent study by Blaimer et al. (2020) another scenario was proposed where inquilinism dominated throughout the early evolution of cynipoids. This means that cynipoids would be derived from gall‐associated inquiline ancestors. Another phylogenetic reconstruction, however, supported the previously suggested parasitoid‐first hypothesis, where the common ancestor of the cynipoids was a parasitoid. Irrespective of the exact lifestyle of the common ancestor, several host shifts have occurred in the cynipoids, including the use of dipteran hosts, as is the case for L. heterotoma.
FIGURE 1.

The amber wasp Leptopilina heterotoma © Hans Smidt
TABLE 1.
Synonyms of L. heterotoma (from Lue et al., 2016)
| Eucoila heterotoma |
| Ganaspis subnuda |
| Ganaspis monilicornis |
| Erisphagia philippinensis |
| Pseudeucoila (Pseudeucoila) bochei |
| Cothonaspis (Erisphagia) philippinensis |
| Pseudeucoila bochei |
| Leptopilina monilicornis |
| Leptopilina philippinensis |
| Leptopilina subnuda |
| Leptopilina bochei |
Leptopilina heterotoma belongs to the figitid family (Figitidae) and the eucoline subfamily (Eucolinae). While delimitations of the figitids have not been well established, the eucoilines are easily identifiable by the possession of a clear synapomorphy: a scutellar plate with a glandular pit (with unknown function) surmounting the mesothoracic scutellum (Figure 2; Fontal‐Cazalla et al., 2002). Female antennae typically have 13 segments, while the male's antennae have 15 segments. Females also possess a clip at the end of their ovipositor, which is a unique feature of most figitid wasps in the subfamilies Figitinae and Eucolinae (see Section 5; Buffington, 2007). Eucoline adult sizes range from 1 to 5 mm, and the body, brown or black, is shiny. When a L. heterotoma individual is viewed from the side and in the light, the body appears to be amber colored. In trying to boost other researchers to work on L. heterotoma, and to ease the transfer of our scientific knowledge to the general public, we here propose “amber wasp” as the common name for the species.
FIGURE 2.

Lateral view of the thorax of Leptopilina heterotoma (a) and Asobara tabida (b) with the scutellum highlighted with a red line. Dorsal view of the scutellum for L. heterotoma (c) and A. tabida (d). The scutellar plate common to eucolines is highlighted with the green line, and the glandular pit with the blue dot.
All eucolines are endoparasitoids of cyclorraphous fly larvae and have a worldwide distribution, with exception of the poles (Buffington et al., 2020). Leptopilina heterotoma can parasitize a range of different host species, mainly in the Drosophila fly genus (see Section 2), which it attacks when the host itself is developing as a larva. An egg is laid inside the host and only a single individual can successfully survive into adulthood, even if multiple eggs are laid within the same host (i.e., L. heterotoma is a solitary parasitoid). L. heterotoma is a koinobiont, meaning that the host continues feeding and growing while the wasp is developing. Interestingly, L. heterotoma initially develops inside the host, but will migrate out of the host's body during later developmental stages (10 days after oviposition; Figure 3). Eucoilines are generally pro‐ovigenic (Ellers & Jervis, 2004), and while L. heterotoma is often referred to as being pro‐ovigenic (Carton et al., 1986; Haccou et al., 1991; Kimura, 2019), for most strains tested so far considerable egg numbers (sometimes more than 300) are matured during adult life even if some eggs are mature at emergence (Vayssade et al., 2012).
FIGURE 3.

Development of Leptopilina heterotoma. Timeline of the developmental stages of Drosophila melanogaster (green), and L. heterotoma developing in D. melanogaster (blue) at 25°C. Numbers indicate the time in days (adapted from van Alphen & Thunnissen, 1983). L. heterotoma goes through three larval stages (Carton et al., 1986; Jenni, 1951) that may differ depending on the temperature and the host species used (Howe, 1967; Jenni, 1951). A female can oviposit in all larval host instars, but survival is highest when eggs are laid in second instar (Jenni, 1951). After ~30–34 h, the embryo possesses 10 segments (corresponding to the three thoracic and seven abdominal segments of the adult) that are clearly visible (Jenni, 1951). The egg then hatches after ~39–49 h, with females hatching approximately 3 h later than males (Eijsackers & Bakker, 1971). The first larval instar possesses caudal and thoracic appendages, and the larva uses its mandibles mainly to consume host hemolymph (Carton et al., 1986; Jenni, 1951). The first molt of the parasitoid takes place at approximately the same time as host pupation (Carton et al., 1986), which may have a similar hormonal basis (Kopelman & Chabora, 1984). From the second instar onwards, larvae use their mandibles to feed on the host's tissues (Carton et al., 1986). At the time of the second molt, the parasitoid leaves the host's body and lies in between the pupa and the puparium feeding as an ectoparasitoid (Carton et al., 1986). The third larval instar has a much rounder shape than the earlier instars and does not bear any appendages anymore. In the pre‐pupal stage, the larva loses its mandibles (Jenni, 1951) and excretes pellets (meconia) that become visible at the posterior end of the host puparium (Carton et al., 1986; Jenni, 1951). Pupation lasts approximately 9 days (Jenni, 1951) and the parasitoid becomes gradually pigmented (Jenni, 1951; van Alphen & Thunnissen, 1983). The time of emergence is ~21 days after oviposition for males, and ~23 days for females. After emerging from its own puparium, the adult L. heterotoma remains within the host's puparium for ~24 h before emergence (van Alphen & Thunnissen, 1983).
Drosophila parasitoids, including L. heterotoma, have been reviewed in the past, most extensively in the book chapters of Carton et al. (1986) and Fleury et al. (2009). More recently, Wertheim (2022) has synthesized the work on host‐parasitoid co‐evolution in the context of virulence and immunity, including L. heterotoma. No review has yet been dedicated solely to the wasp L. heterotoma that, together with several other species in the Leptopilina genus, has been a staple of research in ecology and evolution since the 1950s. With this review, we synthesize key findings obtained with L. heterotoma as a model system, highlighting the major contribution this species has made to research in ecology and evolution. We further suggest avenues for future research to enthuse others to use this intriguing species as a model system.
2. HOST SUITABILITY, HOST RESISTANCE, AND PARASITOID VIRULENCE
The amber wasp L. heterotoma predominantly parasitizes hosts in the Drosophila genus, a very diverse and rich taxon, but also other drosophilid species, such as Zaprionus flies (Table S1). L. heterotoma does, however, not perform equally well on all these species, due to differences in suitability, and species‐specific immune reactions. Following oviposition of a wasp, a parasitized host can indeed initiate an immune response in <48 h in an attempt to kill the wasp's egg (Mortimer, 2013; Nappi, 1975; Poyet et al., 2013). While ovipositing, the female will also inject venom fluids that can suppress the host's immune response to increase the chances of successful parasitoid development. Adaptations and counter‐adaptations in wasp virulence and host immunity leads to an evolutionary arms race that has been particularly well studied in parasitoids (Wertheim, 2022). The interactions between L. heterotoma, as well as L. boulardi, and their hosts has greatly contributed to our understanding of both insect immunity and venom evolution in parasitoids. Several reviews have already discussed this in great detail (Mortimer, 2013; Nappi, 2010; Poirié et al., 2009, 2014; Wertheim, 2022; Yang et al., 2020); hence here we emphasize the work done on host suitability, host immunity and L. heterotoma virulence.
2.1. Host species suitability and phenology
Drosophila species can feed on a wide variety of substrates, including fruits, flowers, tree sap, cacti and mushrooms, generally in a state of decay (Markow & O'Grady, 2008). Drosophila mostly feed on the microbial community associated with decaying substrates, in addition to the substrate itself (Markow & O'Grady, 2008). Generalist flies can oviposit and utilize a wide range of substrates (e.g., D. melanogaster, D. simulans, and D. immigrans), while specialists are typically restricted to a single substrate (Carton et al., 1991; Markow & O'Grady, 2008). For example, D. phalerata breeds in decaying stinkhorn mushrooms (Driessen et al., 1990), while D. sechellia is specialized on rotting morinda fruits that are toxic for other species in the melanogaster group (Markow & O'Grady, 2005). L. heterotoma predominantly attacks drosophilid larvae in fermenting fruits and sap fluxes, including D. melanogaster (Carton et al., 1991; Fleury et al., 2004; Janssen, 1989; Rizki et al., 1990), D. simulans (Carton et al., 1991; Janssen, 1989; Lynch et al., 2016; Papaj & Vet, 1990; Ris et al., 2004) and D. suboscura (Fleury et al., 2004; Janssen, 1989; Ris et al., 2004), and to a lesser extent Drosophila species breeding in decaying plant matter and fungi (e.g., D. phalerata; Janssen et al., 1988).
Leptopilina heterotoma can parasitize many different host species, but host suitability varies between species (Table S1). In a study by Janssen (1989), D. kuntzei was found to be the most suitable host for L. heterotoma with 89% of L. heterotoma offspring surviving, while D. immigrans was least suitable (2% wasp survival). In this study, D. immigrans was the only species (out of 9 species in total) where more hosts than L. heterotoma survived; hence D. immigrans was the least suitable host. Drosophila immigrans is indeed abundant in Europe but is rarely parasitized (Kraaijeveld & Godfray, 2009). The resistance of D. immigrans to parasitism was, however, suggested to result from its thick cuticle rather than the more typical immune response after parasitism (see below; Ideo et al., 2008; Kraaijeveld & Godfray, 2009; van Alphen & Janssen, 1982). In another study, development on D. melanogaster led to the highest percentage of surviving offspring (47%) compared to D. suboscura (30%), as well as D. immigrans and D. suzukii (<1%). Highest survival percentages (>85%) have been recorded on D. melanogaster, D. hydei, D. kuntzei, D. pseudoobscura, and D. suboscura (Table S1). Only very few L. heterotoma individuals survived when development occurred on Zaprionus vittiger, D. suzukii and D. immigrans (but see Hedlund et al., 1996) and no offspring survived when eggs were laid on D. ananassae, D. biarmipes, D. paralutea and D. busckii (Table S1). Survival on D. melanogaster, one of the preferred hosts of L. heterotoma (Carton et al., 1986, 1991; Fleury et al., 2004, 2009; Rouault, 1979) varies considerably between 26% and 93%, a difference that can be explained by several factors, including whether or not tested species shared an ecological history (hosts and wasps were collected from the same area at the same time), as well as genotype and geographic location (i.e., local adaptation; Fleury et al., 2004).
Leptopilina heterotoma and its drosophilid hosts are polyvoltine with multiple generations per year depending on habitat type, resource availability, and temperature (Fleury et al., 2009). Both wasps and hosts are thus present and/or active throughout most of the year, with the exception of winter (Fleury et al., 2009; Wertheim et al., 2006). L. heterotoma abundance is highest during summer, when higher temperatures lead to quicker development of both the wasps and their hosts. A field study by Godfray and Hardy (1990), for example, showed that wasps were abundant from June to September, with the highest number of individuals caught in June (i.e., up to 23 individuals caught per day), and a general decrease in numbers throughout July (13 per day) and August (9 per day). A more recent study by Knoll et al. (2017) in Switzerland also found that wasp abundance decreased from spring to autumn. Contrary to findings of Godfray and Hardy (1990) in the United Kingdom and of Mazzetto et al. (2016) in Italy where almost no individuals were found in September and October, respectively, Fleury et al. (2004) still found a high abundance of L. heterotoma in October in France. A study on the abundance of Drosophila and its parasitoids in Lyon, Valence, and Hyères (France) by Fleury et al. (2004) suggested that the seasonal abundance of L. heterotoma fluctuates in accordance with the abundance of the host D. melanogaster. Wasp abundance was found to depend on the respective location, with L. heterotoma being most abundant in Lyon where D. melanogaster also predominates. Remarkably low numbers of L. heterotoma have also been recorded, for example in the Southern sites in France (Valence and Hyères), resulting from a steep decrease in D. melanogaster numbers (Fleury et al., 2009). In Tunisia, L. heterotoma also nearly disappears when competitive interactions are high, with D. simulans and D. buzzati being the main hosts used (Carton et al., 1991). Abundance of L. heterotoma thus largely depends on geographic location, seasonality, local climatic conditions, host demography, and competition.
2.2. Host immunity
Encapsulation, a cellular immune response, is a process during which specialized haemocytes aggregate around the parasitoid egg and adhere to its surface to form a capsule. In the melanogaster host subgroup, these haemocytes are called lamellocytes, but within the larger Drosophilidae, several taxa evolved distinct types of haemocytes (e.g., pseudopodocytes in the obscura subgroup; see Wertheim, 2022 for a review). Melanization, which is part of the humoral immune response, entails the synthesis of melanin by lamellocytes that are encapsulating the parasitoid egg. This process occurs by the action of phenoloxidases that originate from haemocytes (Kacsoh & Schlenke, 2012; Nappi, 1975; Poyet et al., 2013). The combination of encapsulation by haemocytes and melanization prevents the parasitoid egg from hatching, eventually killing it (Streams, 1968).
Most Drosophila hosts fail to ignite an effective cellular (Nappi & Streams, 1969; Streams, 1968) and humoral immune response (Schlenke et al., 2007), and can thus not prevent the wasp embryo from developing (Poyet et al., 2013). Some host species, such as D. suzukii and D. algonquin, however, do have a strong immune response (Nappi, 1975; Poyet et al., 2013). Host resistance to parasitism likely depends on the level of circulating haemocytes, with more resistant species having higher haemocyte levels (Kacsoh & Schlenke, 2012; Poyet et al., 2013). Resistant hosts, such as D. euronotus and D. algonquin (Table S2), also possess immune pathways associated with the secretion of antimicrobial proteins and peptides, and other immune activities to inhibit egg/larval development inside the host (Nappi, 1970, 1975). An example is the induced changes in levels of a cell‐signaling molecule, nitric oxide, following parasitism (Carton et al., 2009). Even in species susceptible to parasitism by L. heterotoma or other parasitoids, laboratory experiments and observations with natural populations have shown that parasitism resistance is under strong selection and can increase in populations subjected to high parasitism risks (see Wertheim, 2022 for a review). Indeed, despite the high virulence of L. heterotoma, some hosts can acquire increased resistance through the evolution of novel genes, such as lectin‐24A in the D. melanogaster and D. simulans clade, which is implicated in the humoral response following parasitism by L. boulardi and Asobara tabida (Keebaugh & Schlenke, 2012). Increased parasitism resistance comes at cost, however, leading to trade‐offs, for example with host larval competitive ability or larval survival (Wertheim, 2022). Although the underlying immune response mechanisms of resistant hosts are now well understood, it has remained largely unclear how the host is able to recognize parasitoid eggs or larvae.
Among resistant host species, larvae of D. suzukii, known as the spotted wing Drosophila, are particularly efficient in killing L. heterotoma due to their high haemocyte load (Kacsoh & Schlenke, 2012; Poyet et al., 2013). D. suzukii mostly encapsulates developing wasps at the larval stage, rather than the egg stage (i.e., between 48 and 72 h post‐parasitism; Lacovone et al., 2018). D. suzukii originates from Asia and is a pest of economically important fruits, such as cherry, raspberry, blueberry, but also wild and ornamental plants (Kenis et al., 2016; Lee et al., 2015; Poyet et al., 2015). The fact that D. suzukii females lay their eggs on fresh fruits at a time very close to harvest makes the use of classic insecticides a risk for human health. Biocontrol agents are thus a preferable option (Rossi Stacconi et al., 2015). The efficient immune response of D. suzukii makes L. heterotoma almost unable to parasitize the larvae and is, therefore, not an ideal biocontrol agent against D. suzukii (Chabert et al., 2012; Girod, Rossignaud, et al., 2018; Kacsoh & Schlenke, 2012; Knoll et al., 2017; Poyet et al., 2013; Rossi Stacconi et al., 2017). Other parasitoids (e.g., Trichopria drosophilae, Pachycrepoideus vindemmiae), including those from the native region of D. suzukii in Asia (e.g., Asobara japonica, Ganaspis brasiliensis) seem able to parasitize and develop in this pest. These species can be investigated further for their potential use as biocontrol agents (Daane et al., 2016).
Immune responses can largely vary and depend both on biotic factors, e.g., age, developmental stage (Siva‐Jothy et al., 2005), and abiotic factors, e.g., temperature (Nappi & Silvers, 1984) or ethanol concentration (which is relevant because most Drosophila species develop on fermenting fruits; Lynch et al., 2017; Milan et al., 2012). An increased immune response can also be triggered by maternal effects, because Drosophila females produce offspring with increased lamellocyte production when oviposition occurs in the presence of L. heterotoma (Bozler et al., 2020). The Drosophila‐endosymbiont Spiroplasma (see Section 4) also plays a major role in Drosophila resistance against L. heterotoma (Corbin et al., 2021; Higareda Alvear et al., 2021; Paredes et al., 2016; Xie et al., 2011, 2014). By producing ribosome‐inactivating proteins, Spiroplasma seems to suppress development of the juvenile parasitoid by deactivating wasp ribosomes (Ballinger & Perlman, 2017). The protection conferred by Spiroplasma is temperature‐dependent, however, and is absent at 18°C (Corbin et al., 2021). The endosymbiont Wolbachia also increases Drosophila resistance to parasitism by L. heterotoma, albeit weak (Xie et al., 2014).
2.3. Parasitoid virulence
To overcome the host's immune response, some parasitoids inject venom during oviposition (Wertheim, 2022). In L. heterotoma, venom is known to affect host immunity leading to lysis of the host lymph gland (the organ responsible for the production of lamellocytes), thereby preventing the production of haemocytes (Ramroop et al., 2021). Venom fluids contain several components, including kinases, esterases and hydrolases (Heavner et al., 2013), but only few proteins have been accurately characterized up to now. Aspartylglucosaminidase (AGA) could be an important component of L. heterotoma venom (Colinet et al., 2013). This protein is abundant in A. tabida venom, where it is suspected to be involved in host paralysis during oviposition (Moreau et al., 2004). Haemocyte capsule formation around the parasitoid egg requires the glycosylation of proteins. AGA possesses deglycolsylation properties and may thus be involved in encapsulation prevention (Colinet et al., 2013). A recent study showed that a newly described protein, Lar (lymph gland apoptosis‐related protein), was abundant in L. heterotoma venom, promoting lysis of the host lymph gland (Huang et al., 2021). L. heterotoma venom also contains several other proteins, such as Elongation factor 1‐alpha (EF‐1α; Colinet et al., 2013), but its role in inhibiting the host's immune response has not yet been elucidated.
In many parasitoid species, including L. heterotoma, venom also includes virus‐like particles (Chiu et al., 2006; Colinet et al., 2013; Coulette et al., 2017; Goecks et al., 2013; Morales et al., 2005; Rizki et al., 1990). Virus‐like particles are produced in an accessory gland, also called the long gland or venom gland (Ferrarese et al., 2009; Rizki et al., 1990), and matured in a separate reservoir within the female wasp's reproductive system (Chiu et al., 2006; Morales et al., 2005). Virus‐like particles appear to be devoid of nucleic acids, but contain various proteins, among which the most abundant protein, p40, is located on the surface and spikes of mature particles (Chiu et al., 2006). The genes encoding virus‐like particles in L. heterotoma are embedded in the wasp genome (Huang et al., 2021; Wey et al., 2020) and could have originated from an ancestral virus (Di Giovanni et al., 2020). Other authors have, however, argued for a non‐viral origin of virus‐like particles and prefer the term mixed‐strategy extracellular vesicles (Heavner et al., 2013; Wey et al., 2020). Although the exact nature of the particles is still under debate, it is clear that virus‐like particles actively repress the host's immune response through several mechanisms. The particles are able to inhibit the functioning of lamellocyte adherence needed for encapsulation (Rizki et al., 1990; Rizki & Rizki, 1991) and to disrupt the generation of lamellocytes through lysing lymph glands (Chiu & Govind, 2002; Huang et al., 2021). Rizki and Rizki (1991) showed that virus‐like particles can enter lamellocytes and promote their lysis. The particles are also able to reduce the number of sessile haemocytes, another origin of lamellocytes (Anderl et al., 2016; Markus et al., 2009). The guaranteed immune suppression through virus‐like particles allows L. heterotoma to avoid encapsulation of its developing larvae by host lamellocytes and are thus essential for successful development.
3. HOST LOCATION, LEARNING, AND ADAPTIVE PATCH EXPLOITATION STRATEGIES
To produce offspring, female parasitoids need to be able to accurately locate and parasitize hosts. Successful parasitism results from a sequence of behaviors that include host habitat and patch location, host location within a patch, host acceptance, and host suitability (see Section 2; Godfray, 1994). Hosts are often distributed in isolated patches in the environment. To deal with such fragmented environments, parasitoid females need to divide their foraging efforts between different patches that can vary in host abundance during their lifetime, but also between generations (e.g., seasonal variation). Furthermore, in contrast to prey that become unavailable for competing predators, parasitized hosts remain on a patch and can subsequently be encountered by con or hetero‐specific female competitors. Most parasitoid females can discriminate hosts already parasitized by a conspecific, but discrimination of hosts parasitized by hetero‐specifics seems to be less common in parasitoids (Ardeh et al., 2005; Strien‐van Liempt & van Alphen, 1981). When encountering a parasitized host, the female can either reject it and continue to look for unparasitized hosts, or decide to lay an egg, a behavior known as superparasitism. While superparasitism is restricted to interactions with conspecifics, acceptance of a host parasitized by a hetero‐specific is referred to as multiparasitism. Superparasitism and multiparasitism, therefore, represents a combination of extrinsic (i.e., between females for access to hosts) and intrinsic competition (i.e., among parasitoid larvae within a host). Superparasitism comes at a risk though, because in solitary parasitoids only one adult can emerge from a single host, and the second egg generally has the lowest chance of survival (Bakker et al., 1985).
Since the 70's, the behavioral ecology of the amber wasp L. heterotoma has been extensively studied, mostly in the context of optimal foraging theory. This theory states that the time allocated and choices made while foraging for a resource are shaped by natural selection, maximizing fitness (Charnov, 1976). Research using L. heterotoma as a model revealed the importance of associative learning in patch and host selection in parasitoids. Due to its risky nature, superparasitism was long thought to be detrimental to fitness, but superparasitism can lead to fitness benefits for parasitoid females. Studies with L. heterotoma were instrumental to increasing our understanding of this phenomenon (Bakker et al., 1985). This section aims to present the sequence of L. heterotoma female behaviors, ranging from patch location to time allocated for foraging in a patch, illuminating the contribution L. heterotoma made to understanding how female parasitoid behaviors are shaped by natural selection.
3.1. Patch location
Leptopilina heterotoma females are attracted to the substrates on which Drosophila feed and oviposit (van Lenteren & Bakker, 1978; Vet & van Opzeeland, 1985), particularly by the presence and quantity of yeast and fermentation products (such as ethanol) resulting from substrate decay (Dicke et al., 1984; van Batenburg et al., 1983; van Lenteren & Bakker, 1978). These cues allow long‐distance detection of suitable host habitats (i.e., more than 1.5 m; Dicke et al., 1984), even when actual hosts are not present on the patch (Dicke et al., 1984; van Lenteren & Bakker, 1978). As host habitat odors do not necessarily imply the presence of hosts, these cues are not completely reliable. In addition to host habitat cues, L. heterotoma females can also eavesdrop to detect and locate host patches based on a host‐emitted pheromone: the Drosophila aggregation pheromone (Lof et al., 2013; Wertheim et al., 2003; Wiskerke et al., 1993). Aggregation pheromones (with cis‐vaccenyl acetate being the primary active compound; Bartelt et al., 1985) are deposited during oviposition by several Drosophila species to attract conspecific females (Bartelt et al., 1985; Wertheim et al., 2006). The aggregation pheromone is, therefore, a highly reliable cue indicating host presence for L. heterotoma females (Bartelt et al., 1985; Wertheim et al., 2006). Wertheim et al. (2003) showed that host aggregation pheromones indeed help L. heterotoma in finding host patches on smaller and larger spatial scales (i.e., a 40 cm wind tunnel and orchards, respectively). Attraction to host aggregation pheromones is further positively correlated with the concentration of yeast in the patch. Wasps were attracted by the aggregation pheromone of D. melanogaster when the yeast concentration was 2 g yeast/75 g of food medium, but wasps were not attracted when the yeast was less concentrated (1 g/75 g). To effectively locate host patches, L. heterotoma females thus use both habitat and host cues that in the natural environment may be amplified when combined, increasing their signal reliability for host finding.
3.2. Host location and choice within a patch
Once a female identifies and reaches a suitable patch, she starts to search for hosts by walking over the surface of the food substrate while rhythmically moving her antennae up and down and probing the substrate with her ovipositor (van Batenburg et al., 1983; van Lenteren, 1976; Vet & Bakker, 1985). She determines the exact location of the host when her ovipositor touches or pierces the host cuticle (van Lenteren, 1976; Vet & Bakker, 1985). Interestingly, the antennae seem of only little importance in these final steps of host location, because removal of the antennae does not prevent females from finding larvae, at least under laboratory conditions (van Lenteren, 1976). Once the female probes into a host, she can either reject it (i.e., withdraw her ovipositor in <6 s) or proceed to oviposit (lasting between 16 and 25 s; Haccou et al., 1991; van Lenteren, 1976; Varaldi et al., 2005). When a host's cuticle is pierced by the ovipositor, the host larva tries to escape by rotating itself and then moving away (van Lenteren et al., 1998). The ovipositor of L. heterotoma possesses a physical structure resembling a “clip” (see figure 1 in van Lenteren et al., 1998) that allows the wasp to constrain the larva and stop it from moving away while the female is injecting her venom (van Lenteren, 1976; van Lenteren et al., 1998). Following oviposition, the female then preens her ovipositor and genitalia. For a behavioral observer, this preening phase (in addition to oviposition duration) represents the second line of evidence that a female successfully laid an egg (Haccou et al., 1991; van Lenteren, 1976; Varaldi et al., 2005).
Once a female starts foraging in a patch, the presence of host aggregation pheromone is no longer of importance (Wertheim et al., 2003). To determine the presence and quantity of hosts feeding within a patch, females use host‐emitted kairomones (Dicke et al., 1985; van Alphen et al., 1984; Vet et al., 1993). Kairomones are semiochemicals that trigger a response from another species that are only beneficial to the receiver, not the emitter (i.e., the parasitoid and host larva, respectively; Grasswitz & Jones, 2002). Attraction to host kairomones is innate in L. heterotoma, because inexperienced females probe the substrate faster when host kairomones are present (Vet & Groenewold, 1990). When investigating a patch with host kairomones, L. heterotoma females intensify their searching behavior by spending more time on the area containing kairomones, and increasing the frequency of ovipositor probing (van der Hoeven & Vet, 1984). Host kairomones have not yet been identified chemically and could actually be compounds that originate from the adult flies or the larvae, such as cuticular hydrocarbons (CHCs) or feces (Dicke et al., 1985; van Alphen et al., 1984). Host‐produced kairomones are only detectable within a patch. This was substantiated by experiments performed on larger and smaller spatial scales: in a larger space (climate room), L. heterotoma did not visit patches containing hand‐deposited fly larvae (without aggregation pheromones, but with host kairomones; Dicke et al., 1984), while in a small space (5 cm Petri dishes), females were more attracted to patches on which larvae were feeding and crawling compared to host‐free patches (van Alphen et al., 1984). These studies highlight that while yeast odors and aggregation pheromone are of great importance for detecting patches from a distance of several meters (Dicke et al., 1984; Wertheim et al., 2003), host kairomones are critical for host location on a small spatial scale.
3.3. The role of associative learning in patch selection
Host patch selection by L. heterotoma females is not only influenced by chemical cues, but also by previous oviposition experiences, similar to other parasitoid species (Meiners, 2003; Sobhy et al., 2019). Through associative learning, females are more attracted to substrate odors on which they already had a successful oviposition experience (Simons et al., 1992; Vet et al., 1998; Vet & Schoonman, 1988; Vet & van Opzeeland, 1985). L. heterotoma females can use associative learning, for example, to differentiate between distinct substrates, e.g., apple‐yeast versus mushroom (Papaj et al., 1994; Papaj & Vet, 1990; Simons et al., 1992), and more similar substrates, e.g., pear versus apple (Vet et al., 1998). Females are not able, however, to differentiate between two different apple varieties (Vet et al., 1998). Associative learning also plays a role in finding host patches in the field. By doing experiments in a forest in the Netherlands, Papaj and Vet (1990) showed that experienced females tended to find artificial patches (containing apple‐yeast or mushroom substrates, without hosts) faster and more often than naive females. Experienced females were also more attracted to substrate types with which they had a previous parasitism experience. Overall, females seem capable of dynamically adjusting their search strategies in response to variability in environmental stimuli, including the availability and distribution of hosts in their environment (Vet et al., 1998).
Increased efficiency in patch finding with experience seems to result from a change in search activity: Vet and Papaj (1992) reused their protocol with apple‐yeast and mushroom substrates and tested how female experience affected searching behavior in terms of walking speed and direction. Experienced females changed direction less often and walked faster and straighter in the direction of an odor that they had previously experienced. Female preferences acquired through associative learning can, however, be reversed by an unsuccessful parasitism experience (i.e., not finding hosts) on an initially rewarding substrate, meaning that females are able to actively and rapidly adjust their search strategies depending on experience (Papaj et al., 1994). Associative learning also took place, but to a lesser extent, if the previous experience was not successful parasitism, but simply contact with host kairomones (Vet & Groenewold, 1990). Strong kairomone cues for host presence in the substrate thus also reinforces associative learning (Vet & Groenewold, 1990). Learning through processes other than association, like habituation or sensibilization to an environmental cue, do not lead to modifications of female preference (Vet & Groenewold, 1990). Altogether, laboratory and field experiments suggest that associative learning using cues based on host substrate and presence is adaptive when females face variable environments, playing an important role in microhabitat detection and selection under natural conditions.
3.4. The role of associative learning in parasitism success and superparasitism decisions
Learning is essential for the host location process, but also for parasitism success. Naive L. heterotoma females are less successful parasitizing hosts compared to experienced females, and a past oviposition experience decreases oviposition duration (Samson‐Boshuizen et al., 1974). L. heterotoma females are able to distinguish unparasitized hosts from hosts parasitized by conspecifics (Bakker et al., 1967, 1972; Hemerik et al., 2002; Visser, 1995) or themselves (Visser, 1993, 1995), also known as host discrimination. Females further have the ability to estimate the number of eggs already present in a host (Bakker et al., 1972, 1990; Hemerik et al., 2002; Visser, 1995). Like most other parasitoids (Ardeh et al., 2005), L. heterotoma females seem unable to discriminate hosts that are parasitized by other species, such as A. tabida, to avoid multiparasitism (Strien‐van Liempt & van Alphen, 1981; see Section 5 on competitive interactions). Host discrimination allows a female to estimate the quality of hosts within investigated patches, informing her about current oviposition conditions that can also have an effect on future oviposition opportunities (van Alphen & Visser, 1990). Early studies stated that females need a first experience of parasitism on already parasitized hosts to efficiently discriminate hosts (van Lenteren, 1972; van Lenteren & Bakker, 1975), but later work argued that hosts discrimination is innate in L. heterotoma (Henneman et al., 1995; van Alphen et al., 1987). In any case, host discrimination is due to chemosensory sensilla located on the distal part of the ovipositor (Ruschioni et al., 2015; van Lenteren, 1972; van Lenteren et al., 2007). When these sensilla come into contact with D. melanogaster hemolymph, the connected gustatory neurons produce action potentials (van Lenteren et al., 2007). These neurophysiological responses are dependent on the parasitism status of the host, as the number of action potentials differs significantly between unparasitized, singly, and doubly parasitized hosts (Ruschioni et al., 2015).
When two parasitoid eggs are deposited in the same host, the oldest individual within the host generally survives (Bakker et al., 1985; Eijsackers & Bakker, 1971), because it attacks and kills its competitor (Eijsackers & Bakker, 1971). In L. heterotoma, survival probability of the second larva is about 40% when laid shortly after the first larva (i.e., within 3 h), while the second larva never survives when laid after more than 24 h (Bakker et al., 1985; Visser, Luyckx, et al., 1992). Females mostly avoid superparasitism within the 3‐h window (Visser, Luyckx, et al., 1992). A potential explanation is that L. heterotoma marks its hosts during oviposition to prevent other females from superparasitizing. This mark does not, however, last more than 24 h. Similar to a previous experience with an unparasitized host (see above), an experience with a superparasitized host can modify subsequent oviposition decisions through learning (Henneman et al., 1995; van Alphen et al., 1987; Visser, van Alphen, et al., 1992). Visser et al. (1992) showed that oviposition experience on a patch containing only parasitized hosts leads to a higher rate of superparasitism on a new patch that contains both parasitized and unparasitized hosts after 24 h. When females forage alone, L. heterotoma does not superparasitize often (Varaldi et al., 2005), but the tendency to superparasitize increases when females investigate the patch simultaneously with other conspecifics (Bakker et al., 1985; Visser, 1995; Visser, Luyckx, et al., 1992; Visser, van Alphen, et al., 1992). Superparasitism rates further increase with the number of females simultaneously foraging in a patch (Visser et al., 1990). When a female is exposed to conspecifics before an experiment, but is subsequently left to forage on a patch alone, she also tends to superparasitize more than when she is kept alone (Visser, 1995).
Acceptance of previously parasitized hosts seemingly comes at a huge fitness cost for the female, but under extrinsic competitive pressure, superparasitism can be adaptive. When competition and the number of parasitized hosts in a patch are high, having at least some offspring that survive superparasitism is more advantageous than leaving the patch at the risk of not finding any hosts later on. Females are also more inclined to superparasitize hosts containing one of their own eggs (up to 30% of eggs were self‐superparasitized in Visser, 1995) when they are in competition with a conspecific female in the same patch (Visser, 1993, 1995; Visser et al., 1990). Here, self‐superparasitism could be adaptive, because it increases the fitness of the female by decreasing the probability that the host will be superparasitized by another competing female (van Alphen & Visser, 1990). By gathering information while searching for hosts, as well as learning from past oviposition experiences, L. heterotoma females can adaptively adjust their parasitism strategies in response to their environment. Research on L. heterotoma has emphasized that learning is of importance for parasitoids to choose patches that are more likely to contain hosts and to adjust superparasitism decisions, with a positive impact on fitness. Recent studies have, however, shown that learning in insects can come at a cost (de Bruijn et al., 2021; Mery & Kawecki, 2005), potentially leading to tradeoffs between learning capacities and life histories. Considering the extensive knowledge on learning in L. heterotoma, studying the cost of learning and potential trade‐offs can represent an interesting avenue for future research using this species.
3.5. Patch time allocation decisions: Learning from past experiences
A female can exploit multiple patches for oviposition during her lifetime; hence the time she spends within a patch can have a major effect on fitness (Hubbard & Cook, 1978). For example, if a new patch does not contain any hosts or only parasitized hosts, the female would have had a higher fitness if she had continued exploiting a previous, more suitable patch. Based on the marginal value theorem of optimal foraging (Charnov, 1976), patch allocation time depends on the fitness gain within a patch and the potential fitness gain expected on future patches available within the environment (Hubbard & Cook, 1978).
Similar to other parasitoids, patch time allocation in L. heterotoma is influenced by local conditions on the patch, including the number and quality of hosts encountered. For instance, the presence of kairomones, host encounters, and successful oviposition on a patch increase the time a female investigates that patch (Dicke et al., 1985; Haccou et al., 1991; van Alphen et al., 1984; van Lenteren & Bakker, 1978; Varaldi et al., 2005; Vet et al., 1993). Furthermore, females increase foraging efforts in new patches containing substrates on which they had a previous successful parasitism experience (Simons et al., 1992; Vet & Schoonman, 1988). Patch residence time further increases when superparasitism occurs, as it is adaptive for females to allocate more time to a patch with conspecifics to increase (self‐)superparasitism (Visser et al., 1990). In contrast, when the time between ovipositions increases (Haccou et al., 1991) or parasitized host encounters are getting more frequent (van Alphen & Vet, 1986; van Lenteren, 1991; Varaldi et al., 2005), females have a higher tendency to leave a patch. When females experience such poor conditions, they will spend more time finding hosts in a new, different type of substrate (Visser, van Alphen, et al., 1992).
Most optimal foraging models rely on oversimplified assumptions, such as a global knowledge of the organism's environment in terms of prey/host density, distance between patches, etc… Such assumptions are clearly unrealistic, leading to some criticism within the scientific community (Pierce & Ollason, 1987). These earlier studies were, however, necessary for new optimal foraging studies to build upon (King & Marshall, 2022). More recent optimal foraging models include the notion that foraging behaviors are dynamic and change within the lifetime of an individual (King & Marshall, 2022). For example, patch entering decisions and time allocated to a patch depend on the internal physiological state of females, including energetic reserves, age and mating status (Zhang et al., 2022), as well as climatic conditions (Roitberg et al., 1992), and learning. L. heterotoma would be a great model to test more recent optimal foraging models to further develop optimal foraging theory.
3.6. Influence of seasonal factors on parasitism strategies and fitness
Seasonal changes can have major effects on insect behavior and fitness (Abram et al., 2017), including parasitism strategies. In L. heterotoma, females are indeed known to adjust parasitism strategies in preparation for winter (Roitberg et al., 1992). For example, changes in photoperiod modify host patch exploitation, as wasps reared under autumn‐like light conditions (16 L:8D, 22°C) investigate host patches longer and superparasitize more often compared to wasps reared under summer conditions (12 L:12D, 22°C). These behavioral adjustments could be due to the shorter life expectancy of autumn females, leading to a riskier oviposition strategy (Roitberg et al., 1992), following the relative fitness rule. This rule states that when facing deleterious environmental conditions, parasitoids should adopt a riskier strategy maximizing the chances that their genes will be represented in the next generation (Giraldeau & Boivin, 2008). Furthermore, L. heterotoma survival in multi‐parasitized D. melanogaster larvae is lower at a cold (15°C) compared to a higher temperature (25°C; Strien‐van Liempt, 1983). Host choice in terms of host species can also affect thermal stress resistance. For example, survival and female fecundity at low (14–18°C) or high (26°C) temperatures are lower when wasps are developing on less suitable hosts (see Section 2 on host suitability), such as D. simulans or D. subobscura (compared to D. melanogaster; Fleury et al., 2004; Ris et al., 2004).
4. THE EVOLUTION OF FAT ACCUMULATION AND CONSEQUENCES FOR LIFE HISTORIES
The ability to accumulate fat is a highly conserved metabolic process across all domains of life (Birsoy et al., 2013; Wältermann & Steinbüchel, 2005). During periods of food abundance, animals, including insects, use dietary nutrients to meet acute energetic demands, while excess sugars and other carbohydrates are converted to fat for long‐term energy storage. Fat is thus a critical source of energy for insects to invest in survival and reproduction, particularly when faced with harsh environmental conditions (Arrese & Soulages, 2010; Hahn & Denlinger, 2011; Sinclair & Marshall, 2018). Fat is further important for other traits, such as locomotor activity, desiccation resistance, and as a macronutrient in eggs (Arrese & Soulages, 2010; Muller et al., 2017). Body size (a proxy for fat reserves, because size and fat content are generally correlated in arthropods; Enriquez et al., 2022; Lease & Wolf, 2011), longevity, and reproductive output are common life history traits for assessing fitness in insects (Roff, 2001). Trade‐offs between longevity and dispersal (e.g., flight), as well as longevity and reproduction have been well documented (Blacher et al., 2017; Chang et al., 2021), where fat allocation underpins both trade‐offs. The tight relationship between fat reserves and fitness thus makes the study of fat accumulation of importance for both ecological and evolutionary studies.
4.1. Fat accumulation
Despite the critical importance of fat reserves, it was only in the early 1990s that adult parasitoids were found unable to accumulate fat including the amber wasp L. heterotoma (Eijs et al., 1998; Ellers, 1996). Using laboratory‐reared individuals, Eijs et al. (1998) were the first to test the effect of multiple food resources (natural, non‐breeding, and artificial substrates) on fat accumulation of adult L. heterotoma. Fat content of L. heterotoma was highest at emergence and declined despite continuous feeding on honey, and irrespective of the Drosophila host used for development. Together with data on other parasitoid species, this lack of fat accumulation was hypothesized to result from the parasitoid lifestyle (Visser & Ellers, 2008). Only parasitoid insects were thus expected to lack fat accumulation, because sufficient fat for allocation into life history traits could be carried over from the host during development. A comparative study with more than 90 insect species then showed that the ability for fat accumulation was indeed lost during the course of evolution, but only in parasitoid lineages (including flies, a beetle, as well as parasitic hymenopterans) and not in other insects (Visser et al., 2010). For L. heterotoma, the results of Visser et al. (2010) differed from those obtained by Eijs et al. (1998), because in the former fat content significantly increased during life, showing that fat had been accumulated. In at least two other parasitoid clades the fat accumulation phenotype seemed to have re‐appeared in generalists, suggesting that adult fat accumulation could have re‐evolved in wasps with a large host range, including L. heterotoma (see Section 2 and Table S1). A repetitive loss and regain of fat accumulation suggests a modification of gene expression, rather than genetic changes in coding sequences of fat synthesis and accumulation genes (Visser et al., 2012). In addition, Moiroux et al. (2010) found that fat accumulation ability differed between geographically distinct L. boulardi populations (reared on the same host species), suggesting local adaptation depending on the environmental settings.
Following the contradictory findings in L. heterotoma and the intra‐specific differences observed in L. boulardi, Visser et al. (2018) conducted a large‐scale study on the ability of 19 field‐caught Leptopilina populations (belonging to different species) to accumulate fat in 2016. Thirteen out of 19 populations were L. heterotoma and these populations were compared to earlier work on 9 geographically distinct L. heterotoma populations collected from the field in 2013. For the populations collected in 2013, similar results were obtained as in Moiroux et al. (2010): some populations lacked fat accumulation, while other populations significantly increased fat content during life. In contrast, the populations obtained in 2016 (as well as the other species tested) all lacked fat accumulation. That puzzling finding resulted from differences between the Drosophila host strains used. The D. melanogaster strain used for the 2013 populations was collected from the field and was much leaner compared to the laboratory‐reared strain used for testing the 2016 populations. This became evident when fat content of recently emerged L. heterotoma females were compared between years: females contained almost twice as much fat in 2016 compared to 2013, explaining why no fat accumulation was observed in any of the 2016 L. heterotoma populations or the other species.
Variation in fat accumulation between L. heterotoma populations was hypothesized to be the result of phenotypic plasticity (i.e., fat accumulation depends on host fat content). A recent study with L. heterotoma indeed confirmed that fatty acid synthesis and fat accumulation depend on host fat content (that can easily be manipulated in the laboratory; Enriquez et al., 2022). Fatty acid synthesis and fat accumulation mainly occurred when the wasps developed on lean hosts, but was shut off on fat hosts (Visser et al., 2021). Reaction norms for fatty acid synthesis also differed considerably between L. heterotoma populations, suggesting that fat synthesis regulation can occur rapidly when host fat content varies and is dependent on the wasp's genotype. L. heterotoma thus represents an interesting example of a parasitoid that shows adaptive phenotypic plasticity in a key physiological trait.
Fat synthesis, accumulation and plasticity therein in L. heterotoma is currently the core theme of our own research and there are many exciting prospects for further research on this topic (Visser et al., 2022). For example, we still need to better understand how plasticity of fat synthesis and accumulation affects life histories and fitness (see the subsection below). We can further use field‐caught populations to elucidate how phenotypic plasticity itself evolves in different natural environments. So far, explicit tests for plasticity of fat synthesis and accumulation have only been done with L. heterotoma (using genetically similar individuals). To determine if plasticity of fat synthesis and accumulation evolved also in other parasitoids, many more parasitoid species now need to be tested (Visser et al., 2022). We can also now start digging into the genomics and transcriptomics of plastic fat synthesis in L. heterotoma to understand the mechanisms at play in generating distinct fat accumulation phenotypes.
4.2. Life histories
Evidence for the close link between fat reserves, critical as a long‐term energy source, and key life history traits in parasitoids comes largely from earlier work on the Drosophila‐parasitizing braconid wasp A. tabida. The importance of fat reserves for A. tabida reproductive functions was demonstrated by the positive correlation between the quantity of fat and female egg load (i.e., fatter females have more eggs in their ovarioles at emergence; Ellers, 1996; Le Lann et al., 2014). Moreover, a higher fat content leads to higher adult survival (Ellers, 1996). Fat reserves also fuel A. tabida locomotion, as fat reserves decreased with increasing dispersal distance (Ellers et al., 1998). Similar to most other parasitoids, A. tabida does not accumulate fat (Ellers, 1996), limiting the amount of fat reserves available for fitness functions. Fat content of A. tabida indeed decreases quickly during the first week of life, when many eggs are laid (Ellers, 1996). During this time, fat reserves are thus mostly allocated towards reproduction, leading to trade‐offs with other life history traits (Ellers, 1996). Although more studies on L. heterotoma are now appearing, particularly concerning fat synthesis and accumulation (see above), very little is known about the link between fat content, life histories, and trade‐offs. Preliminary work using L. heterotoma confirms the major importance of fat reserves, at least for survival, because fat content at emergence determines longevity under starvation for males (i.e., fatter males have a longer lifespan) (B. Visser, unpublished data, Table 2).
TABLE 2.
Life history trait measurements of L. heterotoma (B. Visser, unpublished data)
| Population | Host diet | Male longevity under starvation (days) | Offspring number | Offspring sex ratio (number of males/total offspring) | |||
|---|---|---|---|---|---|---|---|
| Mean | 1 SE | Mean | SE | Mean | 1 SE | ||
| Lh8, Japan | Lean | 5.95 | 0.22 | 73.67 | 10.05 | 1 | 0 |
| Control | 8.21 | 0.62 | 33.88 | 4.43 | 0.47 | 0.12 | |
| Fat | 10 | 0.32 | 15.33 | 3.96 | 0.47 | 0.16 | |
| Lh9, UK | Lean | 5.47 | 0.37 | 36.88 | 4.63 | 0.41 | 0.13 |
| Control | 7.44 | 0.34 | 41 | 6.36 | 0.53 | 0.14 | |
| Fat | 9.45 | 0.21 | 38.25 | 7.05 | 0.29 | 0.07 | |
| Lh10, UK | Lean | 5.58 | 0.43 | 39.63 | 4.36 | 0.70 | 0.14 |
| Control | 6.86 | 0.43 | 37.44 | 4.60 | 0.37 | 0.09 | |
| Fat | 8.60 | 0.76 | 53.50 | 8.98 | 0.47 | 0.16 | |
| Lh13, Belgium | Lean | 6.33 | 0.23 | 73.29 | 3.99 | 0.46 | 0.14 |
| Control | 8.14 | 0.28 | 54.50 | 8.64 | 0.42 | 0.09 | |
| Fat | 9.13 | 0.29 | 56.71 | 7.97 | 0.29 | 0.03 | |
Note: For each trait the mean (±1 SE) is provided. Data were obtained from wasps reared at 23°C with females ovipositing on lean, control, and fat D. melanogaster hosts (obtained as in Enriquez et al., 2022; Visser et al., 2021). Longevity under starvation was determined for males that developed on lean or fat hosts.
Offspring sex ratios of parasitoid wasps have been of particular interest in the context of local mate competition theory (see Section 7; Godfray & Cook, 1997; Hamilton, 1967), but host quality can also affect sex allocation patterns (Charnov, 1979, 1982; Clark, 1978; Godfray, 1994; Hardy, 1994; Visser et al., 2014). Charnov theorized that sex allocation of parasitoid females depends on host quality (typically measured as host size) when host quality affects the fitness of sons and daughters differently (Charnov, 1979; Charnov et al., 1981). Charnov's model assumes that host body size (and associated fat content, see above) is a key determinant of both female and male fitness. The relationship between size and fitness is even more important for females, as they benefit more from being large compared to males (i.e., a higher reproductive success and fecundity are typically proportional to host size). Males are then laid in smaller hosts, while females are laid in larger hosts, optimizing host exploitation. For several parasitoid species, the proportion of males was indeed shown to decrease with increasing host size (Charnov, 1982; Godfray, 1994; King, 1993). In L. heterotoma, sex allocation also seems to be dependent on host quality: sex ratios are generally male‐biased when females lay eggs on lean hosts, and female‐biased on fat hosts (B. Visser, unpublished data; Table 2). Variation in L. heterotoma offspring sex ratios also appears to be dependent on the wasp population (Table 2). It remains unclear, however, if and how parasitoid females can estimate host size, which can vary largely in time and space.
Clark (1978) proposed that local resource competition can also affect sex allocation. If resources are locally limited, parasitoid females may be forced to compete with each other females for access to resources (Visser et al., 2014). Under such circumstances, mothers limit competition among daughters and allocate more resources by producing sons that can disperse (male‐biased sex ratio). As a result of increasing temperatures, L. boulardi, a major competitor of L. heterotoma, is migrating towards more northern parts of Europe, replacing L. heterotoma. The presence of L. boulardi results in higher mortality and lower host availability for L. heterotoma (Fleury et al., 2004). To cope with increased competition, higher fecundity and investment in mobility (to be able to find more suitable hosts), coupled with a shorter life span (that is traded‐off) are expected based on the balanced mortality assumptions of Price (1974). However, no clear distinction in life history traits between L. heterotoma populations, with or without L. boulardi, was found (Vayssade et al., 2012; Vuarin et al., 2012). Moreover, host‐ (e.g., age, sex, or species) or wasp‐ (e.g., species, genotype, maternal size, age, diet, or microhabitat) related traits need to be considered in future studies on parasitic wasp sex ratios, including L. heterotoma (Chabora et al., 1979; King, 1987).
Endosymbionts can have a major impact on their host, including life histories (see Section 7 on cytoplasmic incompatibility). For L. heterotoma attacking Spiroplasma‐infected and uninfected Drosophila, no differences were, however, found in the number of eggs laid (Paredes et al., 2016; Xie et al., 2010, 2014). A recent study on Spiroplasma showed that this endosymbiont actually subverts specific host lipids and its proliferation is limited by the availability of host hemolymph‐lipids (Herren et al., 2014). Spiroplasma and wasp thus seem to compete for Drosophila host resources, a pattern already reported for L. boulardi (Paredes et al., 2016). The presence of Spiroplasma in some Drosophila hosts can thus have major consequences for lipid availability during development of L. heterotoma, a factor known to affect fat acumulation in adults.
Availability and quality of resources, as well as abiotic factors, such as temperature, are fluctuating at different temporal scales in the environment (between years, seasons, days,…). Temperature is known to have a major effect on female parasitoid behavioral decisions (i.e., foraging, host choice; Amat et al., 2006; Moiroux et al., 2015) that can affect offspring nutrient acquisition during development and consequently fat accumulation and fitness. L. heterotoma occurrence is widespread, which is typically associated with a high tolerance to a wide range of temperatures (Addo‐Bediako et al., 2000; Sunday et al., 2012). Life histories in L. heterotoma seem to be optimal between 20 and 23°C. Indeed, survival of developing L. heterotoma (Ris et al., 2004; Rossi Stacconi et al., 2017), fecundity of females (Fleury et al., 2004; Le Lann et al., 2014; Ris et al., 2004), and parasitism success (Rossi Stacconi et al., 2017) decrease at lower (14–18°C) or higher (25–35°C) temperatures. Temperature further has a significant effect on resource‐use strategies of L. heterotoma: females reared at 20°C accumulated a significant amount of fat reserves, whereas individuals at 23°C did not accumulate fat (Le Lann et al., 2014). More studies are now needed to fully appreciate how temperature, and fluctuations therein, affect resource acquisition, use (i.e., fat accumulation phenotypes), as well as life histories and trade‐offs in L. heterotoma.
5. PARASITOID SPECIES COEXISTENCE
There are currently more than 2000 recorded species within the host fly subfamily Drosophilinae (O'Grady & DeSalle, 2018). Within the genus Drosophila, there have been several major adaptive radiations, and some lineages have high diversification rates related to resource‐use (Markow & O'Grady, 2008). Although the number of parasitoids known to attack Drosophila species are underestimated (Lue et al., 2021), there is already high intra‐ and interspecific competition for hosts within the guild of parasitoids associated with Drosophila. In this section, we introduce the guild of Drosophila parasitoids and discuss species abundances in Europe and Asia. We further describe how competition for host resources can lead to potential speciation, and how spatial and temporal resource partitioning allows species coexistence.
The amber wasp L. heterotoma belongs to a large guild of parasitoids attacking Drosophila species, with a current count of 108 species belonging to 20 genera (Carton et al., 1986; Lue et al., 2021; Table S2). The use of Drosophila hosts evolved independently in the superfamilies Ichneumonoidea, Cynipoidea, Chalcidoidea, and Diaprioidea. Hosts are attacked either during the larval stage (e.g., Leptopilina, Ganaspis, Asobara, Opius) or the pupal stage (e.g., Pachycrepoideus, Spalangia, Trichopria; Carton et al., 1986). All larval parasitoids of Drosophila are endoparasitoids, while the pupal parasitoids are either ectoparasitoids (i.e., Pteromalidae) or endoparasitoids (i.e., Diapriidae; Figure 4; Carton et al., 1986). P. vindemmiae and Spalangia sp. were further found as secondary parasitoids, also called hyperparasitoids, on primary hymenopteran (e.g., Leptopilina and Asobara species) or dipteran hosts (Gibson, 2009; van Alphen & Thunnissen, 1982). In terms of developmental strategies, all braconids attacking Drosophila are koinobionts (i.e., allowing host growth after parasitism), while species in the subfamilies Pteromalinae and Spalangiinae are idiobionts (arresting host development). The guild of parasitoid species associated with Drosophila thus shows great diversity in host exploitation strategies.
FIGURE 4.
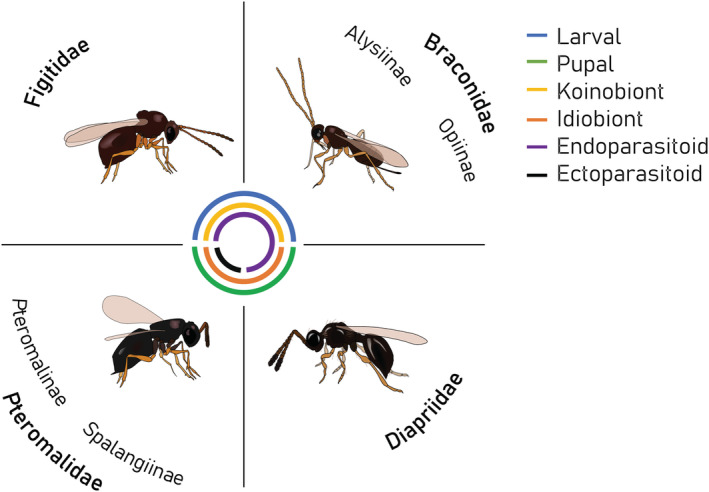
Lifestyle characteristics of the four main wasp families parasitizing Drosophila. Each family is visually represented by a common species: Figitidae—Leptopilina heterotoma, Braconidae—Asobara tabida, Pteromalidae—Pachycrepoideus vindemmiae, Diapriidae—Trichopria drosophilae. Eggs of ectoparasitoids are laid on the outside of the host, whereas those of endoparasitoids are laid inside the host. Endoparasitoid larvae may, however, develop some time outside the host body, depending on the species (see Figure 3 for L. heterotoma where this occurs; Harvey & Strand, 2002).
In Europe, the larval endoparasitoids L. heterotoma, L. boulardi and A. tabida are common (Fleury et al., 2009; Knoll et al., 2017; Mazzetto et al., 2016), sharing different host species, such as D. melanogaster, D. simulans, and D. subobscura (Fleury et al., 2004, 2009; Kraaijeveld & Godfray, 1999). D. phalerata is the most abundant fungal‐feeding host and is parasitized mainly by L. clavipes (Driessen et al., 1990). Among the pupal parasitoids, P. vindemmiae, T. drosophilae, and the genus Spalangia, are the most common in Europe (Delpuech & Allemand, 2011; Fleury et al., 2009; Kremmer et al., 2017). Data on the occurrence of Drosophila parasitoids and their hosts are relatively scarce outside Europe and Asia (but see Abram et al., 2022; Lue et al., 2018 for data in North America, and Jeffs et al., 2021 for data in Oceania). P. vindemmiae and T. drosophilae, which are cosmopolitan, are the main pupal parasitoids in Asia (Daane et al., 2016; Giorgini et al., 2019; Mitsui et al., 2007). In Japan, the most common drosophilids feeding on fruits in temperate regions are the native D. lutescens, D. suzukii, and the exotic D. simulans and D. immigrans (Kimura et al., 1994; Mitsui et al., 2007; Mitsui & Kimura, 2010). Parasitoids attacking these species are A. japonica, which has a remarkably large host range, and G. brasiliensis (currently considered as a cryptic species; Kimura & Mitsui, 2020; Mitsui et al., 2007; Mitsui & Kimura, 2010). The same species are found in South Korea (Daane et al., 2016), while in China G. brasiliensis, L. japonica and A. mesocauda are the most common parasitoids (Giorgini et al., 2019; Girod, Borowiec, et al., 2018). The G. brasiliensis lineage that specializes on D. suzukii could represent a suitable biocontrol agent against this pest (Nomano et al., 2017), once the species within this complex are formally described (Seehausen et al., 2020).
5.1. Cryptic species
When resources, such as hosts, are limited competition between species intensifies. An outcome of intense competition is competitive exclusion, where one of the competing species ultimately goes extinct (Losos, 2000). Alternatively, natural selection can favor phenotypes within a population that avoid resource competition. Populations can thus diverge in resource use, lowering competition and allowing species coexistence, potentially leading to speciation. L. heterotoma belongs to a species‐rich genus, containing more than 30 species, that is divided into several groups, including a L. heterotoma group (Figure 5). Two species within this group have a broad distribution (L. heterotoma, L. victoriae), while the other species are restricted to Asia (L. pacifica, L. ryukyuensis, L. japonica, L. tokioensis) or Africa (L. guineaensis). Some of these species have only recently been described and their biology still remains largely unknown (Novkovic et al., 2011; Wachi et al., 2015). L. heterotoma is distributed across the temperate regions of Europe, Asia, North America and Oceania. It has been observed up to Sendai in Japan, although the records furthest South (Tokyo) were recently proposed to be cryptic species (based on sequencing of neutral mitochondrial and nuclear markers; Kimura & Mitsui, 2020; Novkovic et al., 2011).
FIGURE 5.

Phylogeny of the genus Leptopilina, redrawn from Novkovic et al. (2011) and Buffington et al. (2020).
Considering that hymenopteran parasitoids belong to one of the most diverse insect orders (Forbes et al., 2018), it is not surprising that an increasing number of cryptic parasitoid species are being discovered (Gokhman, 2018). L. heterotoma from Sendai and Sapporo appear genetically most similar to European strains, two potential cryptic species were identified in Tokyo, and the genetically most divergent species was caught on the islands Iriomote and Amami‐oshima (Novkovic et al., 2011; Visser et al., 2018; Figure 5). The three cryptic species indeed appear to have shifted host use, with one of the Tokyo species parasitizing mainly D. bizonata breeding on mushrooms, the other Tokyo strain parasitizing Scaptodrosophila coracina breeding on fruits, and the island species mainly parasitizing Lissocephala species that breed on figs. It is still unclear whether the strains identified can still interbreed, but these potentially cryptic species offer interesting opportunities to study speciation in action (Struck et al., 2018).
5.2. Niche partitioning
Over shorter time scales, competition for hosts can be reduced through temporal or spatial niche partitioning (Germain et al., 2018; Harvey et al., 2014; Kronfeld‐Schor & Dayan, 2003). Due to its broad distribution across the world, L. heterotoma interacts with and can face severe competition from other wasp species, mainly those attacking frugivorous Drosophila, such as its congener L. boulardi and the braconid A. tabida. Indeed, no clear spatial niche differentiation seems to be apparent for these three species (Fleury et al., 2009). A study performed in the UK showed that A. tabida and L. heterotoma are abundant and co‐occur from May to September (Godfray & Hardy, 1990), while in the Southeast of France L. heterotoma and L. boulardi dominate with relatively few A. tabida individuals emerging from April to September (Fleury et al., 2009). In Tunisia, L. heterotoma faces intense competition from L. boulardi, which is probably causing L. heterotoma's competitive exclusion during most of the season (Carton et al., 1991). Abundance of competing parasitoids in the Southeast of France seems to follow that of the different host species (Fleury et al., 2009). The geographic range of L. boulardi is restricted to the Mediterranean, where the host D. simulans dominates, while in the North L. heterotoma thrives developing on D. melanogaster. L. heterotoma abundance also reaches only a few percent when L. boulardi is present. Under such intense competition, L. heterotoma seems to persist as a result of phenological differences between parasitoid species, with L. heterotoma being present and most abundant only very early and late in the season. This is possible, because unlike L. boulardi, L. heterotoma does not diapause in winter (Carton et al., 1991; Kimura, 2019).
Leptopilina heterotoma seems to have a competitive advantage when L. boulardi females are infected by a virus, the L. boulardi filamentous virus (LbFV) that increases the rate of superparasitism (Section 3). As a consequence, fewer offspring of infected L. boulardi females reach adulthood allowing L. heterotoma to predominate, at least in laboratory experiments (Patot et al., 2012). In the field, 55%–95% of L. boulardi females may be infected with LbFV, depending on the location, with infection increasing towards the South (and being absent in the North). Considering the drastic effect of LbFV infection on L. boulardi's parasitism strategy, it can be expected that competitiveness is lowered in infected L. boulardi also in natural populations, but this remains to be tested.
On even smaller spatial scales, L. heterotoma can avoid competition using chemical cues to select a preferred microhabitat for oviposition. For example, Vet and van Opzeeland (1985) showed that L. heterotoma prefers substrates that are in a later stage of decay, compared to A. tabida that prefers substrates at an early stage of decay (Vet et al., 1984). These findings confirmed anecdotal field observations where A. tabida appeared near substrates about the same time as the hosts, while L. heterotoma appeared only later (Vet & van Opzeeland, 1985). Due to differences in the temporality of parasitism between A. tabida and L. heterotoma, where hosts parasitized by A. tabida are likely already at the pupal stage, multiparasitism and direct competition can be avoided. Furthermore, circadian rhythms leading to temporal segregation can also contribute to coexistence between the three main competing Drosophila parasitoids. In a study by Fleury, Allemand, et al. (2000), the authors compared the circadian rhythms of L. boulardi, A. tabida and L. heterotoma, revealing that within a single day, L. heterotoma and A. tabida are active and ovipositing earlier than L. boulardi. Both L. heterotoma and A. tabida can thereby avoid competition with L. boulardi, the strongest intrinsic competitor (see the next subsection; Allemand et al., 1999; Carton et al., 1991).
Once competitors do arrive at the same patch, competition can still be avoided: Janssen et al. (1995) showed that when L. heterotoma interacts with its congener L. clavipes on decaying stinkhorn patches, L. heterotoma avoids patches where L. clavipes is present. The same avoidance strategy was found when L. heterotoma would encounter patches with L. boulardi. Weiss et al. (2013) indeed showed that L. heterotoma females avoided host patches that were already occupied or exploited by both conspecific and heterospecific female wasps, as well as wasp extracts. L. heterotoma females thus use different environmental factors to avoid competition on larger and smaller spatial and temporal scales.
5.3. Intrinsic competition
When competitors cannot be avoided and egg laying occurs in the same patch, L. boulardi outcompetes L. heterotoma. When both species were allowed to lay eggs at the same time with access to the same host (D. simulans), L. heterotoma parasitism rate was reduced from 50% (parasitizing alone) to 30% (together with L. boulardi; Carton et al., 1991). Furthermore, L. heterotoma developmental success was also reduced, from 51% to 37%. Similar patterns were found by Fleury et al. (2009) using two host species (D. melanogaster, D. simulans), although genotypes originating from more Southern populations in France were better at competing with L. boulardi (~30% L. heterotoma emergence) compared to Northern populations (~10% L. heterotoma emergence). This suggests that there is local adaptation for increased competitive ability in populations where L. heterotoma and L. boulardi co‐occur.
Once a host contains more than one developing parasitoid, intense intrinsic competition is unavoidable, because only one parasitoid can utilize and survive on one host. In experiments where A. tabida and L. heterotoma were laid in the same host (D. melanogaster), generally one of the competitors is eliminated through physical attack by the first hatched larva (Strien‐van Liempt, 1983). Which species survives depends on several factors, including the time interval between oviposition, temperature, and multiparasitism. Studying coexistence and competition between Drosophila parasitoids is now particularly relevant in the context of climate warming, as L. boulardi is migrating northwards, leading to population (and potentially genetic) differentiation in thermal reaction norms of life histories in marginal populations (Delava et al., 2022). Future studies on the consequences of the recent range expansion of L. boulardi on competitive interactions can help to better understand and predict the effects of climate change.
6. CHEMICAL COMMUNICATION AND SEMIOCHEMICAL PARSIMONY
Chemical communication probably constitutes the oldest and most widespread form of communication, occurring in all domains of life (Wyatt, 2014). Although several hundred sex pheromone components (i.e., molecules involved in mating behavior or related processes between individuals of the same species; Wyatt, 2010) have been identified (El‐Sayed, 2022), the origin and evolution of sex pheromones are still not well understood for most animals. Most insects produce sex pheromones to stimulate mating behavior through sexual attraction. The release of sex pheromones may be related either to the attraction of the opposite sex, generally via highly volatile compounds released by females to attract males over long distances, or as a part of male courtship behavior at closer range, generally via low volatile compounds (Ayasse et al., 2001; Kohl et al., 2015; Renou, 2014).
In the amber wasp L. heterotoma, iridoids play a key role in the mate‐finding process (i.e., in the attraction of males to females). Weiss et al. (2013) highlighted that the sex pheromone of L. heterotoma is mainly composed of (−)‐irodomyrmecin (i.e., a type of monoterpenoids), a highly volatile compound produced by female wasps. Four additional minor iridoid components, ((+)‐isoiridomyrmecin, two irodials and a third stereoisomer of iridomyrmecin), appear to be essential for the sex pheromone to be completely bioactive and highly attractive to males (Weiss, Hofferberth, et al., 2015, Weiss et al., 2013; Table 3). These compounds are produced and stored in a cephalic gland, more specifically in a pair of mandibular glands (Stökl et al., 2012; Stökl & Herzner, 2016). A recent study with several Leptopilina species, including L. heterotoma, tested the attraction of males towards patches with the odor of the opposite sex or the odor of hosts (Böttinger & Stökl, 2020). Males were only attracted to patches if females were present and were not attracted by host odors (living Drosophila larvae on the host patch). Females were more attracted to patches containing host odors than to conspecific male odors, irrespective of their mating status (virgin or mated; Böttinger & Stökl, 2020). This result is consistent with earlier studies showing that L. heterotoma females can eavesdrop on adult Drosophila pheromone communication, to which females are attracted to locate larval laying sites (Wertheim et al., 2003; Wiskerke et al., 1993).
TABLE 3.
List of iridoid compounds produced by L. heterotoma males and females (a), with or without ant predator attack (b), in mated and virgin females (c), and mate attraction quantities (d).
| Mean amount in ng (±1 SE) | Reference | ||
|---|---|---|---|
| Male | Female | ||
| a. Iridoid compounds found in L. heterotoma | |||
| (−)‐iridomyrmecin | – | 236.3 ± 20.6/110.1 ± 16.6 | Stökl et al. (2012), Weiss, Hofferberth, et al. (2015) |
| +)‐isoiridomyrmecin | 39.4 ± 3.8 | 22.1 ± 4.8/5.8 ± 3 | Stökl et al. (2012), Weiss, Hofferberth, et al. (2015) |
| Iridodial 1 | Trace | 26.1 ± 3.3/10.9 ± 2.8 | Stökl et al. (2012), Weiss, Hofferberth, et al. (2015) |
| Iridodial 2 | – | 9.1 ± 0.8/5 ± 1.6 | Stökl et al. (2012), Weiss, Hofferberth, et al. (2015) |
| Third stereoisomere of iridomyrmecin | – | 4.9 ± 1.2 | Stökl et al. (2012), Weiss, Hofferberth, et al. (2015) |
| b. Total amount of iridomyrcecins released by L. heterotoma | |||
| Females (10), not attacked | 3 ± 1.2 | Stökl et al. (2012) | |
| Females (10), attacked by Myrmica rubra | 370 ± 70 | Stökl et al. (2012) | |
| Females (3), attacked by Cardiocondyla obscurior | Trace | Stökl et al. (2015) | |
| Females (3), attacked by M. scabrinodis | 18.2* | Stökl et al. (2015) | |
| Males (10), not attacked | 5.8 ± 2.2 | Stökl et al. (2012) | |
| Males (10), attacked by M. rubra | 61.8 ± 20.6 | Stökl et al. (2012) | |
| c. Total amount of (−)‐iridomyrmecin released by L. heterotoma | |||
| Mated females (10) | 3 ± 1.2 | Stökl et al. (2012), Weiss et al. (2013) | |
| Virgin females (10) | 15.5* | Weiss et al. (2013) | |
| d. Amount of L. heterotoma female iridomyrmecins (in ng) | Attraction of males | ||
| 60 | Yes | Weiss et al. (2013) | |
| 30 | Yes | Weiss et al. (2013) | |
| 15 | Yes | Weiss et al. (2013) | |
| 8 | Yes | Weiss et al. (2013) | |
| 4 | No | Weiss et al. (2013) | |
Note: The mean (±1 SE) or median (*) amounts are provided in ng based on reported values in the cited references.
Sex pheromones can also play a key role for species recognition and mate choice on a short range. Within the Leptopilina genus, females of most species, including L. victoriae (Weiss, Ruther, et al., 2015), L. clavipes (Pfeiffer et al., 2018) and L. ryukyuensis (Böttinger et al., 2019) rely on cuticular hydrocarbons and/or iridoids, to attract males. In contrast, L. heterotoma solely relies on iridoids (Böttinger et al., 2021). The specificity of mate attraction in L. heterotoma remains unclear, however, as contrasting results have been obtained (Fauvergue et al., 1999; Weiss et al., 2013). The question is whether males are still able to discriminate against heterospecifics during courtship. To test this, Weiss et al. (2013) compared wing fanning times (i.e., part of the courtship sequence; see Section 7 on mating behaviors) of L. heterotoma males when presented with paper filters impregnated with either L. heterotoma or L. boulardi female iridoids. Male wing fanning lasted significantly longer when L. heterotoma males were exposed to iridoids from conspecific females, meaning that males recognized conspecific females and were prepared to mate. Mate recognition (as opposed to mate attraction) is thus species‐specific and mediated by a blend of iridoid compounds characteristic for L. heterotoma (Weiss et al., 2013; Weiss, Hofferberth, et al., 2015; Weiss, Ruther, et al., 2015).
Sex pheromone communication was proposed to have evolved from precursor molecules initially used for other purposes, i.e., the sender‐precursor hypothesis (Stökl & Steiger, 2017; Wyatt, 2014). This hypothesis states that any compound released by one individual and detected by another individual of the same species can evolve into a sex pheromone if there is a selective advantage for both sender and receiver (Wyatt, 2014). Chemical compounds are generally synthesized in limited quantities and assumed to be costly, so reusing existing compounds for chemical communication may be favored by selection, a phenomenon referred to as “semiochemical parsimony” (Blum, 1996). For a long time, data supporting the sender‐precursor hypothesis remained rare in insects, mainly because most studies only experimentally tested a pheromone's function, while neglecting the study of primary functions.
Sex pheromones in L. heterotoma have attractive properties, but also seem to be repellent. L. heterotoma females indeed emit a defensive secretion composed of (−)‐iridomyrmecin (around 80% of the secretion) and minor amounts of the four other iridoid compounds (Stökl et al., 2012; Weiss et al., 2013). In males, this secretion is composed of a single compound: (+)‐isoiridomyrmecin (Stökl et al., 2012). The pheromone secretion is released during an attack from natural enemies, such as ants, but in much higher quantities compared to use as sex pheromones (Stökl et al., 2012, 2015; Table 3). Due to the larger size of female L. heterotoma mandibular glands, females can release larger amounts of iridoid compounds than males (Stökl & Herzner, 2016). Females are also able to discriminate between predator species and to control and adjust the amount of iridoids to release accordingly (Stökl et al., 2012, 2015; Stökl & Herzner, 2016; Table 3).
The threefold use of (−)‐iridomyrmecin by L. heterotoma as sex pheromone, for defense, and competition avoidance (see Section 5 on competitive interactions; Weiss et al., 2013), represents an example of a semiochemical parsimony that reinforces the sender‐precursor hypothesis (Stökl & Steiger, 2017; Wyatt, 2014). The use of (−)‐iridomyrmecin might have evolved from a defensive compound to a competition avoidance cue to a female sex pheromone (Stökl & Steiger, 2017). In this context, the costs and benefits for males responding to iridoids must be evaluated, because (−)‐iridomyrmecin attraction can both increase the probability of finding a female and thus mating success, but at a risk of being harmed by a predator if the defensive chemical compounds released by the female are not sufficient to repulse it. Assessing predation risk and the use of defensive compounds in natural populations or recently field‐caught Leptopilina wasps would help to determine the selective pressure on males to better understand the evolution of sex pheromones.
7. MATING‐RELATED TRAITS AND POPULATION STRUCTURING
Mate finding, dispersal, and mate choice decisions can have major evolutionary consequences that have often been studied in parasitoids by examining patterns of sex allocation (Hardy, 1994). Key theoretical advancements were made by Fisher's frequency dependent selection for equal sex ratios (Fisher, 1930), and Hamilton's local mate competition theory predicting female‐biased sex ratios because related males compete for mates (Hamilton, 1967). Depending on the system, the ecology of mating can lead to clear population structuring (local mating) or panmixis (random mating) at the extremes, although intermediate mating structures, such as partial local mating, may actually be most common (Hardy, 1994). In this section, we look at research concerned with mate‐finding, dispersal, mating, and sex ratio distortion in the amber wasp L. heterotoma.
In many animals, mate finding is a crucial step for producing viable offspring, but in haplodiploids, such as Hymenoptera, mating is not a necessity (Cook, 1993; Godfray, 1988, 1990; Godfray & Grafen, 1988; Hardy, 1994). In haplodiploids, including L. heterotoma, unfertilized eggs develop into haploid males and fertilized eggs into diploid females (Heimpel & de Boer, 2008). Virgin females are thus able to reproduce, but generate exclusively male offspring (so‐called “constrained sex allocation”; Godfray, 1990), whereas mated females can control the sex ratio of offspring by choosing whether to fertilize an egg before oviposition or not. Another consequence of haplodiploidy is that virgin females face a trade‐off between mate‐searching (to be able to produce daughters) and host‐searching (to immediately produce sons only; Godfray, 1990). In contrast to female reproductive success (e.g., the number of eggs produced), male reproductive success depends on the number of fertile females he can mate with, leading to distinct reproductive strategies for both sexes.
7.1. Dispersal
In a recent study, Böttinger and Stökl (2020) investigated mate finding and dispersal from the natal patch in males and females of four Leptopilina species, including L. heterotoma. On average, L. heterotoma males emerged about 2 days before females (but see Eijsackers and Bakker (1971) and Fauvergue et al. (1999) showing within‐brood emergence is similar for males and females). This daily rhythm could be an adaptation to competition between males, as the first emerging males can court and mate with more females (Fagerström & Wiklund, 1982; Fauvergue et al., 1999; Pompanon et al., 1995). Dispersal of both males and females occurred directly after emergence from the natal patch. Males thus start dispersing before conspecific females emerge on the same patch. Fauvergue et al. (1999) indeed already showed that about 20% of both males and females emerged without a potential mate present. Moreover, dispersal of L. heterotoma females was up to three times higher compared to the other Leptopilina species, where a similar proportion of males and females dispersed (Böttinger & Stökl, 2020; Fauvergue et al., 1999). Individuals that emerge (and disperse) in the absence of conspecifics may favor off‐patch matings and reduce local mate‐competition, but on the other hand may compete with males present on another patch. Dispersal of L. heterotoma thus appears to differ from other Leptopilina species and other parasitoid wasp species, where males wait for the emergence of conspecific females to mate on the natal patch (Carton et al., 1986; Godfray, 1994; Godfray & Hardy, 1990). Post‐emergence dispersal of L. heterotoma males appears to be beneficial and does not pose a risk in finding mating partners.
Variation in dispersal within the Leptopilina genus was recently found to be related to chemical compounds released by females (Böttinger & Stökl, 2020). The volatility of sex pheromones can be an important determinant of male and female wasp dispersal behavior (Böttinger & Stökl, 2020). For Leptopilina species that use highly volatile sex pheromones (i.e., iridoids; see Section 6 on chemical communication), such as L. heterotoma or L. japonica, males started to disperse immediately after emergence and the presence of females does not affect the dispersal rate (Böttinger & Stökl, 2020). L. heterotoma females also showed a significantly higher dispersal rate compared to heterospecific females emitting sex pheromones that are less volatile. Furthermore, whether hosts are present or not can affect dispersal propensity. L. heterotoma males were more attracted to patches with females, whereas virgin and mated females dispersed towards patches containing host odors (Böttinger & Stökl, 2020). Moreover, L. heterotoma males were found to be attracted to volatiles emitted only by their conspecific virgin females (i.e., there was no attraction to mated females) both in the field and in the laboratory (Fauvergue et al., 1999). Based on similar findings in another wasp (Lysiphlebus testaceipes), we can hypothesize that L. heterotoma virgin females are able to search for hosts while emitting sex pheromones to attract males (Fauvergue et al., 2008). Dispersal of males would then be driven solely by mate‐searching (and feeding), which is indeed easier for species that emit highly volatile iridoid sex pheromones, such as L. heterotoma. The high dispersal rate of L. heterotoma males may increase their mating opportunities and success, as they can mate several times in nature, while females seem to mate only once (see below). Although nothing is known about competition between L. heterotoma conspecific males for mating opportunities, we can assume that the high dispersal rate also decreases fights among male wasps (Godfray, 1994). Such a strategy, where males disperse rapidly from the natal patch in search of females guided by volatile pheromones deviates from expectations under local mate competition in haplodiploid species (Hamilton, 1967; Hardy, 1994).
Parasitoids can lay a single (solitary) or multiple eggs (gregarious) inside a single host. When hosts are aggregated on patches, however, solitary parasitoids can be considered “quasi‐gregarious”. L. heterotoma is indeed quasi‐gregarious, due to the high aggregation of Drosophila larvae on single patches (Fauvergue et al., 1999). Mating in L. heterotoma was assumed to be restricted to a local patch, where brothers compete for females, leading to strict local mate competition and female‐biased offspring sex ratios (Hamilton, 1967). The mating system of some parasitoid species, including L. heterotoma, does, however, not seem to follow Hamilton's predictions (Fauvergue et al., 1999; Hardy, 1994). Reviewing the mating structure of 22 parasitoid species, Hardy (1994) concluded that complete local mating is exceptional, rather than the norm in gregarious and quasi‐gregarious parasitoids. Moreover, Fauvergue et al. (1999) reviewed the literature on long‐distance volatile sex pheromones in parasitoids and found that 21 species, including L. heterotoma, use sex pheromones for mate finding, including gregarious, quasi‐gregarious and solitary species. Volatile sex pheromones aim to facilitate dispersal and off‐patch matings, which in turn reduce local mate competition, sib‐mating, the risk of inbreeding, and competition between males. The conclusions of Böttinger and Stökl (2020) align well with the suggestion of Hardy (1994) and results of Fauvergue et al. (1999) that L. heterotoma shows partial local mate competition, with both on‐patch and off‐patch mating. These observations reinforce the conclusion that off‐patch mating may be frequent in gregarious and quasi‐gregarious parasitoids, but more data is needed to develop hypotheses on the evolution of such mating structures.
7.2. Courtship and mating
Once a potential mate has been located, L. heterotoma shows a stereotypical courtship sequence, like many other insects (described in more details in Isidoro et al., 1999; van den Assem, 1969). Both males and females are sexually receptive immediately after emergence. Courtship starts with the male rapidly fanning (i.e., vibrating) his wings, without making actual contact with the female. While wing fanning takes place, the male will position his antennae forward and will start following the female. Once the male is in close enough proximity, he will make physical contact with the female, initially only with his antennae. He will then attempt to mount the female and place his antennae parallel with those of the female. The male will then ‘paddle’ the club‐shaped part of the female's antennae with his own antennae. A receptive female will subsequently extrude her ovipositor to expose her genital aperture. The male then ceases wing fanning and paddling, moves backwards and spreads his wings before copulating with the female, which typically requires more than one attempt. Once the male dismounts, both male and female will start preening different body parts, while the female will again conceal her genitalia. The male may attempt a new courtship sequence, but a female will generally not mate more than once, at least not in the laboratory (van den Assem, 1969).
If a L. heterotoma male is unsuccessful in copulating with a female despite several attempts, the male will dismount while the female continues to show typical behaviors observed during copulation (absence of movement; van den Assem, 1969). Such unfertilized females will conceal their genital area after the typical duration of a successful copulation and will not copulate again; hence behaviorally these females respond as if copulation was successful, also called “pseudo‐virgins” (Godfray, 1994). Placing males and females together is thus no guarantee that a female will have successfully mated, although it is not clear how frequently courtship is unsuccessful for males. Unmated females will have suffered at least some of the costs associated with mating (e.g., the cost of being courted, a reduction in time she can dedicate to finding and parasitizing hosts) without having the benefits (to produce female offspring). The question is how common pseudo‐virgins are in the field and whether these females will mate again. Mated females will generally only become receptive again after several weeks in the laboratory (van den Assem, 1969), and sex allocation patterns suggest that sperm may be depleted 6 days after mating as only males are produced (Chabora et al., 1979). Under optimal conditions in the field (e.g., sufficient host availability), multiple mating may thus not be necessary when actual mating has occurred.
Comparing the same L. heterotoma strain as van den Assem (1969) with another strain from the USA, Veerkamp (1982) showed that the latter differed considerably in the timing of mating‐related behaviors, offspring numbers, and sex allocation patterns. This would suggest that mating‐related behaviors may depend largely on the local environment, leading to local adaptation and population differentiation. Ridley (1993) suggested that solitary hymenopteran species are primarily monandrous (females mate once), while gregarious species are mainly polyandrous (females mate multiple times). Considering the strong effects of local environmental conditions, different patterns of dispersal observed between populations (described above), and a quasi‐gregarious host distribution, we could expect at least some multiple mating to occur when siblings are competing at the natal patch. This remains strictly hypothetical for L. heterotoma, but for other monandrous species, such as Nasonia vitripennis, Aphelinus asychis, Trichogramma evanescens (Boulton et al., 2015, 2019; Damiens & Boivin, 2005; Jacob & Boivin, 2005; Ridley, 1988; Wang et al., 2021), multiple mating is occasionally observed in the field. It would be very interesting to compare mating‐related traits between distinct, natural wasp populations, a task for which L. heterotoma is particularly well suited.
7.3. Wolbachia and cytoplasmic incompatibility
Factors unrelated to mating structure can also have a large effect on sex allocation patterns, including the intracellular alpha‐proteobacterium Wolbachia pipientis. Wolbachia is inherited maternally (i.e., vertical transmission), but it can occasionally also be acquired horizontally from a conspecific (Frost et al., 2014) or another species (Ahmed et al., 2016). The density and location of Wolbachia inside L. heterotoma is sex‐dependent: females harbor a greater number of bacteria per cell compared to males, and in females Wolbachia is mainly located in the abdomen compared to the head and thorax in males (Mouton et al., 2003). L. heterotoma can harbor three different Wolbachia strains, wLhet1, wLhet2 and wLhet3, and these strains all belong to the Wolbachia A clade (Vavre et al., 1999, 2000; Werren et al., 1995; Zhou et al., 1998). Infection with the three distinct wLhet strains appears to predominate in nature, although double‐, mono‐ and non‐infected individuals have also been recorded in natural populations (Mouton, 2004). In contrast to A. tabida that requires Wolbachia for oogenesis (Mouton et al., 2009), none of the three strains is obligatory for L. heterotoma (Vavre et al., 2000). However, wLhet1 does seem to be required for persistence of the other strains, because mono‐infection with wLhet2 or wLhet3 could not be established in the laboratory (Mouton et al., 2003). The density of each Wolbachia strain remains constant regardless of the presence of other strains, suggesting an absence of competition between strains (Mouton et al., 2003). The relative proportion of the three strains does not vary depending on temperature or host genotype: wLhet3 is always the most abundant, while wLhet2 is the least abundant (Mouton et al., 2003, 2007). Temperature and host genotype do, however, affect the total Wolbachia load (Mouton et al., 2007).
Wolbachia can have various effects on host fitness, including cytoplasmic incompatibility: a reproductive incompatibility resulting in embryonic death (Shropshire et al., 2020). In diploids, where all eggs are fertilized, a complete cytoplasmic incompatibility leads to loss of all progeny. In haplodiploids, two types of cytoplasmic incompatibility have been described: “Female Mortality” and “Male Development” (Figure 6). For Female Mortality cytoplasmic incompatibility, fertilized eggs cannot develop; hence only males are produced from unfertilized eggs (Figure 6). For Male Development cytoplasmic incompatibility, fertilized eggs lose the paternal chromosome and develop into haploid males. While the genes underlying cytoplasmic incompatibility have been discovered, the molecular mechanisms and differences between the two incompatibility types have not yet been elucidated (Shropshire et al., 2020). In general, the incompatibility type and number of offspring resulting from an incompatible cross depends on several factors, such as host species, host genotype, as well as Wolbachia strain and Wolbachia load (Bordenstein et al., 2003; Raychoudhury & Werren, 2012). In L. heterotoma, cytoplasmic incompatibility induced by the three strains (wLhet1/wLhet2/wLhet3 male × Wolbachia‐free female) resulted in a Female Mortality type incompatibility (Vavre et al., 2000, 2001). Curiously, crosses between L. heterotoma individuals containing only one or two Wolbachia strains revealed different types of cytoplasmic incompatibility, intermediate between Female Mortality and Male Development incompatibility, where part of the offspring died, while some developed as haploid males. The percentage of haplodized eggs decreased with the number of strains involved from ~41% to ~18% (Mouton et al., 2005). This clearly indicates that cytoplasmic incompatibility is dependent on the number of strains and/or Wolbachia density in L. heterotoma. The three wLhet strains are further bidirectionally incompatible, meaning that any cross between individuals bearing different strains would result in embryonic death (Mouton et al., 2005).
FIGURE 6.

The two types of cytoplasmic incompatibility induced by Wolbachia in haplodiploid insects: the Male Development type described in Nasonia vitripennis (Breeuwer & Werren, 1990) and the Female Mortality type described in Leptopilina heterotoma (Vavre et al., 2000, 2001). f: Wolbachia‐free, w: infected with Wolbachia (wNvitA and wNvitB for N. vitripennis; wLhet1, wLhet2 and wLhet3 for L. heterotoma).
In addition to the clear negative fitness consequences of cytoplasmic incompatibility, Wolbachia can further reduce L. heterotoma fitness by reducing locomotor performance, survival under starvation, and egg production (Fleury, Vavre, et al., 2000). This has, however, only been tested with triply infected individuals; hence the effects of each strain on fitness are not known (Fleury, Vavre, et al., 2000). The eggs of Wolbachia‐cured females showed a lower encapsulation rate by D. simulans larvae, revealing that immunity‐related traits are also likely affected (Fytrou et al., 2006; see Section 2 on host immunity). No effect was found of Wolbachia infection on circadian rhythm or development time (Fleury, Vavre, et al., 2000). When both parents were triply infected, sex ratios remained unchanged, indicating that Wolbachia is not feminizing nor male‐killing in L. heterotoma (Fleury, Vavre, et al., 2000). So far, no positive fitness effects of Wolbachia have been found for L. heterotoma (Fleury, Vavre, et al., 2000). Considering its strong negative effects on fitness and its high prevalence in natural populations, Wolbachia is particularly deleterious for L. heterotoma.
8. KEY FEATURES OF L. HETEROTOMA AS A MODEL SYSTEM
The incredible knowledge‐base on the amber wasp L. heterotoma described largely in this review highlights the major contribution that this model system has made to research in ecology and evolution. L. heterotoma phenotypes often lie in between the most extreme life history syndromes. For example, development occurs as an endo‐ and ectoparasitoid, fat accumulation is plastic and dependent on the host environment, there seems to be partial local mate competition, and cytoplasmic incompatibility involves both types (Male Development and Female Mortality). This makes L. heterotoma an excellent system for comparative studies.
Previous work on L. heterotoma further paves the way for the development of novel research in different fields. For example, L. heterotoma would be an excellent system to determine the cost of learning and the trade‐offs between learning ability and life histories in changing environments. We further know very little about the species' basic population dynamics (e.g., rate of increase, density‐dependence), knowledge that could be of use for linking individual‐level and population‐level processes. There is still a major gap of knowledge on mate choice decisions and sexual selection in L. heterotoma, which could play an important role in population differentiation and speciation. Indeed, aside from the work on local mate competition, we still know relatively little about genetic differentiation between populations, including dispersal distance, migration, and gene flow. Sequencing neutral markers revealed large gene flow and minor sequence differences between L. heterotoma populations (Visser et al., 2018), but phenotypically we see major intra‐specific differences in diverse traits, such as mating behaviors, egg numbers, and fat accumulation phenotypes.
Recently, a high quality and annotated genome sequence of L. heterotoma became available (Di Giovanni et al., 2020; Huang et al., 2021; Wey et al., 2020). The genome was originally sequenced to study the evolution of virus‐like particles, and genomic tools have indeed mostly been used in studies on virulence and immunity (Wertheim, 2022). Genetic tools, such as gene‐targeted knock‐down RNA interference (RNAi) and CRISPR‐Cas9 have been widely used to characterize gene functions, also in parasitoids (Dalla Benetta et al., 2020; Li et al., 2012, 2017; Lynch, 2006; Werren, 2009). A successful RNAi method was already developed for L. boulardi by injecting dsRNA directly into dissected late larval instars (Colinet et al., 2014). Using a similar method, a recent study with L. heterotoma also showed high RNAi efficiency (Huang et al., 2021). This provides an exciting prospect for future studies on gene function in L. heterotoma.
Another asset is L. heterotoma's close association with Drosophila species, where D. melanogaster is itself an important model in genetics, developmental biology and genomics (Gompel & Carroll, 2003; Kuntz & Eisen, 2014; Prud'homme & Gompel, 2010; Ugur et al., 2016). There are thus a plethora of resources available for the host, e.g., mutants, the Drosophila Genetic Reference Panel (Mackay et al., 2012), and the more recent literature has increased focus also on Drosophila ecology (O'Grady & DeSalle, 2018). Most hosts (Bombin & Reed, 2016; Klepsatel et al., 2018, 2020; Krams et al., 2020; Wertheim et al., 2006) and parasitoids (Fleury et al., 2004, 2009; Mazzetto et al., 2016) are further easily observed and caught in the field, as well as reared in the lab (i.e., using artificial media, short generation times, high offspring numbers). Altogether, this makes the L. heterotoma‐Drosophila system an excellent eco‐evolutionary model system for studying host‐parasitoid dynamics and interactions, also in natural populations.
AUTHOR CONTRIBUTIONS
Maude Quicray: Validation (equal); writing – original draft (equal); writing – review and editing (equal). Léonore Wilhelm: Validation (equal); writing – original draft (equal); writing – review and editing (equal). Thomas Enriquez: Validation (equal); writing – original draft (equal); writing – review and editing (equal). Shulin He: Validation (equal); writing – original draft (equal). Mathilde Scheifler: Validation (equal); writing – original draft (equal); writing – review and editing (equal). Bertanne Visser: Conceptualization (lead); funding acquisition (lead); project administration (lead); supervision (lead); validation (equal); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Table S1.
Table S2.
ACKNOWLEDGMENTS
We would like to thank Cécile Le Lann and Joan van Baaren for providing their datasets on life history traits. We are further grateful to Cécile Le Lann, two anonymous reviewers, and the associate editor for constructive feedback on previous versions of this manuscript. BV, TE, SH, MS and MQ were funded by the Fonds National de Recherche Scientifique F.R.S.‐FNRS (grants “J.0190.21”, “T.0186.20”, “FC 41573”). TE was further funded by the Fondation Fyssen. We would like to thank the University Foundation of Belgium for its contribution to publication fees.
Quicray, M. , Wilhelm, L. , Enriquez, T. , He, S. , Scheifler, M. , & Visser, B. (2023). The Drosophila‐parasitizing wasp Leptopilina heterotoma: A comprehensive model system in ecology and evolution. Ecology and Evolution, 13, e9625. 10.1002/ece3.9625
Maude Quicray and Léonore Wilhelm are co‐first authors.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- Abram, P. K. , Boivin, G. , Moiroux, J. , & Brodeur, J. (2017). Behavioural effects of temperature on ectothermic animals: Unifying thermal physiology and behavioural plasticity. Biological Reviews, 92(4), 1859–1876. 10.1111/brv.12312 [DOI] [PubMed] [Google Scholar]
- Abram, P. K. , Wang, X. , Hueppelsheuser, T. , Franklin, M. T. , Daane, K. M. , Lee, J. C. , Lue, C.‐H. , Girod, P. , Carrillo, J. , Wong, W. H. L. , Kula, R. R. , Gates, M. W. , Hogg, B. N. , Moffat, C. E. , Hoelmer, K. A. , Sial, A. A. , & Buffington, M. L. (2022). A coordinated sampling and identification methodology for larval parasitoids of spotted‐wing Drosophila . Journal of Economic Entomology, 115, 922–942. 10.1093/jee/toab237 [DOI] [PubMed] [Google Scholar]
- Addo‐Bediako, A. , Chown, S. L. , & Gaston, K. J. (2000). Thermal tolerance, climatic variability and latitude. Proceedings of the Royal Society B, 267(1445), 739–745. 10.1098/rspb.2000.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, M. Z. , Breinholt, J. W. , & Kawahara, A. Y. (2016). Evidence for common horizontal transmission of Wolbachia among butterflies and moths. BMC Evolutionary Biology, 16(1), 118. 10.1186/s12862-016-0660-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allemand, R. , Fleury, F. , Lemaitre, C. , & Boulétreau, M. (1999). Dynamique des populations et interactions compétitives chez deux espèces de Leptopilina, parasitoïdes, dans la vallée du Rhône (Hymenoptera: Figitidae). Annales de la Société entomologique de France, 35, 97–103. [Google Scholar]
- Amat, I. , Castelo, M. , Desouhant, E. , & Bernstein, C. (2006). The influence of temperature and host availability on the host exploitation strategies of sexual and asexual parasitic wasps of the same species. Oecologia, 148(1), 153–161. 10.1007/s00442-005-0332-9 [DOI] [PubMed] [Google Scholar]
- Anderl, I. , Vesala, L. , Ihalainen, T. O. , Vanha‐aho, L.‐M. , Andó, I. , Rämet, M. , & Hultmark, D. (2016). Transdifferentiation and proliferation in two distinct hemocyte lineages in Drosophila melanogaster larvae after wasp infection. PLoS Pathogens, 12(7), e1005746. 10.1371/journal.ppat.1005746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeh, M. J. , de Jong, P. W. , & van Lenteren, J. C. (2005). Intra‐ and interspecific host discrimination in arrhenotokous and thelytokous Eretmocerus spp. Biological Control, 33(1), 74–80. 10.1016/j.biocontrol.2005.01.006 [DOI] [Google Scholar]
- Arrese, E. L. , & Soulages, J. L. (2010). Insect fat body: Energy, metabolism, and regulation. Annual Review of Entomology, 55, 207–225. 10.1146/annurev-ento-112408-085356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayasse, M. , Paxton, R. J. , & Tengö, J. (2001). Mating behaviour and chemical communication in the order hymenoptera. Annual Review of Entomology, 46, 31–78. 10.1146/annurev.ento.46.1.31 [DOI] [PubMed] [Google Scholar]
- Bakker, K. , Bagchee, S. N. , Zwet, W. R. , & Meelis, E. (1967). Host discrimination in Pseudocoila bochei (Hymenoptera: Cynipidae). Entomologia Experimentalis et Applicata, 10(3–4), 295–311. 10.1111/j.1570-7458.1967.tb02449.x [DOI] [Google Scholar]
- Bakker, K. , Eijsackers, H. J. P. , van Lenteren, J. C. , & Meelis, E. (1972). Some models describing the distribution of eggs of the parasite Pseudeucoila bochei (Hym., Cynip.) over its hosts, larvae of Drosophila melanogaster . Oecologia, 10(1), 29–57. 10.1007/BF00822760 [DOI] [PubMed] [Google Scholar]
- Bakker, K. , Peulet, P. , & Visser, M. E. (1990). The ability to distinguish between hosts containing different numbers of parasitoid eggs by the solitary parasitoid Leptopilina heterotoma (Thomson) (Hym., Cynip.). Netherlands Journal of Zoology, 40(3), 514–520. 10.1163/156854290X00064 [DOI] [Google Scholar]
- Bakker, K. , van Alphen, J. J. M. , van Batenburg, F. H. D. , van der Hoeven, N. , Nell, H. W. , van Strien‐van Liempt, W. T. F. H. , & Turlings, T. C. J. (1985). The function of host discrimination and superparasitization in parasitoids. Oecologia, 67, 572–576. 10.1007/BF00790029 [DOI] [PubMed] [Google Scholar]
- Ballinger, M. J. , & Perlman, S. J. (2017). Generality of toxins in defensive symbiosis: Ribosome‐inactivating proteins and defense against parasitic wasps in Drosophila . PLoS Pathogens, 7(13), e1006431. 10.1371/journal.ppat.1006431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt, R. J. , Schaner, A. M. , & Jackson, L. L. (1985). Cis‐vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster . Journal of Chemical Ecology, 11, 1747–1756. 10.1007/BF01012124 [DOI] [PubMed] [Google Scholar]
- Birsoy, K. , Festuccia, W. T. , & Laplante, M. (2013). A comparative perspective on lipid storage in animals. Journal of Cell Science, 126(7), 1541–1552. 10.1242/jcs.104992 [DOI] [PubMed] [Google Scholar]
- Blacher, P. , Huggins, T. J. , & Bourke, A. F. G. (2017). Evolution of ageing, costs of reproduction and the fecundity‐longevity trade‐off in eusocial insects. Proceedings of the Royal Society B, 284, 20170380. 10.1098/rspb.2017.0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaimer, B. B. , Gotzek, D. , Brady, S. G. , & Buffington, M. L. (2020). Comprehensive phylogenomic analyses re‐write the evolution of parasitism within cynipoid wasps. BMC Evolutionary Biology, 20(1), 155. 10.1186/s12862-020-01716-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum, M. S. (1996). Semiochemical parsimony in the Arthropoda. Annual Review of Entomology, 41(1), 353–374. 10.1146/annurev.en.41.010196.002033 [DOI] [PubMed] [Google Scholar]
- Bombin, A. , & Reed, L. K. (2016). The changing biodiversity of Alabama Drosophila: Important impacts of seasonal variation, urbanization, and invasive species. Ecology and Evolution, 6(19), 7057–7069. 10.1002/ece3.2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein, S. R. , Uy, J. J. , & Werren, J. H. (2003). Host genotype determines cytoplasmic incompatibility type in the haplodiploid genus Nasonia . Genetics, 164, 223–233. 10.1093/genetics/164.1.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttinger, L. C. , Hofferberth, J. , Ruther, J. , & Stökl, J. (2019). Semiochemicals mediating defense, intraspecific competition, and mate finding in Leptopilina ryukyuensis and L. japonica (hymenoptera: Figitidae), parasitoids of Drosophila . Journal of Chemical Ecology, 45, 241–252. 10.1007/s10886-019-01052-w [DOI] [PubMed] [Google Scholar]
- Böttinger, L. C. , Hüftlein, F. , & Stökl, J. (2021). Mate attraction, chemical defense, and competition avoidance in the parasitoid wasp Leptopilina pacifica . Chemoecology, 31, 101–114. 10.1007/s00049-020-00331-3 [DOI] [Google Scholar]
- Böttinger, L. C. , & Stökl, J. (2020). Dispersal from natal patch correlates with the volatility of female sex pheromones in parasitoid wasps. Frontiers in Ecology and Evolution, 8, 557527. 10.3389/fevo.2020.557527 [DOI] [Google Scholar]
- Boulton, R. A. , Collins, L. A. , & Shuker, D. M. (2015). Beyond sex allocation: The role of mating systems in sexual selection in parasitoid wasps: Sexual selection in parasitoid wasps. Biological Reviews, 90(2), 599–627. 10.1111/brv.12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton, R. A. , Cook, N. , Greenway, E. V. , Glaser, G. L. , Green, J. , & Shuker, D. M. (2019). Local mate competition modifies the costs of mating in a mostly monandrous parasitoid wasp. Behavioral Ecology, 30, 417–425. 10.1093/beheco/ary181 [DOI] [Google Scholar]
- Bozler, J. , Kacsoh, B. Z. , & Bosco, G. (2020). Maternal priming of offspring immune system in Drosophila . G3, 10(1), 165–175. 10.1534/g3.119.400852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeuwer, J. A. J. , & Werren, J. H. (1990). Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature, 346(6284), 558–560. 10.1038/346558a0 [DOI] [PubMed] [Google Scholar]
- Buffington, M. L. (2007). The occurrence and phylogenetic implications of the ovipositor clip within the Figitidae (Insecta: Hymenoptera: Cynipoidea). Journal of Natural History, 41(33–36), 2267–2282. 10.1080/00222930701579732 [DOI] [Google Scholar]
- Buffington, M. L. , Giorgini, M. , Lue, C.‐H. , Formisano, G. , Cascone, P. , Forshage, M. , Driskell, A. , & Guerrieri, E. (2020). Description of the aberrant Leptopilina lasallei n. sp., with an updated phylogeny of Leptopilina Förster (hymenoptera: Figitidae: Eucoilinae). Journal of Natural History, 54(9–12), 565–583. 10.1080/00222933.2020.1754483 [DOI] [Google Scholar]
- Carton, Y. , Bouletreau, M. , van Alphen, J. J. M. , & van Lenteren, J. C. (1986). The Drosophila parasitic wasps. In Ashburner M., Carson H. L., & Thompson J. N. (Eds.), The genetics and biology of Drosophila (Vol. 3, pp. 348–394). Academic Press. [Google Scholar]
- Carton, Y. , Frey, F. , & Nappi, A. J. (2009). Parasite‐induced changes in nitric oxide levels in Drosophila paramelanica . Journal of Parasitology, 95(5), 1134–1141. 10.1645/GE-2091.1 [DOI] [PubMed] [Google Scholar]
- Carton, Y. , Haouas, S. , Marrakchi, M. , & Hochberg, M. (1991). Two competing parasitoid species coexist in sympatry. Oikos, 60(2), 222–230. 10.2307/3544869 [DOI] [Google Scholar]
- Chabert, S. , Allemand, R. , Poyet, M. , Eslin, P. , & Gibert, P. (2012). Ability of European parasitoids (hymenoptera) to control a new invasive Asiatic pest, Drosophila suzukii . Biological Control, 63(1), 40–47. 10.1016/j.biocontrol.2012.05.005 [DOI] [Google Scholar]
- Chabora, P. C. , Smolin, S. J. , & Kopelman, A. H. (1979). The life history of Pseudeucoila sp., a protelian parasite of Drosophila . Annals of the Entomological Society of America, 72(4), 495–499. 10.1093/aesa/72.4.495 [DOI] [Google Scholar]
- Chang, H. , Guo, X. , Guo, S. , Yang, N. , & Huang, Y. (2021). Trade‐off between flight capability and reproduction in Acridoidea (Insecta: Orthoptera). Ecology and Evolution, 11(23), 16849–16861. 10.1002/ece3.8317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov, E. L. (1976). Optimal foraging, the marginal value theorem. Theoretical Population Biology, 9(2), 129–136. 10.1016/0040-5809(76)90040-X [DOI] [PubMed] [Google Scholar]
- Charnov, E. L. (1979). The genetical evolution of patterns of sexuality: Darwinian fitness. The American Naturalist, 113(4), 465–480. 10.1086/283407 [DOI] [Google Scholar]
- Charnov, E. L. (1982). The theory of sex allocation. (MPB‐18), 18. Princeton University Press. 10.2307/j.ctvx8b6km [DOI] [PubMed] [Google Scholar]
- Charnov, E. L. , Los‐den Hartogh, R. , Jones, W. J. , & van den Assem, J. (1981). Sex ratio evolution in a variable environment. Nature, 289, 27–33. 10.1038/289027a0 [DOI] [PubMed] [Google Scholar]
- Chiu, H. , & Govind, S. (2002). Natural infection of D. melanogaster by virulent parasitic wasps induces apoptotic depletion of hematopoietic precursors. Cell Death and Differentiation, 9, 1379–1381. 10.1038/sj.cdd.4401134 [DOI] [PubMed] [Google Scholar]
- Chiu, H. , Morales, J. , & Govind, S. (2006). Identification and immuno‐electron microscopy localization of p40, a protein component of immunosuppressive virus‐like particles from Leptopilina heterotoma, a virulent parasitoid wasp of Drosophila . Journal of General Virology, 87(2), 461–470. 10.1099/vir.0.81474-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, A. B. (1978). Sex ratio and local resource competition in a prosimian primate. Science, 201(4351), 163–165. 10.1126/science.201.4351.163 [DOI] [PubMed] [Google Scholar]
- Colinet, D. , Deleury, E. , Anselme, C. , Cazes, D. , Poulain, J. , Azema‐Dossat, C. , Belghazi, M. , Gatti, J.‐L. , & Poirié, M. (2013). Extensive inter‐ and intraspecific venom variation in closely related parasites targeting the same host: The case of Leptopilina parasitoids of Drosophila . Insect Biochemistry and Molecular Biology, 43(7), 601–611. 10.1016/j.ibmb.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Colinet, D. , Kremmer, L. , Lemauf, S. , Rebuf, C. , Gatti, J.‐L. , & Poirié, M. (2014). Development of RNAi in a Drosophila endoparasitoid wasp and demonstration of its efficiency in impairing venom protein production. Journal of Insect Physiology, 63, 56–61. 10.1016/j.jinsphys.2014.02.011 [DOI] [PubMed] [Google Scholar]
- Cook, J. M. (1993). Sex determination in the hymenoptera: A review of models and evidence. Heredity, 71, 421–435. 10.1038/hdy.1993.157 [DOI] [Google Scholar]
- Corbin, C. , Jones, J. E. , Chrostek, E. , Fenton, A. , & Hurst, G. D. D. (2021). Thermal sensitivity of the Spiroplasma – Drosophila hydei protective symbiosis: The best of climes, the worst of climes. Molecular Ecology, 30(5), 1336–1344. 10.1111/mec.15799 [DOI] [PubMed] [Google Scholar]
- Coulette, Q. , Lemauf, S. , Colinet, D. , Prévost, G. , Anselme, C. , Poirié, M. , & Gatti, J.‐L. (2017). Biochemical characterization and comparison of aspartylglucosaminidases secreted in venom of the parasitoid wasps Asobara tabida and Leptopilina heterotoma . PLoS One, 12(7), e0181940. 10.1371/journal.pone.0181940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daane, K. M. , Wang, X.‐G. , Biondi, A. , Miller, B. , Miller, J. C. , Riedl, H. , Shearer, P. W. , Guerrieri, E. , Giorgini, M. , Buffington, M. , van Achterberg, K. , Song, Y. , Kang, T. , Yi, H. , Jung, C. , Lee, D. W. , Chung, B.‐K. , Hoelmer, K. A. , & Walton, V. M. (2016). First exploration of parasitoids of Drosophila suzukii in South Korea as potential classical biological agents. Journal of Pest Science, 89, 823–835. 10.1007/s10340-016-0740-0 [DOI] [Google Scholar]
- Dalla Benetta, E. , Chaverra‐Rodriguez, D. , Rasgon, J. L. , & Akbari, O. S. (2020). Pupal and adult injections for RNAi and CRISPR gene editing in Nasonia vitripennis . Journal of Visualized Experiements, 166, e61892. 10.3791/61892 [DOI] [PubMed] [Google Scholar]
- Damiens, D. , & Boivin, G. (2005). Male reproductive strategy in Trichogramma evanescens: Sperm production and allocation to females. Physiological Entomology, 30, 241–247. 10.1111/j.1365-3032.2005.00453.x [DOI] [Google Scholar]
- de Bruijn, J. A. C. , Vosteen, I. , Vet, L. E. M. , Smid, H. M. , & de Boer, J. G. (2021). Multi‐camera field monitoring reveals costs of learning for parasitoid foraging behaviour. Journal of Animal Ecology, 90(7), 1635–1646. 10.1111/1365-2656.13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delava, E. , Fleury, F. , & Gibert, P. (2022). Differentiation of thermal reaction norms between marginal and core populations of a northward expanding parasitoid. BioRxiv. 10.1101/2022.04.26.489532 [DOI] [Google Scholar]
- Delpuech, J.‐M. , & Allemand, R. (2011). Side effects of fungicides on the abundance and the species diversity of the natural populations of Drosophila and their hymenopterous parasitoids in orchards. Phytoparasitica, 39, 429–435. 10.1007/s12600-011-0180-6 [DOI] [Google Scholar]
- Di Giovanni, D. , Lepetit, D. , Guinet, B. , Bennetot, B. , Boulesteix, M. , Couté, Y. , Bouchez, O. , Ravallec, M. , & Varaldi, J. (2020). A behavior‐manipulating virus relative as a source of adaptive genes for Drosophila parasitoids. Molecular Biology and Evolution, 37, 2791–2807. 10.1093/molbev/msaa030 [DOI] [PubMed] [Google Scholar]
- Dicke, M. , van Lenteren, J. C. , Boskamp, G. J. F. , & van Dongen‐van Leeuwen, E. (1984). Chemical stimuli in host‐habitat location by Leptopilina heterotoma (Thomson) (hymenoptera: Eucoilidae), a parasite of Drosophila . Journal of Chemical Ecology, 37(10), 695–712. 10.1007/BF00988537 [DOI] [PubMed] [Google Scholar]
- Dicke, M. , van Lenteren, J. C. , Boskamp, G. J. F. , & Van Voorst, R. (1985). Intensification and prolongation of host searching in Leptopilina heterotoma (Thomson) (hymenoptera: Eucoilidae) through a kairomone produced by Drosophila melanogaster . Journal of Chemical Ecology, 11, 125–135. 10.1007/bf00987611 [DOI] [PubMed] [Google Scholar]
- Driessen, G. , van Alphen, J. J. M. , & Hemerik, L. (1990). Drosophila species, breeding in the stinkhorn (Phallus impudicus Pers.) and their larval parasitoids. Netherlands Journal of Zoology, 40(3), 409–427. 10.1163/156854290X00019 [DOI] [Google Scholar]
- Eijs, I. E. M. , Ellers, J. , & van Duinen, G.‐J. (1998). Feeding strategies in drosophilid parasitoids: The impact of natural food resources on energy reserves in females. Ecological Entomology, 23(2), 133–138. 10.1046/j.1365-2311.1998.00117.x [DOI] [Google Scholar]
- Eijsackers, H. J. P. , & Bakker, K. (1971). Elimination by physical attack of supernumerary larvae of Pseudeucoila bochei Weld (Cynipidae) in their hosts, larvae of Drosophila . Netherlands Journal of Zoology, 21(2), 205–207. 10.1163/002829671X00168 [DOI] [Google Scholar]
- Ellers, J. (1996). Fat and eggs: An alternative method to measure the trade‐off between survival and reproduction in insect parasitoids. Netherlands Journal of Zoology, 46(3–4), 227–235. 10.1163/156854295X00186 [DOI] [Google Scholar]
- Ellers, J. , & Jervis, M. A. (2004). Why are so few parasitoid wasp species pro‐ovigenic? Evolutionary Ecology, 6(7), 993–1002. [Google Scholar]
- Ellers, J. , van Alphen, J. J. M. , & Sevenster, J. G. (1998). A field study of size‐fitness relationship in the parasitoid Asobara tabida. Journal of Animal Ecology, 67(2), 318–324. http://www.jstor.org/stable/2647500 [Google Scholar]
- El‐Sayed, A. (2022). The Pherobase: Database of pheromones and semiochemicals . http://www.pherobase.com
- Enriquez, T. , Lievens, V. , Nieberding, C. M. , & Visser, B. (2022). Pupal size as a proxy for fat content in laboratory‐reared and field‐collected Drosophila species. Scientific Reports, 12, 12855. 10.1038/s41598-022-15325-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström, T. , & Wiklund, C. (1982). Why do males emerge before females? Protandry as a mating strategy in male and female butterflies. Oecologia, 52(2), 164–166. 10.1007/bf00363830 [DOI] [PubMed] [Google Scholar]
- Fauvergue, X. , Fleury, F. , Lemaitre, C. , & Allemand, R. (1999). Parasitoid mating structures when hosts are patchily distributed: Field and laboratory experiments with Leptopilina boulardi and L. heterotoma . Oikos, 86(2), 344–356. 10.2307/3546451 [DOI] [Google Scholar]
- Fauvergue, X. , Lo Genco, A. , & Lo Pinto, M. (2008). Virgins in the wild: Mating status affects the behavior of a parasitoid foraging in the field. Oecologia, 156(4), 913–920. 10.1007/s00442-008-1037-7 [DOI] [PubMed] [Google Scholar]
- Ferrarese, R. , Morales, J. , Fimiarz, D. , Webb, B. A. , & Govind, S. (2009). A supracellular system of actin‐lined canals controls biogenesis and release of virulence factors in parasitoid venom glands. Journal of Experimental Biology, 212(14), 2261–2268. 10.1242/jeb.025718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R. A. (1930). The genetical theory of natural selection. Clarendon. 10.5962/bhl.title.27468 [DOI] [Google Scholar]
- Fleury, F. , Allemand, R. , Vavre, F. , Fouillet, P. , & Boulétreu, M. (2000). Adaptive significance of a circadian clock: Temporal segregation of activities reduces intrinsic competitive inferiority in Drosophila parasitoids. Proceedings: Biological Sciences, 267(1447), 1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury, F. , Gibert, P. , Ris, N. , & Allemand, R. (2009). Chapter 1: Ecology and life history evolution of frugivorous Drosophila parasitoids. Advances in Parasitology, 70, 3–44. 10.1016/S0065-308X(09)70001-6 [DOI] [PubMed] [Google Scholar]
- Fleury, F. , Ris, N. , Allemand, R. , Fouillet, P. , Carton, Y. , & Bouletreau, M. (2004). Ecological and genetic interactions in Drosophila‐parasitoids communities: A case study with D. melanogaster, D. simulans and their common Leptopilina parasitoids in South‐Eastern France. Genetica, 120(1–3), 181–194. [DOI] [PubMed] [Google Scholar]
- Fleury, F. , Vavre, F. , Ris, N. , Fouillet, P. , & Boulétreau, M. (2000). Physiological cost induced by the maternally‐transmitted endosymbiont Wolbachia in the Drosophila parasitoid Leptopilina heterotoma . Parasitology, 121(5), 493–500. 10.1017/S0031182099006599 [DOI] [PubMed] [Google Scholar]
- Fontal‐Cazalla, F. M. , Buffington, M. L. , Nordlander, G. , Liljeblad, J. , Ros‐Farre, P. , Nieves‐Aldrey, J. L. , Pujade‐Villar, J. , & Ronquist, F. (2002). Phylogeny of the Eucoilinae (hymenoptera: Cynipoidea: Figitidae). Cladistics, 18(2), 154–199. 10.1111/j.1096-0031.2002.tb00147.x [DOI] [PubMed] [Google Scholar]
- Forbes, A. A. , Bagley, R. K. , Beer, M. A. , Hippee, A. C. , & Widmayer, H. A. (2018). Quantifying the unquantifiable: Why hymenoptera, not Coleoptera, is the most speciose animal order. BMC Ecology, 18(21), 1–11. 10.1186/s12898-018-0176-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, C. L. , Pollock, S. W. , Smith, J. E. , & Hughes, W. O. H. (2014). Wolbachia in the flesh: Symbiont intensities in germ‐line and somatic tissues challenge the conventional view of Wolbachia transmission routes. PLoS One, 9(7), e95122. 10.1371/journal.pone.0095122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fytrou, A. , Schofield, P. G. , Kraaijeveld, A. R. , & Hubbard, S. F. (2006). Wolbachia infection suppresses both host defence and parasitoid counter‐defence. Proceedings of the Royal Society B, 273(1588), 791–796. 10.1098/rspb.2005.3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain, R. M. , Williams, J. L. , Schluter, D. , & Angert, A. L. (2018). Moving character displacement beyond characters using contemporary coexistence theory. Trends in Ecology and Evolution, 33(2), 74–84. 10.1016/j.tree.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Gibson, G. A. P. (2009). Revision of new world Spalangiinae (hymenoptera: Pteromalidae). Zootaxa, 2259(1), 1–159. 10.11646/zootaxa.2259.1.1 [DOI] [Google Scholar]
- Giorgini, M. , Wang, X.‐G. , Wang, Y. , Chen, F.‐S. , Hougardy, E. , Zhang, H.‐M. , Chen, Z.‐Q. , Chen, H.‐Y. , Liu, C.‐X. , Cascone, P. , Formisano, G. , Carvalho, G. A. , Biondi, A. , Buffington, M. , Daane, K. M. , Hoelmer, K. A. , & Guerrieri, E. (2019). Exploration for native parasitoids of Drosophila suzukii in China reveals a diversity of parasitoid species and narrow host range of the dominant parasitoid. Journal of Pest Science, 92, 509–522. 10.1007/s10340-018-01068-3 [DOI] [Google Scholar]
- Giraldeau, L.‐A. , & Boivin, G. (2008). Risk assessment and host exploitation strategies in insect parasitoids. In Wajnberg E., Bernstein C., & van Alphen J. J. M. (Eds.), Behavioral ecology of insect parasitoids: From theoretical approaches to field applications (pp. 212–227). Blackwell Publishing. 10.1002/9780470696200.ch10 [DOI] [Google Scholar]
- Girod, P. , Borowiec, N. , Buffington, M. , Chen, G. , Fang, Y. , Kimura, M. T. , Peris‐Felipo, F. J. , Ris, N. , Wu, H. , Xiao, C. , Zhang, J. , Aebi, A. , Haye, T. , & Kenis, M. (2018). The parasitoid complex of D. suzukii and other fruit feeding Drosophila species in Asia. Scientific Reports, 8(1), 11839. 10.1038/s41598-018-29555-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod, P. , Rossignaud, L. , Haye, T. , Turlings, T. C. J. , & Kenis, M. (2018). Development of Asian parasitoids in larvae of Drosophila suzukii feeding on blueberry and artificial diet. Journal of Applied Entomology, 142(5), 483–494. 10.1111/jen.12496 [DOI] [Google Scholar]
- Godfray, H. C. J. (1988). Virginity in haplodiploid populations: A study on fig wasps. Ecological Entomology, 13(3), 283–291. [Google Scholar]
- Godfray, H. C. J. (1990). The causes and consequences of constrained sex allocation in haplodiploid animals. Journal of Evolutionary Biology, 3(1–2), 3–17. 10.1046/j.1420-9101.1990.3010003.x [DOI] [Google Scholar]
- Godfray, H. C. J. (1994). Parasitoids: Behavioral and evolutionary ecology. In Krebs J. R. & Clutton‐Brock T. (Eds.), Monographs in behavior and ecology (Vol. 67). Princeton University Press. 10.2307/j.ctvs32rmp [DOI] [Google Scholar]
- Godfray, H. C. J. , & Cook, J. M. (1997). Mating systems of parasitoid wasps. In Choe J. C. & Crespi B. J. (Eds.), The evolution of mating systems in insects and arachnids (pp. 211–225). Cambridge University Press. 10.1017/CBO9780511721946.013 [DOI] [Google Scholar]
- Godfray, H. C. J. , & Grafen, A. (1988). Unmatedness and the evolution of eusociality. The American Naturalist, 131(2), 303–305. [Google Scholar]
- Godfray, H. C. J. , & Hardy, I. C. W. (1990). Estimating the frequency of constrained sex allocation in field populations of hymenoptera. Behaviour, 114(1), 137–147. 10.1163/156853990X00086 [DOI] [Google Scholar]
- Goecks, J. , Mortimer, N. T. , Mobley, J. A. , Bowersock, G. J. , Taylor, J. , & Schlenke, T. A. (2013). Integrative approach reveals composition of endoparasitoid wasp venoms. PLoS One, 8(5), e64125. 10.1371/journal.pone.0064125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhman, V. E. (2018). Dimensions and borderlines of parasitoid hymenoptera species: A paradigm shift? Biology Bulletin Reviews, 8(3), 227–233. 10.1134/S2079086418030052 [DOI] [Google Scholar]
- Gompel, N. , & Carroll, S. B. (2003). Genetic mechanisms and constraints governing the evolution of correlated traits in drosophilid flies. Nature, 424(6951), 931–935. 10.1038/nature01787 [DOI] [PubMed] [Google Scholar]
- Grasswitz, T. R. , & Jones, G. R. (2002). Chemical ecology. Encyclopedia of Life Sciences. 10.1038/npg.els.0003265 [DOI] [Google Scholar]
- Haccou, P. , Vlas, S. J. D. , Alphen, J. J. M. V. , & Visser, M. E. (1991). Information processing by foragers: Effects of intra‐patch experience on the leaving tendency of Leptopilina heterotoma . The Journal of Animal Ecology, 60(1), 93–106. 10.2307/5447 [DOI] [Google Scholar]
- Hahn, D. A. , & Denlinger, D. L. (2011). Energetics of insect diapause. Annual Review of Entomology, 56(1), 103–121. 10.1146/annurev-ento-112408-085436 [DOI] [PubMed] [Google Scholar]
- Hamilton, W. D. (1967). Extraordinary sex ratios: A sex‐ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science, 156(3774), 477–488. 10.1126/science.156.3774.477 [DOI] [PubMed] [Google Scholar]
- Hardy, I. C. W. (1994). Sex ratio and mating structure in the parasitoid hymenoptera. Oikos, 69(1), 3–20. 10.2307/3545278 [DOI] [Google Scholar]
- Harvey, J. A. , Snaas, H. , Malcicka, M. , Visser, B. , & Bezemer, T. M. (2014). Small‐scale spatial resource partitioning in a hyperparasitoid community. Arthropod‐Plant Interactions, 8(5), 393–401. 10.1007/s11829-014-9319-y [DOI] [Google Scholar]
- Harvey, J. A. , & Strand, M. R. (2002). The developmental strategies of endoparasitoid wasps vary with host feeding ecology. Ecology, 83(9), 2439–2451. 10.1890/0012-9658(2002)083[2439:tdsoew]2.0.co;2 [DOI] [Google Scholar]
- Heavner, M. E. , Gueguen, G. , Rajwani, R. , Pagan, P. E. , Small, C. , & Govind, S. (2013). Partial venom gland transcriptome of a Drosophila parasitoid wasp, Leptopilina heterotoma, reveals novel and shared bioactive profiles with stinging hymenoptera. Gene, 526(2), 195–204. 10.1016/j.gene.2013.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund, K. , Vet, L. E. M. , & Dicke, M. (1996). Generalist and specialist parasitoid strategies of using odours of adult drosophilid flies when searching for larval hosts. Oikos, 77(3), 390–398. 10.2307/3545929 [DOI] [Google Scholar]
- Heimpel, G. E. , & de Boer, J. G. (2008). Sex determination in the hymenoptera. Annual Review of Entomology, 53(1), 209–230. 10.1146/annurev.ento.53.103106.093441 [DOI] [PubMed] [Google Scholar]
- Hemerik, L. , Van Der Hoeven, N. , & van Alphen, J. J. M. (2002). Egg distributions and the information a solitary parasitoid has and uses for its oviposition decisions. Acta Biotheoretica, 50(3), 167–188. 10.1023/a:1016543310896 [DOI] [PubMed] [Google Scholar]
- Henneman, M. L. , Papaj, D. R. , Figueredo, A. J. , & Vet, L. E. M. (1995). Egg‐laying experience and acceptance of parasitized hosts by the parasitoid, Leptopilina heterotoma (hymenoptera: Eucoilidae). Journal of Insect Behavior, 8(3), 331–342. 10.1007/BF01989362 [DOI] [Google Scholar]
- Herren, J. K. , Paredes, J. C. , Schüpfer, F. , Arafah, K. , Bulet, P. , & Lemaitre, B. (2014). Insect endosymbiont proliferation is limited by lipid availability. eLife, 3, e02964. 10.7554/eLife.02964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higareda Alvear, V. M. , Mateos, M. , Cortez, D. , Tamborindeguy, C. , & Martinez‐Romero, E. (2021). Differential gene expression in a tripartite interaction: Drosophila, Spiroplasma and parasitic wasps. PeerJ, 9, e11020. 10.7717/peerj.11020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe, R. W. (1967). Temperature effects on embryonic development in insects. Annual Review of Entomology, 12(1), 15–42. 10.1146/annurev.en.12.010167.000311 [DOI] [PubMed] [Google Scholar]
- Huang, J. , Chen, J. , Fang, G. , Pang, L. , Zhou, S. , Zhou, Y. , Pan, Z. , Zhang, Q. , Sheng, Y. , Lu, Y. , Liu, Z. , Zhang, Y. , Li, G. , Shi, M. , Chen, X. , & Zhan, S. (2021). Two novel venom proteins underlie divergent parasitic strategies between a generalist and a specialist parasite. Nature Communications, 12, 234. 10.1038/s41467-020-20332-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard, S. F. , & Cook, R. M. (1978). Optimal foraging by parasitoid wasps. The Journal of Animal Ecology, 47, 593–604. 10.2307/3803 [DOI] [Google Scholar]
- Ideo, S. , Watada, M. , Mitsui, H. , & Kimura, M. T. (2008). Host range of Asobara japonica (hymenoptera: Braconidae), a larval parasitoid of drosophilid flies. Entomological sciences, 11(1), 1–6. 10.1111/j.1479-8298.2007.00244.x [DOI] [Google Scholar]
- Isidoro, N. , Bin, F. , & Romani, R. (1999). Diversity and function of male antennal glands in Cynipoidea (hymenoptera). Zoologica Scripta, 28(1–2), 165–174. 10.1046/j.1463-6409.1999.00013.x [DOI] [Google Scholar]
- Jacob, S. , & Boivin, G. (2005). Costs and benefits of polyandry in the egg parasitoid Trichogramma evanescens Westwood (hymenoptera: Trichogrammatidae). Biological Control, 32(2), 311–318. 10.1016/j.biocontrol.2004.10.009 [DOI] [Google Scholar]
- Janssen, A. (1989). Optimal host selection by Drosophila parasitoids in the field. Functional Ecology, 3(4), 469–479. 10.2307/2389621 [DOI] [Google Scholar]
- Janssen, A. , Driessen, G. , Haan, M. D. , & Roodbol, N. (1988). The impact of parasitoids on natural populations on temperate woodland Drosophila . Netherlands Journal of Zoology, 38(1), 61–73. 10.1163/156854288x00049 [DOI] [Google Scholar]
- Janssen, A. , van Alphen, J. J. M. , Sabelis, M. W. , & Bakker, K. (1995). Specificity of odour‐mediated avoidance of competition in Drosophila parasitoids. Behavioral Ecology and Sociobiology, 36, 229–235. 10.1007/BF00165831 [DOI] [Google Scholar]
- Jeffs, C. T. , Terry, J. C. D. , Higgie, M. , Jandová, A. , Konvičková, H. , Brown, J. J. , Lue, C. H. , Schiffer, M. , O'Brien, E. K. , Bridle, J. , Hrček, J. , & Lewis, O. T. (2021). Molecular analyses reveal consistent food web structure with elevation in rainforest Drosophila – Parasitoid communities. Ecography, 44(3), 403–413. 10.1111/ecog.05390 [DOI] [Google Scholar]
- Jenni, W. (1951). Beitrag zur morphologie und biologie der cynipide Pseudeucoila bochei weld, eines larvenparasiten von Drosophila melanogaster meig. Acta Zoologica, 32(3), 177–254. 10.1111/j.1463-6395.1951.tb00468.x [DOI] [Google Scholar]
- Kacsoh, B. Z. , & Schlenke, T. A. (2012). High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster . PLoS ONE, 7(4), e34721. 10.1371/journal.pone.0034721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh, E. S. , & Schlenke, T. A. (2012). Adaptive evolution of a novel Drosophila lectin induced by parasitic wasp attack. Molecular Biology and Evolution, 29(2), 565–577. 10.1093/molbev/msr191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenis, M. , Tonina, L. , Eschen, R. , van der Sluis, B. , Sancassani, M. , Mori, N. , Haye, T. , & Helsen, H. (2016). Non‐crop plants used as hosts by Drosophila suzukii in Europe. Journal of Pest Science, 89, 735–748. 10.1007/s10340-016-0755-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, M. T. (2019). Overwintering of reproductively mature females of a pro‐ovigenic parasitic wasp, Leptopilina heterotoma (hymenoptera: Figitidae). Entomological Science, 22(3), 264–269. 10.1111/ens.12365 [DOI] [Google Scholar]
- Kimura, M. T. , & Mitsui, H. (2020). Drosophila parasitoids (hymenoptera) of Japan. Entomological Science, 23(4), 359–368. 10.1111/ens.12432 [DOI] [Google Scholar]
- Kimura, M. T. , Ohtsu, T. , Yoshida, T. , Awasaki, T. , & Lin, F.‐J. (1994). Climatic adaptations and distributions in the Drosophila takahashii species subgroup (Diptera: Drosophilidae). Journal of Natural History, 28(2), 401–409. 10.1080/00222939400770181 [DOI] [Google Scholar]
- King, A. J. , & Marshall, H. H. (2022). Optimal foraging. Current Biology, 32(12), R680–R683. 10.1016/j.cub.2022.04.072 [DOI] [PubMed] [Google Scholar]
- King, B. (1987). Offspring sex ratios in parasitoid wasps. Quarterly Review of Biology, 62(4), 367–396. 10.1086/415618 [DOI] [Google Scholar]
- King, B. (1993). Sex ratio manipulation by parasitoid wasps. In Wrensch D. L. & Ebbert M. A. (Eds.), Evolution and diversity of sex ratio in insects and mites (pp. 418–441). Chapman & Hall. [Google Scholar]
- Klepsatel, P. , Knoblochová, D. , Girish, T. N. , Dircksen, H. , & Gáliková, M. (2020). The influence of developmental diet on reproduction and metabolism in Drosophila . BMC Evolutionary Biology, 20(1), 93. 10.1186/s12862-020-01663-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepsatel, P. , Procházka, E. , & Gáliková, M. (2018). Crowding of Drosophila larvae affects lifespan and other life‐history traits via reduced availability of dietary yeast. Experimental Gerontology, 110, 298–308. 10.1016/j.exger.2018.06.016 [DOI] [PubMed] [Google Scholar]
- Knoll, V. , Ellenbroek, T. , Romeis, J. , & Collatz, J. (2017). Seasonal and regional presence of hymenopteran parasitoids of Drosophila in Switzerland and their ability to parasitize the invasive Drosophila suzukii . Scientific Reports, 7, 40697. 10.1038/srep40697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl, J. , Huoviala, P. , & Jefferis, G. S. (2015). Pheromone processing in Drosophila . Current Opinion in Neurobiology, 34, 149–157. 10.1016/j.conb.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman, A. H. , & Chabora, P. C. (1984). Immature stages of Leptopilina boulardi (hymenoptera: Eucoilidae), a rotelean arasite of Drosophila spp. (Diptera: Drosophilidae). Annals of the Entomological Society of America, 77(3), 264–269. 10.1093/aesa/77.3.264 [DOI] [Google Scholar]
- Kraaijeveld, A. R. , & Godfray, H. C. J. (1999). Geographic patterns in the evolution of resistance and virulence in Drosophila and its parasitoids. The American Naturalist, 153(S5), S61–S74. 10.1086/303212 [DOI] [PubMed] [Google Scholar]
- Kraaijeveld, A. R. , & Godfray, H. C. J. (2009). Chapter 10: Evolution of host resistance and parasitoid counter‐resistance. In Prevost G. (Ed.) Advances in parasitology (Vol. 70, pp. 257–280). Elsevier. 10.1016/S0065-308X(09)70010-7 [DOI] [PubMed] [Google Scholar]
- Krams, I. A. , Krams, R. , Jõers, P. , Munkevics, M. , Trakimas, G. , Luoto, S. , Eichler, S. , Butler, D. M. , Merivee, E. , Must, A. , Rantala, M. J. , Contreras‐Garduño, J. , & Krama, T. (2020). Developmental speed affects ecological stoichiometry and adult fat reserves in Drosophila melanogaster . Animal Biology, 71(1), 1–20. 10.1163/15707563-bja10043 [DOI] [Google Scholar]
- Kremmer, L. , Thaon, M. , Borowiec, N. , David, J. , Poirié, M. , Gatti, J.‐L. , & Ris, N. (2017). Field monitoring of Drosophila suzukii and associated communities in south eastern France as a pre‐requisite for classical biological control. Insects, 8(4), 124. 10.3390/insects8040124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfeld‐Schor, N. , & Dayan, T. (2003). Partitioning of time as an ecological resource. Annual Review of Ecology, Evolution and Systematics, 34(1), 153–181. 10.1146/annurev.ecolsys.34.011802.132435 [DOI] [Google Scholar]
- Kuntz, S. G. , & Eisen, M. B. (2014). Drosophila embryogenesis scales uniformly across temperature in developmentally diverse species. PLoS Genetics, 10(4), e1004293. 10.1371/journal.pgen.1004293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacovone, A. , Ris, N. , Poirié, M. , & Gatti, J.‐L. (2018). Time‐course analysis of Drosophila suzukii interaction with endoparasitoid wasps evidences a delayed encapsulation response compared to D. melanogaster . PLoS ONE, 13(8), e0201573. 10.1371/journal.pone.0201573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Lann, C. , Lodi, M. , & Ellers, J. (2014). Thermal change alters the outcome of behavioural interactions between antagonistic partners. Ecological Entomology, 39(5), 578–588. 10.1111/een.12135 [DOI] [Google Scholar]
- Lease, H. M. , & Wolf, B. O. (2011). Lipid content of terrestrial arthropods in relation to body size, phylogeny, ontogeny and sex. Physiological Entomology, 36(1), 29–38. 10.1111/j.1365-3032.2010.00767.x [DOI] [Google Scholar]
- Lee, J. C. , Dreves, A. J. , Cave, A. M. , Kawai, S. , Isaacs, R. , Miller, J. C. , Van Timmeren, S. , & Bruck, D. J. (2015). Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: Drosophilidae). Annals of the Entomological Society of America, 108(2), 117–129. 10.1093/aesa/sau014 [DOI] [Google Scholar]
- Li, K.‐M. , Au, L. Y. C. , Douglah, D. , Chong, A. , White, B. J. , Ferree, P. M. , & Akbari, O. S. (2017). Generation of heritable germline mutations in the jewel wasp Nasonia vitripennis using CRISPR/Cas9. Scientific Reports, 7(1), 901. 10.1038/s41598-017-00990-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, K.‐M. , Ren, L.‐Y. , Zhang, Y.‐J. , Wu, K.‐M. , & Guo, Y.‐Y. (2012). Knockdown of microplitis mediator odorant receptor involved in the sensitive detection of two chemicals. Journal of Chemical Ecology, 38(3), 287–294. 10.1007/s10886-012-0085-y [DOI] [PubMed] [Google Scholar]
- Lof, M. E. , De Gee, M. , Dicke, M. , Gort, G. , & Hemerik, L. (2013). Exploitation of chemical signaling by parasitoids: Impact on host population dynamics. Journal of Chemical Ecology, 39(6), 752–763. 10.1007/s10886-013-0298-8 [DOI] [PubMed] [Google Scholar]
- Losos, J. B. (2000). Ecological character displacement and the study of adaptation. Proceedings of the National Academy of Sciences of the United States of America, 97(11), 5693–5695. 10.1073/pnas.97.11.5693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue, C.‐H. , Borowy, D. , Buffington, M. L. , & Leips, J. (2018). Geographic and seasonal variation in species diversity and community composition of frugivorous Drosophila (Diptera: Drosophilidae) and their Leptopilina (hymenoptera: Figitidae) parasitoids. Environmental Entomology, 47(5), 1096–1106. 10.1093/ee/nvy114 [DOI] [PubMed] [Google Scholar]
- Lue, C.‐H. , Buffington, M. L. , Scheffer, S. , Lewis, M. , Elliott, T. A. , Lindsey, A. R. I. , Driskell, A. , Jandova, A. , Kimura, M. T. , Carton, Y. , Kula, R. R. , Schlenke, T. A. , Mateos, M. , Govind, S. , Varaldi, J. , Guerrieri, E. , Giorgini, M. , Wang, X. , Hoelmer, K. , … Hrcek, J. (2021). DROP: Molecular voucher database for identification of Drosophila parasitoids. Molecular Ecology Resources, 21(7), 2437–2454. 10.1111/1755-0998.13435 [DOI] [PubMed] [Google Scholar]
- Lue, C.‐H. , Driskell, A. C. , Leips, J. , & Buffington, M. L. (2016). Review of the genus Leptopilina (hymenoptera, Cynipoidea, Figitidae, Eucoilinae) from the eastern United States, including three newly described species. Journal of Hymenoptera Research, 53, 35–76. 10.3897/jhr.53.10369 [DOI] [Google Scholar]
- Lynch, J. (2006). A method for parental RNA interference in the wasp Nasonia vitripennis . Nature Protocols, 1(1), 486–494. 10.1038/nprot.2006.70 [DOI] [PubMed] [Google Scholar]
- Lynch, Z. R. , Schlenke, T. A. , Morran, L. T. , & de Roode, J. C. (2017). Ethanol confers differential protection against generalist and specialist parasitoids of Drosophila melanogaster . PLoS ONE, 12(7), e0180182. 10.1371/journal.pone.0180182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, Z. R. , Schlenke, T. A. , & Roode, J. C. (2016). Evolution of behavioural and cellular defences against parasitoid wasps in the Drosophila melanogaster subgroup. Journal of Evolutionary Biology, 29(5), 1016–1029. 10.1111/jeb.12842 [DOI] [PubMed] [Google Scholar]
- Mackay, T. F. C. , Richards, S. , Stone, E. A. , Barbadilla, A. , Ayroles, J. F. , Zhu, D. , Casillas, S. , Han, Y. , Magwire, M. M. , Cridland, J. M. , Richardson, M. F. , Anholt, R. R. H. , Barrón, M. , Bess, C. , Blankenburg, K. P. , Carbone, M. A. , Castellano, D. , Chaboub, L. , Duncan, L. , … Gibbs, R. A. (2012). The Drosophila melanogaster genetic reference panel. Nature, 482(7384), 173–178. 10.1038/nature10811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markow, T. A. , & O'Grady, P. M. (2005). Evolutionary genetics of reproductive behavior in Drosophila: Connecting the dots. Annual Review of Genetics, 39(1), 263–291. 10.1146/annurev.genet.39.073003.112454 [DOI] [PubMed] [Google Scholar]
- Markow, T. A. , & O'Grady, P. M. (2008). Reproductive ecology of Drosophila . Functional Ecology, 22(5), 747–759. 10.1111/j.1365-2435.2008.01457.x [DOI] [Google Scholar]
- Markus, R. , Laurinyecz, B. , Kurucz, E. , Honti, V. , Bajusz, I. , Sipos, B. , Somogyi, K. , Kronhamn, J. , Hultmark, D. , & Ando, I. (2009). Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster . Proceedings of the National Academy of Sciences of the United States of America, 106(12), 4805–4809. 10.1073/pnas.0801766106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzetto, F. , Marchetti, E. , Amiresmaeili, N. , Sacco, D. , Francati, S. , Jucker, C. , Dindo, M. L. , Lupi, D. , & Tavella, L. (2016). Drosophila parasitoids in northern Italy and their potential to attack the exotic pest Drosophila suzukii . Journal of Pest Science, 89(3), 837–850. 10.1007/s10340-016-0746-7 [DOI] [Google Scholar]
- Meiners, T. (2003). Associative learning of complex odours in parasitoid host location. Chemical Senses, 28(3), 231–236. 10.1093/chemse/28.3.231 [DOI] [PubMed] [Google Scholar]
- Mery, F. , & Kawecki, T. J. (2005). A cost of long‐term memory in Drosophila . Science, 308(5725), 1148. 10.1126/science.1111331 [DOI] [PubMed] [Google Scholar]
- Milan, N. F. , Kacsoh, B. Z. , & Schlenke, T. A. (2012). Alcohol consumption as self‐medication against blood‐borne parasites in the fruit fly. Current Biology, 22(6), 488–493. 10.1016/j.cub.2012.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui, H. , & Kimura, M. T. (2010). Distribution, abundance and host association of two parasitoid species attacking frugivorous drosophilid larvae in Central Japan. European Journal of Entomology, 107(4), 535–540. 10.14411/eje.2010.061 [DOI] [Google Scholar]
- Mitsui, H. , Van Achterberg, K. , Nordlander, G. , & Kimura, M. T. (2007). Geographical distributions and host associations of larval parasitoids of frugivorous Drosophilidae in Japan. Journal of Natural History, 41(25–28), 1731–1738. 10.1080/00222930701504797 [DOI] [Google Scholar]
- Moiroux, J. , Boivin, G. , & Brodeur, J. (2015). Temperature influences host instar selection in an aphid parasitoid: Support for the relative fitness rule. Biological Journal of the Linnean Society, 115(4), 792–801. 10.1111/bij.12545 [DOI] [Google Scholar]
- Moiroux, J. , Le Lann, C. , Seyahooei, M. A. , Vernon, P. , Pierre, J.‐S. , van Baaren, J. , & van Alphen, J. J. M. (2010). Local adaptations of life‐history traits of a Drosophila parasitoid, Leptopilina boulardi: Does climate drive evolution? Ecological Entomology, 35(6), 727–736. 10.1111/j.1365-2311.2010.01233.x [DOI] [Google Scholar]
- Morales, J. , Chiu, H. , Oo, T. , Plaza, R. , Hoskins, S. , & Govind, S. (2005). Biogenesis, structure, and immune‐suppressive effects of virus‐like particles of a Drosophila parasitoid, Leptopilina victoriae . Journal of Insect Physiology, 51(2), 181–195. 10.1016/j.jinsphys.2004.11.002 [DOI] [PubMed] [Google Scholar]
- Moreau, S. J. M. , Cherqui, A. , Doury, G. , Dubois, F. , Fourdrain, Y. , Sabatier, L. , Bulet, P. , Saarela, J. , Prévost, G. , & Giordanengo, P. (2004). Identification of an aspartylglucosaminidase‐like protein in the venom of the parasitic wasp Asobara tabida (hymenoptera: Braconidae). Insect Biochemistry and Molecular Biology, 34(5), 485–492. 10.1016/j.ibmb.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Mortimer, N. T. (2013). Parasitoid wasp virulence: A window into fly immunity. Fly, 7(4), 242–248. 10.4161/fly.26484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton, L. (2004). Diversité et densité bactériennes dans les symbioses à infections multiples: Régulation et effets sur l'hôte, cas des associations Wolbachia‐insectes. Université Claude Bernard‐Lyon, 1. [Google Scholar]
- Mouton, L. , Henri, H. , Bouletreau, M. , & Vavre, F. (2003). Strain‐specific regulation of intracellular Wolbachia density in multiply infected insects. Molecular Ecology, 12(12), 3459–3465. 10.1046/j.1365-294X.2003.02015.x [DOI] [PubMed] [Google Scholar]
- Mouton, L. , Henri, H. , Boulétreau, M. , & Vavre, F. (2005). Multiple infections and diversity of cytoplasmic incompatibility in a haplodiploid species. Heredity, 94(2), 187–192. 10.1038/sj.hdy.6800596 [DOI] [PubMed] [Google Scholar]
- Mouton, L. , Henri, H. , Charif, D. , Boulétreau, M. , & Vavre, F. (2007). Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biological Letters, 3(2), 210–213. 10.1098/rsbl.2006.0590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton, L. , Henri, H. , & Fleury, F. (2009). Interactions between coexisting intracellular genomes: Mitochondrial density and Wolbachia infection. Applied Environmental Microbiology, 75(7), 1916–1921. 10.1128/AEM.02677-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, D. , Giron, D. , Desouhant, E. , Rey, B. , Casas, J. , Lefrique, N. , & Visser, B. (2017). Maternal age affects offspring nutrient dynamics. Journal of Insect Physiology, 101, 123–131. 10.1016/j.jinsphys.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Nappi, A. J. (1970). Defense reactions of Drosophila euronotus larvae against the Hymenopterous parasite Pseudeucoila bochei . Journal of Invertebrate Pathology, 16, 408–418. 10.1016/0022-2011(70)90160-6 [DOI] [PubMed] [Google Scholar]
- Nappi, A. J. (1975). Cellular immune reactions of larvae of Drosophila algonquin . Parasitology, 70, 189–194. 10.1017/S0031182000049659 [DOI] [PubMed] [Google Scholar]
- Nappi, A. J. (2010). Cellular immunity and pathogen strategies in combative interactions involving Drosophila hosts and their endoparasitic wasps. Invertebrate Survival Journal, 7(2), 198–210. [Google Scholar]
- Nappi, A. J. , & Silvers, M. (1984). Cell surface changes associated with cellular immune reactions in Drosophila . Science, 225, 1166–1168. 10.1126/science.6433482 [DOI] [PubMed] [Google Scholar]
- Nappi, A. J. , & Streams, F. A. (1969). Haemocytic reactions of Drosophila melanogaster to the parasites Pseudocoila mellipes and P. bochei . Journal of Insect Physiology, 15(9), 1551–1566. 10.1016/0022-1910(69)90175-9 [DOI] [Google Scholar]
- Nomano, F. Y. , Kasuya, N. , Matsuura, A. , Suwito, A. , Mitsui, H. , Buffington, M. L. , & Kimura, M. T. (2017). Genetic differentiation of Ganaspis brasiliensis (hymenoptera: Figitidae) from east and Southeast Asia. Applied Entomological Zoology, 52, 429–437. 10.1007/s13355-017-0493-0 [DOI] [Google Scholar]
- Nordlander, G. (1980). Revision of the genus Leptopilina Förster, 1869, with notes on the status of some other genera (hymenoptera, Cynipoidea: Eucoilidae). Insect Systematics and Evolution, 11(4), 428–453. 10.1163/187631280794710024 [DOI] [Google Scholar]
- Novkovic, B. , Mitsui, H. , Suwito, A. , & Kimura, M. T. (2011). Taxonomy and phylogeny of Leptopilina species (hymenoptera: Cynipoidea: Figitidae) attacking frugivorous drosophilid flies in Japan, with description of three new species. Entomological Science, 14(3), 333–346. 10.1111/j.1479-8298.2011.00459.x [DOI] [Google Scholar]
- O'Grady, P. M. , & DeSalle, R. (2018). Phylogeny of the genus Drosophila . Genetics, 209(1), 1–25. 10.1534/genetics.117.300583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaj, D. R. , Snellen, H. , Swaans, K. , & Vet, L. E. M. (1994). Unrewarding experiences and their effect on foraging in the parasitic wasp Leptopilina heterotoma (hymenoptera: Eucoilidae). Journal of Insect Behaviour, 7(4), 465–481. 10.1007/BF02025444 [DOI] [Google Scholar]
- Papaj, D. R. , & Vet, L. E. M. (1990). Odor learning and foraging success in the parasitoid, Leptopilina heterotoma . Journal of Chemical Ecology, 16(11), 3137–3150. 10.1007/BF00979616 [DOI] [PubMed] [Google Scholar]
- Paredes, J. C. , Herren, J. K. , Schüpfer, F. , & Lemaitre, B. (2016). The role of lipid competition for endosymbiont‐mediated protection against parasitoid wasps in Drosophila . mBio, 7(4), 1–8. 10.1128/mBio.01006-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patot, S. , Allemand, R. , Fleury, F. , & Varaldi, J. (2012). An inherited virus influences the coexistence of parasitoid species through behaviour manipulation: A symbiont mediates interspecific competition. Ecology Letters, 15(6), 603–610. 10.1111/j.1461-0248.2012.01774.x [DOI] [PubMed] [Google Scholar]
- Pfeiffer, L. , Ruther, J. , Hofferberth, J. , & Stökl, J. (2018). Interference of chemical defence and sexual communication can shape the evolution of chemical signals. Scientific Reports, 8(1), 321. 10.1038/s41598-017-18376-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, G. J. , & Ollason, J. G. (1987). Eight reasons why optimal foraging theory is a complete waste of time. Oikos, 49(1), 111–117. 10.2307/3565560 [DOI] [Google Scholar]
- Poirié, M. , Carton, Y. , & Dubuffet, A. (2009). Virulence strategies in parasitoid hymenoptera as an example of adaptive diversity. Comptes Rendus Biologies, 332(2–3), 311–320. 10.1016/j.crvi.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Poirié, M. , Colinet, D. , & Gatti, J.‐L. (2014). Insights into function and evolution of parasitoid wasp venoms. Current Opinion in Insect Science, 6, 52–60. 10.1016/j.cois.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Pompanon, F. , Fouillet, P. , & Bouletreau, M. (1995). Emergence rhythms and protandry in relation to daily patterns of locomotor activity in Trichogramma species. Evolutionary Ecology, 9(5), 467–477. 10.1007/bf01237829 [DOI] [Google Scholar]
- Poyet, M. , Havard, S. , Prevost, G. , Chabrerie, O. , Doury, G. , Gibert, P. , & Eslin, P. (2013). Resistance of Drosophila suzukii to the larval parasitoids Leptopilina heterotoma and Asobara japonica is related to haemocyte load. Physiological Entomology, 38(1), 45–53. 10.1111/phen.12002 [DOI] [Google Scholar]
- Poyet, M. , Le Roux, V. , Gibert, P. , Meirland, A. , Prévost, G. , Eslin, P. , & Chabrerie, O. (2015). The wide potential trophic niche of the asiatic fruit fly Drosophila suzukii: The key of its invasion success in temperate Europe? PLoS ONE, 10(11), e0142785. 10.1371/journal.pone.0142785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, P. W. (1974). Strategies for egg production. Evolution, 28(1), 76–84. 10.1111/j.1558-5646.1974.tb00728.x [DOI] [PubMed] [Google Scholar]
- Prud'homme, B. , & Gompel, N. (2010). Genomic hourglass. Nature, 468(7325), 768–769. 10.1038/468768a [DOI] [PubMed] [Google Scholar]
- Ramroop, J. R. , Heavner, M. E. , Razzak, Z. H. , & Govind, S. (2021). A parasitoid wasp of Drosophila employs preemptive and reactive strategies to deplete its host's blood cells. PLoS Pathogens, 17(5), e1009615. 10.1371/journal.ppat.1009615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychoudhury, R. , & Werren, J. H. (2012). Host genotype changes bidirectional to unidirectional cytoplasmic incompatibility in Nasonia longicornis . Heredity, 108(p), 105–114. 10.1038/hdy.2011.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renou, M. (2014). Chapter 2: Pheromones and general odor perception in insects. In Mucignat‐Caretta C. (Ed.), Neurobiology of chemical communication. CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Ridley, M. (1988). Mating frequency and fecundity in insects. Biological Reviews, 63(4), 509–549. 10.1111/j.1469-185X.1988.tb00669.x [DOI] [Google Scholar]
- Ridley, M. (1993). Clutch size and mating frequency in parasitic hymenoptera. The American Naturalist, 142, 893–910. 10.1086/285579 [DOI] [Google Scholar]
- Ris, N. , Allemand, R. , Fouillet, P. , & Fleury, F. (2004). The joint effect of temperature and host species induce complex genotype‐by‐environment interactions in the larval parasitoid of Drosophila, Leptopilina heterotoma (hymenoptera: Figitidae). Oikos, 106(3), 451–456. 10.1111/j.0030-1299.2004.13274.x [DOI] [Google Scholar]
- Rizki, R. M. , & Rizki, T. M. (1991). Effects of lamellolysin from a parasitoid wasp on Drosophila blood cells in vitro. Journal of Experimental Zoology, 257(2), 236–244. 10.1002/jez.1402570214 [DOI] [PubMed] [Google Scholar]
- Rizki, T. M. , Rizki, R. M. , & Carton, Y. (1990). Leptopilina heterotoma and L. boulardi: Strategies to avoid cellular defense responses of Drosophila melanogaster . Experimental Parasitology, 70(4), 466–475. 10.1016/0014-4894(90)90131-U [DOI] [PubMed] [Google Scholar]
- Roff, D. A. (2001). Life history, evolution. Encyclopedia of Biodiversity, 4, 631–641. 10.1016/B978-0-12-384719-5.00087-3 [DOI] [Google Scholar]
- Roitberg, B. D. , Mangel, M. , Lalonde, R. G. , Roitberg, C. A. , van Alphen, J. J. M. , & Vet, L. (1992). Seasonal dynamic shifts in patch exploitation by parasitic wasps. Behavioral Ecology, 3(2), 156–165. 10.1093/beheco/3.2.156 [DOI] [Google Scholar]
- Ronquist, F. (1995). Phylogeny and early evolution of the Cynipoidea (hymenoptera). Systematic Entomology, 205(4), 309–335. 10.1111/j.1365-3113.1995.tb00099.x [DOI] [Google Scholar]
- Ronquist, F. (1999). Phylogeny, classification and evolution of the Cynipoidea. Zoologica Scripta, 28(1–2), 139–164. 10.1046/j.1463-6409.1999.00022.x [DOI] [Google Scholar]
- Rossi Stacconi, M. V. , Buffington, M. , Daane, K. M. , Dalton, D. T. , Grassi, A. , Kaçar, G. , Miller, B. , Miller, J. C. , Baser, N. , Ioriatti, C. , Walton, V. M. , Wiman, N. G. , Wang, X. , & Anfora, G. (2015). Host stage preference, efficacy and fecundity of parasitoids attacking Drosophila suzukii in newly invaded areas. Biological Control, 84, 28–35. 10.1016/j.biocontrol.2015.02.003 [DOI] [Google Scholar]
- Rossi Stacconi, M. V. , Panel, A. , Baser, N. , Ioriatti, C. , Pantezzi, T. , & Anfora, G. (2017). Comparative life history traits of indigenous Italian parasitoids of Drosophila suzukii and their effectiveness at different temperatures. Biological Control, 112, 20–27. 10.1016/j.biocontrol.2017.06.003 [DOI] [Google Scholar]
- Rouault, J. (1979). Rôle des parasites entomophages dans la compétition entre espèces jumelles de Drosophiles. Comptes Rendus de l'Académies des Sciences Paris, 289, 643–646. [Google Scholar]
- Ruschioni, S. , van Loon, J. J. A. , Smid, H. M. , & van Lenteren, J. C. (2015). Insects can count: Sensory basis of host discrimination in parasitoid wasps revealed. PLoS ONE, 10(10), e0138045. 10.1371/journal.pone.0138045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson‐Boshuizen, M. , Bakker, K. , & van Lenteren, J. C. (1974). Success of parasitization of Pseudeucoila bochei weld (Hym., Cynip.): A matter of experience. Netherlands Journal of Zoology, 24(1), 67–85. 10.1163/002829674X00174 [DOI] [Google Scholar]
- Schlenke, T. A. , Morales, J. , Govind, S. , & Clark, A. G. (2007). Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster . PLoS Pathogens, 3, 1486–1501. 10.1371/journal.ppat.0030158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen, L. M. , Ris, N. , Driss, L. , Racca, A. , Girod, P. , Warot, S. , Borowiec, N. , Toševski, I. , & Kenis, M. (2020). Evidence for a cryptic parasitoid species reveals its suitability as a biological control agent. Scientific Reports, 10(1), 19096. 10.1038/s41598-020-76180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shropshire, J. D. , Leigh, B. , & Bordenstein, S. R. (2020). Symbiont‐mediated cytoplasmic incompatibility: What have we learned in 50 years? eLife, 9, e61989. 10.7554/eLife.61989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, M. T. T. P. , Suverkropp, B. P. , Vet, L. E. M. , & Moed, G. (1992). Comparison of learning in related generalist and specialist eucoilid parasitoids. Entomologia Experimentalis et Applicata, 64(2), 117–124. 10.1111/j.1570-7458.1992.tb01601.x [DOI] [Google Scholar]
- Sinclair, B. J. , & Marshall, K. E. (2018). The many roles of fats in overwintering insects. Journal of Experimental Biology, 221(Suppl_1), jeb161836. 10.1242/jeb.161836 [DOI] [PubMed] [Google Scholar]
- Siva‐Jothy, M. T. , Moret, Y. , & Rolff, J. (2005). Insect immunity: An evolutionary ecology perspective. Advances in Insect Physiology, 32, 1–48. 10.1016/s0065-2806(05)32001-7 [DOI] [Google Scholar]
- Sobhy, I. S. , Goelen, T. , Herrera‐Malaver, B. , Verstrepen, K. J. , Wäckers, F. , Jacquemyn, H. , & Lievens, B. (2019). Associative learning and memory retention of nectar yeast volatiles in a generalist parasitoid. Animal Behaviour, 153, 137–146. 10.1016/j.anbehav.2019.05.006 [DOI] [Google Scholar]
- Stökl, J. , & Herzner, G. (2016). Morphology and ultrastructure of the allomone and sex‐pheromone producing mandibular gland of the parasitoid wasp Leptopilina heterotoma (hymenoptera: Figitidae). Arthropod Structure & Development, 45(4), 333–340. 10.1016/j.asd.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Stökl, J. , Hofferberth, J. , Pritschet, M. , Brummer, M. , & Ruther, J. (2012). Stereoselective chemical defense in the Drosophila parasitoid Leptopilina heterotoma is mediated by (−)‐Iridomyrmecin and (+)‐Isoiridomyrmecin. Journal of Chemical Ecology, 38(4), 331–339. 10.1007/s10886-012-0103-0 [DOI] [PubMed] [Google Scholar]
- Stökl, J. , Machacek, Z. , & Ruther, J. (2015). Behavioural flexibility of the chemical defence in the parasitoid wasp Leptopilina heterotoma . The Science of Nature, 102(11–12), 67. 10.1007/s00114-015-1317-0 [DOI] [PubMed] [Google Scholar]
- Stökl, J. , & Steiger, S. (2017). Evolutionary origin of insect pheromones. Current Opinion in Insect Science, 24, 36–42. 10.1016/j.cois.2017.09.004 [DOI] [PubMed] [Google Scholar]
- Streams, F. A. (1968). Defense reactions of Drosophila species (Diptera: Drosophilidae) to the parasite Pseudeucoila bochei (hymenoptera: Cynipidae). Annals of the Entomological Society of America, 61(1), 158–164. 10.1093/aesa/61.1.158 [DOI] [Google Scholar]
- van Strien‐van Liempt, W. T. F. H. (1983). The competition between Asobara tabida Nees von Esenbeck, 1834 and Leptopilina Heterotoma (Thomson, 1862) in multiparasitized hosts. Netherlands Journal of Zoology, 33(2), 125–163. 10.1163/002829683X00066 [DOI] [Google Scholar]
- van Strien‐van Liempt, W. T. F. H. , & van Alphen, J. J. M. (1981). The absence of interspecific host discrimination in Asobara tabida Nees and Leptopilina heterotoma (Thomson), coexisting larval parasitoids of Drosophila species. Netherlands Journal of Zoology, 31(4), 701–712. 10.1163/002829681X00239 [DOI] [Google Scholar]
- Struck, T. H. , Feder, J. L. , Bendiksby, M. , Birkeland, S. , Cerca, J. , Gusarov, V. I. , Kistenich, S. , Larsson, K.‐H. , Liow, L. H. , Nowak, M. D. , Stedje, B. , Bachmann, L. , & Dimitrov, D. (2018). Finding evolutionary processes hidden in cryptic species. Trends in Ecology and Evolution, 33(3), 153–163. 10.1016/j.tree.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Sunday, J. M. , Bates, A. E. , & Dulvy, N. K. (2012). Thermal tolerance and the global redistribution of animals. Nature Climate Change, 2, 686–690. 10.1038/nclimate1539 [DOI] [Google Scholar]
- Ugur, B. , Chen, K. , & Bellen, H. J. (2016). Drosophila tools and assays for the study of human diseases. Disease Models & Mechanisms, 9(3), 235–244. 10.1242/dmm.023762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen, J. J. M. , Eebes, H. , van Lenteren, J. C. , & Nell, H. W. (1984). The response of a polyphagous parasitoid (Leptopilina heterotoma (Thomson)) to a kairomone produced by one of its hosts (Drosophila melanogaster Meigen). Netherlands Journal of Zoology, 34(2), 215–219. 10.1163/002829684X00164 [DOI] [Google Scholar]
- van Alphen, J. J. M. , & Janssen, A. R. M. (1982). Host selection by Asobara tabida Nees (Braconidae; Alysiinae) a larval parasitoid of fruit inhabiting Drosophila species. Netherlands Journal of Zoology, 32(2), 215–231. 10.5281/zenodo.805740 [DOI] [Google Scholar]
- van Alphen, J. J. M. , & Thunnissen, I. (1983). Host selection and sex allocation by Pachycrepoideus vindemiae Rondani (Pteromalidae) as a facultative hyperparasitoid of Asobara tabida Nees (Braconidae; Alysiinae) and Leptopilina heterotoma (Cynipoidea; Eucoilidae). Netherlands Journal of Zoology, 33(4), 497–514. 10.1163/002829683X00228 [DOI] [Google Scholar]
- van Alphen, J. J. M. , van Dijken, M. J. , & Waage, J. K. (1987). A functional approach to superparasitism: Host discrimination needs not be learnt by. Netherlands Journal of Zoology, 37(2), 167–179. 10.1163/002829686X00045 [DOI] [Google Scholar]
- van Alphen, J. J. M. , & Vet, L. (1986). An evolutionary approach to host finding and selection. In Waage J. K. & Greathead D. J. (Eds.), Insect parasitoids (pp. 23–61). Academic Press. [Google Scholar]
- van Alphen, J. J. M. , & Visser, M. E. (1990). Superparasitism as an adaptive strategy for insect parasitoids. Annual Review of Entomology, 35, 59–79. 10.1146/annurev.en.35.010190.000423 [DOI] [PubMed] [Google Scholar]
- van Batenburg, F. H. D. , Bakker, K. , van Lenteren, J. C. , & van Alphen, J. J. M. (1983). Searching for and parasitization of larvae of Drosophila melanogaster (Dipt.: Drosophilidae) by Leptopilina heterotoma (Hym.: Eucoilidae): A Monte Carlo simulation model and the real situation. Netherlands Journal of Zoology, 33(3), 306–336. 10.1163/002829683X00156 [DOI] [Google Scholar]
- van den Assem, J. (1969). Reproductive behaviour of Pseudeucoila bochei (hymenoptera: Cynipidae). Netherlands Journal of Zoology, 19(4), 641–648. 10.1163/002829669X00080 [DOI] [Google Scholar]
- van der Hoeven, R. , & Vet, L. E. M. (1984). Comparison of the behavioural response of two Leptopilina species (hymenoptera: Eucoilidae), living in different microhabitats, to kairomone of their host (Drosophilidae). Netherlands Journal of Zoology, 34(2), 220–227. 10.1163/002829684X00173 [DOI] [Google Scholar]
- van Lenteren, J. C. (1972). Contact‐chemoreceptors on the ovipositor of Pseudeucoila bochei Weld (Cynipidae). Netherlands Journal of Zoology, 22(3), 347–350. 10.1163/002829672X00158 [DOI] [Google Scholar]
- van Lenteren, J. C. (1976). The development of host discrimination and the prevention of superparasitism in the parasite Pseudeucoila bochei Weld (Hym.: Cynipidae). Netherlands Journal of Zoology, 26(1), 1–83. 10.1163/002829676X00055 [DOI] [Google Scholar]
- van Lenteren, J. C. (1991). Encounters with parasitized hosts: To leave or not to leave a patch. Netherlands Journal of Zoology, 41(2–3), 144–157. 10.1163/156854291X00090 [DOI] [Google Scholar]
- van Lenteren, J. C. , & Bakker, K. (1975). Discrimination between parasitised and unparasitised hosts in the parasitic wasp Pseudeucoila bochei: A matter of learning. Nature, 254(5499), 417–419. 10.1038/254417a0 [DOI] [PubMed] [Google Scholar]
- van Lenteren, J. C. , & Bakker, K. (1978). Behavioural aspects of the functional responses of a parasite (Pseudeucoila Bochei Weld) to its host (Drosophila melanogaster). Netherlands Journal of Zoology, 28(2), 213–233. 10.1163/002829678X00062 [DOI] [Google Scholar]
- van Lenteren, J. C. , Isidoro, N. , & Bin, F. (1998). Functional anatomy of the ovipositor clip in the parasitoid Leptopilina heterotoma (Thompson) (hymenoptera: Eucoilidae), a structure to grip escaping host larvae. International Journal of Insect Morphology and Embryology, 27(3), 263–268. 10.1016/S0020-7322(98)00019-1 [DOI] [Google Scholar]
- van Lenteren, J. C. , Ruschioni, S. , Romani, R. , van Loon, J. J. A. , Qiu, Y. T. , Smid, H. M. , Isidoro, N. , & Bin, F. (2007). Structure and electrophysiological responses of gustatory organs on the ovipositor of the parasitoid Leptopilina heterotoma . Arthropod Structure & Development, 36(3), 271–276. 10.1016/j.asd.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Varaldi, J. , Fouillet, P. , Boulétreau, M. , & Fleury, F. (2005). Superparasitism acceptance and patch‐leaving mechanisms in parasitoids: A comparison between two sympatric wasps. Animal Behaviour, 69(6), 1227–1234. 10.1016/j.anbehav.2004.09.012 [DOI] [Google Scholar]
- Vavre, F. , Dedeine, F. , Quillon, M. , Fouillet, P. , Fleury, F. , & Boulétreau, M. (2001). Within‐species diversity of Wolbachia‐induced cytoplasmic incompatibility in haplodiploid insects. Evolution, 55(8), 1710–1714. 10.1111/j.0014-3820.2001.tb00691.x [DOI] [PubMed] [Google Scholar]
- Vavre, F. , Fleury, F. , Lepetit, D. , Fouillet, P. , & Bouletreau, M. (1999). Phylogenetic evidence for horizontal transmission of Wolbachia in host‐parasitoid associations. Molecular Biology and Evolution, 16(12), 1711–1723. 10.1093/oxfordjournals.molbev.a026084 [DOI] [PubMed] [Google Scholar]
- Vavre, F. , Fleury, F. , Varaldi, J. , Fouillet, P. , & Bouleatreau, M. (2000). Evidence for female mortality in Wolbachia‐mediated cytoplasmic incompatibility in haplodiploid insects: Epidemiologic and evolutionary consequences. Evolution, 54(1), 191–200. 10.1111/j.0014-3820.2000.tb00019.x [DOI] [PubMed] [Google Scholar]
- Vayssade, C. , Martel, V. , Moiroux, J. , Fauvergue, X. , van Alphen, J. J. M. , & van Baaren, J. (2012). The response of life‐history traits to a new species in the community: A story of Drosophila parasitoids from the Rhône and Saône valleys: Shift of life‐history trade‐off. Biological Journal of the Linnean Society, 107(1), 153–165. 10.1111/j.1095-8312.2012.01918.x [DOI] [Google Scholar]
- Veerkamp, F. A. (1982). Genetic variation in the pattern of initial oviposition behaviour of Leptopilina heterotoma Thomson (=Pseudeucoila bochei weld), a parasite of Drosophila melanogaster . Netherlands Journal of Zoology, 32(1), 88–107. 10.1163/002829682X00067 [DOI] [Google Scholar]
- Vet, L. E. M. , & Bakker, K. (1985). A comparative functional approach to the host detection behaviour of parasitic wasps. 2. A quantitative study on eight eucoilid species. Oikos, 44(3), 487–498. 10.2307/3565790 [DOI] [Google Scholar]
- Vet, L. E. M. , De Jong, A. G. , Franchi, E. , & Papaj, D. R. (1998). The effect of complete versus incomplete information on odour discrimination in a parasitic wasp. Animal Behaviour, 55(5), 1271–1279. 10.1006/anbe.1997.0686 [DOI] [PubMed] [Google Scholar]
- Vet, L. E. M. , & Groenewold, A. W. (1990). Semiochemicals and learning in parasitoids. Journal of Chemical Entomology, 16(11), 3119–3135. 10.1007/BF00979615 [DOI] [PubMed] [Google Scholar]
- Vet, L. E. M. , Janse, C. , van Achterberg, C. , & van Alphen, J. J. M. (1984). Microhabitat location and niche segregation in two sibling species of Drosophilid parasitoids: Asobara tabida (Nees) and A. rufescens (Foerster) (Braconidae: Alysiinae). Oecologia, 61(2), 182–188. 10.1007/BF00396757 [DOI] [PubMed] [Google Scholar]
- Vet, L. E. M. , & Papaj, D. R. (1992). Effects of experience on parasitoid movement in odour plumes. Physiological Entomology, 17(1), 90–96. 10.1111/j.1365-3032.1992.tb00994.x [DOI] [Google Scholar]
- Vet, L. E. M. , & Schoonman, G. (1988). The influence of previous foraging experience on microhabitat acceptance in Leptopilina heterotoma . Journal of Insect behaviour, 1(4), 387–392. 10.1007/BF01054501 [DOI] [Google Scholar]
- Vet, L. E. M. , Sokolowski, M. B. , MacDonald, D. E. , & Snellen, H. (1993). Responses of a generalist and a specialist parasitoid (hymenoptera: Eucoilidae) to Drosophilid larval kairomones. Journal of Insect Behaviour, 6(5), 615–624. 10.1007/BF01048127 [DOI] [Google Scholar]
- Vet, L. E. M. , & van Opzeeland, K. (1985). Olfactory microhabitat selection in Leptopilina heterotoma (Thomson) (Hym.: Eucoilidae), a parasitoid of Drosophilidae. Netherlands Journal of Zoology, 35(3), 497–504. 10.1163/002829685X00352 [DOI] [Google Scholar]
- Visser, B. , Alborn, H. T. , Rondeaux, S. , Haillot, M. , Hance, T. , Rebar, D. , Riederer, J. M. , Tiso, S. , van Eldijk, T. J. B. , Weissing, F. J. , & Nieberding, C. M. (2021). Phenotypic plasticity explains apparent reverse evolution of fat synthesis in parasitic wasps. Scientific Reports, 11, 7751. 10.1038/s41598-021-86736-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser, B. , & Ellers, J. (2008). Lack of lipogenesis in parasitoids: A review of physiological mechanisms and evolutionary implications. Journal of Insect Physiology, 54(9), 1315–1322. 10.1016/j.jinsphys.2008.07.014 [DOI] [PubMed] [Google Scholar]
- Visser, B. , Hance, T. , Noël, C. , Pels, C. , Kimura, M. T. , Stökl, J. , Geuverink, E. , & Nieberding, C. M. (2018). Variation in lipid synthesis, but genetic homogeneity, among Leptopilina parasitic wasp populations. Ecology and Evolution, 8, 7355–7364. 10.1002/ece3.4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser, B. , Le Lann, C. , den Blanken, F. J. , Harvey, J. A. , van Alphen, J. J. M. , & Ellers, J. (2010). Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. Proceedings of the National Academy of Sciences of the United States of America, 107(19), 8677–8682. 10.1073/pnas.1001744107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser, B. , Le Lann, C. , Nieberding, C. M. , Lammers, M. , Hahn, D. A. , Alborn, H. T. , Enriquez, T. , Scheifler, M. , Harvey, J. A. , & Ellers, J. (2022). Why do many parasitoids lack adult triglyceride accumulation, despite functioning fatty acid biosynthesis machinery? EcoEvoRxiv. 10.32942/osf.io/zpf4j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser, B. , Le Lann, C. , Snaas, H. , Hardy, I. , & Harvey, J. (2014). Consequences of resource competition for sex allocation and discriminative behaviors in a hyperparasitoid wasp. Behavioral Ecology and Sociobiology, 68, 105–113. 10.1007/s00265-013-1627-1 [DOI] [Google Scholar]
- Visser, B. , Roelofs, D. , Hahn, D. A. , Teal, P. E. A. , Mariën, J. , & Ellers, J. (2012). Transcriptional changes associated with lack of lipid synthesis in parasitoids. Genome Biology and Evolution, 4(8), 864–874. 10.1093/gbe/evs065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser, M. E. (1993). Adaptive self‐ and conspecific superparasitism in the solitary parasitoid Leptopilina heterotoma (hymenoptera: Eucoilidae). Behavioral Ecology, 4(1), 22–28. 10.1093/beheco/4.1.22 [DOI] [Google Scholar]
- Visser, M. E. (1995). The effect of competition on oviposition decisions of Leptopilina heterotoma (hymenoptera: Eucoilidae). Animal Behaviour, 49(6), 1677–1687. 10.1016/0003-3472(95)90089-6(6) [DOI] [Google Scholar]
- Visser, M. E. , Luyckx, B. , Nell, H. W. , & Boskamp, G. J. F. (1992). Adaptive superparasitism in solitary parasitoids: Marking of parasitized hosts in relation to the pay‐off from superparasitism. Ecological Entomology, 17(1), 76–82. 10.1111/j.1365-2311.1992.tb01042.x [DOI] [Google Scholar]
- Visser, M. E. , van Alpen, J. J. M. , & Nell, H. K. (1990). Adaptive superparasitism and patch time allocation in solitary parasitoids: the influence of the number of parasitoids depleting patch. Behaviour, 114(164), 23–35. [Google Scholar]
- Visser, M. E. , van Alphen, J. J. M. , & Nell, H. W. (1992). Adaptive superparasitism and patch time allocation in solitary parasitoids: The influence of pre‐patch experience. Behavioural Ecology and Sociobiology, 31(3), 163–171. 10.1007/BF00168643 [DOI] [Google Scholar]
- Vuarin, P. , Allemand, R. , Moiroux, J. , van Baaren, J. , & Gibert, P. (2012). Geographic variations of life history traits and potential trade‐offs in different populations of the parasitoid Leptopilina heterotoma . Naturwissenschaften, 99(11), 903–912. 10.1007/s00114-012-0972-7 [DOI] [PubMed] [Google Scholar]
- Wachi, N. , Nomano, F. Y. , Mitsui, H. , Kasuya, N. , & Kimura, M. T. (2015). Taxonomy and evolution of putative thelytokous species of Leptopilina (hymenoptera: Figitidae) from Japan, with description of two new species: Thelytokous Leptopilina wasps from Japan. Entomological Science, 18(1), 41–54. 10.1111/ens.12089 [DOI] [Google Scholar]
- Wältermann, M. , & Steinbüchel, A. (2005). Neutral lipid bodies in prokaryotes: Recent insights into structure, formation, and relationship to eukaryotic lipid depots. Journal of Bacteriology, 187(11), 3607–3619. 10.1128/JB.187.11.3607-3619.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Wang, L. , Liu, J. , Zhang, D. , & Liu, T. (2021). Multiple mating of Aphelinus asychis enhance the number of female progeny but shorten the longevity. Insects, 12(9), 823. 10.3390/insects12090823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, I. , Hofferberth, J. , Ruther, J. , & Stökl, J. (2015). Varying importance of cuticular hydrocarbons and iridoids in the species‐specific mate recognition pheromones of three closely related Leptopilina species. Frontiers in Ecology and Evolution, 3, 19. 10.3389/fevo.2015.00019 [DOI] [Google Scholar]
- Weiss, I. , Rössler, T. , Hofferberth, J. , Brummer, M. , Ruther, J. , & Stökl, J. (2013). A nonspecific defensive compound evolves into a competition avoidance cue and a female sex pheromone. Nature Communications, 4, 2767. 10.1038/ncomms3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, I. , Ruther, J. , & Stökl, J. (2015). Species specificity of the putative male antennal aphrodisiac pheromone in Leptopilina heterotoma, Leptopilina boulardi, and Leptopilina victoriae . BioMed Research International, 2015, 202965. 10.1155/2015/202965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weld, L. H. (1944). Description of new Cynipidae including two new genera (hymenoptera). Proceedings of the Entomological Society of Washington, 46(p), 55–66. [Google Scholar]
- Werren, J. (2009). Larval RNAi in Nasonia (parasitoid wasp). Cold Spring Harbor Protocols, 2009(10), 1–6. 10.1101/pdb.prot5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren, J. H. , Windsor, D. , & Guo, L. (1995). Distribution of Wolbachia among neotropical arthropods. Proceedings of the Royal Society of London, 262(1364), 197–204. 10.1098/rspb.1995.0196 [DOI] [Google Scholar]
- Wertheim, B. (2022). Adaptations and counter‐adaptations in Drosophila host‐parasitoid interactions: Advances in the molecular mechanisms. Current Opinion in Insect Science, 51, 100896. 10.1016/j.cois.2022.100896 [DOI] [PubMed] [Google Scholar]
- Wertheim, B. , Allemand, R. , Vet, L. E. M. , & Dicke, M. (2006). Effects of aggregation pheromone on individual behaviour and food web interactions: A field study on Drosophila . Ecological Entomology, 31(3), 216–226. 10.1111/j.1365-2311.2006.00757.x [DOI] [Google Scholar]
- Wertheim, B. , Vet, L. E. M. , & Dicke, M. (2003). Increased risk of parasitism as ecological costs of using aggregation pheromones: Laboratory and field study of Drosophila‐Leptopilina interaction. Oikos, 100(2), 269–282. 10.1034/j.1600-0706.2003.11579.x [DOI] [Google Scholar]
- Wey, B. , Heavner, M. E. , Wittmeyer, K. T. , Briese, T. , Hopper, K. R. , & Govind, S. (2020). Immune suppressive extracellular vesicle proteins of Leptopilina heterotoma are encoded in the wasp genome. G3, 10(1), 1–12. 10.1534/g3.119.400349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerke, J. S. C. , Dicke, M. , & Vet, L. E. M. (1993). Larval parasitoid uses aggregation pheromone of adult hosts in foraging behaviour: A solution to the reliability‐detectability problem. Oecologia, 93(1), 145–148. 10.1007/BF00321204 [DOI] [PubMed] [Google Scholar]
- Wyatt, T. D. (2010). Pheromones and signature mixtures: Defining species‐wide signals and variable cues for identity in both invertebrates and vertebrates. Journal of Comparative Physiology A, 196(10), 685–700. 10.1007/s00359-010-0564-y [DOI] [PubMed] [Google Scholar]
- Wyatt, T. D. (2014). Pheromones and animal behavior: Chemical signals and signature mixtures. Cambridge University Press. 10.1017/CBO9781139030748 [DOI] [Google Scholar]
- Xie, J. , Butler, S. , Sanchez, G. , & Mateos, M. (2014). Male killing Spiroplasma protects Drosophila melanogaster against two parasitoid wasps. Heredity, 112(4), 399–408. 10.1038/hdy.2013.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J. , Tiner, B. , Vilchez, I. , & Mateos, M. (2011). Effect of the Drosophila endosymbiont Spiroplasma on parasitoid wasp development and on the reproductive fitness of wasp‐attacked fly survivors. Evolutionary Ecology, 25(5), 1065–1079. 10.1007/s10682-010-9453-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J. , Vilchez, I. , & Mateos, M. (2010). Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma . PLoS ONE, 5(8), e12149. 10.1371/journal.pone.0012149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L. , Qiu, L. , Fang, Q. , Stanley, D. W. , & Ye, G. (2020). Cellular and humoral immune interactions between Drosophila and its parasitoids. Insect Sci., 28(5), 1208–1227. 10.1111/1744-7917.12863 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Qian, B. , Ilyas, A. , Gong, X. , Xu, J. , Liu, P. , & Hu, H. (2022). Influence of parasitoid states on the propensity to enter and the stay in a patch. Journal of Insect Behaviour, 35, 56–64. 10.1007/s10905-022-09799-z [DOI] [Google Scholar]
- Zhou, W. , Rousset, F. , & O'Neill, S. (1998). Phylogeny and PCR‐based classification of Wolbachia strains using wsp gene sequences. Proceedings of the Royal Society of London, 265(1395), 509–515. 10.1098/rspb.1998.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


