Abstract
Background
Dysfunctional breathing (DB) has been shown to negatively affect asthma control in adults, but for children and adolescents, the knowledge is scarce. DB is among others characterized by dyspnea and hyperventilation. The Nijmegen Questionnaire (NQ) is often used as a marker for DB. We conducted a cross‐sectional survey to estimate the prevalence of DB in patients with asthma in a pediatric outpatient clinic and to determine the impact of DB on asthma control.
Methods
Patients between 10 and 17 years were invited to complete the NQ and the Asthma Control Questionnaire (ACQ) and report the use of beta2 agonist (β2). Spirometry data and prescribed asthma medications were noted from the patient record.
Results
Three hundred and sixty‐three patients (180 boys) completed the survey. Sixty‐seven patients (18%) scored ≥23 points in the NQ predicting DB. The DB group was older (median (range)) 15.6 (10.5–17.9) vs. 13.7 (10.0–17.9) years) (p < .01), and girls were overrepresented (84%) (p < .01). FEV1% exp. was higher in the DB group (mean (SD)) (89.4 (9.0) vs. 85.7 (11.8)) (p < .02). ACQ score (median (range)) (2.0 (0–4) vs. 0.6 (0–3.4)) (p < .01) and the use of β2 (median (range)) (2 (0–56) vs. 0 (0–20) puffs/week) (p < .01) were higher. Inhaled corticosteroid dose (mean (SD) (416 (160) vs. 420 (150) mcg) and the use of a second controller were equal between the groups.
Conclusion
Dysfunctional breathing was a frequent comorbidity, especially in adolescent girls. DB correlated with poorer asthma control and higher use of β2 and may be an important cofactor in difficult‐to‐treat asthma.
Keywords: adolescent, asthma, asthma control, child, dysfunctional breathing
Key Message.
Dysfunctional breathing was frequently observed in adolescents with asthma. Dysfunctional breathing impacted perceived asthma control. Clinicians should be aware of dysfunctional breathing as it may lead to overestimated asthma severity and over‐treatment.
1. INTRODUCTION
Dysfunctional breathing (DB) or breathing pattern disorder has been shown to negatively impact asthma control and asthma‐related quality of life in adults. 1 DB is characterized by intermittent or persistent symptoms of breathing difficulties that cannot be explained by any underlying disease. Symptoms can be dyspnea, hyperventilation, chest pain, sighing, yawning, or shortness of breath. 2 Being a clinical diagnosis, no gold standard method for diagnosing DB exists; however, the Nijmegen Questionnaire (NQ) 3 is used in many studies as a diagnostic marker for DB. 1 , 4 , 5 The NQ was originally developed for diagnosing hyperventilation syndrome and has later been suggested by its inventors as a tool for detecting functional respiratory complaints. 6
In adults with difficult‐to‐treat asthma, DB has been identified as one of the more possible explanations of poor asthma control 4 , 7 Other explanations may include poor treatment adherence, or severe asthma requiring more advanced treatment. In the group of difficult‐to‐treat asthma, it is important to identify DB, to avoid over‐treatment and facilitate breathing retraining, in the other hand it is also important to identify patients with severe asthma in need of more specialized medical treatment.
Little is known about the prevalence and patient characteristics of DB in children and adolescents with asthma, though a study in a population from a Dutch hospital outpatient clinic found a prevalence of 5%. The patients with DB experienced poorer asthma control despite no difference in lung function, FENO, or allergen sensitization compared to patients without DB. 8
We conducted a cross‐sectional survey to estimate the prevalence of DB in children followed with asthma in the pediatric outpatient clinic and to describe patterns of this group regarding demographics, asthma diagnosis, and treatment including compliance. Moreover, the aim was also to determine the impact of DB on asthma control.
2. METHODS
2.1. Patients
Children and adolescents between 10 and 17 years were invited to participate in the survey when attending the Pediatric and Adolescent Outpatient Clinic, University Hospital of Lillebaelt, Kolding for a scheduled asthma follow‐up visit. A one‐year study period was planned, starting in February 2021. However, because of many canceled and rescheduled visits due to COVID‐19 in January and February 2022, we decided to extend the study period to 15 months.
Inclusion criteria for this study were asthma diagnosed by a physician in accordance with guidelines, 9 treatment with inhaled corticosteroids during at least the last 3 months prior to inclusion, and age 10–17 years.
Exclusion criteria were significant cardiopulmonary, neurologic, or musculoskeletal disorder.
2.2. Nijmegen questionnaire
The NQ 3 is a 16‐item questionnaire developed to detect hyperventilation and intended as a measurement of “functional respiratory complaints.” 6 , 10
Each symptom is given a score from 0 (never happens) to 4 (happens very often). The summarized score ranges from 0 to 64 points, where a score ≥23 points predicts DB. NQ is validated to predict hyperventilation in adults with asthma. 11 Although the NQ has not been formally validated for use in children and adolescents it has been used in previous studies of DB in this age group. 8 , 12 A recent study found a positive correlation both between NQ and dyspnea during a cardiopulmonary exercise test, and between NQ and anxiety score in children with suspected DB. 10
2.3. Asthma control questionnaire
The participants were asked to answer the Asthma Control Questionnaire (ACQ5), 13 which has been validated for children from 6 years of age. 14 ACQ5 is a 5‐item questionnaire scoring asthma symptoms from 0 (best) to 6 (worst) points. The ACQ score is calculated as an average of the five items.
Allergen sensitization was recorded from the patient medical record as a specific IgE >0.35 IU or a skin prick test >3 mm for pollen, furred animals, house dust mites, or molds.
2.4. Height, weight, and body mass index
Height and weight were measured at the beginning of each consultation on a stand‐up scale (model 704, Seca, Germany) and a stadiometer (model 204 altimeter, Seca, Germany), respectively. Body mass index (BMI) was electronically calculated as weight (kg)/height2 (m). Body mass index‐standard deviation score (BMI‐SDS) was automatically given by the electronical height and weight schedule based on published Danish growth Charts. 15
2.5. Spirometry
Lung function test by spirometry was performed using Medicro spirometry software, in accordance with guidelines. 16 The forced expiratory volume in 1 s (FEV1) was recorded as percent of expected values. 17 The ratio of FEV1 to the forced ventilatory capacity (FEV1/FVC) was recorded.
2.6. Criteria for asthma diagnosis
The diagnostic criteria for asthma were taken from the patient medical record and noted as either: (1) significant change in FEV1 in either reversibility to beta2, exercise challenge, or mannitol challenge in accordance with guidelines, 9 (2) hospitalization with acute severe asthma, and/or (3) clinical assessment based on classical asthma symptoms and effect of inhaled corticosteroids (ICS).
When asthma was diagnosed by reversibility or exercise challenge, the change in FEV1 in percent was noted. When diagnosed with mannitol challenge, the dose of mannitol used to provoke a 15% fall in FEV1 (PD15) was noted. It was also noted when an exercise challenge failed because the participant was not able to fulfill the 8‐min treadmill run. When asthma was diagnosed by hospitalization, spirometry during the admission and within 4 weeks after discharge was performed in most cases. During the journal audit, the airflow limitation and variability were checked to ensure that the diagnosis of asthma was correct.
2.7. Asthma medication
Inhaled corticosteroids daily dose was recorded in mcg. Participants were asked about the use of bronchial reliever medication during the last week before the visit, and the answer was noted as puffs/week. Use of add‐on controller medications, i.e., long‐acting beta 2 agonist (LABA) or montelukast and use of nasal steroids were recorded. The asthma plan was noted as either step up, unchanged, or step down in medication.
Compliance with ICS or combined ICS/LABA was calculated as the percent of prescribed doses bought from pharmacies as registered by the Danish electronic Shared Medical Record.
2.8. Statistical methods
Study data were managed and collected in REDCap hosted at OPEN (Odense Patient Data Network, Odense University Hospital, Odense, Denmark).
Statistical analysis was performed using STATA16 (StataCorp, College Station). For group comparison of continuous data, the two‐sample Student's t‐test or Mann–Whitney rank‐sum test were used for normally and non‐normally distributed data, respectively. χ 2‐test was performed for binary outcomes.
To investigate the effect of predictors of DB, a logistic regression analysis was performed. Chosen predictors were sex, age, BMI‐SDS, ACQ, allergy status, failure to perform exercise challenge, FEV1%, FEV1/FVC, and type of diagnosis. The model was fitted with predictors with p < .3 using backward elimination.
To investigate the effect of NQ on asthma control a multiple regression model was developed with ACQ as dependent and sex, age, BMI‐SDS, NQ, B2, allergy status, FEV1%, FEV1/FVC, and add‐on medication as independent variables. The model was fitted with predictors with p < .3 using backward elimination.
The model was rerun using a modified Nijmegen score leaving out the three questions (Nq6, NQ7, NQ8) that relate to symptoms that overlap with asthma symptoms.
For all analyses, p < .05 was considered statistically significant.
2.9. Ethics
The processing of personal data was notified to and approved by the Region of Southern Denmark and listed in the internal record (Journal no.: 20/44798). Permission to gather and store patient information was given by participants aged 15 years or older. For children under 15 years of age, permission was given by a legal guardian.
3. RESULTS
Table 1 presents the demographic and patient characteristics of participants with and without dysfunctional breathing, and of the total cohort.
TABLE 1.
Demographics and asthma description in children and adolescents with and without asthma
| Non‐DB | DB | All | |
|---|---|---|---|
| Participants (n, %) | 296 (82) | 67 (18) | 363 |
| Age (median, range) | 13.7 (10.1–17.9) | 15.6 (10.5–17.9)*** | 13.8 (10–18) |
| Sex (n, % boys) | 169, 57% | 11, 16%*** | 145, 49% |
| BMI‐SDS (mean, SD) | 0.6 (0.07) |
0.3 (0.14) (p = .05) |
|
| Atopic (n, %) | 200 (68) | 32 (49)** | 232 (64) |
| FEV1% exp (mean, SD) | 85.7 (11.9) | 89.4 (9.0)* | 86.4 (11.5) |
| FEV1/FVC (mean, SD) | 0.82 (0.09) | 0.87 (0.07)* | 0.83 (0.09) |
| NQ score (median, 25%–75% range) | 9 (5–14) | 29 (24–35)*** | 11 (5–19) |
| ACQ score (median, 25%–75% range) | 0.6 (0.4–1.2) | 2.0 (1.4–2.6)*** | 0.8 (0.4–1.6) |
| Budesonide¤ mcg (mean, SD) | 420 (149) | 416 (160) | 419 (151) |
| LABA (n, %) | 91 (31) | 24 (36) | 115 (32) |
| Beta2, puffs/week (median, 25%–75% range) | 0 (0–2) | 2 (0–5)*** | 0 (0–3) |
|
Compliance with prescribed ICS (%) (median, 25%–75% range) |
85 (55–99) |
80 (63–99) |
85 (56–99) |
| Criteria for asthma diagnosis | |||
| Reversibility to Beta2 (n, %) | 122 (41) | 23 (34) | 145 (40) |
| Change in FEV1% (median, 25%–75% range) | 17 (13–22) | 14 (12–18) | |
| Exercise challenge (n, %) | 28 (9.5) | 9 (13) | 37 (10) |
| Change in FEV1% (median, 25%–75% range) | −18 (−16 to −25) | −16 (−15 to −18) | |
| Failed exercise challenge, n (% of performed) | 10 (14) | 9 (35) * | |
| Mannitol challenge, (n, %) | 54 (18) | 18 (26) | 72 (20) |
| pD15 mcg (median, 25%–75% range) | 200 (96–430) | 358.5 (305–521) (p = .05) | |
| Acute asthma hospitalization (n, %) | 33 (11) | 2 (3) | 35 (10) |
| Treatment trial (n, %) | 59 (20) | 15 (22) | 74 (20) |
| Astmaplan | |||
| Step up (n, %) | 29 (9.8) | 10 (14.9) | 39 (10.7) |
| Unchanged (n, %) | 186 (62.8) | 43 (64.2) | 229 (63.0) |
| Step down (n, %) | 81 (27.3) | 14 (21) | 95 (26.3) |
Note: *p < .05, **p < .01, ***p < .001, ¤ or other ICS in equivalent dose.
Of the 363 patients (180 boys) completing the survey, 67 patients (18%) scored ≥23 points in the NQ predicting DB. The DB group was older and girls were overrepresented. Mean BMI‐SDS was perisignificantly higher in the non‐DB group.
Asthma characterization: FEV1% exp. and FEV1/FVC were lower, and more patients were sensitized to one or mere aeroallergens in the non‐DB group. Inhaled corticosteroid dose, use of a second controller, and use of nasal steroids were equal between the groups. Compliance with the prescribed ICS or ICS/LABA combination was equal between the groups.
Asthma control questionnaire score and use of β2 agonist were higher in the DB group, predicting poorer perceived asthma control.
There were no differences in diagnostic methods between the groups. Response in FEV1 to Beta2 agonist and exercise challenge at the time of diagnosis were equal, whereas there was a perisignificantly higher PD15 in participants with DB, diagnosed by mannitol challenge. Participants in the DB group more often failed to fulfill an exercise challenge. Of the 35 patients diagnosed by hospitalization, the diagnosis was ensured by a significant FEV1 variability during hospitalization in 29 patients, and on a later occasion in four patients.
Predictors of DB were older age (p < .01), female sex (p < .01), higher ACQ score (p < .001), and higher FEV1 (p < .05). BMI‐SDS correlated negatively with DB (p < .05).
Predictors of ACQ were NQ (p < .001), use of beta2 agonist (p < .001), and BMI‐SDS (p < .01) correlated positively with ACQ meaning that higher NQ, higher BMI‐SDS, and higher use of bronchial reliever medication predicted poorer asthma control as estimated by ACQ. No effect was found of atopic status or use of add‐on controller medication, whereas a tendency for a negative correlation with FEV1% exp. was found (p = .1).
When running the model with a modified NQ score without NQ5‐7 similar results were found.
Figure 1 shows the relationship between ACQ and NQ and the modified NQ, respectively.
FIGURE 1.
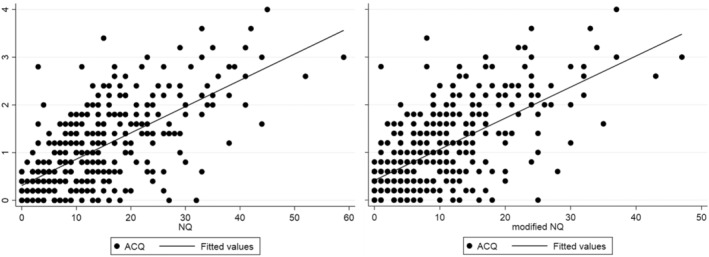
ACQ correlated with both NQ and a modified NQ without three asthma overlap symptoms.
4. DISCUSSION
4.1. DB prevalence
In our study, we investigated DB in a cohort of children and adolescents with asthma in a secondary care outpatient clinic. The patients were generally well‐controlled. In this study, we found a compliance with the prescribed ICS of more than 80%, which is considerably higher than reported in other studies reporting compliance of only 40%–70% for this age group. 18 , 19 , 20
We found DB as evaluated by the NQ to be common in children and adolescents with asthma, as 18% of our cohort screened positive. This is in accordance with D'Alba et al. 12 who studied dysfunctional breathing in adolescents with and without self‐reported asthma and found a DB frequency of 25% of participants with asthma, whereas de Groot et al. 8 found a frequency of only 5% in children and adolescents with asthma. The reason for the discrepancy can probably be explained by differences in age as the participants in the latter study were younger.
To our knowledge, these two studies are the only ones having studied the prevalence and patterns of dysfunctional breathing in a cohort of children and adolescents with asthma previously. In addition, some adult studies support our findings as they also report relatively high prevalences of DB in patients with asthma. 1 , 21
4.2. Characterization of the DB group
It has been suggested that DB in difficult‐to‐treat asthma may act as a confounder for asthma symptoms, leading to an overestimation of asthma severity resulting in over‐treatment. 4 , 22
In our study, the DB group was older and with a female overrepresentation both of which are consistent with previous studies both in children and adults. 1 , 12 , 21 We also found a higher FEV1%exp and a lower fraction of allergic sensitization in the DB group, whilst there were no differences in the amount of controller asthma medication used. However, the DB group had a higher consumption of bronchial reliever medication. Moreover, despite the lower rate of allergic sensitization in the DB group, an equal fraction of the groups used nasal steroids. Compliance with prescribed ICS was equal. All variables were independent predictors of DB in the regression model. Moreover, there were no differences in airflow variation at the diagnostic tests. Our findings thus support that patients with asthma and DB do not suffer from more severe asthma than patients without DB as suggested by others. 4 However, we found that a high fraction of the DB group was unable to fulfill an exercise challenge, which may indicate that this group had a high degree of exercise intolerance, due to other reasons than asthma.
Surprisingly, we found lower BMI‐SD in the DB group than in the non‐DB group. This is in contrast to an adult study, 4 whereas differences in BMI‐SD were not reported by the other adolescent studies. 8 , 12
4.3. Predictors of asthma control
Nijmegen Questionnaire was an independent predictor of poor ACQ, indicating that DB contributed to poorer perceived asthma control. As three of the symptoms in the NQ overlap with asthma symptoms, it could be speculated that it is in fact these asthma symptoms that drive the higher NQ score in some of the DB patients. To investigate this, we calculated a modified NQ score without these three asthma overlap symptoms. This did not change the correlation between NQ and ACQ, which is in accordance with Sedeh et al. 4 , who also found the correlation between ACQ and NQ to be independent of the asthma overlap symptoms in the NQ. Additionally, we found no evidence of more severe asthma in our DB group. Therefore, there is a risk, that some of the symptoms in the DB group are misinterpreted as asthma symptoms, despite not being markers of more severe asthma in this group of patients.
Body mass index‐standard deviation score was correlated to both DB and asthma control; however, the effects on those were divergent. Thus high BMI‐SDS correlated to poor perceived asthma control, whereas low BMI‐SD correlated to DB. It is quite surprising that the DB group was both leaner and with poorer perceived asthma control. This indicates that overweight and DB are different risk factors for poor perceived asthma control.
A strength of our study is the thorough description of our cohort when it comes to asthma diagnosis, medication, medical compliance, and asthma plan. A limitation is that we did not record any information regarding comorbidities (including psychiatric or functional symptoms) thus we do not know from our data whether these were overrepresented in DB as reported by others. 2 Another limitation is that our diagnostic tool, the NQ, is not validated for children and adolescents.
In conclusion, we found DB to be a frequent comorbidity of adolescent asthma and to impact the perceived asthma control negatively. We suggest that DB may be a confounder of asthma symptoms, leading to a misinterpretation of asthma control as worse than is actually the case. Based on our study, we suggest routine screening for DB in difficult‐to‐treat pediatric and adolescent asthma in order to avoid overtreatment. As patients with DB often experience poor treatment effects, it may be a relief for the patient to address DB as an explanation of the symptoms. Furthermore, as the symptoms of DB are a burden for the patients, treatment options of this condition may be the subject for future studies.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13909.
ACKNOWLEDGEMENT
We thank OPEN, Odense Patient data Explorative Network, and Odense University Hospital, Odense, Denmark for providing data management to the study.
Vahlkvist S, Jürgensen L, Hell TD, Petersen TH, Kofoed P‐E. Dysfunctional breathing and its impact on asthma control in children and adolescents. Pediatr Allergy Immunol. 2023;34:e13909. doi: 10.1111/pai.13909
Editor: Ömer Kalayci
Clinical Trial registration: NCT04734795
REFERENCES
- 1. Veidal S, Jeppegaard M, Sverrild A, Backer V, Porsbjerg C. The impact of dysfunctional breathing on the assessment of asthma control. Respir Med. 2017;123:42‐47. [DOI] [PubMed] [Google Scholar]
- 2. Connett GJ, Thomas M. Dysfunctional breathing in children and adults with asthma. Front Pediatr. 2018;6:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res. 1985;29(2):199‐206. [DOI] [PubMed] [Google Scholar]
- 4. Sedeh FB, von Bülow A, Backer V, et al. The impact of dysfunctional breathing on the level of asthma control in difficult asthma. Respir Med. 2020;163:105894. [DOI] [PubMed] [Google Scholar]
- 5. Stanton AE, Vaughn P, Carter R, Bucknall CE. An observational investigation of dysfunctional breathing and breathing control therapy in a problem asthma clinic. J Asthma. 2008;45(9):758‐765. [DOI] [PubMed] [Google Scholar]
- 6. van Dixhoorn J, Folgering H. The Nijmegen questionnaire and dysfunctional breathing. ERJ Open Res. 2015;1(1):1‐2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rogliani P, Sforza M, Calzetta L. The impact of comorbidities on severe asthma. Curr Opin Pulm Med. 2020;26(1):47‐55. [DOI] [PubMed] [Google Scholar]
- 8. de Groot EP, Duiverman EJ, Brand PL. Dysfunctional breathing in children with asthma: a rare but relevant comorbidity. Eur Respir J. 2013;41(5):1068‐1073. [DOI] [PubMed] [Google Scholar]
- 9. Global Initiative for Asthma . Global strategy for asthma management and prevention. 2020. https://ginasthma.org/. Assessed 07.September 2022.
- 10. Peiffer C, Pautrat J, Benzouid C, et al. Diagnostic tests and subtypes of dysfunctional breathing in children with unexplained exertional dyspnea. Pediatr Pulmonol. 2022;57:2428‐2436. [DOI] [PubMed] [Google Scholar]
- 11. Grammatopoulou EP, Skordilis EK, Georgoudis G, et al. Hyperventilation in asthma: A validation study of the Nijmegen Questionnaire – NQ. J Asthma. 2014;51(8):839‐846. [DOI] [PubMed] [Google Scholar]
- 12. D'Alba I, Carloni I, Ferrante AL, Gesuita R, Palazzi ML, de Benedictis FM. Hyperventilation syndrome in adolescents with and without asthma. Pediatr Pulmonol. 2015;50(12):1184‐1190. [DOI] [PubMed] [Google Scholar]
- 13. Juniper EF, O'byrne PM, Guyatt G, Ferrie P, King D. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902‐907. [DOI] [PubMed] [Google Scholar]
- 14. Juniper EF, Gruffydd‐Jones K, Ward S, Svensson K. Asthma control questionnaire in children: validation, measurement properties, interpretation. Eur Respir J. 2010;36(6):1410‐1416. [DOI] [PubMed] [Google Scholar]
- 15. Tinggaard J, Aksglaede L, Sørensen K, et al. The 2014 Danish references from birth to 20 years for height, weight and body mass index. Acta Paediatr. 2014;103(2):214‐224. [DOI] [PubMed] [Google Scholar]
- 16. Miller MR, Hankinson JA, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319‐338. [DOI] [PubMed] [Google Scholar]
- 17. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi‐ethnic reference values for spirometry for the 3‐95‐yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vasbinder E, Dahhan N, Wolf B, et al. The association of ethnicity with electronically measured adherence to inhaled corticosteroids in children. Eur J Clin Pharmacol. 2013;69(3):683‐690. [DOI] [PubMed] [Google Scholar]
- 19. Burgess SW, Sly PD, Morawska A, Devadason SG. Assessing adherence and factors associated with adherence in young children with asthma. Respirology. 2008;13(4):559‐563. [DOI] [PubMed] [Google Scholar]
- 20. Krishnan JA, Bender BG, Wamboldt FS, et al. Adherence to inhaled corticosteroids: an ancillary study of the childhood asthma management program clinical trial. J Allergy Clin Immunol. 2012;129(1):112‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas M, McKinley R, Freeman E, Foy C. Prevalence of dysfunctional breathing in patients treated for asthma in primary care: cross sectional survey. BMJ. 2001;322(7294):1098‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heaney LG, Robinson DS. Severe asthma treatment: need for characterising patients. Lancet. 2005;365(9463):974‐976. [DOI] [PubMed] [Google Scholar]


