Abstract
Case series summary
Twenty-nine cats from different institutions with confirmed or highly suspected primary hyperaldosteronism treated by unilateral adrenalectomy were retrospectively included in this study. The most frequent clinical signs were lethargy (n = 20; 69%) and neck ventroflexion (n = 17; 59%). Hypokalaemia was present in all cats, creatinine kinase was elevated in 15 and hyperaldosteronism was documented in 24. Hypertension was frequently encountered (n = 24; 89%). Preoperative treatment included potassium supplementation (n = 19; 66%), spironolactone (n = 16; 55%) and amlodipine (n = 11; 38%). There were 13 adrenal masses on the right side, 15 on the left and, in one cat, no side was reported. The median adrenal mass size was 2 × 1.5 cm (range 1–4.6 × 0.4–3.8); vascular invasion was present in five cats, involving the caudal vena cava in four cats and the renal vein in one. Median duration of surgery was 57 mins. One major intraoperative complication (3%) was reported and consisted of haemorrhage during the removal of a neoplastic thrombus from the caudal vena cava. In 4/29 cats (14%), minor postoperative complications occurred and were treated medically. One fatal complication (3%) was observed, likely due to disseminated intravascular coagulation. The median duration of hospitalisation was 4 days; 97% of cats survived to discharge. The potassium level normalised in 24 cats within 3 months of surgery; hypertension resolved in 21/23 cats. Follow-up was available for 25 cats with a median survival of 1082 days. Death in the long-term follow-up was mainly related to worsening of comorbidities.
Relevance and novel information
Adrenalectomy appears to be a safe and effective treatment with a high rate of survival and a low rate of major complications. Long-term medical treatment was not required.
Keywords: Adrenal mass, aldosteronoma, adrenal tumour, surgery
Introduction
Primary hyperaldosteronism (PHA) is considered to be rare in cats, although its true prevalence is unknown. 1 In the majority of cases, feline PHA is due to a unilateral adrenocortical tumour, which produces an excess of aldosterone. The main systemic consequences of hyperaldosteronism are systemic hypertension and hypokalaemia. 2 If technically feasible, surgical removal of the adrenal tumour is potentially curative.1,2 To our knowledge, a total of 50 feline cases with hyperaldosteronism treated by adrenalectomy have been described in the veterinary literature.3–11
Perioperative mortality after adrenalectomy in cats ranges from 20% to 40%.4,6,7 The most frequent perioperative complication is moderate/severe haemorrhage. Progressive lethargy and anorexia have also been reported with high frequency in the postoperative period.4,6,7 Female sex and increased duration of anaesthesia have been reported as negative prognostic factors for survival after surgery.6,7
The aim of the present multicentre study was to retrospectively evaluate the type and frequency of complications and the survival rate after unilateral adrenalectomy in cats with PHA. Owing to the improvement in perioperative management in veterinary medicine and the increased awareness of the PHA in cats, our hypothesis was that overall survival after adrenalectomy for PHA has improved in comparison to previous studies.
Case series description
Cats from multiple institutions with a high suspicion of PHA, treated by adrenalectomy and with histological confirmation of an adrenocortical tumour, were retrospectively enrolled in the study.
The inclusion criteria were a suspicion of PHA with clinical signs consistent with hypertension (eg, retinal detachment) and/or hypokalaemic myopathy (eg, weakness and ventroflexion of the neck); confirmation of hypertension and/or hypokalaemia and/or elevated concentration of aldosterone in serum; evidence of an adrenal mass on diagnostic imaging; treatment with adrenalectomy and information regarding the postoperative period; and histological confirmation of an adrenocortical tumour. Exclusion criteria were a lack of data regarding the suspicion of hyperaldosteronism (aldosteronaemia or blood pressure or hypokalaemia); no surgical treatment; or a lack of information regarding the postoperative period.
Information retrieved from the records included signalment, clinical history, examination findings, blood pressure, preoperative blood test results, urinalysis, serum aldosterone concentration, the presence of comorbidities, intraoperative and postoperative complications, surgical and anaesthetic time, duration of hospitalisation and medical treatments. Information regarding the adrenal mass included preoperative diagnostic imaging findings, screening for distant metastasis performed by ultrasound of the abdomen and three-view thoracic radiographs or total body CT, dimensions, the side and histopathological findings. Survival time and cause of death were also recorded.
Intraoperative complications were divided as anaesthesia- or surgery-related. The postoperative complications were classified as minor in the case that medical treatment was required, major in the case a second surgical intervention was required and fatal if they led to death of the animal. 12
Follow-up (>3 months) was carried out with a clinical check-up or telephone interview with the owner or the referring veterinarian.
Data were reported as median (range). Median survival time was calculated using the Kaplan–Meier product limit method. Survival time was defined as the interval between the adrenal surgery and the date at which the cat was last known to be alive, or the date of its death from any cause. The data were analysed using a commercially available software program (MedCalc). The significance level was set at P <0.05.
Thirty-five cats treated by adrenalectomy were identified from the database of 12 veterinary hospitals. Six cats were excluded due to a lack of information regarding the diagnosis of hyperaldosteronism, leaving a study population of 29 cats. There were 25 domestic shorthairs, one Maine Coon, one Turkish Van, one Siamese and one Siamese mixed breed. Median age was 12 years (range 6–16); 17 were castrated males and 12 were spayed females. Median body weight was 4.5 kg (range 2.5–7.3).
The most frequent clinical signs are summarised in Table 1.
Table 1.
Clinical signs of 29 cats with confirmed or highly suspected primary hyperaldosteronism
| Clinical signs | Cats |
|---|---|
| Weakness/lethargy | 20 (69) |
| Neck ventroflexion | 17 (59) |
| Gastrointestinal signs | 10 (33) |
| Polyuria/polydipsia | 7 (22) |
| Blindness | 2 (7) |
Data are presented as n (%)
On clinical examination, a systolic heart murmur (grade II–IV/VI) was appreciated in 16 cats (55%). An examination of the fundus was performed in 10 cats and revealed tortuous retinal vessels in two and/or retinal haemorrhage in two. Systemic arterial hypertension was documented in 24/27 cats for which pressure measurements (14 obtained by oscillometric and 13 by Doppler systems) were available in the files (82%). The comorbidities reported were cardiopathies (n = 7), diabetes mellitus (n = 2), chronic enteropathy (n = 2), chronic kidney disease (n = 2) and hyperthyroidism (n = 1).
The most common clinicopathological abnormalities and aldosterone measurements are listed in Table 2.
Table 2.
Clinicopathological results of 29 cats with confirmed or highly suspected primary hyperaldosteronism
| Parameter | No. of cats with parameters measured | No. of cats with increased parameters | No. of cats with decreased parameters | Range | Mean |
|---|---|---|---|---|---|
| Haematocrit (%) | 27 | 0 | 9 | 23–38 | 30 |
| Platelets (mm3) | 27 | 2 | 0 | 159,000–768,000 | 338,000 |
| WBCs (mm3) | 22 | 2 | 2 | 7790–16,400 | 9370 |
| K (mEq/l) | 29 | 0 | 29 | 2.06–3.2 | 2.6 |
| Na (mEq/l) | 26 | 8 | 0 | 150–168 | 154 |
| Creatinine (mg/dl) | 26 | 6 | 0 | 0.8–6.5 | 1.5 |
| Urea (mg/dl) | 25 | 13 | 0 | 30–481 | 23 |
| CK (U/l) | 21 | 15 | 0 | 122–20,100 | 2464 |
| Aldosterone (pmol/l) | 24 | 24 | 0 | 594 to >5000 | |
| USG | 21 | 2 | 18 | 1008–1050 | 1022 |
| Proteinuria | 15 | 8 | 0 | 0.15–3 | 0.9 |
| PT (s) | 7 | 1 | 0 | 8.3–12.3 | 10.2 |
| aPTT (s) | 7 | 0 | 1 | 14.7–31.9 | 15.8 |
WBCs = white blood cells; CK = creatinine kinase; USG = urine specific gravity; PT = prothrombin time; aPTT = activated partial thromboplastin time
In five cats, serum aldosterone measurements were not available. However, hypokalaemia, increased creatinine kinase and hypertension were reported in all five cats.
Ultrasound was the most common diagnostic imaging modality used to diagnose an adrenal mass, performed in 22 cats (76%). Three-view thoracic radiographs were performed in 10 cats (34%) and total body CT in 16 cats (55%). Radiographic or CT evaluation of the thorax was unavailable in three cats. A right adrenal mass was observed in 13 cats and a left adrenal mass in 15; in one cat, the side was not reported. The median maximal dimensions of the adrenal mass were 2 × 1.5 cm (range 1–4.6 × 0.4–3.8). Vascular invasion was observed in five cats, a thrombus was present in the caudal vena cava originating from a mass on the right adrenal gland in four cats and, in one case, the thrombus invaded the left renal vein and originated from a mass of the left adrenal gland. In seven cats, compression of the caudal vena cava was suspected due to a mass effect.
Preoperative medical treatment was started in all but two cats; it included supplementation with potassium gluconate (n = 19; 2 mEq/4.5 kg q12h PO) and antihypertensive medications, such as spironolactone (n = 16; 1–2 mg/kg q12h PO), amlodipine (n = 11; 0.625–1.25 mg/kg q24h) and the angiotensin-converting enzyme inhibitor benazepril (n = 5; 0.25–0.5 mg/kg q24h PO).
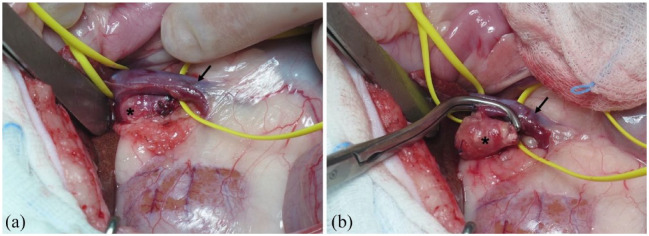
The median anaesthetic time, reported in 25 cats, was 112 mins (range 40–280). Duration of anaesthesia was >4 h in only four cats; the median surgical time (available in 27 cats) was 57 mins (range 18–150). The anaesthesia protocol varied between different institutions. Antibiotic prophylaxis (cephazolin 22 mg/kg IV or ampicillin and sulbactam 20 mg/kg IV) was administered to all cats at induction and repeated every 2 h during surgery. Seven cats (24%) received an antibiotic postoperatively. Venotomy of the caudal vena cava was performed in four cats (14%; Figure 1), and venotomy of the left renal vein was required in one cat (3%) to remove the tumour thrombus.
Figure 1.
(a) Intraoperative image taken during adrenalectomy of a right adrenal mass of 2 × 0.6 × 0.4 cm (*) in a cat with a small thrombus extending through the phrenicoabdominal vein and minimally in the caudal vena cava. Note the caudal vena cava isolated (arrow). (b) After isolation of the adrenal mass, Satinsky forceps have been applied after retracting the neoplastic thrombus in the phrenicoabdominal vein to partially occlude the blood flow in the caudal vena cava (arrow) before venotomy, thrombus removal and suture
Anaesthesia was uncomplicated in 18 cats. Intraoperative anaesthetic complications were reported in nine cats (31%) and included hypotension in seven cats (24%) and episodes of hypertension during manipulation of the adrenal gland in two cats (7%).
Intraoperative surgery-related complications were reported in one cat (3%) and consisted of haemorrhage during dissection of a right adrenal gland with tumour thrombus in the caudal vena cava, requiring a packed red blood cell transfusion. No infiltration of surrounding organs was reported (Figure 2)
Figure 2.

Intraoperative image taken during isolation of a left adrenal mass of 3.5 × 2 × 3 cm (arrow) in contact with the left kidney (*) in which no infiltration was present
In the postoperative period, six complication events occurred in five cats (17%). One fatal complication occurred (3%); this cat had a severely prolonged clotting time, hypoglycaemia, dyspnoea, neurological signs (rotatory nystagmus and seizures) and cardiopulmonary arrest 4 h postoperatively, with unsuccessful resuscitation and death of the cat. A disseminated intravascular coagulopathy was suspected; however, no necropsy was performed. Five minor complications occurred (17%): temporary elevation of urea and creatinine (two cats), mild hyperthermia (two cats) and obstipation (one cat; the same cat also experienced transient azotaemia).
Twenty-eight cats (97%) survived to discharge; the median duration of hospitalisation was 4 days (range 1.5–12).
The adrenal masses were classified as cortical adenoma in 9/28 (32%) cats and as carcinoma in 19/28 (68%); no information regarding the type of tumour was available for one cat.
Spironolactone was suspended immediately after surgery in nine cats and stopped at discharge in the remaining seven cats. Potassium supplementation was suspended in 15 cats immediately after surgery, while it was tapered off over the first week and then stopped in 13 cats.
The serum potassium level was within normal limits 2 weeks to 3 months after surgery in 24 cats. Blood pressure returned to normal limits in 21/23 cats (91%) for which information was available. Hypertension persisted in 2/23 cats (9%) with concomitant chronic kidney failure and treatment with amlodipine was continued.
Follow-up was available for 25 cats; three cats were lost to follow-up. Eleven cats were alive at the time of writing. Information regarding the cause of death was available for 7/14 cats that died or were euthanased after discharge. Two cats underwent euthanasia for chronic kidney disease 9 months and 4 years after surgery, respectively; two cats died after worsening of hypertrophic cardiomyopathy that led to congestive heart failure after 6 and 21 months, respectively. One cat died after 2 years from a neoplasia in the neck and one cat was euthanased due to an abdominal mass in the left abdomen, consistent with a suspected enlarged lymph node with metastasis from an epithelial neoplasia. The cat had previously been diagnosed with a cortical adenoma of the left adrenal gland. Recurrence of the adrenal neoplasia could not be completely excluded; however, it was considered to be unlikely. The recurrence of clinical signs was reported by the owner in one cat; however, additional investigation was refused and the cat died 9 months postoperatively. Median survival time after surgery was 1082 days (range 183–1551; Figure 3).
Figure 3.
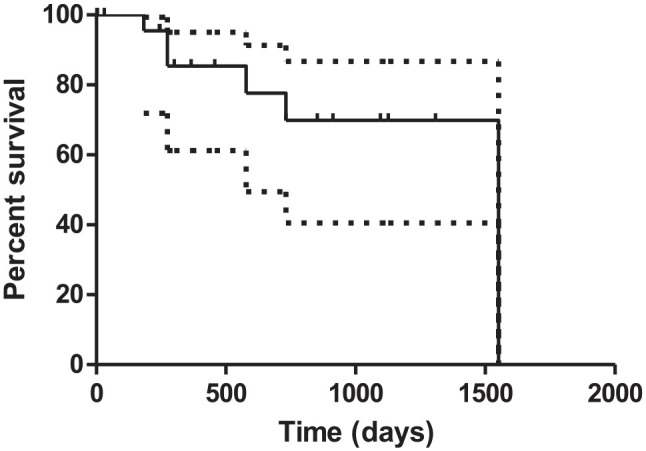
Kaplan–Meier survival curve for 29 cats with aldosteronoma treated surgically. The solid line represents median survival time and the dashed lines the 95% confidence interval. Fourteen cats were censored as they were still alive or lost to follow-up
Discussion
This study confirmed our hypothesis that adrenalectomy was associated with resolution of PHA and with prolonged median survival in cats with a unilateral adrenal mass, with a higher survival rate (97%) than previously reported.4,6,7 In previous studies, survival to discharge ranged from 60% to 80%; the main complications encountered were intra- or postoperative haemorrhage and, less frequently, lethargy.4,6,7 The reason for the improved outcome may be related to the increased awareness of feline PHA and improved perioperative management. It is not possible to exclude the possibility that, in the cats included in the present study, the disease was at an earlier stage than for cases in previous studies.
In the present study, hypotension was the most frequent anaesthetic complication (26%). Lo et al 6 reported this complication in all cats included in their study that underwent adrenalectomy for PHA; they also found that a duration of anaesthesia beyond 4 h increased the risk of death after adrenalectomy. In the present study, only four cats remained anaesthetised for >4 h; the median duration of anaesthesia was 122 mins vs 220 mins in a previous study. 6 This difference may reflect less complicated surgeries in the cats included in the present study and, consequently, a shorter duration of anaesthesia and a decreased risk of death. Another hypothesis is that, thanks to experience and technology, surgeons have, over time, become more accustomed to routinely removing adrenal masses, thus speeding up surgical procedures.
Preoperative medical treatment (potassium supplementation and antihypertensive medications, spironolactone and amlodipine) may represent an important step in stabilising the cardiovascular system before anaesthesia and surgery.
Haemorrhage was the only intraoperative complication reported in one cat with a tumour thrombus in the caudal vena cava. Haemorrhage is a well-recognised complication of adrenalectomy in dogs and cats due to the elevated vascularisation of the adrenal mass and the anatomical proximity to large vessels, namely the caudal vena cava, renal vessels and the associated phrenico-abdominal vein.4,7 Moreover, the presence of vascular neoplastic thrombi makes the surgery more difficult with the need for temporary partial or complete occlusion of the caudal vena cava, venotomy and possible blood loss or anaesthetic complications, such as hypotension and arrhythmias. In dogs, there is contrasting evidence regarding the greater chances of complications and mortality after venotomy for tumour thrombus removal.13–16 In cats, there is no specific information.
The only fatal postoperative complication – observed in one cat – was probably due to the development of disseminated intravascular coagulation. Coagulation abnormalities have been reported in previous studies; in particular, thromboembolism is a well-known complication in dogs that undergo adrenalectomy for hypercortisolism.7,17,18 Currently, there are no clear guidelines for preventing it.
Regarding the minor complications encountered in the postoperative period, hyperthermia was observed in two cats treated with antibiotics. Sepsis has infrequently been reported after adrenalectomy in cats with PHA, 4 while it is a more frequent complication after adrenalectomy for hypercortisolism or bilateral adrenalectomy in cats. 18
In the present study, antibiotic prophylaxis was administered preoperatively in all cats; however, it is not known if preoperative antibiotics are needed or useful in reducing the rate of complications, in particular infection, after adrenalectomy.
In two cats, transient azotaemia or worsening of azotaemia were reported postoperatively, potentially linked to a period of intraoperative hypotension, in cats with chronic kidney disease. 19
Multiple corticosteroid abnormalities have recently been described in cats with hyperaldosteronism;5,7,20 tumours secreting multiple hormones, including cortisol and sex hormones, have been reported.9,21,22 These cats may experience hypocortisolism after adrenalectomy, which can cause lethargy and require corticosteroid supplementation. 7 In the present study, endocrine tests to rule out hypercortisolism were not routinely carried out. The presence of concurrent hypercortisolism was considered unlikely owing to the lack of the typical clinical signs (eg, skin abnormalities and abdominal enlargement), the absence of diabetes mellitus and the atrophy of the non-neoplastic adrenal gland. Additional endocrine tests may be helpful in identifying multiple hormonal abnormalities and for optimal management during the postoperative period. Phaeochromocytoma is rare in cats; however, it represents a differential diagnosis in cats with an adrenal mass and vague clinical signs. 7
Outcome information was available in 25 cats, with 16 being alive or having died from causes unrelated to the adrenal mass. As previously reported, death in cats with PHA successfully undergoing adrenalectomy is usually not related to the adrenal mass itself. These cats frequently suffer from comorbidities, such as cardiomyopathies and chronic renal disease, which can worsen over time and be the cause of death, as confirmed in the present report. 6 Death may have been related to the recurrence of PHA in two cats, but no confirmation was possible.
In this population, the majority of the adrenal masses were cortical carcinomas (69%); it has previously been described that malignancy in the adrenal glands does not appear to be correlated to a worse long-term prognosis after adrenalectomy, which is different to human medicine.4,23 This study corroborated this finding, given the long median survival time (1082 days) for the entire population of included cats. However, the histological classification between adenoma and carcinoma may be challenging and misclassification can occur. One of the cats included in the study with a diagnosis of adrenal adenoma developed a possible lymph node metastasis from an epithelial mass during the follow-up, raising the suspicion of a possible malignant adrenal tumour. Unfortunately, this suspicion of metastasis could not be confirmed with further diagnostic investigations.
The main limitations of the study were inherent to its retrospective and multicentric nature, with underestimation of the complication rate and non-homogenous long-term follow-up, and the low number of cases due to the low prevalence of the disease.
Measurement of aldosteronaemia was unavailable for five cats. In these cases, the clinical signs, blood work abnormalities, presence of an adrenal mass compatible on histology with a cortical tumour, and resolution of the clinical signs, hypokalaemia and hypertension after adrenalectomy were considered sufficient for them to be included in the study.
The cases were collected at different institutions; this could be considered a limitation as different approaches and protocols may have been used. However, it better reflects the different realities in which veterinarians can operate and manage these cases.
Conclusions
Adrenalectomy remains an effective and safe option for the treatment of feline PHA caused by a single adrenal mass. In the present study, adrenalectomy had an excellent outcome with a low rate of complications. In the majority of cats, the clinical signs resolved after surgery, with no long-term treatment needed.
Acknowledgments
The authors would like to thank Professor Francesco Dondi for his support of this study.
Footnotes
Accepted: 5 October 2022
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognised high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Sara Del Magno  https://orcid.org/0000-0002-2900-6989
https://orcid.org/0000-0002-2900-6989
Federico Fracassi  https://orcid.org/0000-0003-3121-2199
https://orcid.org/0000-0003-3121-2199
References
- 1. Kooistra HS. Primary hyperaldosteronism in cats: an underdiagnosed disorder. Vet Clin North Am Small Anim Pract 2020; 50: 1053–1063. [DOI] [PubMed] [Google Scholar]
- 2. Schulman RL. Feline primary hyperaldosteronism. Vet Clin Small Anim 2010; 40: 353–359. [DOI] [PubMed] [Google Scholar]
- 3. Kirkwood N, Boland L, Brunel L, et al. Acute adrenal haemorrhage in two cats with aldosterone-secreting adenocarcinomas. JFMS Open Rep 2019; 5. DOI: 10.1177/2055116919840828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ash RA, Harvey AM, Tasker S. Primary hyperaldosteronism in the cat: a series of 13 cases. J Feline Med Surg 2005; 7: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harro CC, Refsal KR, Shaw N, et al. Retrospective study of aldosterone and progesterone secreting adrenal tumors in 10 cats. J Vet Intern Med 2021; 35: 2159–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lo AJ, Holt DE, Brown DC, et al. Treatment of aldosterone-secreting adrenocortical tumors in cats by unilateral adrenalectomy: 10 cases (2002–2012). J Vet Intern Med 2014; 28: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daniel G, Mahomy OM, Markovich JE, et al. Clinical findings, diagnostics and outcome in 33 cats with adrenals neoplasia (2002–2013). J Feline Med Surg 2016; 18: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Willi B, Kook PH, Quante S, et al. Primary hyperaldosteronism in cats. Schweiz Arch Tierheilkd 2012; 154: 529–537. [DOI] [PubMed] [Google Scholar]
- 9. Yu J, Lenord J, Lau M, et al. Gynaecomastia in a male neutered cat with an adrenal tumour and associated hyperprogesteronism, hypercortisolism and hyperaldosteronism. JFMS Open Rep 2021; 7. DOI: 10.1177/20551169211045640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rose SA, Kyles AE, Labelle P, et al. Adrenalectomy and caval thrombectomy in a cat with primary hyperaldosteronism. J Am Anim Hosp Assoc 2007; 43: 209–214. [DOI] [PubMed] [Google Scholar]
- 11. Del Magno S, Pisoni L, Magarotto J, et al. Two cases of suspect primary hyperaldosteronism in cats. Veterinaria 2012; 26: 41–50. [Google Scholar]
- 12. Follette CM, Giuffrida MA, Balsa IM, et al. A systematic review of criteria used to report complications in soft tissue and oncologic surgical clinical research studies in dogs and cats. Vet Surg 2020; 49: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Massari F, Nicoli S, Romanelli G, et al. Adrenalectomy in dogs with adrenal gland tumors: 52 cases (2002–2008). J Am Vet Med Assoc 2011; 239: 216–221. [DOI] [PubMed] [Google Scholar]
- 14. Barrera JS, Bernard F, Ehrhart EJ, et al. Evaluation of risk factors for outcome associated with adrenal gland tumors with or without invasion of the caudal vena cava and treated via adrenalectomy in dogs: 86 cases (1993–2009). J Am Vet Med Assoc 2013; 242: 1715–1721. [DOI] [PubMed] [Google Scholar]
- 15. Knight RC, Lamb CR, Brockman DJ, et al. Variations in surgical technique for adrenalectomy with caudal vena cava venotomy in 19 dogs. Vet Surg 2019; 48: 751–759. [DOI] [PubMed] [Google Scholar]
- 16. Mayhew PD, Boston SE, Zwingenberger AL, et al. Perioperative morbidity and mortality in dogs with invasive adrenal neoplasms treated by adrenalectomy and cavotomy. Vet Surg 2019; 48: 742–750. [DOI] [PubMed] [Google Scholar]
- 17. Adrin AA, Nelson RW. Adrenal glands. In: Johnston SA, Tobias KM. (eds). Veterinary Surgery small animal. 2nd ed. St Louis, MO: Elsevier, 2018, pp 2281–2291. [Google Scholar]
- 18. Duesberg CA, Nelson RW, Feldman EC, et al. Adrenalectomy for treatment of hyperadrenocorticism in cats: 10 cases (1988–1992). J Am Vet Med Assoc 1995; 207: 1066–1070. [PubMed] [Google Scholar]
- 19. Javadi S, Djajadiningrat-Laanen SC, Kooistra HS, et al. Primary hyperaldosteronism, a mediator of progressive renal disease in cats. Domest Anim Endocrin 2005; 28: 85–104. [DOI] [PubMed] [Google Scholar]
- 20. Langlois DK, Mazaki-Tovi M, Harro CC, et al. Multiple corticosteroid abnormalities in cats with hyperaldosteronism. J Vet Intern Med 2021; 35: 2152–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeClue AE, Breshears LA, Pardo ID, et al. Hyperaldosteronism and hyperprogesteronism in a cat with an adrenal cortical carcinoma. J Vet Intern Med 2005; 19: 355–358. [DOI] [PubMed] [Google Scholar]
- 22. Guerios SD, de Melo Souza CH, Bacon NJ. Adrenocortical tumor in a cat secreting more than one type of corticosteroid. JFMS Open Rep 2015; 1. DOI: 10.1177/2055116915617970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kerkhofs TMA, Verhoeven RHA, van der Zwan JM, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer 2013; 49: 2579–2586. DOI: 10.1016/j.ejca.2013.02.034. [DOI] [PubMed] [Google Scholar]