Abstract
A 17-year-old male elite athlete presented for evaluation after an abnormal pre-competitive college screening electrocardiogram. Subsequent evaluation revealed the presence of hypertrophic cardiomyopathy. He remained asymptomatic throughout four years of follow-up. Through shared decision making, he continued to play competitively and is now a professional athlete. (Level of Difficulty: Advanced.)
Key Words: competitive athlete, hypertrophic cardiomyopathy, shared decision making
Abbreviations and Acronyms: CMR, cardiac magnetic resonance imaging; ECG, electrocardiogram; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; LGE, late gadolinium enhancement; LVOT, left ventricular outflow tract; SCD, sudden cardiac death; SDM, shared decision making; TTE, transthoracic echocardiography
Central Illustration
History of Presentation
Both the patient and his family have given their written consent to the presentation and publication of this case in this deidentified format.
Learning Objectives
-
•
To understand risk stratification and indications for prophylactic ICD in hypertrophic cardiomyopathy.
-
•
To appreciate the role of shared decision making for the appropriate patient in making complex clinical decisions.
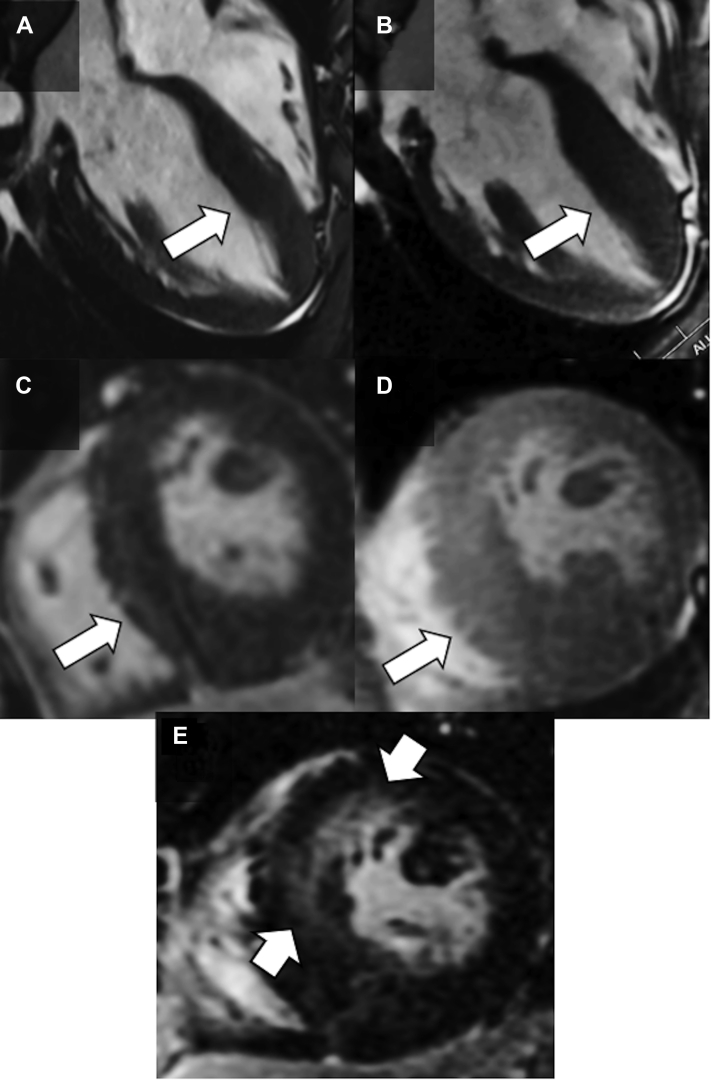
A 17-year-old male athlete presented for evaluation after an abnormal precompetitive college screening exam. His first ever 12-lead electrocardiogram (ECG) revealed left ventricular hypertrophy with T-wave inversions in the inferior and precordial leads (Figure 1). Follow-up transthoracic echocardiography (TTE) suggested hypertrophic cardiomyopathy (HCM). Cardiac magnetic resonance imaging (CMR) showed a maximum wall thickness of 23 mm at the apical inferior region of the left ventricle, no left ventricular outflow tract (LVOT) gradient, normal cavity size, normal left ventricular ejection fraction of 68%, and minimal late gadolinium enhancement (LGE) of <5% of myocardial mass (Figures 2A and 2C). With Valsalva, the LVOT gradient increased to 45 mm Hg with mild systolic anterior motion of the mitral valve; with exercise, the LVOT gradient increased to >60 mm Hg. However, the patient remained asymptomatic with excellent exercise capacity, normal blood pressure, and no ventricular arrhythmias.
Figure 1.
12-Lead Electrocardiogram
Normal sinus rhythm, voltage criteria for left ventricular hypertrophy, and deep T-wave inversions in leads II, III, aVF, and V3-V6. Note normalization of T-wave in lead aVR, which is classic in apical hypertrophic cardiomyopathy.
Figure 2.
Cardiac Magnetic Resonance Imaging
(A and C) Initial CMR showed a maximum wall thickness of 23 mm at the apical inferior region of the left ventricle and a total late gadolinium enhancement (LGE) volume of <5%. (B and D) Follow-up CMR 3 years later showed progression of hypertrophy with a maximum wall thickness of 28 mm and (E) a total LGE volume of 15%.
Initially, the patient was disqualified from competitive sports. He sought a second opinion from Dr Ackerman. He denied any symptoms including syncope, exertional chest pain or dyspnea, and palpitations. Vital signs and examination were unremarkable. The ECG was consistently abnormal, and repeated CMR confirmed the initial diagnosis of apical-variant HCM. The 24-hour Holter monitoring showed 2 premature ventricular complexes and no nonsustained ventricular tachycardia. His absolute risk of sudden cardiac death (SCD) was estimated to be approximately 0.5% to 1% per year according to the European Society of Cardiology risk calculator1 and the American Heart Association (AHA) HCM SCD Calculator (https://professional.heart.org/en/guidelines-and-statements/hcm-risk-calculator), insufficient to recommend prophylactic placement of an implantable cardioverter-defibrillator (ICD). After comprehensive evaluation and extensive discussions with the patient and his parents, a full return-to-play was supported and pursued using a shared decision making (SDM) model of care.
Past Medical History
There was no significant past medical history. A detailed family history was unremarkable, including no family history of premature or unexpected SCD.
Investigations
Genetic testing showed a splice site alteration in the MYBPC3 gene that encodes for cardiac myosin-binding protein C (c.3330+5G>C-MYBPC3), which was thought to be the likely pathologic variant. Subsequent screening of appropriate first-degree relatives confirmed parental inheritance. The MYBPC3-positive parent has been lifelong asymptomatic and with normal TTE.
Surveillance TTE and CMR every 7 to 8 months for the patient were unchanged, and he was cleared to play competitive sports at the elite level. However, 3 years after initial evaluation, his maximum wall thickness increased to 28 mm according to both TTE and CMR (Figures 2B and 2D). Left atrial volume was increased at 40 mL/m2, and B-type natriuretic peptide was measured at 594 pg/mL compared with 385 pg/mL at the time of diagnosis. Global longitudinal strain was reduced at −8%, most prominently in the apical region. Total volume of LGE had also increased to 15%, primarily at the apex (Figure 2E). Biventricular systolic function remained normal without LVOT obstruction at rest or with Valsalva. Ambulatory 24-hour ECG monitoring was again unremarkable.
Management
There were several important issues to consider in this patient’s management. First, regarding medical therapy, the patient was started on low-dose metoprolol succinate as a precautionary measure, which he tolerated without side-effects. Second, the risk of SCD and option for prophylactic ICD were addressed regularly with the patient and his parents. The patient did not have canonic risk factors for HCM-mediated SCD as defined by the American College of Cardiology (ACC) and AHA,2 and he remained asymptomatic with stable imaging through the initial years of follow-up. An implantable loop recorder was proposed as a monitoring strategy to distinguish between neurally mediated syncope vs nonfatal arrhythmia should he develop syncope, but it was not pursued. Thus, the patient, his parents, and cardiologist (Dr Ackerman) initially proceeded for 3 years without an ICD, based on perceived relatively low risk status. The detectable increase in hypertrophy and LGE on surveillance imaging subsequently compelled a recommendation for a prophylactic ICD, independently from his athlete status. Continuing with the SDM model of care regarding the type of ICD itself, a “minimally invasive” epicardial ICD,3 which may be particularly useful in competitive athletes, was proposed. Ultimately, there remained the question of whether the patient should continue with competitive sports given progression of disease and its unknown relationship between playing competitive sports and risk of death. He and his parents accepted the nonzero—albeit relatively low—risk of SCD, and the patient wished to continue pursuing his athletic career. We supported this decision and ensured that all parties at the university and professional levels were kept informed and maintained a well practiced emergency action plan.
Discussion
The management of this complex case was guided by SDM, representing a shift from historically more paternalistic practice within sports cardiology and genetic cardiology.4 A binary style of decision making has been traditional for competitive athletes with risk factors for SCD even as opinions differed on whether that approach was judicious.5,6 Indeed, the 2011 ACC/AHA guidelines recommend that patients with HCM not participate in intense competitive sports.7 During our care of this patient, the 2020 ACC/AHA HCM guidelines were released, denoting a change from the previous recommendation discouraging participation in sports to one prioritizing SDM.2 This timely update, in addition to consultation with members of the ACC/AHA writing committee (Dr Martinez), contributed to our initial support of the patient’s desire to continue with competitive sports.
The SDM model requires that the patient be able to engage in sophisticated conversation regarding risk and responsibility of risk. It should be acknowledged that assessing ability for risk interpretation can be challenging.8 Other important variables not captured in risk calculators that may modify SCD risk for athletes with cardiovascular disease include the type of sport, the relative endurance and strength components experienced during training and competition,9 and the athlete’s specific demographics. Moreover, depending on the sport and level of play, stakeholders including university athletic departments (and their lawyers), team physicians, professional organizations, and players’ representatives may need to share in the discussion.8 Nonetheless, despite the interest of these other parties and their ability to influence approval to play, the fundamental decision to accept risk is one that must be driven by patient preference. For minors, the decision making may be even more complex as parental control assumes a larger role.
In our case, through years of follow-up and with steadfast parental involvement, the patient consistently expressed a thoughtful understanding of his diagnosis and demonstrated the ability to rationalize his decision making. Our discussions employed a framework10 based on review of data, disclosure of the uncertainty in estimating absolute risk for elite athletes, and serial reassessment of his specific risk profile led by a multidisciplinary team of experts. Based on his presenting disease characteristics and calculated relatively low absolute risk of SCD during his initial evaluations, we supported his decisions. Once there was discernible increase in hypertrophy and LGE, we recommended an ICD, knowing it was a Class IIb recommendation and that the final decision resided with the patient. It is important to highlight that our recommendation was independent from the opinion regarding his sports eligibility. Whether for an athlete, artist, or academic, the imaging changes alone compelled our recommendation for an ICD. To our knowledge, it is unclear how much being a competitive athlete may increase the absolute risk of SCD compared with a nonathlete.
Finally, it should be recognized that precompetitive cardiac screening unequivocally altered this patient’s life. While it did allow for earlier detection of disease, it also led to initial disqualification from collegiate sports and arguably unnecessary hardships endured by the patient and his family to advocate for himself and the pursuit of his athletic career.
Follow-Up
The patient decided to proceed with a minimally invasive epicardial ICD. He was deemed medically eligible to continue his sport at the professional level and remains asymptomatic to date. During the first year since his epicardial ICD implantation, there have been no device-related complications, no shocks, and no recordings of any concerning ventricular arrhythmias. He remains in close follow-up with ongoing surveillance for disease progression and risk reevaluation.
Conclusions
An elite competitive athlete was diagnosed with asymptomatic apical-variant HCM and now competes professionally with a prophylactic ICD. This case highlights the role of shared decision making and close surveillance in the management of complex patients, especially within the fields of sports cardiology and genetic cardiology.
Funding Support and Author Disclosures
This work was supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program (to Dr Ackerman). Dr Ackerman is a consultant for Abbott, Boston Scientific, Bristol Myers Squibb, Daiichi Sankyo, Invitae, LQT Therapeutics, Medtronic, and UpToDate, and with the Mayo Clinic has equity, intellectual property, or royalty relationships with AliveCor, Anumana, ARMGO Pharma, and Pfizer, none of which were involved in this manuscript in any way. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Michael Papadakis, MD, served as Guest Associate Editor for this article.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.O‘Mahony C., Jichi F., Pavlou M., et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD) Eur Heart J. 2014;35(30):2010–2020. doi: 10.1093/eurheartj/eht439. [DOI] [PubMed] [Google Scholar]
- 2.Ommen S.R., Mital S., Burke M.A., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76(25):e159–240. doi: 10.1016/j.jacc.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 3.Schneider A.E., Burkhart H.M., Ackerman M.J., Dearani J.A., Wackel P., Cannon B.C. Minimally invasive epicardial implantable cardioverter-defibrillator placement for infants and children: an effective alternative to the transvenous approach. Heart Rhythm. 2016;13(9):1905–1912. doi: 10.1016/j.hrthm.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Levine B.D., Stray-Gundersen J. The medical care of competitive athletes: the role of the physician and individual assumption of risk. Med Sci Sports Exerc. 1994;26(10):1190–1192. [PubMed] [Google Scholar]
- 5.Baggish A.L., Ackerman M.J., Lampert R. Competitive sport participation among athletes with heart disease: a call for a paradigm shift in decision making. Circulation. 2017;136(17):1569–1571. doi: 10.1161/CIRCULATIONAHA.117.029639. [DOI] [PubMed] [Google Scholar]
- 6.Maron B.J., Nishimura R.A., Maron M.S. Shared decision-making in HCM. Nat Rev Cardiol. 2017;14(3):125–126. doi: 10.1038/nrcardio.2017.6. [DOI] [PubMed] [Google Scholar]
- 7.Gersh B.J., Maron B.J., Bonow R.O., et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2020;76(25):3022–3055. doi: 10.1016/j.jacc.2020.08.044. [DOI] [PubMed] [Google Scholar]
- 8.Kim J.H., Dickert N.W. Athletes with cardiovascular disease and competitive sports eligibility: progress and challenges ahead. JAMA Cardiol. 2022;7(7):663–664. doi: 10.1001/jamacardio.2022.0806. [DOI] [PubMed] [Google Scholar]
- 9.Levine B.D., Baggish A.L., Kovacs R.J., Link M.S., Maron M.S., Mitchell J.H. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 1: Classification of Sports: Dynamic, Static, and Impact: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132(22):e262–266. doi: 10.1161/CIR.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 10.Martinez M.W., Kim J.H., Shah A.B., et al. Exercise-induced cardiovascular adaptations and approach to exercise and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78(14):1453–1470. doi: 10.1016/j.jacc.2021.08.003. [DOI] [PubMed] [Google Scholar]