Abstract
Background
Interleukin‐1β (IL‐1β) is a pro‐inflammatory cytokine mainly produced by monocytes and macrophages with a wide range of biological effects. Evidence has shown that IL‐1β plays a vital role in the process of apoptosis; however, the specific mechanisms, by which IL‐1β induces apoptosis, vary due to different cellular and experimental conditions. Therefore, this present reviewstudy aimed to systematically review the association between the molecular mechanisms of IL‐1β‐induced apoptosis in pathological processes and the role of signaling pathways. This article also sought to briefly investigate the potential of signaling pathway‐targeted therapy in the prevention and treatment of disease.
Methods
This is a literature review article. The present discourse aim is first to scrutinize and assess the available literature on IL‐1β and apoptosis. The relevant studies using the keywords of “IL‐1β‐induced apoptosis” and “signaling pathways” were searched in the databases of PubMed, Scopus, Google Scholar, and Web of Science. Gathered relevant material, and extracted information was then assessed.
Results
IL‐1β can induce apoptosis in various types of cells under different external stimuli via the mitochondrial pathway, death receptor pathway and endoplasmic reticulum pathway, and that the different pathways are often interconnected. The NF‐kB signaling pathway, p38MAPK, and JNK signaling pathways mainly play a proapoptotic part, and the ERK1/2 pathway has a bidirectional role in regulating apoptosis, while activation of the PI3K‐Akt signaling pathway can inhibit apoptosis.
Conclusion
This review indicates that IL‐1β‐induced apoptosis plays an important role in pathogenesis and development of pathology of many inflammatory diseases. Elucidating the role of the signaling pathways will aid the development of targeted therapeutic treatments.
Keywords: apoptosis, interleukin‐1β, MAPKs signaling pathway, NF‐κB signaling pathway, PI3K/Akt signaling pathway
Evidence has shown that interleukin‐1β (IL‐1β) plays a vital role in the process of apoptosis. This paper reviews the molecular mechanisms of IL‐1β‐induced apoptosis in pathological processes, focusing on the role of signaling pathways. This article also briefly describes the potential of signal pathway‐targeted therapy in the prevention and treatment of disease, using the application of signal pathways in the treatment of disc degeneration and osteoarthritis as examples.

1. INTRODUCTION
Interleukin‐1β (IL‐1β) is a quintessential pro‐inflammatory cytokine expressed primarily by monocytes and macrophages. The biological functions of IL‐1β mainly include mediating inflammatory responses and innate immunity, 1 and IL‐1β's ability to induce DNA methylation is closely related to the occurrence and development of tumors. 2 , 3 In addition, with the discovery of the relationship between osteoarthritis and IL‐1β‐induced chondrocyte apoptosis, 4 there is increasing evidence that IL‐1β can induce apoptosis, such as cardiomyocyte, 5 nucleus pulposus cells, 6 human umbilical vein endothelial cells, 7 β‐cells, 8 and fibrosus cells. 9
Apoptosis is the autonomous and orderly death of cells under certain physiological or pathological conditions, which is controlled by a series of activation, expression, and regulation of genes. Currently, the three well‐known pathways for apoptosis are mitochondria pathway, death receptor pathway, and endoplasmic reticulum stress pathway, whose ability to mediate IL‐1β‐induced apoptosis have been proven by studies. The difference between the mechanism of the three pathways in regulating the apoptosis process may result from the type, origin, growth environment of cells, and the external factors to initiate apoptosis, based on which the signal transduction pathways involved also differ.
1.1. IL‐1β overview
1.1.1. The interleukin‐1 superfamily
The interleukin‐1 superfamily includes IL‐1α, ‐1Ra, ‐18, ‐33, ‐36Ra, ‐36α, ‐36β, ‐3 6γ, ‐37, and ‐38, of which IL‐1β is one of the earliest discovered and most important members. The IL‐1β gene is encoded by seven exons and six introns. The abundance of AU sequence on the 3′ untranslated zone of the seventh exon causes IL‐1β to be extremely unstable and easily degradable by enzymes, 10 which is a prerequisite for IL‐1β to be cleavable.
1.1.2. Activation and action of IL‐1β
IL‐1β is not through the classical endoplasmic reticulum‐Golgi secretion pathway but sequential activation of vesicular or gasdermin d‐mediated secretory pathways. 11
In the cytoplasm, there is usually inactive pro‐interleukin‐1 (pro‐IL‐1β), which is the processed by the IL‐1β‐converting enzyme (ICE) and converted into active and mature IL‐1. The mature IL‐1βthen binds to the type 1 IL‐1 receptor (IL‐1R1) to exert biological functions.
1.2. Molecular mechanism of IL‐1β‐induced apoptosis
1.2.1. Mitochondria pathway
Mitochondria are organelles critical to the process of apoptosis. In experiments related to IL‐1β‐induced apoptosis of nucleus pulposus cells 12 and intervertebral disc cells 13 changes in protein expression levels of Bcl‐2, Bax, caspase‐9, and caspase‐3 can be detected, so it is inferred that IL‐1β significantly increases the proapoptotic protein Bax and decreases the antiapoptotic protein Bcl‐2. 6 , 14 The ratio of Bax to Bcl‐2 affects the mitochondrial membrane potential leaving the mitochondrial permeability transition pore open and the mitochondria highly impaired. 15 Then cytochrome‐c (cyt‐c) is released into the cytoplasm 16 triggering the downstream caspase‐9‐mediated endogenous apoptotic pathway. 17 Hydrogen sulfide 18 and appropriate mechanical stress 19 were found to improve apoptosis that is associated with mitochondrial dysfunction.
1.2.2. Death receptor pathway
In recent years, Hui et al. 8 found that the IL‐1β/Fas/caspase‐8 apoptotic pathway plays a role in amyloid‐induced β‐cell apoptosis in an in vitro cultured human islet amyloid models. Similarly, Park et al. found that the increase in IL‐1β levels is closely related to the upregulation of Fas and the activation of caspase‐8. 20 Therefore, it can be assumed that IL‐1β can induce the exogenous apoptosis pathway mediated by death receptors. Structural changes occur after the binding of Fas with the ligand FasL. Then, the activated Fas binds with the death domain, which aggregates Fas death domain‐associated protein to form a dimer. The dimer then further activates caspase‐8 triggering a downstream caspase‐3 cascade reaction, which finally leads to apoptosis. In addition, Kim et al. found that caspase‐8 cleaved the proapoptotic protein Bid into tBid, which is accompanied by the release of cyt‐c and the production of reactive oxygen species (ROS). Kim's discovery linked the death receptor pathway and the mitochondrial pathway. 21
1.2.3. Endoplasmic reticulum pathway
Dong et al. 22 found that the process of IL‐1β‐induced neuronal apoptosis was accompanied by Ca2+ imbalance and upregulation of endoplasmic reticulum stress‐related proteins GRP78 and CHOP. Li et al. 23 also found that IL‐1β‐induced chondrocytes underwent endoplasmic reticulum stress‐mediated apoptosis, along with elevated expression of GRP78, CHOP, and cleaved caspase‐12. All of the above experiments demonstrate that IL‐1β can induce endoplasmic reticulum stress (ERS)‐mediated apoptosis through the GRP78/CHOP/caspase‐12 pathway, the process of which often requires the involvement of Ca2+. 24 Recently, it has been found that drugs targeting this pathway are effective in reducing endoplasmic reticulum stress and IL‐1β‐induced apoptosis, thereby alleviating inflammatory disease. 25
For IL‐1β to induce apoptosis, a single pathway can work in a stand‐alone fashion or interact with other pathways to take effect collaboratively. Some scholars suggest that IL‐1β upregulates Bax, cyt‐c, caspase‐9, and caspase‐3 to induce apoptosis via the mitochondrial damage pathway. 9 , 15 Conversely, Park et al. 26 stated that apoptosis of intervertebral disc cells occurs only through the membrane receptor pathway and that the mitochondrial injury pathway is not involved in this process. However, Heyde's study found that both the membrane receptor pathway and the mitochondrial injury pathway are involved in apoptosis of intervertebral disc cells. 27 Additionally, IL‐1β not only activates the caspase‐dependent pathway but also indirectly promotes the occurrence of apoptosis by inhibiting the expression of members of the inhibitors of apoptosis proteins family. 28 The different findings may be due to different concentrations and duration of action of IL‐1β, and the specific mechanisms are to be validated in more different types of cells.
1.3. Signaling pathways involved in the regulation of IL‐1β‐induced apoptosis
IL‐1β‐induced apoptosis is the result of the interaction and joint regulation of multiple signaling pathways including nuclear factor‐κB (NF‐κB) signaling pathway, mitogen‐activated protein kinases (MAPKs) signaling pathway, and phosphoinositol‐3 kinase (PI3K)/Akt signaling pathway, etc. Among them, NF‐κB signaling pathway, p38 mitogen‐activated protein kinase (p38MAPK) signaling pathway, and c‐ Jun N‐ terminal kinase (JNK) signaling pathway mainly play a pro‐apoptotic role, and the extracellular signal‐regulated protein kinase (ERK) 1/2 pathway has a bidirectional role in regulating apoptosis, while activation of the PI3K‐Akt signaling pathway can inhibit apoptosis (Figure 1).
Figure 1.
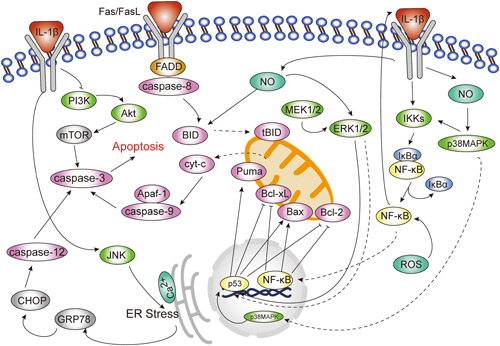
Role of signal transduction pathways in interleukin‐1β (IL‐1β)‐induced apoptosis. The nuclear factor‐κB (NF‐κB) signaling pathway, p38 mitogen‐activated protein kinase (p38MAPK), and c‐Jun N‐terminal kinase (JNK) signaling pathways mainly play a proapoptotic part, and the extracellular signal‐regulated protein kinase (ERK1/2) pathway has a bidirectional role in regulating apoptosis, while activation of the PI3K‐Akt signaling pathway can inhibit apoptosis.
1.3.1. NF‐κB signaling pathway
NF‐κB has been reported to be involved in regulation of the IL‐1β‐induced apoptosis in different diseases. 29 , 30 , 31 , 32 There are two different pathways for NF‐κB signaling activation: the classical pathway and the alternative pathway. 33 IL‐1β acts as an immune mediator to induce the classical NF‐κB pathway, where IL‐1β and IL‐1R binds to activate the IκB kinases (IKKs) complex. IKKs then degrade downstream inhibitor of NF‐κB (IκB) molecules to translocate NF‐κΒ into the nucleus to affect the transcription of related target genes. 34 , 35 Yun et al. used the inhibitor PS1145 to verify that activation of NF‐κB is essential to the process of IL‐1β‐inducing apoptosis, 32 and if phosphorylation of NF‐κB p65 is inhibited or reversed, 36 , 37 , 38 the activation of NF‐κB and nuclear transposition are affected, 39 , 40 , 41 thus, exerting antiapoptotic effects. To further explore the specific mechanism, Guo et al. found that the expression of NF‐κB‐regulated gene products and the level of proapoptotic protein caspase‐3 were significantly increased in the IL‐1β treatment group of synovial cells in rheumatoid arthritis rats, while the expression of antiapoptotic proteins Bcl‐2 and Bcl‐xL was significantly decreased. 31
NF‐κB is central to the action of many pro‐inflammatory factors. NF‐κB is activated by pro‐inflammatory factors, which in turn directly or indirectly upregulates their levels to create a positive feedback. 33 IL‐1β is one of these pro‐inflammatory factors to activate NF‐κB. The activation of NF‐κB upregulates the release of IL‐1β, IL‐6, 42 and tumor necrosis factor‐α (TNF‐α), 43 which fuels apoptosis 44 , 45 and inflammation. 46 , 47 ROS activated by IL‐1β is another pro‐inflammatory factors. 48 NF‐κB, as a downstream molecule of ROS 49 when activated, stimulates the release of pro‐inflammatory factors such as cyclo‐oxygenase‐2 (COX‐2), prostaglandin E2 (PGE2), and NO. 50 Then, the NO in turn activates NF‐κB in a p38MAPK‐dependent manner, forming a positive feedback pathway that promotes apoptosis. Some research focus on reducing the production of ROS and NO, and so on, so as to inhibit IL‐1β‐induced apoptosis and inflammation progression. 51 , 52 , 53 , 54 However, it has been suggested that the effect of ROS on NF‐κB may be related to the duration of oxidative stress, so the sustained oxidative stress may instead inhibit NF‐κB activity. 55
1.3.2. MAPKs signaling pathway
Extensive studies have shown that the MAPKs signaling pathway is activated by various physical, chemical, biological, and other stimuli, and plays a key role in apoptosis. Typical MAPK family signaling pathways mainly include ERK, JNK, and p38MAPK. There is extensive traffic between the pathways, and these kinases integrate signals at different sites through transcription‐dependent and transcription‐independent mechanisms, ultimately converging on caspase activation to lead to apoptosis. Tu et al. found that the phosphorylation levels of JNK, p38MAPK, and ERK were significantly higher than those in the control group after IL‐1β stimulation for 48 h. 39 One of the mechanisms by which drugs can suppress the progression of arthritis is by reducing IL‐1β‐induced phosphorylation of MAPKs. 18 , 56 Therefore, it can be assumed that IL‐1β activates the MAPKs pathway, which induces apoptosis.
p38MAPK signaling pathway
The p38MAPK signaling pathway is involved in regulating the IL‐1β‐induced apoptosis 57 with nitric oxide (NO) 58 and NF‐κB 59 being important molecules involved in this pathway. Inducible nitric oxide synthase (iNOS) and NO are present in apoptotic cells induced by IL‐1β. iNOS is an upstream signaling molecule for NO. NO can be antiapoptotic or pro‐apoptotic, depending on its concentration, source, and duration.
NO of High concentrations can promote apoptosis in a variety of cells, and the proapoptotic mechanisms may include the following three: (1) NO promotes apoptosis by regulating Bax/Bcl‐2 proteins and upregulating caspase‐3 through a p38/p53‐dependent pathway, 60 , 61 while blocking the p38/p53 pathway to prevent IL‐1β‐induced neuronal apoptosis. 62 Upregulation of p53 specifically involves two pathways: first, p38 phosphorylates the upstream kinase of IκB, which activates NF‐κB and increases p53 transcription; second, p38 can directly phosphorylate Serine 15 residues of p53, thereby stabilizing p53 protein content. 63 (2) NO forms ONOO‐ with O2‐, leading to mitochondrial dysfunction, cleavage of Bid, and activation of Fas to enable cascade reaction with downstream caspase‐8. 64 (3) In an in vitro experiment on rat pheochromocytoma cells, Li et al. found that NO upregulated COX‐2 expression by enhancing the binding of activator protein‐1 (AP‐1) to DNA leading to p53 accumulation and apoptosis. 65
The antiapoptotic mechanisms of NO of low concentrations include inducing the formation of heat shock protein 70, 66 enhancing Bcl‐2 expression, 67 and directly inhibiting caspase‐8 protease activity, 68 and so on. In mammals, the antiapoptotic properties of NO are often associated with cyclic guanylate cyclase (cGMP) dependence. 69 Although NO is associated with apoptosis, the available studies on the involvement of NO in the regulation of IL‐1β‐induced apoptosis are mainly related to p53. So, it remains unclear whether the other related mechanisms mentioned above play a role in IL‐1β‐induced apoptosis.
JNK signaling pathway
IL‐1β induces JNK signaling, 37 but intervention with the drug 70 , 71 or the JNK inhibitor SP600125 72 , 73 can inhibit IL‐1β‐induced JNK1/2 activation and apoptosis. A range of evidence links the JNK signaling pathway to IL‐1β‐induced apoptosis. The specific mechanisms may be: (1) The JNK pathway may be an upstream pathway of endoplasmic reticulum stress. Previous studies have shown that IL‐1β induces endoplasmic reticulum stress and apoptosis in pancreatic β‐cells in a JNK‐dependent manner. 74 (2) Promote apoptosis via the JNK/p53 pathway. 75 Ling et al. verified that lower JNK levels and reduced p53 phosphorylation were associated with inhibition of neuronal apoptosis. 76 One explanation might be that the phosphorylated JNK activates p53, which phosphorylates itself at the proline‐rich structural domain Threonine 81 site. Then Phosphorylated p53 upregulates the expression of various proapoptotic target genes such as Puma 77 and Bax, 78 while decreasing the expression of antiapoptotic genes such as Bcl‐2 72 and Bcl‐xL, resulting in cyt‐c release and apoptosis. However, in some cases, p53 can promote apoptosis independently of JNK activation. 79 At present, it is unclear whether JNK nondependent activation of p53 is associated with the process of IL‐1β‐induced apoptosis.
ERK signaling pathway
The ERK1/2 pathway has both antiapoptotic and proapoptotic effects, depending on the experimental conditions and/or cell types. One of the mechanisms involved in IL‐1β‐induced apoptosis may be through the MEK/ERK signaling pathway, 80 which increases the expression of Bax and caspase‐3 and decreases the expression of Bcl‐2. Inhibition of ERK1/2 may block IL‐1β‐induced mitochondrial damage and apoptosis. 81 Upregulation of p53 levels may also be one of the mechanisms. The experiments of Sun et al. 82 and Lee et al. 83 found that ERK1/2, an upstream signal of p53, induces apoptosis by activating the ERK1/2‐p53 pathway and upregulating caspase‐3. However, it has also been shown that after activation of ERK1/2 by NO, ERK1/2 instead inhibits p53 phosphorylation thereby protecting chondrocytes from apoptosis. 84 All of the above experiments can demonstrate that the ERK1/2‐p53 pathway plays an important role in apoptosis, but it is still unclear how this pathway affects IL‐1β‐induced apoptosis so further experimental studies are needed.
PI3K/Akt signaling pathway
In recent years, there has been increasing evidence that regulation of the cell cycle through the PI3K/Akt signaling pathway can inhibit IL‐1β‐induced apoptosis and inflammatory. 85 , 86 First, Cai et al. found that compared to normal cells and tissues, apoptosis was increased in IL‐1β‐treated articular chondrocytes accompanied by downregulation of PI3K mRNA levels, 4 which demonstrated that PI3K is associated with apoptosis. Next, Guo et al. found that 17β‐estradiol attenuated IL‐1β‐induced early apoptosis in nucleus pulposus cells by increasing the expression of activated mechanistic target of rapamycin (mTOR) and decreasing the expression of activated caspase‐3, in intervertebral disc lesions. 87 Yan et al. 88 showed that IL‐1β‐induced chondrocyte apoptosis is due to the weakening of the PI3K/Akt/mTOR pathway and that activation of this pathway is protective against apoptosis. In addition, Xu et al. 89 suggested that the suppression of apoptosis by drugs through the PI3K/Akt pathway may also be associated with altered mitochondrial membrane potential.
1.4. Targeted therapeutic applications
IL‐1β has been shown to play an integral role in the pathology of many inflammatory diseases, including the induction of apoptosis and the mediation of inflammatory responses. The clarification of the mechanisms of disease progression provides the molecular basis for research into new drugs and opens up new rationales. Signaling pathway‐targeted therapies have been studied more centrally in the treatment of disc degeneration and osteoarthritis, and as such are illustrated below as an example.
A recent review revealed that the activation of the PI3K/Akt signaling pathway alleviated intervertebral disc degeneration and focused attention on the therapeutic effects of estrogen. 90 Resveratrol increased the ratio of IL‐1β‐induced p‐Akt protein expression to Akt protein expression in myeloid cells with partial inhibition of apoptosis‐related molecules, and its protective effect against IL‐1β‐induced apoptosis in NP cells was diminished when the inhibitor LY294002 was further added. 14 Likewise, other studies also suggested the effect of 17β‐estradiol, 91 proanthocyanidins, 92 and omentin‐1 93 against apoptosis induced by IL‐1β in NP cells via the PI3K/Akt pathway. In the other hand, the NF‐κB pathway is also an effective intervention site. IL‐1β‐mediated phosphorylation of p65 and translocation of NF‐κB were attenuated by the addition of berberine, indicating that activation of the NF‐κB pathway was inhibited by berberine. In addition, berberine prevented the degradation of the extracellular matrix of nucleus pulposus cells and has therapeutic potential in the prevention or treatment of intervertebral disc degeneration. 40
In IL‐1β‐induced chondrocyte injury, NaHS partially inhibited IL‐1β‐induced phosphorylation of the MAPK cascade, significantly reversing apoptosis associated with mitochondrial dysfunction 18 ; LMP‐1 suppressed the expression of p‐p65 and p‐JNK, reducing apoptosis by regulating NF‐κB and MAPK/JNK pathways. 71 Tricetin repressed the expression of MMPs and IL‐1β‐induced NO and PGE2 production, while markedly attenuating IL‐1β‐induced phosphorylation of JNK and p38, exerting antiapoptotic effects via Bax/Bcl‐2/caspase‐3. 94 Tetramethylpyrazine protects chondrocytes by reducing endoplasmic reticulum stress through inhibition of IL‐1β‐induced GRP78 and CHOP expression, 95 which is likely to be a potential therapeutic agent for osteoarthritis.
Apart from the currently reported drugs, numerous other biotherapeutic studies have been published. circular RNAs 96 , 97 , 98 and Long noncoding RNAs 99 , 100 , 101 are currently a hot topic of research, and their antiapoptotic mechanisms are not identical to those of traditional signal pathways, pending further in‐depth studies at the molecular level.
2. CONCLUSION AND PROSPECTS
This paper reviews various literature and studies to indicate that IL‐1β can induce apoptosis in various types of cells under different external stimuli via the mitochondrial pathway, death receptor pathway and endoplasmic reticulum pathway, and that the different pathways are often interconnected. The NF‐κB signaling pathway, p38MAPK, and JNK signaling pathways mainly play a proapoptotic part, and the ERK1/2 pathway has a bidirectional role in regulating apoptosis, while activation of the PI3K‐Akt signaling pathway can inhibit apoptosis. As apoptosis is a complex process, the molecules, substrates, mechanisms of action and regulation of the signal transduction pathway remain to be further investigated. The study of signal pathways has led to a clearer understanding of the pathophysiology of disease and has provided a theoretical basis and new ideas for the development of targeted therapeutics that interfere specifically with the expression of molecules in one or more signal pathways to stop disease progression accurately and effectively. We look forward to a future where the treatment of IL‐1β and its signal pathways may become a new target for the treatment of inflammatory diseases, and inhibitors of the caspase downstream pathway are promising new loci for treatment. At present, many of these targeted therapeutics remain in in vitro models with a long way to go before they can be used clinically. In addition, the research of normal physiological activity is no less important than the study of the onset of disease.
In the future, it may be possible to explore the role of IL‐1β in inducing apoptosis during physiological processes, such as physiological root resorption in deciduous teeth, based on an understanding of the IL‐1β signal pathway, which has great potential for preventive applications.
CONFLICT OF INTEREST
The authors declare no competing interests.
ACKNOWLEDGMENT
This study did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Wang P, Qian H, Xiao M, Lv J. Role of signal transduction pathways in IL‐1β‐induced apoptosis: pathological and therapeutic aspects. Immun Inflamm Dis. 2023;11:e762. 10.1002/iid3.762
DATA AVAILABILITY STATEMENT
All the information included in this manuscript is available upon request by contact with the corresponding author.
REFERENCES
- 1. Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin‐1 and related cytokines in the regulation of inflammation and immunity. Immunity. 2019;50(4):778‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim HJ, Kim N, Park JH, Choi S, Shin CM, Lee OJ. Helicobacter pylori eradication induced constant decrease in interleukin‐1B expression over more than 5 years in patients with gastric cancer and dysplasia. Gut Liver. 2020;14(6):735‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hernandez‐Vargas H, Goldsmith C, Mathot P, Dante R. Stromal‐associated cytokines bias the interplay between gene expression and DNA methylation in human breast cancers. Epigenetics. 2020;15(5):511‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai C, Min S, Yan B, et al. MiR‐27a promotes the autophagy and apoptosis of IL‐1β treated‐articular chondrocytes in osteoarthritis through PI3K/AKT/mTOR signaling. Aging. 2019;11(16):6371‐6384. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5. Zhao Z, Du S, Shen S, Wang L. microRNA‐132 inhibits cardiomyocyte apoptosis and myocardial remodeling in myocardial infarction by targeting IL‐1β. J Cell Physiol. 2020;235(3):2710‐2721. [DOI] [PubMed] [Google Scholar]
- 6. Wang K, Chen T, Ying X, et al. Ligustilide alleviated IL‐1β induced apoptosis and extracellular matrix degradation of nucleus pulposus cells and attenuates intervertebral disc degeneration in vivo. Int Immunopharmacol. 2019;69:398‐407. [DOI] [PubMed] [Google Scholar]
- 7. Zhang L, Wang F, Zhang Q, et al. Anti‐inflammatory and anti‐apoptotic effects of stybenpropol A on human umbilical vein endothelial cells. Int J Mol Sci. 2019;20(21):5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hui Q, Asadi A, Park YJ, et al. Amyloid formation disrupts the balance between interleukin‐1β and interleukin‐1 receptor antagonist in human islets. Mol Metab. 2017;6(8):833‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang X, Wang L, Yuan Z, et al. Interleukin‐1β induces apoptosis in annulus fibrosus cells through the extracellular signal‐regulated kinase pathway. Connect Tissue Res. 2018;59(6):593‐600. [DOI] [PubMed] [Google Scholar]
- 10. Dinarello CA, Donath MY, Mandrup‐Poulsen T. Role of IL‐1β in type 2 diabetes. Curr Opin Endocrinol Diab Obes. 2010;17(4):314‐321. [DOI] [PubMed] [Google Scholar]
- 11. Semino C, Carta S, Gattorno M, Sitia R, Rubartelli A. Progressive waves of IL‐1β release by primary human monocytes via sequential activation of vesicular and gasdermin D‐mediated secretory pathways. Cell Death Dis. 2018;9(11):1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun K, Zhu J, Sun J, et al. Neuropeptide Y prevents nucleus pulposus cells from cell apoptosis and IL‑1β‑induced extracellular matrix degradation. Cell Cycle. 2021;20(10):960‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li B, Lu ZY, Jiang SD, Jiang LS, Zheng XF. Smad7 is highly expressed in human degenerative discs and participates in IL‐1β‐induced apoptosis of rat AF cells via the mitochondria pathway. Oxid Med Cell Longevity. 2022;2022:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang Y, Xie Z, Yu J, Fu L. Resveratrol inhibits IL‐1β‐mediated nucleus pulposus cell apoptosis through regulating the PI3K/Akt pathway. Biosci Rep. 2019;39(3):BSR20190043. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Shen J, Xu S, Zhou H, et al. IL‐1β induces apoptosis and autophagy via mitochondria pathway in human degenerative nucleus pulposus cells. Sci Rep. 2017;7(1):41067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qian H, Huang Q, Chen Y, Liu Q, Fang J, Ye M. Caspase‑9 was involved in cell apoptosis in human dental pulp stem cells from deciduous teeth. Mol Med Rep. 2018;18:1067‐1073. [DOI] [PubMed] [Google Scholar]
- 17. Vico TA, Marchini T, Ginart S, et al. Mitochondrial bioenergetics links inflammation and cardiac contractility in endotoxemia. Basic Res Cardiol. 2019;114(5):38. [DOI] [PubMed] [Google Scholar]
- 18. Wang B, Shao Z, Gu M, et al. Hydrogen sulfide protects against IL‐1β‐induced inflammation and mitochondrial dysfunction‐related apoptosis in chondrocytes and ameliorates osteoarthritis. J Cell Physiol. 2021;236(6):4369‐4386. [DOI] [PubMed] [Google Scholar]
- 19. Zhang J, Hao X, Chi R, Qi J, Xu T. Moderate mechanical stress suppresses the IL‐1β‐induced chondrocyte apoptosis by regulating mitochondrial dynamics. J Cell Physiol. 2021;236(11):7504‐7515. [DOI] [PubMed] [Google Scholar]
- 20. Park YJ, Warnock GL, Ao Z, et al. Dual role of interleukin‐1β in islet amyloid formation and its β‐cell toxicity: implications for type 2 diabetes and islet transplantation. Diabetes, Obes Metab. 2017;19(5):682‐694. [DOI] [PubMed] [Google Scholar]
- 21. Kim WS, Lee KS, Kim JH, et al. The caspase‐8/Bid/cytochrome c axis links signals from death receptors to mitochondrial reactive oxygen species production. Free Radic Biol Med. 2017;112:567‐577. [DOI] [PubMed] [Google Scholar]
- 22. Dong Y, Kalueff AV, Song C. N‐methyl‐d‐aspartate receptor‐mediated calcium overload and endoplasmic reticulum stress are involved in interleukin‐1beta‐induced neuronal apoptosis in rat hippocampus. J Neuroimmunol. 2017;307:7‐13. [DOI] [PubMed] [Google Scholar]
- 23. Li ZZ, Wang F, Liu S, Li H, Wang Y. Ablation of PKM2 ameliorated ER stress‐induced apoptosis and associated inflammation response in IL‐1β‐treated chondrocytes via blocking Rspo2‐mediated Wnt/β‐catenin signaling. J Cell Biochem. 2020;121(10):4204‐4213. [DOI] [PubMed] [Google Scholar]
- 24. Zhang IX, Raghavan M, Satin LS. The endoplasmic reticulum and calcium homeostasis in pancreatic beta cells. Endocrinology. 2020;161(2):bqz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng Y, Chen X, Lan T, Yan B, Zhang R. Panax notoginseng saponin reduces IL‐1β‐stimulated apoptosis and endoplasmic reticulum stress of nucleus pulposus cells by suppressing miR‐222‐3p. Ann Transl Med. 2022;10(13):748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park JB, Park IC, Park SJ, et al. Anti‐apoptotic effects of caspase inhibitors on rat intervertebral disc cells. J Bone Joint Surg. 2006;88(4):771‐779. [DOI] [PubMed] [Google Scholar]
- 27. Heyde CE, Tschoeke SK, Hellmuth M, Hostmann A, Ertel W, Oberholzer A. Trauma induces apoptosis in human thoracolumbar intervertebral discs. BMC Clin Pathol. 2006;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen Y, Qin J, Bu P. Pathways involved in interleukin‐1β‐mediated murine cardiomyocyte apoptosis. Tex Heart Inst J. 2015;42(2):109‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu S, Li Y, Wang B, Peng K. TRIM38 protects chondrocytes from IL‐1β‐induced apoptosis and degeneration via negatively modulating nuclear factor (NF)‐κB signaling. Int Immunopharmacol. 2021;99:108048. [DOI] [PubMed] [Google Scholar]
- 30. Zhu C, Jiang W, Cheng Q, Hu Z, Hao J. Hemeoxygenase‐1 suppresses IL‐1β‐induced apoptosis through the NF‐κB pathway in human degenerative nucleus pulposus cells. Cell Physiol Biochem. 2018;46(2):644‐653. [DOI] [PubMed] [Google Scholar]
- 31. Guo JT, Cao XQ, Wu LL, et al. Effect of IL‐1β on apoptosis of synovial cells in rheumatoid arthritis rats via the NF‐κB pathway. Eur Rev Med Pharmacol Sci. 2019;23(23):10211‐10217. [DOI] [PubMed] [Google Scholar]
- 32. Yun JH. Interleukin‐1β induces pericyte apoptosis via the NF‐κB pathway in diabetic retinopathy. Biochem Biophys Res Commun. 2021;546:46‐53. [DOI] [PubMed] [Google Scholar]
- 33. Lepetsos P, Papavassiliou KA, Papavassiliou AG. Redox and NF‐κB signaling in osteoarthritis. Free Radic Biol Med. 2019;132:90‐100. [DOI] [PubMed] [Google Scholar]
- 34. Oliver KM, Garvey JF, Ng CT, et al. Hypoxia activates NF‐κB–dependent gene expression through the canonical signaling pathway. Antioxid Redox Signaling. 2009;11(9):2057‐2064. [DOI] [PubMed] [Google Scholar]
- 35. Surai PF, Kochish II, Kidd MT. Redox homeostasis in poultry: regulatory roles of NF‐κB. Antioxidants. 2021;10(2):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ji B, Guo W, Ma H, et al. Isoliquiritigenin suppresses IL‐1β induced apoptosis and inflammation in chondrocyte‐like ATDC5 cells by inhibiting NF‐κB and exerts chondroprotective effects on a mouse model of anterior cruciate ligament transection. Int J Mol Med. 2017;40:1709‐1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nano E, Petropavlovskaia M, Rosenberg L. Islet neogenesis associated protein (INGAP) protects pancreatic β cells from IL‐1β and IFNγ‐induced apoptosis. Cell Death Discov. 2021;7(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen T, Zhou R, Chen Y, et al. Curcumin ameliorates IL‐1β‐induced apoptosis by activating autophagy and inhibiting the NF‐κB signaling pathway in rat primary articular chondrocytes. Cell Biol Int. 2021;45(5):976‐988. [DOI] [PubMed] [Google Scholar]
- 39. Rao Z, Wang S, Wang J. Protective effects of psoralidin on IL‑1β‑induced chondrocyte apoptosis. Mol Med Rep. 2018;17(2):3418‐3424. [DOI] [PubMed] [Google Scholar]
- 40. Lu L, Hu J, Wu Q, et al. Berberine prevents human nucleus pulposus cells from IL‑1β‑induced extracellular matrix degradation and apoptosis by inhibiting the NF‑κB pathway. Int J Mol Med. 2019;43(4):1679‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tu J, Li W, Zhang Y, et al. Simvastatin inhibits IL‐1β‐induced apoptosis and extracellular matrix degradation by suppressing the NF‐kB and MAPK pathways in nucleus pulposus cells. Inflammation. 2017;40(3):725‐734. [DOI] [PubMed] [Google Scholar]
- 42. Arora R, Van Theemsche KM, Van Remoortel S, Snyders DJ, Labro AJ, Timmermans JP. Constitutive, basal, and β‐alanine‐mediated activation of the human mas‐related G protein‐coupled receptor D induces release of the inflammatory cytokine IL‐6 and is dependent on NF‐κB signaling. Int J Mol Sci. 2021;22(24):13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen T, Zhang X, Zhu G, et al. Quercetin inhibits TNF‐α induced HUVECs apoptosis and inflammation via downregulating NF‐kB and AP‐1 signaling pathway in vitro. Medicine. 2020;99(38):e22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie Q, Yang Z, Huang X, et al. Ilamycin C induces apoptosis and inhibits migration and invasion in triple‐negative breast cancer by suppressing IL‐6/STAT3 pathway. J Hematol Oncol. 2019;12(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ezquerro S, Mocha F, Frühbeck G, et al. Ghrelin reduces TNF‐α‐induced human hepatocyte apoptosis, autophagy, and pyroptosis: role in obesity‐associated NAFLD. J Clin Endocrinol Metab. 2019;104(1):21‐37. [DOI] [PubMed] [Google Scholar]
- 46. Guo C, He L, Hu N, et al. Aconiti lateralis radix praeparata lipid‐soluble alkaloids alleviates IL‐1β‐induced inflammation of human fibroblast‐like synoviocytes in rheumatoid arthritis by inhibiting NF‐κB and MAPKs signaling pathways and inducing apoptosis. Cytokine. 2022;151:155809. [DOI] [PubMed] [Google Scholar]
- 47. Zhu F, Duan W, Zhong C, Ji B, Liu X. The protective effects of dezocine on interleukin‐1β‐induced inflammation, oxidative stress and apoptosis of human nucleus pulposus cells and the possible mechanisms. Bioengineered. 2022;13(1):1399‐1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jia X, Luo Z, Gao Y, et al. EGCG upregulates UCP3 levels to protect MIN6 pancreatic islet cells from interleukin‐1β‐induced apoptosis. DDDT. 2020;14:4251‐4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. An Y, Zhang H, Wang C, et al. Activation of ROS/MAPKs/NF‐κB/NLRP3 and inhibition of efferocytosis in osteoclast‐mediated diabetic osteoporosis. FASEB J. 2019;33(11):12515‐12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xue EX, Lin JP, Zhang Y, et al. Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway. Oncotarget. 2017;8(26):41988‐42000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rao Z, Wang S, Wang J. Peroxiredoxin 4 inhibits IL‐1β‐induced chondrocyte apoptosis via PI3K/AKT signaling. Biomed Pharmacother. 2017;90:414‐420. [DOI] [PubMed] [Google Scholar]
- 52. Xian Bo S, Yan Jie W, De Chao C, et al. An inducible nitric oxide synthase dimerization inhibitor prevents the progression of osteoarthritis. Front Pharmacol. 2022;13:861183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kang H, Dong Y, Peng R, et al. Inhibition of IRE1 suppresses the catabolic effect of IL‐1β on nucleus pulposus cell and prevents intervertebral disc degeneration in vivo. Biochem Pharmacol. 2022;197:114932. [DOI] [PubMed] [Google Scholar]
- 54. Liu C, Chen Y. Ketorolac tromethamine alleviates IL‐1β‐induced chondrocyte injury by inhibiting COX‐2 expression. Exp Ther Med. 2022;23(5):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu M, Bian Q, Liu Y, et al. Sustained oxidative stress inhibits NF‐κB activation partially via inactivating the proteasome. Free Radic Biol Med. 2009;46(1):62‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shen Y, Teng L, Qu Y, et al. Anti‐proliferation and anti‐inflammation effects of corilagin in rheumatoid arthritis by downregulating NF‐κB and MAPK signaling pathways. J Ethnopharmacol. 2022;284:114791. [DOI] [PubMed] [Google Scholar]
- 57. Cai L, Lei C, Li R, Chen WN, Li CM. Aquaporin‐4 blockage by siRNA protects rat articular chondrocytes from IL‐1β‐induced apoptosis by inhibiting p38 MAPK signal pathway. Ann Clin Lab Sci. 2017;47(5):563‐571. [PubMed] [Google Scholar]
- 58. Fei J, Liang B, Jiang C, Ni H, Wang L. Luteolin inhibits IL‐1β‐induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed Pharmacother. 2019;109:1586‐1592. [DOI] [PubMed] [Google Scholar]
- 59. Kim HL, Lee HJ, Lee DR, Choi BK, Yang SH. Anti‐osteoarthritic effects of an herbal composition LI73014F2 on Interleukin‐1β‐induced primary human articular chondrocytes. Molecules. 2020;25(9):2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. FU HY, Shen L, Gao XS, Cui DX, Cui ZY. SF1670 inhibits apoptosis and inflammation via the PTEN/Akt pathway and thus protects intervertebral disc degeneration. Eur Rev Med Pharmacol Sci. 2020;24:8694‐8702. [DOI] [PubMed] [Google Scholar]
- 61. Choi JB, Kim JH, Lee H, Pak JN, Shim BS, Kim SH. Reactive oxygen species and p53 mediated activation of p38 and caspases is critically involved in kaempferol induced apoptosis in colorectal cancer cells. J Agricult Food Chem. 2018;66(38):9960‐9967. [DOI] [PubMed] [Google Scholar]
- 62. Shan H, Bian Y, Shu Z, et al. Fluoxetine protects against IL‐1β‐induced neuronal apoptosis via downregulation of p53. Neuropharmacology. 2016;107:68‐78. [DOI] [PubMed] [Google Scholar]
- 63. Kim SJ, Hwang SG, Shin DY, Kang SS, Chun JS. p38 kinase regulates nitric oxide‐induced apoptosis of articular chondrocytes by accumulating p53 via NFκB‐dependent transcription and stabilization by serine 15 phosphorylation. J Biol Chem. 2002;277(36):33501‐33508. [DOI] [PubMed] [Google Scholar]
- 64. Cui ZG, Kondo T, Matsumoto H. Enhancement of apoptosis by nitric oxide released from α‐phenyl‐tert‐butyl nitrone under hyperthermic conditions. J Cell Physiol. 2006;206(2):468‐476. [DOI] [PubMed] [Google Scholar]
- 65. Li MH, Jang JH, Surh YJ. Nitric oxide induces apoptosis via AP‐1‐driven upregulation of COX‐2 in rat pheochromocytoma cells. Free Radic Biol Med. 2005;39(7):890‐899. [DOI] [PubMed] [Google Scholar]
- 66. Mazzei L, Cuello‐Carrión FD, Docherty N, Manucha W. Heat shock protein 70/nitric oxide effect on stretched tubular epithelial cells linked to WT‐1 cytoprotection during neonatal obstructive nephropathy. Int Urol Nephrol. 2017;49(10):1875‐1892. [DOI] [PubMed] [Google Scholar]
- 67. Chanvorachote P, Nimmannit U, Stehlik C, et al. Nitric oxide regulates cell sensitivity to cisplatin‐induced apoptosis through S‐nitrosylation and inhibition of Bcl‐2 ubiquitination. Cancer Res. 2006;66(12):6353‐6360. [DOI] [PubMed] [Google Scholar]
- 68. Kim YM, Kim TH, Chung HT, Talanian RV, Yin XM, Billiar TR. Nitric oxide prevents tumor necrosis factor α–induced rat hepatocyte apoptosis by the interruption of mitochondrial apoptotic signaling through S‐nitrosylation of caspase‐8. Hepatology. 2000;32(4 Pt 1):770‐778. [DOI] [PubMed] [Google Scholar]
- 69. Khan FH, Dervan E, Bhattacharyya DD, McAuliffe JD, Miranda KM, Glynn SA. The role of nitric oxide in cancer: master regulator or not? Int J Mol Sci. 2020;21(24):9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhong L, Cao L, Song R, et al. Glutamine exerts a protective effect on osteoarthritis development by inhibiting the Jun N‐terminal kinase and nuclear factor Kappa‐b signaling pathways. Sci Rep. 2022;12(1):11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ou D, Liu S, Tong C, Tan H, Yang Y, He C. LIM mineralization protein‐1 inhibits IL‐1β‐induced human chondrocytes injury by altering the NF‐κB and MAPK/JNK pathways. Exp Ther Med. 2021;23(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang Q, Yin Z, Zhang F, Cao K, Sun H. CTHRC1 mediates IL‑1β‑induced apoptosis in chondrocytes via JNK1/2 signaling. Int J Mol Med. 2018;41:2270‐2278. [DOI] [PubMed] [Google Scholar]
- 73. Zhang C, Yang Y, Gao Y, Sun D. NaF‐induced neurotoxicity via activation of the IL‐1β/JNK signaling pathway. Toxicology. 2022;469:153132. [DOI] [PubMed] [Google Scholar]
- 74. Wang Q, Zhang H, Zhao B, Fei H. IL‐1β caused pancreatic β‐cells apoptosis is mediated in part by endoplasmic reticulum stress via the induction of endoplasmic reticulum Ca2+ release through the c‐Jun N‐terminal kinase pathway. Mol Cell Biochem. 2009;324(1‐2):183‐190. [DOI] [PubMed] [Google Scholar]
- 75. Lu L, Ma J, Sun M, et al. Melatonin ameliorates MI‐induced cardiac remodeling and apoptosis through a JNK/p53‐dependent mechanism in diabetes mellitus. Oxid Med Cell Longevity. 2020;2020:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ling GQ, Li XF, Lei XH, et al. C‑Jun N‑terminal kinase inhibition attenuates early brain injury induced neuronal apoptosis via decreasing p53 phosphorylation and mitochondrial apoptotic pathway activation in subarachnoid hemorrhage rats. Mol Med Rep. 2019;19(1):327‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Song H, Wei M, Liu W, Shen S, Li J, Wang L. Cisplatin induced apoptosis of ovarian cancer A2780s cells by activation of ERK/p53/PUMA signals. Histol. Histopathol. 2018;33(1):73‐79. [DOI] [PubMed] [Google Scholar]
- 78. Wolf ER, McAtarsney CP, Bredhold KE, Kline AM, Mayo LD. Mutant and wild‐type p53 form complexes with p73 upon phosphorylation by the kinase JNK. Sci Signal. 2018;11(524):eaao4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hu K, Gong X, Ai Q, et al. Endogenous AMPK acts as a detrimental factor in fulminant hepatitis via potentiating JNK‐dependent hepatocyte apoptosis. Cell Death Dis. 2017;8(3):e2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhou B, Chen D, Xu H, Zhang X. Proliferation of rabbit chondrocyte and inhibition of IL‐1β‐induced apoptosis through MEK/ERK signaling by statins. In Vitro Cell Dev Biol Anim. 2017;53(2):124‐131. [DOI] [PubMed] [Google Scholar]
- 81. Ansari MY, Novak K, Haqqi TM. ERK1/2‐mediated activation of DRP1 regulates mitochondrial dynamics and apoptosis in chondrocytes. Osteoarth Cartil. 2022;30(2):315‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sun CY, Zhu Y, Li XF, et al. Scutellarin increases cisplatin‐induced apoptosis and autophagy to overcome cisplatin resistance in non‐small cell lung cancer via ERK/p53 and c‐met/AKT signaling pathways. Front Pharmacol. 2018;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lee HJ, Oh SY, Jo I. Zearalenone induces endothelial cell apoptosis through activation of a cytosolic Ca2+/ERK1/2/p53/Caspase 3 signaling pathway. Toxins. 2021;13(3):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kim SJ, Ju JW, Oh CD, et al. ERK‐1/2 and p38 kinase oppositely regulate nitric oxide‐induced apoptosis of chondrocytes in association with p53, caspase‐3, and differentiation status. J Biol Chem. 2002;277(2):1332‐1339. [DOI] [PubMed] [Google Scholar]
- 85. Wu G, Xue P, Jin H, et al. Upregulation of P2Y12 inhibits chondrocyte apoptosis in lumbar osteoarthritis through the PI3K/AKT signaling pathway. Mol Biol Rep. 2022;49(7):6459‐6466. [DOI] [PubMed] [Google Scholar]
- 86. Shi X, Jie L, Wu P, et al. Calycosin mitigates chondrocyte inflammation and apoptosis by inhibiting the PI3K/AKT and NF‐κB pathways. J Ethnopharmacol. 2022;297:115536. [DOI] [PubMed] [Google Scholar]
- 87. Guo HT, Yang SD, Zhang F, et al. 17β‑Estradiol protects against interleukin‑1β‑induced apoptosis in rat nucleus pulposus cells via the mTOR/caspase‑3 pathway. Mol Med Rep. 2019;20(2):1523‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yan S, Jiang C, Li H, Li D, Dong W. FAM3A protects chondrocytes against interleukin‐1β‐induced apoptosis through regulating PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun. 2019;516(1):209‐214. [DOI] [PubMed] [Google Scholar]
- 89. Xu Z, Li X, Shen G, et al. The protective effect of ginsenoside Rg1 on apoptosis in human ankle joint traumatic arthritis chondrocytes. Evid Based Complement Alter Med. 2022;2022:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yang S, Zhang F, Ma J, Ding W. Intervertebral disc ageing and degeneration: the antiapoptotic effect of oestrogen. Ageing Res Rev. 2020;57:100978. [DOI] [PubMed] [Google Scholar]
- 91. Yang SD, Ma L, Yang DL, Ding WY. Combined effect of 17β‐estradiol and resveratrol against apoptosis induced by interleukin‐1β in rat nucleus pulposus cells via PI3K/Akt/caspase‐3 pathway. PeerJ. 2016;4:e1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen HW, Liu MQ, Zhang GZ, et al. Proanthocyanidins inhibit the apoptosis and aging of nucleus pulposus cells through the PI3K/Akt pathway delaying intervertebral disc degeneration. Connect Tissue Res. 2022;63:650‐662. [DOI] [PubMed] [Google Scholar]
- 93. Cabral VLF, Wang F, Peng X, et al. Omentin‐1 promoted proliferation and ameliorated inflammation, apoptosis, and degeneration in human nucleus pulposus cells. Arch Gerontol Geriat. 2022;102:104748. [DOI] [PubMed] [Google Scholar]
- 94. Sun FF, Hu PF, Xiong Y, Bao JP, Qian J, Wu LD. Tricetin protects rat chondrocytes against IL‐1β‐Induced inflammation and apoptosis. Oxid Med Cell Longevity. 2019;2019:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hu S, Wang S, He J, Bian Y. Tetramethylpyrazine alleviates endoplasmic reticulum stress‑activated apoptosis and related inflammation in chondrocytes. Mol Med Rep. 2021;25(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Li Y, Xie W, Zheng Y, et al. The miR‐548d‐5p/SP1 signaling axis regulates chondrocyte proliferation and inflammatory responses in osteoarthritis. Int Immunopharmacol. 2022;110:109029. [DOI] [PubMed] [Google Scholar]
- 97. Fu G, Yin F, Zhao J. Depletion of circ_0128846 ameliorates interleukin‐1β‐induced human chondrocyte apoptosis and inflammation through the miR‐940/PTPN12 pathway. Int Immunopharmacol. 2022;110:108996. [DOI] [PubMed] [Google Scholar]
- 98. Fu S, Fan Q, Xu J, et al. Circ_0008956 contributes to IL‐1β‐induced osteoarthritis progression via miR‐149‐5p/NAMPT axis. Int Immunopharmacol. 2021;98:107857. [DOI] [PubMed] [Google Scholar]
- 99. Huang Y, Chen D, Yan Z, et al. LncRNA MEG3 protects chondrocytes from IL‐1β‐induced inflammation via regulating miR‐9‐5p/KLF4 axis. Front Physiol. 2021;12:617654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xu J, Pei Y, Lu J, et al. LncRNA SNHG7 alleviates IL‐1β‐induced osteoarthritis by inhibiting miR‐214‐5p‐mediated PPARGC1B signaling pathways. Int Immunopharmacol. 2021;90:107150. [DOI] [PubMed] [Google Scholar]
- 101. Yuan A, Wu P, Zhong Z, He Z, Li W. Long non‐coding RNA Gm37494 alleviates osteoarthritis chondrocyte injury via the microRNA‐181a‐5p/GABRA1 axis. J Orthop Surg. 2022;17(1):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the information included in this manuscript is available upon request by contact with the corresponding author.


