Abstract
Introduction:
Maxillary premolars, may be more susceptible to fracture due to their anatomy; especially when there is loss of tooth structure. Therefore, it is necessary to evaluate materials and techniques that may increase fracture resistance during and post root canal treatment. This in vitro study aimed to evaluate root fracture resistance of maxillary premolars when filled with three root canal sealers as well as whether this resistance would be increased by passive ultrasonic irrigation (PUI).
Methods and Materials:
Sixty-four maxillary premolars with two roots were randomly divided into one negative control group (intact canals; n = 8), one positive control group (instrumented, unsealed canals; n = 8), and six experimental groups (n = 8), which were instrumented with ProTaper Next rotary system up to X2 file and subdivided according to final irrigation (with or without PUI) and type of sealer used (AH-Plus [AH], MTA Fillapex [MTA], or EndoSequence BC Sealer [ES]). The specimens were subjected to fracture strength test in a universal testing machine at a speed of 1 mm/min until fracture. The maximum force required to induce fracture was recorded (N). The Kruskal-Wallis test and DUNN test were used for analysis.
Results:
The lowest force required to cause root fracture was observed in the positive control group (310.48 ± 54.08 N); this was significantly different from the other groups (P < 0.05). There was no significant difference between experimental groups obturated with the same sealer, whether with or without PUI (AH with PUI: 558.80 ± 87.12 N; AH without PUI: 508.75 ± 97.55 N; MTA with PUI: 507.27 ± 174.55 N; MTA without PUI: 516.69 ± 96.56 N; ES with PUI: 526.76 ± 143.97 N; ES without PUI: 628.40 ± 94.74 N) (P > 0.05). There was also no significant difference between the experimental groups and the negative control group (P > 0.05).
Conclusions:
In this in vitro study PUI did not increase the fracture resistance of maxillary premolars, while AH Plus, MTA Fillapex, EndoSequence sealers increased fracture resistance of instrumented root canals.
Key Words: Endodontically Treated Teeth, Fracture Strength, Root Canal Obturation, Root Canal Preparation, Smear Layer
Introduction
Endodontically treated maxillary premolars with narrow roots in the mesiodistal direction are prone to longitudinal root fractures [1-3]. A number of factors can affect root fracture resistance, including excessive tooth structure removal, dentin dehydration, access cavity preparation, root canal irrigation and instrumentation, excessive pressure during obturation, and root canal preparation for the placement of intraradicular posts [4].
Irrigation is an essential step in the treatment, because it promotes the elimination of microorganisms and the removal of debris and smear layer from the root canal system. Smear layer prevents the penetration of intracanal medicament and sealer into dentinal tubules and negatively affects the adhesion of the filling material to the canal wall [5]. It is composed of organic and inorganic substances, fragments of odontoblastic processes, microorganisms and their byproducts, and necrotic tissue [6].
Syringe irrigation is a standard procedure, although it is a limited method. Therefore, other techniques have been suggested and the activation of the irrigant should be considered as it results in better cleaning of the canals, increasing tissue dissolution and significantly reducing the numbers of bacteria [7].
Passive ultrasonic irrigation (PUI) is considered an effective adjuvant for final cleaning by increasing the effectiveness of the irrigant in the removal of smear layer and debris from inaccessible areas. In addition, acoustic microstreaming and hydrodynamic cavitation contribute to an increased effectiveness of the irrigant [5, 8]. The efficacy of additional cleaning protocols, such as PUI, in increasing the fracture strength of endodontically treated teeth by increasing root canal cleanliness, dentin permeability, penetration of the sealer into the dentinal tubules, and mechanical bonding strength remains to be clarified.
After cleaning and shaping of the root canal system, filling is required to prevent infiltration of fluids and microorganisms into the cervical and apical regions. The most commonly used filling material is gutta-percha cone associated with root canal sealer [9]. Sealers are capable of filling the voids between gutta-percha cones and the gap between gutta-percha and dentinal canal walls [9]. It has been suggested that the adhesion power of the sealer to the root canal dentin surface can maintain the integrity of the cement-dentin interface during mechanical stress, thus increasing fracture strength. In addition, sealer penetration into dentinal tubules is desirable, because it improves bonding between dentin and sealer, increases sealing ability and mechanical blockage [10, 11].
The MTA Fillapex (Angelus, Londrina, Brazil) is a mineral trioxide aggregate (MTA)-based sealer. It has high radiopacity, low solubility when in contact with tissue fluids, low expansion during setting, and excellent viscosity for insertion [10]. AH-Plus (Dentsply, Konstanz, Germany) is an epoxy-based root canal sealer. It is often used in research for comparison with other sealers because of its good physicochemical capacity and adhesion to the root canal walls [12]. EndoSequence BC Sealer (Brasseler, Savannah, GA, USA) is a premixed bioceramic root canal sealer. It has high biocompatibility, antimicrobial activity and sealing ability, high pH (12.8), high radiopacity and produce hydroxyapatite crystals during setting time, creating a chemical bond between the filling material and root dentine [13].
The purpose of this in vitro study was to evaluate root fracture resistance of maxillary premolars filled with gutta-percha and AH-Plus, MTA Fillapex or EndoSequence root canal sealers and whether this resistance would be increased by PUI. The null hypothesis was that there would be no significant difference between the sealers and final irrigation protocols in increasing root fracture resistance.
Materials and Methods
Specimen selection and preparation
The protocol for this study was approved by the Research Ethics Committee of São Leopoldo Mandic (approval number: 2019 462).
A total of 64 extracted human maxillary premolars were selected and stored in saline solution until use to preserve humidity of dentinal tubules, but no longer than 6 months after extraction. Periapical mesiodistal and buccopalatal radiographs were taken to analyze the internal anatomy and only teeth with two roots and two straight root canals (one buccal and one palatal) were used. Teeth with root caries, cracks, internal or external resorption, calcified root canals or endodontic treatment were excluded from the study. All teeth were inspected under an operating microscope under 25× magnification (Alliance, São Carlos, Brazil).
The crowns of all the teeth were removed by Zekrya drill (Dentsply MailleferBallaigues, Switzerland) with a high-speed rotary handpiece (Kavo do Brasil, Joinvile, Brazil) under copious water irrigation to adjust the lengh of the roots to a standardized length of 13 mm. To standardize tooth size, only teeth with a mesiodistal length of 6 (±1) mm and a buccopalatal length of 8.5 (±1) mm, measured at the cementoenamel junction with a caliper, were selected.
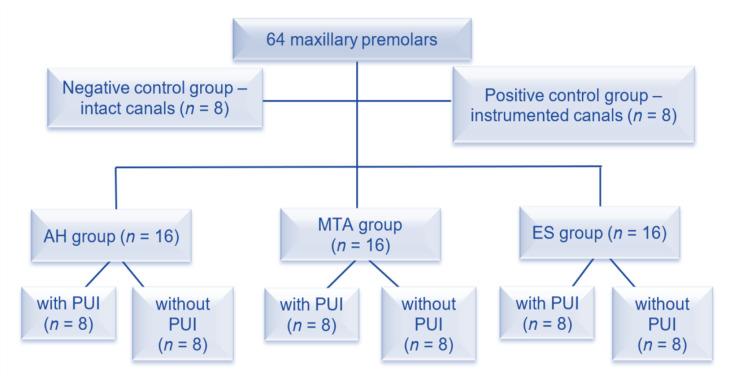
The teeth were randomly divided into one negative control group (intact root canals; n = 8), one positive control group (instrumented, unsealed root canals; n = 8), and three experimental groups (n = 16) according to the sealer used (AH-Plus [AH], MTA Fillapex [MTA], and EndoSequence [ES]), which were then divided into six subgroups each n = 8; according to the final irrigation protocol used before root canal filling (with PUI or without PUI), as shown in Figure 1.
Figure 1.
Distribution of control and experimental groups has been shown (AH: AH-Plus sealer, MTA: MTA Fillapex sealer, ES: EndoSequence BC Sealer, PUI: passive ultrasonic irrigation)
Root canals, except for those in the negative control group, were first explored with #10 and #15 K-files. The canals were instrumented with ProTaper Next rotary system (Dentsply, Maillefer, Ballaigues, Switzerland) up to X2 file (25/0.06) driven by an X-Smart engine (Dentsply Maillefer, Ballaigues, Switzerland), operating at 300 rpm and torque of 3 N/cm. Preflaring was performed with ProTaper Next X1 file (17/0.04), 5 mm short of the initial tooth length. Working length was measured visually by introducing a #10 K-file into the root canal until its tip became visible at the apical foramen. The working length was determined 1 mm short of this measurement. The canals were then instrumented with X1 and X2 files at the working length. At each instrument change, apical patency was confirmed with a #10 K-file 1 mm passing through the foramen. Irrigation was performed with 5 mL of 2.5% sodium hypochlorite (NaOCl) (Biofarm, Vitória, Brazil) using a disposable syringe and 0.55×20 needle.
After chemical mechanical preparation, in the groups with PUI, the canals were again irrigated with 5 mL of 2.5% NaOCl, agitated with a TRA 31 T ultrasonic insert (Trinks, São Paulo, Brazil), driven by a Jet Sonic BP ultrasound system (Gnatus, São Paulo, Brazil), with 20% output power, 2 mm short of the working length, for 20 sec, and aspirated with a 0.014-inch-diameter capillary tip. The smear layer was then removed by irrigation of the canal with 5 mL of 17% ethylenediaminetetraacetic acid (EDTA) (Biofarm, Vitória, Brazil), followed by agitation with the ultrasonic insert for 20 sec and aspiration. The canals were again irrigated with 5 mL of 2.5% NaOCl, agitated with the ultrasonic insert for 20 sec, and aspirated with the capillary tip. The irrigation solutions were changed and agitated at 3 cycles of 20 sec each, totaling 1 min, as recommended by van der Sluis et al. [14]. The canals were dried with sterile absorbent paper points.
In the groups without PUI, after irrigation with NaOCl, the canals were irrigated with 17% EDTA and again with NaOCl, without agitation, based on the same sequence of steps, time and volume used in the groups with PUI. As the groups with agitation, the irrigation solutions were changed at 3 cycles of 20 sec each, totaling 1 min. The canals were dried with sterile absorbent paper points.
All teeth were filled with Fine Medium gutta-percha cones (Odous de Deus, Juiz de Fora, Brazil) calibrated until apical adjustment to the corresponding diameter of the X2 file, at the working length, by the single-cone technique. The sealers AH-Plus and MTA Fillapex were mixed according to the manufacturer, in equal volumes (1:1) of pastes A and B on a mixing pad to a homogenous consistency and introduced into the canals with Lentulo (Dentsply, Maillefer, Ballaigues, Switzerland). The EndoSequence BC Sealer was introduced into the root canal through its intracanal tip. The canals were then filled with the sealer, and the previously calibrated cones were inserted. Then the cone was cut off at the level of the orifice and lightly condensed with a plugger. Periapical radiographs were performed to evaluate the quality of the obturation. The access cavities were sealed with Z100 composite resin (3M ESPE, Seefeld, Germany). The teeth were stored at 37°C in 100% humidity for 30 days to allow for sealer setting.
Preparation for the mechanical test
To simulate the periodontal ligament space, the apical 5 mm of the roots were covered with melted wax (Lisanda, São Paulo, Brazil) until a 0.2 to 0.3-mm thick layer was obtained, measured by inserting a periodontal probe into the wax, before being embedded in acrylic resin for the fracture strength test. Copper rings were filled with self-curing acrylic resin (Jet, São Paulo, Brazil), where the teeth were mounted vertically, exposing 8 mm of the cervical third of the root canal. As soon as polymerization of the acrylic resin started, the roots were removed from the resin and the wax was cleaned from the root surfaces by using a curette. The root surfaces were then covered with a thin layer of polyvinylsiloxane impression material (Coltene, Whaledent, Altstätten, Switzerland) and again embedded in the acrylic resin (Jet, Clássico, São Paulo, Brazil).
Mechanical testing was performed in an EMIC DL 2000 universal testing machine (EMIC Ensaio de Materiais Indústria e Comércio, São José dos Pinhais, Brazil), with Trd 26 load cell and compression of 2000 kgf, at a speed of 1 mm/min. The top plate contained a 3-mm-diameter spherical metal tip. The tip was centered on the roots, and vertical forces were applied in an increasing manner (1 mm/min) until fracture. Fracture was defined as the moment at which a sudden drop in force was observed on the testing machine monitor. The maximum force required to fracture each specimen was recorded in Newtons (N).
Statistical analysis
Data were analyzed by the Kruskal-Wallis test in order to evaluate possible differences between the groups and DUNN test was used for pairwise comparison of the groups. Statistical analysis was performed using SPSS software, version 24.0 (SPSS, Chicago, IL, USA). The level of significance was set at 5% for all analyses.
Results
The mean (SD), maximum and minimum fracture strength values obtained for all eight groups are shown in Table 1.
Table 1.
The mean (SD), maximum and minimum values of fracture resistance of teeth
| Groups | Mean (SD) | Minimum | Maximum |
|---|---|---|---|
| Negative Control | 611.65 (166.14) | 300.94 | 787.24 |
| Positive Control | 310.48 (54.08) | 241.67 | 402.06 |
| AH with PUI | 558.80 (87.12) | 377.06 | 648.40 |
| AH without PUI | 508.75 (97.55) | 333.36 | 618.80 |
| MTA with PUI | 507.27 (174.55) | 317.15 | 763.28 |
| MTA without PUI | 516.69 (96.56) | 365.08 | 657.56 |
| ES with PUI | 526.76 (143.97) | 238.92 | 683.84 |
| ES without PUI | 628.40 (94.74) | 496.16 | 758.34 |
AH: AH-Plus sealer, MTA: MTA Fillapex sealer, ES: EndoSequence BC Sealer, PUI: passive ultrasonic irrigation
The lowest force required to fracture the specimens was observed in the positive control group, with a significant difference when compared to the experimental groups (P<0.05) and to the negative control group (P=0.003). Among the experimental groups, there was no significant difference between the groups obturated with the same sealer, whether with or without PUI (P>0.05). There was also no significant difference between the experimental groups and the negative control group (P>0.05).
The fracture pattern found in all groups was a vertical fracture extending from the buccal to the palatal aspect.
Discussion
The present in vitro study showed that the roots of maxillary premolars filled with EndoSequence BC, AH-Plus and MTA Fillapex sealers had their fracture resistance increased, when compared to the positive control group, where the roots were only instrumented, and this resistance was not changed when the irrigating substances were agitated by the PUI technique, to promote better cleaning of the canal walls and consequent better penetration of the sealer in the dentinal tubules [15].
For this research, the specimens were standardized only in height and mesiodistal and buccopalatal widths. Ertas et al. [16] concluded that, for fracture resistance studies, the volume and weight should also be standardized and Katanec et al. [17] reported that the age of the specimens can also influence the results, as well as the storage period and the immersion solution. The immersion solution used in this study was saline solution, resembling the natural environment in the body. The sample storage period for this study was about 6 months, longer than another studies [17-19]. Other solutions may change the microhardness of dentin, such as de-ionized water and sodium hypochlorite [17, 20].
ProTaper Next files up to X2 file were used for instrumentation of the root canals so as not to extrapolate the clinical approach. Some studies have investigated the formation of microcracks during the preparation of root canals. Katanec et al. [17] evaluated, by means of micro-computed tomography (micro-CT), microcrack formation after root canal preparation with Self-adjunting File, Reciproc Blue and ProTaper Next instruments on young premolars and concluded that the instruments do not cause dentinal microcracks. Stringheta et al. [21] compared the methods of micro-CT and cross-sectioning followed by stereomicroscopy in assessing dentinal defects after instrumentation with Reciproc, ProTaper Next, Wave One Gold and ProDesign Logic. None of the evaluated instrumentation systems led to the formation of new dentin defects. All of the defects identified in the stereomicroscopic analysis were already present before instrumentation, or were absent at both time points in the micro-CT analysis, indicating that the formation of new defects resulted from the sectioning procedure performed before stereomicroscopy and not from instrumentation.
As irrigant solution, 2.5% sodium hypochlorite was used to remove debris and tissue dissolution at each file change and in the final cleaning. 17% EDTA was used to remove smear layer for 1 min to prevent affecting the dentin microhardeness and the modulus of elasticity [20]. This finding has relevance for the present methodology. Some authors have evaluated the influence of tooth fracture resistance after some irrigation regimes. Lantigua Domínguez et al. [19] evaluated the effects of saline solution, 2.5% NaOCl+17% EDTA, 2% chlorhexidine (CHX) gel+17% EDTA and a mixture of 5% NaOCl+18% hydroxyethylidene bisphosphonate (HEBP) on susceptibility to root fracture resistance and concluded that the mixture of 5% NaOCl+18% HEBP resulted in a lower fracture resistance. Turk et al. [22] compared distilled water (DW), 5% EDTA+2.5% NaOCl, 17% EDTA+2.5% NaOCl, 5% EDTA+2.5% NaOCL+DW+2% CHX, 17% EDTA+2.5% NaOCl+DW+2% CHX and concluded that intra-canal CHX rinse of EDTA/NaOCl treated root dentine enhanced the fracture resistance of roots. Sungur et al. [23] evaluated 5.25% NaOCl and 2% CHX and concluded that final irrigation with NaOCl reduced the fracture resistance. 5.25% NaOCl significantly decreases the elastic modulus and flexural strength of human dentin because of the proteolytic action of concentrated hypochlorite on the collagen matrix of dentin [20].
A more effective cleaning of the dentin walls attributed to the agitation of the irrigating substances leads to greater penetration of the sealers into the dentinal tubules, which is considered potentially beneficial because it increases the interface between the material and dentin wall [12]. Mohammadian et al. [24] evaluated the dentin-sealer interface in three different sealers using scanning electron microscopy and concluded that BC Sealer and AH-Plus had less gaps than Dorifill in coronal area, despite not having agitated the irrigating substance in their methodology. This can increase mechanical retention through the interconnection of the cement plugs inside the tubules [25] and increase the cement-dentin connection, thereby improving sealing capacity and cement retention, which can be acquired mechanically [8].
One of the purposes of this study was to evaluate whether improved root canal cleaning attributed to PUI [6, 26-28] would increase root fracture resistance as a result of better penetration of the sealer into the dentinal tubules although some studies indicate that other agitation techniques promote better cleaning of the apical third [5, 29-31]. Zand et al. [15] compared the effects of final agitation of 5.25% NaOCl with XP-finisher file, PUI, Er: YAG laser and conventional needle irrigation on the penetration of a bioceramic sealer into dentinal tubules and concluded that the PUI technique resulted in deeper penetration of the sealer. Our results showed no difference between the experimental groups. PUI did not contribute to increase the fracture resistance of the roots, despite promoting better removal of debris and smear layer, consequently improving the penetration of sealer in dentinal tubules, favoring its antimicrobial action and improving the interface between filling material and dentin [32, 33]. Previous studies with the same methodology were not found in the literature for direct comparisons. However, adjuvant cleaning methods, such as self-adjusting file (SAF), PUI, photon-induced photoacoustic streaming (PIPS), and erbium-doped yttrium aluminum garnet (Er: YAG) or neodymium-doped yttrium aluminum garnet (Nd: YAG) lasers, have been shown not to alter root fracture resistance when used as additional cleaning techniques to remove residual filling material during retreatment [34].
In the present study, the specimens were filled with gutta-percha and three different sealers. Sealers in the form of a powder and liquid were avoided because of their larger particle size could influence their penetration in to the dentinal tubules [4]. All specimens fractured vertically from the buccal to the palatal aspect, in agreement with the fracture pattern observed in previous studies [8, 35]. The direction of the force applied can influence the direction of the fractures, reason why fractures in the buccopalatal direction are commonly observed in studies that apply vertical forces [36]. Although forces applied in ex vivo studies cannot be completely simulated as in clinical situations, standardization of the force applied in all experimental groups is necessary to allow for comparison between the root-strengthening capacity of different sealing materials [4]. The type of sealer used did not affect the type of fracture, but the application of forces at different angles along the long axis of the tooth would better simulate the occlusal forces in clinical conditions [10].
Despite the lack of significant differences between the experimental groups and the negative control group to support the hypothesis that the sealers reinforce the dentin structure, the same does not apply to the comparison with the positive control group. There was a decrease in fracture strength when the teeth were instrumented but not obturated. Although, clinically, this situation does not really occur in endodontic treatment, this group was extremely important for the perception of a decrease in root fracture resistance against tooth wear resulting from instrumentation [10, 11, 36]. When comparing the experimental groups, there was no difference between the groups when the roots were filled with different sealers. The same result was observed by Sagsen et al. [10], comparing the fracture resistance of roots filled with AH-Plus, iRoot SP and MTA Fillapex. Dibaji et al. [18] assessed the fracture resistance of roots following the application of different sealers including Epiphany, iRoot sealer and AH-Plus and concluded that the different sealers did not change the fracture resistance of roots compared to unprepared root canals. However, in contrast to our findings, some studies shown different results. The difference between these results and ours may be due to difference in methodologies. The dental group and the endodontic sealers used to fill the canals were not the same. Ghoneim et al. [37] concluded that iRoot SP increased the resistance to the fracture of roots, when compared to ActiV GP. In the study by Kazandag et al. [38] roots filled with AH-Plus+Gutta-percha had greater resistance to fracture than Resilon+Epiphany, ActiV GP cone+ActiV GP sealer and ActiV GP sealer+Gutta-percha. Topçuoglu et al. [4] concluded that EndoSequence BC and AH-Plus increased the force to fracture mandibular premolars, when compared to Tech Biosealer.
Conclusion
Within the limitations of an in vitro study, it can be concluded that PUI did not increase root fracture resistance of maxillary premolars. Moreover, AH-Plus, EndoSequence, and MTA Fillapex sealers reinforced endodontically treated roots, when compared with roots that had only been instrumented, and these sealers/fillers had no significant differences between them. This confirms the need for an adequate filling of the root canal system.
Conflict of Interest:
‘None declared’.
References
- 1.Jafari Navimipour E, Ebrahimi Chaharom ME, Alizadeh Oskoee P, Mohammadi N, Bahari M, Firouzmandi M. Fracture resistance of endodontically-treated maxillary premolars restored with composite resin along with glass fiber insertion in different positions. J Dent Res Dent Clin Dent Prospects. 2012;6(4):125–30. doi: 10.5681/joddd.2012.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammadi N, Kahnamoii MA, Yeganeh PK, Navimipour EJ. Effect of fiber post and cusp coverage on fracture resistance of endodontically treated maxillary premolars directly restored with composite resin. J Endod. 2009;35(10):1428–32. doi: 10.1016/j.joen.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S, Berman LH, Blanco L, Bakland L, Kim JS. A demographic analysis of vertical root fractures. J Endod. 2006;32(12):1160–3. doi: 10.1016/j.joen.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Topcuoglu HS, Tuncay O, Karatas E, Arslan H, Yeter K. In vitro fracture resistance of roots obturated with epoxy resin-based, mineral trioxide aggregate-based, and bioceramic root canal sealers. J Endod. 2013;39(12):1630–3. doi: 10.1016/j.joen.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 5.Saber Sel D, Hashem AA. Efficacy of different final irrigation activation techniques on smear layer removal. J Endod. 2011;37(9):1272–5. doi: 10.1016/j.joen.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Blank-Goncalves LM, Nabeshima CK, Martins GH, Machado ME. Qualitative analysis of the removal of the smear layer in the apical third of curved roots: conventional irrigation versus activation systems. J Endod. 2011;37(9):1268–71. doi: 10.1016/j.joen.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt TF, Teixeira CS, Felippe MC, Felippe WT, Pashley DH, Bortoluzzi EA. Effect of ultrasonic activation of irrigants on smear layer removal. J Endod. 2015;41(8):1359–63. doi: 10.1016/j.joen.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Martins Justo A, Abreu da Rosa R, Santini MF, Cardoso Ferreira MB, Pereira JR, Hungaro Duarte MA, Reis So MV. Effectiveness of final irrigant protocols for debris removal from simulated canal irregularities. J Endod. 2014;40(12):2009–14. doi: 10.1016/j.joen.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Lee KW, Williams MC, Camps JJ, Pashley DH. Adhesion of endodontic sealers to dentin and gutta-percha. J Endod. 2002;28(10):684–8. doi: 10.1097/00004770-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Sagsen B, Ustun Y, Pala K, Demirbuga S. Resistance to fracture of roots filled with different sealers. Dent Mater J. 2012;31(4):528–32. [PubMed] [Google Scholar]
- 11.Celikten B, Uzuntas CF, Gulsahi K. Resistance to fracture of dental roots obturated with different materials. Biomed Res Int. 2015;2015:591031. doi: 10.1155/2015/591031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akcay M, Arslan H, Durmus N, Mese M, Capar ID. Dentinal tubule penetration of AH Plus, iRoot SP, MTA fillapex, and guttaflow bioseal root canal sealers after different final irrigation procedures: A confocal microscopic study. Lasers Surg Med. 2016;48(1):70–6. doi: 10.1002/lsm.22446. [DOI] [PubMed] [Google Scholar]
- 13.Candeiro GT, Correia FC, Duarte MA, Ribeiro-Siqueira DC, Gavini G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J Endod. 2012;38(6):842–5. doi: 10.1016/j.joen.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 14.van der Sluis LW, Vogels MP, Verhaagen B, Macedo R, Wesselink PR. Study on the influence of refreshment/activation cycles and irrigants on mechanical cleaning efficiency during ultrasonic activation of the irrigant. J Endod. 2010;36(4):737–40. doi: 10.1016/j.joen.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Zand V, Milani AS, Yavari HR, Majidi AR. The effects of different agitation techniques of canal irrigant on tubular penetration of a bioceramic sealer. Iran Endod J. 2019;14(4):289–95. doi: 10.22037/iej.v14i4.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ertas H, Sagsen B, Arslan H, Er O, Ertas ET. Effects of physical and morphological properties of roots on fracture resistance. Eur J Dent. 2014;8(2):261–4. doi: 10.4103/1305-7456.130631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katanec T, Miletić I, Baršić G, Kqiku-Bliblikaj L, Žižak M, Krmek SJ. Incidence of dentinal micro cracks during root canal preparation with self adjusting file, reciproc blue, and protaper next. Iran Endod J. 2020;15(1):6–11. doi: 10.22037/iej.v15i1.26667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dibaji F, Afkhami F, Bidkhori B, Kharazifard MJ. Fracture Resistance of Roots after Application of Different Sealers. Iran Endod J. 2017;12(1):50–4. doi: 10.22037/iej.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lantigua Dominguez MC, Feliz Pedrinha V, Oliveira Athaide da Silva LC, Soares Ribeiro ME, Loretto SC, de Almeida Rodrigues P. Effects of Different Irrigation Solutions on Root Fracture Resistance: An in Vitro Study. Iran Endod J. 2018;13(3):367–72. doi: 10.22037/iej.v13i3.19247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zehnder M. Root canal irrigants. J Endod. 2006;32(5):389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Stringheta CP, Pelegrine RA, Kato AS, Freire LG, Iglecias EF, Gavini G, Bueno C. Micro-computed Tomography versus the Cross-sectioning Method to Evaluate Dentin Defects Induced by Different Mechanized Instrumentation Techniques. J Endod. 2017;43(12):2102–7. doi: 10.1016/j.joen.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Turk T, Kaval ME, Sarikanat M, Hulsmann M. Effect of final irrigation procedures on fracture resistance of root filled teeth: an ex vivo study. Int Endod J. 2017;50(8):799–804. doi: 10.1111/iej.12680. [DOI] [PubMed] [Google Scholar]
- 23.Sungur DD, Altundasar E, Uzunoglu E, Yilmaz Z. Influence of different final irrigation regimens and various endodontic filling materials on vertical root fracture resistance. Niger J Clin Pract. 2016;19(2):267–71. doi: 10.4103/1119-3077.164334. [DOI] [PubMed] [Google Scholar]
- 24.Mohammadian F, Farahanimastary F, Dibaji F, Kharazifard MJ. Scanning Electron Microscopic Evaluation of the Sealer-Dentine Interface of Three Sealers. Iran Endod J. 2017;12(1):38–42. doi: 10.22037/iej.2017.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shokouhinejad N, Sabeti M, Gorjestani H, Saghiri MA, Lotfi M, Hoseini A. Penetration of Epiphany, Epiphany self-etch, and AH Plus into dentinal tubules: a scanning electron microscopy study. J Endod. 2011;37(9):1316–9. doi: 10.1016/j.joen.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 26.van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40(6):415–26. doi: 10.1111/j.1365-2591.2007.01243.x. [DOI] [PubMed] [Google Scholar]
- 27.Leoni GB, Versiani MA, Silva-Sousa YT, Bruniera JF, Pecora JD, Sousa-Neto MD. Ex vivo evaluation of four final irrigation protocols on the removal of hard-tissue debris from the mesial root canal system of mandibular first molars. Int Endod J. 2017;50(4):398–406. doi: 10.1111/iej.12630. [DOI] [PubMed] [Google Scholar]
- 28.Al-Jadaa A, Paque F, Attin T, Zehnder M. Acoustic hypochlorite activation in simulated curved canals. J Endod. 2009;35(10):1408–11. doi: 10.1016/j.joen.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Kato AS, Cunha RS, da Silveira Bueno CE, Pelegrine RA, Fontana CE, de Martin AS. Investigation of the efficacy of passive ultrasonic irrigation versus irrigation with reciprocating activation: an environmental scanning electron microscopic study. J Endod. 2016;42(4):659–63. doi: 10.1016/j.joen.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Duque JA, Duarte MA, Canali LC, Zancan RF, Vivan RR, Bernardes RA, Bramante CM. Comparative effectiveness of new mechanical irrigant agitating devices for debris removal from the canal and isthmus of mesial roots of mandibular molars. J Endod. 2017;43(2):326–31. doi: 10.1016/j.joen.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Karade P, Chopade R, Patil S, Hoshing U, Rao M, Rane N, Chopade A, Kulkarni A. Efficiency of different endodontic irrigation and activation systems in removal of the smear layer: a scanning electron microscopy study. Iran Endod J. 2017;12(4):414–8. doi: 10.22037/iej.v12i4.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kocak S, Bagci N, Cicek E, Turker SA, Can Saglam B, Kocak MM. Influence of passive ultrasonic irrigation on the efficiency of various irrigation solutions in removing smear layer: a scanning electron microscope study. Microsc Res Tech. 2017;80(5):537–42. doi: 10.1002/jemt.22829. [DOI] [PubMed] [Google Scholar]
- 33.Kokkas AB, Boutsioukis A, Vassiliadis LP, Stavrianos CK. The influence of the smear layer on dentinal tubule penetration depth by three different root canal sealers: an in vitro study. J Endod. 2004;30(2):100–2. doi: 10.1097/00004770-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Kamalak A, Uzun I, Arslan H, Keles A, Doganay E, Keskin C, Akcay M. Fracture resistance of endodontically retreated roots after retreatment using self-adjusting file, passive ultrasonic irrigation, photon-induced photoacoustic streaming, or laser. Photomed Laser Surg. 2016;34(10):467–72. doi: 10.1089/pho.2016.4121. [DOI] [PubMed] [Google Scholar]
- 35.Lertchirakarn V, Timyam A, Messer HH. Effects of root canal sealers on vertical root fracture resistance of endodontically treated teeth. J Endod. 2002;28(3):217–9. doi: 10.1097/00004770-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Zamin C, Silva-Sousa YT, Souza-Gabriel AE, Messias DF, Sousa-Neto MD. Fracture susceptibility of endodontically treated teeth. Dent Traumatol. 2012;28(4):282–6. doi: 10.1111/j.1600-9657.2011.01087.x. [DOI] [PubMed] [Google Scholar]
- 37.Ghoneim AG, Lutfy RA, Sabet NE, Fayyad DM. Resistance to fracture of roots obturated with novel canal-filling systems. J Endod. 2011;37(11):1590–2. doi: 10.1016/j.joen.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Kazandag MK, Sunay H, Tanalp J, Bayirli G. Fracture resistance of roots using different canal filling systems. Int Endod J. 2009;42(8):705–10. doi: 10.1111/j.1365-2591.2009.01571.x. [DOI] [PubMed] [Google Scholar]