Abstract
Objective
Cognitive impairment is prevalent in systemic lupus erythematosus (SLE). There remain gaps in understanding cognition and SLE longitudinally. We studied intraindividual change in cognition in SLE over time.
Methods
Data were from the University of California, San Francisco Lupus Outcome Study, which included 1281 adults with SLE. The Hopkins Verbal Learning Test‐Revised (HVLT‐R) and the Controlled Oral Word Association Test (COWAT) were administered annually over 7 years. A two‐state Markov analysis was used to model transition intensities for probabilities of change in cognition. Logistic regression examined the association between clinical variables and cognitive change.
Results
Minimal transition between cognitive states was observed in the Markov analysis. Using the COWAT, higher levels of self‐reported depression were associated with decreased likelihood of cognitive improvement (Relative Risk [RR]: 0.98; 95% confidence interval [CI]: 0.96‐0.99), and higher self‐reported disease severity was associated with cognitive decline (RR: 1.05; 95% CI: 1.02‐1.09). Using the HVLT‐R, increasing age (RR: 1.02; 95% CI: 1.01‐1.03) and higher education level (RR: 1.82; 95% CI: 1.28‐2.58) were associated with cognitive improvement, and higher self‐reported disease severity (RR: 1.02; 95% CI: 1.01‐1.03) and depression (RR: 1.05; 95% CI: 1.03‐1.07) were associated with cognitive decline.
Conclusion
Most individuals with SLE did not transition between states of high (Z score ≥ −1.5) or low (Z score < −1.5) cognition in a Markov analysis over a 7‐year assessment period, highlighting a degree of relative stability in cognition over time. Increasing age and higher education levels were associated with greater likelihood of cognitive improvement. Greater self‐reported SLE disease severity and depression were associated with cognitive decline.
SIGNIFICANCE & INNOVATIONS.
The high prevalence of cognitive impairment and systemic lupus erythematosus (SLE) is well established using cross‐sectional data. Cognitive impairment is known to negatively impact social role participation and health‐related quality of life in SLE.
This study is the first to examine cognitive function in SLE at an intraindividual level by comparing individual participant cognitive test scores on repeated assessments over time. Moreover, this is among the largest longitudinal studies on SLE and cognition to date and includes 1281 participants observed over 7 years.
Study results demonstrated that most individuals with SLE did not transition between states of high (Z score ≥ −1.5) or low (Z score < −1.5) cognition in a Markov analysis over a 7‐year assessment period, highlighting a degree of relative stability in cognition over time. Our regression analysis suggests that treating comorbid depression and severe illness may foster cognitive improvement and limit decline overtime. These findings can be considered when creating targeted therapies to preserve and improve cognition in patients with SLE.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease known to affect multiple body systems in the setting of autoantibody deposition and complement protein activation (1). In 1999, the American College of Rheumatology (ACR) published a set of case definitions known as neuropsychiatric systemic lupus erythematosus to describe how SLE affects the central nervous system (CNS) (2). A growing body of literature has since highlighted cognitive impairment as a prevalent neuropsychiatric manifestation of SLE with an estimated prevalence of 38% (95% confidence interval [CI]: 33%‐43%; range 3%‐81%) among studies that used at least one standardized objective measure or a comprehensive battery to assess cognition (3). However, the data are primarily cross‐sectional, and there remain gaps in understanding how SLE affects cognition at the intraindividual level over time.
Cognitive impairment encompasses significant deficits in several domains: memory, language, simple and complex attention, visual–spatial processing, executive skills, and psychomotor processing (2). Although there is no single unifying pattern of cognitive impairment identified in SLE, verbal and nonverbal learning and working memory deficits are among the most commonly affected domains (4, 5). Among patients with SLE, cognitive impairment is associated with unemployment, disability (6), and poor mental health (7) and is negatively related to social role participation (8). Therefore, it is important to characterize how cognitive impairment in SLE fluctuates over time to identify opportunities to mitigate, stabilize, and potentially reverse existing impairment.
The objectives of this study are twofold: 1) to examine how cognitive function changes over time in adults with SLE by analyzing repeated assessments of verbal learning and memory and verbal fluency domains of cognition and modeling transition intensities for probabilities of change using a Markov analysis and 2) to understand what clinically relevant factors are associated with change in cognition.
MATERIALS AND METHODS
Patient population and data collection
Data were from the University of California San Francisco (UCSF) Lupus Outcome Study (9), a single center longitudinal study of 1281 adults with SLE. Participants were recruited from academic medical centers, community rheumatology clinics, and nonclinical sources, including conferences, patient support groups, and advertisements. Participant diagnoses were confirmed by medical chart review using the ACR SLE classification criteria. Participants were observed annually beginning in 2002 and assessed remotely using structured telephone interview guides implemented by trained surveyors. Non–English‐speaking participants were excluded because all measures of cognition and demographic data were administered in English. The study protocol was approved by the UCSF Committee on Human Research, and all participants provided informed consent (IRB #11‐05717). Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
The first cognitive measure was the Hopkins Verbal Learning Test‐Revised (HVTL‐R) (10), an assessment of verbal learning and memory. The HVLT‐R contains six forms, each listing 12 nouns selected from three different semantic categories (eg, vegetables, animals). The words are presented in three learning trials in which the participant attempts to repeat the words immediately after presentation. Approximately 25 minutes later, tests of delayed recall and recognition are performed. The second cognitive measure was the Controlled Oral Word Association Test (COWAT) (11), which assesses verbal fluency. For the COWAT, participants are given three letters and asked to produce as many words as possible that begin with the provided letter within 1 minute, with some exclusion criteria (eg, proper nouns).
Covariate measures included the Center of Epidemiologic Studies Depression Scale (CESD) as a self‐reported measure of depressive symptoms and the Systemic Lupus Erythematosus Activity Questionnaire (SLAQ) as a measure of patient‐reported disease activity. The CESD has a score range of 0‐60, with greater scores corresponding to more severe symptoms of depression in the last week (12). The SLAQ contains 24 items pertaining to SLE disease symptoms and includes a question about overall SLE disease severity within the past 3 months (13). The SLAQ and CESD were administered annually during patient follow‐up assessments.
Primary Markov analysis
Analysis was based on a Markov model for the change in cognitive function; because the model is based on change, analysis was restricted to individuals with at least two assessments of cognition within the 7‐year study period. Please refer to Supplementary Table 1 for a breakdown of the number of assessments completed by participants, including the number of participants who had less than two assessments and were excluded from the analysis. The likelihood function for Markov analyses is based on probabilities governing observed transitions between states at consecutive observation times. Conditional on the state of the response at a given assessment, the probability of being in the state observed at the subsequent assessment is computed, and the sequence of all such probabilities is multiplied to obtain the probability for the full path of observed cognitive states for each individual. The overall likelihood for a given data set is then computed by aggregating information over the entire sample by taking the product of individuals likelihoods. Markov models were fit for cognitive function as measured by COWAT and HVLT‐R over time in parallel analyses. COWAT and HVLT‐R scores were transformed into age‐normed Z scores. Two states of cognition were defined based on the Z scores of the respective outcomes: state 1 reflected the state of lower cognitive function (Z score < −1.5), and state 2 reflected higher cognitive function (Z score ≥ −1.5) (Figure 1). Separate analyses were created for each cognitive test (ie, COWAT and HVLT‐R).
Figure 1.
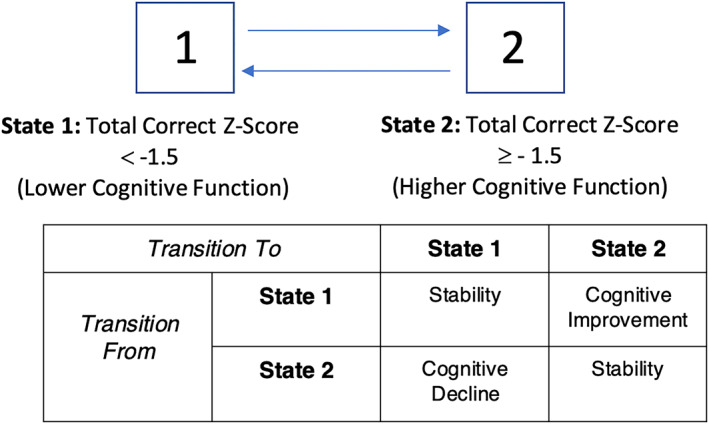
Two‐state reversible Markov model for the Hopkins Verbal Learning Test‐Revised and Controlled Oral Word Association Test to model the probabilities of transition between higher and lower cognitive states.
The methodology for Markov analyses used (14) in this study is sufficiently flexible that a variable number of assessments across different participants is readily handled. When individuals contribute to more than two assessments for Markov processes, this increases the information and hence enhances the precision of estimates and power of tests. Variation in the times between assessments (across follow‐up windows both within participants and between participants) is likewise permissible because the likelihood contributions are based on the transition probability functions evaluated at arbitrary time points (14).
The observable data consist of the states that are occupied at the assessment times, but cognition changes continuously between assessments. To reflect this, the transition probabilities governing the observed cognition responses are derived based on continuous‐time Markov models and their associated transition intensity functions. Transition intensity functions characterize the instantaneous risk of transition in an analogous way that the hazard function characterizes the instantaneous risk of failure in survival analyses. Using an intensity‐based framework, covariate effects can be modeled by adopting multiplicative models wherein covariates scale up or down the transition intensities. Such multiplicative intensity‐based models can be viewed as generalizations of the proportional hazards model used for survival analysis. In the multistage setting, relative risks are obtained for covariate effects, which convey whether the intensities for transitions are increased or decreased by some multiplicative amount according to covariate values. We considered the covariates age, level of education, CESD score, SLAQ score, treatment with intravenous (IV) glucocorticoids during the study period, and SLE disease duration at study entry.
Secondary Markov analysis
To study the relation between the two cognitive measures, we fitted a four‐state Markov model to study how changes in the COWAT state altered the risk of changes in HVLT‐R and vice versa (see Figure 2 for definitions of the four states). For example, if an individual had high cognitive scores on both the HVLT‐R and the COWAT, we examined the probability of declining scores over time compared to those who had low scores on either the COWAT or HVLT‐R at baseline.
Figure 2.
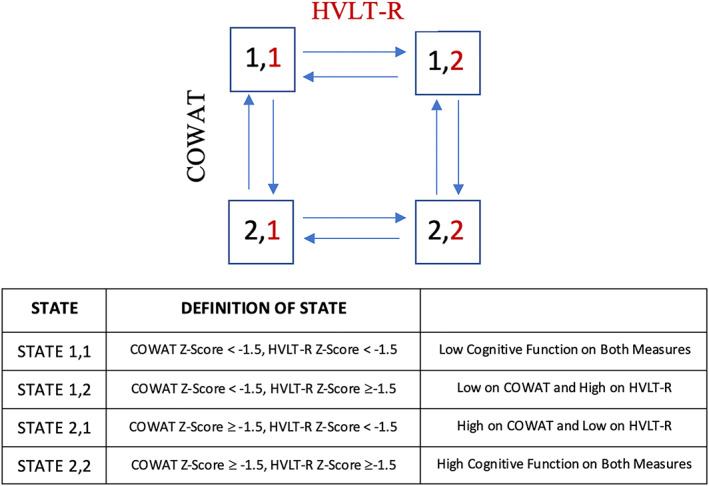
Four‐state reversible Markov model to model the association between the Hopkins Verbal Learning Test‐Revised (HVLT‐R) and Controlled Oral Word Association Test (COWAT) over time.
RESULTS
Sample characteristics
The sample comprised 1281 participants. At least two assessments with the same measure were required to be included in all analyses. This permitted the inclusion of 911 of the 1281 participants for the COWAT and 1023 of 1281 participants for the HVLT‐R. A total of 888 participants had at least two scores measured using both the COWAT and the HVLT‐R for inclusion in the secondary analysis. See Table 1 for demographic data.
Table 1.
Distribution of demographic variables
| At study entry (N = 1281) | At first assessment with COWAT (n = 917) | At first assessment with HVLT‐R (n = 1030) | At first assessment with both COWAT and HVLT‐R (n = 895) | |
|---|---|---|---|---|
| Age, y, mean (SD) | 45.7 (13.6) | 48.3 (13.1) | 47.0 (13.2) | 48.2 (13.1) |
| Female sex, n (%) | 1172 (91.5) | 847 (92.4) | 950 (92.2) | 826 (92.3) |
| Education, n (%) | ||||
| High school diploma or less | 280 (21.9) | 121 (13.2) | 145 (14.1) | 115 (12.8) |
| College or associate degree or trade or vocational school | 541 (42.2) | 431 (47.0) | 489 (47.5) | 421 (47.0) |
| Bachelor's degree or higher | 460 (35.9) | 365 (39.8) | 396 (38.4) | 359 (40.2) |
| Income below 125% of the US Federal Poverty Level, n (%) | 216 (17.2) | 117 (13.0) | 145 (14.2) | 112 (12.7) |
| Race, n (%) | ||||
| White | 644 (50.3) | 525 (57.3) | 594 (57.7) | 519 (58.0) |
| Black | 125 (9.8) | 80 (8.7) | 93 (9.0) | 76 (8.5) |
| Latino | 89 (6.9) | 60 (6.5) | 70 (6.8) | 60 (6.7) |
| Asian | 108 (8.4) | 79 (8.6) | 86 (8.3) | 77 (8.6) |
| Other (Native American, Hawaiian, and mixed race) | 315 (24.6) | 173 (18.9) | 187 (18.2) | 163 (18.2) |
| Received corticosteroids IV, n (%) | 441 (36.3) | 111 (12.3) | 121 (11.9) | 106 (12.1) |
| SLAQ score, mean (SD) | 12.5 (8.2) | 12.6 (7.8) | 12.59 (8.0) | 12.47 (7.8) |
| CESD score, mean (SD) | 16.4 (12.8) | 14.0 (12.2) | 15.22 (12.7) | 13.19 (12.2) |
| Disease duration at study entry, y, mean (SD) | 12.2 (8.5) | 12.2 (8.4) | 12.29 (8.5) | 12.24 (8.4) |
Abbreviations: CESD, Center of Epidemiologic Studies Depression Scale; COWAT, Controlled Oral Word Association Test; HVLT‐R, Hopkins Verbal Learning Test‐Revised; IV, intravenous; SLAQ, Systemic Lupus Erythematosus Activity Questionnaire.
Primary Markov analysis
COWAT
Supplementary Table 1 demonstrates the number of transitions between cognitive states using either the COWAT or HVLT‐R. Overall, most participants demonstrated stability in cognition on repeated assessments using the COWAT. Of the 2186 pairs of consecutive participant assessments, 1917 pairs of assessments showed stability in higher cognitive function (state 2 to state 2) and 96 pairs of assessments showed stability in lower cognitive function (state 1 to state 1).
CESD and SLAQ scores were significantly associated with change in cognition as assessed by the COWAT over time (Table 2). Each unit increase in the CESD score (higher levels of depression) was associated with a 2% decreased intensity for improvement in cognition (Relative Risk [RR]: 0.98; 95% CI: 0.96‐0.99). Each unit increase in the SLAQ score (greater disease severity) was associated with a 5% increased intensity of decline in cognition (RR: 1.05; 95% CI: 1.02‐1.09). Age, level of education, treatment with IV glucocorticoids, and SLE disease duration were not significantly associated with change in COWAT scores over time.
Table 2.
Univariate and multivariate regression models to assess for variables associated with change in cognitive states in the Markov analysis when using the COWAT as the primary measure of cognition
| Transitions | Covariate | Univariate regression model | Multivariate regression model | ||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% CI | P | ||
| 1 to 2 (improvement in cognition) | Age: per 1 year | — | — | — | 0.99 | 0.98‐1.02 | 0.89 |
| Education: college or vocational or bachelor's degree or greater vs. high school diploma or less | — | — | — | 1.58 | 0.96‐2.62 | 0.07 | |
| Corticosteroids IV: yes vs. no | 0.88 | 0.50‐1.52 | 0.64 | 1.01 | 0.57‐1.78 | 0.98 | |
| SLAQ score: per 1 unit | 0.99 | 0.96‐1.02 | 0.44 | 1.00 | 0.98‐1.03 | 0.51 | |
| CESD score: per 1 unit | 0.98 | 0.97‐1.00 | 0.02* | 0.98 | 0.96‐0.99 | 0.02* | |
| Disease duration at study entry: per 1 year | 0.99 | 0.96‐1.01 | 0.28 | 0.98 | 0.95‐1.01 | 0.12 | |
| 2 to 1 (decline in cognition) | Age: per 1 year | — | — | — | 0.99 | 0.97‐1.02 | 0.53 |
| Education: college or vocational or bachelor's degree or greater vs. high school diploma or less | — | — | — | 0.65 | 0.36‐1.19 | 0.16 | |
| Corticosteroids IV: yes vs. no | 1.62 | 0.80‐3.25 | 0.18 | 1.25 | 0.61‐2.57 | 0.55 | |
| SLAQ score: per 1 unit | 1.05 | 1.02‐1.09 | 0.00* | 1.05 | 1.01‐1.09 | 0.00* | |
| CESD score: per 1 unit | 1.02 | 1.00‐1.04 | 0.07 | 1.00 | 0.97‐1.02 | 0.78 | |
| Disease duration at study entry: per 1 year | 1.01 | 0.98‐1.04 | 0.76 | 1.00 | 0.97‐1.04 | 0.83 | |
Abbreviations: CESD, Center of Epidemiologic Studies Depression Scale; CI, confidence interval; COWAT, Controlled Oral Word Association Test; IV, intravenous; RR, XXX; SLAQ, Systemic Lupus Erythematosus Activity Questionnaire.
Statistically significant (P‐value <0.05).
HVLT‐R
Most participants demonstrated stability in cognition as measured by the HVLT‐R on repeated assessments (Supplementary Table 1). Of the 4260 pairs of consecutive participant assessments, 3269 pairs of assessments showed stability in higher cognitive function (state 2 to state 2) and 315 pairs of assessments showed stability in lower cognitive function (state 1 to state 1).
Age, level of education, CESD scores, and SLAQ scores were significantly associated with change in cognition as assessed by the HVLT‐R over time (Table 3). Every increase in age by 1 year was associated with a 2% greater chance of improving in cognitive function (RR: 1.02; 95% CI: 1.01‐1.03). Having completed a higher level of education was associated with an 82% greater chance of improving in cognitive function over time (RR: 1.82; 95% CI: 1.28‐2.58). Each unit increase in the CESD score was associated with a 5% chance of declining in cognition (RR: 1.05; 95% CI: 1.03‐1.07), and each unit increase in the SLAQ score was associated with a 2% chance of declining in cognitive function (RR: 1.02; 95% CI: 1.01‐1.03). Treatment with IV corticosteroids and disease duration were not associated with change in HVLT‐R scores over time.
Table 3.
Univariate and multivariate regression models to assess for variables associated with change in cognitive states in the Markov analysis when using the HVLT‐R as the primary measure of cognition
| Transitions | Covariate | Univariate regression model | Multivariate regression model | ||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% CI | P | ||
| 1 to 2 (improvement in cognition) | Age: per 1 year | — | — | — | 1.02 | 1.01‐1.03 | 0.00 |
| Education: college or vocational or bachelor's degree or greater vs. high school diploma or less | — | — | — | 1.82 | 1.28‐2.58 | 0.00 | |
| Corticosteroids IV: yes vs. no | 1.18 | 0.77‐1.83 | 0.47 | 1.17 | 0.73‐1.87 | 0.51 | |
| SLAQ score: per 1 unit | 1.01 | 0.99‐1.03 | 0.38 | 1.02 | 1.00‐1.04 | 0.11 | |
| CESD score: per 1 unit | 1.00 | 0.99‐1.01 | 0.54 | 0.99 | 0.98‐1.00 | 0.13 | |
| Disease duration at study entry: per 1 year | 1.00 | 0.99‐1.02 | 0.63 | 1.01 | 0.00‐1.02 | 0.52 | |
| 2 to 1 (decline in cognition) | Age: per 1 year | — | — | — | 0.99 | 0.97‐1.01 | 0.93 |
| Education: college or vocational or bachelor's degree or greater vs. high school diploma or less | — | — | — | 0.71 | 0.50‐1.00 | 0.05 | |
| Corticosteroids IV: yes vs. no | 1.81 | 1.14‐2.89 | 0.01* | 1.30 | 0.79‐2.14 | 0.30 | |
| SLAQ score: per 1 unit | 1.07 | 1.05‐1.08 | 0.00* | 1.05 | 1.02‐1.07 | 0.00* | |
| CESD score: per 1 unit | 1.04 | 1.03‐1.05 | 0.00* | 1.02 | 1.00‐1.03 | 0.00 | |
| Disease duration at study entry: per 1 year | 1.01 | 0.99‐1.02 | 0.55 | 1.01 | 0.99‐1.03 | 0.25 | |
Abbreviations: CESD, Center of Epidemiologic Studies Depression Scale; CI, confidence interval; COWAT, Controlled Oral Word Association Test; HVLT‐R, Hopkins Verbal Learning Test‐Revised; IV, intravenous; RR, Relative Risk; SLAQ, Systemic Lupus Erythematosus Activity Questionnaire.
Statistically significant (P‐value <0.05).
Secondary Markov analysis
Those who were in a higher cognitive state on both the HVLT‐R and COWAT at baseline were 67% less likely to transition to a lower cognitive state on the HVLT‐R than those who were only at a higher cognitive state on the COWAT [2,2 to 2,1 vs. 1,2 to 2,1] (intensity ratio: 0.33; 95% CI: 0.14‐0.61) (Table 4). Similarly, those who were in a higher cognitive state on both the HVLT‐R and COWAT at baseline [2,2] were 67% less likely to transition to a lower cognitive state on the COWAT [2,2 to 1,2 vs. 2,1 to 1,2] (intensity ratio: 0.33; 95% CI: 0.16‐0.80). Participants who were in a higher cognitive state on the HVLT‐R but a lower cognitive state on the COWAT [2,1] were 2.33 times more likely to improve on their COWAT scores over time [2,1 to 2,2] than those who were in a lower cognitive state on both measures at baseline [1,1 to 2,2] (intensity ratio: 2.33, 95% CI: 1.01‐5.38). Those who started in a higher cognitive state on the COWAT and a lower cognitive state on the HVLT‐R did not have a greater chance of improving in their HVLT‐R scores over time than those who started in a lower cognitive state on both scores at baseline (intensity ratio: 1.23; 95% CI: 0.66‐2.32).
Table 4.
A multistate regression model with both COWAT and HVLT‐R [COWAT Z score, HVLT‐R Z score]
| Baseline transition | Intensity ratio | 95% CI | P value |
|---|---|---|---|
| [1,1] to [1,2] | 0.14 | 0.05‐0.44 | |
| [2,1] to [2,2] | 0.18 | 0.07‐0.46 | |
| Ratio: [2,1] to [2,2]/ [1,1] to [1,2] | 1.24 | 0.66‐2.32 | 0.506 |
| [1,2] to [2,1] | 0.12 | 0.03‐0.45 | |
| [2,2] to [2,1] | 0.04 | 0.01‐0.11 | |
| Ratio: [2,2] to [2,1]/ [1,2] to [2,1] | 0.33 | 0.14‐0.61 | 0.001 |
| [1,1] to [2,1] | 0.46 | 0.14‐1.46 | |
| [1,2] to [2,2] | 1.07 | 0.36‐3.21 | |
| Ratio: [1,2] to [2,2]/ [1,1] to [2,1] | 2.33 | 1.01‐5.38 | 0.047 |
| [2,1] to [1,1] | 0.09 | 0.02‐0.34 | |
| [2,2] to [1,2] | 0.03 | 0.01‐0.13 | |
| Ratio: [2,2] to [1,2]/ [2,1] to [1,1] | 0.33 | 0.16‐0.80 | 0.012 |
Note: All models are adjusted for age and education.
Abbreviations: CI, confidence interval; COWAT, Controlled Oral Word Association Test; HVLT‐R, Hopkins Verbal Learning Test‐Revised.
DISCUSSION
Prior longitudinal assessments of cognition in patients with SLE have been limited by small sample sizes and short periods of follow‐up (3). Our study included 1023 participants with SLE who completed the HVLT‐R and 911 patients who completed the COWAT on at least two occasions. These measures were administered annually over a 7‐year period. By examining intraindividual change in participants’ scores, we incorporated 4260 pairs of consecutive assessments using the HVLT‐R and 2186 pairs of consecutive assessments using the COWAT into the Markov analyses. This is the largest sample size and number of individual assessments from a single study examining cognitive function longitudinally in SLE to date.
We used Markov analyses to model transition intensities for probabilities of change between states of high and low cognitive function. The results demonstrated that most individuals with SLE did not fluctuate between states of high and low cognitive function over a 7‐year time span. Specifically, those initially assessed to have higher cognitive function tended to maintain higher cognitive test scores (Z score ≥ −1.5) on repeated assessments, whereas those with lower cognitive scores at baseline tended not to improve to a state of higher cognitive scores. The finding that adults with SLE tend to have relatively stable cognitive function over time replicates other previous studies with smaller sample sizes and shorter durations of follow‐up (3, 15, 16, 17). Data demonstrating that cognition is a relatively stable construct in SLE inspires confidence that therapeutic programs can be developed to foster cognitive stability and potentially promote improvement.
Several clinically relevant variables were found to be associated with change in cognition: age, level of education, self‐reported depression, and self‐reported disease severity. Older age was associated with an increased likelihood of improving in cognition overtime such that each yearly increase in age was associated with greater probability of transition from a lower to higher cognitive state. This association remained true after adjusting for level of education, SLE disease duration, disease severity, and depression. This is in keeping with prior cross‐sectional studies that showed younger patients with SLE had more cognitive impairment on neuropsychiatric testing than elder patients (18, 19, 20). Patients with SLE may develop adaptive strategies to living with the cognitive stressors associated with SLE overtime, translating into preserved or improved cognitive test scores. More research is needed to determine how clinicians can tailor treatments based on age to improve cognition in patients with SLE.
Higher level of education was associated with a greater likelihood of improving in cognition and had a protective effect against declining in cognition in the verbal memory and learning domain but not in the verbal fluency domain. Higher level of education may support patients with SLE in developing adapting strategies to mitigate the effect of cognitive stressors associated with the disease. This result supports prior studies that demonstrated an association between cognitive impairment in SLE and social determinants of health, such as employment (6).
Greater self‐reported level of SLE disease severity by SLAQ was associated with an increased likelihood of declining in cognition. This remained true after adjusting for age, education, depression, disease duration at study entry, and treatment with IV corticosteroids during the study period. The severity of SLE disease activity is likely associated with great inflammatory effects on the CNS. This suggests that maintaining patients with SLE in periods of remission may prevent further cognitive decline overtime.
Lastly, a higher depression score on the CESD was associated with a lower likelihood of improving in the verbal fluency domain and a greater likelihood of declining in the memory and learning domains overtime. A recent systematic review and meta‐analysis found a 35% pooled prevalence of depression in patients with SLE across 69 studies, with a 95% CI between 30% and 40% (21). If depression both is a barrier to cognitive improvement and is associated with significant cognitive decline, as seen in our study, it is essential to treat comorbid depression in SLE patient populations to promote cognitive stability and prevent decline. Issues with concentration and loss of interest in hobbies and activities are well known symptoms of depression, which may exacerbate underlying cognitive impairment in patients with SLE.
For our secondary analysis, a four‐state Markov model was used to examine the association between the HVLT‐R and the COWAT. These two measures were significantly associated with each other over time, and the scores of one test affected how likely the scores in the other test were to change. For example, participants who were in a high cognitive state on both the HVLT‐R and the COWAT were less likely to decline in either measure than those who had lower cognition on either the HVLT‐R or the COWAT at baseline. Similarly, those in a high cognitive state on the HVLT‐R but a low cognitive state on the COWAT were more likely to improve on the COWAT over time than those who were in a low state on both measures at baseline. To summarize, the HVLT‐R and COWAT were associated with each other over time in a predictable manner. This suggests that understanding how patients with SLE vary in verbal fluency may be associated with how they vary on verbal learning and memory. Future research comparing different measures of cognition over time is needed to understand how various domains of cognition vary in SLE longitudinally.
Relatively few studies have assessed cognitive impairment in patients with SLE over time, although with small sample sizes (16, 17, 22, 23). A 2018 10‐year prospective study on a cohort of 43 White patients with SLE demonstrated that 50% of patients with cognitive impairment at baseline improved over time, whereas 10% of patients experienced cognitive decline (23). In contrast, observing a cohort of 755 patients with SLE, Touma et al described the joint trajectories of cognitive function and depressive symptoms overtime and demonstrated that patients with low cognitive impairment at baseline did not improve over a 7‐year period and those with normal cognition did not decline (17). Other studies have reported mixed results, showing either stability (16), improvement (22), or worsening cognition over time (24). These differences may relate to varying sample sizes, baseline patient characteristics, measures of cognition, and variable follow‐up.
It should be noted that although we used the HVLT‐R and COWAT as measures of cognitive function, they are tests of verbal learning and memory (HVLT‐R) and verbal fluency (COWAT) rather than comprehensive tests of cognition. Specific tests of visual–spatial and psychomotor domains of cognition were not administered because of the remote nature of assessing patients by telephone. Therefore, our findings that suggest that little transition between states of high and low cognition in participants with SLE occurs on repeated assessments reflect only cognitive domains of learning, memory, and verbal fluency. However, previous studies showed evidence of the validity of telephone‐administered screening tests in SLE when compared to a comprehensive neuropsychological battery (15, 25). The HVLT‐R has shown adequate sensitivity (74%) and specificity (68%) in detecting cognitive impairment in patients with SLE compared to the modified ACR SLE battery. Moreover, the HVLT‐R and COWAT are amenable to use in clinics, and their reliability and validity have been assessed in patients with SLE (26).
An additional limitation is the use of Z scores to dichotomize patient tests into higher and lower cognitive states rather than incorporating cognitive scores as a continuous variable. There is a chance that participants with COWAT and HVLT‐R scores close to the chosen Z score cut offs (outliers) would transition between states without reflecting meaningful clinical change in their cognition. Dichotomization of Z scores was done for the Markov analysis to create states of cognition and model probabilities of transition between states over time.
Participants had a relatively long disease duration at study entry (average of 12 years), which may make the results less generalizable to those with newly diagnosed SLE. Additionally, the results reflect stability in cognitive performance in a follow‐up period of up to 7 years and should not be extrapolated to reflect cognitive stability over the lifetime of an individual with SLE. Individuals with severe cognitive impairment who were unable to provide informed consent or participate in the interviews were excluded. There was no measure of physician‐assessed disease severity, and self‐reported disease severity by SLAQ may not always correspond with physician‐assessed measures.
This study suggests that individuals with SLE who demonstrate high cognition at baseline in domains of verbal learning and memory and verbal fluency tend to maintain high cognition over time and that those who demonstrate low cognition at baseline tend to remain low over time. Specifically, most individuals with SLE did not transition between predefined states of high (Z score ≥ −1.5) and low (Z score < −1.5) cognition in the Markov analysis over a 7‐year assessment period, highlighting a degree of relative stability in cognition over time. Increasing age and higher education levels were associated with a greater chance of cognitive improvement. Higher level of self‐reported SLE disease severity and depression were barriers to experiencing cognitive improvement and were risk factors for experiencing cognitive decline in both assessments. By using a large cohort with frequent reassessments of cognition over time, we have added to the understanding of the short‐term disease trajectory of cognitive impairment in SLE. These findings may be considered when creating targeted therapies to preserve and improve cognition in patients with SLE.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Drs. Touma, Cook, and Katz had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Perera, Cook, Lee, Katz, Touma.
Acquisition of data
Perera, Cook, Lee, Katz, Touma.
Analysis and interpretation of data
Perera, Cook, Lee, Katz, Touma.
Supporting information
Disclosure Form
Supplementary Table S1: ‐ Breakdown of the number of HVLT‐R and COWAT assessments completed by participants over the seven‐year study period (N = 1281)
ACKNOWLEDGMENTS
Dr. Touma's laboratory is supported by donations from the Kathi and Peter Kaiser family, the Lou and Marissa Rocca family, the Mark and Diana Bozzo family, the Stacey and Mark Krembil family, and the Schroeder Arthritis Institute, Toronto Western Hospital.
The Lupus Outcomes Study was supported by grant AR‐053308 from the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases. The Toronto Western Hospital Lupus Clinic is supported by a grant from Lupus Ontario and the Schroeder Arthritis Institute at University Health Network. Dr. Touma's work was supported by the Department of Medicine, University of Toronto.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr2.11529&file=acr211529‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2016;16;2:16039. [DOI] [PubMed] [Google Scholar]
- 2. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999;42:599–608. [DOI] [PubMed] [Google Scholar]
- 3. Rayes HA, Tani C, Kwan A, et al. What is the prevalence of cognitive impairment in lupus and which instruments are used to measure it? A systematic review and meta‐analysis. Semin Arthritis Rheum 2018;48:240–55. [DOI] [PubMed] [Google Scholar]
- 4. Monastero R, Bettini P, del Zotto E, et al. Prevalence and pattern of cognitive impairment in systemic lupus erythematosus patients with and without overt neuropsychiatric manifestations. J Neurol Sci 2001;184:33–9. [DOI] [PubMed] [Google Scholar]
- 5. Calderón J, Flores P, Babul M, et al. Systemic lupus erythematosus impairs memory cognitive tests not affected by depression. Lupus 2014;23:1042–53. [DOI] [PubMed] [Google Scholar]
- 6. Appenzeller S, Cendes F, Costallat LT. Cognitive impairment and employment status in systemic lupus erythematosus: a prospective longitudinal study. Arthritis Rheum 2009;61:680–7. [DOI] [PubMed] [Google Scholar]
- 7. Tam LS, Wong A, Mok VC, et al. The relationship between neuropsychiatric, clinical, and laboratory variables and quality of life of Chinese patients with systemic lupus erythematosus. J Rheumatol 2008;35:1038–45. [PubMed] [Google Scholar]
- 8. Mendelsohn S, Khoja L, Alfred S, et al. Cognitive impairment in systemic lupus erythematosus is negatively related to social role participation and quality of life: a systematic review. Lupus 2021;30:1617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yelin E, Trupin L, Katz P, et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum 2007;57:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol 1991;5:125–42. [Google Scholar]
- 11. Benton AL. Problems of test construction in the field of aphasia. Cortex 1967;3:32–58. [Google Scholar]
- 12. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 13. Karlson EW, Daltroy LH, Rivest C, et al. Validation of a systemic lupus activity questionnaire (SLAQ) for population studies. Lupus 2003;12:280–6. [DOI] [PubMed] [Google Scholar]
- 14. Kalbfleisch JD, Lawless JF. The analysis of panel data under a Markov assumption. J Am Stat Assoc 1985;80:863–71. [Google Scholar]
- 15. Yuen K, Green R, Bingham K, et al. Metrics and definitions used in the assessment of cognitive impairment in systemic lupus erythematosus: a systematic review. Semin Arthritis Rheum 2021;51:819–30. [DOI] [PubMed] [Google Scholar]
- 16. Carlomagno S, Migliaresi S, Ambrosone L, et al. Cognitive impairment in systemic lupus erythematosus: a follow‐up study. J Neurol 2000;247:273–9. [DOI] [PubMed] [Google Scholar]
- 17. Touma Z, Moghaddam B, Su J, et al. Cognitive function trajectories in association with the depressive symptoms trajectories in systemic lupus erythematosus over time. Arthritis Care Res (Hoboken) 2021;73:1436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whitelaw DA, Spangenberg JJ, Rickman R, et al. The association between the antiphospholipid antibody syndrome and neuropsychological impairment in SLE. Lupus 1999;8:444–8. [DOI] [PubMed] [Google Scholar]
- 19. Holliday SL, Navarrete MG, Hermosillo‐Romo D, et al. Validating a computerized neuropsychological test battery for mixed ethnic lupus patients. Lupus 2003;12:697–703. [DOI] [PubMed] [Google Scholar]
- 20. Nantes SG, Su J, Dhaliwal A, et al. Performance of screening tests for cognitive impairment in systemic lupus erythematosus. J Rheumatol 2017;44:1583–9. [DOI] [PubMed] [Google Scholar]
- 21. Moustafa AT, Moazzami M, Engel L, et al. Prevalence and metric of depression and anxiety in systemic lupus erythematosus: a systematic review and meta‐analysis. Semin Arthritis Rheum 2020;50:84–94. [DOI] [PubMed] [Google Scholar]
- 22. Hanly JG, Cassell K, Fisk JD. Cognitive function in systemic lupus erythematosus: results of a 5‐year prospective study. Arthritis Rheum 1997;40:1542–3. [DOI] [PubMed] [Google Scholar]
- 23. Ceccarelli F, Perricone C, Pirone C, et al. Cognitive dysfunction improves in systemic lupus erythematosus: results of a 10 years prospective study. PLoS One 2018;13:e0196103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao Y, Lau EY, Wan JH, et al. Systemic lupus erythematosus patients with past neuropsychiatric involvement are associated with worse cognitive impairment: a longitudinal study. Lupus 2016;25:637–44. [DOI] [PubMed] [Google Scholar]
- 25. Julian LJ, Yazdany J, Trupin L, et al. Validity of brief screening tools for cognitive impairment in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2012;64:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuen K, Bingham K, Tayer‐Shifman OE, et al. Measures of cognition in rheumatic diseases. Arthritis Care Res (Hoboken) 2020;72 Suppl 10:660–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Supplementary Table S1: ‐ Breakdown of the number of HVLT‐R and COWAT assessments completed by participants over the seven‐year study period (N = 1281)


