Abstract
Background
Equid herpesvirus (EHV) commonly affects horses causing neurologic and respiratory symptoms beside spontaneous abortions, meaning huge economic losses for equine industry worldwide. In foals, the virus can facilitate secondary infections by Rhodococcus equi, important in morbidity and mortality in equines. A total of five genotypes of EHV were previously described in Brazil including EHV-1, EHV-2, EHV-3, EHV-4, and EHV-5. EHV-2 genotype had only been previously described in Brazil in asymptomatic animals. We report the investigation of the dead of 11 foals in Middle-west region of Brazil showing respiratory and neurological symptoms, as well as several abortions in mares from the same farm.
Methods
Clinical and laboratory exams were performed in this case study. Lung, whole blood, serum, and plasma samples were analyzed by necroscopic and histopathologic techniques followed by molecular assays (conventional and qPCR and Sanger sequencing).
Results and conclusion
Laboratory exams revealed neutrophilia leukocytosis. Necroscopic and histopathologic findings were suppurative bronchopneumonia and ulcerative enteritis. Molecular assays point to the absence of the bacteria Rhodococcus equi and other viruses (including other EHV). The presence of EHV-2 DNA was confirmed by sequencing in serum sample from one foal. This is the first confirmed outbreak of EHV-2 causing disease in Brazilian horses with confirmed presence of the virus, and which highlight the important role of EHV-2 in equine respiratory disease and spontaneous abortions in equid in Brazil.
Keywords: Horse, Foal, Herpesvirus, Pneumonia, Neurological signs, Abortion, EHV-2
Background
Horses are the natural host of five distinct equid herpesvirus (EHV), members of the Herpesviridae family (Order Herpesvirales) distributed in two groups alphaherpesvirus and gammaherpesvirus [1]. EHV-1, EHV-3, EHV-4, EHV-8, and EHV-9 are members of the Alphaherpesvirinae subfamily, genus Varicellovirus, and EHV-2, EHV-5, and EHV-7 are classified in the Gammaherpesvirinae subfamily in the Percavirus genus (EHV-2 and EHV-5) and EHV-7 in the Rhadinovirus genus (ICTV 2018).
Equid gammaherpesvirus 2 (EHV-2) is known as the cause of acute infections and long latency in horses. This virus has been frequently correlated to immunosuppression in foals, upper respiratory tract and chronic pulmonary disease, keratoconjunctivitis, general malaise, abortion [2], poor performance, and granulomatous dermatitis [3, 4].
EHV-2 infection usually occurs soon after birth [5]. However, the detection of EHV-2 DNA in the lungs of the aborted fetus indicate that this virus may be capable to cross the placental barrier and infect the foal in gestational period [6]. Beside the absence of an effective vaccine against EHV-2, Belák et al. [7] showed that producing hyperimmune serum of a local strain of EHV-2 and vaccinating foals with repeated applications may prevent the disease.
Apparently, EHV-2 disease presents a two-phase evolution in young foals. The initial phase begins with an occasional appearance of nasal discharge and slight increase in body temperature followed by a temporary recover and the development of a severe respiratory disease, pneumonia, pulmonary abscesses, high fever, and death. The second phase is characterized by complications aggravated by the bacteria Rhodococcus equi with the invasion of the respiratory tract [7, 8]. This secondary infection causes bronchopneumonia, intestinal manifestations, septic arthritis and osteomyelitis, uveitis, and sepsis jeopardizing the foal athletic career or even leading to death [9, 10].
EHV-2 is endemic in horse population worldwide being identified in horses without clinical symptoms or presenting respiratory disease [4, 11–13]. In South America, a study conducted in Argentina reports the circulation of EHV-2 in thoroughbred horses presenting respiratory symptoms also indicating a high prevalence in this equine population [11]. The presence of EHV-2 in Brazilian horse herd was only recently confirmed by the detection of herpesvirus DNA in nasal swabs of asymptomatic adult horses [12].
Despite the link between EHV-2 and acute infections, abortions, and the possible link with poor performance in horses, the potential relation of EHV-2 in the development of clinical diseases in horses from Brazil was never analyzed. Here, we report the potential relation of EHV-2 on an outbreak causing the dead of 11 foals from Middle-west region of Brazil.
Methods
Case description
From the 14 foals that were born in a breeding farm from Middle-west region of Brazil during the year of 2018, 11 foals from “Mangalarga Marchador” breed (with age raging between 1 and 3 months) present respiratory symptoms such as dyspnea and pulmonary edema evolving to neurological signs (circling walking, lost look) and flexural limb deformities leading to death. Besides that, four mares in the same farm and the same period had abortions.
One of the foals showing respiratory and neurological symptoms, a Brazilian saddle horse male (86 days age), was accompanied during this study. At the clinical exam, this foal showed heart rate 104 bpm, respiratory rate 42 mpm, pale oral mucosa, capillary refill time 3 s, estimate dehydration 8%, reduced gut motility in all tract, and rectal temperature 39.8 °C. Furthermore, the foal showed mild to marked dyspnea, nasal serobolous secretion, entropion in both eyes, conjunctival hyperemia, miosis, corneal opacity, presence of hypopyon, and fibrin on eye anterior chamber. The complete blood count (CBC) disclosed leukocytosis (18,100/mm3) by neutrophilia (12,100/mm3) and hyperfibrinogenemia (800 mg/dL).
Ceftiofur 10 mg/kg, amikacin 20 mg/kg, and flunixin meglumine 0.25 mg/kg IV were administered. 1% prednisolone acetate eye drops and 0.4% ketorolac tromethamine drops, one drop in each eye, every 4 h was administered. Ringer lactato was the choice for IV fluids 5 mL/kg/h, after 2 h treating the patient died.
Sample collection, necropsy, and histopathology
Necropsy was performed following biosecurity standard procedures. Samples of serum, total blood, liquor, and tissue from the brain, gut, liver, and spleen were collected and sent to histopathological analysis and pathogen molecular screening assays.
Serum, whole blood, liquor, and brain, gut, liver, and spleen from a foal previously described in item 2.1 were collected, and serum and whole blood were sent to Clinical and Molecular Virology Lab ICB-II/USP (LabVCM).
Molecular assays
Quantitative polymerase chain reaction (qPCR) assay in the detection of virulent Rhodococcus equi and EHV-1 and EHV-4 was performed in commercial laboratory with methodology protected by commercial confidentiality agreement.
Serum and whole blood samples were transported in cold-chain logistic to the Laboratory of Clinical and Molecular Virology (LabVCM) from University of São Paulo. Total nucleic acids were extracted using NucliSENS® easyMAG® automatized platform (BioMerieux) following manufacturer’s instructions.
The polymerase herpesvirus genetic material was amplified by PCR and nested-PCR assay targeting highly conserved region between all herpesvirus subfamilies and capable to detect all species of EHV as described by VandeVanter et al. [14]. The positive sample was sent to Sanger sequencing. Samples were also analyzed for the presence of distinct Flavivirus and Alphavirus by Pan-Flavivirus [15] and Pan-Alphavirus [16] Real-time SybrGreen qPCR assays.
The nucleotide sequences obtained were aligned using the Geneious software (version Prime). Phylogenetic analyses were performed using the MEGA7 software (version 7.0.2) using maximum likelihood (ML) with neighbor-joining (NJ) and GTR + Ꝩ + I model.
Results
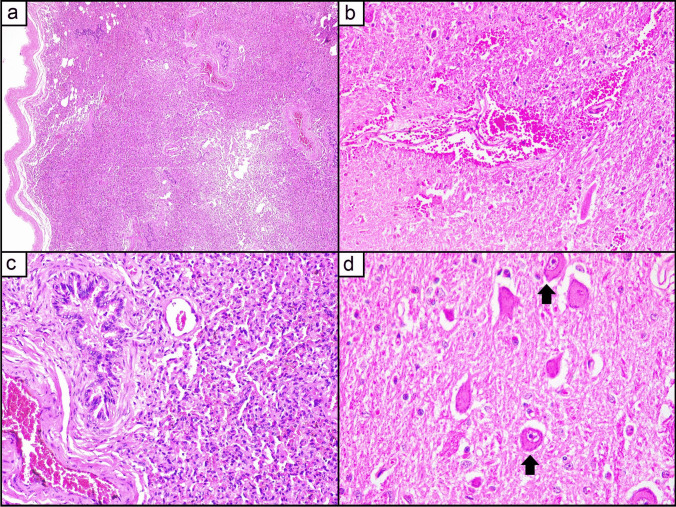
Histopathological evaluation of lung tissue indicates bronchi and bronchioles with hyaline membranes in their lumen (Fig. 1). These findings are consistent with an acute, multifocal interstitial pneumonia, a marked coalescent associated with multifocal atelectasis. Bronchi and bronchioles have hyaline membranes in their lumen. In less affected areas, there is a source of eosinophilic fibrillar material, possibly associated with edema, in an alveolar space and slight alveolar wall thickening. Multifaceted areas of marked alveolar wall rupture are observed in apical regions. Distinct tissues obtained from one of the 11 dead foals revealed no morphological changes in the liver, spleen, kidney, and heart.
Fig. 1.
Histopathologic analysis of lung and brain. a Lung. Thickening of the pleura is observed (on the left of the image), markedly altered lung parenchyma due to a large inflammatory infiltrate in the alveolar septa. HE, 40 × . b Brain. Perivascular hemorrhage in the Virchow-Robin space, expanding to the neuroparenchyma. HE, 200 × . c Lung. Alveolar septa infiltrated by a large number of mononuclear inflammatory cells (lymphocytes and histiocytes); additionally, the alveoli present atelectasis. A bronchiole is seen to be filled with proteinaceous material. HE, 200 × . d Brain. Neuronal central chromatolysis; the arrow identifies the affected neurons with typical features of cytoplasmic pallor and eccentricity of the nucleus
During necropsy exam, serobullous content was observed in the tracheal lumen, consistent with pulmonary edema; the lung was congested with areas suggestive of hepatization, in addition was observed to circular and whitish lesions of approximately 1 cm; engorgement of the mesenteric vessels, and cecal mucosa with marked hyperemia and diffuse hemorrhage (Fig. 2).
Fig. 2.
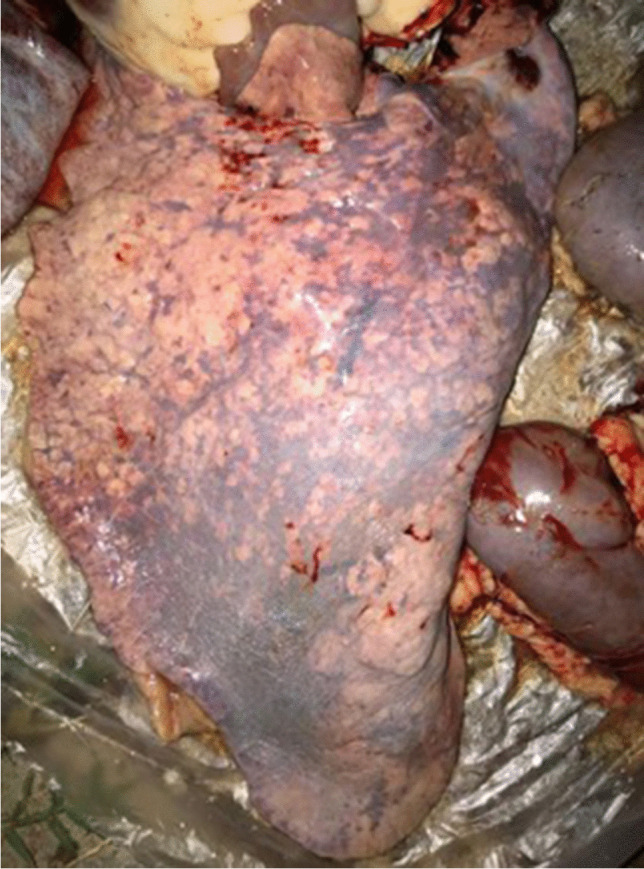
Necropsy exam. Foal lung presenting bronchopneumonia during necropsy exam
Real-time quantitative polymerase chain reaction (qPCR) assays targeting virulent Rhodococcus equi and EHV-1 and EHV-4 were negative for all samples tested.
EHV-2 DNA was detected in serum sample collected from one of the 11 the dead foal components of this study. Whole blood analyzed was negative in the same test. Serum and whole blood were also negative for the presence of Flavivirus and Alphavirus RNA.
Serum shows amplification of 315 bp fragment in PCR-nested reaction and the presence of equid gammaherpesvirus 2 was confirmed by sequencing and phylogenetic analysis (Fig. 3).
Fig. 3.
Maximum likelihood phylogenetic tree. Tree reconstructed using a MEGA7 software with heuristic search and “GTR + gamma + I” algorithm. Bootstrap (10,000 bootstrap replicates) values greater than 50% are depicted in the figure and visualized in the FigTree software (version 1.4.4). The analyzed sample (GenBank accession number MN537721) is highlight in red segregating in gammaherpesvirus clade
Discussion
In this study, we describe at first time the detection of EHV-2 in serum from equines presenting clinical symptoms in Brazil. In this mysterious outbreak disease related with death and abortions, the epidemiologic data indicate the role of EHV-2 once the virus was detected in one serum sample.
The necropsy of the 11 horses indicate the same characteristics and cause of death. Samples related to one of this 11 deaths were collected and tested for distinct potential etiologic agents including Rhodococcus equi bacteria, herpesvirus, Flavivirus, and Alphavirus. The unique pathogen detected was EHV-2.
The genetic analysis confirm that sequences segregated in equine herpesvirus 2 clade and distance analysis revealed homogeneity (100% bootstrap values) with equid gammaherpesvirus 2, EHV-2 (U20824 and KY628992), and shown 16.4% of distance with equid herpesvirus 5 (KU315429) and 14.5% distance of equid gammaherpesvirus 7 (EU165547). The obtained sequence was compared with herpesviruses from different hosts (equines, humans, monkeys, dolphins, bats, rodents, and other mammals) representatives of the three main herpesviruses lineages (alpha, beta, and gamma) available in GenBank databases.
The EHV-2 presence in South America was previously reported in horses presenting respiratory clinical symptoms in Argentina [11] and only recently in health horses randomly sampled in Brazil [12]. Until our data, the relation of EHV-2 with horse diseases and abortion was reported only in Italy [17] and the role of EHV-2 in abortion is still unclear [18].
However, Marenzoni et al. [19] detected EHV-1 and EHV-5 in 14% (9 animals) with different reproductive problems, including one co-infection with EHV-1 and EHV-2. The same authors detected the presence of EHV-5 in non-pregnant uterus, implying that it may have a role in reproductive dysfunction and have a negative consequence on the pregnant uterus.
EHV-2 can immunosuppress foals and allowed other bacterial infection and death. This represents an important economic, and affective lost for horse owners worldwide [20]. In Brazil [21], we have a high prevalence of positive farms for Rhodococcus equi, a bacteria that causes several disease in foals and can have infectivity and pathogenesis increased by the presence of EHV-2.
EHV-1 is well known as a cause of abortion in mares [22]; however, EHV-2 transplacental transmission was demonstrated previously [6]. The present study shows that not only EHV-1 but also EHV-2 can be an important cause of abortion in mares. In this report, several mares aborted, even been vaccinated with commercial vaccines against equine herpesvirus. It demonstrates the necessity of a correct diagnostic in abortion cases and an effective vaccine against EHV-2 that can prevent it since commercial vaccines available in Brazil and worldwide only protect against EHV-1 and 4. The relation between all the abortions with EHV-2 cannot be confirmed once samples from these animals were not collected. Otherwise, the necropsy of 10 foals indicate similar results with the observations from the foal sampled. All foals died maybe due to this strain being more pathogenic, since no other agent was isolated and we are not prepared to vaccinate and prevent future EHV-2 outbreaks.
Our study has several limitations including (i) sampling of only one foal fatal case, (ii) absence of pulmonary tissue for molecular detection of EHV-2, (iii) the sequencing of only one sample obtained from fatal case in a distinct organ related with the probability cause of death, and (iv) absence of immunohistochemical analysis. The strengths of our study include (i) sampling of distinct tissues of a dead foal from a farm presenting consecutive horse abortions and foal deaths, an outbreak with unidentified pathogen or cause; (ii) sensitive methodology targeting major respiratory/neurologic pathogens; and (iii) the combination of histopathological, necropsy, and virological data.
Conclusion
We report the potential relation of EHV-2 with horse disease outbreak of respiratory and neurologic disease in a farm of Brazil leading to the death of 11 foals. This is the first EHV-2 outbreak in Brazil showing disease and leading to dead, confirmed by the PCR method. The acute characteristics and high morbidity highlight the importance of this virus circulation. Furthermore, conventional treatment was unsuccessful, maybe due to a higher pathogenicity of this strain, since no other agent was isolated.
This case reports it is an alert of what can become a huge problem for horse breeders and veterinarians as this virus can spread through Brazil and South America continent since it is a border region. Our results confirm the presence of EHV-2 working an important role in equid respiratory disease.
Funding
Post-doc fellowship São Paulo Research Foundation-FAPESP 2014/15090–8 (ACAC), scholarship São Paulo Research Foundation-FAPESP 2020/01487–4 (CVM) and 2018/18867–4 (CMO), São Paulo Research Foundation-FAPESP technical training scholarship 2018/05346–6 (VBS), scholarship project National Council for Scientific and Technological Development-CNPq 133013/2019–7 (SC) and 152365/2019–2 (LGBG), and Project São Paulo Research Foundation-FAPESP 2016/08727–5 (DBLO).
Declarations
Ethics approval
The process was referred to the “Ethics Committee on Animal Use” from Biomedical Sciences Institute—University of Sao Paulo (CEUA-ICB-USP).
Conflict of interest
The authors declare no competing interests.
Information pre-print
This work had a pre-print version on Biorxiv, available on https://www.biorxiv.org/content/10.1101/809053v1
Footnotes
Responsible Editor: Luis Nero
The original online version of this article was revised: The first article note has been corrected
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author summary
Equid gammaherpesvirus 2 (EHV-2) can cause respiratory infection, abortion, and in extreme cases neurologic symptoms and death.
Outbreak of EHV-2 never was described in Brazil and can be critical to losses in equid industry. Our report provides evidence for a direct link between the EHV-2 strain and the outbreak in foals. We proved the clinical, histopathological, and phylogenetic analyses of segment of EHV-2 polymerase.
In summary, our study confirm that gammaherpesvirus 2 is circulating in Brazil and the EHV-2 is a cause of the first outbreak in foals showing the important role of EHV-2 in equid respiratory disease.
Angélica Cristine de Almeida Campos, Sofa Cicolo contributed equally to this article.
Carla Bargi Belli and Danielle Bruna Leal Oliveira contributed equally to this article as senior researcher.
Change history
2/6/2023
The first article note has been corrected.
Change history
2/10/2023
A Correction to this paper has been published: 10.1007/s42770-023-00917-3
Contributor Information
Angélica Cristine de Almeida Campos, Email: camposac@alumni.usp.br.
Carla Bargi Belli, Email: cbbelli@usp.br.
References
- 1.Azab W, Bedair S, Abdelgawad A, et al. Detection of equid herpesviruses among different Arabian horse populations in Egypt. Vet Med Sci. 2019;5:361–371. doi: 10.1002/vms3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kershaw O, Von Oppen T, Glitz F, Deegen E, Ludwig H, Borchers K. Detection of equine herpesvirus type 2 (EHV-2) in horses with keratoconjunctivitis. Virus Res. 2001;80(1–2):93–99. doi: 10.1016/S0168-1702(01)00299-4. [DOI] [PubMed] [Google Scholar]
- 3.Sledge DG, Miller DL, Styer EL, Hydrick HA, Baldwin CA. Equine herpesvirus 2-associated granulomatous dermatitis in a horse. Vet Pathol. 2006;43(4):548–552. doi: 10.1354/vp.43-4-548. [DOI] [PubMed] [Google Scholar]
- 4.Schlocker N, Gerber-Bretscher R, von Fellenberg R (1995) Equine herpesvirus 2 in pulmonary macrophages of horses. Am J Vet Res 56(6):749–754. https://europepmc.org/article/med/7653883 [PubMed]
- 5.Harden TJ, Bagust TJ, Pascoe RR, Spradbrow PB. Studies on equine herpesviruses 5. Isolation and characterisation of slowly cytopathic equine herpesviruses in Queensland. Aust Vet J. 1974;50(11):483–488. doi: 10.1111/j.1751-0813.1974.tb14052.x. [DOI] [PubMed] [Google Scholar]
- 6.Galosi CM, De La Paz VC, Fernández LC, et al. Isolation of equine herpesvirus-2 from the lung of an aborted fetus. J Vet Diagnostic Investig. 2005;17(5):500–502. doi: 10.1177/104063870501700520. [DOI] [PubMed] [Google Scholar]
- 7.Belák S, Pálfi V, Tuboly S, Bartha L. Passive immunization of foals to prevent respiratory disease caused by equine herpesvirus type 2. Zentralblatt fur Vet R B J Vet Med Ser B. 1980;27(9–10):826–830. doi: 10.1111/j.1439-0450.1980.tb02037.x. [DOI] [PubMed] [Google Scholar]
- 8.Pálfi V, Belák S, Molnár T. Isolation of equine herpesvirus type 2 from foals, showing respiratory symptoms. Zentralblatt fur Vet R B J Vet Med Ser B. 1978;25(2):165–167. doi: 10.1111/j.1439-0450.1978.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 9.Giguère S, Prescott JF. Clinical manifestations, diagnosis, treatment, and prevention of Rhodococcus equi infections in foals. Vet Microbiol. 1997;56(3–4):313–334. doi: 10.1016/S0378-1135(97)00099-0. [DOI] [PubMed] [Google Scholar]
- 10.Giguère S, Cohen ND, Keith Chaffin M, et al. Rhodococcus equi: clinical manifestations, virulence, and immunity. J Vet Intern Med. 2011;25(6):1221–1230. doi: 10.1111/j.1939-1676.2011.00804.x. [DOI] [PubMed] [Google Scholar]
- 11.Craig MI, Barrandeguy ME, Fernández FM. Equine herpesvirus 2 (EHV-2) infection in thoroughbred horses in Argentina. BMC Vet Res. 2005;1:1–5. doi: 10.1186/1746-6148-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dall Agnol AM, Beuttemmuller EA, Pilz D et al. (2019) Detection of equid gammaherpesvirus 2 and 5 DNA in the upper respiratory tract of asymptomatic horses from Southern Brazil. Brazilian J Microbiol 2–5. 10.1007/s42770-019-00100-7 [DOI] [PMC free article] [PubMed]
- 13.Negussie H, Gizaw D, Tesfaw L et al. (2017) Detection of equine herpesvirus ( EHV ) -1 , -2 , -4 and -5 in Ethiopian equids with and without respiratory problems and genetic characterization of EHV-2 and EHV-5 strains. 64:1970–1978. 10.1111/tbed.12601 [DOI] [PubMed]
- 14.Vandevanter DR, Warrener P, Bennett L et al (1996) Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol 34(7):1666–1671. 10.1128/JCM.34.7.1666-1671.1996 [DOI] [PMC free article] [PubMed]
- 15.Johnson N, Wakeley PR, Mansfield KL, et al. Assessment of a novel real-time pan-flavivirus RT-polymerase chain reaction. Vector-Borne Zoonotic Dis. 2010;10(7):665–671. doi: 10.1089/vbz.2009.0210. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Seco MP, Rosario D, Domingo C, et al. Generic RT-nested-PCR for detection of flaviviruses using degenerated primers and internal control followed by sequencing for specific identification. J Virol Methods. 2005;126(1–2):101–109. doi: 10.1016/j.jviromet.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Marenzoni ML, Bietta A, Lepri E, et al. Role of equine herpesviruses as co-infecting agents in cases of abortion, placental disease and neonatal foal mortality. Vet Res Commun. 2013;37(4):311–317. doi: 10.1007/s11259-013-9578-6. [DOI] [PubMed] [Google Scholar]
- 18.Léon A, Fortier G, Fortier C, et al. Detection of equine herpesviruses in aborted foetuses by consensus PCR. Vet Microbiol. 2008;126(1–3):20–29. doi: 10.1016/j.vetmic.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Marenzoni M, Stefanetti V, Danzetta ML, Timoney PJ. Gammaherpesvirus infections in equids: a review. Vet Med Res Reports. 2015;6:91. doi: 10.2147/VMRR.S39473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woźniakowski G, Samorek-Salamonowicz E. Animal herpesviruses and their zoonotic potential for cross-species infection. Ann Agric Environ Med. 2015;22(2):191–194. doi: 10.5604/12321966.1152063. [DOI] [PubMed] [Google Scholar]
- 21.Garcia Ribeiro M, Seki I, Yasuoka K, et al. Molecular epidemiology of virulent Rhodococcus equi from foals in Brazil: virulence plasmids of 85-kb type I, 87-kb type I, and a new variant, 87-kb type III. Comp Immunol Microbiol Infect Dis. 2005;28(1):53–61. doi: 10.1016/j.cimid.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Patel JR, Bateman H, Williams J, Didlick S. Derivation and characterisation of a live equid herpes virus-1 (EHV-1) vaccine to protect against abortion and respiratory disease due to EHV-1. Vet Microbiol. 2003;91(1):23–39. doi: 10.1016/S0378-1135(02)00259-6. [DOI] [PubMed] [Google Scholar]