Graphical abstract
Keywords: Left atrial appendage occlusion, Watchman device, Procedural transesophageal echocardiography, Deployment complication
Highlights
-
•
Watchman device buckling is a previously undescribed deployment complication.
-
•
Both 2D and 3D TEE assist in detection of buckling during and after the procedure.
-
•
Challenging LAA anatomy may play a significant role in buckling risk.
-
•
An acute angle between the deployment catheter and the LAA ostium may confer risk.
-
•
With diligent TEE guidance, buckling may be corrected during the procedure.
Introduction
There is growing enthusiasm regarding left atrial appendage (LAA) occlusion (LAAO) in the prevention of atrial fibrillation- (AF-) related stroke. A meta-analysis comparing 5-year outcomes of the Watchman (Boston Scientific) LAAO device versus warfarin found that LAAO was comparable in stroke prevention and had less risk of bleeding, intracranial hemorrhage (ICH), and mortality.1 Additionally, the recent Left Atrial Appendage Occlusion Study III trial added enthusiasm about the stroke reduction capacity of LAAO.2 In 2015 the Food and Drug Administration approved the use of an LAAO device for stroke prevention in patients for whom systemic anticoagulation was suboptimal.
Percutaneous LAAO is not without risks. Complications of device placement include air embolism, cardiac perforation, and device embolism.3 To minimize risk and ensure implantation success, the procedure is typically guided by transesophageal echocardiography (TEE). Usually, multiplanar two-dimensional (2D) TEE is used in conjunction with three-dimensional (3D) TEE, which can improve assessment of the spatial relationship between left atrial structures and the LAAO device.4 The Watchman device trials (Protect5 2009 and Prevail6 2014) suggested that TEE be used to confirm device stability, appropriate device compression, and the absence of peridevice leaks greater than 5 mm. Although our clinical experience suggests that the feet of the device often bend and mold to the shape of the appendage, no literature has shown evidence of buckling of the exposed surface of the device during or after implant.
Here we present a case series of 3 patients who experienced a unique periprocedural deployment complication of a particular LAAO device, Watchman (Boston Scientific), that relies on radial compression to maintain a secure position within the LAA. In each case we describe the LAAO device buckling, leading to the formation of a buckle, with one side of the device collapsing in upon itself. This was ultimately recognized intraprocedurally in 2 cases and was rectified. In the first case, the structural flaw was not easily recognized on 2D TEE and was missed until follow-up TEE more clearly visualized the buckle on 3D TEE. This complication, while rare, represents an undescribed complication of LAAO device placement that is easily rectified if recognized and should be screened for during the procedure. While we report on 3 similar deployment complications associated with a particular LAAO device (Watchman), we suspect this may not be unique as other LAAO devices come to market that rely on radial compression within the LAA ostium. We hypothesize and will discuss potential etiologies of device buckling related to the noncoaxial angle of device deployment relative to the sheath position and the anatomy of the LAA.
Case Presentation 1
The first case is an 83-year-old man with a medical history of permanent AF, coronary artery disease, heart failure, and hypertension. The patient had an ICH while on warfarin. Following this ICH, oral anticoagulation was discontinued, and the patient had a presumed cardioembolic stroke on aspirin monotherapy. After a stroke off oral anticoagulation, the patient underwent Watchman (first generation) implantation in November 2017. Preprocedural TEE prior to implantation revealed that the patient's LAA was cauliflower in morphology with a prominent superior lobe that would be used for implantation. LAA measured (width × depth) 2.1 × 2.9 cm at 0°, 2.5 × 2.7 cm at 45°, 2.6 × 2.9 cm at 90°, and 2.7 × 2.6 cm at 135°. After deployment, the device measured (width) 1.8 cm at 0°, 1.9 cm at 45°, 2.3 cm at 90°, and 2.3 cm at 135°. The transseptal puncture was mid in the short axis and mid bicaval views, respectively, on TEE. Immediately following implantation, the 30-mm device was well seated and showed no evidence of periprocedural leak (Figure 1A–C, Videos 1-3). Although the device was noted to measure ovoid, no structural abnormalities were recognized.
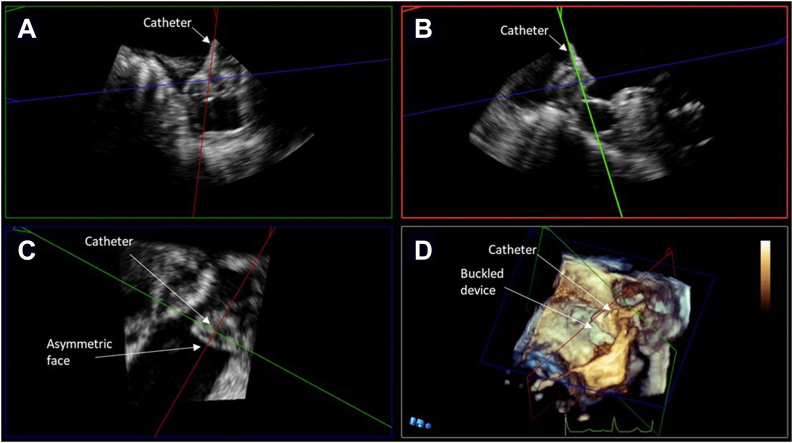
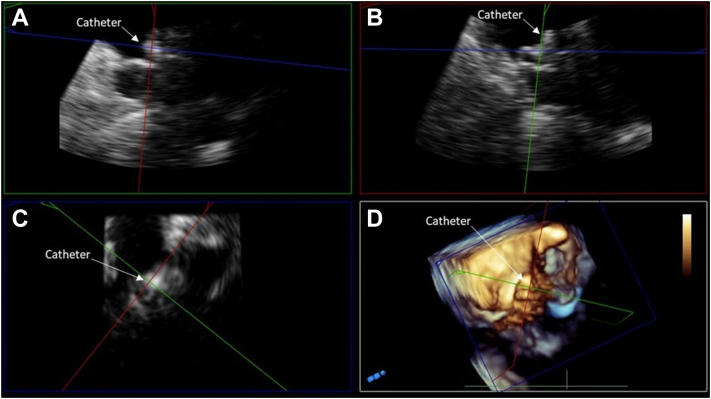
Figure 1.
Midesophageal TEE Images from case 1: (A) 2D x-plane view at 45° showing the acute angle of deployment catheter positioned into the LAA; (B) 2D x-plane view at 45° showing LAAO device buckle immediately after deployment; (C) 3D view at 25° showing collapse of device shoulder immediately after deployment; (D) 2D view with color Doppler at 44° showing the buckle at 45 days postimplantation; (E) 2D x-plane view at 115° showing device buckling and color Doppler evidence of peridevice leak at 1-year postimplantation; (F) 3D view at 45° showing device the buckle at 1-year postimplantation. AV, aortic valve; LA, left atrium; PA, pulmonary artery.
Routine TEE evaluating device placement at 45 days postimplant showed the device remained ovoid, measuring 2.2 × 1.8 cm in major and minor dimensions, and well seated, but a small 3-mm peridevice leak was noted. No structural abnormality was recognized at this 45-day mark using either 2D or 3D TEE (Figure 1D and E). The patient's anticoagulation was stopped and—per protocol—was switched to aspirin and clopidogrel and then aspirin monotherapy at 6 months. One-year follow-up TEE again confirmed device stability, but a buckle was recognized during this study (Figure 1F, Videos 4 and 5). What was recognized during the 1-year study prompted further inspection of previous imaging studies. On closer examination, the device buckle was evident on both the intraprocedural TEE at the site of the peridevice leak and at 45-day follow-up. In part owing to improved imaging technology (upgrade to Philips X8-2T Transesophageal Echocardiography Probe), the buckling was well imaged, was noted to encompass approximately 30% of the face of the device, and was shown to be associated with a persistent leak. A 2- to 3-mm leak could be seen emanating from the buckled portion of the device face in addition to another small leak that was not well measured. This leak was related to a gap between the device and the LAA orifice walls, likely exacerbated by the malformed device. On the 1-year TEE, the device was more clearly no longer circular. Comparing the buckle to the imaging from implant and 45-day TEE, the buckling appeared to worsen in the interim period between implantation and 1-year follow-up and was well visualized on 3D TEE (Figure 1F, Video 5). Device buckling was confirmed on postacquisition multiplanar reconstruction (MPR) imaging (Figure 2A–D). The structural defect was not intervened upon and was not believed to impact the patient's clinical course, although it was presumed to have contributed to the leak as the buckling resulted in a gap between the device and the LAA wall.
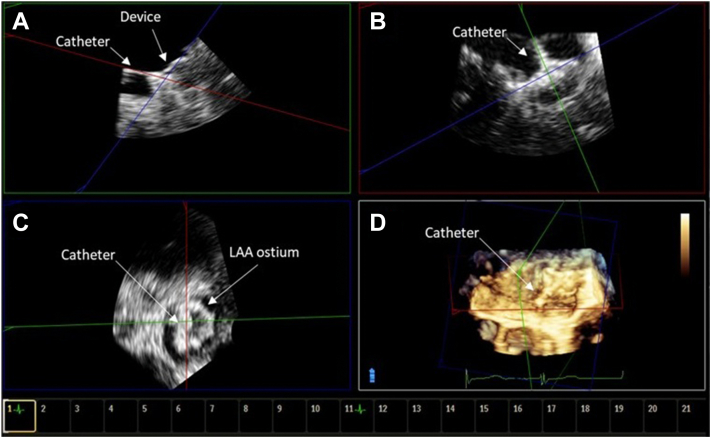
Figure 2.
TEE MPR image from case 1. (A) Orthogonal postacquisition MPR images showing noncoaxial orientation of the catheter relative to the axis of the LAA. (B) The deployment catheter is coaxial to the LAA ostium. (C) Lack of coaxial orientation, which is noted in panel A, led to an asymmetric shape of the device face. (D) Three-dimensional view showing noncoaxial deployment catheter orientation.
Case Presentation 2
The second case is an 80-year-old man with a medical history of AF, anemia, coronary artery disease, hypertension, and obesity. The patient was diagnosed with AF and was anticoagulated with apixaban. However, following initiation of anticoagulation, he was hospitalized with severe gastrointestinal bleeding secondary to arteriovenous malformations, and anticoagulation was stopped. Following the patient's hospital discharge, LAAO device implantation was recommended to mitigate stroke risk in the absence of anticoagulation.
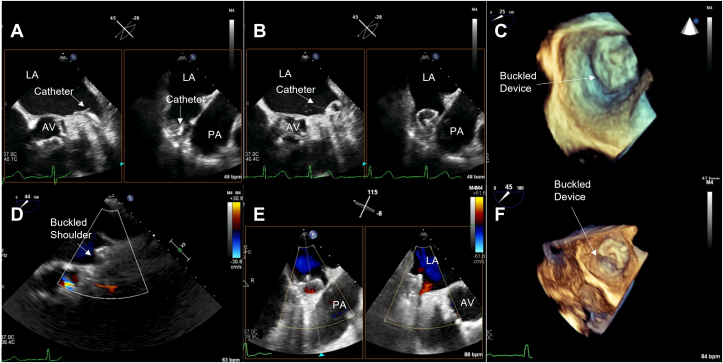
Intraprocedural TEE prior to implantation revealed that the LAA was chicken wing in morphology with a prominent posterior lobe. The LAA measurements (width × depth) were recorded as 2.1 × 2.2 cm at 0°, 1.8 × 2.6 cm at 45°, 1.8 × 2.4 cm at 90°, and 1.7 × 2.2 cm at 135°. The transseptal puncture was mid in the bicaval view and somewhat posterior in the short-axis view. After initial device (Watchman FLX) deployment, the mitral shoulder of the device at 60° appeared to buckle, appearing to collapse in on itself under the forward and possibly downward (toward the left circumflex) pressure of the deployment catheter attached to the device at a relatively acute angle (Figure 3A–C, Videos 6 and 7). After recognition of the buckle, using a combination of 2D and 3D TEE, the catheter was relocated more proximally and posteriorly to make the sheath more coaxial with the device and the device was tugged proximally while fully expanded, a move akin to the “tug test.” With continued gentle proximal traction, the device expanded into a normal circular shape (Figure 3D and E, Video 8). After stable deployment was established, the 27-mm device measured (width) 2.2 cm at 0°, 2.1 cm at 45°, 2.1 cm at 90°, and 2.1 cm at 135°. The device was noted to be well seated with no peridevice leak (Figure 3E and F, Video 8). Device buckling before correction was confirmed on postacquisition MPR imaging (Figure 4A–D). In this case, the buckle occurred during initial device expansion but—in light of comprehensive 2D and 3D imaging recognizing the issue—the structural anomaly was corrected with retraction and adjustment of the deployed device. A TEE imaging at 45-day and 1-year follow-up confirmed good device position and no peridevice leak.
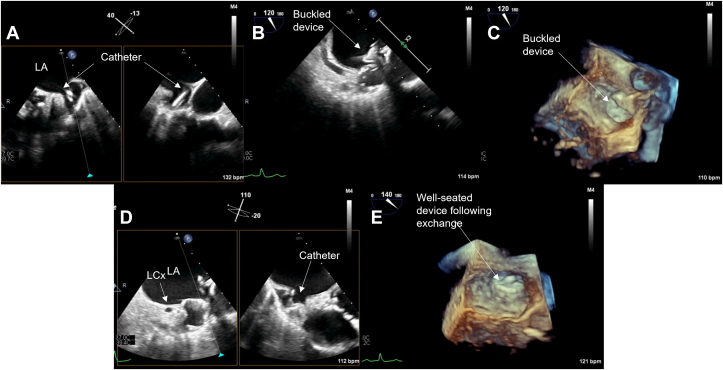
Figure 3.
Midesophageal TEE images from case 2: (A) 2D x-plane view at 60° showing acute angle of deployment catheter into LAA; (B) 2D x-plane view at 60° showing LAAO device buckling immediately after deployment; (C) 3D view at 45° showing buckling immediately after deployment; (D) 2D x-plane view at 60° showing proximal tug to relieve collapsed portion of the device face; (E) 2D x-plane view at 60° showing relieved buckle; (F) 3D view at 60° showing well-seated device. AV, aortic valve; LA, left atrium; LCx, left circumflex coronary artery; PA, pulmonary artery.
Figure 4.
TEE MPR image from case 2. (A) Postacquisition MPR images showing noncoaxial orientation of the deployment catheter to the LAA ostium. (B) Note that the deployment catheter appears more coaxial following a proximal tug. (C) Asymmetric device face noted due to buckle on initial deployment before proximal tug. (D) Three-dimensional image showing buckled device face before proximal tug maneuver.
Case Presentation 3
The third case is a 67-year-old woman with a medical history of AF, prior atrioventricular nodal reentrant tachycardia status postablation, and a history of gastric bypass surgery. The patient developed gastrointestinal bleeding secondary to a gastric ulcer. The ulcer required percutaneous embolization to control the bleeding. As a result, anticoagulation for AF was discontinued. After the patient was treated for gastrointestinal bleeding, LAAO device implantation was recommended to mitigate stroke risk.
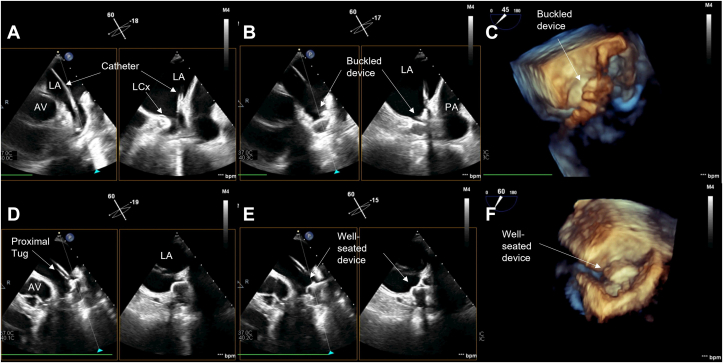
Intraprocedural TEE prior to implantation revealed that the LAA was cauliflower in appearance with a prominent inferior lobe. During periprocedural TEE, LAA measurements (width × depth) were recorded as 2.3 × 3.3 cm at 0°, 2.2 × 3.5 cm at 45°, 2.3 × 3.1 cm at 90°, and 2.1 × 2.0 cm at 135°. The transseptal puncture was mid in the short-axis view and mid in the bicaval view. During initial device implantation using a 31-mm device (Watchman FLX), a large buckle was noted in which almost 50% of the face of the device collapsed in on itself potentially under the downward (toward the left circumflex) pressure of the deployment catheter (Figure 5A–C, Videos 9-11). As the feet of the device were positioned toward the prominent inferior lobe, the angle between the catheter and the face of the device was also relatively acute. The 31-mm device was successfully recaptured and removed, and a subsequent deployment attempt was made with a 27-mm device. The device was deployed relatively deeper in the LAA, and attempts were made to keep the catheter more coaxial to the face of the device. This resulted in a successful deployment, and it measured (width) as 2.1 cm at 0°, 2.2 cm at 45°, 2.2 cm at 90°, and 2.1 cm at 135°. The device was well seated in the LAA, and no peridevice leak was noted (Figure 5D and E, Videos 12 and 13). Device buckling was confirmed on postacquisition MPR imaging (Figure 6A–D). At 45-day follow-up, the device was noted to be well seated, although a very small submillimeter leak was identified along the mitral aspect of the device at 45°. One-year follow-up showed appropriate device position as well.
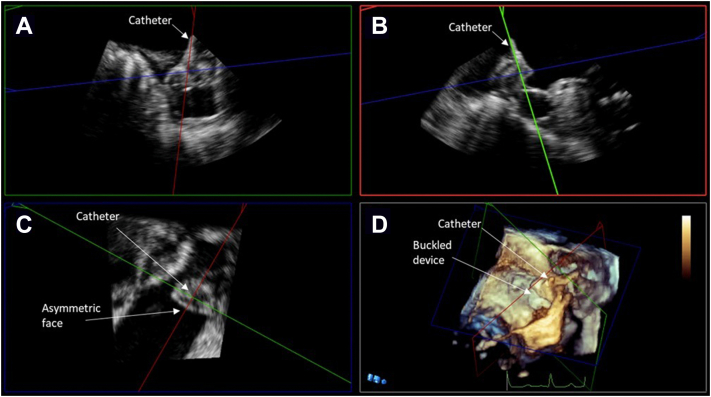
Figure 5.
Midesophageal TEE images from case 3: (A) 2D x-plane view at 40° showing acute angle of deployment catheter into LAA; (B) 2D view at 120° showing LAAO device buckle immediately after deployment; (C) 3D view at 120° showing buckling immediately after deployment; (D) 2D x-plane view at 110° showing less acute deployment catheter angle and well-seated device after initial device recapture; (E) 3D view at 140° showing well-seated device. LA, Left atrium; LCx, left circumflex coronary artery.
Figure 6.
TEE MPR image from case 3. (A) Postacquisition MPR images showing noncoaxial orientation of the deployment catheter to the LAA ostium. (B) Noncoaxial orientation associated with buckled device. (C) Asymmetric device face before smaller device was exchanged. (D) Three-dimensional image of buckling with defect toward the mitral shoulder of the device.
Discussion
In the current case series, we present 3 cases of LAAO device buckling during device deployment. The buckle occurred with 2 different implanters, and 2 of the cases occurred with our most experienced implanter, with well over 100 implants at the time of the second case. Notably, the echocardiographer was the same in each case, increasing the potential to recognize this complication. In case 1, periprocedural imaging showed the 30-mm device was well seated in the LAA, a buckle was present but unrecognized, and there was no peridevice leak at the time of implantation. Forty-five days following device placement, a 3-mm leak was detected. However, the cause of the leak was not initially appreciated. One year following implantation, a buckle in the device affecting 30% of its face was detected and appeared to be the underlying cause of the persistent 3-mm peridevice leak. Detection of the deformity was likely not appreciated until 1-year follow-up due to the less sophisticated imaging technology that was used during the procedure, which may have limited visualization of a relatively small defect. Additionally, this phenomenon is rarely seen and is not something that was routinely screened for on intraprocedural TEE. Based on TEE imaging, the buckling appeared to worsen in the interim period from implantation to 1-year follow-up. Worsening of the peridevice leak may also have contributed to recognition of the buckle as well. Furthermore, the peridevice leak could have worsened because of suboptimal device integrity, which reduced radial tension across the device face. Similarly, a lack of robust radial tension could have promoted a worsening of the buckling over time. Although cardiac CT was not used to evaluate this device buckling, CT could be an alternative or additive imaging modality if further evaluation of a potentially malformed device were needed.
In contrast to case 1, during cases 2 and 3 the device defect was detected by TEE during the procedure. In case 2 after initial deployment, the mitral shoulder of the 27-mm device collapsed on itself. With the assistance of 2D and 3D, TEE the deployment catheter was repositioned more coaxial with the LAA axis and the device was gently retracted. Proper orientation and proximal tugging caused the device to expand and become well seated on subsequent deployment. Finally, in case 3, a 31-mm device was initially deployed and a buckle affecting 50% of the device face was noted on TEE. The 31-mm device was recaptured and replaced successfully with a 27-mm device, which allowed more coaxial deployment. In all 3 cases, the patients did not experience any clinical complications.
The etiology of LAAO device buckling in each case appears to be related to the angle of the deployment catheter relative to the axis of the LAA and the ultimate axis of device deployment. Our experience was related to the Watchman LAAO device, but we suspect this complication may not be entirely unique as other devices relying on similar radial compression come to market. For device deployment, coaxial alignment between the deployment catheter and LAA is ideal.7 This can typically be achieved with a standard transseptal puncture that is inferior in the bicaval view and mid in the short-axis view. This transseptal puncture site may need to be adjusted, however, depending on the orientation of the ostium of the LAA and the planned device deployment angle. If a suboptimal transeptal puncture occurs, this may result in a noncoaxial alignment of the deployment catheter and the LAA ostium, which may increase the likelihood of device buckling. An example of a transeptal puncture that led to a noncoaxial alignment is provided (Figure 7, Video 14). Furthermore, proceduralists should consider the left atrial pressure when planning transeptal puncture as an elevated left atrial pressure could cause bowing of the interatrial septum and make coaxial alignment of the catheter with the LAA ostium more difficult. In addition, a high left atrial pressure may impact the LAA ostial size, leading to an oversized device selection, and contribute to our findings.
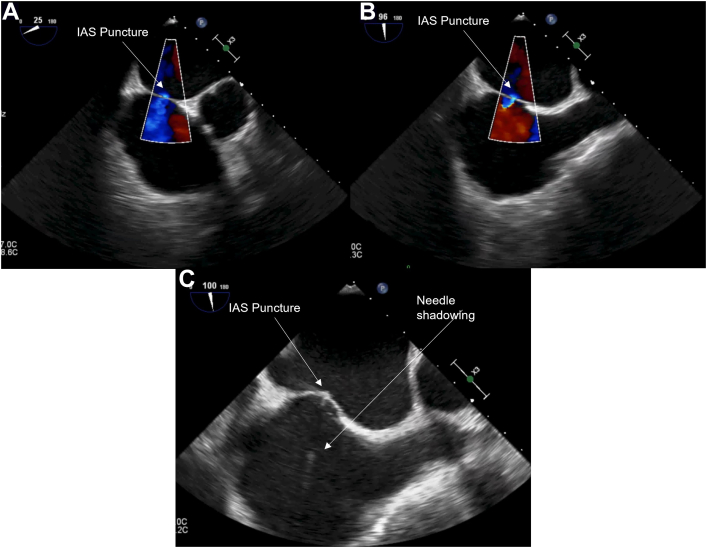
Figure 7.
Midesophageal images of transseptal puncture: (A) 2D view at 25° showing the transeptal puncture and associated color Doppler; (B) 2D view at 96° showing the transeptal puncture and associated color Doppler; (C) 2D view at 100° showing the transeptal puncture with needle shadowing present. IAS, interatrial septum.
In all 3 cases presented, the catheter angle was more acute than normally desired, and we suspect this predisposed to the buckling in each case. The development of the acute angles can be attributed to either the transeptal puncture location or the orientation of the LAA ostium and planned implantation axis (Figure 8). In case 1, LAA had cauliflower morphology with a prominent superior lobe. Transeptal puncture was mid in the short axis and mid in the bicaval views on TEE. This resulted in an acute angle of the deployment catheter compared to the LAA ostium and deployment axis. Although the buckle was not detected early on, with improved imaging or increased echocardiographer awareness of this complication, it may have been noticed intraprocedurally as in the subsequent 2 cases. In case 2, the patient's LAA was noted to have a prominent posterior lobe and a posteriorly oriented LAA ostium on preprocedural TEE. Transeptal puncture was posterior in the short-axis view and mid in the bicaval view. Like case 1 (despite an adjustment of the transseptal puncture site from the standard), the deployment catheter was nonetheless at an acute angle relative to the axis of the LAA. The device buckling was noted and corrected under intraprocedural imaging guidance during initial deployment. In case 3, preprocedural TEE was again significant for an LAA with a prominent inferior lobe. Transeptal puncture was mid in the short axis and mid in the bicaval views. Again, the deployment catheter was at an acute angle. The device abnormality was noted and corrected by catheter adjustment and exchanging for a smaller device under imaging guidance.
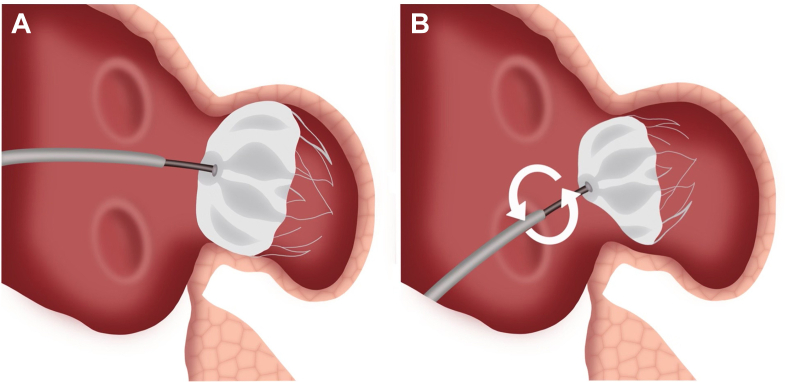
Figure 8.
Buckling graphic. (A) Correct demonstration of LAAO device insertion. Deployment catheter and device face are coaxial to the axis of the LAA and the LAA ostium. The device is well seated. (B) Suboptimal transeptal puncture leads to acute angle between deployment catheter and device face within the LAA. Under torque applied to improve device seating, the device can buckle, resulting in collapse shoulder and an apparent buckle in the face of the device. This could lead to a peridevice leak.
Fortunately, in our limited experience, device buckling resulted in no change in clinical outcome, although the persistent buckle did appear to cause a persistent 3-mm peridevice leak. While corrective intervention may not be needed, prevention through preprocedural imaging and periprocedural technique is manageable and should be pursued. Cardiac computerized tomography is rapidly becoming the standard preprocedure imaging modality and can easily define both the LAA anatomy and orientation of the LAA ostium to aid in LAAO planning.8,9 Careful intraprocedural TEE should also be used prior to transseptal puncture to confirm or identify LAA anatomy and plan the transseptal puncture accordingly. It seems that a prominent posterior or inferior lobe or any LAA anatomy leading to an acute, noncoaxial deployment angle may carry more risk of buckling. In the case of a prominent posterior lobe or a posteriorly oriented LAA ostium, a more posterior transseptal puncture in the short-axis view and more mid in bicaval view could allow for a more coaxial orientation of the deployment catheter with the LAA ostium. If a superiorly oriented ostium is noted, a more anterior and inferior transseptal puncture could maintain coaxial orientation. Periprocedural 2D and 3D TEE allows for detection of buckling after initial deployment. During the procedure, if a buckle develops, there are 2 possible methods that seem to correct the abnormality. As seen in case 2, a proximal tug on the device with a more coaxial catheter orientation may pull the shoulder out and shift the device into a fully expanded position. Alternatively, as seen in case 3, a maneuver to improve coaxial deployment such as adjusting the size of the device or the location of the transseptal puncture could improve deployment and prevent buckling.
Conclusion
Device buckling is a rare LAAO deployment complication. We hypothesize that this phenomenon can be attributed to an acute angle between the deployment catheter and the LAA ostial axis. Potential risks for an acute deployment angle are suboptimal transseptal puncture location, atypical LAA ostium or deployment lobe orientation, or LAA ostium that is particularly difficult to approach coaxially. Routine real-time 3D TEE analysis during deployment confirming coaxial orientation of the catheter and normal device appearance should be used to prevent this complication. While device buckling did not impact clinical outcomes in the presented cases, the clinical significance of buckling remains unknown. The defect can be detected with 2D and 3D TEE. If buckling is detected, the structural abnormality can be corrected intraprocedurally under careful TEE guidance with a gentle proximal tug or device redeployment with a more coaxial orientation of the catheter to the axis of the LAA.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent Statement
The authors declare that since this was a non-interventional, retrospective, observational study utilizing de-identified data, informed consent was not required from the patient under an IRB exemption status.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Acknowledgments
We thank Natalie Shrader for her help with figure creation and video editing.
Footnotes
Supplementary data to this article can be found online at 10.1016/j.case.2022.10.004.
Supplementary Data
Two-dimensional TEE ME x-plane video at 45° and –28° showing the acute angle of deployment catheter positioned into the LAA. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 45° showing LAAO device buckle immediately after deployment. ME, midesophageal.
Three-dimensional TEE ME video at 25° showing buckling of device shoulder immediately after deployment. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 115° showing device buckling and color Doppler evidence of peridevice leak at 1 year postimplantation. ME, midesophageal.
Three-dimensional TEE ME video at 45° showing device malformation at 1 year postimplantation. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 60° showing acute angle of deployment catheter into LAA. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 60° showing LAAO device buckling immediately after deployment. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 60° showing relieved buckle. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 40° showing acute angle of deployment catheter into LAA. ME, midesophageal.
Two-dimensional TEE ME video at 120° showing LAAO device buckle immediately after deployment. ME, midesophageal.
Three-dimensional TEE ME video at 120° showing buckle immediately after deployment. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 110° showing less acute deployment catheter angle and well-seated device after initial device recapture. ME, midesophageal.
Three-dimensional TEE ME video at 140° showing well-seated device. ME, midesophageal.
Two-dimensional TEE ME video at 96° showing the transeptal puncture with needle shadowing present. ME, midesophageal.
References
- 1.Reddy V., Doshi S., Kar S., Gibson D., Price M., Huber k., et al. 5-year outcomes after left atrial appendage closure. J Am Coll Cardiol. 2017;70:2964–2975. doi: 10.1016/j.jacc.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Whitlock R., Healey J., Vincent J., Brady K., Teoh K., Royse A., et al. Rationale and design of the left atrial appendage occlusion study (LAAOS) III. Ann Cardiothorac Surg. 2014;3:45–54. doi: 10.3978/j.issn.2225-319X.2013.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Möbius-Winkler S., Majunke N., Sandri M., Mangner N., Linke A., Stone G., et al. Percutaneous left atrial appendage closure: technical aspects and prevention of periprocedural complications with the watchman device. World J Cardiol. 2015;7:65–75. doi: 10.4330/wjc.v7.i2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chue C.D., de Giovanni J., Steeds R.P. The role of echocardiography in percutaneous left atrial appendage occlusion. Eur J Echocardiogr. 2011;12:i3–i10. doi: 10.1093/ejechocard/jer090. [DOI] [PubMed] [Google Scholar]
- 5.Holmes D.R., Reddy V., Turi Z., Doshi S., Sievert H., Buchbinder M., et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 6.Holmes D.R., Kar S., Price M., Whisenant B., Sievert H., Doshi S., et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Saw J., Lempereur M. Percutaneous left atrial appendage closure. JACC Cardiovasc Interv. 2014;7:1205–1220. doi: 10.1016/j.jcin.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Rajwani A., Nelson A., Shirazi M., Disney P., Teo K., Wong D., et al. CT sizing for left atrial appendage closure is associated with favourable outcomes for procedural safety. Eur Heart J Cardiovasc Imaging. 2017;18:1361–1368. doi: 10.1093/ehjci/jew212. [DOI] [PubMed] [Google Scholar]
- 9.Korsholm K., Berti S., Iriart X., Saw J., Wang D., Cochet H., et al. Expert recommendations on cardiac computed tomography for planning transcatheter left atrial appendage occlusion. JACC Cardiovasc Interv. 2020;13:277–292. doi: 10.1016/j.jcin.2019.08.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TEE ME x-plane video at 45° and –28° showing the acute angle of deployment catheter positioned into the LAA. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 45° showing LAAO device buckle immediately after deployment. ME, midesophageal.
Three-dimensional TEE ME video at 25° showing buckling of device shoulder immediately after deployment. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 115° showing device buckling and color Doppler evidence of peridevice leak at 1 year postimplantation. ME, midesophageal.
Three-dimensional TEE ME video at 45° showing device malformation at 1 year postimplantation. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 60° showing acute angle of deployment catheter into LAA. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 60° showing LAAO device buckling immediately after deployment. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 60° showing relieved buckle. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 40° showing acute angle of deployment catheter into LAA. ME, midesophageal.
Two-dimensional TEE ME video at 120° showing LAAO device buckle immediately after deployment. ME, midesophageal.
Three-dimensional TEE ME video at 120° showing buckle immediately after deployment. ME, midesophageal.
Two-dimensional TEE ME x-plane video at 110° showing less acute deployment catheter angle and well-seated device after initial device recapture. ME, midesophageal.
Three-dimensional TEE ME video at 140° showing well-seated device. ME, midesophageal.
Two-dimensional TEE ME video at 96° showing the transeptal puncture with needle shadowing present. ME, midesophageal.