Abstract
Purpose
Minimally invasive surgery is the gold standard treatment for adrenal masses, but it may be a challenging procedure in the case of pheochromocytoma (PHEO). The aim of the present study is to report the results of transperitoneal laparoscopic adrenalectomy (TLA) in cases of PHEO in comparison to other types of adrenal lesions.
Methods
From 1994 to 2021, 629 patients underwent adrenalectomy. Twenty-two and thirty-five patients, respectively, were excluded because they underwent bilateral and open adrenalectomy, leaving 572 patients for inclusion. Of these, 114 patients had PHEO (Group A), and 458 had other types of lesions (Group B). To adjust for potential baseline confounders, a propensity score matching (PSM) analysis was conducted.
Results
After PSM, 114 matched pairs of patients were identified from each group. Statistically significant differences were not observed when comparing the median operative time (85 and 90 min in Groups A and B, respectively, p = 0.627), conversion rate [6 (5.3%) in each group, p = 1.000], transfusion rate [4 (3.5%) and 3 (2.6%) in Groups A and B, respectively, p = 1.000], complication rate [7 (6.1%) and 9 (7.9%) in Groups A and B, respectively, p = 0.796), median postoperative hospital stay (3.9 and 3.6 days in Groups A and B, respectively, p = 0.110), and mortality rate [1 (0.9%) in each group, p = 1.000].
Conclusions
Based on this analysis, the results of TLA for PHEO are equivalent to those of TLA for other types of adrenal lesions, but the fundamental requirements are multidisciplinary patient management and adequate surgeon experience. Further prospective studies are required to draw definitive conclusions.
Keywords: Pheochromocytoma (PHEO), Adrenal lesions, Transperitoneal laparoscopic adrenalectomy (TLA), Open adrenalectomy (OA), Propensity score matching (PSM)
Introduction
Pheochromocytoma (PHEO) is a rare catecholamine-producing neuroendocrine tumour arising from chromaffin cells of the adrenal medulla [1–3]. PHEO is a life-threatening condition due to excessive catecholamine production and is characterized by clinical symptoms, including sweating, headaches, palpitations, perspiration, tremors, and facial pallor [4, 5]. These symptoms are often paroxysmal or can be induced by a variety of events, such as strenuous physical exertion, delivery, trauma, anaesthesia, and surgery [4, 5].
In patients with PHEO, adrenalectomy is the gold standard treatment, although resection may be challenging for surgeons due to a high risk of intraoperative haemodynamic instability from excessive catecholamine release during the induction of anaesthesia and with intraoperative surgical manipulation of the gland [6–9].
Since the first report of transperitoneal laparoscopic adrenalectomy (TLA) in 1992 [10], minimally invasive surgery (MIS) has largely replaced open adrenalectomy (OA) as the standard procedure, but its role in the case of large PHEOs and cancer is still debated [6, 11–14].
Although laparoscopic adrenalectomy (LA) and robotic adrenalectomy (RA) versus OA for the management of PHEO have been reported to be associated with better postoperative outcomes in terms of lower estimated blood loss, a shorter hospital stay, and less haemodynamic instability [15, 16], the effectiveness of MIS compared to OA for PHEO is still controversial [11, 17]. Furthermore, the influence of tumour type and activity on postoperative outcomes has not yet been well established [18].
Several studies have compared the postoperative outcomes after LA for PHEO versus other types of adrenal lesions [18, 19]. Some authors reported that PHEO and cortisol-producing adenoma are associated with more demanding surgery and poor postoperative outcomes [19–22]. Other authors reported no differences in postoperative complications based on the type of adrenal lesions [18, 23–25]. However, most of these comparative studies include a relatively small number of patients and a paucity of postoperative complications [18–21, 23–26]. The aim of the present propensity score-matched (PSM) analysis is to compare the 30-day surgical outcomes of TLA for PHEO compared to other types of adrenal lesions.
Materials and methods
This study is a retrospective analysis of prospectively collected data. Institutional review board approval (number: 1/2022) and informed consent were obtained from all participants.
From 1994 to 2021, 629 patients underwent adrenalectomy in two centres (Department of General Surgery and Surgical Specialties, Policlinico Umberto I, Sapienza University of Rome, and Department of General Surgery, Università Politecnica delle Marche, Ancona, Italy).
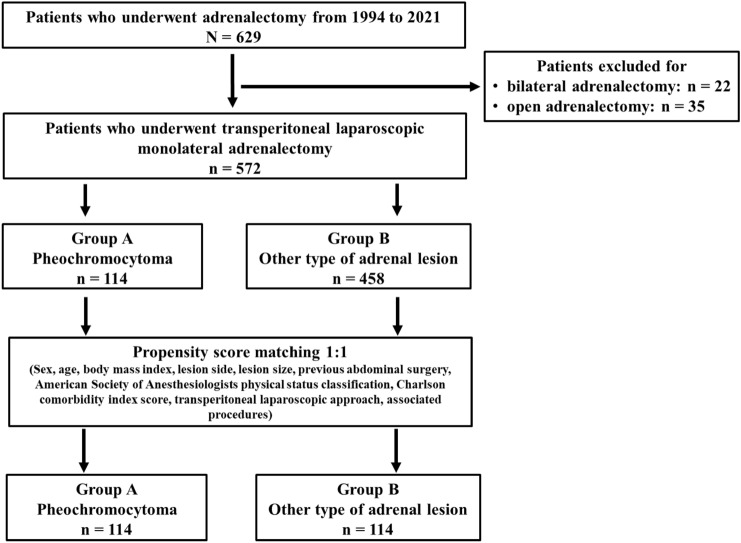
Patients who underwent bilateral [29] and OA were excluded (22 and 35 patients, respectively), leaving 572 patients for inclusion in the study (Fig. 1).
Fig. 1.
Patient selection
Out of the 572 patients, 114 (19.9%) and 458 (80.1%) patients underwent LA for PHEO and for other types of lesions, respectively (Fig. 1).
In all patients, the same medical treatment protocol was followed (which has changed over the years in accordance with the guidelines), and they underwent an identical surgical approach (which, on the contrary, has remained unchanged over the years), as previously reported [27–32].
The patients were evaluated together with the endocrinologist and anaesthesiologist consultants by physical examination and hormonal assessment, which currently includes plasma free or urinary fractionated metanephrines to exclude PHEO, 1 mg overnight dexamethasone suppression test to exclude cortisol excess, aldosterone/renin ratio to exclude primary aldosteronism in case of concomitant hypertension or unexplained hypokalaemia and measurement of sex hormones and steroid precursors in case of clinical or imaging features suggestive of adrenocortical carcinoma, as recommended [1, 17]. Magnetic resonance imaging (MRI) and computerized tomography (CT) scans were performed for each patient [30–32]. Scintigraphy with 131I-metabenzilguanidine or 6-18F-fluoro-l-dopa positron emission tomography was performed in any case of equivocal CT and MRI or discrepancy between imaging and biochemical tests [30–32].
A total body CT scan was performed for all patients who had PHEO.
Last, in all the patients with secreting adrenal lesions, arterial hypertension, chronic heart disease and electrocardiographic abnormalities, echocardiography was performed to rule out any underlying cardiac disease, according to our endocrinological and anaesthesiological protocols.
In the case of PHEO, the goals of perioperative management have always been blood pressure control and normalization of intravascular volume, although in this study, this has been achieved with different protocols over the years [30–32].
Currently, all patients with PHEO start preoperative pharmacological preparation with alpha-blockers (doxazosin: starting dose 2 mg/day) 14 days before surgery. If the patient is not affected by hypertension, the patient still starts with a low dose of an alpha-blocker, which is then modulated by the endocrinologists based on the blood pressure levels, and the final dose can be as high as 32 mg/day, according to the current guidelines [17, 30]. Beta-blockers (atenolol: starting dose 25 mg/day, final dose 50 mg/day) are added in case of tachycardia episodes, after at least three days of alpha blockade administration, to avoid hypertensive crises due to the beta blockade without alpha blockade [17, 30].
The preoperative treatment also includes administration of normal saline 2000 cc the evening before surgery (500 or 1000 cc in patients with heart or renal failure) to reverse catecholamine-induced blood volume contraction and to prevent severe hypotension after tumour removal, as previously reported and as recommended [17, 30–32].
Blood pressure, heart rate, and blood glucose level monitoring with adjustment of associated therapies were performed in the immediate postoperative period, as recommended [17, 30–32], and these were administered based on close collaboration with the anaesthesiology team.
Study design
Patient characteristics [including sex, age and body mass index (BMI)], American Society of Anaesthesiologists (ASA) grade, Charlson comorbidity index (CCI) score [33], previous abdominal surgery, lesion side, type of TLA (anterior or flank approach on the right, anterior, flank or submesocolic retropancreatic approach on the left), associated procedures, conversion rate, operative time, intra- and 30-day postoperative complications (graded according to the Clavien–Dindo classification [34]), adrenal lesion size and histology, hospital stay and 30-day mortality were stored in a Microsoft Excel program (Microsoft Corporation, Redmond, Washington, USA).
Statistical analysis
Continuous data are expressed as medians and 95% confidence intervals (CIs), while categorical variables are expressed as frequencies and percentages. The Mann–Whitney U test and Fisher’s exact test were used for the comparisons between the groups for continuous and categorical variables, respectively. A p value lower than 0.05 was considered statistically significant. Statistical analyses were carried out with SPSS software 22.0 (SPSS Inc., Chicago, Illinois, USA).
To minimize the selection bias and potential confounding factors in this retrospective cohort study, a propensity score matching (PSM) analysis 1:1 was performed to match the Group A patients with the Group B patients. A logistic regression model was applied to evaluate the propensity score of each patient according to the following covariables: sex, age, BMI, ASA grade, CCI score, lesion side, lesion size, previous abdominal surgery, type of TLA, and associated procedures. Two well-matched patient cohorts were obtained using a 1:1 nearest neighbour matching algorithm that pairs patients with the closest PS.
Results
The patient characteristics and surgical outcomes of the entire series after PSM comparing Group A versus Group B are reported in Table 1.
Table 1.
Results after propensity score matching
| Group A | Group B | p value | |
|---|---|---|---|
| n = 114 (50%) | n = 114 (50%) | ||
| Sex ratio, n (%) | |||
| - Men | 49 (43) | 35 (30.7) | 0.074 |
| - Women | 65 (57) | 79 (69.3) | |
| Median age, years (CI 95%) | 52.5 (49.3–55.1) | 53 (50.7–56.4) | 0.706 |
| Median body mass index, kg/m2 (CI 95%) | 25.5 (24.7–26.4) | 26 (26.2–28.2) | 0.714 |
| ASA grade, n (%) | |||
| - I–II | 93 (81.6) | 90 (78.9) | 0.618 |
| - III–IV | 21 (18.4) | 24 (21.1) | |
| CCI score, n (%) | |||
| - 0–1 | 61 (53.5) | 60 (52.6) | 0.894 |
| - ≥ 2 | 53 (46.5) | 54 (47.4) | |
| Previous abdominal surgery, n (%) | 8 (7) | 6 (5.3) | 0.784 |
| Lesion side, n (%) | |||
| - Right | 63 (55.3) | 70 (61.4) | 0.420 |
| - Left | 51 (44.7) | 44 (38.6) | |
| TLA, n (%) | |||
| - Anterior | 69 (60.5) | 80 (70.2) | 0.164 |
| - Left anterior submesocolic retropancreatic | 45 (39.5) | 33 (28.9) | 0.124 |
| - Flank | – | 1 (0.9) | 0.122 |
| Associated procedures, n (%) | 4 (3.5) | 5 (4.4) | 1.000 |
| Conversion rate, n (%) | 6 (5.3) | 6 (5.3) | 1.000 |
| - Adhesions from previous surgery | 1 (0.9) | 1 (0.9) | 1.000 |
| - Adhesion to pancreas | – | – | 1.000 |
| - Adhesion to liver | 1 (0.9) | 1 (0.9) | 1.000 |
| - Bleeding | 2 (1.8) | 2 (1.8) | 1.000 |
| - Respiratory failure from pneumoperitoneum | 1 (0.9) | 1 (0.9) | 1.000 |
| - Retrocaval mass growth | 1 (0.9) | 1 (0.9) | 1.000 |
| Median operative time, minutes (CI 95%) | 85 (88.6–105.9) | 90 (92–112.5) | 0.627 |
| Postoperative complications, n (%, Clavien–Dindo classification grade) | 10 (8.8) | 10 (8.8) | 1.000 |
| Surgical complications | 7 (6.1) | 6 (5.3) | 1.000 |
| - Acute urinary retention | – | – | 1.000 |
| - Ileus | – | – | 1.000 |
| - Abdominal abscess | – | – | 1.000 |
| - Anaemia | 4 (3.5, II) | 3 (2.6, II) | 1.000 |
| - Fever | 1 (0.9, II) | 2 (1.8, II) | 1.000 |
| - Wound infection | 1 (0.9, II) | 1 (0.9, II) | 1.000 |
| - Colonic fistula | – | – | 1.000 |
| - Chylous ascites | 1 (0.9, III-a) | – | 1.000 |
| - Hemoperitoneum | – | – | 1.000 |
| Medical complications | 3 (2.6) | 4 (3.5) | 1.000 |
| - Pleural effusion | 1 (0.9, I) | 1 (0.9, I) | 1.000 |
| - Pneumonia | 2 (1.8, II) | 2 (1.8, I) | 1.000 |
| - Atrial fibrillation | – | – | 1.000 |
| - Acute myocardial infarction | – | 1 (0.9, II) | 1.000 |
| Median lesion size at definitive histology, cm (CI 95%) | 4.3 (4–4.7) | 4.1 (3.9–4.8) | 0.151 |
| Definitive histology (benign: malignant), n % | |||
| Benign lesions | 101 (88.6) | 100 (87.7) | 1.000 |
| - Pheochromocytoma | 101 (88.6) | – | < 0.001 |
| - Secreting adenoma | – | 40 (35.1) | < 0.001 |
| - Non-secreting adenoma | – | 30 (26.3) | < 0.001 |
| - Hyperplasia | – | 18 (15.8) | < 0.001 |
| - Myelolipoma | – | 10 (8.8) | 0.002 |
| - Adrenal cyst | – | 2 (1.8) | 0.450 |
| - Angiomyolipoma | – | – | 1.000 |
| Malignant lesions | 13 (11.4) | 14 (12.3) | 1.000 |
| - Pheochromocytoma | 13 (11.4) | – | < 0.001 |
| - Adrenal carcinoma | – | 10 (8.8) | 0.002 |
| - Adrenal metastases | – | 4 (3.5) | 0.123 |
| Median hospital stay, days (CI 95%) | 4 (4.1–5) | 4.1 (3.6–4.5) | 0.110 |
| Mortality, n (%) | 1 (0.9) | 1 (0.9) | 1.000 |
ASA American Society of Anaesthesiologists, CCI Charlson comorbidity index, TLA transperitoneal laparoscopic adrenalectomy, CI confidence intervals
Statistically significant differences in bold
After PSM, two homogeneous groups were obtained, without any statistically significant differences regarding the preoperative characteristics.
Comparing the postoperative outcomes, no statistically significant differences were observed in median operative time (85 and 90 min in Groups A and B, respectively, p = 0.627), conversion rate [6 (5.3%) in each group, p = 1.000], transfusion rate [4 (3.5%) and 3 (2.6%) in Groups A and B, respectively, p = 1.000], complication rate [7 (6.1%) and 9 (7.9%) in Groups A and B, respectively, p = 0.796], median postoperative hospital stay (3.9 and 3.6 days in Groups A and B, respectively, p = 0.110), and mortality rate [1 (0.9%) in each group, p = 1.000].
Concerning the type of TLA, the anterior approach was mostly used in both groups [69 cases (61%) versus 80 cases (70%), p = 0.164], followed by the left submesocolic retropancreatic approach [45 cases (40%) versus 33 cases (29%), p = 0.0124], while the flank approach was the least adopted in both groups [0 cases versus 1 case (1%), p = 0.122].
Comparing the incidence of metastatic versus benign lesions at the time of surgery, statistically significant differences were not observed between the two groups. A negative resection margin was achieved in all cases.
An associated surgical procedure was performed in four patients (3.5%) in Group A [cholecystectomy in 3 (2.6%) and pedunculated uterine fibroid resection in 1 (0.9%) patients] and in three patients (2.6%) in Group B [cholecystectomy in 1 (0.9%) and umbilical hernia repair in 2 (1.8%) patients].
In Group A, one (0.9%) human immunodeficiency virus (HIV)-positive, ASA III, male patient, who had undergone a previous coronary artery bypass graft died from an acute myocardial infarction on postoperative Day 4. In Group B, 1 (0.9%) female patient with ASA III died from acute respiratory failure on postoperative Day 4.
Discussion
The present PSM analysis demonstrates that TLA for PHEO is associated with the same 30-day surgical outcomes as for other types of lesions, with no statistically significant differences.
As reported in the literature some preoperative characteristics (high BMI, previous abdominal surgery, and large adrenal masses) are related to a higher risk of intra- and postoperative complications [12, 14, 35]. Therefore, a PSM analysis was conducted to balance potential confounders and to obtain two homogeneous groups.
As reported in the European Society for Medical Oncology (ESMO) guidelines, MIS is the gold standard treatment in cases of PHEO up to 6 cm. However, OA is still recommended if complete resection cannot be achieved by MIS [1, 17]. Some authors report that LA for PHEO might result in higher intra- and postoperative complication rates, more blood loss, a longer operative time and a longer hospital stay, but these issues are still debated [19, 26, 36, 37]. The present analysis, however, does not support these words of caution, and our results are in line with those reported by other authors [7, 11, 24, 38]. In a recent study by Arolfo et al., including 114 patients, MIS has showed to be safe and effective even in cases of PHEOs greater than 5 cm in diameter [38]. A recent meta-analysis by Li et al., including more than 700 patients, also reported the superiority of LA compared to OA in the case of PHEO in terms of estimated blood loss, transfusion rate, haemodynamic instability, postoperative complication rate, and length of hospital stay [11]. Regardless, this meta-analysis included only one randomized controlled trial, and it compared heterogeneous patient groups, as the tumour size was smaller [11]. However, the BMI was higher in the LA group than in the OA group [11]. Moreover, some preoperative variables, such as previous abdominal surgery and associated procedures, are missing, which might influence the surgical outcomes [11].
It should be emphasized that in this study the most commonly used approach of TLA was the anterior approach. As reported in the literature [20, 25, 26], early identification and ligation of the adrenal vein performed prior to any gland manipulation and careful dissection with no adrenal capsule disruption are the recommended strategies to reduce the intraoperative catecholamine release, and this represents one of the main advantages of the anterior and submesocolic TLA [22, 27, 30–32].
Since there is no clear superiority of one approach over another [39–42], the recommendations of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) suggest that the surgeon should employ the surgical approach for LA that he or she is most familiar with [43]. For this reason, even if TLA by the anterior approach is an uncommon procedure worldwide, in our experience we have continued to use it. For us, it is more familiar in comparison to other approaches, and our results are similar to those reported in the literature by other authors using the lateral or posterior approaches in terms of operative time, conversion rates and morbidity rates [22, 27, 30–32, 39–41].
These data suggest that, regardless of the adopted approach, the fundamental requirements are adequate surgical experience in advanced MIS and multidisciplinary patient management in high-volume centres [1, 44–50].
Our study suggests that TLA for PHEO using the transperitoneal anterior and submesocolic approach is as safe and effective as TLA performed for other types of tumours. This further supports the concept that other preoperative parameters are probably useful to predict the difficulty of laparoscopic adrenalectomy, such as sex, previous surgery, BMI, site and size of the lesion, associated procedures, and comorbidities [12, 35, 37, 51–53].
The main limitations of the present study are its retrospective nature and the lack of data regarding intraoperative monitoring values for blood pressure, heart rate and glucose in all patients. The long study period also represents a limitation as it involves a certain heterogeneity of the study population, since the recommendations in the management of patients with adrenal lesions have changed considerably over the years [17, 53–56]. The diagnosis and management of patients with adrenal lesions differed between the patients treated in the first and last years of the present study (e.g. the diagnosis of PHEO was initially based on catecholamines and vanillylmandelic acid, while currently, it is based on urinary or plasma metanephrines [17, 55, 56]. Also, our perioperative medical management has undergone changes over the years [13, 22, 30–32]). However, this heterogeneity concerns both the study and the control group; moreover, we did not find differences with regard to the surgical approach over the years which is the real purpose of the study. Furthermore, to the best of our knowledge, the present study is the first and largest one comparing the surgical outcomes of LA performed for PHEO versus other types of lesions, and this study used PSM analysis to account for potential confounders.
In conclusion, based on the present study, the surgical outcomes of TLA for PHEO are equivalent to those of TLA for other types of tumours with the same preoperative characteristics. Adequate surgical experience in advanced MIS and disease-specific LA, coupled with a multidisciplinary patient management including consultations with the endocrinologist and the anaesthesiologist, are essential requirements. Further prospective randomized controlled trials with a larger number of patients are required to draw definitive conclusions.
Author contributions
DC: concept and design, data collection, data analysis, data interpretation, manuscript writing, critical revision, overall supervision, approval for publication. AB: concept and design, data collection, data interpretation, manuscript writing, critical revision, approval for publication. LP: data collection, data interpretation, critical revision, approval for publication. IS: data analysis, data interpretation, critical revision, approval for publication. MO: data collection, data interpretation, critical revision, approval for publication. MG: data interpretation, critical revision, approval for publication. AMP: concept and design, data interpretation, manuscript writing, critical revision, overall supervision, approval for publication.
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets generated analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Diletta Corallino, Andrea Balla, Livia Palmieri, Isabella Sperduti, Monica Ortenzi, Mario Guerrieri and Alessandro M. Paganini declare that they have no conflict of interest or financial ties to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional national research committee and with the 1964 Helsinki Declaration.
Research involving human participants and/or animals
Not applicable.
Informed consent
Informed consent was obtained from all participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fassnacht M, Assie G, Baudin E, Eisenhofer G, de la Fouchardiere C, Haak HR, de Krijger R, Porpiglia F, Terzolo M, Berruti A, ESMO Guidelines Committee Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1476–1490. doi: 10.1016/j.annonc.2020.08.2099. [DOI] [PubMed] [Google Scholar]
- 2.Ma W, Mao Y, Zhuo R, Dai J, Fang C, Wang C, Zhao J, He W, Zhu Y, Xu D, Sun F. Surgical outcomes of a randomized controlled trial compared robotic versus laparoscopic adrenalectomy for pheochromocytoma. Eur J Surg Oncol. 2020;46(10 Pt A):1843–1847. doi: 10.1016/j.ejso.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Picchetto A, Paganini AM, Balla A, Quaresima S, Cantisani V, D'Ambrosio G, Lezoche E. Rare extra-adrenal paraganglioma mimicking a painful Schwannoma: case report. Ann Ital Chir. 2014;85(ePub):S2239253X14022907. [PubMed] [Google Scholar]
- 4.Bihain F, Klein M, Nomine-Criqui C, Brunaud L. Robotic adrenalectomy in patients with pheochromocytoma: a systematic review. Gland Surg. 2020;9(3):844–848. doi: 10.21037/gs-2019-ra-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai S, Wu B, Yao Z, Zhu X, Jiang Y, Wang H. Development and validation of a clinical model to predict intraoperative hemodynamic instability in patients with pheochromocytomas surgery. Endocr J. 2020;67(1):81–89. doi: 10.1507/endocrj.EJ19-0278. [DOI] [PubMed] [Google Scholar]
- 6.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665–75. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 7.Fu SQ, Wang SY, Chen Q, Liu YT, Li ZL, Sun T. Laparoscopic versus open surgery for pheochromocytoma: a meta-analysis. BMC Surg. 2020;20(1):167. doi: 10.1186/s12893-020-00824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang AM, Rosen J, Saidian A, Bae S, Tanno FY, Chambo JL, Bloom J, Gordetsky J, Srougi V, Phillips J, Rais-Bahrami S. Perioperative outcomes of laparoscopic, robotic, and open approaches to pheochromocytoma. J Robot Surg. 2020;14(6):849–854. doi: 10.1007/s11701-020-01056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiernan CM, Du L, Chen X, Broome JT, Shi C, Peters MF, Solorzano CC. Predictors of hemodynamic instability during surgery for pheochromocytoma. Ann Surg Oncol. 2014;21(12):3865–71. doi: 10.1245/s10434-014-3847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med. 1992;327(14):1033. doi: 10.1056/NEJM199210013271417. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Wang Y, Chang X, Han Z. Laparoscopic adrenalectomy (LA) vs open adrenalectomy (OA) for pheochromocytoma (PHEO): a systematic review and meta-analysis. Eur J Surg Oncol. 2020;46(6):991–998. doi: 10.1016/j.ejso.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Alberici L, Paganini AM, Ricci C, Balla A, Ballarini Z, Ortenzi M, Casole G, Quaresima S, Di Dalmazi G, Ursi P, Alfano MS, Selva S, Casadei R, Ingaldi C, Lezoche G, Guerrieri M, Minni F, Tiberio GAM. Development and validation of a preoperative "difficulty score" for laparoscopic transabdominal adrenalectomy: a multicenter retrospective study. Surg Endosc. 2022;36(5):3549–3557. doi: 10.1007/s00464-021-08678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balla A, Palmieri L, Meoli F, Corallino D, Ortenzi M, Ursi P, Guerrieri M, Quaresima S, Paganini AM. Are adrenal lesions of 6 cm or more in diameter a contraindication to laparoscopic adrenalectomy? A case-control study. World J Surg. 2020;44(3):810–818. doi: 10.1007/s00268-019-05287-2. [DOI] [PubMed] [Google Scholar]
- 14.Balla A, Corallino D, Ortenzi M, Palmieri L, Meoli F, Guerrieri M, Paganini AM. Cancer risk in adrenalectomy: are adrenal lesions equal or more than 4 cm a contraindication for laparoscopy? Surg Endosc. 2022;36(2):1131–1142. doi: 10.1007/s00464-021-08380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai S, Yao Z, Zhu X, Li Z, Jiang Y, Wang R, Wu B. Comparison of transperitoneal laparoscopic versus open adrenalectomy for large pheochromocytoma: a retrospective propensity score-matched cohort study. Int J Surg. 2019;61:26–32. doi: 10.1016/j.ijsu.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Zhu W, Wang S, Du G, Liu H, Lu J, Yang W. Comparison of retroperitoneal laparoscopic versus open adrenalectomy for large pheochromocytoma: a single-center retrospective study. World J Surg Oncol. 2019;17(1):111. doi: 10.1186/s12957-019-1649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr; Endocrine Society Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–42. doi: 10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- 18.Limberg J, Stefanova D, Ullmann TM, Thiesmeyer JW, Buicko JL, Finnerty BM, Zarnegar R, Fahey TJ, 3rd, Beninato T. Not all laparoscopic adrenalectomies are equal: analysis of postoperative outcomes based on tumor functionality. Surg Endosc. 2021;35(6):2601–2606. doi: 10.1007/s00464-020-07678-2. [DOI] [PubMed] [Google Scholar]
- 19.Gotoh M, Ono Y, Hattori R, Kinukawa T, Ohshima S. Laparoscopic adrenalectomy for pheochromocytoma: morbidity compared with adrenalectomy for tumors of other pathology. J Endourol. 2002;16(4):245–9. doi: 10.1089/089277902753752223. [DOI] [PubMed] [Google Scholar]
- 20.Kalady MF, McKinlay R, Olson JA, Jr, Pinheiro J, Lagoo S, Park A, Eubanks WS. Laparoscopic adrenalectomy for pheochromocytoma. A comparison to aldosteronoma and incidentaloma. Surg Endosc. 2004;18(4):621–5. doi: 10.1007/s00464-003-8827-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Scholten A, Chomsky-Higgins K, Nwaogu I, Gosnell JE, Seib C, Shen WT, Suh I, Duh QY. Risk factors associated with perioperative complications and prolonged length of stay after laparoscopic adrenalectomy. JAMA Surg. 2018;153(11):1036–1041. doi: 10.1001/jamasurg.2018.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balla A, Quaresima S, Palmieri L, Ortenzi M, Sbardella E, Puliani G, Isidori AM, Guerrieri M, Paganini AM. Is laparoscopic left adrenalectomy with the anterior submesocolic approach for Conn's or Cushing's syndrome equally safe and effective as the lateral and anterior ones? Surg Endosc. 2019;33(9):3026–3033. doi: 10.1007/s00464-018-6601-6. [DOI] [PubMed] [Google Scholar]
- 23.Kiernan CM, Shinall MC, Jr, Mendez W, Peters MF, Broome JT, Solorzano CC. Influence of adrenal pathology on perioperative outcomes: a multi-institutional analysis. Am J Surg. 2014;208(4):619–25. doi: 10.1016/j.amjsurg.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toniato A, Boschin I, Bernante P, Opocher G, Guolo AM, Pelizzo MR, Mantero F. Laparoscopic adrenalectomy for pheochromocytoma: is it really more difficult? Surg Endosc. 2007;21(8):1323–6. doi: 10.1007/s00464-006-9190-8. [DOI] [PubMed] [Google Scholar]
- 25.Mellon MJ, Sundaram CP. Laparoscopic adrenalectomy for pheochromocytoma versus other surgical indications. JSLS. 2008;12(4):380–384. [PMC free article] [PubMed] [Google Scholar]
- 26.Natkaniec M, Pędziwiatr M, Wierdak M, Białas M, Major P, Matłok M, Budzyński P, Dworak J, Buziak-Bereza M, Budzyński A. Laparoscopic adrenalectomy for pheochromocytoma is more difficult compared to other adrenal tumors. Wideochir Inne Tech Maloinwazyjne. 2015;10(3):466–71. doi: 10.5114/wiitm.2015.52869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scoglio D, Balla A, Paci M, Guerrieri M, Lezoche G, D'Ambrosio G, Fabiani B, Ursi P, Paganini AM. Laparoscopic transperitoneal anterior adrenalectomy. Ann Ital Chir. 2013;84(4):411–416. [PubMed] [Google Scholar]
- 28.Ortenzi M, Balla A, Ghiselli R, Vergari R, Silecchia G, Guerrieri E, Maria Paganini A, Guerrieri M. Minimally invasive approach to the adrenal gland in obese patients with Cushing's syndrome. Minim Invasive Ther Allied Technol. 2019;28(5):285–291. doi: 10.1080/13645706.2018.1536669. [DOI] [PubMed] [Google Scholar]
- 29.Balla A, Ortenzi M, Palmieri L, Corallino D, Meoli F, Ursi P, Puliani G, Sbardella E, Isidori AM, Guerrieri M, Quaresima S, Paganini AM. Laparoscopic bilateral anterior transperitoneal adrenalectomy: 24 years experience. Surg Endosc. 2019;33(11):3718–3724. doi: 10.1007/s00464-019-06665-6. [DOI] [PubMed] [Google Scholar]
- 30.Paganini AM, Balla A, Guerrieri M, Lezoche G, Campagnacci R, D'Ambrosio G, Quaresima S, Antonica MV, Lezoche E. Laparoscopic transperitoneal anterior adrenalectomy in pheochromocytoma: experience in 62 patients. Surg Endosc. 2014;28(9):2683–9. doi: 10.1007/s00464-014-3528-4. [DOI] [PubMed] [Google Scholar]
- 31.Balla A, Quaresima S, Ortenzi M, Palmieri L, Meoli F, Corallino D, Guerrieri M, Ursi P, Paganini AM. Results after laparoscopic left anterior transperitoneal submesocolic adrenalectomy for the treatment of pheochromocytoma. Ann Ital Chir. 2019;90:220–224. [PubMed] [Google Scholar]
- 32.Paganini AM, Guerrieri M, Balla A, Quaresima S, Isidori AM, Iafrate F, D'Ambrosio G, Lezoche G, Lezoche E. Management of adrenal incidentaloma by laparoscopic transperitoneal anterior and submesocolic approach. Langenbecks Arch Surg. 2016;401(1):71–9. doi: 10.1007/s00423-015-1367-y. [DOI] [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 34.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 35.Srougi V, Barbosa JAB, Massaud I, Cavalcante IP, Tanno FY, Almeida MQ, Srougi M, Fragoso MC, Chambô JL. Predictors of complication after adrenalectomy. Int Braz J Urol. 2019;45(3):514–522. doi: 10.1590/S1677-5538.IBJU.2018.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parikh PP, Rubio GA, Farra JC, Lew JI. Nationwide review of hormonally active adrenal tumors highlights high morbidity in pheochromocytoma. J Surg Res. 2017;215:204–210. doi: 10.1016/j.jss.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Tiberio GA, Solaini L, Arru L, Merigo G, Baiocchi GL, Giulini SM. Factors influencing outcomes in laparoscopic adrenal surgery. Langenbecks Arch Surg. 2013;398(5):735–43. doi: 10.1007/s00423-013-1082-5. [DOI] [PubMed] [Google Scholar]
- 38.Arolfo S, Giraudo G, Franco C, Parasiliti Caprino M, Seno E, Morino M. Minimally invasive adrenalectomy for large pheochromocytoma: not recommendable yet? Results from a single institution case series. Langenbecks Arch Surg. 2022;407(1):277–283. doi: 10.1007/s00423-021-02312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nigri G, Rosman AS, Petrucciani N, Fancellu A, Pisano M, Zorcolo L, Ramacciato G, Melis M. Meta-analysis of trials comparing laparoscopic transperitoneal and retroperitoneal adrenalectomy. Surgery. 2013;153(1):111–119. doi: 10.1016/j.surg.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 40.Jiang YL, Qian LJ, Li Z, Wang KE, Zhou XL, Zhou J, Ye CH. Comparison of the retroperitoneal versus transperitoneal laparoscopic adrenalectomy perioperative outcomes and safety for pheochromocytoma: a meta-analysis. BMC Surg. 2020;20(1):12. doi: 10.1186/s12893-020-0676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gavriilidis P, Camenzuli C, Paspala A, Di Marco AN, Palazzo FF. Posterior retroperitoneoscopic versus laparoscopic transperitoneal adrenalectomy: a systematic review by an updated meta-analysis. World J Surg. 2021;45(1):168–179. doi: 10.1007/s00268-020-05759-w. [DOI] [PubMed] [Google Scholar]
- 42.Cardinali L, Skrami E, Catani E, Carle F, Ortenzi M, Balla A, Guerrieri M. Laparoscopic transperitoneal adrenalectomy: a comparative study of different techniques for vessel sealing. Surg Endosc. 2021;35(2):673–683. doi: 10.1007/s00464-020-07432-8. [DOI] [PubMed] [Google Scholar]
- 43.Stefanidis D, Goldfarb M, Kercher KW, Hope WW, Richardson W, Fanelli RD; Society of Gastrointestinal and Endoscopic Surgeons SAGES guidelines for minimally invasive treatment of adrenal pathology. Surg Endosc. 2013;27(11):3960–80. doi: 10.1007/s00464-013-3169-z. [DOI] [PubMed] [Google Scholar]
- 44.Park HS, Roman SA, Sosa JA. Outcomes from 3144 adrenalectomies in the United States: which matters more, surgeon volume or specialty? Arch Surg. 2009;144(11):1060–7. doi: 10.1001/archsurg.2009.191. [DOI] [PubMed] [Google Scholar]
- 45.Bergamini C, Martellucci J, Tozzi F, Valeri A. Complications in laparoscopic adrenalectomy: the value of experience. Surg Endosc. 2011;25(12):3845–51. doi: 10.1007/s00464-011-1804-0. [DOI] [PubMed] [Google Scholar]
- 46.Kazaure HS, Sosa JA. Volume-outcome relationship in adrenal surgery: a review of existing literature. Best Pract Res Clin Endocrinol Metab. 2019;33(5):101296. doi: 10.1016/j.beem.2019.101296. [DOI] [PubMed] [Google Scholar]
- 47.Kercher KW, Novitsky YW, Park A, Matthews BD, Litwin DE, Heniford BT. Laparoscopic curative resection of pheochromocytomas. Ann Surg. 2005;241(6):919–926. doi: 10.1097/01.sla.0000164175.26785.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yip L, Duh QY, Wachtel H, Jimenez C, Sturgeon C, Lee C, Velázquez-Fernández D, Berber E, Hammer GD, Bancos I, Lee JA, Marko J, Morris-Wiseman LF, Hughes MS, Livhits MJ, Han MA, Smith PW, Wilhelm S, Asa SL, Fahey TJ, 3rd, McKenzie TJ, Strong VE, Perrier ND. American association of endocrine surgeons guidelines for adrenalectomy: executive summary. JAMA Surg. 2022 doi: 10.1001/jamasurg.2022.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adrenal (MTG1) Endo-ERN. https://endo-ern.eu/it/competenze-specifiche/ghiandola-surrenale-mtg1/. Accessed 4 Nov 2022
- 50.ENS@T European network for the study of adrenal tumors. http://www.ensat.org/ens@t-centers-of-excellence. Accessed 4 Nov 2022
- 51.Wang H, Wu B, Yao Z, Zhu X, Jiang Y, Bai S. Nomogram for predicting severe morbidity after pheochromocytoma surgery. Endocr Connect. 2020;9(4):309–317. doi: 10.1530/EC-20-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inversini D, Manfredini L, Galli F, Zhang D, Dionigi G, Rausei S. Risk factors for complications after robotic adrenalectomy: a review. Gland Surg. 2020;9(3):826–830. doi: 10.21037/gs.2020.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodríguez-Hermosa JI, Planellas-Giné P, Cornejo L, Gironès J, Recasens M, Ortega FJ, Moreno-Navarrete JM, Latorre J, Fernandez-Real JM, Codina-Cazador A. Comparison of outcomes between obese and nonobese patients in laparoscopic adrenalectomy: a cohort study. Dig Surg. 2021;38(3):237–246. doi: 10.1159/000515589. [DOI] [PubMed] [Google Scholar]
- 54.Chen H, Sippel RS, O’Dorisio MS, Vinik AI, Lloyd RV, Pacak K, North American Neuroendocrine Tumor Society (NANETS) The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas. 2010;39(6):775–783. doi: 10.1097/MPA.0b013e3181ebb4f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manger WM, Gifford RW., Jr Pheochromocytoma: current diagnosis and management. Clevel Clin J Med. 1993;60(5):365–378. doi: 10.3949/ccjm.60.5.365. [DOI] [PubMed] [Google Scholar]
- 56.Manu P, Runge LA. Biochemical screening for pheochromocytoma. Superiority of urinary metanephrines measurements. Am J Epidemiol. 1984;120(5):788–790. doi: 10.1093/oxfordjournals.aje.a113947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated analysed during the current study are available from the corresponding author on reasonable request.