Abstract
Background
Acne is the eighth‐most prevalent inflammatory skin disease with no optimal treatment. Photodynamic therapy (PDT) is an effective treatment for severe acne.
Aims
The effect of PDT on the composition and diversity of skin microflora in severe acne patients was studied.
Materials and Methods
A total of 18 patients with severe acne and 8 healthy individuals were selected for this study. Patients were treated with 5‐aminolevulinic acid‐mediated PDT once a week three times in total; the skin microbiome was measured by 16S ribosomal RNA gene sequencing before and after treatment (1 week after each PDT).
Results
The microflora composition was different between healthy controls and patients, and between patients before and after treatment. Alpha diversity indices were lower in patients than those in control. There were 15 bacterial genera with high relative abundance that had noticeable changes during treatment. At the genus level,particularly Cutibacterium acnes (C. acnes formerly Propionibacterium acnes), there was no statistically significant difference among different group. The abundances of Staphylococcus epidermidis and Staphylococcus aureus were low.
Discussion
The microbial composition is different between severe acne patients acne patients and healthy individuals. The therapeutic efficacy of severe acne treated with PDT is associated with the composition and diversity of skin microbiota.
Conclusion
The skin microbial composition changes after PDT treatment. PDT is an effective method for the treatment of severe acne.
Keywords: 16S sequencing, Cutibacterium acnes, photodynamic therapy, severe acne, skin microbiota
1. INTRODUCTION
Acne vulgaris is a chronic inflammatory skin disease of sebaceous gland units and is the eighth‐most prevalent inflammatory skin disease in the world. 1 It includes mild, moderate, and severe acne, in which severe acne is usually characterized by a large number of lesions, including nodules and cysts, and permanently causes disfiguration and scarring, resulting in low self‐esteem and psychological distress, 2 , 3 impacting the quality of life. 4 The pathogenesis of this disease involves four mechanisms: excessive sebum secretion, hyperkeratosis of the hair follicle sebaceous ducts, Cutibacterium acnes overgrowth, and inflammation. 5 , 6 C. acnes promotes inflammation through toll‐like receptor 2 activation, neutrophil chemotactic factors, and complement pathways, which also secrete lipases, proteases, and hyaluronidases that damage the pilosebaceous unit. 7 , 8 , 9 Because of the critical role of C. acnes in the pathogenesis of acne, C. acnes has become a primary target of acne treatment. Presently, through the popularization of high‐throughput sequencing technology, 10 , 11 , 12 researchers have found that the incidence of acne is related to microbiome diversity. 13 Other microbes, including Staphylococcus, 14 Demodex, 15 , 16 and Malassezia, 17 may be involved in the pathogenesis of acne, making the status of skin microflora in the pathogenesis of acne unclear. 8 , 18 , 19
Photodynamic therapy (PDT) combines a photosensitizer and light source to generate reactive oxygen species. 20 PDT is a safe and effective method for the treatment of severe acne. 21 , 22 , 23 In 2016, The American Academy of Dermatology pointed out that PDT is the most evidence‐based photoelectric therapy for acne. 24 However, the exact mechanism of its action has not been fully characterized. Current studies believe that PDT achieves acne treatment by inhibiting sebaceous gland secretion, alleviating hair follicle mouth obstruction, killing C. acnes, and reducing inflammation. 25 Given the current research, acne may be associated with microbiomes other than C. acnes. Studies suggest that PDT can kill gram‐positive and gram‐negative bacteria, fungi, parasites, viruses, and other microbes by producing singlet oxygen and free radicals singlet oxygen, 26 as has been shown for Staphylococcus epidermidis, 27 Staphylococcus aureus, 28 and Pseudomonas aeruginosa. 29 Therefore, PDT may also influence microflora to improve patient clinical symptoms. Therefore, we aimed to understand the effect of PDT on the skin microflora of patients with severe acne to clarify further the mechanism of photodynamic therapy for severe acne.
2. MATERIALS AND METHODS
2.1. Study participants
Eighteen patients with severe acne were selected from the Dermatology Department of the First Affiliated Hospital of Shantou University Medical College from October 2018 to October 2019. In addition, eight volunteers without acne were selected as the healthy group. Their clinical data are listed in Table 1. Inclusion criteria included (1) severe acne (grade IV on the Pillsbury Scale), (2) age >12 years old, gender was not limited, (3) no use of topical hormones, retinoids, antibiotics, and other drugs within 2 weeks before the baseline study visit, (4) did not take steroids, retinoids, antibiotics, and other drugs within 1 month before the baseline study visit, (5) no physical or chemical therapy was given within 1 month before the baseline study visit, and (6) patients voluntarily agreed to participate in the trial. Exclusion criteria included (1) severe acne patients with other facial skin diseases (such as herpes simplex), (2) had photosensitive skin diseases or diseases aggravated by light, (3) people who used photosensitive drugs, (4) patients with severe visceral diseases or autoimmune diseases, (5) pregnant and lactating women or women who were planning to become pregnant, and (6) patients judged by clinicians to be unsuitable for PDT.
TABLE 1.
Clinical data of severe acne patients and health group
| Severe acne patients | Healthy group | |
|---|---|---|
| Total number | 18 | 8 |
| Sex (M/F) | 13/5 | 5/3 |
| Fitzpatrick skin type (III/IV) | 15/3 | 6/2 |
| Age (years) | 21.72 ± 4.80 | 22.25 ± 2.60 |
| Disease duration (months) | 28.22 ± 12.38 | / |
| Total skin lesions (number) | 157.44 ± 42.00 | / |
2.2. Photodynamic therapy and sample collection
Participants received photodynamic therapy once a week, three times in total. A 5% 5‐aminolevulinic acid solution (Shanghai Fudan‐Zhangjiang Bio‐Pharmaceutical, Shanghai, China) was topically applied to the patient's face for a 1–1.5 h incubation. The face was later exposed to an LED light panel (630 ± 5 nm, Led 635, Wuhan Yage Co, Ltd, Wuhan, China) at a dose of 60 J/cm2 for 20 min. A sterile swab was used to collect the sample from 8 healthy individuals on the cheeks and 18 patients with severe acne on the acne skin lesion surface over a cheek area of about 4 cm2, swabbing the skin area 10 times in both vertical and horizontal directions. Patient samples were collected before treatment (0 week) as PTB group, 1 week after the first (1 week) as PTT1 group, second (2 weeks) as PTT2 group, and third (3 weeks) treatments as PTT3 group. The number of acne lesions in all patients was recorded by the same dermatologist. The effective rate was calculated using the following formula30: clearance rate of skin lesions (%) = ((the total number of skin lesions before treatment − the total number of skin lesions after treatment)/the total number of skin lesions before treatment) × 100. Evaluation criteria of acne treatment efficacy were classified as cure response, ≥90% clearance; excellent response, 60%–89% clearance; good response, 20%–59% clearance; poor response, ≤19% clearance. The effective rate (%) = ((the cases of the cure response + the cases of the excellent response)/total cases) × 100.
2.3. DNA extraction, PCR amplification, and 16S rDNA sequencing
Microbial DNA was extracted using a Stool DNA Kit (D4015‐01; Omega). The total DNA was tested using 1% agarose gel electrophoresis. Using the total DNA as a template and the primers (338F 5′‐ACTCCTACGGGAGGCAGCAG‐3′; 806R 5′‐GGACTACHVGGGTWTCTAAT‐3′), we amplified the V3–V4 regions of the bacterial 16S rRNA and sequenced them on the MiSeq 300PE platform using the standard Illumina sequencing primers.
2.4. Data analysis
For data quality control, FASTP (version 0.12.4) software 31 was used to filter the joint pollution sequence, including low‐quality reads. Then QIIME (1.9.1) software 32 was used to cluster tags of more than 97% identity into operational taxonomic units (OTUs) with the available reference method, and then the taxonomy of OTUs was assigned by UCLUST based on Greengenes database (13.8), 33 and a phylogenetic tree was constructed using FastTree. The alpha diversity represented species diversity in a single sample and was evaluated by several diversity indices, such as the Chao1 value, ACE value, Shannon index, and Simpson index. All alpha diversity indexes were calculated in QIIME. The difference in alpha index comparison between the two groups was calculated by a t‐test. Multivariate parameters, including principal component analysis, principal coordinate analysis (PCoA), and analysis of similarity (ANOSIM), were analyzed using SPSS 24.0 software. For all statistical comparisons, p‐values below 0.05 were considered statistically significant.
3. RESULTS
3.1. Clinical evaluation
A total of 18 patients had finished 3 sessions of treatment, and their clinical outcomes are listed in Table 2. The effective rate from the first week to the third week increased exponentially, from 27.78% to 55.56%, and then to 83.33%, demonstrating the effectiveness of photodynamic treatment for acne.
TABLE 2.
Clinical outcomes of patients
| No | Cure | Excellent | Good | Poor | The effective rate (%) | |
|---|---|---|---|---|---|---|
| 1 week | 18 | 0 | 5 | 9 | 4 | 27.78 |
| 2 weeks | 18 | 3 | 7 | 5 | 3 | 55.56 |
| 3 weeks | 18 | 8 | 7 | 3 | 0 | 83.33 |
3.2. Taxonomic assignment
Of the 72 skin samples obtained from 8 healthy individuals and 18 patients, 8 samples were excluded from the analysis because of either a low concentration of extracted DNA or low read numbers from 16S rRNA gene sequencing. We eventually identified 49 phyla, 115 classes, 198 orders, 331 families, 690 genera, and 444 species that were unique and present in at least 1 sample. At the genus level, the dominant bacterium was Cutibacterium, which occupied 20.5% of all samples (Figure 1). Except for a few bacteria, most were at low levels. A heatmap revealed the top 30 skin microflora, of patients and healthy individuals, at the genus level (Figure 2).
FIGURE 1.
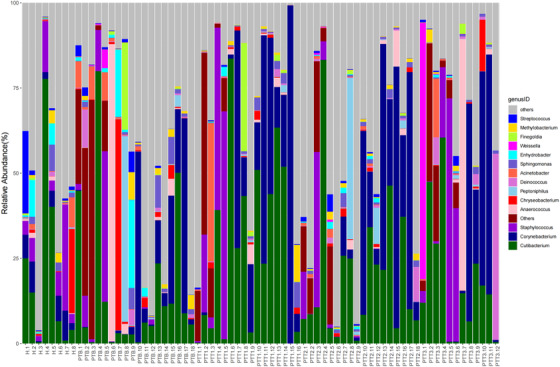
Taxonomy plot of the microbial communities of all samples at the genus level (X: samples, H [n = 8], healthy control; PTB [n = 18], pre photodynamic therapy group; PTT1 [n = 16], the first photodynamic therapy group; PTT2 [n = 18], the second photodynamic therapy group; PTT3 [n = 12], the third photodynamic therapy group; Y: the relative abundance of bacteria in different samples at the genus level)
FIGURE 2.
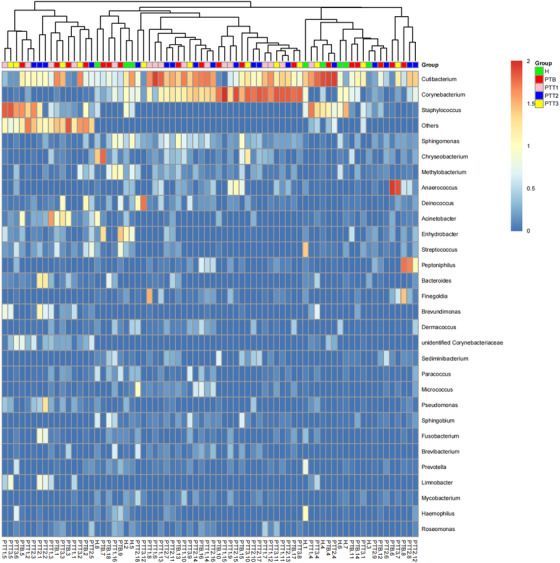
Heatmap revealed the top 30 skin microflora of patients and healthy individuals in the genus level. The horizontal coordinate is sample; the vertical coordinate is genus. The colors range from red to blue refers that the log10 value of the relative abundance of different isolates in different samples is from 2 to 0. (H group [green], healthy control; PTB group [red], pre photodynamic therapy; PTT1 group [pink], the first photodynamic therapy group; PTT2 group [blue], the second photodynamic therapy group; PTT3 group [yellow], the third photodynamic therapy group)
3.3. Microflora diversity in patients and healthy individuals
A Venn diagram analysis was performed for microflora comparison among the various groups (Figure 3). A total of 154 genera were common in all 5 groups. There were 32 genera only observed in healthy skin samples, whereas 28 genera were mainly present in the PTB group, 52 genera in the PTT1 group, 40 genera in the PTT2 group, and 18 genera in the PTT3 group. The microflora diversity was compared between patients and healthy individuals, including α and β diversity that estimated gut microbiota richness and evenness. The α diversity was based on the observed OTU, Chao1, ACE, Shannon, and Simpson's indices (Figure 4). In this study, the α diversity indices were lower in patients in both the pre‐ and posttreatment groups than in healthy individuals. The Chao1 and ACE indexes are good indicators of community richness, whereas Shannon's and Simpson's indexes are good indicators of community diversity. A beta matrix heatmap was used to reflect the similarity of samples at the genus level (Figure 5). Samples with similar β diversity were clustered together. The closer the distance value is to 0, the greater the consistency of species types and abundances between the two samples. The result of the PCoA (Figure 6) was consistent with the results of the UPGMA cluster tree (Figure 7). Samples from different groups were mixed together without forming significant clusters. The reason why samples from the same group did not significantly cluster into the same branch may be due to the difference in flora being noticeable between different individuals. ANOSIM analysis was done to examine the difference in microbial community composition among these groups at the genus level (Figure 8) and the difference between different individuals (Figure 9). Our results indicated that the difference of microbial community composition at the genus level between groups was significant. The difference of microbial community composition at the genus level between individuals was also statistically significant.
FIGURE 3.
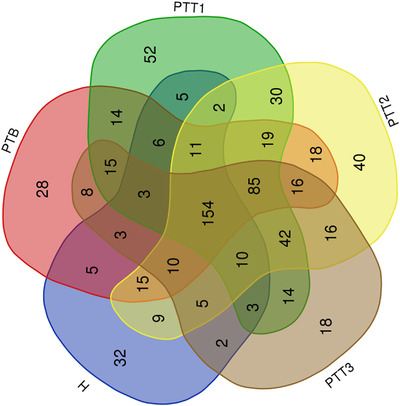
The features of the comparison of microflora between various groups at the genus level (H group, healthy control; PTB group, pre photodynamic therapy group; PTT1 group, the first photodynamic therapy group; PTT2 group, the second photodynamic therapy group; PTT3 group, the third photodynamic therapy group)
FIGURE 4.
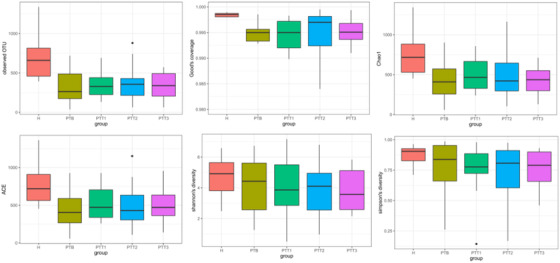
Microbiota α diversity index (observed OTU [operational taxonomic unit], Chao1, ACE, Shannon, and Simpson indices) in different groups (H group, healthy control; PTB group, pre photodynamic therapy group; PTT1, the first photodynamic therapy group; PTT2, the second photodynamic therapy group; PTT3, the third photodynamic therapy group)
FIGURE 5.
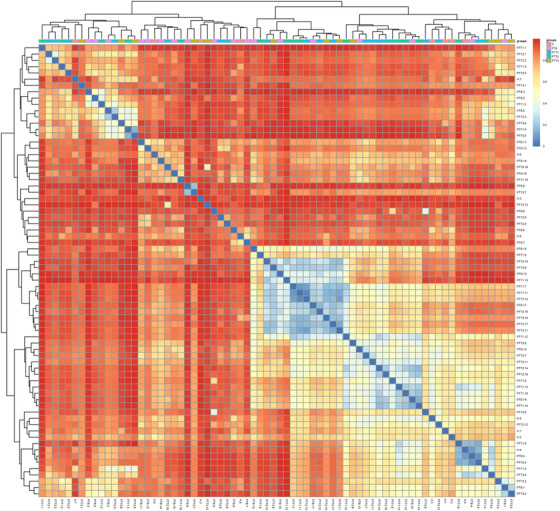
Distance heatmap at the genus level. The horizontal and vertical coordinates are the different samples. The colors range from red to blue refers that the Bray–Curtis distance value of different samples is from 0.8 to 0. (H group, healthy control; PTB group, pre photodynamic therapy group; PTT1 group, the first photodynamic therapy group; PTT2 group, the second photodynamic therapy group; PTT3 group, the third photodynamic therapy group)
FIGURE 6.
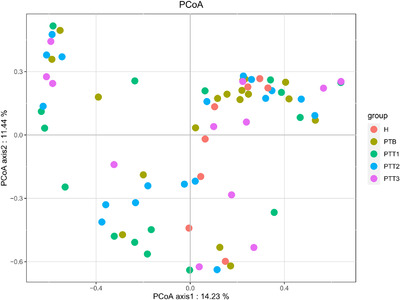
Principal coordinate analysis (PCoA) plots of all skin samples stratified by different groups (H group, healthy control; PTB group, pre photodynamic therapy; PTT1 group, the first photodynamic therapy group; PTT2 group, the second photodynamic therapy group; PTT3 group, the third photodynamic therapy group)
FIGURE 7.
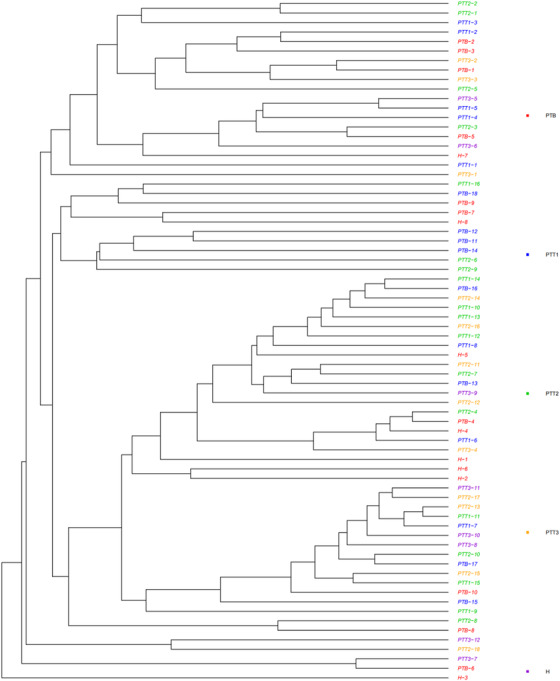
UPGMA clustering of skin samples from different groups (H group, healthy control; PTB group, pre photodynamic therapy group; PTT1 group, the first photodynamic therapy group; PTT2 group, the second photodynamic therapy group; PTT3 group, the third photodynamic therapy group)
FIGURE 8.
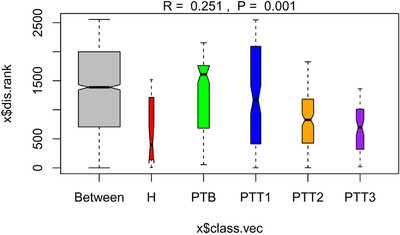
Analysis of similarity (ANOSIM) analysis was used to examine the differences in microbial community composition among these groups at the genus level. (H group, healthy control; PTB group, pre photodynamic therapy; PTT1 group, the first photodynamic therapy group; PTT2 group, the second photodynamic therapy group; PTT3 group, the third photodynamic therapy group)
FIGURE 9.
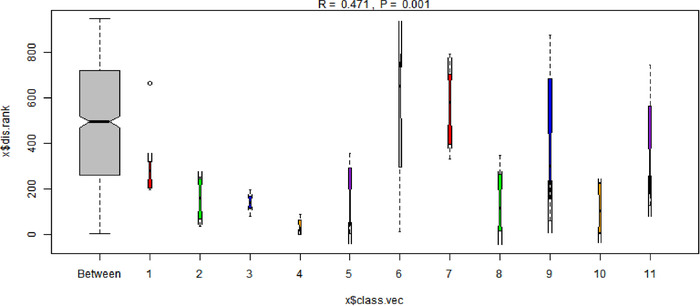
Analysis of similarity (ANOSIM) analysis was used to examine the differences in microbial community composition among different individuals. (X: different individuals, those patients who were not involved in all three treatments were eliminated in this figure.)
3.4. Comparison of microflora between healthy controls and patients before treatment
The taxonomic composition distribution histograms in the five groups are shown at the levels of phylum, order, class, family, genus, and species (Figure 10). At the phylum level, the dominant florae were Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes. Actinobacteria and Proteobacteria were higher in group H than in group PTB. In contrast, Firmicutes was higher in PTB group. At the class level, the dominant florae were Gammaproteobacteria, Clostridia, Alphaproteobacteria, Bacilli, and Actinobacteria. Bacilli, Alphaproteobacteria, and Gammaproteobacteria were higher in PTB, whereas Actinobacteria was higher in the H group. At the order level, the dominant florae were Actinomycetales, Clostridiales, and Bacillales. Actinomycetales was higher in the H group than in PTB group. Bacillales, Propionibacteriales, and Pseudomonadales were higher in PTB than in H group. At the family level, the dominant florae were Staphylococcaceae, Corynebacteriaceae, and Propionibacteriaceae. Propionibacteriaceae and Staphylococcaceae were higher in the H group than in the PTB group, whereas Planococcaceae was higher in the PTB group than in the H group. At the genus level, the dominant florae were Staphylococcus, Corynebacterium, and Cutibacterium. Staphylococcus and Cutibacterium were higher in the H group than in the PTB group, whereas Anaerococcus and Enhydrobacter were higher in PTB than in H. At the species level, the dominant flora was Propionibacterium acnes. Its abundance was higher in the H group. Our results demonstrate that skin microbial composition is significantly different between the pretreatment and the healthy control groups.
FIGURE 10.
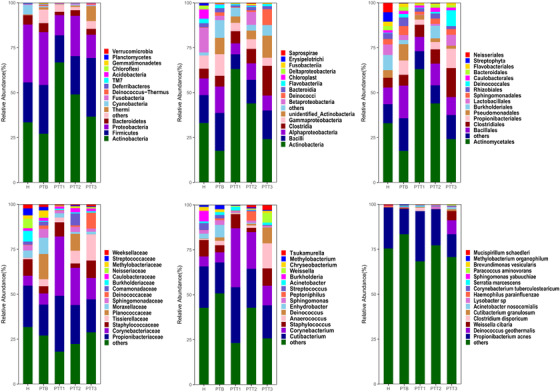
The taxonomic composition distribution histograms in five groups at the levels of phylum, class, order, family, genus, and species (H group, healthy control; PTB group, pre photodynamic therapy group; PTT1, the first photodynamic therapy group; PTT2, second photodynamic therapy group; PTT3, third photodynamic therapy group)
3.5. Comparison of microflora between patients before and after treatment
At the phylum level, the dominant phyla were Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes. Actinobacteria was higher in PTT1–PTT3 than in PTB, and its abundance gradually decreased from PTT1 to PTT3. In contrast, the relative abundance of Firmicutes and Bacteroidetes gradually increased from PTT1 to PTT3. At the class level, the dominant florae were Gammaproteobacteria, Clostridia, Alphaproteobacteria, Bacilli, and Actinobacteria. The abundance of Actinobacteria gradually decreased from PTT1 to PTT3, whereas the abundance of Bacilli and Clostridia gradually increased from PTT1 to PTT3. At the order level, the dominant florae were Actinomycetales, Clostridiales, and Bacillales. Actinomycetales was higher in the PTT1–PTT3 groups than in PTB patients, but its abundance gradually decreased from PTT1 to PTT3. The abundance of Bacillales was higher in PTB patients than in groups after treatment. At the family level, the dominant florae were Staphylococcaceae, Corynebacteriaceae, and Propionibacteriaceae. The abundances of Propionibacteriaceae and Corynebacteriaceae were higher posttreatment than before treatment but gradually decreased from PTT1 to PTT3. At the genus level, the dominant flora was Staphylococcus, Corynebacterium, and Cutibacterium. In the posttreatment groups, the abundance of Corynebacterium and Cutibacterium decreased from PTT1 to the PTT3 group. At the species level, the dominant flora was P. acnes, but whose abundance gradually decreased from PTT1 to the PTT3. These results demonstrate that the skin microbial composition changes significantly after treatment and show significant differences among the three posttreatment groups.
3.6. Relative abundance of individual bacterial taxa
At the genus level, we identified 15 genera with high relative abundance across all samples and with statistically significant changes over time (Figure 11). According to the trends of change of relative abundance among these 15 genera, we identified two patterns. In one, eight genera, involving Enhydrobacter, Haemophilus, Blautia, Cetobacterium, Prevotella, Streptococcus, Veillonella, and Gordonia, showed a statistically significant difference between the H and PTT3 groups. The other pattern, which included Pseudomonas, Acinetobacter, Granulicatella, Neisseria, Leptotrichia, and Mycobacterium, showed no statistically significant difference between the H and PTT3 groups. Four genera, Gordonia, Pseudomonas, Leptotrichia, and Mycobacterium, were statistically significantly different between the H and PTB groups. At the species level, we mainly focused on the change in abundance of three bacteria between different groups (Figure 12). For C. acnes, there was no statistically significant difference among the healthy control, pretreatment, and posttreatment groups. S. epidermidis and S. aureus were at low concentration levels.
FIGURE 11.
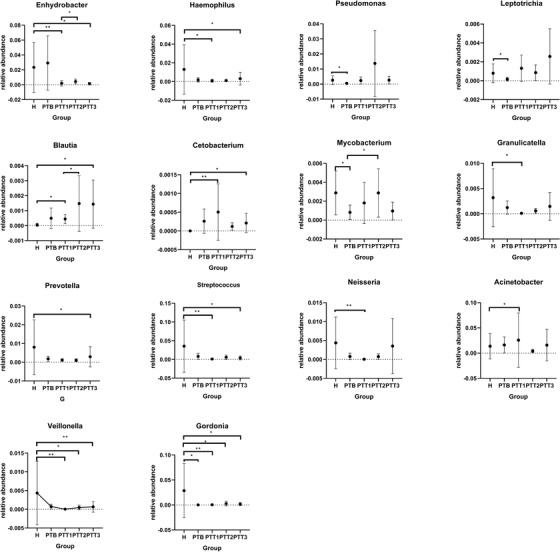
Relative abundance of selected bacterial genera at various groups (H group, healthy control; PTB group, pre photodynamic therapy group; PTT1, the first photodynamic therapy group; PTT2, second photodynamic therapy group; PTT3, third photodynamic therapy group)
FIGURE 12.
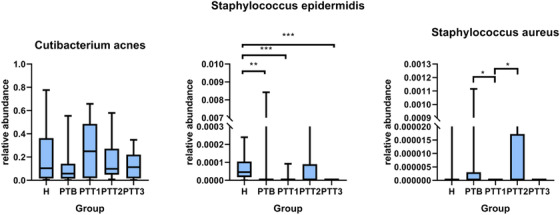
The change in abundance of Cutibacterium acnes, Staphylococcus epidermidis, and Staphylococcus aureus between different groups (H group, healthy control; PTB group, pre photodynamic therapy group; PTT1, the first photodynamic therapy group; PTT2, the second photodynamic therapy group; PTT3, the third photodynamic therapy group)
4. DISCUSSION
Acne treatment is mainly based on severity, in which severe acne is treated mainly with oral isotretinoin and antibiotics, and could take months or even years for completion of treatment. 34 Long‐term antibiotic use may cause side effects, including dysbacteriosis and resistance to symbiotic bacteria, increasing the probability of opportunistic pathogen infection. 35 , 36 Long‐term use of isotretinoin may induce skin and mucous membrane dryness, elevated blood lipids, abnormal liver function, teratogenicity, depression, and suicidal tendencies. Therefore, a variety of safe and effective physical therapies gradually have become the new trend in acne treatment. In the latest edition of the European Guidelines for Clinical Indications for PDT, severe acne is classified as a class IB indication for PDT. 37 Compared with systematic drug therapy, PDT treatment for severe acne has the advantages of rapid onset, good efficacy, high selectivity, no systemic adverse reactions, no drug resistance, and low recurrence rate, based on the nearly 20 years of PDT treatment for severe acne. 38 , 39 , 40 , 41 Our study shows that the therapeutic efficiency of PDT is 27.78%, 55.56%, and 83.33%, after the first, second, and third treatments, respectively. Follow‐up (by telephone or WeChat) indicated that the therapeutic efficacy continued to increase, indicating that PDT could become an alternative therapy or supplement to traditional systemic therapy for severe acne.
We further explored the underlying mechanism of PDT. Current studies mainly focus on the anti‐inflammatory and immunomodulatory mechanism of PDT treatment 42 , 43 and its effect on sebaceous glands. 44 In terms of microorganisms due to the status of C. acnes in the pathogenesis of acne, previous studies have focused on the effect of PDT on C. acnes. Through literature search, we found that there are very few studies in this field, with only one relevant study, 45 which showed the role of the microbiome in acne patients treated with PDT. The novelty of our study was that we analyzed the microflora abundance before, during, and after treatment.
There are hundreds of millions of microorganisms on the skin and its appendages, representing one of the four largest bacterial libraries in the human body. 46 Using a 16S RNA high‐throughput sequencing method, we found that human skin bacteria are mainly Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetea, consistent with a previous study. 47 However, at the genus level in patients with severe acne, Pseudomonas, Gordonia, Leptotrichia, and Mycobacterium are lower than those in healthy individuals. After PDT, levels of the above four microbes gradually increased, suggesting that their reduced levels might be related to the pathogenesis of severe acne, and also suggesting PDT treatment of severe acne may involve the regulation of microbial homeostasis.
Although C. acnes has been associated with the pathogenesis of acne for more than 100 years, we did not find a significant difference in C. acne levels between the healthy group and the severe acne group, which is similar to that of many previous studies. 48 , 49 , 50 , 51 However, studies have further demonstrated that acne‐related strains carry extra virulence genes, 52 generate more porphyrin, 53 and induce inflammatory responses, 54 , 55 whereas healthy strains do not. We did not further classify C. acnes, which is a limitation of our study. Notably, the relative abundance of C. acnes in the healthy group was higher than that in the severe acne group in our study, which is contrary to previous studies. 56 The reason may be related to the higher number of male patients than female patients in our study because a higher relative abundance of C. acnes is found in females. 57 However, the results of Dagnelie et al. 58 and Barnard et al. 59 studies are similar to ours, suggesting that C. acnes proliferation may not necessarily be the only determinant in the pathogenesis of acne vulgaris. Dagnelie et al. suggested that host factors are more critical pathogenic factors, whereas Barnard et al. suggested that microbiome imbalance and its overall virulence changes are critical pathogenic factors. Moreover, of note, there was no significant difference in the relative abundance of C. acnes before and after PDT, which is similar to the studies of Pollock et al. 60 and Hörfelt et al. 61 These two studies also found no significant change in sebaceous excretion rate before and after treatment, suggesting that there are other mechanisms in severe acne. Interestingly, we found that the relative abundance of C. acnes is increased after the first PDT compared with the baseline. Previous studies have suggested that PDT can ameliorate the obstruction of hair follicle sebaceous glands, 62 where C. acnes mostly live, especially in the occulted hair follicle sebaceous glands. 63 That clear increase in C. acnes levels after the first PDT may be due to the opening of the hair follicle sebaceous gland 64 and release of C. acnes to the surface, because the samples in this study were mainly swabbed from the epidermis. Although there was no statistical difference in the relative abundance of C. acnes in the subsequent treatments, a gradual decreasing trend was observed, which may be related to the fact that PDT can cause atrophy and reduce the secretion of the sebaceous glands, 65 thus changing to a more favorable environment for the survival of C. acnes. However, the follow‐up time of our study was short, and we did not analyze the secretion of sebaceous glands in patients. Further study is needed to understand the relationship among photodynamics, sebaceous glands, and C. acnes.
In our study, the α diversity in the healthy group was insignificantly higher than that in patients in both the pre‐ and posttreatment groups, which is consistent with studies by Dagnelie et al. 58 and Kelhälä et al., 66 although our results differ from Kelhälä et al. in that the α diversity in our study was reduced after treatment and gradually decreased with the increasing number of treatments. These results are similar to those of Chien et al., 67 who treated patients with minocycline. We consider that the gradual decrease in α diversity is related to the gradual decrease in acne severity in our patients because the α diversity of grade IV acne patients was higher than that of grade I–III acne patients, suggesting that the decrease of bacterial diversity following PDT may be related to the decrease of severity from grade IV to grade I–III, which is inconsistent with the trend of C. acnes. This suggests that PDT may not only target C. acnes but also cause changes in bacterial diversity in skin microbial communities.
In terms of β diversity, ANOSIM analysis gave an R = 0.535 and p = 0.001 when individuals were grouped, indicating individuals have unique microbial characteristics. ANOSIM analysis, of PTB, PTT1–PTT3 groups, following classification by time gave an R = 0.546 and p = 0.001, which also showed a specific aggregation. In addition, the R‐value was higher than the R‐value of individual groups, which is different from previous studies. 57 , 67 This could be related to our relatively large sample size and short follow‐up time.
5. LIMITATIONS
The sample size included in this study was small, which suggests that statistically significant changes in bacterial classification over time may be driven by individual patients. Moreover, the follow‐up time of our study was short. However, one of the most significant advantages of PDT is the low recurrence rate, and short‐term follow‐up cannot elucidate the relationship between the photodynamic therapy‐dependent low recurrence rate and microflora. We did not analyze the secretion of sebaceous glands in patients and thus could not further elucidate the relationship between photodynamics, sebaceous glands, and microorganisms.
6. CONCLUSION
Our results show that PDT is an effective method for the treatment of severe acne. The skin microbial composition changes after PDT treatment, which may treat severe acne by regulation of microbial homeostasis. Therefore, understanding the composition and overall structure of microbiota in severe acne patients before therapeutic protocol enactment is crucial. The skin microbial composition may be used as a biomarker for treatment pre‐evaluation. Studies with larger sample sizes are needed to test our results.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
The ethics committee of Shantou University Medical College and the First Affiliated Hospital of Shantou University Medical College approved this study (approval number 2020–100). Written informed consent in compliance with the Helsinki Declaration was obtained from all patients. The parents of patients under 18 years have signed a written consent on their behalf.
ACKNOWLEDGMENTS
The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: The present study was supported by the Shantou Science and Technology Project (Grant number 200622175261048).
Guo Y, Zeng M, Yuan Y, et al. Photodynamic therapy treats acne by altering the composition of the skin microbiota. Skin Res Technol. 2023;29:1–14. 10.1111/srt.13269
Yangmin Guo and Mi Zeng contributed equally to this work and share first authorship.
Key Message: Therapeutic efficacy of severe acne treated with PDT is associated with the composition and diversity of skin microbiota.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134(6):1527‐1534. [DOI] [PubMed] [Google Scholar]
- 2. Stamu‐O'Brien C, Jafferany M, Carniciu S, Abdelmaksoud A. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20(4):1080‐1083. [DOI] [PubMed] [Google Scholar]
- 3. Samuels DV, Rosenthal R, Lin R, Chaudhari S, Natsuaki MN. Acne vulgaris and risk of depression and anxiety: a meta‐analytic review. J Am Acad Dermatol. 2020;83(2):532‐541. [DOI] [PubMed] [Google Scholar]
- 4. Durovic MR, Durovic M, Jankovic J, Jankovic S. Quality of life in Montenegrin pupils with acne. PLoS One. 2021;16(4):e0250155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toyoda M, Morohashi M. Pathogenesis of acne. Med Electron Microsc. 2001;34(1):29‐40. [DOI] [PubMed] [Google Scholar]
- 6. Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20(3):335‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Selway JL, Kurczab T, Kealey T, Langlands K. Toll‐like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dessinioti C, Katsambas AD. The role of Propionibacterium acnes in acne pathogenesis: facts and controversies. Clin Dermatol. 2010;28(1):2‐7. [DOI] [PubMed] [Google Scholar]
- 9. Bernales Salinas A. Acne vulgaris: role of the immune system. Int J Dermatol. 2021;60:1076‐1081. [DOI] [PubMed] [Google Scholar]
- 10. Dagnelie MA, Montassier E, Khammari A, Mounier C, Corvec S, Dreno B. Inflammatory skin is associated with changes in the skin microbiota composition on the back of severe acne patients. Exp Dermatol. 2019;28(8):961‐967. [DOI] [PubMed] [Google Scholar]
- 11. O'Neill AM, Gallo RL. Host‐microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome. 2018;6(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gollnick HP, Dreno B. Pathophysiology and management of acne. J Eur Acad Dermatol Venereol. 2015;29(Suppl 4):1‐2. [DOI] [PubMed] [Google Scholar]
- 13. Ramasamy S, Barnard E, Dawson TL Jr., Li H. The role of the skin microbiota in acne pathophysiology. Br J Dermatol. 2019;181(4):691‐699. [DOI] [PubMed] [Google Scholar]
- 14. Dreno B, Martin R, Moyal D, Henley JB, Khammari A, Seite S. Skin microbiome and acne vulgaris: Staphylococcus, a new actor in acne. Exp Dermatol. 2017;26(9):798‐803. [DOI] [PubMed] [Google Scholar]
- 15. Zhao YE, Hu L, Wu LP, Ma JX. A meta‐analysis of association between acne vulgaris and Demodex infestation. J Zhejiang Univ Sci B. 2012;13(3):192‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akcinar UG, Unal E, Dogruman Al F. Demodex spp. as a possible aetiopathogenic factor of acne and relation with acne severity and type. Postepy Dermatol Alergol. 2018;35(2):174‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akaza N, Akamatsu H, Numata S, et al. Microorganisms inhabiting follicular contents of facial acne are not only Propionibacterium but also Malassezia spp. J Dermatol. 2016;43(8):906‐911. [DOI] [PubMed] [Google Scholar]
- 18. Ochsendorf FR. Cutibacterium acnes in acne pathophysiology—the chicken or the egg? Br J Dermatol. 2019;181(4):657‐658. [DOI] [PubMed] [Google Scholar]
- 19. Dreno B, Pecastaings S, Corvec S, Veraldi S, Khammari A, Roques C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol. 2018;32(Suppl 2):5‐14. [DOI] [PubMed] [Google Scholar]
- 20. Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42(7):804‐827. [DOI] [PubMed] [Google Scholar]
- 21. Nicklas C, Rubio R, Cardenas C, Hasson A. Comparison of efficacy of aminolaevulinic acid photodynamic therapy vs. adapalene gel plus oral doxycycline for treatment of moderate acne vulgaris—a simple, blind, randomized, and controlled trial. Photodermatol Photoimmunol Photomed. 2019;35(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 22. Posadzki P, Car J. Light therapies for acne. JAMA Dermatol. 2018;154(5):597‐598. [DOI] [PubMed] [Google Scholar]
- 23. Tang X, Li C, Ge S, Chen Z, Lu L. Efficacy of photodynamic therapy for the treatment of inflammatory acne vulgaris: a systematic review and meta‐analysis. J Cosmet Dermatol. 2020;19(1):10‐21. [DOI] [PubMed] [Google Scholar]
- 24. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945‐973.e33. [DOI] [PubMed] [Google Scholar]
- 25. Sosa‐Moreno A, Comstock SS, Sugino KY, et al. Perinatal risk factors for fecal antibiotic resistance gene patterns in pregnant women and their infants. PloS One. 2020;15(6):e0234751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris F, Pierpoint L. Photodynamic therapy based on 5‐aminolevulinic acid and its use as an antimicrobial agent. Med Res Rev. 2012;32(6):1292‐1327. [DOI] [PubMed] [Google Scholar]
- 27. Li X, Guo H, Tian Q, et al. Effects of 5‐aminolevulinic acid‐mediated photodynamic therapy on antibiotic‐resistant staphylococcal biofilm: an in vitro study. J Surg Res. 2013;184(2):1013‐1021. [DOI] [PubMed] [Google Scholar]
- 28. Zhao KQ, Wu Y, Yi YX, et al. An in vitro model to study the effect of 5‐aminolevulinic acid‐mediated photodynamic therapy on Staphylococcus aureus biofilm. J Vis Exp. 2018;134:57604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bohm GC, Gandara L, Di Venosa G, Mamone L, Buzzola F, Casas A. Photodynamic inactivation mediated by 5‐aminolevulinic acid of bacteria in planktonic and biofilm forms. Biochem Pharmacol. 2020;177:114016. [DOI] [PubMed] [Google Scholar]
- 30. Ma L, Xiang LH, Yu B, et al. Low‐dose topical 5‐aminolevulinic acid photodynamic therapy in the treatment of different severity of acne vulgaris. Photodiagn Photodyn. 2013;10(4):583‐590. [DOI] [PubMed] [Google Scholar]
- 31. Chen SF, Zhou YQ, Chen YR, Gu J. fastp: an ultra‐fast all‐in‐one FASTQ preprocessor. Bioinformatics. 2018;34(17):884‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high‐throughput community sequencing data. Nat Methods. 2010;7(5):335‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera‐checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microb. 2006;72(7):5069‐5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945‐973. [DOI] [PubMed] [Google Scholar]
- 35. Dessinioti C, Katsambas A. Propionibacterium acnes and antimicrobial resistance in acne. Clin Dermatol. 2017;35(2):163‐167. [DOI] [PubMed] [Google Scholar]
- 36. Nakase K, Yoshida A, Saita H, et al. Relationship between quinolone use and resistance of Staphylococcus epidermidis in patients with acne vulgaris. J Dermatol. 2019;46(9):782‐786. [DOI] [PubMed] [Google Scholar]
- 37. Morton CA, Szeimies RM, Basset‐Seguin N, et al. European dermatology forum guidelines on topical photodynamic therapy 2019 Part 2: emerging indications ‐ field cancerization, photorejuvenation and inflammatory/infective dermatoses. J Eur Acad Dermatol. 2020;34(1):17‐29. [DOI] [PubMed] [Google Scholar]
- 38. Nicklas C, Rubio R, Cardenas C, Hasson A. Comparison of efficacy of aminolaevulinic acid photodynamic therapy vs. adapalene gel plus oral doxycycline for treatment of moderate acne vulgaris‐A simple, blind, randomized, and controlled trial. Photodermatol Photo. 2019;35(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 39. Liu LH, Fan X, An YX, Zhang J, Wang CM, Yang RY. Randomized trial of three phototherapy methods for the treatment of acne vulgaris in Chinese patients. Photodermatol Photo. 2014;30(5):246‐253. [DOI] [PubMed] [Google Scholar]
- 40. Posadzki P, Car J. Light therapies for acne. JAMA Dermatology. 2018;154(5):597+. [DOI] [PubMed] [Google Scholar]
- 41. Tang XQ, Li CC, Ge SQ, Chen ZW, Lu LM. Efficacy of photodynamic therapy for the treatment of inflammatory acne vulgaris: a systematic review and meta‐analysis. J Cosmet Dermatol. 2020;19(1):10‐21. [DOI] [PubMed] [Google Scholar]
- 42. Ma Y, Chen QY, Liu Y, Wang QQ, Huang Z, Xiang LH. Effects of 5‐aminolevulinic acid photodynamic therapy on TLRs in acne lesions and keratinocytes co‐cultured with P‐acnes. Photodiagn Photodyn. 2016;15:172‐181. [DOI] [PubMed] [Google Scholar]
- 43. Zhang L, Yang J, Liu X, et al. 5‐Aminolaevulinic acid photodynamic therapy amplifies intense inflammatory response in the treatment of acne vulgaris via CXCL8. Exp Dermatol. 2021;30:923‐931. [DOI] [PubMed] [Google Scholar]
- 44. Tuo J, Wang Q, Zouboulis CC, et al. ALA‐PDT suppressing the cell growth and reducing the lipogenesis in human SZ95 sebocytes by mTOR signaling pathway in vitro. Photodiagnosis Photodyn Ther. 2017;18:295‐301. [DOI] [PubMed] [Google Scholar]
- 45. Tao S, Wang Z, Quan C, Ge Y, Qian Q. The effects of ALA‐PDT on microbiota in pilosebaceous units of patients with severe acne: a metagenomic study. Photodiagnosis Photodyn Ther. 2021;33:102050. [DOI] [PubMed] [Google Scholar]
- 46. Group NHW, Peterson J, Garges S, et al. The NIH human microbiome project. Genome Res. 2009;19(12):2317‐2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grice EA, Kong HH, Conlan S, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324(5931):1190‐1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fitz‐Gibbon S, Tomida S, Chiu BH, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133(9):2152‐2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kelhala HL, Aho VTE, Fyhrquist N, et al. Isotretinoin and lymecycline treatments modify the skin microbiota in acne. Exp Dermatol. 2018;27(1):30‐36. [DOI] [PubMed] [Google Scholar]
- 50. Pecastaings S, Roques C, Nocera T, et al. Characterisation of Cutibacterium acnes phylotypes in acne and in vivo exploratory evaluation of Myrtacine((R)). J Eur Acad Dermatol Venereol. 2018;32(Suppl 2):15‐23. [DOI] [PubMed] [Google Scholar]
- 51. Dreno B, Pecastaings S, Corvec S, Veraldi S, Khammari A, Roques C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol. 2018;32:5‐14. [DOI] [PubMed] [Google Scholar]
- 52. Tomida S, Nguyen L, Chiu BH, et al. Pan‐genome and comparative genome analyses of Propionibacterium acnes reveal its genomic diversity in the healthy and diseased human skin microbiome. mBio. 2013;4(3):e00003‐e00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johnson T, Kang DZ, Barnard E, Li HY. Strain‐level differences in porphyrin production and regulation in Propionibacterium acnes Elucidate Disease Associations. mSphere. 2016;1(1):e00023‐e00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Agak GW, Kao S, Ouyang K, et al. Phenotype and antimicrobial activity of Th17 cells induced by Propionibacterium acnes Strains Associated with healthy and acne skin. J Invest Dermatol. 2018;138(2):316‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu Y, Champer J, Agak GW, Kao S, Modlin RL, Kim J. Different Propionibacterium acnes phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J Invest Dermat. 2016;136(11):2221‐2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16(3):143‐155. [DOI] [PubMed] [Google Scholar]
- 57. Park SY, Kim HS, Lee SH, Kim S. Characterization and analysis of the skin microbiota in acne: impact of systemic antibiotics. J Clin Med. 2020;9(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dagnelie MA, Montassier E, Khammari A, Mounier C, Corvec S, Dreno B. Inflammatory skin is associated with changes in the skin microbiota composition on the back of severe acne patients. Exp Dermatol. 2019;28(8):961‐967. [DOI] [PubMed] [Google Scholar]
- 59. Barnard E, Shi BC, Kang DZ, Craft N, Li HY. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci Rep. 2016;6:39491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pollock B, Turner D, Stringer MR, et al. Topical aminolaevulinic acid‐photodynamic therapy for the treatment of acne vulgaris: a study of clinical efficacy and mechanism of action. Brit J Dermatol. 2004;151(3):616‐622. [DOI] [PubMed] [Google Scholar]
- 61. Hörfelt C, Stenquist B, Larko O, Faergemann J, Wennberg AM. Photodynamic therapy for acne vulgaris: a pilot study of the dose‐response and mechanism of action. Acta Derm‐Venereol. 2007;87(4):325‐329. [DOI] [PubMed] [Google Scholar]
- 62. Megna M, Fabbrocini G, Marasca C, Monfrecola G. Photodynamic therapy and skin appendage disorders: a review. Skin Appendage Disord. 2016;2(3‐4):166‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schommer NN, Gallo RL. Structure and function of the human skin microbiome. Trends Microbiol. 2013;21(12):660‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gozali MV, Yi F, Zhang JA, et al. Photodynamic therapy inhibit Fibroblast Growth Factor‐10 induced keratinocyte differentiation and proliferation through ROS in Fibroblast Growth Factor Receptor‐2b pathway. Sci Rep. 2016;6:27402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tuo J, Wang QQ, Zouboulis CC, et al. ALA‐PDT suppressing the cell growth and reducing the lipogenesis in human SZ95 sebocytes by mTOR signaling pathway in vitro. Photodiagn Photodyn. 2017;18:295‐301. [DOI] [PubMed] [Google Scholar]
- 66. Kelhälä HL, Aho VTE, Fyhrquist N, et al. Isotretinoin and lymecycline treatments modify the skin microbiota in acne. Exp Dermatol. 2018;27(1):30‐36. [DOI] [PubMed] [Google Scholar]
- 67. Chien AL, Tsai J, Leung S, et al. Association of systemic antibiotic treatment of acne with skin microbiota characteristics. JAMA Dermatol. 2019;155(4):425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


